This 24-week RCT reveals significant improvement in communication skills for young children with autism who received clinician-delivered PRT and parent training.
Abstract
Video Abstract
OBJECTIVES:
Our aim was to conduct a randomized controlled trial to evaluate a pivotal response treatment package (PRT-P) consisting of parent training and clinician-delivered in-home intervention on the communication skills of children with autism spectrum disorder.
METHODS:
Forty-eight children with autism spectrum disorder and significant language delay between 2 and 5 years old were randomly assigned to PRT-P (n = 24) or the delayed treatment group (n = 24) for 24 weeks. The effect of treatment on child communication skills was assessed via behavioral coding of parent-child interactions, standardized parent-report measures, and blinded clinician ratings.
RESULTS:
Analysis of child utterances during the structured laboratory observation revealed that, compared with the delayed treatment group, children in PRT-P demonstrated greater improvement in frequency of functional utterances (F1,41 = 6.07; P = .026; d = 0.61). The majority of parents in the PRT-P group (91%) were able to implement pivotal response treatment (PRT) with fidelity within 24 weeks. Children receiving PRT-P also demonstrated greater improvement on the Brief Observation of Social Communication Change, on the Clinical Global Impressions Improvement subscale, and in number of words used on a parent-report questionnaire.
CONCLUSIONS:
This is the first 24-week randomized controlled trial in which community treatment is compared with the combination of parent training and clinician-delivered PRT. PRT-P was effective for improving child social communication skills and for teaching parents to implement PRT. Additional research will be needed to understand the optimal combination of treatment settings, intensity, and duration, and to identify child and parent characteristics associated with treatment response.
What’s Known on This Subject:
There is growing support for naturalistic developmental behavioral interventions for improving social communication competence in young children with autism spectrum disorder. However, rigorous empirical testing of promising interventions is essential for allocating finite treatment resources and improving patient outcomes.
What This Study Adds:
This study reveals the efficacy of a pivotal response treatment package combining parent training and clinician-delivered intervention for young children with autism spectrum disorder. The intervention resulted in significant improvements in functional utterances, vocabulary, and social communication behaviors.
The high prevalence of autism spectrum disorder (ASD) in the pediatric population reveals a need for effective treatment options. There is growing support for naturalistic developmental behavioral interventions (NDBIs), which incorporate both applied behavior analysis (ABA) and developmental principles,1 for remediating symptoms in young children with ASD. However, rigorous empirical testing of interventions remains essential for allocating finite treatment resources and improving patient outcomes. Pivotal response treatment (PRT)2 is an NDBI designed to increase child motivation to interact by focusing on the child’s interests and rewarding effort with natural reinforcement.3 The treatment involves modeling appropriate language during play and waiting for the child to attempt communication before providing access to the preferred activity. It also targets pivotal areas of a child’s development to promote more generalized behavioral improvements. PRT involves training parents to perform the intervention, thereby increasing the child’s exposure to intervention across daily routines.
Although early behavioral interventions are designed to combine clinician-delivered treatment with parent training, in community practice, children often receive primarily clinician-delivered treatment, and providers have limited training in parent-mediated approaches.4 Importantly, empirical evidence from randomized controlled trials (RCTs) has emerged regarding the efficacy of both clinician-delivered PRT and parent training to effectively administer PRT to the child. An RCT revealed that children with ASD showed greater improvement in the mean length of utterance after 3 months of clinician-delivered PRT compared with a structured ABA approach.5 Another recent RCT revealed that, compared with a psychoeducation control group, children with ASD whose parents participated in a 12-week PRT training group showed improvements in frequency of utterances and adaptive communication skills.6 Finally, given evidence that parent fidelity of treatment implementation can decline after training ends,7 a model to support maintenance beyond an initial 12-week period is warranted.
Here we report results from a 24-week RCT of PRT combining parent training and clinician-delivered in-home treatment compared with a delayed treatment group (DTG) receiving stable community-based interventions. The pivotal response treatment package (PRT-P) included a 12-week intensive phase followed by an additional 12-week maintenance phase. In this pilot investigation, the effect of the treatment on child communication skills is assessed via behavioral coding of parent-child interactions, standardized parent-report measures, and blinded clinician ratings. The benefits of a 24-week PRT intervention have not yet been compared with those of community treatment in a controlled trial.
Methods
Study Design
This investigation involved a 24-week RCT in which we examined the efficacy of a PRT-P consisting of parent training and clinician-delivered in-home intervention in targeting functional communication deficits in young children with ASD. This study was approved by Stanford University's Institutional Review Board and was registered in the clinical trials database (clinicaltrials.gov; identifier NCT02037022). The full protocol is available on request. De-identified individual participant data (including data dictionaries) will be made available in addition to study protocols, the statistical analysis plan, and the informed consent form. The data will be made available after publication to investigators who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to ggengoux@stanford.edu. Requests for data will be available until 5 years after the article publication.
Inclusion and Exclusion Criteria
Participants included children 2 to 5 years old with ASD and significant language delay. An ASD diagnosis was based on the Autism Diagnostic Interview–Revised,8 the Autism Diagnostic Observation Schedule, Second Edition,9 Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria, and expert clinical judgment. Consistent with a previous PRT trial,6 the expressive language score on the Preschool Language Scale, Fifth Edition (PLS-5)10 had to be at least 1 SD below the mean for 2- and 3-year-old children, 2 SDs below the mean for 4-year-old children, and 3 SDs below the mean for 5-year-old children. To limit the influence of concomitant communication interventions, children were excluded if they had >1 hour of weekly individual speech therapy, >15 hours of weekly 1:1 ABA treatment, or unstable interventions 1 month before baseline or anticipated treatment changes during the trial. Additional exclusion criteria included other severe psychiatric disorders, genetic abnormality, an active medical problem, a primary language other than English, or living >50 miles away. One parent was required to participate in parent training. No changes in inclusion and exclusion criteria were applied during the study.
Participants
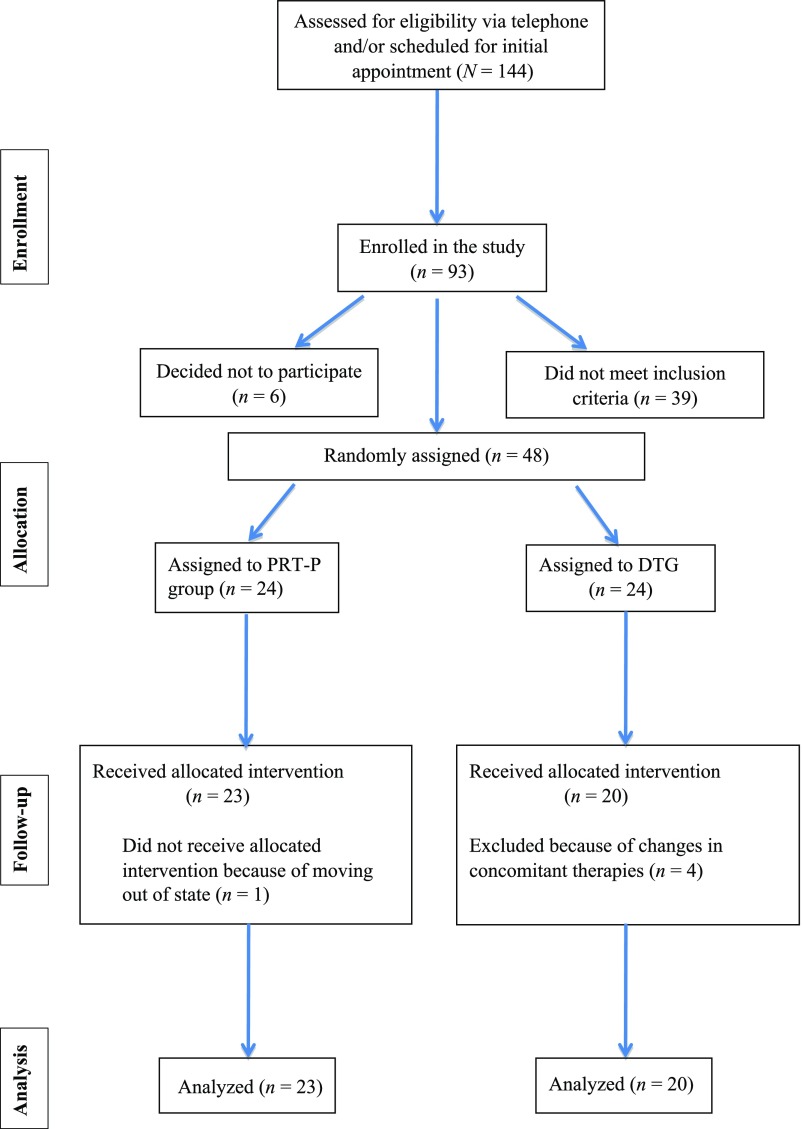
Participants were referred by local professionals or recruited through flyer distribution and word of mouth and were enrolled between December 2013 and July 2016. One hundred forty-four potential subjects were screened (see Fig 1); 93 families enrolled by signing a consent form. Thirty-nine did not meet eligibility criteria on the basis of baseline measures, and 6 families decided not to participate before random assignment. Forty-eight subjects were randomly assigned (PRT-P group: n = 24; DTG: n = 24), and 43 families (PRT-P group: n = 23; DTG: n = 20) completed the 24-week trial. One participant in the PRT-P group withdrew when the family moved out of state; 4 participants in the DTG were excluded from final analyses after significant changes were made to concomitant therapies during the trial. The target sample size for ending the trial (48) was determined by power analysis on the basis of a previous study of PRT parent training.6
FIGURE 1.
Consolidated Standards of Reporting Trials form flowchart.
Approximately 81% of participating children were receiving ABA treatment (mean hours per week = 8.34; SD = 5.6). Ninety-five percent were in school, primarily in special education classes (79%), with 16 hours of school per week on average (range: 0–37.5 hours). Language ability in the sample ranged from nonverbal to phrase speech, and almost all of the children were receiving speech therapy (98%), with an average of 45 minutes of individual therapy per week. Participating parents were primarily women (79%), and the majority were college graduates (84%). The sample was ethnically diverse, with 28% of participants white, 56% Asian American, 7% Hispanic, 2% native Hawaiian, and 7% biracial or other race. There were no significant differences between groups at baseline on any child measures, with the exception of the MacArthur-Bates Communicative Development Inventories (CDI) (Table 1). Differences in the CDI were found for the CDI words out of 396 measure (PRT-P: 118.2 ± 110.9; DTG: 59.0 ± 73.6; t1,41 = −2.09; P = .044) but not for the CDI words out of 680 measure (PRT-P: 141.9 ± 129.9; DTG: 85.6 ± 105.6; t1,41 = −1.544; P = .130).
TABLE 1.
Baseline Comparison of Participants With Autism in the PRT-P Group and DTG
| PRT-P Group | DTG | |
|---|---|---|
| n | 23 | 20 |
| Male/female sex, n | 21/2 | 17/3 |
| Age, mean (SD), mo | 49.5 (11.2) | 47.2 (10.0) |
| MSEL composite score, mean (SD) | 49.9 (1.8) | 50.9 (5.7) |
| SRS-2 raw score, mean (SD) | 95.8 (26.4) | 98.3 (25.6) |
| SLO total utterances, mean (SD) | 49.9 (30.7) | 52.8 (23.9) |
| CGI-S score, mean (SD) | 5.4 (0.5) | 5.4 (0.6) |
| CDI words out of 396, mean (SD)a | 118.2 (110.9) | 59.0 (73.6) |
| CDI words out of 680, mean (SD) | 141.9 (129.9) | 85.6 (105.6) |
| 1-on-1 ABA treatment, mean (SD), h per wk | 8.9 (5.2) | 7.7 (6.1) |
PRT-P group ethnicity: white: 6; Asian American: 12 (includes Southeast Asians); native Hawaiian: 1; Hispanic: 2; biracial: 1; and other: 1; DTG ethnicity: white: 6; Asian: 12 (includes Southeast Asians); Hispanic: 1; and other: 1.
No statistical differences between the PRT-P group and DTG on any of the baseline measures, with the exception of the CDI words out of 396; t1,41 = −2.09; P = .044.
Procedures
After informed consent, families participated in a comprehensive evaluation that included psychological assessments and a review of the medical history to confirm eligibility. Eligible children were stratified on the basis of sex and were randomly assigned (1:1) to the treatment (PRT-P) or control (DTG) group via electronic generation of random numbers (www.randomizer.org) by a senior investigator not involved in the trial. All measures were collected at baseline and at week 24; the primary measure and some secondary measures were also obtained at week 12. Data were managed by using Research Electronic Data Capture,11 hosted at Stanford University’s Center for Clinical Informatics.
PRT-P
The PRT-P treatment consisted of an intensive phase from week 1 to week 12, during which parents received weekly 60-minute parent training sessions and children received 10 hours per week of clinician-delivered in-home treatment, and a maintenance phase from week 12 to week 24, during which parents received monthly 60-minute parent training sessions and children received 5 hours per week of in-home treatment. The parent training curriculum was based on a standard set of PRT teaching materials and video examples.6,7,12,13 Parent training was provided by master’s-level clinicians who were supervised by the first author. In-home treatment was provided by bachelor’s-level clinicians who had demonstrated fidelity of implementation of PRT and who received weekly supervision (see Supplemental Information for details).
DTG
Children assigned to the DTG continued with stable community treatments for the 24-week trial and returned to the clinic at weeks 12 and 24 for assessments. After completion of all study measures, families were offered PRT parent training and in-home treatment, similar to the PRT-P intensive phase.
Measures
Diagnostic and Screening Instruments
The Autism Diagnostic Interview–Revised and the Autism Diagnostic Observation Schedule were administered at baseline to confirm ASD diagnosis for all study participants. The PLS-5 was used to verify significant language delay at baseline and as a secondary outcome measure at week 24.
Primary Outcome
Child frequency of functional utterances was assessed during a 10-minute structured laboratory observation (SLO) at baseline, week 12, and week 24, during which parents were instructed to try to get the child to communicate as much as possible. Consistent with previous research,6,7 raters blind to group assignment tallied the child’s total functional verbal utterances and also specified utterance type (ie, unintelligible, imitative, verbally prompted, nonverbally prompted, or spontaneous). Multiple-word utterances were scored as a single instance of communication (see Supplemental Information for details). Two raters independently scored at least 30% of the videos randomly selected from the total set. For functional utterances, intraclass correlation coefficients (ICCs) indicated excellent (ICC2,1 = 0.94) to acceptable agreement (unintelligible = 0.83; imitative = 0.98; verbally prompted = 0.97; nonverbally prompted = 0.89; spontaneous = 0.74).14
Secondary Outcomes
SLO videos were also scored by using the Brief Observation of Social Communication Change (BOSCC) coding guidelines,15 with high levels of agreement between independent raters (ICC = 0.863). The BOSCC provides a systematic method for blinded raters to code video-recorded interactions and assess change across 16 items in 2 domains (ie, social communication symptoms and repetitive behavior). The BOSCC yields a summary score and a social communication subscore (items 1–8 only). Higher scores indicate greater impairment; therefore, reduction over time represents symptom improvement.
Additional secondary measures focused on language and communication, as well as socialization and global development, included the following: the CDI16 Words and Gestures, the CDI Words and Sentences, the Vineland Adaptive Behavior Scales, Second Edition (Vineland-II)17 communication subscale, the PLS-5,10 the Mullen Scales of Early Learning (MSEL),18 the Social Responsiveness Scale, Second Edition (SRS-2),19 and the Clinical Global Impressions20 Severity (CGI-S) and the Clinical Global Impressions Improvement (CGI-I) subscales. The Clinical Global Impressions (CGI) ratings were completed by a senior investigator blind to group assignment and were focused on social communication skills.6 SLO videos were also scored for parent fidelity of PRT implementation, following published methods.6,21 Ratings of parent fidelity were made on the basis of the same SLO videos used for assessment of utterances but by a different set of raters trained independently. Ratings were completed by raters blind to group assignment and time point. Agreement was 87%, and κ22 was calculated to correct for chance agreement (κ = 0.72). Parents completed a brief questionnaire to report their children’s existing autism interventions at baseline and at week 24 along with a weekly concomitant therapies log to document changes.
Statistical Analyses
Statistical analyses were completed by using IBM SPSS Statistics version 24 (IBM SPSS Statistics, IBM Corporation, Armonk, NY). A mixed-effects regression model with treatment group (PRT-P versus DTG), time (baseline and week 24), and their interaction as fixed-effects covariates was used to examine differences between the 2 groups on primary and secondary outcome measures. Mixed-effects regression models were separately computed for each specific type of utterance. Additionally, a 3-level model for time was also examined for the primary outcome measure (number of utterances) and for the BOSCC because week 12 data were also available.
By using the same modeling approach, secondary analyses were computed for the number of words produced out of 396 on the CDI Words and Gestures form, the number of words produced out of 680 on the CDI Words and Sentences form, the CGI-S and CGI-I subscales, Vineland-II communication standard scores, the Vineland-II expressive v-scale score (mean of 15 and standard deviation of 3), the PLS-5, the MSEL expressive language raw score, the MSEL composite, and the SRS-2 social communication raw score. These analyses were repeated, controlling for baseline differences between groups when they existed (ie, CDI words produced out of 396). The association between several baseline characteristics, including sex, age, and developmental level, and the key outcome variables (total child utterances and BOSCC total score) were also investigated by examining Spearman correlations between these variables.
The percentage of parents meeting 80% fidelity of implementation criteria was also computed for each group. Because the sample size was modest and because the primary purpose was to understand the nature of the treatment effects for planning future studies, a type 1 error rate of 0.05 was used for all analyses.
Results
Outcome Measures
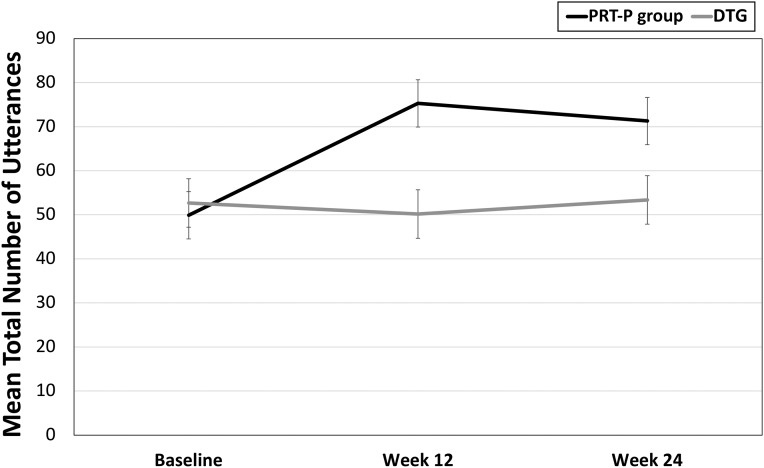
Children participating in the PRT-P showed significantly greater overall improvement between baseline and week 24 in total number of utterances (F1,41 = 6.07; P = .026) compared with children in the DTG (Table 2). Similar treatment effects are evident across the 3 time points (baseline, week 12, and week 24; F2,40 = 3.70; P = .034; Fig 2), with differences between the groups beginning at week 12 (F1,41 = 7.224; P = .010). Group differences were driven primarily by the significant increase in nonverbally prompted utterances in the PRT-P group (F1,41 = 16.409; P < .001; see Supplemental Table 3).
TABLE 2.
Treatment Response of Participants in the PRT-P Group or DTG
| Mean (SD) | Group × Time Interaction | Cohen’s d (Week 24) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | F | P | ||||
| PRT-P Group (n = 23) | DTG (n = 20) | PRT-P Group (n = 23) | DTG (n = 20) | ||||
| SLO | |||||||
| Total utterances | 49.9 (30.7) | 52.8 (23.9) | 71.3 (27.3) | 53.4 (28.8) | 5.808 | .026 | 0.64 |
| BOSCC | |||||||
| Total score | 34.3 (7.5) | 34.6 (4.2) | 26.5 (6.2) | 34.4 (4.9) | 28.794 | <.001 | 1.41 |
| Social communication subscore | 23.8 (4.3) | 25.1 (4.0) | 18.3 (4.8) | 24.63 (3.9) | 9.562 | .004 | 1.45 |
| CDI | |||||||
| Words produced out of 396 | 118.2 (110.9) | 54.2 (72.3) | 194.9 (133.7) | 84.4 (93.5) | 5.663 | .022 | 0.96 |
| Words produced out of 680 | 141.9 (129.9) | 80.2 (105.7) | 256.6 (200.1) | 112.9 (148.1) | 6.039 | .018 | 0.82 |
| Vineland-II | |||||||
| Expressive v-scale score | 7.23 (1.9) | 6.7 (1.3) | 7.6 (2.4) | 6.2 (1.6) | 3.587 | .066 | 0.58 |
| Communication standard score | 63.8 (14.8) | 62.5 (11.6) | 64.9 (16.8) | 62.6 (13.9) | 0.230 | .634 | 0.15 |
| PLS-5 | |||||||
| Expressive standard score | 58.4 (8.9) | 57.9 (7.9) | 58.7 (10.2) | 56.9 (10.5) | 0.384 | .539 | 0.17 |
| MSEL | |||||||
| MSEL expressive language raw score | 18.2 (7.3) | 14.9 (7.6) | 21.0 (8.7) | 17.3 (6.9) | 0.082 | .775 | 0.47 |
| MSEL composite score | 49.9 (1.8) | 50.9 (5.7) | 51.1 (3.9) | 53.8 (10.1) | 0.425 | .519 | 0.35 |
| SRS-2 | |||||||
| Social communication raw score | 95.8 (26.4) | 98.3 (25.6) | 91.9 (22.8) | 93.1 (27.4) | 0.061 | .806 | 0.05 |
| CGI | |||||||
| CGI-S | 5.4 (0.5) | 5.4 (0.6) | 5.13 (0.7) | 5.40 (0.5) | 5.914 | .019 | 0.44 |
| CGI-I | — | — | 2.6 (0.8) | 3.4 (0.7) | 6.858 | <.001 | 1.06 |
CDI: df (1, 40), controlling for baseline difference; CDI 396: df (1, 39), F = 4.459, P = .041; CDI 680: df (1, 39), F = 4.134, P = .049; Vineland-II: df (1; 39); PLS-5: df (1, 41); MSEL: df (1, 41); CGI: df (1, 41), focused on social and communication symptoms. df, degrees of freedom; —, not applicable.
FIGURE 2.
Number of utterances by group during SLO at baseline (PRT-P group: mean ± SE = 49.9 ± 6.13; DTG: mean ± SE = 52.7 ± 5.08), week 12 (PRT-P group: mean ± SE = 75.3 ± 4.31; DTG: mean ± SE = 50.2 ± 5.45), and week 24 (PRT-P group: mean ± SE = 71.3 ± 5.66; DTG: mean ± SE = 53.4 ± 5.96). F2,40 = 3.765; P = .032.
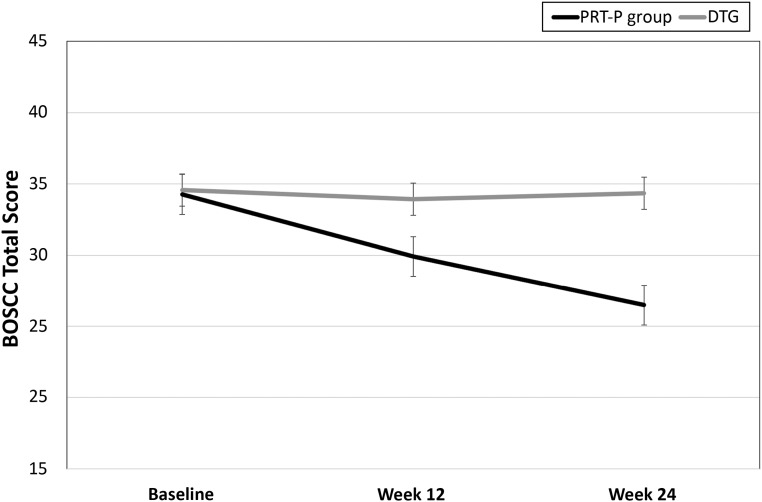
Improvement in the PRT-P group was similarly observed on the BOSCC social communication subscale (Table 2) and in the BOSCC total score (Fig 3). Results remained unchanged for the BOSCC total score when analyses were completed across the 3 time points (baseline, week 12, and week 24; F2,39 = 17.597; P < .001; Fig 3), with improvement being observed at week 12 (F1,40 = 4.345; P = .044). A significant treatment effect was also observed for the CDI words produced out of 396 and CDI words produced out of 680 measures, even when controlling for baseline differences. The treatment effect was also significant on the CGI-S subscale for social communication symptoms (F1,41 = 5.91; P = .019). Significant group differences at week 24 were also evident on the CGI-I subscale (F1,41 = 6.86; P ≤ .001). There was no treatment effect for the PLS-5, the MSEL, the SRS-2, or the Vineland-II communication subscale. Although not statistically significant, effect-size calculations suggested a medium-size treatment effect for the Vineland-II expressive v-scale score. No adverse effects were noted in either group.
FIGURE 3.
BOSCC total score from SLO by group at baseline (PRT-P group: mean ± SE = 34.3 ± 1.6; DTG: mean ± SE = 34.6 ± 0.93), week 12 (PRT-P group: mean ± SE = 29.9 ± 1.25; DTG: mean ± SE = 33.93 ± 1.35), and week 24 (PRT-P group: mean ± SE = 26.5 ± 1.35; DTG: mean ± SE = 34.4 ± 1.13). F2,39 = 17.597; P < .001. Improvement was observed at week 12 (F1,40 = 4.345; P = .044).
No parent met fidelity of PRT implementation at baseline. At week 24, 21 of 23 parents (91%) in the PRT-P group met fidelity of PRT implementation. Only 1 parent in the DTG met PRT fidelity at week 24; although this parent did not meet fidelity at baseline, she did show some PRT skills at study entry.
Predictors of Response
Exploratory analyses were used to examine whether demographic or clinical characteristics predict outcome in the PRT-P group, as measured by changes in the total number of utterances (SLO) and the BOSCC total score. There were no effects of age, sex, or baseline developmental characteristics (ie, MSEL composite or visual reception t score) on treatment outcomes (P > .10 across all measures). A correlation was observed between the baseline developmental quotient (MSEL age equivalent divided by chronological age) and changes on the BOSCC total score (N = 22; R = 0.483; P = .023). This association was likely related to the contribution of the nonverbal subscales (nonverbal developmental quotient: N = 22; R = 0.685; P < .001). Importantly, the positive correlation indicates that lower MSEL scores at baseline were associated with greater improvement on the BOSCC.
Discussion
The results of this RCT of PRT-P support the efficacy of combining parent training with clinician-delivered in-home treatment for improving functional communication skills of young, minimally verbal children with ASD. The PRT-P resulted in greater improvement in total frequency of child functional utterances and social communication behaviors during SLO, greater increase in the number of words produced on the basis of a parent checklist, and greater improvement in social communication function, as assessed by a blinded clinician ratings. The PRT-P retention rate (96% over 24 weeks) suggests strong acceptability of this treatment in our diverse sample. These positive findings, on the basis of multiple metrics, support PRT as an efficacious early-intervention approach, adding to the growing literature supporting PRT as an established intervention for young children with ASD.23
Improvement in total functional utterances in this controlled trial corroborates the previous findings that PRT-group parent training improves functional utterances.6 Significant results were driven primarily by improvement in intelligible nonverbally prompted utterances, and it is encouraging that PRT helped children generate novel language without verbal prompting. The current study was also 1 of the first efforts to employ the BOSCC coding scheme as a measure of treatment response in a clinical trial. Children receiving the PRT-P demonstrated a significant decrease in BOSCC scores (indicating improvement in social communication behaviors) during both the intensive phase and the maintenance phase of the trial, whereas children in the DTG did not show significant change despite continued involvement in community-based treatments. Evidence that overall social communication skills improved, even when parents were taught primarily how to elicit functional verbal communication, is consistent with previous PRT research revealing broad collateral improvements from targeting the pivotal area of motivation for communication.24 Although, in the current study, we applied the BOSCC coding algorithm to existing videos of parent-child interaction,15 our findings also support the promise of the BOSCC coding scheme as a sensitive measure for capturing changes in social communication behaviors as a result of a behavioral treatment.
Additional evidence of improvement after the PRT-P came from parent report of greater change in the number of words produced and from blinded clinician ratings of greater social communication improvement. Similar gains have been documented in a previous PRT study for the CDI25 and for the CGI.6 The CDI is a quantitative communication measure widely used in research on language development, and evidence of CDI improvement suggests that the language gains made by children in the study were recognizable and commonly used words.26,27 This is particularly important given that children showed a high level of unintelligible speech at baseline (∼70% of utterances on the basis of the SLO). Finally, the improvement observed on the CDI, a standardized language measure, is consistent with the changes observed during the SLO and supports the use of ratings derived from laboratory observation of adult-child interactions as an outcome measure in intervention studies used to target language deficits.
No benefits from the PRT-P compared with the DTG were observed on the Vineland-II communication subscale, the MSEL, or the SRS-2. These observations are not consistent with previous studies of PRT in which improvement on the Vineland-II communication subscale,6 the MSEL,7 and the SRS-228 were found. However, medium-size treatment effect was observed on the Vineland-II expressive subscale. Mixed findings have been reported in previous studies of parent-training and clinician-delivered intervention.29–31 Trials of greater treatment intensity or even longer duration may be necessary, especially in children who are severely affected. Interestingly, in this study, lower MSEL scores at baseline, particularly in the nonverbal domain, were found to predict greater positive response on the BOSCC. This finding is different from previous reports of predictors of response to PRT6,32,33 but suggests optimism that the combined parent and clinician-delivered model may have promise for children who have significant developmental delays at baseline.
A high percentage of parents in the PRT-P group met PRT fidelity of implementation criteria after treatment. This finding is consistent with previous research documenting that parents can learn PRT in a relatively short amount of time.6,34 Although many existing early-intervention programs provide minimal parent training, the addition of this critical component may be key to child progress and is cost-efficient relative to intensive clinician-delivered programs. The hybrid parent-training and clinician-delivered intervention is understudied despite having unique potential advantages, including immediate access to trained clinicians, generalization across daily routines, and long-term cost savings, compared with clinician-delivered service models.
There were several limitations in the trial. The combination of parent-training and clinician-delivered PRT makes it impossible to determine which component had the greatest effect on child progress. The sample size was moderate, which limited power to evaluate complex patterns and predictors of treatment response. Children in the control group were offered PRT immediately after the conclusion of the 24-week trial; therefore, the long-term effects of the control condition were not evaluated. Also, group differences in words produced on the CDI at baseline necessitated controlling for this variable in the analyses. Evaluation of generalized changes in child utterances, social communication skills (BOSCC), and parent fidelity was limited by use of the same SLO video probe to evaluate each variable. Finally, in the current study, we used PRT primarily to target communication skills, although PRT may be effective in addressing other aspects of autism symptomotology29,35,36 as well as comorbid symptoms.37
Conclusions
The current study advances support for NDBIs by demonstrating that children with ASD and significant language delay benefit from the combination of parent training and clinician-delivered PRT. Compared with stable community treatment, the PRT-P provided measurable benefits for enhancing child social communication skills across a diverse set of objective outcome measures. Furthermore, children who received PRT showed greater overall improvement in social communication function on the BOSCC, suggesting that PRT that was focused on improving functional verbal utterances produced generalized effects in a range of social communication behaviors. Additional research will be needed to understand the optimal combination of treatment providers and intensity as well as to identify which children and parents are most likely to benefit. Given promising preliminary data from uncontrolled trials, future RCTs should also be used to examine the effects of PRT on other symptom areas28,35,37 and on potential biomarkers of treatment response.38–40
Acknowledgments
We acknowledge the study clinicians and participating families for their contributions to the project.
Glossary
- ABA
applied behavior analysis
- ASD
autism spectrum disorder
- BOSCC
Brief Observation of Social Communication Change
- CDI
MacArthur-Bates Communicative Development Inventories
- CGI
Clinical Global Impressions
- CGI-I
Clinical Global Impressions Improvement
- CGI-S
Clinical Global Impressions Severity
- DTG
delayed treatment group
- ICC
intraclass correlation coefficient
- MSEL
Mullen Scales of Early Learning
- NDBI
naturalistic developmental behavior intervention
- PLS-5
Preschool Language Scale, Fifth Edition
- PRT
pivotal response treatment
- PRT-P
pivotal response treatment package
- RCT
randomized controlled trial
- SLO
structured laboratory observation
- SRS-2
Social Responsiveness Scale, Second Edition
- Vineland-II
Vineland Adaptive Behavior Scales, Second Edition
Footnotes
Drs Gengoux and Hardan conceptualized and designed the study, supervised data collection and treatment implementation, and drafted, reviewed, and revised the manuscript; Dr Abrams assisted in the analysis and interpretation of data, drafted the initial manuscript, and critically reviewed the full manuscript for important intellectual content; Ms Schuck, Ms Millan, Ms Libove, and Ms Ardel coordinated treatment implementation and data acquisition and reviewed and revised the manuscript; Dr Phillips contributed to study design, acquisition of data by supervising completion of blinded assessment measures, and revision of the manuscript for important intellectual content; Ms Fox contributed to data acquisition and interpretation, completed analyses of the Brief Observation of Social Communication Change outcome measure, and revised the manuscript for important content; Dr Frazier provided statistical expertise to aid in the analysis and interpretation of data and critically reviewed the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02037022).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a grant from the National Institute on Deafness and Other Communication Disorders (DC01368902; principal investigator: Dr Hardan). Dr Abrams received additional support from a National Institute of Mental Health K01 Mentored Research Scientist Development Award (MH102428). Data management was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health through grant UL1 TR001085. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Frazier is employed by Autism Speaks; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Schreibman L, Dawson G, Stahmer AC, et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J Autism Dev Disord. 2015;45(8):2411–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koegel RL, Koegel LK. The PRT Pocket Guide: Pivotal Response Treatment for Autism Spectrum Disorders. Baltimore, MD: Paul H. Brookes Publishing Co; 2012 [Google Scholar]
- 3.Koegel LK, Koegel RL, Harrower JK, Carter CM. Pivotal response intervention I: overview of approach. Res Pract Persons Severe Disabl. 1999;24(3):174–185 [Google Scholar]
- 4.Wainer AL, Pickard K, Ingersoll BR. Using web-based instruction, brief workshops, and remote consultation to teach community-based providers a parent-mediated intervention. J Child Fam Stud. 2017;26(6):1592–1602 [Google Scholar]
- 5.Mohammadzaheri F, Koegel LK, Rezaee M, Rafiee SM. A randomized clinical trial comparison between pivotal response treatment (PRT) and structured applied behavior analysis (ABA) intervention for children with autism. J Autism Dev Disord. 2014;44(11):2769–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardan AY, Gengoux GW, Berquist KL, et al. A randomized controlled trial of Pivotal Response Treatment Group for parents of children with autism. J Child Psychol Psychiatry. 2015;56(8):884–892 [DOI] [PubMed] [Google Scholar]
- 7.Gengoux GW, Berquist KL, Salzman E, et al. Pivotal response treatment parent training for autism: findings from a 3-month follow-up evaluation. J Autism Dev Disord. 2015;45(9):2889–2898 [DOI] [PubMed] [Google Scholar]
- 8.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685 [DOI] [PubMed] [Google Scholar]
- 9.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule (ADOS-2). 2nd ed. Torrance, CA: Western Psychological Services; 2012 [Google Scholar]
- 10.Zimmerman IL, Steiner VG, Pond RA. PLS-5: Preschool Language Scale-5. 5th ed. San Antonio, TX: Psychological Corporation; 2011 [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minjarez MB, Williams SE, Mercier EM, Hardan AY. Pivotal response group treatment program for parents of children with autism. J Autism Dev Disord. 2011;41(1):92–101 [DOI] [PubMed] [Google Scholar]
- 13.Koegel LK. Pivotal Response Treatment: Using Motivation as a Pivotal Response. Santa Barbara, CA: University of California; 2011 [Google Scholar]
- 14.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428 [DOI] [PubMed] [Google Scholar]
- 15.Grzadzinski R, Carr T, Colombi C, et al. Measuring changes in social communication behaviors: preliminary development of the Brief Observation of Social Communication Change (BOSCC). J Autism Dev Disord. 2016;46(7):2464–2479 [DOI] [PubMed] [Google Scholar]
- 16.Fenson L, Marchman VA, Thal DJ, Dale PS, Reznick JS, Bates E. MacArthur-Bates Communicative Development Inventories. Baltimore, MD: Paul H. Brookes Publishing Co; 2007 [Google Scholar]
- 17.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2nd ed. San Antonio, TX: Pearson; 2005 [Google Scholar]
- 18.Mullen EM. Mullen Scales of Early Learning: AGS Edition. Circle Pines, MN: American Guidance Service; 1995 [Google Scholar]
- 19.Constantino JN. Social Responsiveness Scale. 2nd ed. Torrance, CA: Western Psychological Services; 2012 [Google Scholar]
- 20.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; 1976 [Google Scholar]
- 21.Bryson SE, Koegel LK, Koegel RL, Openden D, Smith IM, Nefdt N. Large scale dissemination and community implementation of pivotal response treatment: program description and preliminary data. Res Pract Persons Severe Disabl. 2007;32(2):142–153 [Google Scholar]
- 22.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46 [Google Scholar]
- 23.National Autism Center Findings and Conclusions: National Standards Project, Phase 2. Randolph, MA: National Autism Center; 2015. Available at: https://www.nationalautismcenter.org/national-standards-project/phase-2/. Accessed July 10, 2019 [Google Scholar]
- 24.Koegel RL, Koegel LK. Pivotal Response Treatments for Autism: Communication, Social, & Academic Development. Baltimore, MD: Paul H. Brookes Publishing Co; 2006 [Google Scholar]
- 25.Schreibman L, Stahmer AC. A randomized trial comparison of the effects of verbal and pictorial naturalistic communication strategies on spoken language for young children with autism. J Autism Dev Disord. 2014;44(5):1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luyster R, Lopez K, Lord C. Characterizing communicative development in children referred for autism spectrum disorders using the MacArthur-Bates Communicative Development Inventory (CDI). J Child Lang. 2007;34(3):623–654 [DOI] [PubMed] [Google Scholar]
- 27.Charman T, Drew A, Baird C, Baird G. Measuring early language development in preschool children with autism spectrum disorder using the MacArthur Communicative Development Inventory (Infant Form). J Child Lang. 2003;30(1):213–236 [DOI] [PubMed] [Google Scholar]
- 28.Ventola P, Friedman HE, Anderson LC, et al. Improvements in social and adaptive functioning following short-duration PRT program: a clinical replication. J Autism Dev Disord. 2014;44(11):2862–2870 [DOI] [PubMed] [Google Scholar]
- 29.Duifhuis EA, den Boer JC, Doornbos A, Buitelaar JK, Oosterling IJ, Klip H. The effect of pivotal response treatment in children with autism spectrum disorders: a non-randomized study with a blinded outcome measure. J Autism Dev Disord. 2017;47(2):231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith T, Groen AD, Wynn JW. Randomized trial of intensive early intervention for children with pervasive developmental disorder [published corrections appear in Am J Ment Retard. 2000;105(6):508 and Am J Ment Retard. 2001;106(3):208]. Am J Ment Retard. 2000;105(4):269–285 [DOI] [PubMed] [Google Scholar]
- 31.Roberts J, Williams K, Carter M, et al. A randomised controlled trial of two early intervention programs for young children with autism: centre-based with parent program and home-based. Res Autism Spectr Disord. 2011;5(4):1553–1566 [Google Scholar]
- 32.Schreibman L, Stahmer AC, Barlett VC, Dufek S. Brief report: toward refinement of a predictive behavioral profile for treatment outcome in children with autism. Res Autism Spectr Disord. 2009;3(1):163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherer MR, Schreibman L. Individual behavioral profiles and predictors of treatment effectiveness for children with autism. J Consult Clin Psychol. 2005;73(3):525–538 [DOI] [PubMed] [Google Scholar]
- 34.Coolican J, Smith IM, Bryson SE. Brief parent training in pivotal response treatment for preschoolers with autism. J Child Psychol Psychiatry. 2010;51(12):1321–1330 [DOI] [PubMed] [Google Scholar]
- 35.Ventola PE, Yang D, Abdullahi SM, Paisley CA, Braconnier ML, Sukhodolsky DG. Brief report: reduced restricted and repetitive behaviors after pivotal response treatment. J Autism Dev Disord. 2016;46(8):2813–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreibman L, Stahmer AC, Pierce KL. Alternative applications of pivotal response training: teaching symbolic play and social interaction skills In: Koegel LK, Koegel RL, Dunlap G, eds. Positive Behavioral Support: Including People With Difficult Behavior in the Community. Baltimore, MD: Paul H. Brookes Publishing Co; 1996:353–371 [Google Scholar]
- 37.Lei J, Sukhodolsky DG, Abdullahi SM, Braconnier ML, Ventola P. Brief report: reduced anxiety following pivotal response treatment in young children with autism spectrum disorder. Res Autism Spectr Disord. 2017;43–44:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foss-Feig JH, McGugin RW, Gauthier I, Mash LE, Ventola P, Cascio CJ. A functional neuroimaging study of fusiform response to restricted interests in children and adolescents with autism spectrum disorder. J Neurodev Disord. 2016;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang D, Pelphrey KA, Sukhodolsky DG, et al. Brain responses to biological motion predict treatment outcome in young children with autism. Transl Psychiatry. 2016;6(11):e948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkataraman A, Yang DY, Dvornek N, et al. Pivotal response treatment prompts a functional rewiring of the brain among individuals with autism spectrum disorder. Neuroreport. 2016;27(14):1081–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]