Abstract
Background:
Stepping in place (SIP) is a useful locomotor training intervention. The purpose of this study was to investigate the effects of single auditory-cued SIP training on cortical excitability, rhythmic movements and walking ability in patients with Parkinson's disease
(PD).
Methods:
Cross-over randomized control trial. Each participant completed two interventions with at least one-week washout period in between: (1) SIP with concurrent auditory cues (AC condition) and (2) SIP without auditory cues (NC condition).
Results:
In the primary outcome, the cortical silent period (CSP) duration increased (P = .005), whereas short intracortical inhibition (SICI) decreased after training (P = .001). Freezers demonstrated enhanced inhibition in the resting motor threshold and CSP duration. SICI and intracortical facilitation were modulated in both groups under the AC condition. In the secondary outcomes, the stepping variability decreased significantly (AC: P = .033; NC: P = .009), whereas walking cadence increased after training (AC: P = .019; NC: P = .0023).
Conclusions:
Auditory-cued SIP training improved the lower-limb movement variability and modulated the cortical excitability in patients with PD. Freezers may benefit more from this training than nonfreezers.
Keywords: auditory cueing, Parkinson's disease, step in place, transcranial magnetic stimulation
1. Introduction
Parkinson's disease (PD) is a common neurodegenerative disease with dominant movement dysfunction.[1,2] Patients with PD may experience difficulty in performing repetitive rhythmic movements such as finger tapping and walking.[3,4] To ameliorate the rhythmic movement problem, auditory cues are often applied to gait training. Numerous long-term studies have investigated the effects of auditory cues on walking, with most showing an improvement in walking ability[5–7] and a reduction in gait variability.[8,9]
Freezing of gait (FOG) is a unique phenomenon. When comparing patients with or without FOG, some differences are noted. The ability to maintain walking rhythm and regulate timing of bilateral movements is more impaired in freezers (PD patient with freezing of gait) than in nonfreezers (PD patient without freezing of gait). The stride–time and stepping in place (SIP) variability is significantly larger in freezers than in nonfreezers.[4,10] Furthermore, additional pathology occurs in the pedunculopontine nucleus of the mesencephalic locomotor region in freezers.[11,12] Meanwhile, freezers and nonfreezers exhibit different responses to auditory cues.[8]
The surrounding environment can affect the walking ability of patients with PD.[13] Weather, fear of falling, and limited activity space may hinder patients from completing auditory-cued walking training. SIP is a repetitive rhythmic movement containing a reciprocal and rhythmic lower extremity movement pattern, which is similar to gait.[14] It is an alternative training method which can be applied when the space is limited, such as at bedsides or in hospital wards. Hence, it can be an alternative intervention to walking for patients with PD. A study showed that freezers and nonfreezers had different performance when executing SIP movements.[10] The SIP movement might be helpful to explore the difference between freezers and non-freezers.
Long-term auditory cueing training for walking has demonstrated beneficial effects on walking performance and variability. The motor improvements after long-term training have been attributed to functional changes in the brain, originating from accumulative brain reorganization in a single training session. Appending auditory cues to SIP exercise has shown benefits on stroke patients but no study discusses about this training in PD.[15] Therefore, this study investigated the effects of a single auditory-cued SIP training session on lower rhythmic movements and cortical excitability in patients with PD and compared the performance of freezers and nonfreezers. We hypothesized that the effects of auditory cues could modulate cortical excitability and that patients with PD demonstrate more improvement after auditory-cued SIP training than after SIP training without auditory cues.
2. Methods
2.1. Participants
The study protocols had been approved by the Institutional Review Board and Research Ethics Committee of National Taiwan University Hospital.
The inclusion criteria were
-
(1)
age > 20 years,
-
(2)
diagnosed with idiopathic PD (Hoehn and Yahr stages I to III),
-
(3)
no hearing impairment,
-
(4)
can walk independently for at least 10 m without an assistive device, and
-
(5)
can understand and follow instructions (Mini–Mental State Examination [MMSE] score above 24).
Patients were assigned to the freezer or nonfreezer group based on their score in the first question of the new Freezing of Gait (FOG) questionnaire.[16]
Patients were excluded if they met any of the following criteria:
-
(1)
other neurological or psychological diseases,
-
(2)
diagnosed with dementia,
-
(3)
inability to follow instructions, or
-
(4)
contraindications for transcranial magnetic stimulation (TMS) assessment.[17]
The patients were recruited from the Department of Neurology, the Parkinson Center, and Physical Therapy Center at National Taiwan University Hospital. Each subject provided informed consent and completed a TMS safety questionnaire before the first experiment.
2.2. Intervention procedures
To eliminate the influence of medications, patients were asked to stop their dopaminergic drugs at least 8 hours before the experiment.
After baseline data collection was completed, all participants attended two SIP trainings under two conditions in random sequence (through computer-generated random-number allocation), with a washout interval of at least 1 week between two conditions. The SIP training involved 10 rounds of 50-step SIP movements with the arms swinging spontaneously. We used a metronome sound as an auditory cue to guide the SIP movement for the auditory-cueing condition (AC condition). The auditory cue was set at 110% of the usual cadence, which was retrieved from the pretest walking assessment. The participants were asked to match their steps with each beep as accurately as possible. In the non-cued condition (NC condition), the participants performed the SIP movement at their speed. Post-training assessments were completed immediately after the training.
2.3. Primary outcome measures
Cortical excitability was assessed using TMS. A double-cone TMS coil was used to stimulate the motor area of the tibialis anterior, which was connected to a Magstim BiStim2 stimulator (Magstim Co. Ltd., Whitland, UK). Electromyography (EMG) was performed through surface electrodes over the tibialis anterior muscle. The most affected hemisphere was assessed.
During TMS recording, the resting motor threshold (RMT) was determined according to the Rossini method[18]: the lowest intensity that induces 50 μV of MEP in 50% of the testing trials. The following measurements were included in this study.
-
1.
MEP measured at the intensity of 130% of RMT under a resting condition.
-
2.
Cortical silent period (CSP) and active MEP (AMEP) measured at the intensity of 130% of RMT under active contraction.
-
3.
SICI and intracortical facilitation (ICF) obtained through paired-pulse TMS under resting condition. The conditioning stimulus was set at 80% of RMT and the testing stimulus was set at 130% of RMT. The interstimulus intervals (ISIs) were 2, 3, 7, 10, and 12 ms. ISIs of 2 and 3 ms represented SICIs, whereas that of 10 and 12 ms represented ICFs. An ISI of 7 ms was the neutral point. The SICI and ICF values were obtained through the peak-to-peak value of the EMG response, divided by the mean value of MEP, expressed as the percentage of MEP.
2.4. Secondary outcome measures
Movement assessments consisted of the SIP test and walking.
-
1.
SIP performance was measured using the Kistler force plate (type 9260AA, Kistler AG, Winterthur, Switzerland). The patients were asked to step on the force plate at a comfortable speed. Stepping data were recorded for 15 seconds.
-
2.
The patients were asked to walk on the GAITRite electronic walkway at a comfortable speed. Walking variability, walking speed, cadence, and stride length were recorded throughout the system.
2.5. Data analyses
The stepping variability was calculated from the duration between each peak vertical force, which was detected by the force plate. Walking variability was obtained from the step time recorded using the GAITRite walkway. Both the stepping variability and walking variability were represented by the coefficient of variation (CV) = (standard deviation of step time)/(mean of the step time) of each step time. All statistical analyses were performed in SPSS (version 22.0; SPSS Inc., Chicago, IL). The patient characteristics are expressed using descriptive analysis and compared using the independent t test. Within- and between-group comparisons were performed using the Wilcoxon signed-rank test and Mann–Whitney U test, respectively; α-level was set at 0.05.
3. Results
3.1. Participants
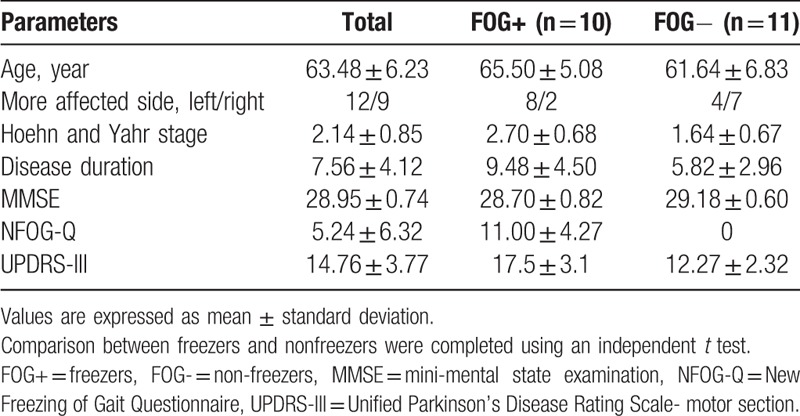
In total, 21 patients with PD (10 freezers and 11 nonfreezers) were recruited (Fig. 1 flowchart). Table 1 shows the demographic data of the participants and a comparison between the freezer and nonfreezer groups.
Figure 1.
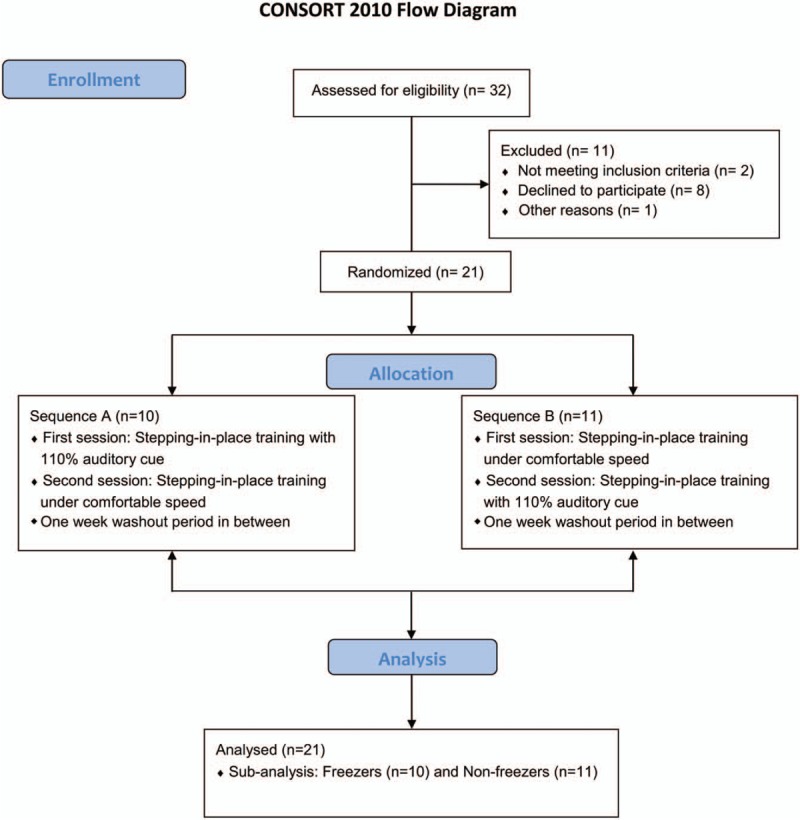
Flowchart of the participants in this study.
Table 1.
Demographic data of the participants.
3.2. TMS assessments
The results of the single-pulse TMS are shown in Table 2. The results of SICI and ICF are presented in Figure 2. No difference in RMT, MEP, and AMEP was observed between the pretest and posttest assessments and between the experimental conditions in general. Under the AC condition, the CSP increased after training and this phenomenon was not observed in NC condition. A significant condition effect was noted between the two conditions (P = .006). For pair-pulse TMS, under the AC condition, the total curve of five interstimulus intervals demonstrated an upward trend after training (Fig. 2A). Moreover, the MEP of SICI (2ms) increased significantly after training from 17.5% to 32% of the unconditioned MEP (P = .001). Under the NC condition, no obvious change after the training was noted in both SICI and ICF (Fig. 2B).
Table 2.
Results of single-pulse transcranial magnetic stimulation and movement assessments of the participants.
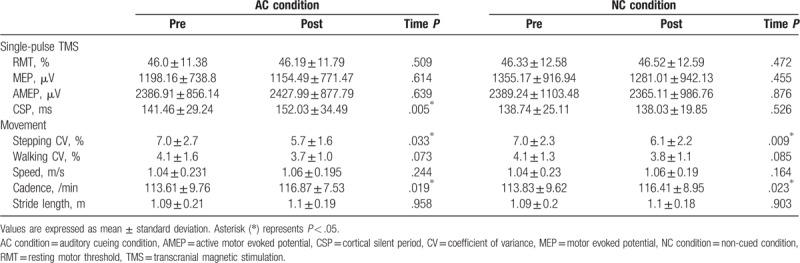
Figure 2.
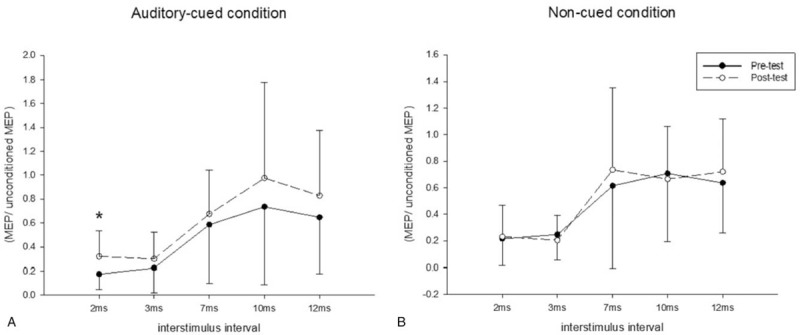
Pair-pulse transcranial magnetic stimulation of all the participants under the (A) auditory-cued and (B) noncued condition. The solid line represents pretest and the dotted line represents posttest results. A significant decrease was found at short intracortical inhibition (2 ms) under the auditory-cued condition (A); no difference was found under the non-cued condition (B). ∗P < .05.
A significant condition effect was found at SICI (3 ms): inhibition decreased under the AC condition, but increased under the NC condition.
3.3. Movement assessments
The CV results of the stepping and walking performance of the participants under the two training conditions are shown in Table 2. No difference in the baseline stepping and walking CVs were noted between the AC and NC conditions (P = .821 and .958, respectively). In both the AC and NC condition, the stepping CV decreased significantly after training. There was no difference between the pretest and posttest walking CV. For walking performance, in both the AC and NC conditions, the patients tended to walk under higher frequency after training (P < .05). However, the difference in the walking speed and stride length was nonsignificant. No between-group difference was identified.
3.4. Comparison between freezers and nonfreezers
The details of the single-pulse TMS in the freezer and nonfreezer groups are presented in Table 3. Group data of SICI and ICF are shown in Figure 3. No difference was found in the baseline values between the groups.
Table 3.
Results of single-pulse transcranial magnetic stimulation and movement variability of the freezers and nonfreezers.
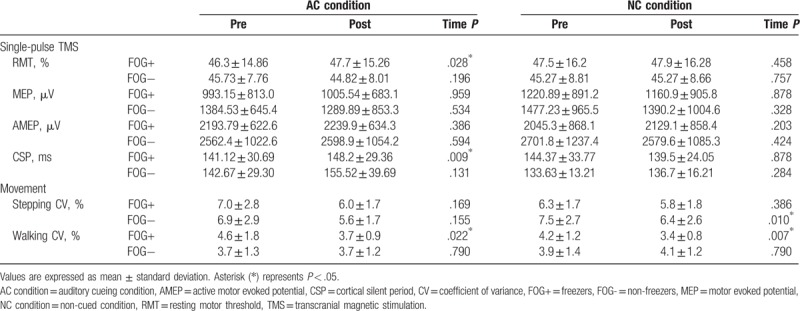
Figure 3.
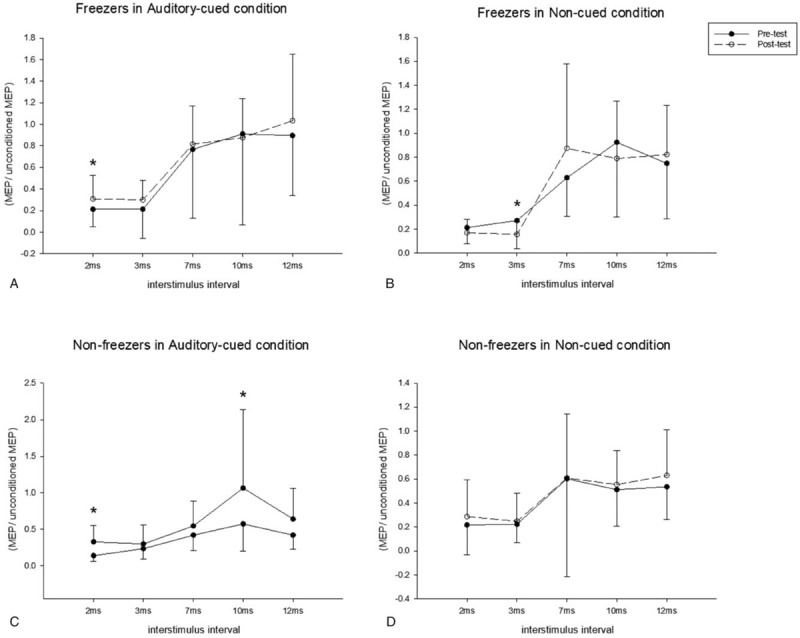
Pair-pulse transcranial magnetic stimulation in the freezer group (A and B) and nonfreezer group (C and D). The solid line represents the pretest and the dotted line represents the posttest results. A significant decrease was found at short intracortical inhibition (2 ms) in the freezers in the auditory-cued condition (A); a significant increase was found at short intracortical inhibition (3 ms) in the freezers in the non-cued condition (B); in the nonfreezers, increased excitation was found at at short intracortical inhibition (2 ms) and intracortical facilitation (10 ms) in the auditory-cued condition (C), and no difference was noted between the pretest and posttest in the non-cued condition (D). ∗P < .05.
In the single-pulse TMS assessments, the freezers showed a significant increase in RMT under the AC condition. The changes in RMT after training were significantly larger in the freezer group than in the nonfreezer group. The SP of the freezer group increased significantly.
In the pair-pulse measurements, significant decreases at SICI(2ms) was noted in both the freezer and nonfreezer groups under the AC condition (P = .047 and .008, respectively; Fig. 3A and C, respectively). An increase in ICF(10ms) was also found in the nonfreezer group under the AC condition (P = .016, Fig. 3C). Increases were noted at SICI (3 ms) in the freezer group under the NC condition (P = .037, Fig. 3B).
The results of the stepping and walking CV are shown in Table 3. Under the AC condition, no significant time effect on the stepping CV was noted in both groups. The freezer group showed more improvement in walking CV after the AC training than did the nonfreezer group. Under the NC condition, the nonfreezer group demonstrated a significant reduction in the stepping CV, whereas a reduction in the walking CV was found in the freezer group.
4. Discussion
4.1. Effects of auditory cues on cortical excitability
The pathological changes of PD could alter the motor performance and cortical excitabilities.[19] Regarding cortical excitability, two major findings were noted in our study. First, the CSP duration increased significantly after SIP training under the AC condition. This result is consistent with the findings of Fisher et al.[19] In their study, CSP duration increased with improvement in gait performance in patients with PD who received high-intensity treadmill training.
The CSP duration is considered shorter in patients with PD than in healthy controls, which represents the failure in the cortical inhibition mechanism, and is the most consistent finding in most TMS studies on PD.[19] It is thought to be related to GABA-B receptors.[27] The CSP duration is sensitive to interventions. Interventions such as medications, high-intensity exercises, and repetitive TMS can increase the CSP duration and aid motor improvements.[20] Therefore, our study suggested that appending auditory cues to SIP training could modulate the GABA-B-related inhibition circuits.
Second, SICI (2ms) decreased significantly after training under the AC condition, representing less inhibition after auditory-cued SIP training. Our result is consistent with one study, which examined the effects of a single session of ankle motor learning training on cortical excitability over the tibialis anterior motor area in healthy subjects.[21] The researchers found that SICI (2.5 ms) decreased significantly with improvements in motor performance in subjects who received ankle skill training. The reduced SICI may be associated with motor learning process, representing the plasticity in primary motor cortex. However, two studies discussed the SICI changes in PD after intervention exhibited different results. One study showed increased SICI after single-session treadmill training regardless of applying transcranial direct stimulation or not.[22] The other interventional study demonstrated an increase in SICI with increased CSP after long-term interventions.[23] It is hard to explain the result as reduced SICI is considered as the manifestation of abnormal neurophysiology in PD. From the abovementioned study about ankle skill training, we surmised that appending auditory cues to movements, unlike walking on the treadmill, was new to patients with PD and therefore may result in different neurophysiological performance.
4.2. Effects of auditory cues on lower extremity rhythmic movements
In our study, decreased stepping CV and increased cadence were noted after the SIP training with and without the auditory cues. The motor improvements might be due to learning effect. In other words, the patients may have become accustomed to the SIP movements and developed an improved strategy to execute them. Increases in the cadence may have been from a transfer effect of the SIP training, causing the patients to walk under more normal cadence.
Research regarding auditory-cued training on the lower extremities can be divided into two categories:
-
(1)
cued walking interventions are performed for a long period, and
-
(2)
concomitant auditory cues are applied to walking with the walking performance under auditory cues being examined.
This is the first study to examine the effects of a single auditory-cued SIP training session. However, the effects of auditory cues could not be determined using kinematic data, such as CV and walking performance. This suggests that patients with PD may require more training sessions to embed the learned rhythm into their autonomic rhythmic movements, such as SIP or walking.
4.3. Comparison between freezers and nonfreezers
Freezers are considered to have additional pathology and more impairment in the inhibitory circuits compared with nonfreezers. Some studies have shown differences between freezers and nonfreezers in neural images, motor performance, and responses to interventions. However, few studies have investigated the differential effects of an auditory-cued intervention between freezers and nonfreezers.
In the TMS assessments, the freezers rather than the nonfreezers had a significant increase in RMT and a notable increase in CSP after training under the AC condition. Thus, the inhibitory circuits mediated by GABA-B receptors were reinforced by the auditory cues in the freezers. In the pair-pulse measurements, after the auditory-cued SIP training, both the freezers and nonfreezers showed a decreased inhibition at SICI (2 ms) and the nonfreezers had additional excitation at ICF (10 ms). General elevated excitability was also found in the nonfreezers. Therefore, we propose that the auditory cues had modulating effects on the intracortical circuits in both groups.
In the movement assessments, the freezers significantly improved in the walking CV under both conditions; a similar, but nonsignificant, improvement was noted in the stepping CV. The nonfreezers only demonstrated a decreased stepping CV under the AC and NC conditions. From these results, we propose that the SIP training was beneficial to both groups and that the freezers benefited more from the SIP training than did the nonfreezers. Task difficulty might explain the performance differences between the groups. Although the SIP movement is similar to regular walking, it requires more balanced control because of a longer single-leg standing time. Hence, the SIP training might have been challenging but acceptable for the freezers and relatively easy for the nonfreezers.
4.4. Clinical implications
An impaired walking capacity is a major concern for patients with PD. Patients with PD have difficulty maintaining regular rhythmicity during walking. Auditory cues are useful for the temporal deficit problem in patients with PD in terms of guiding their rhythm when executing repetitive rhythmic movements. By probing into the neurophysiological changes in the patients, we could understand the effectiveness of the interventions and identify the mechanism behind the motor improvements.
In this study, the SIP training is a simple exercise and can be carried out without worries of falling. The results showed reduced SIP movement variability after session regardless of auditory cues. From some studies on stroke patients, long-term SIP training can help to improve the coordination[24] and walking ability[25]. Irregularity of step is a typical symptom in patients with PD. The findings in this study and other related research may imply the plausibility of SIP training to improve the gait performance in PD, if doing this exercise for long term.
This study showed that the CSP and SICI were modulated after single session of auditory-cued training. The results implied that auditory cues could cause immediate plastic change inside the brain, as noted in one fMRI study that rhythmic auditory cues may play a role in activating compensatory mechanism between the motor execution and perceptual networks in PD.[26] Moreover, this study probed into the differential effects between freezers and non-freezers in advance. We noted that appending auditory cues to SIP training had more beneficial effects in the freezers with modulated cortical excitability. Although some changes in motor and TMS outcomes were observed in the nonfreezers, the improvements were not as significant as those in the freezers. This may imply that freezers depend on auditory cues more than non-freezers.
4.5. Study limitations
This study has several limitations. First, due to insufficient sample size and mild disease severity, the results may not be able to apply to all population with PD. The theoretical sample size based on the CSP effect size from a TMS interventional study[23] is fourteen subjects. Although the participants were sufficient, the effect size was around mild to moderate. More participants were required for reaching a more reliable power and generalization. Furthermore, more freezers and non-freezers are needed for drawing a clearer conclusion on their differential responses to auditory cues. Second, studies on SIP are limited, so it is unclear what training dosage is suitable for patients with PD. Therefore, the training dosage potentially not being adequate for the nonfreezers might explain the differences in the results between the groups. Future studies should consider exploring the effects of SIP. Furthermore, research on long-term SIP training should be considered in future studies.
5. Conclusion
In this study, we investigated the immediate effects of applying rhythmic auditory cues to SIP training and the differences between freezers and nonfreezers in step variability, walking ability, and cortical excitability. The results showed that after SIP training, both the freezers and nonfreezers had improvements in lower extremity variability. Appending auditory cues to training also caused modulating effects on cortical excitability. Furthermore, the freezers demonstrated better improvements in lower extremity variability and more enhanced inhibition in the primary motor cortex than did the nonfreezers. Therefore, the auditory-cued SIP training may be a suitable alternative for walking training for patients with PD, particularly freezers. Further research is needed to examine the optimal dosage of SIP training as well as long-term outcomes.
Author contributions
Conceptualization: Hsiu-Yun Chang, Ya-Yun Lee, Yea-Ru Yang, Jer-Junn Luh.
Data curation: Hsiu-Yun Chang, Ya-Yun Lee, Ruey-Meei Wu, Jer-Junn Luh.
Formal analysis: Hsiu-Yun Chang, Jer-Junn Luh.
Funding acquisition: Jer-Junn Luh.
Investigation: Hsiu-Yun Chang, Jer-Junn Luh.
Methodology: Hsiu-Yun Chang, Ya-Yun Lee, Yea-Ru Yang, Jer-Junn Luh.
Project administration: Jer-Junn Luh.
Resources: Ruey-Meei Wu, Jer-Junn Luh.
Software: Hsiu-Yun Chang.
Supervision: Ruey-Meei Wu, Jer-Junn Luh.
Validation: Ruey-Meei Wu.
Writing – original draft: Hsiu-Yun Chang, Ya-Yun Lee, Ruey-Meei Wu, Yea-Ru Yang, Jer-Junn Luh.
Writing – review & editing: Hsiu-Yun Chang.
Footnotes
Abbreviations: AC condition = auditory-cueing condition, AMEP = active motor evoked potential, CSP = cortical silent period, CV = coefficient of variance, FOG = freezing of gait, ICF = intracortical facilitation, MEP = motor evoked potential, NC condition = non-cued condition, PD = Parkinson's disease, RMT = resting motor threshold, SICI = short intracortical inhibition, SIP = stepping in place, TMS = transcranial magnetic stimulation.
How to cite this article: Chang HY, Lee YY, Wu RM, Yang YR, Luh JJ. Effects of rhythmic auditory cueing on stepping in place in patients with parkinson's disease. Medicine. 2019;98:45(e17874).
This study was partially funded by the Ministry of Science and Technology, Taiwan, R.O.C. (Grant no. MOST 103–2221-E-002 -103 and MOST 106-2221-E-002 -092). Some results were partially presented at the 7th World Congress on Physical Medicine and Rehabilitation.
The authors report no conflicts of interest.
References
- [1].Hirtz D, Thurman DJ, Gwinn-Hardy K, et al. How common are the “common” neurologic disorders? Neurology 2007;68:326–37. [DOI] [PubMed] [Google Scholar]
- [2].Bella SD, Benoit CE, Farrugia N, et al. Effects of musically cued gait training in Parkinson's disease: beyond a motor benefit. Ann N Y Acad Sci 2015;1337:77–85. [DOI] [PubMed] [Google Scholar]
- [3].Freeman JS, Cody FW, Schady W. The influence of external timing cues upon the rhythm of voluntary movements in Parkinson's disease. J Neurol Neurosurg Psychiatry 1993;56:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hausdorff JM, Schaafsma JD, Balash Y, et al. Impaired regulation of stride variability in Parkinson's disease subjects with freezing of gait. Exp. Brain Res 2003;149:187–94. [DOI] [PubMed] [Google Scholar]
- [5].Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry 2007;78:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spaulding SJ, Barber B, Colby M, et al. Cueing and gait improvement among people with Parkinson's disease: a meta-analysis. Arch Phys Med Rehabil 2013;94:562–70. [DOI] [PubMed] [Google Scholar]
- [7].Thaut MH, McIntosh GC, Rice RR, et al. Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Mov Disord 1996;11:193–200. [DOI] [PubMed] [Google Scholar]
- [8].Willems AM, Nieuwboer A, Chavret F, et al. The use of rhythmic auditory cues to influence gait in patients with Parkinson's disease, the differential effect for freezers and non-freezers, an explorative study. Disabil Rehabil 2006;28:721–8. [DOI] [PubMed] [Google Scholar]
- [9].Baker K, Rochester L, Nieuwboer A. The effect of cues on gait variability–reducing the attentional cost of walking in people with Parkinson's disease. Parkinsonism Relat Disord 2008;14:314–20. [DOI] [PubMed] [Google Scholar]
- [10].Nantel J, de Solages C, Bronte-Stewart H. Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson's disease. Gait Posture 2011;34:329–33. [DOI] [PubMed] [Google Scholar]
- [11].Peterson DS, Pickett KA, Duncan R, et al. Gait-related brain activity in people with Parkinson disease with freezing of gait. PLoS One 2014;9:e90634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fling BW, Cohen RG, Mancini M, et al. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 2013;136:2405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jones D, Rochester L, Birleson A, et al. Everyday walking with Parkinson's disease: understanding personal challenges and strategies. Disabil Rehabil 2008;30:1213–21. [DOI] [PubMed] [Google Scholar]
- [14].Garcia RK, Nelson AJ, Ling W, et al. Comparing stepping-in-place and gait ability in adults with and without hemiplegia. Arch Phys Med Rehabil 2001;82:36–42. [DOI] [PubMed] [Google Scholar]
- [15].Wright RL, Masood A, MacCormac ES, et al. Metronome-cued stepping in place after hemiparetic stroke: comparison of a one- and two-tone beat. ISRN Rehabil 2013;2013:1–5. [Google Scholar]
- [16].Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture 2009;30:459–63. [DOI] [PubMed] [Google Scholar]
- [17].Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee Electroencephalogr Clin Neurophysiol 1994;91:79–92. [DOI] [PubMed] [Google Scholar]
- [19].Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil 2008;89:1221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lefaucheur JP. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson's disease: influence of antiparkinsonian treatment and cortical stimulation. Clin Neurophysiol 2005;116:244–53. [DOI] [PubMed] [Google Scholar]
- [21].Perez MA, Lungholt BK, Nyborg K, et al. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp. Exp Brain Res 2004;159:197–205. [DOI] [PubMed] [Google Scholar]
- [22].Fernandez-Lago H, Bello O, Mora-Cerda F, et al. Treadmill walking combined with anodal transcranial direct current stimulation in parkinson disease: a pilot study of kinematic and neurophysiological effects. Am J Phys Med Rehabil 2017;96:801–8. [DOI] [PubMed] [Google Scholar]
- [23].Yang Y, Tseng C, Chiou S, et al. Combination of rTMS and treadmill training modulates corticomotor inhibition and improves walking in Parkinson disease: a randomized trial. Neurorehabil Neural Repair 2013;27:79–86. [DOI] [PubMed] [Google Scholar]
- [24].Murata K, Asai H, Inaoka PT, et al. Walking gait changes after stepping-in-place training using a foot lifting device in chronic stroke patients. J Phys Ther Sci 2016;28:1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wright RL, Brownless SB, Pratt D, et al. Stepping to the beat: feasibility and potential efficacy of a home-based auditory-cued step training program in chronic stroke. Front Neurol 2017;8:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Braunlich K, Seger CA, Jentink KG, et al. Rhythmic auditory cues shape neural network recruitment in Parkinson's disease during repetitive motor behavior. Eur J Neurosci 2019;49:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hallett M. Transcranial magnetic stimulation: a primer. Neuron 2007;55:187–99. [DOI] [PubMed] [Google Scholar]


