Abstract
During decidualization, endometrial stromal cells differentiate into a secretory phenotype to modulate the uterine microenvironment and promote embryo implantation. This highly metabolic process relies on ovarian steroid receptors and glucose transporters. Canonical Notch signaling is mediated by the transcription factor Recombination Signal Binding Protein for Immunoglobulin Kappa J Region (RBPJ). Loss of RBPJ in the mouse uterus (Pgrcre/+Rbpjflox/flox; Rbpj c-KO) results in subfertility in part due to an abnormal uterine–embryonic axis during implantation and, as described herein, decidualization failure. Induced in vivo decidualization in Rbpj c-KO mice was impaired with the downregulation of decidual markers and decreased progesterone receptor (Pgr) signaling. Consistent with in vivo mouse data, RBPJ knockdown during in vitro Human uterine fibroblast (HuF) cell decidualization results in the reduced expression of decidual marker genes along with PGR. Expression of the glucose transporter, SLC2A1, was decreased in the RBPJ-silenced HuF cells, which corresponded to decreased Slc2a1 in the secondary decidual zone of Rbpj c-KO mouse uteri. Exogenous administration of pyruvate, which bypasses the need for glucose, rescues PRL expression in RBPJ-deficient HuF cells. In summary, Notch signaling through RBPJ controls both ovarian steroid receptor PGR and glucose transporter SLC2A1 expression during decidualization, and this dysregulation likely contributes to embryo implantation failure.
Keywords: decidualization, RBPJ, notch, progesterone receptor, glucose transport
Introduction
Receptivity of the uterus to an active blastocyst is initiated through both hormonal regulation by the corpus luteum and embryonic signals, which favor embryo attachment and trophoblast invasion.1–3 The process of invasion and embryonic cues trigger transformation of endometrial stromal fibroblasts into secretory, epithelioid-like decidual cells, termed decidualization.4–6 Decidualized stromal cells migrate toward and encapsulate the implanting embryo, where they simultaneously support and control trophoblast invasion.7–10 Clinical consequences of decidualization defects include recurrent pregnancy loss, preeclampsia, preterm labor, and intrauterine growth restriction.11,12 Implantation defects contribute to ∼75% of failed human pregnancies, suggesting a need to identify mechanisms regulating the early events, including decidualization, during the establishment of a successful pregnancy.13
The evolutionarily conserved Notch signaling pathway mediates key developmental and homeostatic functions, including cellular differentiation, survival, and apoptosis.14,15 Notch signaling consists of 4 heterodimeric transmembrane receptors (NOTCH1-4) whose 5 ligands (Delta-like1, 3, 4 and Jagged1, 2) are expressed on the cell membrane of adjacent cells, thereby initiating receptor–ligand interactions through a juxtacrine mechanism. Upon ligand binding, activated Notch receptors undergo an ADAM protease and gamma-secretase-mediated cleavage cascade that results in the intracellular release of the Notch intracellular domain (NICD), which is the active form of Notch.16 In canonical Notch signaling, the NICD translocates to the nucleus and binds to the Notch family transcription factor recombination signal binding protein-Jκ (RBPJ), converting it from a basal transcriptional repressor to an activator of downstream target genes, such as the “Hairy enhancer of split” (HES) and Hes-related (HEY) transcription factor families.17–20
During the implantation window, NOTCH1 expression is induced in endometrial stromal cells of the nonhuman primate and in women in response to the embryonic signal Chorionic Gonadotropin (CG), which promotes uterine receptivity and decidualization.1,21–24 We have shown that Notch signaling, specifically NOTCH1, is indispensable for the initiation of decidualization in both mice and women.23,25 Further, Notch receptors, ligands, and target genes are all reduced in eutopic endometrium of baboons and women with endometriosis, contributing to their disease-specific decidualization failure.26 In mice, during implantation, Notch1 is initially induced in the stromal cells of the primary decidual zone and then subsequently increases in the secondary decidual zone.25 Ablation of Notch1 in the mouse uterus using a progesterone receptor (Pgr)-driven cre-expressing (Pgrcre/+Notch1f/f; Notch1 c-KO) drastically inhibited their response to an in vivo artificial decidualization stimulus.25 Decidualization failure in Notch1 c-KO mice was associated with the downregulation of genes crucial for progesterone-induced stromal cell differentiation and pathways related to cell proliferation, cell cycle progression, and estrogen receptor α (Esr1) signaling.25 Notch1 c-KO mice produce reduced litter sizes in the first litter but, otherwise, exhibited normal fecundity,25 suggesting compensation by other Notch receptors may have rescued fertility in these mice, since other Notch receptors have been implicated in decidualization.27 Disruption of all potential Notch receptor signaling through ablation of the shared transcription factor, Rbpj, in the mouse uterus (Pgrcre/+Rbpjf/f; Rbpj c-KO) resulted in subfertility due to an abnormal embryonic–uterine axis and impaired decidual remodeling.28 More recently, we identified secondary infertility in Rbpj c-KO mice that results from impaired postpartum uterine repair and enhanced inflammatory signaling.29 While secondary infertility was also associated with impaired in vivo artificial decidualization response, it was unclear whether decidualization defects occur in nulliparous Rbpj c-KO mice contributing to their subfertility. In the current study, we sought to determine the role that RBPJ plays in decidual function beyond the initial implantation phase and its relevance in the setting of human decidualization.
Growing evidence supports the importance of endometrial glucose transporters during decidualization, and their dysregulation results in infertility.30–34 Facilitative glucose transporters (known as the GLUT or SLC2 family) regulate cellular glucose uptake and have distinct tissue-specific and temporal expression patterns.35 SLC2A1 (GLUT1) is ubiquitously expressed in the uteri of both women and rodents.30 Additionally, SLC2A1 is further induced during early gestation and decidualization of mouse and human endometrial stromal cells.32,36 Progesterone administration increases SLC2A1 expression in stromal cells in both women and mice.31–33 Further, Rbpj mediates SLC2A1 expression in memory CD4+ T cells, which is essential for theirsurvival in the mouse,37 suggesting that this mechanism may be important for decidualization.
We hypothesized that Notch signaling through RBPJ mediates differentiation of endometrial stromal cells during the establishment of pregnancy. In the current study, we induced in vivo artificial decidualization in Rbpj c-KO and control mice (Pgr+/+Rbpjf/f). Rbpj c-KO mice displayed reduced decidualization associated with altered Pgr signaling and downregulation of glucose transporter Slc2a1 during decidualization. Our mouse studies were performed in parallel with in vitro decidualization of human uterine fibroblast (HuF) cells after siRNA silencing of RBPJ. Similar to Rbpj c-KO mice, the decidualization response was impaired when RBPJ was silenced and was also associated with reduced Pgr and SLC2A1 expression. Finally, supplementation of pyruvate, bypassing the necessity for glucose, restored the decidualization response in RBPJ-silenced HuF cells. Through these translational studies, we have determined that RBPJ is essential for decidualization in both the mouse and the human, in part, through regulation of progesterone signaling and glucose transporter expression.
Materials and Methods
Mouse Models
All studies performed using animals were approved by the Institutional Animal Care and Use Committee of Michigan State University, East Lansing, Michigan. To avoid the embryonic lethality of a complete Rbpj knockout,38 we generated a uterine-specific, conditional Rbpj knockout mouse. Rbpjf/f mice contain LoxP sites flanking the sixth and seventh exons, corresponding to the DNA binding domain.39 We crossed Pgrcre/+ mice, containing a knock-in of Cre recombinase in 1 copy of the Pgr gene while maintaining normal fecundity and Pgr function,40,41 with Rbpjf/f (control) mice to generate Pgrcre/+Rbpjf/f (Rbpj c-KO) mice, in order to ablate Rbpj in Pgr-positive tissues as described previously.28,29
In Vivo Artificial Decidualization Model
Induction of artificial decidualization was performed on 6-week-old Rbpj c-KO and control mice as described previously.25 Mice were ovariectomized and then primed to mimic the hormonal milieu of early pregnancy with daily E2 (100 ng; Sigma-Aldrich, St. Louis, Missouri) for 3 days followed by 2 days of rest and then daily P4 (1 mg; Sigma-Aldrich) plus E2 (6.7 ng). Six hours following the third E2 plus P4 injection, a mechanical scratch of the antimesometrial luminal epithelium of the left uterine horn (S; stimulated) was performed using a blunted needle to induce decidualization. The unscratched right horn served as the hormonal control (US; unstimulated). Subsequently, daily injections of E2 and P4 were provided with sacrifice 3 or 5 days post-scratch (n = 3-5 mice/group). Uterine tissues were collected, weighed, and either snap frozen in liquid nitrogen or fixed in 4% paraformaldehyde for paraffin embedding. Decidual response was calculated based on the ratio of uterine decidualized to control horn weight.
Immunohistochemistry
Paraformaldehyde 4% fixed paraffin-embedded tissues were sectioned to 6-μm thickness and placed onto microscope slides (Thermo Fisher Scientific, Waltham, Massachusetts). For immunohistochemistry, each tissue section was dewaxed, rehydrated with a graded alcohol series, followed by heat-mediated antigen retrieval in citrate buffer (Antigen unmasking solution; Vector Laboratories, Burlingame, California) and 10% hydrogen peroxide treatment. Sections were blocked for 1 hour in 10% Normal Horse Serum (Vector Laboratories) in phosphate-buffered saline (PBS) incubated overnight at 4°C in one of the following primary antibodies: rabbit anti-PGR (1:500; DAKO, Carpinteria, California), mouse anti-SLC2A1 (1:100; Santa Cruz Biotechnology, Dallas, Texas). Next, sections were incubated in respective biotinylated secondary antibodies for antimouse IgG or anti-rabbit IgG (Vector Laboratories) followed by HRP-conjugated streptavidin. Detection for immunoreactivity was achieved using the DAB Substrate Kit (Vector Laboratories) followed by hematoxylin counterstaining. Staining intensity of each section was quantified by image analysis software ImageJ (NIH) resulting in a Digital HSCORE (D-HSCORE) of staining intensity as previously reported.42
Isolation and Culture of Human Uterine Fibroblast Cells
Human uterine fibroblast cells were isolated from the decidua parietalis from term placentas.23,43 Tissues were procured after patient informed consent was obtained under a protocol approved by the Institutional Review Board at Michigan State University. Corresponding clinical information for each cell line used in this study are summarized in Supplemental Table S1. After trypsinization, cells were propagated in phenol-red-free RPMI 1640 (Thermo Fisher Scientific) supplemented with 10% Charcoal: Dextran Stripped-Fetal Bovine Serum (CDS-FBS). Each cell line obtained from a single placenta is considered a single sample for statistical analysis; therefore, at least 3 HuF cell lines plated in triplicate were used for each experiment. Further, cell lines beyond 6 passages were not used for experiments due to the potential for diminished decidualization response.
Transfection of RBPJ Small-Interfering RNA in HuF cells
Human uterine fibroblast cells were seeded in 6-well dishes at 1.5 × 105 cells per well in 10% CDS-FBS in RPMI. Once cells reached ∼30% to 50% confluence, they were washed with sterile PBS then acclimated to serum and antibiotic-free Opti-MEM media (Thermo Fisher Scientific) for at least 30 minutes prior to transfection. Subsequently, nontargeting (Control; ON-TARGETplus Non-targeting pool; GE Dharmacon, Lafayette, CO) and RBPJ small-interfering RNA (siRNAs; SMARTpool ON-TARGETplus RBPJ siRNA; GE Dharmacon, Lafayette, Colorado) were dissolved in Lipofectamine RNAiMAX (Thermo Fisher Scientific) diluted in Opti-MEM. This mixture was added to the cells to achieve a final concentration of 40 nmol/L of either control or RBPJ siRNA, and cells were incubated with the siRNA mixture for 5 hours. Finally, cells were washed with PBS and then incubated overnight in fresh RPMI with 10% CDS-FBS and antibiotics prior to any future experiments.
In Vitro Decidualization of HuF Cells
In vitro decidualization of HuF cells was performed through administration of an EPC cocktail (a combination of 36 nmol/L estradiol-17β, 1 μmol/L medroxyprogesterone acetate [MPA] and 0.5 mmol/L di-butyryl-cyclic adenosine monophosphate [dbcAMP; dbcAMP; Sigma]) in 2% CDS-FBS in RPMI.23,43,44 Human uterine fibroblast cells were treated with EPC or vehicle (Veh) for 5 days with replacement of treatment and media every 2 days. For in vitro decidualization studies with glucose and pyruvate supplementation, 8 mmol/L d-Glucose or 16 mmol/L Sodium Pyruvate (Sigma) in RPMI, respectively, were used for dilution of EPC or vehicle. Glucose and pyruvate concentrations were chosen based on dose–response experiments using supra-physiologic concentrations.
RNA Isolation and Real Time-Quantitative PCR (RT-qPCR)
Total RNA was isolated from snap-frozen mouse tissues or cultured cells using TRIzol reagent (Invitrogen, Carlsbad, California) followed by reverse transcription to produce cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, California). Differential gene expression was determined using RT-qPCR performed with either TaqMan Universal Master Mix II (Applied Biosystems) or SYBR Green PCR Master Mix (Applied Biosystems) using the ViiA 7 qPCR System (Applied Biosystems). RPL17 and Rpl19 were used for normalization of cDNA from human and mouse samples, respectively. Primer sequences used for RT-qPCR are provided (Supplemental Table S2).
Protein Isolation and Western Blot Analysis
Total protein was isolated from HuF cells using RIPA lysis buffer, and the concentrations were calculated using the bicinchoninic acid (BCA) method (Thermo Fisher Scientific). Eight micrograms of total protein were separated on SDS/PAGE gels (Invitrogen) and then transferred to a PVDF membrane (Millipore, Burlington, Massachusetts). The membranes were then blocked and incubated overnight with primary antibodies: rabbit anti-PGR (1:2000; Thermo Fisher Scientific), mouse anti-SLC2A1 (1:1000; Santa Cruz Biotechnology), and rabbit anti-β-ACTIN (1:10,000; Sigma-Aldrich). After incubation with respective anti-rabbit IgG or anti-mouse IgG secondary antibodies labeled with HRP (Vector Laboratories, Burlingame, CA), immune complexes were visualized using enhanced chemiluminescence (GE Life Sciences, Pittsburgh, PA).
Statistical Analysis
Variation between groups was determined using 2-way analysis of variance with repeated measures followed by an Holm-Sidak post hoc test to determine group-specific differences. Statistical significance was defined as P < .05. All statistical analyses were performed by GraphPad Prism 6.0 (GraphPad Software, San Diego, California).
Results
Recombination Signal Binding Protein-Jκ is Essential for Decidualization in the Mouse and Drives Progesterone Receptor Signaling
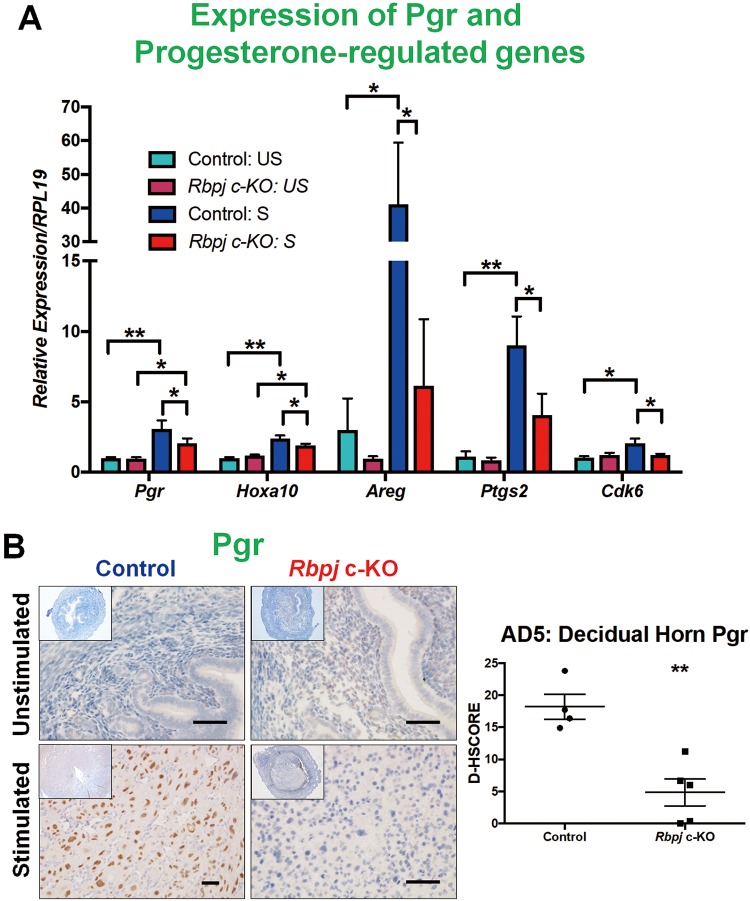
To induce in vivo decidualization, Rbpj c-KO and control mice were ovariectomized, hormonally primed to mimic early gestation and induced to decidualize via a mechanical scratch with collection as described previously.25,29 Decidualization was significantly reduced in Rbpj c-KO mice on both day 3 and day 5 of artificial decidualization (AD) based on stimulated (S) to US uterine horn weight ratio (Figure 1A). We chose to focus our studies on AD5 because this time point corresponded to day 9.5 postconception in natural mouse gestation, when initial embryo loss begins in Rbpj c-KO mice potentially contributing to their subfertility.28 Impaired decidualization in Rbpj c-KO mice on AD5 was associated with reduced uterine mRNA expression of decidual markers Bmp2 and Wnt4 (Figure 1B). Orchestrated ovarian steroid hormone receptor expression is necessary for promoting stromal survival and, ultimately, mediating the epithelial to stromal proliferation shift that occurs during implantation and decidualization.45 Thus, expression patterns of Pgr and downstream signaling effectors were investigated in the uteri of artificially decidualized mice (Figure 2). Pgr mRNA levels were significantly decreased in the stimulated uterine horns of Rbpj c-KO mice compared control mice (Figure 2A). Consistent with this finding, decreased levels of Pgr downstream effectors Hoxa10 and its target Cdk6, Areg, and Ptgs2 were decreased in the stimulated horn of Rbpj c-KO mice compared to the stimulated horn of control mice (Figure 2A). Cdk6 plays an important role in mediating the Hoxa10-induced differentiation of stromal cells toward the decidual phenotype.46 Immunostaining revealed reduced levels of Pgr protein in the stimulated horn of the Rbpj c-KO mouse (Figure 2B). The Notch pathway regulates cell survival and differentiation,14,15 and failed initiation of stromal cell differentiation for decidualization was a consequence of reduced Pgr signaling in the absence of Notch signaling transcriptional factor Rbpj.
Figure 1.
Decreased in vivo artificial decidualization response in Rbpj c-KO mice. Control and Rbpj c-KO mice (n = 3-5/group) were ovariectomized, hormonally primed and then mechanically induced to decidualize. (A) Decreased decidual response in Rbpj c-KO mice was present both 3 and 5 days following mechanical decidualization based on stimulated to unstimulated uterine horn weight. (B) Impaired decidualization was associated with reduced mRNA expression of decidual marker genes, Bmp2 and Wnt4, in Rbpj c-KO mice on AD5. Data represented as mean ± standard error of the mean (SEM). **P < .01; scale bar = 0.5 cm.
Figure 2.
Downregulation of Pgr signaling in Rbpj c-KO mice during in vivo artificial decidualization. Ovarian steroid hormone receptor levels were investigated in Rbpj c-KO and control mice by RT-qPCR and immunohistochemistry. (A) Pgr mRNA was significantly increased in the decidual horn of control mice compared to the unstimulated horn, which was not the case in Rbpj c-KO mice (n = 3-5/group). Additionally, Pgr target genes, Hoxa10, Areg, Ptgs2 and Cdk6 were significantly decreased in the decidual horn of Rbpj c-KO mice compared to control mice. (B) Reduced Pgr expression was localized to the decidua of Rbpj c-KO mice. Data represented as Mean ± standard error of the mean (SEM). Comparisons were made using Two-Way ANOVA. *P < .05, **P < .01; scale bar = 50 μm. US indicates unstimulated, non-decidualized uterine horn, S, stimulated, decidualized uterine horn.
Endometrial Loss of Rbpj Reduces Slc2a1 Induction During Decidualization
Glucose metabolism plays an important role in rapidly proliferating and differentiating cells, and there is mounting evidence that supports a role for endometrial glucose transporters during decidualization and embryo implantation.46 We examined the mRNA levels of glucose transporter Slc2a1 on AD5, which has been described extensively in the setting of natural pregnancy and in vitro decidualization of mouse endometrial stromal cells.32,47 However, to our knowledge, its expression has not been investigated in the setting of in vivo artificial decidualization. Slc2a1 was up regulated in the stimulated horns of Rbpj c-KO and control mice (Figure 3A). There was no statistically significant difference between stimulated horn mRNA levels of Slc2a1, although there was a trend toward decreased expression in Rbpj c-KO mice. Since glucose transporters are often transiently induced in a temporospatial manner, we performed immunostaining to determine both expression and localization of SLC2A1 in the mouse decidua on AD5. Consistent with the trend of decreased Slc2a1 mRNA, Slc2a1 protein levels were reduced in the decidual horn of Rbpj c-KO mice compared to control mice (Figure 3B). Interestingly, Slc2a1 staining in control mice more specifically localized within the secondary decidual zone.
Figure 3.
Reduced glucose transporter, Slc2a1, expression is associated with Rbpj loss during in vivo artificial decidualization. Glucose transporter mRNA and protein levels were investigated by RT-qPCR and immunohistochemistry, respectively, during artificial decidualization. (A) Slc2a1 was significantly induced in the decidual horns of the control and Rbpj c-KO mice compared to control horns. Slc2a1 mRNA expression was reduced in the control horn of Rbpj c-KO mice compared to control mice (n = 3-5/group). (B) Slc2a1 protein localization was significantly reduced in the decidual horn of Rbpj c-KO mice, particularly in the secondary decidual zone (n = 4-5/group). Data represented as Mean ± standard error of the mean (SEM). Comparisons were made using Two-Way ANOVA for more than 2 groups or using a Student t-test for 2 groups. *P < .05, **P < .01; Scale Bar = 100 μm; US indicates unstimulated, non-decidualized uterine horn; S, stimulated, decidualized uterine horn; SDZ, secondary decidual zone; PDZ, primary decidual zone.
Recombination Signal Binding Protein-Jκ is Indispensable for Progesterone Receptor and Glucose Transporter Expression During Decidualization in HuF Cells
Human uterine fibroblast cells represent a proliferating population of undifferentiated stromal fibroblasts, which can be decidualized in vitro.23,43,44 To determine the role of RBPJ in human decidualization and the translational relevance of our work in the Rbpj-ablated mouse, HuF cells were decidualized for 5 days in vitro with a well-described hormone cocktail consisting of estradiol, medroxyprogesterone acetate (MPA), and db-cAMP (EPC) following siRNA-mediated knockdown of RBPJ.23 We confirmed that RBPJ expression was reduced in siRNA silenced HuF cells (Supplemental Figure S1A). Expression of human decidual markers PRL and IGFBP1 were significantly reduced following RBPJ knockdown (Figure 4A). Similar to the Rbpj c-KO mouse, impaired decidualization was coupled with decreased PGR expression (Figure 4B). We investigated mRNA levels of glucose transporter SLC2A1, which was reduced in Rbpj c-KO mice. As expected, RBPJ siRNA knockdown in decidualized HuF cells resulted in decreased mRNA expression of SLC2A1 compared to control siRNA treatment (Figure 4C). Subsequently, we confirmed that gene expression levels correlated with protein levels by Western blot analysis. PGR A and B along with SLC2A1 protein levels were significantly reduced with loss of RBPJ during decidualization in HuF cells (Figure 4D). Altogether, our findings indicate that similar to the mouse, RBPJ expression coordinates both PGR and glucose transporter expression in human endometrial fibroblasts, which are essential for decidualization.
Figure 4.
Loss of RBPJ in HuF cells phenocopies the mouse during decidualization. Human uterine fibroblast cells were transfected with either control or RBPJ siRNA and subsequently treated with either vehicle or EPC [estradiol, medroxyprogesterone (MPA), and di-butyryl-cyclic adenosine monophosphate (dbcAMP)] to induce in vitro decidualization. Decidual markers, ovarian steroid hormone receptors and glucose transport molecules were investigated by RT-qPCR. (A) In response to EPC, mRNA expression of human decidual markers PRL and IGFBP1 were significantly reduced with RBPJ siRNA knockdown compared to control siRNA treated cells. (B) Similar to the Rbpj c-KO mouse, Pgr expression was significantly reduced with loss of RBPJ during decidualization. (C) Glucose transporter, SLC2A1, mRNA expression was reduced in EPC-treated HuF cells with RBPJ knockdown. However, pyruvate transporter SLC16A1 was unaffected by RBPJ loss with EPC treatment. (D) PGR A and B, along with SLC2A1, protein levels are significantly reduced with RBPJ siRNA knockdown during decidualization in HuF cells. Data represented as Mean ± SEM (n = 3-4 HuF cell lines performed in triplicates); comparisons were made using Two-Way ANOVA. *P < 0.05.
Pyruvate Supplementation Rescues Decidualization Failure With RBPJ Loss in HuF Cells
Both in Rbpj c-KO mouse endometrium and isolated HuF cells where RBPJ was suppressed, decidualization was dramatically altered and was associated with decreased expression of glucose transporter SLC2A1. Therefore, we hypothesized that supplementation of additional glucose or pyruvate, which bypasses the necessity for glucose, might restore decidualization associated with loss of RBPJ in HuF cells. First, we confirmed that pyruvate transporter, SLC16A1, expression was unaffected by RBPJ loss (Figure 4C). Next, we supplemented the control and RBPJ knockdown HuF cells with additional glucose or pyruvate during in vitro decidualization of HuF cells. Dosages of glucose and pyruvate (8 mmol/L and 16 mmol/L, respectively) selected for this experiment were determined based on physiological dose–response experiments conducted previously in our laboratory and were ultimately chosen based on their ability to favor decidualization without negatively affecting cellular osmotic pressure. Remarkably, administration of pyruvate but not glucose restored expression of decidual marker PRL in RBPJ knockdown HuF cells during decidualization (Figure 5A). Additionally, Pgr mRNA levels were also restored through supplementation of glucose during decidualization with loss of RBPJ in HuF cells (Figure 5B).
Figure 5.
Pyruvate supplementation rescues decidual marker gene and PGR expression in the setting of RBPJ loss. Human uterine fibroblast cells were transfected with either control or RBPJ siRNA and subsequently treated with either vehicle or EPC [estradiol, medroxyprogesterone (MPA), and di-butyryl-cyclic adenosine monophosphate (dbcAMP)] to induce in vitro decidualization with cell culture media supplementation of 8 mM Glucose, 16 mM Pyruvate, or neither. (A) Administration of pyruvate but not glucose restored expression of decidual marker PRL in EPC-treated RBPJ knockdown HuF cells. (B) Pyruvate supplementation increased PGR expression in decidualized RBPJ knockdown HuF cells compared to no glucose or glucose treatment. Data represented as Mean ± standard error of the mean (SEM) (n = 3-4 HuF cell lines performed in triplicates). The effect of pyruvate in increasing PRL and PGR levels in RBPJ knockdown cells during decidualization has been highlighted with bars. Comparisons were made using Two-Way ANOVA. Significantly changed mRNA levels between two groups are represented by matching letters, where P < .05.
Discussion
Notch is an arbiter of cell fate, and, based on our previous work, its expression regulates endometrial transformation during embryo implantation and more specifically, decidualization.23,26 In the current study, we demonstrate that ablation of RBPJ in the mouse uterus and in HuF cells reduces their capacity to differentiate toward the decidual phenotype. Contributors to impaired decidualization in both models included dysregulated Pgr expression and reduced glucose transporter Slc2a1 expression. Additionally, supplemental pyruvate partially restores the expression of decidual markers in HuF Cells in which RBPJ was silenced during decidualization. Our findings support a previously undescribed contributor to subfertility in Rbpj c-KO mice, where implantation failure was shown to result from abnormal embryonic–uterine axis.28 However, in the current study, we determined that Rbpj c-KO mice display a reduced in vivo response to artificial decidualization based on decidual horn wet weight and differentiation marker expression, which is independent of the presence of an embryo. Additionally, we determined a translational role for RBPJ in women, using an in vitro decidualization HuF cell model.
Similar to Notch1, Rbpj is dynamically expressed in a temporospatial manner in the mouse uterus during the establishment of pregnancy. Embryonic signals induce NOTCH1 expression, which include CG in primates or physical contact in mice.23–25 During implantation in mice, Notch1 is initially expressed in the subepithelial stromal cells of the primary decidua, and as pregnancy progresses there is a shift of expression to the secondary decidua.25 Consistent with the mouse, NOTCH1 initially increases in decidualizing HuF cells and subsequently becomes downregulated at the completion of decidualization.23 Expression of Notch1 is tightly regulated, where overactivation of Notch1 signaling impairs decidualization and causes in infertility.48 Similar to Notch1, Rbpj is initially induced in the primary decidual zone in the mouse and subsequently downregulated, at which point its expression is localized within the secondary decidual zone.28 In the current study, Slc2a1 staining was concentrated within cells of the secondary decidual zone of control mice, which did not occur in the Rbpj c-KO mice. Overall, these findings suggest Slc2a1 expression is induced to a greater extent in stromal cells during the initiation of decidualization rather than upon completion of differentiation, which parallels the increase and the subsequent downregulation of Notch1 and Rbpj expression during decidualization.23,25,28
During implantation, Pgr drives stromal proliferation and promotes transformation of endometrial fibroblasts toward a secretory phenotype while suppressing epithelial Esr1.45 In part, Pgr signaling through Hoxa10 promotes differentiation of stromal cells through induction of cell cycle regulators, such as Cdk6.46,49 In the Rbpj c-KO mice, broad downregulation of the Pgr signaling pathway was evident. A limitation to this study is the potential for haploinsufficiency of the Pgr gene in Rbpj c-KO mice, which could contribute to decreased Pgr expression. However, other studies in Pgr-cre mice have shown these mice display preserved Pgr expression.40 Further, Pgr expression was decreased with RBPJ knockdown during decidualization of HuF cells, where haploinsufficency does not occur.
Based on our prior and present work, a close relationship between Pgr and Notch signaling exists. P4-targets were decreased in Notch1 c-KO mice during decidualization, including Cdk6, and Pgr mediates cleavage of Notch1 to its active form.23,25 Further, overactivation of Notch signaling indirectly suppresses Pgr expression via recruitment of DNA methyltransferases and hypermethylation of its promoter in an Rbpj-dependent manner.48 Since Rbpj basally suppresses Notch target expression, its loss can mimic either downregulation or upregulation of Notch signaling.50 Therefore, RBPJ mediates Notch receptor-independent functions, which may include regulation of Pgr signaling.
Glucose transporter SLC2A1 expression was downregulated during in vivo and in vitro decidualization in the absence of RBPJ. In women, stromal SLC2A1 expression is low in the proliferative phase and increases during the mid-secretory phase, corresponding to the period of uterine receptivity and increased progesterone levels.31 Further, inhibition of glucose transporters drastically reduced PRL secretion during in vitro decidualization of human endometrial stromal cells.31 In rat, endometrial stromal Slc2a1 expression pattern during early pregnancy mirrors that of Rbpj.28,47 Initially, Slc2a1 is induced in the stromal cells of the primary decidual zone and as implantation progresses, expression shifts to the secondary decidual zone.47 In the current study, Slc2a1 staining was concentrated in the stromal cells of the secondary decidual zone on AD5 in control mice, which was reduced in the Rbpj c-KO mouse decidual horn. We confirmed decreased Slc2a1 expression in the mouse with loss of Rbpj occurs with RBPJ knockdown during decidualization in HuF cells. Based on our findings, we hypothesize that Rbpj acts upstream of Slc2a1 and that both are early mediators of proliferation and differentiation of stromal fibroblasts into decidual cells. After completion of differentiation during decidualization, both Notch1 and Rbpj are reduced in the primary decidual zone, which may subsequently reduce Slc2a1 expression in the primary decidual zone.23,25
In CD4+ memory T cells, Rbpj mediates survival through regulation of Slc2a1 expression and loss of Rbpj could be overcome by administration of pyruvate, which bypasses the requirement for glucose.37 In the current study, we found that supplementation of pyruvate rescued expression of PGR and the decidual marker PRL associated with RBPJ knockdown in decidualizing HuF cells. Dysregulated glucose transporter expression represents an understudied contributor to infertility despite reduced levels of SLC2A1 in midsecretory biopsies of patients with idiopathic versus clear anatomical causes for infertility.31 A positive impact of pyruvate supplementation on embryo development in culture media used for in vitro fertilization patients has been previously reported.51 However, to our knowledge, the effect of supplementing pyruvate in IVF culture media during embryo transfer on implantation rates in the setting of impaired uterine receptivity has not been determined. We have shown that impaired decidualization can be restored with supplementation of low levels of pyruvate, through potentially bypassing the necessity for glucose. Future studies are indicated to determine whether endometrial receptivity can be restored in the setting of infertility through supplementation of pyruvate.
Supplemental Material
Supplemental_figures_and_tables for The Notch Family Transcription Factor, RBPJκ, Modulates Glucose Transporter and Ovarian Steroid Hormone Receptor Expression During Decidualization by Michael R Strug, Ren-Wei Su, Tae Hoon Kim, Jae-Wook Jeong and Asgerally Fazleabas in Reproductive Sciences
Acknowledgments
The authors thank Dr. Francesco DeMayo (National Institute of Environmental Health Sciences) and Dr. John Lydon (Baylor College of Medicine) for kindly providing the Pgrcre/+ mice and Dr. Nadia Carlesso (City of Hope, Duarte, CA) and Dr.Tasuku Honjo (University of Kyoto, Japan) for kindly providing the Rbpjflox/flox mice. The authors also thank Ms. Samantha Bond, Ms. Sharra Poncil, Ms. Ariadna Ochoa, and Mr. Mark Olson for their excellent technical assistance. Funding for this study was provided by NICHD R01 HD042280 (ATF) and NICHD F30 HD082951 (MS).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NICHD R01 HD042280 (ATF), NICHD F30 HD082951 (MS).
Supplemental Material: Supplemental material is available for this article online.
References
- 1. Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci U S A. 1999;96(5):2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fazleabas AT, Kim JJ, Strakova Z. Implantation: embryonic signals and the modulation of the uterine environment--a review. Placenta. 2004; 25(suppl A):S26–S31. [DOI] [PubMed] [Google Scholar]
- 3. Banerjee P, Fazleabas AT. Endometrial responses to embryonic signals in the primate. Int J Dev Biol. 2010;54(2-3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finn CA. The biology of decidual cells. Adv Reprod Physiol. 1971;5:1–26. [PubMed] [Google Scholar]
- 5. Jayatilak PG, Glaser LA, Warshaw ML, Herz Z, Gruber JR, Gibori G. Relationship between luteinizing hormone and decidual luteotropin in the maintenance of luteal steroidogenesis. Biol Reprod. 1984;31(3):556–564. [DOI] [PubMed] [Google Scholar]
- 6. Lala PK, Graham CH. Mechanisms of trophoblast invasiveness and their control: the role of proteases and protease inhibitors. Cancer Metastasis Rev. 1990;9(4):369–379. [DOI] [PubMed] [Google Scholar]
- 7. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. [DOI] [PubMed] [Google Scholar]
- 8. Weimar CH, Macklon NS, Post Uiterweer ED, Brosens JJ, Gellersen B. The motile and invasive capacity of human endometrial stromal cells: implications for normal and impaired reproductive function. Hum Reprod Update. 2013;19(5):542–557. [DOI] [PubMed] [Google Scholar]
- 9. Anacker J, Segerer SE, Hagemann C, et al. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol Hum Reprod. 2011;17(10):637–652. [DOI] [PubMed] [Google Scholar]
- 10. Grewal S, Carver J, Ridley AJ, Mardon HJ. Human endometrial stromal cell rho GTPases have opposing roles in regulating focal adhesion turnover and embryo invasion in vitro. Biol Reprod. 2010;83(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plaks V, Rinkenberger J, Dai J, et al. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci U S A. 2013;110(27):11109–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salker M, Teklenburg G, Molokhia M, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5(4):e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. [DOI] [PubMed] [Google Scholar]
- 15. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. [DOI] [PubMed] [Google Scholar]
- 16. D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194(3):237–255. [DOI] [PubMed] [Google Scholar]
- 19. Tamura K, Taniguchi Y, Minoguchi S, et al. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-J kappa/Su(H). Curr Biol. 1995;5(12):1416–1423. [DOI] [PubMed] [Google Scholar]
- 20. Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res. 1999;9(3):179–188. [DOI] [PubMed] [Google Scholar]
- 21. Kim JJ, Jaffe RC, Fazleabas AT. Insulin-like growth factor binding protein-1 expression in baboon endometrial stromal cells: regulation by filamentous actin and requirement for de novo protein synthesis. Endocrinology. 1999;140(2):997–1004. [DOI] [PubMed] [Google Scholar]
- 22. Jasinska A, Strakova Z, Szmidt M, Fazleabas AT. Human chorionic gonadotropin and decidualization in vitro inhibits cytochalasin-D-induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology. 2006;147(9):4112–4121. [DOI] [PubMed] [Google Scholar]
- 23. Afshar Y, Miele L, Fazleabas AT. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology. 2012;153(6):2884–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strug MR, Su R, Young JE, et al. Intrauterine human chorionic gonadotropin infusion in oocyte donors promotes endometrial synchrony and induction of early decidual markers for stromal survival: a randomized clinical trial. Hum Reprod. 2016;31(7):1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Afshar Y, Jeong JW, Roqueiro D, et al. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J. 2012;26(1):282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su RW, Strug MR, Joshi NR, et al. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab. 2015;100(3):E433–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Otti GR, Saleh L, Velicky P, Fiala C, Pollheimer J, Knofler M. Notch2 controls prolactin and insulin-like growth factor binding protein-1 expression in decidualizing human stromal cells of early pregnancy. PLoS One. 2014;9(11):e112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang S, Kong S, Wang B, et al. Uterine Rbpj is required for embryonic-uterine orientation and decidual remodeling via Notch pathway-independent and -dependent mechanisms. Cell Res. 2014;24(8):925–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strug MR, Su RW, Kim TH, et al. RBPJ mediates uterine repair in the mouse and is reduced in women with recurrent pregnancy loss. FASEB J. 2018;32(5):2452–2466. fj201701032 R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frolova AI, Moley KH. Glucose transporters in the uterus: an analysis of tissue distribution and proposed physiological roles. Reproduction. 2011;142(2):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Wolff M, Ursel S, Hahn U, Steldinger R, Strowitzki T. Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2003;88(8):3885–3892. [DOI] [PubMed] [Google Scholar]
- 32. Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology. 2009;150(3):1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim ST, Moley KH. Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium. Biol Reprod. 2009;81(1):188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frolova AI, Moley KH. Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology. 2011;152(5):2123–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Augustin R. The protein family of glucose transport facilitators: it’s not only about glucose after all. IUBMB Life. 2010;62(5):315–333. [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi M, Sakata M, Ogura K, Miyake A. Gestational changes of glucose transporter gene expression in the mouse placenta and decidua. J Endocrinol Invest. 1996;19(8):567–569. [DOI] [PubMed] [Google Scholar]
- 37. Maekawa Y, Ishifune C, Tsukumo S, Hozumi K, Yagita H, Yasutomo K. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med. 2015;21(1):55–61. [DOI] [PubMed] [Google Scholar]
- 38. Oka C, Nakano T, Wakeham A, et al. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development. 1995;121(10):3291–3301. [DOI] [PubMed] [Google Scholar]
- 39. Han H, Tanigaki K, Yamamoto N, et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. International immunology. 2002;14(6):637–645. [DOI] [PubMed] [Google Scholar]
- 40. Lee K, Jeong J, Kwak I, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38(10):1204–1209. [DOI] [PubMed] [Google Scholar]
- 41. Soyal SM, Mukherjee A, Lee KY, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. [DOI] [PubMed] [Google Scholar]
- 42. Fuhrich DG, Lessey BA, Savaris RF. Comparison of HSCORE assessment of endometrial beta3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal Quant Cytopathol Histpathol. 2013;35(4):210–216. [PMC free article] [PubMed] [Google Scholar]
- 43. Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod. 1998;59(1):160–168. [DOI] [PubMed] [Google Scholar]
- 44. Strakova Z, Srisuparp S, Fazleabas AT. Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology. 2000;141(12):4664–4670. [DOI] [PubMed] [Google Scholar]
- 45. Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358(2):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rahman MA, Li M, Li P, Wang H, Dey SK, Das SK. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev Biol. 2006;290(1):105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Korgun ET, Demir R, Hammer A, et al. Glucose transporter expression in rat embryo and uterus during decidualization, implantation, and early postimplantation. Biol Reprod. 2001;65(5):1364–1370. [DOI] [PubMed] [Google Scholar]
- 48. Su RW, Strug MR, Jeong JW, Miele L, Fazleabas AT. Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc Natl Acad Sci U S A. 2016;113(8):2300–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111(1-2):99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kulic I, Robertson G, Chang L, et al. Loss of the Notch effector RBPJ promotes tumorigenesis. J Exp Med. 2015;212(1):37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Conaghan J, Handyside AH, Winston RM, Leese HJ. Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro. J Reprod Fertil. 1993;99(1):87–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_figures_and_tables for The Notch Family Transcription Factor, RBPJκ, Modulates Glucose Transporter and Ovarian Steroid Hormone Receptor Expression During Decidualization by Michael R Strug, Ren-Wei Su, Tae Hoon Kim, Jae-Wook Jeong and Asgerally Fazleabas in Reproductive Sciences