Abstract
Background and Aims:
To examine whether Heart Rate Variability (HRV) measures can be used to detect Neurocardiogenic Injury (NCI).
Methods:
326 consecutive admissions with aneurysmal subarachnoid hemorrhage (SAH) met criteria for the study. 56 of 326 subjects (17.2%) developed NCI which we defined by wall motion abnormality with ventricular dysfunction on transthoracic echocardiogram or cardiac troponin-I > 0.3 ng/mL without ECG evidence of coronary artery insufficiency. HRV measures (in time and frequency domains, as well as nonlinear technique of detrended fluctuation analysis) were calculated over the first 48 hours. We applied longitudinal multilevel linear regression to characterize the relationship of HRV measures with NCI and examine between group differences at baseline and over time.
Results:
There was decreased vagal activity in NCI subjects with a between group difference in Low/High Frequency Ratio (beta 3.42, SE 0.92, p=0.0002), with sympathovagal balance in favor of sympathetic nervous activity. All time-domain measures were decreased in SAH subjects with NCI. An ensemble machine learning approach translated these measures into a classification tool that demonstrated good discrimination using the area under the receiver operating characteristic curve (AUROC 0.82), the area under precision recall curve (AUPRC 0.75), and a correct classification rate of 0.81.
Conclusions:
HRV measures are significantly associated with our label of NCI and a machine learning approach using features derived from HRV measures can classify SAH patients that develop NCI.
Keywords: Data Mining, Neurocardiogenic, Heart Rate Variability, Subarachnoid Hemorrhage, Myocardial Stunning, Machine Learning
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) patients are at risk for neurocardiogenic injury (NCI) via a neurally mediated process of excessive catecholamine release by cardiac sympathetic nerve terminals.1, 2 Myocardial necrosis occurs with a release of cardiac enzymes unrelated to coronary artery insufficiency.2-4 It is a mostly reversible condition that can occur in the first 3 days in 15-22% of SAH patients3, 5-11 In its severe form, regional wall motion abnormalities (RWMA) and/or ventricular dysfunction (VD) can be seen on transthoracic echocardiogram (TTE).5, 8, 12 This is known as neurogenic stunned myocardium (NSM).13 The diagnosis of NCI is dependent on clinical triggers for cardiac troponin-I (cTI) and TTE testing; cTI indications include ECG changes or chest pain, while TTE indications include cTI levels > 0.3 ng/mL,3, 14 ECG abnormalities, symptoms of hypotension, chest pain, or arrhythmia.3, 15, 16 Studies of NSM expectedly suffer from biased samples in which not all SAH patients meet the clinical triggers to undergo TTE. NSM can be seen with cTI levels of < 0.3 ng/mL.7, 17
ECG monitoring is universal in the intensive care unit. Online computational analyses already enable ectopy and arrhythmia detection and alarm-setting for goal-directed clinical attention.18-20 A large body of literature suggests that HRV measures may play a role in classifying outcome in a variety of disease types including sepsis,21 stroke,22 trauma,23 and others.24, 25 In small studies of subjects with26 and without27 SAH (both n = 13), time-varying HRV measures were found to be associated with NSM.
We sought to test the association of HRV measures with NCI in SAH patients in a large dataset with consecutive SAH subjects with universal TTE per protocol. In addition, we explored whether HRV features could be used to build a machine learning classifier for NCI in SAH patients. There is no consensus definition of NCI; the label used to train our classifers was defined as an ejection fraction < 55% (abnormal) with hypokinesis/akinesis of any cardiac wall on TTE and without a history of cardiomyopathy or congestive heart failure, or cTI > 0.3 ng/mL without ECG evidence for coronary artery insufficiency.14, 17, 28
Materials and Methods
Patient Cohort
Consecutive patients with SAH admitted to the neurologic intensive care unit (NICU) were prospectively enrolled in an observational cohort study designed to identify novel risk factors for secondary injury and poor outcome. Inclusion criteria were SAH secondary to ruptured aneurysms that were secured (by clipping or coiling). We excluded patients with SAH secondary to perimesencephalic bleeds, trauma, arteriovenous malformation, patients < 18 years old, or history of congestive heart failure with decreased ejection fraction on initial transthoracic echocardiogram (only 1 patient). The study was approved by the Columbia University Medical Center Institutional Review Board. In all cases, written informed consent was obtained from the patient or a surrogate.
Cardiac Testing
All patients had an ECG and cTI on admission. cTI was repeated in all patients with an abnormal ECG (Q waves, QTc prolongation, ST-segment abnormalities, or T-wave inversion) or clinical signs or symptoms of potential cardiovascular dysfunction (pulmonary edema, hypertension or hypotension demonstrated by systolic blood pressure > 160 or < 100 mmHg respectively, arrhythmia, or chest pain).3, 15, 29 Standard practice at our institution was to obtain TTE within 3 days of admission on all SAH patients.15 All echocardiograms were interpreted by an attending cardiologist who was not blinded to the clinical status of the patient (diagnosis of SAH may have been known). Echocardiograms were performed using two-dimensional sector scanning, Doppler color flow mapping, and pulsed and continuous wave Doppler interrogation. Standard parasternal long axis, short axis, and apical 2- and 4- chamber views were obtained for analysis of WMA. Left ventricular ejection fraction (LVEF) was visually estimated and rated as normal (LVEF ≥ 55%), mildly (LVEF 40-54%), moderately (LVEF 30-39%), or severely (LVEF < 30%) depressed. Repeat TTE was performed as clinically indicated, as a result of new ECG changes, cTI > 0.3 ng/mL, arrhythmias, unexplained hemodynamic changes (e.g. falling blood pressure), or unexplained pulmonary edema.
Data Collection
Baseline demographic and clinical information were collected (Table 1), including age, sex, underlying conditions that influence autonomic nervous system activity or HRV interpretation (history of acute myocardial infarction, congestive heart failure, diabetes, history of beta-blocker use prior to hospitalization, and atrial fibrillation or arrhythmia), aneurysm location, ICU length of stay, Hunt Hess (HH) grade, Modified Fisher Scale (MFS), Glasgow Coma Scale (GCS), World Federation of Neurological Surgeons Grading System (WFNS), and DCI. ECG data was acquired using a high-resolution acquisition system (BedmasterEX; Excel Medical Electronics Inc, Jupiter, FL, USA) from General Electric Solar 8000i monitors (Port Washington, NY, USA; 2006-2013) at 240 Hz, from ICU admission to discharge. HRV analysis was restricted to non-overlapping 300-s (5-min) epochs of ECG data, as is standard practice. Features were created from summary statistics of 48 hours of HRV analyses since admission.
Table 1.
Characteristics of SAH patients with and without NCI
| Patient Characteristics | NCI+ (n=56) |
NCI− (n=270) |
p value a |
|---|---|---|---|
| Age, years (mean ± SD) | 53.23 ± 15.82 | 55.01 ±13.71 | 0.46 |
| Female Sex, % | 75.00 (42/56) |
68.52 (185/270) |
0.42 |
| Length of Intensive Care Unit Stay (mean ± SD) | 15.69 ± 8.15 | 12.84 ± 7.06 | 0.014 |
| History of Congestive Heart Failure, % | 1.79 (1/56) |
1.14 (3/264) |
0.54 |
| History of Beta-Blockers, % | 12.50 (7/56) |
12.96 (35/270) |
1.00 |
| History of Atrial Fibrillation/ Arrhythmia, % | 3.57 (2/56) |
0.76 (2/264) |
0.14 |
| History of Acute Myocardial Infarction, % | 1.79 (1/56) |
2.65 (7/264) |
1.00 |
| History of Diabetes, % | 5.36 (3/56) |
8.68 (23/265) |
0.59 |
| Aneurysm Location, % | 0.17 | ||
| Anterior | 53.57 (30/56) |
63.70 (172/270) |
|
| Posterior | 46.43 (26/56) |
36.30 (98/270) |
|
| Hunt Hess Grade, % | <.00001 | ||
| 1-3 | 37.50 (21/56) |
78.65 (210/267) |
|
| 4-5 | 62.50 (35/56) |
21.35 (57/267) |
|
| Modified Fisher Scale, % | 0.29 | ||
| 0-2 | 20.41 (10/49) |
28.92 (72/249) |
|
| 3-4 | 79.59 (39/49) |
71.08 (177/249) |
|
| Glasgow Coma Scale, % | < .00001 | ||
| 3-8 | 60.71 (34/56) |
20.97 (56/267) |
|
| 9-12 | 16.07 (9/56) |
9.74 (26/267) |
|
| 13-15 | 23.21 (13/56) |
69.29 (185/267) |
|
| World Federation of Neurological Surgeons Grading System, % | < .00001 | ||
| 1-3 | 23.21 (13/56) |
69.29 (185/267) |
|
| 4-5 | 76.79 (43/56) |
30.71 (82/267) |
|
| Delayed Cerebral Ischemia, % | 35.71 (20/56) |
24.44 (66/270) |
0.12 |
p values were calculated using Fisher exact test for frequency comparisons of categorical variables and Mann-Whitney U test for two-group comparisons of continuous variables. All statistical tests were two-tailed. Significant values are highlighted in bold. SAH = subarachnoid hemorrhage; NCI = neurocardiogenic injury; SD = standard deviation.
The targeted classification outcome was NCI, which currently lacks a consensus definition. We defined NCI as an ejection fraction < 55% (abnormal) with hypokinesis/akinesis of any cardiac wall on TTE and without a history of cardiomyopathy or congestive heart failure, or cTI > 0.3 ng/mL without ECG evidence for coronary artery insufficiency.14, 17, 28
Statistical Procedures
Frequency comparisons for categorical variables were performed by Fisher exact test. Two-group comparisons of continuous variables were performed with the non-parametric Mann-Whitney U test, to avoid any assumption about the distribution of the data. All statistical tests were two-tailed and a p-value < 0.05 was considered statistically significant.
To assess whether NCI discrimination could be achieved via commonly acquired clinical data, median HR, oxygen saturation (SPO2), systolic (SBP), and diastolic blood pressure (DBP) were compared.
R-wave detection was achieved by wavelet decomposition of ECG signal, using an algorithm verified on over 10,000 clinical studies.30 The algorithm includes artifact detection for the selection of artifact-free segments. More details of ECG analysis are provided in Appendix A. HRV analysis, per standard practice, was restricted to non-overlapping 300-s (5-min) epochs of ECG data.31 Various time- and frequency-domain methods were used to quantify HRV (descriptions in Appendix B). Time-domain measures included mean of normal R-R intervals (MNN), standard deviation of interbeat intervals (SDNN), coefficient of variation of interbeat intervals (CV of HR), root mean square of successive differences of interbeat intervals (RMSSD), and the Poincare plot statistics (SD1, SD2, and their ratio). Frequency-domain measures included normalized low frequency (LF) power, normalized high frequency (HF) power, and LF/HF ratio. A description of these methods is found in a consensus guide for the measurement and physiologic interpretation of ECG for clinical use.31 Detrended fluctuation analysis (DFA) is a nonlinear technique which quantifies the long-range correlation behavior in nonstationary physiological time series data.32 More details of DFA are provided in Appendix C.
We created longitudinal multilevel linear regression models where each HRV measure was the outcome (resulting in 12 separate models), and NCI was the exposure of interest. We studied whether expected values of the intercepts and slopes were affected by NCI; that is, the inter-group difference and intra-subject variability (accounting for multiple measurements per person) over time. These models enabled us to show if HRV is different between groups (NCI+/ NCI−), and whether the shape of the HRV trajectory changes over time. An alpha level of 0.05 was significant for these models. Statistical analysis was performed using SAS software (SAS Institute 2011, Cary, NC). See Appendix D for further explanation of the multilevel linear regression method.
While traditional statistical approaches can demonstrate significant between-group differences, they do not function as a translatable tool for classification in an individual.33, 34 In other words, while there may be significant between-group HRV differences, there is enough overlap of data points for the two groups that precludes distinction of a clinical diagnosis.35 To translate our findings into a tool for classification, we applied an ensemble machine learning approach. We computed summary statistics (mean, median, standard deviation, entropy, and slope) for each of the 12 HRV measures for the first 48 hours from the time of their admission resulting in 60 features. We applied minimal redundancy and maximal relevance (mRMR)36 criterion to select the features that maximally stratify these two classes. We maximized the mutual information between features and classes (i.e. relevance) while minimizing the mutual information among features (i.e. redundancy). These features were ranked in order of relevance and 25 features were selected empirically to maximize the performance of the classifier. We then built L1-regularized logistic regression (LR), linear and kernel support vector machine (SVM-L, SVM-K), random forest (RF), and ensemble classifiers. Ensemble classifiers (EC) are based on soft voting, which predicts the class labels based on the predicted probabilities for classifiers, i.e.,
Where is the computed probability by the voting classifier, Wj is the weight assigned to the jth classifier and i ∈ {0,1} for a binary classifier.
We performed nested five-fold cross-validation to tune model parameters and to report the accuracy. More details of how nested cross-validation is used to prevent over-fitting are provided in Appendix E. All classifiers were evaluated for good discrimination using the area under the receiver operating characteristic curve (AUROC). We report the median AUROC, ± standard deviation (SD). AUROC can generate overly optimistic estimates of discriminative performance when the frequency of events is rare.37-39 We therefore also calculated precision recall curves40 and confusion matrices to show the overall correct classification rates. Computation of features was performed in Matlab (Mathworks, Natick, MA) and machine learning models were built in Python (www.python.org).
Results
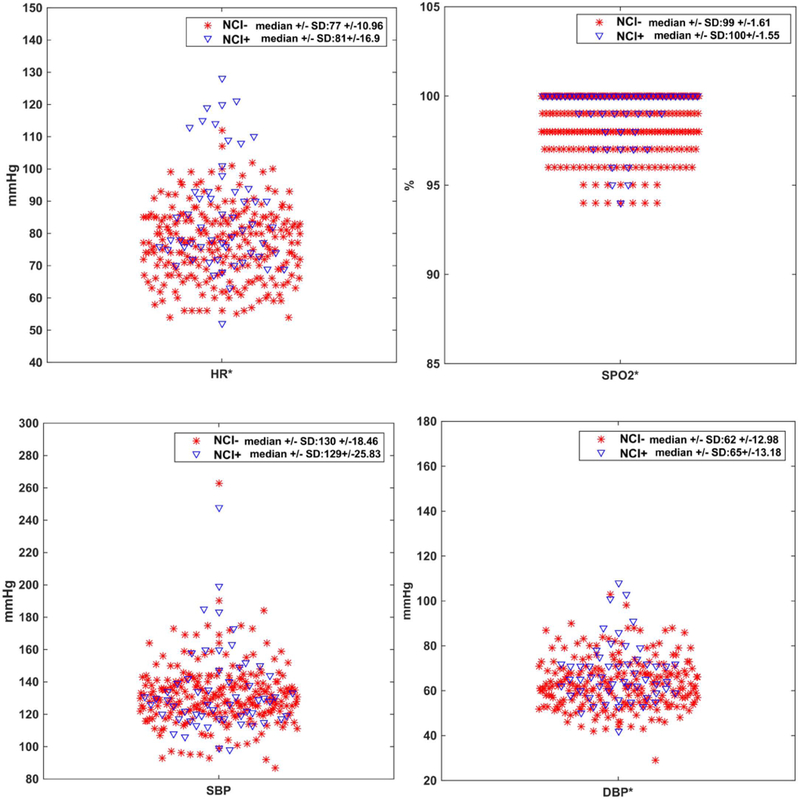
326 SAH subjects from April 2006 to January 2013 were included for analysis. 56 (17.18%) subjects had NCI. The mean age was 55.70 (± 14.06) years, 227 were women (69.63%), and 217 were non-white (66.56%). Underlying diseases that influence autonomic nervous activity were balanced between those with and without NCI, including history of acute myocardial infarction (1.79 vs 2.65%, p=1), congestive heart failure (1.79 vs 1.14%, p=0.54), diabetes (5.36 vs 8.68%, p=0.59), prior beta-blocker use (12.50 vs 12.96%, p=1), and atrial fibrillation or arrhythmia (3.57 vs 0.76%, p=0.14). There were no differences in aneurysm location. Subjects with NCI had higher scores on clinical scales quantifying neurological impairment on admission (HH, GCS, and WFNS). There were no differences in a grading scale on admission for DCI (MFS) or for incidence of DCI. Average length of ICU stay was longer for NCI+ subjects (15.69 vs. 12.84 days, p=0.014). (Table 1). 294 of 326 subjects (90.18%) underwent TTE, on average 1.65 days after admission. 321 of 326 subjects (98.47%) had cTI levels. 40 of 56 NCI+ subjects (71.4%) met TTE criteria for NSM. Using Mann-Whitney U test, median HR, SPO2, and DBP were different between groups of NCI+ and NCI− subjects, while SBP was not (Appendix F). There was notable overlap in the distribution of simple vitals data, indicating the need for more sophisticated methods to classify individual patients (Figure 1). For summary statistics of HRV features for NCI+ and NCI− groups, refer to Appendix G.
Figure 1.
Distribution of commonly acquired vital signs. While heart rate (HR), oxygen saturation (SPO2), and diastolic blood pressure (DBP) are statistically different between NCI+ and NCI− groups, none of these standard vital signs can be used to distinguish individual subjects’ NCI classification because of overlap. * indicates statistical significance, using Mann-Whitney U test.
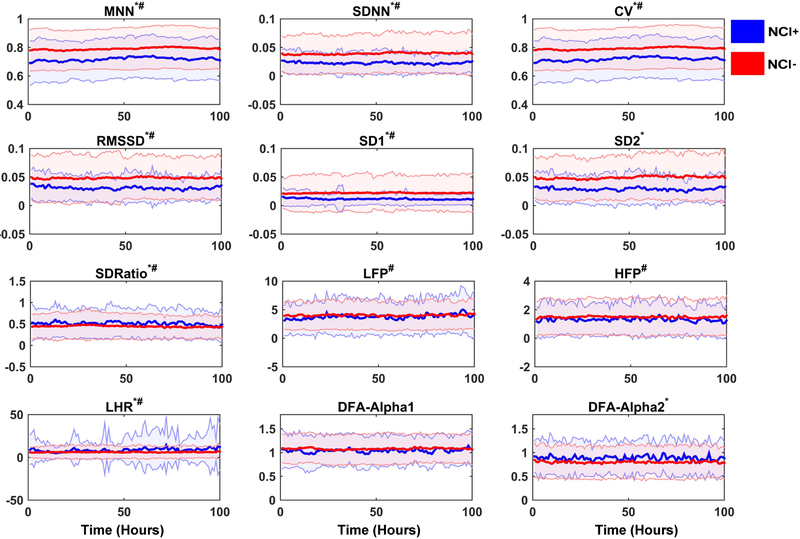
Using multilevel linear regression models to account for repeated measures over time within each subject, we found an association between many of the HRV measures and NCI (Table 2 and Figure 2). There were significant between-group differences for all HRV measures except HFP, LFP, and DFA Alpha 1. Overall, subjects with NCI had decreased HRV values, as indicated by the Beta (MNN, SDNN, CV, RMSSD, SD1, SD2) reported in (Table 2). LHR, SD Ratio, and DFA Alpha 2 were higher in those with NCI. All HRV measures had significant changes over time in NCI+ subjects, except for SD2, DFA Alpha 1, and DFA Alpha 2. We found that while there were no significant differences for LFP and HFP values between NCI groups, the change over time in these measures (LFP, HFP) was statistically significant. The SD2 and DFA Alpha 2 values were significantly different between NCI groups. However, the change in these HRV measures (SD2, DFA Alpha 2) over time were not statistically significant. There was no statistically significant association for DFA Alpha 1, either between NCI groups or over time.
Table 2.
Results of Longitudinal Multilevel Linear Regression
| Heart Rate Variability Measures |
Baseline Values | Changes Over Time for NCI+ | ||||
|---|---|---|---|---|---|---|
| Beta | Standard Error |
p-value | Beta | Standard Error |
p-value | |
| MNN | −0.10 | 0.018 | <0.0001 | 0.00015 | 0.000016 | <0.0001 |
| SDNN | −0.016 | 0.0045 | 0.0003 | 0.0000053 | 0.0000025 | 0.0354 |
| CV | −0.102 | 0.018 | <0.0001 | 0.00028 | 0.000011 | <0.0001 |
| RMSSD | −0.016 | 0.005 | 0.0022 | −0.00001 | 0.0000029 | <0.0001 |
| SD1 | −0.010 | 0.004 | 0.0194 | 0.0000094 | 0.0000017 | <0.0001 |
| SD2 | −0.020 | 0.0048 | <0.0001 | 0.0000028 | 0.0000033 | 0.4013 |
| SD Ratio | 0.079 | 0.031 | 0.0103 | −0.00010 | 0.000030 | 0.0007 |
| HFP | −0.221 | 0.119 | 0.0646 | 0.00036 | 0.00012 | <0.0001 |
| LFP | −0.185 | 0.233 | 0.4271 | −0.0013 | 0.00029 | 0.0035 |
| LHR | 3.42 | 0.92 | 0.0002 | −0.015 | 0.0011 | <0.0001 |
| DFA Alpha 1 | −0.049 | 0.031 | 0.1132 | 0.0000061 | 0.000032 | 0.0560 |
| DFA Alpha 2 | 0.0867 | 0.019 | <0.0001 | −0.00003 | 0.000043 | 0.4936 |
NCI = neurocardiogenic injury; MNN = mean of normal RR intervals; SDNN = mean of the standard deviations for all normal to normal RR intervals; CV = coefficient of variation of the RR interval time series; RMSSD = square root of the mean squared differences of successive NN intervals; SD1 = quantitative interpretation of Poincare plot and the standard deviation of the minor axis; SD2 = quantitative interpretation of Poincare plot and the standard deviation of the major axis; SD Ratio = SD1/SD2; HFP = high frequency power; LFP = low frequency power; LHR = LFP/HFP; DFA = detrended fluctuation analysis. For explanations of these terms, see Appendix B and C.
Figure 2.
Difference of HRV measures between NCI groups and changes over time. Asterisk(*) indicates there are significant between-group differences. Hashtag(#) indicates the HRV measures change over time with statistical significance. (HRV = heart rate variability; NCI = neurocardiogenic injury). The x-axis represents the time since admission and y-axis represents the normalized values of different HRV measures, solid lines indicate median values and shaded parts indicate the variance.
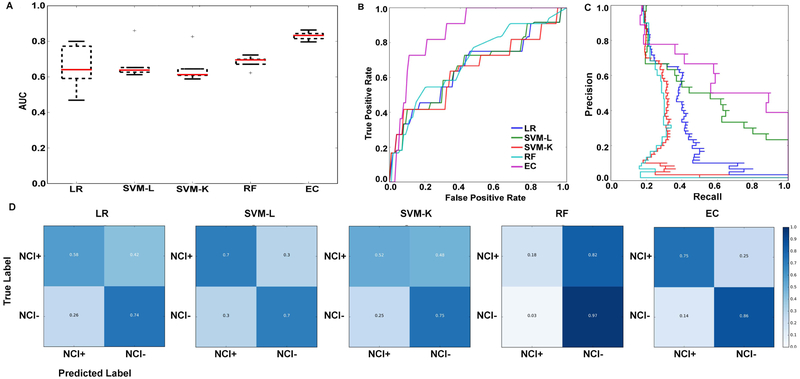
Machine learning approaches were applied to ascertain the power of these HRV measures for classifying NCI. The top 25 ranking features selected by MRMR are listed in Appendix H. Model evaluation for the five different classifiers are reported in Appendix I and Figure 3. Ensemble classifier performed the best with AUROC of 0.82 ± 0.05; AUPRC of 0.75 ± 0.10 (EC). EC was able to classify NC1+ with an accuracy of 75 ± 13% (median ± SD) and NCI− with an accuracy of 86 ± 8% (median ± SD), indicating that the classifier is not biased to one group (NCI+ or NCI−).
Figure 3.
Illustrating the performance of five different classifiers and the feature weights associated with, e.g., the LR classifier. (A) cross-validation results of five classifiers displayed using box plots, (B) area under the receiver operating characteristic curves, (C) area under precision recall curves, (D) confusion matrices of the five classifiers (LR = logistic regression, SVM-L = support vector machine – linear, SVM-K = support vector machine – kernel, RF = random forest, EC = ensemble classifier).
Discussion
Early and passive recognition of NCI after SAH would enable identification of patients at increased risk for cardiopulmonary complications, delayed cerebral ischemia (DCI), death, and poor functional outcome.3 Those that also have NSM could benefit from enlightened vasopressor choices and awareness of obstacles to maintaining cerebral perfusion in cases of DCI.41-46
In a prior study of 13 patients with takotsubo cardiomyopathy, HRV measured shortly after hospital admission demonstrated lower values of the time-domain features SDNN and SDANN. In a pilot study of 13 high grade SAH patients, the time-domain feature RMSSD had significant between group differences for NSM,26 the severe form of NCI with RWMA or VD. In our analysis of patient data from a prospectively collected observational cohort, we found that HRV measures are significantly influenced by NCI in SAH patients. To our knowledge, this is the largest human study of HRV in SAH patients with NCI, and the only one to use machine learning approaches to classify NCI.
We were able to show that HRV measures are significantly influenced by NCI (both baseline values and changes over time). Notably, all time domain HRV values were lower in NCI subjects compared to those without NCI. Also, LHR values were higher in NCI subjects compared to those without NCI. It is known that there is increased blood catecholamine concentrations in the first two days of SAH.47 The between group difference in LHR suggests a sympathovagal imbalance tipped in favor of enhanced sympathetic nervous activity, vagal withdrawal, or relative lack of augmentation in vagal activity specific to those who develop NCI.
To translate our findings into a tool for classification, we applied an ensemble machine learning approach, which showed good discrimination with an AUROC of 0.82, an AUPRC of 0.75, and a correct classification rate of 0.81. The threshold of accuracy required of a clinical decision-making tool is dependent on clinical context. For a critical and unique laboratory test which requires utmost accuracy, favoring zero false positives and negatives, an AUROC should approach 1.0. For a clinical prediction tool which is meant to support clinical decision making and allow better cognitive framing of an individual patient for which one must allocate invasive testing or monitoring resources, a threshold of 0.8 may be sufficient. For example, current and foreseeable models of breast cancer risk achieve AUCs of 0.6 to 0.7 and yet are useful for counseling and prevention activities48.
The challenge to timely detection of syndromes such as NCI is the insidious or subtle nature of symptom onset, and the dependency on obtaining a TTE. The development of an automated operator-independent monitoring tool and non-invasive biomarker for early detection has the potential to improve patient outcomes. This approach is increasingly pursued for other subclinical or subtle syndromes, such as neonatal decline49, sepsis50-57, pre-cardiac arrest58, or vasospasm.59 The universal availability of telemetry in critical care units positions ECG and heart rate variability (HRV) analyses as a high-yield target for clinical decision support.
Our study has several limitations. First, there is no consensus definition of NCI, and the criteria we have used for NCI has not been validated. This limits immediate generalization of the findings, but as our definition is based on data typically collected as part of routine medical care this will allow validation on an independently collected dataset. Second, while the rigorous definition of NCI would necessitate the exclusion of coronary artery disease, this has proved impractical in a clinical setting because SAH patients are not typically stable enough to undergo coronary catheterization nor qualify for anticoagulation, so diagnostic maneuvers are delayed until a future time of stability and/or allowance for treatment (if indicated). At that future state, NCI has typically declared itself by its reversibility. This practice is supported by a well-defined literature showing that the phenomenon of catecholamine-mediated neurocardiogenic injury, specifically neurogenic stunned myocardium, occurs in the presence of normal coronary arteries (by autopsy or angiography)10, 60-63. In the clinical setting, practical criteria are used to differentiate myocardial infarction from NCI after SAH17. As cardiac injury may influence HRV64, it is possible that our model may have the potential to also identify patients with non-NCI cardiac injury. While our model is not designed to identify all patients with any cardiac injury, if non-NCI cardiac dysfunction was identified, this lack of specificity would not be harmful. Third, there are challenges to overcome when using HRV for clinical diagnosis, including the influence of noise and artefacts inherent within ECG signals collected within the in-hospital setting, the lack of specificity, the lack of “normal” or threshold values, high inter-subject variability, and unpredictable responses to standard physiological interventions.35,65,71 Excluding artefactual segments from HRV analysis and using a machine learning approach for classification minimizes the impact of these issues. Fourth, other factors that affect autonomic tone were not included in our prospectively collected data (i.e. precise doses of sedation medication). While timing and dosing of medications may influence our ability to interpret the precise interactions between NCI and HRV features, the advantage of employing machine learning methods is that full understanding of causality is not necessary for classification; the presence of a relationship between HRV features and NCI is sufficiently reflected by the successful performance of the model.
The availability of cardiac telemetry in critical care units makes HRV a high-yield target for further studies. Future effort will explore a more robust feature set and advanced machine learning models to improve the performance of our classifiers. We also plan to implement and validate a temporal classification model as a clinical decision support tool delivered to relevant clinicians (treating physicians and nurse) for the early detection of NCI after SAH, perhaps as a trigger for diagnostic TTE.
Conclusions
A consensus definition of NCI does not exist, but it is recognized that it increases risk for cardiopulmonary complications, delayed cerebral ischemia (DCI), death, and poor functional outcome after SAH. HRV measures are significantly associated with our definition of NCI (between groups and within group changes over time). A machine learning approach using features derived from HRV measures can classify SAH patients that develop neurocardiogenic injury.
Supplementary Material
Acknowledgments
Funding: This study was funded by National Institute of Health (NIH), grant number : NIEHS K01-ES026833-02 (SP)
Footnotes
Conflicts-of-Interest/Disclosures:
None.
Ethics approval and consent to participate: The study was approved by the Columbia University Medical Center Institutional Review Board. In all cases, written informed consent was obtained from the patient or a surrogate.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Wybraniec MT, Mizia-Stec K and Krzych L. Neurocardiogenic injury in subarachnoid hemorrhage: A wide spectrum of catecholamin-mediated brain-heart interactions. Cardiol J 2014; 21: 220–228. DOI: 10.5603/CJ.a2014.0019. [DOI] [PubMed] [Google Scholar]
- 2.Tung P, Kopelnik A, Banki N, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke; a journal of cerebral circulation 2004; 35: 548–551. DOI: 10.1161/01.STR.0000114874.96688.54. [DOI] [PubMed] [Google Scholar]
- 3.Naidech AM, Kreiter KT, Janjua N, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation 2005; 112: 2851–2856. DOI: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- 4.Parekh N, Venkatesh B, Cross D, et al. Cardiac troponin I predicts myocardial dysfunction in aneurysmal subarachnoid hemorrhage. Journal of the American College of Cardiology 2000; 36: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 5.Banki N, Kopelnik A, Tung P, et al. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. Journal of neurosurgery 2006; 105: 15–20. DOI: 10.3171/jns.2006.105.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Kothavale A, Banki NM, Kopelnik A, et al. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocritical care 2006; 4: 199–205. DOI: 10.1385/NCC:4:3:199. [DOI] [PubMed] [Google Scholar]
- 7.Malik AN, Gross BA, Rosalind Lai PM, et al. Neurogenic Stress Cardiomyopathy After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg 2015; 83: 880–885. DOI: 10.1016/j.wneu.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee VH, Connolly HM, Fulgham JR, et al. Tako-tsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: an underappreciated ventricular dysfunction. Journal of neurosurgery 2006; 105: 264–270. DOI: 10.3171/jns.2006.105.2.264. [DOI] [PubMed] [Google Scholar]
- 9.Lee VH, Oh JK, Mulvagh SL, et al. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocritical care 2006; 5: 243–249. DOI: 10.1385/NCC:5:3:243. [DOI] [PubMed] [Google Scholar]
- 10.Kono T, Morita H, Kuroiwa T, et al. Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. Journal of the American College of Cardiology 1994; 24: 636–640. [DOI] [PubMed] [Google Scholar]
- 11.Kawai S, Suzuki H, Yamaguchi H, et al. Ampulla cardiomyopathy ('Takotusbo' cardiomyopathy)--reversible left ventricular dysfunction: with ST segment elevation. Jpn Circ J 2000; 64: 156–159. [DOI] [PubMed] [Google Scholar]
- 12.Zaroff JG, Rordorf GA, Ogilvy CS, et al. Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: evidence for neurally mediated cardiac injury. J Am Soc Echocardiogr 2000; 13: 774–779. [DOI] [PubMed] [Google Scholar]
- 13.Murthy SB, Shah S, Rao CP, et al. Neurogenic Stunned Myocardium Following Acute Subarachnoid Hemorrhage: Pathophysiology and Practical Considerations. Journal of intensive care medicine 2015; 30: 318–325. DOI: 10.1177/0885066613511054. [DOI] [PubMed] [Google Scholar]
- 14.Kilbourn KJ, Levy S, Staff I, et al. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 2013; 115: 909–914. DOI: 10.1016/j.clineuro.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Temes RE, Tessitore E, Schmidt JM, et al. Left ventricular dysfunction and cerebral infarction from vasospasm after subarachnoid hemorrhage. Neurocriticai care 2010; 13: 359–365. DOI: 10.1007/s12028-010-9447-x. [DOI] [PubMed] [Google Scholar]
- 16.Kilbourn KJ, Ching G, Silverman DI, et al. Clinical outcomes after neurogenic stress induced cardiomyopathy in aneurysmal sub-arachnoid hemorrhage: a prospective cohort study. Clin Neurol Neurosurg 2015; 128: 4–9. DOI: 10.1016/j.clineuro.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Bulsara KR, McGirt MJ, Liao L, et al. Use of the peak troponin value to differentiate myocardial infarction from reversible neurogenic left ventricular dysfunction associated with aneurysmal subarachnoid hemorrhage. Journal of neurosurgery 2003; 98: 524–528. DOI: 10.3171/jns.2003.98.3.0524. [DOI] [PubMed] [Google Scholar]
- 18.Spann JF Jr., Moellering RC Jr., Haber E, et al. Arrhythmias in Acute Myocardial Infarction; a Study Utilizing an Electrocardiographic Monitor for Automatic Detection and Recording of Arrhythmias. N Engl J Med 1964; 271: 427–431. DOI: 10.1056/NEJM196408272710901. [DOI] [PubMed] [Google Scholar]
- 19.Julian DG, Valentine PA and Miller GG. Disturbances of Rate, Rhythm and Conduction in Acute Myocardial Infarction: A Prospective Study of 100 Consecutive Unselected Patients with the Aid of Electrocardiographic Monitoring. Am J Med 1964; 37: 915–927. [DOI] [PubMed] [Google Scholar]
- 20.Stock E, Goble A and Sloman G. Assessment of arrhythmias in myocardial infarction. Br Med J 1967; 2: 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorman JR, Delos JB, Flower AA, et al. Cardiovascular oscillations at the bedside: early diagnosis of neonatal sepsis using heart rate characteristics monitoring. Physiol Meas 2011; 32: 1821–1832. DOI: 10.1088/0967-3334/32/11/S08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binici Z, Mouridsen MR, Kober L, et al. Decreased nighttime heart rate variability is associated with increased stroke risk. Stroke; a journal of cerebral circulation 2011; 42: 3196–3201. DOI: 10.1161/STROKEAHA.110.607697. [DOI] [PubMed] [Google Scholar]
- 23.Ryan ML, Ogilvie MP, Pereira BM, et al. Heart rate variability is an independent predictor of morbidity and mortality in hemodynamically stable trauma patients. J Trauma 2011; 70: 1371–1380. DOI: 10.1097/TA.0b013e31821858e6. [DOI] [PubMed] [Google Scholar]
- 24.Mazzeo AT, La Monaca E, Di Leo R, et al. Heart rate variability: a diagnostic and prognostic tool in anesthesia and intensive care. Acta Anaesthesiol Scand 2011; 55: 797–811. DOI: 10.1111/j.1399-6576.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 25.DeGiorgio CM, Miller P, Meymandi S, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav 2010; 19: 78–81. DOI: 10.1016/j.yebeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S, Kaffashi F, Loparo KA, et al. The use of heart rate variability for the early detection of treatable complications after aneurysmal subarachnoid hemorrhage. Journal of clinical monitoring and computing 2013; 27: 385–393. DOI: 10.1007/s10877-013-9467-0. [DOI] [PubMed] [Google Scholar]
- 27.Waldenborg M, Soholat M, Kahari A, et al. Multidisciplinary assessment of tako tsubo cardiomyopathy: a prospective case study. BMC Cardiovasc Disord 2011; 11: 14 DOI: 10.1186/1471-2261-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deibert E, Barzilai B, Braverman AC, et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. Journal of neurosurgery 2003; 98: 741–746. DOI: 10.3171/jns.2003.98.4.0741. [DOI] [PubMed] [Google Scholar]
- 29.Mayer SA, LiMandri G, Sherman D, et al. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. Journal of neurosurgery 1995; 83: 889–896. DOI: 10.3171/jns.1995.83.5.0889. [DOI] [PubMed] [Google Scholar]
- 30.Syed TU, Kaffashi F, Loparo KA, et al. System, apparatus and method for diagnosing seizures. 2014. [Google Scholar]
- 31.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996; 17: 354–381. [PubMed] [Google Scholar]
- 32.Peng CK, Buldyrev SV, Havlin S, et al. Mosaic organization of DNA nucleotides. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 1994; 49: 1685–1689. [DOI] [PubMed] [Google Scholar]
- 33.Johnson AE, Ghassemi MM, Nemati S, et al. Machine Learning and Decision Support in Critical Care. Proc IEEE Inst Electr Electron Eng 2016; 104: 444–466. DOI: 10.1109/JPROC.2015.2501978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bzdok D, Altman N and Krzywinski M. Points of Significance: Statistics versus Machine Learning. Nature Methods 2018: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rickards CA, Ryan KL, Ludwig DA, et al. Is heart period variability associated with the administration of lifesaving interventions in individual prehospital trauma patients with normal standard vital signs? Critical care medicine 2010; 38: 1666–1673. DOI: 10.1097/CCM.0b013e3181e74cab. [DOI] [PubMed] [Google Scholar]
- 36.Peng H, Long F and Ding C. Feature selection based on mutual information: criteria of max-dependency, max-relevance, and min-redundancy. IEEE transactions on pattern analysis and machine intelligence 2005; 27: 1226–1238. DOI: 10.1109/TPAMI.2005.159. [DOI] [PubMed] [Google Scholar]
- 37.Davis J and Goadrich M. The relationship between Precision-Recall and ROC curves. Proceedings of the 23rd international conference on Machine learning Pittsburgh, Pennsylvania, USA: ACM, 2006, p. 233–240. [Google Scholar]
- 38.Claesen M, Smet FD, Suykens JAK, et al. A robust ensemble approach to learn from positive and unlabeled data using SVM base models. Neurocomput 2015; 160: 73–84. DOI: 10.1016/j.neucom.2014.10.081. [DOI] [Google Scholar]
- 39.Goadrich M, Oliphant L and Shavlik J. Gleaner: Creating ensembles of first-order clauses to improve recall-precision curves. Mach Learn 2006; 64: 231–261. DOI: 10.1007/s10994-006-8958-3. [DOI] [Google Scholar]
- 40.Leisman DE. Rare Events in the ICU: An Emerging Challenge in Classification and Prediction. Critical care medicine 2018; 46: 418–424. DOI: 10.1097/CCM.0000000000002943. [DOI] [PubMed] [Google Scholar]
- 41.Mosley WJ 2nd, Manuchehry A, McEvoy C, et al. Takotsubo cardiomyopathy induced by dobutamine infusion: a new phenomenon or an old disease with a new name. Echocardiography 2010; 27: E30–33. DOI: 10.1111/j.1540-8175.2009.01089.x. [DOI] [PubMed] [Google Scholar]
- 42.Saito R, Takahashi T, Noshita N, et al. Takotsubo cardiomyopathy induced by dobutamine infusion during hypertensive therapy for symptomatic vasospasm after subarachnoid hemorrhage -case report. Neurol Med Chir (Tokyo) 2010; 50: 393–395. [DOI] [PubMed] [Google Scholar]
- 43.Taccone FS, Brasseur A, Vincent JL, et al. Levosimendan for the treatment of subarachnoid hemorrhage-related cardiogenic shock. Intensive care medicine 2013; 39: 1497–1498. DOI: 10.1007/s00134-013-2945-5. [DOI] [PubMed] [Google Scholar]
- 44.Santoro F, Ieva R, Ferraretti A, et al. Safety and feasibility of levosimendan administration in takotsubo cardiomyopathy: a case series. Cardiovasc Ther 2013; 31: e133–137. DOI: 10.1111/1755-5922.12047. [DOI] [PubMed] [Google Scholar]
- 45.Yaman M, Arslan U, Kaya A, et al. Levosimendan accelerates recovery in patients with takotsubo cardiomyopathy. Cardiol J 2016; 23: 610–615. DOI: 10.5603/CJ.a2016.0100. [DOI] [PubMed] [Google Scholar]
- 46.Mayer SA, Lin J, Homma S, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke; a journal of cerebral circulation 1999; 30: 780–786. [DOI] [PubMed] [Google Scholar]
- 47.Kawahara E, Ikeda S, Miyahara Y, et al. Role of autonomic nervous dysfunction in electrocardio-graphic abnormalities and cardiac injury in patients with acute subarachnoid hemorrhage. Circ J 2003; 67: 753–756. [DOI] [PubMed] [Google Scholar]
- 48.Gail MH and Pfeiffer RM. Breast Cancer Risk Model Requirements for Counseling, Prevention, and Screening. J Natl Cancer Inst 2018; 110: 994–1002. DOI: 10.1093/jnci/djy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saria S, Rajani AK, Gould J, et al. Integration of early physiological responses predicts later illness severity in preterm infants. Sci Transl Med 2010; 2: 48ra65 DOI: 10.1126/scitranslmed.3001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mani S, Ozdas A, Aliferis C, et al. Medical decision support using machine learning for early detection of late-onset neonatal sepsis. Journal of the American Medical Informatics Association : JAMIA 2014; 21: 326–336. DOI: 10.1136/amiajnl-2013-001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gultepe E, Green JP, Nguyen H, et al. From vital signs to clinical outcomes for patients with sepsis: a machine learning basis for a clinical decision support system. Journal of the American Medical Informatics Association : JAMIA 2014; 21: 315–325. DOI: 10.1136/amiajnl-2013-001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nachimuthu SK and Haug PJ. Early detection of sepsis in the emergency department using Dynamic Bayesian Networks. AMIA Annual Symposium proceedings / AMIA Symposium AMIA Symposium 2012; 2012: 653–662. [PMC free article] [PubMed] [Google Scholar]
- 53.Henry KE, Hager DN, Pronovost PJ, et al. A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med 2015; 7: 299ra122 DOI: 10.1126/scitranslmed.aab3719. [DOI] [PubMed] [Google Scholar]
- 54.Calvert JS, Price DA, Chettipally UK, et al. A computational approach to early sepsis detection. Comput Biol Med 2016; 74: 69–73. DOI: 10.1016/j.compbiomed.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Stanculescu I, Williams CK and Freer Y. Autoregressive hidden Markov models for the early detection of neonatal sepsis. IEEE J Biomed Health Inform 2014; 18: 1560–1570. DOI: 10.1109/JBHI.2013.2294692. [DOI] [PubMed] [Google Scholar]
- 56.Tang CH, Middleton PM, Savkin AV, et al. Non-invasive classification of severe sepsis and systemic inflammatory response syndrome using a nonlinear support vector machine: a preliminary study. Physiol Meas 2010; 31: 775–793. DOI: 10.1088/0967-3334/31/6/004. [DOI] [PubMed] [Google Scholar]
- 57.Taylor RA, Pare JR, Venkatesh AK, et al. Prediction of In-hospital Mortality in Emergency Department Patients With Sepsis: A Local Big Data-Driven, Machine Learning Approach. Acad Emerg Med 2016; 23: 269–278. DOI: 10.1111/acem.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ong ME, Lee Ng CH, Goh K, et al. Prediction of cardiac arrest in critically ill patients presenting to the emergency department using a machine learning score incorporating heart rate variability compared with the modified early warning score. Critical care 2012; 16: R108 DOI: 10.1186/cc11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roederer A, Holmes JH, Smith MJ, et al. Prediction of significant vasospasm in aneurysmal subarachnoid hemorrhage using automated data. Neurocritical care 2014; 21: 444–450. DOI: 10.1007/s12028-014-9976-9. [DOI] [PubMed] [Google Scholar]
- 60.Mayer SA, Fink ME, Homma S, et al. Cardiac injury associated with neurogenic pulmonary edema following subarachnoid hemorrhage. Neurology 1994; 44: 815–820. [DOI] [PubMed] [Google Scholar]
- 61.Pollick C, Cujec B, Parker S, et al. Left ventricular wall motion abnormalities in subarachnoid hemorrhage: an echocardiographic study. J Am Coll Cardiol 1988; 12: 600–605. [DOI] [PubMed] [Google Scholar]
- 62.Sato K, Masuda T, Kikuno T, et al. [Left ventricular asynergy and myocardial necrosis accompanied by subarachnoid hemorrhage: contribution of neurogenic pulmonary edema]. J Cardiol 1990; 20: 359–367. [PubMed] [Google Scholar]
- 63.Yamaguchi T, Shimizu Y, Ono N, et al. A case of subarachnoid hemorrhage with electrocardiographic and echocardiographic changes simulating transmural myocardial infarction. Jpn J Med 1991; 30: 142–145. [DOI] [PubMed] [Google Scholar]
- 64.Hillebrand S, Gast KB, de Mutsert R, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace 2013; 15: 742–749. 2013/January/30 DOI: 10.1093/europace/eus341. [DOI] [PubMed] [Google Scholar]
- 65.Rickards CA, Ryan KL and Convertino VA. Characterization of common measures of heart period variability in healthy human subjects: implications for patient monitoring. Journal of clinical monitoring and computing 2010; 24: 61–70. DOI: 10.1007/s10877-009-9210-z. [DOI] [PubMed] [Google Scholar]
- 66.Ryan KL, Rickards CA, Ludwig DA, et al. Tracking central hypovolemia with ecg in humans: cautions for the use of heart period variability in patient monitoring. Shock 2010; 33: 583–589. DOI: 10.1097/SHK.0b013e3181cd8cbe. [DOI] [PubMed] [Google Scholar]
- 67.Sethuraman G, Ryan KL, Rickards CA, et al. Ectopy in trauma patients: cautions for use of heart period variability in medical monitoring. Aviat Space Environ Med 2010; 81: 125–129. [DOI] [PubMed] [Google Scholar]
- 68.Hinojosa-Laborde C, Rickards CA, Ryan KL, et al. Heart Rate Variability during Simulated Hemorrhage with Lower Body Negative Pressure in High and Low Tolerant Subjects. Front Physiol 2011; 2: 85 DOI: 10.3389/fphys.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salomao E Jr., Otsuki DA, Correa AL, et al. Heart Rate Variability Analysis in an Experimental Model of Hemorrhagic Shock and Resuscitation in Pigs. PLoS One 2015; 10: e0134387 DOI: 10.1371/journal.pone.0134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryan ML, Thorson CM, Otero CA, et al. Clinical applications of heart rate variability in the triage and assessment of traumatically injured patients. Anesthesiol Res Pract 2011; 2011: 416590 DOI: 10.1155/2011/416590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sacha J and Pluta W. Alterations of an average heart rate change heart rate variability due to mathematical reasons. International journal of cardiology 2008; 128: 444–447. DOI: 10.1016/j.ijcard.2007.06.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.