Abstract
Cuticular hydrocarbons (CHCs) form the boundary between insects and their environments and often act as essential cues for species, mate, and kin recognition. This complex polygenic trait can be highly variable both among and within species, but the causes of this variation, especially the genetic basis, are largely unknown. In this study, we investigated phenotypic and genetic variation of CHCs in the seaweed fly, Coelopa frigida, and found that composition was affected by both genetic (sex and population) and environmental (larval diet) factors. We subsequently conducted behavioral trials that show CHCs are likely used as a sexual signal. We identified general shifts in CHC chemistry as well as individual compounds and found that the methylated compounds, mean chain length, proportion of alkenes, and normalized total CHCs differed between sexes and populations. We combined these data with whole genome resequencing data to examine the genetic underpinnings of these differences. We identified 11 genes related to CHC synthesis and found population‐level outlier SNPs in 5 that are concordant with phenotypic differences. Together these results reveal that the CHC composition of C. frigida is dynamic, strongly affected by the larval environment, and likely under natural and sexual selection.
Keywords: Coelopa frigida, cuticular hydrocarbons, diet, population differentiation, sexual signal
We examined the cuticular hydrocarbons of the seaweed fly (Coelopa frigida) for the first time and found significant variation due to sex, population, and diet. Using a whole genome resequencing resource, we identified 11 genes related to CHC synthesis and found outlier SNPs in 5 of them. Overall our study shows that the CHC composition of the seaweed fly is highly dynamic and likely under natural and sexual selection.

1. INTRODUCTION
In insects, cuticular hydrocarbons (CHCs) are a primary adaptation to life on land because they protect against desiccation (Blomquist & Bagnères, 2010; Wigglesworth, 1945). However, in many solitary and social insects, cuticular hydrocarbons are also used as one of the primary cues to recognize, and possibly discriminate between species, sexes, and among kin (Bagneres, Lorenzi, Dusticier, Turillazzi, & Clement, 1996; Blomquist & Bagnères, 2010; Ferveur, 2005; Van Oystaeyen et al., 2014). The multifarious use of CHCs in adaptation and communication means that the composition is frequently under both natural (Blomquist & Bagnères, 2010; Foley & Telonis‐Scott, 2011; Howard & Blomquist, 2005; Rajpurohit et al., 2017) and sexual selection (Blomquist & Bagnères, 2010; Ferveur, 2005; Howard & Blomquist, 2005; Peterson et al., 2007; Steiger et al., 2013; Thomas & Simmons, 2009).
De novo synthesis of CHCs is well defined and conserved across insects (Blomquist & Bagnères, 2010; Howard & Blomquist, 2005): Synthesis takes place in oenocytes and starts with acetyl‐CoA which is then elongated by a fatty acid synthase (FAS) forming a long‐chained fatty acyl‐CoA. FASs may also add methyl groups to these fatty acids (Blomquist et al., 1994; Chung et al., 2014; Juarez, Ayala, & Brenner, 1996; de Renobales, Woodin, & Blomquist, 1986). Elongases further lengthen these fatty acyl‐CoAs and double bonds or triple bonds are added by desaturases (Blomquist & Bagnères, 2010; Howard & Blomquist, 2005). These are reduced to aldehydes by reductases, and then, these aldehydes are converted to hydrocarbons by a decarboxylation reaction, which is mediated by a cytochrome P450 (Qiu et al., 2012). Coding or expression variation in any number of these enzymes can lead to significant changes in CHC composition (ex: Chen et al., 2016; Chung et al., 2014; Dembeck et al., 2015; Qiu et al., 2012; Reed, Quilici, Blomquist, & Reitz, 1995; de Renobales et al., 1986).
Changes in gene expression (Chertemps et al., 2007; Dallerac et al., 2000; Feldmeyer, Elsner, & Foitzik, 2014; Shirangi, Dufour, Williams, & Carroll, 2009) and/or coding sequences (Badouin et al., 2013; Keays, Barker, Wicker‐thomas, & Ritchie, 2011; Kulmuni, Wurm, & Pamilo, 2013) can impact both the overall composition of CHCs and the amount of specific compounds. Multiple environmental factors may also influence CHC composition. For example, CHCs can vary under different temperature regimes (Rouault, Marican, Wicker‐Thomas, & Jallon, 2004; Savarit & Ferveur, 2002; Toolson & Kupersimbron, 1989), diets (Liang & Silverman, 2000; Stennett & Etges, 1997; Stojkovic, Savkovic, Dordevic, & Tucic, 2014), or other climatic variables (Howard, Howard, & Colquhoun, 1995; Kwan & Rundle, 2010). Moreover, multiple studies have found that CHC composition varies between natural populations (ex: Etges & Ahrens, 2001; Frentiu & Chenoweth, 2010; Haverty, Nelson, & Page, 1990; Perdereau, Dedeine, Christidès, & Bagnères, 2010), but it is unknown how much of this variation is due to plasticity versus genetic divergence. The contribution of different factors can be teased apart using laboratory studies. The role of genetic factors can, for example, be assessed by raising different populations in the same environment. Likewise, plasticity can be assessed by raising the same population in multiple environments (i.e., common garden and reciprocal transplant experiments). Determining the causes and consequences of CHC variation is an important general step toward understanding the evolution of CHCs and their co‐option as sexual signals.
The seaweed fly Coelopa frigida presents an attractive system in which to investigate causes and consequences of variation in CHCs. These flies live in highly dynamic environments (Berdan, Rosenquist, Larson, & Wellenreuther, 2018) making it likely that both genetic changes and phenotypic plasticity affect CHC composition. Coelopa frigida lives in “wrackbeds,” accumulations of decomposing seaweed on shorelines that act as both habitat and food source for larvae and adults. Local adaptation in response to wrackbed composition has been demonstrated in Swedish C. frigida (Wellenreuther, Rosenquist, Jaksons, & Larson, 2017). Furthermore, CHCs may be under sexual selection as it is possible that CHCs are used as a mating signal in this system. Mating in C. frigida is characterized by intense sexual conflict with no courtship behavior but evidence of female choice. Males will approach and forcefully mount females. Females are reluctant to mate and use a variety of responses to dislodge the male, such as downward curling of the abdomen (to prevent contact), kicking of the legs, and shaking from side to side (Blyth & Gilburn, 2011; Day, Foster, & Engelhard, 1990). It has been consistently reported that mating attempts by large males are more likely to result in copulation (Butlin, Read, & Day, 1982; Gilburn, Foster, & Day, 1992, 1993) but the signals used for mate choice are unknown.
Here, we investigate the cuticular hydrocarbons of C. frigida. Specifically, we examine variation in CHC signatures due to sex (possible signature of sexual selection), population (possible signature of natural selection), and larval environment/diet (phenotypic plasticity). We combine this with behavioral trials to test the significance of CHCs in a mate choice context. We also investigate genetic variation that may underlie this phenotypic variation. We discuss our findings in light of sexual selection and adaptation in this species and others and outline future research areas that deserve attention.
2. METHODS
2.1. Study species
Coelopa frigida belongs to the group of acalyptrate flies which exclusively forage on decomposing seaweed (Cullen, Young, & Day, 1987). This species is found along the seashores of Northern Europe (Mcalpine, 1991) and plays a vital role in coastal environmental biodiversity (Griffin et al., 2018) and health by accelerating the decomposition of algae, allowing for faster release of nutrients (Cullen et al., 1987).
2.2. Sampling
Fly larvae were collected in April and May 2017 from two Norwegian populations, Skeie (58.69733, 5.54083) and Østhassel (58.07068, 6.64346), and two Swedish populations, Stavder (57.28153, 12.13746) and Ystad (55.425, 13.77254). These populations are situated along an environmental cline in salinity, wrackbed (piles of rotting seaweed on the shoreline) composition, and wrackbed microbiome (E. L. Berdan, M. Wellenreuther, & K. Johannesson, unpublished data; Day, Dawe, Dobson, & Hillier, 1983) from the North Sea to the Baltic Sea (Figure 1). Larvae were transported to Tjärnö Marine Laboratory of the University of Gothenburg where they completed development in an aerated plastic pot filled with their own field wrack in a temperature controlled room at 25°C with a 12‐hr/12‐hr light–dark cycle. After adults eclosed in the laboratory, they were transferred to a new pot filled with standard wrack consisting of 50% Fucus spp. and 50% Saccharina latissima which had been chopped, frozen, and then defrosted. The exception to this is a subset of Ystad adults that were allowed to mate on the same material they had been collected in (field wrack). Thus, we had five treatment groups: all four populations (Skeie, Østhassel, Stavder, and Ystad) on standard wrack and Ystad also on field wrack. Adult flies were allowed to lay eggs on the provided wrack, and this second generation was raised entirely in a temperature controlled room at 25°C with a 12‐hr/12‐hr light–dark cycle. As larvae pupated, they were transferred to individual 2‐ml tubes with a small amount of cotton soaked in 5% (w/v) glucose to provide moisture and food for the eclosing adult. This ensured virginity in all eclosing flies. The tubes were checked every day, and the date of eclosure of each fly was noted. Flies mature approximately 24 hr after eclosion at 25 degrees so two days after eclosure adult flies were frozen at −80°C and kept there until CHC extraction.
Figure 1.

Map of Coelopa frigida populations used in this study. Base map from dmaps (http://d-maps.com/carte.php?num_car=5972)
2.3. CHC analysis
Frozen flies were placed in 12‐well porcelain plates to defrost and dry, as moisture can affect lipid dissolution. Each fly was then placed in a 1.5‐ml high recovery vial containing 300 µl of n‐hexane, vortexed at a low speed for 5 s, and extracted for 5 min. Afterward, flies were removed from the vial and allowed to air‐dry on the porcelain plates before they were weighed (Sartorius Quintix 124‐1S microbalance) to the nearest 0.0001 g and sexed. Extracts were evaporated until dry under nitrogen gas and then stored at −20°. Before analysis, 20 µl of n‐hexane containing 1 µg/ml n‐nonane was added as an internal standard to each vial, which was then vortexed at maximum speed for 10 s.
The extracts from 20 male and 20 female flies from every treatment group were analyzed on a GC (Agilent GC 6890) coupled to a MS (Agilent 5973 MSD) using a HP‐5MS capillary column (Agilent) (see Table S1 for instrument settings).
The total ion chromatograms were quality checked, de‐noised, and Savitzky‐Golay filtered before peak detection and peak area integration in OpenChrom®. Peaks were then aligned using the R package GCalignR (Ottensmann, Stoffel, Nichols, & Hoffman, 2018) prior to statistical analysis. Peaks were tentatively identified by their mass spectra and retention time index based on a C21‐C40 n‐alkane standard solution (Sigma‐Aldrich).
2.4. Statistical analysis
The effects of sex and population on CHC profile were assessed using flies raised on standard wrack using a balanced sampling design (N = 13 for each combination), and 127 peaks were identified by the R package GCalignR. The effects of sex and diet (i.e., wrack type) during the larval stage on CHC profiles were analyzed using a balanced design of flies from the Ystad population (N = 12 for each combination), and 111 peaks were identified by R package GCalignR. Peak areas were normalized on the peak area of the internal standard and the weight of the fly before being auto‐scaled prior to statistical analysis. Clustering of samples was visualized with a principal component analysis (PCA), and group differences were analyzed using a PERMANOVA followed by multiple group comparisons using the PRIMER‐E 7 software. We also analyzed group differences via (O)PLS‐DA using the ropls packages in R (Thevenot, Roux, Xu, Ezan, & Junot, 2015). Candidate compounds for group differentiation were determined from variables of importance for OPLS‐DA projection (VIP scores) (Galindo‐Prieto, Eriksson, & Trygg, 2014), which were further assessed by univariate analysis using a false discovery‐adjusted significance level of α = .05 using the Benjamini and Hochberg's FDR‐controlling procedure (Benjamini & Hochberg, 1995).
We noticed that in general the Norwegian populations tended to cluster together and differ from Swedish populations. Thus, we used country rather than population for our further analyses. We examined sex and country effects in more depth to examine shifts in chemistry that could be linked to our genetic data (see below). Our results above indicated that the differences in CHC profiles between sexes and populations of C. frigida were due to quantitative rather than qualitative differences (i.e., changes in relative amounts rather than presence/absence of certain compounds). Thus, we looked for general shifts in CHC chemistry by examining (a) the total normalized peak area (i.e., the sum of all peaks used in analysis), (b) the proportion of alkenes, and (c) the proportion of methylated compounds. For #1, we used all 127 peaks used in the previous analysis, and for #2 and #3, we used the subset of these peaks that could be accurately categorized (116 peaks). For the total peak area, we transformed the data with a log transformation and then used the “dredge” function from the MuMIn package (Barton, 2017) to compare AIC values for nested glm (Gaussian distribution, link=identity) models with the terms sex, country, and country × sex. If there were two models that differed in AIC by <1, we chose the simpler model. For alkenes and methylated compounds, our data were proportional and did not include 1 or 0 so we used the “betareg” function from the betareg package (Grun, Kosmidis, & Zeileis, 2012). We again used the “dredge” function to compare AIC values for nested models with the terms sex, country, and country × sex and took the simpler model in case of an AIC difference of <1.
We also analyzed the distribution of chain lengths in our samples. To do this, we subsetted all peaks where we had accurate length information (107 peaks) and then calculated the weighted mean chain length as
where li is the length of a carbon chain and pi is the proportional concentration of all chains of that length. We also calculated dispersion around this mean as
We analyzed these in the same manner as described above, using AIC values to conduct model choice from glm models (Gaussian distribution, link=identity).
2.5. Behavioral trials
Given experimental restrictions, we were unable to accurately test for the use of CHCs in female choice, and thus, we tested for the use CHCs in male choice. Behavioral trials were conducted with flies from the Østhassel population that were collected in March 2018 and cultured in the laboratory for several generations. To collect adults for testing, we selected pupae and kept them in individual Eppendorf tubes (to ensure virginity) containing a small piece of cotton soaked in a 0.5% mannitol solution. When a fly eclosed, the Eppendorf was moved to the refrigerator to slow down aging and kept there for up to 4 weeks which is a common practice in this species (Gilburn, Foster, & Day, 1992; ex: Crean, Dunn, Day, & Gilburn, 2000). In all behavioral trials, five males (marked individually with nail polish) were introduced to a small petri dish (Ø 55 mm) with a small amount of minced Saccharina (≈1 g) and two dead flies mounted on insect pins. We conducted two different types of trials: I. Males were able to choose between a dead male and a dead female and II. Males were able to choose between two dead females one of which had been extracted in hexane (see above for methods). Type 1 trials were used to confirm that our methodology was working, and type II trials were used to test whether CHCs play a role in male choice. For type II trials, we checked that extracted females were mostly devoid of CHCs by re‐extracting five females and performing GC‐MS analysis as described above. The hexane extraction reduced the total amount of CHCs on the flies by 72% ± 6%. All trials lasted for 30 min, and we recorded all mountings and how long they lasted. We used a paired Wilcoxon rank‐sum test on our data, with male as our unit of replication, to determine whether males preferentially mounted one of the two proffered flies.
2.6. Transcriptome
Adult C. frigida were collected from wrackbeds at two locations, Delp (68.4°N, 14.52°E) and Tårnvik (67.59°N, 15.05°E), in Norway. Fly collection was done with nets that were gently moved slightly above the wrackbed to catch adult flies. Upon capture, each fly was identified to species and sexed and then placed in individual Eppendorf tubes filled with RNAlater (Invitrogen). Once in the tube, a clean scalpel was used to squash each fly to ensure that the RNAlater could easily permeates tissues to stabilize and protect cellular RNA. Three males and three females were collected per location. Library preparation was done with the Illumina TruSeq RNA Sample Prep Kit. Sequencing was conducted at BGI Tech Solutions (Hong Kong) on one lane of HiSeq 2000.
All reads were assembled together using Trinity version r20140717 (Grabherr et al., 2011) with the default parameters but specifying the strand specificity of sequencing reads.
2.7. Candidate gene analysis
Based on a literature search for genes involved in CHC synthesis, we identified 37 reference protein sequences, which were downloaded from FlyBase (http://flybase.org) in October 2017. We used tblastn with the default settings to identify orthologs in the C. frigida transcriptome. We then used gmap (Wu & Watanabe, 2005) to find areas of the C. frigida genome (M. Wellenreuther, C. Mérot, & L. Bernatchez, unpublished) that might contain these genes. We output all alignments as a gff3 file. We have a previously made a VCF file created from 30x whole genome resequencing of 46 C. frigida across five populations in Scandinavia (E. L. Berdan & M. Wellenreuther, unpublished data). This VCF file was made by aligning raw reads to the C. frigida genome using bwa‐mem (Li & Durbin, 2010). Duplicate reads were marked using “picard” (http://picard.sourceforge.net) and SNPs were called with the Genome Analysis Toolkit (GATK; DePristo et al., 2011; Van der Auwera et al., 2013) specifically the GATK‐module “UnifiedGenotyper” (Van der Auwera et al., 2013). VCF filtering was done as described in Berdan, Mazzoni, Waurick, Roehr, & Mayer, 2015. We used this VCF file and the gff3 output from gmap in SNPeff (Cingolani et al., 2012) to annotate the SNPs in these genes.
To find which SNPs are diverged between populations, we used vcftools (Danecek et al., 2011) to calculate Cockerham and Weir's F ST estimate (Weir & Cockerham, 1984) for all SNPs in our reference genome. We retained the top 5% of this distribution (F ST ≥ 0.142, mean F ST = 0.024) as SNPs that were potentially divergent. We then subset the VCF file to include only outlier SNPs located in our previously identified genomic regions.
3. RESULTS
3.1. CHC analysis
A diverse blend of hydrocarbons with more than 100 different compounds was found in the cuticular extract of C. frigida. The majority of CHCs ranged in chain length from 23 to 33 carbon atoms. They contained odd numbered n‐alkanes, methyl‐, dimethyl‐, and trimethyl‐alkanes, as well as odd numbered alkenes. Some even numbered alkanes were also present but in lower quantities.
3.2. Sex and population effects
Results of the PERMANOVA indicated significant differences between females and males and between populations but also a significant interaction between sex and population (Table 1A). Pairwise tests on the interaction showed a significant difference between males and females for all populations except Ystad (Table S2). Multiple comparisons of populations showed that the Norwegian populations (Skeie and Østhassel) were similar, while the Swedish populations (Ystad and Stavder) differed for males but not for females. In general, the Norwegian populations tended to differ from the Swedish and a similar pattern was also shown in the results of the PCA and PLS‐DA (Figure 2a,c, Table S3). After combining the Swedish and Norwegian populations, the PERMANOVA indicated highly significant differences between females and males as well as between Sweden and Norway (Table 1B). OPLS‐DA models for both sex and country were significant and lead to separate clusters of females and males, and Sweden and Norway, respectively (Figure 2b,d, Table S3). The OPLS‐DA model revealed 41 peaks that contributed with a VIP score >1 to the differentiation between females and males. Of these, 31 were also significant in t tests with a FDR‐adjusted p‐values (Table 2). Generally, compounds were more abundant in males with the exception of only three peaks that were more abundant in females. Looking at country differentiation between Swedish and Norwegian populations, we found 43 compounds with a VIP score >1, of which 29 were significant in t tests with FDR‐adjusted p‐values and consisted of an equal mix of compounds more abundant in either of the countries (Table 2). Differences in CHC profiles between sexes and populations were due to quantitative differences, affecting a wide range of compounds instead of qualitative differences, that is, the presence/absence of a few distinct compounds.
Table 1.
PERMANOVA and PERMDISP results for (A) sex and population, (B) sex and country, and (C) sex and wrack type
| (A) | PERMANOVA: sex and population | PERMDISP | |||||
|---|---|---|---|---|---|---|---|
| df | SS | MS | F | p‐Value | |||
| Sex | 1 | 658.1 | 658.1 | 5.86 | <0.001*** | See Table S2 | |
| Population | 3 | 1,150.3 | 383.4 | 3.41 | <0.001*** | ||
| Sex:Population | 3 | 486.9 | 162.3 | 1.44 | 0.015* | ||
| Residuals | 96 | 10,786.0 | 112.4 | ||||
| Total | 103 | 13,081 | |||||
| (B) | PERMANOVA: sex and country | PERMDISP | |||||
|---|---|---|---|---|---|---|---|
| df | SS | MS | F | p‐Value | F | p‐Value | |
| Sex | 1 | 658.0 | 658.1 | 5.60 | <0.001*** | 8.94 | 0.003** |
| Country | 1 | 517.8 | 517.8 | 4.41 | <0.001*** | 1.19 | 0.279 |
| Sex:Country | 1 | 154.1 | 154.1 | 1.31 | 0.128 | ||
| Residuals | 100 | 11,751.0 | 117.5 | ||||
| Total | 103 | 13,081 | |||||
| (C) | PERMANOVA: sex and wrack type | PERMDISP | |||||
|---|---|---|---|---|---|---|---|
| df | SS | MS | F | p‐Value | F | p‐Value | |
| Sex | 1 | 360.9 | 360.9 | 3.73 | <0.001*** | 2.36 | 0.129 |
| Wrack | 1 | 481.3 | 481.3 | 4.98 | <0.001*** | 2.29 | 0.139 |
| Sex:Wrack | 1 | 122.3 | 122.3 | 1.27 | 0.1629 | ||
| Residuals | 44 | 4,252.5 | 96.7 | ||||
| Total | 47 | 5,217 | |||||
p < .05
p < .01
p < .001.
Figure 2.

(a) PCA of all samples from the balanced data set indicating population (gray—Østhassel, orange—Skeie, blue—Stavder, green—Ystad) and sex (triangle—female, star—male). (b) OPLS‐DA for sex using all samples from the balanced data set, (c) PLS‐DA for population using all samples from the balanced data set, and (d) OPLS‐DA for country using all samples from the balanced data set (yellow—Sweden, purple—Norway). Circles indicate 95% confidence regions
Table 2.
List of compounds that are significantly important for differentiation of at least factor (sex, diet, or country)
| Compound number | Retention Index | Identified as | Methyl group position | Importance in differentiation for factor | |||
|---|---|---|---|---|---|---|---|
| Sex in sex & country | Country in sex & country | Sex in sex & diet | Wrack type in sex & diet | ||||
| 1 | 2,096 | Unknown alkane | * | ||||
| 2 | 2,101 | C21 | * | ||||
| 3 | 2,176 | Unknown alkane | * | ||||
| 5 | 2,202 | C22 | * | ||||
| 7 | 2,300 | C23 | * | * | * | ||
| 8 | 2,344 | Unknown alkane | * | * | |||
| 9 | 2,365 | 2‐ME‐C23 | * | ||||
| 11 | 2,400 | C24 | * | * | * | ||
| 14b | 2,478 | 25:x‐C25 | * | ||||
| 16 | 2,500 | C25 | * | ||||
| 17 | 2,524 | Unknown alkane | * | ||||
| 20 | 2,564 | 2‐ME‐C25 | * | ||||
| 21 | 2,576 | 3‐ME‐C25 | * | ||||
| 22 | 2,584 | 5,X‐Me‐C25 | X = 9,11,13,15 | * | * | ||
| 23 | 2,600 | C26 | * | ||||
| 24 | 2,638 | Unknown alkane | * | ||||
| 26 | 2,665 | 2‐Me‐C26 | * | ||||
| 27 | 2,680 | 27:x‐C27 | * | * | |||
| 29 | 2,701 | C27 | * | * | |||
| 30 | 2,712 | Unknown alkane | * | ||||
| 31 | 2,735 | 11+13‐Me‐C27 | * | * | |||
| 33 | 2,765 | 2‐Me‐C27+11,15‐Me‐C27 | * | * | |||
| 34 | 2,775 | 3‐ME‐C27 | * | ||||
| 37a | 2,807 | 3,X‐Me‐C27 | X = 15,13,11,7 | * | |||
| 40 | 2,850 | 29:x,x‐C29 | * | ||||
| 41 | 2,857 | 29:x,x‐C29 | * | ||||
| 42 | 2,865 | 2‐ME‐C28 or x:C29 | * | * | |||
| 44 | 2,880 | 29:x‐C29 | * | * | |||
| 45 | 2,882 | 29:x‐C29 | * | ||||
| 46 | 2,891 | 4,12‐Me‐C28 | * | ||||
| 47 | 2,902 | C29 | * | * | |||
| 48 | 2,933 | 15+13+11‐Me‐C29 | * | * | |||
| 50 | 2,961 | 11,X‐ME‐C29 | X = 15,17 | * | * | ||
| 51 | 2,972 | 7,X‐ME‐C29 | X = 11,13,15 | * | * | * | |
| 52 | 2,982 | 5,13‐Me‐C29 | * | * | |||
| 53 | 3,007 | 3,13‐ME‐C29 | * | * | |||
| 54 | 3,033 | 3,X,15‐Me‐C29 | X = 11,13 | * | * | ||
| 55 | 3,042 | 3,7,15‐Me‐C29 | * | ||||
| 56 | 3,054 | 31:x,x‐C31 | * | ||||
| 57 | 3,058 | 12,16‐Me‐C30 | * | ||||
| 60 | 3,074 | 31:x‐C31 or 6,16‐Me‐C30 | * | ||||
| 61 | 3,082 | 31:x‐C31 | * | * | |||
| 62 | 3,090 | 31:x‐C31 or 4,16‐Me‐C30 | * | ||||
| 63 | 3,101 | C31 | * | * | * | * | |
| 64 | 3,118 | Unknown alkane | * | * | * | ||
| 65 | 3,131 | 15+13+11+9‐Me‐C31 | * | * | * | ||
| 66 | 3,157 | 15,19‐Me‐C31 | * | * | |||
| 67 | 3,163 | 9,X‐Me‐C31 | X = 13,15 | * | * | * | |
| 68 | 3,170 | 7,X‐Me‐C31 | X = 15,17 | * | * | ||
| 69 | 3,180 | 5,15‐Me‐C31 | * | * | |||
| 71 | 3,194 | Unknown alkane | * | * | |||
| 72 | 3,205 | 3,X‐Me‐C31 | X = 9,11,13 | * | * | ||
| 73a | 3,231 | 3,X,Y‐Me‐C31 | X = 9,11; Y = 13,15 | * | * | ||
| 74 | 3,246 | 33:x,x,x,x‐C33 | * | ||||
| 75 | 3,256 | 33:x,x‐C33 | * | ||||
| 76 | 3,272 | 33:x‐C33 | * | ||||
| 77 | 3,284 | 33:x‐C33 | * | ||||
| 78 | 3,300 | Unknown alkane | * | * | * | * | |
| 79 | 3,315 | Unknown alkene | * | ||||
| 80 | 3,330 | 17+15+13+11‐Me‐C33 | * | * | * | ||
| 81 | 3,353 | 11,X‐Me‐C33 | X = 13,15,17 | * | |||
| 82 | 3,359 | 9,X‐Me‐C33 | X = 11,13,15 | * | |||
| 83a | 3,365 | 7,X‐Me‐C33 | X = 11,13,15,17 | * | |||
| 83b | 3,368 | 7,X‐Me‐C33 | X = 11,13,15,17 | * | * | ||
| 84 | 3,377 | 5,X‐Me‐C33 | X = 15,17 | * | * | ||
| 85a | 3,391 | 7,13,17‐Me‐C33 | * | * | |||
| 85b | 3,393 | 7,13,17‐Me‐C33 | * | ||||
If compounds are significant for differentiation of any category in any comparison a * appears under that factor. Color indicates the direction of the differentiation (red—higher in females, blue—higher in males, gray—higher in Norwegian populations, green—higher in Swedish populations, yellow—higher in flies raised on standard wrack, purple—higher in flies raised on field wrack).
Significant t test with FDA α = .05
3.3. Sex and diet effects
PERMANOVA demonstrated a significant difference between females and males in the Ystad population and also a significant effect of the larval diet (wrack type; Table 1C). Both OPLS‐DA models for either sex or wrack type were significant and lead to separate clusters of females and males and flies raised on standard or field wrack (Figure 3b,c, Table S3). The OPLS‐DA model revealed 38 peaks that contributed to the differentiation between females and males with a VIP score >1. Of these, 24 compounds were also significant in a t test with FDR‐adjusted p‐values (Table 2). All of these, except for three peaks, were more abundant in males. Overall 65% (26 compounds) were shared between this analysis and the previous analysis investigating sex and population effects. Looking at differentiation between flies raised on a diet consisting of standard or field wrack, we found 33 compounds with a VIP score >1. Of these, 27 compounds were significant using a t test with FDR‐adjusted p‐values, all with higher amounts in flies reared on field wrack (Table 2). Flies reared on field wrack had more CHCs per gram body weight than flies reared on standard wrack (normalized totals: females field wrack 7.96 ± 0.94, females standard wrack 4.09 ± 0.47, males field wrack 11.24 ± 1.03, males standard wrack 7.09 ± 1.21). Differences in CHC profiles between sexes and diets were exclusively due to quantitative differences, affecting a wide range of compounds instead of qualitative differences, that is, the presence/ absence of a few distinct compounds.
Figure 3.

(a) PCA of samples from Ystad indicating diet (blue—field, yellow—standard) and sex (triangle—female, star—male). (b) OPLS‐DA for sex using only Ystad samples. (c) OPLS‐DA for diet using only Ystad samples. Circles indicate 95% confidence regions
3.4. Shifts in chemistry
As our results above indicated an overall effect of country rather than population, we used country and sex for our terms in the glm models. For total normalized peak area, the best model contained only sex as a main effect (for a full AIC comparison for all models, see Table S4a–e). In general, males had more CHCs per gram body weight than females (Figure 4a). For the proportion of alkenes, the best model contained country and sex as main effects (for type II analysis of deviance tables for all best models, see Table S5a–e). Females tended to have more alkenes than males and individuals from Norway had more alkenes than individuals from Sweden (Figure 4b). The best model for the proportion of methylated compounds contained sex and country as the main effects. As with alkenes, females tended to have more methylated compounds than males and individuals from Norway tended to have more methylated compounds than individuals from Sweden (Figure 4c).
Figure 4.
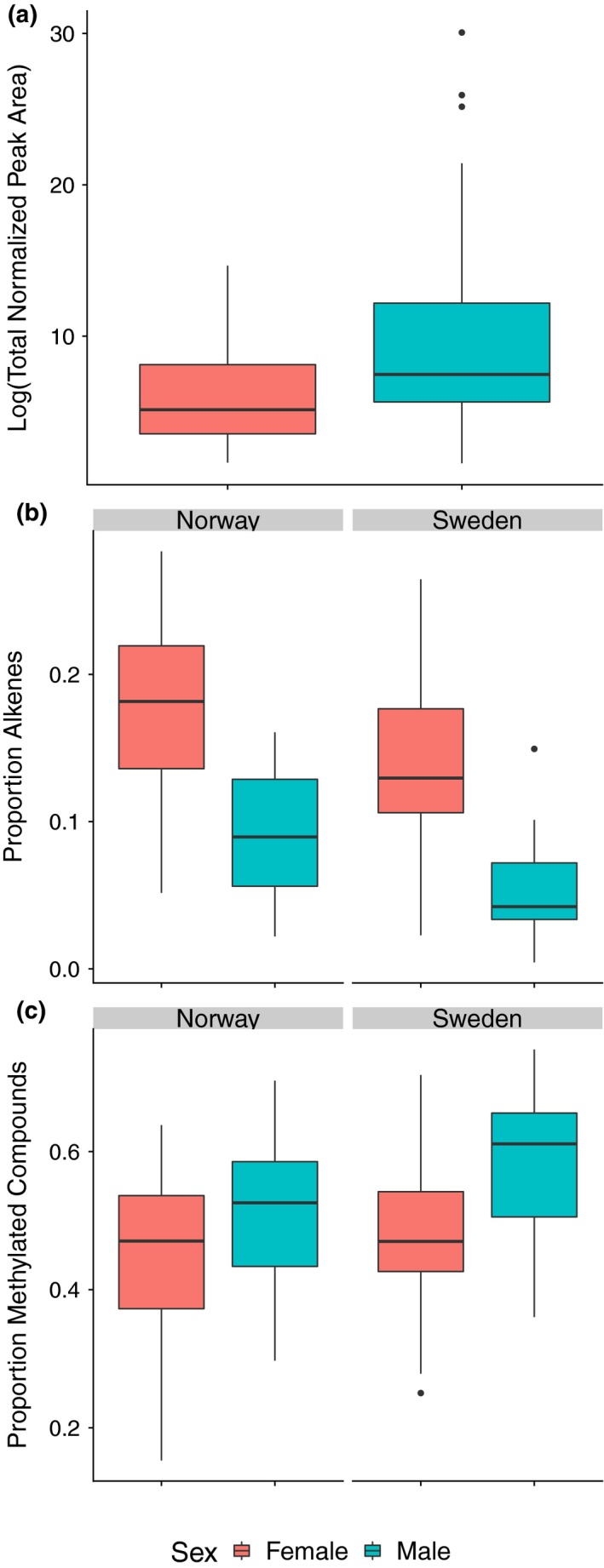
Boxplot showing differences in (a) Log transformed total peak area, (b) proportion of compounds that are alkenes, and (c) proportion of methylated compounds. Females are in red, and males are in blue
The best model for weighted mean chain length was country and sex. Overall, males had longer mean chain lengths than females and individuals from Sweden had longer mean chain lengths than individuals from Norway (Figure 5a). The best model for dispersion around mean chain length was also country and sex. Males had higher dispersion than females, and individuals from Norway had higher dispersion than individuals from Sweden (Figure 5b).
Figure 5.

Boxplot showing differences in (a) Weighted mean chain length and (b) dispersion around the mean. Females are in red, and males are in blue
3.5. Behavioral trials
We conducted 20 trials of type I (female vs. male) and 12 trials of type II (female vs. extracted female). We only retained data from males that mounted at least one of the proffered flies leaving us with data from 28 males (type I) and 33 males (type II). In type I trials, males preferred the female to the male and on average spent 73% (±7%) of their “mating time” mounting her (Wilcoxon signed‐rank test with continuity correction, V = 220, p = .0132). In type II trials, males preferred the control (i.e., non‐extracted) female and on average spent 82% (±6%) of their “mating time” mounting her (Wilcoxon signed‐rank test with continuity correction, V = 493, p = .0002).
3.6. Genetic analysis
Our assembled transcriptome contained 41,787 contigs, which made up 25,683 “genes” (clusters of transcripts with similar genetic content). The N50 contig length was 2,530. Using this transcriptome, we identified 15 isoforms from 13 transcripts with matches to our query proteins. These transcripts mapped to 16 unique areas of the genome assembly. We blasted these areas against the NCBI nr database (accessed in April 2018) to confirm our annotation. Five of these areas had either no match or matched to an unrelated protein and were thus removed. The remaining genes include two putative fatty acid synthases, two putative desaturases, five putative elongases, one putative cytochrome P450 reductase, and one putative cytochrome P450‐4G1 (Table 3). We found 1,042 SNPs within these genes (including 5K upstream and 5K downstream), of these 11 had an F ST value >0.142 (i.e., in the top 5% of the F ST distribution; Table 4).
Table 3.
Putative genes involved in Cuticular hydrocarbon synthesis in Coelopa frigida
| Scaffolda | Gene positiona | Best blast hitb |
|---|---|---|
| scaffold_129 | 185034–197861 | Desaturase 1 |
| scaffold_155 | 109640–112144 | Cytochrome P450‐4G1 |
| scaffold_206 | 225893–231312 | Cytochrome P450 reductase |
| scaffold_480 | 98147–106166 | CG5326 (elongase family) |
| scaffold_480 | 2453–12223 | CG31522 (elongase family) |
| scaffold_545 | 1553–3217 | CG5326 (elongase family) |
| scaffold_340 | 1141–16368 | Baldspot (elongase family) |
| scaffold_149 | 7122–16098 | Fatty Acid Synthase 3 |
| scaffold_51 | 250672–259608 | Fatty Acid Synthase 1 |
| scaffold_716 | 36957–55451 | CG2781 (elongase family) |
| scaffold_994 | 14376–17314 | CG9743 (contains fatty acid desaturase domain and acyl‐CoA desaturase) |
From unpublished genome assembly (Wellenreuther et al.).
From a BLAST against D. melanogaster annotated nucleotides on http://flybase.org.
Table 4.
Outlier SNPs putatively involved in geographic differences
| Scaffolda | Positiona | Variant typeb | Best blast hitc | F ST d |
|---|---|---|---|---|
| scaffold_129 | 195,074 | Missense variant | Desaturase 1 | 0.16 |
| scaffold_155 | 116,876 | Downstream gene variant | Cytochrome P450‐4G1 | 0.22 |
| scaffold_206 | 225,645 | Downstream gene variant | Cytochrome P450 reductase | 0.17 |
| scaffold_206 | 226,804 | Missense variant | Cytochrome P450 reductase | 0.17 |
| scaffold_206 | 229,444 | Missense variant | Cytochrome P450 reductase | 0.20 |
| scaffold_206 | 230,032 | Intron variant | Cytochrome P450 reductase | 0.30 |
| scaffold_480 | 105,896 | Synonymous variant | CG5326 (elongase family) | 0.22 |
| scaffold_480 | 110,918 | Downstream gene variant | CG5326 (elongase family) | 0.16 |
| scaffold_994 | 16,976 | Missense variant | CG9743 (contains fatty acid desaturase domain and acyl‐CoA desaturase) | 0.16 |
| scaffold_994 | 18,844 | Downstream gene variant | CG9743 (contains fatty acid desaturase domain and acyl‐CoA desaturase) | 0.20 |
| scaffold_994 | 19,574 | Downstream gene variant | CG9743 (contains fatty acid desaturase domain and acyl‐CoA desaturase) | 0.20 |
From unpublished genome assembly (Wellenreuther et al.).
Annotated in SnpEff (see text for details).
From a BLAST against Drosophila melanogaster annotated nucleotides on http://flybase.org.
Calculated from a separate unpublished data set of WGS from 46 Coelopa frigida.
4. DISCUSSION
Here, we explored causes of variation in cuticular hydrocarbons (CHCs) in the seaweed fly C. frigida. We detected considerable phenotypic CHC variation attributable to sex, population, and diet and were able to describe genetic variation in candidate genes involved in CHC synthesis that was attributable to population. We show that this trait is likely used as a signal in male mate choice indicating that CHC composition may be under both natural and sexual selection in this system. We discuss these results and their consequences below.
Males and females differed in their CHC composition. Not only did the composition between males and females differ (Table 2, Figures 2, 3, 4, 5) but, in addition to that, males consistently had more CHC per gram body weight than females (Figure 4a). Our behavioral trials demonstrate that males preferentially mount females with an intact signal (i.e., CHC cocktail), indicating that CHCs are likely used in male mate choice. Although we were unable to test female choice, it is likely that females also use CHCs in mate choice. A role for CHCs in sexual selection in C. frigida would be in line with the general finding that CHCs play a major role in communication in insects, specifically in Diptera (Blomquist & Bagnères, 2010; Ferveur & Cobb, 2010; Howard & Blomquist, 2005). Differences in CHCs have been shown to be involved in a wide variety of behaviors in Diptera such as courtship, aggregation, and dominance (Ferveur & Cobb, 2010). For instance, 11‐cis‐vaccenyl acetate, a male‐specific hydrocarbon in Drosophila melanogaster, increases male–male aggression while suppressing male mating (Wang & Anderson, 2010). The same compound is also involved in aggregation behavior in both D. simulans and D. melanogaster (Bartelt, Schaner, & Jackson, 1985; Schaner, Bartelt, & Jackson, 1987). Other aggregation hydrocarbons, such as (Z)‐10‐heneicosene, in D. virilis have been shown to attract males and females of certain ages (Bartelt & Jackson, 1984). Further behavioral tests in C. frigida can determine if CHCs are additionally used in aggregation and male–male interactions.
We found a shift in the composition between sexes, populations, and diets rather than differences in the presence/absence of specific compounds. This type of pattern differs from some dipterans where sex and population differences are mostly qualitative (Carlson & Schlein, 1991; Dallerac et al., 2000; Everaerts, Farine, Cobb, & Ferveur, 2010; Ferveur & Sureau, 1996; Gomes, Trigo, & Eiras, 2008) although many dipterans also show quantitative differences (ex: Byrne, Camann, Cyr, Catts, & Espelie, 1995; Jallon & David, 1987). This discrepancy is likely to be partially explained by the distribution of compounds that make up the CHCs in C. frigida. While C. frigida chain lengths and compound classes are comparable with other dipteran species (Ferveur & Cobb, 2010), the distribution of these compounds is somewhat different. Many dipterans have principal CHC components that make up a large proportion of the CHC composition (Etges & Jackson, 2001; Ferveur & Sureau, 1996; Gomes et al., 2008; Jallon & David, 1987), while the most abundant compound (the C25 alkane) observed in this study only made up on average 15% of the CHCs in C. frigida.
We found strong geographic signatures in CHC composition when flies were raised in a common garden, which indicates a shift in genetic variation. We examined coding variation to determine whether we could tie population‐level phenotypic differences to genetic differences. We observed country‐level variation with strong differences between Norway and Sweden (Figures 2d, 4, and 5). We found potential outlier SNPs in desaturases, elongases, a cytochrome P450‐4G1, and a cytochrome P450 reductase. Desaturases add double bonds or triple bonds to alkanes (Blomquist & Bagnères, 2010; Howard & Blomquist, 2005). We found an outlier missense variant in a putative desaturase 1 as well as a missense and two downstream outlier variants in a putative desaturase (Table 4). This aligns with our phenotypic data showing differences in the proportion of alkanes versus alkenes between Norway and Sweden. Elongases lengthen fatty acyl‐CoAs and are necessary for chains longer than 16 carbon atoms (Blomquist & Bagnères, 2010). We found both synonymous and downstream outlier variants in putative C. frigida elongases (Table 4) and corresponding differences in both the mean chain length and variance of the chain length (dispersion around the mean) due to country of origin. P450 reductases are responsible for reducing fatty acids to aldehydes (Blomquist & Bagnères, 2010). Knockdown of P450 reductases in D. melanogaster leads to a striking reduction of cuticular hydrocarbons (Qiu et al., 2012). P450‐4G genes, which are unique to insects, encode an oxidative decarboxylase that catalyzes the aldehyde to hydrocarbon reaction. Work in other insect species has found that a knockdown or knockout of this gene leads to a decrease in overall hydrocarbon levels (Chen et al., 2016; Qiu et al., 2012; Reed et al., 1995). We found a downstream outlier variant in our cytochrome P450‐4G1 and multiple kinds of outlier variants in a cytochrome P450 reductase. However, the total amount of CHCs did not differ between populations, only sexes. As males and females share a genome (with the exception of the sex chromosome), it is unlikely that these SNPs affect this difference. Finally, we also found variation between Sweden and Norway in the proportion of methylated compounds. Other studies suggest that differences in the proportion of methylated compounds are often caused by either variation in fatty acid synthase (FAS, Blomquist et al., 1994; Juarez et al., 1996; de Renobales et al., 1986) or multiple copies of FAS with different functions (Chung et al., 2014). We found two different FASs in our genome (Table 3) but neither of them contained divergent SNPs. Future studies are needed to examine whether these genetic and phenotypic changes are related. Specifically, investigations into both RNA expression and tests of function of these loci will be necessary to provide a direct link. However, the association between the known function of several of these genes (elongases, desaturases) and the corresponding differences in populations are marked. Given that we see shifts in multiple chemical aspects (length, methylation, and alkene/alkane ratio), it is likely that multiple loci are responsible for these patterns.
We also detected plasticity in CHC composition due to the environment; the wrackbed itself had a strong influence on the CHC composition (Figure 3c). Wrackbed composition and microbiome (i.e., the food source for C. frigida larvae) vary across C. frigida populations in Europe (E. L. Berdan, M. Wellenreuther, & K. Johannesson, unpublished data, Butlin & Day, 1989; Day et al., 1983; Wellenreuther et al., 2017). Consequently, the CHC composition of C. frigida is likely to differ between natural populations in accordance with other research showing that larval diet impacts CHC composition (Etges & de Oliveira, 2014; Liang & Silverman, 2000; Rundle, Chenoweth, Doughty, & Blows, 2005; Stojkovic et al., 2014).
Together, our results indicate that both genetic and environmental factors influence the CHC composition in C. frigida. This variation may lead to several potential evolutionary scenarios: (a) Natural selection may select for a different CHC composition in different environments, and natural polymorphism in CHC genes may be under direct selection due to this. For example, Drosophila serrata and D. birchii differ in the environment they inhabit (habitat generalist vs. habitat specialist for humid rainforests). These two species also differ in the concentration of methyl branched CHCs (especially important in desiccation resistance) caused, in part, by changes in the cis‐regulatory sequence likely under natural selection (Chung et al., 2014). (b) Natural populations differ strongly in their CHC composition due to environmental effects (i.e., plasticity). If assortative mating or selection on preferences is present, this could lead to locally distinct traits and preferences (Chung & Carroll, 2015). This would reduce effective migration between populations, as foreign males would be at a disadvantage. Differences in CHC composition and corresponding preferences have been shown to cause behavioral isolation between many different Drosophila species or populations (Coyne, Crittenden, & Mah, 1994; Etges & de Oliveira, 2014; Rundle et al., 2005).
In conclusion, the analysis of CHC extracts from male and female C. frigida from multiple populations revealed a complex and variable mix of more than 100 different compounds. We confirmed that CHCs are likely used in mate choice and found extensive phenotypic variation attributable to diet, sex, and country as well as associated genetic variation attributable to the latter. This reveals that CHC composition is dynamic, strongly affected by the larval environment, and most likely under natural and sexual selection. Further work is needed to explore the evolutionary consequences of these differences.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
ELB conceived and designed the project. ELB, SE, GAM, and GMN did the laboratory work. SE and GMN analyzed the chemical data and ELB analyzed the genetic data. SE, ELB, and MW wrote the paper with input from the other authors. HP supervised the project.
Supporting information
ACKNOWLEDGMENTS
We thank the two reviewers for helpful comments which improved the manuscript. We thank the members of the Marine Chemical Ecology group at Gothenburg University for helpful comments on experimental design. E.B. was supported by a Marie Skłodowska‐Curie fellowship 704920—ADAPTIVE INVERSIONS—H2020‐MSCA‐IF‐2015. MW and the research were supported by grants from Vetenskapsrådet to MW (2012‐3996), from the Mistra Foundation to HP (AquaAgriKelp), and from the Swedish Foundation for Strategic Research to HP (Sweaweed).
Berdan E, Enge S, Nylund GM, Wellenreuther M, Martens GA, Pavia H. Genetic divergence and phenotypic plasticity contribute to variation in cuticular hydrocarbons in the seaweed fly Coelopa frigida . Ecol Evol. 2019;9:12156–12170. 10.1002/ece3.5690
DATA AVAILABILITY STATEMENT
All data are available on Dryad (https://doi.org/10.5061/dryad.943mp7) including
The raw GC‐MS output from all of the samples
Fasta file of the genomic regions containing the candidate genes
The transcriptome.
REFERENCES
- Badouin, H. , Belkhir, K. , Gregson, E. , Galindo, J. , Sundström, L. , Martin, S. J. , … Smadja, C. M. (2013). Transcriptome characterisation of the ant Formica exsecta with new insights into the evolution of desaturase genes in social hymenoptera. PLoS ONE, 8, e68200 10.1371/journal.pone.0068200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagneres, A. G. , Lorenzi, M. C. , Dusticier, G. , Turillazzi, S. , & Clement, J. L. (1996). Chemical usurpation of a nest by paper wasp parasites. Science, 272, 889–892. 10.1126/science.272.5263.889 [DOI] [PubMed] [Google Scholar]
- Bartelt, R. , & Jackson, L. (1984). Hydrocarbon component of the Drosophila virilis (Diptera: Drosophilidae) aggregation pheromone:(Z)‐10‐heneicosene. Annals of the Entomological Society of America, 77, 364–371. 10.1093/aesa/77.4.364 [DOI] [Google Scholar]
- Bartelt, R. J. , Schaner, A. M. , & Jackson, L. L. (1985). cis‐Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster . Journal of Chemical Ecology, 11, 1747–1756. 10.1007/BF01012124 [DOI] [PubMed] [Google Scholar]
- Barton, K. (2017). MuMIn. Retrieved from https://CRAN.R-project.org/package=MuMIn [Google Scholar]
- Berdan, E. L. , Mazzoni, C. J. , Waurick, I. , Roehr, J. T. , & Mayer, F. (2015). A population genomic scan in Chorthippus grasshoppers unveils previously unknown phenotypic divergence. Molecular Ecology, 24, 3918–3930. [DOI] [PubMed] [Google Scholar]
- Berdan, E. , Rosenquist, H. , Larson, K. , & Wellenreuther, M. (2018). Inversion frequencies and phenotypic effects are modulated by the environment: Insights from a reciprocal transplant study in Coelopa frigida . Evolutionary Ecology, 32, 683–698. [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Blomquist, G. J. , & Bagnères, A.‐G. (2010). Insect hydrocarbons: Biology, biochemistry, and chemical ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Blomquist, G. J. , Guo, L. , Gu, P. , Blomquist, C. , Reitz, R. C. , & Reed, J. R. (1994). Methyl‐branched fatty acids and their biosynthesis in the housefly, Musca domestica L. (Diptera: Muscidae). Insect Biochemistry and Molecular Biology, 24, 803–810. [Google Scholar]
- Blyth, J. E. , & Gilburn, A. S. (2011). The function of female behaviours adopted during premating struggles in the seaweed fly, Coelopa frigida . Animal Behavior, 81, 77–82. 10.1016/j.anbehav.2010.09.013 [DOI] [Google Scholar]
- Butlin, R. , & Day, T. (1989). Environmental correlates of inversion frequencies in natural populations of seaweed flies (Coelopa frigida). Heredity, 62, 223 10.1038/hdy.1989.32 [DOI] [Google Scholar]
- Butlin, R. K. , Read, I. L. , & Day, T. H. (1982). The effects of a chromosomal inversion on adult size and male mating success in the seaweed fly, Coelopa frigida . Heredity, 49, 51–62. 10.1038/hdy.1982.64 [DOI] [Google Scholar]
- Byrne, A. , Camann, M. , Cyr, T. , Catts, E. , & Espelie, K. (1995). Forensic implications of biochemical differences among geographic populations of the black blow fly, Phormia regina (Meigen). Journal of Forensic Science, 40, 372–377. [PubMed] [Google Scholar]
- Carlson, D. , & Schlein, Y. (1991). Unusual polymethyl alkenes in tsetse flies acting as abstinon in Glossina morsitans . Journal of Chemical Ecology, 17, 267–284. 10.1007/BF00994332 [DOI] [PubMed] [Google Scholar]
- Chen, N. , Fan, Y. L. , Bai, Y. , Li, X. D. , Zhang, Z. F. , & Liu, T. X. (2016). Cytochrome P450 gene, CYP4G51, modulates hydrocarbon production in the pea aphid, Acyrthosiphon pisum . Insect Biochemistry and Molecular Biology, 76, 84–94. 10.1016/j.ibmb.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Chertemps, T. , Duportets, L. , Labeur, C. , Ueda, R. , Takahashi, K. , Saigo, K. , & Wicker‐Thomas, C. (2007). A female‐biased expressed elongase involved in long‐chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America, 104, 4273–4278. 10.1073/pnas.0608142104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, H. , & Carroll, S. B. (2015). Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays, 37, 822–830. 10.1002/bies.201500014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, H. , Loehlin, D. W. , Dufour, H. D. , Vaccarro, K. , Millar, J. G. , & Carroll, S. B. (2014). A single gene affects both ecological divergence and mate choice in Drosophila. Science, 343, 1148–1151. 10.1126/science.1249998 [DOI] [PubMed] [Google Scholar]
- Cingolani, P. , Platts, A. , Wang, L. L. , Coon, M. , Nguyen, T. , Wang, L. , … Ruden, D. M. (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w(1118); iso‐2; iso‐3. Fly, 6, 80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A. , Crittenden, A. P. , & Mah, K. (1994). Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila . Science, 265, 1461–1464. 10.1126/science.8073292 [DOI] [PubMed] [Google Scholar]
- Crean, C. S. , Dunn, D. W. , Day, T. H. , & Gilburn, A. S. (2000). Female mate choice for large males in several species of seaweed fly (Diptera: Coelopidae). Animal Behavior, 59, 121–126. 10.1006/anbe.1999.1268 [DOI] [PubMed] [Google Scholar]
- Cullen, S. J. , Young, A. M. , & Day, T. H. (1987). Dietary requirements of seaweed flies (Coelopa frigida). Estuarine, Coastal and Shelf Science, 24, 701–710. 10.1016/0272-7714(87)90108-9 [DOI] [Google Scholar]
- Dallerac, R. , Labeur, C. , Jallon, J. M. , Knipple, D. C. , Roelofs, W. L. , & Wicker‐Thomas, C. (2000). A Δ9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America, 97, 9449–9454. 10.1073/pnas.150243997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek, P. , Auton, A. , Abecasis, G. , Albers, C. A. , Banks, E. , DePristo, M. A. , … Grp, G. P. A. (2011). The variant call format and VCFtools. Bioinformatics, 27, 2156–2158. 10.1093/bioinformatics/btr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, T. H. , Dawe, C. , Dobson, T. , & Hillier, P. C. (1983). A chromosomal inversion polymorphism in Scandinavian populations of the seaweed fly, Coelopa frigida . Hereditas, 99, 135–145. 10.1111/j.1601-5223.1983.tb00738.x [DOI] [PubMed] [Google Scholar]
- Day, T. H. , Foster, S. P. , & Engelhard, G. (1990). Mating behavior in seaweed flies (Coelopa frigida). Journal of Insect Behavior, 3, 105–120. 10.1007/BF01049198 [DOI] [Google Scholar]
- de Renobales, M. , Woodin, T. S. , & Blomquist, G. J. (1986). Drosophila melanogaster fatty acid synthetase: Characteristics and effect of protease inhibitors. Insect Biochemistry, 16, 887–894. 10.1016/0020-1790(86)90061-2 [DOI] [Google Scholar]
- Dembeck, L. M. , Boroczky, K. , Huang, W. , Schal, C. , Anholt, R. R. H. , & Mackay, T. F. C. (2015). Genetic architecture of natural variation in cuticular hydrocarbon composition in Drosophila melanogaster . eLife, 4, e09861 10.7554/eLife.09861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo, M. A. , Banks, E. , Poplin, R. , Garimella, K. V. , Maguire, J. R. , Hartl, C. , … McKenna, A. (2011). A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics, 43(5), 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges, W. J. , & Ahrens, M. A. (2001). Premating isolation is determined by larval‐rearing substrates in cactophilic Drosophila mojavensis. V. Deep geographic variation in epicuticular hydrocarbons among isolated populations. The American Naturalist, 158, 585–598. [DOI] [PubMed] [Google Scholar]
- Etges, W. J. , & de Oliveira, C. C. (2014). Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. X. Age‐specific dynamics of adult epicuticular hydrocarbon expression in response to different host plants. Ecology and Evolution, 4, 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges, W. J. , & Jackson, L. L. (2001). Epicuticular hydrocarbon variation in Drosophila mojavensis cluster species. Journal of Chemical Ecology, 27, 2125–2149. [DOI] [PubMed] [Google Scholar]
- Everaerts, C. , Farine, J. P. , Cobb, M. , & Ferveur, J. F. (2010). Drosophila cuticular hydrocarbons revisited: Mating status alters cuticular profiles. PLoS ONE, 5, e9607 10.1371/journal.pone.0009607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer, B. , Elsner, D. , & Foitzik, S. (2014). Gene expression patterns associated with caste and reproductive status in ants: Worker‐specific genes are more derived than queen‐specific ones. Molecular Ecology, 23, 151–161. 10.1111/mec.12490 [DOI] [PubMed] [Google Scholar]
- Ferveur, J.‐F. (2005). Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behavior Genetics, 35, 279 10.1007/s10519-005-3220-5 [DOI] [PubMed] [Google Scholar]
- Ferveur, J.‐F. , & Cobb, M. (2010). Behavioral and evolutionary roles of cuticular hydrocarbons in Diptera In Blomquist G. J., & Bagnères A.‐G. (Eds.), Insect hydrocarbons: Biology, biochemistry, and chemical ecology (pp. 325–343). Cambridge, NY: Cambridge University Press. [Google Scholar]
- Ferveur, J. F. , & Sureau, G. (1996). Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex‐mosaic Drosophila melanogaster . Proceedings of the Royal Society B: Biological Sciences, 263, 967–973. [DOI] [PubMed] [Google Scholar]
- Foley, B. , & Telonis‐Scott, M. (2011). Quantitative genetic analysis suggests causal association between cuticular hydrocarbon composition and desiccation survival in Drosophila melanogaster . Heredity, 106, 68 10.1038/hdy.2010.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentiu, F. D. , & Chenoweth, S. F. (2010). Clines in cuticular hydrocarbons in two Drosophila species with independent population histories. Evolution, 64, 1784–1794. 10.1111/j.1558-5646.2009.00936.x [DOI] [PubMed] [Google Scholar]
- Galindo‐Prieto, B. , Eriksson, L. , & Trygg, J. (2014). Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS). Journal of Chemometrics, 28, 623–632. [Google Scholar]
- Gilburn, A. , Foster, S. , & Day, T. (1992). Female mating preference for large size in Coelopa frigida (seaweed fly). Heredity, 69, 209–216. 10.1038/hdy.1992.118 [DOI] [Google Scholar]
- Gilburn, A. , Foster, S. , & Day, T. (1993). Genetic correlation between a female mating preference and the preferred male character in seaweed flies (Coelopa frigida). Evolution, 47, 1788–1795. [DOI] [PubMed] [Google Scholar]
- Gomes, C. C. G. , Trigo, J. R. , & Eiras, Á. E. (2008). Sex pheromone of the American warble fly, Dermatobia hominis: The role of cuticular hydrocarbons. Journal of Chemical Ecology, 34, 636. [DOI] [PubMed] [Google Scholar]
- Grabherr, M. G. , Haas, B. J. , Yassour, M. , Levin, J. Z. , Thompson, D. A. , Amit, I. , … Regev, A. (2011). Full‐length transcriptome assembly from RNA‐Seq data without a reference genome. Nature Biotechnology, 29, 644 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, C. , Day, N. , Rosenquist, H. , Wellenreuther, M. , Bunnefeld, N. , & Gilburn, A. (2018). Tidal range and recovery from the impacts mechanical beach grooming. Ocean and Coastal Management, 154, 66–71. [Google Scholar]
- Grun, B. , Kosmidis, I. , & Zeileis, A. (2012). Extended beta regression in R: Shaken, stirred, mixed, and partitioned. Journal of Statistical Software, 48, 1–25. [Google Scholar]
- Haverty, M. I. , Nelson, L. J. , & Page, M. (1990). Cuticular hydrocarbons of four populations of Coptotermes formosanus Shiraki in the United States. Journal of Chemical Ecology, 16, 1635–1647. 10.1007/BF01014096 [DOI] [PubMed] [Google Scholar]
- Howard, R. W. , & Blomquist, G. J. (2005). Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annual Review of Entomology, 50, 371–393. 10.1146/annurev.ento.50.071803.130359 [DOI] [PubMed] [Google Scholar]
- Howard, R. W. , Howard, C. D. , & Colquhoun, S. (1995). Ontogenetic and environmentally induced changes in cuticular hydrocarbons of Oryzaephilus surinamensis (Coleoptera: Cucujidae). Annals of the Entomological Society of America, 88, 485–495. 10.1093/aesa/88.4.485 [DOI] [Google Scholar]
- Jallon, J. M. , & David, J. R. (1987). Variations in cuticular hydrocarbons among the eight species of the Drosophila melanogaster subgroup. Evolution, 41, 294–302. [DOI] [PubMed] [Google Scholar]
- Juarez, M. P. , Ayala, S. , & Brenner, R. R. (1996). Methyl‐branched fatty acid biosynthesis in Triatoma infestans . Insect Biochemistry and Molecular Biology, 26, 599–605. 10.1016/S0965-1748(96)00021-5 [DOI] [Google Scholar]
- Keays, M. C. , Barker, D. , Wicker‐thomas, C. , & Ritchie, M. G. (2011). Signatures of selection and sex‐specific expression variation of a novel duplicate during the evolution of the Drosophila desaturase gene family. Molecular Ecology, 20, 3617–3630. 10.1111/j.1365-294X.2011.05208.x [DOI] [PubMed] [Google Scholar]
- Kulmuni, J. , Wurm, Y. , & Pamilo, P. (2013). Comparative genomics of chemosensory protein genes reveals rapid evolution and positive selection in ant‐specific duplicates. Heredity, 110, 538–547. 10.1038/hdy.2012.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, L. , & Rundle, H. D. (2010). Adaptation to desiccation fails to generate pre‐ and postmating isolation in replicate Drosophila melanogaster laboratory populations. Evolution, 64, 710–723. [DOI] [PubMed] [Google Scholar]
- Li, H. , & Durbin, R. (2010). Fast and accurate long‐read alignment with Burrows‐Wheeler transform. Bioinformatics, 26, 589–595. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, D. , & Silverman, J. (2000). “You are what you eat”: Diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile . Naturwissenschaften, 87, 412–416. 10.1007/s001140050752 [DOI] [PubMed] [Google Scholar]
- Mcalpine, D. K. (1991). Review of the Australian kelp flies (Diptera, Coelopidae). Systematic Entomology, 16, 29–84. 10.1111/j.1365-3113.1991.tb00573.x [DOI] [Google Scholar]
- Ottensmann, M. , Stoffel, M. A. , Nichols, H. J. , & Hoffman, J. I. (2018). GCalignR: An R package for aligning gas‐chromatography data for ecological and evolutionary studies. PLoS ONE, 13, e0198311 10.1371/journal.pone.0198311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdereau, E. , Dedeine, F. , Christidès, J.‐P. , & Bagnères, A.‐G. (2010). Variations in worker cuticular hydrocarbons and soldier isoprenoid defensive secretions within and among introduced and native populations of the subterranean termite, Reticulitermes flavipes . Journal of Chemical Ecology, 36, 1189–1198. 10.1007/s10886-010-9860-9 [DOI] [PubMed] [Google Scholar]
- Peterson, M. A. , Dobler, S. , Larson, E. L. , Juárez, D. , Schlarbaum, T. , Monsen, K. J. , & Francke, W. (2007). Profiles of cuticular hydrocarbons mediate male mate choice and sexual isolation between hybridising Chrysochus (Coleoptera: Chrysomelidae). Chemoecology, 17, 87–96. 10.1007/s00049-007-0366-z [DOI] [Google Scholar]
- Qiu, Y. , Tittiger, C. , Wicker‐Thomas, C. , Le Goff, G. , Young, S. , Wajnberg, E. , … Feyereisen, R. (2012). An insect‐specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 109, 14858–14863. 10.1073/pnas.1208650109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpurohit, S. , Hanus, R. , Vrkoslav, V. , Behrman, E. L. , Bergland, A. O. , Petrov, D. , … Schmidt, P. S. (2017). Adaptive dynamics of cuticular hydrocarbons in Drosophila . Journal of Evolutionary Biology, 30, 66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, J. R. , Quilici, D. R. , Blomquist, G. J. , & Reitz, R. C. (1995). Proposed mechanism for the cytochrome P450‐catalyzed conversion of aldehydes to hydrocarbons in the house fly, Musca domestica . Biochemistry, 34, 16221–16227. 10.1021/bi00049a038 [DOI] [PubMed] [Google Scholar]
- Rouault, J. D. , Marican, C. , Wicker‐Thomas, C. , & Jallon, J. M. (2004). Relations between cuticular hydrocarbon (HC) polymorphism, resistance against desiccation and breeding temperature; A model for HC evolution in D. melanogaster and D. simulans . Genetica, 120, 195–212. 10.1023/B:GENE.0000017641.75820.49 [DOI] [PubMed] [Google Scholar]
- Rundle, H. D. , Chenoweth, S. F. , Doughty, P. , & Blows, M. W. (2005). Divergent selection and the evolution of signal traits and mating preferences. Plos Biology, 3, 1988–1995. 10.1371/journal.pbio.0030368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarit, F. , & Ferveur, J. F. (2002). Temperature affects the ontogeny of sexually dimorphic cuticular hydrocarbons in Drosophila melanogaster . Journal of Experimental Biology, 205, 3241–3249. [DOI] [PubMed] [Google Scholar]
- Schaner, A. M. , Bartelt, R. J. , & Jackson, L. L. (1987). (Z)‐11‐octadecenyl acetate, an aggregation pheromone in Drosophila simulans . Journal of Chemical Ecology, 13, 1777–1786. 10.1007/BF00980218 [DOI] [PubMed] [Google Scholar]
- Shirangi, T. R. , Dufour, H. D. , Williams, T. M. , & Carroll, S. B. (2009). Rapid evolution of sex pheromone‐producing enzyme expression in Drosophila . PLoS Biology, 7, e1000168 10.1371/journal.pbio.1000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger, S. , Ower, G. D. , Stökl, J. , Mitchell, C. , Hunt, J. , & Sakaluk, S. K. (2013). Sexual selection on cuticular hydrocarbons of male sagebrush crickets in the wild. Proceedings of the Royal Society B: Biological Sciences, 280, 20132353 10.1098/rspb.2013.2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennett, M. D. , & Etges, W. J. (1997). Premating isolation Is determined by larval rearing substrates in cactophilic Drosophila mojavensis. III. Epicuticular hydrocarbon variation is determined by use of different host plants in Drosophila mojavensis and Drosophila arizonae . Journal of Chemical Ecology, 23, 2803–2824. [Google Scholar]
- Stojkovic, B. , Savkovic, U. , Dordevic, M. , & Tucic, N. (2014). Host‐shift effects on mating behavior and incipient pre‐mating isolation in seed beetle. Behavioral Ecology, 25, 553–564. 10.1093/beheco/aru015 [DOI] [Google Scholar]
- Thevenot, E. A. , Roux, A. , Xu, Y. , Ezan, E. , & Junot, C. (2015). Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. Journal of Proteome Research, 14, 3322–3335. 10.1021/acs.jproteome.5b00354 [DOI] [PubMed] [Google Scholar]
- Thomas, M. L. , & Simmons, L. W. (2009). Sexual selection on cuticular hydrocarbons in the Australian field cricket, Teleogryllus oceanicus . BMC Evolutionary Biology, 9, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolson, E. C. , & Kupersimbron, R. (1989). Laboratory evolution of epicuticular hydrocarbon composition and cuticular permeability in Drosophila pseudoobscura – Effects on sexual dimorphism and thermal‐acclimation ability. Evolution, 43, 468–473. [DOI] [PubMed] [Google Scholar]
- Van der Auwera, G. A. , Carneiro, M. O. , Hartl, C. , Poplin, R. , Del Angel, G. , Levy-Moonshine, A. , … Banks, E. (2013). From FastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Current Protocols in Bioinformatics, 43(1), 11–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oystaeyen, A. , Oliveira, R. C. , Holman, L. , van Zweden, J. S. , Romero, C. , Oi, C. A. , … Wenseleers, T. (2014). Conserved class of queen pheromones stops social insect workers from reproducing. Science, 343, 287–290. 10.1126/science.1244899 [DOI] [PubMed] [Google Scholar]
- Wang, L. M. , & Anderson, D. J. (2010). Identification of an aggression‐promoting pheromone and its receptor neurons in Drosophila . Nature, 463, 227–231. 10.1038/nature08678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, B. S. , & Cockerham, C. C. (1984). Estimating F‐Statistics for the analysis of population structure. Evolution, 38, 1358–1370. [DOI] [PubMed] [Google Scholar]
- Wellenreuther, M. , Rosenquist, H. , Jaksons, P. , & Larson, W. (2017). Local adaptation along an environmental cline in a species with an inversion polymorphism. Journal of Evolutionary Biology, 30, 1068–1077. 10.1111/jeb.13064 [DOI] [PubMed] [Google Scholar]
- Wigglesworth, V. (1945). Transpiration through the cuticle of insects. Journal of Experimental Biology, 21, 97–114. [Google Scholar]
- Wu, T. D. , & Watanabe, C. K. (2005). GMAP: A genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics, 21, 1859–1875. 10.1093/bioinformatics/bti310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available on Dryad (https://doi.org/10.5061/dryad.943mp7) including
The raw GC‐MS output from all of the samples
Fasta file of the genomic regions containing the candidate genes
The transcriptome.


