Abstract
We investigated the impact of hypertension on circulatory responses to exercise and the role of the exercise pressor reflex in determining the cardiovascular abnormalities characterizing patients with hypertension. Following a 7-day drug washout, 8 hypertensive (HTN; mean arterial pressure [MAP] 130±4mmHg; 65±3yrs) and 8 normotensive (NTN; MAP 117±2mmHg; 65±2yrs) individuals performed single-leg knee-extensor exercise (7W, 15W, 50%, 80%-Wpeak) under control conditions and with lumbar intrathecal fentanyl impairing feedback from μ-opioid receptor-sensitive leg muscle afferents. Femoral artery blood flow (QL), MAP (femoral artery), leg vascular conductance (LVC), and changes in cardiac output (CO) were continuously measured. While the increase in MAP from rest to control exercise was significantly greater in HTN compared to NTN, the exercise-induced increase in CO was comparable between groups, and QL and LVC responses were ~18% and ~32% lower in the hypertensive patients (P<0.05). The blockade-induced decreases in MAP were significantly larger during exercise in HTN (~11mmHg) compared to NTN (~6mmHg). Afferent blockade attenuated the central hemodynamic response to exercise similarly in both groups resulting in a ~15% lower CO at each workload. With no effect in NTN, afferent blockade significantly raised the peripheral hemodynamic response to exercise in HTN, resulting in ~14% and ~23% higher QL and LVC during exercise. Finally, QL and MAP during fentanyl-exercise in HTN were comparable to that of NTN under control conditions (P>0.2). These findings suggest that exercise pressor reflex abnormalities largely account for the exaggerated MAP response and the impaired peripheral hemodynamics during exercise in hypertension.
Keywords: autonomic control, cardiovascular disease, blood flow, blood pressure, exercise intolerance
INTRODUCTION
Patients with hypertension (HTN) are, compared to their healthy counterpart (NTN), characterized by exaggerated sympathoexcitation, increased vascular resistance, compromised limb blood flow, and higher blood pressures during exercise 1–4. While the cardiovascular risks associated with these irregularities are well recognized 5, the reasons why they occur are not completely understood. For example, some studies found the absolute increases in mean arterial blood pressure (MAP; in mmHg) from rest to exercise (arms or legs) to be similar in HTN and NTN 4, 6, 7, suggesting that the pressor abnormalities during exercise are predominantly accounted for by the chronically elevated baseline levels in hypertensive patients. Other studies, also not particularly limited to a specific limb (arms or legs), muscle mass (small or large), or contractile regime ( isometric or dynamic exercise), contradict these observations and document that the absolute changes in MAP and sympathoexcitation from rest to exercise are substantially larger in HTN compared to NTN 2, 5, 8, 9. This suggests that the pressor abnormalities during exercise in hypertensive patients result from the combination of larger exercise-induced increases, superimposed on chronically elevated baseline levels. The reason as to why in some studies HTN patients display normal exercise-induced cardiovascular changes while in others HTN patients are characterized by exaggerated responses remains unclear.
The cardiovascular response at the onset of, and during, exercise is determined by three autonomic neurocirculatory control mechanisms. These include a feed-forward system, called central command 10, and two feedback systems, namely, the baroreflexes 11, and the exercise pressor reflex (EPR) 12. Findings from recent animal studies unanimously suggest that the inappropriate cardiovascular response to exercise in hypertension is largely determined by an overactive EPR emanating from both heightened mechano- and metaboreflexes 13, 14. Briefly, the EPR is triggered by muscle contraction-induced mechanical (i.e. the mechanoreflex component of the EPR) and chemical (i.e. metaboreflex component) stimuli activating group III/IV muscle afferents which stimulate neural circuits in the nucleus tractus solitarii and the ventrolateral medulla. This results in an increase in both cardiac and muscle sympathetic nerve activity and parasympathetic withdrawal which, in turn, causes substantial hemodynamic changes 15, 16.
In contrast to the findings in animal studies, the literature compiled from work in humans is rather unclear with some studies documenting metaboreflex-based EPR abnormalities in HTN 8, 17, whereas others suggest normal cardiovascular responses to exercise while simultaneously emphasizing EPR dysfunction in these patients 4, 6. At least some of these inconsistencies might result from the fact that human investigations on EPR function have traditionally utilized post-exercise circulatory occlusion techniques (PECO) following isometric exercise utilizing a small muscle mass. Since there are two subtypes of group III/IV muscle afferents, with one responding to metabolites associated with normal exercise and one only responding to a metabolic milieu seen during ischemic contractions 18, 19, studies employing PECO may not entirely focus on EPR function as evoked by normal exercise. Furthermore, findings based on the PECO approach are also limited as this technique is utilized at rest with a different autonomic background activity compared to exercise, and neglects the influence of central command and potential interactions between the mechano- and the metaboreflex component of the EPR 20, 21.
To circumvent these issues, previous studies used intrathecal fentanyl to attenuate feedback from group III/IV muscle afferents during exercise 22. This pharmacological approach does not alter muscle force generating capacity 23, but attenuates both the mechano- and the metaboreceptive components of the reflex arc allowing for a functionally relevant investigation of the EPR in health 22, 24 and disease 25, 26. Of note, with respect to hypertension, such an attenuation of muscle afferent feedback during leg exercise in hypertensive subjects was recently documented to curb their exercising MAP to that of normotensive individuals 2. However, as afferent blockade was not applied in the normotensive participants, in whom this procedure is known to attenuate MAP during exercise 24, the potential for abnormal EPR function in hypertension was not actually addressed.
Therefore, the purpose of the current study was to evaluate a) the impact of hypertension on the circulatory response to exercise, and b) the role of the EPR in determining the cardiovascular abnormalities often characterizing hypertensive individuals. We hypothesized that the leg vascular conductance and blood flow response to knee-extensor exercise is impaired in HTN and that abnormal EPR function account for much of these irregularities.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. Eight normotensive (NTN) and eight age-, BMI-, and activity-matched hypertensive (HTN) males participated in this study (Table 1). None of the subjects were diagnosed with diabetes, pulmonary disease or heart failure. HTN subjects who were on pharmacological treatment of hypertension (5 out of 8), discontinued their medication, with a 7 day wash out period, prior to data collection. Hypertension was defined as a systolic BP >140 mmHg and/or diastolic BP >90 mmHg 27. All experimental procedures conformed to the Declaration of Helsinki and were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center. All participants were provided with and signed an informed consent.
Table 1.
Subject Characteristics
| Characteristic | HTN | NTN |
|---|---|---|
| Number of subjects | 8 | 8 |
| Age (years) | 65 ± 3 | 66 ± 3 |
| Weight (kg) | 81 ± 5 | 91 ± 5 |
| Height (cm) | 179 ± 2 | 180 ± 3 |
| Wpeak (Watts) | 34 ± 6 | 44 ± 3* |
| 50% Wpeak (Watts) | 17 ± 2 | 22 ± 3* |
| 80% Wpeak (Watts) | 28 ± 3 | 36 ± 5* |
| Body mass index (kg m−2) | 25.3 ± 1.2 | 28.2 ± 1.6 |
| Right thigh muscle mass (kg) | 2.1 ± 0.2 | 2.2 ± 0.1 |
| Systolic blood pressure (mmHg) | 154 ± 5 | 123 ± 9* |
| Diastolic blood pressure (mmHg) | 92 ± 3 | 80 ± 5* |
| Mean arterial pressure (mmHg) | 130 ± 4 | 117 ± 2* |
| Antihypertensive medication (number of participants out of 8) | 5 1: Lisinopril 2: Losartan 3: Hydrochlorothiazide |
0 |
Experimental protocol
Subjects were familiarized with the experimental procedures and the exercise modality in the first session. In a follow-up session, subjects performed an incremental single-leg knee-extensor test (0 ± 5 W/min) to task failure to determine their maximum work rate (Wpeak). After 48 to 72 hours, subjects returned to the laboratory where their right femoral artery and vein were catheterized (18 gauge) using the Seldinger technique. Following 15 minutes of rest, CO2 sensitivity was evaluated by determining the ventilatory response to three levels of inspired CO2. This test was conducted to assess the potential migration of intrathecal fentanyl to the level of the brainstem, which, if apparent, could confound the results of the study as fentanyl can bind to the medullary opioid receptors and directly affect the neurons that are involved in cardiovascular and ventilatory response 28.
Following a short break, measurements of femoral artery blood flow (QL), cardiovascular variables, and arterial/venous blood samples were taken at rest. Thereafter, dynamic right leg knee-extensor exercise (3 min duration, separated by 2 min of rest, 60 revolutions min−1) at two absolute intensity work rates (7 and 14W) and two relative intensity work rates (50% Wpeak: HTN 17±2 W; NTN 22±3 W; P=0.2; 80% Wpeak: HTN 28±3 W; NTN 36±5 W; P=0.2) was performed. Cardiovascular variables and QL were recorded continuously throughout exercise, while arterial/venous blood samples were taken during the final minute of each work rate. Subjects were asked to rate their perceived exertion on a modified Borg scale of 0 – 10 29.
Following a 2-h rest period, the subjects were seated in an upright position and 1 ml of intrathecal fentanyl (0.025 mg ml−1), an opioid analgesic with no effect on the force generating capacity of the quadriceps 23, 30, was delivered intrathecally at vertebral interspace L3-L4 22. Ten minutes later, the CO2 sensitivity test and the exercise protocol were repeated with attenuated feedback from group III/IV muscle afferents (FENT).
Measurements
Cardiovascular responses
QL (Doppler ultrasound), heart rate (HR; 12-lead ECG), stroke volume (SV; Finapres), and cardiac output (CO; calculated as HR x SV) were quantified as previously described 31. Specifically, for the measurement of QL, blood velocity (cm/s) and vessel diameter were measured simultaneously in 12-s clips during the final minute of exercise in the common femoral artery. QL (l/min) was calculated as: blood velocity*π*(vessel diameter/2)2*60. Blood velocity measurements were performed with a handheld probe positioned firmly to maintain an insonation angle of 60 deg or less at rest and during exercise. Arterial and venous blood pressure measurements were achieved utilizing pressure transducers in line with the femoral arterial and venous catheters 31. MAP: diastolic pressure + 1/3 (systolic pressure – diastolic pressure); mean venous blood pressure (MVP): average of venous systolic and diastolic pressure; perfusion pressure (PP): MAP – MVP; leg vascular conductance (LVC): QL/PP.
Blood derived variables
Femoral arterial and venous blood samples were anaerobically collected and analyzed using a co-oximeter and blood gas analyzer (GEM 4000; Instrumentation Laboratory Co., Bedford, MA, USA). Arterial (CaO2) and venous (CvO2) oxygen content was calculated as (1.39 (Hb) (oxyhemoglobin saturation/100)) + (0.003 PO2 ). Percentage O2 extraction was calculated as: [(CaO2 - CvO2) / CaO2 ] 100. Oxygen delivery was calculated as the product of QL and CaO2 and leg oxygen consumption (VO2) as the product of CaO2 - CvO2 difference and QL.
Steady-state CO2 response test
Measurements were carried out as previously described31. Briefly, after baseline responses to eupneic air were recorded for 5 min, ventilatory responses to two different concentrations of inspired CO2 (3 and 6% CO2, 70% O2, and N2 balanced; 4 minutes each) were measured in all subjects. Arterial blood samples were collected during the final 30 s of each condition and analyzed for PCO2. Breathing frequency (f R) and tidal volume (VT) were averaged over the final minute in each condition.
Data Analysis
Data points over the last minute of exercise were averaged for all dependent variables. The assumptions of a linear regression model were found to be upheld by inspection of scatter plots and histograms of residuals and predicted values. A 3-way interaction linear mixed model analyses with repeated measures were used to investigate the effect of group (i.e. NTN and HTN), work rate (7W, 15W; 50%Wpeak, 80%Wpeak) and feedback condition (control, Fentanyl) on all the calculated responses. Posthoc pairwise comparisons were performed to compare data across all three variables. Results are expressed as mean ± SEM and Cohen’s effects sizes (dz) are provided when appropriate. Statistical significance was set at P ≤ 0.05.
RESULTS
Hypercapnic ventilatory response test
Intrathecal fentanyl had no effect on eupneic air breathing, as demonstrated by the comparable breathing pattern and PaCO2 in all subjects in CTRL and FENT. Exposure to the two levels of hypercapnia did not result in a difference in PaCO2 and ventilatory responses in CTRL and FENT or between the HTN and NTN groups (Table S1, online data supplement).
MAP and PP
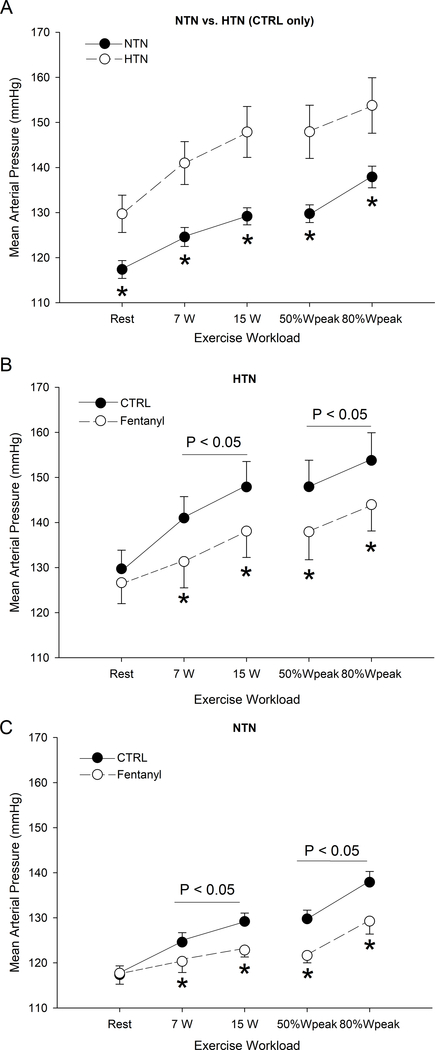
Resting MAP (130±4 mmHg vs 117±2 mmHg; P<0.05) and PP (104±4 mmHg vs 95±2 mmHg; P<0.05) were higher in HTN compared to NTN. Under CTRL conditions, both the absolute (mmHg) and relative (percent) increase in MAP from rest to exercise, at any intensity, was higher in HTN compared to NTN (Figure 1; P<0.05). At any given absolute and relative work rate, MAP was higher in HTN compared to NTN (Figure 1B and C) (P<0.01). With no effect at rest (P=0.6; dz=0.53), intrathecal fentanyl attenuated MAP and PP during exercise in HTN (P<0.01), resulting in exercising blood pressures that were no longer different to those of the normotensive individuals during the CTRL condition (P>0.1; dz<0.54) (Figure 1). Although fentanyl also reduced MAP during exercise in NTN (P<0.05), the blockade-induced decrease at the two absolute intensities (~6 mmHg) was significantly attenuated compared to that exhibited in HTN (~10 mmHg), but the effect was not different (P>0.1; dz=0.31) between the two groups during exercise at the two relative intensities (~8 mmHg in NTN and ~10 mmHg in HTN). In the fentanyl condition, there was a larger absolute (P<0.05) and relative (P<0.05) increase in MAP from rest to exercise in the hypertensive patients (Figure 1).
Figure 1. Mean arterial pressure (MAP) at rest and during the final minute of each work rate during the control (Ctrl) and fentanyl exercise in hypertensive (HTN) and normotensive (NTN) participants.
There was an effect of HTN on MAP during CTRL (P < 0.05). *P < 0.05 vs. HTN (panel A) and vs. CTRL (panels B and C).
Central hemodynamics (HR and CO)
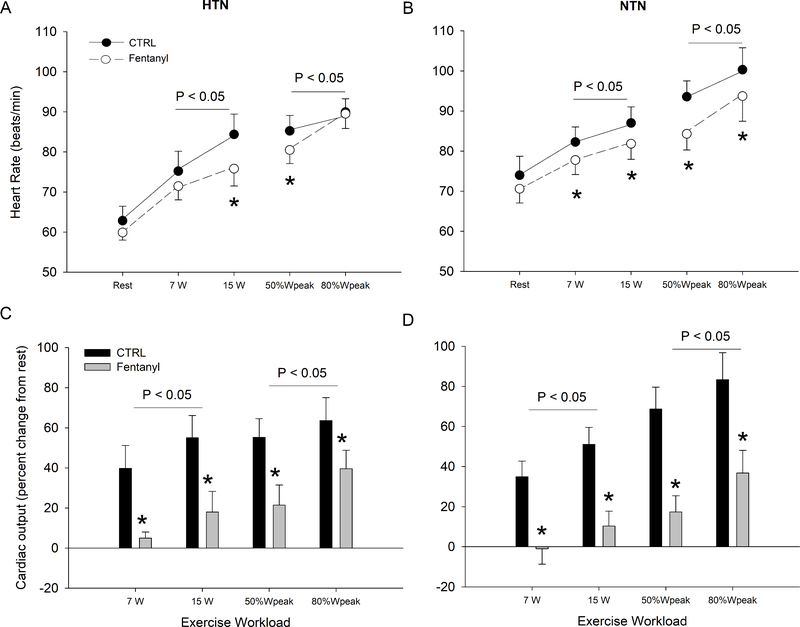
Resting HR and CO (NTN: 5.0 ± 0.4 L.min−1, HTN: 5.9 ± 0.9 L.min−1 P>0.06; dz<0.94) were not different between the two groups. The increase in HR and CO from rest to exercise of a given absolute intensity was not different between HTN and NTN (Figure 2; P>0.10; dz<0.46). HR was not different between the two groups at the two absolute work rates (P>0.1; dz<0.60), but was higher in NTN during the two relative work rates (P<0.05). With no effect at rest, intrathecal fentanyl significantly attenuated HR during absolute and relative work rates in both groups. With fentanyl blockade in both groups, the percent increase in HR from rest to exercise, of any intensity, was larger in HTN compared to NTN (P<0.05). Furthermore, without affecting resting values, intrathecal fentanyl significantly attenuated the CO response to exercise in both groups. Consequently, in both groups, with fentanyl blockade, the percent increase in CO from rest to exercise was not different (P>0.47; dz<0.50; Figure 2C and D).
Figure 2. Heart rate at rest, during the final minute of each intensity, and percent increase in cardiac output from rest during control (Ctrl) and fentanyl exercise in hypertensive (HTN) and normotensive (NTN) participants.
HR was not different between the two groups at the absolute work rates (P = 0.2), but higher in NTN compared to HTN during relative work rates (P < 0.05). The percent increase in cardiac output from rest was not different between the two groups at both absolute and relative work rates during CTRL exercise (P > 0.3). P values on graph indicate main effect of fentanyl. *P < 0.05 vs. CTRL.
Peripheral hemodynamics (QL and LVC)
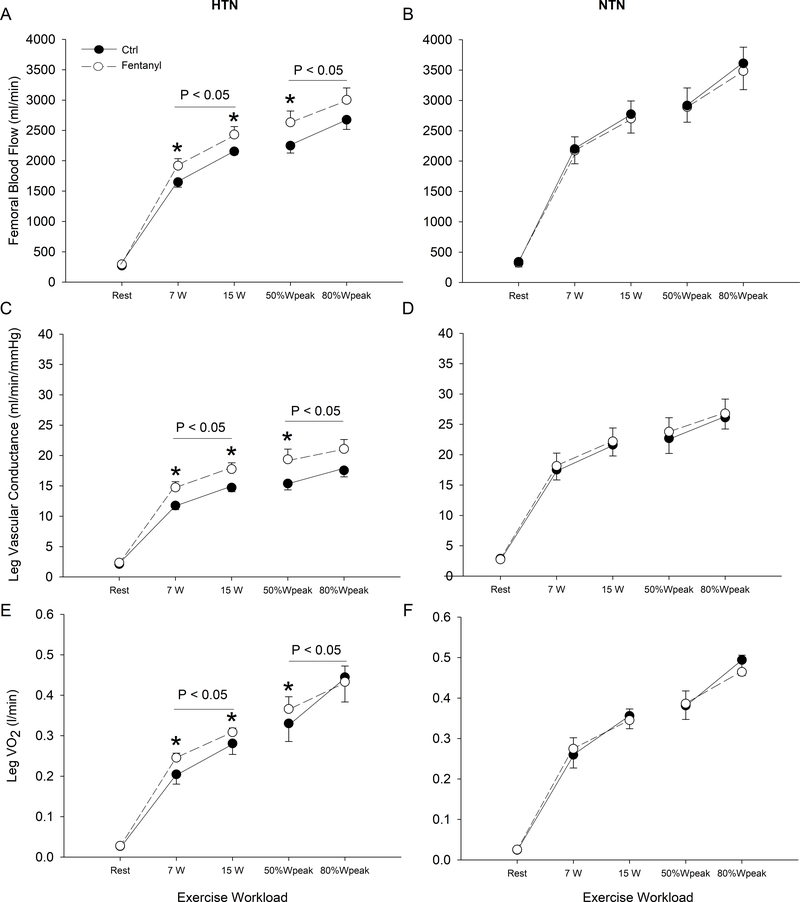
Resting QL and LVC were not different between the two groups (P>0.7; dz<0.13). The increase in QL and LVC from rest to exercise during CTRL conditions was ~18% and ~35% lower, respectively, in HTN compared to NTN (P<0.01). During CTRL exercise, at the same absolute and relative work rates, QL and LVC were 14–23% lower in HTN compared to NTN (P<0.01; Figure 3). Intrathecal fentanyl had no effect on resting QL and LVC in either group (P>0.8; dz<0.10). During exercise, fentanyl significantly increased (P<0.05) QL (12–16%) and LVC (20–25%) at the two absolute and the two relative work rates in HTN (Figure 3A and C), resulting in no difference (P>0.2; dz<0.74) in the QL response to 7 and 15W compared to NTN individuals during the CTRL condition. Intrathecal fentanyl did not change peripheral hemodynamics in NTN (P>0.5; dz<0.51; Figure 3B and D).
Figure 3. Femoral blood flow, leg vascular conductance, and leg VO2 at rest and during the final minute of each work rate performed under control (CTRL) and Fentanyl conditions in hypertensive (HTN) and normotensive (NTN) participants.
During CTRL exercise at the same absolute and relative work rates, femoral blood flow, leg vascular conductance, and leg VO2 were lower in HTN compared to NTN (P < 0.05). P values on graph indicate main effect of fentanyl. *P < 0.05 vs. CTRL.
Leg O2 Supply and O2 Utilization
Detailed results from the femoral arterial and venous O2 delivery and gas exchange measurements are presented in Table S2 of the online data supplement. Resting leg O2 delivery and leg VO2 (Figure 3E and F) were not different (P>0.80; dz<0.63) between the two groups. The increase in arteriovenous O2 difference, leg O2 delivery and leg VO2 from rest to exercise (7W and 14 W) in CTRL conditions was lower in HTN compared to NTN (P<0.05). During CTRL exercise, leg O2 delivery was lower in HTN compared to NTN (P<0.01) and arteriovenous O2 difference was not different between the two groups (P>0.80; dz<0.38), resulting in a ~21% lower leg VO2 at the absolute work rates in HTN compared to NTN (P<0.001). Intrathecal fentanyl had no effect on resting leg O2 delivery and leg VO2 in either group (P>0.14; dz<0.13). In NTN, leg O2 delivery, arteriovenous O2 difference, and leg VO2 during exercise were not altered by fentanyl (P>0.60; dz<0.28). In contrast, in HTN, while fentanyl had no effect on arteriovenous O2 difference (P>0.31; dz<0.10), fentanyl significantly increased leg O2 delivery (P<0.01) and leg VO2 (P<0.05) during absolute work rates, resulting in no difference (P>0.20; dz<0.63) in leg VO2 at 7 and 15W compared to NTN individuals assessed during the CTRL condition.
DISCUSSION
We investigated the impact of hypertension on the circulatory response to exercise and the role of the EPR in determining the cardiovascular abnormalities characterizing patients with hypertension. Compared to NTN, unmedicated HTN patients were, in addition to exhibiting an elevated resting blood pressure, characterized by a substantially greater exercise-induced increase in MAP and a compromised hyperemic response, as reflected by a lower QL and LVC at all work rates. Intrathecal fentanyl temporarily attenuated the EPR by partially blocking leg muscle afferent feedback, providing valuable insight into the role of this autonomic control mechanism in determining the observed circulatory abnormalities in HTN. Utilizing this approach, we determined that the fentanyl-induced decrease in MAP during exercise was greater in HTN than NTN and that afferent blockade curbed the increase in MAP during exercise in HTN to that seen in NTN under CTRL conditions. Furthermore, while invariant in NTN, afferent blockade facilitated the peripheral hemodynamic response to exercise in HTN and normalized QL and LVC to the levels of NTN. These observations suggest that individuals with hypertension are characterized by irregular EPR function and that this reflex abnormality likely accounts for most of the altered hemodynamic responses to exercise exhibited by this population.
Circulatory Responses to Exercise in Hypertension
While the literature from both human and animal studies suggest that MAP, vascular resistance, and sympathoexcitation are greater during exercise in hypertension 1–4, the impact of the disease on the hemodynamic response to exercise is less conclusive. Some human studies report MAP and/or HR changes from rest to exercise to be comparable in hypertensive and normotensive individuals 4, 6, 7, while others contradict these observations and document exaggerated responses in hypertensive patients 2, 5, 8, 9. It should be recognized that the heightened blood pressure during exercise in hypertensive individuals might simply result from normal exercise-induced increases, and therefore appropriate autonomic control, added on top of an elevated baseline. It is therefore important for studies focusing on the impact of hypertension on neurocirculatory control mechanisms (e.g. EPR) to consider the adequacy of the patients’ circulatory response during the transition from rest to exercise and not simply on levels during exercise. The patients in the current study were, compared to NTN, characterized by a substantially greater exercise-induced increase in MAP, significantly smaller exercise-induced increases in QL and LVC, but similar increases in CO and HR (Figure 1–3). Unlike several prior investigations 4, 6, 7, we considered these abnormal responses to exercise to be a prerequisite for this study, focused on the hypothesis that EPR dysfunction may account for the altered hemodynamic response to exercise in patients with hypertension.
Impact of Hypertension on Exercise Pressor Reflex Function
This study suggests that altered EPR function predominantly account for the circulatory abnormalities to exercise often observed in hypertension. This conclusion is based on the observations that a) muscle afferent blockade increased the LVC and QL response to exercise in HTN with no effect in NTN (Figure 3), and b) the afferent blockade-induced decrease in MAP was substantially greater in HTN compared to NTN (Figure 1). Although afferent blockade eliminated the majority of the difference in the MAP response to exercise between the groups (i.e., comparing both groups in the blocked condition), the increase was still larger in HTN. This remaining inequality suggests that, in addition to a dysfunctional EPR, other factors also contribute to the abnormal MAP response to exercise in hypertension. Indeed, disease-related impairments of other autonomic control mechanisms, such as the arterial baroreflex 32,33 and the chemoreflex 34, but also impaired functional sympatholysis 3 and vascular dysfunction 35, 36, have previously been identified to contribute to the cardiovascular abnormalities in hypertension. These findings suggest that hypertension alters EPR function and that this accounts for the compromised QL and LVC response associated with this disease and for most, but not all, of the exaggerated MAP response to exercise in this population.
Of note, leg VO2 during CTRL exercise was substantially lower in HTN compared to NTN. While this difference could theoretically be explained by a disease-related shift towards a greater reliance on non-oxidative metabolism to perform muscular contractions, the similar muscle lactate efflux in both groups (Table S2, online supplement) does not support this contention. Alternative explanations for this discrepancy include a lower O2 cost of contraction in hypertensive individuals and/or a certain degree of plasticity in the O2 consumption process 37, 38. Additional investigations are needed to address the mechanism underlying the observed group difference in leg VO2 during exercise. Interestingly, with no effect in NTN, afferent blockade raised leg VO2 in HTN and this gain was mainly achieved by an increase in O2 delivery (Table S2, online supplement). While the blockade-induced increase in leg VO2 is potentially reflective of a shift back toward a greater reliance on oxidative metabolism, the invariant lactate efflux does not support this idea.
The central hemodynamic (CO and HR) response to exercise at a given work rate was comparable between HTN and NTN (Figure 2). Afferent blockade attenuated the CO response similarly in both groups reflecting normal EPR control of cardiac output in hypertension. This indirectly implies that the abnormal EPR control of MAP in hypertensive patients is mainly accounted for by an increased systemic vascular tone or resistance, which likely also contributes to the attenuated hyperemic response to exercise in these patients. The significant effect of afferent feedback on the CO response in the current hypertensive patients contrasts with a recent study demonstrating no effect of afferent blockade on cardiac output in such patients during cycling exercise 2. Given the documented significance of the EPR in determining the CO response to exercise in healthy young 38 and old 24 individuals, and in patients with heart failure 25, this discrepancy is puzzling, but possibly related to differences in the modality of exercise.
Although afferent blockade attenuated the HR response to exercise in both groups, the increase from rest to exercise (performed with fentanyl) was significantly larger in HTN compared to NTN. Given the lack of a difference in the CO responses to fentanyl exercise in both groups, it is possible that the chronotropic effect of the EPR is compromised in hypertension while the inotropic effect of the EPR might be enhanced. Although speculative, the compromised chronotropic effect of the EPR might, potentially, be the result of a down-regulation, or desensitization, of β1-adrenergic receptors, a structural alteration associated with cardiovascular disease 39. The increased inotropic effect of the EPR might therefore just be a compensatory response to meet the cardiac output requirement to perform exercise.
While the current study provides direct evidence for a dysfunctional EPR in hypertension, the exact mechanism underlying this change remains uncertain. It is, for example, reasonable to hypothesize that disease-related alterations in intrinsic muscle characteristics 40 may exacerbate the intramuscular metabolic perturbation at a given absolute work rate, which, in turn, increases stimulation of metabosensitive muscle afferents and facilitates the metaboreflex component of the EPR. However, in the current study, inferences based upon absolute work rate are confounded by the impact of a lower Wpeak in HTN compared to NTN, which means that a given absolute work rate would be relatively more challenging and, therefore, likely, result in a greater intracellular perturbation in the HTN group. Of note, subjects also performed two relative work rates (50% and 80% Wpeak), where there should not be group differences in intramuscular perturbation, but EPR abnormalities were still evident (Figures 1 and 3). This casts doubt on exacerbated intramuscular perturbation as the mechanism responsible for the augmented EPR in HTN compared to NTN. Other potential explanations for the dysfuntional EPR in hypertension include increased afferent sensitivity, altered CNS processing of afferent signals, and altered end-organ responsiveness to sympathetic activity 14, 41, 42.
Finally, intrathecal fentanyl is specific to μ-opioid receptors and only reduces approximately 60% of group III/IV muscle afferent feedback from the exercising lower limb 43. Considering the partial nature of this block, the observed role of the EPR in determining the cardiovascular response to exercise is likely an underestimation in both subject groups. Furthermore, assuming the intramuscular metabolic perturbation (and therefore group III/IV-mediated afferent feedback) was more severe at the two absolute intensities (7 W and 15 W) in HTN compared to NTN, the partial nature of the blockade would have resulted in greater residual group III/IV muscle afferent feedback in the patients. This might have caused a larger underestimation of EPR function in HTN compared to NTN. It is therefore reasonable to speculate that the actual degree of EPR dysfunction in hypertension is, potentially, considerably more severe than reflected in the current set of data. It should be acknowledged that the findings of this study are based on male participants who were either not medicated or studied after a 7-day drug washout period. It is possible that in the presence of medication, and in female participants, the outcomes of this work would be challenged.
Conclusion
Individuals with hypertension frequently present with irregular EPR function that largely account for the patients’ altered hemodynamic response to exercise. These findings have important implications for the development of future treatment strategies aimed at reducing the risk of acute cardiac events and stroke during exercise and for improving exercise tolerance which can, ultimately, facilitate an improved quality of life and reduce mortality.
PERSPECTIVES
This study exposed the abnormal EPR-mediated hemodynamic control in hypertensive individuals as a potential therapeutic target. Specifically, afferent blockade normalized major cardiovascular abnormalities in these patients and resulted in MAP and QL values that were no longer different to those documented in NTN under control conditions. These observations are of substantial clinical significance as they suggest a novel mechanism that could be targeted to minimize the risk of acute cardiac events and stroke associated with an exaggerated blood pressure response to exercise 44. Indeed, this study also offers a target to ameliorate peripheral blood flow restriction and therefore exercise limitations that hinder the success of physical rehabilitation, a highly effective non-pharmacological treatment strategy for hypertension 45, 46. Taken together, the present findings suggest that reversing EPR-mediated hemodynamic abnormalities in hypertension may facilitate safer exercise and the potential to perform physical activity in a more effective way. In practical terms, ischemic preconditioning and repeated exposure to high concentrations of intramuscular metabolites have been hypothesized to blunt the sensitivity of metaboreceptors47, 48 and might offer a potential strategy to attenuate group III/IV muscle afferent feedback and therefore, perhaps, EPR abnormalities in hypertension. However, the habitual use of these strategies as effective therapeutic modalities in hypertension is currently unknown.
Supplementary Material
NOVELTY & SIGNIFICANCE.
What Is New?
Patients with hypertension are characterized by an exaggerated blood pressure response to physical activities and attenuated leg blood flow during exercise
This study focused on the exercise pressor reflex (EPR), an autonomic neurocirculatory control mechanism, as a potential determinant of the patients abnormal hemodynamic responses to, and during, physical activities
What Is Relevant?
Hypertensive patients suffer from abnormal EPR function
Importantly, temporary pharmacological attenuation of the EPR largely reversed the circulatory abnormalities and normalized exercising blood pressure and leg blood flow in hypertensive patients to levels documented in normotensive individuals
Summary
Individuals with hypertension are characterized by irregular EPR function and this reflex abnormality largely accounts for the abnormal hemodynamic response to exercise in the hypertensive patients.
Acknowledgments
Sources of Funding
This study was supported by the National Heart, Lung, and Blood Institute (HL-116579); the Salt Lake City VAMC Geriatrics Research, Education and Clinical Center; and the Department of Veterans Affairs (E1697-R, E6910-R, and E9275-L).
Footnotes
Conflict of interest
The authors declare no conflict of interest
REFERENCES
- 1.Lund-Johansen P Twenty-year follow-up of hemodynamics in essential hypertension during rest and exercise. Hypertension. 1991;18:III54–61 [DOI] [PubMed] [Google Scholar]
- 2.Barbosa TC, Vianna LC, Fernandes IA, Prodel E, Rocha HN, Garcia VP, Rocha NG, Secher NH, Nobrega AC. Intrathecal fentanyl abolishes the exaggerated blood pressure response to cycling in hypertensive men. The Journal of physiology. 2016;594:715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. The Journal of physiology. 2011;589:1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: Effect of high-intensity aerobic training. The Journal of physiology. 2012;590:1481–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: Role of cardiorespiratory fitness. J Cardiopulm Rehabil. 2002;22:178–183 [DOI] [PubMed] [Google Scholar]
- 6.Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am J Hypertens. 2006;19:951–957 [DOI] [PubMed] [Google Scholar]
- 7.Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. European journal of applied physiology. 2009;105:351–356 [DOI] [PubMed] [Google Scholar]
- 8.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: Role of the muscle metaboreflex. American journal of physiology. Heart and circulatory physiology. 2010;299:H1318–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greaney JL, Edwards DG, Fadel PJ, Farquhar WB. Rapid onset pressor and sympathetic responses to static handgrip in older hypertensive adults. J. Hum. Hypertens. 2015;29:402–408 [DOI] [PubMed] [Google Scholar]
- 10.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. The Journal of physiology. 1972;226:173–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Experimental physiology. 2012;97:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. The Journal of physiology. 1972;224:173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. American journal of physiology. Heart and circulatory physiology. 2008;295:H1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the trpv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. The Journal of physiology. 2011;589:6191–6204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Experimental physiology. 2006;91:89–102 [DOI] [PubMed] [Google Scholar]
- 16.Secher NH, Amann M. Human investigations into the exercise pressor reflex. Exp. Physiol. 2012;97:59–69 [DOI] [PubMed] [Google Scholar]
- 17.Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: Role of purinergic receptors. American journal of physiology. Heart and circulatory physiology 2014;306:H132–141 [DOI] [PubMed] [Google Scholar]
- 18.Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp. Physiol 2014;992:368–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, atp, and lactate mediated by asic, p2x, and trpv1. Journal of neurophysiology. 2008;100:1184–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degtyarenko AM, Kaufman MP. Fictive locomotion and scratching inhibit dorsal horn neurons receiving thin fiber afferent input. American journal of physiology. Regulatory, integrative and comparative physiology. 2000;279:R394–403 [DOI] [PubMed] [Google Scholar]
- 21.O’Leary DS. Altered reflex cardiovascular control during exercise in heart failure: Animal studies. Exp. Physiol 2006;91:73–77 [DOI] [PubMed] [Google Scholar]
- 22.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group iii and iv muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. Journal of applied physiology. 2010;109:966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. The Journal of physiology. 2009;587:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidhu SK, Weavil JC, Venturelli M, Rossman MJ, Gmelch BS, Bledsoe AD, Richardson RS, Amann M. Aging alters muscle reflex control of autonomic cardiovascular responses to rhythmic contractions in humans. American journal of physiology. Heart and circulatory physiology. 2015;309:H1479–H1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Wray WD, Stehlik J, Richardson RS. Group iii/iv muscle afferents impair limb blood in patients with chronic heart failure. Int. J. Cardiol. 2014;174:368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW, Morgan DE, Stehlik J, Richardson RS. The mechanoreflex and hemodynamic response to passive leg movement in heart failure. Med. Sci. Sports Exerc. 2016;48:368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B, Management of Arterial Hypertension of the European Society of H, European Society of C. 2007 guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the european society of hypertension (esh) and of the european society of cardiology (esc). J Hypertens. 2007;25:1105–1187 [DOI] [PubMed] [Google Scholar]
- 28.Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respiratory physiology & neurobiology. 2008;164:160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borg G Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics; 1998 [Google Scholar]
- 30.Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE, Amann M. Group iii/iv locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol. 2017;128:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J, Richardson RS. Group iii/iv muscle afferents impair limb blood in patients with chronic heart failure. International journal of cardiology. 2014;174:368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancia G, Ludbrook J, Ferrari A, Gregorini L, Zanchetti A. Baroreceptor reflexes in human hypertension. Circ. Res. 1978;43:170–177 [DOI] [PubMed] [Google Scholar]
- 33.Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, Bernardi L. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714–718 [DOI] [PubMed] [Google Scholar]
- 34.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612 [DOI] [PubMed] [Google Scholar]
- 35.Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing-implications in hypertension. J. Mol. Cell. Cardiol 2015;83:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743 [DOI] [PubMed] [Google Scholar]
- 37.Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. The Journal of physiology. 2007;581:853–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group iii and iv muscle afferents to the circulatory response to rhythmic exercise in humans. The Journal of physiology. 2011;589:3855–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, Femminella GD, Leosco D, Bonaduce D. Beta-adrenergic receptor responsiveness in aging heart and clinical implications. Frontiers in physiology. 2014;4:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez N, Torres SH, Vera O, De Sanctis JB, Flores E. Muscle fiber composition and capillarization in relation to metabolic alterations in hypertensive men. J Med. 2001;32:67–82 [PubMed] [Google Scholar]
- 41.Brock JA, Van Helden DF. Enhanced excitatory junction potentials in mesenteric arteries from spontaneously hypertensive rats. Pflugers Arch. 1995;430:901–908 [DOI] [PubMed] [Google Scholar]
- 42.Smith SA, Leal AK, Murphy MN, Downey RM, Mizuno M. Muscle mechanoreflex overactivity in hypertension: A role for centrally-derived nitric oxide. Autonomic neuroscience : basic & clinical. 2015;188:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hureau TJ, Weavil JC, Thurston TS, Broxterman RM, Nelson AD, Bledsoe AD, Jessop JE, Richardson RS, Wray DW, Amann M. Identifying the role of group iii/iv muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. The Journal of physiology. 2018;596:1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SA. Exercise in hypertension: Do skeletal muscle reflexes make this a dangerous proposition? American journal of physiology. Heart and circulatory physiology 2010;299:H1302–1303 [DOI] [PubMed] [Google Scholar]
- 45.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of myocardial infarction onset study investigators. N. Engl. J. Med 1993;329:1677–1683 [DOI] [PubMed] [Google Scholar]
- 46.Sharman JE, La Gerche A, Coombes JS. Exercise and cardiovascular risk in patients with hypertension. Am. J. Hypertens 2015;28:147–158 [DOI] [PubMed] [Google Scholar]
- 47.Cruz RS, Pereira KL, Lisboa FD, Caputo F. Could small-diameter muscle afferents be responsible for the ergogenic effect of limb ischemic preconditioning? J Appl Physiol (1985). 2016:jap 00662 02016 [DOI] [PubMed] [Google Scholar]
- 48.Zghal F, Cottin F, Kenoun I, Rebai H, Moalla W, Dogui M, Tabka Z, Martin V. Improved tolerance of peripheral fatigue by the central nervous system after endurance training. Eur. J. Appl. Physiol 2015;115:1401–1415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.