Abstract
Study Objectives:
Comparable health effects of mandibular advancement device (MAD) and continuous positive airway pressure (CPAP) therapy have been attributed to higher adherence with MAD compared with CPAP therapy. The objective of this study was to make a direct comparison of the objective adherence between MAD and CPAP in patients with moderate obstructive sleep apnea (OSA).
Methods:
Adherence was monitored for 12 months in 59 patients with moderate OSA (apnea-hypopnea index 15–30 events/h) as part of a randomized controlled trial. Objective adherence with MAD was assessed using the TheraMon microsensor. Objective adherence with CPAP was assessed using the built-in registration software with readout on SD card. Self-reported adherence with both therapies was assessed using a questionnaire.
Results:
Forty patients (68%) completed the study with the therapy to which they were randomly assigned. Median (interquartile range) objective adherence (h/night) in the 3rd month was 7.4 (5.2–8.2) for MAD and 6.8 (5.7–7.6) for CPAP (P = .41), compared to 6.9 (3.5–7.9) with MAD and 6.8 (5.2–7.6) with CPAP (P = .85) in the 12th month. There were no significant changes between the 3rd and 12th month for both MAD (P = .21) and CPAP (P = .46). Changes in adherence were not significantly different between MAD and CPAP (P = .51). Self-reported adherence was significantly higher with MAD than CPAP at all follow-ups. Self-reported adherence with CPAP was lower than objective CPAP adherence at the 6th and 12th month (P = .02).
Conclusions:
Objective adherence with MAD and CPAP is comparable and consistent over time. Self-reported adherence is higher with MAD than with CPAP giving rise to interesting discrepancy between objective and self-reported adherence with CPAP.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Identifier: NCT01588275
Citation:
de Vries GE, Hoekema A, Claessen JQPJ, Stellingsma C, Stegenga B, Kerstjens HAM, Wijkstra PJ. Long-term objective adherence to mandibular advancement device therapy versus continuous positive airway pressure in patients with moderate obstructive sleep apnea. J Clin Sleep Med. 2019;15(11):1655–1663.
Keywords: sleep apnea, patient adherence, randomized controlled trial
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although current evidence suggests higher adherence with a mandibular advancement device (MAD) than with continuous positive airway pressure (CPAP) therapy, a direct comparison between the objective adherence profiles of both treatment modalities has not yet been performed in patients with moderate obstructive sleep apnea (OSA).
Study Impact: This study shows that objective adherence with MAD and CPAP therapy is comparable and consistent over time. Self-reported adherence is higher with MAD than with CPAP therapy, and objective adherence with CPAP is higher than self-reported adherence with CPAP. This study enhances the knowledge about adherence rates of two regularly used treatment modalities in moderate OSA. The results do not support the general idea that adherence with MAD is higher compared with CPAP therapy.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder1 characterized by repeated upper airway collapse during sleep resulting in a complete cessation or a substantial reduction in airflow. The repetitive airflow limitation causes intermittent hypoxia, which in turn sets off a chain of events, including activation of the sympathetic nervous system, brief awakenings from sleep (arousals) and sleep fragmentation. Other consequences may include excessive daytime sleepiness, impaired quality of life, an increased risk to become involved in occupational2,3 and traffic accidents,4,5 sick leave and work disability.6 Ultimately, cardiovascular consequences of OSA may include an increased risk of developing systemic hypertension7–9 and cardiovascular disease, such as myocardial infarction, cardiac arrhythmias, and stroke.10–18
As OSA has a large impact on individual health and societal costs, it is important that patients receive appropriate treatment in order to reduce symptoms, comorbidities and its economic burden. Treatment with continuous positive airway pressure (CPAP) prevents upper airway collapse by pneumatically “splinting” the upper airway19 and is the most frequently prescribed treatment for OSA.20 Oral appliance therapy has become an attractive alternative, especially in mild and moderate OSA.21 Mandibular advancement devices (MAD) are oral appliances that advance the mandible in a forward position, thereby aiming at relieving upper airway collapse by modifying the position of the mandible, tongue, and pharyngeal structures. MADs are now recommended in mild and moderate patients who prefer MADs or for patients who do not respond to or fail CPAP therapy.22,23
In moderate OSA (apnea-hypopnea index [AHI] 15–30 events/h) MAD24,25 and CPAP can be considered as primary interventions as both have been proven effective in reducing the AHI. In terms of AHI reduction, MAD is considered less efficacious than CPAP.26–30 However, MAD and CPAP show comparable results on behavioral and other health-related outcomes.31 This comparable effectiveness is attributed to a suggested higher adherence with MAD than with CPAP. The latest systematic review by Schwartz et al27 showed that adherence with MAD was significantly higher than with CPAP, where adherence with MAD was completely based on self-reported usage. Unfortunately, MAD often lacks the technology to objectively assess daily adherence. Recently, objective adherence monitors have become available for MAD therapy. Although evidence suggests a higher (objective) adherence with MAD than with CPAP therapy,30,32,33 a direct comparison between the objective adherence profiles of MAD and CPAP has not yet been performed.34 Therefore, the aim of this study is to assess the long-term objective and self-reported adherence of MAD versus CPAP in patients with moderate OSA.
METHODS
Study design
Patients (aged ≥ 18 years) with an AHI of 15–30 events/h (primarily of the obstructive type) were invited to take part in a parallel multicenter randomized controlled trial, assessing the clinical and cost-effectiveness of MAD versus CPAP. Details about this study, including the complete inclusion and exclusion criteria and data on the total participating group can be found in a separate article.35 This current article describes the adherence monitored in patients of two of the three participating centers. The randomized controlled trial study was approved by the local Ethical Committee (University Medical Center Groningen: METc2010/355, NL34138.042.10) and is registered at ClinicalTrials.gov (NCT01588275). All patients provided written informed consent.
Randomization and Masking
The randomization procedure was performed using a computer program, thereby concealing the allocation sequence from the investigators. Minimization was applied to minimize the imbalance between the number of patients in each group (MAD versus CPAP) regarding cardiovascular parameters (ie hypercholesterolemia, diabetes and hypertension status at baseline). It was not possible to blind patients to the intervention they received.
MAD and CPAP
MAD
Patients randomized to the MAD group were treated with a custom-made titratable bibloc MAD (SomnoDent MAS, SomnoMed Australia/Europe AG). To start, the mandible was set at 70% of the patient’s maximum advancement. The forward position of the mandible with the appliance was adjusted to the convenience of the patient until symptoms abated or until further advancement caused discomfort.
CPAP
Patients randomized to the CPAP group underwent autoCPAP (Philips Respironics REMstar Auto A-Flex, provided by VitalAire BV The Netherlands) for 3 weeks, after which the appropriate fixed CPAP pressure for each individual patient (device provided by the health care provider of the patient) was set by a skilled specialized nurse (ie, highest pressure derived from the Hoffstein formula36 or the 90% criterion (mean pressure ≤ 90% of the time) of the autoCPAP). Patients were fitted with a comfortable mask before titration of the CPAP pressure. Patients were allowed to change their mask during the study and to use chinstraps or a humidifier if required.
Adherence Measurements
Objective adherence with MAD was assessed using a microsensor, the TheraMon Orthosmart BV microsensor (MC Technology GmbH, Hargelsberg, Austria). The safety and feasibility of this method has been shown in a previous study.32 It is 9.0 × 13.0 × 4.5 mm in size, fully covered by acrylic and embedded in the lower part of the MAD. The microsensor measures the existing temperature (setting: minimum 33.5°C and maximum 39.5°C measured every 15 minutes) and stores these values in internal memory. The memory can store the measurement data of approximately 100 days. Objective adherence with CPAP was recorded via the software readout of a built in SD card. Self-reported adherence was assessed using a questionnaire after 3, 6 and 12 months after the start of therapy and asked patients how many days per week and hours per night they generally use their therapy.
Calculation of Adherence
Readouts were collected and questionnaires filled in 3, 6 and 12 months after the start of the therapy. Night to night usage was retrieved for 365 days. The following variables were calculated for the 3rd, 6th and 12th month: (1) objective adherence (h/night) calculated over all registered nights, (2) objective adherence (h/night) calculated over the nights the device was used (ie, > 0 hours), (3) self-reported adherence (h/night), (4) percentage of nights the device was used (ie, > 0 hours) over all registered nights, (5) percentage of nights the device was used ≥ 4 hours calculated over all registered nights, (6) percentage of nights the device was used ≥ 4 hours calculated over those nights the device was used (ie, > 0 hours).
Polysomnography
Patient underwent (ambulatory) polysomnography (PSG) to assess the effectiveness of the therapy at 3 months, and 1 year after the start of therapy. When adjustments were made to the therapy based on the PSG results after 3 months, an extra PSG was performed with the adjusted therapy.
Statistical Analysis
Descriptive statistics are presented as median and interquartile range (IQR) or mean ± standard deviation (SD) for continuous variables dependent on normality. Categorical variables are presented in terms of proportions. Mann-Whitney U tests were performed to assess the difference in adherence between MAD and CPAP at each follow-up (3rd, 6th and 12th month), and to assess the difference between MAD and CPAP in adherence measures over time. Wilcoxon signed rank tests were performed to assess the change in adherence over time for each therapy separately.
In the primary per protocol analysis only patients who completed the entire study period of 1 year using their randomized therapy were included. A second analysis (ie, an intention to treat analysis) was performed, also including the patients who dropped out of the study and patients who switched to the alternative therapy during the study. Adherence for dropouts and patients who switched was scored according to a worst case scenario (ie, adherence after dropping out and switching was scored as zero). Patients who did not start therapy were coded as having missing data.
Data were analyzed with SPSS 23.0 statistical software (IBM, Armonk, New York). A 2-sided P value of < .05 was considered to be statistically significant.
RESULTS
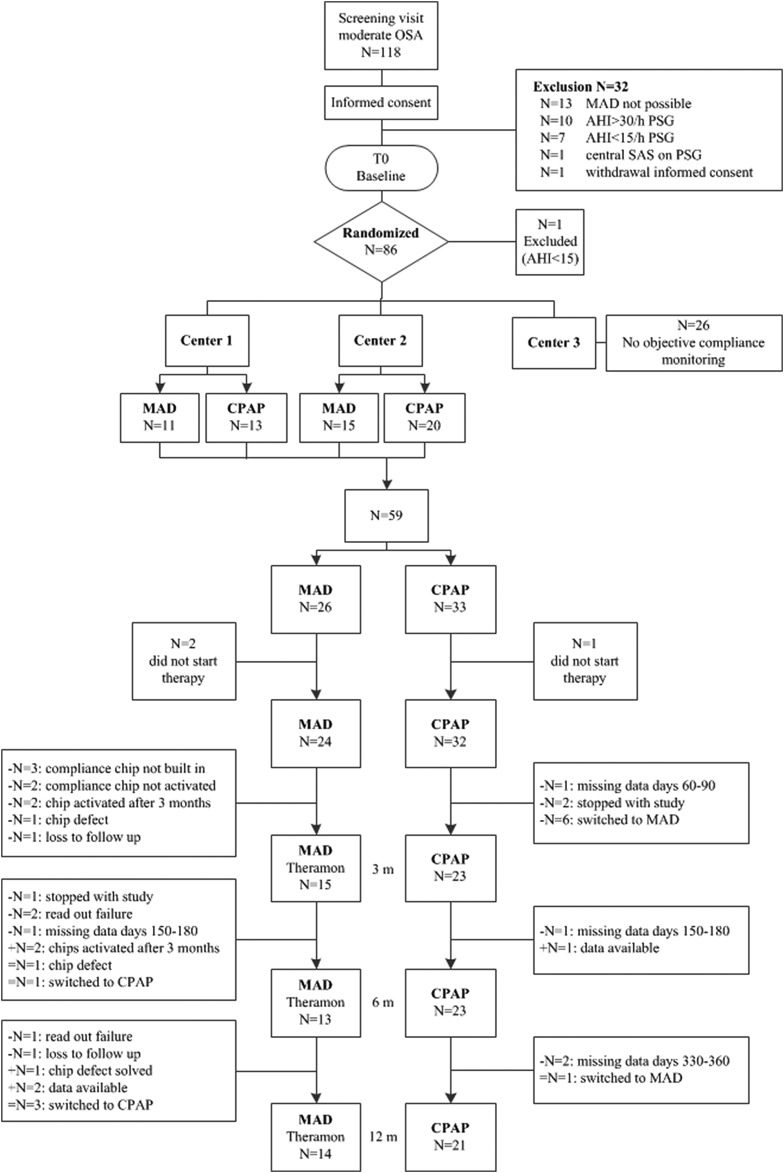
From June 2012 to September 2016, 86 patients were randomized. Daily adherence of both MAD and CPAP was monitored in two of the three participating centers (n = 59). For those 59 participants (age 51.1 ± 9.7 years, AHI 21.3 ± 4.4 events/h, BMI 30.4 ± 4.9 kg/m2, men/women: 50/9) objective and self-reported adherence data were collected (MAD n = 26, CPAP n = 33). There were no significant differences in age, AHI, BMI and Epworth Sleepiness Scale (ESS) score at baseline between both intervention groups and between the 59 patients from the two centers where objective adherence was assessed compared with the patients from the third center.
Three patients did not start with therapy due to different reasons (n = 2 MAD, n = 1 CPAP). In total 56 patients started with the therapy they were randomly assigned to (Figure 1). Three patients stopped during the study (n = 1 MAD, n = 2 CPAP), and two patients were lost to follow-up (n = 2 MAD). Four of the 24 (17%) patients who started MAD switched to CPAP therapy (all treatment failures, n = 1 after 3 months of therapy, n = 3 after 6 months of therapy). In the CPAP group seven of the 32 (22%) patients switched to MAD therapy (all adherence failures, n = 6 after baseline, n = 1 after 6 months of therapy). All patients who switched to the other therapy completed the study.
Figure 1. Flowchart available objective data MAD and CPAP therapy.
AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, MAD = mandibular advancement device, OSA = obstructive sleep apnea, PSG = polysomnography, SAS = sleep apnea syndrome.
Of the 59 randomized patients, 40 patients (68%; MAD n = 17, CPAP n = 23) completed the study with the therapy to which they were randomly assigned. There were no significant differences at baseline in age, AHI, BMI and ESS between both groups. There were no differences at baseline between patients who switched or dropped out versus patients who completed the entire study with their randomized therapy. Figure 1 shows the flowchart for the availability of objective data for the MAD and CPAP group.
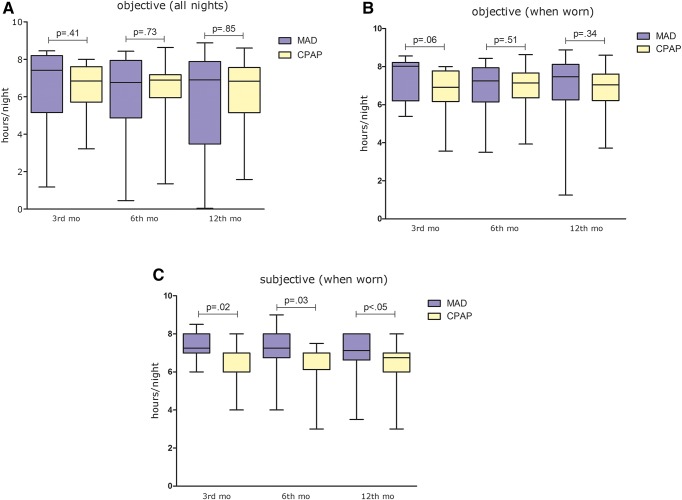
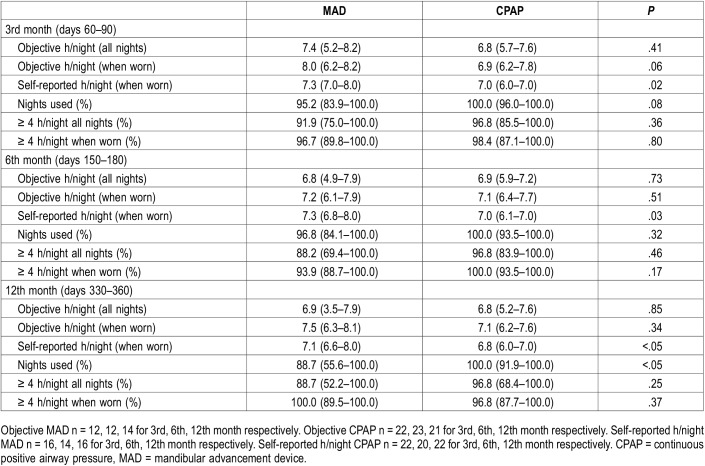
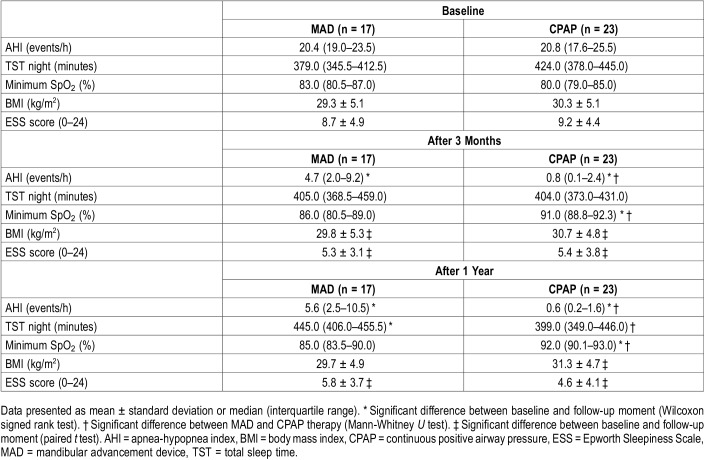
The median (IQR) objective adherence (h/night) in the 3rd month was 7.4 (5.2–8.2) for MAD and 6.8 (5.7–7.6) for CPAP (P = .41). Objective adherence in the 12th month was 6.9 (3.5–7.9) for MAD and 6.8 (5.2–7.6) for CPAP (P = .85) (Figure 2). There were no significant differences in objective adherence between MAD and CPAP at each specific time period, except for the percentage of nights the device was used during the 12th month, which was higher for CPAP when compared with MAD (P < .05). The percentage of nights the device was used for at least 4 hours did not differ between MAD and CPAP therapy (Table 1).
Figure 2. Objective and self-reported adherence in the 3rd, 6th and 12th month with MAD and CPAP.
Boxplots represent the median and interquartile ranges, whiskers represent the minimum and maximum. (A) Median h/night measured over all nights. (B) Median h/night measured over the nights when device was used. (C) Self-reported median h/night measured over the nights when device was used. CPAP = continuous positive airway pressure, MAD = mandibular advancement device.
Table 1.
Objective and self-reported adherence in the 3rd, 6th and 12th month with MAD and CPAP therapy (per protocol analysis).
The objective usage (h/night) was stable over time and there were no significant changes (all nights) between the 3rd and 12th month for both MAD (P = .21) and CPAP therapy (P = .46). Changes in adherence were not significantly different between MAD and CPAP therapy (P = .51). Self-reported adherence was significantly higher with MAD when compared with CPAP at all follow-ups (P = .02, P = .03, P < .05 respectively). There was a significant underestimation of CPAP adherence at the 6th and 12th month (ie, self-reported adherence with CPAP was lower when compared with objective CPAP adherence) (P = .02 for 6 and 12 months).
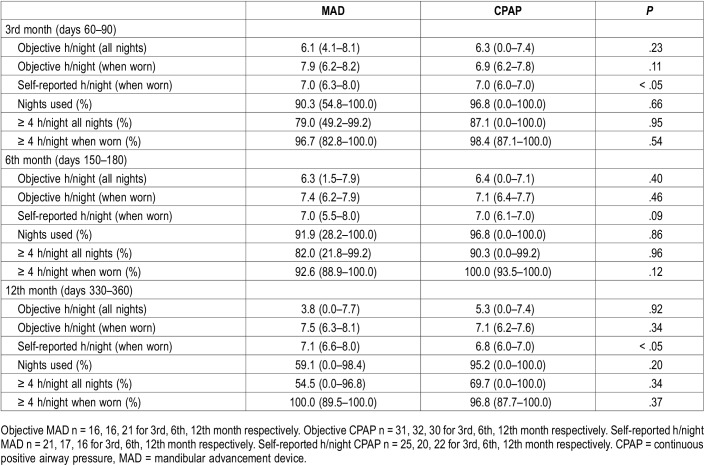
The intention to treat analysis (worst case scenario) showed no significant differences between MAD and CPAP in median objective h/night, percentage nights used > 0 hours and the percentage of nights used ≥ 4 hours (Table 2).
Table 2.
Objective and self-reported adherence for the 3rd, 6th and 12th month with MAD and CPAP therapy (intention to treat analysis).
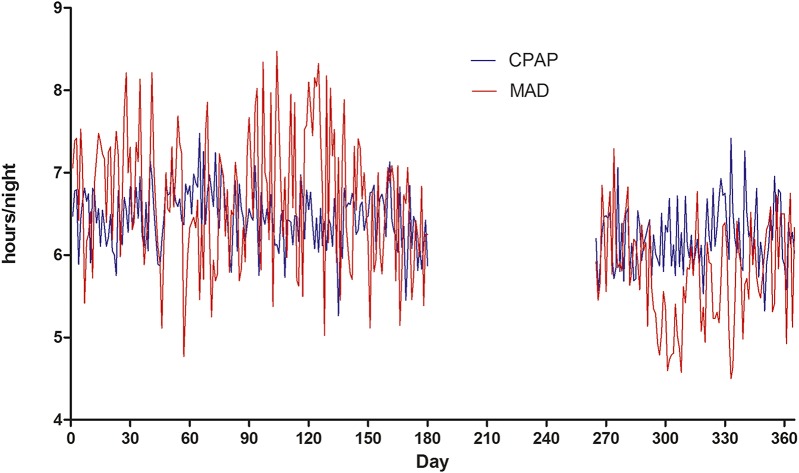
Figure 3 shows the mean objective adherence (h/night) with MAD and CPAP therapy, over a time period of 1 year. Days 181–264 of MAD could not be displayed as the memory card can only store from the preceding 100 days and therefore data is missing for this specific time period.
Figure 3. Mean usage for MAD and CPAP therapy over 365 days.
Days 181-264 of MAD could not be displayed as the memory card can only store from the preceding 100 days and therefore data is missing for this specific time period. CPAP = continuous positive airway pressure, MAD = mandibular advancement device.
AHI significantly reduced with both MAD and CPAP. However, the AHI reduction with CPAP was significantly larger than with MAD (Table 3).
Table 3.
Polysomnographic outcomes, body mass index and ESS scores for baseline, and 3 and 12 months with therapy (n = 40).
DISCUSSION
To the best of our knowledge, this is the first study showing that objective adherence with MAD and CPAP is comparable and consistent over time. Self-reported adherence is higher with MAD than with CPAP and objective adherence with CPAP is higher than self-reported adherence with CPAP.
Objective and Self-Reported Adherence
In general, nonadherence with therapy is an important and well-known problem. In our study 8 patients dropped out of the study, and in total 11 patients switched to the other therapy (n = 4, MAD to CPAP; n = 7 CPAP to MAD). After 1 year, 17 (65%) patients could be considered continuing users of MAD and 23 (70%) patients as continuing users of CPAP.
In the group of continuing users we did not find significant differences in adherence between MAD and CPAP therapy in both the 3rd and 12th month. The percentage of nights the device was used for at least 4 hours was high for both MAD and CPAP and did not significantly differ between both treatment modalities.
The objective MAD adherence at the 3-month follow-up is comparable with the results found by Vanderveken et al32 and Dieltjens et al33 (median [IQR] of 7.0 [5.9–7.6]). Furthermore, adherence (h/night) was stable over time as there were no significant changes between the 3rd and 12th month for both MAD (P = .21) and CPAP therapy (P = .46). This result is comparable with the results found by Dieltjens et al,33 who also showed a stable median use rate over 1 year in continuing MAD users.
When comparing the adherence rate of CPAP found in our study, we observed a higher therapeutic adherence when compared with most other studies. In our study, CPAP was used for a median (IQR) of 6.8 (5.7–7.6) h/night in the 3rd month (mean ± SD 6.6 ± 1.2 h/night), while the mean CPAP use, based on 66 studies, reported by Rotenberg et al37 was 4.6 h/night. When taking the dropouts and patients who switched to MAD into account (intention to treat analysis—worst-case scenario) CPAP was used for a median (IQR) of 6.3 (0.0–7.4) h/night (mean ± SD 4.7 ± 3.2 h/night). The higher CPAP adherence rate in our group of continuing users might be explained by the fact that patients were not blinded to the aims of the study and were present when read outs were performed. The continued and close follow-up of patients could have led to higher adherence rates. Furthermore, all participants were willing to be randomized to either MAD or CPAP. This entails that patients did not have an a priori aversion against CPAP, which might have led to the higher adherence rates compared with other studies, where in some cases patients only had CPAP therapy as a treatment option. Although more patients randomized to CPAP experienced comfort (and thereby adherence) problems, the intention to treat analysis, including those patients who dropped out and switched to the other therapy, showed the same results as the per protocol analysis. It can be concluded that when the patient accepts CPAP, adherence is comparable with MAD.
Self-reported adherence was significantly higher with MAD than with CPAP at all follow-ups (P = .02, P = .03, P < .05 for 3, 6 and 12 months, respectively). This difference could largely be explained by the significant underestimation of CPAP usage durintt the 6th and 12th month. This finding contrasts to what is generally observed in literature, namely a higher self-reported adherence when compared with objective adherence. The mismatch in CPAP users in our study might be the result of a decrease in total sleep time (TST) with CPAP (while TST in the MAD group increased), giving the patient using CPAP a feeling of shorter usage time.
Strengths and Limitations
The strength of this study is that objective adherence was obtained in a randomized controlled trial, giving us the opportunity to directly compare adherence rates of MAD versus CPAP. Furthermore, data were collected over a period of 1 year.
Our study has some limitations. In our study patients were not blinded and were aware of the fact that adherence was monitored. The patients were physically present when readouts were collected as it was part of the outpatient control visits and patients were instantly informed about their adherence rates. The consequence of this procedure could be that patients use their device more frequently and during longer periods. However, the results of this study show comparable adherence rates found in other studies assessing objective MAD adherence. For CPAP however, this could have led to a higher adherence rate compared with other studies.
Future Perspectives
There is still debate whether the commonly used cutoff value of 4 h/night38 is clinically relevant. The evidence for the use of dichotomizing patients in two groups based on this cutoff value and assessing long-term outcomes in those two groups is limited. Although this study is an important step forward in the knowledge of objective adherence of MAD compared to CPAP therapy, more studies are needed to assess the effects of MAD (and CPAP) adherence on long-term (ie, 10 years) quality of life and cardiovascular outcomes.39
CONCLUSIONS
This study is the first to directly compare long-term objective night-to-night adherence with MAD versus CPAP in patients with moderate OSA. Objective adherence with MAD and CPAP was comparable and consistent over time. Self-reported adherence was higher with MAD than with CPAP and objective adherence with CPAP was higher than self-reported adherence with CPAP.
DISCLOSURE STATEMENT
The deceased author qualifies as having made an authorship-worthy contribution to the paper. The deceased author knew the paper was being submitted and agreed to that prior to death. This study was funded by SomnoMed Goedegebuure and VitalAire Nederland BV. GEV, AH and PJW report grants from SomnoMed Goedegebuure and from VitalAire Nederland BV, during the conduct of the study. AH reports personal fees for being Medical advisor from Somnomed, Airway Management, and Zephyr Sleep Technologies, outside the submitted work. PJW reports grants and personal fees from Philips, grants and personal fees from RESMED, grants from VIVISOL, personal fees from Synapse, and from Bresotec, outside the submitted work. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Figures: GEDV, PJW; study design: GEDV, AH, BS, HAMK, PJW; data collection: GEDV, JQPJC, CS, PJW; data analysis: GEDV, AH, BS, HAMK, PJW; data interpretation: GEDV, AH, HAMK, PJW; writing: GEDV, AH, JQPJC, CS, BS, HAMK, PJW.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- MAD
mandibular advancement device
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindberg E, Carter N, Gislason T, Janson C. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164(11):2031–2035. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 3.Sjösten N, Kivimaki M, Oksanen T, et al. Obstructive sleep apnoea syndrome as a predictor of work disability. Respir Med. 2009;103(7):1047–1055. doi: 10.1016/j.rmed.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Rodenstein D. Sleep apnea: traffic and occupational accidents–individual risks, socioeconomic and legal implications. Respiration. 2009;78(3):241–248. doi: 10.1159/000222811. [DOI] [PubMed] [Google Scholar]
- 5.Terán-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. cooperative group burgos-santander. N Engl J Med. 1999;340(11):847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 6.Sivertsen B, Overland S, Glozier N, Bjorvatn B, Maeland JG, Mykletun A. The effect of OSAS on sick leave and work disability. Eur Respir J. 2008;32(6):1497–1503. doi: 10.1183/09031936.00044908. [DOI] [PubMed] [Google Scholar]
- 7.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Floras JS. Hypertension and sleep apnea. Can J Cardiol. 2015;31(7):889–897. doi: 10.1016/j.cjca.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166(2):159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 11.Bradley TD, Floras JS. Sleep apnea and heart failure: part I: obstructive sleep apnea. Circulation. 2003;107(12):1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 12.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 13.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 14.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 16.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 17.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126(12):1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 18.Al-Falahi Z, Williamson J, Dimitri H. Atrial fibrillation and sleep apnoea: guilt by association? Heart Lung Circ. 2017;26(9):902–910. doi: 10.1016/j.hlc.2017.05.127. [DOI] [PubMed] [Google Scholar]
- 19.Schwab RJ, Pack AI, Gupta KB, et al. Upper airway and soft tissue structural changes induced by CPAP in normal subjects. Am J Respir Crit Care Med. 1996;154(4 Pt 1):1106–1116. doi: 10.1164/ajrccm.154.4.8887615. [DOI] [PubMed] [Google Scholar]
- 20.Giles TL, Lasserson TJ, Smith BH, White J, Wright JJ, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;1:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF, de Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008;87(9):882–887. doi: 10.1177/154405910808700917. [DOI] [PubMed] [Google Scholar]
- 22.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 23.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serra-Torres S, Bellot-Arcis C, Montiel-Company JM, Marco-Algarra J, Almerich-Silla JM. Effectiveness of mandibular advancement appliances in treating obstructive sleep apnea syndrome: a systematic review. Laryngoscope. 2016;126(2):507–514. doi: 10.1002/lary.25505. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Long H, Jian F, et al. The effectiveness of oral appliances for obstructive sleep apnea syndrome: a meta-analysis. J Dent. 2015;43(12):1394–1402. doi: 10.1016/j.jdent.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Marklund M. Update on oral appliance therapy for OSA. Curr Sleep Med Rep. 2017;3(3):143–151. doi: 10.1007/s40675-017-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz M, Acosta L, Hung YL, Padilla M, Enciso R. Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Breath. 2018;22(3):555–568. doi: 10.1007/s11325-017-1590-6. [DOI] [PubMed] [Google Scholar]
- 28.Sharples L, Glover M, Clutterbuck-James A, et al. Clinical effectiveness and cost-effectiveness results from the randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO) and long-term economic analysis of oral devices and continuous positive airway pressure. Health Technol Assess. 2014;18(67) doi: 10.3310/hta18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharples LD, Clutterbuck-James AL, Glover MJ, et al. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev. 2016;27:108–124. doi: 10.1016/j.smrv.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutherland K, Phillips CL, Cistulli PA. Efficacy versus effectiveness in the treatment of obstructive sleep apnea: CPAP and oral appliances. J Dent Sleep Med. 2015;2(4):175–181. [Google Scholar]
- 31.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 32.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91–96. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dieltjens M, Braem MJ, Vroegop AVMT, et al. Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013;144(5):1495–1502. doi: 10.1378/chest.13-0613. [DOI] [PubMed] [Google Scholar]
- 34.Hamoda MM, Kohzuka Y, Almeida FR. Oral appliances for the management of OSA: an updated review of the literature. Chest. 2018;153(2):544–553. doi: 10.1016/j.chest.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 35.de Vries GE, Hoekema A, Vermeulen KM, et al. Clinical- and cost-effectiveness of a mandibular advancement device versus continuous positive airway pressure in moderate obstructive sleep apnea. J Clin Sleep Med. 2019 doi: 10.5664/jcsm.7980. Sep 5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miljeteig H, Hoffstein V. Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea. Am Rev Respir Dis. 1993;147(6 Pt 1):1526–1530. doi: 10.1164/ajrccm/147.6_Pt_1.1526. [DOI] [PubMed] [Google Scholar]
- 37.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1) doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 39.de Vries GE, Wijkstra PJ, Houwerzijl EJ, Kerstjens HAM, Hoekema A. Cardiovascular effects of oral appliance therapy in obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:55–68. doi: 10.1016/j.smrv.2017.10.004. [DOI] [PubMed] [Google Scholar]