Summary
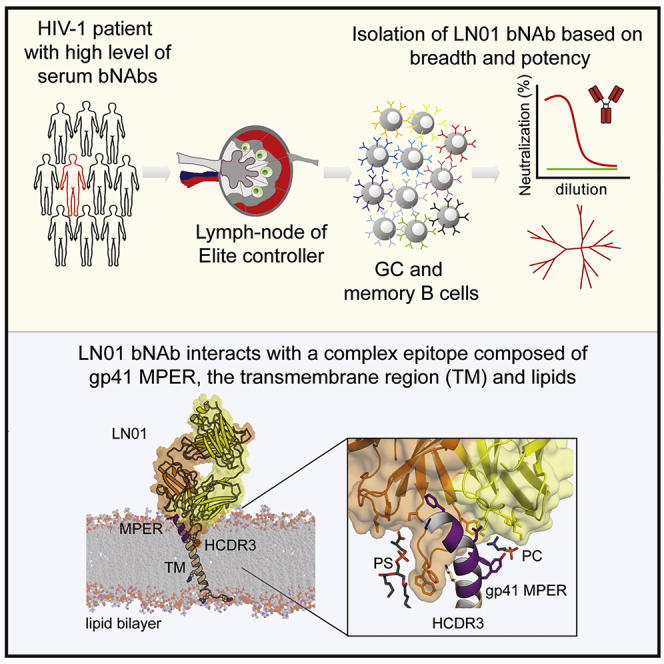
Potent and broadly neutralizing antibodies (bnAbs) are the hallmark of HIV-1 protection by vaccination. The membrane-proximal external region (MPER) of the HIV-1 gp41 fusion protein is targeted by the most broadly reactive HIV-1 neutralizing antibodies. Here, we examine the structural and molecular mechansims of neutralization by anti-MPER bnAb, LN01, which was isolated from lymph-node-derived germinal center B cells of an elite controller and exhibits broad neutralization breadth. LN01 engages both MPER and the transmembrane (TM) region, which together form a continuous helix in complex with LN01. The tilted TM orientation allows LN01 to interact simultaneously with the peptidic component of the MPER epitope and membrane via two specific lipid binding sites of the antibody paratope. Although LN01 carries a high load of somatic mutations, most key residues interacting with the MPER epitope and lipids are germline encoded, lending support for the LN01 epitope as a candidate for lineage-based vaccine development.
Keywords: HIV-1, Env, gp41, MPER, broadly neutralizing antibody, LN01, 4E10, 10E8, membrane interaction
Graphical Abstract
Highlights
-
•
bNAb LN01 neutralizes 92% of a 118-strain virus panel
-
•
LN01 targets the HIV-1 gp41 MPER, the TM region, and lipids
-
•
LN01-complexed MPER forms a continuous helix with TM
-
•
Most LN01 paratope residues interacting with MPER-TM and lipids are germline encoded
The gp41 membrane-proximal external region (MPER) is a highly conserved region of HIV-1 Env. Pinto et al. characterize the broadly neutralizing anti-MPER mAb LN01, which shows low autoreactivity. LN01 interacts with a complex epitope comprising MPER, the transmembrane region, and lipids, providing insights for vaccine design.
Introduction
The key to HIV-1 vaccine development is the induction of broadly neutralizing antibodies (bnAbs). The currently known classes of bnAbs target six functional regions on the envelope glycoprotein encompassing the V2 apex, the V3 glycan site, the CD4 binding site, the gp120-gp41 interface region, the gp120 silent face, and the membrane proximal external region (MPER) of Env gp41 (Kwong and Mascola, 2018, Sok and Burton, 2018). MPER-specific bnAbs 4E10, 10E8, DH511, and VRC42 target the same helical linear epitope, which precedes the transmembrane (TM) region (Cardoso et al., 2005, Huang et al., 2012, Krebs et al., 2019, Williams et al., 2017, Zwick et al., 2001), and neutralizes more than 90% of multiclade strains (Krebs et al., 2019, Sok and Burton, 2018).
A hallmark of MPER bnAbs are long, heavy-chain CDR3 (HCDR3) loops carrying hydrophobic residues at their tips, whose interaction with membrane is required for neutralization (Alam et al., 2009, Julien et al., 2010, Ofek et al., 2010, Scherer et al., 2010). MPER bnAbs show various degrees of autoreactivity linked to immune tolerance mechanisms (Chen et al., 2013, Doyle-Cooper et al., 2013) that could impair MPER bnAb development. BnAbs 4E10 and VRC42.01 show the most significant non-specific interaction with lipids and membrane (Alam et al., 2007, Krebs et al., 2019), while 10E8 and DH511 lineage bnAbs lack important non-specific membrane binding (Huang et al., 2012, Krebs et al., 2019, Williams et al., 2017), indicating that non-specific membrane autoreactivity is not a prerequisite of bnAbs targeting MPER. However, specific interaction with membrane is important and structures of 4E10 and 10E8 revealed lipid binding of both bnAbs (Irimia et al., 2016, Irimia et al., 2017). Based on these structures, models of the Fab-MPER-membrane interface have been generated and have shown that the MPER epitope helix lies nearly perpendicular to the membrane (Irimia et al., 2016, Irimia et al., 2017, Rujas et al., 2016). Although MPER bnAbs 4E10, 10E8, and DH511 recognize the same epitope, their approach angles vary (Williams et al., 2017). In contrast, VRC42.04 is a close variant of 4E10 that recognizes the same epitope with the same approach angle as 4E10. Interestingly, the latter bnAbs have been independently generated in clade B-and clade CRF016AE-infected patients (Krebs et al., 2019, Zwick et al., 2001).
MPER epitopes are likely only poorly accessible on native Env trimers, and full epitope accessibility requires at least some degree of receptor/co-receptor-binding-induced conformational changes in Env (Chakrabarti et al., 2011, Lee et al., 2016, Rathinakumar et al., 2012). Consequently, MPER bnAbs bind with high affinity to the fusion intermediate conformation of gp41 (Chen et al., 2014, Frey et al., 2008, Lai et al., 2014). This conformation forms during the receptor-binding-induced transitions from native Env (Pancera et al., 2014) to the postfusion conformation (Buzon et al., 2010), thereby facilitating exposure of the linear MPER sequence motif in a membrane context. This transient conformation is suitable for bipartite anti-MPER bnAb binding of the MPER and membrane.
Dependent on the patient cohort investigated, MPER-specific Abs are either present in a large number of patient sera with broad neutralizing activity (Doria-Rose et al., 2017, Huang et al., 2012, Molinos-Albert et al., 2014) or are reported to be rare (Landais et al., 2016, Pietzsch et al., 2010). Importantly, their presence increases the potency and breadth of the sera substantially in comparison to V2 apex bnAbs (Jacob et al., 2015). Furthermore, antibody cocktails including MPER antibodies are more potent (Kong et al., 2015), and in vitro the presence of 10E8 in a 4-antibody cocktail reduced significantly the amount of incomplete neutralization (Wagh et al., 2016). Finally, both 4E10 and 10E8 protect animals from SHIV infection by passive immunization (Hessell et al., 2010) (Pegu et al., 2014).
Here, we have isolated a broad and potent anti-MPER neutralizing Ab, LN01, derived from lymph-node germinal center B cells of an elite controller infected with a clade B strain. This antibody uses a heavy-chain germline V gene and thus extends the B cell repertoire for the induction of MPER bnAbs. We have determined the reactivity of the unmutated common ancestor (UCA) and the role of the extensive load of somatic mutations for neutralization. We show that in addition to the MPER epitope, LN01 binding requires part of the TM for interaction. Structural studies have revealed the role of the TM and that of specific lipid-binding pockets. It is noteworthy that MPER forms a continuous helix with the complete gp41 TM region. In synergy with molecular dynamics simulation, we propose a model of LN01 interaction with its monomeric epitope and with the membrane, revealing important implications for gp41 immunogen design targeting the LN01 lineage.
Results
LN01 Isolation and Characterization
Among a cohort of chronically HIV-1-infected patients, naïve to antiretroviral therapy, we identified a patient (SA003) who showed high level of serum bnAbs, as assessed on a panel of 9 HIV-1 pseudoviruses (PVs) from the Global Panel of HIV-1 Env reference strains (Figure S1A). Of note, SA003 donor is an elite controller with viremia <50 HIV-1 RNA copies per mL of plasma (infected with clade B HIV-1). From patient SA003, we isolated lymph node mononuclear cells (LNMC) and sorted IgG memory B cells (CD19+IgA−IgM−CD27+CD38−) and IgG germinal center (GC) B cells (CD19+IgA−IgM−CD27+CD38+). The two B cell subsets were immortalized with Epstein-Barr virus (EBV) in the presence of anti-B-cell-receptor polyclonal antibodies and cultured for 14 days on a monolayer of mesenchymal stromal cells (MSCs) together with a cocktail of stimuli composed of IL2, IL21, IL6, and the TLR-9 agonist CpG-2006. The supernatants of B cell cultures were screened for their ability to neutralize 2 HIV-1 PVs from the Global Panel, BJOX2000 (clade CFR07) and CE1176 (clade C). One B cell supernatant from the IgG GC B cell library showed a high percentage of neutralization against both PVs tested (>70% for BJOX2000 and >90% for CE1176) (Figure S1B). The VH and VL regions of the monoclonal antibody produced by this B cell clone were sequenced and expressed as recombinant IgG1 monoclonal antibody, hereafter referred to as LN01.
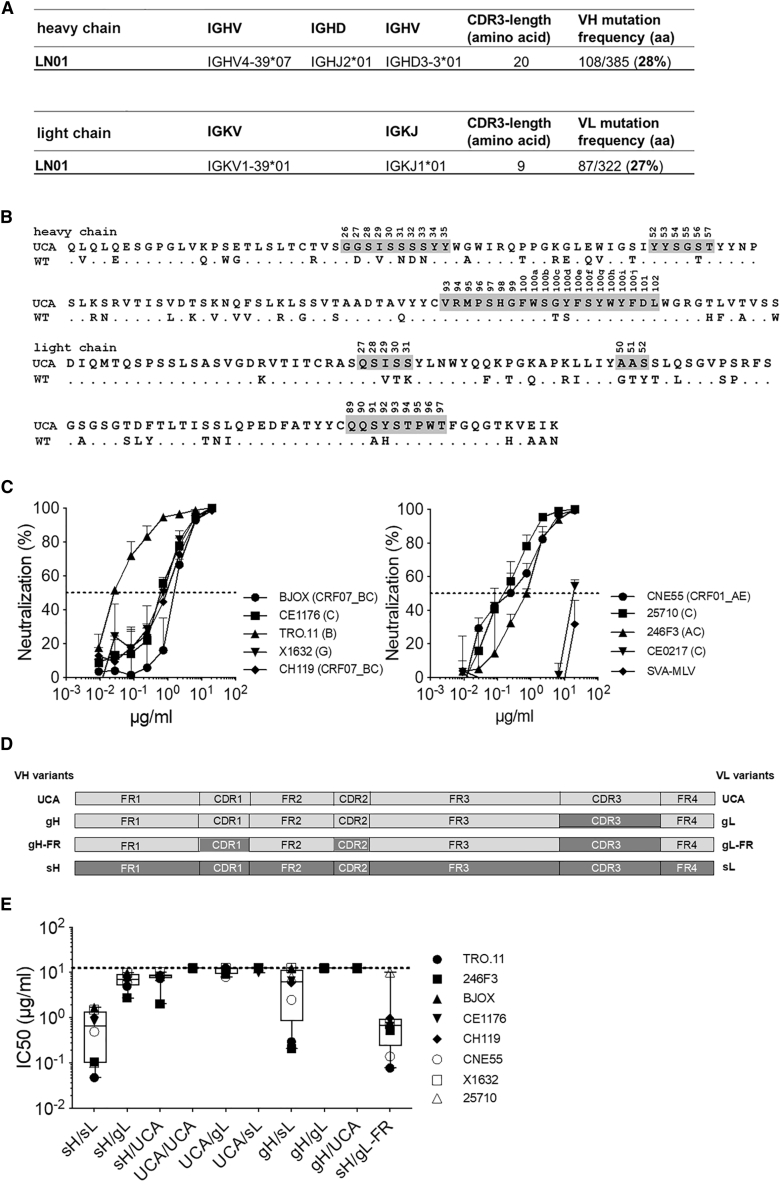
The sequence analysis revealed that LN01 was originally an IgG3 antibody encoding the IGHV4-39 and IGKV1-39 VH and VK germline genes. Two common features of HIV-1 bnAbs were also found in LN01 mAb: high frequency of somatic mutations in the heavy and light chain variable regions compared to the germline sequence (28% and 27%, respectively) and a long HCDR3 loop made of 20 amino acids (Figure 1A). The alignment of LN01 amino acid sequences with the unmutated common ancestor (UCA) sequences showed a high degree of mutations in HCDR1, framework 2 (FR-H2), FR-H4, LCDR1, LCDR2, and FR-L4 (Figure 1B).
Figure 1.
Analysis of LN01 mAb Sequence and Neutralization
(A) Analysis of LN01 sequences showing the inferred germline genes and alleles encoding the variable region of the heavy and light chains, the amino acid length of the CDR3 regions and the mutation frequency of the variable regions of the light and heavy chains (aa, amino acid).
(B) Alignment of the amino acid sequences of the variable regions of LN01 wild-type and LN01 UCA. The CDRs regions are highlighted in gray.
(C) LN01 IgG1 activity was tested in vitro in neutralization assay using TZM-bl cells. Different concentrations of the antibody were tested against nine pseudoviruses (PVs) of the Global Panel plus a control PV (SVA-MLV). Shown on y axes is the % of neutralization and the standard deviation (SD) calculated on quadruplicates.
(D) Schematic of LN01 unmutated common ancestor (UCA) and variants created for investigation of the neutralization requirements of LN01 germlined variants. Light gray areas represent sequence from UCA; dark gray regions are from the somatic, mature antibody. Wild-type, somatically mutated heavy (sH), or somatically mutated light (sL) chains; gH or gL, germline V-gene revertants of sH or sL in which HCDR3 or LCDR3 are mature; gH-FR or gL-FR, germline V-gene revertants of sH or sL in which HCDRs or LCDRs are mature.
(E) Box-and-whisker plots showing the neutralizing activity of LN01 germline variants against a panel of eight PVs of the Global Panel as measured using a neutralization assay on TZM-bl cells.
The neutralizing activity of recombinant LN01 IgG1 mAb was initially tested against a small panel of 9 HIV-1 PVs (and a control PV, SVA-MLV). LN01 was found to neutralize 8 PVs with a median IC50 value of 0.57 μg/mL (ranging between 0.03 μg/mL of TRO.11 and 1.61 μg/mL of BJOX2000) (Figure 1C). We also checked the neutralizing activity of LN01 mAb expressed in the IgG3 and Fab formats. Of note, both IgG3 and Fab formats showed the same neutralizing activity as compared with LN01 IgG1 (Figure S2).
To further characterize the LN01 mAb activity we determined whether FcγRI expressed on target cells could augment the potency of Ab-mediated neutralization of HIV-1 PVs, an effect that was previously described to occur with gp41 MPER-specific neutralizing Abs and not with gp120-specific mAbs such as b12 and 2G12 (Perez et al., 2009). Hence, we tested side by side the LN01 IgG1 neutralizing activity on parental TZM-bl cells versus TZM-bl expressing FcγRI in parallel with two anti-MPER bnAbs, 10E8 and 4E10. Differences in neutralization potencies of ≥3-log between parental target cells and FcγRI cells were observed for 10E8 and 4E10, as well as for LN01 (Figure S3). This improved potency may result from a kinetic advantage unique to antibodies whose epitopes are difficult to access or exposed for only a short time, such as the MPER displayed on intermediate conformations of the Env protein during an early stage of fusion. Taken together, these results show that LN01 is a bnAb with high breadth and potency against a multi-clade HIV-1 PVs panel.
Neutralization by LN01 Is Dependent on Somatic Mutations
To investigate the role of somatic mutations on the neutralizing activity of LN01, we generated nine LN01 variants with FR and CDR regions completely reverted to the germline in VH and VK and compared their neutralizing activity to that of the fully mutated LN01 mAb (Figures 1D and 1E). The germlining of all somatic mutations in the heavy chain (UCA VH variant) completely abolished LN01-neutralizing activity against the HIV-1 PVs tested, regardless of which light-chain variants were used (UCA/UCA, UCA/gL, and UCA/sL variants). Similarly, the germlining of all somatic mutations in the light chain (UCA VL variant) also abolished LN01 neutralizing activity, independently of which heavy-chain variants were used (sH/UCA, gH/UCA, UCA/UCA variants).
However, when the mature light chain is paired to the heavy chain completely reverted to the germline except for HCDR3 (gH/sL variant), LN01-neutralizing activity is impaired but not abolished. Of note, when the mature heavy chain is paired to the light chain in which all the FRs are reverted to the germline and the CDRs are mature (sH/gL-FR), the LN01 neutralizing activity is comparable to that of the fully mutated Ab (sH/sL) (Figure 1E). These results show that with respect to the light chain only the somatic mutations in the CDRs (8 mutations in a total of 29 mutations) are required for LN01 activity. Thus, this raises the question whether other somatic mutations can be as well reverted to germline while maintaining the LN01 neutralizing activity.
Analysis of LN01 Autoreactivity
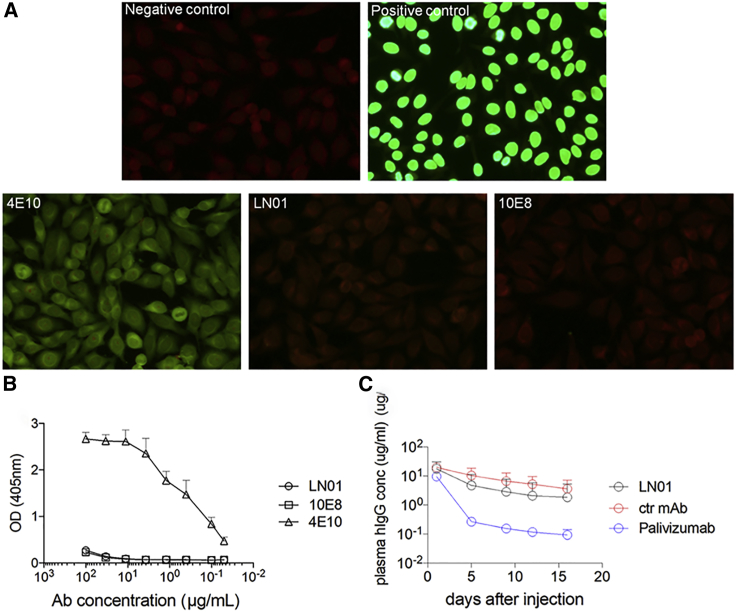
A common feature of several HIV-1 bnAbs, e.g. 2F5, 4E10, and 10E8, is that they cross-react with self-antigens. To evaluate LN01 mAb autoreactivity, we tested its binding to HEp-2 epithelial cells and to cardiolipin. LN01 showed a low binding to HEp-2 cells at the highest concentration tested comparable to the staining with 10E8 but significantly lower than 4E10 (Figure 2A). No binding was observed to cardiolipin (Figure 2B). To further characterize the LN01 bnAb, we performed the pharmacokinetic study in huFcRn transgenic mice. Following intravenous administration, the LN01 serum concentration declined over the 2 weeks of observation with kinetics similar to those of a control mAb specific for an irrelevant antigen and superior to those of the clinical stage mAb palivizumab (Figure 2C). These results show that LN01, based on the preliminary assessment, seems to be non-autoreactive and to have a favorable pharmacokinetic profile. More in-depth analysis is needed to confirm the safety profile of LN01.
Figure 2.
Analysis of LN01 Autoreactivity
(A) Immunofluorescence on Hep-2 cells. BnAbs LN01, 4E10, and 10E8 as well as positive and negative controls provided by the diagnostic kit were tested at 50 μg/mL.
(B) ELISA to measure the binding to the self-antigen cardiolipin. Assay performed according to manufacturer instructions. Shown are OD values of duplicates at 405 nm.
(C) Pharmacokinetic analysis performed in huFcRn transgenic mice (Tg276, Jackson Laboratory). LN01, palivizumab and a control mAb specific for an irrelevant antigen were administered i.v. at 10 mg/kg (n = 5). The concentration of human mAbs in plasma ± standard deviation (SD) was determined at multiple time points using a total human IgG ELISA, as described in STAR Methods.
LN01 Neutralization Breadth, Potency, and Effector Function
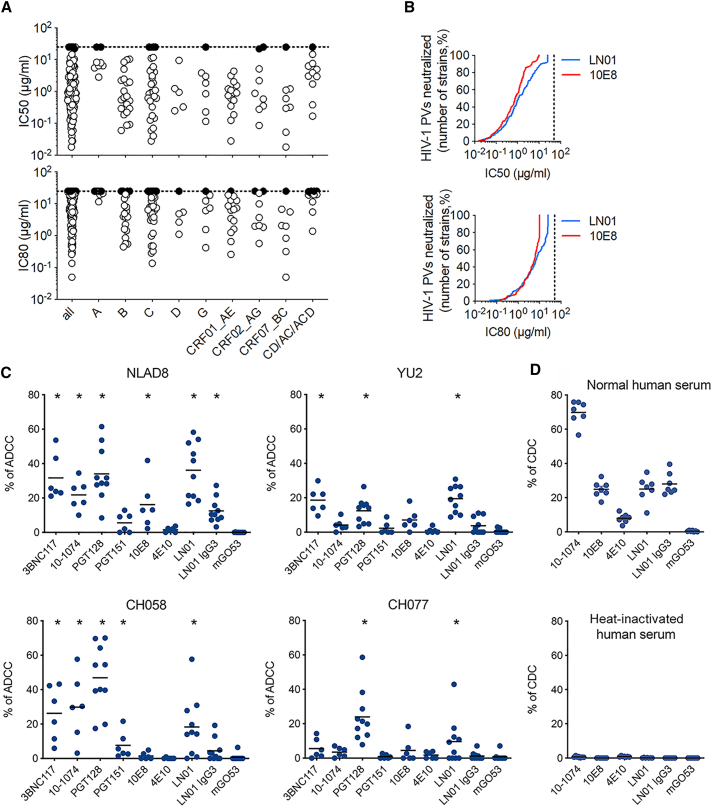
Since the LN01 mAb showed high breadth and potency against a small panel of HIV-1 PVs, it was then tested against an 118-isolate Env-PVs panel in parallel with bnAb 10E8. LN01 neutralized 92% of the tested PVs compared to 95% for 10E8; the median IC50 were 1.1 and 0.8 μg/mL for LN01 and 10E8, respectively. When the IC80 values are considered, LN01 and 10E8 neutralized 83% and 85% of the tested PVs with a median of 7.4 and 6.1 μg/mL for LN01 and 10E8, respectively (Figure 3A). Of note, the neutralizing activity of LN01 was not skewed to specific clades, albeit it seems to be less effective on clade A (the SA003 patient was infected by a clade B HIV-1) (Table S1). Thus, LN01 mAb mediates broad and potent neutralization against a large panel of HIV-1 viruses comparable to bnAb 10E8 (Figure 3B).
Figure 3.
LN01 Neutralization Breadth, Potency, and Effector Function Killing of HIV-1-Infected Lymphocytes
(A) The neutralizing activity of LN01 IgG1 tested against a cross-clade panel of 118 HIV-1 PVs. The IC50 (top panel) and IC80 (bottom panel) expressed in mg/ml were determined in TZM-bl-cell-based micro-neutralization assay as described in STAR Methods.
(B) Neutralization breadth-potency curves for LN01 and 10E8, with breadth shown as percentage of PVs neutralized at each IC50 (top panel) or IC80 (bottom panel) cutoff (25 μg/mL for LN01 and 10 μg/mL for 10E8).
(C) ADCC killing of HIV-1 infected lymphocytes performed with bnAbs at 15 μg/mL on CEM-NKR-CCR5 cells infected with NLAD8, YU2, CH058, or CH077 HIV-1 strains. ADCC was calculated as the disappearance of Gag+ cells with or without antibodies (n = 6–10), with each dot representing an individual donor of primary NK cell. ADCC responses of each tested antibody were compared to that of the isotype control mGO53 in the Wilcoxon test (∗p < 0.05).
(D) CDC-mediated cell killing performed with bnAbs at 15 μg/mL incubated with a Raji-YU2 Env cell line in the presence of normal human serum from six individual donors or heat-inactivated human serum. The mGO53 antibody was used as a negative control in (C) and (D).
The targeted killing of HIV-1 infected lymphocytes is an effector function held by some but not all bnAbs. Antibody-dependent cellular cytotoxicity (ADCC) is mediated through the binding of the Fc portion of the antibody to Fc receptors that are expressed on effector cells including natural killer (NK) cells. In complement-dependent cytotoxicity (CDC), antibody binding to the target cell induces cell lysis that is triggered by the binding of C1q subunits to the Fc antibody region. For in vitro ADCC evaluations, CEM-NKR-CCR5 CD4 T cells infected with one of four HIV-1 viral strains were cultured with primary NK cells in the presence or absence of different antibodies (Figure 3C). ADCC activities of IgG1 and IgG3 versions of LN01 were compared to that of bnAbs 10E8 and 4E10. BnAbs 3BNC117, 10–1074, PGT128, PGT151 (all IgG1) and the isotype control mG053 were used as controls. One lab-adapted (NLAD8) and three primary strains of HIV-1 (YU2, CH058, and CH077) were tested. In this assay, LN01 IgG1 displays significant ADCC against the four HIV-1 strains tested at levels that are comparable to control bnAbs. LN01 IgG3 showed a reduced response. 10E8 displayed ADCC against 1 out of 4 strains and 4E10 showed no response (Figure 3C). CDC-mediated cell lysis induced through bnAbs was evaluated using Raji cells engineered to stably express cell surface levels of HIV-1 YU2 envelope. Following the incubation of cells with human serum and antibodies, the appearance of dead cells was monitored by flow cytometry with heat-inactivated human serum used as negative control. LN01 IgG1 and IgG3 antibodies both exhibited specific CDC-mediated killing of the envelope positive cells at equivalent levels observed for 10E8, while 4E10 showed substantially reduced CDC activity (Figure 3D).
LN01 Epitope Specificity and Binding
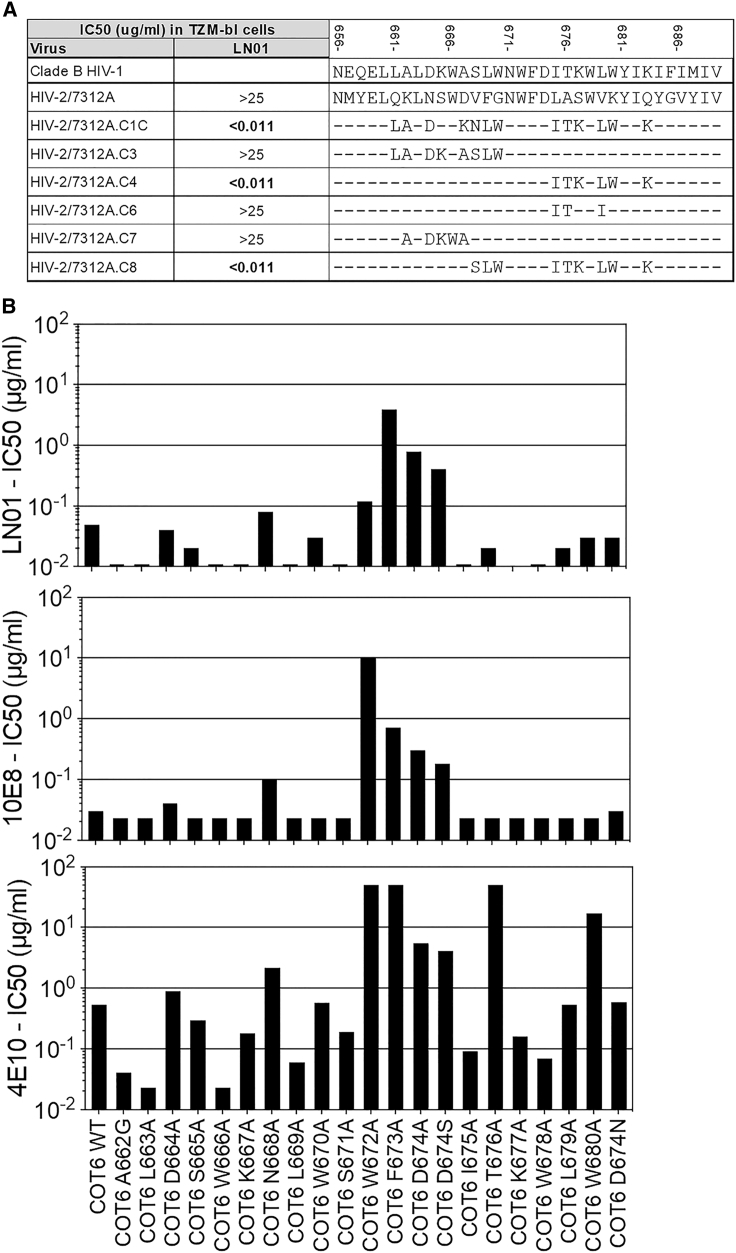
The results described above suggested that LN01 could target the MPER of HIV-1 Env. To address this hypothesis, we used a panel of HIV-2/HIV-1 chimeric PVs containing various segments of the HIV-1 MPER replacing the parental HIV-2/7312A sequences (Tomaras et al., 2011). Importantly, IgG1 LN01 antibody did not neutralize the parental HIV-2 7312A strain. IgG1 LN01 antibody was found to potently neutralize the chimeric virus 7312A.C4 in which six residues from HIV-1 were replaced in the HIV-2 MPER region (LASWVKYIQ replaced by ITKWLWYIK) but not the chimeric virus 7312A.C6 in which only three residues in the same region were replaced (LASWVKYIQ was replaced by ITSWIKYIQ) (Figure 4A). A similar finding was observed with the chimeric virus 7312A.C1C where the same six mutations of 7312A.C4 were combined with additional seven mutations in the N-terminal region of MPER. These results indicate that residues in the C-terminal region of the gp41 MPER (in particular L679, W680, and K683) are involved in IgG1 LN01 antibody binding and neutralization activity, thus indicating that LN01 is a MPER-specific bnAb.
Figure 4.
Relevant Residues on HIV-1 PVs for LN01 Binding
(A) HIV-2 chimera containing HIV-1 MPER region and parental HIV-2 were used to determined the binding of recombinant LN01. In the table are shown the IC50 (mg/mL) calculated based on the neutralization assay with TZM-bl cells (middle column), and the different mutations in the sequence carried by MPER of each chimeras (right column).
(B) The activities of LN01, 10E8, and 4E10 mAbs against several MPER mutants of the COT6.15 virus were assessed. Shown are the IC50 values expressed in μg/mL.
To better define the specificity of LN01 we used a peptide microarray formed by 1423 15-mer peptides, overlapping by 12 amino acids, that cover the full length of the consensus HIV-1 Env gp160 sequences for clades A, B, C, D, group M, CRF01_AE, and CRF02_AG. LN01 was tested in parallel with a control antibody called 7B2 (that is specific for the immunodominant region of gp41) for binding to the peptide microarray. Of note, IgG1 LN01 antibody did not react with any of the peptides in this library, while 7B2 strongly reacted with 190–195 peptides that spanned the gp41 immunodominant region (Figure S4A). In addition, LN01 did not interact with a 28-amino-acid peptide spanning the entire MPER region (Figure S4B).
To further refine the epitope mapping, we tested LN01 and two other MPER-specific mAbs, 10E8 and 4E10, against a panel of COT6.15 viruses (clade C) encoding MPER mutants (Tomaras et al., 2011). LN01 neutralizing activity was reduced by >8-fold against viruses with the mutants F673A, D674A and D674S (both F673 and D674 are shared in HIV-2), while only marginally reduced against the W672A mutant (Figure 4B). Of note, 10E8 shared with LN01 the reduced activity against the F673A, D674A, and D674S mutants, but its activity was reduced by 2-log against the W672A mutant, indicating that LN01 and 10E8 share a similar interaction with F673 and D674 but are likely to interact differently with W672.
Because LN01 did not interact with a MPER-peptide-containing residues 671–683, longer MPER-containing peptides, MPER-TM1 (residues 671–689 including seven TM residues) and MPER-TM2 (residues 630–711, containing the complete TM region) (Figure S5A) were tested in binding studies. MPER-TM2, solubilized in a buffer containing β-D-octyl glucoside (β-OG), forms trimers (Figure S5B) but does not interact with LN01. However, when solubilized in a buffer containing Fos-Choline-12, MPER-TM2 is monomeric and forms a complex with LN01 Fabs (Figure S5C). Binding kinetics were analyzed both in β-OG and in Fos-Choline-12-containing buffers by surface plasmon resonance (SPR) and compared to 10E8 interaction with MPER-TM1 and MPER-TM2. From this analysis, Kds were measured to be 160.8 nM for LN01 binding to MPER-TM1 in β-OG and 170.0 nM for interaction with MPER-TM1 in Fos-Choline-12, showing similar binding in both detergents. The interaction with MPER-TM2 is tighter, resulting in a Kd of 13.0 nM, underlining a role for the complete TM region. Binding of 10E8 yielded Kds of 62.0 and 36.9 nM for the interaction with MPER-TM1 and again a slightly higher affinity of 24.2 nM for the interaction with MPER-TM2 (Figure S5D). We conclude that the LN01 epitope extends from MPER into the TM region.
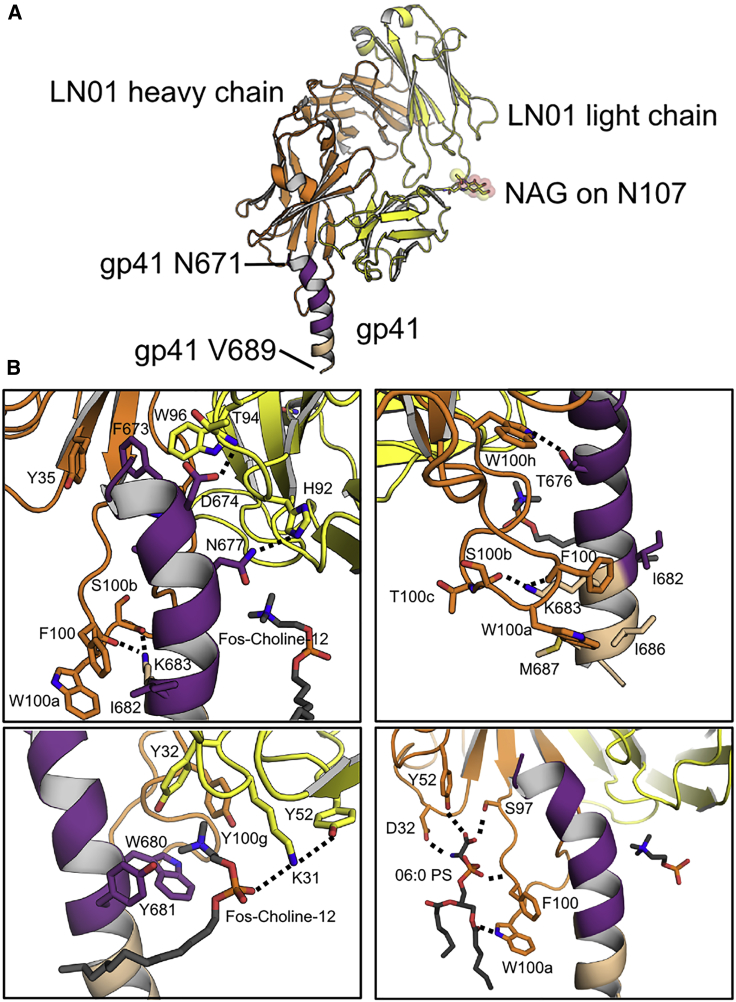
LN01 Structures in Complex with MPER-TM
Crystal structures of LN01 were determined in complex with MPER-TM1, in complex with MPER-TM1 and phosphatidylserine (06:0 PS), and in complex with MPER-TM2 to resolutions of 3.2 Å, 3.1 Å, and 3.9 Å, respectively (Table S2). Two identical complexes of LN01/MPER-TM1 were present in the asymmetric unit. MPER-TM1 residues 671–689 adopt a slightly bent helical conformation in complex with LN01 (Figure 5A). Main contacts are mediated by polar interactions, D674 to LCDR3 T94, N677 to LCDR3 H92, T676 to HCDR3 W100h, and K683 to the carbonyls of HCDR3 F100 and S100b (Figure 5B). Furthermore, gp41 F673 inserts into a hydrophobic pocket composed of HCDR3 W100h, HCDR1 Y35, and LCDR3 W96. In addition, numerous hydrophobic contacts include long-range interactions of HCDR3 residues, F100 and W100a, to gp41 I682, I686, and M687, thereby positioning the tip of HCDR3 close to TM residues (Figure 5B; Table S3). The overall contact interface of the Fab and MPER-TM1 spans 683 Å2. The structure also reveals the presence of a Fos-Choline-12 detergent molecule (Figure S6A); its phosphate group interacts with LCDR1 K31 and the cation of the choline inserts into a cation box (Morita et al., 2016, Roderick et al., 2002) made up of gp41 W680 and Y681 and LCDR1 Y32 and HCDR3 Y100g (Figure 5B). Co-crystallization of MPER-TM1-LN01 with 06:0 phosphatidylserine (PS) (Figure S6B) revealed a network of polar interactions coordinating one PS molecule. HCDR3 S97 and HCDR2 Y52 hydrogen bond to the carbonyl of PS, HCDR1 D32 contacts the amide of PS, the amide of HCDR3 F100 hydrogen bonds to the phosphate group of PS, and HCDR3 W100a to the carbonyl of the acyl chain (Figures 5B and S6B). This interaction network reveals how lipid interaction coordinates the HCDR3 extended conformation, allowing W100a immersion into the lipid bilayer. Notably, most residues coordinating epitope recognition, Fos-Choline-12, and PS interaction in LN01 are present in the LN01 UCA. Exceptions are LCDR1 K31 (Fos-Choline-12, phosphocholine head group), which itself is coordinated by LCDR2 Y52 (Figure 5B), LCDR3 H92 (contacting MPER), HCDR1 D32, and HCDR2 Y52 (contacting PS), as well as LCDR3 H92 (contacting gp41) (Figures 1B and 5B). Furthermore, replacement of UCA HCDR3 G100c by wild-type (WT) HCDR3 T100c likely influences the HCDR3 loop conformation and its capacity to reach the lipid bilayer. Thus, LN01 UCA does not neutralize, mainly because it lacks specific lipid-binding sites.
Figure 5.
Structure of LN01 in Complex with gp41 MPER-TM1 and with Lipid
(A) Structure of the LN01-MPER-TM1 complex. LN01 is colored in yellow (light chain) and orange (heavy chain) and the N-acetyl-β-d-glucosamine (NAG) on Asn107 of the light chain is shown with spheres. Gp41 is colored in purple (MPER) and beige (TM). The same coloring was used in all figures.
(B) Close up of the interactions with gp41 MPER, Fos-Choline-12, and PS revealing two lipid-binding sites on either side of the MPER helix. Fos-Choline-12, PS, and residues involved in polar contacts and important hydrophobic contacts are indicated and represented in sticks; hydrogen bond interactions are represented as dashed lines. The upper two panels show the interactions of LN01 with gp41 MPER from two different orientations. The lower left panel shows the interaction with Fos-Choline-12, a putative lipid-binding site accommodating phosphatidylcholine, and the lower right panel shows the hydrogen bond network that coordinates 06:0 PS forming a second lipid-binding pocket.
The neutralization analyses of LN01 variants having different heavy- and light-chain combinations (Figures 1D and 1E) agree with the molecular details of LN01 epitope recognition. In order to define the importance of the interaction with the peptidic epitope, we analyzed the LN01 variants with respect to MPER-TM2 interaction (Figure S7). Using bio-layer interferometry (BLI), WT (sH/sL) LN01 showed a KD of 2.3 ± 0.01 nM, which is a factor of 5 higher than the KD determined by SPR (Figure S5D). LN01 sH/gL-FR (Figure 1E) binds the gp41 epitope with a KD of 7.0 ± 0.03 nM indicating that light-chain framework mutations affect binding slightly while maintaining neutralization potency, except for strain 2570 (Figure 1E). LN01 gH/sL (Figure 1D) binds with a KD of 6.2 ± 0.02 nM but shows no or lower potency in neutralization (Figure 1E) in agreement with the importance of HCDR1 D32 and HCDR2 Y52 for PS coordination (Figure 5B). LN01 gH/gL and LN01 gH/UCA (Figure 1D) both bind with low micromolar affinity (KD = 1.4 ± 03 and 4.7 ± 1.7 μM) explaining their lack of neutralization. LN01 sH/gL has a 100-fold lower KD of 453.3 ± 82 nM and exerts only some low potency neutralization (Figure 1E), which may be explained by the absence of the PS and Fos-Choline12-binding sites as well as the lower affinity due to potential long-range effects of light-chain CDR residues. LN01 UCA/UCA, UCA/sL, and UCA/gL (Figure 1D) showed no binding, in agreement with no neutralization (Figure 1E), highlighting the importance of HCDR3 interactions with gp41 (Figure 5B). This thus confirms the importance of both high-affinity gp41 peptide epitope interaction and lipid binding for neutralization.
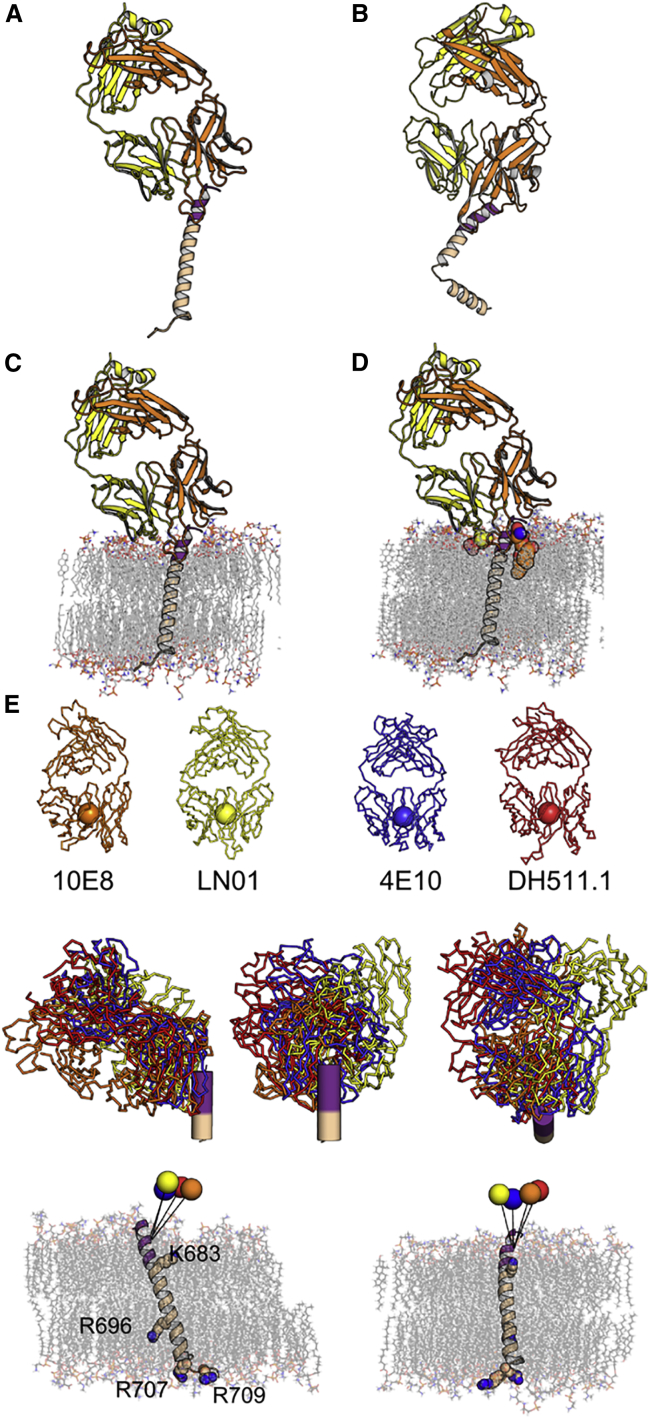
The structure of LN01 in complex with MPER-TM2 containing the complete TM region reveals that MPER forms a continuous helix with the TM comprising residues 684–711 (Figure 6A). The asymmetric unit contained four complexes, two with a continuous straight MPER-TM helix and two with a 90° and 110° kink at the conserved Gly 691 in the middle of the TM region (Figures 6B and S5E). LN01 interactions with MPER are the same in all four complexes and similar to the LN01-MPER-TM1 structures. Molecular dynamics (MD) simulations of the LN01-MPER-TM2 complex placed into a bilayer with a lipid composition analogous to that of the viral envelope (Brügger et al., 2006) revealed a 25° tilted orientation of the TM segment with respect to that of the bilayer (Figure 6C). This tilt orients the LN01 Fab on the membrane such that the HCDR3 dips into the bilayer and positions the lipid-binding sites to interact with lipid head groups (Figure 6D). Furthermore, the tilt angle of the TM allows residue K683 to interact with lipid head groups of one leaflet and R696 to contact head groups of the opposite leaflet; residues R707 and R709 terminating the TM helix interact as well with lipid head groups in the simulation (Figure S6C).
Figure 6.
Structure of LN01 in Complex with gp41 MPER-TM2 Reveals a Continuous Helix of MPER and TM
(A) Structure of the LN01-MPER-TM2 complex with the TM in the straight conformation. Color coding is the same as in Figure 5. Gp41 MPER residues 650 to 670 are disordered in the LN01-MPER-TM2 structure.
(B) Structure of the LN01-MPER-TM2 complex with the TM in the bent conformation.
(C) Orientation of the LN01-MPER-TM2 complex in the lipid bilayer based on the MD simulation result (lipids are represented in sticks).
(D) LN01-MPER-TM2 complex with modeled phosphatidylcholine and 06:0 PS (based on the LN01-MPER-TM1 structure) demonstrates that both lipids are well positioned to be part of the bilayer. Phosphatidylcholine and 06:0 PS are shown in spheres.
(E) The angle of approach of MPER bnAbs. The upper panel shows the bnAbs LN01 (yellow), 10E8 (orange), 4E10 (blue), and DH511.1 (red), represented with a sphere at the center of the variable domain for each antibody.
The middle panel shows their orientations upon recognition of the linear helical epitope as determined by Cα superposition of the MPER peptide of the four complexes (two side views and one top view looking down the helical axis of MPER).
The lower panel shows the representation of the angle of approach of the different bnAbs on the MPER-TM domain inserted in the lipid bilayer (lipids and basic residues are shown as sticks). The trajectory between the center of each antibody and gp41 T676 is depicted by a straight line.
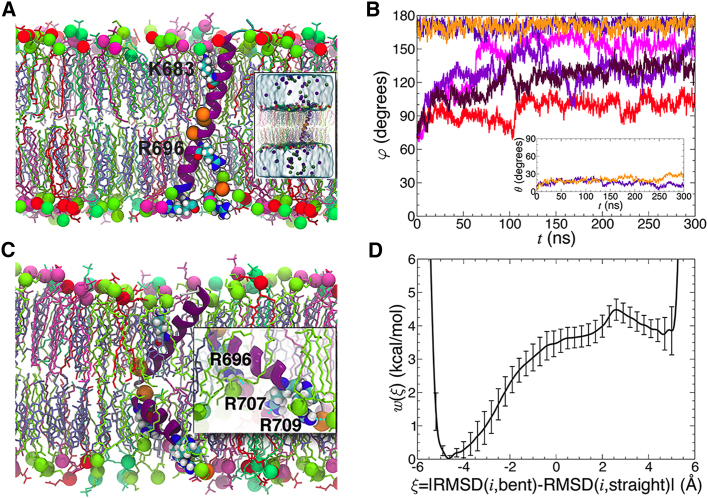
Although some of the LN01-interacting MPER-TM1 residues overlap with the contacts observed for bnAbs 10E8, 4E10, and DH511.1 (DH511.2) (Figure S5E), all four antibodies approach the MPER epitope with different angles. Comparison of the approach angles, based on the orientation of the MPER-TM2 in the membrane as determined by MD simulation (Figure 6C), showed that Fabs 10E8, 4E10, and DH511.1 are rotated by 30°, 14°, and 34°, respectively, compared with the LN01 position (Figure 6E). Taken together, the different angles of approach of MPER bnAbs suggest that half of the MPER surface is immunologically silent and not accessible in any of the different conformational states of Env. In order to determine whether TM tilting in the membrane is induced by LN01 binding, we performed MD simulation to position the straight TM alone in the lipid bilayer. Furthermore, we included the bent TM conformation in the simulation to test its potential physiological relevance. Six 0.3-μs MD trajectories were generated, two of them starting from a straight TM helix, and four initiated with a bent TM helix. In the simulations starting from a straight TM helix, TM did not interconvert into a bent form (Figures 7A and 7B). Analysis of the trajectories indicates that the TM is steadily anchored to the lipid bilayer by interactions of K683 with the head groups of one leaflet, and residues R707/709 interacting with head groups of the other leaflet (Figure 7A) as shown in the LN01-MPER-TM2 simulation (Figure S6C). Measuring the tilt of the α helix with respect to the membrane plane as a function of time revealed average tilt angles of 18° ± 5°, slightly smaller than the tilt angle imposed by the LN01 Fab interaction (Figures 5C and S6C). This conformation indicates that the guanidine group of the strictly conserved central TM residue R696 can contact polar head groups (Figures 7A and S6C). In contrast, simulations initiated with the bent TM revealed a propensity to interconvert toward a straight conformation. Out of four independent simulations, only one preserved a bent TM conformation, with a near 90° bending angle. In one simulation, the α helix rapidly retracted to a nearly straight form, while in the remaining two, the TM appears to navigate between the two conformations (Figure 7B). The central R696 can contact polar head groups in the bent conformation as well, and the anchoring residues at the membrane boundaries K683 and R707/709 squeeze the membrane, reducing its diameter locally (Figure 7C). The MD trajectories further suggest that the straight and the bent TM helices in a membrane are metastable states of the free-energy landscape, the straight conformation likely corresponding to a lower free-energy state than the bent form. To verify this hypothesis, the potential of mean force (PMF) underlying the transition between the two conformations was determined in an 11-μs multiple-walker adaptive-biasing-force (MW-ABF) simulation (Comer et al., 2014). The one-dimensional free-energy profile features two minima separated by a 4.5 kcal/mol barrier, the straight and the bent conformations being 3.8 kcal/mol in favor of the former (Figure 7D). Thus, starting from a bent α helix, the free-energy barrier to overcome is only 0.7 kcal/mol. These observations indicate that depending on the initial conditions, the bent α helix can rapidly interconvert to a straight α helix. Conversely, at 310K (37°C), the straight α helix is very unlikely to bend to an elbow-shaped α helix, in line with our equilibrium brute-force MD simulations. We conclude that the straight TM region is the lowest energy state, which most likely represents the TM conformation present upon LN01 binding in vivo.
Figure 7.
Molecular Dynamics Simulation of the Straight and Bent TM Conformations in a Lipid Bilayer
The lipid bilayer is composed of POPC (turquoise), POPE (yellow-green), POPS (red), SSM (pink), and cholesterol (mauve). The lipid head groups are represented as van der Waals spheres of the corresponding colorcolour. The G residues of the α helix are highlighted as orange spheres.
(A) MD simulation of the straight TM conformation reveals a tilt angle of 17°. The inset shows the complete assay, featuring water and the K+ (violet) and Cl− (green) ions.
(B) MD simulation of the bent TM conformation demonstrates that the bent form thins the membrane locally.
(C) Time evolution of the bending angle, φ, formed by the long axes of the α-helical segments spanning residues I675 to M687 and I697 to N706 determined from six independent MD simulations. The inset of (C) depicts the time evolution of the tilt angle, θ, formed by the long axis of gp41 and the normal to the lipid bilayer.
(D) Free-energy profile characterizing the transition between the straight (left minimum) and the bent (right minimum) conformations of gp41 in a membrane. The error bars correspond to the standard deviation measured from the eight walkers of the 11-μs MW-ABF simulation.
Discussion
Here, we report the isolation as well as functional and structural characterization of a bnAb (LN01) isolated from lymph-node GC B cells of an elite controller. LN01, originally isolated as IgG3, uses IGHV4-39 and IGKV1-39 VH, and VK genes and neutralizes 92% of a 118-strain virus panel. We show that LN01 binds to the same gp41 MPER epitope as bnAbs 10E8, 4E10, DH511, and VRC42.04 (Cardoso et al., 2005, Huang et al., 2012, Krebs et al., 2019, Williams et al., 2017). However, LN01 interaction with the MPER epitope requires, in addition to MPER, amino acid residues in adjacent helical turns of the transmembrane region for interaction. Thus, at least one helical turn of the TM is an integral part of the LN01 epitope. Furthermore, the affinity of LN01 is 10 times higher when MPER is fused to the complete TM segment, which may provide a more stable TM structure. In line with a role of the TM for anti-MPER bnAb function, the affinity of 10E8 is 100 times higher when the MPER epitope is linked to part of the TM (Rujas et al., 2016).
The MPER epitope adopts the same helical structure in complex with 10E8, 4E10, DH511, VRC42, and LN01, although the contact residues differ because four of the five bnAbs approach the epitope from different angles. Together, they cover approximately 180° of the cylindrical MPER helix surface, which is inconsistent with a tight trimeric conformation. Indeed, a trimeric NMR model of MPER-TM indicates that the MPER epitope is not accessible in this conformation (Dev et al., 2016). The cryoelectron microscopy (cryo-EM) structure of native Env in complex with PGT151 suggests that glycans N88 and N625 sterically hinder 10E8 binding to native Env trimers. Thus, in order to interact with native Env, 10E8 interaction requires lifting the trimer off the membrane surface, resulting in a conformation similar to the one induced by CD4 splaying MPER-TM apart (Huang et al., 2014, Lee et al., 2016). Although these early conformational states of Env may provide initial binding, the binding affinity of 10E8 or other MPER antibodies to such an early conformational intermediate has not been investigated. The real target of bnAbs targeting MPER is most likely the gp41 fusion intermediate conformation (Frey et al., 2008, Lai et al., 2014), whereas anti-MPER bnAbs prevent refolding into the fusion-active conformation (Weissenhorn et al., 1997). This is also in agreement with the long mean half-life of neutralization (Williams et al., 2017) linked to the prolonged MPER epitope exposure (Shen et al., 2010) and in line with the increased neutralization potencies observed between parental cells and FcγRI cells as reported here.
Most MPER antibodies except VRC42 (Krebs et al., 2019) have been isolated as the IgG3 subclass, whose more flexible hinge region may favour MPER epitope recognition, although no differences in neutralization or CDC activity were observed between LN01 IgG1 and IgG3 subclasses. In contrast, LN01 IgG1 showed a trend toward improved ADCC mediated killing of HIV-1 infected lymphocytes compared to the IgG3. These potential differences between the LN01 antibody subclasses will require further ADCC investigation with a concentration response analysis. Interestingly, using four different strains of HIV-1, LN01 showed a higher ADCC activity than 10E8 and 4E10, the latter being negative in ADCC as reported previously (Bruel et al., 2016).
We further show that LN01 binds lipids similar to 4E10 and 10E8 (Irimia et al., 2016, Irimia et al., 2017), which is likely to be a general feature of MPER bnAbs. The long HCDR3 with hydrophobic residues at its tip, which are essential for neutralization (Alam et al., 2009, Chen et al., 2014, Julien et al., 2010, Lutje Hulsik et al., 2013, Ofek et al., 2010, Scherer et al., 2010), is another common feature of MPER bnAbs. Our MD simulations reveal that LN01 bound to MPER-TM is positioned such that HCDR3 can insert into the membrane, thereby interacting simultaneously with the first turn of the α-helical TM and lipids via at least two lipid-binding pockets. Thus, the membrane is part of a bipartite epitope, which increases the binding affinity (Reichart et al., 2016) and transforms MPER bnAbs into “conformational” Abs (Mouquet et al., 2010). Although membrane interaction is essential for neutralization, LN01 autoreactivity with membranes in the absence of the gp41 epitope is below the detection level similar to that reported for 10E8 (Huang et al., 2012) and VRC42.04 (Krebs et al., 2019) and thus is not as prominent as described for 4E10 (Alam et al., 2007, Haynes et al., 2005). Nevertheless, some autoreactivity such as specific lipid binding plays a role in the maturation of MPER bnAbs, in line with the suggestion that their generation requires the ability to overcome immune tolerance mechanisms (Moody et al., 2016) by engaging antibody lineages induced by host proteins or microbiota (Liao et al., 2011, Trama et al., 2014).
The germline VH and VL gene usage of MPER bnAbs shows similarities and differences. 10E8 and DH511 use the germline V gene IGHV3-15, and 4E10 and VRC42 employ IGHV1-69, while LN01 uses yet another one, IGHV4-39, hence expanding the VH repertoire of naïve B cells for MPER antibody generation. In contrast, the light-chain V gene usage of IGKV1-39 is shared between LN01 and the DH511, while 4E10 and VRC42 use IGKV3-20 and 10E8 IGLV3-19 (Finton et al., 2014, Huang et al., 2012, Krebs et al., 2019, Williams et al., 2017). This indicates that these five anti-MPER bnAbs, derived from four different patients, have found common and divergent solutions to the same problem, suggesting that successful targeting MPER might not be exceptional, in line with the presence of MPER-specific Abs in polyclonal sera (Doria-Rose et al., 2017, Huang et al., 2012). The UCA version of LN01 does not neutralize PVs infection, in agreement with no detectable interaction with the gp41 MPER-TM2 epitope. Testing our different LN01 variants for epitope binding revealed that high-affinity peptide epitope binding, as well as the presence of both proposed lipid-binding sites, are important for LN01 neutralization and potency. Furthermore, many of the LN01 somatic mutations within the framework regions seem to be unimportant for neutralization. However, some long-range structural effects cannot be excluded, in agreement with findings that suggest that HIV-1 bnAbs require framework mutations for either increased flexibility and/or direct antigen contact (Klein et al., 2013). In particular, our structural studies suggest that only five of the CDR somatic mutations are implicated in epitope and lipid binding. Similarly, a longitudinal study on the 4E10-related bnAb VRC42 suggests that it becomes broad with a low mutation rate (Krebs et al., 2019). Thus, further investigations will inform on the minimal requirement of mutations in light of improving LN01 developability properties.
The crystal structure of MPER-TM2 containing the complete TM region reveals a continuous helix of MPER and TM similar to the monomeric MPER-TM NMR structure (Chiliveri et al., 2018). Other structural models propose a hinge or helical bent connecting MPER and TM (Apellániz et al., 2015, Kwon et al., 2018), underlining some conformational flexibility within MPER. MD simulation placed the TM domain alone with an approximate 18° tilt angle in the membrane stabilized by the basic residues at both membrane boundaries. This conformation allows the central, strictly conserved R696 to interact with lipid head groups. Thus, R696 may be strictly conserved in order to position and/or stabilize the tilted TM during the conformational transitions of gp41, leading to membrane fusion (Schibli and Weissenhorn, 2004), which is consistent with the proposal that R696 is important for membrane-fusion efficiency (Long et al., 2011). We further observe a bent conformation of the TM region, which has no role in LN01 interaction. Although this may be a pure Fos-Choline-12 effect, bending occurs at Gly positions that are strictly conserved in HIV-1, HIV-2, and SIV TM sequences (Chen and Chou, 2017). MD simulations confirmed that the TM region can navigate between a straight and bent conformation, both being metastable, although the straight form represents the lower free-energy state. Interestingly, the bent conformation compresses the membrane, and membrane thinning of SNARE TMs was proposed to lower the free energy of stalk formation by favoring the local concentration of highly bent stalk structures (Smirnova et al., 2019) formed at early stages of the fusion process (Chernomordik and Kozlov, 2008). It is, therefore, tempting to speculate that a bent Env TM may have a similar role in membrane fusion.
In summary, the functional and structural data indicate that potential MPER immunogens must be linked to the native Env transmembrane region and incorporated in the membrane as the MPER epitope extends into the TM region, thereby generating the functional bipartite gp41 membrane epitope. Since the TM alone orients itself in the membrane with a tilt angle that is similar to the one induced or stabilized by LN01 interaction, MPER-TM inserted in the membrane may present the optimal immunogen. However, since MPER itself has some degree of conformational flexibility and may as well insert into the membrane when linked to the TM domain (Apellániz et al., 2015, Kwon et al., 2018), our structures indicate that stabilization of the continuous MPER-TM helix by chemical (Bird et al., 2014) or other means could favor immunogenicity and thus B cell stimulation.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | Source | Identifier |
|---|---|---|
| Antibodies | ||
| PE-Cy7 Mouse Anti-Human CD19 | BD Biosciences | Cat#341113_clone SJ25-C1 |
| Alexa Fluor 488 goat anti-Human IgM | Invitrogen | Cat#A21215; RRID:AB_2535800 |
| Goat anti-Human IgA FITC | Invitrogen | Cat#A18788; RRID:AB_2535565 |
| Anti-Human CD3 PC5 | Beckman Coulter | Cat#A07749 |
| Anti-Human CD38 APC | BD Pharmingen | Cat#555462_clone HIT2 (RUO); RRID:AB_398599 |
| Anti-Human CD14 PC5 | Beckman Coulter | Cat#A07765 |
| Anti Human CD27 BV650 | BioLegend | Cat#302827_clone O323; RRID:AB_11124941 |
| AffiniPure F(ab’)2 Fragment Goat Anti-Human IgA+IgG+IgM (H+L) | Jackson Immunoresearch | Cat#109-006-064; RRID:AB_2337548 |
| AP-conjugated anti-human IgG secondary antibody | Southern Biotech | Cat#2040-04; RRID: AB_2795643 |
| 10E8 recombinant mAb | IRB and Pasteur; Huang et al., 2012 | PMID:23151583; RRID:AB_2491067 |
| HK20 recombinant mAb | IRB; Sabin et al., 2010 |
PMID:21124990; RRID:AB_2491018 |
| 4E10 recombinant mAb | IRB and Pasteur; Stiegler et al., 2001 | PMID:11788027; RRID:AB_2491029 |
| LN01 Recombinant mAb and All Variants | this paper | N/A |
| Mouse Anti-HIV-1 Gag FITC (Clone KC57) | Beckman Coulter | Cat#6604665 |
| mGO53 | Dr. Hugo Mouquet, Institut Pasteur, Paris; Wardemann et al., 2003 | PMID:12920303 |
| 3BNC117 | Produced by Dr. Hugo Mouquet; Scheid et al., 2011 | PMID: 21764753; RRID:AB_2491033 |
| 10–1074 | Produced by Dr. Hugo Mouquet; Mouquet et al., 2012 | PMID: 23115339; RRID:AB_2491062 |
| PGT128 | Produced by Dr. Hugo Mouquet; Walker et al., 2011 | PMID:21849977; RRID:AB_2491047 |
| PGT151 | Produced by Dr. Hugo Mouquet; Falkowska et al., 2014 | PMID: 24768347 |
| Bacterial and Virus Strains | ||
| HIV-1 PV strain CE1176 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| HIV-1 PV strain BJOX2000 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| HIV-1 PV strain X1632 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| HIV-1 PV strain 25710 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| HIV-1 PV strain TRO.11 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| HIV-1 PV strain CH119 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| HIV-1 PV strain 246F3 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| HIV-1 PV strain CE0217 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| HIV-1 PV strain CNE55 | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| SVA-MLV_control PV | Fraunhofer Institute for Biomedical Engineering IBMT | N/A |
| Epstein Barr Virus | IRB | N/A |
| pCH058.c/2960 | NIH AIDS Reagent Program | Cat#11856 |
| pCH077.t/2627 | NIH AIDS Reagent Program | Cat#11742 |
| pNL(AD8) | NIH AIDS Reagent Program | Cat#11346 |
| pYU2 | NIH AIDS Reagent Program | Cat#1350 |
| Standard panel of reference Env pseudoviruses | Huang et al., 2016 | Multiclade Reference Panel PMID:27315479 |
| OverExpress C41(DE3) chemically competent E. coli cells | Sigma-Aldrich | Cat#CMC0017 |
| Ni-NTA Agarose | Qiagen | Cat#30210 |
| Biological Samples | ||
| HIV-1 Infected ART Naïve Patient LNMCs | Hospital “Sacco” in Milan, Italy | N/A |
| Human serum | Etablissement Français du Sang | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 28amino acids long peptide gp41 MPER | Pepscan | N/A |
| Human recombinant IL-6 | BD Pharmingen | Cat#550071 |
| Human recombinant IL-21 | ImmunoTools | Cat#11340215 |
| Human recombinant IL-2 | IRB | N/A |
| MPER-TM1 Peptide | Smart Bioscience | www.smart-bioscience.com |
| Fos-Choline-12 | anatrace | Cat#F308 |
| n-Octyl-β-D-glucosid n-Octyl-β-D-Glucopyranoside | anatrace | Cat#O311 |
| PEG3350 | Sigma-Aldrich | Cat#88276 |
| PEG8000 | Sigma-Aldrich | Cat#89510 |
| HEPES | Sigma-Aldrich | Cat#H4034 |
| Ethylene glycol | Carl Roth | Cat#6881.1 |
| 06:0 PS | Avanti Polar Lipids | Cat#840030 |
| Critical Commercial Assays | ||
| ImmunoWELL cardiolipin (IgG) test | GenBio | Cat#3090 |
| Pierce Rapid Gold BCA Protein Assay kit | Thermo Fisher Scientific | Cat#A53225PR |
| NK cell isolation kit | Miltenyi Biotec | Cat#130-092-657 |
| LIVE/DEAD Fixable Aqua Dead Cell Stain Kit | Invitrogen | Cat#L34965 |
| ANA HEp-2 Test System | Zeus Scientific | Cat# FA2400 |
| EZ-LinkNHS-PEG4-Biotinylation Kit | Thermo Scientific | Cat#21455 |
| Deposited Data | ||
| MPER-TM1-LN01 complex | this paper | 6SNC |
| MPER-TM1-LN01-PS complex | this paper | 6SND |
| MPER-TM2-LN01 complex | this paper | 6SNE |
| Experimental Models: Cell Lines | ||
| TZM-bl cell line | NIH AIDS Reagent Program | Cat#8129 |
| TZM-bl FcγRI cell line | NIH AIDS Reagent Program | Cat#11798 |
| Expi293F cells | Thermo Fisher Scientific | Cat#A14527 |
| Mesenchimal stromal cells (MSC) | IRB | N/A |
| CEM-NKR-CCR5 | NIH AIDS Reagent Program | Cat# 4376 |
| Raji | ATCC | CCL-86 |
| 293T/17 | ATCC | Cat# CRL-11268 |
| Experimental Models: Organisms/Strains | ||
| Mouse: homozygous human FcRn (huFcRn) transgenic mice_Tg276 strain | Jackson Laboratory | JAX: 004919 |
| Oligonucleotides | ||
| CpG 2006 | Microsynth | N/A |
| Recombinant DNA | ||
| Plasmid: IgG1 expression vector | Tiller et al., 2008 | PMID:17996249 |
| Plasmid: Igk expression vector | Tiller et al., 2008 | PMID:17996249 |
| pMX-YU2-ENVΔCT- IRES-GFP | Provided by Dr. Hugo Mouquet, Pasteur | N/A |
| Plasmid: MPER-TM2 expression vector | This study | N/A |
| Software and Algorithms | ||
| Prism 8 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| CLC Main Workbench 8 | QIAGEN | https://www.qiagenbioinformatics.com/products/clc-main-workbench/ |
| IMGT-the International Immunogenetics Information System | Lefranc et al., 1999 | http://www.imgt.org |
| XDS | Kabsch, 2010 | PMID:20124693 |
| Phaser | McCoy et al., 2007 | PMID:19461840 |
| COOT | Emsley et al., 2010 | PMID:20383002 |
| REFMAC | Murshudov et al., 1997 | PMID:15299926 |
| Phenix | Adams et al., 2010 | PMID:20124702 |
| SBGrid | Morin et al., 2013 | N/A |
| Pymol | Warren DeLano | www.pymol.org |
| VMD (Version 1.9.4) | Humphrey et al., 1996 | https://www.ks.uiuc.edu/Research/vmd/ |
| NAMD (Version 2.13) | Phillips et al., 2005 | https://www.ks.uiuc.edu/Research/namd/ |
| CHARMM-GUI | Jo et al., 2008 | http://www.charmm-gui.org |
| WebLogo | Schneider and Stephens, 1990 | https://weblogo.berkeley.edu/logo.cgi |
| wwPDB OneDep System | https://deposit-pdbe.wwpdb.org | |
| wwPDB Validation Service | https://validate.wwpdb.org/ | |
| BLI Data Analysis software (v11.1.0.25) | Pall FortéBio | https://www.moleculardevices.com |
| SBGrid Consortium | (Morin et al., 2013) | https://sbgrid.org/ |
| Python 3.7 | https://python.org | |
| Biacore T200 Control Software | https://www.gelifesciences.com/biacore | |
Lead Contact and Materials Availability
Plasmids and mAbs generated in this study will be made available on request but may require a payment and/or a completed Material Transfer agreement if there is potential for commercial application. Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Winfried Weissenhorn (winfried.weissenhorn@ibs.fr).
Experimental Model and Subject Details
Cell Lines
TZM-bl wild-type and TZM-bl expressing the FcγRI cells were obtained from NIH-AIDS Research and Reference Reagent Program (ARRRP) and used for neutralisation assays. TZM-bl wild type cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 units of Penicillin and 0.1 mg/ml of Streptomycin while TZM-bl expressing the FcγRI cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 0.025M Hepes, 50 μg/ml of Gentamicin, 20 μg/ml of Blasticidin. Both cell lines were cultivated at 37°C in a humidified 5% CO2 incubator. Cell monolayers were split at 1x106 cells/T175 flask at confluence by treatment with 0.25% trypsin. Raji-Env cells (obtained from the ATCC, ATCC® CCL-86™) were cultured in RPMI supplemented with 10% FCS and 1% penicillin/streptomycin at 37°C in 5% CO2 and were used for CDC experiments. Expi293F cells (ThermoFisher Scientific) were maintained in Expi293 Expression Medium and cultivated at 37°C in a humidified 8% CO2 incubator (shaker with 25-mm shaking diameter, speed set to 140 rpm). Cells were split, once they reached a density of approximately 1-4x106 cells/ml, at 0.3x106 cells/ml. Expi293F cells were used for Ab production. The sex of all cell lines is female.
HIV-1 Primary Viruses
NLAD8, YU2 and transmitted-founder HIV-1 strains (CH058, CH077; obtained from the NIH AIDS Reagent Program) were prepared by the transfection of 293T cells (obtained from the ATCC) along with vesicular stomatitis virus G (VSV-G) to normalize infectivity, as done previously (Casartelli et al., 2010). HIV-1 PVs were provided by the Fraunhofer Institute for Biomedical Engineering IBMT.
Sample Collection
Male donor SA003 (year of birth 1963), ART naïve, was selected among a cohort of HIV-1-infected patients enrolled in the Hospital “Sacco” in Milan due to the ability of its plasma to neutralize eight out of nine HIV-1 PVs of the Global Panels. The aforementioned donor is a Long Term Non-Progressor (LTNP) patient who consented to lymphadenectomy, signed informed written consent and underwent surgery. At that time, he had been infected with HIV-1 for 30 years with CD4 T-cell counts of 474 cells/μl and plasma HIV-1 RNA values of 1600 copies/ml, CDC stage A1. The donor was not involved in previous procedures and he had never received antiretroviral therapy. The protocol was approved by the Ethic Commission Milan Area A (Comitato Etico Interaziendale Milano Area A) protocol Number 0006143 on February 20th 2015.
Animals
Treatment-naive female, 6-10 weeks old, homozygous human FcRn (huFcRn) transgenic mice (Tg276 strain, Jackson Laboratory Bar Harbor, ME) were used to assess the pharmacokinetics of monoclonal antibodies. Mice were bred in the specific pathogen-free (spf) facility at the Institute for Research in Biomedicine Switzzerland under a chow diet. All the in vivo procedures were performed in accordance with the Swiss Federal Veterinary Office guidelines and as authorized by the Cantonal Veterinary Office.
Methods Details
B-cell Isolation and Stimulation
Memory and GC B cells from patient SA003 were isolated from cryopreserved lympho node mononuclear cells (LNMCs) as follows: LNMCs were stained with anti-human CD19 PE-Cy7 (BD Bioscience_341113), anti-human IgM FITC (Invitrogen_A21215), anti-human IgA FITC (Invitrogen_A18788), anti-human CD27 BV650 (Biolegend_302827), anti-human CD38 APC (Beckman Coulter_555462) and anti-human CD14 PC5 (Beckman Coulter_A07765) plus anti-human CD3 PC5 (Beckman Coulter_A07749) on ice for 20 min. The cells were then washed, filtered and sorted using FACSAria (Becton Dickinson). Memory IgG+ B cells were sorted as CD19+CD27+CD38- while GC IgG+ B cells as CD19+CD27+CD38+, both negative for all other markers, resuspended in complete IMDM with 10% FBS and immortalized with Epstein-Barr virus (EBV) in the presence of 2.5 μg/ml CpG 2006, 2.5 μg/ml AffiniPure F(ab’)2 Fragment Goat Anti-Human IgA+IgG+IgM (H+L) (Jackson Immunoresearch), 500 U/ml IL-2, 5 ng/ml IL-6 (BD Pharmingen) and 10 ng/ml IL-21 (ImmunoTolls). The two subsets of B cells were seeded at 0.5 cells per well on a monolayer of Mesenchimal Stromal Cells (MSC) (Corti et al., 2014; Pinto et al., 2013) in 384-well microtiter plates. After 2 weeks, the supernatants were screened for neutralization activity using high throughput micro-neutralization assay against BJOX and CE1176 HIV-1 PVs. The B-cell cultures that neutralized both the PVs were then re-tested in secondary screening against the same PVs plus other two: X1632 and 25710. The B cells that neutralized four out of four HIV-1 PVs were lysed and the variable regions of the heavy and light chain were cloned.
Sequence Analysis of Antibody cDNA
cDNA was synthesized from selected B-cell culture and both the heavy and light chain variable regions (VH and VL) were sequenced as previously described (Tiller et al., 2008). Using the Database: IMGT (http://www.imgt.org), the VH and VL gene family and the number of somatic mutations were determined by analyzing the homology of the VH and VL sequences to known human V, D and J genes. UCA sequences of the VH and VL were constructed using IMGT/V-QUEST.
Production of Recombinant Antibody and Antibody Variants
The variable regions of the heavy and light chain were cloned into IgG1 and IgΚ expression vectors (Tiller et al., 2008) and expressed recombinantly by transient transfection of Expi293F cells (ThermoFisher Scientific) using polyethylenimine (PEI). After 7 days, cell culture supernatants were collected, centrifuged, filtered and purified by protein A chromatography (GE Healthcare). The purified antibodies were quantified using Pierce Rapid Gold BCA Protein Assay kit (ThermoFisher Scientific).
Neutralization Assays
A single-cycle infectivity assay was used to measure the neutralization of luciferase-encoding virions pseudotyped with the desired HIV-1 Env-protein. For the primary screening, the culture supernatants from day 14 were tested using 384-well based HIV-1 PVs microneutralization assay using in parallel two strains, CE1176 and BJOX2000 representative respectively of clade C and CRF07. Neutralization were undertaken on TZM-bl (3000 cell per well). Briefly, 10μl of culture supernatant was pre-incubated with 10 μl of diluted HIV-1 PVs for 1h at 37° (5% CO2) and then added on the top of TZM-bl. After an incubation of 72h at 37°, the cells were lysed with BriteLite reagent (PerkinElmer) and the luciferase activity detected using the EnVision multimode plate reader (PerkinElmer). For the secondary screening, the neutralization was performed following the same protocol but making four dilution of the culture supernatant before adding the HIV-1 PVs (CE1176, BJOX2000, X1632 and 25710). To test the breadth and potency of the recombinant antibodies, the neutralization assay was performed in 96-well plates. Briefly, 35μl of serial diluted mAb were pre-incubated with 35μl of HIV-1 PVs for 1h at 37° and then added on TZM-bl or TZM-bl expressing FcγRI, depending on the experiment, seeded the day before at 10 000 cell per well. The volume of medium was then adjusted to 200 μl per well the day after. After an incubation of 72h at 37°, the cells were lysed, and the luciferase activity measured using the EnVision. IC50 and IC80 were calculated by nonlinear regression analysis using the GraphPad Prism 5 software.
Neutralization against the extended panel of HIV-1 PVs (Huang et al., 2016) was employed to evaluate breadth and potency. Neutralization was assessed after a single round of infection in either TZM-bl or TZM-bl/FcγR1 cells using non-reported PV as described (Montefiori, 2009). A pre-titrated dose of PV was incubated with serial 3-fold dilutions of test sample in duplicate in a total volume of 150 μl for 1 hr at 37°C in 96-well flat-bottom culture plates. Freshly trypsinized cells (10,000 cells in 100 μl of growth medium containing 75 μg/ml DEAE dextran) were added to each well. One set of 8 control wells received cells + virus (virus control) and another set received cells only (background control). After 48 hours of incubation, 100 μl of cells was transferred to a 96-well black solid plate (Costar) for measurements of luminescence using the Britelite Luminescence Reporter Gene Assay System (PerkinElmer Life Sciences). Assay stocks of PV were prepared by transfection in 293T/17 cells (American Type Culture Collection) and titrated in TZM-bl cells as described (Montefiori, 2009).
ADCC Assay
HIV-1-infected target CEM-NKR.CCR5 CD4 T cells were stained using the Far Red DDAO cell tracker (Life Technologies). 2–5 ×104 target cells were plated in U-bottom 96-well plates and incubated with antibodies (15 μg/ml) for 5 min at room temperature. NK cells were added in each well (at a ratio of 1 CEM-NKR-CCR5 : 10 NK). Plates were spun 1 min at 300 g to promote cell contacts and incubated at 37°C for 4 h. Cells were then stained for intra-cellular Gag with the anti-Gag KC57 murine monoclonal antibody. To measure cell viability, the live/dead fixable aqua dead cell marker (1: 1,000 in PBS, Life Technologies) was added 20 min at 4°C before fixation. Data were acquired on a BD FACS CANTO II and analysed using FlowJo software. The frequencies of Gag+ cells among Far-Red+ cells were determined. ADCC was calculated using the following formula: 100 × (% of Gag+ target cells plus NK without antibody - % of Gag+ target cells plus effector with antibody)/(% of Gag+ target cells plus NK without antibody). Negative values were set to zero. Antibodies used in this assay, 3BNC117 (Scheid et al., 2011), 10-1074 (Mouquet et al., 2012), PGT128 (Walker et al., 2011), PGT151 (Falkowska et al., 2014), 4E10 (Stiegler et al., 2001), 10E8 (Huang et al., 2012) and mGO53 (Wardemann et al., 2003) were produced as described above.
Complement-dependent Cytotoxicity Assay
Raji-Env cells were generated with Raji cells (obtained from the ATCC, ATCC® CCL-86™) that were spinoculated (1,000g for 1,5h at 32°C) with a retroviral viral vector carrying Env (pMX-YU2 ENVΔCT-GFP-PuroR) (Pietzsch et al., 2010). Transduced cells (GFP+) were sorted and cultivated in the presence of Puromycin (1μg/ml, Sigma). High level of Env expression was then obtained by subcloning. 0.5 x 105 Raji-Env Cells were cultivated in presence of 50% normal human serum (NHS) or heat-inactivated human serum (HIHS) and with or without antibodies (15 μg/ml). After 24h, cells were washed twice with PBS and stained with the live/dead fixable aqua dead cell marker (1:1,000 in PBS, Life Technologies) for 30 min. Cells were then fixed with 4% PFA for 10min at RT. The frequency of dead cells was measured by flow cytometry (Attune Nxt, ThermoFischer). CDC was calculated using the following formula: 100 x (% of dead cells with antibody - % of dead cells without antibody)/(100 - % of dead cells without antibody).
Pharmacokinetic Analysis
LN01 pharmacokinetic was performed in Tg276 huFcRn transgenic mice in parallel with palivizumab and a control mAb specific for an irrelevant antigen. The mAbs were administered i.v. at 10 mg/ml (number of mice per group = 5). The concentration of human IgG mAbs in plasma were determined at multiple time points: 1-, 5-, 9-, 13- and 16-days post-administration. Mice bleeding was performed from the tail vein of mice.
Autoreactivity Assays
The LN01 mAb autoreactivity was tested by indirect immunofluorescence on human HEp-2 cells and by ELISA against a self-antigen, the human cardiolipin. In the first approach, LN01 mAb was tested at 50 μg/ml on slides coated with HEp-2 cells in parallel to a negative control and the positive control provided by the kit as well as 4E10 and 10E8 at 50 μg/ml. All the procedure was performed according to the kit specifications (Zeus Scientific). In the second approach, the anti-cardiolipin ELISA was performed according to the kit instructions (GenBio). Serial dilutions of the mAbs were tested on the pre-coated 96-well plate.
ELISA Assay
The binding of LN01, 10E8 and HK20 (Sabin et al., 2010) mAbs to the 28 amino acids long peptide encompassing the entire gp41 MPER was tested by ELISA using 96 well plates half-area. Briefly, ELISA plates were coated with 2 μg/ml of the long peptide (Pepscan), blocked with 1% BSA and incubated with titrated antibodies, followed by AP-conjugated anti-human IgG secondary antibody (SouthernBiotech). Plates were then washed and substrate (p-NPP, Sigma) was added. After 1h of incubation, plates were read at 405 nm. For human IgG quantification in the plasma of mice from in vivo experiment, 96 well plates were coated with 10 μg/ml of goat anti-human IgG UNL (SouthernBiotech), blocked with 1% BSA and incubated with titrated plasma, followed by AP-conjugated anti-human IgG secondary antibody (SouthernBiotech). Plates were then washed and substrate (p-NPP, Sigma) was added. After 30 min of incubation, plates were read at 405 nm. As standard to quantify human IgG concentration, serial dilutions of Rituximab were used.
Linear Epitope Mapping
Linear epitope mapping was performed by peptide microarray as previously reported (Gottardo et al., 2013, Shen et al., 2015). The peptides were printed onto 3D-Epoxy glass slides and were analyzed with a GenePix 4000B scanner (Tomaras et al., 2011). The binding of LN01 and 7B2 was detected by incubation with DyLight 649-labeled goat anti-human IgG. Fluorescence intensity was measured using a GenePix 4000B scanner and was analyzed with GenePix software. Signal intensity is the median fluorescence intensity of triplicate spots for each peptide included on array slides.
Gp41 Peptides
The MPER-TM1 peptide (671-NWFDITNWLWYIKLFIMIV-KKKKKK-689) was synthesized (Smart Bioscience, Grenoble). MPER-TM2 contains an N-terminal His-tag, a flag-tag a TEV protease site and gp41 residues 630 to 711 similar to the construct described before (Lutje Hulsik et al., 2013). For crystallization, the construct was engineered to contain a second TEV protease site inserted at position 649, which produced gp41-TM2 containing residues 650 to 711. The cDNAs were cloned into petM20 and expressed in C41 E. coli cells for 3h at 37⁰. Cells were lysed by sonication in a buffer containing 20 mM Tris pH8.0, 100 mM NaCl, 1% CHAPS. The soluble fraction was passed over a Ni2+-chromatography column. Purified gp41 was cleaved by TEV protease at a ratio (w/w) 1:10 passed over an anion exchange column followed by a second Ni2+-chromatography column to remove non cleaved protein. The resulting MPER-TM2 was further purified by size exclusion chromatography on a S200 column in a buffer containing either 25 mM Hepes pH7.5, 150 mM NaCl, 3 mM Fos-Choline-12 or 25 mM Hepes pH7.5, 150 mM NaCl, 1% β-OG (n-Octyl-β-D-glucosid). For complex formation of MPER-TM2 with LN01 Fabs, the complex was purified by SEC on a superdex 200 column in a buffer containing 25 mM Hepes pH7.5, 150 mM NaCl, 3 mM Fos-Choline-12. The complex was concentrated to 10 mg/ml. For complex formation of the MPER-TM1 peptide with LN01 Fabs, the peptide was solubilized in a buffer containing 25 mM Hepes pH7.5, 150 mM NaCl, 6 mM Fos-Choline-12 at 2 mg/ml, mixed with LN01 Fabs in a buffer containing 25 mM Hepes pH7.5, 150 mM NaCl, 3 mM Fos-choline-12 and concentrated to 10 mg/ml. The peptide was added to the LN01 Fabs at the molar ratio of 1:1.5 (Fab : MPER-TM1 peptide).
Surface Plasmon Resonance (SPR) Analysis
Biacore T200 (GE Healthcare, USA) was used for real-time binding interaction studies. Antibodies LN01 and 10E8 were immobilized via amine coupling on CM5 chips (GE Healthcare) in Hepes buffer (pH 7.5) at a flow rate of 10 μl/min until the surface plasmon resonance reached 2000 RU. Analyte concentrations varied from 1 to 2048 nM. Different dilutions of analytes were sequentially injected at a flow rate of 90 μl/min for 180s. MPER-TM1 and MPER-TM2 analytes were applied in a buffer containing 20 mM Tris pH 7.5, 100 mM NaCl, and either 1% β-OG (n-Octyl-β-D-glucosid) or 3 mM Fos-Choline-12. The dissociation time was set for 5 min and 0.1% SDS was injected for regeneration of the sensor chips. The sensogrammes were processed and visualized with Python. SPR profiles were fit to a double exponential equation. The model of biphasic binding was identified as the two state conformational change model, since the concentration dependencies of eigenvalues were linear. Dissociation constants were calculated as described (Tiwari et al., 2015).
Bio-layer Interferometry Binding Analysis
Binding measurements between LN01, LN01 variants and MPER-TM2 were carried out on an Octet Red instrument (ForteBio). For the determination of the binding between LN01 IgG and MPER-TM2, LN01 IgG were labelled with biotin (EZ-Link NHS-PEG4-Biotin) and bound to Streptavidin (SA) biosensors (ForteBio). The biosensors loaded with IgG were equilibrated in the kinetic buffer (25 mM HEPES pH 7.5, 150 mM NaCl and 0.1 % Fos-Choline-12) for 120 sec prior to measuring association with MPER-TM2 for 200 seconds at 25°C. Data were analyzed using the ForteBio analysis software version 11.1.0.25 (ForteBio). For LN01 sH/sL, LN01 gH/sL and LN01 sH/gL-FR the kinetic parameters were calculated using a global fit 1:1 model. For the determination of the binding of LN01 sH/sL, LN01 gH/gL and LN01 gH/UCA, KD were estimated by steady state analysis. For LN01 UCA/sL, LN01 UCA/gL and LN01 UCA/UCA no binding was detected in this experimental setup. All bio-layer interferometry experiments were conducted at least three times.
Crystallization, Data Collection and Structure Determination
Crystal screening was performed at the High Throughput Crystallisation Laboratory (HTX lab, EMBL Grenoble) in 96-well sitting drop vapour diffusion plates (Greiner). Following optimization, crystals used for diffraction studies were grown at 20°C (293 K) in hanging drop vapour diffusion plates. The LN01/MPER-TM1 complex was crystallized in 0.1 M HEPES pH 7.5, 10 %(w/v) PEG 3350. Crystal were grown by mixing 1 μl of the reservoir containing 0.1 M HEPES pH 7.5, 10 %(w/v) PEG 3350, 0.1%(w/v) Fos-Choline-12 and 1 μl of protein at a concentration of 8 mg/ml. The crystal was soaked into a cryo protectant solution containing 0.1 M HEPES pH 7.5, 5 % (w/v) PEG 3350, 30 %(w/v) Ethylene glycol, 0.1 %(w/v) Fos-Choline-12, and flash cooled in liquid N2 at 100 K. For LN01/MPER-TM1, data were collected on the ESRF beamline ID30A at a wavelength of 0.9677 Å. Data were processed with the program XDS (Kabsch, 2010). The LN01/MPER-TM1 crystals belong to space group P 43 21 2 (Table S2) and the structure was solved by molecular replacement using the program Phaser (McCoy et al., 2007) and a model of the LN01 Fab generated by I-Tasser (Zhang, 2008). The model was rebuilt using COOT (Emsley et al., 2010) and refined using REFMAC (Murshudov et al., 1997) and Phenix (Adams et al., 2010). Statistics for data reduction and structure refinement are presented in Table S2.
The crystals of LN01/MPER-TM1 plus 1,2-dihexanoyl-sn-glycero-3-phospho-L-serine (06:0 PS) were obtained by adding 1 mM of 06:0 PS in the protein solution and in the cryoprotectant solution. X-ray data were collected on the ESRF beamline ID23eh1, at a wavelength of 0.98 Å and data were processed with the program XDS (Kabsch, 2010).
The LN01/MPER-TM2 complex was crystallized by mixing 1 μl of 10 mg ml-1 of the LN01/MPER-TM2 complex and 1 μl of the reservoir solution containing 0.1 M HEPES pH 7.5, 9 %(w/v) PEG 8000, 8% Ethylene glycol, 10 mM MnCl2. For cryo protection, crystals were soaked in 0.1 M HEPES pH 7.5, 9 %(w/v) PEG 8000, 25 %(w/v) Ethylene glycol, 10 mM MnCl2, 0.1 %(w/v) Fos-Choline-12 prior to flash cooling in liquid N2 at 100 K. X-ray data were collected on the ESRF beamline ID30B at a wavelength of 0.9763 Å and data were processed with the program XDS (Kabsch, 2010). The LN01/MPER-TM2 crystals belong to space group P 21 21 21 (Table S2). The structure was solved by molecular replacement using the program Phaser (McCoy et al., 2007) and the LN01/MPER-TM1 model coordinates. The model was rebuilt using COOT (Emsley et al., 2010) and refined using REFMAC (Murshudov et al., 1997) and Phenix (Adams et al., 2010). Statistics for data reduction and structure refinement are presented in Table S2. The models were evaluated by using COOT and Phenix validation tools.
Two copies of the LN01/MPER-TM1 complex and 4 copies of the LN01/MPER-TM2 complex are present in the asymmetric units of the respective crystals. Numbering of the Fab was performed according to Kabat. The LN01/MPER-TM1 complex was refined to 3.2 Å data with an R/Rfree of 21.3/25.3%. 96.22% of the residues are within the most favoured regions of a Ramachandran plot. The LN01/MPER-TM1 + 06:0 PS complex was refined to 3.1 Å data with an R/Rfree of 23.8/26.7%. 95.8% of the residues are within the most favoured of a Ramachandran plot. The LN01/MPER-TM2 complex was refined to 3.9 Å data with an R/Rfree of 21.8/26.0% and 95.39% of the residues are within the most favoured regions of a Ramachandran plot. (Table S2). Some of the software packages used in this study were compiled by SBGrid (Morin et al., 2013).
Figure Generation
Molecular graphics figures were generated with PyMOL (W. Delano; The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC, http://www.pymol.org). To determine the angle of approach of antibodies LN01, 10E8, 4E10 and DH511.1 to gp41 MPER a similar method as described (Williams et al., 2017) was employed. Briefly, an axis was drawn from a spatial position midway between the variable region disulfide bond to the Cα atom of residue T676 thereby defining the direction of approach. The representation of the sequence conservation of MPER shown in Figure S6 was generated using WebLogo (Schneider and Stephens, 1990).
Molecular Dynamics (MD) Simulation
The molecular assay consisted of gp41 MPER-TM2 or only TM embedded in a lipid bilayer formed by 20 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), 44 1-palmitoyl-2-oleoyl-phosphatidylethanolamine (POPE), 18 1-palmitoyl-2-oleoyl-phosphatidyl serine (POPS), 28 sphingomyelin d18:0/d16:0 (SSM) and 90 cholesterol units in equilibrium with 11,123 water molecules, corresponding to a cell dimension of approximately 66 × 66 × 120 Å3. K+ and Cl- ions were added to reach an ionic concentration of 150 mM. All molecular dynamics (MD) simulations reported herein were performed employing the parallel, scalable program NAMD 2.12 (Phillips et al., 2005). Periodic boundary conditions (PBCs) were applied in the three directions of Cartesian space. Water was described by the TIP3P model (Jorgensen et al., 1983), and both gp41 and its lipid environment by the all-atom CHARMM36 force field (Jo et al., 2008, Klauda et al., 2010, MacKerell et al., 1998). A mass repartitioning scheme was introduced, allowing the equations of motion to be integrated with a time step of 4 fs, using the r-RESPA multiple time-step algorithm (Tuckerman et al., 1992). Covalent bonds involving hydrogen atoms were constrained to their equilibrium length by means of the RATTLE (Andersen, 1983) and SETTLE algorithms (Miyamoto and Kollman, 1992). The temperature and the pressure were maintained at 303 K and 1 atm, respectively, using Langevin dynamics and the Langevin piston method (Feller et al., 1995). Long-range electrostatic forces were taken into account by means of the particle mesh Ewald algorithm (Darden et al., 1993). A 12-Å cutoff was applied to truncate van der Waals and short-range Coulombic interactions. Visualization and analyses of the MD trajectories were performed with the VMD program (Humphrey et al., 1996). The free-energy landscape underlying the transition of gp41 from its straight to its bent form was determined using a multiple-walker version (Comer et al., 2014) of the MW-ABF algorithm (Comer et al., 2015) with a reaction coordinate model equal to the difference of the distance root mean square deviations (RMSD) with respect to these two conformations and eight walkers. No assumption was made on the initial orientation of the MPER-TM2 segment. The latter was placed such that its longitudinal axis was aligned with the normal to the lipid bilayer, i.e., the z-axis of Cartesian space. Next, the computational assay was suitably thermalized until a plateau in the dimensions of the membrane patch was reached and a steady orientation of the MPER-TM2 segment was observed. The tilt angle reported in this work is, therefore, a consequence of the sequence of the MPER-TM2 segment in relationship with the composition of the lipid bilayer, rather than an initial placement suggested by experiment. The steady orientation of the MPER-TM1 segment served as a starting point for the LN01-MPER-TM2 simulation.
The initial gp41 MPER-TM2 assay was built by aligning gp41 onto its equilibrium conformation sampled from the simulation with the isolated straight TM peptide. Lipids and water molecules were thermalized during 50 ns, while maintaining atoms of the protein complex to their crystallographic positions by means of harmonic restraints. Next, a set of three harmonic restraints over root-mean-square deviation collective variables was used to maintain backbones of LN01 and MPER-TM2 and side chains of LN01/MPER-TM2 contact residues in their reference conformation, respectively. Using this protocol, the orientation of the protein complex with respect to the bilayer was equilibrated during 200 ns. Finally, all the restraints were removed, and a 200 ns trajectory was produced.
Quantification and Statistical Analysis
Antidody EC50 and EC80 and serum ID50 were determined from log-transformed nonlinear regression, dose-response sigmoidal curve fit data using GraphPad 8. Antibody neutralization values higher then 12.5 or 25 μg/ml were considered as negative. The results were reported in Figures 1E and 2A and S1 and S3. Statistical models inherent to REFMAC (Murshudov et al., 1997) and Phenix (Adams et al., 2010) were employed for the structure refinement. All binding and neutralization assays were conducted with at least duplicate measurements. ADCC responses were analyzed in the Wilcoxon test. The results were reported in Figure 3C.
Data and Code Availability
All data generated or analyzed during this study are included in this published article (and its Supplemental Information). Atomic coordinates and structure factors of the reported crystal structures have been deposited in the Protein Data Bank (https://www.rcsb.org; PDB: 6SNC, 6SND, 6SNE).
Acknowledgments
This work was supported by Collaboration for AIDS Vaccine Discovery grants from the Bill and Melinda Gates Foundation (OPP1114725 to G.P. and A.L. and OPP1032144 to D.C.M.). Part of the project (W.W.) has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 681137. W.W. acknowledges the Institute Universitaire de France (IUF) and the platforms of the Grenoble Instruct-ERIC center (ISBG; UMS 3518 CNRS-CEA-UGA-EMBL) within the Grenoble Partnership for Structural Biology (PSB). Platform access was supported by FRISBI (ANR-10-INBS-05-02) and GRAL, a project of the University Grenoble Alpes graduate school (Ecoles Universitaires de Recherche) CBH-EUR-GS (ANR-17-EURE-0003). O.S. acknowledges funding from the Institut Pasteur, the ANRS, the Vaccine Research Institute and the Gilead HIV cure program. We thank the ESRF-EMBL Joint Structural Biology Group for access and support at the ESRF beam lines, J. Marquez (EMBL) from the HTX crystallization facility, J.-B. Reiser and A. Choquet for assistance on the SPR and BLI platforms, C. Mas for assistance on the SEC-MALLS platform, P. Raúl Armoa Ortiz for his contribution to crystallization, H. Mouquet for providing the monoclonal antibodies used in Figures 3C and 3D, and C. Atzori for clinical work.
Author Contributions
A.L., D.C., and G.P. conceived and designed the study. W.W. conceived and designed the structural and biophysical work. D.P. designed and performed experiments, and analyzed data. C.F. contributed to conceiving the project and coordinated profiling. C.S. carried out the neutralization studies. C. Caillat produced and characterized complexes by X-ray crystallography and BLI-binding studies. S.G. performed SPR experiments. F.D. and C. Chipot performed molecular dynamics simulation experiments. S.B. carried out antibodies sequencing and cloning. A.M. carried out in vivo studies. D.J. carried out cell sorting for B cell isolation. A.R. identified and recruited HIV-infected patients for the study. M.T. processed biological samples from patients. O.S., J.D., and T.B. conceived and performed the ADCC and CDC assays. G.D.T. and X.S. conceived and performed the peptide microarray assay. M.S.S. and D.C.M. performed neutralization assays. D.C., D.P., C.C., and W.W. wrote the manuscript with input from all authors.
Declaration of Interests
The authors declare no competing interests. D.P., A.M., and D.C. are employees of Vir Biotechnology and hold shares in Vir Biotechnology. LN01 is covered by patent application no. WO 2017/093985A1. Inventors: G.P. and A.L.
Published: October 22, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.chom.2019.09.016.
Contributor Information
Davide Corti, Email: dcorti@vir.bio.
Giuseppe Pantaleo, Email: giuseppe.pantaleo@chuv.ch.
Winfried Weissenhorn, Email: winfried.weissenhorn@ibs.fr.
Supplemental Information
References
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S.M., McAdams M., Boren D., Rak M., Scearce R.M., Gao F., Camacho Z.T., Gewirth D., Kelsoe G., Chen P. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 2007;178:4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S.M., Morelli M., Dennison S.M., Liao H.X., Zhang R., Xia S.M., Rits-Volloch S., Sun L., Harrison S.C., Haynes B.F. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. USA. 2009;106:20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen H.C. Rattle: a “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 1983;52:24–34. [Google Scholar]
- Apellániz B., Rujas E., Serrano S., Morante K., Tsumoto K., Caaveiro J.M., Jiménez M.Á., Nieva J.L. The atomic structure of the HIV-1 gp41 transmembrane domain and its connection to the immunogenic membrane-proximal external region. J. Biol. Chem. 2015;290:12999–13015. doi: 10.1074/jbc.M115.644351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G.H., Irimia A., Ofek G., Kwong P.D., Wilson I.A., Walensky L.D. Stapled HIV-1 peptides recapitulate antigenic structures and engage broadly neutralizing antibodies. Nat. Struct. Mol. Biol. 2014;21:1058–1067. doi: 10.1038/nsmb.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel T., Guivel-Benhassine F., Amraoui S., Malbec M., Richard L., Bourdic K., Donahue D.A., Lorin V., Casartelli N., Noël N. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat. Commun. 2016;7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brügger B., Glass B., Haberkant P., Leibrecht I., Wieland F.T., Kräusslich H.G. The HIV lipidome: a raft with an unusual composition. Proc. Natl. Acad. Sci. USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon V., Natrajan G., Schibli D., Campelo F., Kozlov M.M., Weissenhorn W. Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 2010;6:e1000880. doi: 10.1371/journal.ppat.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso R.M., Zwick M.B., Stanfield R.L., Kunert R., Binley J.M., Katinger H., Burton D.R., Wilson I.A. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Casartelli N., Sourisseau M., Feldmann J., Guivel-Benhassine F., Mallet A., Marcelin A.G., Guatelli J., Schwartz O. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010;6:e1000955. doi: 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B.K., Walker L.M., Guenaga J.F., Ghobbeh A., Poignard P., Burton D.R., Wyatt R.T. Direct antibody access to the HIV-1 membrane-proximal external region positively correlates with neutralization sensitivity. J. Virol. 2011;85:8217–8226. doi: 10.1128/JVI.00756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Chou J.J. Structure of the transmembrane domain of HIV-1 envelope glycoprotein. FEBS J. 2017;284:1171–1177. doi: 10.1111/febs.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Frey G., Peng H., Rits-Volloch S., Garrity J., Seaman M.S., Chen B. Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41. J. Virol. 2014;88:1249–1258. doi: 10.1128/JVI.02664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang J., Hwang K.K., Bouton-Verville H., Xia S.M., Newman A., Ouyang Y.B., Haynes B.F., Verkoczy L. Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10. J. Immunol. 2013;191:1260–1275. doi: 10.4049/jimmunol.1300770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L.V., Kozlov M.M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiliveri S.C., Louis J.M., Ghirlando R., Baber J.L., Bax A. Tilted, uninterrupted, monomeric HIV-1 gp41 transmembrane helix from residual dipolar couplings. J. Am. Chem. Soc. 2018;140:34–37. doi: 10.1021/jacs.7b10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer J., Gumbart J.C., Hénin J., Lelièvre T., Pohorille A., Chipot C. The adaptive biasing force method: everything you always wanted to know but were afraid to ask. J. Phys. Chem. 2015;119:1129–1151. doi: 10.1021/jp506633n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer J., Phillips J.C., Schulten K., Chipot C. Multiple-replica strategies for free-energy calculations in NAMD: multiple-walker adaptive biasing force and Walker selection rules. J. Chem. Theor. Comput. 2014;10:5276–5285. doi: 10.1021/ct500874p. [DOI] [PubMed] [Google Scholar]
- Darden T., York D., Pedersen L. Particle mesh Ewald: an N⋅log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- Dev J., Park D., Fu Q., Chen J., Ha H.J., Ghantous F., Herrmann T., Chang W., Liu Z., Frey G. Structural basis for membrane anchoring of HIV-1 envelope spike. Science. 2016;353:172–175. doi: 10.1126/science.aaf7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose N.A., Altae-Tran H.R., Roark R.S., Schmidt S.D., Sutton M.S., Louder M.K., Chuang G.Y., Bailer R.T., Cortez V., Kong R. Mapping polyclonal HIV-1 antibody responses via next-generation neutralization fingerprinting. PLoS Pathog. 2017;13:e1006148. doi: 10.1371/journal.ppat.1006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle-Cooper C., Hudson K.E., Cooper A.B., Ota T., Skog P., Dawson P.E., Zwick M.B., Schief W.R., Burton D.R., Nemazee D. Immune tolerance negatively regulates B cells in knock-in mice expressing broadly neutralizing HIV antibody 4E10. J. Immunol. 2013;191:3186–3191. doi: 10.4049/jimmunol.1301285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowska E., Le K.M., Ramos A., Doores K.J., Lee J.H., Blattner C., Ramirez A., Derking R., van Gils M.J., Liang C.H. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity. 2014;40:657–668. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller S.E., Zhang Y.H., Pastor R.W., Brooks B.R. Constant pressure molecular dynamics simulations − the Langevin piston method. J. Chem. Phys. 1995;103:4613–4621. [Google Scholar]
- Finton K.A., Friend D., Jaffe J., Gewe M., Holmes M.A., Larman H.B., Stuart A., Larimore K., Greenberg P.D., Elledge S.J. Ontogeny of recognition specificity and functionality for the broadly neutralizing anti-HIV antibody 4E10. PLoS Pathog. 2014;10:e1004403. doi: 10.1371/journal.ppat.1004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey G., Peng H., Rits-Volloch S., Morelli M., Cheng Y., Chen B. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. USA. 2008;105:3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardo R., Bailer R.T., Korber B.T., Gnanakaran S., Phillips J., Shen X., Tomaras G.D., Turk E., Imholte G., Eckler L. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One. 2013;8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B.F., Fleming J., St Clair E.W., Katinger H., Stiegler G., Kunert R., Robinson J., Scearce R.M., Plonk K., Staats H.F. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Hessell A.J., Rakasz E.G., Tehrani D.M., Huber M., Weisgrau K.L., Landucci G., Forthal D.N., Koff W.C., Poignard P., Watkins D.I. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Kang B.H., Pancera M., Lee J.H., Tong T., Feng Y., Imamichi H., Georgiev I.S., Chuang G.Y., Druz A. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Ofek G., Laub L., Louder M.K., Doria-Rose N.A., Longo N.S., Imamichi H., Bailer R.T., Chakrabarti B., Sharma S.K. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Yu J., Lanzi A., Yao X., Andrews C.D., Tsai L., Gajjar M.R., Sun M., Seaman M.S., Padte N.N. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell. 2016;165:1621–1631. doi: 10.1016/j.cell.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graphics. 1996;14(33–38):27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Irimia A., Sarkar A., Stanfield R.L., Wilson I.A. Crystallographic identification of lipid as an integral component of the epitope of HIV broadly neutralizing antibody 4E10. Immunity. 2016;44:21–31. doi: 10.1016/j.immuni.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Serra A.M., Sarkar A., Jacak R., Kalyuzhniy O., Sok D., Saye-Francisco K.L., Schiffner T., Tingle R., Kubitz M. Lipid interactions and angle of approach to the HIV-1 viral membrane of broadly neutralizing antibody 10E8: insights for vaccine and therapeutic design. PLoS Pathog. 2017;13:e1006212. doi: 10.1371/journal.ppat.1006212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R.A., Moyo T., Schomaker M., Abrahams F., Grau Pujol B., Dorfman J.R. Anti-V3/glycan and anti-MPER neutralizing antibodies, but not anti-V2/glycan site antibodies, are strongly associated with greater anti-HIV-1 neutralization breadth and potency. J. Virol. 2015;89:5264–5275. doi: 10.1128/JVI.00129-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Kim T., Iyer V.G., Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- Jorgensen W.L., Chandrasekhar J., Madura J.D., Impey R.W., Klein M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–935. [Google Scholar]
- Julien J.P., Huarte N., Maeso R., Taneva S.G., Cunningham A., Nieva J.L., Pai E.F. Ablation of the complementarity-determining region H3 apex of the anti-HIV-1 broadly neutralizing antibody 2F5 abrogates neutralizing capacity without affecting core epitope binding. J. Virol. 2010;84:4136–4147. doi: 10.1128/JVI.02357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 2010;66:133–144. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauda J.B., Venable R.M., Freites J.A., O'Connor J.W., Tobias D.J., Mondragon-Ramirez C., Vorobyov I., MacKerell A.D., Jr., Pastor R.W. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., Diskin R., Scheid J.F., Gaebler C., Mouquet H., Georgiev I.S., Pancera M., Zhou T., Incesu R.B., Fu B.Z. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong R., Louder M.K., Wagh K., Bailer R.T., deCamp A., Greene K., Gao H., Taft J.D., Gazumyan A., Liu C. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J. Virol. 2015;89:2659–2671. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs S.J., Kwon Y.D., Schramm C.A., Law W.H., Donofrio G., Zhou K.H., Gift S., Dussupt V., Georgiev I.S., Schätzle S. Longitudinal analysis reveals early development of three MPER-directed neutralizing antibody lineages from an HIV-1-infected individual. Immunity. 2019;50:677–691.e13. doi: 10.1016/j.immuni.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B., Lee M., Waring A.J., Hong M. Oligomeric structure and three-dimensional fold of the HIV gp41 membrane-proximal external region and transmembrane domain in phospholipid bilayers. J. Am. Chem. Soc. 2018;140:8246–8259. doi: 10.1021/jacs.8b04010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong P.D., Mascola J.R. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity. 2018;48:855–871. doi: 10.1016/j.immuni.2018.04.029. [DOI] [PubMed] [Google Scholar]
- Lai R.P., Hock M., Radzimanowski J., Tonks P., Hulsik D.L., Effantin G., Seilly D.J., Dreja H., Kliche A., Wagner R. A fusion intermediate gp41 immunogen elicits neutralizing antibodies to HIV-1. J. Biol. Chem. 2014;289:29912–29926. doi: 10.1074/jbc.M114.569566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landais E., Huang X., Havenar-Daughton C., Murrell B., Price M.A., Wickramasinghe L., Ramos A., Bian C.B., Simek M., Allen S. Broadly neutralizing antibody responses in a large longitudinal sub-Saharan HIV primary infection cohort. PLoS Pathog. 2016;12:e1005369. doi: 10.1371/journal.ppat.1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Ozorowski G., Ward A.B. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science. 2016;351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H.X., Chen X., Munshaw S., Zhang R., Marshall D.J., Vandergrift N., Whitesides J.F., Lu X., Yu J.S., Hwang K.K. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J. Exp. Med. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y., Meng F., Kondo N., Iwamoto A., Matsuda Z. Conserved arginine residue in the membrane-spanning domain of HIV-1 gp41 is required for efficient membrane fusion. Protein Cell. 2011;2:369–376. doi: 10.1007/s13238-011-1051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutje Hulsik D., Liu Y.Y., Strokappe N.M., Battella S., El Khattabi M., McCoy L.E., Sabin C., Hinz A., Hock M., Macheboeuf P. A gp41 MPER-specific llama VHH requires a hydrophobic CDR3 for neutralization but not for antigen recognition. PLoS Pathog. 2013;9:e1003202. doi: 10.1371/journal.ppat.1003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKerell A.D., Bashford D., Bellott M., Dunbrack R.L., Evanseck J.D., Field M.J., Fischer S., Gao J., Guo H., Ha S. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Kollman P.A. SETTLE: an analytical version of the SHAKE and RATTLE algorithms for rigid water models. J. Comput. Chem. 1992;13:952–962. [Google Scholar]
- Molinos-Albert L.M., Carrillo J., Curriu M., Rodriguez de la Concepción M.L., Marfil S., García E., Clotet B., Blanco J. Anti-MPER antibodies with heterogeneous neutralization capacity are detectable in most untreated HIV-1 infected individuals. Retrovirology. 2014;11:44. doi: 10.1186/1742-4690-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D.C. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- Moody M.A., Pedroza-Pacheco I., Vandergrift N.A., Chui C., Lloyd K.E., Parks R., Soderberg K.A., Ogbe A.T., Cohen M.S., Liao H.X. Immune perturbations in HIV-1-infected individuals who make broadly neutralizing antibodies. Sci. Immunol. 2016;1:aag0851. doi: 10.1126/sciimmunol.aag0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A., Eisenbraun B., Key J., Sanschagrin P.C., Timony M.A., Ottaviano M., Sliz P. Collaboration gets the most out of software. Elife. 2013;2:e01456. doi: 10.7554/eLife.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita J., Kano K., Kato K., Takita H., Sakagami H., Yamamoto Y., Mihara E., Ueda H., Sato T., Tokuyama H. Structure and biological function of ENPP6, a choline-specific glycerophosphodiester-phosphodiesterase. Sci. Rep. 2016;6:20995. doi: 10.1038/srep20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H., Scharf L., Euler Z., Liu Y., Eden C., Scheid J.F., Halper-Stromberg A., Gnanapragasam P.N., Spencer D.I., Seaman M.S. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. USA. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H., Scheid J.F., Zoller M.J., Krogsgaard M., Ott R.G., Shukair S., Artyomov M.N., Pietzsch J., Connors M., Pereyra F. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov G.N., Vagin A.A., Dodson E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Ofek G., McKee K., Yang Y., Yang Z.Y., Skinner J., Guenaga F.J., Wyatt R., Zwick M.B., Nabel G.J., Mascola J.R. Relationship between antibody 2F5 neutralization of HIV-1 and hydrophobicity of its heavy chain third complementarity-determining region. J. Virol. 2010;84:2955–2962. doi: 10.1128/JVI.02257-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M., Zhou T., Druz A., Georgiev I.S., Soto C., Gorman J., Huang J., Acharya P., Chuang G.Y., Ofek G. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegu A., Yang Z.Y., Boyington J.C., Wu L., Ko S.Y., Schmidt S.D., McKee K., Kong W.P., Shi W., Chen X. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci. Transl. Med. 2014;6:243ra88. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L.G., Costa M.R., Todd C.A., Haynes B.F., Montefiori D.C. Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: a specific role for antibodies against the membrane-proximal external region of gp41. J. Virol. 2009;83:7397–7410. doi: 10.1128/JVI.00656-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R.D., Kalé L., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzsch J., Scheid J.F., Mouquet H., Seaman M.S., Broder C.C., Nussenzweig M.C. Anti-gp41 antibodies cloned from HIV-infected patients with broadly neutralizing serologic activity. J. Virol. 2010;84:5032–5042. doi: 10.1128/JVI.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinakumar R., Dutta M., Zhu P., Johnson W.E., Roux K.H. Binding of anti-membrane-proximal gp41 monoclonal antibodies to CD4-liganded and -unliganded human immunodeficiency virus type 1 and simian immunodeficiency virus virions. J. Virol. 2012;86:1820–1831. doi: 10.1128/JVI.05489-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichart T.M., Baksh M.M., Rhee J.K., Fiedler J.D., Sligar S.G., Finn M.G., Zwick M.B., Dawson P.E. Trimerization of the HIV transmembrane domain in lipid bilayers modulates broadly neutralizing antibody binding. Angew. Chem. Int. Ed. Engl. 2016;55:2688–2692. doi: 10.1002/anie.201508421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick S.L., Chan W.W., Agate D.S., Olsen L.R., Vetting M.W., Rajashankar K.R., Cohen D.E. Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nat. Struct. Biol. 2002;9:507–511. doi: 10.1038/nsb812. [DOI] [PubMed] [Google Scholar]
- Rujas E., Caaveiro J.M., Partida-Hanon A., Gulzar N., Morante K., Apellániz B., García-Porras M., Bruix M., Tsumoto K., Scott J.K. Structural basis for broad neutralization of HIV-1 through the molecular recognition of 10E8 helical epitope at the membrane interface. Sci. Rep. 2016;6:38177. doi: 10.1038/srep38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin C., Corti D., Buzon V., Seaman M.S., Lutje Hulsik D., Hinz A., Vanzetta F., Agatic G., Silacci C., Mainetti L. Crystal structure and size-dependent neutralization properties of HK20, a human antibody binding to the highly conserved heptad repeat 1 of gp41. PLoS Pathog. 2010;6:e1001195. doi: 10.1371/journal.ppat.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid J.F., Mouquet H., Ueberheide B., Diskin R., Klein F., Oliveira T.Y., Pietzsch J., Fenyo D., Abadir A., Velinzon K. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer E.M., Leaman D.P., Zwick M.B., McMichael A.J., Burton D.R. Aromatic residues at the edge of the antibody combining site facilitate viral glycoprotein recognition through membrane interactions. Proc. Natl. Acad. Sci. USA. 2010;107:1529–1534. doi: 10.1073/pnas.0909680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibli D.J., Weissenhorn W. Class I and class II viral fusion protein structures reveal similar principles in membrane fusion (Review) Mol. Membr. Biol. 2004;21:361–371. doi: 10.1080/09687860400017784. [DOI] [PubMed] [Google Scholar]
- Schneider T.D., Stephens R.M. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Dennison S.M., Liu P., Gao F., Jaeger F., Montefiori D.C., Verkoczy L., Haynes B.F., Alam S.M., Tomaras G.D. Prolonged exposure of the HIV-1 gp41 membrane proximal region with L669S substitution. Proc. Natl. Acad. Sci. USA. 2010;107:5972–5977. doi: 10.1073/pnas.0912381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Duffy R., Howington R., Cope A., Sadagopal S., Park H., Pal R., Kwa S., Ding S., Yang O.O. Vaccine-induced linear epitope-specific antibodies to simian immunodeficiency virus SIVmac239 envelope are distinct from those induced to the human immunodeficiency virus type 1 envelope in nonhuman primates. J. Virol. 2015;89:8643–8650. doi: 10.1128/JVI.03635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova Y.G., Risselada H.J., Müller M. Thermodynamically reversible paths of the first fusion intermediate reveal an important role for membrane anchors of fusion proteins. Proc. Natl. Acad. Sci. USA. 2019;116:2571–2576. doi: 10.1073/pnas.1818200116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D., Burton D.R. Recent progress in broadly neutralizing antibodies to HIV. Nat. Immunol. 2018;19:1179–1188. doi: 10.1038/s41590-018-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler G., Kunert R., Purtscher M., Wolbank S., Voglauer R., Steindl F., Katinger H. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 2001;17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- Tiller T., Meffre E., Yurasov S., Tsuiji M., Nussenzweig M.C., Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari P.B., Wang X., He J., Darici Y. Analyzing surface plasmon resonance data: choosing a correct biphasic model for interpretation. Rev. Sci. Instrum. 2015;86:035001. doi: 10.1063/1.4914027. [DOI] [PubMed] [Google Scholar]
- Tomaras G.D., Binley J.M., Gray E.S., Crooks E.T., Osawa K., Moore P.L., Tumba N., Tong T., Shen X., Yates N.L. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J. Virol. 2011;85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trama A.M., Moody M.A., Alam S.M., Jaeger F.H., Lockwood B., Parks R., Lloyd K.E., Stolarchuk C., Scearce R., Foulger A. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell Host Microbe. 2014;16:215–226. doi: 10.1016/j.chom.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckerman M.E., Berne B.J., Martyna G.J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 1992;97:1990–2001. [Google Scholar]
- Wagh K., Bhattacharya T., Williamson C., Robles A., Bayne M., Garrity J., Rist M., Rademeyer C., Yoon H., Lapedes A. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 Clade C infection. PLoS Pathog. 2016;12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.M., Huber M., Doores K.J., Falkowska E., Pejchal R., Julien J.P., Wang S.K., Ramos A., Chan-Hui P.Y., Moyle M. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H., Yurasov S., Schaefer A., Young J.W., Meffre E., Nussenzweig M.C. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W., Dessen A., Harrison S.C., Skehel J.J., Wiley D.C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- Williams L.D., Ofek G., Schätzle S., McDaniel J.R., Lu X., Nicely N.I., Wu L., Lougheed C.S., Bradley T., Louder M.K. Potent and broad HIV-neutralizing antibodies in memory B cells and plasma. Sci. Immunol. 2017;2 doi: 10.1126/sciimmunol.aal2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M.B., Labrijn A.F., Wang M., Spenlehauer C., Saphire E.O., Binley J.M., Moore J.P., Stiegler G., Katinger H., Burton D.R. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplemental Information). Atomic coordinates and structure factors of the reported crystal structures have been deposited in the Protein Data Bank (https://www.rcsb.org; PDB: 6SNC, 6SND, 6SNE).