Abstract
Dopamine (DA) neuron projections to the striatum are functionally heterogeneous with diverse behavioral roles. We focus here on DA neuron projections to the nucleus accumbens (NAc) medial Shell, their distinct anatomical and functional connections, and discuss their role in motivated behavior. We first review rodent studies showing that a subpopulation of DA neurons in the medial ventral tegmental area (VTA) project to the NAc medial Shell. Using a combinatoric strategy, we show that the majority of DA neurons projecting to the NAc Shell express vesicular glutamate transporter 2 (VGLUT2) making them capable of glutamate co-transmission (DA-GLU neurons). In the NAc dorsal medial Shell, all of the DA neuron terminals arise from DA-GLU neurons, while in the lateral NAc Shell, DA neuron terminals arise from both DA-GLU neurons and DA-only neurons, without VGLUT2. DA-GLU neurons make excitatory connections to the three major cells types, spiny projection neurons, fast-spiking interneuron and cholinergic interneurons (ChIs). The strongest DA-GLU neuron excitatory connections are to ChIs. Photostimulation of DA-GLU neuron terminals in the slice drive ChIs to fire in a burst. Finally, we review studies that address specially the behavioral function of this subpopulation of DA neurons in extinction learning and latent inhibition. Taking into account findings from anatomical and functional connectome studies, we propose that DA-GLU neuron connections to ChIs in the medial Shell play a crucial role in switching behavioral responses under circumstances of altered cue-reinforcer contingencies.
Introduction
Dopamine (DA) neurons in the ventral midbrain are distributed within the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA). Since the first description of these neurons (Ungerstedt, 1971), studies on SNc DA neurons have focused on motor behavior, as loss of SNc DA neurons underpins Parkinson’s disease, while studies on the function of VTA DA neurons have been associated with translation of motivation to action (Mogenson et al., 1980). VTA DA neurons projecting to limbic and cortical areas are known to regulate adaptive responses to both positive and negative reinforcers (Salamone and Correa, 2012; Zahm, 2000). In the past two decades, it has become clear that VTA DA neurons are anatomically and functionally heterogeneous and regulate different aspects of motivated behavior (Bromberg-Martin et al., 2010; Chuhma et al., 2017; Lammel et al., 2014; Morales and Margolis, 2017; Sanchez-Catalan et al., 2014; Salamone and Correa, 2012; Volman et al., 2013). This review focuses on the subpopulation of DA neurons projecting to the nucleus accumbens (NAc) medial Shell and their putative behavioral roles.
The medial Shell of the nucleus accumbens
The majority of VTA DA neurons project to the NAc (Breton et al., 2018; Ikemoto, 2007; Swanson, 1982), which is further divided into three subregions, the medial Shell, lateral Shell and the Core (Groenewegen et al., 1991; Voorn et al., 2004). The NAc medial Shell is distinguished from other NAc subregions by dense afferents from the infralimbic prefrontal cortex, the anterior paraventricular thalamus, the ventral hippocampus, parvocellular basal lateral amygdala, dorsolateral septum, lateral hypothalamus, brainstem nuclei (nucleus of the solitary tract of the hypothalamus, pedunculopontine tegmental nucleus and parabrachial nucleus) and medial VTA (Beier et al., 2015; Berendse et al., 1992; Brog et al., 1993; Delfs et al., 1998; Do-Monte et al., 2017; Groenewegen et al., 1991; H. Groenewegen et al., 1999; Heimer et al., 1991; Hunnicutt et al. 2016; Voorn et al., 2004; Zahm, 2000).
The NAc medial Shell is further differentiated from the NAc lateral Shell and Core by its efferents to the medial ventral pallidum, anterior lateral hypothalamus, lateral preoptic area and VTA (Beier et al., 2015; Groenewegen et al., 1999; Usuda et al.,1998; Yang et al., 2018; Zahm, 2000). The neuronal populations targeted in the NAc projecting regions also differ. For example, NAc medial Shell neurons make strong GABAergic connections to VTA DA neurons, while the NAc lateral Shell neurons make strong GABAergic connections to SN GABA neurons that in turn disinhibit neighboring DA neurons (Yang et al., 2018). Thus, NAc medial Shell neurons directly inhibit VTA DA neuron firing and NAc lateral Shell neurons indirectly increase SN DA neuron firing. Overall, these studies identify the medial Shell as a separate functional unit of the NAc.
DA/GLU neurons preferentially project to the NAc medial Shell
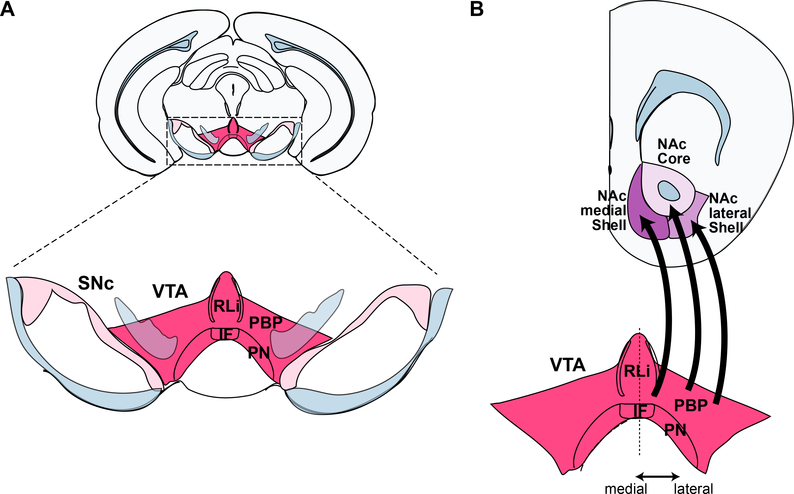
In the rodent, DA neurons comprise between 50–60% of VTA neurons (Breton et al., 2018; Nair-Roberts et al., 2008; Yetnikoff et al., 2014) and are dispersed within several subregions, including the parabrachial pigmented nucleus (PBP), paranigral nucleus (PN), caudal linear nucleus (CLi), interfascicular nucleus (IF) and rostral linear nucleus of the raphe (RLi) (Figure 1A). The dense VTA projections to the NAc subregions are largely ipsilateral and follow a medial-lateral topography (Figure 1B) (Beier et al., 2015; Breton et al., 2018; Ikemoto, 2007; Lammel et al., 2008; Rodríguez-López et al, 2017; Saunders et al., 2018; Swanson, 1982). The NAc medial Shell receives dopaminergic innervation from the posteromedial VTA subdivisions, which include the IF, CLi and PN. The NAc lateral Shell and Core receive dopaminergic innervation from the lateral half of the VTA, which includes the PBP. Simultaneous injections of the retrograde tracer cholera toxin subunit B (CTB) tagged with either Alexa Fluor 594 or 647 in the medial Shell or Core compartments revealed no overlap in the labeled cell population in the rat VTA (Luo et al., 2018). Furthermore, a mouse study using an intersectional strategy to label VTA DA neurons projecting to either the NAc lateral or medial Shell, clearly showed that the labeled axon arbors did not overlap (Beier et al., 2015). Thus, several lines of evidence point to the existence of a distinct group VTA DA neurons projecting selectively to the NAc medial Shell.
Figure 1 – The ventral tegmental area and its projections to the NAc.
A. Coronal section illustrating the location of the VTA in the ventral midbrain (upper), and its subregions (lower). B. VTA projections to the NAc medial Shell, lateral Shell and Corefollow a medial-lateral topography. Due to its more caudal location, the caudal linear nucleus (CLi) is not shown. Color coding: light blue, fibers; light pink, substantia nigra pars compacta (SNc); dark pink, ventral tegmental area (VTA); dark violet, nucleus accumbens (NAc) medial Shell; light violet, NAc lateral Shell; pale violet, NAc core. Abbreviations: IF, interfascicular nucleus; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; RLi, rostral linear nucleus of the raphe; VTA, ventral tegmental area.
Medial VTA DA neurons are distinguished by their ability to co-release glutamate (for review see Trudeau et al., 2014). Studies examining the colocalization of vesicular glutamate transporter 2 (VGLUT2) mRNA and TH immunoreactivity revealed the restricted and high prevalence of dopamine-glutamate (DA-GLU) neurons in the medial VTA (Kawano et al., 2006; Yamaguchi et al., 2011). DA neurons projecting to the NAc medial Shell preferentially express VGLUT2 mRNA in both rat and mouse (Yamaguchi et al., 2011; Yang et al., 2018). A recent mapping study of molecularly defined DA neuron subtypes, confirmed that medial VTA contains DA-GLU neurons and that they project preferentially to the NAc medial Shell (Poulin et al., 2018). DA-GLU neurons were identified using the INTRSECT strategy (Fenno et al., 2014), in which EYFP is expressed only in cells that express Cre and Flp recombinases. When Cre-on (Con) Flp-on (Fon) INTRSECT virus is injected in the VTA of double mutant mice,expressing Cre under the VGLUT2 promotor and Flp under the TH promotor (TH Flp;VGLUT2 Cre mice), YFP expression is restricted to TH positive (+)/VGLUT2+ VTA neurons.
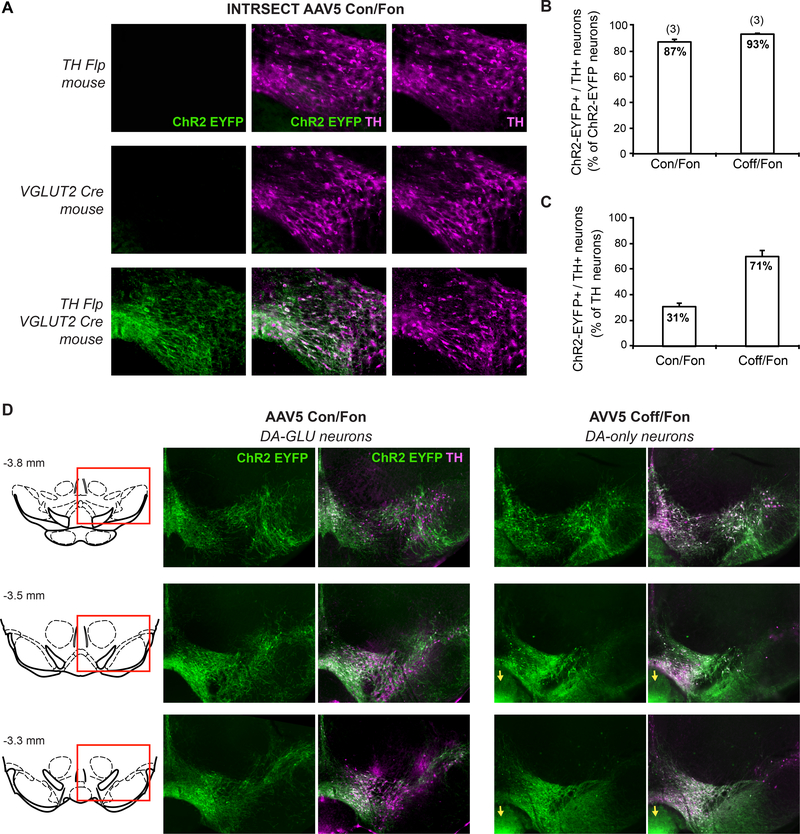
Using the same strategy we have confirmed these observations and shown further the exclusiveness of these projections. TH Flp;VGLUT2 Cre double mutant mice were either injected with the INTRSECT Con/Fon virus (AAV-nEF-Con/Fon hChR2(H134R)-EYFP-WPRE), restricting ChR2-EYFP expression to DA-GLU neurons, or INTRSECT Cre-off (Coff) Fon virus (AAV-nEF-Coff/Fon hChR2(H134R)-EYFP-WPRE), restricting ChR2-EYFP expression to DA-only neurons (TH neurons that do not express VGLUT2). VTA injections used previously described methods (Chuhma et al., 2014; Mingote et al., 2017), and the results are presented in Figure 2 and 3. We show first ChR2-EYFP expression induced by a Con/Fon virus requires expression of both Cre and Flp in DA cells (Figure 2A). We estimated the specificity of each Con/Fon and Coff/Fon virus by counting how many ChR2-EYFP+ VTA cells also coexpressed TH immunoreactivity. Our results show a specificity rate of 93 ± 1.0 % for the Coff/Fon virus and of 87 ± 2.6 % for the Con/Fon virus (Figure 2B). Among all TH immunoreactive cells in the VTA, we found that 71 ± 4.6 % expressed Coff/Fon virus and are thus DA-only neurons, while 31 ± 2.6 % expressed Con/Fon virus and are thus DA-GLU neurons (Figure 2C). This number of VTA DA-GLU neuron is only slightly higher than previously reported (Kawano et al., 2006; Steinkellner et al., 2018; Yamaguchi et al., 2011). DA-GLU neurons were mostly seen in the medial VTA, IF and PN subregions, and DA-only neurons in the lateral PBP (Figure 2D), consistent with previous in situ hybridization studies (Kawano et al., 2006; Steinkellner et al., 2018; Yamaguchi et al., 2011). Although the specificity within the VTA was high for both viruses, TH Flp may label some TH negative cells in the interpeduncular nucleus, as reported previously by Poulin and colleagues (2018). This non-specific expression was seen in mice injected with the Coff/Fon virus (Figure 2D, yellow arrows). Thus, the TH promotor appears to be active in GABAergic neurons in this nucleus. These neurons project to the lateral habenula but do not produce TH protein nor release DA (Lammel et al., 2015). Interestingly, Con/Fon virus did not show this ectopic expression, since interpeduncular nucleus GABAergic neurons do not co-express TH and VGLUT2, further validating the combinatoric strategy.
Figure 2 – Visualization ofDA-GLU and DA-only neurons using the INTRSECT combinatoric strategy.
A. Photomicrographs of the VTA assessing expression of ChR2-EYFP after intra-VTA injections of INTRSECT AAV5 Con/Fon in three different mutant mice: TH Flp, VGLUT2 Cre, and TH Flp;VGLUT2. Cells and processes labeled by the Con/Fon virus and showing EYFP immunoreactivity (left photomicrograph) are only visible in mice that express both Cre and Flp, validating the combinatoric strategy. B. Graphs displaying the specificity of the INTRSECT Con/Fon and Coff/Fon viruses, measured as the percentage of ChR2-EYFP positive neurons expressing TH in the VTA. Numbers of mice used are indicated above the bars. C. Summary of average percentage of TH+neurons in the VTA expressing either INTRSECT AAV5 Con/Fon or Coff/Fon. D. Photomicrographs of the VTA showing the distribution of the cells expressing either AAV5 Con/Fon (DA-GLU neurons; left panels) or AAV5 Coff/Fon (DA-only neurons; right panels). Ectopic expression of the AAV5 Con/Fon in the interpeduncular nucleus is indicated by yellow arrows. Numbers above the schematic coronal slices (left) are distance from bregma. These results are original data that have not been been previously published.
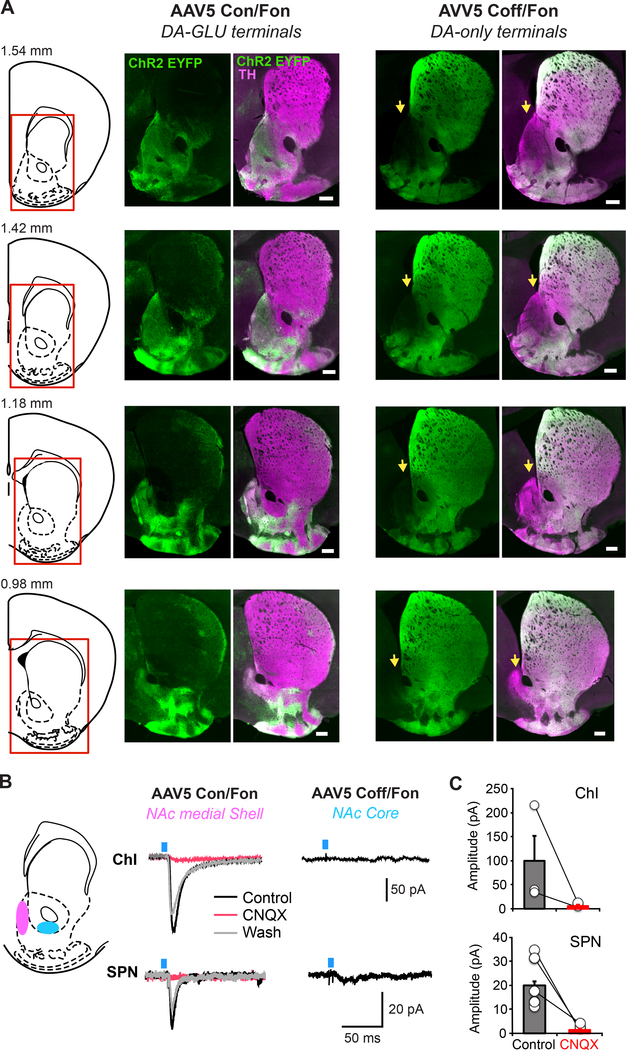
Figure 3 – Projections of DA-GLU and DA-only neurons to the Striatum.
A. Photomicrographs of the striatum taken at different anterior-posterior positions, showing distribution of DA terminals labeled with ChR2-EYFP driven by either INTRSECT AAV5 Con/Fon (DA-GLU neurons; left panels) or Coff/Fon (DA-only neurons; right panels) in the NAc. Numbers above the schematic coronal slices (left) indicate distance from bregma. Areas in the NAc medial dorsal Shell lacking DA-only labeled terminals are indicated by yellow arrows. B. Schematic of a coronal section (1.42 mm from bregma) indicating locations of patch-clamp recordings in the NAc medial Shell (pink) and NAc Core (blue) (left). Representative traces of EPSCs generated by single-pulse photostimulation (blue bar) at 0.1 Hz recorded from ChIs and SPNs are shown. Traces are averages of 10 consecutive recordings. EPSCs were observed when photostimulating DA terminals labeled by Con/Fon virus and recording in the NAc medial Shell. Responses were blocked by bath application of the AMPA receptor antagonist CNQX (40 0μM, red trace; wash, gray trace). EPSCs were not seen when photostimulating DA terminals labeled by Coff/Fon virus. ince the medial Shell lacks DA-only neuron terminals, DA-only neuron terminal stimulation and recording was done in the NAc Core. C. Summary of average EPSC amplitudes in ChIs and SPNs, before and after CNQX in mice injected with the Con/Fon virus is shown. These results are original data that have not been previously published.
Figure 3A shows the distribution of the ChR2-EYFP+ axons labeled by Con/Fon and Coff/Con viruses within the striatum. In agreement with Poulin et al. (2018), the projections from DA-GLU neurons are restricted to the NAc medial Shell and medial olfactory tubercle. Strikingly axons of DA-only neurons almost completely avoid the NAc dorsal medial Shell (Figure 3A, yellow arrows), while still innervating the NAc lateral Shell and Core and most of the dorsal striatum. Thus, not only do DA-GLU neurons target the NAc medial Shell specifically, but they do so exclusively in the dorsal medial Shell. Whole-cell voltage clamp recordings support the specificity of this combinatoric strategy by showing that glutamate-mediated excitatory postsynaptic currents (EPSCs) are observed only when photostimulating DA-GLU neuron axons in the medial Shell, and not when photostimulating DA-only neuron axons in the Core (Figure 3B), which receives the densest innervation from DA-only neurons.
Effects of DA neuron glutamate cotransmission in the NAc Shell
Selective photostimulation of DA neuron terminals in different brain regions recipient to DA neuron projections in DAT-IREScre; Ai32 mice has enabled comprehensive mapping of DA neuron connections, revealing the remarkable complexity of the ventral midbrain DA neuron signals and their regional heterogeneity (Chuhma et al., 2014; Kabanova et al., 2015; Mingote et al., 2015; Pérez-López et al., 2018; Straub et al., 2014; Stuber et al., 2010; Tritsch et al., 2012; Tecuapetla et al., 2010; Wieland et al., 2014). Several new modes of DA neuron signaling have been revealed. First, DA neuron DA transmission can induce fast synaptic responses. DA transmission in the medial dorsal striatum pauses spontaneous firing of cholinergic interneurons (ChIs) by inducing a subsecond hyperpolarization mediated by D2R coupled to a G-protein coupled inward rectifier potassium channels (GIRK channels) (Cai and Ford, 2018; Chuhma et al., 2014, 2018; Straub et al., 2014). Second, DA neurons synthesize and co-release GABA in the NAc and dorsal striatum (Kim et al., 2015; Tritsch et al, 2012; Tritsch et al., 2014). Only a few DA neurons express mRNA for the GABA synthetic enzyme GAD 65 (Kim et al., 2015; González-Hernández et al., 2001; Tritsch et al., 2014). Instead, DA neurons sustain GABA release via plasma membrane uptake of GABA (Tritsch et al., 2014) and synthesis mediated by aldehyde dehydrogenase 1a1 (Kim et al., 2015). DA neurons do not express vesicular GABA transporter; apparently GABA is loaded into vesicles via vesicular monoamine transporter 2 (Tritsch et al, 2012). Finally, DA neurons make glutamate-mediated excitatory connections in the NAc and lateral dorsal striatum, but not to the medial dorsal striatum (Cai and Ford, 2018; Chuhma et al., 2014; Chuhma et al.,2018; Mingote et al., 2015; Stuber et al., 2010; Tecuapetla et al., 2010). It appears that in relation to connections to ChIs, DA-GLU neurons signal via ionotropic glutamate in the medial Shell, while they signal via metabotropic glutamate receptors in the lateral dorsal striatum.
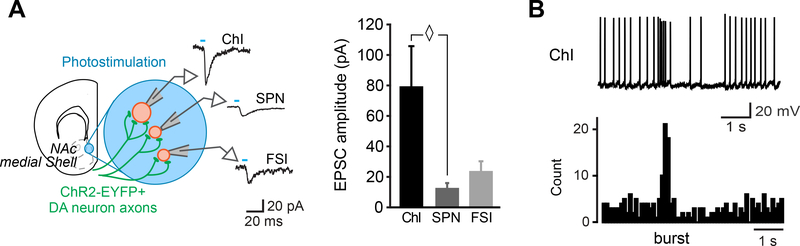
The optogenetic mapping of the DA neuron synaptic connections in the striatum clearly defined the NAc medial Shell as a hotspot for glutamate cotransmission (Chuhma et al., 2014; Mingote et al., 2015). Glutamatergic responses were measured in spiny projection neurons (SPN), fast spiking neurons (FSI), and ChI and the amplitude of these responses gives an estimate of cell-specific connection strength (Figure 4A). SPNs and FSIs showed similarly low connection strength. These weak connections are unlikely to drive SPNs and FSIs to fire given their deep resting membrane potentials, suggesting that DA neurons would only drive firing coincident with other glutamatergic inputs. Indeed, it has been shown that when SPNs are slightly depolarized and at membrane potentials approximating the typical in vivo up state of these neurons, single pulse stimulation of DA neurons is sufficient to drive firing (Tecuapetla et al., 2010). In contrast, the strength of the DA neuron glutamatergic connections to ChIs was several times greater (Figure 4A). Indeed, burst stimulation of DA neurons drives ChIs to burst fire and then pause (Figure 4B). The burst is driven by the activation of AMPA receptors and the pause by activity-dependent activation of SK3 channels and partially by D2 receptors coupled with GIRK channels (Chuhma et al., 2014). DA neurons in the medial Shell make no apparent GABAergic connections to ChIs (Chuhma et al., 2014).
Figure 4 – Functional connectivity of DA/GLU neurons in the NAc medial Shell.
A. Schematic of a coronal slice (1.34 mm from bregma) indicating the location of the patch-clamp recordings in the NAc medial Shell (left). DA neuron excitatory responses evoked by photostimulation (blue circles) were measured from ChIs, SPNs and FSI. On the left is the summary of average EPSC amplitude after single-pulse photostimulation (modified from Chuhma et al. 2014). B. Effect of photostimulation mimicking DA neuron bursting (5 pulses at 20 Hz) on ChI firing. A representative trace is shown on top, with peristimulus histograms summing ten consecutive traces (0.1 s bin) below (modified from Mingote et al., 2017). Abbreviations: ChI, cholinergic interneuron; SPN, spiny projection neuron; FSI, fast spiking interneuron.
Why DA neurons make the strongest glutamatergic connections to ChIs is not clear. A recent paper suggested that DA neuron glutamate-only synapses have high release probability (Silm et al., 2019), but the number of glutamate vesicles per synapse or number of synapses per ChI may also contribute to cell-specific connectivity. DA neurons make widespread axonal arborizations that can broadcast signals to many striatal neurons (Matsuda et al., 2009). In the NAc medial dorsal Shell, which only receives projections from DA-GLU neurons, a single DA neuron could excite multiple ChIs and synchronize their activity. The effects of synchronized ChIs activity on local striatal circuits has been studied extensively in the last decade with optogenetic stimulation (Cachope et al., 2012; English et al., 2012; Faust et al., 2015; Nelson et al., 2011; Threlfell and Cragg, 2011; Threlfell et al., 2012; Witten et al., 2010). These studies have revealed that ChIs modulate DA release and SPN activity.
Synchronized burst firing of ChI directly increases DA release by stimulating presynaptic nicotinic acetylcholine receptors (nAChRs) on DA neuron terminals (Cachope et al., 2012; Threlfell et al., 2012). In the NAc Core and dorsal striatum, the ChI-driven DA release does not show frequency-dependent summation, as single or train stimulation of ChIs produces DA transients of similar amplitude (Shin et al., 2017; Threlfell et al., 2012). The lack of summation is due to desensitization of nAChR during train stimulation. In the NAc shell, nAChRs on DA neuron terminals show less desensitization due to elevated acetylcholinesterase activity (Shin et al., 2017). This reduces the inhibitory effect at higher frequencies, allowing frequency-dependent increases in DA release. Thus, the NAc Shell local circuit and molecular environment appears to be suitable for establishing a positive feedback loop in which DA neuron burst activity induces further DA release through frequency-dependent activation of presynaptic nAChRs. The activation of nAChR will also increase release of cotransmitters, glutamate and GABA. DA neurons make glutamatergic connections to both ChIs and SPNs, and GABA connections to SPNs (Chuhma et al., 2014; Tritsch et al., 2014). However, both GABA and glutamate synapses show short-term depression when DA neurons are stimulated at burst firing frequencies (Mingote et al., 2017; Straub et al., 2014; Tecuapetla et al., 2010; Tritsch et al., 2014), limiting the facilitating effect of nAChR activation. So, the main effect of stimulating DA neuron presynaptic nAChRs will be to increase DA release.
Synchronized activation of ChI also drives inhibitory responses in SPNs both in the NAc and striatum (English et al., 2012; Faust et al., 2015; Nelson et al., 2014; Luo et al., 2013; Witten et al., 2010). This inhibition is disynaptic and recruits local GABAergic circuits. In the dorsal striatum, ChI-driven inhibition is mediated by the activation of presynaptic nAChR in some classes of GABA interneurons and GABA-releasing DA neurons (English et al., 2012; Nelson et al., 2014; Tepper et al., 2018). GABAergic responses induced by nAChR activation are similar in SPNs expressing either D1 or D2 receptors (Luo et al., 2013), suggesting that synchronized ChI activity induces a general inhibition of striatal outputs. In NAc medial Shell, the optogenetic activation of ChIs inhibits 81% of SPNs recorded in vivo (Witten et al., 2010). This effect is blocked by mecamylamine, and thus mediated by nAChR and most likely disynaptic (Witten et al., 2010). The GABA neurons mediating ChI-driven SPN inhibition in the Shell remains unknown. Although nAChR induced GABA release from dorsal striatum DA neuron terminals, the contribution of GABA corelease in NAc mShell is likely to be minimal, because of short-term depression and limited GABA cotransmission in the region (Straub et al., 2014). Further research is necessary to determine the involvement of GABA interneurons, since these cells may play a critical role in mediating the inhibitory effects of ChIs on SPN activity.
As schematized in Figure 5, DA neurons in the NAc Shell make strong glutamatergic connections to ChIs and may synchronize their activity. Synchronized bursting of ChIs triggers a cascade of events affecting the excitability of SPNs. This feed-forward system has two phases; the first phase is a rapid and transient inhibition of SPN activity, followed by a second phase with multiple modulatory components. The initial inhibition of SPNs involves the activation of presynaptic nicotinic receptors on GABA interneurons and GABA-releasing DA neurons. The second phase is mediated by the activation of G-protein coupled muscarinic and DAergic receptors in SPNs. These modulatory effects are less characterized in the NAc Shell (Goldberg et al., 2012; Surmeier et al., 2011). In general, M2-class muscarinic and DA D2 receptor signaling increases the excitability of SPNs, while M1-class muscarinic and DA D1 receptor signaling decreases the excitability SPNs. The effects depend on the divergent expression of these receptors in different subpopulations of SPNs, adding further complexity beyond what is illustrated in the schematic.
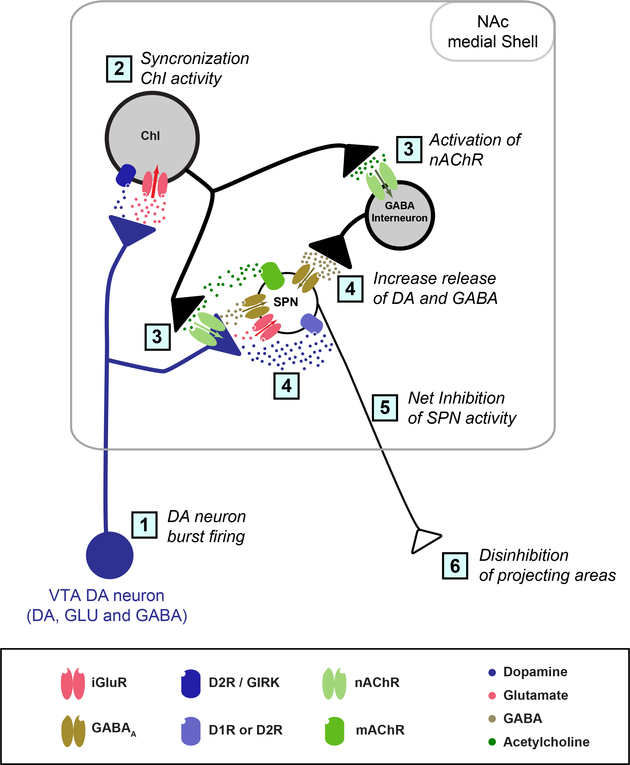
Figure 5 – DA neuron glutamate cotransmission in the NAc medial Shell.
Simplified schematic of the NAc Shell local circuit showing the cascade of events triggered by DA neuron activity. DA neurons evoke DA and glutamate signals at their synaptic connections to ChIs, while they evoke DA, glutamate and GABA signals at their connections to SPNs. The following sequence of events are hypothesized: 1) DA-GLU neuron burst firing; 2) Synchronization of ChIs activity in the NAc medial Shell by DA-GLU excitatory inputs; 3) Increased acetylcholine release and activation of presynaptic nAChRs in DA neurons and GABA interneurons, 4) an overall increase in DA and GABA release; 5) GABAA receptor activation in SPNs induces rapid and transient inhibition of SPN activity. 6). Decrease in GABA release from SPNs leads to disinhibition of NAc Shell projection areas. Transmitter release sites are shown as one presynaptic terminal per postsynaptic NAc cell type. Note that the modulatory effects mediated by muscarinic and DAergic receptors in SPNs, which are hypothesized to alter the excitability of SPN on a longer time scale, are not shown. Abbreviations: ChI, cholinergic interneuron; SPN, spiny projection neuron; ChR2, channelrhodopsin; NpHR, halorhodopsin; iGluR, ionotropic glutamate receptor; D1R, dopamine D1 receptor; D2R, dopamine D2 receptor; D2R / GIRK, dopamine D2 receptor coupled with G protein-activated inward rectifier potassium channels; nAChR, nicotinic receptor; mAChR, muscarinic receptor.
Salient events activate DA neurons projecting to the NAc Shell
DA neurons modulate motivation through their actions in the NAc (Floresco, 2015; Salamone and Correa, 2012). Burst firing of DA neurons is often observed during aversive, appetitive or novel events, and during the presentation of cues in the environment predicting positive or negative reinforcement (Bromberg-Martin et al., 2010; Hamid et al., 2016; Saddoris et al., 2015). In the NAc Shell, activity of DA neurons reflects salience of events and instigates responses directed towards salient stimuli (Saddoris et al., 2015; Wyvell and Berridge, 2000). For example, microdialysis and voltammetry studies have shown increases in DA release in NAc medial Shell when animals consume food or enter a novel environment (Bassareo and Di Chiara, 1997; Gambarana et al., 2003; Rebec et al., 1996; Roitman et al., 2008). Salient aversive events are associated with a slight decrease in DA release, which is immediately followed by a large increase in DA release at the end of the aversive stimulus(Budygin et al., 2012; de Jong et al., 2019). Increases in DA release associated with food and novelty rapidly dissipate with repeated exposure (Bassareo and Di Chiara, 1997; Bassareo and Di Chiara, 1999; Gambarana et al., 2003; Rebec et al., 1996; Segovia et al., 2011), while those associated with inescapable shock do not (Budygin et al., 2012), in agreement with increases in DA release tracking general salience. Cues predicting the delivery of food also increase DA release in the NAc Shell and the amount of DA released is positively correlated with the amount of food delivered (Sackett et al., 2017). Thus, an important factor controlling the activity of DA neurons projecting to the NAc medial Shell is the relevance of stimuli in the environment, which incorporate different dimensions of salience related to novelty and previous experience with an appetitive or aversive reinforcer. As such, DA neurons projecting to the NAc medial Shell convey alerting signals that track alterations in the environment to promote changes in behavioral output.
DA neurons projecting to the NAc medial Shell signal changes in contingencies and promote behavioral switching
DA neuron control of motivated behavior involves the capacity to facilitate switching between behaviors (Eveden and Robbins, 1983; Oades, 1985; Weiner and Feldon, 1997; Redgrave et al., 1999). Changes in DA transmission in the NAc Shell modulate the degree to which competing behavioral repertoires interfere with ongoing behavior. For example, DA receptor antagonism in the medial Shell does not block food consumption (Baldo et al., 2002; Berridge and Robinson, 1998; Nowend et al., 2001; Salamone and Correa, 2012) but alters the microstructure of feeding; animals eat the same amount of food in fewer and longer bouts, without engaging in other common behaviors, such as grooming or locomotion (Baldo et al., 2002). Reduced DA transmission decreases switching, while increased DA is associated with switching to a new behavioral strategy. In a decision-making paradigm, increases in DA release in the NAc medial Shell are greater when rats made choices under ambiguous conditions (St. Onge et al., 2012). Similarly, increases in DA release observed during performance of a set-shifting task suggest a role in shifting but not in acquisition (Stefani and Moghaddam, 2006).
Evidence that DA-GLU neurons in the medial Shell modulate behavioral switching is supported by studies using paradigms in which stimulus-reinforcer contingencies are altered, such as extinction. During extinction, a stimulus that was previously associated with a primary reinforcer is presented several times without consequence. This situation requires a shift in behavior and new learning so that the animal stops responding to the stimulus and deems it irrelevant. Animals form two separate memories, one about stimulus-reinforcer association and another about the stimulus-nothing association. Behavioral responses to the stimulus after conditioning depend on how strongly the stimulus elicits one or the other memory and produces a behavioral switch (Westbrook and Bouton, 2010).
There is evidence that in extinction increasing activity of DA neurons projecting to the NAc mShell facilitate switching, while inhibiting them disrupts switching. During fear extinction, a subpopulation of VTA DA neurons increases their activity with omission of the expected aversive event, i.e. at offset of the presentation of the conditioned stimulus (Bromberg-Martin et al., 2010; Salinas-Hernández et al., 2018). In mice trained to associate a tone with a shock and during extinction, photostimulation of VTA DA neurons at tone offset promotes a switch from fear responding to extinction and facilitates extinction learning (Salinas-Hernández et al., 2018). The photoinhibition of VTA DA neurons during the same period disrupts switching from fear responding to extinction and impairs extinction learning. DA-GLU neurons projecting to the NAc Shell are activated by the omission of an expected shock during extinction (Badrinarayan et al., 2012; de Jong et al., 2019). Inhibiting DA-GLU neurons projecting to the NAc Shell, but not the NAc Core, impairs extinction learning (Luo et al., 2018). These observations suggest that DA-GLU neurons projecting to the NAc Shell modulate extinction. Figure 6 illustrates hypothesized changes in the NAc medial Shell during fear extinction. DA-GLU neuron burst firing during shock omission may synchronize ChI activity, triggering a cascade of events which produce prolonged increases in DA release and the inhibition of SPNs activity (Figure 5).
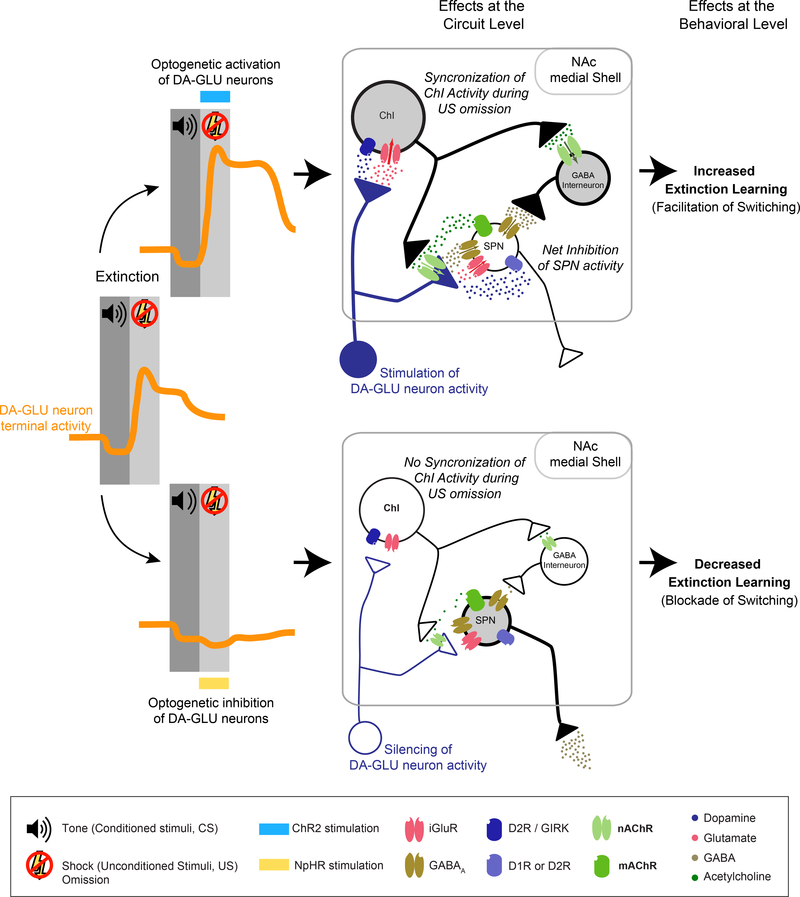
Figure 6 – Modulating DA-GLU activity in the NAc medial Shell during fear conditioning.
The panel on the left shows the activity of DA-GLU neuron terminals (orange line) in the NAc medial Shell during fear extinction. Tone presentation (dark gray bar) is associated with a slight decrease in DA-GLU neuron activity, while shock omission (light gray bar) is associate with a prolonged increase in their activity (based on findings from Badrinarayan et al., 2012; de Jong et al., 2019; Salinas-Hernández et al., 2018). The upper panel on the right illustrates the activity of DA-GLU neurons after optogenetic stimulation during shock omission and hypothesized circuit and behavioral effects. At the circuit level, the burst firing of DA-GLU neurons synchronizes ChIs activity. The net effect on SPN excitability is a rapid and transient inhibition of all SPNs (based on findings from Witten et al., 2010). At the behavioral level, the photostimulation of DAGLU neurons facilitates switching and increases extinction learning (based on findings from Luo et al., 2018; Salinas-Hernández et al., 2018). The lower panel, illustrates the activity of DA-GLU neurons after optogenetic inhibition. At the circuit level, the inhibitory manipulation prevents DA-GLU neurons from synchronizing ChIs and the subsequent net inhibition of SPNs. At the behavioral level, the inhibition of DA-GU neurons during shock omission blocks switching and slows extinction (based on findings from Luo et al., 2018; Salinas-Hernández et al., 2018). For detailed information on the local circuit diagrams refer to Figure 5 caption.
Extinction of reward-associated conditioned responses may undergo similar modulation by Shell-projecting DA neurons. During extinction of conditioned responses to food, DA release measured by microdialysis is increased in the NAc Shell but not in the Core (Bassareo et al., 2017). A voltammetry study showed large and prolonged increases in DA release in the NAc Shell during the early phases of extinction (Saddoris et al., 2015). These prolonged increases in DA release were also observed in fear extinction (Badrinarayan et al., 2012; de Jong et al., 2019) and fit the positive feedback loop described in Figure 5 in which DA-GLU neurons drive the synchronization of ChI activity and induce further increase in DA release through activation of nAChRs. Nevertheless, the involvement of DA neurons in extinction of appetitive responses remains controversial. A subpopulation of DA neurons shows a pause in firing during reward omission (Cohen et al., 2012; Schultz, 2007) and opposing that pause by optogenetic stimulation of VTA DA neurons during reward omission slows extinction (Steinberg et al., 2013), suggesting that the neurons code for a negative reward prediction error (Cohen et al., 2012; Schultz, 2007; Steinberg et al., 2013).
Subpopulations of DA neurons projecting to different subregions of the NAc serve different motivational functions (Bromberg-Martin et al., 2010). Measuring DA release voltammetrically during a learned instrumental chain schedule showed that Shell-projecting DA neurons respond to salient and alerting events, while Core-projecting DA neurons track changes in prediction errors (Saddoris et al., 2015). DA neurons that show an increase in firing may target the NAc Shell selectively, and facilitate extinction by alerting to the presence of unexpected events and promoting switching behavior; while DA neurons that show a pause in firing may target the NAc core selectively and facilitate extinction by signaling a negative error prediction. Thus, photostimulation NAc-Shell would facilitate extinction learning (as described in aversive conditions by Luo et al. 2018), while photostimulation of NAc-projecting DA neurons would slow extinction (as described in appetitive conditions by Steinberg et al., 2013). Future research should test this hypothesis by directly comparing the stimulation of these two subpopulations of neurons during extinction in both aversive and appetitive conditions.
Studies on latent inhibition support a role for DA-GLU neurons in behavioral switching. As in extinction, animals in a latent inhibition experiment are exposed to conflicting contingencies; a stimulus is first presented several times until it becomes irrelevant and it is then paired with a primary reinforcer. In this circumstance, animals need to shift from a stimulus-nothing association to a stimulus-reinforcer association; however, the pre-exposure interferes with this process and reduces the associative strength between the stimulus and reinforcer, revealing latent inhibition (Lubow, 2010). Latent inhibition has been mostly studied using aversive stimuli. In the pre-exposure phase, a tone is presented several times without consequences; in a following conditioning phase, the tone is paired with a mild shock; and in final test phase, the tone is present alone and the amount of freezing is measured. Animals that are pre-exposed to the tone freeze less to the tone in comparison with animals that were not pre-expose to the tone and just received tone-shock pairings. The decrease in freezing in pre-exposed animals reflects latent inhibition (Moser et al., 2000; Weiner and Feldon, 1997).
Latent inhibition is modulated by DA release in the NAc Shell during conditioning. An in vivo microdialysis showed that presentation of a tone paired with a shock increases DA release in the Shell. However, this increase in DA release during conditioning was eliminated when animals were pre-expose to the tone and showed latent inhibition (Murphy et al., 2000). Intra-NAc injections of amphetamine during the conditioning phase, which increase DA levels, disrupt latent inhibition and facilitate switching (Young et al., 2005; Moser et al., 2000). Intra-NAc haloperidol injections during conditioning, which block DAergic signals through D2 receptors, enhance latent inhibition and block switching (Joseph et al., 2000). Thus, the extent of latent inhibition expression depends on the activity of Shell-projecting DA-GLU neurons during the conditioning phase, when the animal first encounters conflicting contingencies. Only a few studies examined latent inhibition using appetitive stimuli and it is not clear how DA neurons modulate this type of latent inhibition (Killcross et al., 1994; Moser et al., 2000). Impairing DA neuron glutamate cotransmission enhances latent inhibition and blocks switching (Mingote et al., 2017), suggesting that changing DA-GLU neurons into DA-only neurons interferes with learning a new role in situations of conflict.
As reviewed above, there is evidence that Shell-projecting DA-GLU neurons track salient events, such as changes in stimulus-reinforcer contingencies. Studies on fear conditioning and latent inhibition point to a critical role of Shell-projecting DA-GLU neurons in situations of conflict, helping determine how quickly or efficiently learning of new contingencies develops.
How DA neuron GLU cotransmission in the NAc medial Shell might facilitate behavioral switching
Studies examining behavioral effects of lesions or inactivation of the NAc Shell suggest that a major role of the NAc Shell is to suppress competing behaviors that interfere with ongoing goal-directed responses (Floresco, 2015). Thus, in fully predicted circumstances, activation of SPNs in the NAc Shell promotes a Stay on task mode by inhibiting projection areas and blocking competing behavior patterns (Figure 7A). However, in ambiguous circumstances, inhibition of SPNs promotes a Switch task mode by disinhibiting projecting areas (Figure 7B). This hypothesis is supported by several studies showing that NAc medial Shell, but not Core, lesions disrupt latent inhibition and facilitate switching (Gal et al., 2005; Jongen-Rêlo et al., 2002; Pothuizen et al., 2005). With disinhibition of NAc Shell projection areas, previously blocked competing behaviors can be expressed, allowing animals to test new behavioral responses, setting an opportunity for new learning. Selecting an optimal strategy from unlocked competing behaviors requires other parallel striatal circuits, such as the NAc core and the Striatum (Floresco, 2015; Sharpe et al., 2019). Once a new goal-directed behavior is established, the NAc Shell goes back to Stay on task mode.
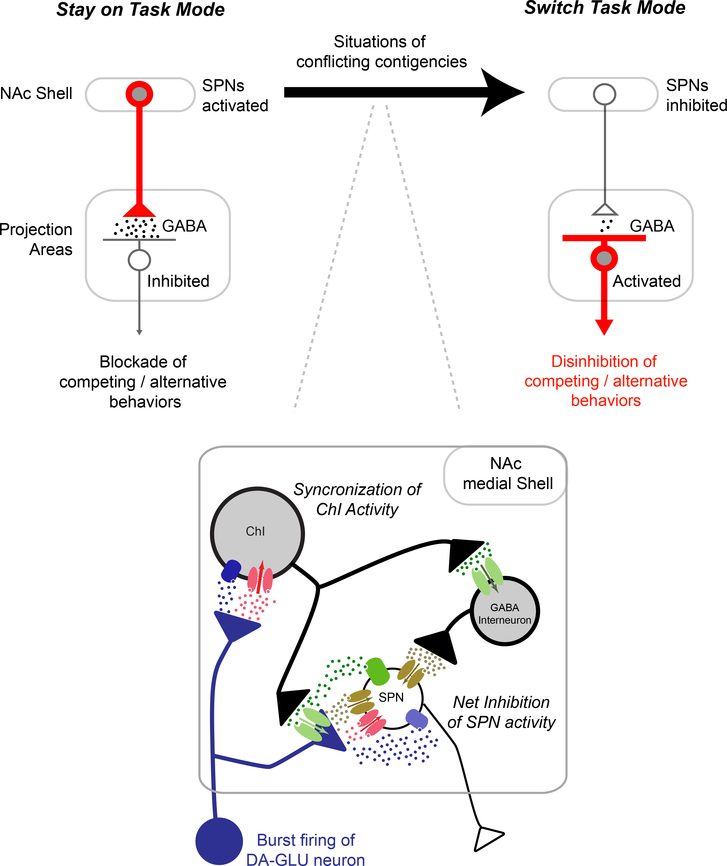
Figure 7 – How DA/GLU neurons facilitate behavioral switching.
Schematic on the left shows NAc Shell connections to projection areas when animals engage in well-predicted goal directed behaviors, which promote Stay on Task (based on suggested function by (Floresco, 2015). In this mode, SPNs in the NAc Shell are active, inhibiting projection areas and blocking expression of competing behaviors that could interfere with the ongoing task. Situations of conflicting contingencies (black arrow) activate DA-GLU neurons and gate the NAc Shell into a Switch Task mode. The NAc Shell local circuit diagram (inset) shows how DA-GLU neurons synchronize ChIs activity and produce a rapid and transient inhibition of SPN activity. (For detailed information on the local circuit changes induced by DA-GLU neurons refer to Figure 5 caption). So in the Switch Task mode, most of the SPNs in the NAc Shell are silent, leading to less GABA release in projection areas. The disinhibition of these areas releases previously blocked behaviors and allows for the exploration of new behavioral strategies.
We hypothesize that transition between the two modes is driven by the activation of Shell-projection DA-GLU neurons (Figure 7). These neurons are activated by conflicting contingencies and promote a Switch task mode by synchronizing ChI activity and inducing a rapid inhibition of SPNs. The role of NAc Shell ChIs in behavioral switching is supported by a recent study of ChIs in extinction in a cocaine-context association (Lee et al., 2016). Optogenetic activation of ChIs enhanced extinction and facilitated switching, while inhibition suppressed extinction and blocked switching. In our model, we propose that ChI activation leads to inhibition SPN activity and disinhibition of NAc Shell projection areas. As new learning progresses and a new behavioral response develops, DA-GLU neuron activity would decrease and promote Stay on task. This hypothesis is supported by fear conditioning studies revealing an increase in DA neuron activity in the early stages of extinction and a decrease in activity during late stages (Badrinarayan et al., 2012; Salinas-Hernández et al., 2018).
Overall, the studies reviewed here support a role for DA-GLU neurons in behavioral switching, however several links between cell activity and behaviors are still missing. For example, DA-GLU neuron induced synchronization of ChI activity in the NAc Shell should be assessed in vivo. A recent study recorded the activity of NAc cells while photostimulating DA neurons and found that 25% of the recorded cells increased their firing within 50 ms of stimulation, most likely mediated by monosynaptic connections (Wang et al., 2017). The effect was eliminated in mice lacking VGLUT2 in DA neurons, showing that the postsynaptic effects depend on glutamate cotransmission. However, the postsynaptic cells were not identified in this study. Future studies should take advantage of in vivo calcium imaging techniques to measured ChI synchronized activity and its links to DA neuron activity (Rehani et al., 2019). Stimulation of DA-GLU neurons induces a subsequent ChI-driven inhibition of SPNs, which is supported by work done in the dorsal striatum and in vivo recordings from the NAc Shell by Witten and colleagues (2010). Nevertheless, further research is required to identify which GABAergic neurons are activated by acetylcholine and mediate the overall inhibition of SPNs activity.
Finally, perhaps the biggest challenge will be to determine the neural circuits in NAc Shell that are disinhibited by DA-GLU neuron burst activity in situations of conflicting contingencies, such as in extinction or in latent inhibition. Exploiting the ability of optogenetic techniques to suppress the activity of specific NAc outputs selectively may be very useful. Indeed, this technology has already been use to dissect NAc Shell outputs controlling feeding (Baldo and Kelley, 2007; Maldonado-Irizarry et al., 1995). Optogenetic inhibition of D1-expressing SPNs rapidly stimulates feeding by disinhibiting the lateral hypothalamus, while stimulation blocks feeding (O’Connor et al., 2015). The work of Berridge and colleagues, further revealed that rostral NAc Shell and DA D1 receptors control feeding, while the caudal NAc Shell and DA D1 and D2 receptors control fearful behaviors (Faure et al., 2008; Richard and Berridge, 2011). Other studies have shown that different subregions of the NAc Shell control either aversive or appetitive responses (Al-Hasani et al., 2015) and this may reflect divergent input-output relationships within the NAc Shell (Groenewegen et al., 1999; Reed et al., 2018; Yang et al., 2018). Dissecting these neural circuits will require a systematic analysis of the effects of silencing different outputs along the anterior-posterior axis of the NAc Shell during behaviors associated with either appetitive or aversive outcomes.
Since the first report of glutamate cotransmission in DA neurons in the late 1990’s (Sulzer et al., 1998), the function has been gradually elucidated. The recent development of new technologies to manipulate subpopulation of DA neurons selectively revealed that DA-GLU neurons are located in the medial VTA, preferentially project to the NAc medial Shell and control the activity of ChIs. A better understanding of how DAGLU neurons modulate NAc Shell associated-circuits will be crucial in establishing the function of DA-GLU neurons in motivated behavior. Here we have described a series of testable hypotheses about the functions of DA-GLU neurons, both at the synaptic and behavioral levels, which should promote more research in this area and advance our understanding of the DA system.
Highlights.
Dopamine-glutamate neurons project selectively to the Nucleus medial Shell
Dopamine-glutamate neurons in the NAc medial Shell make ChI burst fire
Dopamine-glutamate neurons are involved in behavioral switching
Proposed mechanism through which dopamine-glutamate neurons gate a “switching task mode”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hasani Ream, McCall Jordan G., Gunchul Shin, Gomez Adrian M., Schmitz Gavin P., Bernardi Julio M., Pyo Chang-O., et al. 2015. “Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward.” Neuron 87 (5): 1063–77. 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, and Aragona BJ. 2012. “Aversive Stimuli Differentially Modulate Real-Time Dopamine Transmission Dynamics within the Nucleus Accumbens Core and Shell.” Journal of Neuroscience 32 (45): 15779–90. 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo Brian A., and Kelley Ann E.. 2007. “Discrete Neurochemical Coding of Distinguishable Motivational Processes: Insights from Nucleus Accumbens Control of Feeding.” Psychopharmacology 191 (3): 439–59. 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Baldo Brian A, Ken Sadeghian, Basso Ana Maria, and Kelley Ann E. 2002. “Effects of Selective Dopamine D1 or D2 Receptor Blockade within Nucleus Accumbens Subregions on Ingestive Behavior and Associated Motor Activity.” Behavioural Brain Research 137 (1–2): 165–77. 10.1016/S01664328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Bassareo Valentina, Cucca Flavia, Frau Roberto, and Di Chiara Gaetano. 2017. “Changes in Dopamine Transmission in the Nucleus Accumbens Shell and Core during Ethanol and Sucrose Self-Administration.” Frontiers in Behavioral Neuroscience 11 (May). 10.3389/fnbeh.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo Valentina, and Di Chiara Gaetano. 1997. “Differential Influence of Associative and Nonassociative Learning Mechanisms on the Responsiveness of Prefrontal and Accumbal Dopamine Transmission to Food Stimuli in Rats Fed Ad Libitum.” The Journal of Neuroscience 17 (2): 851–61. 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo Valentina, and Di Chiara Gaetano. 1999. “Modulation of Feeding-Induced Activation of Mesolimbic Dopamine Transmission by Appetitive Stimuli and Its Relation to Motivational State: Limbic Dopamine and Feeding.” European Journal of Neuroscience 11 (12): 4389–97. 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Beier Kevin T., Steinberg Elizabeth E., DeLoach Katherine E., Stanley Xie, Kazunari Miyamichi, Lindsay Schwarz, Gao Xiaojing J., Kremer Eric J., Malenka Robert C., and Liqun Luo. 2015. “Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping.” Cell 162 (3): 622–34. 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse Henk W., Galis-De Graaf Yvonne, and Groenewegen Henk J.. 1992. “Topographical Organization and Relationship with Ventral Striatal Compartments of Prefrontal Corticostriatal Projections in the Rat.” The Journal of Comparative Neurology 316 (3): 314–47. 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berridge Kent C, and Robinson Terry E. 1998. “What Is the Role of Dopamine in Reward: Hedonic Impact, Reward Learning, or Incentive Salience?” Brain Research Reviews 28 (3): 309–69. 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Breton Jocelyn M., Charbit Annabelle R., Snyder Benjamin J., Fong Peter T. K., Dias Elayne V., Himmels Patricia, Lock Hagar, and Margolis Elyssa B.. 2018. “Relative Contributions and Mapping of Ventral Tegmental Area Dopamine and GABA Neurons by Projection Target in the Rat.” Journal of Comparative Neurology, December 10.1002/cne.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog Judith S., Salyapongse Aimee, Deutch Ariel Y., and Zahm Daniel S.. 1993. “The Patterns of Afferent Innervation of the Core and Shell in the ?Accumbens? Part of the Rat Ventral Striatum: Immunohistochemical Detection of Retrogradely Transported Fluoro-Gold.” The Journal of Comparative Neurology 338 (2): 255–78. 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin Ethan S., Matsumoto Masayuki, and Hikosaka Okihide. 2010. “Dopamine in Motivational Control: Rewarding, Aversive, and Alerting.” Neuron 68 (5): 815–34. 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, and Wightman RM. 2012. “Aversive Stimulus Differentially Triggers Subsecond Dopamine Release in Reward Regions.” Neuroscience 201 (January): 331–37. 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope Roger, Mateo Yolanda, Mathur Brian N., Irving James, Wang Hui-Ling, Morales Marisela, Lovinger David M., and Cheer Joseph F.. 2012. “Selective Activation of Cholinergic Interneurons Enhances Accumbal Phasic Dopamine Release: Setting the Tone for Reward Processing.” Cell Reports 2 (1): 33–41. 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Yuan, and Ford Christopher P.. 2018. “Dopamine Cells Differentially Regulate Striatal Cholinergic Transmission across Regions through Corelease of Dopamine and Glutamate.” Cell Reports 25 (11): 3148–3157.e3. 10.1016/j.celrep.2018.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma Nao, Mingote Susana, Kalmbach Abigail, Yetnikoff Leora, and Rayport Stephen. 2017. “Heterogeneity in Dopamine Neuron Synaptic Actions Across the Striatum and Its Relevance for Schizophrenia.” Biological Psychiatry 81 (1): 43–51. 10.1016/j.biopsych.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma Nao, Mingote Susana, Moore Holly, and Rayport Stephen. 2014. “Dopamine Neurons Control Striatal Cholinergic Neurons via Regionally Heterogeneous Dopamine and Glutamate Signaling.” Neuron 81 (4): 901–12. 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma Nao, Mingote Susana, Yetnikoff Leora, Kalmbach Abigail, Ma Thong, Ztaou Samira, Sienna Anna-Claire, et al. 2018. “Dopamine Neuron Glutamate Cotransmission Evokes a Delayed Excitation in Lateral Dorsal Striatal Cholinergic Interneurons,” eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Jeremiah Y., Haesler Sebastian, Vong Linh, Lowell Bradford B., and Uchida Naoshige. 2012. “Neuron-Type-Specific Signals for Reward and Punishment in the Ventral Tegmental Area.” Nature 482 (7383): 85–88. 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs Jill M, Yan Zhu, Druhan Jonathan P, and Aston-Jones Gary S. 1998. “Origin of Noradrenergic Afferents to the Shell Subregion of the Nucleus Accumbens: Anterograde and Retrograde Tract-Tracing Studies in the Rat.” Brain Research 806 (2): 127–40. 10.1016/S0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Do-Monte Fabricio H., Angélica Minier-Toribio, Kelvin Quiñones-Laracuente, Medina-Colón Estefanía M., and Quirk Gregory J.. 2017. “Thalamic Regulation of Sucrose Seeking during Unexpected Reward Omission.” Neuron 94 (2): 388–400.e4. 10.1016/j.neuron.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English Daniel F, Osvaldo Ibanez-Sandoval, Eran Stark, Fatuel Tecuapetla, György Buzsáki, Karl Deisseroth, Tepper James M, and Tibor Koos. 2012. “GABAergic Circuits Mediate the Reinforcement-Related Signals of Striatal Cholinergic Interneurons.” Nature Neuroscience 15 (1): 123–30. 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveden JL, and Robbins TW. 1983. “Increased Response Switching, Perseveration and Perseverative Switching Following d-Amphetamine in the Rat.” Psychopharmacology 80 (1): 67–73. [DOI] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, and Berridge KC. 2008. “Mesolimbic Dopamine in Desire and Dread: Enabling Motivation to Be Generated by Localized Glutamate Disruptions in Nucleus Accumbens.” Journal of Neuroscience 28 (28): 7184–92. 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust Thomas W., Assous Maxime, Shah Fulva, Tepper James M., and Tibor Koós. 2015. “Novel Fast Adapting Interneurons Mediate Cholinergic-Induced Fast GABA A Inhibitory Postsynaptic Currents in Striatal Spiny Neurons.” European Journal of Neuroscience 42 (2): 1764–74. 10.1111/ejn.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno Lief E, Joanna Mattis, Charu Ramakrishnan, Minsuk Hyun, Lee Soo Yeun, Miao He, Jason Tucciarone, et al. 2014. “Targeting Cells with Single Vectors Using Multiple-Feature Boolean Logic.” Nature Methods 11 (7): 763–72. 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco Stan B. 2015. “The Nucleus Accumbens: An Interface Between Cognition, Emotion, and Action.” Annual Review of Psychology 66 (1): 25–52. 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Gal Gilad, Schiller Daniela, and Weiner Ina. 2005. “Latent Inhibition Is Disrupted by Nucleus Accumbens Shell Lesion but Is Abnormally Persistent Following Entire Nucleus Accumbens Lesion: The Neural Site Controlling the Expression and Disruption of the Stimulus Preexposure Effect.” Behavioural Brain Research 162 (2): 246–55. 10.1016/j.bbr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Gambarana C, Masi F, Leggio B, Grappi S, Nanni G, Scheggi S, De Montis MG, and Tagliamonte A. 2003. “Acquisition of a Palatable-Food-Sustained Appetitive Behavior in Satiated Rats Is Dependent on the Dopaminergic Response to This Food in Limbic Areas.” Neuroscience 121 (1): 179–87. 10.1016/S0306-4522(03)00383-X. [DOI] [PubMed] [Google Scholar]
- Goldberg Joshua A., Ding Jun B., and Surmeier D. James. 2012. “Muscarinic Modulation of Striatal Function and Circuitry” In Muscarinic Receptors, edited by Fryer Allison D., Arthur Christopoulos, and Nathanson Neil M., 208:223–41. Berlin, Heidelberg: Springer Berlin Heidelberg; 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- González-Hernández Tomás, Pedro Barroso-Chinea, Abraham Acevedo, Salido Eduardo, and Manuel Rodríguez. 2001. “Colocalization of Tyrosine Hydroxylase and GAD65 MRNA in Mesostriatal Neurons: DA/GABA Mesostriatal Cotransmission.” European Journal of Neuroscience 13 (1): 57–67. 10.1111/j.1460-9568.2001.01371.x. [DOI] [PubMed] [Google Scholar]
- Groenewegen Henk J., Berendse Henk W., Wolters Jan G., and Lohman Anthony H.M.. 1991. “Chapter 5 The Anatomical Relationship of the Prefrontal Cortex with the Striatopallidal System, the Thalamus and the Amygdala: Evidence for a Parallel Organization” In Progress in Brain Research, 85:95–118. Elsevier; 10.1016/S0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Groenewegen Henk J., Wright Christopher I., Beijer Arno V.J., and Pieter Voorn. 1999. “Convergence and Segregation of Ventral Striatal Inputs and Outputs.” Annals of the New York Academy of Sciences 877: 49–63. 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Groenewegen Henkj, Mulder Antonius B, Beijer Arno V J, and Wright Christopher. 1999. “Hippocampal and Amygdaloid Interactions in the Nucleus Accumbens.” Psychobiology 27 (2): 149–164. [Google Scholar]
- Hamid Arif A, Pettibone Jeffrey R, Mabrouk Omar S, Hetrick Vaughn L, Robert Schmidt, Vander Weele Caitlin M, Kennedy Robert T, Aragona Brandon J, and Berke Joshua D. 2016. “Mesolimbic Dopamine Signals the Value of Work.” Nature Neuroscience 19 (1): 117–26. 10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, and Wohltmann C. 1991. “Specificity in the Projection Patterns of Accumbal Core and Shell in the Rat.” Neuroscience 41 (1): 89–125. 10.1016/0306-4522(91)90202-Y. [DOI] [PubMed] [Google Scholar]
- Hunnicutt Barbara J, Jongbloets Bart C, Birdsong William T, Gertz Katrina J, Haining Zhong, and Tianyi Mao. 2016. “A Comprehensive Excitatory Input Map of the Striatum Reveals Novel Functional Organization.” eLife 5 (November). 10.7554/eLife.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto Satoshi. 2007. “Dopamine Reward Circuitry: Two Projection Systems from the Ventral Midbrain to the Nucleus Accumbens–Olfactory Tubercle Complex.” Brain Research Reviews 56 (1): 27–78. 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong, de Johannes W., Seyedeh Atiyeh Afjei, Iskra Pollak Dorocic, Peck James R., Christine Liu, Kim Christina K., Lin Tian, Karl Deisseroth, and Stephan Lammel. 2019. “A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System.” Neuron 101 (1): 133–151.e7. 10.1016/j.neuron.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen-Rêlo AL, Kaufmann S, and Feldon J. 2002. “A Differential Involvement of the Shell and Core Subterritories of the Nucleus Accumbens of Rats in Attentional Processes.” Neuroscience 111 (1): 95–109. 10.1016/S0306-4522(01)00521-8. [DOI] [PubMed] [Google Scholar]
- Joseph MH, Peters SL, Moran PM, Grigoryan GA, Young AMJ, and Gray JA. 2000. “Modulation of Latent Inhibition in the Rat by Altered Dopamine Transmission in the Nucleus Accumbens at the Time of Conditioning.” Neuroscience 101 (4): 921–30. 10.1016/S0306-4522(00)00437-1. [DOI] [PubMed] [Google Scholar]
- Kabanova Anna, Pabst Milan, Lorkowski Markus, Braganza Oliver, Boehlen Anne, Nikbakht Negar, Pothmann Leonie, et al. 2015. “Function and Developmental Origin of a Mesocortical Inhibitory Circuit.” Nature Neuroscience 18 (6): 872–82. 10.1038/nn.4020. [DOI] [PubMed] [Google Scholar]
- Kawano Michihiro, Kawasaki Akiko, Hiromi Sakata-Haga, Yoshihiro Fukui, Kawano Hitoshi, Nogami Haruo, and Hisano Setsuji. 2006. “Particular Subpopulations of Midbrain and Hypothalamic Dopamine Neurons Express Vesicular Glutamate Transporter 2 in the Rat Brain.” The Journal of Comparative Neurology 498 (5): 581–92. 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- Killcross AS, Dickinson A, and Robbins TW. 1994. “Amphetamine-Induced Disruptions of Latent Inhibition Are Reinforcer Mediated: Implications for Animal Models of Schizophrenic Attentional Dysfunction.” Psychopharmacology 115 (1– 2): 185–95. 10.1007/BF02244771. [DOI] [PubMed] [Google Scholar]
- Kim J-I, Ganesan S, Luo SX, Wu Y-W, Park E, Huang EJ, Chen L, and Ding JB. 2015. “Aldehyde Dehydrogenase 1a1 Mediates a GABA Synthesis Pathway in Midbrain Dopaminergic Neurons.” Science 350 (6256): 102–6. 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel Stephan, Hetzel Andrea, Olga Häckel, Ian Jones, Liss Birgit, and Jochen Roeper. 2008. “Unique Properties of Mesoprefrontal Neurons within a Dual Mesocorticolimbic Dopamine System.” Neuron 57 (5): 760–73. 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel Stephan, Byung Kook Lim, and Malenka Robert C.. 2014. “Reward and Aversion in a Heterogeneous Midbrain Dopamine System.” Neuropharmacology 76 (January): 351–59. 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel Stephan, Steinberg Elizabeth E., Csaba Földy Nicholas R. Wall, Beier Kevin, Luo Liqun, and Malenka Robert C.. 2015. “Diversity of Transgenic Mouse Models for Selective Targeting of Midbrain Dopamine Neurons.” Neuron 85 (2): 429–38. 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Junuk, Finkelstein Joel, Jung Yoon Choi, and Witten Ilana B.. 2016. “Linking Cholinergic Interneurons, Synaptic Plasticity, and Behavior during the Extinction of a Cocaine-Context Association.” Neuron 90 (5): 1071–85. 10.1016/j.neuron.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE. 2010. “A Short History of Latent Inhibition.” In Latent Inhibition. Cognition, Neuroscience and Applications, 1–19. Cambridge. [Google Scholar]
- Luo Ray, Uematsu Akira, Weitemier Adam, Aquili Luca, Koivumaa Jenny, McHugh Thomas J., and Johansen Joshua P.. 2018. “A Dopaminergic Switch for Fear to Safety Transitions.” Nature Communications 9 (1). 10.1038/s41467-018-04784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Ruixi, Janssen Megan J., Partridge John G., and Vicini Stefano. 2013. “Direct and GABA-Mediated Indirect Effects of Nicotinic ACh Receptor Agonists on Striatal Neurones: Nicotinic Receptors in Striatal Interneurones.” The Journal of Physiology 591 (1): 203–17. 10.1113/jphysiol.2012.241786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Irizarry Cs, Swanson Cj, and Kelley Ae. 1995. “Glutamate Receptors in the Nucleus Accumbens Shell Control Feeding Behavior via the Lateral Hypothalamus.” The Journal of Neuroscience 15 (10): 6779–88. 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, and Kaneko T. 2009. “Single Nigrostriatal Dopaminergic Neurons Form Widely Spread and Highly Dense Axonal Arborizations in the Neostriatum.” Journal of Neuroscience 29 (2): 444–53. 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Chuhma N, Kusnoor SV, Field B, Deutch AY, and Rayport S. 2015. “Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions.” Journal of Neuroscience 35 (49): 16259–71. 10.1523/JNEUROSCI.1674-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote Susana, Chuhma Nao, Kalmbach Abigail, Thomsen Gretchen M, Yvonne Wang, Mihali Andra, Sferrazza Caroline, Ilana Zucker-Scharff, Anne-Claire Siena, Welch Martha G, Jose Lizardi-Ortiz, Sulzer David, Moore Holly, Inna Gaisler-Salomon, Stephen Rayport. 2017. “Dopamine Neuron Dependent Behaviors Mediated by Glutamate Cotransmission,” eLife; 6 pii: e27566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson G, Jones D, and Yim C. 1980. “From Motivation to Action: Functional Interface between the Limbic System and the Motor System.” Progress in Neurobiology 14 (2–3): 69–97. 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Morales Marisela, and Margolis Elyssa B.. 2017. “Ventral Tegmental Area: Cellular Heterogeneity, Connectivity and Behaviour.” Nature Reviews Neuroscience 18 (2): 73–85. 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- Moser, Paul C, Hitchcock Janice M, Sarah Lister, and Moran Paula M. 2000. “The Pharmacology of Latent Inhibition as an Animal Model of Schizophrenia.” Brain Research Reviews 33 (2–3): 275–307. 10.1016/S0165-0173(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Pezze M-A, Feldon J, and Heidbreder C. 2000. “Differential Involvement of Dopamine in the Shell and Core of the Nucleus Accumbens in the Expression of Latent Inhibition to an Aversively Conditioned Stimulus.” Neuroscience 97 (3): 469–77. 10.1016/S0306-4522(00)00043-9. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, and Ungless MA. 2008. “Stereological Estimates of Dopaminergic, GABAergic and Glutamatergic Neurons in the Ventral Tegmental Area, Substantia Nigra and Retrorubral Field in the Rat.” Neuroscience 152 (4): 1024–31. 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, Thur KE, Horsley RR, Spicer C, Marsden CA, and Cassaday HJ. 2011. “Reduced Dopamine Function within the Medial Shell of the Nucleus Accumbens Enhances Latent Inhibition.” Pharmacology Biochemistry and Behavior 98 (1): 1–7. 10.1016/j.pbb.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson Alexandra B., Hammack Nora, Yang Cindy F., Shah Nirao M., Seal Rebecca P., and Kreitzer Anatol C.. 2014. “Striatal Cholinergic Interneurons Drive GABA Release from Dopamine Terminals.” Neuron 82 (1): 63–70. 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, and Salamone JD. 2001. “D1 or D2 Antagonism in Nucleus Accumbens Core or Dorsomedial Shell Suppresses Lever Pressing for Food but Leads to Compensatory Increases in Chow Consumption.” Pharmacology Biochemistry and Behavior 69 (3–4): 373–82. 10.1016/S0091-3057(01)00524-X. [DOI] [PubMed] [Google Scholar]
- Oades RD. 1985. “The Role of Noradrenaline in Tuning and Dopamine in Switching between Signals in the CNS.” Neuroscience & Biobehavioral Reviews 9 (2): 261–82. [DOI] [PubMed] [Google Scholar]
- O’ Connor, Eoin C, Kremer Yves, Lefort Sandrine, Harada Masaya, Pascoli Vincent, Rohner Clément, and Christian Lüscher. 2015. “Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding.” Neuron 88 (3): 553–64. 10.1016/j.neuron.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Pérez-López José Luis, Rubén Contreras-López, Ramírez-Jarquín Josué O., and Tecuapetla Fatuel. 2018. “Direct Glutamatergic Signaling From Midbrain Dopaminergic Neurons Onto Pyramidal Prefrontal Cortex Neurons.” Frontiers in Neural Circuits 12 (August). 10.3389/fncir.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuizen Helen H. J., Jongen-Rêlo Ana L., Joram Feldon, and Yee Benjamin K.. 2005. “Double Dissociation of the Effects of Selective Nucleus Accumbens Core and Shell Lesions on Impulsive-Choice Behaviour and Salience Learning in Rats.” European Journal of Neuroscience 22 (10): 2605–16. 10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]
- Poulin Jean-Francois, Caronia Giuliana, Hofer Caitlyn, Cui Qiaoling, Helm Brandon, Ramakrishnan Charu, Chan C. Savio, Dombeck Daniel A., Karl Deisseroth, and Rajeshwar Awatramani. 2018. “Mapping Projections of Molecularly Defined Dopamine Neuron Subtypes Using Intersectional Genetic Approaches.” Nature Neuroscience 21 (9): 1260–71. 10.1038/s41593-018-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Grabner CP, Johnson M, Pierce RC, and Bardov MT. 1996. “Transient Increases in Catecholaminergic Activity in Medial Prefrontal Cortex and Nucleus Accumbens Shell during Novelty.” Neuroscience 76 (3): 707–14. 10.1016/S0306-4522(96)00382-X. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, and Gurney K. 1999. “The Basal Ganglia: A Vertebrate Solution to the Selection Problem?” Neuroscience 89 (4): 1009–23. 10.1016/S0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Reed Sean J., Lafferty Christopher K., Mendoza Jesse A., Yang Angela K., Davidson Thomas J., Grosenick Logan, Deisseroth Karl, and Britt Jonathan P.. 2018. “Coordinated Reductions in Excitatory Input to the Nucleus Accumbens Underlie Food Consumption.” Neuron 99 (6): 1260–1273.e4. 10.1016/j.neuron.2018.07.051. [DOI] [PubMed] [Google Scholar]
- Rehani Rotem, Atamna Yara, Tiroshi Lior, Chiu Wei-Hua, de Jesús Aceves Buendía José, Martins Gabriela J., Jacobson Gilad A., and Goldberg Joshua A.. 2019. “Activity Patterns in the Neuropil of Striatal Cholinergic Interneurons in Freely Moving Mice Represent Their Collective Spiking Dynamics.” Eneuro 6 (1): ENEURO.0351–18.2018. 10.1523/ENEURO.0351-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, and Berridge KC. 2011. “Nucleus Accumbens Dopamine/Glutamate Interaction Switches Modes to Generate Desire versus Dread: D1 Alone for Appetitive Eating But D1 and D2 Together for Fear.” Journal of Neuroscience 31 (36): 12866–79. 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-López Claudia, Francisco Clascá, and Lucía Prensa. 2017. “The Mesoaccumbens Pathway: A Retrograde Labeling and Single-Cell Axon Tracing Analysis in the Mouse.” Frontiers in Neuroanatomy 11 (March). 10.3389/fnana.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman, Mitchell F, Wheeler Robert A, Wightman R Mark, and Carelli Regina M. 2008. “Real-Time Chemical Responses in the Nucleus Accumbens Differentiate Rewarding and Aversive Stimuli.” Nature Neuroscience 11 (12): 1376–77. 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett Deirdre A., Saddoris Michael P., and Carelli Regina M.. 2017. “Nucleus Accumbens Shell Dopamine Preferentially Tracks Information Related to Outcome Value of Reward.” Eneuro 4 (3): ENEURO.0058–17.2017. 10.1523/ENEURO.0058-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris Michael P., Cacciapaglia Fabio, Wightman R. Mark, and Carelli Regina M.. 2015. “Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation.” The Journal of Neuroscience 35 (33): 11572–82. 10.1523/JNEUROSCI.2344-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone John D., and Correa Mercè. 2012. “The Mysterious Motivational Functions of Mesolimbic Dopamine.” Neuron 76 (3): 470–85. 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Hernández, Ximena I, Pascal Vogel, Sebastian Betz, Raffael Kalisch, Torfi Sigurdsson, and Sevil Duvarci. 2018. “Dopamine Neurons Drive Fear Extinction Learning by Signaling the Omission of Expected Aversive Outcomes.” ELife 7 10.7554/eLife.38818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Kaufling J, Georges F, Veinante P, and Barrot M. 2014. “The Antero-Posterior Heterogeneity of the Ventral Tegmental Area.” Neuroscience 282: 198–216. 10.1016/j.neuroscience.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Saunders Benjamin T., Richard Jocelyn M., Margolis Elyssa B., and Janak Patricia H.. 2018. “Dopamine Neurons Create Pavlovian Conditioned Stimuli with Circuit-Defined Motivational Properties.” Nature Neuroscience 21 (8): 1072–83. 10.1038/s41593-018-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz Wolfram. 2007. “Multiple Dopamine Functions at Different Time Courses.” Annual Review of Neuroscience 30 (1): 259–88. 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Segovia KN, Correa M, and Salamone JD. 2011. “Slow Phasic Changes in Nucleus Accumbens Dopamine Release during Fixed Ratio Acquisition: A Microdialysis Study.” Neuroscience 196: 178–88. 10.1016/j.neuroscience.2011.07.078. [DOI] [PubMed] [Google Scholar]
- Sharpe Melissa J., Stalnaker Thomas, Schuck Nicolas W., Killcross Simon, Schoenbaum Geoffrey, and Niv Yael. 2019. “An Integrated Model of Action Selection: Distinct Modes of Cortical Control of Striatal Decision Making.” Annual Review of Psychology 70 (1): 53–76. 10.1146/annurev-psych-010418-102824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Jung Hoon, Adrover Martin F., and Alvarez Veronica A.. 2017. “Distinctive Modulation of Dopamine Release in the Nucleus Accumbens Shell Mediated by Dopamine and Acetylcholine Receptors.” The Journal of Neuroscience 37 (46): 11166–80. 10.1523/JNEUROSCI.0596-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silm Kätlin, Yang Jing, Marcott Pamela F., Asensio Cedric S., Eriksen Jacob, Guthrie Daryl A., Newman Amy H., Ford Christopher P., and Edwards Robert H.. 2019. “Synaptic Vesicle Recycling Pathway Determines Neurotransmitter Content and Release Properties.” Neuron, April 10.1016/j.neuron.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Onge, J. R., Ahn S, Phillips AG, and Floresco SB. 2012. “Dynamic Fluctuations in Dopamine Efflux in the Prefrontal Cortex and Nucleus Accumbens during Risk-Based Decision Making.” Journal of Neuroscience 32 (47): 16880–91. 10.1523/JNEUROSCI.3807-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, and Moghaddam B. 2006. “Rule Learning and Reward Contingency Are Associated with Dissociable Patterns of Dopamine Activation in the Rat Prefrontal Cortex, Nucleus Accumbens, and Dorsal Striatum.” Journal of Neuroscience 26 (34): 8810–18. 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg Elizabeth E, Ronald Keiflin, Boivin Josiah R, Witten Ilana B, Karl Deisseroth, and Janak Patricia H. 2013. “ A causal link between prediction errors, dopamine neurons and learning”. Nature Neuroscience 16: 966–973. doi: 10.1038/nn.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner Thomas, Zell Vivien, Farino Zachary J., Sonders Mark S., Villeneuve Michael, Freyberg Robin J., Przedborski Serge, Lu Wei, Freyberg Zachary, and Hnasko Thomas S.. 2018. “Role for VGLUT2 in Selective Vulnerability of Midbrain Dopamine Neurons.” Journal of Clinical Investigation 128 (2): 774–88. 10.1172/JCI95795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C, Tritsch NX, Hagan NA, Gu C, and Sabatini BL. 2014. “Multiphasic Modulation of Cholinergic Interneurons by Nigrostriatal Afferents.” Journal of Neuroscience 34 (25): 8557–69. 10.1523/JNEUROSCI.0589-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, and Bonci A. 2010. “Dopaminergic Terminals in the Nucleus Accumbens But Not the Dorsal Striatum Corelease Glutamate.” Journal of Neuroscience 30 (24): 8229–33. 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer David, Joyce Myra P., Lin Ling, Geldwert Daron, Haber Suzanne N., Hattori Toshiaki, and Rayport Stephen. 1998. “Dopamine Neurons Make Glutamatergic Synapses In Vitro.” The Journal of Neuroscience 18 (12): 4588–4602. 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Carrillo-Reid L, and Bargas J. 2011. “Dopaminergic Modulation of Striatal Neurons, Circuits, and Assemblies.” Neuroscience 198 (December): 3–18. 10.1016/j.neuroscience.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larry Swanson. 1982. “The Projections of the Ventral Tegmental Area and Adjacent Regions: A Combined Fluorescent Retrograde Tracer and Immunofluorescence Study in the Rat.” Brain Research Bulletin 9: 321–353. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Karl Deisseroth, Rice Margaret E, Tepper James M, and Tibor Koos. 2010. “Glutamatergic Signaling by Mesolimbic Dopamine Neurons in the Nucleus Accumbens.” Journal of Neuroscience 30 (20): 7105–10. 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper James M., Tibor Koós, Osvaldo Ibanez-Sandoval, Tecuapetla Fatuel, Faust Thomas W., and Assous Maxime. 2018. “Heterogeneity and Diversity of Striatal GABAergic Interneurons: Update 2018.” Frontiers in Neuroanatomy 12 (November). 10.3389/fnana.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell Sarah, Lalic Tatjana, Platt Nicola J, Jennings Katie A, Karl Deisseroth and Stephanie Jane Cragg. 2012. “Striatal Dopamine ReleaseIs Triggered by Synchronized Activity in Cholinergic Interneurons.” Neuron 75: 58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Threlfell Sarah, and Stephanie Jane Cragg. 2011. “Dopamine Signaling in Dorsal Versus Ventral Striatum: The Dynamic Role of Cholinergic Interneurons.” Frontiers in Systems Neuroscience 5 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch Nicolas X., Ding Jun B., and Sabatini Bernardo L.. 2012. “Dopaminergic Neurons Inhibit Striatal Output through Non-Canonical Release of GABA.” Nature 490 (7419): 262–66. 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch, Nicolas X, Won-Jong Oh, Chenghua Gu, and Sabatini Bernardo L. 2014. “Midbrain Dopamine Neurons Sustain Inhibitory Transmission Using Plasma Membrane Uptake of GABA, Not Synthesis.” ELife 3 (April). 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau Louis-Eric, Hnasko Thomas S., Åsa Wallén-Mackenzie Marisela Morales, Rayport Steven, and Sulzer David. 2014. “The Multilingual Nature of Dopamine Neurons” In Progress in Brain Research, 211:141–64. Elsevier; 10.1016/B978-0-444-63425-2.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt Urban. 1971. “Stereotaxic Mapping of the Monoamine Pathways in the Rat Brain*.” Acta Physiologica Scandinavica 82 (S367): 1–48. 10.1111/j.1365-201X.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Usuda Iwao, Tanaka Koichi, and Chiba Tanemichi. 1998. “Efferent Projections of the Nucleus Accumbens in the Rat with Special Reference to Subdivision of the Nucleus: Biotinylated Dextran Amine Study.” Brain Research 797 (1): 73–93. 10.1016/S0006-8993(98)00359-X. [DOI] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, and Lobo MK. 2013. “New Insights into the Specificity and Plasticity of Reward and Aversion Encoding in the Mesolimbic System.” Journal of Neuroscience 33 (45): 17569–76. 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn Pieter, Vanderschuren Louk J.M.J, Groenewegen Henk J, Robbins Trevor W, and Pennartz Cyriel M.A. 2004. “Putting a Spin on the Dorsal–Ventral Divide of the Striatum.” Trends in Neurosciences 27 (8): 468–74. 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang Dong V., Viereckel Thomas, Zell Vivien, Åsa Konradsson-Geuken, Broker Carl J., Talishinsky Aleksandr, Yoo Ji Hoon, et al. 2017. “Disrupting Glutamate CoTransmission Does Not Affect Acquisition of Conditioned Behavior Reinforced by Dopamine Neuron Activation.” Cell Reports 18 (11): 2584–91. 10.1016/j.celrep.2017.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I, and Feldon J. 1997. “The Switching Model of Latent Inhibition: An Update of Neural Substrates.” Behavioural Brain Research 88 (1): 11–25. 10.1016/S0166-4328(97)02314-0. [DOI] [PubMed] [Google Scholar]
- Westbrook RF, and Bouton ME. 2010. “Latent Inhibition and Extinction: Their Signature Phenomena and the Role of Prediction Error.” In Latent Inhibition. Cognition, Neuroscience and Aplications for Schizophrenia, 23–29. Cambridge. [Google Scholar]
- Wieland S, Du D, Oswald MJ, Parlato R, Kohr G, and Kelsch W. 2014. “Phasic Dopaminergic Activity Exerts Fast Control of Cholinergic Interneuron Firing via Sequential NMDA, D2, and D1 Receptor Activation.” Journal of Neuroscience 34 (35): 11549–59. 10.1523/JNEUROSCI.1175-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin S-C, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, and Deisseroth K. 2010. “Cholinergic Interneurons Control Local Circuit Activity and Cocaine Conditioning.” Science 330 (6011): 1677–81. 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell Cindy L., and Berridge Kent C.. 2000. “Intra-Accumbens Amphetamine Increases the Conditioned Incentive Salience of Sucrose Reward: Enhancement of Reward ‘Wanting’ without Enhanced ‘Liking’ or Response Reinforcement.” The Journal of Neuroscience 20 (21): 8122–30. 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang H-L, Li X, Ng TH, and Morales M. 2011. “Mesocorticolimbic Glutamatergic Pathway.” Journal of Neuroscience 31 (23): 8476–90. 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Hongbin, de Jong Johannes W., YeEun Tak, James Peck, Bateup Helen S., and Stephan Lammel. 2018. “Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations.” Neuron 97 (2): 434–449.e4. 10.1016/j.neuron.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, and Zahm DS. 2014. “An Update on the Connections of the Ventral Mesencephalic Dopaminergic Complex.” Neuroscience 282 (December): 23–48. 10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, Andrew MJ, Moran Paula M., and Joseph Michael H.. 2005. “The Role of Dopamine in Conditioning and Latent Inhibition: What, When, Where and How?” Neuroscience & Biobehavioral Reviews 29 (6): 963–76. 10.1016/j.neubiorev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Zahm Daniel S. 2000. “An Integrative Neuroanatomical Perspective on Some Subcortical Substrates of Adaptive Responding with Emphasis on the Nucleus Accumbens.” Neuroscience & Biobehavioral Reviews 24 (1): 85–105. 10.1016/S0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]