ABSTRACT
A previous meta-analysis provided convincing evidence for an inverse association between adherence to a Mediterranean diet (MedDiet) and the risk of all-cause mortality. Since then, 19 prospective studies have been published. We updated the evidence from these prospective studies and conducted a dose-response meta-analysis to test the linear and potential nonlinear dose-response associations between adherence to a MedDiet and the risk of all-cause mortality. The PubMed, Scopus, ISI Web of Knowledge, and Embase bibliographic databases were systematically searched up to August 24, 2018. Summary HRs were estimated with the use of a random-effects meta-analysis to assess the association between a 2-point increment in MedDiet adherence and the risk of all-cause mortality. Sensitivity and subgroup analyses were performed and potential publication bias was tested. Twenty-nine prospective studies with 1,676,901 participants and 221,603 cases of all-cause mortality were included in the final analysis. The pooled HR of all-cause mortality was 0.90 (95% CI: 0.89, 0.91; I2 = 81.1%) for a 2-point increment in adherence to a MedDiet. Subgroup analyses showed that a significant inverse association was stronger in participants who lived in the Mediterranean region compared with non-Mediterranean areas (HRs: 0.82 compared with 0.92, respectively), and in studies that used the Panagiotakos MedDiet score. A nonlinear dose-response meta-analysis indicated that the risk of all-cause mortality linearly decreased with the increase in adherence to a MedDiet. The robustness of findings was confirmed in the sensitivity analyses. In conclusion, low-quality evidence from prospective cohort studies suggests an inverse association between adherence to a MedDiet and the risk of all-cause mortality, especially in Mediterranean regions. An inverse linear dose-response relation was also observed between adherence to a MedDiet and the risk of all-cause mortality.
Keywords: Mediterranean diet, mortality, meta-analysis, prospective cohort studies, dose-response
Introduction
Dietary patterns rather than single nutrients are a more helpful strategy to better understand the complexity of diet–disease associations (1). Over the past 2 decades, several epidemiologic studies have been conducted to identify the associations between dietary patterns and survival rate (2–6). In this regard, the Mediterranean diet (MedDiet) has been one of the best-studied dietary patterns, for which a beneficial effect on lifespan (3, 7) was first suggested in the 1960s (8).
The MedDiet is characterized by higher intakes of olive oil, legumes, fruits, vegetables, nuts, and fish; moderate intake of dairy products; and low consumption of meat and processed meat products. This combination of dietary habits results in high dietary antioxidant capacity, as well as high intakes of dietary fibers, unsaturated fats, and various healthy phytochemicals (9). Several interventional studies have presented convincing evidence that greater adherence to a MedDiet can reduce traditional cardiometabolic risk factors, and may be associated with a lower risk of developing type 2 diabetes and some types of cancers (10–14). Since the 1960s, a growing body of evidence from epidemiologic studies has been published in relation to the MedDiet and survival. A previous meta-analysis of 16 prospective cohort studies has suggested an 8% reduction in the risk of all-cause mortality per each 2-point increase in the adherence to a MedDiet (3). Since then, several prospective cohort studies have been published. However, to our knowledge, the dose-response relation between adherence to a MedDiet and the risk of all-cause mortality has not been previously determined. Therefore, we updated the evidence from prospective studies and conducted a dose-response meta-analysis to test the linear and potential nonlinear dose-response associations between adherence to a MedDiet and the risk of all-cause mortality.
Methods
Search strategy
We followed the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines for reporting the current meta-analysis (10). The study protocol is available at https://www.crd.york.ac.uk/PROSPERO (registration number CRD42018094059). A systematic search of the literature was carried out in the PubMed, Scopus, ISI Web of Knowledge, and Embase bibliographic databases up to November 15, 2017, followed by an updated search up to August 24, 2018. The following search terms were used: (“Mediterranean diet” OR “dietary pattern” OR “Mediterranean”) AND (“survival” OR “survive” OR “mortality” OR “fatal” OR “death”) and (“follow up” OR “nested case-control” OR “longitudinal studies” OR “cohort studies” OR “prospective studies”) (Supplemental Table 1). No restrictions in terms of the language of publications were considered.
Study selection
Studies were considered eligible for inclusion in the current meta-analysis if they met the following criteria: 1) were original prospective cohort studies conducted in healthy populations aged ≥18 y or older; 2) reported adherence to a MedDiet as exposure and risk of all-cause mortality as the outcomes of interest; 3) provided estimates of RRs, HRs, ORs, or rate ratios with corresponding 95% CIs for ≥3 quantitative categories of a MedDiet score; and 4) reported the number of cases and noncases or person-years in each category of a MedDiet score. Studies that reported the association between continuous MedDiet score and all-cause mortality risk were also included for linear analysis. Two independent investigators (SS and AJ) carried out an initial screening of all titles and abstracts from retrieved papers to identify eligible studies that should be included in the analysis.
Data extraction and quality assessment
Two independent reviewers (SS and AJ) recorded the following characteristics from the identified studies: first author's last name, date of publication, country, sex, study participants, number of cases, duration of follow-up, method of assessment of MedDiet index scoring, dietary assessment tools, HRs, RRs, or ORs for all-cause mortality, and list of variables that were entered into the multivariable model as potential confounders. We contacted authors by e-mail to ask for appropriate data. Sjogren et al. (11) kindly provided the requested data, but we were unable to obtain data from others (12). In line with the Cochrane Risk-of-Bias tool for Nonrandomized Studies (ROBINS-I) assessment scale, 2 independent investigators (SS and AJ) performed the quality assessment to examine the possible risk of bias associated with each of the included studies (13). Disagreements were solved by consulting the principal investigator (SS-B).
Rating the quality of evidence
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to rate the quality of the evidence, i.e., the confidence in the effect estimates (14, 15). GRADE consists of a rating system where the quality of the evidence for each outcome ranges from very low to high. Observational studies start as low quality, and can be downgraded or upgraded based on judgments about 8 different criteria (14, 15). Downgrading decisions depend on the presence of risk of bias, inconsistency, indirectness, imprecision and publication bias, whereas upgrading decisions depend on the observation of large magnitude of effect, a dose-response gradient, and an effect of plausible residual confounding (14, 15). Three independent investigators (SS, AJ, and NBT) rated the quality of the evidence, and disagreements were solved by consensus. The final decision of quality evidence was assessed with the use of GRADEpro software (Grade Working Group).
Statistical methods
The reported RRs or ORs in the primary studies were considered to be equal to HRs. The primary estimate effect of interest was the pooled HR of all-cause mortality for a 2-point increment in adherence to the traditional MedDiet (ranging from 0 to 9). If, in a given study, the effect size was directly reported per each 2-point increment in a traditional MedDiet score (ranging from 0 to 9), the reported risk estimate was directly included in the meta-analysis. For studies in which the risk estimates were reported for a 1- or a 1.5-unit increment in a traditional MedDiet score, we recalculated the reported risk estimates for a 2-point increment in a MedDiet score. For studies that reported the results for a 1-unit increase in a MedDiet score, but did not use a traditional MedDiet score (e.g., reported the result for a 1-unit increment in the MedDiet score, ranging from 0–8, 0–17, 11–55, or 0–14), we recalculated the reported risk estimates for a 2-point increment in a traditional MedDiet score (ranging from 0 to 9). For studies that did not report the continuous MedDiet score and only reported the results across categories of a MedDiet, we used a previously described method (16, 17) to calculate study-specific HRs (linear slopes) and 95% CIs from the natural logarithms of the extracted HRs and 95% CIs across categories of adherence to the MedDiet. For those studies that only reported a MedDiet score as categoric and did not use a traditional MedDiet score (ranging from 0 to 9), we transformed the scores to a 9-point scale. If studies reported results separately for men and women or other subgroups, we combined the subgroup-specific estimates through the use of a fixed-effects model to generate an overall estimate so that each study was only represented once in the main analysis. To test the potential effect of each study on pooled effect size, sensitivity analysis was performed by the stepwise exclusion of each study at a time. To determine the potential sources of heterogeneity, subgroup analyses were conducted based on gender, geographic location, dietary assessment method, follow-up duration, number of participants, definition of a MedDiet, and adjustments for main confounders. Between-study heterogeneity was explored through the use of Cochrane's Q test and quantified by the I2 statistic (P < 0.05) (18). Publication bias was assessed by inspection of funnel plot asymmetry and tested by Egger's asymmetry test (19) and Begg's test (20) (P < 0.10). The influence of any potential publication bias on results was investigated through the use of the Duval and Tweedie (21) trim-and-fill methods.
We also performed an additional analysis to test the potential nonlinear dose-response relation between adherence to a MedDiet and risk of all-cause mortality. A 2-stage hierarchic regression model was used to test the potential nonlinear association, in which the difference between category-specific and reference-specific doses, expressed in quadratic terms, was calculated (22). Then, the dose-response relation, considering within- and between study variances, was estimated through the use of spline transformations. This method requires the distribution of cases and noncases across >3 categories of adherence to a MedDiet, the median value, and the adjusted RRs with their 95% CIs for each category of exposure. For studies that did not use a traditional MedDiet score (ranging from 0 to 9), we transformed the scores to a 9-point scale. Two-sided statistical significance was set at P < 0.05. All statistical analyses were conducted with Stata version 13 (Stata Corp.).
Results
Literature research
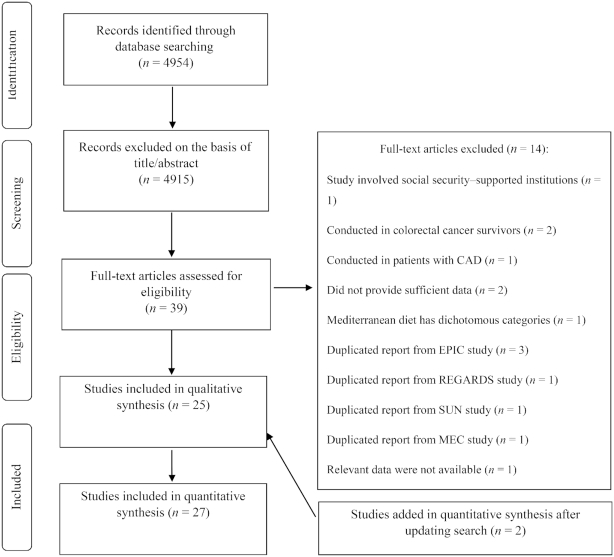
We identified 4954 articles from a systematic search of electronic databases. We excluded 4915 articles after screening the title and abstract (Figure 1). After reviewing the full texts of 39 studies, 14 papers were excluded for the following reasons: 1 study was conducted in subjects living in 2 institutions supported by state social security (23); 2 studies were conducted among long-term colorectal cancer survivors (24, 25); and 1 study included patients with stable coronary artery disease (26), who were excluded because they might have modified their habitual diets after diagnosis of the disease. Moreover, the participants in these studies were not representative of the general population. Two studies were excluded because the required information could not be provided even after contacting the authors (12). Another study was excluded because it reported RRs for adherence to the MedDiet as a dichotomous variable (27). From the European Prospective Investigation into Cancer and Nutrition (EPIC) study, 4 different reports were published that assessed adherence to a MedDiet in relation to total risk of mortality (28–31), and we included the Lassale et al. (30) report because it had the greatest number of participants. However, this study did not report the results across the categories of a MedDiet and were not included in the nonlinear dose-response meta-analysis; thus, another publication from the EPIC study which reported sufficient information was included in the nonlinear dose-response meta-analysis (and not in the main analysis) (31). The study by Booth et al. (32), conducted in participants who were candidates for primary prevention of cardiovascular disease with statin therapy from the REasons for Geographic and Racial Differences in Stroke (REGARDS) study was excluded because the study population overlapped with that of the most recent study by Whalen et al. (33). In addition, of the 2 different reports from the Seguimiento University of Navarra (SUN) cohort study (34, 35), we only included the most recent one by Alvarez-Alvarez et al. (35). From the Multiethnic Cohort (MEC) study, 2 reports were published (36, 37), of which the Shvetsov et al. (36) study was included in our analysis. Two studies were added to the eligible studies after an updated search on August 24, 2018 (38, 39).
FIGURE 1.
Flow diagram for study selection process. CAD, coronary artery disease; EPIC, European Prospective Investigation into Cancer and Nutrition; ES, effect size; MEC, Multiethnic Cohort; REGARDS, REasons for Geographic and Racial Differences in Stroke study; SUN, Seguimiento University of Navarra cohort study.
Of the remaining 26 publications, 1 reported the results in 3 different countries, and was therefore regarded as 3 different studies (40). Finally, 29 prospective cohort studies (27 publications) with 221,603 cases with all-cause mortality among 1,676,901 participants were included in this meta-analysis (9, 11, 30, 31, 33, 35, 36, 38–57). Seven studies did not provide sufficient data (the number of deaths or effect estimates in each category of a MedDiet score), and thus were not included in the nonlinear dose-response meta-analysis (9, 30, 45, 49, 51–53).
Study characteristics
Twenty-nine prospective cohort studies were published between 1995 and 2018 with a follow-up time ranging between 4 and 32 y (Table 1 and Supplemental Table 2). Six studies were from the United States (33, 36, 43, 44, 48, 50), 18 were from Europe (9, 11, 30, 31, 35, 38, 40–42, 46, 47, 49, 51–55, 57), 2 were from Australia (45, 56), and 1 study was from China (39). In most of the studies, dietary intake was measured by an FFQ (9, 30, 31, 33, 35, 36, 38–42, 44–50, 52, 54, 56, 57), in 1 study by diet history (53), in 3 by dietary record (11, 43, 51), and in 1 by dietary recall (55).
TABLE 1.
General characteristics of included prospective studies in meta-analysis of adherence to Mediterranean diet and risk of all-cause mortality1
| Author | Year | Study name, country | Follow-up duration (y) | Number of participants/cases (all-cause mortality) | Age, y2 | Diet assessment method (items) | Mediterranean dietary pattern score range/component (definition based) |
|---|---|---|---|---|---|---|---|
| Trichopoulou et al. (9) | 1995 | Village cohort, Greece | 4–5 | 182/53 | ≥70 | FFQ (190) | Score range: 0–8 (tMedDiet) ↑ vegetables, ↑ fruits and nuts, ↑ legumes, ↑ (MUFAs/SFAs), ↑ cereals, ↔ alcohol, ↓ meat, ↓ dairy |
| Kouris-Blazos et al. (45) | 1999 | Melbourne cohort study, Australia | 4–6 | 330/36 | ≥70 | FFQ (250) | Score range: 0–8 (tMedDiet) ↑ vegetables, ↑ fruits and nuts, ↑ cereals, ↑ legumes, ↑ (MUFAs/SFAs), ↓ meat and meat products, ↓ dairy products, ↔ alcohol |
| Trichopoulou et al. (31) | 2005 | EPIC-elderly, 6 countries in Europe | 7.4 | 52621/2675 | ≥60 | FFQ | Score range: 0–9 (mtMedDiet) ↑ legumes, ↑ cereals, ↑ fruits/nuts, ↑ fish, ↑ vegetables, ↑ (MUFAs + PUFAs)/SFAs, ↓ meat and poultry, ↓ dairy products, ↔ alcohol |
| Lagiou et al. (46) | 2006 | SWLHC Sweden | 12 | 42,237/572 | 30–49 | FFQ | Score range: 0–9 (mtMedDiet) ↑ legumes, ↑ cereals, ↑ fruits/nuts, ↑ fish, ↑ vegetables, ↑ (MUFAs/SFAs), ↓ meat and meat products, ↓ dairy products, ↔ alcohol |
| Sjogren et al. (11) | 2010 | ULSAM Sweden | 10.1 | 924/215 | 71 | 7-d dietary records | Score range: 0–9 (mtMedDiet) ↑ vegetables and legumes, ↑ cereals and potatoes, ↑ fruits, ↑ fish, ↑ (MUFAs/SFAs), ↑ milk and milk products, ↓ meat and meat products, ↔ alcohol |
| Tognon et al. (53) | 2011 | GGPSG, Sweden | 8.5 | 1037/622 | ≥70 | Diet history | Score range: 0–9 (mtMedDiet) ↑ vegetables and potatoes, ↑ fruits, ↑ whole-grain cereals, ↑ fish, ↑ legumes, ↑ nuts and seeds, ↑ (MUFAs + PUFAs)/SFAs, ↓ meat, meat products, and eggs, ↓ dairy, ↔ alcohol |
| van den Brandt (54) | 2011 | NLSC, Netherlands | 4.9 | 120,852/11,506 | 55–69 | FFQ | Score range: 0–9 (mtMedDiet) ↑ vegetables, ↑ fruits, ↑ nuts, ↑ legumes, ↑ fish, ↑ whole grains, ↑ (MUFAs/SFAs), ↓ red meat, ↔ alcohol |
| Tognon et al. (52) | 2012 | VIP, Sweden | 10 | 77,151/2376 | 30–60 | FFQ | Score range: 0–8 (mMedDiet) ↑ vegetables and potatoes, ↑ fruits and juices, ↑ whole grain cereals, ↑ fish and fish products, ↑ (PUFAs + MUFAs)/SFAs, ↓ meat and meat products, ↓ dairy products, ↔ alcohol intake |
| Tognon et al. (51) | 2014 | MONICA, Denmark | 11 | 1849/553 | ≥35 | 7-d food records | Score range: 0–9 (mMedDiet) ↑ (PUFAs + MUFAs)/SFAs, ↑ vegetables, ↑ fruits, ↑ cereals, ↑ fish and fish products, ↓ dairy products, ↓ meat/meat products/eggs, ↔ alcohol |
| Cuenca-Garcia et al. (43) | 2014 | ACLS, USA | 11.6 | 12,449/358 | 20–84 | 3-d diet records | Score range: 0–9 (mtMedDiet) ↑ vegetables, ↑ fruits and nuts, ↑ cereals, ↑ fish and seafood, ↑ legumes, ↑ (MUFAs/SFAs), ↓ meat and meat products, ↓ dairy products, ↔ alcohol |
| George et al. (44) | 2014 | WHI, USA | 12.9 | 63,805/5692 | 50–79 | FFQ (122) | Score range: 0–9 (aMedDiet) ↑ vegetables, ↑ fruits, ↑ nuts, ↑ fish, ↑ legumes, ↑ (MUFAs/SFAs), ↑ whole-grains, ↔ alcohol, ↓ red meat |
| Reedy et al. (50) | 2014 | NIH-AARP, USA | 15 | 424,663/86,419 | 50–71 | FFQ (124) | Score range: 0–9 (aMedDiet) ↑ vegetables, ↑ whole-grains, ↑ fruits, ↑ nuts, ↑ fish, ↑ legumes, ↑ (MUFAs/SFAs), ↓ red and processed meat, ↔ alcohol |
| Vormund et al. (55) | 2014 | Two-cohort study, Switzerland | 32 | 17,800/3934 | ≥16 | 24-h recall checklist | Score range: 0–9 (mtMedDiet) ↑ salad, ↑ vegetables, ↑ fruits, ↑ whole-grains, ↑ fish, ↑ MUFAs, ↓ dairy products, ↓ meat and meat products, ↔ wine |
| Stefler et al. (40) | 2015 | HAPIEE study, Eastern Europe | 7 | 19,263/1314 | 40–70 | FFQ (136, 147, 148 items) | Score range: 0–17 (Sofi MedDiet) ↑ vegetables, ↑ fruits, ↑ legumes, ↑ cereals, ↑ fish, ↓ meat and processed meat, ↓ dairy, ↔ alcohol |
| Prinelli et al. (49) | 2015 | Northern Italy cohort, Italy | 17.4 | 974/193 | 40–74 | FFQ (158) | Score range: 19–45 (Panagiotakos) ↑ vegetables, ↑ fruits, ↑ vegetables, ↑ fish, ↑ nonrefined cereals, ↑ legumes, ↑ olive oil, ↓ poultry, ↓ meat and meat products, ↓ full-fat dairy products, ↔ alcohol |
| Park et al. (48) | 2016 | NHANES III, USA | 18.5 | 1739/386 | 20–88 | FFQ and the 24-h dietary recall | Score range: 0–50 (Panagiotakos) ↑ grains, ↑ legumes, ↑ fruits, ↑ vegetables, ↑ fish, ↑ (MUFAs/SFAs), ↓ red meat, ↓ poultry, ↓ dairy, ↔ alcohol |
| Bo et al. (41) | 2016 | Asti cohort, Italy | 12 | 1658/220 | 45–64 | FFQ (148) | Score range: 0–9 (mtMedDiet) ↑ legumes, ↑ cereals, ↑ fruits/nuts, ↑ fish, ↑ vegetables, ↑ (MUFAs/SFAs), ↓ meat and ↓ dairy products, ↔ alcohol |
| Lassale et al. (30) | 2016 | EPIC, Europe | 10 | 451,256/15,200 | 25–70 | FFQ | Score range: 0–9 (mtMedDiet) ↑ vegetables, ↑ fruits and nuts, ↑ fish, ↑ cereals, ↑ legumes, ↑ (MUFAs/SFAs), ↓ meat and meat products, ↓ dairy products, ↔ alcohol |
| Bonaccio et al. (42) | 2016 | MOLI-SANI study, Italy | 4 | 1995/109 | ≥35 | Epic FFQ (188) | Score range: 0–9 (mtMedDiet) ↑ legumes, ↑ cereals, ↑ fruits/nuts, ↑ fish, ↑ vegetables, ↑ (MUFAs + PUFAs)/SFAs, ↓ meat and poultry, ↓ dairy products, ↔ alcohol |
| Alvarez-Alvarez et al. (35) | 2017 | SUN study, Spain | 10.3 | 19,467/305 | ≥35 | FFQ (136) | Score range: 0–9 (mtMedDiet) ↑ legumes, ↑ cereals, ↑ fruits/nuts, ↑ fish, ↑ vegetables, ↑ (MUFAs + PUFAs)/SFAs, ↓ meat and poultry, ↓ dairy products, ↔ alcohol |
| Limongi et al. (47) | 2017 | ILSA, Italy | 7.1 | 4232/655 | 65–84 | FFQ (49) | Score range: 0–44 (Goulet MedDiet) ↑ grains, ↑ fruits, ↑ vegetables, ↑ legumes, ↑ nuts and seeds, ↑ olive oil, ↑ fish, ↓ red meat/processed meat, ↓ sweets, ↓ eggs, ↓ dairy products |
| Shvetsov et al. (36) | 2017 | MEC, USA | 18 | 193,527/51,702 | 45–75 | FFQ (182) | Score range: 0–9 (aMedDiet) ↑ vegetables, ↑ fruits, ↑nut, ↑ legumes, ↑ fish, ↑ whole grains, ↑ (MUFAs/SFAs), ↓ meat and meat products, ↔ alcohol |
| Whalen et al. (33) | 2017 | REGARDS, USA | 6.25 | 21,423/2513 | ≥45 | FFQ (109) | Score range: 11–55 (aMedDiet) ↑ vegetables, ↑ fruits, ↑nut, ↑ lean meat, ↑ fish, ↑ (MUFAs/SFAs), ↓ sodium, ↓ red and processed meat, ↔ alcohol, ↔ dairy products, ↔ grains and starches |
| Cardenas-Fuentes et al. (38) | 2018 | PREDIMED, Spain | 6.8 | 7356/498 | 20–70 | FFQ (137) | Modified score Mediterranean Diet Adherence Screener |
| Hodge et al. (56) | 2018 | MCCS, Australia | 19 | 41,513/7757 | 40–69 | FFQ (121) | Score range: 0–9 (mtMedDiet) ↑ vegetables, ↑ fruits, ↑ cereals, ↑ fish, ↑ legumes, ↑ (MUFAs/SFAs), ↓ meat and meat products, ↓ dairy, ↔ alcohol |
| Lemming et al. (57) | 2018 | SMC, Sweden | 17 | 33,341/10,478 | 40–70 | FFQ (96) | Score range: 0–8 (tMedDiet) ↑ vegetables and potatoes, ↑ fruits and juices, ↑ whole-grain cereals, ↑ fish and fish products, ↑ (PUFAs + MUFAs)/SFAs, ↓ meat and meat products, ↓ dairy products, ↔ alcohol intake |
| Neelakantan et al. (39) | 2018 | SCHS, China | 17 | 63,257/15,262 | 45–74 | FFQ (165) | Score range: 0–9 (aMedDiet) ↑ vegetables, ↑ fruits, ↑nut, ↑ legumes, ↑ fish, ↑ whole grains, ↑ (MUFAs/SFAs), ↓ meat and meat products, ↔ alcohol |
ACLS, Aerobics Center Longitudinal Study; aMedDiet, Alternative Mediterranean diet (Fung); EPIC, European Prospective Investigation into Cancer and Nutrition; GGPSG, Gerontological and Geriatric Population Studies in Gothenburg; HAPIEE, Health, Alcohol, and Psychosocial Factors In Eastern Europe; ILSA, Italian Longitudinal Study on Aging; MCCS, Melbourne Collaborative Cohort Study; MEC, Multiethnic Cohort study; mMedDiet, modified Mediterranean diet (Knoop); MOLI-SANI, Mediterranean Diet and Low-grade Subclinical Inflammation; MONICA, Multinational Monitoring of trends and determinants in Cardiovascular disease; mtMedDiet, modified traditional Mediterranean diet; NIH-AARP, National Institutes of Health–American Association of Retired Persons; NLSC, Netherlands Cohort Study; PREDIMED, PREvención con DIeta MEDiterránea study; Q, quartile/quintile; REGARDS, REasons for Geographic and Racial Differences in Stroke study; SCHS, Singapore Chinese Heath Study; SMC, Swedish Mammography Cohort; SUN, Seguimiento University of Navarra cohort study; SWLHC, Swedish components of the Scandinavian Women's Lifestyle and Health Cohort; T, tertile; tMedDiet, traditional Mediterranean diet; ULSAM, Uppsala Longitudinal Study of Adult Men cohort; VIP, Vasterbotten Intervention; WHI, Women's Health Initiative study. ↑: increased, ↓ decreased, ↔: moderate.
Values are ranges.
Eight different definitions for the MedDiet, with different scoring criteria, were used in eligible studies, including the traditional MedDiet (n = 3) (9, 45, 57), a modified traditional MedDiet score (n = 11) (11, 30, 31, 35, 41–43, 46, 53–56), a modified MedDiet score (Knoop) (n = 2) (51, 52), an alternative MedDiet score (Fung) (n = 5) (33, 36, 39, 44, 50), the Panagiotakos definition (48, 49) (n = 2), the definition from Goulet et al. (47) (n = 1), a MedDiet adherence screener (38), and in 3 studies the Sofi definition (40).
Meta-analysis
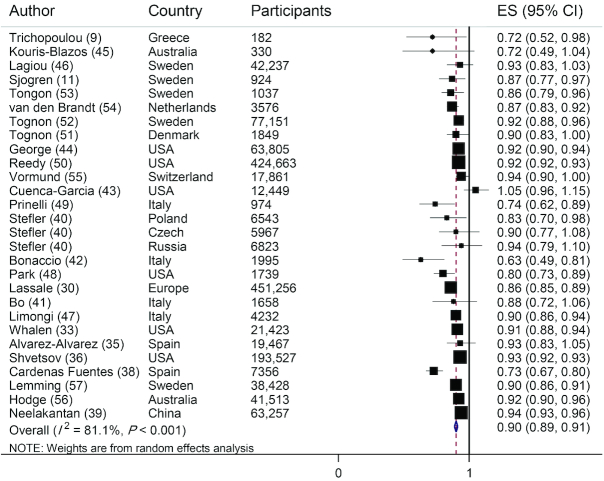
Twenty-eight studies (26 publications) were included in the linear analysis of adherence to a MedDiet and risk of all-cause mortality (9, 11, 30, 33, 35, 36, 38–57). There was a 10% reduction in the risk of all-cause mortality for each 2-point increment in a score of adherence to a MedDiet, with a high heterogeneity between studies (HR: 0.90; 95% CI: 0.89, 0.91; I2 = 81.1%; P-heterogeneity < 0.001) (Figure 2).
FIGURE 2.
Risk of all-cause mortality associated with each 2-point increment in adherence to MedDiet. The black square and horizontal line represents the study-specific HR and 95% CI, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The center of the open diamond presents the pooled HR and its width represents the pooled 95% CI. Weights are from random-effects analysis.
Sensitivity analysis and subgroup analysis
In the sensitivity analysis, summary results did not materially change when each study was sequentially excluded from the main analysis (HR ranged between 0.89 and 0.91). The association remained significant when results were stratified based on gender, study location, follow-up duration, sample size, definition of MedDiet, and dietary assessment methods (apart from studies that used dietary record) (Table 2). The significant inverse association of adherence to a MedDiet and risk of all-cause mortality revealed a stronger relation in the subgroup with participants living in Mediterranean regions compared with other regions (HR: 0.82, 95% CI: 0.86, 0.98; n = 8 compared with HR: 0.92, 95% CI: 0.91, 0.93; n = 20, respectively) as well as in studies that used the Panagiotakos score (HR: 0.78; 95% CI: 0.72, 0.85; n = 2 studies) (48, 49). In the subgroup analysis by dietary assessment method, an inverse association was found only in those studies that used an FFQ as compared with other methods (Table 2).
TABLE 2.
Subgroup analyses of adherence to Mediterranean diet and risk of all-cause of mortality1
| Meta-analysis | Heterogeneity | |||||
|---|---|---|---|---|---|---|
| Subgroup | Studies (n) | HR (95%CI) | Q statistic | I 2 (%) | P-heterogeneity | P-between |
| Total | 0.90 (0.89, 0.91) | 142.96 | 81.1 | <0.001 | ||
| Sex | 0.34 | |||||
| Male | 1 | 0.87 (0.77, 0.97) | 0.00 | — | — | |
| Female | 3 | 0.91 (0.90, 0.93) | 1.62 | 0.00 | 0.445 | |
| Both | 24 | 0.90 (0.898, 0.91) | 142.96 | 81.1 | <0.001 | |
| Region | < 0.001 | |||||
| Mediterranean area | 8 | 0.82 (0.76, 0.88) | 32.88 | 78.7 | <0.001 | |
| Non-Mediterranean area | 20 | 0.92 (0.91, 0.93) | 46.47 | 59.1 | 0.001 | |
| Dietary assessment method | 0.40 | |||||
| FFQ | 23 | 0.90 (0.88, 0.91) | 132.14 | 83.4 | <0.001 | |
| Diet history | 1 | 0.86 (0.77, 0.94) | 0.00 | — | — | |
| Dietary recall | 1 | 0.94 (0.89, 0.99) | 0.00 | — | — | |
| Dietary record | 3 | 0.94 (0.83, 1.05) | 7.88 | 74.6 | 0.019 | |
| Study duration, y | <0.001 | |||||
| <11 | 12 | 0.85 (0.81, 0.89) | 39.29 | 72 | <0.001 | |
| >11 | 16 | 0.90 (0.89, 0.91) | 42.01 | 64.3 | 0.001 | |
| Study participants | <0.001 | |||||
| <10,000 | 15 | 0.83 (0.79, 0.87) | 36.67 | 61.8 | 0.001 | |
| >10,000 | 13 | 0.92 (0.91, 0.93) | 63.37 | 81.1 | <0.001 | |
| Mediterranean definition2 | <0.001 | |||||
| Traditional score | 3 | 0.83 (0.69, 0.96) | 3.92 | 49 | 0.141 | |
| Modified traditional score | 11 | 0.90 (0.86, 0.93) | 39.49 | 74.7 | <0.001 | |
| MedDiet adherence screener | 1 | 0.73 (0.66, 0.79) | 0.00 | — | — | |
| Modified MedDiet score (Knoop) | 2 | 0.92 (0.88, 0.95) | 0.17 | 0.00 | 0.676 | |
| Alternative score (Fung) | 5 | 0.92 (0.91, 0.93) | 12.66 | 68.4 | 0.013 | |
| Panagiotakos score | 2 | 0.78 (0.72, 0.85) | 0.56 | 0.00 | 0.454 | |
| Goulet score | 1 | 0.90 (0.86, 0.94) | 0.00 | — | — | |
| Sofi score | 3 | 0.87 (0.80, 0.97) | 1.11 | 0.00 | 0.573 | |
| Adjustment for confounders | ||||||
| Smoking | 0.13 | |||||
| Yes | 24 | 0.90 (0.88, 0.91) | 126.44 | 83.6 | <0.001 | |
| No | 4 | 0.88 (0.83, 0.94) | 8.49 | 64.7 | 0.037 | |
| BMI | 0.01 | |||||
| Yes | 18 | 0.91 (0.88, 0.92) | 118.97 | 80.2 | <0.001 | |
| No | 10 | 0.88 (0.85, 0.92) | 18.21 | 50.6 | 0.033 | |
| Energy intake | <0.001 | |||||
| Yes | 22 | 0.90 (0.87, 0.91) | 99.57 | 80.1 | <0.001 | |
| No | 6 | 0.90 (0.85, 0.94) | 16.19 | 81.5 | 0.001 | |
| Physical activity | <0.001 | |||||
| Yes | 19 | 0.88 (0.87, 0.91) | 111.25 | 89.5 | <0.001 | |
| No | 9 | 0.91 (0.89, 0.94) | 16.96 | 52.8 | 0.031 | |
| Alcohol consumption | 0.66 | |||||
| Yes | 6 | 0.92 (0.91, 0.94) | 7.24 | 50.4 | 0.203 | |
| No | 22 | 0.87 (0.86, 0.90) | 132.89 | 80.6 | <0.001 | |
Meta-analysis values are HR with 95% CIs.P-heterogeneity within subgroups was calculated with the use of a random-effects model. P-heterogeneity between subgroups was calculated with the use of a fixed-effects model. MedDiet, Mediterranean diet.
Alternative score (Fung) (0–9) (33, 36, 39, 44, 50); Goulet score (0–44) (47); MedDiet adherence screener (0–14) (38); modified MedDiet score (Knoop) (0–8) (51, 52); modified traditional score (0–9) (11, 30, 31, 35, 41–43, 46, 53–56); Panagiotakos score (0–50) (48, 49); Sofi score (0–17) (40); traditional score (0 to 8) (9, 45, 57).
Among the 29 prospective cohort studies, 22 studies (20 publications) met the inclusion criteria for the nonlinear dose-response analysis (11, 31, 33, 35, 36, 38–44, 46–48, 50, 54–57). There was a linear inverse association between the MedDiet score and risk of all-cause mortality (P-nonlinearity = 0.18) (Figure 3).
FIGURE 3.
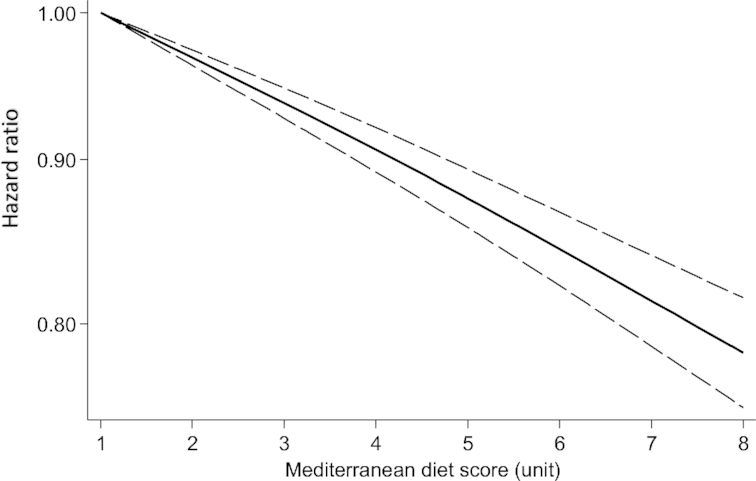
Dose-response analysis of risk of all-cause mortality and adherence to a Mediterranean diet. The solid line and the long-dashed line represent the estimated HR and its 95% CI; the solid line represents the linear relation.
Publication bias and quality assessment
There was no evidence for publication bias based on Begg's test (P = 0.236); however, the Egger's test revealed some indications of publication bias (P = 0.008) (Supplemental Figure 1). An analysis based on the Duval and Tweedie trim-and-fill method did not appreciably change the findings (result not shown). Most of the studies included in the analysis have a moderate to serious risk of bias according to the ROBINS-I tool (Supplemental Table 3).
Overall quality of the evidence (GRADE assessment)
Supplemental Table 4 displays the GRADE assessment for the association between adherence to a MedDiet and all-cause mortality. Although we upgraded the evidence because of a linear dose-response relation, the certainty of the evidence was rated as low because of downgrading for serious risk of bias and very serious inconsistency.
Discussion
The current systematic review and meta-analysis of 29 prospective cohort studies shows that each 2-point increment in the adherence to a MedDiet is associated with a 10% reduction in the risk of all-cause mortality. We selected all-cause mortality as the outcome of interest instead of an individual outcome such as different cancer types or cardiovascular disease because it is a more reliable endpoint, which is not subject to bias. Its accuracy only depends on the number of identified deaths. On the contrary, for specific-cause mortality, the accuracy of recording depends on the correct identification of the cause of death. Moreover, all-cause mortality gives an overview about how adherence to a MedDiet improves overall survival.
In the subgroup analyses, the association remained significant when results were stratified based on gender, study location, follow-up duration, sample size, a MedDiet definition, and dietary assessment method (apart from studies that used dietary record). The reduction in mortality risk was evident with respect to both Mediterranean and non-Mediterranean regions, although it was stronger in Mediterranean countries. The beneficial effect of a MedDiet seemed to be higher when the Panagiotakos MedDiet score was used. In addition, the nonlinear dose-response meta-analysis demonstrated a linear inverse association between adherence to a MedDiet and risk of all-cause mortality. The present study supports the prior meta-analysis findings, which suggested a lower risk of all-cause mortality for subjects who had a greater adherence to a MedDiet (3).
Key components of a MedDiet, including high intakes of fruits, vegetables, nuts, and fish, are associated with lower inflammation and less oxidative stress—biochemical pathways that are linked with risk of developing cardiovascular disease and cancer (58). A recent meta-analysis of 6 randomized controlled trials has shown that a higher adherence to a MedDiet can reduce systolic and diastolic blood pressure, as well as the concentrations of fasting plasma glucose, total cholesterol, and high-sensitivity C-reactive protein, which suggests that the protective effect of this dietary pattern on human health is partly mediated by improvements in metabolic profile (59). Moreover, a recent meta-analysis of >100 prospective studies indicated that an increased intake of individual components of a MedDiet, such as whole grains, vegetables, fruits, nuts, and fish, was associated with a lower risk of all-cause mortality (60). However, findings from 54 clinical trials showed that olive oil, one of the most prominent components of the MedDiet, was not more effective in reducing lipid profile than other unsaturated fatty acids (61).
Up to now, several a priori–defined MedDiet indexes with different scoring systems have been developed for measuring the degree of adherence to this healthy dietary pattern (62). Mediterranean diet indexes comprise dietary components that tend to reflect local food intake patterns (63). Moreover, some food groups were combined into one component; for example, nuts were included in fruit groups, or legumes in vegetable groups (62). Existing differences in the classification of dietary constituents and in different scoring criteria (based on the amount of food consumed and a wide range of cutoffs for food groups) among different MedDiet indexes could considerably change the degree of adherence. Therefore, the specification of the exact mechanisms by which a MedDiet could exert its health benefits in different populations is difficult.
It is important to highlight that only in the PREDIMED study, assessing the effect of the MedDiet on all causes of death, was no effect shown (64). Therefore, other clinical trials are warranted in the future to assess the effect of MedDiet on total mortality.
The subgroup analyses revealed that the association was stronger when the Panagiotakos MedDiet score was used to measure adherence to a MedDiet. This score consists of 11 food items (65), in which poultry was considered as an independent unhealthy component, instead of including it in the meat products group (65). Moreover, potato was not included in the vegetable group, and olive oil was used instead of the MUFA:SFA ratio (65). A recent review by Zaragoza-Martí et al. (66) revealed that the score created by Panagiotakos, although not considered the gold standard measure of MedDiet adherence, provides one of the best overall evaluations of diet in terms of applicability. However, only 2 studies used the Panagiotakos MedDiet score, and a number of studies varied substantially in terms of the MedDiet definition used. Thus, it may be hard to compare the results across different definitions of a MedDiet appropriately.
Our results suggested that a MedDiet has a stronger inverse association with all-cause mortality in Mediterranean populations compared with those living in other geographic regions. One possible explanation could be that high adherence to a MedDiet in non-Mediterranean regions differs from the pattern found with the traditional MedDiet followed in Mediterranean regions, where the consumption of olive oil, fish, vegetables, and legumes is higher than in other regions (28). In addition, the MedDiet scores use sex-specific median intake as cutoffs for each component. Therefore, because median values could differ depending on the study population, those individuals with a high adherence to a MedDiet in non-Mediterranean populations may be classified as poorly adherent in Mediterranean regions.
A nonlinear dose-response meta-analysis showed a linear inverse association between adherence to a MedDiet and risk of all-cause mortality. These findings show that the benefits of a MedDiet on human health increases monotonically as the adherence to this healthy dietary pattern increases. A recent meta-analysis of prospective cohort studies on food groups and risk of all-cause mortality showed different trends in the association of food groups and risk of all-cause mortality (60). For instance, the risk of mortality linearly decreased with the increase in fish, legumes, and whole-grain consumption, whereas a U-shaped association was observed between the consumption of vegetables and nuts and risk of all-cause mortality (60). A MedDiet score is a reliable indicator of overall diet quality. Thus, with the use of a nonlinear dose-response analysis, we have presented, for the first time, a more comprehensive understanding of the association between overall diet quality and risk of all-cause mortality.
The present study has several strengths. First, it included 19 new articles published in the past 4 y, reporting an additional 1,381,567 participants and 203,548 cases that were not included in the previous meta-analyses. Second, we tested the association across several subgroups, which indicated that the association stayed significant when the results were stratified according to the characteristics of the different studies and their participants. Third, for the first time, we tested the nonlinear dose-response association of adherence to a MedDiet with risk of all-cause mortality, which presented a deeper insight into the association between adherence to a MedDiet and survival. Fourth, we used the GRADE system to rate the overall quality of the evidence. Lastly, we only included prospective studies, which allows us to reduce the likelihood of recall and potential selection biases.
There are also some limitations that need to be addressed. First, food preparation techniques, meal patterns, food group compositions, and evaluation of the adherence to a MedDiet by non-Mediterranean populations may be different across studies (63), which could affect the observed results. Second, a high degree of interstudy heterogeneity was observed in the main analysis, which reduces our confidence in the effect estimates. Third, we did not test the potential effect of each individual component of a MedDiet on risk of all-cause mortality, which could help to explain which food groups are mainly responsible for the observed association. Fourth, our confidence in the effect estimates is low due to a serious risk of bias and major inconsistencies caused by the presence of unexplained interstudy heterogeneity.
In conclusion, in the present meta-analysis of prospective cohort studies, a 2-point increase in the adherence to a MedDiet is associated with a 10% lower risk of all-cause mortality. The present results add new information demonstrating a linear inverse relation between adherence to a MedDiet and the risk of all-cause mortality. However, the overall quality of the evidence was rated as low, and therefore further studies are likely to change our confidence in the effect estimates.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—AJ and SS-B: designed the project; SS and AJ: conducted the literature search and wrote the first draft of the manuscript; AJ and SS: analyzed data and interpreted the results; JSS and NBT: revised the subsequent drafts for important intellectual content; SS-B is the guarantor; and all authors: have read and approved the final manuscript.
Notes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author disclosures: None of the authors have any conflicts of interest to declare.
Supplemental Tables 1–4 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: EPIC, European Prospective Investigation into Cancer and Nutrition; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MEC, Multiethnic Cohort study; MedDiet, Mediterranean diet; MOOSE, Meta-Analysis of Observational Studies in Epidemiology; REGARDS, REasons for Geographic and Racial Differences in Stroke study; ROBINS-I, Risk of Bias tool for Nonrandomized Studies; SUN, Seguimiento University of Navarra cohort study.
References
- 1. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 2. Li F, Hou LN, Chen W, Chen PL, Lei CY, Wei Q, Tan WL, Zheng SB. Associations of dietary patterns with the risk of all-cause, CVD and stroke mortality: a meta-analysis of prospective cohort studies. Br J Nutr. 2015;113(1):16–24. [DOI] [PubMed] [Google Scholar]
- 3. Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17(12):2769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vrieling A, Buck K, Seibold P, Heinz J, Obi N, Flesch-Janys D, Chang-Claude J. Dietary patterns and survival in German postmenopausal breast cancer survivors. Br J Cancer. 2013;108(1):188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu Y, Wu H, Wang PP, Savas S, Woodrow J, Wish T, Jin R, Green R, Woods M, Roebothan B et al.. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open. 2013;3(2):e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118(1):74–100. e11. [DOI] [PubMed] [Google Scholar]
- 7. Pérez-López FR, Chedraui P, Haya J, Cuadros JL. Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas. 2009;64(2):67–79. [DOI] [PubMed] [Google Scholar]
- 8. Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. 1997;55(11):383–9. [DOI] [PubMed] [Google Scholar]
- 9. Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, Vassilakou T, Lipworth L, Trichopoulos D. Diet and overall survival in elderly people. BMJ. 1995;311(7018):1457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 11. Sjögren P, Becker W, Warensjö E, Olsson E, Byberg L, Gustafsson I-B, Karlström B, Cederholm T. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: a cohort study in Sweden. Am J Clin Nutr. 2010;92(4):967–74. [DOI] [PubMed] [Google Scholar]
- 12. McNaughton SA, Bates CJ, Mishra GD. Diet quality is associated with all-cause mortality in adults aged 65 years and older. J Nutr. 2011;142(2):320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I et al.. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H et al.. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
- 15. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–28. [DOI] [PubMed] [Google Scholar]
- 17. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6(1):40. [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 21. Duval S, Tweedie R.. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98. [Google Scholar]
- 22. Liu Q, Cook NR, Bergström A, Hsieh C-C. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose-response data. Comput Stat Data Anal. 2009;53(12):4157–67. [Google Scholar]
- 23. Lasheras C, Fernandez S, Patterson AM. Mediterranean diet and age with respect to overall survival in institutionalized, nonsmoking elderly people. Am J Clin Nutr. 2000;71(4):987–92. [DOI] [PubMed] [Google Scholar]
- 24. Jacobs S, Harmon BE, Ollberding NJ, Wilkens LR, Monroe KR, Kolonel LN, Le Marchand L, Boushey CJ, Maskarinec G. Among 4 diet quality indexes, only the alternate Mediterranean diet score is associated with better colorectal cancer survival and only in African American women in the multiethnic cohort—3. J Nutr. 2016;146(9):1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ratjen I, Schafmayer C, di Giuseppe R, Waniek S, Plachta-Danielzik S, Koch M, Nöthlings U, Hampe J, Schlesinger S, Lieb W. Postdiagnostic Mediterranean and healthy Nordic dietary patterns are inversely associated with all-cause mortality in long-term colorectal cancer survivors. J Nutr. 2017;147(4):636–44. [DOI] [PubMed] [Google Scholar]
- 26. Stewart RA, Wallentin L, Benatar J, Danchin N, Hagström E, Held C, Husted S, Lonn E, Stebbins A, Chiswell K. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high-risk patients with stable coronary heart disease. Eur Heart J. 2016;37(25):1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knoops KT, de Groot LC, Kromhout D, Perrin A-E, Moreiras-Varela O, Menotti A, Van Staveren WA. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292(12):1433–9. [DOI] [PubMed] [Google Scholar]
- 28. Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ. 2009;338:b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tong TY, Wareham NJ, Khaw K-T, Imamura F, Forouhi NG. Prospective association of the Mediterranean diet with cardiovascular disease incidence and mortality and its population impact in a non-Mediterranean population: the EPIC-Norfolk study. BMC Med. 2016;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lassale C, Gunter MJ, Romaguera D, Peelen LM, Van der Schouw YT, Beulens JW, Freisling H, Muller DC, Ferrari P, Huybrechts I. Diet quality scores and prediction of all-cause, cardiovascular and cancer mortality in a pan-European cohort study. PLoS One. 2016;11(7):e0159025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocké MC, Peeters PH, van der Schouw YT, Boeing H, Hoffmann K, Boffetta P. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005;330(7498):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Booth JN III, Colantonio LD, Howard G, Safford MM, Banach M, Reynolds K, Cushman M, Muntner P. Healthy lifestyle factors and incident heart disease and mortality in candidates for primary prevention with statin therapy. Int J Cardiol. 2016;207:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whalen KA, Judd S, McCullough ML, Flanders WD, Hartman TJ, Bostick RM. Paleolithic and Mediterranean diet pattern scores are inversely associated with all-cause and cause-specific mortality in adults. J Nutr. 2017;147(4):612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martínez-González MA, Guillén-Grima F, De Irala J, Ruíz-Canela M, Bes-Rastrollo M, Beunza JJ, López del Burgo C, Toledo E, Carlos S, Sánchez-Villegas A. The Mediterranean diet is associated with a reduction in premature mortality among middle-aged adults. J Nutr. 2012;142(9):1672–8. [DOI] [PubMed] [Google Scholar]
- 35. Alvarez-Alvarez I, Zazpe I, de Rojas JP, Bes-Rastrollo M, Ruiz-Canela M, Fernandez-Montero A, Hidalgo-Santamaría M, Martínez-González MA. Mediterranean diet, physical activity and their combined effect on all-cause mortality: the Seguimiento Universidad de Navarra (SUN) cohort. Prev Med. 2017;106:45–52. [DOI] [PubMed] [Google Scholar]
- 36. Shvetsov YB, Harmon BE, Ettienne R, Wilkens LR, Le Marchand L, Kolonel LN, Boushey CJ. The influence of energy standardisation on the alternate Mediterranean diet score and its association with mortality in the Multiethnic Cohort. Br J Nutr. 2016;116(9):1592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101(3):587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cardenas-Fuentes G, Subirana I, Martinez-Gonzalez MA, Salas-Salvado J, Corella D, Estruch R, Fito M, Munoz-Bravo C, Fiol M, Lapetra J et al.. Multiple approaches to associations of physical activity and adherence to the Mediterranean diet with all-cause mortality in older adults: the PREvencion con DIeta MEDiterranea study. Eur J Nutr. 2018;25:1–10. [DOI] [PubMed] [Google Scholar]
- 39. Neelakantan N, Koh W-P, Yuan J-M, van Dam RM. Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among Chinese Adults. J Nutr. 2018;148(8):1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stefler D, Malyutina S, Kubinova R, Pajak A, Peasey A, Pikhart H, Brunner EJ, Bobak M. Mediterranean diet score and total and cardiovascular mortality in Eastern Europe: the HAPIEE study. Eur J Nutr. 2017;56(1):421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bo S, Ponzo V, Goitre I, Fadda M, Pezzana A, Beccuti G, Gambino R, Cassader M, Soldati L, Broglio F. Predictive role of the Mediterranean diet on mortality in individuals at low cardiovascular risk: a 12-year follow-up population-based cohort study. J Transl Med. 2016;14(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bonaccio M, Di Castelnuovo A, Costanzo S, Persichillo M, De Curtis A, Donati MB, De Gaetano G, Iacoviello L; MOLI-SANI Study Investigators. Adherence to the traditional Mediterranean diet and mortality in subjects with diabetes. Prospective results from the MOLI-SANI study. Eur J Prev Cardiol. 2016;23(4):400–7. [DOI] [PubMed] [Google Scholar]
- 43. Cuenca-García M, Artero EG, Sui X, Lee D-C, Hebert JR, Blair SN. Dietary indices, cardiovascular risk factors and mortality in middle-aged adults: findings from the Aerobics Center Longitudinal Study. Ann Epidemiol. 2014;24(4):297–303.. e2. [DOI] [PubMed] [Google Scholar]
- 44. George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180(6):616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kouris-Blazos A, Gnardellis C, Wahlqvist ML, Trichopoulos D, Lukito W, Trichopoulou A. Are the advantages of the Mediterranean diet transferable to other populations? A cohort study in Melbourne, Australia. Br J Nutr. 1999;82(1):57–61. [DOI] [PubMed] [Google Scholar]
- 46. Lagiou P, Trichopoulos D, Sandin S, Lagiou A, Mucci L, Wolk A, Elisabete E, Adami H-O. Mediterranean dietary pattern and mortality among young women: a cohort study in Sweden. Br J Nutr. 2006;96(2):384–92. [DOI] [PubMed] [Google Scholar]
- 47. Limongi F, Noale M, Gesmundo A, Crepaldi G, Maggi S, Group IW. Adherence to the Mediterranean diet and all-cause mortality risk in an elderly Italian population: data from the ILSA study. J Nutr Health Aging. 2017;21(5):505–13. [DOI] [PubMed] [Google Scholar]
- 48. Park Y, Steck S, Fung T, Zhang J, Hazlett L, Han K, Merchant A. Mediterranean diet and mortality risk in metabolically healthy obese and metabolically unhealthy obese phenotypes. Int J Obes. 2016;40(10):1541–9. [DOI] [PubMed] [Google Scholar]
- 49. Prinelli F, Yannakoulia M, Anastasiou CA, Adorni F, Di Santo SG, Musicco M, Scarmeas N, Leite MLC. Mediterranean diet and other lifestyle factors in relation to 20-year all-cause mortality: a cohort study in an Italian population. Br J Nutr. 2015;113(6):1003–11. [DOI] [PubMed] [Google Scholar]
- 50. Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144(6):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tognon G, Lissner L, Sæbye D, Walker KZ, Heitmann BL. The Mediterranean diet in relation to mortality and CVD: a Danish cohort study. Br J Nutr. 2014;111(1):151–9. [DOI] [PubMed] [Google Scholar]
- 52. Tognon G, Nilsson LM, Lissner L, Johansson I, Hallmans G, Lindahl B, Winkvist A. The Mediterranean diet score and mortality are inversely associated in adults living in the subarctic region. J Nutr. 2012;142(8):1547–53. [DOI] [PubMed] [Google Scholar]
- 53. Tognon G, Rothenberg E, Eiben G, Sundh V, Winkvist A, Lissner L. Does the Mediterranean diet predict longevity in the elderly? A Swedish perspective. Age. 2011;33(3):439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Van den Brandt PA. The impact of a Mediterranean diet and healthy lifestyle on premature mortality in men and women. Am J Clin Nutr. 2011;94(3):913–20. [DOI] [PubMed] [Google Scholar]
- 55. Vormund K, Braun J, Rohrmann S, Bopp M, Ballmer P, Faeh D. Mediterranean diet and mortality in Switzerland: an alpine paradox?. Eur J Nutr. 2015;54(1):139–48. [DOI] [PubMed] [Google Scholar]
- 56. Hodge AM, Bassett JK, Dugue PA, Shivappa N, Hebert JR, Milne RL, English DR, Giles GG. Dietary inflammatory index or Mediterranean diet score as risk factors for total and cardiovascular mortality. Nutr Metab Cardiovasc Dis. 2018;28(5):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Warensjo Lemming E, Byberg L, Wolk A, Michaelsson K. A comparison between two healthy diet scores, the modified Mediterranean diet score and the Healthy Nordic Food Index, in relation to all-cause and cause-specific mortality. Br J Nutr. 2018;119(7):836–46. [DOI] [PubMed] [Google Scholar]
- 58. Bosma-den Boer MM, van Wetten M-L, Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr Metab. 2012;9(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nordmann AJ, Suter-Zimmermann K, Bucher HC, Shai I, Tuttle KR, Estruch R, Briel M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am J Med. 2011;124(9):841–51.. e2. [DOI] [PubMed] [Google Scholar]
- 60. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–73. [DOI] [PubMed] [Google Scholar]
- 61. Schwingshackl L, Bogensberger B, Benčič A, Knüppel S, Boeing H, Hoffmann G. Effects of oils and solid fats on blood lipids: a systematic review and network meta-analysis. J Lipid Res. 2018;59(9):1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean diet; a literature review. Nutrients. 2015;7(11):9139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hoffman R, Gerber M.. Evaluating and adapting the Mediterranean diet for non-Mediterranean populations: a critical appraisal. Nutr Rev. 2013;71(9):573–84. [DOI] [PubMed] [Google Scholar]
- 64. Salas-Salvadó J, Fernández-Ballart J, Ros E, Martínez-González M-A, Fitó M, Estruch R, Corella D, Fiol M, Gómez-Gracia E, Arós F. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. 2008;168(22):2449–58. [DOI] [PubMed] [Google Scholar]
- 65. Panagiotakos DB, Milias GA, Pitsavos C, Stefanadis C. MedDietScore: a computer program that evaluates the adherence to the Mediterranean dietary pattern and its relation to cardiovascular disease risk. Comput Methods Programs Biomed. 2006;83(1):73–7. [DOI] [PubMed] [Google Scholar]
- 66. Zaragoza-Martí A, Cabañero-Martínez MJ, Hurtado-Sánchez JA, Laguna-Pérez A, Ferrer-Cascales R. Evaluation of Mediterranean diet adherence scores: a systematic review. BMJ Open. 2018;8(2):e019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.