Abstract
Background:
Organ scarcity continues in solid organ transplantation, such that the availability of organs limits the number of people able to benefit from transplantation. Medical advancements in managing end-stage organ disease have led to an increasing demand for multi-organ transplant, wherein a patient with multi-organ disease receives more than one organ from the same donor. Current allocation schemes give priority to multi-organ recipients over single-organ transplant recipients, which raises ethical questions regarding equity and utility.
Methods:
We use simultaneous liver-kidney (SLK) transplant, a type of multi-organ transplant, as a case study to examine the tension between equity and utility in multi-organ allocation. We adapt the health economics willingness-to-pay threshold to a solid organ transplant setting by coining a new metric: the willingness-to-transplant (WTT) threshold.
Results:
We demonstrate how the WTT threshold can be used to evaluate different SLK allocation strategies by synthesizing utility and equity perspectives.
Conclusions:
We submit that this new framework enables us to distill the question of SLK allocation down to: what is the minimum amount of benefit we require from a deceased donor kidney to allocate it for a particular indication? Addressing the above question will prove helpful to devising a rational system of SLK allocation and is applicable to other transplant settings.
Introduction
Multi-organ transplantation, in which multiple organs from the same donor are allocated to the same patient, has become increasingly common. Ongoing organ scarcity has led to renewed examination of the ethics of this practice.1,2 Take simultaneous liver and kidney (SLK) transplants as an example. In 2015, 5% of all deceased donor kidneys transplanted were allocated to SLK recipients.3 Prior to 2017, no standardized eligibility criteria existed for SLK listing, leading to great inter-institutional variability. In 2017, the Organ Procurement and Transplantation Network (OPTN) implemented two major changes.4 First, the OPTN established medical eligibility criteria for SLK candidates based on severity of kidney disease. Secondly, they established a “Safety Net” to prioritize liver-alone transplant (LAT) recipients with sustained or new kidney failure within one year post transplant, the rationale being that LAT recipients have an increased medical urgency for kidney-alone transplant (KAT) compared to non-LAT recipients awaiting KAT.
While establishing the SLK eligibility criteria improved consistency among transplant programs, there remain significant ethical questions about prioritization of patients with multi-organ diseases over single-organ transplant candidates in the allocation of the nonprimary organ.1 In 2018, in recognition of the importance of this point, the OPTN/United Network of Organ Sharing (UNOS) Ethics Committee completed a White Paper regarding the ethics of multi-organ transplantation.2 This paper identifies the prioritization of multi-organ over single-organ transplantation as an ethical dilemma with a potential impact on equity and utility. In this paper, we summarize the ethical principles guiding organ allocation policies, evaluate these principles with respect to SLK allocation in particular, and propose a framework to incorporate some considerations of equity into utility to create a concrete approach to deciding how to allocate kidneys among competing patient populations.
Ethical principles governing organ allocation
The three ethical principles that govern the allocation of organs for transplantation are equity, utility, and respect for persons.5 We will focus on equity and utility, as they most frequently come into conflict in multi-organ transplantation.2 Equity is based on the principle of justice, which requires that equals are treated equally and, where the demand outstrips supply, unequals unequally.6 Its practical application to allocation requires specification of what it means to be equal and unequal using material principles of justice. These principles set criteria for priority such as urgency or waiting time. An equitable allocation system aims to develop criteria to queue patients to maintain transparency and grant those with equal claim to transplantation an equal opportunity to receive a transplant.
The principle of utility states that an action is right if it promotes as much or more aggregate good than an alternative action.5 In terms of allocating organs, aggregate good can be measured in the maximal good gained from each organ, e.g. allograft survival, patient survival, or quality-adjusted survival. Allocating organs according to the principle of utility requires a focus on achieving the best outcomes for the aggregate of organs being transplanted.
In an ideal world, the principles of utility and equity would align such that the most equitable distribution of organs also creates the best transplant outcomes. However, such alignment is not always the case. The sickest patients in most need of organs frequently have higher post transplant morbidity and mortality than less-sick patients, so organs allocated according to urgency often result in lower gains in utility than alternative strategies.2 SLK allocation exemplifies this conflict between equity and utility.
Equity in SLK allocation
For single-organ, deceased donor transplantation, each organ has a unique allocation scheme. In multi-organ transplants, a primary organ drives allocation and the secondary organ “follows” the primary organ.2 For SLK, the liver is the primary organ, so allocation follows the MELD system, based on the urgent need for a liver, and not a kidney, transplant. In brief, the urgency principle of liver allocation takes precedence over the waiting time principle of kidney allocation. The key question that arises with respect to equity is: Does the urgency for liver transplant extend to the kidney transplant in the setting of SLK? Several studies comparing outcomes in SLK versus LAT recipients shed light on this issue. One study of the United States (US) solid organ transplant registry 2002–2009 examined this question using propensity score matching to account for observed differences among SLK and LAT recipients. These investigators observed significantly lower mortality (roughly half) in the first year post transplant when comparing SLK to LAT, translating to a modest but statistically significant 1–4 month gain in 5-year mean post transplant survival for SLK over LAT.7 Another study using the same database over roughly the same period compared the same two groups, but restricted the analysis to patients listed for SLK in an effort to compare a more homogeneous group of patients.8 In this study, SLK also provided a survival benefit compared to LAT (76% vs 55% at 5 years).
Together, these studies suggest that SLK possibly confers a survival benefit for patients over LAT, but the kidney is likely life-enhancing or life-saving only in the intermediate-to-long term rather than immediately life-saving.2 Maintaining this distinction is important to defining medical urgency. In the case of SLK, there is insufficient evidence to support or refute extending urgency principle for liver allocation to the allocation of a kidney from the same donor.
Utility in SLK allocation
In SLK, outcomes pertaining to the utility of the transplanted kidney include mortality and allograft failure. The overarching question from a utility standpoint is whether the outcomes of kidney transplant in SLK are acceptable as compared to the alternative use of these kidneys in KAT recipients.
Two donor-matched pair analyses of kidney allografts, in which one kidney from the donor went to an SLK recipient and the other a KAT recipient, found significantly lower patient and graft survival in SLK versus KAT and quantified the difference between adjusted 10-year mean allograft lifespan for KAT versus SLK transplant as 1.25 years, a difference driven mostly by the high post transplant mortality in SLK recipients.3,9
Furthermore, the quality of kidneys allocated to SLK patients is generally excellent. Approximately half of the kidneys allocated to SLK recipients belong to the highest quality 35% of kidneys transplanted.4 These kidneys are typically reserved for pediatric and the healthiest KAT candidates most in need of a long-functioning kidney allograft.
Taken together, these studies show that SLK recipients have inferior kidney allograft survival compared to KAT recipients. They also have higher rates of kidney allograft loss from primary nonfunction or early death, wherein no benefit from the kidney transplant is obtained. Allocating higher quality kidneys to recipients with demonstrably worse outcomes seems incongruent with the goal of maximizing utility in kidney transplantation.
Available Allocation Strategies for Liver Transplant Candidates
The medical eligibility criteria of OPTN are fairly liberal in terms of the definition of kidney disease in the setting of end-stage liver disease warranting kidney transplantation, as highlighted by two recent editorials.10,11 For our analysis, we will therefore term the 2017 OPTN SLK policy the “Liberal SLK + Safety Net” strategy. We term a narrower set of allocation criteria—only patients with sustained dialysis needs (dialysis-dependent≥6 weeks) or such metabolic disorders as primary hyperoxaluria are afforded SLK transplant, with LAT recipients relying on the Safety Net if their kidney function deteriorates after transplant—the “Few SLK + Safety Net” strategy. Finally, the most conservative system limits SLK and gives no weight to the medical urgency of LAT recipients (the “Few SLK + No Safety Net” strategy). The rest of this manuscript will present a framework for quantitatively incorporating the equity and utility principles outlined above.
Methods
Willingness-to-Transplant Threshold: A New Theoretic Construct
Health economists have long grappled with choosing health interventions within budgetary constraints. The most commonly used criterion is the willingness-to-pay (WTP) threshold: the maximum amount a health system is willing to pay for a quality-adjusted life-year (QALY). The WTP threshold reflects12:
Society’s monetary valuation of health gains, estimating the marginal benefits to consumers of healthcare services (“demand”);
The opportunity cost resulting from displacement of competing strategies, estimating the marginal costs of healthcare spending (“supply”).
We can translate the above framework into the context of deceased donor organ allocation. Just as the medical system is constrained by resources and not by demand, so the number of deceased donor transplants is limited by organ supply and not by organ demand. An equivalent of the WTP threshold in transplantation, using a deceased donor organ as a “currency” in the setting of scarcity, should therefore reflect the marginal benefits to the recipient of that organ (“demand”) and the opportunity cost resulting from not using the organ for another indication (“supply”). We define a willingness-to-transplant (WTT) threshold as the minimum number of QALYs resulting from a deceased donor organ transplant for which we are willing to allocate the organ. The WTT and WTP thresholds are mathematical inverses: WTT is the minimum benefit acceptable for a given unit of cost (in deceased donor organs), whereas the WTP is the maximal cost (in healthcare dollars) acceptable for a given unit of benefit. To keep our conversation limited to organ allocation, we are restricting considerations of costs in transplantation to “organ cost” and excluding considerations of monetary costs related to organ replacement therapies. In principle, our WTT framework is extensible to include these as well.
The WTP threshold enables ranking of strategies. To do so, the net benefit of each strategy, expressed in monetary terms (net monetary benefit, or NMB), is calculated as13:
The NMB is a theoretical construct that yields the population-level gain of a given strategy in monetary terms less the cost. At each WTP threshold, the strategy producing the highest NMB is preferred.14 Consider the hypothetical example in Table 1, in which three interventions are evaluated for outcome (QALYs) and costs, and where WTP threshold is set to be $10,000 or $50,000 per QALY gained. Strategy 3 yields the most QALYs at the highest cost. Where the WTP threshold is $50,000 per QALY, strategy 3 is preferred; its higher costs are offset by its better outcomes. When the WTP threshold is lowered to $10,000 per QALY, as it may be in a more resource-restrained setting, strategy 2 yields the highest NMB and is preferred. In no case is the least expensive strategy preferred, but should we lower the WTP threshold further, strategy 1 will eventually be preferred. The WTP analysis, is therefore an intuitive way to integrate health outcomes, cost, and willingness to exchange one for the other.
Table 1.
A hypothetical example illustrating how to rank medical strategies based on net monetary benefit. The highest NMB for each WTP threshold is highlighted in grey.
| Strategy | Outcome (QALY) | Cost | NMB (WTP=$10,000) | NMB (WTP=$50,000) |
|---|---|---|---|---|
| 1 | 6.00 | $12,000 | $48,000 | $288,000 |
| 2 | 8.00 | $30,000 | $50,000 | $370,000 |
| 3 | 10.00 | $80,000 | $20,000 | $420,000 |
By analogy, we can set the net benefit in terms of QALY resulting from in transplantation (net transplant benefit, or NTB) as:
The NTB is an analog of the NMB; the terms are shifted as the WTP/WTT thresholds are mathematical inverses. We can think about the NTB as the population-level gain in QALY (for all recipients of that organ) less the “cost” in the number of organs at a set WTT threshold. Consider the hypothetical example in Table 2, in which 3 transplant allocation strategies are evaluated for outcome (QALYs) and organ use, and where the WTT threshold is set to be 1.0 or 5.0 QALY per organ. Strategy 3 leads to the best population-level outcomes but uses the most organs. Where the WTT threshold is 1 QALY per organ, strategy 3 is preferred. When the WTT threshold is increased to 5, i.e., when we choose to only allocate an organ where the expected benefit is at least 5 QALYs, strategy 2, which uses fewer organs than strategy 3, is preferred. Should we increase the WTT threshold further, strategy 1 would eventually be preferred, as the strategy most parsimonious with organs.
Table 2.
A hypothetical example illustrating how to rank medical strategies based on net transplant benefit. The highest NTB for each WTT threshold is highlighted in grey.
| Strategy | Outcome (QALY) | Cost (Organs) | NTB (WTT=1.0 QALY/organ) |
NTB (WTT=5.0 QALY/organ) |
|---|---|---|---|---|
| 1 | 6.00 | 1.00 | 5.00 | 1.00 |
| 2 | 8.00 | 1.20 | 6.80 | 2.00 |
| 3 | 10.00 | 1.80 | 8.20 | 1.00 |
Applying the Willingness-to-Transplant Threshold to Liver-Kidney Allocation Strategies
In our previous work, we performed a modified cost-effectiveness analysis of various liver-kidney transplant strategies. Details of the model may be found in our original paper and the online supplemental materials.15 Briefly, the study consisted of two parts:
We developed a Markov model for liver transplant candidates, using a cohort assembled from Scientific Registry of Transplant Recipients (SRTR) 2002–2013 data (derivation cohort) to recreate post-SLK and LAT trajectories over the same time period. In this Markov model, we followed each theoretical patient for a lifetime, wherein he/she moved through discrete health states post-SLK or LAT (functioning kidney, nonfunctioning kidney requiring dialysis, recipient of transplant kidney, death, etc.). We calculated most of the parameters pertaining to clinical outcomes, including rates of events and time to kidney-after-liver transplant, from this derivation cohort. The remainder of the parameters, mostly related to counterfactual scenarios (what a SLK recipient’s event rates would be had he/she received a LTA and vice versa), we obtained from literature and tested in an extensive series of sensitivity analyses. We validated the model by plotting the actual versus modelled patient outcomes, using SRTR data from different time periods as validation datasets.
We then applied the same model to a theoretically cohort of 55-year-old, first-time liver transplant candidates whose kidney disease profile matched that of SRTR liver transplant (both SLK and LTA) recipients from 2002–2013. We assessed the outcomes and organ utilization of this theoretical cohort under various liver-transplant allocation strategies: the current OPTN system (Liberal SLK + Safety Net), Few SLK + Safety Net, and Few SLK + No Safety Net). Our primary outcome measures were life-years (LYs) and QALYs. Our primary organ utilization measure was the number of deceased donor kidneys deployed per liver transplant recipient. The model highlighted a trade-off between outcomes and organ utilization: per each liver transplant recipient, strategies more liberal with kidney transplants (Liberal SLK + Safety Net) use more deceased donor kidneys and deliver more LYs and QALYs.
In this study, using the aforementioned model, we performed 1 000 000 simulations of the post transplant course of liver transplant recipients under each liver-kidney allocation strategy. In each simulation, we applied the WTT threshold analysis. At each WTT threshold, we estimated the following:
The probability that any given transplant strategy is optimal (percent of simulations in which the strategy yielded the highest NTB);
Whether any strategy yields a greater NTB than the other strategies at the p<0.05 confidence level (assessed by comparing the 95% confidence intervals of the NTB yielded by each strategy).
The first metric allows us to identify the most probably optimal transplant strategy. The second metric allows us to quantify our degree of certainty that identified strategy yields a greater NTB. Our study used de-identified registry data and was approved by the Institutional Review Board at Stanford University in accordance with the Declaration of Helsinki (protocol number IRB-40876).
Results
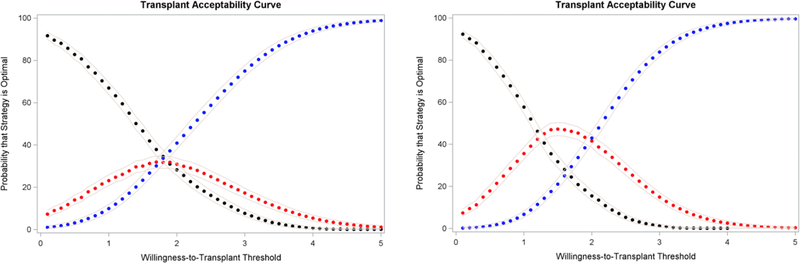
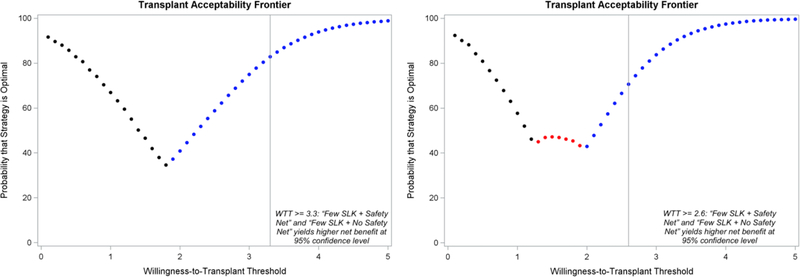
Our results are presented in Figure 1. Whether we use LY (left panel) or QALY (right panel), results are consistent. At lower WTT thresholds (1–2 QALY/LY per kidney), the Liberal SLK + Safety Net strategy (black) yields the highest NTB in a higher percentage of simulations and is preferred. At higher WTT thresholds, the more parsimonious transplant strategies are preferred. The overall transplant acceptability frontier—the optimal kidney transplant strategy at each willingness-to-transplant threshold—is depicted in Figure 2. At WTT thresholds of 3.3 LY or 2.6 QALY per kidney or higher (represented by the grey vertical lines in Figure 2), we can be sure (p<0.05) that the two strategies more parsimonious with SLK, with or without Safety Net (blue and red), yield a higher NTB than the liberal SLK + Safety Net strategy.
Figure 1.
The probability that each strategy is optimal under different WTT thresholds, the effectiveness measure being LY (left panel) or QALY (right panel). Black = Liberal SLK (current OPTN SLK criteria) + Safety Net; Red = Few SLK (only patients with prolonged dialysis or metabolic disorder) + Safety Net; Blue = Few SLK + No Safety Net. Grey lines represent the 95% confidence band.
Figure 2.
The optimal kidney transplant strategy at each willingness-to-transplant threshold, the effectiveness measure being LY (left panel) or QALY (right panel). Black = Liberal SLK (current OPTN SLK criteria) + Safety Net; Red = Few SLK (only patients with prolonged dialysis or metabolic disorder) + Safety Net; Blue = Few SLK + No Safety Net.
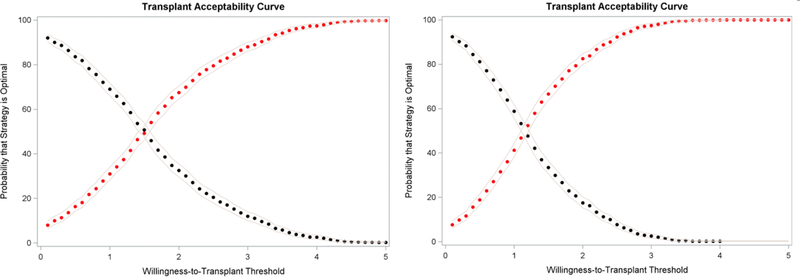
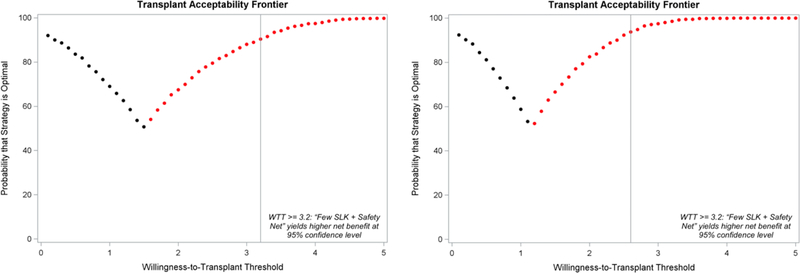
We can restrict this analysis to only allocation strategies considered acceptable. For instance, we can limit analysis to only strategies that contain the Safety Net (Figure 3). At WTT threshold at or lower than 1.5 LY or 1 QALY per kidney used, the “Liberal SLK + Safety Net” strategy yields the highest NTB in a higher percentage of simulations and is preferred; above that WTT threshold, the “Few SLK + Safety Net” strategy is preferred. At WTT thresholds of 3.2 LY or 2.6 QALY or higher per kidney, we can be sure (p<0.05) that the “Few SLK + Safety Net” strategy yields a higher NTB (Figure 4).
Figure 3.
Optimal kidney transplant strategy under different WTT thresholds, the efficacy measure being LY (left panel) or QALY (right panel). Black = Liberal SLK (current OPTN SLK criteria) + Safety Net; Red = Few SLK (only patients with prolonged dialysis or metabolic disorder) + Safety Net. Grey lines represent the 95% confidence band.
Figure 4.
The optimal kidney transplant strategy at each willingness-to-transplant threshold, the effectiveness measure being LY (left panel) or QALY (right panel). Black = Liberal SLK (current OPTN SLK criteria) + Safety Net; Red = Few SLK (only patients with prolonged dialysis or metabolic disorder) + Safety Net.
Discussion
In summary, we present a novel method of framing the liver-kidney allocation debate using a willingness-to-transplant (WTT) threshold. The basic allocation quandary arises from a critical shortage of organ supplies. From the utilitarian perspective, uses of organs leading to the greatest benefits (i.e. gains in LY or QALY) ought to be prioritized, of which SLK is not one. From an equity standpoint, patients with a chance to benefit from a transplant (based on the criteria established by the material principles), including SLK candidates, ought to have equal access to organs. A WTT threshold makes explicit preferences regarding this tradeoff: in essence, it characterizes the smallest benefit gain we are willing to accept under current conditions of organ shortage and state of technology. While its essential framework is utilitarian, it incorporates aspects of the equity principle by establishing an accepted benefit “floor” for strategies: any strategy that can bring its intended population enough benefit to cross the WTT threshold will be considered, even if competing strategies result in higher net benefits (i.e. utility). In an unlimited resource setting, a reasonable WTT threshold would be just above zero—anyone who has an opportunity to benefit, no matter how small, has access to transplant. In a purely utilitarian system, the WTT threshold may be as high as 10 life-years, which is the expected benefit from kidney transplant gained by young patients without diabetes.16 A purely utilitarian allocation system may therefore reserve organs for the candidates most likely to benefit (i.e. young candidates with single-organ diseases) and exclude sicker candidates (i.e. multi-organ transplant candidates). In a setting of severe scarcity, we need to consider the difficult question: What is the appropriate willingness-to-transplant threshold for a kidney?
A reasonable reference point may be the expected LY or QALY gained by the KAT recipient with the lowest expected survival benefit over dialysis. As the expected benefit of the “worst” kidney candidate, this number would best reflect the accepted minimal value of the kidney transplant from a patient and societal perspective. The now-accepted metric for kidney candidate quality is the estimated post transplant survival (EPTS) score, a 4-part metric based on age, dialysis duration, diabetes mellitus and prior solid organ transplant.17 Table 3 summarizes the survival benefits in various KAT subgroups with the highest EPTS scores. The relative mortality risk reduction conferred by KAT (inverse of the hazard ratio) is nearly constant across all these high-risk groups and correspond to a gain from transplant of about 3 life-years.16 This number corresponds to the threshold at which we can be fairly confident that a liver-kidney transplant strategy more sparing of kidneys is preferable to the current strategy. In other words, if we accept a WTT of 3 life-years, the next reasonable incremental policy change would be to increase threshold at which liver transplant candidates are eligible for SLK transplant.
Table 3.
Prior literature on benefit of kidney transplant (relative to dialysis) in high EPTS patient cohorts.
| Study | Population | Cohort | Survival Benefit | |
|---|---|---|---|---|
| Hazard Ratio (reference = waitlist) | LY from Transplant | |||
| Wolfe 199921 | Age 60–74 and diabetes | USRDS 1991–1997 | 0.46 (0.34–0.61) at 18-months post-tx | 3 |
| Wolfe 200821 | Age 65+ and diabetes | USRDS 1987–2006 | - | 3.36 (3.05–3.44) |
| Cassuto 201022 | Prior solid organ tx | UNOS 1995–2008 | 0.37–0.70 depending on organ type | - |
| Jay 201723 | Age 60+ and dialysis-dependent | UNOS 2003–2012 | 0.52 (0.45–0.61) at 12–24 months post-tx | - |
| Rose 201724 | Dialysis >10 years | SRTR 2005–2011 | 0.57 at 12 months post-tx | - |
The full WTT analysis, as we laid out here, requires consideration of the equity principle. The equity principle requires us to define how SLK or LAT patients with persistent kidney failure are different from KAT patients and determine whether these differences ought to translate into different prioritization for kidney transplantation. The key equity question regarding SLK transplantation is whether the medical urgency for liver transplant extends to the kidney transplant. The benefit of SLK versus sequential liver-kidney transplant (i.e. the Few SLK + Safety Net option) is the immediate gain from the simultaneous kidney transplant and thus is the rationale for medical urgency of the kidney transplant. If the kidney confers no immediate benefit, for instance, we would conclude that sequential liver-kidney transplant is the equivalent of SLK transplant and that we should not extend the medical urgency criteria to the simultaneous kidney transplant. Fully applying the WTT, as we have in this paper, therefore requires us to take the additional step of considering differential waitlist prioritization in conjunction with the threshold for transplantation. Even if we are willing to transplant patients who meet the WTT, are we also willing to prioritize them above other patients who also meet the WTT? The WTT for kidney not only sets a lower limit on what outcomes are acceptable for kidney transplantation in multi-organ transplantation, but it also sets a minimum outcome threshold for what patients should be prioritized above KAT patients.
Practically, the concept of the WTT may help clarify some less obvious trade-offs and help reduce ungainly incentives to transplant programs, e.g. according higher priority to multi-organ transplant candidates which may or may not be justified. For instance, the conclusion of UNOS White Paper states that “[w]hen the need for the second organ is unclear, a transplant center should proceed…only if there is a reasonable chance that the second organ is necessary”.2 How reasonable the chance has to be is implicitly determined by how much the organ is in shortage. The decision-analytic model behind WTT allows for that chance to be quantified: one can calculate the expected benefit to be derived from allocating the second organ (versus alternative strategies including sequential transplantation), then comparing it to the WTT.
Conceptually, the WTT may be applied to other organ types. Each organ would have its own WTT that is specific to the magnitude of expected benefit from standard uses of that organ. In addition to multi-organ transplants, single-organ transplants may benefit from consideration of WTT. For instance, the discussion of the candidacy of “marginal” candidates frequently revolves around whether the candidates may still reasonably be expected benefit from a transplant, not the extent of the benefit. Addition of an implicit value judgement (on whether the small benefit to the marginal candidate is sufficient to justify diverting the organ from a more robust candidate) is a consideration in this era of organ shortage, albeit controversial. A pure Homo Economicus stance, as implied by the WTT, is clearly far from the whole picture. Surveys of the general public have indicated a preference toward a pluralistic approach to allocation that includes conflicting principles.22 As public support and perception are indispensable to the practice of solid organ transplantation, studying the public reception of a WTT-based approach to allocation is a key next-step.
Our framework has multiple limitations. First, it is more complex compared to a simplified approach (i.e., comparing allocating a liver/kidney pair separately or to the same liver recipient23). We believe that the simplified approach leaves out important elements our framework incorporates. A patient who receives a liver transplant is not permanently blocked from kidney transplantation. Rather, the timing of the kidney transplant is delayed, and the extent of the delay depends on waitlist conditions. The simplified approach does not allow for this. Also, while the benefit from a SLK transplant is realized by one person, the organ cost of a SLK transplant is spread over the many queueing KAT candidates ahead of whom the SLK recipient received priority. As a result, we submit that our framework incorporates some important elements and, as a derivative of the WTP threshold analysis in health economics literature, also has theoretical grounding.
A second limitation is that our framework is predicated on the ability to model post transplant course. We rely on the best available data, admittedly incomplete and imperfect. For instance, it is difficult to model the course of an expedited kidney-after-liver transplantation under the Safety Net provision and almost impossible to account for the immunologic constraints in sequential organ transplants, since these transplants occurred rarely prior to 2017. Changes in provider and patient behaviors will also be difficult to anticipate or predict, although we can incorporate some of the changes we expect into the model. We anticipate that within a few years, we will have data from these Safety Net transplants to update our model and provide better estimates of outcomes and organ usage. We need better prospective studies, preferably interventional (for instance, assigning patients to SLK versus LAT with Safety Net), to quantify the anticipated effects of each strategy. Our work is meant to supplement, not supplant, these future studies to offer a synthesized framework for a rational and ethical liver-kidney allocation system.
A third limitation is that models can only offer population estimates. Physicians naturally feel uncomfortable reducing individuals to a few parameters and using population estimates for allocation purposes. Indeed, physicians have a professional and ethical responsibility to advocate for individual patients. We contend that, to mitigate the conflict between the duties of physicians toward individual patients versus the transplant patient population as a whole, organ allocation decisions should be made at a national policy level so as to standardize transplantation practices. This is in accordance with the Final Rule and consistent with the principles of distributive justice. As such, population estimates are indispensable.
Conclusion
An ethical analysis using the underlying principles of equity and utility reveals that there is still significant work to do in the area of multi-organ transplant allocation. We propose a new concept: the willingness-to-transplant threshold, which we adopted from the willingness-to-pay threshold in health economics literature. The willingness-to-transplant threshold helps synthesize conflicting ethical principles and distill the problem to: what is the minimum amount of benefit we are willing to accept from a deceased donor organ transplant? The focus of the transplant community should be on answering this question on ethical and empirical grounds. The methodologic framework in this paper can help translate that answer into evaluations of concrete allocation strategies. We submit that this construct will be helpful not only in the SLK allocation debate but may also be extended to other multi-organ allocation strategies.
Acknowledgments
Funding: Research reported here was supported by the John M. Sobrato Gift Fund (J.C.T.) and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K24DK092336 (W.R.K.) and K24 DK085446 (G.M.C.).
Abbreviations
- DDK
Deceased donor kidney
- EPTS
Estimated Post Transplant Survival
- KAT
Kidney alone transplant
- LAT
Liver alone transplant
- LY
Life-year
- LYFT
Life year from transplant
- MELD
Model for End-stage Liver Disease
- NMB
Net monetary benefit
- NTB
Net transplant benefit
- OPTN
Organ Procurement and Transplant Network
- UNOS
United Network of Organ Sharing
- QALY
Quality-adjusted life-year
- SLK
Simultaneous liver-kidney
- US
United States
- WTP
Willingness-to-pay
- WTT
Willingness-to-transplant
Footnotes
Disclosure: The authors declare no conflict of interest. Findings in this manuscript were partly reported in abstract form at the National Kidney Foundation Young Investigator Forum in April, 2017 and American Transplant Congress in May, 2017.
Works Cited
- 1.Reese PP, Veatch RM, Abt PL, et al. Revisiting multi-organ transplantation in the setting of scarcity. Am J Transplant 2014;14(1):21–26. doi: 10.1111/ajt.12557 [DOI] [PubMed] [Google Scholar]
- 2.OPTN/UNOS Ethics Committee. Organ Procurement and Transplantation Network. Ethical Implications of Multi-Organ Transplants January 2019. Available at https://optn.transplant.hrsa.gov/governance/public-comment/ethical-implications-of-multi-organ-transplants/ Published January 2019. Accessed February 1, 2019.
- 3.Cheng XS, Stedman MR, Chertow GM, et al. Utility in Treating Kidney Failure in End-Stage Liver Disease With Simultaneous Liver-Kidney Transplantation. Transplantation 2017;101(5):1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OPTN/UNOS Kidney Transplantation Committee. Simultaneous Liver Kidney (SLK) Allocation Policy Available at https://optn.transplant.hrsa.gov/media/1192/0815-12_SLK_Allocation.pdf Published August 2015. Accessed February 5, 2017.
- 5.Organ Procurement and Transplantation Network. Ethical Principles in the Allocation of Human Organs Available at https://optn.transplant.hrsa.gov/resources/ethics/ethical-principles-in-the-allocation-of-human-organs/ Published June 2015. Accessed November 21, 2018.
- 6.Beauchamp TL, Childress JF. Principles of Biomedical Ethics 6th ed. New York, NY: Oxford University Press; 2009. [Google Scholar]
- 7.Sharma P, Shu X, Schaubel DE, et al. Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pretransplant renal dysfunction. Liver Transpl 2016;22(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hmoud B, Kuo Y-F, Wiesner RH, et al. Outcomes of liver transplantation alone after listing for simultaneous kidney: comparison to simultaneous liver kidney transplantation. Transplantation 2015;99(4):823–828. [DOI] [PubMed] [Google Scholar]
- 9.Choudhury RA, Reese PP, Goldberg DS, et al. A Paired Kidney Analysis of Multiorgan Transplantation: Implications for Allograft Survival. Transplantation 2017;101(2):368–376. [DOI] [PubMed] [Google Scholar]
- 10.Wadei HM, Gonwa TA, Taner CB. Simultaneous Liver Kidney Transplant (SLK) Allocation Policy Change Proposal: Is It Really a Smart Move? Am J Transplant 2016;16(9):2763–2764. [DOI] [PubMed] [Google Scholar]
- 11.Asch WS, Bia MJ. New Organ Allocation System for Combined Liver-Kidney Transplants and the Availability of Kidneys for Transplant to Patients with Stage 4–5 CKD. Clin J Am Soc Nephrol 2017;12(5):848–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallejo-Torres L, García-Lorenzo B, Castilla I, et al. On the Estimation of the Cost-Effectiveness Threshold: Why, What, How? Value Health 2016;19(5):558–566. [DOI] [PubMed] [Google Scholar]
- 13.York Health Economics Consortium. Net Monetary Benefit Available at http://www.yhec.co.uk/glossary/net-monetary-benefit/ Published 2016. Accessed April 6, 2018.
- 14.Suen SC, Goldhaber-Fiebert J. An Efficient, Noniterative Method of Identifying the Cost-Effectiveness Frontier. Med Decis Making 2016;36(1):132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng XS, Kim WR, Tan JC, et al. Comparing Simultaneous Liver-Kidney Transplant Strategies: A Modified Cost-Effectiveness Analysis. Transplantation 2018;102(5):e219–e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe RA, McCullough KP, Schaubel DE, et al. Calculating life years from transplant (LYFT): methods for kidney and kidney-pancreas candidates. Am J Transplant 2008;8(4 Pt 2):997–1011. [DOI] [PubMed] [Google Scholar]
- 17.Clayton PA, McDonald SP, Snyder JJ, et al. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am J Transplant 2014;14(8):1922–1926. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341(23):1725–1730. [DOI] [PubMed] [Google Scholar]
- 19.Cassuto JR, Reese PP, Sonnad S, et al. Wait list death and survival benefit of kidney transplantation among nonrenal transplant recipients. Am J Transplant 2010;10(11):2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jay CL, Washburn K, Dean PG, et al. Survival Benefit in Older Patients Associated With Earlier Transplant With High KDPI Kidneys. Transplantation 2017;101(4):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose C, Gill J, Gill JS. Association of Kidney Transplantation with Survival in Patients with Long Dialysis Exposure. Clin J Am Soc Nephrol 2017;12(12):2024–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biggins SW. Futility and rationing in liver retransplantation: When and how can we say no? J Hepatol 2012;56(6):10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiberd B, Skedgel C, Alwayn I, et al. Simultaneous liver kidney transplantation: a medical decision analysis. Transplantation 2011;91(1):121–127. [DOI] [PubMed] [Google Scholar]