Abstract
Introduction
Cytokines and vascular endothelial growth factors (VEGF) are involved in all aspects of pregnancy: from placentation, through fetal development, parturition and neonatal well-being. Umbilical cord inflammatory cytokines and/or VEGF have not been well studied with respect to dysregulation associated with disorders of pregnancy or maternal/neonatal outcomes.
Methods
Here we have used multiplex ELISA to screen umbilical cord lysates (comprising cord blood, endothelia and Wharton’s jelly, n = 380), for levels of IFN-γ, IL1-β, IL-6, IL-8, IL-10, TNF-α and VEGFs A, C and D and associations with 46 ICD9/10 codes encompassing obstetric, maternal and neonatal variables.
Results
No significant differences were observed for IFNγ, VEGFC or VEGFD with any clinical outcomes. The cytokines IL1-β, IL-6, IL-8, IL-10, and TNF-α showed varying levels of induction and suppression with primarily fetal-placental and neonatal complications. The largest number of significant differences between umbilical cytokines and clinical outcomes were observed for chorioamnionitis (IL1-β, IL-6, IL-8, TNF-α), and meconium passage during birth (IL1-β, IL-6, IL-8) where significant pro-inflammatory responses occurred and sex differences in IL-8 expression were noted. In contrast, gonococcal infection showed suppressed immune response significantly lowering IL1-β, IL-6, IL-8, IL-10 and TNF-α. For 12/46 negative pregnancy outcomes, strong suppression of VEGFA occurred.
Discussion
Angiogenic and inflammatory changes in the umbilical cord could be detrimental by increasing vascular permeability in the umbilical artery or vein and/or altering vascular tone, either of which would alter blood flow affecting delivery and removal of compounds. Further elucidation of inflammatory responses in the umbilical cord may provide mechanistic understanding of adverse pregnancy outcomes.
Introduction
Cytokines and vascular endothelial growth factors (VEGF) are critical molecules in pregnancy and parturition [1]. They are involved in all aspects of pregnancy: from placentation, through fetal and placental development, parturition and neonatal outcomes. They also play major roles when any of these processes are disrupted or abnormal [1]. Increased cytokine levels in pregnancy have been associated with autoimmune diseases (including inflammatory bowel disease, where elevated maternal serum IL-8 is observed (van der Giessen, 2019 #56)), chorioamnionitis and fetal inflammatory response syndrome - elevated IL-6 in fetal plasma [2], gestational diabetes mellitus - elevated IL6 in maternal serum [3], pre-eclampsia elevated materna serum TNF-α [4] and pre-term birth - elevated cord blood IL-6 [5]. Similarly abnormalities in VEGF expression and/or signalling have been associated with gestational diabetes [6], hypertension [7], intra-uterine growth restriction (IUGR) [8], pre-eclampsia [9, 10], pre-term birth [11] and recurrent pregnancy loss [12]. Pregnancy relies on a balance between immune activation and suppression which requires delicate interplay between pro- and anti-inflammatory mediators, hence any dysregulation of these processes has serious implications for continuing pregnancy and the health of the fetus.
Most studies involving umbilical cord have tested cord blood in order to determine circulating endogenous factor levels [1, 7, 13–16], measure fetal drug or chemical exposure [17], or for genetic abnormalities [18]. In comparison to other reproductive tissues, the umbilical cord has received far less attention as a useful tissue for determining endogenous markers of pregnancy outcomes. Some endogenous molecules tested in cord blood have been shown to be reflective of specific syndromes including immune activation and sepsis altering umbilical acute phase reactants [19], the specific cord blood peptidome caused by gestational-diabetes-induced macrosomia [20], mapping the immune response in the fetus (as different to the mother) in cord blood [21], measuring antioxidant status of the newborn in smokers [22], and measuring cord blood TSH as a biomarker of congenital hypothyroidism [15]. However, blood testing can be problematic when trying to quantify exogenous or endogenous molecules as their residence time in blood can be short, so only a snapshot of immediate exposure is available. As an alternative, several researchers have focused on using different reproductive tissues as screening tools for drugs, chemicals, nutrients and other endobiotics [6, 17, 23, 24]. These tissues include neonatal meconium, uterine tissue, placenta and umbilical cord tissue, and each presents advantages and limitations for screening, as compounds’ physicochemical characteristics and pharmacokinetic profiles vary and may cause higher (or lower) affinity for certain tissues. Several authors have published methods of screening in umbilical cord tissues including use of techniques such as ELISA [23–26], gas-chromatography/mass spectrometry [27, 28], liquid chromatography/mass spectrometry [26, 29–34] and radioimmunoassay [35]. It is uncertain whether these results represent accurate results to systemic exposure of either the mother or fetus because the bi-directional flow of endogenous and exogenous compounds across the placenta, and diffusion into the umbilical tissues from both maternal and fetal blood is not well characterized.
Specifically in the case of prior studies of cytokines in umbilical tissues, using freshly extracted human umbilical vein endothelial cells increases in IL6 and decreases in IL8 have been documented due to autoimmune disease (systemic lupus erythematosus) [36], increases in IL8 in response to infection [37], increases in IL-6, IL-8, TNF-α and IFN-γ due to gestational diabetes mellitus [38], and increases in IL-6 and IL-8 due to pre-eclampsia [39] have been observed.
Additionally, several studies of VEGF molecules directly detected in the umbilical tissues have been published. These variously show down-regulation of VEGF in response to hypertension in pregnancy [7], that VEGF and VEGF-receptor levels are higher in pre-eclampsia [40], and umbilical VEGF levels are higher in pre-term birth, very pre-term birth and miscarriage [11, 41]. One critical point is that the umbilical cord may not react the same as other reproductive tissues in the fetal-placental unit. As an example VEGF dysregulation in placental vascular beds in diabetes does not extend to the umbilical cord, where VEGF and vascular function is normal [6], therefore investigation of specific umbilical cord responses in different syndromes of pregnancy and parturition will help to elucidate the mechanisms behind reproductive outcomes.
Umbilical cord has been somewhat overlooked with respect to localized inflammation that may affect vascular permeability and tone, and hence blood flow. This is despite the fact that alterations to blood flow in the umbilical cord will affect all aspects of delivery and removal of substances to and from the fetus. This study has screened a moderately large cohort of umbilical cords (n = 380) to determine [19] whether the UC might present a useful matrix for screening these molecules and (2) whether alterations to inflammatory and angiogenic factors in umbilical cord can associated with adverse obstetric and neonatal outcomes. The purpose of this is to establish whether there are associations between cytokines and/or VEGF and obstetric outcomes that can be detected reliably with cords (that are easy to collect). These associations can be further examined in order to understand if they are diagnostic of reproductive outcomes, and in time; the UC might be a useful rapid pre-screen to assist pathologists.
Materials and Methods
All chemicals were obtained from VWR International Ltd (Mississauga, ON, Canada) unless otherwise stated.
Human umbilical sample collection and processing
The human umbilical cords used in this project (n = 380) were collected at birth, with informed consent from women for inclusion of their tissues into the Hawaii Biorepository, including consent for future investigation after anonymization and de-identification. Exclusion criteria for this study included: age < 18 or > 45, cords collected and stored > 12 months, cords collected > 4 hrs after birth, caesarean section initiated after labor began, emergency caesarean section, fetal or neonatal death, multiple birth. This specific study was approved by the Review Ethics Board at the University of British Columbia (H14–00092) and The University of Hawaii (CHS 15080) and tissues transferred under an executed material transfer agreement M17–00402. The demographics of the cohort as requested by the journal are presented in Table 1 [42].
| N = 380 Mean ± SD (range) |
|
|---|---|
| Maternal Age (years) | 28.2 ± 6.3 (18 – 44) |
| Gestational Age (weeks) | 38.3 ± 2.3 (25 – 42) |
| Ethnicity (%) | |
| Caucasian | 32 |
| Asian | 37 |
| Indigenous | 12 |
| Other or Unknown | 19 |
| Baby weight (g) | 2950 ± 725 (836 – 4250) |
| Maternal BMI (kg/mm2) | 26.2 ± 6.5 (15.2 – 52.7) |
| Maternal weight gain (kg) | 3165 ± 609 (860 – 4692) |
Umbilical cords were collected, washed, snap-frozen in liquid nitrogen and stored at −80°C. Upon request, pieces of umbilical cord tissue (0.2 – 0.5 g) were cut from frozen cords and shipped on dry ice to the University of British Columbia, where they were stored at −80°C until processing to lysates as previously described [24, 25]. Almost all umbilical tissues retained varying levels of cord blood, but externally derived blood was completely washed off. Briefly, tissues were thawed and mechanically homogenized using a Tissue Tearor™ (Daigger Scientific, Vernon Hills, IL, USA) in 1:3 (w:v) Tris-HCl buffer containing 5 mM MgCl2 and 2 mM PMSF. Lysates were separated into 50 μL aliquots and frozen at −80°C until use.
Validation of commercial ELISA for detection in umbilical cord lysates
Commercial ELISAs were from Mesoscale Diagnostics, Rockville, MD, USA and performed as per the manufacturer’s instructions. These ELISAs are multiplex in two platforms, the Cytokine ELISA simultaneously detects the classical pro-inflammatory cytokines TNFα, IL-1β, IL-6, IL-8. IL-10 and IFN-γ and the vascular endothelial growth factor (VEGF) platform simultaneously detects VEGFA, C and D.
To validate the specificity of the ELISA for detection of analytes in individual umbilical cord lysates, samples were blinded by study number and included spiked positives (assay positive control). The bench investigator did not receive the key for analysis until all samples had been screened, including being unaware of the presence of spiked controls.
Demographic and Statistical Analyses
Associations between analytes and 46 ICD9/ICD10 chart fields for obstetric and neonatal outcomes were performed. Normality of the data was determined by D’agostino-Pearson Omnibus test for normality. For clinical conditions and demographics with binary outcomes (n = 38), unpaired sample t-tests were performed between control and cases, with Welches correction for unequal variance, only case groups of 4 or more were considered. For continuous variables (n = 8) correlations were performed using Pearson’s or Spearman’s tests as appropriate. Numbers of cases and controls varied as not all ICD9/10 codes were complete for all samples. All statistical analyses were performed using GraphPad Prism 6.0 for Mac OSX (Graph Pad Prism, San Diego, CA).
Results
Multiplex ELISA utility and validation for umbilical cord lysates
The method requires between 10 and 25 μl of UC lysate to detect cytokines (equivalent to 2.5 – 6.25 mg of tissue) and 25 – 40 μl of UC lysate to detect VEGF molecules (6.25 – 10 mg tissue), allowing for the use of very small pieces of UC to detect up to six and three analytes simultaneously.
When the bench scientist was blinded and tubes were spiked with commercial standard 100% detection of TNF-α, IL-1β and VEGFD were recorded, on each of two separate days. The method has 100% method selectivity, meaning that the bench scientist always detected the spiked samples. When known concentrations of TNF-α, IL-1β and VEGFD were spiked into umbilical lysate, then non-spiked background subtracted, the TNF-α standard was detected at 57 ± 5 % of calculated concentration (1150 ± 98 pg/mL detected vs. 2000 pg/mL expected), the IL-1β detected at 33 ± 10 % of expected (17 ± 5 pg/mL detected vs. 50 pg/mL expected), and VEGFD a spike of 30 ng/mL returned quantitation of 2 ± 1 ng/mL (7 ± 4 % of expected). Detection is lower than absolute levels due to matrix effects. This means lysates with low levels of cytokines/VEGF may be missed (increase of Type II/false negative error). Accordingly for cytokines and VEGF molecules in umbilical cords relative comparisons are valid between groups, but quantitative statements about absolute levels or concentrations of molecules are not supported.
IL1-β, IL-6 and IL-8 were detected in all UC lysates tested. concentration. TNF-α was detected in 376 cords (99%), IL-10 in 280 cords (74%) and IFN-γ detected in 131 cords (34%). VEGF detection was much lower, being observed in 181 cords for VEGFA (48%), 132 cords for VEGFD (35%) and 14 cords for VEGFC (4%).
Demographic differences with sex
Because there were more male than female babies in our sample (170 females: 210 males) we analysed all ICD9/10 codes for differences with sex. Male sex was associated with significantly higher baby weight (3226 ± 629 g) as compared to females (3091 ± 578 g, p = 0.03), significantly more likely to be admitted to NICU ( 11% rate of admission for males as compared to 6% rate for females, p = 0.06), significantly more likely for the mother the have gentational hypertension with a male baby 11% for male babies and 5 % for female babies, p = 0.02. In contrast females approached significantly longer gestational age in weeks (38.52 ± 2.15 vs. 38.07 ± 2.42, p = 0.05) and were more likely to be born to women with chlamydia infection 11% infection rate female babies vs. 5 % in male babies, p = 0.06.
Correlations between cytokines and VEGF molecules and continuous variables
There were no biologically significant correlations observed with any cytokines or VEGF molecules with the continuous variables of maternal age (years), gestational age (days), length of membrane rupture (cm), amount of blood loss (mL), baby weight (grams), baby head circumference (cm), baby chest circumference (cm) or length of hospital stay (days). Although several correlations were statistically significant due to large sample size, the correlation coefficients (r2) were never above 0.2, indicating poor biological significance.
Differences between cytokines and VEGF molecules with discrete variables
For discrete variables no differences in cytokines or VEGF molecules were observed for β-streptococcus infection, chlamydia infection, estimated date of confinement (EDC) based on last menstrual period, 1st, 2nd or 3rd trimester scans; fetal bradycardia, fetal distress (all types), fetal heart trace monitoring, gestational hypertension, intrauterine growth restriction, maternal anemia, mild pre-eclampsia, neonatal respiratory distress syndrome, placental abruption or sex.
No significant differences in IFN-γ, VEGFC or VEGFD were observed for any of the obstetric variables or neonatal outcomes described in our ICD9/10 codes.
Significant differences in obstetric and neonatal outcomes were observed for IL1-β, IL-6, IL-8, IL-10, TNF-α and VEGFA, detailed in Figures 1 – 3. Changes in cytokines detected in the umbilical cord were primarily associated with variables related to the fetal-placental unit, labour and neonatal outcomes. In general maternal variables were not associated with significant differences in cytokine levels in umbilical tissues. In contrast, maternal variables (vaginal bleeding, asthma, and smoking) were observed to affect VEGFA in addition to fetal-placental unit, labour and neonatal variables. Because there were more female than males, sex-based stratification was performed for all significant associations. The only effect that sex had on cytokines was for IL8 levels and meconium passage (as below).
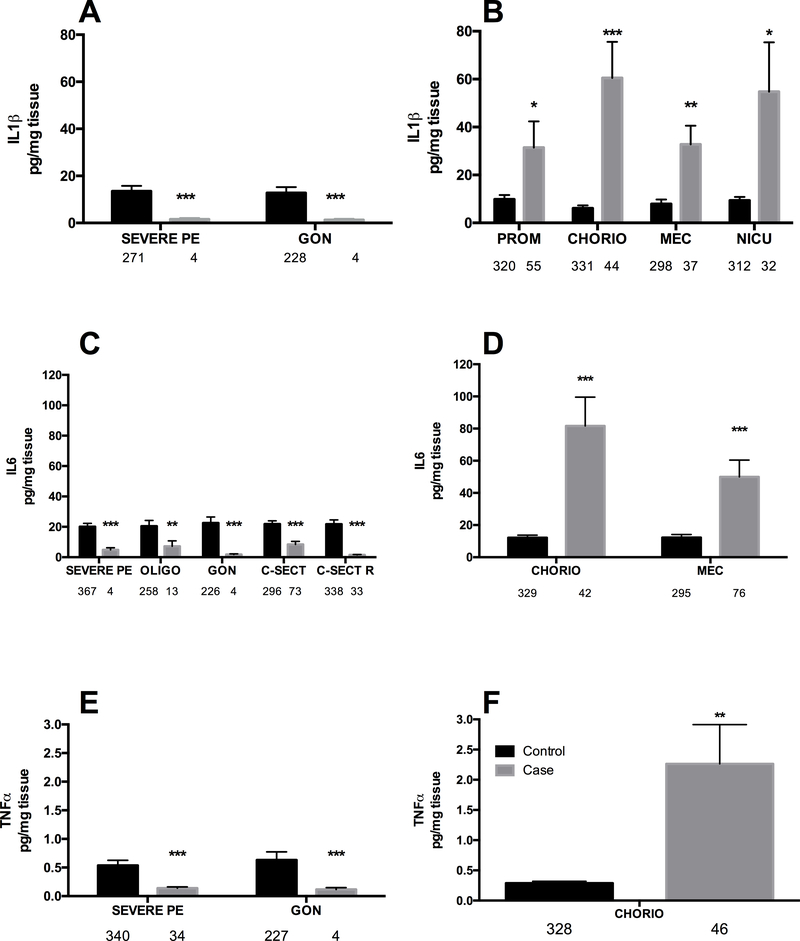
Figure 1: Proinflammatory cytokine levels are decreased (A, C, E) or increased (B, D, F) in umbilical cord lysates by fetal-placental, labour & delivery, and neonatal variables.
A: Suppression of IL1-β. B: Induction of IL1-β. C: Suppression of IL-6. D: Induction of IL-6. E: Suppression of TNF-α. F: Induction of TNF-α. Bars are mean ± SEM. N values are listed below bars. * = p < 0.05, ** = p < 0.01, *** = p < 0.001. CHORIO = chorioamnionitis, CHRONIC HTN = maternal chronic hypertension, C-SECT = current caesarean section; C-SECT R = repeat caesarean section, GEST DIA = gestational diabetes, GON = gonococcus infections, MEC = meconium passage in utero, NICU = neonatal admission to NICU, OLIGO = oligohydramnios, PREM B = premature birth, PREM L = premature labour, PROM = premature rupture of membranes, SEVERE PE = severe pre-eclampsia.
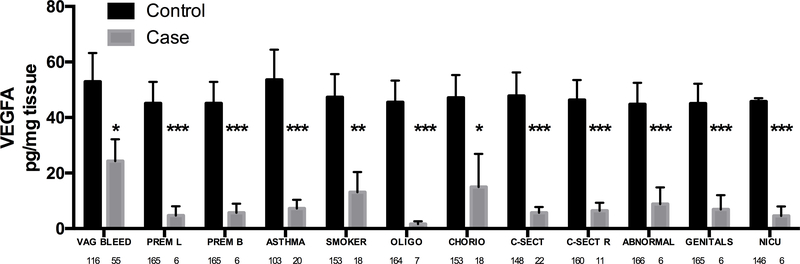
Figure 3: VEGFA levels are suppressed in umbilical cord lysates in association with fetal-placental, maternal, labour & delivery and neonatal variables.
Bars are mean ± SEM. N values are listed below bars. * = p < 0.05, *** = p < 0.001. ABNORMAL = any neonatal abnormality, ASTHMA = maternal asthma, CHORIO = chorioamnionitis, C-SECT = current caesarean section, C-SECT R = repeat caesarean section, GENITALS = abnormalities of the neonatal genitalia, GON = gonococcus infections, MEC = meconium passage in utero, MEMB = membrane rupture, NICU = neonate admitted to the NICU, OLIGO = oligohydramnios, PREM B = premature birth, PREM L = premature labour, PROM = premature rupture of the membranes, VAG BLEED = maternal vaginal bleeding prior to birth.
Briefly, for the pro-inflammatory cytokines; IL1-β was significantly decreased in severe pre-eclampsia and with gonococcus infection (p < 0.001, both ~6-fold decreased, Fig. 1A) and significantly increased when there was premature rupture of the membranes (p < 0.05, 3-fold increase), chorioamnionitis (p < 0.001, 6-fold increase), meconium passage during birth (p < 0.01, 3-fold increase) and admission to the NICU (p < 0.05, 5-fold increase), Figure 1B.
The levels of IL-6 were significantly diminished in severe pre-eclampsia (p < 0.001, 4-fold), oligohydramnios (p < 0.01, 4-fold), and caesarean section (p < 0.001, 3-fold) and profoundly diminished with repeat caesarean section and gonococcus (p < 0.001, 10-fold, both), Figure 1C. For IL-6 significantly higher levels were observed in chorioamnionitis and with passage of meconium during birth (p < 0.001 both, Figure 1D).
Relatively few significant changes in TNF-α were observed, with significantly lower levels in umbilical tissues from severe pre-eclampsia and gonococcus infection (p < 0.001 both, and ~2-fold inhibition both, Figure 1E). Interestingly chorioamnionitis caused a large and significant increase in TNF-α of around 10-fold (p < 0.01, Figure 1F).
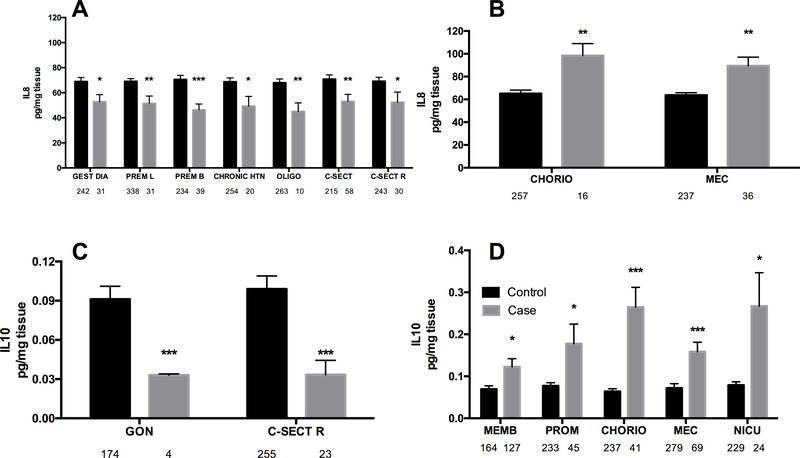
For the chemokine IL-8 (that is not classically pro-inflammatory, but does contribute to the inflammatory response through neutrophil recruitment by chemotaxis) the overwhelming response in umbilical tissues was significantly decreased IL-8 levels of between 20 and 40%. This included in gestational diabetes (p < 0.05), premature labour (p < 0.01), premature birth (p < 0.001), chronic maternal hypertension (p < 0.05), oligohydramnios (p < 0.01), caesarean delivery (p < 0.01) and repeat caesarean (p < 0.05, Figure 2A). In contrast chorioamnionitis and meconium passage during birth were both associated with significantly increased IL8 (p < 0.01, Figure 2B). When meconium passage was further stratified by sex, male and female fetuses demostrated different IL-8 profiles. IL8 in females with meconium passage was significantly lower than in females without meconium (49.0 ± 25.9 vs. 67.2 ± 42.9 pg/mg tissue, p = 0.012) while males with meconium as compared to males without meconium passage did not show IL8 inhibition ( 65.3 ± 39.3 vs. 69.3 ± 2.11, p = 0.74). When female fetuses with meconium passage were compared to males with meconium significantly lower IL-8 was present (49.1 ± 25.9 vs. 65.3 ± 39.3 pg/mg tissue, p = 0.015).
Figure 2: IL-8 and IL-10 levels are decreased (A, C) or increased (B, D) in umbilical cord lysates by fetal-placental, labour & delivery and neonatal variables.
Bars are mean ± SEM. N values are listed below bars. ** = p < 0.01, *** = p < 0.001. CHORIO = chorioamnionitis, C-SECT R = repeat caesarean section, GON = gonococcus infections, MEC = meconium passage in utero, MEMB = membrane rupture, NICU = neonate admitted to the NICU, PROM = premature rupture of the membranes, SEVERE PE = severe pre-eclampsia.
For the lone anti-inflammatory cytokine detected, IL-10; the opposite pattern was observed than for pro-inflammatory cytokines - names that fewer variables cause inhibition of IL-10 (n = 2) and there was more induction of IL-10 (n = 5 variables). IL-10 was significantly decreased in umbilical tissue that tested positive for gonococcus infection and came from caesarean section repeat (p < 0.001, 3-fold decrease, both Figure 2C). Levels of IL-10 were significantly increased in membrane rupture (p < 0.05, 50% increase), premature rupture of membranes (p < 0.05, 100% increase), chorioamnionitis (p < 0.001, with a large ~4-fold increase), meconium passage during birth (p < 0.001, but only a modest 2-fold increase) and admission to the NICU (p < 0.05, with ~4-fold and very variable increase), Figure 2D.
In contrast to the cytokines, VEGFA was decreased in umbilical cords in response to 12/46 variables tested (Figure 3). It was also associated with maternal variables (vaginal bleeding, asthma, smoking), labour and delivery variables (premature labour, premature birth, caesarean section, repeated caesarean section), placental/fetal unit abnormalities (chorioamnionitis, oligohydramnios) and neonatal variables (any neonatal abnormality, abnormal neonatal genitals, NICU admission)
Discussion
We have successfully detected associations between obstetric and neonatal clinical variables and changes in proinflammatory cytokines/VEGFA in umbilical cord lysates. With respect to cytokines, the umbilical cord appears useful to detecting immune responses of the fetal-placental unit, and caused during labour and delivery. For the molecule VEGFA only suppression was observed (no increases) and changes in response to maternal variables of vaginal bleeding, asthma and smoking were observed in addition to fetal-placental outcomes, (whereas cytokine levels were not altered by maternal variables). Asthma and smoking are classical inhibitors of VEGFA and can almost be considered positive controls for the accuracy of the associations we observed [13, 43]. Our specific findings that cytokines present in umbilical cord lysates are significantly elevated in chorioamnionitis, meconium passage during birth, premature rupture of membranes, NICU admission and significantly depressed with gonococcus infection and oligohydramnios are novel. There are, however; several other studies demonstrating associations between cord blood cytokines and pregnancy outcomes, including risk of neonatal hearing test failure [5], neonatal brain injury [44, 45], and maternal illicit drug use [16], supporting the plausibility of our findings.
Although VEGF is generally considered to be derived from the vascular endothelia, here primarily umbilical vein endothelia, with a small contribution from umbilical artery endothelia [7, 40, 46], during pregnancy circulating levels of VEGF in maternal serum almost double [13].
Hence VEGF present in the umbilical cord is likely to be a combination of locally produced and maternally-derived molecules. This fits well with our derived data where VEGFA was associated with maternal variables, including asthma, smoking and vaginal bleeding as well as fetal-placental and neonatal outcomes.
Similarly for inflammatory cytokines there are two ways that they could enter the umbilical cord: they may be produced locally by venous and arterial endothelia [39, 40, 46] or enter by passive diffusion from cord blood. The umbilical cord is highly perfused and IL1-β, IL-8, and TNF-α can increase the permeability of endothelia allowing molecules as large as polymorphonuclear neutrophils (PMNs) to cross into endothelial tissue [47, 48]. As cytokines are smaller than PMNs it is possible that they may also cross into the endothelia throught heir own permeabilization mechanism, but this requires mechanistic confirmation. It is possible that the cytokines detected in lysates are produced locally, and there is some support for this because cytokines were solely associated with variables relating to the fetal-placental unit and the labour and delivery process and not with maternal factors. However to determine the veracity of this statement, blood from umbilical vein and umbilical artery would have to be collected separately and compared for cytokine levels; differences on either side of the circulation would indicate their placental or maternal origin. The VEGF and cytokines concentrations detected may be representative of their concentrations within umbilical arterial and venous blood, and not produced by local endothelia - or it could be a combination of both.
A further consideration of these data demonstrated that the chorioamnionitis (IL1-β, IL-6, IL-8, TNF-α), and meconium passage during birth (IL1-β, IL-6, IL-8) were associated with strong pro-inflammatory responses. The exception to this is IL-8 where stratified analysis indicated that sex plays a role in the inflammatory response to meconium. Female fetuses downregulated IL-8 production, as compared to male fetuses, so the global response is not up-regulation, but rather driven by a sex difference where females are not able to maintain/produce IL-8 with meconium passage. The mechanism behind this sex-based difference remains to be resolved. One potential explanation for the inflammation observed with chorioamnionitis is that when this is coupled with funisitis and/or vasculitis within umbilical cord/chorionic plate fetal inflammatory response syndrome occurs, and elevated IL-6 is always present [2]. However, it is unlikely that umbilical cords from the acute syndrome were included in this study because placentas and their cords from abnormal or clearly compromised pregnancies are sent to pathology and not available for research.
In contrast, gonococcal infection was associated with suppressed immune response across-the-board with significantly decreased IL1-β, IL-6, IL-8, IL-10 and TNF-α. For the other infections agents on which we had data similar trends were observed that did not reach significance due to the small number of coded samples. There were only 91 records of streptococcus B testing of which 17 were positive, likewise of the 213 records coded for Chlamydia sp., only 27 were positive. When ELISA non-detects were removed, the samples sizes were very underpowered, which is likely the reason for not reaching significance. However, global inhibition of immune responses in placental and umbilical tissues by active infection is an area deserving of more study, perhaps in order to develop an early-alert diagnostic for neonates.
In terms of method reliability, umbilical lysates are an accurate tool for investigating these analytes, as blinded bench researchers always detected spiked samples. However, only relative quantitation of molecules could be achieved as a spiking study showed lower levels of analyte than the known concentration added. This is likely due to matrix effects of the umbilical cord lysate itself. This means our data likely contains greater Type II error (false negative). Hence, this platform can only be used for relative quantitation such as in case-control studies of this type. The positive outcome from this validation, is that because there is higher Type II error, our results are conservative meaning that statistically significant results are probably even more biologically important than reported. Additionally, we are missing data on the use of several medications, particularly the use of antibiotics in labour and/or prophylactically for e.g. infection or PROM, and also the use of antenatal steroids, magnesium sulfate and prostaglandins for labour induction were all unknown. These may have a confounding effect on our results.
In summary, the cytokines and angiogenic factors tested are not individually diagnostic of any single syndrome, disease or complication, indeed by their very nature they are generalized response molecules of the immune system. Despite this, our study demonstrated that umbilical cord lysate may provide reliable information about inflammation in the intra-uterine environment during pregnancy and during labour and delivery. In addition to mechanistic considerations of inflammation affecting umbilical arterial and venous tone and permeability with respect to fetal health, with more in depth study it may be possible to confirm specific patterns of cytokines and angiogenic factor dysregulation in the umbilical cord that are together associated with specific syndromes. That would enable these molecules to be used in parallel with pathological examination of reproductive tissues to assist in confirming subtle effects, as part of differential diagnosis or as a preliminary “alert” screening to rule out (or confirm) that further testing of the mother or neonate for example for an active bacterial infection, may be needed.
Highlights.
Umbilical inflammatory responses are altered by negative pregnancy outcomes.
Umbilical angiogenic responses are diminished by negative pregnancy outcomes.
Further study at cell and molecular level may reveal the associations’ mechanisms.
Acknowledgements
The University of Hawaii Human Reproductive Biospecimen Repository was funded by National Institutes of Health RMATRIX - 3U54MD007584. Hugh Kim is supported by a Canadian Institutes of Health Research (CIHR) Clinician-Scientist Salary Award MC2-127872. Hugh Kim and Abby Collier received a joint grant from the Associate Deans, Research of Dentistry and Pharmaceutical Sciences, UBC to support this work.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kalagiri R, Carder T, Choudhury S, Vora N, Ballard A, Govande V, Drever N, Beeram M, Uddin M, Inflammation in Complicated Pregnancy and Its Outcome. American Journal of Perinatology 33(14) (2016) 1337–1356. [DOI] [PubMed] [Google Scholar]
- [2].Kim C, Romero R, Chaemsaithong P, Chaiyasit N, Yoon B, Kim Y, Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance., American Journal of Obstetrics and Gynecology. 213 (2015) S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morisset A, Dubé M, Côté J, Robitaille J, Weisnagel S, Tchernof A, Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obstetrica Gynecologica Scandinavica 90(5) (2011) 524–30.. [DOI] [PubMed] [Google Scholar]
- [4].M AA;, A AI;, Mohamed M, Soliman M, Evaluation of Some Cytokines and Gene Expressions in Pre-eclampsia., Pakistan Journal of Biological Sciences 22(3) (2019) 148–153.. [DOI] [PubMed] [Google Scholar]
- [5].Shim Y, Choi B, Park K, Lee H, Jung Y, Kim Y, Inflammatory and Immune Proteins in Umbilical Cord Blood: Association with Hearing Screening Test Failure in Preterm Neonates. Mediators of Inflammation September 19 (2018) 4209359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leach L, Placental vascular dysfunction in diabetic regnancies: intimations of fetal cardiovascular disease? Microcirculation 18(4) (2011) 263–91. [DOI] [PubMed] [Google Scholar]
- [7].Bhavina K, Radhika J, Pandian S, VEGF and eNOS expression in umbilical cord from pregnancy complicated by hypertensive disorder with different severity. Biomedical Research International (2014) 982159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Padavala S, Pope N, Baker P, Crocker I, An imbalance between vascular endothelial growth factor and its soluble receptor in placental villous explants of intrauterine growth-restricted pregnancies., Journal of the Society for Gynecological Investigation 13(1) (2006) 40–7. [DOI] [PubMed] [Google Scholar]
- [9].Jim B, Karumanchi S, Preeclampsia: Pathogenesis, Prevention, and Long-Term Complications. Seminars in Nephrology 37(4) (2017) 386–397. [DOI] [PubMed] [Google Scholar]
- [10].Baker P, Krasnow J, Roberts J, Yeo K, Elevated serum levels of vascular endothelial growth factor in patients with preeclampsia. Obstetrics and Gynecology 86(5) (1995) 815–21.. [DOI] [PubMed] [Google Scholar]
- [11].Krukier I, Pogorelova T, Production of vascular endothelial growth factor and endothelin in the placenta and umbilical cord during normal and complicated pregnancy., Bulleting of Experimental Biology and Mediciene 141(2) (2006) 216–8. [DOI] [PubMed] [Google Scholar]
- [12].Sun Y, Chen M, Mao B, Cheng X, Zhang X, Xu C, Association between vascular endothelial growth factor polymorphism and recurrent pregnancy loss: A systematic review and meta-analysis., European Journal of Obstetrics, Gynecology and Reproductive Biology 211 (2017) 169–176. [DOI] [PubMed] [Google Scholar]
- [13].Bikov A, Bohacs A, Eszes N, Weiszhar Z, Ivancso I, Muller V, Rigo J Jr., Losonczy G, Tamasi L, Horvath I, Circulating and exhaled vascular endothelial growth factor in asthmatic pregnancy. Biomarkers. 2012 (17) (2012) 7. [DOI] [PubMed] [Google Scholar]
- [14].Hassanein S, El-Farrash R, Hafez H, Hassanin O, Abd E, Rahman NA., Cord blood interleukin-6 and neonatal morbidities among preterm infants with PCR-positive Ureaplasma urealyticum. Journal of Maternal Fetal and Neonatal Medicine 25(10) (2012) 2106–10. [DOI] [PubMed] [Google Scholar]
- [15].John J, Abraham A, Sahu S, Umbilical cord blood TSH: a predictor of congenital hypothyroidism., Indian Journal of Physiology and Pharmacology. 57(4) (2013) 452–3. [PubMed] [Google Scholar]
- [16].Mardini V, Rohde L, Ceresér K,., Gubert C, da Silva E Xavier F Parcianello R Röhsig L Pechansky F Pianca T Szobot C, IL-6 and IL-10 levels in the umbilical cord blood of newborns with a history of crack/cocaine exposure in utero: a comparative study. Trends in Psychiatry and Psychotherapy 38(1) (2016) 40–9. [DOI] [PubMed] [Google Scholar]
- [17].Gray T, Huestis M, Bioanalytical procedures for monitoring in utero drug exposure. Anaytical and Bioanalytical Chemistry 388(7) (2007) 1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Badeau M, Lindsay C, Blais J, Nshimyumukiza L, Takwoingi Y, Langlois S, Légaré F, Giguère Y, Turgeon A, Witteman W, Rousseau F, Genomics-based non-invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women., Cochrane Database Systematic Review. 11:CD011767. doi: 10.1002/14651858.CD011767.pub2. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mithal L, Palac H, Yogev R, Ernst L, Mestan K, Cord Blood Acute Phase Reactants Predict Early Onset Neonatal Sepsis in Preterm Infants. PLoS One. 12(1) (2017) e0168677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu F, Zhao C, Liu L, Ding H, Huo R, Shi Z, Peptidome profiling of umbilical cord plasma associated with gestational diabetes-induced fetal macrosomia. Journal of Proteomics. 139(38–44) (2016). [DOI] [PubMed] [Google Scholar]
- [21].Fragiadakis G, Baca Q, Gherardini P, Ganio E, Gaudilliere D, Tingle M, Lancero H, McNeil L, Spitzer M, Wong R, Shaw G, Darmstadt G, Sylvester K, Winn V, Carvalho B, Lewis D, Stevenson D, Nolan G, Aghaeepour N, Angst M, Gaudilliere B, Mapping the Fetomaternal Peripheral Immune System at Term Pregnancy. Journal of Immunology 197(11) (2016) 4482–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chelchowska M, Ambroszkiewicz J, Gajewska J, Laskowska-Klita T, Leibschang J, The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn., European Journal of Obstetrics, Gynecology and Reproductive Biology 155(2) (2011) 132–6. [DOI] [PubMed] [Google Scholar]
- [23].Collier A, Sato B, Milam K, Wright T, Methamphetamine, smoking, and gestational hypertension affect norepinephrine levels in umbilical cord tissues., Clinical and Experimental Obstetrics and Gynecology. 42(5) (2015) 580–5. [PubMed] [Google Scholar]
- [24].Wright TE, Milam KA, Rougee L, Tanaka MD, Collier AC, Agreement of umbilical cord drug and cotinine levels with maternal self-report of drug use and smoking during pregnancy, Journal of Perinatology 31(5) (2011) 324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Knight S, Smith A, Wright T, Collier A, Detection of opioids in umbilical cord lysates: an antibody-based rapid screening approach., Toxicology Mechanisms and Methods 2018 July 31:1–26. doi: 10.1080/15376516.2018.1506850. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buchi K, Suarez C, Varner M, The prevalence of prenatal opioid and other drug use in Utah., American Journal of Perinatology 30(3) (2013) 241–4. [DOI] [PubMed] [Google Scholar]
- [27].Montgomery D, Plate C, Alder S, Jones M, Jones J, Christensen R, Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium., Journal of Perinatology 26 (1) (2006) 11–4. [DOI] [PubMed] [Google Scholar]
- [28].Montgomery D, Plate C, Jones M, Jones J, Rios R, Lambert D, Schumtz N, Wiedmeier S, Burnett J, Ail S, Brandel D, Maichuck G, Durham C, Henry E, Christensen R, Using umbilical cord tissue to detect fetal exposure to illicit drugs: a multicentered study in Utah and New Jersey., Journal of Perinatology 28(11) (2008) 750–3. [DOI] [PubMed] [Google Scholar]
- [29].Colby J, Comparison of umbilical cord tissue and meconium for the confirmation of in utero drug exposure., Clinical Biochemistry 50(13–14) (2017) 784–790. [DOI] [PubMed] [Google Scholar]
- [30].Jones J, Jones M, Jones B, Sulaiman K, Plate C, Lewis D, Detection of Codeine, Morphine, 6-Monoacetylmorphine, and Meconin in Human Umbilical Cord Tissue: Method Validation and Evidence of In Utero Heroin Exposure Therapeutic Drug Monitoring 37(1) (2015) 45–52.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lendoiro E, Quintela O, de Castro A, Cruz A, López-Rivadulla M, Concheiro M, Target screening and confirmation of 35 licit and illicit drugs and metabolites in hair by LC-MSMS., Forensic Science International 217(1–3) (2012) 207–15.. [DOI] [PubMed] [Google Scholar]
- [32].Jones J, Rios R, Jones M, Lewis D, Plate C, Determination of amphetamine and methamphetamine in umbilical cord using liquid chromatography-tandem mass spectrometry., Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 877(29) (2009) 3701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paniagua-González L, Jiménez-Morigosa C, Lendoiro E, Concheiro M, Cruz A, López-Rivadulla M, de Castro A, Development and validation of a liquid chromatography-tandem mass spectrometry method for the determination of nicotine and its metabolites in placenta and umbilical cord., Drug Testing and Analysis Mar 10. doi: 10.1002/dta.2381 (2018). [DOI] [PubMed] [Google Scholar]
- [34].Kim J, de Castro A, Lendoiro E, Cruz-Landeira A, López-Rivadulla M, Concheiro M, Detection of in utero cannabis exposure by umbilical cord analysis., Drug Testing and Analysis 10(4) (2018) 636–643. [DOI] [PubMed] [Google Scholar]
- [35].Little B, Snell L, Trimmer K, Ramin S, Ghali F, Blakely C, Garret A, Peripartum cocaine use and adverse pregnancy outcome., American journal of Human Biology 11(5) (1999) 598–602. [DOI] [PubMed] [Google Scholar]
- [36].Rodriguez E, Guevara J, Paez A, Zapata E, Collados M, Fortoul T, Lopez-Marure R, Masso F, Montaño L, The altered expression of inflammation-related molecules and secretion of IL-6 and IL-8 by HUVEC from newborns with maternal inactive systemic lupus erythematosus is modified by estrogens., Lupus 17(12) (2008) 1086–95.. [DOI] [PubMed] [Google Scholar]
- [37].Rochelson B, Dowling O, Schwartz N, Metz C, Magnesium sulfate suppresses inflammatory responses by human umbilical vein endothelial cells (HuVECs) through the NFkappaB pathway., The Journal of Reproductive Immunology 73(2) (2007) 101–107.. [DOI] [PubMed] [Google Scholar]
- [38].Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, Longo S, Duncombe G, Mitchell M, Rice G, Illanes S, Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation., Diabetes 65(3) (2016) 598–609. [DOI] [PubMed] [Google Scholar]
- [39].Al-Ofi E, Anumba D, Ligands of toll-like receptors 2/4 differentially alter markers of inflammation, adhesion and angiogenesis by monocytes from women with pre-eclampsia in co-culture with endothelial cells., The Journal of Reproductive Immunology 121 (2017) 26–33. [DOI] [PubMed] [Google Scholar]
- [40].Almasry S, Elfayomy A, Hashem H, Ultrastructure and histomorphometric analysis of human umbilical cord vessels in preeclampsia: a potential role of VEGF, VEGFR-1 and VEGFR-2., Romanian Journal of Morphology and Embryology 57(Suppl 2) (2016) 681–689,. [PubMed] [Google Scholar]
- [41].Kaukola T, Räsänen J, Herva R, Patel D, Hallman M, Suboptimal neurodevelopment in very preterm infants is related to fetal cardiovascular compromise in placental insufficiency. American Journal of Obbstetrics and Gynecology 193(2) (2005) 414–20. [DOI] [PubMed] [Google Scholar]
- [42].Nelson D, Burton G, A technical note to improve the reporting of studies of the human placenta., Placenta 12 (2010) 008. [DOI] [PubMed] [Google Scholar]
- [43].Evans P, Wheeler T, Anthony F, Osmond C, Maternal serum vascular endothelial growth factor during early pregnancy., Clinical Sciences (London) 92(567–71. ) (1997). [DOI] [PubMed] [Google Scholar]
- [44].Lu H, Huang W, Chen X, Wang Q, Zhang Q, Chang M, Relationship between premature brain injury and multiple biomarkers in cord blood and amniotic fluid., Journal of Maternal Fetal and Neonatal Medicine 31(21) (2018) 2898–2904.. [DOI] [PubMed] [Google Scholar]
- [45].Tian C, Cheng L, Gu X, Cord blood TNF-α and IL-6 levels as diagnostic indicators of brain damage in neonates with non-asphyxia fetal distress., Archives of Gynecology and Obstetrics 295(2) (2017) 337–342. [DOI] [PubMed] [Google Scholar]
- [46].Infanger M, Grosse J, Westphal K, Leder A, Ulbrich C, Paul M, Grimm D, Vascular endothelial growth factor induces extracellular matrix proteins and osteopontin in the umbilical artery., Annals of Vascular Surgery 22(2) (2008) 273–84. [DOI] [PubMed] [Google Scholar]
- [47].Biffl W, Moore E, Moore F, Carl V, Franciose R, Banerjee A, Interleukin-8 increases endothelial permeability independent of neutrophils., Journal of Trauma. 39(1) (1995) 98–102. [DOI] [PubMed] [Google Scholar]
- [48].Burke-Gaffney A, Keenan A, Modulation of human endothelial cell permeability by combinations of the cytokines interleukin-1 alpha/beta, tumor necrosis factor-alpha and interferon-gamma., Immunopharmacology. 25(1) (1993) 1–9. [DOI] [PubMed] [Google Scholar]