Abstract
Alzheimer's disease (AD) is a neurodegenerative disease of the central nervous system that is characterized by progressive cognitive dysfunction and which ultimately leads to dementia. Studies have shown that energy dysmetabolism contributes significantly to the pathogenesis of a variety of aging-associated diseases and degenerative diseases of the nervous system, including AD. One focus of research thus has been how to regulate the expression of nicotinamide phosphoribosyltransferase (NAMPT) to prevent against neurodegenerative diseases. Therefore, the present study used 6-month-old APPswe/PS1ΔE9 (APP/PS1) transgenic mice as early AD mouse models and sought to evaluate nicotinamide adenine dinucleotide (NAD+) and FK866 (a NAMPT inhibitor) treatment in APP/PS1 mice to study NAMPT dysmetabolism in the process of AD and elucidate the underlying mechanisms. As a result of this treatment, the expression of NAMPT decreased, the synthesis of ATP and NAD+ became insufficient and the NAD+/NADH ratio was reduced. The administration of NAD+ alleviated the spatial learning and memory of APP/PS1 mice and reduced senile plaques. Administration of NAD+ may also increase the expression of the key protein NAMPT and its related protein sirtuin 1 as well as the synthesis of NAD+. Therefore, increasing NAMPT expression levels may promote NAD+ production. Their regulation could form the basis for a new therapeutic strategy.
Keywords: Alzheimer's disease, nicotinamide adenine dinucleotide, nicotinamide phosphoribosyltransferase
Introduction
Alzheimer's disease (AD) is a neurodegenerative disease of the central nervous system that is characterized by progressive cognitive dysfunction, ultimately leading to dementia. While the pathological hallmarks are mainly the formation of extracellular senile plaques, intracellular neurofibrillary tangles and neuronal loss, its pathogenesis has not been fully elucidated (1). Previous studies have demonstrated that energy dysmetabolism contributes significantly to the pathogenesis of a variety of aging-associated diseases and degenerative disease of the nervous system, including AD (2,3). With aging, the imbalance of energy metabolism in the brain is intensified, which promotes amyloid (A)β production. Aβ further induces energy metabolism disorder in the brain (4,5). When the energy metabolism of neurons in the brain is deregulated, ATP synthesis is insufficient and leads to irreversible damage to neuronal structure and function (6,7). Since research has demonstrated that energy disorders of the brain have an important impact on the development of AD, improving the energy metabolism of nerve cells in the brain is expected to delay the pathogenesis of AD (8–10).
Nicotinamide adenine dinucleotide (NAD+) is an important coenzyme in the redox reaction, which can participate in energy synthesis through glycolysis, the tricarboxylic acid cycle and mitochondrial oxidative phosphorylation (10). The majority of NAD+ is synthesized by a salvage pathway from the conversion of nicotinamide to a nicotinamide mononucleotide, which is subsequently converted to NAD+ (11). Nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme in the NAD+ rescue pathway and is involved in energy metabolism by regulating NAD+ synthesis (12), maintaining the homeostasis of energy metabolism in the brain and thereby increasing the expression of NAD+ (the level of NAMPT has been proven to regulate positively NAD+ levels) (13). However, few studies to date have directly explored the potential therapeutic effect of targeting NAMPT and NAD+ biosynthesis, despite it being known that NAD+ is the starting point of the majority of key metabolic pathways and therefore the key governor of cellular aging and age-related processes (14,15). How to regulate the expression of NAMPT to protect against neurodegenerative diseases such as AD has become the focus of recent research.
The present study used 6-month-old APPswe/PS1ΔE9 (APP/PS1) transgenic mice as early AD mouse models and observed the mechanism of NAMPT-related pathways in the process of AD.
Materials and methods
Ethics statement
All procedures were performed in accordance with the Guide for and Use of Medical Laboratory Animals (Ministry of Health China, 1998) (16) and were approved by the Shanghai University of Traditional Chinese Medicine (Shanghai, China) Laboratory Animal Care and Use Committee (no. PZSHUTCM19011807).
APP/PS1 transgenic mice and drug treatment
In total, 18 male APP/PS1 transgenic mice (age: 24 weeks; weight: 26±3 g) and 6 male C57BL/6 mice (age: 24 weeks; weight: 27±2 g) were used in the present study. The APP/PS1 mouse strain is a double-transgenic hemizygote that expresses a chimeric mouse/human amyloid precursor protein and mutant human presenilin-1. These transgenic mice were used as they develop behavioral and pathology features of AD similar to patients and have been used in previous AD studies worldwide (17–19). Separately, C57BL/6 mice were used as age-matched controls. The APP/PS1 transgenic mice were purchased from the Model Animal Research Center of Nanjing University, while C57BL/6 mice were obtained from the Experimental Animal Center of Shanghai Academy. The NAD+ group were intraperitoneally injected with NAD+ (30 mg/kg, Sigma-Aldrich; Merck KGaA) at 20 weeks of age and once every other day for 4 weeks. The FK866 (NAMPT inhibitor) group were intraperitoneally injected with FK866 (1 mg/kg, Sigma-Aldrich; Merck KGaA) at 20 weeks of age and once every other day for 4 weeks. The mice were housed in a controlled specific-pathogen-free environment (22±3°C; 60% relative humidity; 12-h light/dark cycle) with ad libitum access to water and food.
Morris water maze test
Spatial learning and memory of the mice were assessed using a Morris water maze test (MWM) according to a previous study (20) with minor modifications. The water was made opaque with titanium dioxide and water temperature was kept at 22±2°C. A platform was placed 1 cm under the surface of the water. In the hidden platform experiment, mice received 4 training trials per day for 5 consecutive days. On the first trial of the first day, mice were placed on the platform for 10 sec, after which they were placed in the water. The pool area was conceptually divided into four quadrants of equal size. Taking the quadrant of the platform as the first quadrant, the second and fourth quadrants were taken as the starting point for 2 trials and the mice were placed facing the pool wall. If a mouse failed to find the platform in 70 sec, it was gently guided to the platform location and allowed to stay on it for 30 sec. The time to find the platform was recorded as the escape latency. The experiments were recorded with a camera connected a video recorder and a computerized tracking system, with the automatic timer set to 70 sec.
In the probe trials, at 24 h after the last training trial, the platform was removed and the mice were placed at the second and fourth quadrants, and the time of the target quadrant (first quadrant) and the number of times crossing the location that previously contained the platform within 70 sec were recorded.
Thioflavin S staining
Mice were anesthetized with 5% chloral hydrate at a dose of 400 mg/kg intraperitoneally and saline solution was used for perfusion through the heart, which was followed by 4% paraformaldehyde. The brains of the mice were then removed, fixed in 4% paraformaldehyde for 24 h at room temperature and immersed in 30% sucrose until they sank. The brain tissue was serially sectioned at a 30-µm thickness using a HM1950 freezing microtome. The sections were permeabilized in xylene for 10 min, dehydrated using anhydrous ethanol for 5 min, and then stained with 1% thioflavin S (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. The stained samples were differentiated in 70% alcohol solution for 5 min, mounted with glycerol gel, and observed using fluorescence microscopy (magnification, 200×; Olympus BX51; Olympus Corporation).
NAD+/NADH analysis
A NAD+/NADH quantification kit was used to determine NAD+/NADH levels (cat. no. k337-100; BioVision, Inc.), according to the manufacturer's instructions. Hippocampus tissue (20 mg) was washed with precooled phosphate-buffered saline (PBS), homogenized in 400 µl of the NAD+/NADH extraction buffer and then centrifuged for 5 h at 18,000 × g at 4°C. The resulting supernatant was labeled as total NAD+ sample (NAD+t). Subsequently, 200 µl of the NAD+t sample was heated at 60°C for 30 min (to consume all NAD+ from the sample, leaving only NADH to be analyzed). Following cooling on ice, the sample was centrifuged at 12,000 × g for 30 sec at 4°C and the resulting supernatant was labeled as the NADH sample. NAD+/NADH extraction buffer was added to bring the volume to 50 µl. Subsequently, 100 µl of enzyme reaction mix and 10 µl of NADH developer were added to each well, the mixture was incubated at room temperature for 2 h and the absorbance was measured using a microplate reader (wavelength: 450 nm).
ATP content detection
Cortical or hippocampus tissue (10 mg) was washed with precooled PBS, homogenized in 100 µl of lysis buffer (cat. no. P0013B: Beyotime Institute of Biotechnology) and centrifuged at 12,000 × g at 4°C for 5 min. The resulting supernatant was transferred to a new Eppendorf tube and then the reaction solution (ATP Assay Buffer, ATP probe, ATP Converter and Develop Mix; Sigma-Aldrich; Merck KGaA) was added to bring the volume to 50 µl. The reaction solution was shaken in a shaker for 30 min at room temperature. To correct for the background of the samples, a well per sample was used as the sample background well. The absorbance was measured at a wavelength of 570 nm using a microplate reader.
Immunohistochemical staining
Following a behavioral test, three mice were randomly selected in each group. Following anesthesia, the brains were perfused with 4% paraformaldehyde for 4 h and then transferred to a 30% sucrose gradient at room temperature. After the brains had sunk to the bottom, continuous coronal sectioning was performed (30 µm thickness) using a HM1950 freezing microtome. The sections were permeabilized in xylene for 10 min and dehydrated using anhydrous ethanol for 5 min. The sections were washed with 0.01 M of PBS and blocked with 5% BSA (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. Then the sections were incubated with polyclonal anti-NAMPT antibody (1:200; cat. no. ab45890; Abcam) overnight at 4°C. Following rinsing with PBS, sections were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (1:500; cat. no. SA00001-2; ProteinTech Group, Inc.) for 1 h at 37°C, washed with PBS, and streptavidin-biotin complex (Beyotime Institute of Biotechnology) was added. Then the sections were developed with 3,3′-diaminobenzidine for 10 min at room temperature and dehydrated with gradient alcohol, cleared with xylene, sealed with neutral gum and observed with using a light microscope (magnification, ×200; Olympus BX51; Olympus Corporation).
Western blot analysis
The isolated hippocampus tissue was collected, homogenized in lysis buffer (cat. no. P0013B; Beyotime Institute of Biotechnology), lysed on ice for 30 min and centrifuged at 12,000 × g for 20 min at 4°C. Total protein concentration was determined using a Micro BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). Then, 30 µg of protein sample was heated at 95°C for 5 min and separated by 10% SDS-polyacrylamide gel electrophoresis, after which it was transferred onto a PVDF membrane. The membrane was blocked with 3% BSA at 37°C for 1 h. Following blocking, the membranes were incubated with primary antibodies (anti-NAMPT 1:500, cat. no. ab45890, Abcam; anti-SIRT1, 1:1,000, cat. no. ab110304, Abcam; and GAPDH, 1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.) overnight at 4°C. The membrane was washed with TBST three times for 10 min and then treated with HRP-labeled goat anti-rabbit secondary antibody (1:2,000; cat. no. SA00001-2; ProteinTech Group, Inc.) and HRP-labeled goat anti-mouse secondary antibody (1:2,000; cat. no. SA00001-1; ProteinTech Group, Inc.) for 1 h at 37°C. Blots were washed three times for 10 min in TBST. After incubation with enhanced chemiluminescence chromogenic solution (cat. no. P0018s; Beyotime Institute of Biotechnology) for 1 min at room temperature, quantification of the proteins of interest was determined relative to GAPDH using ImageJ software (version 1.4; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad software 6.0 (GraphPad Software, Inc.). Measurement data are presented as the mean ± standard error of the mean. Two-way ANOVA followed by Bonferroni post hoc test was used to analyze group differences in the escape latency. A one-way ANOVA followed by LSD multiple-range test was used in the probe trial. For the non-normally distributed data or for data with heterogeneous variance, a Kruskal-Wallis test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
APP/PS1 mice exhibit impaired performance and effects of NAD+ and FK866 treatment in the MWM tasks
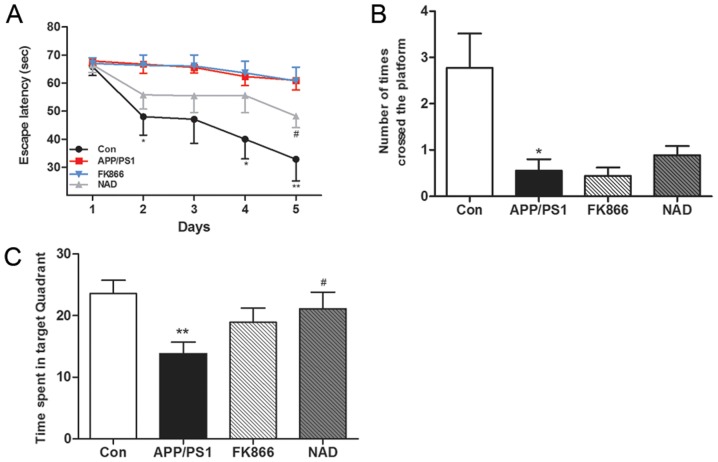
Memory impairment in APP/PS1 transgenic mice was determined using the MWM test in terms of the escape latency. The results demonstrated that, with the increase in the number of training days, the escape latency gradually shortened. On the fifth day, the escape latency of the APP/PS1 group (AD) mice was significantly longer compared with the blank control group (P<0.01), suggesting that the learning and memory abilities of AD mice were significantly impaired. Furthermore, the escape latency of NAD+ mice was significantly shorter than that of APP/PS1 mice after intraperitoneal injection of NAD+ (P<0.01); while, after FK866 intervention, there was no significant change in escape latency in the FK866 mice compared with the APP/PS1 mice (Fig. 1A).
Figure 1.
APP/PS1 mice exhibit decreased performance and effects of NAD+ and FK866 treatment in the Morris water maze tasks. (A) Escape latency (latency to find the hidden platform) was determined in a 5-day trial study. Day 6 was a probe trial, in which (B) the number of platform crossings and (C) time spent in the target quadrant were determined (total time of the trial was 70 sec). Data are expressed as the mean ± standard error of the mean. n=8. *P<0.05, **P<0.01 vs. Con; and #P<0.05 vs. APP/PS1 group. APP/PS1, APPswe/PS1ΔE9; NAD, nicotinamide adenine dinucleotide; FK866, an inhibitor of NAMPT; NAMPT, nicotinamide phosphoribosyltransferase; Con, control.
In the space exploration experiment on the sixth day, after removing the platform it was found that APP/PS1 mice significantly reduced the target quadrant residence time (P<0.01) compared with control group mice, indicating that the spatial memory ability of AD mice was impaired. Following NAD+ treatment, the NAD+ mice spent more time in the target quadrant compared with the APP/PS1 mice (P<0.01); however, there was no significant change in the target quadrant residence time of the mice in the APP/PS1 group compared with the FK866 group (Fig. 1B). In comparison with the control group, the number of platform crossings was significantly reduced in the APP/PS1 group (P<0.05) and the NAD+ mice exhibited an increased number of platform crossings compared with the APP/PS1 mice, but these findings were not statistically significant (P>0.05). Overall, there was no significant change between the APP/PS1 group and FK866 group (Fig. 1C).
NAD+ treatment reduces amyloid plaques of cortex and hippocampus in APP/PS1 transgenic mice
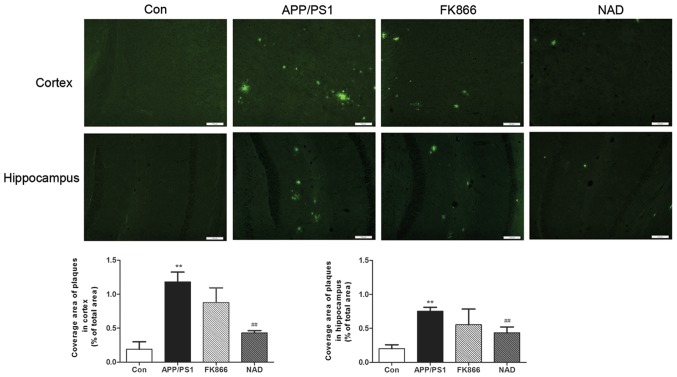
Staining with Thioflavin S for senile plaques in the cortex and hippocampus was conducted. As expected, the AD mice presented numerous plaques at 6 months of age as compared with the control mice in cortex and hippocampus; however, following NAD+ treatment, scattered green fluorescent plaques were observed in the cortex and hippocampus of every group and, after FK866 injection, multiple green fluorescent plaques were also seen in the cortex and hippocampus in AD mice (Fig. 2).
Figure 2.
Histological images of Thioflavin S staining in the cortex and hippocampus and the effect of NAD+ and FK866 on amyloid plaques. Scale bar, 100 µm. **P<0.01 vs. Con; and ##P<0.01 vs. APP/PS1. NAD, nicotinamide adenine dinucleotide; FK866, an inhibitor of NAMPT; NAMPT, nicotinamide phosphoribosyltransferase; Con, control.
NAD+ treatment improves ATP content of cortex and hippocampus in APP/PS1 transgenic mice
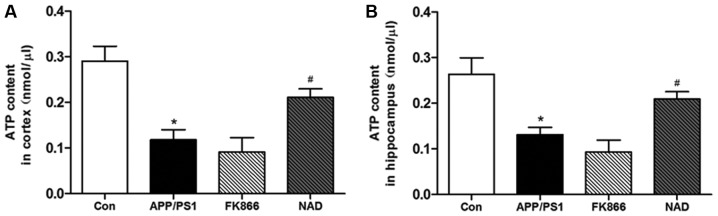
ATP content in the cortex and hippocampus was measured in each group of mice. The results demonstrated that, compared with the control group, the ATP content in the cortex in the APP/PS1 group was significantly decreased (P<0.05), while, in comparison with the APP/PS1 model group, the ATP content in the cortex in the NAD+ group was significantly increased (P<0.05). Following FK866 intervention, the ATP content of the cortex decreased compared with APP/PS1 mice, but without statistical significance (P>0.05). Additionally, in comparison with the control group, the ATP content in the hippocampus in the APP/PS1 group was significantly decreased (P<0.05), while, compared with the APP/PS1 model group, the ATP content in the hippocampus in the NAD+ group was significantly increased (P<0.05). Following FK866 intervention, there was no significant change in the NAD+ group compared with APP/PS1 model group (Fig. 3).
Figure 3.
APP/PS1 mice exhibited decreased ATP content, and the effects of NAD+ and FK866 treatment on ATP content. (A) Change of ATP content in the cortex. (B) Change of ATP content in the hippocampus. All data are expressed as the mean ± standard error of the mean. n=3. *P<0.05, vs. Con; and #P<0.05, vs. APP/PS1. APP/PS1, APPswe/PS1ΔE9; NAD, nicotinamide adenine dinucleotide; FK866, an inhibitor of NAMPT; NAMPT, nicotinamide phosphoribosyltransferase; Con, control.
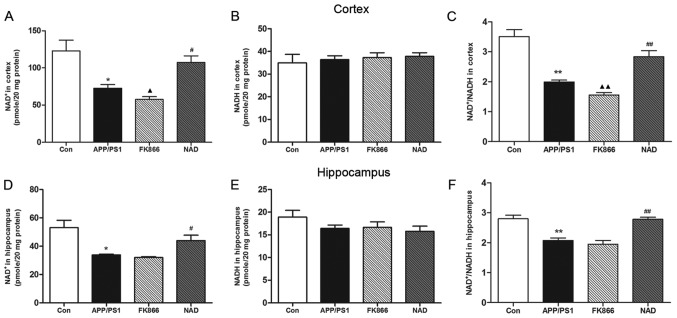
NAD+ treatment increases NAD+ level and NAD+/NADH ratio in APP/PS1 transgenic mice
NAD+ and NADH are essential coenzymes for cell energy metabolism. Under normal physiological conditions, the NAD+/NADH ratio reflects the redox state of cells. When the energy metabolism of the cells is deregulated, the levels of NAD+ and NADH will change, which is reflected as an imbalance in the NAD+/NADH ratio (21). The present study detected NAD+ and NADH levels in the cortex and hippocampus. The NAD+ content in the cortex in the APP/PS1 group was significantly decreased compared with the control group (P<0.05) and the NAD+/NADH ratio was similarly decreased (P<0.01), although the NADH level was not significantly changed. Compared with the APP/PS1 model group, the NAD+ content of the cortex was significantly increased in the NAD+ group (P<0.05) and the NAD+/NADH ratio was significantly increased (P<0.01), while the NADH level was not significantly changed. Separately, in comparison with APP/PS1 mice, NAD+ content in the cortex decreased significantly in the FK866 group (P<0.05) and the NAD+/NADH ratio decreased (P<0.01), although the NADH level did not change significantly between the two groups.
Compared with the control group, the NAD+ content in the hippocampus in the APP/PS1 group was significantly decreased (P<0.05), while the NADH level was not significantly changed and the NAD+/NADH ratio was decreased (P<0.01). Furthermore, compared with the APP/PS1 model group, the NAD+ content in the hippocampus in the NAD+ group was significantly increased (P<0.05) and the NAD+/NADH ratio was significantly increased (P<0.01), while the NADH level was not significantly different. Regarding the NAD+ content, NADH level and NAD+/NADH ratio, there was no significant change in the hippocampus in the FK866 group compared with the other mice (Fig. 4).
Figure 4.
NAD+ level and NAD+/NADH ratio of APP/PS1 mice and effects of NAD+ and FK866 treatment. (A) NAD+ level, (B) NADH level and (C) NAD+/NADH ratio in the cortex in every group. (D) NAD+ level, (E) NADH level and (F) NAD+/NADH ratio in the hippocampus in every group. All data are expressed as the mean ± standard error of the mean (n=4). *P<0.05, **P<0.01 vs. Con; #P<0.05, ##P<0.01 vs. APP/PS1; and ▲P<0.05, ▲▲P<0.01 vs. APP/PS1. NAD, nicotinamide adenine dinucleotide; APP/PS1, APPswe/PS1ΔE9; Con, control.
Expression of NAMPT protein decreases in APP/PS1 transgenic mice and NAD+ treatment improves the expression
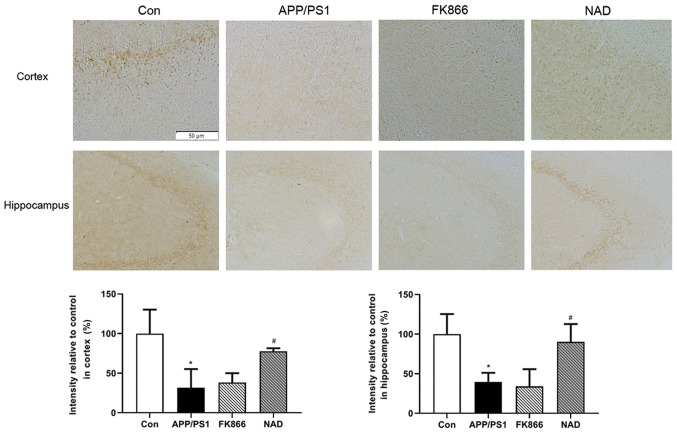
In order to confirm the expression of NAMPT proteins in the cortex and hippocampus in APP/PS1 transgenic mice, immunohistochemical staining was performed. It was observed that the brown positive expression of NAMPT protein in the cortex and hippocampus in APP/PS1 group was decreased compared with the control group (P<0.05). Following intraperitoneal injection of NAD+, the positive expression of NAMPT protein increased compared with the APP/PS1 group (P<0.05), while there was no significant change in NAMPT levels in the cortex and hippocampus in FK866 mice compared with APP/PS1 mice (P>0.05; Fig. 5).
Figure 5.
Immunohistochemical staining of NAMPT expression in the cortex and hippocampus with representative images. Scale bar, 50 µm. *P<0.05 vs. Con; and #P<0.05 vs. APP/PS1. NAMPT, nicotinamide phosphoribosyltransferase; Con, control; APP/PS1, APPswe/PS1ΔE9; FK866, an inhibitor of NAMPT; NAD, nicotinamide adenine dinucleotide.
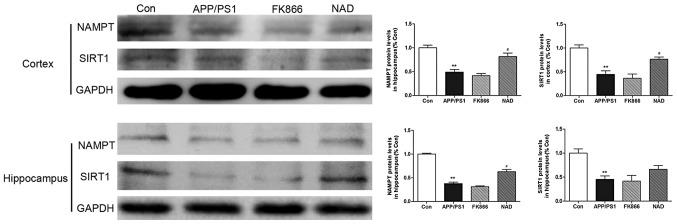
Protein extracts were also prepared and analyzed by western blotting to determine protein levels. The results demonstrated that the protein expression of NAMPT and SIRT1 in the cortex in the APP/PS1 group was significantly decreased when compared with the control group (P<0.01). Intraperitoneally injecting NAD+ increased the expression of NAMPT in the cortex in the NAD+ mice (P<0.05). There was no significant change in NAMPT protein levels in the FK866 mice compared with in APP/PS1 mice (P>0.05). Consistent with the changes in the cortex, the results demonstrated that the NAMPT and SIRT1 level in the hippocampus in the APP/PS1 group was decreased compared with the control group (P<0.01). NAMPT expression levels in the hippocampus in the NAD+ group were also significantly increased after intraperitoneal injection of NAD+ (P<0.05); however, injecting NAD+ did not increase SIRT1 expression levels. Finally, there was no significant change in NAMPT and SIRT1 protein expression levels in the hippocampus in FK866 mice, compared with in APP/PS1 mice (P>0.05; Fig. 6).
Figure 6.
Western blot analysis of NAMPT and SIRT1 protein expression in the cortex and hippocampus in every group and the quantification of NAMPT protein. Data are presented as the mean ± standard error of the mean (n=3). **P<0.01 vs. Con; and #P<0.05 vs. APP/PS1. NAMPT, nicotinamide phosphoribosyltransferase; SIRT, sirtuin; Con, control; APP/PS1, APPswe/PS1ΔE9; FK866, an inhibitor of NAMPT; NAD, nicotinamide adenine dinucleotide.
Discussion
AD is a degenerative disease of the nervous system. Studies have demonstrated that energy metabolism abnormalities are closely related to the development of early AD (22–25). The energy metabolism of failing neurons often precedes, or occurs simultaneously with, cognitive impairment (26). The mechanism may be that the energy metabolism disorder of the brain can upregulate amyloid precursor protein amyloidal digestion pathway to promote the production of Aβ and Aβ deposition, further causing mitochondrial dysfunction and leading to abnormal energy metabolism (27,28). In addition, abnormal energy metabolism of the brain has also been associated with hyperphosphorylation of the protein τ, which can affect synaptic function and aggravate cognitive impairment by activating the p38/mitogen-activated protein kinase-related pathway (29).
NAD+ is required for mitochondrial respiration and ATP synthesis and maintenance of NAD+ level is important for the prevention of mitochondrial dysfunction (30). NAD+ level declines with age in many cell and tissue types, with altered NAD+ metabolism and concurrent alterations in the mitochondrial function being inherent in AD (31). NAD+ can be synthesized via different precursors; the main two synthetic pathways are tryptophan synthetic pathways de novo and salvage pathways (32,33). The de novo synthesis pathway of tryptophan is mainly carried out in the liver and kidneys, which only synthesizes a very small amount of NAD+. The salvage pathway, using nicotinic acid (NA) and nicotinamide (NAM) as raw materials, is the main source of NAD+ synthesis. NA and NAM produce nicotinamide mononucleotide (NMN) through catalyzation by nicotinic acid phosphoribosyltransferase. NAMPT, originally identified as pre-B-cell colony enhancing factor, is the rate-limiting enzyme that catalyzes the first step in the biosynthesis of NAD+ from nicotinamide (33,34). Inhibition of NAMPT results in significant depletion of NAD+ which can modulate the activity of the TCA cycle and most of sirtuins (35).
Previous studies have found that NAD+ precursors such as NMN and NAM can improve the learning and memory abilities and mitochondrial function of AD model mice, suggesting that NAD+ upregulation can reverse the damage of brain energy metabolism (36,37). Other studies have suggested that NAD+ mediates a number of major biological processes, including calcium homeostasis, mitochondrial functions and energy metabolism, aging, and cell death in various tissues including the brain (38–40). NAD+ acts as a neuroprotective agent via several mechanisms, including the prevention of ATP depletion (41) NAMPT, a cytokine secreted by fat cells, is expressed in various tissues including the liver, kidneys, skeletal muscle and brain tissue and is involved in the oxidation of cells due to the biosynthesis of nicotinamide adenine dinucleotide (42). NAMPT affects energy metabolism, protein modification, DNA repair and other important processes by participating in the synthesis of NAD+. As a rate-limiting enzyme of the NAD+ salvage pathway, NAMPT can affect cell metabolism and NAD+ synthesis, interfering with NAD+ dependent-related protein expression (38,42). Recently, studies have demonstrated that NAMPT is associated with central nervous system diseases, in that it can protect neurons and delay the occurrence of axonal degeneration (43,44). With increasing age, the expression of NAMPT in the brain of AD patients is decreased, causing the level of NAD+ and the antioxidant enzyme glutathione to decrease, aggravating neuronal degeneration (45,46). Previous studies have demonstrated that NAMPT is mainly expressed in neurons in the mouse brain (47–49). With aging, the essential cofactor NAD+ decreases; the age-associated decrease in NAD+ levels is due to a decline in protein level NAMPT (50) and AD is an age-related neurodegenerative disease (51).
The present study has demonstrated that cortex and hippocampal NAD+ levels and NAMPT expression declined in early stage AD model mice. Based on previous studies (52,53), it is hypothesized that NAD+ administration could combat NAMPT decrease in the early stage of AD. The present study demonstrated that the decrease of NAMPT lead to NAD+ decrease, while NADH levels were not affected, leading to a decrease in the NAD/NADH ratio. Maintenance of NAD+/NADH ratio is critical for mitochondrial function, such as ATP synthesis in TCA cycle and oxidative phosphorylation in mitochondria (54), and it was identified that ATP decreased in the cortex and hippocampus. As NAD+ is able to travel freely inside the cell, decreased cellular NAD+ levels may result in reduced activity of the enzymes that use NAD+ as a substrate, including SIRT1 (55). In various animal models, age-related metabolic decline is positively correlated with the decline of NAD+, and the change of NAD+/NADH ratio can regulate sirtuin enzymes, including SIRT1. SIRT1 is expressed in both brain and peripheral tissues, and may shuttle to the cytoplasm during neuronal differentiation and neurite outgrowth and apoptosis. SIRT1 has an essential role in regulating cellular homeostasis by influencing neuron survival and mitochondrial biogenesis (56,57). The present study demonstrated that the intraperitoneal injection NAD+ levels in APP/PS1 mice was also associated with increased level of SIRT1. The NAD+ level in APP/PS1 mice after treatment with FK866 was measured and, as expected, FK866 treated APP/PS1 mice contained significantly less total NAD+ in the cortex and a lower NAD+/NADH ratio compared with APP/PS1 mice without any treatment. In the hippocampus, there was no significance difference between the FK866 and APP/PS1 groups in the level of NAD+ and the NAD+/NADH ratio, which may be related to the pathological development of early AD mice model. However, it is considered that these results indicated that NAMPT inhibition caused NAD+ deficiency, which demonstrated that improvement of NAMPT can increase the generation NAD.
The present study demonstrated that NAD+ depletion with decreased NAMPT is reversible and that NAD+ replenishment can reverse the impaired brain-energy metabolism that are implicated in cognitive decline and mitigates neurodegeneration. Given that NAMPT plays an important role in NAD synthesis, combining NAD+ or a drug increasing NAMPT may constitute a therapy for AD in the future.
Acknowledgements
Not applicable.
Funding
Funding was provided by the National Natural Science Foundation of China (grant no. 81503626) and the Shanghai Health Bureau Youth Fund (grant no. 201540254).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SX was the principal investigator and designed the study. YH performed statistical analysis, conducted the study and wrote the manuscript. XH performed experiments. DS performed statistical analysis and data collection. CC contributed to experimental design and manuscript preparation. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All procedures were performed in accordance with the Guide for and Use of Medical Laboratory Animals (Ministry of Health of China, 1998) and approved by the Shanghai University of Traditional Chinese Medicine Laboratory Animal Care and Use Committee (no. PZSHUTCM19011807).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Yin F, Boveris A, Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal. 2014;20:353–371. doi: 10.1089/ars.2012.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer's disease. Free Radic Biol Med. 2016;100:108–122. doi: 10.1016/j.freeradbiomed.2016.04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabezas-Opazo FA, Vergara-Pulgar K, Pérez MJ, Jara C, Osorio-Fuentealba C, Quintanilla RA. Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer's disease. Oxid Med Cell Longev. 2015;2015:509654. doi: 10.1155/2015/509654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han XJ, Hu YY, Yang ZJ, Jiang LP, Shi SL, Li YR, Guo MY, Wu HL, Wan YY. Amyloid β-42 induces neuronal apoptosis by targeting mitochondria. Mol Med Rep. 2017;16:4521–4528. doi: 10.3892/mmr.2017.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R, Li JJ, Diao S, Kwak YD, Liu L, Zhi L, Büeler H, Bhat NR, Williams RW, Park EA, Liao FF. Metabolic stress modulates Alzheimer's β-secretase gene transcription via SIRT1-PPARү-PGC-1 in neurons. Cell Metab. 2013;17:685–694. doi: 10.1016/j.cmet.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu MF, Yin JH, Hwang CS, Tang CM, Yang DI. NAD attenuates oxidative DNA damages induced by amyloid beta-peptide in primary rat cortical neurons. Free Radic Res. 2014;48:794–805. doi: 10.3109/10715762.2014.907889. [DOI] [PubMed] [Google Scholar]
- 8.Seixas da Silva GS, Melo HM, Lourenco MV, Lyra E Silva NM, de Carvalho MB, Alves-Leon SV, de Souza JM, Klein WL, da-Silva WS, Ferreira ST, De Felice FG. Amyloid-β oligomers transiently inhibit AMP-activated kinase and cause metabolic defects in hippocampal neurons. J Biol Chem. 2017;292:7395–7406. doi: 10.1074/jbc.M116.753525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rijpma A, van der Graaf M, Meulenbroek O, Olde Rikkert MGM, Heerschap A. Altered brain high-energy phosphate metabolism in mild Alzheimer's disease: A 3-dimensional 31P MR spectroscopic imaging study. Neuroimage Clin. 2018;18:254–261. doi: 10.1016/j.nicl.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajmohan R, Reddy PH. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer's disease Neurons. J Alzheimers Dis. 2017;57:975–999. doi: 10.3233/JAD-160612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elhassan YS, Philp AA, Lavery GG. Targeting NAD+ in metabolic disease: New insights into an old molecule. J Endocr Soc. 2017;1:816–835. doi: 10.1210/js.2017-00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 14.Ruggieri S, Orsomando G, Sorci L, Raffaelli N. Regulation of NAD biosynthetic enzymes modulates NAD-sensing processes to shape mammalian cell physiology under varying biological cues. Biochim Biophys Acta. 2015;1854:1138–1149. doi: 10.1016/j.bbapap.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang Y, Li X, Zhang M, Lv K. Microarray analysis of circular RNA expression patterns in polarized macrophages. Int J Mol Med. 2017;39:373–379. doi: 10.3892/ijmm.2017.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of alzheimer-like pathology in app/ps1 mice. Ann Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- 18.Janus C, Flores AY, Xu G, Borchelt DR. Behavioral abnormalities in app swe/ps1de9 mouse model of ad-like pathology: Comparative analysis across multiple behavioral domains. Neurobiol Aging. 2015;36:2519–2532. doi: 10.1016/j.neurobiolaging.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Balducci C, Mancini S, Minniti S, La Vitola P, Zotti M, Sancini G, Mauri M, Cagnotto A, Colombo L, Fiordaliso F, et al. Multifunctional liposomes reduce brain β-amyloid burden and ameliorate memory impairment in Alzheimer's disease mouse models. J Neurosci. 2014;34:14022–14031. doi: 10.1523/JNEUROSCI.0284-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caccamo A, Branca C, Talboom JS, Shaw DM, Turner D, Ma L, Messina A, Huang Z, Wu J, Oddo S. Reducing ribosomal protein S6 Kinase 1 expression improves spatial memory and synaptic plasticity in a mouse model of Alzheimer's disease. J Neurosci. 2015;35:14042–14056. doi: 10.1523/JNEUROSCI.2781-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SY, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, Li T, Du F, Öngür D. Redox Dysregulation in schizophrenia revealed by in vivo NAD+/NADHH measurement. Schizophr Bull. 2017;43:197–204. doi: 10.1093/schbul/sbx024.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blass JP, Sheu RK, Gibson GE. Inherent abnormalities in energy metabolism in Alzheimer disease. Interaction with cerebrovascular compromise. Ann N Y Acad Sci. 2000;903:204–221. doi: 10.1111/j.1749-6632.2000.tb06370.x. [DOI] [PubMed] [Google Scholar]
- 23.Lewczuk P, Riederer P, O'Bryant SE, Verbeek MM, Dubois B, Visser PJ, Jellinger KA, Engelborghs S, Ramirez A, Parnetti L, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the consensus of the task force on biological markers in psychiatry of the world federation of societies of biological psychiatry. World J Biol Psychiatry. 2018;19:244–328. doi: 10.1080/15622975.2017.1375556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez MJ, Ponce DP, Aranguiz A, Behrens MI, Quintanilla RA. Mitochondrial permeability transition pore contributes to mitochondrial dysfunction in fibroblasts of patients with sporadic Alzheimer's disease. Redox Biol. 2018;19:290–300. doi: 10.1016/j.redox.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swerdlow RH, Koppel S, Weidling I, Hayley C, Ji Y, Wilkins HM. Mitochondria, Cybrids, Aging, and Alzheimer's disease. Prog Mol Biol Transl Sci. 2017;146:259–302. doi: 10.1016/bs.pmbts.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adiele RC, Adiele CA. Mitochondrial regulatory pathways in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2016;53:1257–1270. doi: 10.3233/JAD-150967. [DOI] [PubMed] [Google Scholar]
- 27.Velliquette RA, O'Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: Possible early events in Alzheimer's disease pathogenesis. J Neurosci. 2005;25:10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Zhu Y, Cao X, Sun S, Zhao B. Mitochondrial toxic effects of Aβ through mitofusins in the early pathogenesis of Alzheimer's disease. Mol Neurobiol. 2014;50:986–996. doi: 10.1007/s12035-014-8675-z. [DOI] [PubMed] [Google Scholar]
- 29.Lauretti E, Li JG, Di Meco A, Pratico D. Glucose deficit triggers tau pathology and synaptic dysfunction in a tauopathy mouse model. Transl Psychiatry. 2017;7:e1020. doi: 10.1038/tp.2016.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang EF. Mitophagy and NAD+ inhibit Alzheimer disease. Autophagy. 2019;15:1112–1114. doi: 10.1080/15548627.2019.1596497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh D, Levault KR, Brewer GJ. Relative importance of redox buffers GSH and NAD+(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell. 2014;13:631–640. doi: 10.1111/acel.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Sauve AA. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. 2016;1864:1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pehar M, Harlan BA, Killoy KM, Vargas MR. Nicotinamide adenine dinucleotide metabolism and Neurodegeneration. Antioxid Redox Signal. 2018;18:1652–1668. doi: 10.1089/ars.2017.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23:420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer's disease-relevant murine model. BMC Neurol. 2015;15:19. doi: 10.1186/s12883-015-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Z, Yang W, Gao Z, Jia P. Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci Lett. 2017;647:133–140. doi: 10.1016/j.neulet.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, Tarim A, Alemifar A, Han X, Wilkins HM, et al. Deletion of NAMPT in projection neurons of adult mice leads to motor dysfunction, Neurodegeneration, and death. Cell Rep. 2017;20:2184–2200. doi: 10.1016/j.celrep.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katsyuba E, Auwerx J. Modulating NAD+ metabolism, from bench to bedside. EMBO J. 2017;36:2670–2683. doi: 10.15252/embj.201797135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol. 2015;50:284–297. doi: 10.3109/10409238.2015.1028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryu KW, Nandu T, Kim J, Challa S, DeBerardinis RJ, Kraus WL. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science. 2018;360(pii):eaan5780. doi: 10.1126/science.aan5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci USA. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- 43.Lin JB, Kubota S, Ban N, Yoshida M, Santeford A, Sene A, Nakamura R, Zapata N, Kubota M, Tsubota K, et al. NAMPT-Mediated NAD(+) Biosynthesis is essential for vision in mice. Cell Rep. 2016;17:69–85. doi: 10.1016/j.celrep.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LoCoco PM, Risinger AL, Smith HR, Chavera TS, Berg KA, Clarke WP. Pharmacological augmentation of nicotinamide phosphoribosyltransferase (NAMPT) protects against paclitaxel-induced peripheral neuropathy. Elife. 2017;6:e29626. doi: 10.7554/eLife.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh D, LeVault KR, Barnett AJ, Brewer GJ. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3×Tg-AD mouse neurons. J Neurosci. 2012;32:5821–5832. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Sysol JR, Singla S, Zhao S, Yamamura A, Valdez-Jasso D, Abbasi T, Shioura KM, Sahni S, Reddy V, et al. Nicotinamide Phosphoribosyltransferase promotes pulmonary vascular remodeling and is a therapeutic target in pulmonary arterial hypertension. Circulation. 2017;135:1532–1546. doi: 10.1161/CIRCULATIONAHA.116.024557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang SN, Miao CY. Targeting NAMPT as a therapeutic strategy against stroke. Stroke Vasc Neurol. 2019;4:83–89. doi: 10.1136/svn-2018-000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and aging. Diabetes Obes Metab. 2013;15(Suppl 3):S26–S33. doi: 10.1111/dom.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiss G, Konrad C, Pour-Ghaz I, Mansour JJ, Németh B, Starkov AA, Adam-Vizi V, Chinopoulos C. Mitochondrial diaphorases as NAD+ donors to segments of the cirtric acid cycle that support substrate-level phosphorylation yielding ATP during respiratory inhibition. FASEB J. 2014;28:1682–1697. doi: 10.1096/fj.13-243030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O'Connell JF, Baptiste BA, et al. NAD+ supplementation normalizes key Alzheimer's features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci USA. 2018;115:1876–1885. doi: 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Li H, Ding S. The effects of NAD+ on apoptotic neuronal death and mitochondrial biogenesis and function after glutamate Excitotoxicity. Int J Mol. 2014;15:20449–20468. doi: 10.3390/ijms151120449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan Y, Wang SR, Huang XZ, Xie QH, Xu YY, Shang D, Han CM. Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J Am Soc Nephrol. 2017;28:2337–2352. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du LL, Xie JZ, Cheng XS, Li XH, Kong FL, Jiang X, Ma ZW, Wang JZ, Chen C, Zhou XW. Activation of sirtuin 1 attenuates cerebral ventricular streptozotocin-induced tau hyperphosphorylation and cognitive injuries in rat hippocampi. Age (Dordr) 2014;36:613–623. doi: 10.1007/s11357-013-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonfili L, Cecarini V, Cuccioloni M, Angeletti M, Berardi S, Scarpona S, Rossi G, Eleuteri AM. SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Mol Neurobiol. 2018;55:7987–8000. doi: 10.1007/s12035-018-0973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.