Abstract
Microbial production of butyrate is impaired in patients with ulcerative colitis (UC); however, this inhibition is not well understood in Japanese UC patients. Therefore, we quantitatively analyzed genes encoding butyryl-CoA:acetate CoA-transferase (but) and butyrate kinase (buk) in the gut microbiota of Japanese patients with UC and healthy volunteers (HVs). But showed higher levels than buk. Moreover, patients with UC showed significantly decreased levels of but associated with Roseburia sp./Eubacterium rectale compared with HVs. But, which is associated with Faecalibacterium sp., was maintained in patients with UC, with an unchanged relative abundance of Faecalibacterium sp. microorganisms in patients with UC compared with HVs.
Keywords: microbiota, ulcerative colitis, butyrate, butyryl-CoA:acetate CoA-transferase, Roseburia sp., Faecalibacterium sp.
Ulcerative colitis (UC) is a chronic, relapsing, immune-mediated disease [1]. Patients with UC exhibit mucosal inflammation that extends from the rectum to the proximal segments of the colon [2]. The human gastrointestinal tract is reported to harbor 3.8 × 1013 bacteria in a 70-kg reference man [3]; these bacteria interact with each other and their host, significantly influencing human health and physiology. Several alterations (dysbiosis) have been reported in the gut microbial profile of patients with UC [4]. A previous study revealed a significant reduction in the numbers of two of the most important groups, Roseburia hominis (belonging to clostridial cluster XIVa) and Faecalibacterium prausnitzii (belonging to clostridial cluster IV), in the intestinal flora of patients with UC compared with healthy individuals [5]. After the relapse of UC, the population of fecal F. prausnitzii recovers in patients who achieve remission [6]. Moreover, butyrate-producing commensals contribute to mitigating intestinal diseases [7].
Bacteria produce short-chain fatty acids such as acetate, propionate, and butyrate which regulate adaptive immune responses [8]. We previously found that butyrate production is reduced in human microbiota models (Kobe University Human Intestinal Microbiota Model [KUHIMM]) of patients with UC compared with healthy individuals [9]. Butyrate contributes to the differentiation of naïve T cells into FoxP3+ regulatory T-cells, which serve as anti-inflammatory effectors [10]. It also inhibits the differentiation of naïve T cells into interferon-γ-producing cells [11]. Thus, butyrate mediates gut homeostasis and epithelium integrity.
The microbiota of healthy people mainly synthesizes butyrate via acetyl-coenzyme A (CoA) to form acetoacetyl-CoA, which is reduced in a stepwise manner to butyryl-CoA [12]. Two pathways execute the final step of butyrate formation from butyryl-CoA via butyryl-CoA:acetate CoA-transferase (encoded by but) or butyrate kinase (encoded by buk) [13]. These genes serve as biomarkers for identifying butyrate-producing communities [14]. In a healthy human colon, the but pathway predominates [15]. In the USA, patients with UC who underwent a colectomy followed by ileal pouch anal anastomosis harbor abnormal butyrate-producing communities predominated by buk [16]. Few detectable levels of but are similar to reference but genes of F. prausnitzii and Roseburia sp. in the intestinal microbiome of US patients with UC [16]. Additionally, differences in diet influence the composition of butyrate-producing bacteria [14, 17]. Thus, the results for US patients may not directly apply to Japanese patients with UC because of dietary differences.
Here, we analyzed the butyrate synthesis pathways that function in fecal microbial communities of Japanese patients with UC and compared the results with those of healthy individuals. We further analyzed butyrate synthesis using the KUHIMM, as this model reproducibly maintains the microbiota composition [18], reflecting the metabolic activity of butyrate production in the human colon [9, 18]. The results contribute to the characterization of Japanese patients with UC from the perspective of gene dynamics.
We studied 12 Japanese patients with a history of UC and 12 healthy volunteers (HVs) as previously described [9]. Written informed consent was obtained from all participants. The study was performed in accordance with the principles of the Declaration of Helsinki and guidelines of our institution and was approved by the Institutional Ethics Review Board of Kobe University (research code, 1902; approved May 10, 2016). The study was performed in accordance with the guidelines approved by the Medical Ethics Committee of Kobe University. The KUHIMM was initiated by inoculation of each fecal sample into a medium-containing vessel, as previously described [18]. Fecal samples were cultured for 30 hr. Microbial genomic DNA was extracted from the fecal samples and fermentation cultures as previously described [19]. Purified DNA was eluted into TE buffer (10 mM Tris-HCl, 1.0 mM EDTA) and stored at −20°C.
The levels of but and buk were determined by quantitative PCR using our primer sets previously designed by Vital et al. [16] (Table 1). Amplification was performed using TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara Bio, Inc., Kusatsu, Japan) with 2 µL template DNA per reaction (total volume 20 µL). Annealing temperatures and final primer concentrations were used according to Vital et al. [16] (primers described in Table 1) as follows: G_buk (64°C, 0.83 μM), G_Fprsn (70°C, 0.83 μM), G_Ros/Eub (62°C, 0.83 μM), G_Ros_R and G_Eub_R (60°C, 0.42 μM each), and total 16S (60°C, 0.67 μM). Thermocycling was performed as follows: 2 min at 50°C, 10 min at 95°C, 45 sec at 95°C; 45 sec at appropriate annealing temperatures, and 45 sec at 72°C. Elongation at 72°C was omitted from the reactions when using the 16S rRNA gene (×40) as a template. Samples were analyzed in duplicate. Genomic DNAs of Clostridium perfringens JCM 1290T, F. prausnitzii JCM 31915, and Roseburia intestinalis JCM 17583T were used to generate standard curves to determine target concentrations. We used an MiSeq Sequencer (Illumina, Inc., San Diego, CA, USA) as previously described to determine the sequences of the 16S rRNA genes [9]. The Mann-Whitney U test was used for statistical analysis. P values <0.05 were considered statistically significant.
Table 1. Primers used in this study are illustrated.
| Primer Name (Forward) |
Base sequence | Primer Name (Reverse) |
Base sequence | Genomic DNA s for standard curvesa | Reference |
|---|---|---|---|---|---|
| G_buk_F | tgctgtWgttggWagaggYgga | G_buk_R | gcaacIgcYttttgatttaatgcatgg | Clostridium perfringens JCM 1290T | [16] |
| G_Fprsn_F | gacaagggccgtcaggtcta | G_Fprsn_R | ggacaggcagatRaagctcttgc | Faecalibacterium prausnitzii JCM31915 | |
| G_RosEub_F | tcaaatcMggIgactgggtWga | G_Ros_R G_Eub_R | tcgataccggacatatgccaKgag tcataaccgcccatatgccatgag | Roseburia intestinalis JCM17583T | |
| 1132F | atggYtgtcgtcagctcgtg | 1108R | Gggttgcgctcgttgc | Faecalibacterium prausnitzii JCM31915 | |
G_buk_F/R – buk genes of Clostridium acetobutylicum, C. butyricum, and C. perfringens; G_Fprsn – but gene of Faecalibacterium prausnitzii; G_RosEub, G_Ros_R, G_Eub_R – but genes of Eubacterium rectale and Roseburia sp.; 1132F, 1108R – universal primers for 16S.
a 16S rRNA gene copy numbers: 10 for Clostridium perfringens [29], 9 for Faecalibacterium prausnitzii [30], and 1 for Roseburia intestinalis (GenBank: FP929049.1).
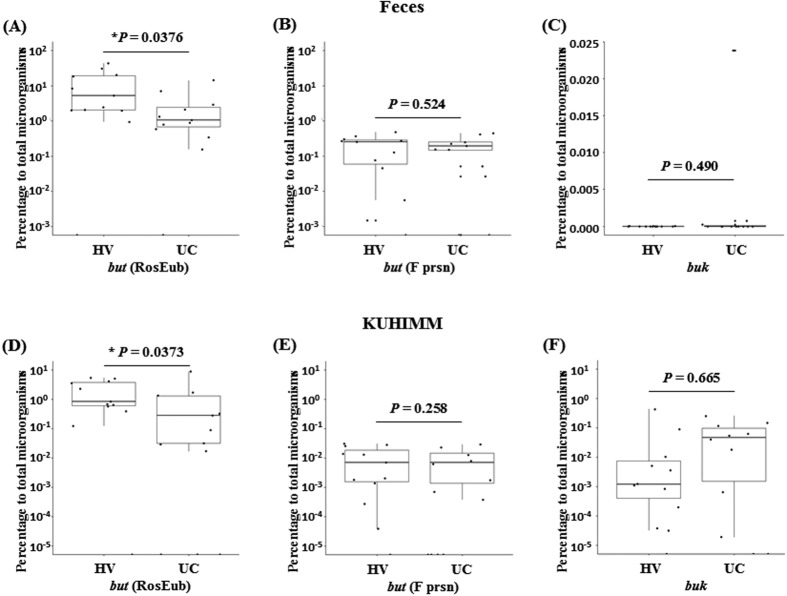
The butyrate-producing bacterial community is associated with functional resistance in patients with UC [12, 16]. Therefore, we performed quantitative PCR analysis to determine the levels of but and buk using fecal samples and KUHIMMs from Japanese HVs and patients with UC. The ratios of the but and buk levels to that of the 16S rRNA gene are shown in Fig. 1. Interestingly, the ratio of but, which is associated with Roseburia sp./E. rectale in patients with UC, was significantly decreased in the fecal community (p=0.0376, Mann-Whitney U test) and in the KUHIMMs (p=0.0373, Mann-Whitney U test) compared with in the HVs. However, the ratios of the but levels in the fecal community associated with F. prausnitzii did not significantly differ in patients with UC and HVs (p=0.524, Mann-Whitney U test) and in the KUHIMM (p=0.258, Mann-Whitney U test). In contrast, in the fecal community, the ratios of buk levels were significantly lower compared to those of but in HVs and patients with UC. These findings were confirmed using the KUHIMM. The ratio of buk did not significantly differ between HVs and patients with UC, in the fecal community and KUHIMM.
Fig. 1.
Quantitative PCR analysis of butyryl-CoA:acetate CoA-transferase (but) and butyrate kinase (buk) genes in feces and in the KUHIMM. (A, D) but in Roseburia sp./E. rectale, but (RosEub) (B, E) but in F. prausnitzii, but (F prsn), and (C,F) buk in C. butyricum, C. acetobutylicum, and C. perfringens. The percentages were calculated by quantitative PCR analyses (= 100 × [but or buk copy numbers]/[16S rRNA gene copy numbers]). Experiments were performed using DNA samples from (A–C) fecal samples and (D–F) KUHIMM fermentation cultures. * indicates significant difference, *p<0.05.
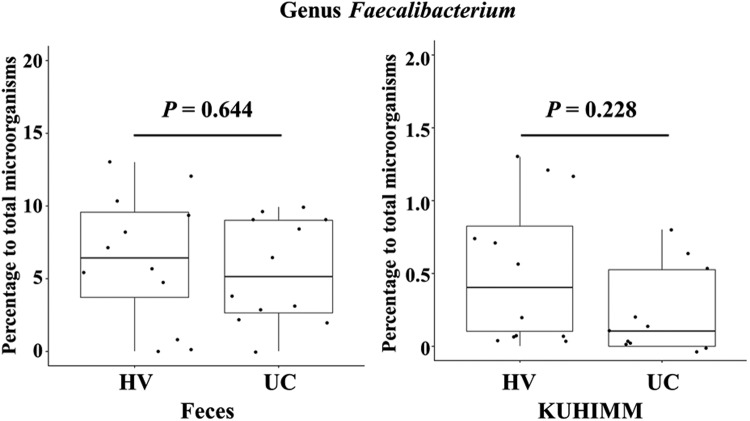
We next analyzed the bacterial 16S rRNA gene sequences of fecal communities and those of the KUHIMMs of HVs and patients with UC. The relative abundance of members of the Lachnospiraceae, which includes Roseburia, was decreased in patients with UC compared with HVs in fecal communities and KUHIMMs, as described previously [9]. These results corresponded to the present results acquired by quantitative PCR for but associated with Roseburia sp. The relative abundance of Faecalibacterium in the fecal communities and KUHIMMs did not significantly differ between those of HVs and patients with UC. These results were consistent with the quantitative PCR results for but in Faecalibacterium sp. (Fig. 2).
Fig. 2.
Relative abundances of members of Faecalibacterium. Relative abundances of members of Faecalibacterium in (A) fecal samples and (B) corresponding fermentation cultures of healthy volunteers (HVs) and patients with UC.
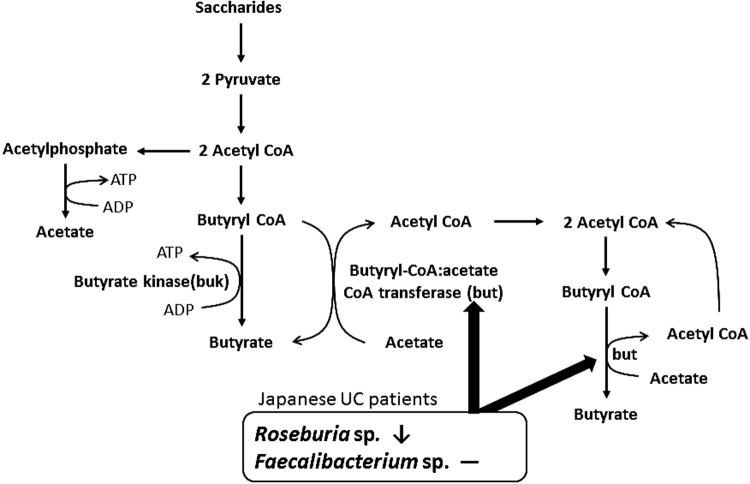
This is the first study to analyze butyrate-producing bacteria in Japanese patients with UC (Fig. 3). But predominated in Japanese patients with UC and HVs. However, buk predominates in US patients with UC [16], and but generally predominates in healthy individuals [17], as described in a study of people residing in the United Kingdom [15]. The levels of these genes associated with serious adverse consequences for US patients with UC were compared with those in their Japanese counterparts because the but pathway yields more butyrate compared with the buk pathway [15]. Moreover, but, which is associated with Roseburia sp./E. rectale (clostridial cluster XIVa), was decreased, while but, which is associated with Faecalibacterium sp. (clostridial cluster IV), was maintained in Japanese patients with UC; however, but levels associated with Roseburia sp./E. rectale and Faecalibacterium sp. are decreased in US patients with UC [16].
Fig. 3.
Relationship between butyrate-producing bacteria and pathways for acetate and butyrate formation in Japanese patients with UC. But associated with Roseburia sp. was decreased, and that associated with Faecalibacterium sp. was maintained. Pathways for acetate and butyrate formation are from Khan et al. [31] and Duncan et al. [23], respectively.
It is important to consider the factors that differentially affect the populations of butyrate-producing bacteria harbored by Japanese and US patients with UC. For example, Van den Abbeelle et al. [20] found that Roseburia sp. is the predominant producer of butyrate in the mucin layer. This result is consistent with findings showing that the colonic mucus thickness was increased in mice fed dietary fiber [21]; additionally, Roseburia sp. is sensitive to the composition of dietary fiber, which is altered after intake of a low-fiber diet by humans [17]. These findings indicate that the mucus layer is degraded in Japanese and US patients with UC, leading to alterations in the levels of but associated with Roseburia sp.
In contrast, the gut microbiome of Japanese people is comprised of a larger population of Bifidobacterium compared with that in people residing in other countries [22]. Further, Roseburia sp. and F. prausnitzii use acetate as a substrate for butyrate production when they are cultured in media containing glucose as the sole carbon source [23]. Moreover, acetate-producing Bifidobacterium longum and acetate-converting, butyrate-producing bacteria cross-feed when cultured in media containing oligofructose [24]. These findings indicate that cross-feeding occurs between species of Bifidobacterium and butyrate-producing bacteria grown on a prebiotic substrate in the human colon. We previously showed that the number of Bifidobacterium was not decreased in Japanese patients with UC (relative abundance: 12.0%) compared with that in healthy individuals (relative abundance: 4.3%), in studies of the human microbiota using 16S rRNA gene amplicons [9]. Bifidobacterium species contribute to the reproduction of Faecalibacterium species, which proliferate in the lumen [25]. In contrast, cross-feeding between species of Bifidobacterium species and Roseburia species, which are reported to colonize the mucus rather than a luminal environment [25] as described above, may be weak in the lesioned mucus layer of patients with UC [26]. This occurs in Japanese patients with UC and thus does not influence the ratio of but associated with Faecalibacterium sp.
In conclusion, the present study revealed a decrease in but associated with Roseburia species rather than with Faecalibacterium species, demonstrating the importance of restoring Roseburia species in Japanese patients with UC. This may be accomplished by dietary consumption of probiotics and prebiotics, which stimulate Bifidobacterium species and butyrate-producing colon bacteria [27, 28].
Acknowledgments
This work was supported by the Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative BioProduction Kobe), Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
REFERENCES
- 1.Sartor RB. 2006. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3: 390–407. [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. 2017. Ulcerative colitis. Lancet 389: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sender R, Fuchs S, Milo R. 2016. Revised estimated for the number of human and bacteria cells in the body. PLoS Biol 14: e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorrentino D. 2017. Microbial dysbiosis in spouses of ulcerative colitis patients: any clues to disease pathogenesis? World J Gastroenterol 23: 6747–6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. 2014. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63: 1275–1283. [DOI] [PubMed] [Google Scholar]
- 6.Varela E, Manichanh C, Gallart M, Torrejón A, Borruel N, Casellas F, Guarner F, Antolin M. 2013. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther 38: 151–161. [DOI] [PubMed] [Google Scholar]
- 7.Tye H, Yu CH, Simms LA, de Zoete MR, Kim ML, Zakrzewski M, Penington JS, Harapas CR, Souza-Fonseca-Guimaraes F, Wockner LF, Preaudet A, Mielke LA, Wilcox SA, Ogura Y, Corr SC, Kanojia K, Kouremenos KA, De Souza DP, McConville MJ, Flavell RA, Gerlic M, Kile BT, Papenfuss AT, Putoczki TL, Radford-Smith GL, Masters SL. 2018. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat Commun 9: 3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki K, Inoue J, Sasaki D, Hoshi N, Shirai T, Fukuda I, Azuma T, Kondo A, Osawa R. 2019. Construction of a model culture system of human colonic microbiota to detect decreased Lachnospiraceae abundance and butyrogenesis in the feces of ulcerative colitis patient. Biotechnol J 14: 1800555. [DOI] [PubMed] [Google Scholar]
- 10.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- 11.Gurav A, Sivaprakasam S, Bhutia YD, Boettger T, Singh N, Ganapathy V. 2015. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem J 469: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vital M, Howe AC, Tiedje JM. 2014. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio 5: e00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis P, Flint HJ. 2017. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 19: 29–41. [DOI] [PubMed] [Google Scholar]
- 14.Vital M, Gao J, Rizzo M, Harrison T, Tiedje JM. 2015. Diet is a major factor governing the fecal butyrate-producing community structure across Mammalia, Aves and Reptilia. ISME J 9: 832–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. 2004. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol 186: 2099–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vital M, Penton CR, Wang Q, Young VB, Antonopoulos DA, Sogin ML, Morrison HG, Raffals L, Chang EB, Huffnagle GB, Schmidt TM, Cole JR, Tiedje JM. 2013. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis P, Flint HJ. 2009. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294: 1–8. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki D, Sasaki K, Ikuta N, Yasuda T, Fukuda I, Kondo A, Osawa R. 2018. Low amounts of dietary fibre increase in vitro production of short-chain fatty acids without changing human colonic microbiota structure. Sci Rep 8: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi R, Sasaki K, Sasaki D, Fukuda I, Tanaka K, Yoshida K, Kondo A, Osawa R. 2016. A single-batch fermentation system to simulate human colonic microbiota for high-throughput evaluation of prebiotics. PLoS One 11: e0160533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Abbeele P, Belzer C, Goossens M, Kleerebezem M, De Vos WM, Thas O, De Weirdt R, Kerckhof FM, Van de Wiele T. 2013. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J 7: 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Núñez G, Martens EC. 2016. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167: 1339–1353.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H, Hattori M. 2016. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res 23: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ. 2002. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol 68: 5186–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falony G, Vlachou A, Verbrugghe K, De Vuyst L. 2006. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol 72: 7835–7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeiren J, Van den Abbeele P, Laukens D, Vigsnaes LK, De Vos M, Boon N, Van de Wiele T. 2012. Decreased colonization of fecal Clostridium coccoides/Eubacterium rectale species from ulcerative colitis patients in an in vitro dynamic gut model with mucin environment. FEMS Microbiol Ecol 79: 685–696. [DOI] [PubMed] [Google Scholar]
- 26.Aliquor M, Zaidi D, Valcheva R, Jovel J, Martinez I, Sergi C, Walter J, Mason AL, Wong GK, Dieleman LA, Carroll MW, Huynh HQ, Wine E. Mucosal barrier depletion and loss of bacterial diversity are primary abonormalitis in paediatric ulcerative colits. J Crohns Colits 10: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. 2016. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7: 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pituch-Zdanowska A, Banaszkiewicz A, Albrecht P. 2015. The role of dietary fibre in inflammatory bowel disease. Prz Gastroenterol 10: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci USA 99: 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bag S, Ghosh TS, Das B. 2017. Complete genome sequence of Faecalibacterium prausnitzii isolated from the gut of a healthy Indian adult. Genome Announc 5: e01286–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan MT, Duncan SH, Stams AJ, van Dijl JM, Flint HJ, Harmsen HJ. 2012. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J 6: 1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]