Abstract
Study Objectives:
Decreased upper-airway muscle responsiveness is one of the major phenotypes of obstructive sleep apnea. Use of α1-adrenergic antagonists is correlated with decreased muscle responsiveness in animal studies, but this association has not yet been demonstrated in humans. This study examined whether use of α1-adrenergic antagonists is an independent risk factor for sleep apnea in humans.
Methods:
Data for this retrospective cohort study were obtained from the National Health Insurance Research Database from Taiwan. Between 2000 and 2012, 25,466 patients with hypertension and 18,930 patients without hypertension were enrolled. These groups were divided into α1-adrenergic antagonist users and nonusers, matched by age, sex, and index year. Individuals were monitored for diagnosis of sleep apnea until 2013.
Results:
After adjusting for propensity score and potential confounders, including age, geographic location, enrollee category, income, urbanization level, comorbidities, and medication, the adjusted hazard ratios (HRs) for development of sleep apnea with α1-adrenergic antagonist use were 2.38 (95% confidence interval [CI] 1.82–3.10) and 2.82 (95% CI 1.79–4.44) in the hypertension and nonhypertension groups, respectively. Similarly, the adjusted HRs for development of severe sleep apnea with α1-adrenergic antagonist use were 2.74 (95% CI 1.78–4.22) and 4.23 (95% CI 1.57–11.40) in hypertension and nonhypertension patient groups, respectively. The interaction between α1-adrenergic-antagonist user and patients with hypertension was tested using multivariable Cox regression. The results showed that there are positive additive interactions for developing sleep apnea and severe sleep apnea, respectively.
Conclusions:
Our study suggests that patients with hypertension using α1-adrenergic antagonists have a higher risk of sleep apnea. Routine sleep apnea screening would be beneficial for patients with hypertension who take α1-adrenergic antagonists.
Citation:
Su P-L, Lin W-K, Lin C-Y, Lin S-H. Alpha-1 adrenergic-antagonist use increases the risk of sleep apnea: a nationwide population-based cohort study. J Clin Sleep Med. 2019;15(11):1571–1579.
Keywords: α 1-adrenergic antagonist, hypertension, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Decreased upper airway muscle responsiveness, particularly genioglossus muscle activity, is one of the phenotypes of obstructive sleep apnea. Although the use of α1-adrenergic antagonist agents has been shown to decrease genioglossus activity through suppression of hypoglossal nucleus motor neuron activity in animal studies, this concept has not yet been examined in humans because of ethical issues.
Study Impact: In this study, we conducted a large-scale population-based cohort study and found that α1-adrenergic antagonist use is associated with a higher risk of sleep apnea, both in patients with hypertension and those without hypertension. As such, early detection of sleep apnea would be beneficial for patients taking α1-adrenergic antagonist agents.
INTRODUCTION
Obstructive sleep apnea (OSA) is an increasingly common disorder, characterized by repetitive pharyngeal narrowing and collapse during sleep,1 which affects about 49.7% and 23.4% of the male and female population, respectively.2 People with OSA may either experience complete pharyngeal collapse, causing apnea, or partial narrowing, causing hypopnea, and then progress to intermittent hypoxemia and sleep fragmentation.1 Although continuous positive airway pressure (CPAP) therapy can resolve sleep-disordered breathing, about half of patients with OSA are intolerant of or poorly adherent to this therapy.1 Thus, a phenotypic approach may help the management of OSA.
Insufficient upper-airway muscle responsiveness to negative pharyngeal pressure is one of the major phenotypes in the pathophysiology of OSA.3–6 Genioglossus muscle activity plays an important role in insufficient upper-airway muscle responsiveness.7 In an animal study, genioglossus muscle activity during sleep was found to increase after injection of norepinephrine into the hypoglossal motor nucleus.8 It implied that norepinephrine could increase genioglossus muscle activity. Previous in vitro studies have also proven that norepinephrine can depolarize and increase the excitability of hypoglossal motor neurons in brainstem tissue slices.9–11 However, genioglossus activity decreased significantly with microdialysis perfusion of terazosin, a type of α1-adrenergic antagonist, into the hypoglossal motor nucleus.12 Another α1-adrenergic antagonist, tamsulosin, also caused exacerbation of OSA, according to a case report.13
There is a high incidence of hypertension among patients with OSA, and conversely, OSA is an independent risk factor for hypertension.14 The α1-adrenergic antagonists are also widely used to control hypertension, especially in refractory cases.15 According to the afore-mentioned literature, patients with hypertension who use α1-adrenergic antagonists may have more severe OSA.
To our knowledge, no large-scale human intervention study has focused on the relationship between the use of α1-adrenergic antagonists and the development of sleep apnea, because of ethical issues. Using data deposited in the National Health Insurance Database, this population-based cohort study aimed to determine whether the use of α1-adrenergic antagonist agents is an independent risk factor for the development of sleep apnea. Furthermore, we investigated the incidence of sleep apnea among patients with hypertension who were taking α1-adrenergic antagonists.
METHODS
Design
This study was designed as a longitudinal, observational, retrospective cohort study. We enrolled a group of people exhibiting hypertension within a defined period as the hypertension study group. From the same population, we enrolled a comparison group of people who did not exhibit hypertension. In each group, we further classified people based on whether they did or did not use α1-adrenergic antagonists. The use of α1-adrenergic antagonist agents was defined as prescription at the outpatient department and patients having prescription records at least three times in one year were included. Therefore, people in both groups (hypertension and nonhypertension) were divided into α1-adrenergic-antagonist user and nonuser subgroups. We then followed the outcomes of these four groups, including sleep apnea and severe sleep apnea, over time. The study was approved by the ethics committee and institutional review board of National Cheng Kung University Hospital (Institutional Review Board number: B-ER-107-302).
Database
The National Health Insurance (NHI) of Taiwan is a universal health insurance plan that has been in operation since 1995. The NHI of Taiwan provides coverage to almost 97% of the nation’s population of 23 million.16 The NHI Research Database (NHIRD) of Taiwan uses the claims data from the NHI to extract numerous datasets for researchers, which are deposited in various databases. The data analyzed in the current study were obtained from the Longitudinal Health Insurance Database 2000, which contains one million insured people randomly selected from the total population. This database covers NHI claims data from 2000 to 2013. There are no statistically significant differences in age, sex, or health care costs between the sample group and the total population. This database has been widely used for studies on epidemiology, prescription use, disease diagnosis, and hospitalization, and has been shown to be of high quality.16 Identifying information for all patients is encrypted to protect personal privacy.
Study Sample
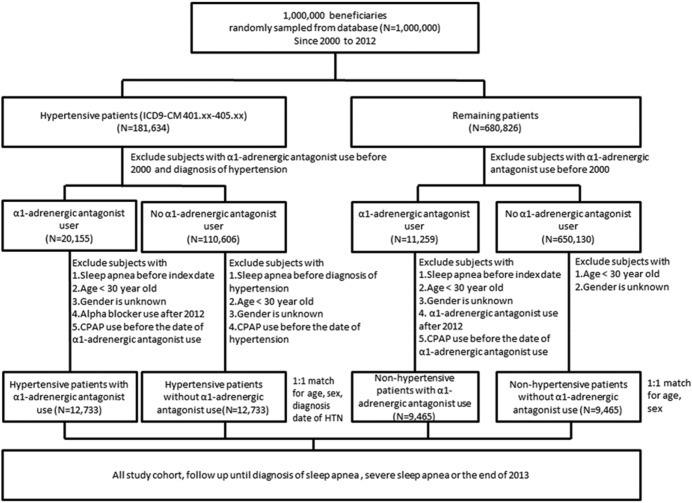
The study sample in this retrospective cohort study was divided into four groups. Diagnosis codes were assigned to patients according to the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM). Initially, patients with diagnosis code HTN (401–405), assigned between January 2000 and December 2012, were included in the hypertension group. When enrolling patients with HTN, we excluded those who were taking alpha blockers, using noninvasive positive pressure ventilation, or had a diagnosis of sleep apnea (ICD-9-CM codes 780.51, 780.53, 780.57) before January 2000 or prior to diagnosis of hypertension. We also excluded patients younger than 30 years, to focus on the higher-risk population of older adults, and assessed the effects of an α1-adrenergic-antagonist use on their risk of developing sleep apnea or severe sleep apnea. A total of 25,466 patients with newly diagnosed hypertension were included in the study (Figure 1). Each individual was followed until 2013.
Figure 1. Study flow chart.
CPAP = continuous positive airway pressure.
Matching
Frequency matching, also known as category or group matching, was used to ensure an equal distribution of variables among study groups. We applied 1:1 frequency matching to ensure that patients who did or did not use α1-adrenergic antagonists were equally distributed among the strata defined by sex, age (three categories, 30–44 years, 45–60 years, and older than 60 years), and year of receiving medical care.17,18
Potential Confounders
Enrollee category (EC) was used as a proxy measure of socioeconomic status to classify participants into four subgroups: EC 1 (full-time or regularly paid personnel of public schools, governmental agencies, or the civil service), EC 2 (employees of privately owned enterprises), EC 3 (other employees or paid personnel, members of farmer or fisher associations, or self-employed individuals), and EC 4 (members of low-income families, substitute-services draftees, and veterans). Relative to income, the cost of health insurance was most expensive for EC 1 members, followed by EC 2, EC 3, and then EC 4.
To evaluate the association between urbanization level and sleep apnea, the participants’ residence locations were divided into three categories: urban, suburban, and rural. Locations were classified based on the following five variables: population density, the percentage of residents who were agricultural workers, the number of physicians per 100,000 people, the percentage of residents with college or higher education, and the percentage of residents who were age 65 years or older.19 In general, residents of rural areas had the lowest socioeconomic status.
We identified the following potential confounding risk factors for sleep apnea across all individuals: hypertension, type 2 diabetes mellitus (DM), atherosclerotic vascular disease (ASVD), hyperlipidemia, asthma, chronic obstructive pulmonary disease (COPD), allergic rhinitis, diseases of the musculoskeletal system and connective tissue, and obesity.20–24 We adjusted for benign prostate hyperplasia and aortic dissection in multivariate analyses, because these diseases are strongly associated with α1-adrenergic antagonist use.25,26 We also used the ATC codes to adjust for the use of the following hypertension medications: beta blockers, thiazide-type diuretics, other diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, and direct renin inhibitors.
Main Outcome Measure
The endpoint of the study was defined as the development of sleep apnea or severe sleep apnea. Drug-related sleep apnea was defined by the following two criteria: (1) an ICD-9-CM examination code of polysomnography, followed by an ICD-9-CM diagnostic code of 780.51, 780.53, or 780.57 in the same year27; and (2) use of an α1-adrenergic antagonist agent for more than 1 month before diagnosis of sleep apnea. The definition of severe sleep apnea was based on the ICD-9-CM code for positive pressure ventilation.28
Validation
We validated the ICD-9-CM codes for the identification of HTN by analyzing the medical records (charts) of patients treated at the National Cheng Kung University Hospital, a 1,200-bed tertiary referral hospital in Taiwan. We randomly selected 200 patients who had HTN ICD-9-CM codes of 401.0, 401.1, or 401.9 from the inpatient and outpatient claims database for January 2008 to December 2010 in National Cheng Kung University Hospital. The contents of this database were similar to those of the NHIRD. The clinical diagnosis of hypertension was ascertained based on the definition used by the American College of Cardiology and the American Heart Association’s Task Force on Clinical Practice Guidelines, which stipulates that systolic blood pressure must be ≥ 130 mmHg or diastolic blood pressure must be ≥ 80 mmHg.29 The results showed a positive predictive value of 95% (95% confidence interval [CI], 88.8–97.9%) for HTN.
Statistical Analysis
Baseline descriptive data are presented as the mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. Pearson chi-square test or Fisher exact test were used to compare the demographic and clinical characteristics between hypertensive patients who did or did not use α1-adrenergic antagonists, as well as between patients without hypertension who did or did not use α1-adrenergic antagonists. The hazard ratios (HRs) were analyzed using multivariable Cox proportional hazards models with adjustment of propensity score and other potential confounders. To estimate the propensity score (ie, the probability of using an α1-adrenergic antagonist agent) for each participant, patient characteristics were entered into a logistic regression model as the independent variables. These characteristics included age, sex, area of residence, enrollee category, monthly income, level of urbanization, and comorbidities. We then analyzed the first adjusted HRs (adjusted HR1) with adjustment of propensity score and the second HRs (adjusted HR2) with adjustment of propensity score and potential confounders. The HRs that violated the assumption of proportional hazards were calculated using a stratified Cox regression model.30 We also used competing-risk models to adjust for risk of death, because death may act as a competing risk for sleep apnea and severe sleep apnea.31 Statistical analysis was carried out using SAS software, version 9.4 (SAS Institute, Cary, North Carolina, USA).
Sensitivity Analysis
To investigate the effect of other potential residual confounding factors on the observed results, we conducted a sensitivity analysis using the R package “obsSens.”32 In this analysis, we added another hypothetical unmeasured confounding factor with a similar risk effect size as our disease variable. We then tested whether this additional factor confounded our observation with respect to differences in prevalence between the α1-adrenergic-antagonist user and nonuser groups.
RESULTS
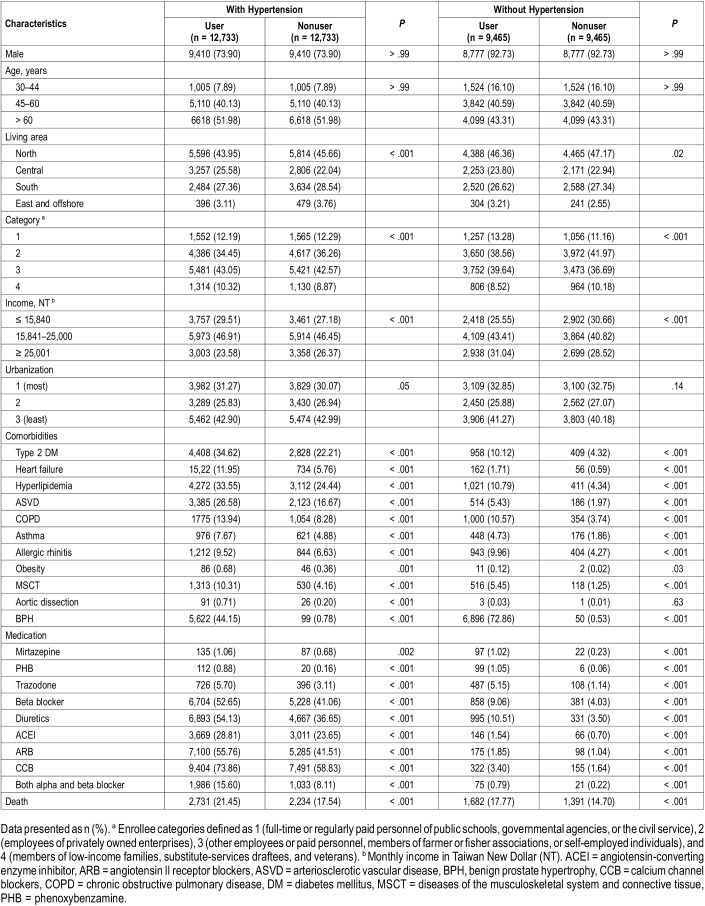
A total of 25,466 patients with hypertension and 18,930 patients without hypertension were included in this study, most of whom were male (81.9%) and a large proportion of whom were older than 60 years (48.3%; Table 1). Patients who used α1-adrenergic antagonists had higher rates of comorbidities, such as DM, hyperlipidemia, and asthma. The use of medication for cardiovascular disease was also higher in patients who used α1-adrenergic antagonists. In the hypertension group, the average follow-up times were 64.5 ± 41.6 and 71.0 ± 42.1 months, respectively, for patients who did or did not use α1-adrenergic antagonists. In the group without hypertension, the average follow-up times were 64.5 ± 42.4 and 72.6 ± 42.3 months, respectively, for patients who did or did not use α1-adrenergic antagonists.
Table 1.
Demographic information and premorbid comorbidities for the cohort of sampled patients, stratified by hypertension and use of α1-adrenergic antagonist.
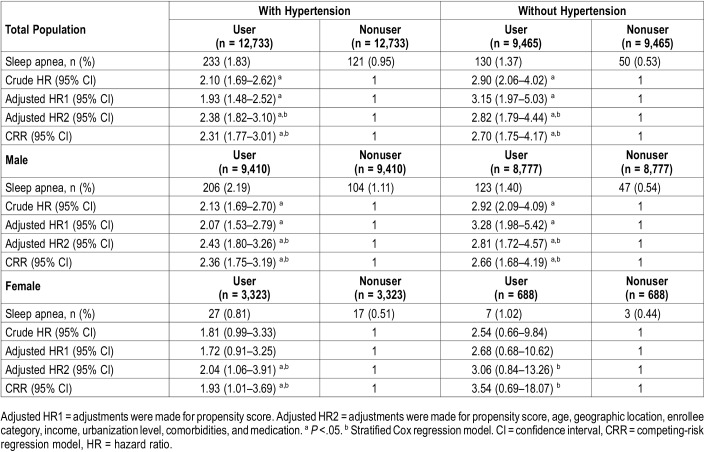
The incidence rate of sleep apnea was 1.39% in the hypertension group and 0.95% in the group without hypertension (Table 2). Sleep apnea was more likely to develop during the follow-up period in patients using α1-adrenergic antagonists, with crude HRs of 2.10 and 2.90 in the hypertension and no hypertension groups, respectively. After adjusting for propensity score and other potential confounders, the HRs for developing sleep apnea with α1-adrenergic antagonist use were 2.38 and 2.82 in the hypertension and no hypertension groups, respectively. Further, when death was considered as a competing risk factor for sleep apnea, the HRs of the competing-risk regression (CRR) model showed similar results. With stratification by sex, α1-adrenergic antagonist use was found to be a significant sleep apnea risk for both male and female patients with hypertension, but only for male patients without hypertension.
Table 2.
Multivariable analysis of hazard ratios of sleep apnea stratified by sex, hypertension and use of α1-adrenergic antagonist.
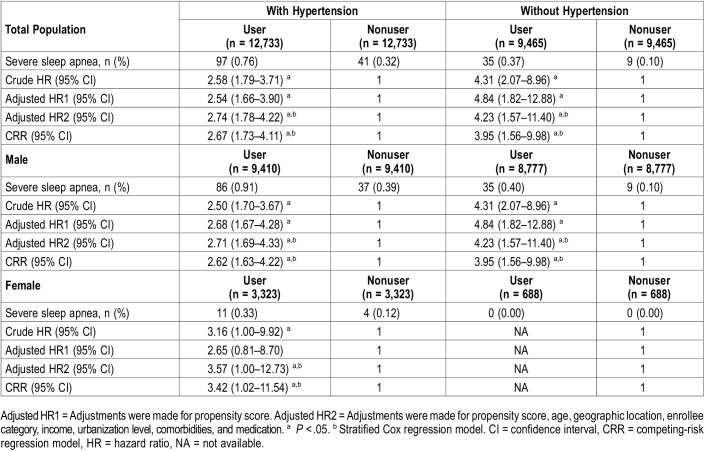
The incidence rates of severe sleep apnea were 0.54% and 0.23% in the hypertension and no hypertension groups, respectively (Table 3). Severe sleep apnea was more likely to develop during the follow-up period in patients using α1-adrenergic antagonists, with crude HRs of 2.58 and 4.31 in patients with hypertension and those without hypertension, respectively. After adjusting for the propensity score and other potential confounders, the HRs for developing severe sleep apnea with α1-adrenergic antagonist use were 2.74 and 4.23 in patients with and those without hypertension, respectively. The HRs of the CRR model also showed similar results when death was considered as a competing risk factor for severe sleep apnea. The α1-adrenergic antagonist use was found to be a significant risk factor for severe sleep apnea among both male and female patients with hypertension, but only for male patients without hypertension. The incidence of severe sleep apnea among female patients without hypertension was too low to estimate the HR.
Table 3.
Multivariable analysis of hazard ratios of severe sleep apnea stratified by sex, hypertension and use of α1-adrenergic antagonist.
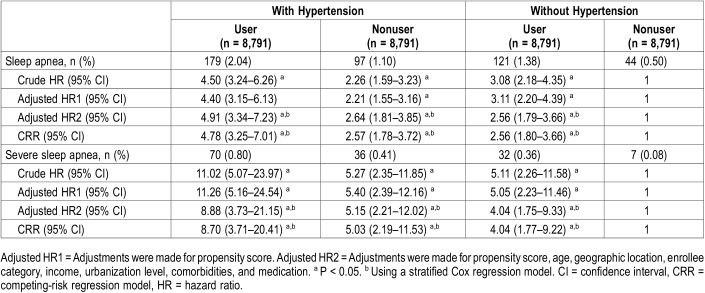
To compare the HRs among patients with and without hypertension, we also used frequency matching between patients with and without hypertension, in α1-adrenergic-antagonist user and nonuser groups. After matching, there were 8791 patients in each group available for analysis (Table 4). The interaction between α1-adrenergic-antagonist user and patients with hypertension was tested using multivariable Cox regression. The results showed that there is a positive additive interaction, but an absence of multiplicative interaction. Using patients without hypertension who did not use α1-adrenergic antagonists as the reference, the crude HRs for developing sleep apnea were 3.08, 2.26, and 4.50, respectively, in the following groups: patients without hypertension using α1-adrenergic antagonists, patients with hypertension not using α1-adrenergic antagonists, and patients with hypertension using α1-adrenergic antagonists. After adjusting for propensity score and other potential confounders, the HRs for developing sleep apnea during the follow-up period were 2.56, 2.64, and 4.91, respectively, in the same three groups. When death was considered as a competing risk factor for sleep apnea, the CRR model showed the similar results. Additionally, the crude HRs for developing severe sleep apnea were 5.11, 5.27, and 11.02, respectively, in the same three groups. After adjusting for propensity score and other potential confounders, the HRs for developing severe sleep apnea during the follow-up period were 4.04, 5.15, and 8.88, respectively. When death was considered as a competing risk factor for severe sleep apnea, the CRR model also showed the similar results.
Table 4.
Multivariable analysis of hazard ratios of sleep apnea and severe sleep apnea, patients without hypertension and nonα1-adrenergic antagonist use cohort as reference.
To evaluate the time-dependent effect on the risk of sleep apnea, we classify the follow-up period into years with α1-adrenergic antagonist use and years without α1-adrenergic antagonist use, then perform the time-dependent survival analysis. The use of α1-adrenergic antagonist is still associated increased risk of sleep apnea (Table S1 and Table S2 in the supplemental material).
To investigate the effect of other potential residual confounding factors on the observed results, we conducted a sensitivity analysis to investigate the trend estimates for the HR of sleep apnea in the hypertension group and no hypertension group using a multivariable-adjusted Cox regression model with the addition of a residual confounding factor (Figure S1, Figure S2, Figure S3 and Figure S4 in the supplemental material). For example, when an additional residual confounder was present in all patients with hypertension not using α1-adrenergic antagonists (the prevalence of the unmeasured confounder was 1.0) and none of the patients with hypertension using α1-adrenergic antagonists had this residual confounder (the prevalence of the unmeasured confounder was 0.0), α1-adrenergic-antagonist use would be a risk for sleep apnea (HR = 5.47, the top line in Figure S1). Figure S1 and Figure S2 show that in all situations, both patients with and without hypertension who received α1-adrenergic antagonists had a higher risk of sleep apnea occurrence relative to patients who did not take α1-adrenergic antagonists, even if an unmeasured confounder existed. Figure S3 and Figure S4 show similar results for the risk of severe sleep apnea.
DISCUSSION
In this large-scale, retrospective cohort study using a nationwide database, we found that patients who used α1-adrenergic antagonists had a significantly higher risk of sleep apnea. Patients with hypertension using α1-adrenergic antagonists had the highest HR (8.70) for the development of severe sleep apnea, relative to patients without hypertension who did not use α1-adrenergic antagonists. Among males, α1-adrenergic-antagonist use significantly increased the risk of sleep apnea in both hypertension and no hypertension groups. Among females, however, this correlation could only be demonstrated for the hypertension group, due to a lower overall rate of sleep apnea.
Based on previous population-based studies, the prevalence of OSA is higher in males than in females.33–35 A meta-analysis including 24 studies also demonstrate higher prevalence of OSA in the male population (13% to 33%) than in the female population (6% to 19%).36 When focusing on the effect of hypertension in the Vitoria Sleep Cohort, there is a significant relationship between hypertension and OSA in the male population but not in the female population.37 In the hypertension group of the current study, the incidence of sleep apnea higher in the male population was twice higher as the incidence in the female population (Table 2). This difference may be related to a relatively poor genioglossus response in males. In a physiologic study, females were found to have higher phasic and tonic genioglossus muscle activity than males when facing an inspiratory load.38 Furthermore, another study using hypoxic challenge showed that females presented better genioglossus muscle responsiveness.39 The relatively poor genioglossus muscle responsiveness in males could explain the higher incidence of sleep apnea in this population.
Intermittent hypoxemia during OSA events increases sympathetic tone, which results in a nondipping blood pressure pattern and daytime resistant hypertension.40 Several studies have focused on the incidence of hypertension among patients with OSA. A longitudinal study has demonstrated that such patients have a significantly higher risk of hypertension, with an HR of 2.67.41 In the Wisconsin Sleep Cohort, a dose-response relationship was found between the risk of development of nondipping blood pressure and the severity of OSA. Compared to patients with an apnea-hypopnea index (AHI) of < 1 event/h, patients with AHI ≥ 15 events/h have a higher relative risk (2.84) of the development of nondipping blood pressure.42 Conversely, 38% of patients with hypertension have OSA, which is ninefold the rate in patients with normal blood pressure (4%).43 In another case-control study, the incidence of OSA was 71% in patients with resistant hypertension, approximately double that in patients with controlled hypertension (38%).44
The genioglossus muscle is the major component of the group of pharyngeal dilator muscles, which act to prevent pharyngeal collapse during sleep.45 Although poor upper-airway muscle responsiveness alone, in the absence of anatomical compromise (critical pressure less than −5 cmH2O), does not result in OSA,7 severe OSA could develop in patients with profound anatomical compromise (critical pressure more than +5 cmH2O) combined with impaired muscle responsiveness.46 Almost all patients with severe OSA had prominent anatomical compromise, the incidence of which was significantly higher than in patients with mild OSA.7 As mentioned previously, genioglossus muscle activity is mainly governed by the hypoglossal motor nucleus, which receives input from brainstem noradrenergic neurons.47,48 The use of noradrenergic blockage was shown to cause a significant decrease in genioglossus activity in animal studies.12,49,50 In the current study, the use of α1-adrenergic antagonists was associated with a higher risk of both sleep apnea and severe sleep apnea.
A strength of our study is the large number of patients, including patients with sleep apnea who used α1-adrenergic antagonists, enrolled from a nationwide database in Taiwan. The retrospective cohort design and large sample size provided considerable statistical power to detect differences between these two groups. Additionally, we adequately adjusted for sleep apnea risk factors, including age and comorbid clinical illnesses such as hypertension, DM, ASVD, hyperlipidemia, asthma, COPD, allergic rhinitis, obesity, and musculoskeletal system and connective tissue diseases.
This study has several limitations. First, the diagnoses of sleep apnea and other comorbid medical conditions were based on administrative claims data; thus, it is possible there was some misclassification. However, based on previous epidemiologic database studies, the quality of the NHIRD data is acceptable, and the hypertension codes were validated by chart review.16,18,27,28 Second, frequency matching was conducted in order to control for potential confounding factors, but the matching process may not eliminate all bias. Third, many items, such as body mass index and neck circumference, were not available in the NHIRD administrative data. These factors may be important determinants of sleep apnea development.
This study is the first to demonstrate the relationship between the use of α1-adrenergic antagonists and the incidence of sleep apnea. Our results suggest that patients with hypertension using α1-adrenergic antagonists have a higher risk of sleep apnea.
DISCLOSURE STATEMENT
All authors have read and approved the paper. The study was supported by the grant NCKUH-10603015 from National Cheng Kung University Hospital, Tainan, Taiwan. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Chih-Hui Hsu, from the Biostatistics Consulting Center, Clinical Medicine Research Center, National Cheng Kung University Hospital, for providing statistical consulting services. The authors thank Anthony Abram (www.uni-edit.net) for editing and proofreading this manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ASVD
arteriosclerotic vascular disease
- COPD
chronic obstructive pulmonary disease
- CRR
competing-risk regression
- DM
diabetes mellitus
- HR
hazard ratio
- OSA
obstructive sleep apnea
REFERENCES
- 1.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol (1985) 2008;105(5):1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 5.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120(12):505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loewen AH, Wellman A, Heinzer RC, et al. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep. 2011;34(8):1061–1073. doi: 10.5665/SLEEP.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin XT, Cui N, Zhong W, Jin X, Wu Z, Jiang C. Pre- and postsynaptic modulations of hypoglossal motoneurons by alpha-adrenoceptor activation in wild-type and Mecp2(-/Y) mice. Am J Physiol Cell Physiol. 2013;305(10):C1080–C1090. doi: 10.1152/ajpcell.00109.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflugers Arch. 1996;431(6):942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- 10.Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol. 1994;72(5):2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- 11.Parkis MA, Bayliss DA, Berger AJ. Actions of norepinephrine on rat hypoglossal motoneurons. J Neurophysiol. 1995;74(5):1911–1919. doi: 10.1152/jn.1995.74.5.1911. [DOI] [PubMed] [Google Scholar]
- 12.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174(11):1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 13.Moran M. Reversible exacerbation of obstructive sleep apnea by alpha1-adrenergic blockade with tamsulosin: A case report. Respir Med Case Rep. 2016;19:181–186. doi: 10.1016/j.rmcr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genta-Pereira DC, Furlan SF, Omote DQ, et al. Nondipping blood pressure patterns predict obstructive sleep apnea in patients undergoing ambulatory blood pressure monitoring. Hypertension. 2018;72(4):979–985. doi: 10.1161/HYPERTENSIONAHA.118.11525. [DOI] [PubMed] [Google Scholar]
- 15.McComb MN, Chao JY, Ng TM. Direct vasodilators and sympatholytic agents. J Cardiovasc Pharmacol Ther. 2016;21(1):3–19. doi: 10.1177/1074248415587969. [DOI] [PubMed] [Google Scholar]
- 16.Lee YC, Hung SY, Wang HK, et al. Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep. 2015;38(2):213–221. doi: 10.5665/sleep.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Graaf MA, Jager KJ, Zoccali C, Dekker FW. Matching, an appealing method to avoid confounding? Nephron Clin Pract. 2011;118(4):c315–c318. doi: 10.1159/000323136. [DOI] [PubMed] [Google Scholar]
- 18.Wang HK, Lin SH, Sung PS, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83(11):1080–1085. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- 19.Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4(1):1–22. [Google Scholar]
- 20.Carneiro G, Zanella MT. Obesity metabolic and hormonal disorders associated with obstructive sleep apnea and their impact on the risk of cardiovascular events. Metabolism. 2018;84:76–84. doi: 10.1016/j.metabol.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Gagnadoux F, Le Vaillant M, Goupil F, et al. Depressive symptoms before and after long-term CPAP therapy in patients with sleep apnea. Chest. 2014;145(5):1025–1031. doi: 10.1378/chest.13-2373. [DOI] [PubMed] [Google Scholar]
- 22.Prasad B, Nyenhuis SM, Weaver TE. Obstructive sleep apnea and asthma: associations and treatment implications. Sleep Med Rev. 2014;18(2):165–171. doi: 10.1016/j.smrv.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Romem A, Iacono A, McIlmoyle E, et al. Obstructive sleep apnea in patients with end-stage lung disease. J Clin Sleep Med. 2013;9:687–693. doi: 10.5664/jcsm.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuda T. Clinical manifestations and overall management strategies for Duchenne muscular dystrophy. Methods Mol Biol. 2018;1687:19–28. doi: 10.1007/978-1-4939-7374-3_2. [DOI] [PubMed] [Google Scholar]
- 25.Mobley D, Feibus A, Baum N. Benign prostatic hyperplasia and urinary symptoms: evaluation and treatment. Postgrad Med. 2015;127(3):301–307. doi: 10.1080/00325481.2015.1018799. [DOI] [PubMed] [Google Scholar]
- 26.Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012;33(1):26–35b. doi: 10.1093/eurheartj/ehr186. [DOI] [PubMed] [Google Scholar]
- 27.Sung PS, Yeh CC, Wang LC, et al. Increased risk of dementia in patients with non-apnea sleep disorder. Curr Alzheimer Res. 2017;14(3):309–316. doi: 10.2174/1567205013666161108104703. [DOI] [PubMed] [Google Scholar]
- 28.Shiao TH, Liu CJ, Luo JC, et al. Sleep apnea and risk of peptic ulcer bleeding: a nationwide population-based study. Am J Med. 2013;126(3):249–255. doi: 10.1016/j.amjmed.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. 3rd ed. New York, NY: Springer; 2012. [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 32.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54(3):948–963. [PubMed] [Google Scholar]
- 33.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98(10):984–989. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 36.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Cano-Pumarega I, Barbé F, Esteban A, Martínez-Alonso M, Egea C, Durán-Cantolla J. Spanish Sleep Network Sleep apnea and hypertension: are there sex differences? The Vitoria Sleep Cohort. Chest. 2017;152(4):742–750. doi: 10.1016/j.chest.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Popovic RM, White DP. Influence of gender on waking genioglossal electromyogram and upper airway resistance. Am J Respir Crit Care Med. 1995;152(2):725–731. doi: 10.1164/ajrccm.152.2.7633734. [DOI] [PubMed] [Google Scholar]
- 39.Jordan AS, Catcheside PG, O'Donoghue FJ, Saunders NA, McEvoy RD. Genioglossus muscle activity at rest and in response to brief hypoxia in healthy men and women. J Appl Physiol (1985) 2002;92(1):410–417. doi: 10.1152/japplphysiol.00461.2001. [DOI] [PubMed] [Google Scholar]
- 40.Phillips CL, Drager LF. Is obstructive sleep apnoea an innocent bystander in the pathophysiology of arterial stiffening? Thorax. 2018;73(12):1099–1100. doi: 10.1136/thoraxjnl-2018-212332. [DOI] [PubMed] [Google Scholar]
- 41.Appleton SL, Vakulin A, Martin SA, et al. Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest. 2016;150(3):495–505. doi: 10.1016/j.chest.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax. 2015;70(11):1062–1069. doi: 10.1136/thoraxjnl-2015-207231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worsnop CJ, Naughton MT, Barter CE, Morgan TO, Anderson AI, Pierce RJ. The prevalence of obstructive sleep apnea in hypertensives. Am J Respir Crit Care Med. 1998;157(1):111–115. doi: 10.1164/ajrccm.157.1.9609063. [DOI] [PubMed] [Google Scholar]
- 44.Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest. 2007;132(6):1858–1862. doi: 10.1378/chest.07-1170. [DOI] [PubMed] [Google Scholar]
- 45.Saboisky JP, Butler JE, Fogel RB, et al. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95(4):2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- 46.Sands SA, Eckert DJ, Jordan AS, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190(8):930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29(6):931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- 48.Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol. 1983;220(3):280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- 49.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172(10):1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532(Pt 2):467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.