Abstract
The presence of multidrug‐resistant bacteria like methicillin‐resistant Staphylococcus aureus (MRSA) in retail meat is one of the current concerns of the public health authorities. Bacterial cross‐contamination and recontamination during household food preparation could play an important role in the dissemination of such bacteria, and therefore could contribute to a serious health problem, more specifically for immunocompromised people. In order to evaluate the importance of such events, a probabilistic model was developed to estimate the likelihood and extent of cross‐contamination and recontamination and the burden of MRSA from contaminated raw chicken meat via hands and kitchen utensils in a serving (consisting on a slice of bread and a piece of grilled chicken meat) during a household barbecue in Germany. A modular design was used, taking into account the chronological order of the routines during the barbecue event, and Monte Carlo simulations were applied. Available data on the prevalence and burden of MRSA in chicken meat at retail in Germany were used as starting point and were incorporated in the model as probability distributions. The probabilities and extent of bacterial transfer between food items and kitchen utensils (referred to as “Objects”) and the routines performed during food preparation (referred to as “Actions”) specified by their probabilities of occurrence were incorporated as the main input parameters. The model was set up in R 3.5.0 and converted to a standardized format (FSKX file). Therefore, the code can be easily accessed, evaluated, modified, and reused for different purposes. The present study contributes to the quantification of consumer exposure to MRSA through food consumption once contaminated food has entered the household kitchen. Even when the MRSA prevalence and bacterial load in retail chicken meat in Germany are low, resistant bacteria can reach the consumer due to cross‐contamination and recontamination events. The results show that the probability of one CFU to be transferred from the contaminated raw chicken meat to the final serving and the number of MRSA bacteria transferred due to cross‐contamination and recontamination events are in general low, being the contamination of the final serving more likely to occur via bread, rather than via grilled chicken. The results show that the prevalence of MRSA at retail highly influences the probability of the final serving to be contaminated. However, this study also highlights the importance of keeping good hygiene practices during the household food manipulation for reducing the spread of MRSA. The provision of the model in a standardized data format will allow an easy incorporation of the developed model into a complete quantitative microbial risk assessment model that will greatly help to estimate the risk of consumer exposure to MRSA through the consumption of contaminated food.
Keywords: chicken, consumer exposure, cross‐contamination and recontamination, food preparation, hygiene practices, methicillin‐resistant Staphylococcus aureus
A probabilistic model was developed to estimate the likelihood and extent of cross‐contamination and recontamination and the burden of methicillin‐resistant Staphylococcus aureus (MRSA) from contaminated raw chicken meat via hands and kitchen utensils in a serving (consisting on a slice of bread and a piece of grilled chicken meat) during a household barbecue in Germany. It contributes to the quantification of consumer exposure to MRSA through food consumption once contaminated food has entered the household kitchen.

1. INTRODUCTION
In recent decades, the presence of multidrug‐resistant bacteria has been considered as a major concern in public health (van Duin & Paterson, 2016), reducing the efficacy of antimicrobial treatments (Li & Webster, 2018) and contributing to more than 25,000 deaths in the European Union (EU) each year (Cassini et al.., 2019). A well‐known example of bacteria that is resistant to a number of antibiotics is the methicillin‐resistant Staphylococcus aureus (MRSA). It is reported from many parts of the world not only as a major cause of healthcare‐associated infections but also, and increasingly, as a cause of community‐acquired (CA) infections (Gastmeier, 2010). In Europe, it still remains an important pathogen although an overall decline of MRSA has been reported between 2013 and 2016 (ECDC, 2017). In Germany, MRSA affects 0.5%–1.5% of the general population and 1.0%–2.5% of patients at hospital admission (Kock et al., 2014).
MRSA has been widely detected in livestock animals, such as pigs, poultry, and cattle (Friese et al., 2013; Tenhagen et al., 2009, 2014). Livestock‐associated (LA‐) MRSA has also emerged among humans indicating a zoonotic transmission from animals to humans. Livestock professionals, such as farmers, veterinarians, and slaughterhouse workers handling live animals, are at increased risk (Kock et al., 2014). LA‐MRSA is relatively rare in urban areas. However, some cases of LA‐MRSA carriage in humans cannot be explained by livestock contact. Due to the fact that during the slaughtering process, the carcasses can be contaminated through contact with the skin, respiratory secretions, feces, urine, and other exudates, MRSA has been also found in raw meat including pork, beef, and chicken meat (de Boer et al., 2009; Boost, Wong, Ho, & O'Donoghue, 2013; Tang et al., 2017). Thus, one could speculate that humans might have acquired such MRSA via contaminated food (Deiters, Günnewig, Friedrich, Mellmann, & Köck, 2015). Available data suggest that the prevalence and level of MRSA in raw chicken meat at retail are in general low (de Boer et al., 2009; Weese, Avery, & Reid‐Smith, 2010). However, even when the risk of exposure to MRSA through consumption of contaminated food appears to be small in comparison with that related to the contact with livestock animals or humans (EFSA, 2009), it has been suggested that commercially distributed meat could play an important role in the presence of MRSA in the community (Ogata et al., 2012). The ingestion of MRSA could result in intestinal colonization and further extra‐intestinal infection or transmission (Weese et al., 2010).
In the EU, 39.3% of the foodborne outbreaks are caused by improper handling of food in households (EFSA, 2017). It is considered that this is mainly due to the fact that the food hygiene routines at consumer level cannot be controlled as it is done, for example, at industrial processing level (Mazengia, Fisk, Liao, Huang, & Meschke, 2015). In particular, during special events like outdoor barbecues, people pay less attention to food safety and hygiene practices (Bearth, Cousin, & Siegrist, 2014), and therefore, such events bear the risk of foodborne disease outbreaks (Allerberger et al., 2003; Mertens et al., 2013). Not washing hands after handling raw meat, putting cooked meat at the same dish as the raw meat, and using the same knife or cutting board for raw meat and ready to eat (RTE) food consumed along with the barbecue have been described as important cross‐contamination routes from raw poultry meat to cooked meat and/or to RTE food (Sampers et al., 2012). In this sense, MRSA cross‐contamination (indirect transfer of bacteria from a contaminated product to a noncontaminated product via kitchen utensils) and recontamination (food contamination after the inactivation process) (Pérez‐Rodríguez, Valero, Carrasco, García, & Zurera, 2008) events occurring during household food preparation could play an important role in the occurrence of MRSA‐related outbreaks or colonization events (Jones, Kellum, Porter, Bell, & Schaffner, 2002; Kadariya, Smith, & Thapaliya, 2014; Sergelidis & Angelidis, 2017). However, nowadays it still remains to be determined to what extent raw chicken meat manipulated during household barbecue possibly contributes to the consumer exposure to MRSA, and therefore, the study of MRSA cross‐contamination and recontamination events is of particular interest.
The use of consumer phase models (CPMs) has been proposed as a valuable tool for assessing the risk for the consumer to get colonized or infected after food preparation and consumption (Nauta & Christensen, 2011). They evaluate the different pathways that contribute to the consumer exposure. Particularly, cross‐contamination models have been widely used for understanding and evaluating the microbial transfer dynamics from contaminated food surfaces to other recipient food surfaces in food‐related environments (Possas, Carrasco, García‐Gimeno, & Valero, 2017). Most of the available cross‐contamination models on the improper handling of raw chicken meat have been developed for Campylobacter spp. (Chapman, Otten, Fazil, Ernst, & Smith, 2016). However, in relation to resistant bacteria just one model describing the cross‐contamination of ESBL/AmpC E. coli in meat can be found in the literature (Evers et al., 2017). So far, there are no models describing the cross‐contamination and recontamination events during food preparation for MRSA.
In order to evaluate one of the possible human exposure pathways to MRSA, a quantitative analysis was carried out to estimate the likelihood and extent of cross‐contamination and recontamination and the burden of MRSA from contaminated raw chicken meat via hands and kitchen utensils in a serving (consisting on a piece of bread and a piece of grilled chicken meat) during a household barbecue. For that reason, a simplified model that is able to describe the dynamics of cross‐contamination and recontamination of MRSA and its impact on the consumer exposure was designed. The probabilistic model created is capable of predicting whether or not transfer of MRSA takes place (probability of one colony‐forming unit (CFU) to be transferred from raw chicken meat to bread and grilled chicken) and the amount of MRSA transferred to bread and grilled chicken (level of transfer) in case the raw chicken meat is contaminated. Based on these predictions, an estimation of the probability of consumer exposure and level of exposure due to cross‐contamination and recontamination from eating one portion of bread and grilled chicken possibly contaminated by these resistant bacteria originating from raw chicken meat was calculated. In addition, the influence of the prevalence of MRSA at retail and the different hygiene practices within the consumer exposure was also evaluated.
2. MATERIAL AND METHODS
2.1. Scenario settings
The scenario contemplates a household barbecue, where a piece of raw chicken meat is cut into two pieces and grilled in a barbeque. In parallel, a slice of sliced bread, which will be consumed together with the grilled chicken, is cut also into two pieces. The bread and the grilled chicken are cut and manipulated, respectively, with the same kitchen utensils that were previously used to cut and manipulate the raw chicken meat. In some cases, it was considered that the utensils (cutting board and dish) could be rinsed after cutting/manipulating the raw chicken meat. Figure 1 and Table 1 present a detailed description of the scenario settings, including the objects involved during each action.
Figure 1.
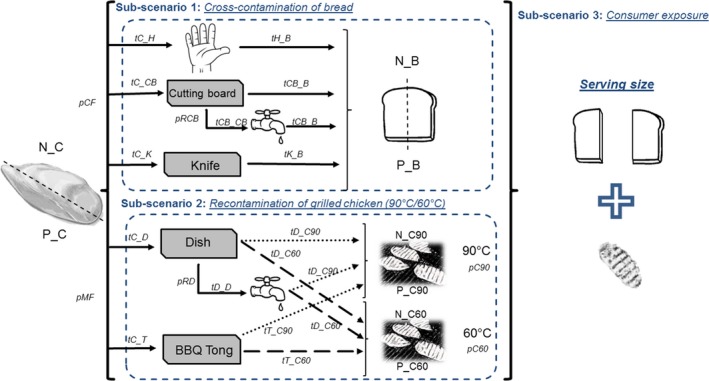
General scheme of the contemplated scenario: objects, transfer coefficients, and probabilities of action occurrence that play a role within the developed model. (tX_Y: transfer coefficient from X to Y; tX_X: persistence coefficient in X; N_X: number of bacteria in X; P_X: prevalence/probability of bacteria in X; C: raw chicken meat; H: hands; D: dish; B: bread; CB: cutting board; K: knife; T: barbecue tong (BBQ Tong); C90: grilled chicken at 90°C; C60: grilled chicken at 60°C; pCF: probability of cutting the raw chicken first; pMF: probability that the raw chicken meat is manipulated first; pRCB: probability of rinsing the cutting board; pRD: probability of rinsing the dish; pC90: probability that the grilled chicken remains warm at 90°C; pC60: probability of the grilled chicken to cool down to 60°C)
Table 1.
Detailed description of the scenario settings contemplated in the model design
| Subscenario | Action | Action description | Objects |
|---|---|---|---|
| 1. Cross‐contamination of bread |
Action 1: Cutting raw chicken meat |
Consumer holds the contaminated raw chicken meat with the hands to cut it with a knife on a cutting board. |
|
| Action 2: Rinsing cutting board | The cutting board can be rinsed in some cases after cutting the raw chicken meat and before cutting the bread. |
|
|
| Action 3: Cutting bread | Later, he/she takes a piece of sliced bread with the contaminated hands, to cut it in two pieces with the same knife and in the same cutting board that have been used for cutting the contaminated raw chicken meat. |
|
|
| 2. Recontamination of grilled chicken (90°C/60°C) | Action 4: Manipulating raw chicken meat |
Once the chicken is cut, the consumer takes the pieces of raw chicken meat with the hands and places them on a dish. The consumer takes a raw chicken piece from the dish with the barbecue tong and places it into the barbecue. |
|
| Action 5: Rinsing dish | The dish can be rinsed in some cases after being used for raw chicken, and before being used for cooked chicken. |
|
|
| Action 6: Manipulating hot grilled chicken (90°C) | The grilled chicken is removed from the barbecue with the contaminated barbecue tong and placed on the contaminated dish (rinsed or not rinsed). |
|
|
| Action 7: Manipulating cooled grilled chicken (60°C) |
When the demand for grilled chicken meat decreases in some moments during the barbecue event, some pieces are removed from the central part of the barbecue, and they cool down to 60°C. The grilled chicken at 60°C is manipulated with the contaminated barbecue tong and placed on a contaminated dish (rinsed or not rinsed). |
|
|
| 3. Consumer Exposure | Action 8: Food consumption | The consumer eats the final serving consisting of a slice of bread and a piece of grilled chicken. |
|
2.2. Technical assumptions
Within this scenario, a number of objects that can be contaminated, together with a number of actions, contributing to cross‐contamination and recontamination between objects, were identified (Table 1).
We considered one of the worst‐case scenarios where (a) hands were not rinsed or washed with soap; (b) the same kitchen utensils were used for raw meat and RTE foods and the replacement by clean ones was considered negligible; (c) kitchen utensils were not washed with soap; and (iv) just the cutting board and the dish were rinsed in some cases.
The epidemiological data on the prevalence and the burden of MRSA measured on retail chicken meat in Germany (BVL, 2017; Pauly et al., 2019) were used as the starting point of this model, as it was assumed that initially only raw chicken meat is contaminated with MRSA.
No predictive microbial models were incorporated within the model to describe bacterial growth, inactivation, or survival. We assumed that during the barbecue event, the raw chicken meat was kept refrigerated until its use and that the time needed to prepare the meal was not enough to allow substantial bacterial growth. A complete inactivation of bacteria was assumed after the grilling process, and undercooking of chicken meat was neglected. Hence, the final concentration of MRSA in the grilled chicken meat was then entirely assigned to the recontamination event after preparation. For contaminated bread and grilled chicken, all MRSA were assumed to survive.
Transfer coefficients (tX_Y) between food and kitchen objects (expressed as the probability for one CFU to be transferred from X to Y during the food preparation [Nauta, Jacobs‐Reitsma, Evers, Pelt, & Havelaar, 2005]) and bacteria persistence coefficients (tX_X) after rinsing the cutting board and dish (denoting the probability that a CFU is still present on an object after rinsing it [Mylius, Nauta, & Havelaar, 2007]) were incorporated as model parameters. As the transfer and persistence coefficients were expressed as colony‐forming unit per square centimeter (CFU/cm2) and per action performed, we assumed that all CFUs were equally distributed in the raw chicken meat and that all of them had the same physiological state and did not differ in adhesion properties or other characteristics. No dependence between the different transfer events was considered.
As just those bacteria in the chicken surface are able to be transmitted to the different surfaces and because one individual cell can only be on one object at one time, we defined different surfaces for the different objects (e.g., piece of raw chicken meat: upper, side, bottom, and internal surfaces) and established a chronological order of actions. Thereby, those cells already transferred from one surface (e.g., from the upper surface of the raw chicken to the hands) were not considered for the next transfer event (e.g., from the upper surface of the chicken to the barbecue tong).
For calculating the level of bacteria in bread and grilled chicken, the weight of the respective portions was not considered, but the surfaces in contact during the food preparation. The size (and therefore the surface) of the objects (chicken meat, bread and kitchen utensils) was considered to be always the same. The surfaces contemplated for the raw chicken meat, the barbecue tong, and the knife were equal to those considered by Fetsch and Tenhagen (2017). Surfaces corresponding to hands, cutting board, dish, bread, and grilled chicken were estimated from different sources.
It was considered that the grilled chicken meat was always consumed together with sliced bread in the form of a sandwich. The consumer exposure to MRSA was then calculated per serving/portion, assuming that the serving consists on a slice of bread and a piece of grilled chicken. We assumed that the manipulation of the grilled chicken before consumption is just possible at two temperatures: 90°C (representing the grilled chicken consumed warm) and 60°C (representing the grilled chicken consumed after some time of storage).
2.3. Model design
A similar approach followed by Mylius et al. (2007) for their model developed for Campylobacter spp. for cross‐contamination during food preparation was used, extending it according to our different cross‐contamination and recontamination pathways and the different assumptions made during the model design.
A probabilistic approach was used, and Monte Carlo simulations were applied. A modular design was applied, taking into account the chronological order of actions during the barbecue event. The probabilities and extent of bacterial transfer between food items and kitchen utensils and the probability of hygiene practices to be performed or not during food preparation specified by their probabilities of occurrence were incorporated as the main input parameters.
2.4. Probability distributions
Assuming that the prevalence of MRSA and its burden at retail level do not have a single and constant value, appropriate probability distribution functions were used. Collected data from the literature on the prevalence and burden of MRSA at retail in raw chicken in Germany were fitted to theoretical distributions using fitdistrplus, an R package for fitting purposes (Delignette‐Muller & Dutang, 2015). Different probability distributions were fitted to these data and the goodness of fitting was evaluated by Kolmogorov–Smirnov test and visual analysis.
2.5. Model simulation
The designed model was set up in R 3.5.0 on a Windows 7 platform. The R package “mc2d” (version 0.1‐18) was used to implement Monte Carlo simulations for the model in order to simulate many repetitions of food preparation. Then, probability distributions were calculated for the probabilities of one CFU of MRSA to be transferred from raw chicken meat and the number of MRSA transferred to bread, grilled chicken, and final serving as a result of cross‐contamination and recontamination events (100,000 iterations).
In addition, in order to get an overview on the influence of the prevalence of MRSA on raw chicken meat at retail and the different hygiene practices contemplated in the model in the consumer exposure to MRSA, different scenarios were simulated. For this purpose, a deterministic approach was applied, using the mean values of the probability distributions applied in the model, and varying the default values of the probabilities of action occurrence between 0 and 1.
2.6. Model reusability and exchange
In order to provide transparency and consistency of the developed model and to facilitate the reuse and exchange of the model script (Plaza‐Rodríguez et al., 2018), the R code was created following the structure required by the standardized data format called Food Safety Knowledge Markup Language (FSK‐ML) (https://foodrisklabs.bfr.bund.de/fsk-ml-food-safety-knowledge-markup-language/), including two R scripts: (a) Model script and (b) Visualization Script. The entire metadata schema was also generated by using the metadata Excel sheet template (https://foodrisklabs.bfr.bund.de/rakip-harmonization-resources/) in order to describe in detail the complete model range of applicability. Finally, the generated model was converted to FSKX file.
3. RESULTS
3.1. Model description
A complete overview of the model, including the description of the dependent variables and the model equations, is provided in Table 2.
Table 2.
Complete overview of the designed model including the description of the dependent variables and the model equations
| Calculation | Description | Dependent variable | Model equation | ||
|---|---|---|---|---|---|
|
Subscenario 1. Cross‐contamination of bread |
1. A. Probability of one CFU being transferred from raw chicken meat to bread | 1.1. Probability of one CFU being transferred from raw chicken meat to bread (pC_B) (via hands, cutting board, and knife) | pC_B | Eq. 1 | pC_B = (tC_H * tH_B) + (tC_CB * tCB_CB * tCB_B) + (tC_K * tK_B) |
| 1.2. Probability of one CFU being transferred from raw chicken meat to bread (pC_B_p) (via hands, cutting board and knife), including type of handling into account. | pC_B_p | Eq. 2 | pC_B_p = ((tC_H * tH_B) + (((tC_CB * pCB) + (tC_CB * tCB_CB * pRCB)) * tCB_B) + (tC_K * tK_B)) * pCF | ||
| 1.3. Probability of one CFU being transferred from raw chicken meat to bread (via hands, cutting board, and knife), including the prevalence of MRSA at retail in raw chicken meat. | PC_B | Eq. 3 | PC_B = (pC_B_p * P_C) | ||
| 1. B. Number of cells transferred from raw chicken meat to bread | 1.4. Number of CFU being transferred per cm2 to bread from contaminated hands (N_H), cutting board (N_CB), rinsed cutting board (N_RCB), and knife (N_K). | N_H | Eq. 4 | N_H = binom (N_C, (tC_H * tH_B)) | |
| N_CB | Eq. 5 | N_CB = binom (N_C, (tC_CB * tCB_B)) | |||
| N_RCB | Eq. 6 | N_RCB = binom (N_CB, (tCB_CB)) | |||
| N_K | Eq. 7 | N_K = binom (N_C, (tC_K * tK_B)) | |||
| 1.5. Number of CFU being transferred per portion of bread (N_B) from contaminated hands (N_Hs), cutting board (N_CBs), rinsed cutting board (N_RCBs), and knife (N_Ks), considering the surface of the bread in contact with hands (SB_H), cutting board (SB_CB), rinsed cutting board (SB_RCB), and knife (SB_K) during food preparation. | N_B | Eq. 8 | N_B = (N_Hs + ( N_CBs *pCB) + ( N_RCBs *pRCB) + N_Ks ) * pCF | ||
| N_Hs | Eq. 9 | N_Hs = N_H * SB_H | |||
| N_CBs | Eq. 10 | N_CBs = N_CB * SB_CB | |||
| N_RCBs | Eq. 11 | N_RCBs = N_RCB * SB_CB | |||
| N_Ks | Eq. 12 | N_Ks = N_K * SB_K | |||
| Subscenario 2. Recontamination of grilled chicken | 2. A. Probability of one cell being transferred from raw chicken meat to grilled chicken | 2.1. Probability of one CFU being transferred from raw chicken meat to grilled chicken (pC_GC) considering the chicken meat at 90°C (pC_C90) and 60°C (pC_C60), via dish and barbecue tong. | pC_GC | Eq. 13 | pC_GC = ( pC_C90 + pC_C60 ) |
| pC_C90 | Eq. 14 | pC_C90 = (tC_D * tD_D * tD_C90) + (tC_T * tT_C90) | |||
| pC_C60 | Eq. 15 | pC_C60 = (tC_D * tD_D * tD_C60) + (tC_T * tT_C60) | |||
| 2.2. Probability of one CFU being transferred from raw chicken meat to grilled chicken (pC_GC_p) (via dish and barbecue tong), including type of handling into account and considering the chicken meat at 90°C (pC_C90_p) and 60°C (pC_C60_p). | pC_GC_p | Eq. 16 | pC_GC_p = ((pC_C90_p * pC90) + (pC_C60_p * pC60)) * pMF | ||
| pC_C90_p | Eq. 17 | pC_C90_p = (((tC_D * pD) + (tC_D * tD_D * pRD)) * tD_C90) + (tC_T * tT_C90) | |||
| pC_C60_p | Eq. 18 | pC_C60_p = (((tC_D * pD) + (tC_D * tD_D * pRD)) * tD_C60) + (tC_T * tT_C60) | |||
| 2.3. Probability of one CFU being transferred from raw chicken meat to grilled chicken (PC_GC) (via dish and barbecue tong), including the prevalence of MRSA at retail in raw chicken meat. | PC_GC | Eq. 19 | PC_GC = (pC_GC_p * P_C) | ||
| 2B. Number of cells transferred from raw chicken meat to grilled chicken | 2.4. Number of CFU being transferred per cm2 to grilled chicken (90°C and 60°C) from contaminated dish (NC_D), rinsed dish (NC_RD), and barbecue tong (NC_T_up, NC_T_down). | NC90_D | Eq. 20 | NC90_D = binom((N_C − N_CB), (tC_D * tD_C90)) | |
| NC90_RD | Eq. 21 | NC90_RD = binom( (NC90_D, tD_D) | |||
|
NC90_T_up NC90_T_down |
Eq. 22 | NC90_T_up = binom ((N_C − N_H), (tC_T * tT_C90)) | |||
| Eq. 23 | NC90_T_down = binom ((N_C − (N_CB + NC90_D)), (tC_T * tT_C90)) | ||||
| NC60_D | Eq. 24 | NC60_D = binom( (N_C − N_CB), (tC_D * tD_C60)) | |||
| NC60_RD | Eq. 25 | NC60_RD = binom( (NC60_D, tD_D) | |||
|
NC60_T_up NC60_T_down |
Eq. 26 | NC60_T_up = binom ((N_C − N_H), (tC_T * tT_C60)) | |||
| Eq. 27 | NC60_T_down = binom ((N_C − (N_CB + NC60_D)), (tC_T * tT_C60)) | ||||
| N_GC | Eq. 28 | N_GC = (( N_C90s * pC90) + ( N_C60s * pC60)) * pMF | |||
| N_C90s | Eq. 29 | N_C90s = ( NC90_Ds * pD) + ( NC90_RDs * pRD) + NC90_Ts_up + NC90_Ts_down | |||
| 2.5. Number of CFU being transferred per portion of grilled chicken (N_GC) (considering the hot grilled chicken (N_C90s) and the cooled grilled chicken (N_C60s)) from contaminated dish (NC_Ds), rinsed dish (NC_RDs), barbecue tong (NC_Ts_up, NC_Ts_down), including the surface of the grilled chicken in contact with the dish and rinsed dish (SGC_D) and barbecue tong (SGC_T_up, SGC_T_down) during food preparation. | NC90_Ds | Eq. 30 | NC90_Ds = NC90_D * SGC_D | ||
| NC90_RDs | Eq. 31 | NC90_RDs = NC90_RD * SGC_D | |||
| NC90_Ts_up | Eq. 32 | NC90_Ts_up = NC90_T_up *SGC_T | |||
| NC90_Ts_down | Eq. 33 | NC90_Ts_down = NC90_T_down * SGC_T | |||
| N_C60s | Eq. 34 | N_C60s = ( NC60_Ds * pD) + ( NC60_RDs * pRD) + NC60_Ts_up + NC60_Ts_down | |||
| NC60_Ds | Eq. 35 | NC60_Ds = NC60_D * SGC_D | |||
| NC60_RDs | Eq. 36 | NC60_RDs = NC60_RD * SGC_D | |||
| NC60_Ts_up | Eq. 37 | NC60_Ts_up = NC60_T_up * SGC_T | |||
| NC60_Ts_down | Eq. 38 | NC60_Ts_down = NC60_T _down * SGC_T | |||
| Subscenario 3. Consumer exposure | 3A. Probability of one cell being transferred from raw chicken meat to the final serving | 3.1. Probability of one CFU being transferred from raw chicken meat to the final serving considering the prevalence of MRSA at retail in raw chicken meat. | P_Ex | Eq. 39 | P_E = pC_B_p * pC_GC_p * P_C |
| 3B. Number of cells transferred from raw chicken meat to the final serving | 3.2. Number of CFU being transferred from the contaminated raw chicken meat to the final serving consisting in a slice of bread and in a piece of grilled chicken. | N_Ex | Eq. 40 | N_Ex = N_B + N_GC |
Abbreviations: tX_Y, transfer coefficient from X to Y; tX_X, persistence coefficient in X; N_X, number of bacteria in X; P_X, prevalence/probability of bacteria in X; C, raw chicken meat; H, hands; D, dish; B, bread; CB, cutting board; K, knife; T, barbecue tong; C90, grilled chicken at 90°C; C60, grilled chicken at 60°C; pCF, probability of cutting the raw chicken first; pMF, probability that the raw chicken meat is manipulated first; pRCB, probability of rinsing the cutting board; pRD, probability of rinsing the dish; pC90, probability that the grilled chicken remains warm at 90°C; pC60, probability of the grilled chicken to cool down to 60°C. The values of the parameters highlighted in bold have been calculated with specific equations.
The model was divided into three main subscenarios:
Calculation of the cross‐contamination of the bread.
Calculation of the recontamination of the grilled chicken.
Calculation of the consumer exposure by consuming a slice of bread and a piece grilled chicken.
Within subscenarios 1 and 2, the following steps were followed:
-
Calculation of the probability that the food (bread/grilled chicken) is contaminated
First, the probability of one CFU of MRSA being transferred from the raw chicken meat purchased at retail to the RTE food (bread/grilled chicken) via hands and kitchen utensils was calculated. As we assumed no dependence between the consecutive transfer events (e.g., transfer from the raw chicken meat to hand, and then from the contaminated hand to bread), transfer coefficients corresponding to each consecutive transfer event were multiplied (Table 2: Eq. 1). For the subscenario 2, the probabilities of bacteria to be transferred to the grilled chicken at 90 and 60°C (Table 2: Eqs. 14 and 15) were calculated independently, and then, they were used to calculate the final probability of bacteria to be transferred to grilled chicken (Table 2: Eq. 13).
Apart from transfer coefficients, we took into account the frequencies of the type of handling during the food preparation. Transfer coefficients between the different surfaces and the probabilities of action occurrence depending on the type of handling were included within the calculation (Table 2: Eq. 2, Eqs. 16–18) in order to obtain the final probability of one CFU of MRSA to be transferred to bread/grilled chicken during the cross‐contamination and recontamination events.
As not all raw chicken meat at retail is contaminated with MRSA, prevalence of MRSA at retail level was included also in the calculation. Therefore, the probability of one CFU of MRSA to be transferred to bread/grilled chicken due to cross‐contamination and recontamination events was calculated by multiplying in the equation (Eq.) the prevalence of this resistant bacteria in raw chicken meat at retail multiplied by the probability of cross‐contamination and recontamination events (Table 2: Eqs. 3, 19).
-
Calculation of the level of contamination of food (bread/grilled chicken) when the food is contaminated
In a first step, we calculated the number of CFU transferred per square centimeter from the raw chicken meat to the surface of one object and then from the contaminated object to the RTE food (bread/grilled chicken). As we assumed that microorganisms were distributed homogeneously throughout the food surface, we applied a binomial distribution (Table 2: Eqs. 4–7, 20–27) by using as a distribution parameter the transfer coefficients (CFU/cm2) and the number of bacteria in the donor surface of the object. Those cells already transferred from the raw chicken meat (donor surface) to the hands, knife, and cutting board in the subscenario 1 (cross‐contamination of bread) were not taken into account to calculate the number of CFU transferred to the barbecue tong and the dish within the subscenario 2 (recontamination of the grilled chicken), and for this purpose, they were subtracted from the initial bacterial concentration in the respective donor surface from the raw chicken meat (Table 2: Eqs. 22–27).
In a further step, the total amount of bacteria transferred to bread/grilled chicken was calculated by multiplying the number of CFU transferred per square centimeter by the surface of the bread/grilled chicken in contact during the cross‐contamination or recontamination events (Table 2: Eqs. 9–12, Eqs. 29–38).
As it was assumed that each consumer eats a serving consisting of a slice of bread and a piece of grilled chicken, within the subscenario 3, the probability that at least one CFU is transferred to the final serving, and therefore the probability of the consumer to be exposed to MRSA, was calculated. For that, we multiplied the calculated probabilities of bacteria in bread (pC_B_p) and in grilled chicken meat (pC_GC_p) due to cross‐contamination and recontamination events with the prevalence of MRSA in raw chicken meat at retail in Germany (Table 2: Eq. 39). The level of consumer exposure (number of bacteria that are transferred to the final serving) was calculated by adding the number of bacteria due to the cross‐contamination of the bread (N_B) to the number of bacteria due to the recontamination of the grilled chicken (N_GC) (Table 2: Eq. 40).
3.2. Model parameters
An exhaustive list with the definitions, default numerical values, and source of the model parameters is provided in Table 3.
Table 3.
Definitions, default numerical values, and sources of the model parameters
| Subscenario | Parameter type | Notation | Parameter | Unita | Descriptionb | Parameter reference |
|---|---|---|---|---|---|---|
| 1–2 | Prevalence and bacterial concentration at retail in raw chicken meat | P_C | Prevalence of MRSA (P) in raw chicken meat (C) at retail | CFU/cm2 | rbeta (0.31, 3.38) | BVL (2017) |
| N_C | Contamination level (N) on raw chicken meat (C) at retail | rlnorm (1.41, 2.22)/1.25 | Pauly et al. (2019) | |||
| 1 | Transfer coefficients and bacterial persistence after rinsing | tC_H | Transfer coefficient (t) from raw chicken meat (C) to hand (H) | 0.53 | Fetsch and Tenhagen (2017) | |
| tC_CB | Transfer coefficient (t) from raw chicken meat (C) to cutting board (CB) | 0.04 | Fetsch and Tenhagen (2017) | |||
| tC_K | Transfer coefficient (t) from raw chicken meat (C) to knife (K) | 0.02 | Fetsch and Tenhagen (2017) | |||
| tH_B | Transfer coefficient (t) from hands (H) to bread (B) | 0.03 | Luber et al. (2006) | |||
| tCB_B | Transfer coefficient (t) from cutting board (CB) to bread (B) | 0.66 | Fetsch and Tenhagen (2017) | |||
| tK_B | Transfer coefficient (t) from knife (K) to bread (B) | 0.66 | Fetsch and Tenhagen (2017) | |||
| tCB_CB | Persistence coefficient of MRSA in cutting board (CB) after rinsing | 0.12 | Fetsch and Tenhagen (2017) | |||
| Probabilities of action occurrence | pCF | Probability (p) that the raw chicken meat (C) is cut first (F) (before cutting the bread) | 0.50 | Nauta et al. (2005) | ||
| pRCB | Probability (p) that the cutting board (CB) is rinsed (R) after cutting the raw chicken meat and before cutting the bread | 0.28 | Voedingscentrum (1999) | |||
| pCB | Probability (p) that the cutting board (CB) is not rinsed after cutting the raw chicken meat and before cutting the bread | 1‐pRCB | Assumption | |||
| Surfaces involved | SB_H | Bread contaminated surface (SB) from hand (H) | cm2 | 90 | Fetsch and Tenhagen (2017) | |
| SB_CB | Bread contaminated surface (SB) from cutting board (CB) | cm2 | runif (63, 80) | Fetsch and Tenhagen (2017) | ||
| SB_K | Bread contaminated surface (SB) from knife (K) | cm2 | 19.60 | Fetsch and Tenhagen (2017) | ||
| 2 | Transfer coefficients and bacterial persistence after rinsing | tC_D | Transfer coefficient (t) from raw chicken meat (C) to dish (D) | 0.01 | Fetsch and Tenhagen (2017) | |
| tC_T | Transfer coefficient (t) from raw chicken meat (C) to barbecue tong (T) | 0.01 | Fetsch and Tenhagen(2017) | |||
| tD_C90 | Transfer coefficient (t) from dish (D) to grilled chicken that remains at 90°C (C90) | 0.01 | Fetsch and Tenhagen (2017) | |||
| tT_C90 | Transfer coefficient (t) from barbecue tong (T) to grilled chicken that remains at 90°C (C90) | 0.05 | Fetsch and Tenhagen (2017) | |||
| tD_C60 | Transfer coefficient (t) from dish (D) to grilled chicken that remains at 60°C (C60) | 0.10 | Fetsch and Tenhagen (2017) | |||
| tT_C60 | Transfer coefficient (t) from barbecue tong (T) to grilled chicken that remains at 60°C (C60) | 0.18 | Fetsch and Tenhagen (2017) | |||
| tD_D | Persistence coefficient of MRSA in dish (D) after rinsing | 0.28 | Fetsch and Tenhagen (2017) | |||
| Probability of action occurrence | pMF | Probability (p) that the raw chicken meat is manipulated (M) first (F) (before grilled chicken is manipulated) | 1 | Assumption | ||
| pC90 | Probability (p) that the grilled chicken remains warm (C90) when is manipulated | 0.60 | Assumption | |||
| pC60 | Probability (p) that the grilled chicken cools to 60°C (C60) before being manipulated | 1‐pC90 | Assumption | |||
| pRD | Probability that the dish (D) is rinsed(R) after being used for raw chicken meat manipulation | 0.28 | Voedingscentrum (1999) | |||
| pD | Probability (p) that the dish (D) is not rinsed after being used for raw chicken meat | 1‐pRD | Assumption | |||
| Surfaces involved | SGC_D | Grilled chicken contaminated surface (SGC) from dish (D) | cm2 | 22.14 | Fetsch and Tenhagen (2017) | |
| SGC_T | Grilled chicken contaminated surface (SGC) from barbecue tong (T) | cm2 | 14.17 | Fetsch and Tenhagen (2017) |
Transfer/persistence coefficients, probabilities, and prevalence values are expressed as a fraction of 1.
The probability data are rounded to two decimals. To see the exact data consult model script.
3.2.1. Prevalence and burden of MRSA
Data on the prevalence and burden of MRSA on raw chicken meat at retail level were collected from scientific literature. Epidemiological data from the annual surveillance of antimicrobial resistance in Germany in 2016 (BVL, 2017) measured as the percentage of raw chicken meat samples contaminated in the different Federal States (obtained from a total 422 samples) were used to calculate the prevalence of MRSA on raw chicken meat at retail.
Burden of MRSA in raw chicken meat at retail was obtained from Pauly et al. (2019). As these data were expressed as the number of CFUs per gram of raw chicken meat, they were transformed to CFU/cm2 previously to its inclusion in the model. For this purpose, we assumed that 25 g of chicken meat correspond to 20 cm2 of chicken surface (based on laboratory measurements: data not shown).
Beta and log‐normal distributions were the most suitable distributions to describe the prevalence and burden of MRSA in raw chicken meat at retail, respectively.
3.2.2. Transfer and persistence coefficients
Most of the transfer and persistence coefficients were obtained from Fetsch and Tenhagen (2017), where skinless chicken breast filets were artificially contaminated with 5–7 log CFU of MRSA (CC398) per 20 cm2 and stored overnight. They played both scenarios contemplated in this work repeating each step 10 times and using one‐time equipment and one filet per step (with a fixed and strict setting in terms of time, crew, and people playing the scenario and taking the samples to avoid interindividual variation). Either swab or food samples were analyzed by classical microbiological colony counting techniques using selective agar plates. Before calculating the transfer coefficients, they normalized the measurements per cm2.
They calculated transfer rates as follows:
The transfer coefficient from hands to bread was obtained from the experiments carried out by Luber, Brynestad, Topsch, Scherer, and Bartelt (2006) in Campylobacter.
Due to the lack of correlated measurements between the donor and receiving surfaces and the wide variability of the transfer and persistence coefficients presented by Fetsch and Tenhagen (2017), it was not possible to fit probability distributions out of them, and the transfer and persistence coefficients were incorporated within the model as mean probabilities of one CFU to be transferred from one surface to other, or to remain in one surface after rinsing. In order to contemplate the variability in the transfer events between the different objects, we applied binomial distributions for the calculation of the number of CFU transferred between two surfaces.
3.2.3. Actions and probability of action occurrence
Within the actions considered (Table 1), the probability that the following hygiene practices were performed was included within the model: (a) probability that the raw chicken meat is cut before cutting the bread (pCF); (b) probability that the cutting board is rinsed after cutting the raw chicken meat and before cutting the bread (pRCB); (c) probability that the raw chicken meat is manipulated before grilled chicken (pMF); (d) probability that the dish is rinsed after being used for raw chicken meat (pRD); probability that the grilled chicken remain warm (pC90); and (e) probability that the grilled chicken cools to 60°C before being manipulated (pC60) (Figure 1).
Data on the frequencies of these hygiene practices were collected from the scientific literature. In some cases, data were estimated from the authors as experts’ opinion.
3.2.4. Surfaces involved
A detailed description of the chronologically ordered list of actions together with the surfaces in contact during the food preparation considered for the model is provided in the Appendix 1. For the calculation of the contaminated surfaces, we took into consideration the area (cm2) of the surface in contact (donor or receiving surface) which had smaller dimensions, so in those cases where the donor surface was higher than the receiving surface, the area of this last surface was considered for calculating the number of CFU transferred. In those cases where the donor surface was smaller than the receiving surface, the first one was considered.
3.3. Estimation of consumer exposure per serving
Figure 2 and Table 4 show, for the default parameters values listed in Table 3, the simulated distributions of the relative frequencies of one CFU to be transferred from raw chicken meat (given the MRSA prevalence at retail) to bread (PC_B), grilled chicken (PC_GC), and the final serving (P_Ex). We see that the probability of one CFU to be transferred from the raw chicken meat is really low in all cases, but it is higher during the cross‐contamination of the bread. The mean value of the probability of bread to be contaminated with MRSA is 1.88 × 10–3, while for the grilled chicken the probability to be contaminated with MRSA is 1.07 × 10–4. In 95% of simulations, the probability of one CFU of MRSA to be transferred to the final serving from the contaminated raw chicken meat and therefore the probability of the consumer to be exposed by at least one cell while consuming a serving would be smaller than 1.07 × 10–5.
Figure 2.
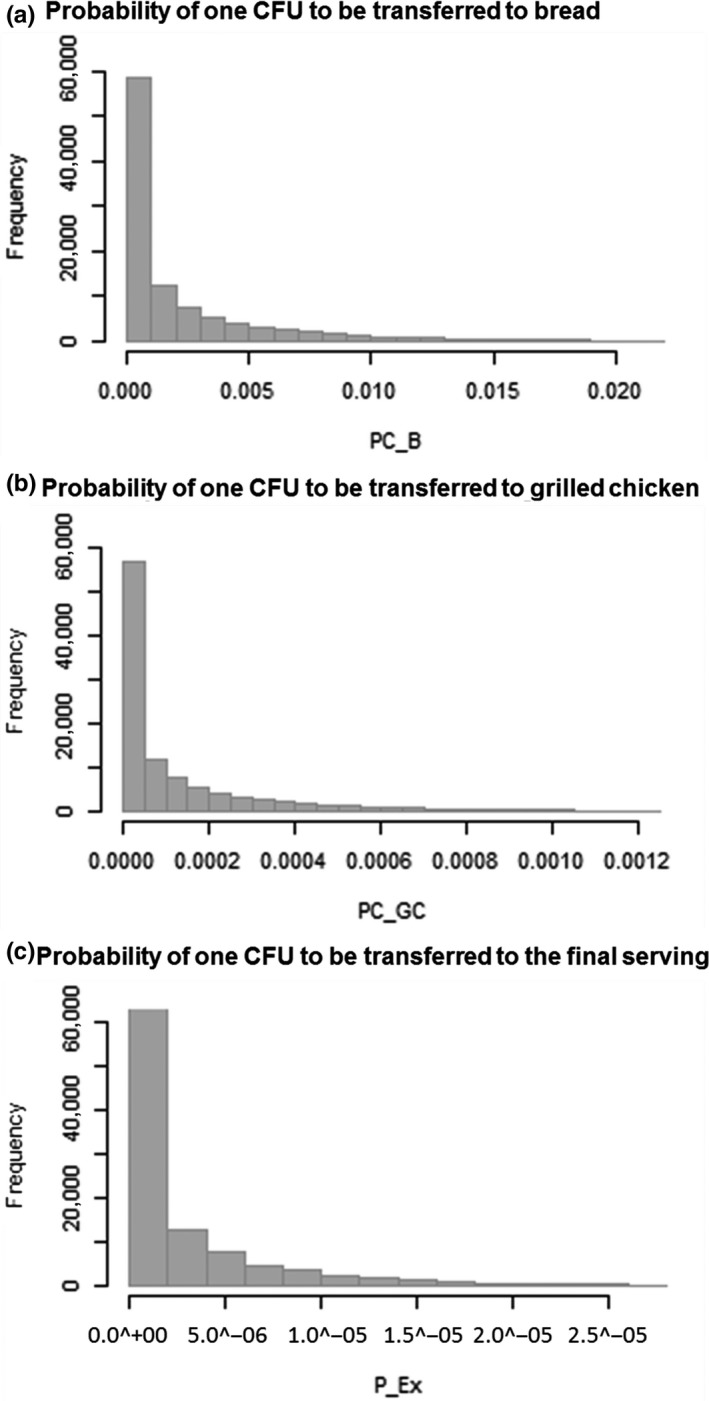
Model output from a series of 105 Monte Carlo simulations of the model, showing the relative frequencies of the probabilities of one CFU to be transferred to (a) bread (PC_B), (b) grilled chicken (PC_GC), and (c) the final serving (P_Ex) due to the cross‐contamination and recontamination events contemplated in the model
Table 4.
Model output from a series of 105 Monte Carlo simulations of the model, showing the probabilities of one CFU to be transferred from raw chicken meat to bread (PC_B), grilled chicken (PC_GC) and final serving (P_Ex), and the number of CFU transferred from raw chicken meat to bread (N_B), grilled chicken (N_GC), and final serving (N_Ex)
| Mean | SD | 1st Qu. | 2nd Qu. | 3rd. Qu. | Max. | ||
|---|---|---|---|---|---|---|---|
| Probability of one CFU being transferred from raw chicken meat (expressed as a fraction of 1) | PC_B | 1.88 × 10–3 | 2.89 × 10–3 | 5.60 × 10–5 | 5.63 × 10–4 | 2.48 × 10–3 | 2.13 × 10–2 |
| PC_GC | 1.07 × 10–4 | 1.65 × 10–4 | 3.19 × 10–6 | 3.21 × 10–5 | 1.41 × 10–4 | 1.22 × 10–3 | |
| P_Ex | 2.44 × 10–6 | 3.75 × 10–6 | 7.27 × 10–8 | 7.31 × 10–7 | 3.21 × 10–6 | 2.77 × 10–5 | |
| Number of CFU transferred from raw chicken meat (CFU/serving) | N_B | 57.8 | 587 | 0 | 0 | 28.2 | 95,627 |
| N_GC | 1.03 | 10.9 | 0 | 0 | 0 | 1,753 | |
| N_Ex | 58.8 | 597 | 0 | 0 | 28.6 | 97,380 |
Focusing on contaminated raw chicken meat, the simulated distributions of the number of MRSA transferred from the raw chicken meat to the bread (N_B), grilled chicken (N_GC), and final serving (N_Ex) are provided in Figure 3 and Table 4. In this case, the number of CFU transferred to bread (mean = 57.8 CFU per slice of bread) is also higher than the number of CFU transferred to grilled chicken (mean = 1.03 CFU per portion of grilled chicken). In 75% of simulations, less than 29 CFU of MRSA would be transferred to the final serving.
Figure 3.
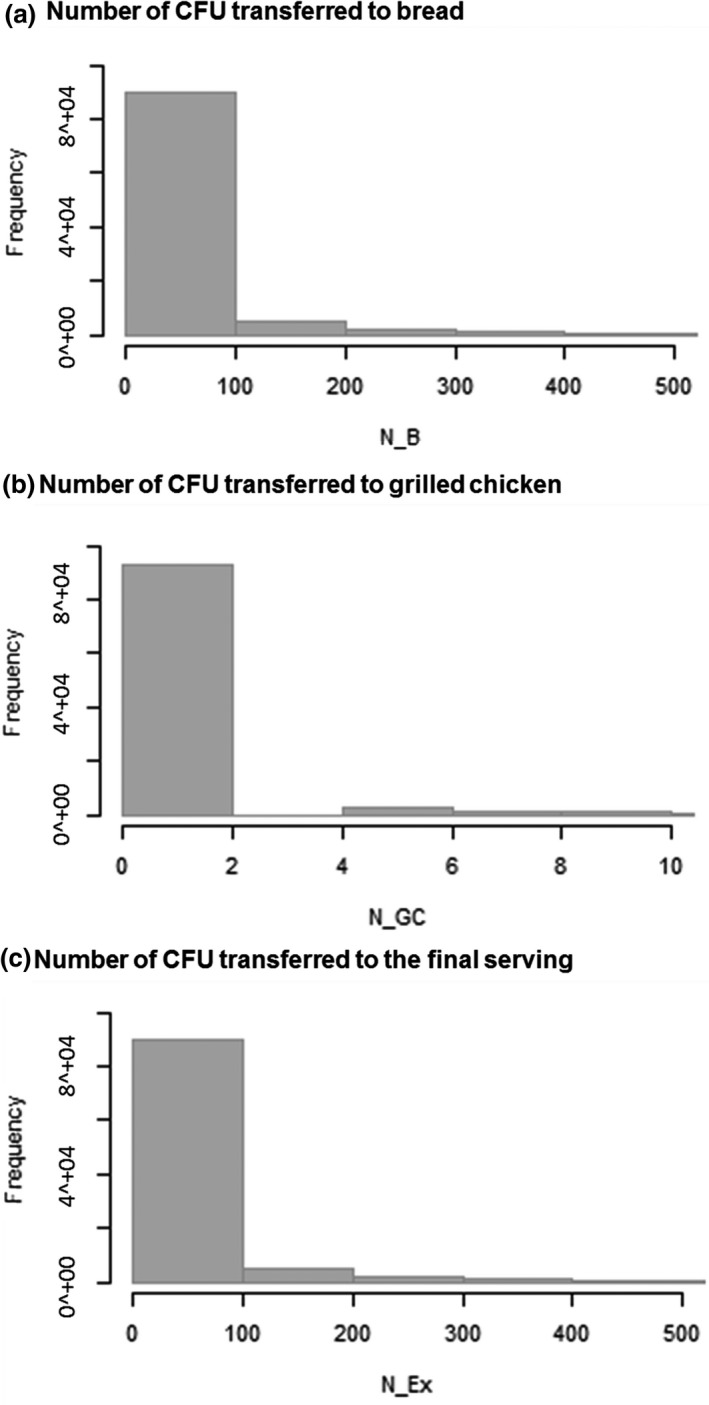
Model output from a series of 105 Monte Carlo simulations of the model, showing the relative frequencies of the number of MRSA transferred from raw chicken meat to (a) bread (N_B), (b) grilled chicken (N_GC), and (c) the final serving (N_Ex) due to the cross‐contamination and recontamination events contemplated in the model
The influence of the temperature of the grilled chicken on the mean probability of one CFU to be transferred and the number of CFU transferred in average is shown in Figure 4. The probability of the grilled chicken to get recontaminated was estimated to be 4.05 × 10–5 in average in case the chicken remains warm (90°C) and 2.07 × 10–4 if it cools down to 60°C. For the grilled chicken at 90°C, the number of CFU transferred was estimated to have a mean value of 0.506 CFU/portion with no bacterial transmission in 95% of cases. For the grilled chicken that cools down to 60°C before being manipulated, 1.81 CFU/portion would be transferred in average and in 95% of cases less than 15 CFU/portion would be transferred.
Figure 4.
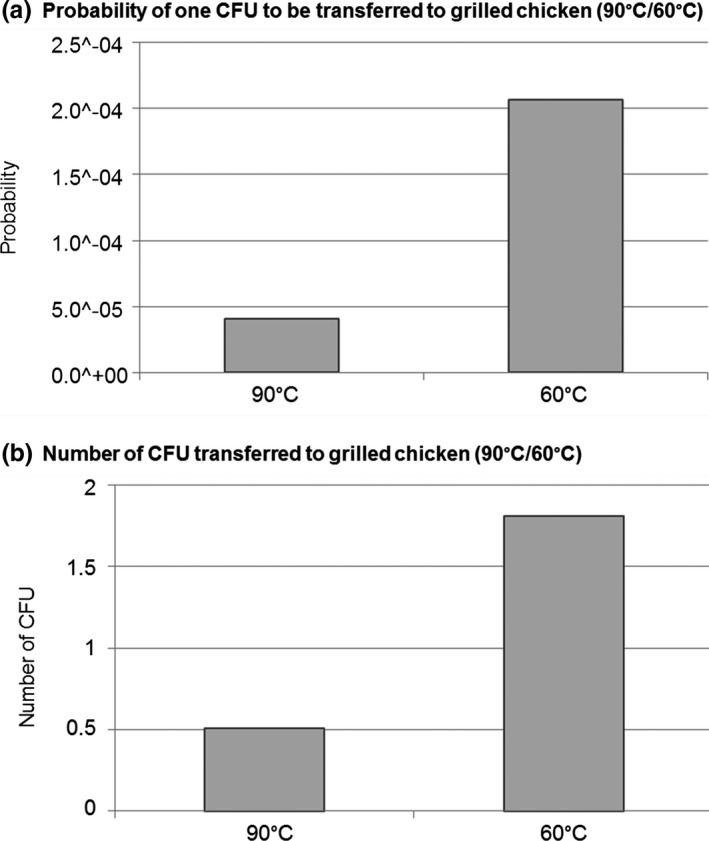
Influence of the temperature of the grilled chicken (90°C/60°C) on the (a) mean probability of one CFU to be transferred to grilled chicken and (b) number of CFU transferred in average to a portion of grilled chicken
3.4. Influence of different hygiene practices within the consumer exposure
The effects of the variation in the MRSA prevalence at retail and the probabilities of action occurrence are presented in Figure 5. Taking into account the design of our model and the deterministic approach applied in this step, a linear correlation was observed in all cases. To show the magnitude of the influence of these factors, Figure 6 presents the value of the regression coefficients and their positive or negative effect on consumer exposure.
Figure 5.
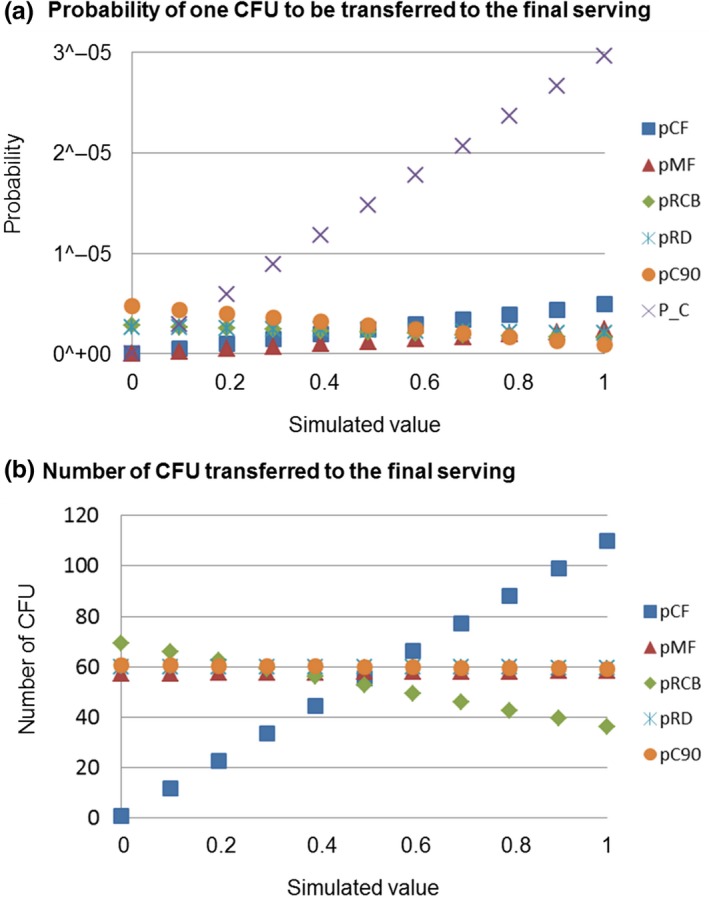
(a) Probability of one CFU of MRSA to be transferred and the (b) number of CFU transferred to the final serving as a function of the (i) probability that the raw chicken meat is cut before cutting the bread (pCF); (ii) probability that the raw chicken meat is manipulated before the grilled chicken meat (pMF); (iii) probability that the cutting board is rinsed after cutting the raw chicken meat and before cutting the bread (pRCB); (iv) probability that the dish is rinsed after being used for raw chicken meat (pRD); probability that the grilled chicken remains warm (pC90); and (v) prevalence of MRSA in chicken meat at retail (P_C)
Figure 6.
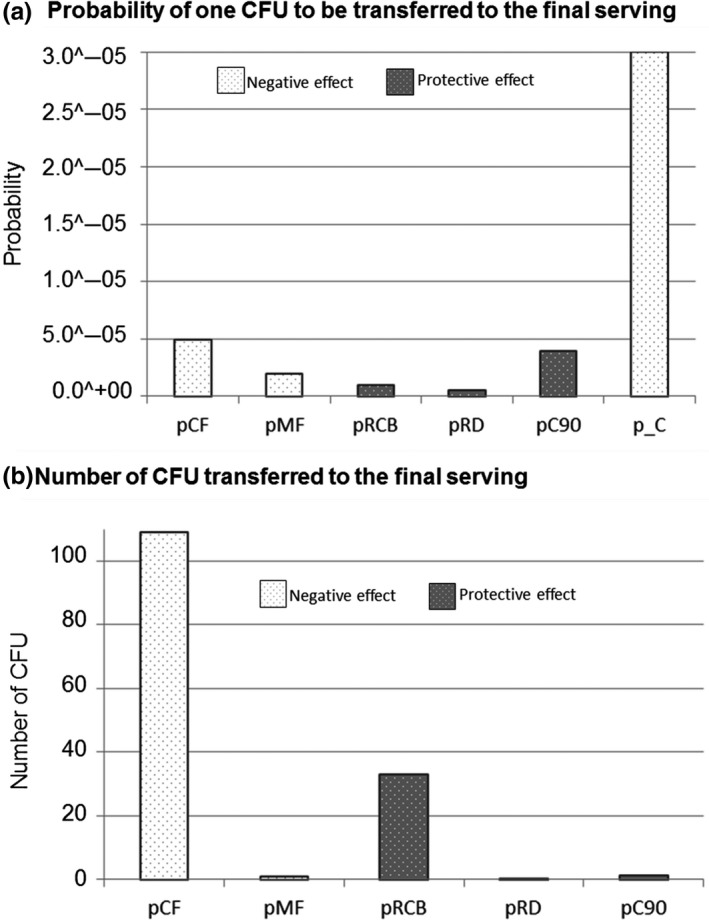
Representation of the regression coefficients between the probabilities of action occurrence and (a) the probability of one CFU to be transferred to the final serving and (b) the number of CFU transferred to the final serving, and its positive or negative effect in the consumer exposure. (pCF: probability that the raw chicken meat is cut before cutting the bread; pMF: probability that the raw chicken meat is manipulated before the grilled chicken meat; pRCB: probability that the cutting board is rinsed after cutting the raw chicken meat and before cutting the bread; pRD: probability that the dish is rinsed after being used for raw chicken meat; pC90: probability that the grilled chicken remains warm; and P_C: prevalence of MRSA in chicken meat at retail)
The results clearly show that the probability that one CFU is transferred from the raw chicken meat to the final serving is proportional to the prevalence of MRSA at retail and to the probability of cutting and manipulating the raw chicken meat first, and is negatively related to the probability of rinsing the cutting board and the dish and to manipulate the chicken warm (Figure 5a). As it can be seen from Figure 6a, the prevalence of MRSA in raw chicken meat at retail is the factor which contributes most in increasing the risk of consumer exposure followed by the probability of cutting the raw chicken first and by the probability of manipulating the raw chicken meat before the RTE products. The probability of manipulating the grilled chicken warm (at 90°C) seems to have a protective effect on the consumer exposure. However, a higher tendency of rinsing the cutting board or the dish seems to be not really effective hygiene measures for reducing the probability of consumer exposure to MRSA.
Focusing in those cases in which the raw chicken meat is contaminated, the most influencing factor on the number of bacteria transferred to the final serving is the probability of the chicken to be cut before the bread (Figures 5b and 6b). However, the probability of manipulating the raw chicken meat before seems not to have a real influence within the number of cells transferred. In order to reduce the number of cells transferred, a higher tendency of rinsing the cutting board is more effective than rinsing the dish or manipulating the chicken warm.
3.5. Model reusability and exchange
The generated model is accessible as FSKX file (https://zenodo.org/record/3240621#.XP1Ds4gzbIU). This file includes the model script, parameters, and metadata including the description of the model's range of applicability.
4. DISCUSSION
Societal changes over the last years in the European countries have led to new eating habits in consumers that should be taken into account in risk prevention (Taché & Carpentier, 2014). In this respect, social events like household barbecues have gained great popularity in countries like Germany (Danesi, 2012). As the practices and activities carried out during food preparation can largely influence the bacterial transfer events (Pérez‐Rodríguez et al., 2008) and due to the fact that during outdoor barbecues, people pay less attention to food safety and hygiene practices (Bearth et al., 2014), the study of the bacterial transfer dynamic during such events is of extreme importance. Although the models are always a simplification of the reality, the development of a probabilistic model for describing the dynamics of cross‐contamination and recontamination events during a household barbecue can be very useful in order to evaluate one of the potential pathways for the human exposure to resistant bacteria like MRSA. However, estimating the occurrence of cross‐contamination and recontamination events is not a simple task mainly due to the complexity of the contemplated scenario and in some cases also the scarcity of available data. In order to achieve a balance between simplicity and complexity, during the model design some assumptions were necessary.
4.1. Technical assumptions
The data on the prevalence and level of MRSA on raw chicken meat used as an input value for the model were based on the results obtained from samples taken at retail points, and bacteria growth before food preparation was neglected. However, during transportation from retail to home and during meat storage before the barbecue event, favorable conditions for the growth of MRSA could be possible. Therefore, the inclusion of predictive microbial models for the growth of MRSA during these previous phases could reflect more realistic conditions during a barbecue event. As a result, our model possibly leads to an underestimation of the levels of MRSA that could grow during these previous stages of food transportation and storage (Kennedy, Blair, McDowell, & Bolton, 2005). In addition, we assumed a complete inactivation of bacteria on the grilled product. However, undercooking of the grilled meat could have been also considered, which would lead to an increase in the number of bacteria in the final serving. It was difficult to find studies that provide quantitative data on MRSA in chicken at retail, and just one of them referred to the situation in Germany.
The assumption that transfer probabilities are independent has been successfully applied previously by other authors (Mylius et al., 2007). Even when it is not realistic, this assumption helps in the simplified interpretation of the scenario studied. In relation to the transfer and persistence coefficients, they were adapted from Fetsch and Tenhagen (2017). In this study, some transfer coefficients showed values greater than 100. However, as no more than the bacteria present in one surface can be transferred to the subsequent surface, these values were assumed to be of 100%. In addition, Fetsch and Tenhagen (2017) did not calculate the transmission of MRSA from hands to bread that could have been of great utility for determining the real transmission in this scenario. In our case, as there were no data available for MRSA, we assumed that this value is the same as that obtained by Luber et al. (2006) for Campylobacter spp.
It is well known that transfer coefficients normally contain a high level of variability due to the large number of factors involved, and also because of the experimental error derived from sampling and the enumeration techniques (Pérez‐Rodríguez et al., 2008). This is why commonly probability distributions are used for cross‐contamination models (Kusumaningrum, Asselt, Beumer, & Zwietering, 2004; Mylius et al., 2007; Nauta et al., 2005). However, due to the lack of correlated measurements between donor and receiving surfaces and the large variability in the transfer events, it was not possible to fit probability distributions to the data provided by Fetsch and Tenhagen (2017). Instead, transfer coefficients were included in the model as the mean probability of one CFU to be transferred from one surface to another and the variability in the transmission events was included by applying binomial distributions. The binomial distribution is suitable for the calculation of the variability of the number of bacteria transferred between surfaces (Nauta & Christensen, 2011), as it is able to describe no bacterial transmission (that it is realistic to expect when low levels of bacteria are found in the chicken meat) (Pérez‐Rodríguez et al., 2008). Further experiments describing the transfer dynamics of MRSA between the surfaces contemplated in this scenario would be necessary to improve the output of our model.
The study of the bacterial dynamics contemplated also needed some simplifications. This is the case of the “Cutting raw chicken meat” and “Cutting bread” actions that could have been subdivided into different subactions: (a) place the chicken/bread on the cutting board, (b) cut it, and (c) move it to the dish. However, since data on the transfer dynamics of these subactions are not available, we considered them as single actions.
Bearth et al. (2014) found that during outdoor barbecues, people pay less attention to food safety and hygiene practices. However, to our knowledge there are no studies describing the specific probabilities of hygiene practices carried out during a barbecue event. Therefore, a refinement of the probabilities of action occurrence by observing the consumer behavior during a barbecue event would allow us to reflect more realistic conditions and to establish relationship between the different consumer hygiene practices. As we wanted to show a worst‐case scenario during a household barbecue, the possibility of using different utensils for raw meat and RTE food was not considered. In addition, we took the probability of rinsing the cutting board and the dish (28%) from Voedingscentrum (1999), assuming that the remaining 72% was the probability of not rinsing the cutting board/dish. However, this assumption does not reflect the actual situation contemplated by them, as they included within this remaining 72% the probability of the kitchen utensils not to be rinsed and the probability of being washed.
The probability of the grilled chicken meat to remain warm/cold was set arbitrarily because to our knowledge, there are no data available on this in the scientific literature. This probability will most probably depend on the time of the barbecue, as at the beginning most of the meat will be manipulated and consumed warm, while as time goes on, and people are more satiated, the meat will cool down and will be manipulated and consumed colder.
As just those bacteria on the surface will be transferred during the cross‐contamination and recontamination events, for our model surfaces were of great importance. Even when many authors use the weight of the final serving for calculating the level of bacterial transfer, other authors have also considered the serving size as square centimeters (Kusumaningrum et al., 2004). However, while they transformed the weight of the serving size into cm2, we established directly the serving size in cm2 by considering just the surfaces of the final serving contaminated during the cross‐contamination and recontamination events (Appendix 1). As we assumed that initially only the raw chicken meat was contaminated (and not the bread) and that the cooking process is able to destroy all the MRSA in the grilled chicken, we think that this assumption is suitable to our scenario. Surfaces contemplated by Fetsch and Tenhagen (2017) for the raw chicken meat, the barbecue tong, and the knife in their experiments were contemplated for our model. Some other surfaces involved were estimated based on the literature review (hands), standard commercial size (cutting board, dish, and sliced bread), or assumed (grilled chicken). For calculating the proportion of these surfaces contaminated, we took into consideration some further assumptions. For the cutting board, we assumed displacement of the meat on the surface of the cutting board during the cutting process, and therefore, this variation was included in the contemplated surface as a probability distribution (Appendix 1). As high temperatures cause protein denaturation and a considerable reduction in the water‐holding capacity of meat (Choi et al., 2016), we assumed that surface of the raw chicken piece suffered a reduction during the cooking process. Choi et al. (2016) determined that the cooking loss of chicken steaks after grilling was around 22%. However, the cooking treatment they applied ended when the meat samples reached 75°C at their thermal center. The grilled process contemplated in our scenario achieved a higher temperature (90°C), so then higher cooking loss can be expected. Because the cooking loss is not only due to the water loss but also due to the shortening of muscle fibers, we assume a 30% of surface reduction with respect to the initial surface of the piece of raw chicken. We are aware that different surfaces could lead to a different model output, but as surfaces are contemplated as model parameters, they can be easily modified according to the specific scenario.
4.2. Model output
Data on the probability of one CFU to be transferred to the final serving and the number of pathogens on contaminated serving due to cross‐contamination and recontamination events presented in this paper are determined with a probabilistic approach, using probability distributions of the prevalence and burden of MRSA at retail level, predefined values for transfer and persistence rates, involved object surfaces and probabilities of action occurrence, as well as by first‐order Monte Carlo simulations.
Our results offer further support to the existing hypotheses (Ogata et al., 2012) that assure that the presence of MRSA in retail meat is a potential source of MRSA for the consumer. Even when the risk of exposure to MRSA through consumption of contaminated food appears to be small, our results show that it is realistic to expect that a fraction of the human exposure to MRSA could originate from cross‐contamination and recontamination events during household food preparation. Since consumers do not know the degree of contamination of the raw meat that they buy at retail, the presence of MRSA cells in the final serving should not be dismissed. Therefore, it is obviously important to handle the raw meat properly, by using separate surfaces or by washing the surfaces during the preparation of raw and cooked foods or RTE foods in order to reduce or eliminate any risk.
Given the results, it seems that the contamination of the final serving is more likely to occur via bread, rather than via grilled chicken. The highest contribution of the contaminated bread to the final consumer exposure could be explained by the fact that higher transfer coefficients are associated to the cross‐contamination of the bread, and also by the fact that the high temperatures of the grilled chicken could lead to the inactivation of a part of bacteria. The results show that recontamination of the grilled chicken depends on the temperature of the chicken when it is manipulated with unwashed contaminated kitchen utensils. This could be due to the fact that bacteria in the contaminated barbecue tong or dish are killed with high temperatures like 90°C. Therefore, the probability of consuming the grilled chicken warm (at 90°C) seems to have a protective effect on the consumer exposure, most probably due to the fact that bacteria transferred to the grilled chicken at those high temperatures during the recontamination event are not able to survive (Kennedy et al., 2005).
In general, the variation in the probabilities of action occurrence seems not to have a great influence on the probability of bacterial transfer and the number of CFU transferred. This could be due to the fact that other parameters like the transfer coefficients could play a more important role within the model output, but their influence has not been evaluated during this study as they are “fixed” for the contemplated scenario and cannot be varied consciously by changing consumer habits.
The results show that the prevalence of MRSA at retail is the factor that mostly contributes to the probability of consumer exposure and that most of the hygiene practices contemplated have not a great influence on the probability and the level of consumer exposure. However, since during a normal cooking treatment all MRSA cells that are present on the chicken surface will be destroyed (Kennedy et al., 2005), consumer exposure through the consumption of cooked chicken will depend only on the cross‐contamination and recontamination events occurring during the food preparation routines. Typical examples are hygienic failures like unwashed hands of persons handling raw meat and deficient or lack of cleanliness like putting cooked meat at the same dish of the raw meat or using the same knife or cutting board for RTE food consumed together (Sampers et al., 2012). The use of washed surfaces or separate surfaces will avoid the cross‐contamination and recontamination events and therefore the exposure of consumers to MRSA through the consumption of contaminated food.
We have to take into consideration that the results provided here in relation to the consumer exposure correspond to the consumption of just one serving. However, higher probabilities and levels of exposure could be expected during a real situation, as during a barbecue event more than just one piece of meat and bread are consumed. In addition, during a normal barbecue more RTE products can be manipulated and consumed together with the meat (such as raw vegetable sticks or fresh salads). Those could be also contaminated due to the same cross‐contamination events contemplated for the contamination of the bread.
The results of our model show that improper handling of MRSA‐contaminated chicken meat may result in consumer exposure to MRSA. However, the consequences for the consumer of this level of exposure are not contemplated in the model. Foodborne outbreaks associated with Staphylococcus aureus are almost exclusively related to the formation of enterotoxins, which are formed before consumption at a certain germ concentration in the food (if the bacterium has the corresponding toxin genes). LA‐MRSA is normally not enterotoxigenic, and therefore, the risk of acquiring infections via contaminated meat is estimated to be low. Thus, in the aftermath of consumption, colonization would only occur without clinical symptoms.
The exact extent to which the exposure of MRSA‐contaminated chicken meet contributes to the occurrence of human colonization, is also not included within the model. In pigs receiving nasal inoculation of bacteria, the lowest dosage for a successful MRSA colonization was 2 × 104 CFU (Jouy et al., 2012). In addition, Angen et al. (2017) showed that short‐term exposure to airborne MRSA poses a substantial risk for pig farms visitors to become nasal carriers, but the carriage is typically cleared within hours to a few days. So, the probability that the person exposed and contaminated through the consumption of contaminated food becomes a carrier seems to be very small, as the transferred bacteria will not be able to prevail against the resident flora.
Therefore, our model does not provide evidences for increased risk of MRSA human colonization or infection following the consumption of the contaminated food contemplated in this scenario.
Even when the developed model could help to quantify the consumer exposure to MRSA through food consumption once contaminated food has entered the household kitchen, the results presented in this paper should be interpreted cautiously, as they are based on a specific scenario, data, and assumptions. Moreover, the model should be validated when new information becomes available.
4.3. Model reusability
One of the current challenges in the microbiological food safety is the difficulty to exploit and apply the knowledge (data or models) generated in previous studies (Plaza‐Rodríguez et al., 2018). In order to avoid this, the model developed in this study has been provided in the standardized data format FSK‐ML (as FSKX file). In this way, it is easier to reproduce results from the paper, for example, by using the software tool FSK‐Lab (de Alba Aparicio et al., 2018). Even when the probabilistic model developed in this study is specific to the contemplated scenario (including the particular assumptions applied), it can vary when new data, assumptions, or cross‐contamination routes are included. Indeed, the structure of the model could be easily adapted to similar scenarios with different food products, microorganisms, or different routes of contamination. Due to the standardized data format used to exchange the model, the script can be easily modified and linked with other types of models in order to construct a complete quantitative microbial risk assessment (QMRA).
5. CONCLUSIONS
The resulting model, although it is a simplification of the proposed scenario, can be helpful for understanding the MRSA transfer dynamics and for quantifying the consumer exposure to MRSA through food consumption once contaminated food has entered the household kitchen. Even when the MRSA prevalence and bacterial load in retail chicken meat in Germany are low, this work has demonstrated that these resistant bacteria can reach the consumer due to cross‐contamination and recontamination events. This study therefore highlights not only the importance of strengthening measures aimed to reduce the prevalence of MRSA on raw chicken meat at retail, but also the need to keep good hygiene practices during the household food manipulation. Washing hands after touching raw meat and before handling RTE food, keeping utensils and serving dishes clean when preparing food and ensuring not to mix those used to prepare raw and RTE dishes, using different utensils, plates, and cutting boards for raw and cooked food might help to reduce the spread of MRSA. The incorporation of the developed model into a complete QMRA model will greatly help to estimate the risk of consumer exposure to MRSA through the consumption of contaminated food, allowing to develop strategies for reducing its spread.
CONFLICT OF INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
Carolina Plaza Rodríguez and Bernd‐Alois Tenhagen conceptualized the study. Carolina Plaza Rodríguez involved in formal analysis. Annemarie Kaesbohrer and Bernd‐Alois Tenhagen acquired the funding. Carolina Plaza Rodríguez wrote the original draft of the manuscript. Carolina Plaza Rodríguez, Annemarie Kaesbohrer and Bernd‐Alois Tenhagen reviewed and edited the manuscript.
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
We wish to thank Dr. Maarten Nauta and Dr. Fernando Pérez Rodríguez for their valuable comments and suggestions during the model design, Dr. Ylanna Kelner‐Burgos for providing the details of the contemplated scenario, and Dr. Michal‐Jan Czyz and Dr. Leticia Ungaretti Haberbeck for their support during the generation of the R code. This work was supported by the German Federal Ministry of Education and Science (BMBF) in the framework of the projects MedVet‐Staph (01KI1014C) and RESET (01KI1313B).
APPENDIX 1.
Detailed description of the surfaces that play a role during the food preparation and that were considered for the calculation of the dynamic of the bacterial transmission from the raw chicken meat to the final serving. [Correction added on 22 July 2019 after first online publication: footnote symbols were missing and have since been added to this table]
| Raw chicken meat | Hands/kitchen utensils | Final contaminated food | |||||
|---|---|---|---|---|---|---|---|
| First donor surface | Receiving surface | Contaminated/second donor surface | Receiving surface | Final contaminated surface (cm2) | |||
| 0. Raw chicken meat |
Upper and side surfaces of the chicken meat 90 cm2 Fetsch and Tenhagen (2017) |
1. Hands |
Hand surface area 448 cm2 Lee, Choi, and Kim (2007) |
90 cm2 | 1. Bread |
Upper, bottom, and one side surface of the bread 93.6 cm2 |
90 |
| 2. Barbecue Tong |
Upper part of the barbecue tong 14.17 cm2 Fetsch and Tenhagen (2017) |
14.17 cm2 | 2. Grilled chicken |
Upper part of the grilled chicken 22.14 cm2 *** |
14.17 | ||
|
Bottom surface of the chicken meat 63 cm2 Fetsch and Tenhagen (2017) |
1. Cutting board |
Upper part of the cutting board 1,200 cm2 Assumed |
63−80 cm2 * | 1. Bread |
Bottom surface of the bread 81 cm2 |
63−80 | |
| 2. Dish |
Dish surface ~350 cm2 Assumed |
63 cm2 | 2. Grilled chicken |
Bottom surface of the grilled chicken 22.14 cm2 *** |
22.14 | ||
| 3. Barbecue Tong |
Bottom part of the barbecue tong 14.17 cm2 Fetsch and Tenhagen (2017) |
14.17 cm2 | 3. Grilled chicken |
Bottom surface of the grilled chicken 22.14 cm2 *** |
14.17 | ||
|
Internal surface of the chicken meat 27 cm2 Fetsch and Tenhagen (2017) |
1. Knife |
Both sides of the knife 28 cm2 Fetsch and Tenhagen (2017) |
21 cm2 ** | 1. Bread |
Inner surface of the bread 19.6 cm2 |
19.6 | |
Corresponds with the bottom surface of the chicken that has been in contact with the cutting board (±1 cm for each side for movements during cutting)
Corresponds with the part of the knife in contact with the inner surface of the chicken meat.
Corresponds to the initial bottom surface of the grilled chicken minus a 30% reduction during grilling.
Plaza‐Rodríguez C, Kaesbohrer A, Tenhagen B‐A. Probabilistic model for the estimation of the consumer exposure to methicillin‐resistant Staphylococcus aureus due to cross‐contamination and recontamination. MicrobiologyOpen. 2019;8:e900 10.1002/mbo3.900
DATA AVAILABILITY STATEMENT
The model script generated during the current study is available in the Zenodo repository at https://zenodo.org/record/3240621#.XP1CyIgzbIU (https://doi.org/10.5281/zenodo.3240620).
REFERENCES
- Allerberger, F. , Al‐Jazrawi, N. , Kreidl, P. , Dierich, M. P. , Feierl, G. , Hein, I. , & Wagner, M. (2003). Barbecued chicken causing a multi‐state outbreak of Campylobacter jejuni enteritis. Infection, 31, 19–23. 10.1007/s15010-002-3088-8 [DOI] [PubMed] [Google Scholar]
- Angen, Ø. , Feld, L. , Larsen, J. , Rostgaard, K. , Skov, R. , Madsen, A. M. , & Larsen, A. R. (2017). Transmission of methicillin‐resistant Staphylococcus aureus to human volunteers visiting a swine farm. Applied and Environment Microbiology, 83, e01489‐01417 10.1128/AEM.01489-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearth, A. , Cousin, M.‐E. , & Siegrist, M. (2014). Poultry consumers' behaviour, risk perception and knowledge related to campylobacteriosis and domestic food safety. Food Control, 44, 166–176. 10.1016/j.foodcont.2014.03.055 [DOI] [Google Scholar]
- Boost, M. V. , Wong, A. , Ho, J. , & O'Donoghue, M. (2013). Isolation of methicillin‐resistant Staphylococcus aureus (MRSA) from retail meats in Hong Kong. Foodborne Pathogens and Disease, 10, 705–710. [DOI] [PubMed] [Google Scholar]
- BVL . (2017). Berichte zur Lebensmittelsicherheit. Zoonosen‐Monitoring 2016. BVL Report. 12.2. Berichte zur Lebensmittelsicherheit. Bundesamt für Verbraucherschutz und Lebensmittelsicherheit, Berlin. [Google Scholar]
- Cassini, A. , Högberg, L. D. , Quattrocchi, A. , Hoxha, A. , Simonsen, G. S. , Colomb‐Cotinat, M. , … Monnet, D. L. (2019). Attributable deaths and disability‐adjusted life‐years caused by infections with antibiotic‐resistant bacteria in the EU and the European Economic Area in 2015: A population‐level modelling analysis. The Lancet Infectious Diseases, 19, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, B. , Otten, A. , Fazil, A. , Ernst, N. , & Smith, B. A. (2016). A review of quantitative microbial risk assessment and consumer process models for Campylobacter in broiler chickens. Microbial Risk Analysis, 2–3, 3–15. 10.1016/j.mran.2016.07.001 [DOI] [Google Scholar]
- Choi, Y.‐S. , Hwang, K.‐E. , Jeong, T.‐J. , Kim, Y.‐B. , Jeon, K.‐H. , Kim, E.‐M. , … Kim, C.‐J. (2016). Comparative study on the effects of boiling, steaming, grilling, microwaving and superheated steaming on quality characteristics of marinated chicken steak. Korean Journal for Food Science of Animal Resources, 36, 1–7. 10.5851/kosfa.2016.36.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesi, G. (2012). Commensality in French and German young adults: An ethnographic study. Hospitality and Society, 1, 153–172. 10.1386/hosp.1.2.153_1 [DOI] [Google Scholar]
- de Alba Aparicio, M. , Buschhardt, T. , Swaid, A. , Valentin, L. , Mesa‐Varona, O. , Günther, T. , … Filter, M. (2018). FSK‐Lab – An open source food safety model integration tool. Microbial Risk Analysis, 10, 13–19. 10.1016/j.mran.2018.09.001 [DOI] [Google Scholar]
- de Boer, E. , Zwartkruis‐Nahuis, J. T. M. , Wit, B. , Huijsdens, X. W. , de Neeling, A. J. , Bosch, T. , … Heuvelink, A. E. (2009). Prevalence of methicillin‐resistant Staphylococcus aureus in meat. International Journal of Food Microbiology, 134, 52–56. 10.1016/j.ijfoodmicro.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Deiters, C. , Günnewig, V. , Friedrich, A. W. , Mellmann, A. , & Köck, R. (2015). Are cases of methicillin‐resistant Staphylococcus aureus clonal complex (CC) 398 among humans still livestock‐associated? International Journal of Medical Microbiology, 305, 110–113. 10.1016/j.ijmm.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Delignette‐Muller, M. L. , & Dutang, C. (2015). fitdistrplus : An R package for fitting distributions. Journal of Statistical Software, 64, 1–34. [Google Scholar]
- ECDC . (2017). Surveillance of antimicrobial resistance in Europe 2016, Annual report of the European Antimicrobial Resistance Surveillance Network (EARS‐Net). Stockholm, Sweden: European Centre for Disease Prevention and Control. [Google Scholar]
- EFSA . (2009). Assessment of the Public Health significance of meticillin resistant Staphylococcus aureus (MRSA) in animals and foods. EFSA Journal, 993, 1–73. [Google Scholar]
- EFSA . (2017). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal, 15, 5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers, E. G. , Pielaat, A. , Smid, J. H. , van Duijkeren, E. , Vennemann, F. B. C. , Wijnands, L. M. , & Chardon, J. E. (2017). Comparative exposure assessment of ESBL‐producing Escherichia coli through meat consumption. PLoS ONE, 12, e0169589 10.1371/journal.pone.0169589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetsch, A. , & Tenhagen, B. A. (2017). How big is the risk? Update on MRSA in the food chain. Berlin, Germany: RESET‐MedVet‐Staph Abschlusssymposium. [Google Scholar]
- Friese, A. , Schulz, J. , Zimmermann, K. , Tenhagen, B. A. , Fetsch, A. , Hartung, J. , & Rösler, U. (2013). Occurrence of livestock‐associated methicillin‐resistant Staphylococcus aureus in turkey and broiler barns and contamination of air and soil surfaces in their vicinity. Applied and Environment Microbiology, 79, 2759–2766. 10.1128/AEM.03939-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastmeier, P. (2010). Healthcare‐associated versus community‐acquired infections: A new challenge for science and society. International Journal of Medical Microbiology, 300, 342–345. 10.1016/j.ijmm.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Jones, T. F. , Kellum, M. E. , Porter, S. S. , Bell, M. , & Schaffner, W. (2002). An outbreak of community‐acquired foodborne illness caused by methicillin‐resistant Staphylococcus aureus . Emerging Infectious Diseases, 8, 82–84. 10.3201/eid0801.010174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouy, E. , Le Roux, A. , Kéranflec’h, A. , Granier, S. A. , Laurent, F. , Kempf, I. , … Chauvin, C. (2012). Methicillin‐resistant Staphylococcus aureus ST398 contamination and transmission in pigs after a low dose inoculation. Letters in Applied Microbiology, 54, 518–523. 10.1111/j.1472-765X.2012.03239.x [DOI] [PubMed] [Google Scholar]
- Kadariya, J. , Smith, T. C. , & Thapaliya, D. (2014). Staphylococcus aureus and staphylococcal food‐borne disease: An ongoing challenge in Public Health. BioMed Research International, 2014, 827965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, J. , Blair, I. S. , McDowell, D. A. , & Bolton, D. J. (2005). An investigation of the thermal inactivation of Staphylococcus aureus and the potential for increased thermotolerance as a result of chilled storage. Journal of Applied Microbiology, 99, 1229–1235. 10.1111/j.1365-2672.2005.02697.x [DOI] [PubMed] [Google Scholar]
- Kock, R. , Ballhausen, B. , Bischoff, M. , Cuny, C. , Eckmanns, T. , Fetsch, A. , … Becker, K. (2014). The impact of zoonotic MRSA colonization and infection in Germany. Berliner und Munchener Tierarztliche Wochenschrift, 127, 384–398. [PubMed] [Google Scholar]
- Kusumaningrum, H. D. , van Asselt, E. D. , Beumer, R. R. , & Zwietering, M. H. (2004). A quantitative analysis of cross‐contamination of Salmonella and Campylobacter spp. via domestic kitchen surfaces. Journal of Food Protection, 67, 1892–1903. 10.4315/0362-028X-67.9.1892 [DOI] [PubMed] [Google Scholar]
- Lee, J.‐Y. , Choi, J.‐W. , & Kim, H. (2007). Determination of hand surface area by sex and body shape using alginate. Journal of Physiological Anthropology, 26, 475–483. [DOI] [PubMed] [Google Scholar]
- Li, B. , & Webster, T. J. (2018). Bacteria antibiotic resistance: New challenges and opportunities for implant‐associated orthopedic infections. Journal of Orthopaedic Research, 36, 22–32. 10.1002/jor.23656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber, P. , Brynestad, S. , Topsch, D. , Scherer, K. , & Bartelt, E. (2006). Quantification of Campylobacter species cross‐contamination during handling of contaminated fresh chicken parts in kitchens. Applied and Environment Microbiology, 72, 66–70. 10.1128/AEM.72.1.66-70.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazengia, E. , Fisk, C. , Liao, G. , Huang, H. , & Meschke, J. (2015). Direct observational study of the risk of cross‐contamination during raw poultry handling: Practices in private homes. Food Protection Trends, 35, 8–23. [Google Scholar]
- Mertens, E. , Kreher, H. , Rabsch, W. , Bornhofen, B. , Alpers, K. , & Burckhardt, F. (2013). Severe infections caused by Salmonella Enteritidis PT8/7 linked to a private barbecue. Epidemiology and Infection, 141, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylius, S. D. , Nauta, M. J. , & Havelaar, A. H. (2007). Cross‐contamination during food preparation: A mechanistic model applied to chicken‐borne Campylobacter . Risk Analysis, 27, 803–813. 10.1111/j.1539-6924.2006.00872.x [DOI] [PubMed] [Google Scholar]
- Nauta, M. , & Christensen, B. (2011). The impact of consumer phase models in microbial risk analysis. Risk Analysis, 31, 255–265. 10.1111/j.1539-6924.2010.01481.x [DOI] [PubMed] [Google Scholar]
- Nauta, M. J. , Jacobs‐Reitsma, W. F. , Evers, E. G. , Van Pelt, W. , & Havelaar, A. H. (2005). Risk assessment of Campylobacter in the Netherlands via broiler meat and other routes. RIVM report 250911006. National Institute for Public Health and the Environment, Bilthoven. [Google Scholar]
- Ogata, K. , Narimatsu, H. , Suzuki, M. , Higuchi, W. , Yamamoto, T. , & Taniguchi, H. (2012). Commercially distributed meat as a potential vehicle for community‐acquired methicillin‐resistant Staphylococcus aureus . Applied and Environment Microbiology, 78, 2797–2802. 10.1128/AEM.07470-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly, N. , Wichmann‐Schauer, H. , Ballhausen, B. , Torres Reyes, N. , Fetsch, A. , & Tenhagen, B. A. (2019). Detection and quantification of methicillin‐resistant Staphylococcus aureus in fresh broiler meat at retail in Germany. International Journal of Food Microbiology, 292, 8–12. 10.1016/j.ijfoodmicro.2018.11.025 [DOI] [PubMed] [Google Scholar]
- Pérez‐Rodríguez, F. , Valero, A. , Carrasco, E. , García, R. M. , & Zurera, G. (2008). Understanding and modelling bacterial transfer to foods: A review. Trends in Food Science & Technology, 19, 131–144. 10.1016/j.tifs.2007.08.003 [DOI] [Google Scholar]
- Plaza‐Rodríguez, C. , Haberbeck, L. U. , Desvignes, V. , Dalgaard, P. , Sanaa, M. , Nauta, M. , … Guillier, L. (2018). Towards transparent and consistent exchange of knowledge for improved microbiological food safety. Current Opinion in Food Science, 19, 129–137. 10.1016/j.cofs.2017.12.002 [DOI] [Google Scholar]
- Possas, A. , Carrasco, E. , García‐Gimeno, R. M. , & Valero, A. (2017). Models of microbial cross‐contamination dynamics. Current Opinion in Food Science, 14, 43–49. 10.1016/j.cofs.2017.01.006 [DOI] [Google Scholar]
- Sampers, I. , Berkvens, D. , Jacxsens, L. , Ciocci, M.‐C. , Dumoulin, A. , & Uyttendaele, M. (2012). Survey of Belgian consumption patterns and consumer behaviour of poultry meat to provide insight in risk factors for campylobacteriosis. Food Control, 26, 293–299. 10.1016/j.foodcont.2012.01.054 [DOI] [Google Scholar]
- Sergelidis, D. , & Angelidis, A. S. (2017). Methicillin‐resistant Staphylococcus aureus: A controversial food‐borne pathogen. Letters in Applied Microbiology, 64, 409–418. [DOI] [PubMed] [Google Scholar]
- Taché, J. , & Carpentier, B. (2014). Hygiene in the home kitchen: Changes in behaviour and impact of key microbiological hazard control measures. Food Control, 35, 392–400. 10.1016/j.foodcont.2013.07.026 [DOI] [Google Scholar]
- Tang, Y. , Larsen, J. , Kjeldgaard, J. , Andersen, P. S. , Skov, R. , & Ingmer, H. (2017). Methicillin‐resistant and ‐susceptible Staphylococcus aureus from retail meat in Denmark. International Journal of Food Microbiology, 249, 72–76. 10.1016/j.ijfoodmicro.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Tenhagen, B. A. , Fetsch, A. , Stuhrenberg, B. , Schleuter, G. , Guerra, B. , Hammerl, J. A. , … Appel, B. (2009). Prevalence of MRSA types in slaughter pigs in different German abattoirs. The Veterinary Record, 165, 589–593. 10.1136/vr.165.20.589 [DOI] [PubMed] [Google Scholar]
- Tenhagen, B. A. , Vossenkuhl, B. , Käsbohrer, A. , Alt, K. , Kraushaar, B. , Guerra, B. , … Fetsch, A. (2014). Methicillin‐resistant Staphylococcus aureus in cattle food chains – Prevalence, diversity, and antimicrobial resistance in Germany. Journal of Animal Science, 92, 2741–2751. [DOI] [PubMed] [Google Scholar]
- van Duin, D. , & Paterson, D. (2016). Multidrug resistant bacteria in the community: Trends and lessons learned. Infectious Disease Clinics of North America, 30, 377–390. 10.1016/j.idc.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voedingscentrum . (1999). Hygiëne privé‐huishouding Voedingscentrum, Den Haag. [Google Scholar]
- Weese, J. S. , Avery, B. P. , & Reid‐Smith, R. J. (2010). Detection and quantification of methicillin‐resistant Staphylococcus aureus (MRSA) clones in retail meat products. Letters in Applied Microbiology, 51, 338–342. 10.1111/j.1472-765X.2010.02901.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The model script generated during the current study is available in the Zenodo repository at https://zenodo.org/record/3240621#.XP1CyIgzbIU (https://doi.org/10.5281/zenodo.3240620).


