ABSTRACT
As there is currently no cure for dementia, there is an urgent need for preventive strategies. The current review provides an overview of the existing evidence examining the associations of the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets and their dietary components with cognitive decline, dementia, and Alzheimer's disease (AD). A systematic search was conducted within Ovid Medline for studies published up to 27 March 2019 and reference lists from existing reviews and select articles were examined to supplement the electronic search results. In total, 56 articles were included. Higher adherence to the Mediterranean diet was associated with better cognitive scores in 9 of 12 cross-sectional studies, 17 of 25 longitudinal studies, and 1 of 3 trials. Higher adherence to the DASH diet was associated with better cognitive function in 1 cross-sectional study, 2 of 5 longitudinal studies, and 1 trial. Higher adherence to the MIND diet was associated with better cognitive scores in 1 cross-sectional study and 2 of 3 longitudinal studies. Evidence on the association of these dietary patterns with dementia in general was limited. However, higher adherence to the Mediterranean diet was associated with a lower risk of AD in 1 case-control study and 6 of 8 longitudinal studies. Moreover, higher adherence to the DASH or MIND diets was associated with a lower AD risk in 1 longitudinal study. With respect to the components of these dietary patterns, olive oil may be associated with less cognitive decline. In conclusion, current scientific evidence suggests that higher adherence to the Mediterranean, DASH, or MIND diets is associated with less cognitive decline and a lower risk of AD, where the strongest associations are observed for the MIND diet.
Keywords: Mediterranean, DASH, MIND, dietary patterns, dietary components, nutrition, cognition, cognitive decline, dementia, Alzheimer's disease
Introduction
In 2015 ∼47 million people worldwide were diagnosed with dementia, which is the seventh leading cause of death worldwide (1). Due to the global aging population, the number of people living with dementia is expected to increase to 75 million by 2030 (2). With estimated global costs of dementia of 818 billion US$ in 2015, representing 1.09% of the global gross domestic product, dementia has a huge impact on societal healthcare costs (2, 3).
As there is currently no cure for dementia, preventative measures are of major importance to reduce the expected rise in dementia cases (2). To date, many studies have examined the role of nutrients and foods in the prevention of cognitive decline, dementia, and Alzheimer's disease (AD) (4–6). Over recent decades, research has shifted towards studying dietary patterns to take into account interactions between nutrients or foods and possible synergic effects of nutrients (7, 8). Three dietary patterns that have been frequently studied in relation to cognitive decline, dementia, or AD are the Mediterranean diet (9), the Dietary Approaches to Stop Hypertension (DASH) diet (10), and the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet (11).
The Mediterranean diet is a dietary pattern that is consumed in countries surrounding the Mediterranean Sea, for example in Greece (9). Meta-analyses indicate that higher adherence to the Mediterranean diet is associated with better global cognition and episodic memory (12), a lower risk of cognitive impairment (13, 14), and a lower risk of neurodegenerative diseases (13, 15). The Mediterranean diet is characterized by a high consumption of fruits, vegetables, and olive oil, with a moderate consumption of alcohol (16, 17). Similar to the Mediterranean diet, the DASH diet also specifies a high consumption of plant-based foods and additionally limits the intake of SFAs, total fat, cholesterol, and sodium (10). The DASH dietary pattern has been developed to prevent and treat hypertension and has been shown to improve cardiovascular disease (CVD) risk factors, including systolic and diastolic blood pressure and total cholesterol (10, 18). The MIND dietary pattern has been developed to protect the brain and prevent against dementia (11). This dietary pattern is a combination of the Mediterranean diet and the DASH diet and is based on dietary components that have been shown to be neuroprotective. The MIND diet emphasizes natural plant-based foods and limited intakes of animal foods and foods high in saturated fat. Uniquely, the MIND diet also specifies the consumption of berries and green leafy vegetables (11).
So far, 10 reviews have discussed the current evidence on the association of the Mediterranean, DASH, and MIND diets with cognitive decline, dementia, or AD (19–28). However, 5 of these reviews only included a brief summary of the available evidence (19, 21–24). Of the 4 extensive reviews on the topic, 1 review only included studies of the past 5 y (27), 2 reviews only included observational studies (20, 25), and 2 also discussed intervention studies (26, 28). However, 1 of the reviews that also discussed intervention studies only included studies until 2015 (26) and the other only included cohort and intervention studies (28).
Therefore, the aim of the current review is to summarize, evaluate, and compare all existing observational and trial evidence published up to 27 March 2019 for the Mediterranean, DASH, and MIND diets and their dietary components in relation to cognitive decline, dementia, and AD in middle-aged and older adults aged ≥40 y.
Methods
Literature search and study design
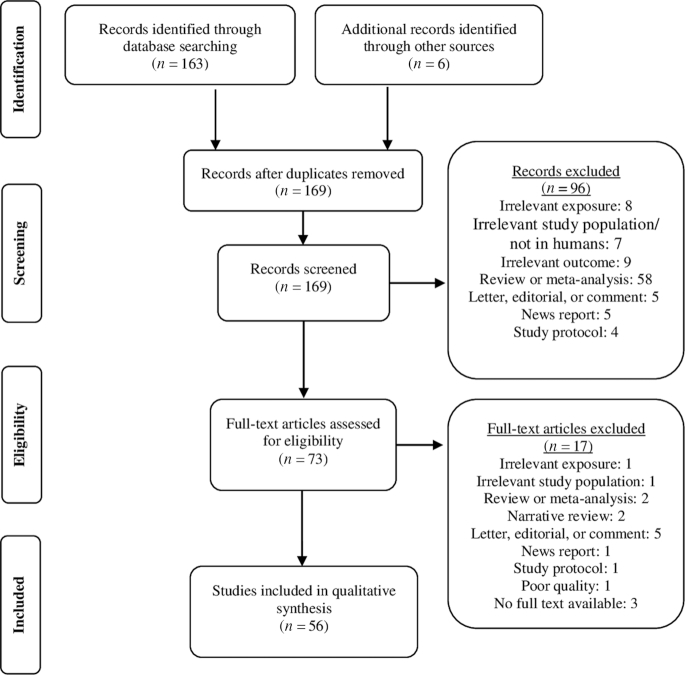
For this review, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed. A systematic search was conducted within Ovid Medline for all studies published in English up to 27 March 2019. Search terms included terms related to cognition, dementia, AD, Mediterranean diet, DASH diet, and MIND diet (Supplemental Table 1). Commentaries, letters, editorials, news, and newspaper articles were not screened. A predefined protocol was not available. The systematic search in Ovid Medline resulted in 163 articles (Supplemental Table 1). Reference lists from existing reviews and select articles were examined to identify studies that were not retrieved by the systematic search in Ovid Medline. This resulted in 6 additional studies. First, titles and abstracts were screened, resulting in 73 potentially relevant articles. Studies were included if: 1) they were performed in adults aged ≥40 y, 2) they measured exposure to ≥1 of the 3 dietary patterns of interest (Mediterranean diet, DASH diet, or MIND diet) or to dietary components as part of these 3 dietary patterns, and 3) the outcome measure was related to cognition, cognitive decline, dementia, or AD. The eligibility criteria of participants aged ≥40 y was selected, because cognitive decline has been shown to be already present in middle age (29). After full-text screening, 56 of the 73 articles were included in this review (Figure 1). For the dietary components of the 3 dietary patterns, evidence is restricted to articles identified using the search strategy for the Mediterranean, DASH, and MIND diets in relation to cognitive decline, dementia, and AD. Study selection and data extraction was performed by 1 researcher and checked by a second researcher.
FIGURE 1.
Flow diagram of the identified and screened studies on the Mediterranean, DASH, and MIND diets and their dietary components in relation to cognitive decline, dementia, or AD.
Quality assessment
The quality of the included studies was assessed according to the following criteria: 1) number of study participants, 2) in the case of prospective studies, duration of follow-up and loss to follow-up, 3) exposure measurement, 4) outcome measurement, and 5) adjustment for potential confounders.
Dietary pattern scores
The Mediterranean, DASH , and MIND diets all have a plant-based origin with moderate to high amounts of fish, but they differ in types and amounts of dietary components. A detailed list of components per diet can be found in Table 1. The assessment of these diets is described below.
TABLE 1.
Overview of the dietary components included in the Mediterranean, DASH, and MIND diets
| Mediterranean diet (16, 17) | DASH diet (10) | MIND diet (11) | |
|---|---|---|---|
| High amounts | Olive oil | — | Olive oil |
| Fish | — | Fish | |
| Breads and other forms of cereals | Grains | Whole grains | |
| Fruits | Fruits | Berries | |
| Vegetables | Vegetables | Green leafy vegetables | |
| — | — | Other vegetables | |
| Legumes | Legumes | — | |
| Nuts | Nuts | Nuts | |
| Beans | — | Beans | |
| Seeds | Seeds | — | |
| — | Low-fat dairy products | — | |
| — | — | Poultry | |
| Moderate amounts | Dairy products | — | — |
| Poultry | Poultry | — | |
| Alcohol | — | Alcohol/wine | |
| — | Fish | — | |
| Restricted amounts | Red meat | Red meat | Red meat and products |
| Processed meat | — | — | |
| Sweets | Sweets | Pastries and sweets | |
| — | Saturated fat | — | |
| — | Total fat | — | |
| — | Cholesterol | — | |
| — | Sodium | — | |
| — | — | Cheese | |
| — | — | Butter/margarine | |
| — | — | Fast fried foods |
Adherence to the Mediterranean diet can be assessed by 2 different scores, namely the original Mediterranean diet score by Trichopoulou et al. (9) and the alternate Mediterranean diet score by Panagiotakos et al. (30). The original score of Trichopoulou et al. ranges from 0 (minimal adherence to the Mediterranean diet) to 9 (maximal adherence) and is based on sex-specific median intake of the study population on 9 components (9). For fish, cereals, fruits + nuts, vegetables, legumes, and MUFA to SFA ratio (MUFA:SFA ratio), a value of 1 is assigned to people who have intake above the median, whereas a value of 0 is assigned to people with intake below the median. For meat and dairy, a value of 1 is assigned to people with intake below the median, whereas a value of 0 is assigned to people with intake above the median. Regarding alcohol, a value of 1 is assigned to people consuming a moderate amount. The alternate score by Panagiotakos et al. ranges from 0 (minimal adherence to the Mediterranean diet) to 55 (maximal adherence) and is based on the consumption of 11 components (30). For each dietary component, a value ranging from 0 to 5 is assigned to people based on predefined cut-offs of intake. For olive oil, fish, nonrefined cereals, fruits, vegetables, legumes, and potatoes a value of 5 is assigned to people with high intake, whereas for meat and meat products, poultry, and full-fat dairy products a score of 5 is assigned to people without consumption. For alcohol, a value of 5 is assigned to people consuming a moderate amount, whereas a value of 0 is assigned to people with either no intake or very high intake.
Adherence to the DASH dietary pattern can also be assessed by 2 different scores, namely the score by Folsom et al. (31) and the score by Fung et al. (32). The score by Folsom et al. is based on predefined cut-offs of intake of 11 components and ranges from 0 (minimal adherence to the DASH diet) to 11 (maximal adherence). For each dietary component a value of 0, 0.5, or 1 is assigned based on intake (31). For total grains, whole grains, fruits, vegetables, dairy foods, and nuts + seeds + dry beans a value of 1 is assigned to people with high intake. For sodium, sweets, percentage kcal from fat, percentage kcal from SFAs, and meats + poultry + fish a value of 1 is assigned to people with low intake. The score by Fung et al. is based on 8 dietary components and for each component a score of 1 to 5 is assigned based on quintile of intake (32). For whole grains, fruits, vegetables, nuts and legumes, and low-fat dairy products a score of 5 is assigned to people with the highest quintile of intake, whereas for sodium, red and processed meats, and sweetened beverages a score of 5 is assigned to people with the lowest quintile of intake. Thus, this score ranges from 8 (minimal adherence to the DASH diet) to 40 (maximal adherence).
The MIND diet score by Morris et al. is based on 15 dietary components and ranges from 0 (minimal adherence to the MIND diet) to 15 (maximal adherence) (11). A value of 0, 0.5, or 1 assigned to people for intake of each dietary component based on predefined cut-offs. For olive oil, fish, whole grains, berries, green leafy vegetables, other vegetables, nuts, beans, and poultry a value of 1 is assigned to people with high intake. For butter + margarine, cheese, red meat and products, fast fried foods, and pastries and sweets a value of 1 is assigned to people with low intake. For wine, a value of 1 is assigned to people with moderate intake.
Results
In total, 50 observational studies and 4 randomized controlled trials were included. Key characteristics and findings of these studies, categorized by dietary pattern and study design (observational or trial) are shown in Tables 2–6. Considerable variations among studies were observed in the country, numbers, age and sex of participants, dietary assessment methods, and dietary components used to measure adherence score to the diets. Of the observational studies and trials, 16 studies were conducted in Mediterranean countries including Spain (33–38), Greece (39–42), Italy (43–45), and France (46–48), and 26 studies were performed in the United States (11, 49–73). The other studies were conducted in Australia (74–78), Sweden (79–81), China (82, 83), Poland (84), Puerto Rico (85), Scotland (86), and Israel (51). Sample sizes ranged from 79 (33) to 27,842 (64) participants. The vast majority of the observational studies and trials were performed in participants aged ≥60 y (n = 38), 6 studies only included women (52, 66, 67, 70, 72, 73), and 2 studies only included men (64, 80). Most studies assessed dietary intake with an FFQ; 9 studies used either repeated 24-h dietary recalls (33, 46, 51, 83), repeated food diaries (80, 81), a diet adherence screener (35), or a combination of these dietary assessment methods (47, 50). Evidence per dietary pattern and study design is discussed below.
TABLE 2.
Characteristics of the included observational human studies on the Mediterranean diet in relation to cognitive decline, dementia, and AD1
| Authors, year, study name | Study design | Population | Follow-up (y) | Exposure | Outcome | Results | Covariates |
|---|---|---|---|---|---|---|---|
| Mosconi et al. (2018) (49), US | Cross-sectional | n = 116 (38% men) age: 50 y clinically and cognitively normal participants aged 30–60 y who were enrolled in observational brain imaging studies | — | FFQ, MeDi score | Memory (immediate and delayed recall), executive function (WAIS), and language (WAIS vocabulary) and MRI-based cortical thickness | Continuous MeDi score was significantly positively associated with MRI-based cortical thickness of the posterior cingulate cortex (standardized β: 0.023; P = 0.004). MeDi score was not significantly associated with memory, executive function, or language | Age, sex, and APOE genotype |
| Anastasiou et al. (2017) (39), Greece HELIAD | Cross-sectional | n = 1864 (41% men) age: 73.0 y elderly >64 y from 2 cities in Greece, random selection from municipality records | — | SFFQ, A-MeDi score, dietary components | Cognitive status (dementia [DSM-IV, NINCDS/ADRDA criteria]) and cognitive performance (memory [GVLT, CFT], language [BNT, CIMS, categories: objects and the letter A], executive functioning [TMT, verbal fluency, ASR, GST, MP, months forwards and backwards], and visuospatial perception [BJLO, CDT, CFT, TMT]) | Participants with a diagnosis of dementia had a significantly lower A-MeDi score compared to participants without dementia (P < 0.001). Continuous A-MeDi score and A-MeDi score quartile were significantly associated with lower risk of dementia (OR: 0.920; 95% CI: 0.870, 0.974; P = 0.004; ORQ4vs.Q1: 0.440; 95% CI: 0.208, 0.969; P-trend = 0.019). A-MeDi score was significantly associated with composite z-score (β: 0.010; P = 0.007), memory (β: 0.012; P = 0.001), language (β: 0.011; P = 0.007), and executive functioning (β: 0.008; P = 0.049), but not with visuospatial perception (P = 0.059) | Age, sex, education, number of clinical comorbidities, and energy intake |
| Bumenthal et al. (2017) (50), US | Cross-sectional | n = 160 (33% men) age: 65.4 y sedentary adults aged ≥55 y with cognitive impairment and CVD risk factors | — | FFQ and 4-d food diary, A-MeDi and A-DASH score | Verbal memory (HVLT-R, ANT), visual memory (CFT), and executive function/processing speed (ST, DST, COWA, TMT, DSST, Ruff 2&7 Test) | Higher MeDi score was not associated with verbal memory (P = 0.167), nor with executive function/processing speed (P = 0.901) nor with visual memory (P = 0.978) | Age, education, sex, ethnicity, total caloric intake, family history of dementia, and chronic use of anti-inflammatory medications |
| Hernández-Galiot & Goñi (2017) (33), Spain, Garrucha Old Age Health Study | Cross-sectional | n = 79 (48% men) age: 81.0 y noninstitutionalized Spanish elderly | — | 3 24-h diet recalls and a face-to- face interview, 14-item MEDAS | Global cognition (MMSE) | Higher tertile of MEDAS score was significantly associated with better cognitive status (P = 0.034) | - |
| McEvoy, Guyer, Langa & Yaffe (2017) (57), US Health and Retirement Study | Cross-sectional | n = 5907 (40% men) age: 67.8 y community dwelling adults from the age of 50 y | — | 163-item SFFQ, A-MeDi score, MIND diet score | Cognitive performance (global cognition score based on immediate and delayed recall, backward counting, and serial seven subtraction) | Higher A-MeDi score tertile was significantly associated with better cognitive performance (P-trend <0.001). Higher tertile of A-MeDi score was significantly associated with lower risk of poor cognitive performance (ORT3vsT1: 0.65; 95% CI: 0.52, 0.81; P < 0.001) | Sex, age, race, low education attainment, current smoking, obesity, total wealth, hypertension, diabetes mellitus, physical inactivity, depression, and total energy intake |
| Bajerska, Wozniewicz, Suwalska & Jeszka (2014) (84), Poland | Cross-sectional | n = 87 (35% men) age: 70.0 y elderly >60 y from rural areas of Wielkopolska from a community with high risk of metabolic syndrome | — | FFQ, A-MeDi score (high vs. low), dietary components | MCI, global cognition (MMSE), attention (TMT), visual memory (PRM), executive function (ST, SOC, SWM, SSP) | High A-MeDi score was significantly associated with lower prevalence of MCI (P < 0.001) and higher global cognition (β: 0.25; P = 0.001), but not with attention, visual memory, or executive function | Gender, age, education level, smoking status, family status, leisure time physical activity, and existence of metabolic syndrome |
| Zbeida et al. (2014) (51), US and Israel National Health and Nutrition Examination Survey (NHANES) (US) and Israeli National Health and Nutrition Survey of Older Adults (MABAT ZAHAV) (Israel) | Cross-sectional | n NHANES = 2791 (50% men) ageNHANES = 71.2 y nZAHAV = 1786 (47% men) ageZAHAV = 74.9 ycommunity dwelling population, elderly | — | 24-h dietary recalls, MeDi score | Cognitive function (NHANES: cognitive function questionnaire score, MABAT ZAHAV: MMSE) | Higher MeDi score tertile was associated with better cognitive function in both the NHANES (P-trend < 0.001) and the MABAT ZAHAV (P-trend = 0.008) survey | — |
| Chan, Chan & Woo (2013) (82), China | Cross-sectional | n = 3670 (52% men) age >65 y Chinese elderly men and women | — | 280-item FFQ, MeDi score | Cognitive function (CSI-D) | No significant association between MeDi score and cognitive function in both men (ORT3vs.T1: 0.89; 95% CI: 0.56, 1.4; P-trend = 0.882) and women (ORT3vs.T1: 1.02; 95% CI: 0.75, 1.41; P-trend = 0.952) | Age, BMI, PASE, energy intake, education level, Hong Kong community ladder, smoking status, alcohol use, number of ADLs, GDS category, and self-reported history of diabetes, hypertension, and CVD/stroke |
| Corley, Starr, McNeill & Deary (2013) (86), Scotland LBC 1936 | Cross-sectional | n = 878 (±50% men) age: 69.5 y independently living men and women of 70 y | — | 168-item FFQ, Mediterranean diet (22 items) | Cognitive function (IQ[MHT], general cognition [WAIS-III LNS, MR, BD, DS, DST backward, SS], processing speed [SS, DS, SCRT, IT], memory LM and VPA immediate and delayed recalls, SSP forwards and backwards, LNS, DST backward, and verbal ability [NART, WTAR]) | Mediterranean diet score was only positively associated with verbal ability measured with NART and measured with WTAR (ηp2NART: 0.006; P = 0.024; ηp2WTAR: 0.013; P = 0.001), but not associated anymore with IQ (P = 0.767), general cognition (P = 0.960), processing speed (P = 0.205), or memory (P = 0.870) | Sex, age, occupational social class, IQ at age of 11 y |
| Crichton, Bryan, Hodgson & Murphy (2013) (74), Australia | Cross-sectional | n = 1183 (36% men) age: 50.6 y participants from 40 to 65 y from Southern Australia | — | 215-item FFQ, MeDi score, dietary components | Self-reported cognitive function (CFQ) on mistakes in tasks on perception, memory, and motor function | MeDi score was not significantly associated with self-reported cognitive function (P-trend = 0.30). | — |
| Katsiardanis et al. (2013) (40), GreeceThe Velestino Study | Cross-sectional | n = 557 (53% men) age >65 y elderly men and women from Velestino | — | 157-item EPIC-Greek SFFQ, A-MeDi score | Cognitive impairment (MMSE) | Continuous A-MeDi score was significantly associated with less cognitive impairment in men (OR: 0.88; 95% CI: 0.80, 0.98; P = 0.02), but more cognitive impairment in women (OR: 1.11; 95% CI: 1.00, 1.22; P = 0.04) | Age, GDS, education, social activity, smoking, metabolic syndrome |
| Ye et al. (2013) (85), Puerto Rico BPRHS | Cross-sectional | n = 1269 age: 57.3 y adults from 45 to 75 y from Puerto Rico | — | SFFQ, MeDi score | Global cognition (MMSE), memory, executive function, attention, and cognitive impairment (MMSE) | Higher quintile of MeDi score was significantly associated with global cognition (β: 0.14; P-trend = 0.012), but not with executive function (P-trend = 0.52), memory (P-trend = 0.39) or attention (P-trend = 0.067). Both continuous MeDi score and highest quintile of MeDi score were significantly associated with lower risk of cognitive impairment (OR: 0.87; 95% CI: 0.80, 0.94; P < 0.001; ORQ5vs.Q1: 0.51; 95% CI: 0.33, 0.79; P-trend < 0.001) | Age, sex, educational attainment, household income below threshold, acculturation score, smoking status, physical activity score, supplement use, taking >5 types of medication within the last 12 mo, BMI, hypertension, diabetes, total cholesterol, high-density lipoprotein cholesterol, and triglycerides |
| Scarmeas, Stern, Mayeux & Luchsinger (2006) (61), US WHICAP | Case-control | n = 1984 (32% men) age: 76.3 y elderly from 2 WHICAP cohorts | — | 61-item SFFQ, MeDi score | AD (DSMIII, NINCDS-ADRDA criteria | Both higher continuous MeDi score and higher tertile of MeDi score were significantly associated with a lower risk of AD (OR: 0.76; 95% CI: 0.67, 0.87; P < 0.001; ORT3vs.T1: 0.31; 95% CI: 0.16, 0.58; P-trend < 0.001). These values hardly changed after adjustment for vascular variables | Cohort, age, sex, ethnicity, education, APOE genotype, caloric intake, smoking, comorbidity index, BMI, history of stroke, diabetes mellitus, hypertension, heart disease, total cholesterol, high-density lipoprotein, triglycerides, and low-density lipoprotein |
| Hosking, Eramudugolla, Cherbuin, & Anstey (2019) (75), Australia, the Personality and Total Health (PATH) through Life Study | Longitudinal | n = 1220 (50% men) age: 60–64 y older Australian adults | 12 | CSIRO-FFQ, MeDi, A-MeDi, and MIND scores, dietary components | Cognitive impairment: MCI/dementia (Winbald criteria, NINCDS-ADRDA criteria) | Higher tertile of MeDi score was not significantly associated with cognitive impairment (ORT3vs.T1: 1.30; 95% CI: 0.79, 2.15; P-trend = 0.29). Higher tertile of A-MeDi score was also not significantly associated with cognitive impairment (ORT3vs.T1: 0.77; 95% CI: 0.43, 1.39; P-trend = 0.40 | Energy intake, age, sex, APOE ε4 allele, education, mental activity, physical activity, smoking status, depression, diabetes, BMI, hypertension, heart disease, and stroke |
| Bhushan et al. (2018) (64), US HPFS | Longitudinal | n = 27,842 (100% men) age: 51 y male health professionals from the US | ±26 | FFQ, MeDi score, dietary components | Subjective cognitive function | Higher quintile of MeDi score was associated with a lower risk of both poor subjective cognitive function (ORQ5vs.Q1: 0.64; 95% CI: 0.55, 0.75; P-trend < 0.001) and moderate subjective cognitive function (ORQ5vs.Q1: 0.76; 95% CI: 0.70, 0.83; P-trend < 0.001) compared with good subjective cognitive function | Age, smoking history, diabetes, hypertension, depression, hypercholesterolemia, physical activity level, and BMI |
| Shakersain et al. (2018) (79), Sweden SNAC-K | Longitudinal | n = 2223 (39% men) age: 69.5 y community residents from Kungsholmen ≥60 y | 6 | 98-item SFFQ, A-MeDi, A-DASH and MIND scores, dietary components | Global cognition (MMSE) | Higher A-MeDi score was significantly associated with less cognitive decline (β: 0.006; 95% CI: 0.002, 0.009; P = 0.002; βT3vs.T1: 0.099; 95% CI: 0.036, 0.163; P = 0.002). A-MeDi score was not significantly associated with a lower risk of MMSE score ≤ 24 (P = 0.204; PT3vs.T1 = 0.263) | Total caloric intake, age, sex, education, civil status, physical activity, smoking, BMI, vitamin/mineral supplement intake, vascular disorders, diabetes, cancer, depression, APOE ε4, and dietary components other than those included in each dietary index |
| Tanaka et al. (2018) (43), Italy Invecchiare in Chianti (InCHIANTI) | Longitudinal | n = 832 age: 75.4 y (44% men) Older adults from the Chianti region in Italy | 10.1 | FFQ, MeDi score, dietary components | Global cognition (MMSE) | Continuous MeDi score was significantly associated with a lower risk of cognitive decline of 5 units in MMSE (HR: 0.89; 95% CI: 0.81, 0.97; P = 0.007). Higher tertile of MeDi score was also significantly associated with a lower risk of cognitive decline of 5 units in MMSE (HRT3vs.T1: 0.62; 95% CI: 0.43, 0.91; P = 0.015) | Age, sex, study site, chronic diseases, years of education, total energy intake, physical activity, BMI, APOE ε4 allele, CRP, and IL-6 |
| Haring et al. (2016) (52), US WHIMS | Longitudinal | n = 6425 (0% men) age: 65–79 y postmenopausal elderly women | 9.11 | Women's Health Initiative (WHI)-FFQ, MeDi score, and DASH score | MCI (MMSE and battery of neuropsychological tests [animal category, BNT, word list memory task, copying and recalling 4 line drawings, TMT]) | A-MeDi score quintile was not significantly associated with reduced risk of MCI (P-trend = 0.08). In a subset of white women with further adjustment for APOE ε4 allele quintile of A-MeDi score was significantly associated with a lower risk of MCI (HRQ5vs.Q1: 0.67; 95% CI: 0.45, 1.00; P-trend = 0.01) | Age, race, education level, WHI hormone trial randomization assignment, baseline 3MS level, smoking status, physical activity, diabetes, hypertension, BMI, family income, depression, history of CVD, and total energy intake |
| Galbete et al. (2015) (34), Spain Sun Project | Longitudinal | n = 823 (71% men) age: 61.9 y Spanish university graduates >55 y | 6–8 | 136-item SFFQ, MeDi score, dietary components | Cognitive function (TICS) | Lower tertile of MeDi score was significantly associated with faster cognitive decline (mean difference(T1+T2)vs.T3: −0.56; 95% CI: −0.99, −0.13; P = 0.011; mean differenceT1vs.T3: −0.43; 95% CI: −0.92, 0.05; P = 0.08; mean differenceT2vs.T3: −0.62; 95% CI: −1.07, −0.18; P = 0.006) | Age, sex, APOE genotype, follow-up time, total energy intake, BMI, smoking status, physical activity, diabetes, hypertension, hypercholesterolemia, history of CVD, and years of university education |
| Koyama et al. (2015) (65), US Health, Aging and Body Composition study | Longitudinal | n = 2326 (49% men) age: 70–79 y African-American and white elderly | 7.9 | 108-item block FFQ via interviews, A-MeDi score (race-specific) | Global cognition (3MS score) | Among African American, but not among whites, A-MeDi score was significantly associated with less cognitive decline (mean differenceAFRICAN-AMERICANS: 0.22; 95% CI: 0.05, 0.39; P = 0.01; PWHITES = 0.14) | Age, sex, education, BMI, current smoking, physical activity, depression, diabetes, total energy intake, and socio-economic status |
| Morris et al. (2015) (53), US Rush MAP | Longitudinal | n = 923 (± 24% men) age: 58–98 y people living in retirement communities or senior public housing units in Chicago | 4.5 | 144-item SFFQ, A-MeDi, A-DASH, and MIND scores | AD (based on NINCDS-ADRDA criteria) | Highest tertile of A-MeDi adherence was significantly associated with lower risk of AD (HRT3vsT1: 0.46; 95% CI: 0.27, 0.79; P-trend = 0.006) | Age, sex, education, APOE ε4, participation in cognitively stimulating activities, physical activity, total energy intake, and cardiovascular conditions |
| Olsson et al. (2015) (80), Sweden ULSAM | Longitudinal | n = 1038 (100% men) age: 70 y men from the municipality of Uppsala | 12 | 7-d food diary, adapted MeDi score | AD (based on NINCDS-ADRDA and DSM-IV criteria), dementia, and cognitive impairment (MMSE) | Continuous MeDi score was not associated with a lower risk of AD, dementia, or cognitive impairment. Higher tertile of MeDi score was also not associated with AD (P-trend = 0.95); dementia (P-trend = 0.70), or cognitive impairment (P-trend = 0.41). In participants with energy intake according to the Goldberg cut-off, highest tertile of MeDi score was significantly associated with a lower risk of cognitive impairment (ORT3vs.T1: 0.32; 95% CI: 0.11, 0.89) | Energy, education, APOE ε4 allele, living alone, smoking, and physical activity |
| Qin et al. (2015) (83), China, China Health and Nutrition Survey | Longitudinal | n = 1650 (±50% men) age ≥55 y Chinese community dwellers | 5.3 | 3-d 24-h recall, adapted MeDi score, dietary components | Decline in global cognition, composite z-scores and verbal memory (modified TICS) | Higher MeDi score was, only in participants ≥ 65 y, significantly associated with slower rate of decline in global cognitive scores (β: 0.10; 95% CI: 0.01, 0.18), composite z-scores (β: 0.014; 95% CI: 0.001, 0.027), and verbal memory scores (β 0.016; 95% CI: 0.001, 0.030). Higher tertile of MeDi score was significantly associated with less decline of global cognitive scores (βT3vs.T1: 0.28; 95% CI: 0.02, 0.54), z-scores (βT3vs.T1: 0.042; 95% CI: 0.002, 0.081) and verbal memory scores (βT3vs.T1: 0.047; 95% CI: 0.003, 0.091) only in participants ≥ 65 y | Age, gender, education, region, urbanization index, annual household income per capita, total energy intake, physical activity, current smoking, time since baseline, BMI, hypertension, and time interactions with each covariate |
| Trichopoulou et al. (2015) (41), Greece EPIC-Greece | Longitudinal | n = 401 (36% men) age >65 y elderly EPIC participants from Athens or the Attica area | 6.6 | 150-item SFFQ, MeDi score, dietary components | Global cognition (MMSE) | MeDi score tertile was significantly associated with less mild cognitive decline (ORT3vs.T1: 0.46, 95% CI: 0.25, 0.87; P-trend = 0.012), substantial cognitive decline (ORT3vs.T1: 0.34; 95% CI: 0.13, 0.89; P-trend = 0.025). Results were even more striking in participants ≥ 75 y | Sex, age, years of education, BMI, physical activity, smoking status, diabetes, hypertension, cohabiting, and total energy intake |
| Tangney et al. (2014) (54), US MAP | Longitudinal | n = 826 (26% men) age: 81.5 y elderly living in Chicago retirement communities and subsidized housing, normal cognitive function | 4.1 | 144-item SFFQ, A-MeDi score, A-DASH score | Global cognition (composite score of 19 tests), episodic memory (logical memory, word list recall, world list recognition, EBS), semantic memory (verbal fluency from CERAD, BNT, 12-item reading test), working memory (DST forward and backward, DO), perceptual speed [SDMT, Number Comparison (NC), Stroop Neuropsychological Screening (SNS)], and visuospatial ability (JLO, SPM) | A-MeDi score was significantly associated with slower rate of change of global cognition (β: 0.002; P = 0.01), episodic memory (β: 0.003; P = 0.02), and semantic memory (β: 0.003; P = 0.02). A-MeDi score tertile was associated with slower rate of change of global cognition (βT3vs.T1: 0.034; P = 0.003), episodic memory (βT3vs.T1: 0.040; P = 0.007), semantic memory (βT3vs.T1: 0.033; P = : 0.01), and working memory (βT3vs.T1: 0.033; P = 0.01) | Total energy intake, age, sex, education, and cognitive activities |
| Gallucci et al. (2013) (44), Italy Treviso Longeva (TRELONG) study | Longitudinal | n = 309 (40% men) age: 79.1 y long-lived elderly from Northern Italy | 7 | FFQ, Mediterranean diet yes/no (based on cereal, fish, vegetables, and fruit intake) | Global cognition (MMSE) | Adherence to Mediterranean diet (yes vs. no) was not significantly associated with less cognitive decline (β: 0.205; P = 0.758) | — |
| Kesse-Guyot et al. (2013) (46), France Stroop Neuropsychological Screening (SU.VI.MAX ) | Longitudinal | n = 3083 (54% men) age: 52.0 middle-aged | 13 | 12 24-h recalls, MeDi score, MSDPS | Cognitive performance (episodic memory [RI-48 cued recall test], lexical-semantic memory [verbal fluency tasks], short-term memory [DST forward and backward], working memory [Forward Digit Span task (FDS), Backward Digit Span task (BDS)], mental flexibility [TMT]) | Higher tertile of MeDi score was only associated with working memory span (P-trend = 0.03), but not with global cognition (P-trend = 0.27), episodic memory (P-trend = 0.50), short-term memory (P-trend = 0.97), mental flexibility (P-trend = 0.77), or semantic memory (P-trendsemantic = 0.51; P-trendphonemic = 0.37). Higher tertile of MSDPS was significantly associated with semantic fluency on the phonemic fluency task (P-trend = 0.048), but not with semantic fluency on semantic fluency task (P-trend = 0.06), global cognition (P-trend = 0.12), episodic memory (P-trend = 0.94), short-term memory (P-trend = 0.67), working memory (P-trend = 0.49), or mental flexibility (P-trend = 0.55) | Age, sex, education, follow-up time, supplementation group during the trial phase, number of 24-h dietary records, total energy intake, BMI, occupational status, smoking status, physical activity, memory difficulties at baseline, depressive symptoms concomitant with the cognitive function assessment, and history of diabetes, hypertension, or CVD |
| Samieri, Okereke, Devore & Grodstein (2013) (66), US Nurses’ Health Study | Longitudinal | n = 16,058 (0% men) mean age: 74.3 y women from the Nurses’ Health Study ≥70 y | 6 | 116-item SFFQ, adapted MeDi score, dietary components | Global cognition (TICS and composite score of TICS, EBMT, CF, DST backward), and verbal memory (immediate and delayed recalls of the EBMT and TICS) | Long-term higher quintile of MeDi score was significantly associated with better performance on TICS (mean differenceQ5vs.Q1: 0.06; 95% CI: 0.01, 0.11; P-trend = 0.004), global cognition (mean differenceQ5vs.Q1: 0.05; 95% CI: 0.01, 0.08; P-trend = 0.002) and verbal memory (mean differenceQ5vs.Q1: 0.06; 95% CI: 0.03, 0.10; P-trend < 0.001) at older age. Quintile of average MeDi score was not significantly associated with change in TICS score (P-trend = 0.31), global cognition (P-trend = 0.84), or verbal memory (P-trend = 0.70) | Age, education, long-term physical activity and total energy intake, BMI, smoking, multivitamin use, and history of depression, diabetes, hypertension, hypercholesterolemia, or myocardial infarction |
| Samieri et al. (2013) (67), US Women's Health Study | Longitudinal | n = 6174 (0% men) age: 72 y subset of participants from the Women's Health study aged ≥65 y | 4 | 131-item SFFQ, adapted MeDi-score, dietary components | Global cognition (TICS, EBMT, CF) and verbal memory (EBMT, delayed recall of TICS 10-word list) | MeDi score quintile was not significantly associated with better average global cognition (P-trend = 0.63) or verbal memory (P-trend = 0.44), nor with change in global cognition (P-trend = 0.26) and verbal memory (P-trend = 0.40) | Treatment arm, age at initial cognitive testing, Caucasian race, high education, high income, energy intake, physical activity, BMI, smoking, diabetes, hypertension, hypercholesterolemia, hormone use, and depression |
| Titova et al. (2013) (81), Sweden A follow-up of PIVUS | Longitudinal | n = 194 (52% men) age: 70.1 y subset of PIVUS participants, cognitive assessment at 75 y | 5 | 7-d food diary, adapted MeDi score, dietary components | Global cognition (7MS), brain volume (3D T1-weighted MRI-scan) | After adjustment continuous MeDi score was not significantly associated with global cognitive function (P = 0.13). Continuous MeDi score was not associated with gray (P = 0.19) or white (P = 0.97) matter volume or total brain volume (P = 0.32) | Gender, energy intake, education, self-reported physical activity, low-density cholesterol, BMI, systolic blood pressure, and HOMA-IR |
| Tsivgoulis et al. (2013) (68), United States Reasons for Geographic and Racial Differences in Stroke (US REGARDS) | Longitudinal | n = 17,478 (43% men) age: 64.4 y oversampling African-American subjects and from the Stroke Belt region, ≥45 y | 4.0 | 98-item block FFQ, MeDi score | Cognitive impairment Six-item-Screener (SIS) | High MeDi adherence was significantly associated with lower risk of ICI (OR: 0.87; 95% CI: 0.76, 1.00; P = 0.0460) compared with low MeDi adherence. There was no interaction between race (P = 0.2928) or Stroke Belt region (P = 0.9978) and the association of MeDi adherence with risk of ICI. Higher tertile of MeDi score was also significantly associated with lower risk of ICI (P-trend = 0.0436) | Age, sex, race, region (Stroke Belt vs. other region), educational level, income, number of packs smoked per year, weekly exercise, diabetes mellitus, hypercholesterolemia, atrial fibrillation, history of heart disease, BMI, waist circumference, systolic and diastolic blood pressure, ACE-inhibitors/angiotensin receptor blockers, β-blockers, other antihypertensive medication, depressive symptoms, and self-reported health status |
| Wengreen et al. (2013) (69), US Cache County Study on Memory, Health, and Aging | Longitudinal | n = 3580 (± 43% men) age ≥65 y mainly non-Hispanic white | 10.6 | 142-item FFQ, MeDi score, DASH score | Global cognition (3MS) | Higher quintile of MeDi score was associated with better average cognition during follow-up (mean differenceQ5vs.Q1: 0.94; P-trend = 0.0022) but was not significantly associated with rate of change of cognitive function | Age, sex, education, BMI, frequency of moderate physical activity, multivitamin and mineral supplement use, history of drinking and smoking, and history of diabetes, heart attack, or stroke |
| Cherbuin & Anstey (2012) (76), Australia PATH through Life Study | Longitudinal | n = 1528 (± 49% men) age: 60–64 y elderly, random selection of residents of Canberra | 4 | 215-item FFQ, MeDi score, dietary components | MCI, cognitive decline, cognitive disorder (CDR), any-MCD (based on MMSE, CVLT, SDMT, PP, SRT) | Continuous MeDi score was not significantly associated with risk of MCI (OR: 1.41; 95% CI: 0.95, 2.10; P = 0.087), CDR 0.5 (OR: 1.18; 95% CI: 0.88, 1.57; P = 0.266), or any-MCD (OR: 1.20; 95% CI: 0.98, 1.47; P = 0.079) | Age, sex, education, APOE ε4 genotype, BMI, physical activity, stroke, diabetes, hypertension, and total energy intake |
| Gardener, et al. (2012) (77), Australia, Australian Imaging, Biomarkers and Lifestyle Study of Ageing | Longitudinal | n = 970 (42% men) age = 71.72 y HC, MCI, and AD Australian subjects ≥60 y | 1.5 | 74-item SFFQ (AD participants had assistance or validation), MeDi score | Global cognition (MMSE), episodic verbal memory (CVLT II), logical memory (WMS), and verbal executive function (D-KEFS) | Higher MeDi score was significantly associated with less change in global cognition (r: 0.098; P = 0.014), but not with change in episodic verbal memory (P = 0.472), logical memory (P = 0.779), or verbal executive function (P = 0.294) | — |
| Vercambre, Grodstein, Berr & Kang (2012) (70), US WACS | Longitudinal | n = 2504 (0% men) age ≥65 y female health professionals with CVD or ≥3 coronary risk factors | 5.4 | 116-item SFFQ, MeDi score | Global composite score, global cognition (TICS), verbal memory (TICS 10-word list, EBMT) and category fluency | MeDi score tertile was not associated with change in global composite score (P-trend = 0.88), global cognition (P-trend = 0.53), verbal memory (P-trend = 0.97), or category fluency (P-trend = 0.64) | Age, education, total energy intake, marital status, physical activity, use of multivitamin supplements, smoking status, BMI, postmenopausal hormone therapy use, aspirin use exceeding 10 d in the previous month, nonsteroidal anti-inflammatory drug use exceeding 10 d in the previous month, history of depression, cardiovascular profile at baseline, diabetes, hypertension, hyperlipidaemia, and randomization assignment for vitamin E, vitamin C, β-carotene, and folate |
| Tangney et al. (2011) (71), US Chicago Health and Aging Project | Longitudinal | n = 3790 (38% men) age: 75.4 y older residents, African Americans and whites | 7.6 | 139-item FFQ, A-MeDi score | Global cognition (immediate and delayed recall of the EBMT, MMSE, and SDMT) | Continuous A-MeDi score was significantly associated with reduced decline in global cognitive function (β: 0.0014; P = 0.0004). The continuous A-MeDi wine score (only wine and no other types of alcohol were taken into account) was also significantly reduced decline in global cognitive function (β: 0.0014; P = 0.0009) | Age, sex, race, education, participation in cognitive activities, total energy intake, and the interaction between time and each dietary quality score |
| Gu, Luchsinger, Stern & Scarmeas (2010) (55), US WHICAP II | Longitudinal | n = 1219 (33% men) age: 76.7 y elderly from northern Manhattan | 3.8 | 61-item SFFQ, MeDi score | AD (DSM-III, NINCDS-ADRDA criteria) | Higher continuous MeDi score was associated with lower risk of AD (HR: 0.87; 95% CI: 0.78, 0.97; P = 0.01). Higher tertile of MeDi score was borderline significantly associated with a lower risk of AD (HRT3vs.T1: 0.68; 95% CI: 0.42, 1.08; P-trend = 0.06) | Age, gender, education, race, hsCRP, fasting insulin, and adiponectin concentrations |
| Roberts et al. (2010) (56), US | Longitudinal | n = 1233 (52% men) age: 70–89 y random selection of elderly residents of Olmsted County | 2.2 | 128-item block FFQ, MeDi score, dietary components | MCI (CDR, neurological evaluation [STMS, HS, LMII, VRII, AVLT, TMT, DSST, BNT, CF, PC, BD]) | Higher MeDi score tertile was not significantly associated with reduced risk of MCI during follow-up (HRT3vs.T1: 0.75; 95% CI: 0.46, 1.21; P = 0.24) | Age, years of education, total caloric intake, sex, stroke, APOE ε4 allele status, coronary artery disease, and depressive symptoms |
| Féart, et al. (2009) (47), France, Three-City cohort | Longitudinal | n = 1410 (± 37% men) age: 75.9 y noninstitutionalized elderly community dwellers from Bordeaux | 4.1 | FFQ, 24-h recall, MeDi score | Global cognition (MMSE), semantic verbal fluency (IST), visual memory (BVRT), and verbal memory FCSRT), and dementia and AD (examination by neurologist and DSM-IV) | Higher MeDi score was only significantly associated with less change in global cognition (β: −0.006; 95% CI: −0.01, −0.0003; P = 0.04), but not with change in semantic verbal fluency (P = 0.32), visual memory (P = 0.90) or verbal memory (P = 0.08). Highest MeDi tertile was not significantly associated with less change in performance on any cognitive test compared with lowest MeDi tertile (PMMSE = 0.06; PIST = 0.49; PBVRT = 0.50; PFCSRT = 0.06). However, in individuals who did not develop dementia during follow-up MeDi score and MeDi tertile were significantly associated with less decline in global cognition (β: −0.006; 95% CI: −0.011, −0.007; P = 0.03; βT3vs.T1: −0.03; 95% CI: −0.05, −0.001; P = 0.04) and verbal memory (β: 0.05; 95% CI: 0.005, 0.010; P = 0.03; βT3vs.T1: 0.21; 95% CI: 0.008, 0.41; P = 0.04). MeDi-score and MeDi tertile were not significantly associated with risk of incident dementia (P = 0.43; PT3vs.T1 = 0.72) or AD (P = 0.96; PT3vs.T1 = 0.71) | Age, sex, education, marital status, total energy intake, practice of physical exercise, taking 5 medications or more, Center for Epidemiological Studies-Depression Scale score, APOE genotype, BMI, hypertension, hypercholesterolemia, diabetes, tobacco use, stroke, and their interaction with time |
| Scarmeas et al. (2009) (59), US WHICAP | Longitudinal | n = 1875 (32% men) age: 76.9 y 2 cohorts, elderly without dementia, northern Manhattan | Normal cognitive subjects: 4.5; MCI subjects: 4.3 | 61-item SFFQ, MeDi score | MCI, MCI with memory impairment, MCI without memory impairment, AD (DSM-III, NINCDS-ADRDA criteria) | Higher MeDi score was significantly associated with a lower risk of MCI (HR: 0.92; 95% CI: 0.85, 0.99; P = 0.04). Higher MeDi score tertile was almost significantly associated with a lower risk of MCI (HR: 0.72; 95% CI: 0.52, 1.00; P-trend = 0.05), not significantly associated with a lower risk of MCI with memory impairment (P = 0.18), nor with a lower risk of MCI without memory impairment (P = 0.13). Higher MeDi score tertile in MCI was significantly associated with a lower risk of AD (HR: 0.52; 95% CI: 0.30, 0.91; P-trend = 0.02), but continuous MeDi score was not (P = 0.09). Higher MeDi score tertile was significantly associated with a lower risk of AD in participants with MCI without memory impairment (HR-trend: 0.50; 95% CI: 0.35, 0.79; P = 0.003), but not in participants with MCI with memory impairment (P = 0.45) | Cohort, age, sex, ethnicity, education, APOE genotype, caloric intake, BMI, and time between the first dietary assessment and the first cognitive assessment |
| Scarmeas et al. (2009) (60), US WHICAP | Longitudinal | n = 1880 (31% men) age: 77.2 y 2 cohorts, elderly without dementia, northern Manhattan | 5.4 | 61-item SFFQ, MeDi score | AD (DSMIII, NINCDS-ADRDA criteria) | Higher tertile of MeDi score was independent from physical activity, significantly associated with reduced risk of AD (HRT3vsT1: 0.60; 95% CI: 0.42, 0.87; HRtrend: 0.79; 95% CI: 0.66, 0.94; P = 0.008) | Cohort, age, sex, ethnicity, education, APOE ε4 allele, caloric intake, BMI, smoking, depression, leisure activities, comorbidity index, baseline clinical dementia rating score, and time between first dietary and first physical activity assessment |
| Psaltopoulou et al. (2008) (42), Greece EPIC-Greece | Longitudinal | n = 732 (35% men) age ≥60 y men and women ≥60 y from Attica | 8.0 | 150-item FFQ, MeDi score, dietary components | Global cognition (MMSE) | Continuous MeDi score was not significantly associated with global cognition after follow-up (P = 0.485) | Gender, age, marital status, years of education, height, BMI, physical activity, smoking, alcohol intake, hypertension, diabetes, geriatric depression score, and energy intake |
| Scarmeas, Luchsinger, Mayeux & Stern (2007) (63), US prospective study of aging and dementia | Longitudinal | n = 192 (22% men) age: 82.9 y community-based participants with AD from New York >65 y | 4.4 | 61-item SFFQ, MeDi score | Mortality in AD | Continuous MeDi score was significantly associated with reduced risk of mortality (HR: 0.76; 95% CI: 0.65, 0.89; P = 0.001). MeDi score tertile was also significantly associated with reduced risk of mortality (HRT3vsT1: 0.27; 95% CI: 0.10, 0.69; P-trend = 0.003) | Period of recruitment, age, gender, ethnicity, education, APOE genotype, caloric intake, smoking, and BMI |
| Scarmeas, Stern, Tang, Mayeux & Luchsinger (2006) (62), US WHICAP | Longitudinal | n = 2258 (32% men) age: 77.2 y 2 cohorts, elderly without dementia, northern Manhattan | 4.0 | 61-item SFFQ, MeDi score | AD (DSMIII, NINCDS-ADRDA criteria) | Higher continuous MeDi score and tertile of MeDi score were significantly associated with lower risk of AD (HR: 0.91; 95% CI: 0.83, 0.98; P = 0.015; HRT3vs.T1: 0.60; 95% CI: 0.42, 0.87; P-trend = 0.007) | Cohort, age, sex, ethnicity, education, APOE genotype, caloric intake, smoking, comorbidity index, and BMI |
AD, Alzheimer's disease; ADL, activities of daily living; A-MeDi, alternate Mediterranean diet; ANT, animal naming test; any-MCD, any mild cognitive disorder; ASR, anomalous sentence repetition; AVLT, auditory verbal learning test; BD, block design; BDS, Backward Digit Span task; BJLO, Benton's Judgement of Line Orientation; BNT, Boston Naming Test; BPRHS, Boston Puerto Rican Health Study; BVRT, Benton Visual Retention Test; CDR, clinical dementia rating; CDT, clock-drawing test; CF, category fluency; CFT, complex figure test; CFQ, cognitive failures questionnaire; CIMS, complex ideational material subtest; COVA, controlled oral word association test; CSI-D, community screening instrument for dementia; CSIRO, Commonwealth Scientific and Industrial Research Organisation; CVD, cardiovascular disease; CVLT, California Verbal Learning Test; DASH, Dietary Approaches to Stop Hypertension; D-KEFS, Delis-Kaplan Executive Function System Verbal Fluency; DO, digit ordering; DS, digit symbol; DST, digit span test; DSM, diagnostic and statistical manual of mental disorders; DSST, digit symbol substitution test; EBMT, East Boston Memory Test; EBS, East Boston Story; EPIC, European Prospective Investigation into Cancer and Nutrition; FCSRT, Free and Cued Selective Reminding Test; FDS, Forward Digit Span task; GDS, geriatric depression scale; GST, graphical sequence test; GVLT, Greek Verbal Learning Test; HC, healthy control; HELIAD, Hellenic Longitudinal Investigation Of Ageing And Diet; HPFS, Health Professionals Follow-up Study; HS, Hachinski Scale; HVLT-R, Hopkins Verbal Learning Test-Revised; ICI, incident cognitive impairment; InCHIANTI, Invecchiare in Chianti; IST, Isaacs Set Test; IT, inspection time; JLO, judgment of line orientation; LBC 1936, Lothian Birth Cohort 1936; LM, logical memory; LNS, letter-number sequencing; MABAT ZANAV, Israeli National Health and Nutrition Survey of Older Adults; MAP, Memory and Aging Project; MCI, mild cognitive impairment; MEDAS, Mediterranean Diet Adherence Screener; MeDi, Mediterranean Diet; MHT, Moray House Test; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; MMSE, Mini-Mental State Examination; MP, motor programming; MR, matrix reasoning; MSDPS, Mediterranean-Style Dietary Pattern Score; NART, National Adult Reading Test; NC, number comparison; NHANES, National Health and Nutrition Examination Survey; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association; PASE, physical activity scale for the elderly; PATH, Personality and Total Health; PC, picture completion; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; PP, Purdue Pegboard; PRM, pattern recognition memory test; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SCRT, simple and choice reaction time; SDMT, symbol digit modality test; SFFQ, semi-quantitative FFQ; SIS, six-item screener; SNAC-K = Swedish National study on Aging and Care in Kungsholmen; SNS, Stroop Neurophysiological Screening; SOC, Stockings of Cambridge test; SPM, standard progressive matrices; SRT, simple reaction time; SS, symbol search; SSP, spatial span test; ST, Stroop Test; STMS, short test of mental status; SU.VI.MAX, Supplementation With Vitamins And Mineral Antioxidants; SWM, spatial working memory test; TICS, Telephone Interview For Cognitive Status; TMT, trail making test; ULSAM, Uppsala Longitudinal Study of Adult Men; VPA, verbal paired associates; VR, visual reproduction; WACS, Women's Antioxidant Cardiovascular Study; WAIS, Wechsler Adult Intelligence Scale; WHI, Women's Health Initiative; WHICAP, Washington Heights-Inwood Columbia Aging Project; WHIMS, Women's Health Initiative Memory Study; WMS, Wechsler Memory Scale; WTA, Wechsler Test of Adult Reading; 3MS, modified mini-mental state; 7MS, 7-minute screen.
TABLE 6.
Characteristics of the included observational human studies on the dietary components of the Mediterranean, DASH, and MIND diets in relation to cognitive decline, dementia, and AD1
| Authors, year, study name | Study design | Population | Follow- up (y) | Exposure | Outcome | Results | Covariates |
|---|---|---|---|---|---|---|---|
| Anastasiou et al. (2017) (39), Greece HELIAD | Cross-sectional | n = 1864 (41% men) age: 73.0 y elderly >64 y from 2 cities in Greece, random selection from municipality records | — | SFFQ, A-MeDi score, dietary components | Cognitive status (dementia [DSM-IV, NINCDS/ADRDA criteria]), cognitive performance (memory [GVLT, CFT]), language (BNT, CIMS, categories: objects and the letter A], executive functioning [TMT, verbal fluency, ASR, GST, MP, months forwards and backwards], and visuospatial perception [JLO, CDT, CFT, TMT]) | Fish consumption was significantly associated with lower risk of dementia (OR: 0.311; 95% CI: 0.147, 0.658, P = 0.002) and consumption of nonrefined cereals was significantly associated with cognitive performance (β: 0.059; P = 0.004). No significant associations for potatoes, fruits, vegetables, legumes, olive oil, red meats, poultry, full-fat dairy, and alcohol with cognitive status or performance | Age, sex, education, number of clinical comorbidities, and energy intake |
| Bajerska, Woźniewicz, Suwalska & Jeszka (2014) (84), Poland | Cross-sectional | n = 87 (35% men) age: 70.0 y elderly >60 y from rural areas of Wielkopolska from a community with high risk of metabolic syndrome | — | FFQ, A-MeDi score (high vs. low), dietary components | MCI, global cognition (MMSE), attention (TMT), visual memory (PRM), executive function (ST, SOC, SWM, SSP) | Consumption of fish, vegetables, and olive or rapeseed oil was positively associated with attention (β: −1.97; P = 0.05), visual memory (β: 0.09; P = 0.01), and executive function, (βSTROOP: −0.33; P = 0.05; βSSP: 0.06; P = 0.05), respectively. Consumption of full-fat dairy products was negatively associated with executive function (βSWM: 0.02; P = 0.05). Consumption of red meat and meat products was negatively associated with executive function (βSOC: −0.02; P = 0.01) and global cognition (β: −0.02; P = 0.01). No significant associations for unrefined cereals, fruit, legumes, potatoes, poultry, and alcohol with global cognition, attention, visual memory, or executive function | Gender, age, education level, smoking status, family status, leisure time physical activity, and existence of metabolic syndrome |
| Crichton, Bryan, Hodgson & Murphy (2013) (74), Australia | Cross-sectional | n = 1183 (36% men) age: 50.6 y adults from 40 to 65 y from Australia | — | 215-item FFQ, adapted MeDi score, dietary components | Self-reported cognitive function (CFQ) on mistakes in tasks on perception, memory, and motor function | Intake of plant food, fish, red meat, cereals, dairy, and poultry was not significantly associated with CFQ | Age, gender, education, BMI, exercise, smoking, and total energy intake |
| Valls-Pedret et al. (2012) (38), Spain, PREDIMED | Cross-sectional | n = 447 (48% men) age: 66.9 y community dwelling people ≥55 y at high risk of CVD | — | 137-item FFQ, intake of many dietary components | Global cognition (MMSE), immediate and delayed episodic verbal memory (RAVLT) and immediate and working memory (DST) | Wine intake significantly associated with better global cognition (β: 0.252; P = 0.044). Total olive oil intake significantly associated with better immediate episodic verbal memory (β: 0.755, P = 0.014), whereas cereal intake was significantly associated with worse immediate episodic verbal memory (β: −0.431; P = 0.032). Intake of virgin olive oil, total olive oil, and coffee were significantly associated with better delayed episodic verbal memory (βVOO: 0.136, P = 0.037; P-valueOO: 0.001; βcoffee: 0.294, P = 0.016), whereas meat and cereal intake were significantly associated with worse delayed episodic verbal memory (βmeat: −0.845, P = 0.020; βcereal: −0.235, P = 0.001). Walnut intake was significantly associated with better working memory (β: 1.191; P = 0.039). Total urinary polyphenol excretion was significantly associated with better immediate episodic verbal memory (β: 1.208, P = 0.015), but not with delayed episodic verbal memory (β: 0.357; P = 0.053). Higher quintile of polyphenol excretion was significantly associated with immediate and delayed episodic verbal memory (P-trend = 0.018 and P-trend = 0.003, respectively). No significant associations for vegetables, legumes, fruits, total nuts, fish, dairy products, and total alcohol | Gender, age, education, BMI, smoking, APOE ε4 allele, energy expenditure in physical activity, diabetes, hypertension, and hyperlipidaemia |
| Roberts et al. (2010) (56), US | Cross-sectional | n = 1233 (52% men) age: 70–89 y random selection of residents of Olmsted County | — | 128-item block FFQ, MeDi score, dietary components | MCI (CDR and neurological evaluation [STMS, HS, LMII, VRII, AVLT, TMT, DSST, BNT, CF, PC, BD]), a-MCI or na-MCI | Vegetable intake was significantly associated with lower risk of MCI (ORT3vs.T1: 0.66; 95% CI: 0.44, 0.99; P = 0.05; P-trend = 0.02). (MUFA + PUFA):SFA ratio was significantly associated with lower risk of MCI (ORT3vs.T1: 0.52; 95% CI: 0.33, 0.81; P = 0.004; P-trend = 0.007). When split into a-MCI and na-MCI only (MUFA + PUFA):SFA ratio was significantly associated with lower risk of a-MCI (ORT3vs.T1: 0.49; 95% CI: 0.30, 0.80; P = 0.004; P-trend = 0.01) after adjustment and MUFA:SFA ratio was significantly associated with increased risk of na-MCI (ORT3vs.T1: 1.70; 95% CI: 0.71, 4.08; P = 0.23; P-trend = 0.04). No significant association for legumes, fruits, dairy, grains and cereals, meat, fish, and alcohol with risk of MCI, a-MCI, or na-MCI | Age, years of education, total caloric intake, sex, stroke, APOE ε4 allele status, coronary artery disease, and depressive symptoms |
| Hosking, Eramudugolla, Cherbuin, & Anstey (2019) (75), Australia PATH Through Life Study | Longitudinal | n = 1220 (50% men) age: 60–64 y older Australian adults | 12 | CSIRO-FFQ, MeDi, A-MeDi, and MIND scores, dietary components | Cognitive impairment: MCI/dementia (Winbald criteria, NINCDS-ADRDA criteria) | Nut consumption was significantly associated with a lower risk of MCI/dementia (OR: 0.42; 95% CI: 0.21, 0.85; P = 0.016). No significant association for processed and fast fried food, sweets and pastries, green leafy vegetables, other vegetables, berries, fish, cheese, grains, beans, poultry, meat, or wine | Energy intake, age, sex, APOE ε4 allele, education, mental activity, physical activity, smoking status, depression, diabetes, BMI, hypertension, heart disease, and stroke |
| Bhushan et al. (2018) (64), US HPFS | Longitudinal | n = 27,842 (100% men) age: 51 y male health professionals from the US | ±26 | FFQ, MeDi score, dietary components | Subjective cognitive function | Higher quintile of intake of vegetables (β: −0.033; P < 0.001), fruits and nuts (β:–0.016; P = 0.005), or fish (β: −0.024; P < 0.001) was significantly associated with better subjective cognitive function. No significant association for legumes, cereals, red meat and meat products, MUFA:SFA ratio, milk and dairy products, or alcohol | Age, smoking history, diabetes, hypertension, depression, hypercholesterolemia, physical activity level, and BMI |
| Shakersain et al. (2018) (79), Sweden SNAC-K | Longitudinal | n = 2223 (39% men) age: 69.5 y community residents from Kungsholmen ≥60 y | 6 | 98-item SFFQ, A-MeDi, A-DASH, and MIND scores, dietary components | Global cognition (MMSE) | Intake of poultry, fish, vegetable oil, wine (red and white), tea, and water was significantly associated with slower cognitive decline, whereas intake of grains (refined grains), dairy products (high-fat dairy products), milk (high-fat milk), butter (margarine), sugar and fruit juice was significantly associated with faster cognitive decline during follow-up. No significant association for vegetables, fruits, legumes, red and processed meat, ice cream, beer, spirits, and carbonated drinks | Total caloric intake, age, sex, education, civil status, physical activity, smoking, BMI, vitamin/mineral supplement intake, vascular disorders, diabetes, cancer, depression APOE ε4, and dietary components other than main exposure in each model |
| Tanaka et al. (2018) (43), Italy InCHIANTI | Longitudinal | n = 832 age: 75.4 y (44% men) older adults from the Chianti region in Italy | 10.1 | FFQ, MeDi score, dietary components | Global cognition (MMSE) | No significant associations for intake of vegetables, legumes, fish, fruits and nuts, cereal, MUFA:SFA ratio, dairy, meat, or alcohol with cognitive decline | Age, sex, study site, chronic diseases, years of education, total energy intake, physical activity, BMI, APOE ε4 allele, C-reactive protein (CRP), and IL-6 |
| Galbete et al. (2015) (34), Spain Sun Project | Longitudinal | n = 823 (71% men) age: 61.9 y Spanish university graduates >55 y | 6–8 | 136-item SFFQ, MeDi score, dietary components | Cognitive function (TICS) | Intake of olive oil and MUFA:SFA ratio above median was significantly associated with less cognitive decline than intake below median (mean differenceOO: −0.37; 95% CI: −0.68, −0.06; P = 0.020; mean differenceMUFA:SFA: −0.53; 95% CI: −0.84, −0.22; P = 0.001). No significant association for fruits and nuts, vegetables, cereals, legumes, fish, meat and meat products, dairy, and alcohol with cognitive decline | Age, sex, APOE genotype, TICS score at final cognitive evaluation, follow-up time between baseline and second cognitive evaluation, total energy intake, BMI, smoking status, physical activity, diabetes, hypertension, hypercholesterolemia, history of CVD, years of university education, and all other items in the MeDi score |
| Qin et al. (2015) (83), China China Health and Nutrition Survey | Longitudinal | n = 1650 (± 50% men) age ≥55 y elder Chinese community dwellers | 5.3 | 3-d 24-h recall, adapted MeDI score, dietary components | Global cognition, composite z-scores and verbal memory (modified TICS) | Fish consumption was, only in participants ≥65 y, associated with slower cognitive decline (mean difference: 0.34; 95% CI: 0.11, 0.56) and animal-source cooking fat was associated with faster cognitive decline (mean difference: −0.31; 95% CI: −0.55, −0.07) compared wiyh no consumption. No significant association for vegetables, legumes and nuts, fruits, fiber-rich grains, dairy products, alcohol, and red meat and processed meat with cognitive decline | Age, gender, education, region, urbanization index, annual household income per capita, total energy intake, physical activity, current smoking, time since baseline, BMI, hypertension, and time interactions with each covariate |
| Trichopoulou et al. (2015) (41), Greece EPIC-Greece | Longitudinal | n = 401 (36% men) age >65 y elderly EPIC participants from Athens or the Attica area | 6.6 | 150-item SFFQ, MeDi score, dietary components | Global cognition (MMSE) | Vegetable consumption was significantly associated with less substantial cognitive decline (OR: 0.39; 95% CI: 0.22, 0.69; P = 0.001), but not with mild cognitive decline (P = 0.244). No significant association for legumes, fruits and nuts, dairy products, cereals, meat, fish, alcohol, and MUFA:SFA with mild, or substantial cognitive decline | Sex, age, years of education, BMI, physical activity, smoking status, diabetes, hypertension, cohabiting, and total energy intake |
| Samieri, Okereke, Devore & Grodstein (2013) (66), US Nurses’ Health Study | Longitudinal | n = 16,058 (0% men) mean age: 74.3 y women from the Nurses’ Health Study ≥70 y | 6 | 116-item SFFQ, adapted MeDi score, dietary components | Global cognition (TICS and composite score of TICS, EBMT, CF, DST backward), and verbal memory (immediate and delayed recalls of the EBMT and TICS) | Vegetable intake was significantly associated with less decline in global cognition (mean differenceQ5vs.Q1: 0.011; 95% CI: 0.001, 0.020; P-trend = 0.04). MUFA:SFA ratio was significantly associated with less decline in global cognition (mean differenceQ5vs.Q1: 0.013; 95% CI: 0.005, 0.021; P-trend < 0.001) and verbal memory (mean differenceQ5vs.Q1: 0.014; 95% CI: 0.004, 0.024; P-trend = 0.001). No significant association for legumes, fruits, whole grains, nuts, fish, red and processed meat, and alcohol with change in global cognition or verbal memory | Age, education, long-term physical activity and energy intake, BMI, smoking, multivitamin use, and history of depression, diabetes, hypertension, hypercholesterolemia, or myocardial infarction |
| Samieri et al. (2013) (67), US Women's Health Study | Longitudinal | n = 6174 (0% men) age: 72 y subset of participants from the Women's Health study aged ≥65 y | 4 | 131-item SFFQ, adapted MeDi score, dietary components | Global cognition (TICS, EBMT, CF) and verbal memory (EBMT, delayed recall of TICS 10-word list) | Higher quintile of MUFA:SFA ratio was associated with slower decline of global cognition (mean differenceQ5vs.Q1: 0.07; 95% CI: 0.01, 0.12; P-trend = 0.03) and verbal memory (mean differenceQ5vs.Q1: 0.07; 95% CI: 0.01, 0.14; P-trend = 0.05). However, higher quintile of MUFA:SFA ratio was associated with worse average global cognition (mean differenceQ5vs.Q1: −0.06; 95% CI: −0.11, −0.02; P-trend = 0.002). Higher quintile of whole grain intake was significantly associated with better average global cognition (mean differenceQ5vs.Q1: 0.07; 95% CI: 0.02, 0.12; P-trend = 0.02). No significant association for fruits, vegetables, legumes, nuts, red and processed meats, and alcohol with decline in global cognition or verbal memory, nor with average global cognition or verbal memory | Treatment arm, age at initial cognitive testing, Caucasian race, high education, high income, energy intake, physical activity, BMI, smoking, diabetes, hypertension, hypercholesterolemia, hormone use, and depression |
| Titova et al. (2013) (81), Sweden A follow-up of PIVUS | Longitudinal | n = 194 (52% men) age: 70.1 y subset of PIVUS participants with cognitive assessment at the age of 75 y | 5 | 7-d food diary, adapted MeDi score, dietary components | Global cognition (7MS), brain volume (3D T1-weighted MRI-scan) | Consumption of meat and meat products was significantly associated with worse global cognitive function (β: −0.26; P < 0.001) and smaller total brain volume (β: −0.16; P = 0.04). No association for alcohol, milk and milk products, PUFA:SFA, vegetables and legumes, fruits, cereals and potatoes, and fish with global cognition, total brain volume, or volume of gray or white matter | Gender, energy intake, education, self-reported physical activity, low-density cholesterol, BMI, systolic blood pressure, and HOMA-IR |
| Wengreen et al. (2013) (69), US Cache County Study on Memory, Health and Aging | Longitudinal | n = 3580 (± 43% men) age ≥65 y mainly non-Hispanic white | 10.6 | 142-item FFQ, MeDi score, DASH score, dietary components | Global cognition (3MS) | Significant better average cognitive function during follow-up for higher quintile of intake of whole grain (mean differenceQ5vs.Q1: 1.19; P-trend = 0.0054), nuts and legumes (mean differenceQ5vs.Q1: 1.22; P-trend < 0.0001), and legumes only (mean differenceQ5vs.Q1: 1.16; P-trend < 0.0001). No significant association for intake of fruit, vegetables, red and processed meat, meat and meat products, low-fat dairy, sweetened beverages, sodium, all grains, fish, full-fat dairy and MUFA:SFA ratio | Age, sex, education, BMI, frequency of moderate physical activity, multivitamin and mineral supplement use, history of drinking and smoking, and history of diabetes, heart attack, or stroke |
| Cherbuin & Anstey (2012) (76), Australia PATH Through Life Study | Longitudinal | n = 1528 (± 49% men) age: 60–64 y random selection of residents of Canberra | 4 | 215-item FFQ, MeDi score, dietary components | MCI, cognitive decline, cognitive disorder (CDR), any-MCD (based on MMSE, CVLT, SDMT, PP, and SRT) | Fish intake was associated with higher risk of MCI (OR: 1.02; 95% CI: 1.00, 1.04; P = 0.048), CDR 0.5 (OR: 1.02; 95% CI: 1.00, 1.04; P = 0.027), and any-MCD (OR: 1.02; 95% CI: 1.00, 1.03; P = 0.012). MUFA intake was significantly associated with risk of MCI (OR: 5.60; 95% CI: 1.66, 18.76; P = 0.005) and CDR 0.5 (OR: 3.10; 95% CI: 1.07, 9.02; P = 0.037). Dairy consumption was significantly associated with risk of MCI (OR: 1.01; 95% CI: 1.00, 1.01; P = 0.030) and any-MCD (OR: 1.01; 95% CI: 1.00, 1.01; P = 0.027). Fruit intake and vegetable intake were associated with risk of MCI (OR: 1.01; 95% CI: 1.00, 1.01; P = 0.022 and OR: 1.01; 95% CI: 1.00, 1.02; P = 0.020, respectively). Cereal intake was significantly associated with higher risk of any-MCD (OR: 1.01; 95% CI: 1.00, 1.01; P = 0.027). No significant association for intake of SFA, meat, legumes, MUFA:SFA ratio, or alcohol | Age, sex, education, APOE ε4 genotype, BMI, physical activity, stroke, diabetes, hypertension, and total energy intake |
| Berr et al. (2009) (48), France Three-city study | Longitudinal | n = 6947 (40% men) age >65 y noninstitutionalized elderly from Bordeaux, Montpellier, and Dijon | ±4 | FFQ, olive oil intake (none, moderate, intensive) | Global cognition (MMSE, BVRT, IST) | Intensive use of olive oil, but not moderate use of olive oil, was significantly associated with reduced risk of decline in visual memory (ORT3vs.T1: 0.83; 95% CI: 0.69, 0.99; P = 0.04). Intensive use of olive oil was not associated with decline in verbal fluency after adjustment (P = 0.10), or with global cognition (P = 0.58). Results remained similar when data from participants with incident dementia during follow-up was removed | Age, sex, centre, education, income, baseline cognitive score, depressive symptoms, APOE ε4 allele, CVD, hypertension, diabetes, hypercholesterolemia, BMI, smoking status, and dietary intake of fruits/vegetables, ω-3 oil, fish, coffee, and alcohol |
| Psaltopoulou et al. (2008) (42), Greece EPIC-Greece | Longitudinal | n = 732 (35% men) age ≥60 y men and women ≥60 y from Attica | 8.0 | 150-item FFQ, MeDi score, dietary components | Global cognition (MMSE) | PUFA intake and seed oil intake were significantly associated with worse global cognition (βPUFA: −0.40; 95% CI: −0.68, −0.13; P = 0.004; βSEED: −0.34; 95% CI: −0.56, −0.12; P = 0.002). No significant association for SFA, MUFA, olive oil, and fish and seafood with global cognition after follow-up | Gender, age, marital status, years of education, height, BMI, physical activity, smoking, alcohol intake, hypertension, diabetes, geriatric depression score, and energy intake |
| Solfrizzi, et al. (2006) (45), Italy, Italian Longitudinal Study on Aging | Longitudinal | n = 278 (55% men) age: 73.01 y older, nondemented, free-living subjects | 8.5 | 77-item SFFQ, protein, carbohydrate, SFA, fiber, energy, fatty acids | Global cognition (MMSE) | High MUFA (β: −0.001; 95% CI: −0.002, −0.0009; P < 0.05), high PUFA (β: −0.006; 95% CI: −0.012, −0.0004; P < 0.05), and higher energy (β: −0.00001, P < 0.05) intake were significantly associated with lower decline in global cognitive function. No significant association for carbohydrates, fibers, SFA, UFA:SFA, and MUFA:SFA with decline in global cognition | Sex, age, education, Charlson comorbitidy index, BMI, MMSE baseline score, total energy intake, |
a-MCI, amnestic mild cognitive impairment; A-MeDi, alternate Mediterranean diet; any-MCD, any mild cognitive disorder; ASR, anomalous sentence repetition; AVLT, auditory verbal learning test; APOE ε4, apolipoprotein E; BD, block design; BNT, Boston Naming Test; BVRT, Benton Visual Retention Test; CDR, clinical dementia rating; CDT, clock-drawing test; CF, category fluency; CFQ, cognitive failures questionnaire; CFT, complex figure test; CIMS, complex ideational material subtest; CRP, C-reactive protein; CVD; cardiovascular disease; CVLT, California Verbal Learning Test; DASH, Dietary Approaches to Stop Hypertension; DSM, diagnostic and statistical manual of mental disorders; DSST, digit symbol substitution test; DST, digit span test; EBMT, East Boston Memory Test; EPIC, European Prospective Investigation into Cancer and Nutrition; GST, graphical sequence test; GVLT, Greek Verbal Learning Test; HELIAD, Hellenic Longitudinal Investigation of Ageing and Diet; HS, Hachinski Scale; InCHIANTI, Invecchiare in Chianti; IST, Isaacs Set Test; JLO, judgement of line orientation; LM, logical memory; MCI, mild cognitive impairment; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; MMSE, Mini-Mental State Examination; MP, motor programming; na-MCI, nonamnestic mild cognitive impairment; PATH, Personality and Total Health; PC, picture completion; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; PP, Purdue Pegboard; PREDIMED, Prevención con Dieta Mediterránea; PRM, pattern recognition memory test; RAVLT, Rey Auditory Verbal Learning; SDMT, symbol-digit modalities test; SFFQ, semi-quantitative FFQ; SNAC-K, Swedish National Study on Aging and Care in Kungsholmen; SOC, Stockings of Cambridge Test; SRT, simple reaction time; SSP, spatial span test; ST, Stroop Test; STMS, short test of mental status; SWM, spatial working memory test; TICS, Telephone Interview For Cognitive Status; TMT, trail making test; VR, visual reproduction; 3MS, modified mini-mental state; 7MS, 7-minute screen.
Mediterranean diet
Cross-sectional studies
The Mediterranean diet in relation to cognitive function, dementia, or AD was investigated in 12 cross-sectional studies and 1 case-control study (Table 2) (33, 39, 40, 49–51, 57, 61, 74, 82, 84–86). In 3 of these studies the Mediterranean diet was associated with better cognitive functioning, including Spanish (n = 79) (33), American (n = 5907) (57), and a combination of Israeli (n = 1786) and American (n = 2791) (51) participants. In addition, in 4 studies, including middle-aged and older Greek (n = 1864) (39), Polish (n = 87) (84), Scottish (n = 878) (86), and Puerto Rican (n = 1269) (85) participants, the Mediterranean diet was associated with better cognitive function in specific domains, including global cognition, memory, language, executive functioning, and verbal ability. Furthermore, in a study of elderly Greek participants (n = 557) each 1-unit increase in Mediterranean diet score was associated with a lower risk of cognitive impairment in men (OR: 0.88; 95% CI: 0.80, 0.98; P = 0.02), but an increased risk of cognitive impairment in women (OR: 1.11; 95% CI: 1.00, 1.22; P = 0.04) (40). Moreover, a study in US middle-aged participants (n = 116) observed an association between higher adherence to the Mediterranean diet and larger cortical thickness of the posterior cingulate cortex (β: 0.023; P = 0.004), whereas no association with cognitive function was observed (49). Adherence to the Mediterranean diet and cognitive function were not associated in the remaining 3 studies among Chinese (n = 3670) (82), Australian (n = 1183) (74), and US (n = 160) (50) participants.
With respect to dementia, 1 Greek cross-sectional study (n = 1864) investigated the association between the Mediterranean diet and dementia and showed an 8% lower risk of dementia (OR: 0.92; 95% CI: 0.87, 0.97; P = 0.004) for a 1-unit increase in Mediterranean diet score and a 56% lower dementia risk in the highest quartile of Mediterranean diet adherence (ORQ4vs.Q1: 0.440; 95% CI: 0.208, 0.969; P-trend = 0.019) (39). In a case-control study (n = 1984), each 1-unit increase in Mediterranean diet score was associated with a 24% lower risk of AD (OR: 0.76; 95% CI: 0.67, 0.87; P < 0.001); additionally, the highest tertile of Mediterranean diet adherence was associated with a 69% lower risk of AD (ORT3vs.T1: 0.31; 95% CI: 0.16, 0.58; P-trend < 0.001) (61).
Longitudinal studies
In total, 31 longitudinal studies investigated the Mediterranean diet in relation to cognitive decline, dementia, or AD (Table 2) (34, 41–44, 46, 47, 52–56, 59, 60, 62–71, 75–77, 79–81, 83). Higher adherence to the Mediterranean diet was associated with less cognitive decline after 4 to 26 y of follow-up in 7 of 23 longitudinal studies in American (n = 3790 ≤ 27,842) (64, 68, 71), Swedish (n = 2223) (79), Spanish (n = 823) (34), Italian (n = 832) (43), and Greek (n = 401) (41) participants. In 6 studies including American (n = 826 ≤ 16,058) (54, 66, 69), Australian (n = 970) (77), and French adults (n = 1410 and 3038) (46, 47), the Mediterranean diet was associated with less cognitive decline in specific cognitive domains after 1.5 to 13 y of follow-up. Participants from the Washington Heights-Inwood Columbia Aging Project (WHICAP) (n = 1880) showed an 8% lower risk of mild cognitive impairment (MCI) for each 1-unit increase in Mediterranean diet score (HR: 0.92; 95% CI: 0.85, 0.99; P = 0.04) after 4.5 y of follow-up (59). However, this association was not significant when studied by Mediterranean diet adherence in tertile (HRT3vs.T1: 0.72; 95% CI: 0.52, 1.00; P-trend = 0.05). Additionally, higher adherence to the Mediterranean diet was associated with less cognitive decline in African-American older adults (mean difference: 0.22; 95% CI: 0.05, 0.39; P = 0.01), but not in white American older adults (n = 2326) (65). Furthermore, stratified analysis suggested a beneficial association in Chinese participants (n = 1650) aged ≥65 y, but not in younger participants (83). Moreover, in Swedish older men (n = 1038) higher adherence to the Mediterranean diet was associated with a lower risk of cognitive impairment (ORT3vs.T1: 0.32; 95% CI: 0.11, 0.89) after 12 y of follow-up in a subpopulation of participants with energy intake according to the Goldberg cut-off only (80). In the other 8 studies including American (n = 1233 ≤ 6425) (52, 56, 67, 70), Italian (n = 309) (44), Greek (n = 732) (42), Australian (n = 1528) (76), and Swedish participants (n = 194) (81) the Mediterranean diet was not associated with cognitive decline after 2.2 y to ≤10.6 y of follow-up. The Mediterranean diet was not associated with total brain volume, gray matter volume, or white matter volume after 5 y of follow-up among Swedish older adults (n = 194) (81).
With respect to dementia, Mediterranean diet adherence was not associated with dementia among French (n = 1410) (47), Swedish (n = 1038) (80), and Australian (n = 1220) (75) older adults after 4.1 y to ≤12 y of follow-up. In 3 studies, 2 studies in participants from the WHICAP (n = 1880 and n = 1984) (60, 61) and 1 study in participants from the Memory and Aging Project (MAP) (n = 923) (53), a significantly lower risk of AD was shown with higher adherence to the Mediterranean diet after 3.8 y to ≤5.4 y of follow-up. In these studies a 40 to 54% lower risk of AD was shown for the highest tertile of Mediterranean diet adherence and a 9% lower risk of AD was found for each 1-unit increase in Mediterranean diet score. The WHICAP also demonstrated a lower AD risk in people with MCI for the highest tertile of adherence (HRT3vs.T1: 0.52; 95% CI: 0.30, 0.91; P-trend = 0.02) after 4.3 y of follow-up (n = 1875), but not when the Mediterranean diet score was analyzed per unit increase (59). Furthermore, in the WHICAP II (n = 1219) each 1-unit increase in Mediterranean diet score was associated with a 13% lower risk of AD (HR: 0.87; 95% CI: 0.78, 0.97; P = 0.01) after 3.8 y of follow-up (55). A borderline nonsignificant association was observed for the highest tertile of Mediterranean diet adherence (HRT3vs.T1: 0.68; 95% CI: 0.42, 1.08; P-trend = 0.06). Higher adherence to the Mediterranean diet was not associated with AD after 4.1 and 12 y of follow-up among French (n = 1410) (47) and Swedish (n = 1038) (80) older adults. Finally, each 1-unit increase in Mediterranean diet score was associated with a 24% lower risk of mortality from AD (HR: 0.76; 95% CI: 0.65, 0.89; P = 0.001) among American older adults (n = 192), where the upper Mediterranean diet adherence tertile was associated with a 73% lower AD risk (HRT3vs.T1: 0.27; 95% CI: 0.10, 0.69; P-trend = 0.003) after 4.4 y of follow-up (63).
Trial evidence
The effect of the Mediterranean diet on cognitive decline was reported in 5 articles, representing 3 randomized controlled trials (Table 3) (35–37, 78, 87). After a 6-mo intervention in Australian healthy elderly participants (n = 137) no significant difference between the Mediterranean diet group and the control group was observed for the cognitive domains executive function, memory, processing speed, or visual-spatial memory (78). The Prevención con dieta Mediterránea (PREDIMED) trial investigated the effect of the Mediterranean diet supplemented with extra-virgin olive oil or mixed nuts on cognitive decline and compared this with a low-fat control diet in Spanish adults at high vascular risk, which resulted in 3 articles based on 3 different population samples (35–37). Higher Mediterranean diet adherence with extra-virgin olive oil during 6.5 y was related to better cognitive performance (n = 522) (36) and a lower risk of MCI (n = 268) (37). Higher adherence to the Mediterranean diet with extra-virgin olive oil was also related to improved global cognition and frontal cognition, but had no effect on memory and MCI after 4.1 y of intervention (n = 334) (35). Evidence on the effect of the Mediterranean diet with nuts on cognition was mixed (35–37). Higher adherence to the Mediterranean diet with nuts did not affect the risk of MCI (35, 36). In 1 of the PREDIMED populations (n = 522) the Mediterranean diet with nuts improved global cognition (mean difference for Mini-Mental State Examination [MMSE]: 0.57; 95% CI: 0.11, 1.03, P = 0.015; mean difference for the clock-drawing test [CDT]: 0.33; 95% CI: 0.003, 0.67; P = 0.048) (36), whereas another PREDIMED population (n = 268) did not show an effect of the Mediterranean diet with nuts (37). In addition, another PREDIMED sample (n = 334) suggested that the Mediterranean diet with nuts particularly improved memory, but not frontal and global cognition (35). The third trial investigated the effect of the Mediterranean diet and a low-fat diet on cognitive performance compared with the waiting-list control diet in 155 participants from the UK with elevated cholesterol concentrations and showed an adverse effect of both the Mediterranean diet and the low-fat diet on attention after 12 wk of intervention (87). No effect of the Mediterranean diet on motor speed, memory, and choice reaction time was found compared with the control diet.
TABLE 3.
Characteristics of the included randomized controlled trials on the Mediterranean and DASH diets in relation to cognitive decline, dementia, and AD1
| Authors, year, study name | Population (sample size, mean age, kind of people) | Follow-up (y) | Exposure | Outcome | Results |
|---|---|---|---|---|---|
| MeDi diet | |||||
| Knight et al. (2016) (78), Australia MedLey | n = 137 (47% men) age: 72.1 healthy men and women >65 y | 0.5 | MeDi, control diet (habitual diet) | Cognitive performance executive function (ST, ILF, ELF, TOL), memory (RAVLT, DST forward and backward, LNS), processing speed (SS and coding core subtests WAIS IV), and visual-spatial memory (BVRT) | MeDi did not significantly change global cognition (P = 0.19), executive function (P = 0.33), memory (P = 0.50), processing speed (P = 0.15), or visual-spatial memory (P = 0.48) compared with the control diet |
| Valls-Pedret et al. (2015) (35), Spain The Prevencion con dieta Mediterranea (PREDIMED) | n = 334 (± 49% men) age: 66.9 y cognitively healthy men and women at high vascular risk ≥55 y | 4.1 | 144-item screener, MeDi with EVOO (1 L/wk), MeDi with mixed nuts (30 g/d), low-fat control | Global cognition (MMSE, RAVLT, ASF, DS subtest, VPA, CTT) divided into memory, frontal and global, MCI | Both RAVLT scores (total learning and delayed recall) significantly improved for all 3 dietary patterns. CTT part 1 significantly improved for MeDi with EVOO (mean change: −5.77; 95% CI: −11.25, −0.28) and CTT part 2 significantly worsened for MeDi with nuts (mean change: 24.23; 95% CI: 1.36, 47.10) and control diet (mean change: 37.56; 95% CI: 18.14, 56.97). Compared with the control, the MeDi with EVOO caused significantly more improvement in total learning RAVLT score (P = 0.04) and less decline in color trail test part 2 (P = 0.045). Frontal cognition significantly improved for MeDi with EVOO (mean change: 0.23; 95% CI: 0.03, 0.43). Compared with the control group, MeDi with EVOO significantly improved in global cognition (P = 0.005) and frontal cognition (P = 0.003) and MeDi with nuts significantly improved memory (P = 0.04). The rate of MCI did not significantly differ between the 3 groups (P = 0.28) |
| Martínez-Lapiscina et al. (2013) (37), Spain PREDIMED-NAVARRA | n = 268 (45% men) age: 74.1 y community dwelling participants at high vascular risk ≥55 y | 6.5 | 137-item FFQ, questionnaire, MeDi with EVOO, MeDi with mixed nuts, low-fat control | Global cognition (MMSE, CDT), cognitive episodic memory [VPA], verbal memory [RAVLT], visual memory [ROCF]), visual-spatial abilities (ROCF), language Boston Naming Test (BNT), ASF, FAS), executive function (attention + immediate memory + working memory [DST, TMT], abstract reasoning [similarities test]) | MeDi with EVOO significantly increased global cognition measured with MMSE, immediate memory, immediate and delayed visual memory, and phonemic fluency compared with control and significantly increased immediate and delayed visual memory and episodic memory compared with MeDi with nuts. MeDi with nuts did not significantly differ from control in cognitive performance. Compared with control, MeDi with EVOO was significantly associated with lower risk of MCI (OR: 0.341; 95% CI: 0.120, 0.969, P = 0.044), whereas MeDi with nuts was not (P = 0.226) |
| Martínez-Lapiscina et al. (2013) (36), Spain PREDIMED-NAVARRA | n = 522 (45% men) age: 74.6 y participants at high vascular risk ≥55 y | 6.5 | MeDi with EVOO, MeDi with mixed nuts, low-fat control | Global cognition (MMSE, CDT) | MeDi with EVOO and was significantly associated with better global cognitive performance compared with the low-fat control diet (mean differenceMMSE: 0.62; 95% CI: 0.18, 1.05; P = 0.005; mean differenceCDT: 0.51; 95% CI: 0.20, 0.82; P = 0.001). MeDi with nuts was also significantly associated with better global cognitive performance (mean differenceMMSE: 0.57, 95% CI: 0.11, 1.03, P = 0.015; mean differenceCDT: 0.33; 95% CI: 0.003, 0.67; P = 0.048) |
| Wardle et al. (2000) (87), UK | n = 155 (55% men) age: 53 y participants with elevated cholesterol concentrations | 0.2 | MeDi (high fruit, vegetables, and fish, low-fat, MUFA), low-fat diet, waiting-list control diet | Cognitive function (motor speed [tapping speed], memory [verbal immediate free recall], choice reaction time, and attention [sustained attention task]) | MeDi and low-fat diet decreased attention compared with control (P < 0.001). MeDi was not significantly associated with motor speed, memory, or choice reaction time compared with the control diet |
| DASH diet | |||||
| Smith et al. (2010) (58), US Exercise and Nutrition Interventions for Cardiovascular Health (ENCORE) | n = 124 (36% men) age: 52.3 overweight participants with high blood pressure | 0.3 | DASH diet, DASH + weight management, control | Executive function memory-learning (TMT, Stroop interference, DS, VFT, VPA, WAT) and psychomotor speed (Ruff 2&7, DSST) | DASH diet alone did not significantly improve EFML compared with the control (P = 0.214), but did significantly improve psychomotor speed (Cohen's D: 0.440; P = 0.036). DASH diet with weight management significantly improved both EFML (Cohen's D: 0.562; P = 0.008) and psychomotor speed (Cohen's D: 0.480; P = 0.023) compared with the control |
ASF, animals semantic fluency; BNT, Boston Naming Test; BVRT, Benton Visual Retention Test; CDT, clock-drawing test; CTT, color trail test; DASH, Dietary Approaches to Stop Hypertension; DS, digit span; DST, digit span test; DSST, digit symbol substitution test; ELF, excluded letter fluency; ENCORE, Exercise and Nutrition Interventions for Cardiovascular Health; EVOO, extra-virgin olive oil; ILF, initial letter fluency; LNS, letter-number sequencing; MCI, mild cognitive disorder; MeDi, Mediterranean Diet; MedLey, Mediterranean diet for cognitive function and cardiovascular health in the elderly; MMSE, Mini-Mental State Examination; PREDIMED, Prevención con Dieta Mediterránea; RAVLT, Rey Auditory Verbal Learning Test; ROCF, Rey-Osterrieth Complex Figure; SS, symbol search; ST, Stroop Test; TMT, trail making test; TOL, Tower of London; VFT, verbal fluency test; VPA, verbal paired associates; WAIS, Wechsler adult intelligence scale; WAT, word association test.
DASH diet
Observational evidence
The DASH diet in relation to cognitive decline, dementia, or AD was examined in 1 cross-sectional study (50) and 6 longitudinal studies (Table 4) (52–54, 69, 72, 79). Each 1-unit increase in DASH diet score was cross-sectionally associated with better verbal memory (β: 0.18; P = 0.018), but not with visual memory nor executive function or processing speed in US participants (n = 160) (50). Longitudinally, the highest quintile of DASH diet adherence was associated with a 28% lower risk of MCI (HR: 0.72; 95% CI: 0.52, 1.02; P-trend = 0.04) in US participants (n = 6425) after 9.11 y of follow-up (52). In addition, each 1-unit increase in DASH diet score was associated with less change in global cognition (β: 0.007; P = 0.03), episodic memory (β: 0.008; P = 0.04), and semantic memory (β: 0.009; P = 0.02) in US participants after 4.1 y of follow-up (n = 826) (54). Furthermore, adherence to the DASH diet was associated with better average cognition in US older adults (n = 3580) (mean differenceQ5vs.Q1: 0.97; P-trend = 0.0001), but not with cognitive decline after 10.6 y of follow-up (69). In 2 other studies including US (n = 16,144) (72) and Swedish (n = 2223) (79) participants no association was found after 4.1 y and ≤10.6 y of follow-up.
TABLE 4.
Characteristics of the included observational human studies on the DASH diet in relation to cognitive decline, dementia, and AD1
| Authors, year, study name | Study design | Population | Follow- up (y) | Exposure | Outcome | Results | Covariates |
|---|---|---|---|---|---|---|---|
| Blumenthal et al. (2017) (50), US | Cross-sectional | n = 160 (33% men) age: 65.4 sedentary adults aged ≥55 y with cognitive impairment and CVD risk factors | — | FFQ and 4-d food diary, A-MeDi and A-DASH score | Verbal memory (HVLT-R, ANT), visual memory (CFT), and executive function/processing speed (ST, DST, COWA, TMT, DSST, Ruff 2&7 Test) | Higher adherence to the DASH diet was associated with better verbal memory (β: 0.18; P = 0.018), but not with executive function/processing speed (P = 0.569) or visual memory (P = 0.248) | Age, education, sex, ethnicity, total caloric intake,, family history of dementia, and chronic use of anti-inflammatory medications |
| Shakersain et al. (2018) (79), Sweden SNAC-K | Longitudinal | n = 2223 (39% men) age: 69.5 y community residents from Kungsholmen ≥60 y | 6 | 98-item SFFQ, A-MeDi, A-DASH and MIND scores, dietary components | Global cognition (MMSE) | DASH score was not associated with cognitive decline (P = 0.568; PT3vs.T1 = 0.472), nor with risk of MMSE score ≤24 (P = 0.970; PT3vs.T1 = 0.746) | Total caloric intake, age, sex, education, civil status, physical activity, smoking, BMI, vitamin/mineral supplement intake, vascular disorders, diabetes, cancer, depression APOE ε4, and dietary components other than those included in each dietary index |
| Berendsen et al. (2017) (72), US Nurses’ Health Study | Longitudinal | n = 16,144 (0% men) age: 74.3 ywomen ≥70 y | 4.1 | 116-item SFFQ, DASH | Global cognition (TICS and composite score of TICS, EBMT, CF, and DST backward) and verbal memory (immediate and delayed recalls of EBMT and TICS 10-word list) | Higher long-term adherence to the DASH diet was associated with better average global cognition (P-trend = 0.009), verbal memory (P-trend = 0.002), and TICS (P-trend = 0.03), but was not significantly associated with change in global cognition (P-trend = 0.51), verbal memory (P-trend = 0.68), or TICS score (P-trend = 0.98) during follow-up | Age, education, physical activity, caloric intake, alcohol intake, smoking status, multivitamin use, BMI, and history of depression, high blood pressure, hypercholesterolemia, myocardial infarction, and diabetes mellitus |
| Haring et al. (2016) (52), US WHIMS | Longitudinal | n = 6425 (0% men) age: 65–79 y postmenopausal women | 9.11 | FFQ, A-MeDi score and DASH score | MCI (MMSE and battery of neuropsychological tests [animal category, BNT, word list memory task, copying and recalling 4 line drawings, TMT]) | Quintile of DASH score was significantly associated with lower risk of MCI (HRQ5vs.Q1: 0.72; 95% CI: 0.52, 1.02; P-trend = 0.04) | Age, race, education level, WHI hormone trial randomization assignment, baseline 3MS level, smoking status, physical activity, diabetes, hypertension, BMI, family income, depression, history of CVD, and total energy intake |
| Morris et al. (2015) (53), US Rush MAP | Longitudinal | n = 923 (± 24% men) age: 58–98 y people living in retirement communities or senior public housing units | 4.5 | 144-item SFFQ, A-MeDi, A-DASH, and MIND scores | AD (based on NINCDS-ADRDA criteria) | For the DASH diet only the highest tertile of adherence was significantly associated with lower risk of AD (HRT3vs.T1: 0.61; 95% CI: 0.38, 0.97; P-trend = 0.07) | Age, sex, education, Apolipoprotein E ( EAPOE) ε4, participation in cognitively stimulating activities, physical activity, total energy intake, and cardiovascular conditions |
| Tangney et al. (2014) (54), US MAP | Longitudinal | n = 826 (26% men) age: 81.5 y elderly living in Chicago retirement communities and subsidized housing, normal cognitive function | 4.1 | 144-item SFFQ, A-MeDi score, A-DASH score | Global cognition (composite score of 19 tests), episodic memory (logical memory, word list recall, word list recognition, EBS), semantic memory (verbal fluency from CERAD, BNT, 12-item reading test), working memory (DST forward and backward, DO), perceptual speed (SDMT, NC, SNS), and visuospatial ability (JLO, SPM) | Continuous DASH score was significantly associated with slower rate of decline in global cognition (β: 0.007; P = 0.03), episodic memory (β: 0.008; P = 0.04), and semantic memory (β: 0.009; P = 0.02). Tertile of DASH score was only significantly associated with a slower rate of change in global cognition (βT3vs.T1: 0.022; P = 0.04). DASH and MeDi scores were almost as predictive for rate of change of global cognition (standardized β: 2.33 and 2.0, respectively) | Total energy intake, age, sex, education, and cognitive activities |
| Wengreen et al. (2013) (69), US Cache County Study on Memory, Health and Aging | Longitudinal | n = 3580 (± 43% men) age ≥65 y mainly non-Hispanic white | 10.6 | 142-item FFQ, MeDi score, DASH score | Global cognition (3MS) | Higher quintile of DASH score was associated with better average cognition during follow-up (mean differenceQ5vs.Q1: 0.97; P-trend = 0.0001), but was not significantly associated with rate of change of cognitive function | Age, sex, education, BMI, frequency of moderate physical activity, multivitamin and mineral supplement use, history of drinking and smoking, and history of diabetes, heart attack, or stroke |
AD, Alzheimer's disease; A-MeDi, alternate Mediterranean diet; ANT, animal naming test; APOE ε4, apolipoprotein E; BNT, Boston Naming Test; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CF, category fluency; CFT, complex figure test; COWA, controlled oral word association test; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DO, digit ordering; DSST, digit symbol substitution test; DST, digit span test; EBMT, East Boston Memory Test; EBS, East Boston Story; HVLT-R, Hopkins Verbal Learning Test-Revised; JLO, judgement of line orientation; MAP, Memory And Aging Project; MCI, mild cognitive impairment; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; MMSE, Mini-Mental State Examination; NC, number comparison; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association; SDMT, Symbol Digit Modalities Test; SFFQ, semi-quantitative FFQ; SNAC-K, Swedish National Study on Aging and Care in Kungsholmen; SNS, Stroop Neuropsychological Screening; SPM, standard progressive matrices; ST, Stroop Test; TICS, Telephone Interview for Cognitive Status; TMT, trail making test; WHI, Women's Health Initiative; WHIMS, Women's Health Initiative Memory Study; 3MS, modified mini-mental state.
In the only longitudinal study on AD in US participants (n = 923) a 39% lower risk was shown for highest DASH diet adherence (HRT3vs.T1: 0.61; 95% CI: 0.38, 0.97; P-trend = 0.07), whereas in this study somewhat stronger inverse associations were shown for the Mediterranean and MIND diets (53).
Trial evidence
A randomized controlled trial investigated the effect of the DASH diet on cognition in US adults over 4 mo (n = 124) (Table 3) (58). Psychomotor function significantly improved among participants assigned to the DASH diet alone (Cohen's D: 0.440; P = 0.036) and among those assigned to the DASH diet with additional weight management (Cohen's D: 0.480; P = 0.023), where the control group consumed their usual diet. However, whereas the DASH diet with additional weight management significantly improved executive function, memory, and learning (Cohen's D: 0.562; P = 0.008), no such effect was observed for the DASH diet alone.
MIND diet
Observational evidence
In 1 cross-sectional (57) and 5 longitudinal (11, 53, 73, 75, 79) studies the MIND diet was investigated in relation to cognitive decline, dementia, or AD (Table 5). Higher adherence to the MIND diet was cross-sectionally associated with better cognitive performance (global cognition score 15.6 ± 0.09 and 14.9 ± 0.10 in the highest and lowest tertile of MIND diet score, respectively; P-trend < 0.001) and a 35% lower risk of poor cognitive performance (ORT3vs.T1: 0.70; 95% CI: 0.56, 0.86; P = 0.001) among US adults (n = 5907) (57). Longitudinally, the association of the MIND diet with cognitive decline was investigated in 3 studies in Swedish older adults (n = 2223) (79) and US adults (n = 960 and n = 16,058) (11, 73). Higher adherence to the MIND diet was associated with less cognitive decline in Swedish older adults (n = 2223) after 6 y of follow-up (79). In addition, in 1 study including US adults (n = 960), higher MIND diet adherence was significantly associated with less cognitive decline after 4.7 y of follow-up in all 5 measured cognitive domains, including episodic memory (β: 0.0090; P = 0.001), semantic memory (β: 0.0113; P < 0.0001), visuospatial ability (β: 0.0077; P = 0.002), perceptual speed (β: 0.0097; P < 0.0001), and working memory (β: 0.0060; P = 0.01) (11). Higher MIND diet score was also associated with less global cognitive decline (β: 0.0106; P < 0.0001; βT3vsT1: 0.0366; P = 0.01) with a stronger inverse association for the MIND diet compared with the Mediterranean (P for MIND compared with MeDi = 0.02) and DASH diet (P for MIND compared with DASH = 0.02) (11). No association between the MIND diet and cognitive decline was observed in US older nurses after 12.9 y of follow-up (n = 16,058) (73).
TABLE 5.
Characteristics of the included observational human studies on the MIND diet in relation to cognitive decline, dementia, and AD1
| Authors, year, study name | Study design | Population | Follow-up (y) | Exposure | Outcome | Results | Covariates |
|---|---|---|---|---|---|---|---|
| McEvoy, Guyer, Langa & Yaffe (2017) (57), US Health and Retirement Study | Cross-sectional | n = 5907 (40% men) age: 67.8 y community dwelling adults from the age of 50 y | — | 163-item SFFQ, A-MeDi score, MIND diet score | Cognitive performance (global cognition score based on immediate and delayed recall, backward counting and serial seven subtraction) | Higher MIND score tertile was significantly associated with better cognitive performance (P-trend < 0.001). Higher tertile of MIND score was significantly associated with lower risk of poor cognitive performance (ORT3vs.T1: 0.70; 95% CI: 0.56, 0.86; P = 0.001) | Sex, age, race, low education attainment, current smoking, obesity, total wealth, hypertension, diabetes mellitus, physical inactivity, depression, and total energy intake |
| Hosking, Eramudugolla, Cherbuin, & Anstey (2019) (75), Australia PATH Through Life Study | Longitudinal | n = 1220 (50% men) age: 60–64 y older Australian adults | 12 | CSIRO-FFQ, MeDi, A-MeDi, and MIND scores, dietary components | Cognitive impairment: MCI/dementia (Winbald criteria, NINCDS-ADRDA criteria) | Higher tertile of MIND score was significantly associated with a lower risk of cognitive impairment (ORT3vs.T1: 0.47; 95% CI: 0.24, 0.91; P-trend = 0.026) | Energy intake, age, sex, APOE ε4 allele, education, mental activity, physical activity, smoking status, depression, diabetes, BMI, hypertension, heart disease, and stroke |
| Berendsen et al. (2018) (73), US Nurses’ Health Study | Longitudinal | n = 16,058 (0% men) age: 74.3 y older women | 12.9 | 116-item FFQ, MIND score | Global cognition (TICS and composite score of TICS, EBMT, CF, and DST backward) and verbal memory (immediate and delayed recalls of EBMT and TICS 10-word list) | Higher adherence to MIND diet was not significantly associated with less decline in global cognition (P-trend = 0.95), verbal memory (P-trend = 0.98), or TICS score (P-trend = 0.73) during follow-up | Age, education, physical activity, caloric intake, alcohol intake, smoking status, multivitamin use, BMI, depression, and history of hypertension, hypercholesterolemia, myocardial infarction, and diabetes mellitus |
| Shakersain et al. (2018) (79), Sweden SNAC-K | Longitudinal | n = 2223 (39% men) age: 69.5 y community residents from Kungsholmen ≥60 y | 6 | 98-item SFFQ, A-MeDi, A-DASH, and MIND scores, dietary components | Global cognition (MMSE) | Higher MIND score was significantly associated with less cognitive decline (β: 0.006; 95% CI: 0.003, 0.009; P < 0.001; βT3vs.T1: 0.126; 95% CI: 0.064, 0.188; P < 0.001) and with lower risk of MMSE score ≤24 (HR: 0.965; 95% CI: 0.941, 0.989; P = 0.005; HRT3vs.T1: 0.468; 95% CI: 0.261, 0.840; P = 0.011) | Total caloric intake, age, sex, education, civil status, physical activity, smoking, BMI, vitamin/mineral supplement intake, vascular disorders, diabetes, cancer, depression APOE ε4, and dietary components other than those included in each dietary index |
| Morris et al. (2015) (53), US Rush MAP | Longitudinal | n = 923 (± 24% men) age: 58–98 y people living in retirement communities or senior public housing units in Chicago | 4.5 | 144-item SFFQ, A-MeDi, A-DASH, and MIND scores | AD (based on NINCDS-ADRDA criteria) | Both middle and high tertile of MIND diet score were significantly associated with lower risk of AD (HRT2vs.T1: 0.65; 95% CI: 0.44, 0.98; HRT3vs.T1: 0.47, 95% CI: 0.29, 0.76; P-trend = 0.002) | Age, sex, education, APOE ε4, participation in cognitively stimulating activities, physical activity, total energy intake, and cardiovascular conditions |
| Morris et al. (2015) (11), US Rush MAP | Longitudinal | n = 960 (25% men) age: 81.4 y elderly living in retirement communities or senior public housing units in Chicago | 4.7 | 144-item SFFQ, MIND diet score | Global cognition, episodic memory, semantic memory, visuospatial ability, perceptual speed and working memory | MIND diet score was significantly associated with slower decline in global cognition (β: 0.0106; P < 0.0001), episodic memory (β: 0.0090; P = 0.001), semantic memory (β: 0.0113; P < 0.0001), visuospatial ability (β: 0.0077; P = 0.002), perceptual speed (β: 0.0097; P < 0.0001), and working memory (β = 0.0060; P = 0.01). Higher tertile of MIND diet score was significantly associated with slower decline of global cognitive score (βT3vs.T1: 0.0366; P = 0.01). MIND diet score was more protective against cognitive decline than the DASH score or MeDi score according to the standardized β coefficients (βMIND: 4.39, βMeDi: 2.46, βDASH: 2.60; PMIND vs. MeDi = 0.02; PMIND vs. DASH = 0.03) | Age, sex, education, participation in cognitive activities, smoking history, physical activity hours per week, total energy intake, (APOE ε4 allele, time, history of stroke, myocardial infarction, diabetes, hypertension, and interaction terms between each covariate and time) |
AD, Alzheimer's disease; A-MeDi, alternate Mediterranean diet; CF, category fluency; CSIRO, Commonwealth Scientific and Industrial Research Organisation; DASH, Dietary Approaches to Stop Hypertension; DST, digit span test; EBMT, East Boston Memory Test; MAP, Memory and Aging Project; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; MMSE, Mini-Mental State Examination; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association; PATH, Personality and Total Health; SFFQ, semi-quantitative FFQ; SNAC-K, Swedish National Study on Aging and Care in Kungsholmen; TICS, Telephone Interview For Cognitive Status.
A longitudinal study in Australian older adults (n = 1220) showed a 53% lower risk of cognitive impairment, which included both MCI and dementia, for highest MIND diet adherence (ORT3vs.T1: 0.47; 95% CI: 0.24, 0.91; P-trend = 0.026) (75). In the only longitudinal study that investigated AD, a 53% lower risk was found for highest MIND diet adherence (HRT3vs.T1: 0.47; 95% CI: 0.29, 0.76) in US adults (n = 923) after 4.5 y of follow-up (53). In addition, a 35% lower AD risk was shown for moderate MIND diet adherence (HRT3vs.T1: 0.65; 95% CI: 0.44, 0.98), whereas for moderate adherence to the Mediterranean and DASH diets no significant association with AD was shown.
Evidence for the association of the dietary components of the Mediterranean, DASH, and MIND diets with cognitive decline, dementia, and AD is discussed below and presented in Table 6.
Dietary components
Fish and ω-3
Fish consumption was cross-sectionally associated with a lower risk of dementia (39) and better attention (84), but not with global cognition (38, 39, 74, 84), visual memory (84), executive function (84), episodic verbal memory (38), working memory (38), or a lower risk of MCI (56, 84). In 2 (64, 79) of 12 (34, 41–43, 64, 66, 69, 75, 76, 79, 81, 83) longitudinal studies, fish consumption was associated with better subjective cognitive function and less cognitive decline; 1 study showed an increased risk of MCI, mild cognitive disorder, and cognitive decline (76). Another longitudinal study demonstrated an association between fish consumption and less cognitive decline for participants aged ≥65 y (83).
Plant-based foods
In 1 cross-sectional study the consumption of plant foods in general was examined, showing no association with self-reported cognitive function (74). In 2 (56, 84) of 4 (38, 39, 56, 84) cross-sectional studies an association between vegetable consumption and better cognitive function was shown; vegetable consumption was associated with better visual memory (84) and a lower risk of MCI (56). Longitudinally, vegetable consumption was associated with better subjective cognitive function and less cognitive decline in 3 (41, 64, 66) of 12 studies (34, 41, 43, 64, 66, 67, 69, 75, 76, 79, 81, 83); 1 study showed a higher risk of MCI, but not of mild cognitive disorder or cognitive decline (76). Green leafy vegetables specifically were not associated with a lower risk of cognitive impairment in a longitudinal study (75). Olive oil consumption was associated with better cognitive function or less cognitive decline for at least some cognitive domains in 2 (38, 84) of 3 (38, 39, 84) cross-sectional studies and 2 (34, 48) of 3 (34, 42, 48) longitudinal studies. Results on vegetable oil and seed oils are mixed, showing inverse (79) as well as adverse associations (42). Nut consumption, specifically walnut consumption, was cross-sectionally associated with better cognitive function (38). Longitudinally, nut consumption in general was associated with better cognitive function and a lower risk of cognitive impairment in 3 (64, 69, 75) of 9 (34, 41, 43, 64, 66, 67, 69, 75, 83) studies. Legume intake, examined in 4 cross-sectional (38, 39, 56, 84) and 12 longitudinal (34, 41, 43, 64, 66, 67, 69, 75, 76, 79, 81, 83) studies, was associated with better cognitive function in only 1 longitudinal study (69). Fruit consumption was not associated with cognitive function in cross-sectional studies (38, 39, 56, 84). In 1 (64) of 11 (34, 41, 43, 64, 66, 67, 69, 76, 79, 81, 83) longitudinal studies, fruit consumption was associated with better subjective cognitive function, but in another longitudinal study fruit consumption was associated with a higher risk of MCI. Consumption of berries specifically was not associated with cognitive impairment in a longitudinal study (75). No association between potatoes and cognitive function was observed in 2 cross-sectional studies (39, 84) and 1 longitudinal study (81). Urinary polyphenol excretion, a biomarker of dietary polyphenol intake, was associated with better immediate episodic verbal memory in a cross-sectional study, but results for delayed episodic verbal memory were mixed (38).
Meat
Meat consumption in general was associated with worse cognitive function on at least some domains in 1 (38) of 2 (38, 84) cross-sectional studies and 1 (81) of 7 (34, 41, 43, 69, 75, 76, 81) longitudinal studies. Red meat consumption specifically was associated with worse executive function in 1 (84) of 3 (39, 74, 84) cross-sectional studies, but longitudinal studies investigating red and processed meat did not show an association with cognitive decline (64, 66, 67, 69, 79, 83). The consumption of poultry was not associated with cognitive function in 3 cross-sectional studies (39, 74, 84), but was associated with less cognitive decline in 1 (79) of 2 (75, 79) longitudinal studies.
Grains and cereals
Grain and cereal consumption together was not associated with risk of MCI in a cross-sectional study (56). Cereal consumption was associated with worse cognitive function on at least some cognitive domains in 1 (38) of 2 (38, 74) cross-sectional and 1 (76) of 6 (34, 41, 43, 64, 76, 81) longitudinal studies, whereas the consumption of nonrefined cereals specifically was associated with better cognitive function in 1 (39) of 2 (39, 84) cross-sectional studies. Grain consumption, specifically refined grains, was associated with more cognitive decline in 1 (79) of 3 (69, 75, 79) longitudinal studies, but whole grain consumption was associated with better average performance on at least some cognitive domains in 2 (67, 69) of 4 (66, 67, 69, 83) longitudinal studies.
Saturated and unsaturated fatty acids
MUFA:SFA ratio was cross-sectionally associated with an increased risk of nonamnestic MCI (56), whereas an association with less cognitive decline was shown in 3 (34, 66, 67) of 9 (34, 41, 43, 45, 64, 66, 67, 69, 76) longitudinal studies. Results for consumption of MUFA or PUFA were mixed, with 1 longitudinal study showing a beneficial association with cognitive decline (45) and other longitudinal studies showing adverse associations (42, 76). A higher (MUFA + PUFA):SFA ratio was associated with a lower risk of MCI, and specifically amnestic MCI, in a cross-sectional study (56). No associations for SFA intake (42, 45, 76), PUFA:SFA ratio (81), or unsaturated fatty acid to SFA ratio (45) with cognitive decline were found.
Dairy
Dairy consumption was associated with worse cognitive function on at least some cognitive domains in 1 (84) of 5 (38, 39, 56, 74, 84) cross-sectional studies and 1 (79) of 8 (34, 41, 43, 64, 69, 76, 79, 83) longitudinal studies. In addition, dairy consumption was associated with a higher risk of MCI and mild neurocognitive disorder in a longitudinal study (76). No association between cheese consumption and cognitive impairment was observed in a longitudinal study (75). Consumption of milk, specifically high-fat milk, was associated with more cognitive decline in 1 (79) of 3 longitudinal (64, 79, 81) studies. In the same study margarine consumption was shown to be associated with more cognitive decline, but no association between consumption of ice cream and cognitive decline was observed (79).
Alcohol
Alcohol consumption was not associated with cognitive function in 4 cross-sectional (38, 39, 56, 84) and 9 longitudinal (34, 41, 43, 64, 66, 67, 76, 81, 83) studies. Wine consumption specifically was associated with better global cognition and less cognitive decline in 1 cross-sectional study (38) and 1 (45) of 2 (45, 75) longitudinal studies. The consumption of spirits or beers was not associated with cognitive decline in a longitudinal study (79).
Other dietary components
The consumption of sugar or fruit juice was associated with more cognitive decline in a longitudinal study (79), but intake of carbohydrates or sweetened beverages in general was not associated with cognitive decline in 2 other longitudinal studies (45, 69). No association between the consumption of fiber (45), sodium (69), processed and fast fried food (75), sweets and pastries (75), or animal-source cooking fat (83) and cognitive decline or cognitive impairment was shown longitudinally.
Discussion
The evidence summarized in this review suggests that higher adherence to the Mediterranean, DASH, or MIND diets is associated with less cognitive decline and a lower risk of AD, which is mainly based on observational evidence. The number of studies examining the impact of the studied diets on dementia risk are scarce and too inconclusive to draw any conclusions. When comparing the potential impact of the different diets in respect to cognitive decline and AD, data suggest stronger inverse associations for the MIND diet compared with the Mediterranean and DASH diets (11, 53, 75). Moreover, inverse associations tend to be stronger for the Mediterranean diet with AD than the DASH diet (53). Compared with previous studies in this research field, the current review particularly included more studies on the recently developed MIND diet.
Due to methodological differences between the included studies, i.e. study design and study population, dietary assessment methodology, and outcome measurement, the overall results should be interpreted with care.
With respect to the study design and study population, the majority of the included studies were observational studies (50/56 studies), which are known to be prone to reverse causation, potential confounding, and over adjustment (88–90). To illustrate the case of reverse causation, it is likely that participants living with dementia or cognitive decline included in a cross-sectional study report less foods than they actually consumed (88, 91). In addition, the possibility of residual confounding has to be considered, as many observational studies did not adjust for one or more potential confounders, such as APOE ε4 allele, sex, ethnicity, socioeconomic status, education, CVD risk factors, depression, comorbidities or medication, smoking, energy intake, BMI or obesity, physical activity, cognitive or social activity, or the use of nutritional supplements. On the other hand, the inclusion of potential intermediates may have led to over adjustment and as such to attenuation of existing true associations. Namely, the Mediterranean diet has been associated with hypertension (92), which, in turn, is associated with cerebrovascular health (93). As such, hypertension may be an intermediate pathway explaining the potential effect of the diet on cognitive decline, warranting careful modeling. Cohort-specific characteristics, e.g. age of assessment, education, sex, race, vascular risk, follow-up duration, loss to follow-up, or community dwelling compared with diagnosed with dementia, may also be responsible for differences in outcomes. For instance, in several studies, a beneficial association of the Mediterranean diet with cognitive decline was only shown for part of the study population, namely participants aged ≥65 y (83), African-American participants (65), or men (40), which limits the external validity. The 4 trials on the effect of the Mediterranean and DASH diets on cognition, dementia, or AD that were included in this review also had several limitations, including lack of baseline outcome measurements, small sample size, and relatively short duration of intervention, so no firm conclusion could be drawn from these trials.
With respect to the dietary assessment methodology, most observational studies used an FFQ and some studies used a food diary or 24-h recall (24hR). FFQs and 24hRs are known to be prone to recall bias related to memory deficits, especially in studies with elderly, cognitively impaired participants, or low-educated participants (94). Compared with 24hRs, FFQs are more limited with respect to the variety of foods assessed but often more likely to reflect the usual intake. As food diaries are usually completed during a day, they are more likely to affect eating behavior than FFQs and recalls. Besides these differences between the methods, they are all prone to measurement error related to socially desirable answers and errors in food composition tables. Differences in exposure quantification also arose from the scoring system applied to calculate adherence to the Mediterranean, DASH, or MIND diets. Most studies examining the impact of the Mediterranean diet used the original score as described by Trichopoulou et al. (9) or the alternate Mediterranean diet score as described by Panagiotakos et al. (30). The original Mediterranean diet score is based on the median intake of the population, does not consider extremes in intake, and includes MUFA:SFA ratio instead of olive oil intake (9). As such, the statistical power may be limited in studies in non-Mediterranean countries. The score by Panagiotakos et al. uses predefined cut-offs for intake, divides intake into quintiles, and includes intake of olive oil specifically (30). DASH diet adherence was calculated by using the score by Fung et al. (32), or the score by Folsom et al. (31). Compared with the score of Folsom et al. (31), the score of Fung et al. (32) is based on relatively few dietary components. In addition, the score of Fung et al. (32) is based on intake of the study population. Finally, irrespective of the diet under study, not all studies were able to 1) capture all dietary components when constructing the dietary pattern score and 2) distinguish between dietary components in the same way (e.g. total cereal intake compared with unrefined cereals). The differences in effect sizes between the diets may for instance be explained by the absence of olive oil in the DASH diet, as olive oil was associated with better cognitive function and less cognitive decline (34, 38, 48, 84). Besides nutrition, the Mediterranean diet pyramid also includes other cultural and lifestyle factors including conviviality, culinary activities, physical activity, and adequate rest (17). Of these, physical activity and social network, which is related to conviviality, have been associated with a lower risk of cognitive decline and dementia (95). In addition, daytime napping, which is related to adequate rest, has been shown to improve alertness and performance (96).
With respect to the outcome measure, several studies only used a global screening tool, such as the MMSE, Telephone Interview for Cognitive Status (TICS), Modified Mini-Mental State Examination (3MS), or seven-minute screen (7MS), whereas other studies applied multiple cognitive tests. A global screening tool may be less sensitive to detect possible associations due to a potential ceiling effect (97), so studies using global screening tools may be more likely to present null-associations. Nevertheless, overall, the results of studies using a global screening tool were rather similar to the results of studies using multiple cognitive tests. In addition, 4 longitudinal studies did not assess cognitive status at baseline, which may have led to residual confounding due to interindividual differences in cognitive function or brain volume. Conversely, including baseline outcome measurements may also lead to an overestimation of the effects due to the possibility of a learning effect with an even larger effect with an increasing number of repetitions.
Investigating the role of nutrients, foods, and dietary patterns in relation to dementia is important as there is no cure for dementia yet. A focus on whole dietary patterns is useful as the effect of a combination of nutrients may be larger than the effect of single nutrients and because possible interactions between nutrients are incorporated. Many observational studies have already examined the Mediterranean diet in relation to cognitive decline, dementia, or AD, but more observational studies on the DASH and MIND diets are recommended. In addition, the number of intervention studies investigating either the Mediterranean, DASH, or MIND diet is very limited, so more intervention studies on each of these dietary patterns are needed. In the United States, an intervention study on the effect of the MIND diet on cognitive decline, dementia, AD, and vascular dementia is ongoing (98). Furthermore, both observational and intervention studies examining the association of the Mediterranean, DASH, or MIND diets with brain structure are recommended, because these studies may provide insights into the exact mechanisms via which the 3 dietary patterns may protect against cognitive decline, dementia, and AD. For example, adherence to the Mediterranean diet has been associated with less decline in the cerebral metabolic rate of glucose and less increase in Pittsburgh compound B in AD-affected regions (99). Moreover, future trials could examine all 3 dietary patterns simultaneously, because this would provide interesting insights into whether the Mediterranean, DASH, or MIND diet is most protective against cognitive decline, dementia, and AD. However, it may be easier for people to change the intake of one nutrient or food than to change their whole diet. Therefore, despite the shift of research from single nutrients and foods towards whole dietary patterns, research on specific nutrients or foods is still useful. An interesting field of study is to investigate the role of olive oil in cognitive decline, dementia, and AD via both observational and intervention studies as this may be one of the most important components driving the association of the Mediterranean and MIND diets with less cognitive decline. Last of all, future research should, if possible, take into account the methodological issues described above.
Conclusions
The results of this review suggest that higher adherence to the Mediterranean, DASH, or MIND diets is associated with less cognitive decline and a lower risk of AD, as demonstrated by 10 out of 14 cross-sectional studies, 1 case-control study, 21 out of 33 longitudinal studies, and 4 out of 6 articles on intervention studies. Evidence for an association with dementia was inconsistent. Observational studies indicate that the MIND diet may be more protective against cognitive decline and AD than the Mediterranean and DASH diets (11, 53, 75), but more evidence on the MIND diet is required to draw a firm conclusion. Furthermore, the Mediterranean diet seems more protective against AD than the DASH diet (53). Based on the studies included in the current review, in which dietary components were assessed as part of 1 of the 3 dietary patterns, olive oil consumption seems to be an important component underlying these associations (34, 38, 48, 84).
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—ACvdB: conceptualized the review, developed the search strategy, conducted the literature search, and wrote the manuscript; EMB-B and AAMB: critically reviewed the manuscript; OvdR: supported the review and writing process and critically reviewed the manuscript; all authors read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: ACvdB, EMB-B, AAMB, and OvdR, no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AD, Alzheimer's disease; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; MCI, mild cognitive impairment; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; MMSE, Mini-Mental State Examination; PREDIMED, Prevención con dieta Mediterránea; WHICAP, Washington Heights-Inwood Columbia Aging Project; 24hR, 24-h recall.
References
- 1. WHO. Global Health Observatory (GHO) data: top 10 causes of death. 2017[last accessed 19 Feb 2018]. Available from: http://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/. [Google Scholar]
- 2. Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International; 2015. [Google Scholar]
- 3. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia - a systematic review. Plant Foods Hum Nutr. 2013;68:279–92. [DOI] [PubMed] [Google Scholar]
- 5. Frank B, Gupta S.. A review of antioxidants and Alzheimer's disease. Ann Clin Psychiatry. 2005;17:269–86. [DOI] [PubMed] [Google Scholar]
- 6. Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Rev Neurol. 2009;5:140–52. [DOI] [PubMed] [Google Scholar]
- 7. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 8. van de Rest O, Berendsen AM, Haveman-Nies A, De Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6:154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trichopoulou A, Martínez-González MA, Tong TY, Forouhi NG, Khandelwal S, Prabhakaran D, Mozaffarian D, de Lorgeril M. Definitions and potential health benefits of the Mediterranean diet: views from experts around the world. BMC Med. 2014;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM et al.. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 11. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimer's Dement. 2015;11:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lehert P, Villaseca P, Hogervorst E, Maki PM, Henderson VW. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric. 2015;18:678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, Roberts RO. Association of Mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;39:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74:580–91. [DOI] [PubMed] [Google Scholar]
- 15. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–96. [DOI] [PubMed] [Google Scholar]
- 16. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6 Suppl):1402S–6S. [DOI] [PubMed] [Google Scholar]
- 17. Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, Medina FX, Battino M, Belahsen R, Miranda G et al.. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14:2274–84. [DOI] [PubMed] [Google Scholar]
- 18. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113:1–15. [DOI] [PubMed] [Google Scholar]
- 19. Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, Schilardi A, D ’introno A, Montagna M La, Calvani M et al.. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer's disease and late-life cognitive disorders: a systematic review. J Alzheimer's Dis. 2017;59:815–49. [DOI] [PubMed] [Google Scholar]
- 21. Abbatecola AM, Russo M, Barbieri M. Dietary patterns and cognition in older persons. Curr Opin Clin Nutr Metab Care. 2018;21:10–3. [DOI] [PubMed] [Google Scholar]
- 22. Walters M, Hackett K, Caesar E, Isaacson R, Mosconi L. Role of nutrition to promote healthy brain aging and reduce risk of Alzheimer's disease. Curr Nutr Rep. 2017;6:63–71. [Google Scholar]
- 23. Wright RS, Gerassimakis C, Bygrave D, Waldstein SR. Dietary factors and cognitive function in poor urban settings. Curr Nutr Rep. 2017;6:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones JM, Korczak R, Peña RJ, Braun HJ. CIMMYT series on carbohydrates, wheat, grains, and health: impact of minerals, phytochemicals, specific grain-based foods, and dietary patterns on mild cognitive impairment, Alzheimer's disease, and Parkinson's disease. Cereal Foods World. 2017;62:104–14. [Google Scholar]
- 25. Custodero C, Valiani V, Agosti P, Schilardi A, D'Introno A, Lozupone M, La Montagna M, Panza F, Solfrizzi V, Sabbà C. Dietary patterns, foods, and food groups: relation to late-life cognitive disorders. Off JGG. 2017; 65:78–89. [DOI] [PubMed] [Google Scholar]
- 26. Marchand NE, Jensen MK.. The role of dietary and lifestyle factors in maintaining cognitive health. Am J Lifestyle Med. 2018;12:(4):268–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gardener SL, Rainey-Smith SR.. The role of nutrition in cognitive function and brain ageing in the elderly. Curr Nutr Rep. 2018;7:139–49. [DOI] [PubMed] [Google Scholar]
- 28. Chen X, Maguire B, Brodaty H, O'Leary F. Dietary patterns and cognitive health in older adults: a systematic review. J Alzheimer's Dis. 2019;67:583–619. [DOI] [PubMed] [Google Scholar]
- 29. Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. Br Med J. 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2006;16:559–68. [DOI] [PubMed] [Google Scholar]
- 31. Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens. 2007;20:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 33. Hernández-Galiot A, Goñi I.. Adherence to the Mediterranean diet pattern, cognitive status and depressive symptoms in an elderly non-institutionalized population. Nutr Hosp. 2017;34:338–44. [DOI] [PubMed] [Google Scholar]
- 34. Galbete C, Toledo E, Toledo JB, Bes-Rastrollo M, Buil-Cosiales P, Marti A, Guillén-Grima F, Martínez-González MA. Mediterranean diet and cognitive function: the SUN project. J Nutr Health Aging. 2015;19:305–12. [DOI] [PubMed] [Google Scholar]
- 35. Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MÁ, Martínez-Lapiscina EH, Fitó M, Pérez-Heras A, Salas-Salvadó J et al.. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175:1094–103. [DOI] [PubMed] [Google Scholar]
- 36. Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, San Julián B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MÁ. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84:1318–25. [DOI] [PubMed] [Google Scholar]
- 37. Martínez-Lapiscina EH, Clavero P, Toledo E, San Julián B, Sanchez-Tainta A, Corella D, Lamuela-Raventós RM, Martínez JA, Martínez-Gonzalez MÁ. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. J Nutr Health Aging. 2013;17:544–52. [DOI] [PubMed] [Google Scholar]
- 38. Valls-Pedret C, Lamuela-Raventós RM, Medina-Remón A, Quintana M, Corella D, Pintó X, Martínez-González A, Estruch R, Ros E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimer's Dis. 2012;29:773–82. [DOI] [PubMed] [Google Scholar]
- 39. Anastasiou CA, Yannakoulia M, Kosmidis MH, Dardiotis E, Hadjigeorgiou GM, Sakka P, Arampatzi X, Bougea A, Labropoulos I, Scarmeas N. Mediterranean diet and cognitive health: initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLoS One. 2017;12:e0182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katsiardanis K, Diamantaras A-A, Dessypris N, Michelakos T, Anastasiou A, Katsiardani K-P, Kanavidis P, Papadopoulos FC, Stefanadis C, Panagiotakos DB et al.. Cognitive impairment and dietary habits among elders: the Velestino Study. J Med Food. 2013;16:343–50. [DOI] [PubMed] [Google Scholar]
- 41. Trichopoulou A, Kyrozis A, Rossi M, Katsoulis M, Trichopoulos D, La Vecchia C, Lagiou P. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr. 2015;54:1311–21. [DOI] [PubMed] [Google Scholar]
- 42. Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European Prospective Investigation into Cancer and Nutrition). Public Health Nutr. 2008;11:1054–62. [DOI] [PubMed] [Google Scholar]
- 43. Tanaka T, Talegawkar SA, Jin Y, Colpo M, Ferrucci L, Bandinelli S, Tanaka T, Talegawkar SA, Jin Y, Colpo M et al.. Adherence to a Mediterraneandiet protects from cognitive decline in the Invecchiare in Chianti Study of Aging. Nutrients. 2018;10:2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gallucci M, Mazzuco S, Ongaro F, Di Giorgi E, Mecocci P, Cesari M, Albani D, Forloni GL, Durante E, Gajo GB et al.. Body mass index, lifestyles, physical performance and cognitive decline: Treviso Longeva (TRELONG) study. J Nutr Health Aging. 2013;17:378–84. [DOI] [PubMed] [Google Scholar]
- 45. Solfrizzi V, Colacicco AM, D'Introno A, Capurso C, Torres F, Rizzo C, Capurso A, Panza F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging. 2006;27:1694–704. [DOI] [PubMed] [Google Scholar]
- 46. Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, Galan P. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. 2013;97:369–76. [DOI] [PubMed] [Google Scholar]
- 47. Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues J-F, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berr C, Portet F, Carriere I, Akbaraly TN, Feart C, Gourlet V, Combe N, Barberger-Gateau P, Ritchie K. Olive oil and cognition: results from the Three-City Study. Dement Geriatr Cogn Disord. 2009;28:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mosconi L, Walters M, Sterling J, Quinn C, Mchugh P, Andrews RE, Matthews DC, Ganzer C, Osorio RS, Isaacson RS et al.. Lifestyle and vascular risk effects on MRI-based biomarkers of Alzheimer's disease: a cross-sectional study of middle-aged adults from the broader New York City area. BMJ Open. 2018;8:e019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Welsh-Bohmer K, Browndyke JN, Lin P-H, Kraus W, Doraiswamy PM, Burke J et al.. Lifestyle and neurocognition in older adults with cardiovascular risk factors and cognitive impairment. Psychosom Med. 2017;79:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zbeida M, Goldsmith R, Shimony T, Vardi H, Naggan L, Shahar DR. Mediterranean diet and functional indicators among older adults in non-Mediterranean and Mediterranean countries. J Nutr Health Aging. 2014;18:411–8. [DOI] [PubMed] [Google Scholar]
- 52. Haring B, Wu C, Mossavar-Rahmani Y, Snetselaar L, Brunner R, Wallace RB, Neuhouser ML, Wassertheil-Smoller S. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the Women's Health Initiative Memory Study. J Acad Nutr Diet. 2016;116:921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimer's Dement. 2015;11:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tangney CC, Li H, Wang Y, Barnes L, Schneider JA, Bennett DA, Morris MC. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83:1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer's disease. J Alzheimers Dis. 2010;22:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roberts R, Geda YE, Cerhan JR, Knopman DS, Cha RH, Christianson TJH, Pankratz VS, Ivnik RJ, Boeve BF, O'Connor HM et al.. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;29:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McEvoy CT, Guyer H, Langa KM, Yaffe K. Neuroprotective diets are associated with better cognitive function: the Health and Retirement Study. J Am Geriatr Soc. 2017;65:1857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA, Sherwood A. Effects of the Dietary Approaches to Stop Hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55:1331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69:1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bhushan A, Fondell E, Ascherio A, Yuan C, Grodstein F, Willett W. Adherence to Mediterranean diet and subjective cognitive function in men. Eur J Epidemiol. 2018;33:223–34. [DOI] [PubMed] [Google Scholar]
- 65. Koyama A, Houston DK, Simonsick EM, Lee JS, Ayonayon HN, Shahar DR, Rosano C, Satterfield S, Yaffe K. Association between the Mediterranean diet and cognitive decline in a biracial population. Journals Gerontol Ser A Biol Sci Med Sci. 2015;70:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Samieri C, Okereke OI, Devore E, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr. 2013;143:493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Mediterranean diet and cognitive function in older age: results from the Women's Health Study. Epidemiology. 2013;24:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, Unverzagt FW, Moy C, Howard VJ, Kissela B et al.. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology. 2013;80:1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, Tschanz JT, Norton MC, Welsh-Bohmer KA. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am J Clin Nutr. 2013;98:1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vercambre MN, Grodstein F, Berr C, Kang JH. Mediterranean diet and cognitive decline in women with cardiovascular disease or risk factors. J Acad Nutr Diet. 2012;112:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Berendsen AM, Kang JH, van de Rest O, Feskens EJM, de Groot LCPGM, Grodstein F. The Dietary Approaches to Stop Hypertension diet, cognitive function, and cognitive decline in American older women. J Am Med Dir Assoc. 2017;18:427–32. [DOI] [PubMed] [Google Scholar]
- 73. Berendsen AM, Kang JH, Feskens EJM, de Groot CPGM, Grodstein F, van de Rest O. Association of long-term adherence to the MIND diet with cognitive function and cognitive decline in American women. J Nutr Health Aging. 2018;22:222–9. [DOI] [PubMed] [Google Scholar]
- 74. Crichton GE, Bryan J, Hodgson JM, Murphy KJ. Mediterranean diet adherence and self-reported psychological functioning in an Australian sample. Appetite. 2013;70:53–9. [DOI] [PubMed] [Google Scholar]
- 75. Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimer's Dement. 2019;15:581–9. [DOI] [PubMed] [Google Scholar]
- 76. Cherbuin N, Anstey KJ.. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH Through Life Study. Am J Geriatr Psychiatry. 2012;20:635–9. [DOI] [PubMed] [Google Scholar]
- 77. Gardener S, Gu Y, Rainey-Smith SR, Keogh JB, Clifton PM, Mathieson SL, Taddei K, Mondal A, Ward VK, Scarmeas N et al.. Adherence to a Mediterranean diet and Alzheimer's disease risk in an Australian population. Transl Psychiatry. 2012;2:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Knight A, Bryan J, Wilson C, Hodgson J, Davis C, Murphy K. The Mediterranean diet and cognitive function among healthy older adults in a 6-month randomised controlled trial: the MedLey Study. Nutrients. 2016;8:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shakersain B, Rizzuto D, Larsson S, Faxén-Irving G, Fratiglioni L, Xu W-L. The Nordic prudent diet reduces risk of cognitive decline in the Swedish older adults: a population-based cohort study. Nutrients. 2018;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Olsson E, Karlström B, Kilander L, Byberg L, Cederholm T, Sjögren P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimer's Dis. 2015;43:109–19. [DOI] [PubMed] [Google Scholar]
- 81. Titova OE, Ax E, Brooks SJ, Sjögren P, Cederholm T, Kilander L, Kullberg J, Larsson E-M, Johansson L, Åhlström H et al.. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol. 2013;48:1443–8. [DOI] [PubMed] [Google Scholar]
- 82. Chan R, Chan D, Woo J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J Nutr Health Aging. 2013;17:757–65. [DOI] [PubMed] [Google Scholar]
- 83. Qin B, Adair LS, Plassman BL, Batis C, Edwards LJ, Popkin BM, Mendez MA. Dietary patterns and cognitive decline among Chinese older adults. Epidemiology. 2015;26:758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bajerska J, Woźniewicz M, Suwalska A, Jeszka J. Eating patterns are associated with cognitive function in the elderly at risk of metabolic syndrome from rural areas. Eur Rev Med Pharmacol Sci. 2014;18:3234–45. [PubMed] [Google Scholar]
- 85. Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, Healthy Eating Index 2005, and cognitive function in middle-aged and older Puerto Rican adults. J Acad Nutr Diet. 2013;113:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Corley J, Starr JM, McNeill G, Deary IJ. Do dietary patterns influence cognitive function in old age?. Int Psychogeriatrics. 2013;25:1393–407. [DOI] [PubMed] [Google Scholar]
- 87. Wardle J, Rogers P, Judd P, Taylor MA, Rapoport L, Green M, Nicholson Perry K. Randomized trial of the effects of cholesterol-lowering dietary treatment on psychological function. Am J Med. 2000;108:547–53. [DOI] [PubMed] [Google Scholar]
- 88. Ikeda M, Brown J, Holland AJ, Fukuhara R, Hodges JR. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Walls AWG, Steele JG.. The relationship between oral health and nutrition in older people. Mech Ageing Dev. 2004;125:853–7. [DOI] [PubMed] [Google Scholar]
- 90. Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, Pahor M, Furberg CD. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–54. [DOI] [PubMed] [Google Scholar]
- 91. Morris CH, Hope RA, Fairburn CG. Eating habits in dementia: a descriptive study. Br J Psychiatry. 1989;154:801–6. [DOI] [PubMed] [Google Scholar]
- 92. Panagiotakos DB, Pitsavos ChH, Chrysohoou C, Skoumas J, Papadimitriou L, Stefanadis C, Toutouzas PK. Status and management of hypertension in Greece: role of the adoption of a Mediterranean diet in the Attica study. J Hypertens. 2003;21:1483–9. [DOI] [PubMed] [Google Scholar]
- 93. Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McPherson RS, Hoelscher DM, Alexander M, Scanlon K, Serdula M. Dietary assessment methods among school-aged children: validity and reliability. Prev Med (Baltim). 2000;31:S11–33. [Google Scholar]
- 95. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53. [DOI] [PubMed] [Google Scholar]
- 96. Ficca G, Axelsson J, Mollicone DJ, Muto V, Vitiello MV. Naps, cognition and performance. Sleep Med Rev. 2010;14:249–58. [DOI] [PubMed] [Google Scholar]
- 97. Franco-Marina F, García-González JJ, Wagner-Echeagaray F, Gallo J, Ugalde O, Sánchez-García S, Espinel-Bermúdez C, Juárez-Cedillo T, Rodríguez MÁV, García-Peña C. The Mini-Mental State Examination revisited: ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int Psychogeriatrics. 2010;22:72–81. [DOI] [PubMed] [Google Scholar]
- 98. Morris MC. ClinicalTrials.gov: MIND diet intervention and cognitive decline (MIND). 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02817074?term=MIND&cond=Dementia&cntry=US&state=US%3AIL&city=Chicago&rank=1, [Date last accessed 21 Feb 2019]. [Google Scholar]
- 99. Berti V, Walters M, Sterling J, Quinn CG, Logue M, Andrews R, Matthews DC, Osorio RS, Pupi A, Vallabhajosula S et al.. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018;90:e1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.