Abstract
In conditions of acute illness, patients present with reduced plasma T3 concentrations without a concomitant rise in TSH. In contrast, plasma concentrations of the inactive hormone rT3 increase, whereas plasma concentrations of T4 remain low-normal. This constellation of changes, referred to as nonthyroidal illness syndrome (NTIS), is present across all ages, from preterm neonates and over-term critically ill infants and children to critically ill adults. Although the severity of illness strongly correlates with the severity of the NTIS phenotype, the causality of this association remains debated, and pathophysiological mechanisms remain incompletely understood. In the acute phase of illness, NTIS appears to be caused predominantly by an increased peripheral inactivation of thyroid hormones, in which reduced nutritional intake plays a role. Current evidence suggests that these acute peripheral changes are part of a beneficial adaptation of the body to reduce expenditure of energy and to activate the innate immune response, which is important for survival. In contrast, in more severely ill and prolonged critically ill patients, an additional central suppression of the thyroid hormone axis alters and further aggravates the NTIS phenotype. Recent studies suggest that this central suppression may not be adaptive. Whether treatment of this central component of NTIS in prolonged critically ill patients, with the use of hypothalamic releasing factors, improves outcome remains to be investigated in large randomized control trials.
Keywords: thyroid hormone, critical illness, sepsis, pediatric, adult
1. Thyroid Axis Alterations in Response to Critical Illness
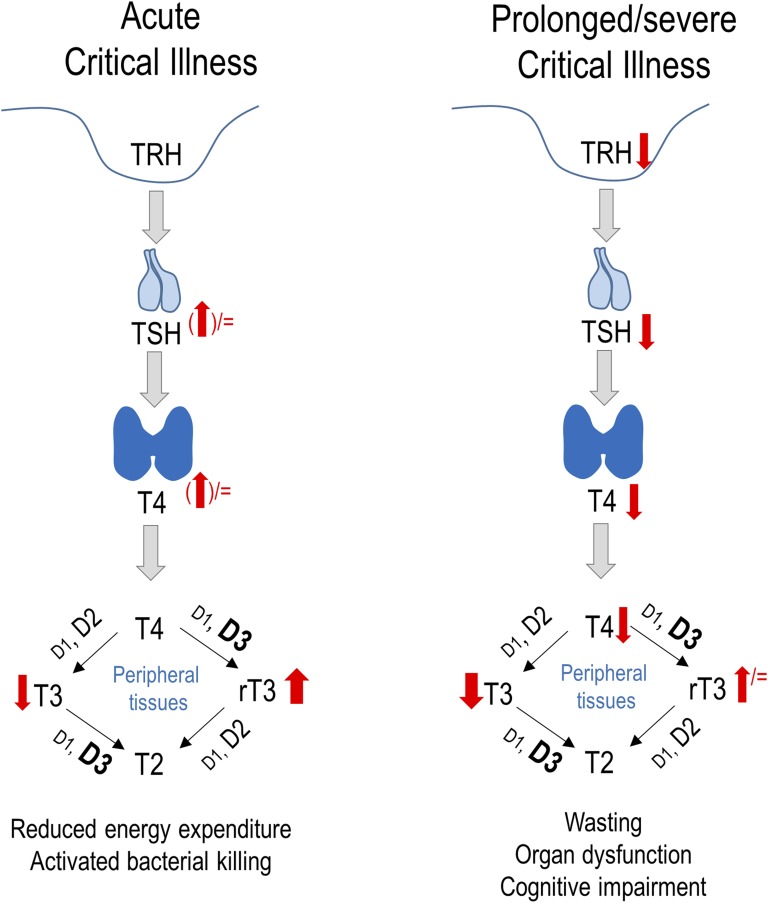
Thyroid hormones are important regulators of thermoregulation, energy expenditure, and cellular metabolism, but they also directly affect the cardiovascular and immune systems [1, 2]. In healthy circumstances, the release of thyroid hormones by the thyroid gland is regulated by a classic neuroendocrine feedback loop. Whenever thyroid hormone levels are low, the hypothalamus releases TRH, which stimulates the anterior pituitary to synthesize and release TSH. TSH stimulates the thyroid gland to produce and release T4 and to a lesser extent T3. The source of the active thyroid hormone T3 is predominantly peripheral deiodination of T4. Negative feedback of T4 and T3 on TRH and TSH secretion further controls homeostasis of thyroid hormone activity. In conditions of acute illness, patients present with reduced plasma T3 concentrations without a concomitant rise in TSH. In contrast, plasma concentrations of the inactive hormone rT3 increase, whereas plasma concentrations of T4 remain low-normal [3–5]. This constellation of changes is referred to as nonthyroidal illness syndrome (NTIS) (Fig. 1).
Figure 1.
Schematic diagram of the biphasic changes observed in NTIS present in response to critical illness. Changes in the hypothalamus–pituitary–thyroid axis and in peripheral thyroid hormone metabolism. D1, D2, and D3 are iodothyronine deiodinase type 1, type 2, and type 3, respectively.
The aim of this review is to provide an update on the current knowledge of NTIS across the ages. The review discusses NTIS in the most extensively studied population of adult critically ill patients and summarizes insights into NTIS in critically ill infants and children and in premature newborns.
2. NTIS in Critically Ill Adults Treated in the Intensive Care Unit
Low plasma T3 and high rT3 concentrations are found in adults suffering from acute illnesses (e.g., infectious diseases, metabolic disorders, cardiovascular, pulmonary diseases, or gastrointestinal diseases) and in adults suffering from burns, trauma, and major surgery [6–10]. The severity of the illness is reflected by the magnitude of the changes in serum T3 and rT3 [4, 8, 11]. Patients with mild to moderate NTIS usually have normal plasma T4 and TSH concentrations, whereas patients with more severe and prolonged illness also display low serum T4 and TSH, both of which are indicative of a poor prognosis [12].
Plasma TSH transiently rises only in the first hours of critical illness, followed by a transient increase in plasma T4, whereas plasma T3 concentrations may already be low and rT3 concentrations high due to acute changes in peripheral thyroid hormone metabolism (Fig. 1) [9]. The expression of the inactivating outer-ring deiodinase D3, which is responsible for conversion of T4 to inactive rT3, was found to be upregulated in liver and skeletal muscle biopsies harvested from nonsurviving critically ill adults [13]. Also, within granulocytes, critical illness upregulates D3 activity, which may contribute to the bacterial killing capacity of these cells [14, 15]. However, increased D2 expression has also been reported in activated macrophages. Activated macrophages can locally increase T3, which could activate phagocytosis and the release of cytokines [16]. In contrast, hepatic activity of the activating inner-ring deiodinase D1 is suppressed by critical illness, which results in decreased deiodination of T4 into T3 [13, 17]. Cytokines and hypoxia are among the possible drivers of such peripherally altered thyroid hormone metabolism [18, 19]. In addition, lowered levels of thyroid hormone–binding globulin and albumin contribute to altered peripheral thyroid hormone availability at target tissues [20, 21].
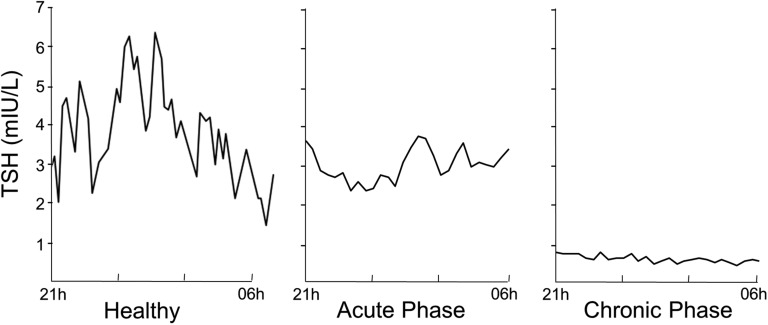
When patients require prolonged intensive care, plasma T3 concentrations remain suppressed and accompanied by low plasma T4 and TSH concentrations (Fig. 2) [22]. This can be explained by reduced hypothalamic TRH expression, as was observed in postmortem biopsies harvested from prolonged critically ill patients [23]. Indeed, the low TRH mRNA correlated with low plasma TSH and T3 concentrations [23]. At the onset of recovery from illness, a rise in TSH and in total and free T4 has been observed, followed by normalization of circulating T4 and T3 levels [24, 25]. Such a rise in TSH preceding recovery suggests reversal of reduced TRH stimulation of the thyrotropes during prolonged critical illness. In prolonged critical illness, peripheral tissues appear to activate compensatory mechanisms to restore T3 availability, for example by upregulating D2 activity, as has been shown in skeletal muscle biopsies [26]. Also, the thyroid hormone transporter monocarboxylate transporter 8 was found to be upregulated in muscle and liver of prolonged critically ill patients [27].
Figure 2.
Illustration of the biphasic response of TSH to critical illness. Representative nocturnal TSH serum concentration profiles of a healthy volunteer and of two critically ill patients are shown to illustrate the differences between the acute and chronic phases of critical illness. [Adapted with permission from Van den Berghe G, de Zegher F, Bouillon R: Clinical review 95: Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83(6):1827-1834.]
The changes within the thyroid axis are part of a broader neuroendocrine response to illness that encompasses all major endocrine axes, which together alter the balance between anabolism and catabolism [28]. Whereas the changes that occur acutely in response to critical illness are considered beneficial, reflecting an attempt to provide energy and limit unnecessary energy expenditure to promote survival, the changes that occur in prolonged critical illness may be harmful, with potentially detrimental consequences such as wasting, organ dysfunction, and impaired cognition. Such differences may explain the ongoing controversy about whether patients with NTIS should be treated to improve the outcome of critical illness. In patients undergoing cardiac surgery, treatment with T3 either did or did not improve postoperative cardiac function [29–31], but patient-centered clinical outcomes were never affected [31–34]. Furthermore, T3 treatment did not affect mortality in patients suffering from acute burn injury [35]. T3 infusion in mechanically ventilated patients with NTIS also did not affect respiratory muscle function [36]. In acutely ill patients with low serum T4 levels, T4 treatment did not affect mortality [37] or even increased it [38]. However, increasing circulating thyroid hormone levels in the acute phase of illness does not necessarily normalize tissue levels [39]. Furthermore, the often supraphysiological doses of T3 or T4 that were tested further suppressed circulating TSH levels, which could hamper the recovery of the pituitary TSH secretion [37–39]. Interestingly, a small proof-of-concept study of prolonged critically ill patients investigated the impact of a continuous infusion of TRH combined with a growth hormone secretagogue and found not only normalized thyroid hormone levels but also reduced catabolism and increased anabolism [40]. Larger studies investigating clinical outcome are currently lacking.
Hypothyroidism during critical illness can be very difficult to diagnose due to the superimposed NTIS. High plasma TSH and low plasma T4 are indicative of hypothyroidism, but the absence of elevated TSH does not exclude hypothyroidism in the context of critical illness [11]. It is generally accepted that patients with premorbid hypothyroidism, which is present in ∼7% of the elderly population, should continue to receive thyroxine replacement while in the intensive care unit (ICU) [41]. Hyperthyroidism is characterized by suppressed plasma TSH in the face of high plasma free T4 and T3, but this constellation can be altered by superimposed NTIS during critical illness [42, 43]. Physical examination for goiter and proptosis and screening for thyroid antibodies can provide further information and help distinguish hyperthyroidism from NTIS. Thyroid storm can be lethal when untreated; however, its symptoms and signs, such as fever, supraventricular tachycardia, gastrointestinal problems, and altered mentation, are atypical in the context of critical illness [42, 43].
3. NTIS in Critically Ill Infants and Children Treated in the Pediatric ICU
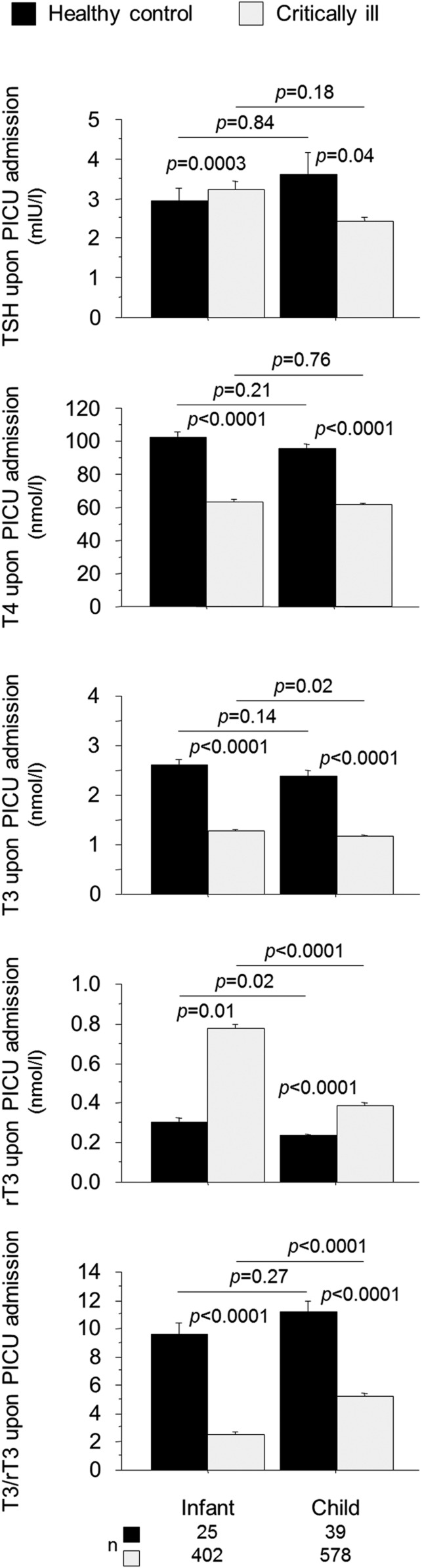
In critically ill infants and in children, NTIS is also present with normal or low T4, low T3, high rT3 plasma concentrations, and a low T3/rT3 ratio in the absence of elevated TSH (Fig. 3). NTIS in children has been described in pediatric ICU (PICU) populations with varying diagnostic categories [44, 45], in patients undergoing cardiac surgery [46–49], in patients suffering from sepsis or septic shock [50–53], and in those with oncological diseases [54, 55]. The critical illness–induced rise in rT3 was found to be more pronounced in infants than in older children and resulted in a lower T3/rT3 ratio [44]. As in adults, the severity of NTIS in children has been associated with poor clinical outcomes of critical illnesses [44, 45, 50, 52, 56]. Lower T4 and lower T3 have been associated with delayed discharge from PICU [45, 52] and with risk of infections [44] and death [44, 50, 52, 56, 57]. Two small studies found higher TSH plasma concentrations in nonsurvivors than in survivors [58, 59], but this could not be confirmed in two large, more diverse PICU populations [44, 45]. Detailed studies documenting the time course of NTIS—from the acute phase through the chronic phase of pediatric critical illness—are currently lacking.
Figure 3.
Impact of critical illness on thyroid hormone concentrations upon PICU admission in infants and children. Infants are younger than 1 y old. Bars represent means, and whiskers represent the SE. The black boxplots represent healthy children, and the light-gray boxes represent critically ill patients. [Reproduced with permission from Jacobs A, Derese I, Vander Perre S, van Puffelen E, Verstraete S, Pauwels L, Verbruggen S, Wouters P, Langouche L, Garcia Guerra G et al: Non-thyroidal illness syndrome in critically ill children: prognostic value and impact of nutritional management. Thyroid. 2019;29(4):480–492.]
Whether treating critically ill children with thyroid hormone is beneficial or not remains unclear. Thyroid hormone substitution has mainly been tested in acute critical illness after cardiac surgery with cardiopulmonary bypass. In most studies, T3 treatment did not improve outcome [60–63]. In one randomized control trial (RCT), T3 infusion improved myocardial function and reduced the need for postoperative intensive care [64]. One study of oral T3 also reported shortened time to extubation [65]. No studies have investigated the impact of treatment with thyroid hormone in the chronic phase of pediatric critical illness.
4. NTIS in Premature Newborns Treated in the Neonatal ICU
Premature infants with low birth weight represent a particular and separate subgroup of patients treated in the neonatal intensive care environment. Preterm infants often present with transient hypothyroxinemia of prematurity (THOP), which is characterized by low circulating T4 and T3 without a concomitant rise in TSH [66, 67]. Although the changes in T4, T3, and TSH are quite similar to what is observed in NTIS in adults, children, and term infants, the underlying mechanisms are even more complex, given that the hypothalamus–pituitary–thyroid axis matures until the end of full-term pregnancy. Hence, thyroid axis immaturity likely contributes to the thyroid hormone alterations observed in preterm newborns [66, 67]. The most immature newborns, who suffer from the highest morbidity rates, also have the most pronounced THOP [68]. Also, in the preterm infant population, studies on the impact of thyroxine supplementation did not provide sufficient evidence in favor of thyroid hormone substitution. Indeed, supplementation of preterm infants with levothyroxine during the first 48 hours of life did not improve morbidity or mortality; nor did it improve longer-term neurodevelopment [69, 70]. A post hoc analysis of one of these studies, however, showed a neurocognitive benefit among infants born at 25 to 26 weeks gestational age [70]. Levothyroxine treatment of preterm infants suffering from THOP also did not improve acute outcome [71], whereas a neurodevelopmental benefit was observed until a corrected age of 3 years [72].
5. Iatrogenic and Nutritional Factors Interfering With NTIS
Adult, pediatric, and neonatal critically ill patients often receive high doses of glucocorticoids, which can lower serum TSH [73–75]. Moreover, endogenously elevated cortisol levels in response to stress may exert a suppressive effect on TSH [76]. Also, dopamine infusion, prescribed as an inotrope and/or vasopressor for critically ill patients, can severely suppress TSH secretion in adults, children, and infants [77, 78]. Although dopamine is no longer a preferred drug for adults, it is still extensively used in pediatric and neonatal ICUs. Less commonly used drugs, such as antiepileptic medications [79–81], certain antiarrhythmic drugs [82], and lithium, can also affect the hypothalamus–pituitary–thyroid axis. Iatrogenic iodine intoxication [83], through the use of iodine-containing contrast fluids or antiseptic dressings, can affect thyroid hormone availability [84].
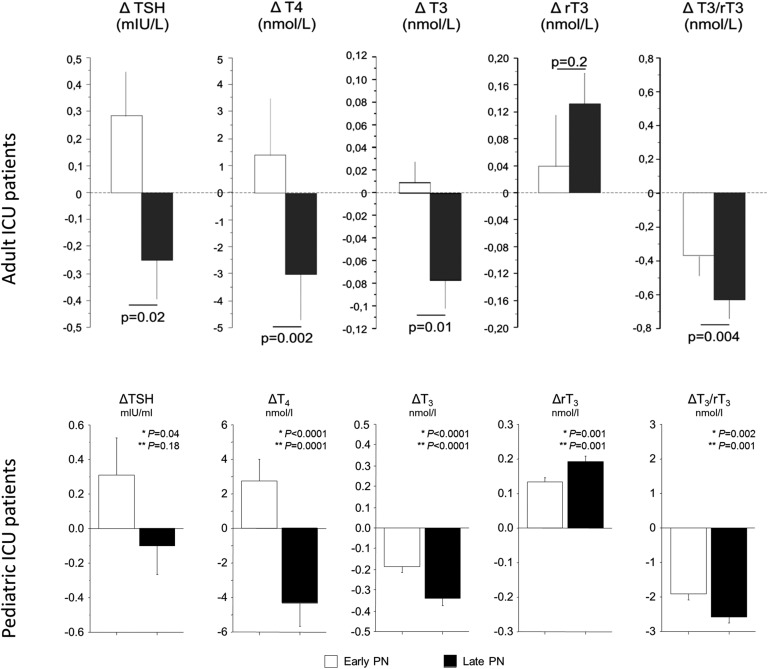
Fasting in healthy individuals induces changes in the thyroid axis that are similar to those in NTIS: plasma T3 decreases, whereas plasma rT3 concentrations rise; these changes rapidly return to baseline upon refeeding [85]. Restricted nutrition during adult critical illnesses appears to contribute to NTIS [86–88]. More recently, two large RCTs in adults and in children demonstrated that tolerating a nutritional deficit during the first week of critical illness as compared with the early administration of supplemental parenteral nutrition resulted in fewer complications and accelerated recovery [89, 90]. Although restricting macronutrient administration in the first week of critical illness reduced complications and accelerated recovery of patients with NTIS, it aggravated the NTIS by further lowering TSH, total T4, T3, and the T3/rT3 ratio (Fig. 4) [44, 88]. Importantly, the clinical benefit of not forcefully feeding early was statistically in part explained by the early further suppression of T3 and T3/rT3 ratio in both the adult and pediatric populations, suggesting that the acute peripheral changes of NTIS likely represent a beneficial adaptation to illness [44, 88]. Such an effect on the peripheral component of NTIS was also shown to be evoked by targeting normal fasting blood glucose levels in critically ill children [45]. This further suppression of T3 and T3/rT3 also in part explained the mortality benefit of tight blood glucose control in this population [45, 91]. In prolonged critically ill adults, however, targeting fasting blood glucose levels did not affect NTIS [8, 92]. The further aggravation of the central component of the NTIS, with further suppression of TSH-induced T4 release, in critically ill adults and children evoked by not forcefully feeding early appeared to counteract the outcome benefits of the intervention [44, 88]. The central component of the NTIS may thus be a maladaptive response, unlike the acute peripheral component. Whereas virtually all ill patients acutely present with low T3 and high rT3 levels, which is interpretable as an immediate adaptation to stress, low T4 levels are especially pronounced in the more severely ill and prolonged critically ill patients [93, 94]. Because current evidence suggests a potential beneficial peripheral component but a harmful central component of NTI, future RCTs should focus on treatment of the central part of the thyroid axis in prolonged critically ill patients. Treatment with hypothalamic releasing factors would theoretically also be a safer option than treatment with T3 or T4 because the normal negative feedback exerted by thyroid hormones at the pituitary level would be maintained and excessively elevated thyroid hormone levels could hereby be avoided.
Figure 4.
Effect of accepting a very low macronutrient intake, by omitting parenteral nutrition (PN) until beyond the first week in ICU (Late-PN), as compared with early provision of full feeding with PN (early-PN) on NTIS. Bars (mean – SE) represent the changes (referred to as Δ) from the admission values to day 3 in the adult or pediatric ICU (or to the last day for patients with shorter ICU stay) in serum TSH, T4, T3, rT3, and the T3/rT3 ratio. The open and filled bars represent the patients randomized to the early-PN and late-PN groups, respectively. [Reproduced with permission from Jacobs A, Derese I, Vander Perre S, van Puffelen E, Verstraete S, Pauwels L, Verbruggen S, Wouters P, Langouche L, Garcia Guerra G et al: Non-Thyroidal Illness Syndrome in Critically Ill Children: Prognostic Value and Impact of Nutritional Management. Thyroid 2019, 29(4):480-492; and Langouche L, Vander Perre S, Marques M, Boelen A, Wouters PJ, Casaer MP, Van den Berghe G: Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab. 2013;98(3):1006-1013.]
Conclusion
Acute critical illness causes low plasma T3 concentrations and increased rT3 plasma concentrations without a concomitant rise in TSH. This constellation, referred to as NTIS, is present across all ages, from preterm neonates to term critically ill infants and children to critically ill adults. Although the severity of illness strongly correlates with the severity of the NTIS phenotype, causality of this association remains debated, and the pathophysiological mechanisms remain incompletely understood. In the acute phase of illness, NTIS appears to be caused predominantly by an increased peripheral inactivation of thyroid hormones in which reduced nutritional intake plays a role. Current evidence suggests that these acute peripheral changes are part of a beneficial adaptation of the body to reduce expenditure of scare energy and to activate the innate immune response that is essential for survival. In contrast, in more severely ill and prolonged critically ill patients, an additional central suppression of the thyroid hormone axis alters and further aggravates the NTIS phenotype. Recent studies indicate that this central suppression may not be adaptive. Whether treatment of this central component of NTIS in prolonged critically ill patients, with use of hypothalamic releasing factors, improves outcome remains to be investigated in large RCTs.
Search Strategy
We searched the PubMed database for articles published in English with different combinations of the search terms “critically ill,” “intensive care,” “sepsis,” “paediatric,” “neonatal,” “thyroid,” and “nonthyroidal illness.”
Acknowledgments
Financial Support: This work was supported by the ERC Advanced Grant AdvG-2017-785809 from the Horizon 2020 Programme (to G.V.d.B.) and by Methusalem programme of the Flemish Government (through the University of Leuven) Grant METH14/06 (to G.V.d.B. and L.L.).
Glossary
Abbreviations:
- ICU
intensive care unit
- NTIS
nonthyroidal illness syndrome
- PICU
pediatric intensive care unit
- RCT
randomized control trial
- THOP
transient hypothyroxinemia of prematurity
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35(2):375–388. [DOI] [PubMed] [Google Scholar]
- 3. Meyer S, Schuetz P, Wieland M, Nusbaumer C, Mueller B, Christ-Crain M. Low triiodothyronine syndrome: a prognostic marker for outcome in sepsis? Endocrine. 2011;39(2):167–174. [DOI] [PubMed] [Google Scholar]
- 4. Wiersinga WM, Lie KI, Touber JL. Thyroid hormones in acute myocardial infarction. Clin Endocrinol (Oxf). 1981;14(4):367–374. [DOI] [PubMed] [Google Scholar]
- 5. Phillips RH, Valente WA, Caplan ES, Connor TB, Wiswell JG. Circulating thyroid hormone changes in acute trauma: prognostic implications for clinical outcome. J Trauma. 1984;24(2):116–119. [DOI] [PubMed] [Google Scholar]
- 6. Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf). 1993;39(5):499–518. [DOI] [PubMed] [Google Scholar]
- 7. Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev. 2011;32(5):670–693. [DOI] [PubMed] [Google Scholar]
- 8. Peeters RP, Wouters PJ, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. Serum rT3 and T3/rT3 are prognostic markers in critically ill patients and are associated with post-mortem tissue deiodinase activities. J Clin Endocrinol Metab. 2005;90(8):4559–4565. [DOI] [PubMed] [Google Scholar]
- 9. Michalaki M, Vagenakis AG, Makri M, Kalfarentzos F, Kyriazopoulou V. Dissociation of the early decline in serum T(3) concentration and serum IL-6 rise and TNFalpha in nonthyroidal illness syndrome induced by abdominal surgery. J Clin Endocrinol Metab. 2001;86(9):4198–4205. [DOI] [PubMed] [Google Scholar]
- 10. Bello G, Pennisi MA, Montini L, Silva S, Maviglia R, Cavallaro F, Bianchi A, De Marinis L, Antonelli M. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest. 2009;135(6):1448–1454. [DOI] [PubMed] [Google Scholar]
- 11. Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015;3(10):816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wehmann RE, Gregerman RI, Burns WH, Saral R, Santos GW. Suppression of thyrotropin in the low-thyroxine state of severe nonthyroidal illness. N Engl J Med. 1985;312(9):546–552. [DOI] [PubMed] [Google Scholar]
- 13. Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88(7):3202–3211. [DOI] [PubMed] [Google Scholar]
- 14. Boelen A, Boorsma J, Kwakkel J, Wieland CW, Renckens R, Visser TJ, Fliers E, Wiersinga WM. Type 3 deiodinase is highly expressed in infiltrating neutrophilic granulocytes in response to acute bacterial infection. Thyroid. 2008;18(10):1095–1103. [DOI] [PubMed] [Google Scholar]
- 15. van der Spek AH, Jim KK, Karaczyn A, van Beeren HC, Ackermans MT, Darras VM, Vandenbroucke-Grauls CMJE, Hernandez A, Brouwer MC, Fliers E, van de Beek D, Boelen A. The thyroid hormone inactivating type 3 deiodinase is essential for optimal neutrophil function: observations from three species. Endocrinology. 2018;159(2):826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Spek AH, Surovtseva OV, Jim KK, van Oudenaren A, Brouwer MC, Vandenbroucke-Grauls CMJE, Leenen PJM, van de Beek D, Hernandez A, Fliers E, Boelen A. Regulation of intracellular triiodothyronine is essential for optimal macrophage function. Endocrinology. 2018;159(5):2241–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peeters RP, Kester MHA, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Increased thyroxine sulfate levels in critically ill patients as a result of a decreased hepatic type I deiodinase activity. J Clin Endocrinol Metab. 2005;90(12):6460–6465. [DOI] [PubMed] [Google Scholar]
- 18. Kwakkel J, Wiersinga WM, Boelen A. Differential involvement of nuclear factor-kappaB and activator protein-1 pathways in the interleukin-1beta-mediated decrease of deiodinase type 1 and thyroid hormone receptor beta1 mRNA. J Endocrinol. 2006;189(1):37–44. [DOI] [PubMed] [Google Scholar]
- 19. Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon MJ, Larsen PR, Bianco AC, Huang SA. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest. 2008;118(3):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langouche L, Lehmphul I, Perre SV, Köhrle J, Van den Berghe G. Circulating 3-T1AM and 3,5-T2 in critically ill patients: a cross-sectional observational study. Thyroid. 2016;26(12):1674–1680. [DOI] [PubMed] [Google Scholar]
- 21. Docter R, van Toor H, Krenning EP, de Jong M, Hennemann G. Free thyroxine assessed with three assays in sera of patients with nonthyroidal illness and of subjects with abnormal concentrations of thyroxine-binding proteins. Clin Chem. 1993;39(8):1668–1674. [PubMed] [Google Scholar]
- 22. Van den Berghe G, de Zegher F, Veldhuis JD, Wouters P, Gouwy S, Stockman W, Weekers F, Schetz M, Lauwers P, Bouillon R, Bowers CY. Thyrotrophin and prolactin release in prolonged critical illness: dynamics of spontaneous secretion and effects of growth hormone-secretagogues. Clin Endocrinol (Oxf). 1997;47(5):599–612. [DOI] [PubMed] [Google Scholar]
- 23. Fliers E, Guldenaar SE, Wiersinga WM, Swaab DF. Decreased hypothalamic thyrotropin-releasing hormone gene expression in patients with nonthyroidal illness. J Clin Endocrinol Metab. 1997;82(12):4032–4036. [DOI] [PubMed] [Google Scholar]
- 24. Hamblin PS, Dyer SA, Mohr VS, Le Grand BA, Lim CF, Tuxen DV, Topliss DJ, Stockigt JR. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab. 1986;62(4):717–722. [DOI] [PubMed] [Google Scholar]
- 25. Feelders RA, Swaak AJ, Romijn JA, Eggermont AM, Tielens ET, Vreugdenhil G, Endert E, van Eijk HG, Berghout A. Characteristics of recovery from the euthyroid sick syndrome induced by tumor necrosis factor alpha in cancer patients. Metabolism. 1999;48(3):324–329. [DOI] [PubMed] [Google Scholar]
- 26. Mebis L, Langouche L, Visser TJ, Van den Berghe G. The type II iodothyronine deiodinase is up-regulated in skeletal muscle during prolonged critical illness. J Clin Endocrinol Metab. 2007;92(8):3330–3333. [DOI] [PubMed] [Google Scholar]
- 27. Mebis L, Paletta D, Debaveye Y, Ellger B, Langouche L, D’Hoore A, Darras VM, Visser TJ, Van den Berghe G. Expression of thyroid hormone transporters during critical illness. Eur J Endocrinol. 2009;161(2):243–250. [DOI] [PubMed] [Google Scholar]
- 28. Preiser JC, Ichai C, Orban JC, Groeneveld AB. Metabolic response to the stress of critical illness. Br J Anaesth. 2014;113(6):945–954. [DOI] [PubMed] [Google Scholar]
- 29. Novitzky D, Cooper DK, Barton CI, Greer A, Chaffin J, Grim J, Zuhdi N. Triiodothyronine as an inotropic agent after open heart surgery. J Thorac Cardiovasc Surg. 1989;98(5 Pt 2):972–977, discussion 977–978. [PubMed] [Google Scholar]
- 30. Mullis-Jansson SL, Argenziano M, Corwin S, Homma S, Weinberg AD, Williams M, Rose EA, Smith CR. A randomized double-blind study of the effect of triiodothyronine on cardiac function and morbidity after coronary bypass surgery. J Thorac Cardiovasc Surg. 1999;117(6):1128–1134. [DOI] [PubMed] [Google Scholar]
- 31. Choi YS, Shim JK, Song JW, Song Y, Yang SY, Kwak YL. Efficacy of perioperative oral triiodothyronine replacement therapy in patients undergoing off-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2013;27(6):1218–1223. [DOI] [PubMed] [Google Scholar]
- 32. Klemperer JD, Klein I, Gomez M, Helm RE, Ojamaa K, Thomas SJ, Isom OW, Krieger K. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med. 1995;333(23):1522–1527. [DOI] [PubMed] [Google Scholar]
- 33. Bennett-Guerrero E, Jimenez JL, White WD, D’Amico EB, Baldwin BI, Schwinn DA. Cardiovascular effects of intravenous triiodothyronine in patients undergoing coronary artery bypass graft surgery: a randomized, double-blind, placebo-controlled trial.JAMA. 1996;275(9):687–692. [PubMed] [Google Scholar]
- 34. Vavouranakis I, Sanoudos G, Manios A, Kalogeropoulou K, Sitaras K, Kokkinos C. Triiodothyronine administration in coronary artery bypass surgery: effect on hemodynamics. J Cardiovasc Surg (Torino). 1994;35(5):383–389. [PubMed] [Google Scholar]
- 35. Becker RA, Vaughan GM, Ziegler MG, Seraile LG, Goldfarb IW, Mansour EH, McManus WF, Pruitt BA Jr, Mason AD Jr. Hypermetabolic low triiodothyronine syndrome of burn injury. Crit Care Med. 1982;10(12):870–875. [DOI] [PubMed] [Google Scholar]
- 36. Bello G, Spinazzola G, Giammatteo V, Montini L, De Pascale G, Bisanti A, Annetta MG, Troiani E, Bianchi A, Pontecorvi A, et al. . Effects of thyroid hormone treatment on diaphragmatic efficiency in subjects with nonthyroidal illness syndrome and on ventilation. Respir Care. 2019. [DOI] [PubMed] [Google Scholar]
- 37. Brent GA, Hershman JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63(1):1–8. [DOI] [PubMed] [Google Scholar]
- 38. Acker CG, Singh AR, Flick RP, Bernardini J, Greenberg A, Johnson JP. A trial of thyroxine in acute renal failure. Kidney Int. 2000;57(1):293–298. [DOI] [PubMed] [Google Scholar]
- 39. Debaveye Y, Ellger B, Mebis L, Visser TJ, Darras VM, Van den Berghe G. Effects of substitution and high-dose thyroid hormone therapy on deiodination, sulfoconjugation, and tissue thyroid hormone levels in prolonged critically ill rabbits. Endocrinology. 2008;149(8):4218–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van den Berghe G, Baxter RC, Weekers F, Wouters P, Bowers CY, Iranmanesh A, Veldhuis JD, Bouillon R. The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clin Endocrinol (Oxf). 2002;56(5):655–669. [DOI] [PubMed] [Google Scholar]
- 41. Vacante M, Biondi A, Basile F, Ciuni R, Luca S, Di Saverio S, Buscemi C, Vicari ESD, Borzì AM. Hypothyroidism as a predictor of surgical outcomes in the elderly. Front Endocrinol (Lausanne). 2019;10:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ringel MD. Management of hypothyroidism and hyperthyroidism in the intensive care unit. Crit Care Clin. 2001;17(1):59–74. [DOI] [PubMed] [Google Scholar]
- 43. Papi G, Corsello SM, Pontecorvi A. Clinical concepts on thyroid emergencies. Front Endocrinol (Lausanne). 2014;5:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacobs A, Derese I, Vander Perre S, van Puffelen E, Verstraete S, Pauwels L, Verbruggen S, Wouters P, Langouche L, Garcia Guerra G, Joosten K, Vanhorebeek I, Van den Berghe G. Non-thyroidal illness syndrome in critically ill children: prognostic value and impact of nutritional management. Thyroid. 2019;29(4):480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gielen M, Mesotten D, Wouters PJ, Desmet L, Vlasselaers D, Vanhorebeek I, Langouche L, Van den Berghe G. Effect of tight glucose control with insulin on the thyroid axis of critically ill children and its relation with outcome. J Clin Endocrinol Metab. 2012;97(10):3569–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marks SD, Haines C, Rebeyka IM, Couch RM. Hypothalamic-pituitary-thyroid axis changes in children after cardiac surgery. J Clin Endocrinol Metab. 2009;94(8):2781–2786. [DOI] [PubMed] [Google Scholar]
- 47. Allen DB, Dietrich KA, Zimmerman JJ. Thyroid hormone metabolism and level of illness severity in pediatric cardiac surgery patients. J Pediatr. 1989;114(1):59–62. [DOI] [PubMed] [Google Scholar]
- 48. Bettendorf M, Schmidt KG, Tiefenbacher U, Grulich-Henn J, Heinrich UE, Schönberg DK. Transient secondary hypothyroidism in children after cardiac surgery. Pediatr Res. 1997;41(3):375–379. [DOI] [PubMed] [Google Scholar]
- 49. Lynch BA, Brown DM, Herrington C, Braunlin E. Thyroid dysfunction after pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2004;127(5):1509–1511. [DOI] [PubMed] [Google Scholar]
- 50. Yildizdaş D, Onenli-Mungan N, Yapicioğlu H, Topaloğlu AK, Sertdemir Y, Yüksel B. Thyroid hormone levels and their relationship to survival in children with bacterial sepsis and septic shock. J Pediatr Endocrinol Metab. 2004;17(10):1435–1442. [PubMed] [Google Scholar]
- 51. Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, Falagas ME. Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol. 2011;164(2):147–155. [DOI] [PubMed] [Google Scholar]
- 52. den Brinker M, Dumas B, Visser TJ, Hop WC, Hazelzet JA, Festen DA, Hokken-Koelega AC, Joosten KF. Thyroid function and outcome in children who survived meningococcal septic shock. Intensive Care Med. 2005;31(7):970–976. [DOI] [PubMed] [Google Scholar]
- 53. den Brinker M, Joosten KF, Visser TJ, Hop WC, de Rijke YB, Hazelzet JA, Boonstra VH, Hokken-Koelega AC. Euthyroid sick syndrome in meningococcal sepsis: the impact of peripheral thyroid hormone metabolism and binding proteins. J Clin Endocrinol Metab. 2005;90(10):5613–5620. [DOI] [PubMed] [Google Scholar]
- 54. Lee YJ, Lee HY, Ahn MB, Kim SK, Cho WK, Lee JW, Chung NG, Cho B, Suh BK. Thyroid dysfunction in children with leukemia over the first year after hematopoietic stem cell transplantation. J Pediatr Endocrinol Metab. 2018;31(11):1241–1247. [DOI] [PubMed] [Google Scholar]
- 55. Duyu A, Çıtak EC, Ak E, Küpeli S, Yağcı Küpeli B, Bayram İ, Sezgin G, Eskendari G, Sezer K. Prevalence and related factors of euthyroid sick syndrome in children with untreated cancer according to two different criteria. J Clin Res Pediatr Endocrinol. 2018;10(3):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Das BK, Agarwal P, Agarwal JK, Mishra OP. Serum cortisol and thyroid hormone levels in neonates with sepsis. Indian J Pediatr. 2002;69(8):663–665. [DOI] [PubMed] [Google Scholar]
- 57. El-Ella SSA, El-Mekkawy MS, El-Dihemey MA. [Prevalence and prognostic value of non-thyroidal illness syndrome among critically ill children]. An Pediatr (Barc). 2019;90(4):237–243. [DOI] [PubMed] [Google Scholar]
- 58. Joosten KF, de Kleijn ED, Westerterp M, de Hoog M, Eijck FC, Voort EV, Hazelzet JA, Hokken-Koelega AC, Hokken-Koelega AC; Hop WCJ. Endocrine and metabolic responses in children with meningoccocal sepsis: striking differences between survivors and nonsurvivors. J Clin Endocrinol Metab. 2000;85(10):3746–3753. [DOI] [PubMed] [Google Scholar]
- 59. Lodha R, Vivekanandhan S, Sarthi M, Arun S, Kabra SK. Thyroid function in children with sepsis and septic shock. Acta Paediatr. 2007;96(3):406–409. [DOI] [PubMed] [Google Scholar]
- 60. Portman MA, Slee A, Olson AK, Cohen G, Karl T, Tong E, Hastings L, Patel H, Reinhartz O, Mott AR, Mainwaring R, Linam J, Danzi S; TRICC Investigators. Triiodothyronine supplementation in infants and children undergoing cardiopulmonary bypass (TRICC): a multicenter placebo-controlled randomized trial: age analysis. Circulation. 2010;122(11Suppl)S224–S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chowdhury D, Ojamaa K, Parnell VA, McMahon C, Sison CP, Klein I. A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. J Thorac Cardiovasc Surg. 2001;122(5):1023–1025. [DOI] [PubMed] [Google Scholar]
- 62. Mackie AS, Booth KL, Newburger JW, Gauvreau K, Huang SA, Laussen PC, DiNardo JA, del Nido PJ, Mayer JE Jr, Jonas RA, McGrath E, Elder J, Roth SJ. A randomized, double-blind, placebo-controlled pilot trial of triiodothyronine in neonatal heart surgery. J Thorac Cardiovasc Surg. 2005;130(3):810–816. [DOI] [PubMed] [Google Scholar]
- 63. Portman MA, Fearneyhough C, Ning XH, Duncan BW, Rosenthal GL, Lupinetti FM. Triiodothyronine repletion in infants during cardiopulmonary bypass for congenital heart disease. J Thorac Cardiovasc Surg. 2000;120(3):604–608. [DOI] [PubMed] [Google Scholar]
- 64. Bettendorf M, Schmidt KG, Grulich-Henn J, Ulmer HE, Heinrich UE. Tri-iodothyronine treatment in children after cardiac surgery: a double-blind, randomised, placebo-controlled study. Lancet. 2000;356(9229):529–534. [DOI] [PubMed] [Google Scholar]
- 65. Marwali EM, Boom CE, Budiwardhana N, Fakhri D, Roebiono PS, Santoso A, Sastroasmoro S, Slee A, Portman MA. Oral triiodothyronine for infants and children undergoing cardiopulmonary bypass. Ann Thorac Surg. 2017;104(2):688–695. [DOI] [PubMed] [Google Scholar]
- 66. Kratzsch J, Pulzer F. Thyroid gland development and defects. Best Pract Res Clin Endocrinol Metab. 2008;22(1):57–75. [DOI] [PubMed] [Google Scholar]
- 67. Eerdekens A, Langouche L, Van den Berghe G, Verhaeghe J, Naulaers G, Vanhole C. Review shows that thyroid hormone substitution could benefit transient hypothyroxinaemia of prematurity but treatment strategies need to be clarified. Acta Paediatr. 2019;108(5):792–805. [DOI] [PubMed] [Google Scholar]
- 68. Fisher DA. Thyroid system immaturities in very low birth weight premature infants. Semin Perinatol. 2008;32(6):387–397. [DOI] [PubMed] [Google Scholar]
- 69. Vanhole C, Aerssens P, Naulaers G, Casneuf A, Devlieger H, Van den Berghe G, de Zegher F. L-thyroxine treatment of preterm newborns: clinical and endocrine effects. Pediatr Res. 1997;42(1):87–92. [DOI] [PubMed] [Google Scholar]
- 70. van Wassenaer AG, Kok JH, de Vijlder JJ, Briët JM, Smit BJ, Tamminga P, van Baar A, Dekker FW, Vulsma T. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks’ gestation. N Engl J Med. 1997;336(1):21–26. [DOI] [PubMed] [Google Scholar]
- 71. Chowdhry P, Scanlon JW, Auerbach R, Abbassi V. Results of controlled double-blind study of thyroid replacement in very low-birth-weight premature infants with hypothyroxinemia. Pediatrics. 1984;73(3):301–305. [PubMed] [Google Scholar]
- 72. Uchiyama A, Kushima R, Watanabe T, Kusuda S. Effect of L-thyroxine supplementation on very low birth weight infants with transient hypothyroxinemia of prematurity at 3 years of age. J Perinatol. 2017;37(5):602–605. [DOI] [PubMed] [Google Scholar]
- 73. Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest. 1969;48(11):2096–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cavalieri RR. The effects of nonthyroid disease and drugs on thyroid function tests. Med Clin North Am. 1991;75(1):27–39. [DOI] [PubMed] [Google Scholar]
- 75. Alkemade A, Unmehopa UA, Wiersinga WM, Swaab DF, Fliers E. Glucocorticoids decrease thyrotropin-releasing hormone messenger ribonucleic acid expression in the paraventricular nucleus of the human hypothalamus. J Clin Endocrinol Metab. 2005;90(1):323–327. [DOI] [PubMed] [Google Scholar]
- 76. Benker G, Raida M, Olbricht T, Wagner R, Reinhardt W, Reinwein D. TSH secretion in Cushing’s syndrome: relation to glucocorticoid excess, diabetes, goitre, and the ‘sick euthyroid syndrome’. Clin Endocrinol (Oxf). 1990;33(6):777–786. [DOI] [PubMed] [Google Scholar]
- 77. Van den Berghe G, de Zegher F. Anterior pituitary function during critical illness and dopamine treatment. Crit Care Med. 1996;24(9):1580–1590. [DOI] [PubMed] [Google Scholar]
- 78. Van den Berghe G, de Zegher F, Lauwers P. Dopamine suppresses pituitary function in infants and children. Crit Care Med. 1994;22(11):1747–1753. [PubMed] [Google Scholar]
- 79. Hirfanoglu T, Serdaroglu A, Camurdan O, Cansu A, Bideci A, Cinaz P, Gucuyener K. Thyroid function and volume in epileptic children using carbamazepine, oxcarbazepine and valproate. Pediatr Int. 2007;49(6):822–826. [DOI] [PubMed] [Google Scholar]
- 80. Miller J, Carney P. Central hypothyroidism with oxcarbazepine therapy. Pediatr Neurol. 2006;34(3):242–244. [DOI] [PubMed] [Google Scholar]
- 81. Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab. 2009;23(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Trohman RG, Sharma PS, McAninch EA, Bianco AC. Amiodarone and thyroid physiology, pathophysiology, diagnosis and management. Trends Cardiovasc Med. 2019;29(5):285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fairbrother F, Petzl N, Scott JG, Kisely S. Lithium can cause hyperthyroidism as well as hypothyroidism: a systematic review of an under-recognised association. Aust N Z J Psychiatry. 2019;53(5):384–402. [DOI] [PubMed] [Google Scholar]
- 84. Thaker VV, Leung AM, Braverman LE, Brown RS, Levine B. Iodine-induced hypothyroidism in full-term infants with congenital heart disease: more common than currently appreciated? J Clin Endocrinol Metab. 2014;99(10):3521–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beer SF, Bircham PM, Bloom SR, Clark PM, Hales CN, Hughes CM, Jones CT, Marsh DR, Raggatt PR, Findlay AL. The effect of a 72-h fast on plasma levels of pituitary, adrenal, thyroid, pancreatic and gastrointestinal hormones in healthy men and women. J Endocrinol. 1989;120(2):337–350. [DOI] [PubMed] [Google Scholar]
- 86. Chourdakis M, Kraus MM, Tzellos T, Sardeli C, Peftoulidou M, Vassilakos D, Kouvelas D. Effect of early compared with delayed enteral nutrition on endocrine function in patients with traumatic brain injury: an open-labeled randomized trial. JPEN J Parenter Enteral Nutr. 2012;36(1):108–116. [DOI] [PubMed] [Google Scholar]
- 87. Ouchi K, Matsubara S, Matsuno S. Effects of supplementary parenteral nutrition on thyroid hormone patterns in surgical patients with liver cirrhosis. Nutrition. 1991;7(3):189–192. [PubMed] [Google Scholar]
- 88. Langouche L, Vander Perre S, Marques M, Boelen A, Wouters PJ, Casaer MP, Van den Berghe G. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab. 2013;98(3):1006–1013. [DOI] [PubMed] [Google Scholar]
- 89. Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506–517. [DOI] [PubMed] [Google Scholar]
- 90. Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, Debaveye Y, Vlasselaers D, Desmet L, Casaer MP, Garcia Guerra G, Hanot J, Joffe A, Tibboel D, Joosten K, Van den Berghe G. Early versus late parenteral nutrition in critically ill children. N Engl J Med. 2016;374(12):1111–1122. [DOI] [PubMed] [Google Scholar]
- 91. Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373(9663):547–556. [DOI] [PubMed] [Google Scholar]
- 92. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. [DOI] [PubMed] [Google Scholar]
- 93. Maldonado LS, Murata GH, Hershman JM, Braunstein GD. Do thyroid function tests independently predict survival in the critically ill? Thyroid. 1992;2(2):119–123. [DOI] [PubMed] [Google Scholar]
- 94. Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome.” Endocr Rev. 1982;3(2):164–217. [DOI] [PubMed] [Google Scholar]
- 95. Van den Berghe G, de Zegher F, Bouillon R. Clinical review 95: acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83(6):1827–1834. [DOI] [PubMed] [Google Scholar]