Abstract
Background:
Intrauterine growth restriction is often accompanied by placental vascular disease, of which histologic maternal vascular malperfusion is prominent. Maternal vascular malperfusion is characterized by accelerated villous maturation consistent with placental aging. Alpha klotho is an anti-aging protein produced by the placenta. We hypothesize that cord blood alpha klotho varies with maternal vascular malperfusion and small for gestational age infants through dysregulated angiogenesis.
Methods:
Nested case-control study of 54 preterm infants (N=22 small for gestational age infants, 32 appropriate for gestational age infants, mean gestational age=33.7±2.7 weeks) and validation sample (N=39) from a longitudinal birth cohort at Prentice Women’s Hospital, Chicago, IL. Cord blood alpha klotho was measured via enzyme-linked immunoassay; concentrations were linked to multiplex data of cord blood angiogenic growth factors.
Results:
Median cord blood alpha klotho was decreased in small for gestational age infants (1200 [859, 2083] pg/mL) versus controls (3193 [1703, 3963] pg/mL; p<0.01) and with severe maternal vascular malperfusion (1170 [760, 2645] pg/mL; P<0.01), consistent with validation sample. Alpha klotho was decreased with maternal vascular malperfusion sublesions signifying accelerated villous maturation, including increased syncytial knots (1230 [805, 3606] pg/mL; p<0.05) and distal villous hypoplasia (1170 [770, 3390] pg/mL; p<0.05). Among 15 angiogenic markers, alpha klotho correlated directly with angiopoietin-2 (beta-coefficient=2.6, p=0.01).
Conclusions:
Cord blood alpha klotho is decreased with small for gestational infants and maternal vascular malperfusion sublesions of accelerated placental villous maturation, and correlated with angiopoietin-2. Alpha klotho may play a role in vascular-mediated accelerated placental aging leading to intrauterine growth restriction.
Keywords: alpha klotho, small for gestational age, intrauterine growth restriction, placental aging, maternal vascular malperfusion, angiopoietin-2, cord blood
INTRODUCTION
Intrauterine growth restriction (IUGR), defined as birth weight for gestational age (BW-for-GA) less than the 10th percentile with evidence of delayed intrauterine growth parameters, occurs in 5-16% of all pregnancies worldwide and is a major cause of stillbirth and death in early infancy [1]. BW-for-GA less than the 10th percentile is associated with neonatal complications such as postnatal growth failure, bronchopulmonary dysplasia, and pulmonary hypertension [2–4], as well as long term health consequences such as neurodevelopmental delays, cardiopulmonary disease, and metabolic syndrome due to fetal programming mechanisms that remain poorly understood [5, 6]. Maternal risk factors for small for gestational age (SGA) infants include preeclampsia, hypertensive disease, undernutrition, smoking, and diabetes among others [7].
The role of the placenta in mediating fetal growth and its consequences of growth restriction is not well-understood. Placental growth and weight often parallel fetal growth, but in many cases fetal growth is preserved with placental growth restriction and vice versa. A common histologic lesion that accompanies pathologic IUGR is maternal vascular malperfusion (MVM) [8]. Two key features of MVM are vessel changes representing dysregulated angiogenesis and villous changes characterized by accelerated villous maturation. These findings may be mechanistic indicators of abnormally advanced placenta aging [9]. The impact of placental aging on the developing fetus is poorly understood, but premature cellular senescence/apoptosis of vascular and villous structures could account for dysregulated angiogenesis leading to IUGR and associated morbidities.
Alpha klotho (AK) is an anti-aging protein produced by the placenta [10]. AK is the cleaved soluble form of the protein, which exerts its effects on distal organs to support vasculature and reduce oxidative stress. In animal and cell models, decreased AK leads to endothelial dysfunction, impaired angiogenesis, increased oxidative stress, and cellular senescence and apoptosis [11–13]. As such, AK has become an increasingly recognized mediator in adult cardiopulmonary, renal, metabolic, and bone diseases [14, 15], but its much earlier role in placentally-mediated fetal growth restriction surrounding birth remains unclear. The objective of this study was to explore the influence of placental maternal vascular malperfusion and SGA on cord blood concentrations of alpha klotho. We hypothesized that cord blood AK is decreased in SGA infants who have evidence of maternal vascular disease, dysregulated angiogenesis, and accelerated placental maturation.
METHODS
Study design and patient sample
We conducted a nested case-control study in which patients were drawn from an ongoing longitudinal cohort study conducted at Northwestern Prentice Women’s Hospital in Chicago, IL, USA from 2008-present. Eligible patients of the parent study are live births ranging from 23–41 weeks of gestation with available cord blood. Exclusion from the parent study include known or suspected major congenital anomalies, those without placental pathology or cord blood available, those where gestational age cannot be reliably determined, and adults unable to consent. For this study, a subset of mother-infant dyads were selected from an existing study sample with complete clinical, placental, and cord blood angiogenic biomarker data in 2012–2013 [16]. For this study, mothers with known comorbid conditions including renal or cardiovascular disease apart from hypertension were excluded. The SGA cases were GA-matched and gender-matched to appropriate for gestational age (AGA) controls with existing angiogenic biomarker data. Thus, a total of 54 patients (22 cases and 32 controls) were included in this study. SGA was defined as birth weight-for-gestational age (BW-for-GA) less than the 10th percentile according to Fenton growth curves [17]. AGA infants were between the 10th and 90th percentiles for BW-for-GA. This study was approved by the Institutional Review Board of Northwestern University. Signed informed consent was obtained from all mothers prior to participation.
Validation Sample
We conducted a validation sample of 39 additional infants recruited from the ongoing longitudinal cohort study restricted to those less than 29 weeks gestation from 2017-present, which included all infant less than 29 weeks with available cord blood in this epoch. This sample was used to test the association of BW-for-GA with cord blood alpha klotho as well as the association between AK and placental MVM. These maternal-infant dyads had the same inclusion/exclusion criteria except for the limitation of gestational age. Alpha klotho ELISA was performed using the same kits (Immuno-Biological Laboratories, MN) using the same method as the larger sample.
Perinatal and placental covariates
Maternal and infant clinical data were obtained using standardized abstraction methods performed by research personnel and the Northwestern Electronic Data Warehouse. Detailed information on prenatal care, intrapartum management, pregnancy complications, and birth outcomes are routinely collected per research protocol. Maternal preeclampsia and other hypertensive disorders of pregnancy are defined according to the American College of Obstetrics and Gynecology Committee criteria [18]. Placental weight and histology are recorded from standardized gross and histopathologic examination by a perinatal pathologist masked to the study information. SGA placental weight is determined using published nomograms [19]. Placental histology included evaluation for presence of lesions along 4 major domains: acute inflammation, chronic inflammation, fetal vascular pathology and MVM. The standardized methods for reporting and analyzing the placental histologic findings are based upon guidelines from the Amsterdam Placental Workshop Group [8] and have been described previously for this cohort [16, 20]. We further classified MVM severity based upon internal criteria previously described and published for this cohort: mild MVM was defined as the presence of either vascular or villous lesions with or without accompanying placental SGA. Severe MVM was defined as the presence of both vascular and villous lesions with accompanying placental SGA [20]. Villous sublesions of MVM included villous infarcts, increased syncytial knots, villous agglutination, increased intervillous fibrin, and distal villous hypoplasia/small terminal villi. Vascular sublesions of MVM included fibrinoid necrosis/acute atherosis, mural hypertrophy of the membrane arterioles, and decidual vascular thrombosis. Accelerated placental aging was defined as signs of accelerated villous maturation including any villous change of MVM as described above.
Cord blood AK and multiplex immunoassay
Venous cord blood was collected at delivery by trained staff. Samples were aliquoted and centrifuged for 10 minutes at 4°C in a tabletop refrigerated centrifuge at 3,000 rpm. Plasma was removed from the cell pellet by pipetting and aliquots stored at −80°C. Cord blood plasma samples were retrieved from the Prentice Birth Cohort Repository and thawed on ice. Only samples without hemolysis were used. Alpha Klotho enzyme linked immunoassay (Immuno-Biological Laboratories, MN) was completed on cord blood plasma by protocol as written by the manufacturer using 1:4 dilutions in duplicate. Multiplex immunoassay using Luminex with magnetic bead platform on commercially available kits (Human Angiogenesis Panel, EMD Millipore, MA) was previously completed on the corresponding cord blood samples from these patients. This multiplex panel included 15 inflammatory and angiopoetic growth factors: Angiopoietin-2 (Ang-2), Epidermal Growth Factor, Hepatocyte Growth Factor, Heparin Binding Epidermal Growth Factor, Endoglin, Vascular Endothelial Growth Factors A, C, and D, Placental Growth Factor, Granulocyte Stimulating Growth Factor, Interleukin-8, Endothelin-1, Leptin, and Fibroblast Growth Factors 1 and 2. These factors were selected, based on literature review, for their direct or indirect association with preeclampsia or fetal growth restriction and potential effect on placental vascular disease, as previously published for this cohort. [16]. The rationale for testing these markers was that in placental insufficiency with MVM, there will be decreased angiogenesis with decreased angiogenic factors as well as decreased AK.
Placental alpha klotho immunohistochemistry
Formalin-fixed placental tissues were cut and placed on slides. Immunohistochemistry was performed using the Vectastain Elite ABC Kit (Vector Labs, Burlingame, CA) according to the manufacturer’s protocol. The slides were placed in a hybridizer at 56–60°C for 30 minutes. They were then washed with tissue clearing agent and rehydrated in series. They were placed in 0.1M sodium citrate buffer, heated for 20 minutes, then allowed to cool to room temperature. Slides were then incubated with 1.5% H2O2 in PBS, then blocked with 2.5% goat serum for 60 minutes. The slides were incubated overnight in primary antibody (Abcam, rabbit anti klotho AB181373) at 1/200 dilution with 2.5% goat serum in PBS. The following day, the slides were washed with PBS and then incubated in secondary antibody (Vector Labs, Burlingame, CA; biotinylated goat anti-rabbit) for 1 hour at room temperature in 2.5% goat serum in PBS. The slides were washed in PBS again, then incubated with Vectastain ABC Reagent at room temperature for 30 minutes. After washing the slides were incubated with DAB substrate and rinsed in water to stop the reaction after about 2 minutes. They were counterstained with hematoxylin. The slides were then dehydrated and mounted. Slides were reviewed by an experienced placental pathologist using a semi-quantitative scoring system. 0 represented negative staining, 1 represented weak staining, 2 represented strong focal, and 3 represented diffuse staining. Villous trophoblasts (VT), extravillous trophoblasts (EVT), and fetal endothelial cells (EC) were scored. Investigators were masked to all maternal and neonatal data including MVM, BW, BW-for-GA, and AK concentration.
Statistical analysis
AK data were linked to the multiplex 15-biomarker panel and clinical data on maternal health and peripartum complications. The primary analysis was cord blood (CB) AK concentration by SGA status. Secondary analyses included AK by MVM status and its corresponding sublesions, MVM sublesion limited to SGA infants, AK by placental weight, maternal, and infant covariates, and correlation with angiogenic biomarker data. Data were analyzed using non-parametric tests when comparing median biomarker concentrations as these were not normally distributed as tested by the Shapiro-Wilk test. Multivariable linear and logistic regression of log-transformed biomarker concentrations were conducted adjusting for relevant maternal and infant covariates (mode of delivery, preterm labor, preeclampsia, SGA status, and GA). In the validation sample, the correlation of BW-for-GA and AK was tested using linear regression. Non-parametric testing was used to test AK and MVM and MVM sublesions in this sample. Students t-tests were used for semi-quantitative comparison testing. P-value <0.05 was considered statistically significant. All analyses were conducted using IBM SPSS Statistics for Macintosh, Version 25 (IBM, Chicago, IL).
RESULTS
Clinical characteristics of the sample are shown in Table 1. Mean GA for the patient sample was 33.7 ± 2.7 weeks with no difference between SGA and AGA groups. There was an equal mix of males and females, maternal age was equal between the groups, and maternal race did not differ significantly between groups. Birth weight was significantly different between the groups as would be expected based on stratification. Preeclampsia and cesarean section were significantly higher in SGA infants, while preterm labor was significantly higher in AGA infants. There were no differences in Apgar scores.
Table 1.
Clinical Characteristics of the Patient Sample
| AGA (n=32) | SGA (n=22) | P value | |
|---|---|---|---|
| Gestational Age (weeks) | 33.4 ± 3 | 34.3 ± 2.6 | NS |
| Birth Weight (grams) | 2118±613 | 1575±361 | <0.01 |
| Sex (% male) | 16 (50) | 11 (50) | NS |
| Maternal Age (years) | 31.5 ± 5.3 | 31.6 ± 6.8 | NS |
| Maternal Race | NS | ||
| Black | 8 (25) | 8 (36.4) | |
| White | 12 (37.5) | 8 (36.4) | |
| Other | 12 (37.5) | 6 (27.3) | |
| Preeclampsia | 7 (22) | 14 (64) | <0.01 |
| Chronic Hypertension | 1 (3) | 6 (27.3) | <0.01 |
| Preterm Labor | 21 (66) | 3 (14) | <0.001 |
| C-section | 7 (22) | 14 (64) | <0.01 |
| APGAR score | |||
| 1 minute (mean ± SD) | 7 ± 1.9 | 6.4 ± 2.4 | NS |
| 5 minute (mean ± SD) | 8.2 ± 1.3 | 8 ± 1.7 | NS |
Numbers reported are mean ± SD or number (%)
Association of AK with perinatal factors
As shown in Table 2, CB AK was lower in the SGA group (median [IQR] 1200 [859, 2083] pg/mL vs 3193 [1703, 3963] pg/mL, p<0.01) compared to the AGA group. Additionally, CB AK was significantly higher with preterm labor and lower with cesarean section and preeclampsia. CB AK was not significantly different with gender, premature rupture of membranes, prolonged rupture of membranes, and Apgar scores. Further analysis of infant covariates using linear regression adjusting for mode of delivery and preterm labor showed CB log transformed AK directly correlated with BW (Standardized Beta=0.4, SE=0, R2=0.34, p<0.001) and BW-for-GA (Standardized beta 0.44, SE=0.003, R2=0.19, p=0.001), but not GA (Standardized Beta=0.11, SE=0.39, R2=0.01, p=0.43).
Table 2.
Median AK Level by Perinatal Covariate
| N | AK Level pg/ml Median [IQR] | P value | |
|---|---|---|---|
| Small for Gestational Age | |||
| No | 32 | 3193 [1703, 3963] | <0.01 |
| Yes | 22 | 1200 [859, 2082] | |
| Male Sex | |||
| No | 27 | 1640 [1050, 3390] | NS |
| Yes | 27 | 2370 [1250, 3810] | |
| Preeclampsia | |||
| No | 33 | 2475 [1373, 4210] | NS |
| Yes | 21 | 1345 [963, 3022] | |
| Chronic Hypertension | |||
| No | 47 | 2305 [1230, 3530] | NS |
| Yes | 7 | 1345 [750, 2370] | |
| Preterm Labor | |||
| No | 30 | 1310 [1006, 2509] | <0.05 |
| Yes | 24 | 3193 [1868, 4310] | |
| C-section | |||
| No | 33 | 2654 [1567, 3816] | <0.05 |
| Yes | 21 | 1250 [963, 2973] | |
| Premature Rupture of Membranes | NS | ||
| No | 35 | 1610 [1050, 3390] | |
| Yes | 19 | 3065 [1640, 4410] | |
| Prolonged Rupture of Membranes | NS | ||
| No | 48 | 2075 [1159, 2075] | |
| Yes | 6 | 3392 [1535, 4592] | |
P-values computed using Kruskal-Wallis non-parametric testing
Cord blood AK and placental characteristics
CB AK concentrations by placental characteristics are shown in Table 3. AK was significantly lower with SGA placenta. Further linear regression analysis did not show a significant correlation between cord blood AK with placental weight (Standardized Beta=0.1, SE=0.04, p>0.05) after adjustment for gestational age, SGA status, mode of delivery, and preterm labor. Median cord blood AK was significantly lower in those with MVM compared to controls (1200 [859, 2082] pg/mL vs 3193 [1703, 3963] pg/mL, p<0.01). Mild MVM did not show significant difference in CB AK when compared to controls, while severe MVM showed lower CB AK compared to those without severe MVM. AK concentrations according to specific MVM sublesions showed that any vascular sublesion had significantly lower CB AK. Specifically, muscularization of the basal plate arterioles showed significantly lower CB AK compared to those without. Any villous sublesion also had significantly lower CB AK. Villous sublesions of increased syncytial knots and distal villous hypoplasia had significantly decreased CB AK. There were no differences with other domains of placental histology.
Table 3.
Cord blood Alpha Klotho according to Placental Characteristics
| N | AK Level pg/ml Median [IQR] | P-Value | |
|---|---|---|---|
| Placental weight small for gestational age | |||
| No | 24 | 3066 [2180, 3822] | <0.001 |
| Yes | 30 | 1240 [859, 2588] | |
| MVM Status | |||
| Any MVM | |||
| No | 29 | 2475 [1657, 3510] | <0.05 |
| Yes | 25 | 1230 [805, 3606] | |
| Mild MVM | |||
| No | 50 | 2075 [1208, 3410] | NS |
| Yes | 4 | 4146 [1586, 4686] | |
| Severe MVM | |||
| No | 33 | 2654 [1657, 3816] | <0.01 |
| Yes | 21 | 1170 [760, 2645] | |
| MVM sublesion | |||
| Vascular Sublesions | |||
| Any Vascular | |||
| No | 29 | 2475 [1657, 3510] | <0.05 |
| Yes | 25 | 1230 [805, 3606] | |
| Fibrinoid necrosis/acute atherosis | |||
| No | 43 | 2370 [1275, 3530] | NS |
| Yes | 11 | 1050 [600, 3390] | |
| Mural hypertrophy of membrane arterioles | |||
| No | 44 | 2415 [1376, 3740] | NS |
| Yes | 10 | 958 [671, 1766] | |
| Muscularization of basal plate arterioles | |||
| No | 38 | 2468 [1575, 3600] | <0.05 |
| Yes | 16 | 1200 [794, 2804] | |
| Decidual vascular thrombosis | |||
| No | 53 | 2180 [1226, 3510] | NS |
| Yes | 1 | 242 | |
| Villous Sublesions | |||
| Any Villous | <0.05 | ||
| No | 29 | 2475 [1657, 3510] | |
| Yes | 25 | 1230 [805, 3606] | |
| Villous infarcts | |||
| No | 44 | 2338 [1235, 3485] | NS |
| Yes | 10 | 1070 [657, 3869] | |
| Increased syncytial knots | |||
| No | 29 | 2475 [1657, 3510] | <0.05 |
| Yes | 25 | 1230 [805, 3606] | |
| Villous agglutination | |||
| No | 46 | 2243 [1245, 3600] | NS |
| Yes | 8 | 1163 [624, 3353] | |
| Increased intervillous fibrin | |||
| No | 45 | 2305 [1263, 3670] | NS |
| Yes | 9 | 1170 [648, 3315] | |
| Distal villous hypoplasia/small terminal villi | |||
| No | 31 | 2475 [1640, 3530] | <0.05 |
| Yes | 23 | 1170 [770, 3390] | |
| Other domains | |||
| Acute inflammation | |||
| No | 38 | 1862 [1050, 3485] | NS |
| Yes | 16 | 2338 [1366, 3730] | |
| Chronic inflammation | |||
| No | 27 | 1640 [865, 3294] | NS |
| Yes | 27 | 2460 [1275, 4410] | |
| Fetal vascular pathology | |||
| No | 38 | 2243 [1245, 3869] | NS |
| Yes | 16 | 1385 [784, 2811] | |
P-values computed using Kruskal-Wallis non-parametric testing compared to the “no” group. MVM=maternal vascular malperfusion
Cord blood AK with SGA stratified by MVM sublesion
We tested the interaction effects of SGA with MVM on AK concentrations. AK was decreased further when placental aging lesions and SGA were both present. Median AK concentration in the subgroup of infants with SGA and distal villous hypoplasia and/or increased syncytial knots was significantly lower than the subgroup with SGA but without distal villous hypoplasia and/or increased syncytial knots (median AK=870 [638, 1166] vs 1895 [1316, 3394], p<0.002). Furthermore, AK was significantly lower when muscularization of the basal plate arterioles was present with SGA compared to SGA without muscularization of the basal plate arterioles (medial= 958 [469, 1166] vs 1540 [1006, 2392], p=0.02).
Angiogenic growth factors and cord blood AK
Table 4 shows CB AK by angiogenic growth factors. Using multivariate linear regression, log-transformed AK was directly correlated with log-transformed angiopoietin-2 (Beta=0.64, (95% CI 0.15–1.12), SE=0.25, p=0.01) after adjustment for the other 14 angiogenic markers, SGA status, gestational age, mode of delivery, and preterm labor. In contrast, the other 14 biomarkers were not significantly correlated with AK.
Table 4.
Angiogenesis Multiplex Assay correlated with Cord Blood Alpha Klotho
| Biomarker | Standardized β-coefficient | SE | P-Value |
|---|---|---|---|
| Epidermal Growth Factor | −0.03 | 0.07 | NS |
| Angiopoeitin-2 | 0.64 | 0.25 | 0.01 |
| Granulocyte Colony Stimulating Factor | 0.02 | 0.11 | NS |
| Endoglin | −0.25 | 0.19 | NS |
| Endothelin 1 | −0.09 | 0.09 | NS |
| Leptin | −0.03 | 0.09 | NS |
| Fibroblast Growth Factor 1 | 0.06 | 0.20 | NS |
| Fibroblast Growth Factor 2 | 0.08 | 0.12 | NS |
| Interleukin-8 | −0.13 | 0.15 | NS |
| Hepatocyte Growth Factor | −0.03 | 0.17 | NS |
| Heparin-Binding Epidermal Growth Factor | 0.25 | 0.17 | NS |
| Placental Growth Factor | 0.05 | 0.10 | NS |
| Vascular Endothelial Growth Factor A | −0.07 | 0.07 | NS |
| Vascular Endothelial Growth Factor C | 0.03 | 0.10 | NS |
| Vascular Endothelial Growth Factor D | 0.11 | 0.06 | NS |
Multiple linear regression was performed in log-transformed variables compared to log-alpha klotho, adjusted for other 14 biomarkers, gestational age, intrauterine growth restriction status, mode of delivery, and preterm labor.
AK expression in the placenta
We performed IHC staining on representative placental tissue slides from cases and controls, with and without placental MVM, as depicted in Figure 1. Using a semi-quantitative scoring system, AGA, non-MVM control placentas had negative to weak staining of EVT, VT, and EC (Figure 1a). AGA infants exposed to placental MVM (Figure 1b) had significantly higher AK staining (p=0.04) compared to controls. There was a trend toward decreased AK endothelial staining in SGA infants with (Figure 1c) and without MVM (Figure 1d) compared to AGA infants with MVM. There were no significant differences between villous and extravillous trophoblast AK staining between the groups.
Figure 1.
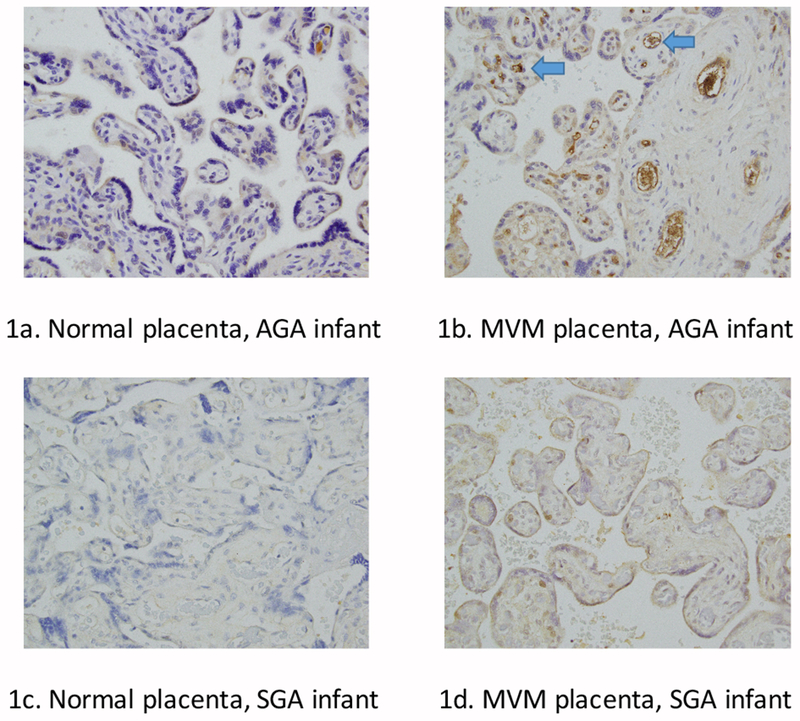
normal placenta, AGA infant shows minimal AK staining. Figure 1b: MVM placenta, AGA infant shows increased endothelial AK staining (blue arrows), suggesting placental endothelial AK plays a protective role in intrauterine growth restriction. Figure 1c: Normal placenta, SGA infant shows low staining of AK in the endothelial cells. Figure 1d: MVM, SGA infant shows low endothelial staining of AK. In the MVM placental slides, distal villous hypoplasia, small terminal villi, and syncytial knots are also apparent.
Validation sample
In this sample, there was one patient who was SGA. Using linear regression, BW-for-GA was directly correlated with cord blood log-AK (Standardized beta 0.67, SE=0.003, R2=0.45, p=0.001). AK was found to be significantly lower with severe MVM (n=4, 681 [395, 1020] vs. 2524 [1347, 3229], p=0.003) and distal villous hypoplasia (n=27, 1521 [1111, 2686] vs 3266 [1626, 3684], p=0.009).
DISCUSSION
We found that cord blood AK was decreased with evidence of accelerated placental villous maturation and in SGA infants. Villous lesions of MVM including increased syncytial knots and distal villous hypoplasia had lower CB AK supporting the finding of placental aging with these sublesions associated with accelerated villous maturation, confirmed in a separate validation sample. There was no association with other domains of placental pathology including acute or chronic inflammation and fetal vascular pathology, supporting a selective association between AK and MVM. Lastly, among 15 angiogenic cytokines and growth factors in cord blood, we found a direct correlation between Ang-2 and AK, suggesting an interactive role for AK in supporting placental and fetal angiogenesis. Collectively, the above findings have important implications for our understanding of the role of accelerated aging and angiogenesis in placentally-mediated fetal growth restriction.
Alpha klotho, named after the Greek goddess who spins the thread of life, is the cleaved soluble form of the anti-aging protein produced by the placenta, kidney, and germinal matrix. It exerts its effects on distal organs to support vasculature and reduce oxidative stress. Decreased AK leads to endothelial dysfunction, impaired angiogenesis, increased oxidative stress, and cellular senescence and apoptosis, which in turn lead to cardiovascular, pulmonary, renal, metabolic, and bone diseases [14, 15]. AK−/− mouse knockout models show a phenotype of accelerated aging with shortened lifespan, arteriosclerosis, osteoporosis, pulmonary emphysema, pulmonary hypertension, ectopic calcification, and skin atrophy. [21] Heterozygous AK+/− knockout mice show only pulmonary emphysema resembling bronchopulmonary dysplasia and associated pulmonary hypertension without other signs of premature aging [22]. In a mouse model of pulmonary hypertension, administration of exogenous AK ameliorates signs and symptoms pulmonary hypertension [23] and in wild type mice, exogenous AK administration increases lifespan by 20–30% [24]. These studies suggest that AK may play a key role in pathologic development of the fetus and organogenesis.
Our cord blood and placental findings support an association between AK and accelerated aging, as advanced villous maturation for gestational age at birth was accompanied by low circulating concentration of AK. Previous studies have shown decreased placental AK mRNA and protein expression as well as decreased CB and maternal serum AK in preeclampsia compared to normal pregnancies [25, 26]. In contrast, another study showed increased placental AK expression with accelerated placental villous maturation [27], a finding that supports our immunohistochemical findings in placental tissues of those infants who are AGA exposed to MVM (Figure 1b). The increased AK endothelial staining with AGA infants exposed to MVM suggests that endothelial AK may be protective of intrauterine growth and/or abnormal development of fetal endothelial vessels in face of MVM and that the placenta may serve as a mediator to protect against the consequences of fetal AK depletion or represent abnormal processing or retention of the soluble protein. Maternal concentration of AK was also shown to be higher in PE that study, which may be due to higher concentrations of placental AK production in maternal circulation. We speculate that AK concentrations are reflective of placental insufficiency and/or chronic hypoxia associated with MVM and PE, which may be regulated by additional hypoxia induced factors including HIF-2alpha [28]. Additional studies are needed to identify the upstream regulators of placental AK expression, but perhaps maternal diseases such as preeclampsia play an early inciting role [29].
A hallmark of placental MVM is abnormal trophoblast implantation which is thought to be a precursor to preeclampsia. Spiral artery remodeling is disrupted leading to malperfusion of the placental bed during pregnancy. This may trigger compensatory increases in Ang-2 and perhaps AK to preserve placental angiogenic and anti-apoptotic functions. Preservation of placental growth and development may lead to depletion of fetal AK concentration as seen in CB at birth. Although these hypothesized mechanisms require further investigation, if true, CB AK may serve as an important marker of SGA mediated by early onset placental villous and vascular dysfunction.
AK has been shown, both in vitro and in vivo, to support angiogenesis [14, 30, 31]. When comparing CB concentration to a panel of angiogenic factors, AK was directly correlated with Ang-2, a marker associated with vessel remodeling and placental trophoblast migration. The understanding of the role of Ang-2 in the placenta is evolving. Literature shows decreased Ang-2 protein expression in IUGR compared with control human placentas [32], restricted to the cytotrophoblasts. When Ang-2 was administered exogenously to trophoblast cell lines, Ang-2 upregulated DNA synthesis and increased nitric oxide signaling. Since CB Ang-2 was directly correlated with CB AK, we hypothesize that presence of Ang-2 may play a role in promoting placental angiogenesis and, in turn, fetal growth. Interaction of Ang-2 and AK needs to be further investigated to elicit a potential mechanism in IUGR.
MVM shows both accelerated villous maturation and dysregulated vascular growth, which are signs of aging in placenta [9]. Previous studies have shown a distinct epigenetic signature of placental aging [33] as well as increased cell senescence, apoptotic markers, and decreased telomere length in placentas from IUGR infants compared to controls [34, 35]. Alpha klotho may provide a mechanistic link between placental aging and the vascular processes that can contribute to placental vascular dysfunction. AK plays a role in placental villous and vascular maturation, and decreased AK may contribute to development of fetal growth restriction in placentas with MVM.
Our study was limited by the small sample size. With a larger sample size, the study would have more power to correlate AK with additional perinatal, placental, and biochemical covariates in a more inclusive regression model. We used a validation sample to confirm our initial findings, which strengthens the association and validity. Additionally, we were limited to the angiogenic factors present in the human angiogenesis multiplex immunoassay. Future epigenetic and proteomic studies may be able to assess the interaction and complexity of angiogenesis related to placental aging and IUGR. An additional limitation is that we are not able to determine the site of production of cord blood AK in this human study. We believe that the total concentration of reflective of the pathophysiology regardless of the site of origin and may be mediated by placental insufficiency. The results of our study are not evidence of causality; additional studies are needed to determine the influence of AK on placental vascular development and its effect on the fetus.
To our knowledge, this is the first study to comprehensively study the clinical, biochemical and placental correlates of AK at birth. The information gained is important as cord blood alpha klotho may be a useful marker to identify infants at risk for the consequences of placental-mediated fetal growth restriction even when IUGR is not apparent. Further studies are warranted to target mechanisms of AK-mediated placental growth and fetal angiogenesis, and to identify factors that regulate its expression and function. In conjunction with placental histology, alpha klotho may be developed as an early biomarker of neonatal outcomes.
Highlights:
Cord blood alpha klotho is decreased in small for gestational age infants.
Klotho is decreased with placental sublesions of accelerated villous maturation.
Angiopoetin-2 is correlated with alpha klotho in cord blood.
Klotho may play a role in vascular-mediated placental aging and fetal growth.
Acknowledgements:
We would like to thank the Northwestern Comprehensive Metabolic Core and Northwestern Enterprise Data Warehouse for their contributions.
Statement of Financial Support: This work was supported by the National Institute of Health [R01-HL139798 (PI Mestan)] and the Stanley Manne Research Institute [Fellow grant (PI Franklin)].
Abbreviations:
- IUGR
intrauterine growth restriction
- BW-for-GA
birth weight for gestational age
- MVM
maternal vascular malperfusion
- AK
alpha klotho
- ang-2
angiopoietin-2
- SGA
small for gestational age
- AGA
appropriate for gestational age
- CB
cord blood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
The authors declare no conflict of interest.
Disclosure Statement: The authors declare no conflict of interest.
REFERENCES
- [1].Brodsky D, Christou H, Current concepts in intrauterine growth restriction, J Intensive Care Med 19(6) (2004) 307–19. [DOI] [PubMed] [Google Scholar]
- [2].Rosenberg A, The IUGR newborn, Semin Perinatol 32(3) (2008) 219–24. [DOI] [PubMed] [Google Scholar]
- [3].Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK, Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia, J Perinatol 33(7) (2013) 553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Voller SB, Chock S, Ernst LM, Su E, Liu X, Farrow KN, Mestan KK, Cord blood biomarkers of vascular endothelial growth (VEGF and sFlt-1) and postnatal growth: a preterm birth cohort study, Early Hum Dev 90(4) (2014) 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wixey JA, Chand KK, Colditz PB, Bjorkman ST, Review: Neuroinflammation in intrauterine growth restriction, Placenta 54 (2017) 117–124. [DOI] [PubMed] [Google Scholar]
- [6].Chen J, Chen P, Bo T, Luo K, Cognitive and Behavioral Outcomes of Intrauterine Growth Restriction School-Age Children, Pediatrics 137(4) (2016). [DOI] [PubMed] [Google Scholar]
- [7].Cetin I, Mando C, Calabrese S, Maternal predictors of intrauterine growth restriction, Curr Opin Clin Nutr Metab Care 16(3) (2013) 310–9. [DOI] [PubMed] [Google Scholar]
- [8].Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AE, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PG, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, van der Voorn JP, van Lijnschoten I, Gordijn SJ, Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement, Arch Pathol Lab Med 140(7) (2016) 698–713. [DOI] [PubMed] [Google Scholar]
- [9].Fox H, Aging of the placenta, Arch Dis Child Fetal Neonatal Ed 77(3) (1997) F171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kuro-o M, Klotho and aging, Biochim Biophys Acta 1790(10) (2009) 1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, Kurabayashi M, Kuroo M, Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases, Cell Mol Life Sci 57(5) (2000) 738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dalton GD, Xie J, An SW, Huang CL, New Insights into the Mechanism of Action of Soluble Klotho, Front Endocrinol (Lausanne) 8 (2017) 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bian A, Neyra JA, Zhan M, Hu MC, Klotho, stem cells, and aging, Clin Interv Aging 10 (2015) 1233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R, Klotho protein protects against endothelial dysfunction, Biochem Biophys Res Commun 248(2) (1998) 324–9. [DOI] [PubMed] [Google Scholar]
- [15].Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M, Regulation of oxidative stress by the anti-aging hormone klotho, J Biol Chem 280(45) (2005) 38029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mestan KK Gotteiner N, Porta N, Grobman W, Su EJ, Ernst LM, Cord Blood Biomarkers of Placental Maternal Vascular Underperfusion Predict Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension, J Pediatr 185 (2017) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R, Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant, BMC Pediatr 13 (2013) 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].A.C.o.O. Practice, ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists, Int J Gynaecol Obstet 77(1) (2002) 67–75. [PubMed] [Google Scholar]
- [19].Pinar H, Sung CJ, Oyer CE, Singer DB, Reference values for singleton and twin placental weights, Pediatr Pathol Lab Med 16(6) (1996) 901–7. [DOI] [PubMed] [Google Scholar]
- [20].Mestan KK, Check J, Minturn L, Yallapragada S, Farrow KN, Liu X, Su E, Porta N, Gotteiner N, Ernst LM, Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension, Placenta 35(8) (2014) 570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI, Mutation of the mouse klotho gene leads to a syndrome resembling ageing, Nature 390(6655) (1997) 45–51. [DOI] [PubMed] [Google Scholar]
- [22].Suga T, Kurabayashi M, Sando Y, Ohyama Y, Maeno T, Maeno Y, Aizawa H, Matsumura Y, Kuwaki T, Kuro OM, Nabeshima Y, Nagai R, Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life, Am J Respir Cell Mol Biol 22(1) (2000) 26–33. [DOI] [PubMed] [Google Scholar]
- [23].Varshney R, Ali Q, Wu C, Sun Z, Monocrotaline-Induced Pulmonary Hypertension Involves Downregulation of Antiaging Protein Klotho and eNOS Activity, Hypertension 68(5) (2016) 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M, Suppression of aging in mice by the hormone Klotho, Science 309(5742) (2005) 1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fan C, Wang Y, Wang J, Lei D, Sun Y, Lei S, Hu M, Tian Y, Li R, Wang S, Clinic significance of markedly decreased alpha-klothoin women with preeclampsia, Am J Transl Res 8(5) (2016) 1998–2010. [PMC free article] [PubMed] [Google Scholar]
- [26].Miranda J, Romero R, Korzeniewski SJ, Schwartz AG, Chaemsaithong P, Stampalija T, Yeo L, Dong Z, Hassan SS, Chrousos GP, Gold P, Chaiworapongsa T, The anti-aging factor alpha-klotho during human pregnancy and its expression in pregnancies complicated by small-for-gestational-age neonates and/or preeclampsia, J Matern Fetal Neonatal Med 27(5) (2014) 449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Loichinger MH, Towner D, Thompson KS, Ahn HJ, Bryant-Greenwood GD, Systemic and placental alpha-klotho: Effects of preeclampsia in the last trimester of gestation, Placenta 41 (2016) 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rajakumar A, Whitelock KA, Weissfeld LA, Daftary AR, Markovic N, Conrad KP, Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia, Biol Reprod 64(2) (2001) 499–506. [DOI] [PubMed] [Google Scholar]
- [29].Cecati M, Giannubilo SR, Saccucci F, Sartini D, Ciavattini A, Emanuelli M, Tranquilli AL, Potential Role of Placental Klotho in the Pathogenesis of Preeclampsia, Cell Biochem Biophys 74(1) (2016) 49–57. [DOI] [PubMed] [Google Scholar]
- [30].Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R, In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome, Biochem Biophys Res Commun 276(2) (2000) 767–72. [DOI] [PubMed] [Google Scholar]
- [31].Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T, Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse, Circulation 110(9) (2004) 1148–55. [DOI] [PubMed] [Google Scholar]
- [32].Dunk C, Shams M, Nijjar S, Rhaman M, Qiu Y, Bussolati B, Ahmed A, Angiopoietin-1 and angiopoietin-2 activate trophoblast Tie-2 to promote growth and migration during placental development, Am J Pathol 156(6) (2000) 2185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mayne BT, Leemaqz SY, Smith AK, Breen J, Roberts CT, Bianco-Miotto T, Accelerated placental aging in early onset preeclampsia pregnancies identified by DNA methylation, Epigenomics 9(3) (2017) 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Paules C, Dantas AP, Miranda J, Crovetto F, Eixarch E, Rodriguez-Sureda V, Dominguez C, Casu G, Rovira C, Nadal A, Crispi F, Gratacos E, Premature placental aging in term small-for-gestational-age and fetal-growth-restricted fetuses, Ultrasound Obstet Gynecol (2018). [DOI] [PubMed] [Google Scholar]
- [35].Londero AP, Orsaria M, Marzinotto S, Grassi T, Fruscalzo A, Calcagno A, Bertozzi S, Nardini N, Stella E, Lelle RJ, Driul L, Tell G, Mariuzzi L, Placental aging and oxidation damage in a tissue micro-array model: an immunohistochemistry study, Histochem Cell Biol 146(2) (2016) 191–204. [DOI] [PubMed] [Google Scholar]


