Summary
Macrophages are professional antigen‐presenting cells relying on the expression of class II major histocompatibility complex (MHC II) genes. Interferon‐γ (IFN‐γ) activates MHC II transcription via the assembly of an enhanceosome centred on class II trans‐activator (CIITA). In the present study, we investigated the role of the forkhead transcription factor FOXO3a in IFN‐ γ‐induced MHC II transcription in macrophages. Knockdown of FOXO3a, but not FOXO1 or FOXO4, diminished IFN‐γ‐induced MHC II expression in RAW cells. On the contrary, over‐expression of FOXO3a, but neither FOXO1 nor FOXO4, enhanced CIITA‐mediated trans‐activation of the MHC II promoter. IFN‐γ treatment promoted the recruitment of FOXO3a to the MHC II promoter. Co‐immunoprecipitation and RE‐ChIP assays showed that FOXO3a was a component of the MHC II enhanceosome forming interactions with CIITA, RFX5, RFXB and RFXAP. FOXO3a contributed to MHC II transcription by altering histone modifications surrounding the MHC II promoter. Of interest, FOXO3a was recruited to the type IV CIITA promoter and directly activated CIITA transcription by interacting with signal transducer of activation and transcription 1 in response to IFN‐γ stimulation. In conclusion, our data unveil a novel role for FOXO3a in the regulation of MHC II transcription in macrophages.
Keywords: epigenetics, FOXO, macrophage, transcriptional regulation
Introduction
Antigen‐dependent activation of T lymphocytes is a hallmark event in adaptive immunity.1 Presentation of processed antigens in the so‐called antigen‐presenting cells (APCs) is delegated to the major histocompatibility complex (MHC). Class I MHC molecules stimulate CD8+ cytotoxic T lymphocytes whereas class II MHC (MHC II) molecules are responsible for activating CD4+ helper T lymphocytes. Therefore, MHC II levels are directly correlated with the ability of the host to mount an effective attack against invading pathogens. Accordingly, disturbances of MHC II expression are invariably associated with immune deficiencies that render the host susceptible to infection. For instance, patients with a rare hereditary disease called bare lymphocyte syndrome lack circulating CD4+ T lymphocytes, suffer from severe immune deficiency, and often die before adulthood.2 Genetic and biochemical studies have demonstrated that bare lymphocyte syndrome pathogenesis can be accounted for by the lack of MHC II expression in APCs.3 On the other hand, aberrant MHC II activation is synonymous with hyperactive T lymphocyte reaction and chronic inflammation, which underscores the pathogenesis of a number of human diseases such as atherosclerosis and asthma.4
Previous investigations have led to the discovery of a multi‐protein complex that regulates MHC II transcription in APCs.5 This complex, which includes regulatory factor for X box (RFX) proteins, nuclear factor Y proteins, and class II trans‐activator (CIITA), binds to the proximal promoter regions of the MHC II genes and recruits basal transcription machinery to activate MHC II transcription. CIITA is considered the core of this MHC II‐activating enhanceosome and hence the master regulator of MHC II transcription.6 CIITA expression itself is tightly regulated, and its loss results in bare lymphocyte syndrome in humans.7 CIITA transcription, driven by type I and type III promoters, is constitutively activated in professional APCs (e.g. dendritic cells and B lymphocytes) allowing the assembly of the MHC II enhanceosome and subsequently MHC II expression by default. Alternatively, interferon‐γ (IFN‐γ) can induce CIITA transcription, via type IV promoter, in both non‐professional APCs (e.g. smooth muscle cells) and professional APCs (e.g. macrophages), thereby prompting the enhanceosome set‐up and MHC II transcription.8 Several transcription factors, including signal transducer and activator of transcription 1 (STAT1) and interferon regulatory factor 1, have been documented to play essential roles in IFN‐γ‐dependent CIITA type IV transcription.9
Forkhead box (FOX) proteins constitute a superfamily of transcriptional regulators that can be further categorized into different subgroups based on sequence homologies. FOXO factors, including FOXO1, FOXO3a, FOXO4 and FOXO6, belong to one of such subfamilies.10 Among the members of this family, FOXO3a is preferentially expressed in myeloid cells (e.g. macrophage).11 Previous studies have shown that FOXO3a regulates a myriad of macrophage‐related functions. For instance, it has been demonstrated that FOXO3a may either promote or antagonize, depending on the context, macrophage autophagy and inflammatory response.12, 13 Several reports suggest that FOXO3a may mediate macrophage apoptosis under stress conditions.14, 15, 16 On the contrary, FOXO3a can also sustain the pro‐inflammatory state of macrophages by regulating the extracellular signal‐regulated kinase signalling pathway to curb reactive oxygen species production without altering cell death during host defence response.17 Nie et al. have demonstrated that FOXO3a represses interleukin‐13 transcription in macrophages to protect against bleomycin‐induced pulmonary inflammation and fibrosis.18 In addition, it has been shown that FOXO3a is capable of inhibiting the NLRP3 inflammasome by potentiating the transcription of Bim in liver macrophages (Kupffer cells).19 Recently, genome‐wide meta‐analysis indicates that interplay between FOXO3a and certain MHC II molecules (e.g. HLA‐DQ) may be involved in the regulation of longevity in humans.20 It is not known, however, whether FOXO3a might contribute to IFN‐γ‐induced MHC II transcription in macrophages. We report here that FOXO3a participates in IFN‐γ‐induced MHC II transcription as a component of the CIITA‐centred enhanceosome. In addition, FOXO3a is directly involved in CIITA type IV transcription by interacting with STAT1. Therefore, our data unveil a novel role for FOXO3a in the regulation of MHC II transcription in macrophages.
Materials and methods
Cell culture, transient transfection and reporter assay
RAW 264.7 cells (hereafter referred to as RAW, purchased from the American Type Culture Collection, ATCC, Manassas, VA) and HEK293 cells (purchased from Invitrogen, Carlsbad, CA) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Primary murine bone marrow‐derived macrophages were isolated and differentiated as previously described.21 FLAG‐tagged human CIITA, FLAG‐tagged human RFX5, FLAG‐tagged human RFXB, His‐tagged human RFXAP, Myc‐tagged human SIRT1, type IV human CIITA promoter‐luciferase construct and the human DRA300 reporter have been described previously.22, 23, 24, 25, 26, 27, 28 Mouse FOXO1 cDNA, mouse FOXO3a and mouse FOXO4 were generously provided by Dr Hao Li (Nanjing Medical University);29 these cDNAs were subcloned into the pEGFP (Clontech, Mountain View, CA) vector to add an N‐terminal green fluorescent protein (GFP) tag. Small interfering RNAs were purchased from Dharmacon (Lafayette, CO). Transient transfections were performed with Lipofectamine 2000. The cells were harvested 24–48 hr after transfection and reporter activity was measured using a luciferase reporter assay system (Promega, Madison, WI) as previously described.30, 31 Briefly, cells were plated in 12‐well culture dishes (~60 000 cells/well). The next day, equal amounts (0·1 µg) of reporter construct and effector construct were transfected into each well. DNA content was normalized by the addition of an empty vector (pcDNA3). For monitoring transfection efficiency and for normalizing luciferase activity, 0·02 µg of GFP construct was transfected into each well. Luciferase activities were normalized by both protein concentration and GFP fluorescence. Data are expressed as relative luciferase unit compared with the control group arbitrarily set as 1.
RNA isolation and real‐time PCR
RNA was extracted with the RNeasy RNA isolation kit (Qiagen, Mansfield, MA) as previously described.32 Reverse transcriptase reactions were performed using a SuperScript First‐strand Synthesis System (Invitrogen). Real‐time polymerase chain reactions (PCR) were performed on an ABI Prism 7500 system with the following primers: Foxo1, 5′‐ACATTTCGTCCTCGAACCAGCTCA‐3 and 5′‐ATTTCAGACAGACTGGGCAGCGTA‐3′; Foxo3a, 5′‐AGTGGATGGTGCGCTGTGT‐3′ and 5′‐CTGTGCAGGGACAGGTTGT‐3′; Foxo4, 5′‐GGTGCCCTACTTCAAGGACA‐3′ and 5′‐GGTTCAGCATCCACCAAGAG‐3′; H2‐Ieb, 5′‐ CAGAACAAGATGTTGAGTGGAGTTG‐3′ and 5′‐TTCTGGTTCCTGAAGTAGATGAACAG‐3′; Ciita type IV, 5′‐GAACAGCGGCAGCTCACA‐3′ and 5′‐TCTCCAGCCAGGTCCATCTG‐3′.
Protein extraction and Western blot
Whole‐cell lysates were obtained by re‐suspending cell pellets in RIPA buffer (50 mm Tris–HCl pH 7·4, 150 mm NaCl, 1% Triton‐X‐100) with freshly added protease inhibitor (Roche, Branford, CT) as previously described.33 Specific antibodies or pre‐immune IgGs were added to and incubated with cell lysates overnight before being absorbed by Protein A/G‐plus Agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Precipitated immune complex was released by boiling with 1× sodium dodecyl sulphate (SDS) electrophoresis sample buffer. Western blot analyses were performed with anti‐STAT1 (Santa Cruz Biotechnology, sc‐346), anti‐RFX5 (Rockland Immunochemicals, Pottstown, PA, 200‐401‐194), anti‐GFP (Proteintech, Wuhan, China, 50430‐2), anti‐HIS (Proteintech, 66005‐1), anti‐FLAG (Sigma, St Louis, MO, F3165), anti‐CIITA (Santa Cruz Biotechnology, sc‐13556), anti‐H2‐Ieβ (eBioscience, Frankfurt am Main, Germany, 14‐5321‐82), or β‐actin (Sigma, A2228).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed essentially as described previously.34, 35, 36, 37, 38, 39, 40, 41, 42 In brief, chromatin in control and treated cells was cross‐linked with 1% formaldehyde. Cells were incubated in lysis buffer (150 mm NaCl, 25 mm Tris–HCl pH 7·5, 1% Triton‐X‐100, 0·1% SDS, 0·5% deoxycholate) supplemented with protease inhibitor tablet and phenylmethylsulphonyl fluoride. DNA was fragmented into ~200 bp pieces using a Branson 250 sonicator. Aliquots of lysates containing 200 μg of protein were used for each immunoprecipitation reaction with anti‐FOXO3a (Santa Cruz Biotechnology, sc‐11351), anti‐CBP (Santa Cruz Biotechnology, sc‐584), anti‐WDR5 (Bethyl Laboratories, Montgomery, TX, A302‐429A), anti‐ASH2 (Bethyl Laboratories, A300‐489A), anti‐trimethyl H3K4 (Millipore, Syracuse, NY, 07‐449), anti‐acetyl H3 (Millipore, 06‐599), anti‐symmetric dimethyl H3R2 (Abcam, Hong Kong ab194684), anti‐PRMT5 (Millipore, 07‐405), anti‐CIITA (Santa Cruz Biotechnology, sc‐13556), anti‐STAT1 (Santa Cruz Biotechnology, sc‐346), anti‐RFX5 (Rockland Immunochemicals, 200‐401‐194), or pre‐immune IgG. For re‐ChIP, the immune complexes were eluted with the elution buffer (1% SDS, 100 mm NaCO3), diluted with the re‐ChIP buffer (1% Triton‐X‐100, 2 mm ethylenediamine tetraacetic acid, 150 mm NaCl, 20 mm Tris–HCl, pH 8·1), and subjected to immunoprecipitation with a second antibody of interest. Precipitated DNA was amplified with the following primers: H2‐Ieb promoter, 5′‐GCCTCCTGAGTGCTGGGATA‐3′ and 5′‐ACTGAGTATCCATGTAATGAAGAGAACTG‐3′; Ciita type IV proximal promoter (‐165/‐20), 5′‐CACTGTGAGGAACCGACTGGAG‐3′ and 5′‐TGGAGCAACCAAGCACCTACTG‐3′; Ciita type IV distal promoter (‐762/‐634), 5′‐AGATAGAGTGAATAGAG‐3′ and 5′‐ACCCCTCCCTACAGGAGATC‐3′; Gapdh promoter, 5′‐ATCACTGCCACCCAGAAGACTGTGGA‐3′ and 5′‐CTCATACCAGGAAATGAGCTTGACAAA‐3′.
Statistical analysis
Two‐tailed Student's t‐test (for comparisons involving two groups) or one‐way analysis of variance with post hoc Scheffé analyses (for comparisons involving three or more groups) were performed using SPSS software (IBM SPSS v18·0, Chicago, IL). Unless otherwise specified, values of P < 0·05 were considered statistically significant.
Results
FOXO3a contributes to IFN‐γ‐induced MHC II transcription in macrophages
To investigate the involvement of FOXO factors in IFN‐γ‐induced MHC II transcription, RAW cells were transfected with specific small interfering RNAs targeting individual FOXO proteins. IFN‐γ treatment robustly stimulated the expression of H2‐Ieb, the mouse orthologue of the human MHC II molecule (HLA‐DRA).43 Depletion of FOXO3a, but not FOXO1 or FOXO4, significantly dampened IFN‐γ‐induced MHC II expression (Fig. 1a). Similar observations were made in primary murine bone marrow‐derived macrophages (Fig. 1b). Consistently, over‐expression of FOXO3a, although by itself it did not impact the MHC II (DRA300) promoter activity, enhanced the MHC II promoter activity in the presence of CIITA (Fig. 1c). Neither FOXO1 nor FOXO4 altered the MHC II promoter activity with or without CIITA.
Figure 1.
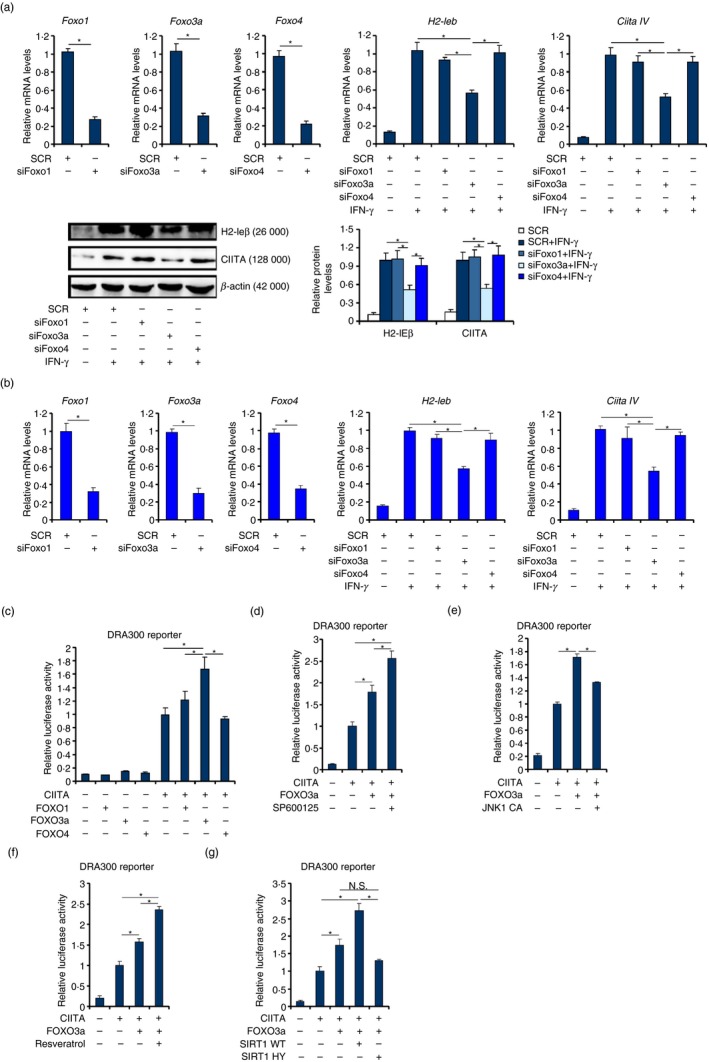
FOXO3a contributes to interferon‐γ (IFN‐γ) ‐induced MHC II transcription in macrophages. (a) RAW cells were transfected with small interfering RNAs (siRNAs) targeting specific FOXO or scrambled siRNA (SCR) followed by treatment with IFN‐γ. Gene expression levels were examined by quantitative PCR and Western blotting. (b) Bone marrow‐derived macrophages were transfected with siRNAs targeting specific FOXO or scrambled siRNA (SCR) followed by treatment with IFN‐γ. Gene expression levels were examined by quantitative PCR. (c) An MHC II promoter luciferase construct (DRA300) was transfected into HEK293 cells with FOXO and CIITA expression constructs. Luciferase activities were normalized by both protein concentration and GFP fluorescence. (d) An MHC II promoter luciferase construct (DRA300) was transfected into HEK293 cells with FOXO3a and CIITA expression constructs followed by treatment with a Jun N‐terminal kinase (JNK) inhibitor (SP600125). Luciferase activities were normalized by both protein concentration and GFP fluorescence. (e) An MHC II promoter luciferase construct (DRA300) was transfected into HEK293 cells with FOXO3a, JNK and CIITA expression constructs. Luciferase activities were normalized by both protein concentration and GFP fluorescence. (f) An MHC II promoter luciferase construct (DRA300) was transfected into HEK293 cells with FOXO3a and CIITA expression constructs followed by treatment with a SIRT1 agonist (resveratrol). Luciferase activities were normalized by both protein concentration and GFP fluorescence. (g) An MHC II promoter luciferase construct (DRA300) was transfected into RAW cells with FOXO3a, SIRT1 and CIITA expression constructs. Luciferase activities were normalized by both protein concentration and GFP fluorescence. N.S., not statistically significant. Data represent averages of three independent experiments and error bars represent SEM. *P ≤ 0·05.
FOXO3a activity is known to be modulated by post‐translational modification‐dependent cytoplasm‐nucleus shuttling. Jun N‐terminal kinase (JNK) has been demonstrated to inhibit FOXO3a by promoting its nuclear export.44 Indeed, treatment with a JNK inhibitor (SP600125) further augmented the MHC II promoter activity driven by CIITA and FOXO3a (Fig. 1d). In contrast, co‐expression of a constitutively active JNK abrogated the enhancement of MHC II transcription by FOXO3a (Fig. 1e). The class III deacetylase SIRT1 activates FOXO3a by promoting its nuclear transport.45 In accordance, treatment with a SIRT1 agonist (resveratrol) amplified the induction of the MHC II promoter by simultaneous expression of CIITA and FOXO3a (Fig. 1f). Similarly, co‐expression of wild‐type SIRT1 but not enzyme‐deficient (HY) SIRT1 further augmented the MHC II promoter activity (Fig. 1g). Together, these data suggest that FOXO3a may play a role in IFN‐γ‐induced MHC II transcription in macrophages.
FOXO3a is a component of the CIITA‐centred MHC II enhanceosome
When RAW cells were treated with IFN‐γ, FOXO3a was recruited to the MHC II (H2‐Ieb) promoter: FOXO3a started to occupy the MHC II promoter 6 hr after IFN‐γ treatment and remained associated with the MHC II promoter 24 hr after the addition of IFN‐γ (Fig. 2a). No conserved forkhead box motif was found within the MHC II promoter region (data not shown), suggesting that FOXO3a might act as a co‐factor and a component of the MHC II enhanceosome. Co‐immunoprecipitation assay showed that FOXO3a formed a complex with CIITA, RFX5, RFXB and RFXAP (Fig. 2b). Further, Re‐ChIP assay confirmed that IFN‐γ treatment augmented the interaction between FOXO3a, RFX5 and CIITA on the MHC II promoter, but not the GAPDH promoter, in RAW cells (Fig. 2c). When RFX5 was depleted with small interfering RNA (Fig. 2d), FOXO3a binding on the MHC II promoter was severely compromised (Fig. 2e). Reciprocally, FOXO3a depletion disrupted the interaction between RFX5 and CIITA on the MHC II promoter (Fig. 2f). Combined, these data suggest that FOXO3a may be an integral part of the MHC II enhanceosome.
Figure 2.
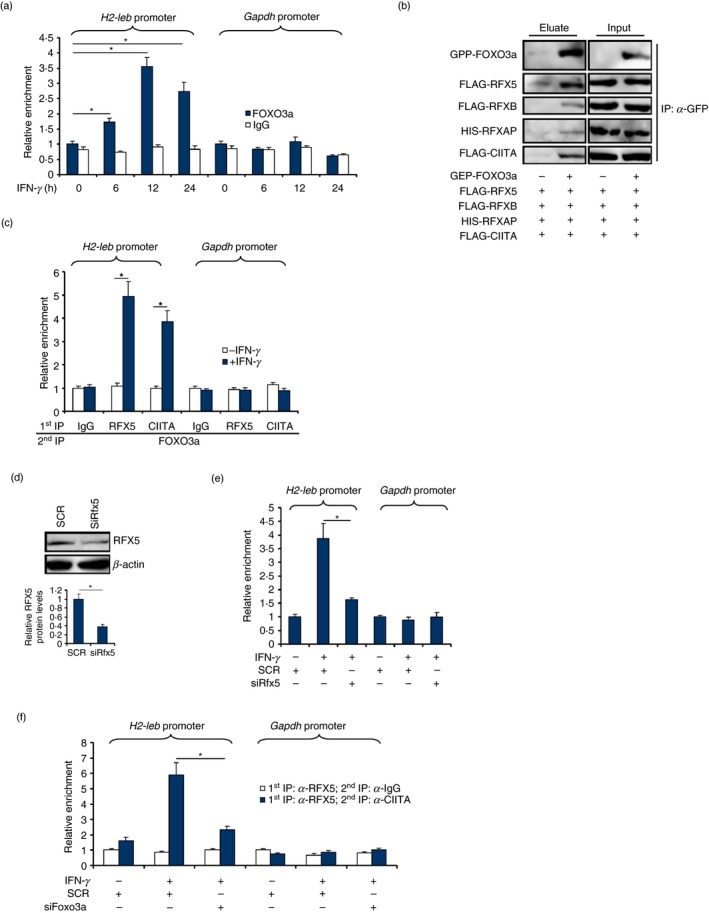
FOXO3a is a component of the CIITA‐centred MHC II enhanceosome. (a) RAW cells were treated with interferon‐γ (IFN‐γ) and harvested at the indicated time‐points. ChIP assay was performed with anti‐FOXO3a or IgG. (b) HEK293 cells were transfected with indicated expression constructs. Immunoprecipitation was performed with anti‐GFP. (c) RAW cells were treated with IFN‐γ for 12 hr. Re‐ChIP assay was performed with the indicated antibodies. (d, e) RAW cells were transfected with small interfering RNA (siRNA) targeting RFX5 or scrambled siRNA (SCR) followed by treatment with IFN‐γ for 12 hr. Knockdown efficiency was examined by Western blotting. ChIP assay was performed with anti‐FOXO3a. (f) RAW cells were transfected with siRNA targeting FOXO3a or SCR followed by treatment with IFN‐γ for 12 hr. Re‐ChIP assay was performed with the indicated antibodies. Data represent averages of three independent experiments and error bars represent SEM. *P ≤ 0·05.
FOXO3a contributes to MHC II transcription by modulating histone modification
To tackle the epigenetic mechanism whereby FOXO3a contributes to MHC II transcription, a series of ChIP assays were performed with antibodies that recognize modified histones and histone‐modifying enzymes. When RAW cells were treated with IFN‐γ, active histone modifications including acetylated histone H3 (AcH3, Fig. 3a), trimethylated histone H3K4 (H3K4Me3, Fig. 3b) and symmetrical dimethylated histone H3R2 (H3R2Me2s, Fig. 3c) were all up‐regulated surrounding the MHC II promoter. FOXO3a knockdown, to various extents, dampened the accumulation of active histone marks on the MHC II promoter. Consistent with these observations, ChIP assays revealed that histone‐modifying enzymes involved in catalysing active histone marks, including the acetyltransferases CBP (Fig. 3d), the H3K4 trimethyltransferase complex components ASH2 (Fig. 3e) and WDR5 (Fig. 3f), and the H3R2 arginine methyltransferase PRMT5 (Fig. 3g) were recruited to the MHC II promoter following IFN‐γ treatment. FOXO3a silencing, however, weakened the occupancies of these factors. Collectively, these data suggest that FOXO3a may contribute to MHC II transcription by modulating recruitment of epigenetic factors to influence histone modifications.
Figure 3.
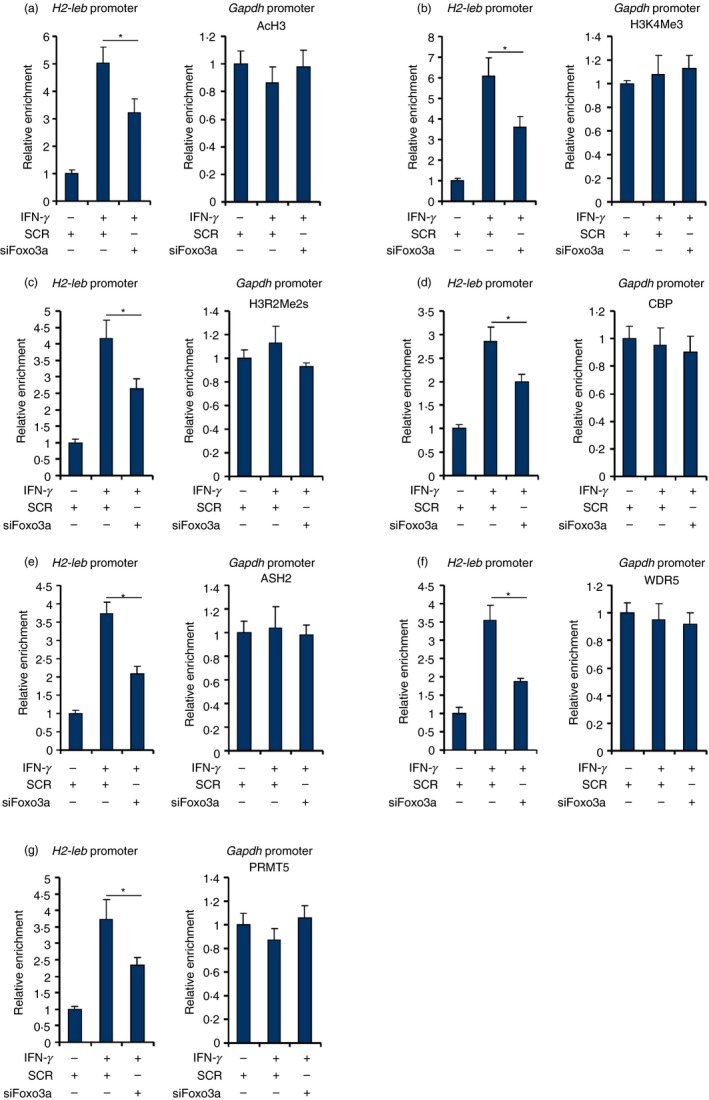
FOXO3a contributes to MHC II transcription by modulating histone modification. (a–g) RAW cells were transfected with small interfering RNA targeting FOXO3a or SCR followed by treatment with interferon‐γ (IFN‐γ) for 12 hr. ChIP assay was performed with anti‐acetyl histone H3 (a), anti‐trimethyl H3K4 (b), anti‐symmetrical dimethyl H3R2 (c), anti‐CBP (d), anti‐ASH2 (e), anti‐WDR5 (f), or anti‐PRMT5 (g). Data represent averages of three independent experiments and error bars represent SEM. *P ≤ 0·05.
FOXO3a directly regulates type IV CIITA transcription
FOXO3a depletion resulted in a decrease in CIITA type IV (Ciita IV), the major IFN‐inducible CIITA isoform,46 in macrophages (Fig. 1a,b). This observation prompted us to investigate the possibility that FOXO3a might directly regulate IFN‐γ‐induced CIITA transcription. Reporter assay showed that FOXO3a over‐expression enhanced induction of the CIITA IV promoter activity by IFN‐γ treatment (Fig. 4a). ChIP assay confirmed that FOXO3a was recruited to the proximal (−165/−20), but not distal (−762/−634), CIITA IV promoter following IFN‐γ treatment (Fig. 4b). In addition, FOXO3a formed a complex with STAT1 on the CIITA promoter only when cells were treated with IFN‐γ, suggesting that FOXO3a might rely on STAT1 to bind to the CIITA promoter (Fig. 4c). Indeed, STAT1 knockdown (Fig. 4d) impaired FOXO3a binding on the CIITA promoter (Fig. 4e).
Figure 4.
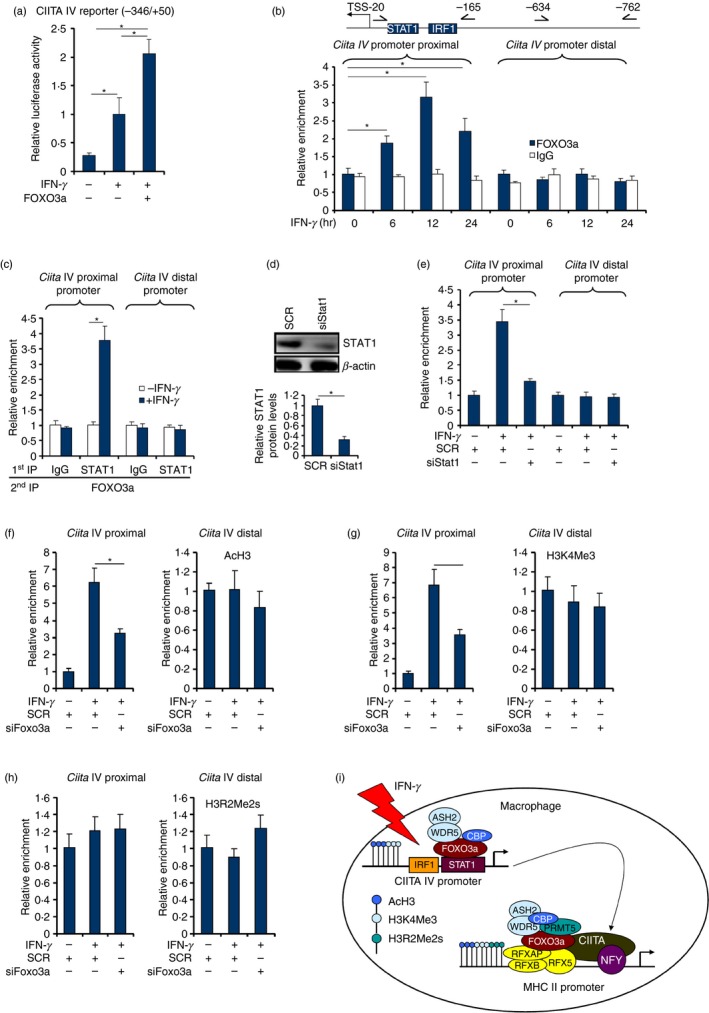
FOXO3a directly regulates type IV CIITA transcription. (a) A CIITA type IV promoter luciferase construct (DRA300) was transfected into HEK293 cells with FOXO followed by treatment with interferon‐γ (IFN‐γ). Luciferase activities were normalized by both protein concentration and GFP fluorescence. (b) RAW cells were treated with IFN‐γ and harvested at the indicated time‐points. ChIP assay was performed with anti‐FOXO3a or IgG. (c) RAW cells were treated with IFN‐γ for 12 hr. Re‐ChIP assay was performed with the indicated antibodies. (d, e) RAW cells were transfected with small interfering RNA (siRNA) targeting STAT1 or scrambled siRNA (SCR) followed by treatment with IFN‐γ for 12 hr. Knockdown efficiency was examined by Western blotting. ChIP assay was performed with anti‐FOXO3a. (f–h) RAW cells were transfected with siRNA targeting FOXO3a or SCR followed by treatment with IFN‐γ for 12 hr. Re‐ChIP assay was performed with indicated antibodies. Data represent averages of three independent experiments and error bars represent SEM. (i) A schematic model. *P ≤ 0·05.
We finally asked whether the same epigenetic mechanism by which FOXO3a contributes to IFN‐γ‐induced MHC II transcription may underscore CIITA transcription. FOXO3a depletion was found to be associated with down‐regulation of acetyl H3 (Fig. 4f) and trimethyl H3K4 (Fig. 4g) but not symmetrical dimethyl H3R2 (Fig. 4h). We therefore conclude that FOXO3a may contribute to MHC II induction by directly activating CIITA transcription in macrophages.
Discussion
MHC II transcription is tightly regulated in macrophages to ensure a robust host defence response while at the same time minimizing aberrant inflammation. Here we propose a new model wherein the forkhead transcription factor FOXO3a contributes to IFN‐γ‐induced MHC II transcription (Fig. 4i). On the one hand, FOXO3a interacts with the MHC II enhanceosome to bind to the MHC II promoter and activate MHC II transcription. On the other hand, FOXO3a directly activates CIITA isoform IV transcription by interacting with STAT1. Therefore, FOXO3a may represent a novel regulator of macrophage‐mediated immunity.
We show here that FOXO3a depletion attenuates IFN‐γ‐induced transcription of MHC II and CIITA type IV genes in macrophages (Fig. 1). This is consistent with a previous report by Fallarino et al. that shows the rescue of IFN‐γ‐dependent phagocyte function by FOXO3a in NOD mice.47 It is unclear to what extent FOXO3a mediates IFN‐γ‐induced gene expression genome‐wide in macrophages. Yang et al. have recently described the regulation of type I CIITA by FOXO1, a closely related paralogue of FOXO3a, in macrophages.48 CIITA type I is the CIITA isoform predominantly found in dendritic cells and is not typically induced by IFN‐γ treatment.49 Of interest, dendritic cell‐restricted deletion of FOXO1 renders the mice susceptible to pathogen infection, probably owing to impaired antigen presentation.50 In contrast, deletion of FOXO3a in dendritic cells did not appear to influence (constitutive) MHC II expression.51 Therefore, it is possible that different FOXO family members may participate in the regulation of MHC II transcription in different cell types via distinct mechanisms.
We show that the ability of FOXO3a to regulate MHC II and CIITA transcription seems to rely on, at least in part, its interaction with and recruitment of various epigenetic factors. Indeed, FOXO3a has been reported to interact with a host of epigenetic factors, including histone acetyltransferases,52 deacetylases,53 demethylases54 and chromatin remodelling proteins,55 to regulate transcription. In addition, it has been thought that FOXO1 and perhaps other members of the FOXO family including FOXO3a can open up chromatin structure to facilitate transcription.56 A caveat in this model is that the same set of enzymes recruited by FOXO3a to modify histones may directly catalyse the post‐translational modification of FOXO3a itself. For instance, acetylation of FOXO3a by CBP has been shown to repress its transcriptional activity whereas arginine methylation of FOXO3a by PRMT is documented to enhance its activity.57, 58 Hence, it is not immediately clear whether dynamic modifications of FOXO3a, in addition to histone modifications, by CBP, PRMT5 and/or ASH2 may contribute to transcriptional activation of MHC II and CIITA in macrophages.
A tempting hypothesis prompted by our observations as reported here is that FOXO3a deficiency in macrophages may impair host defence in response to pathogen invasion but protect against inflammation‐associated disorders. The literature seems to offer conflicting evidence for this hypothesis. For example, Kang et al. have shown that macrophage‐specific deletion of FOXO3a protects against arthritis in mice.59 On the contrary, Accili and colleagues have reported that simultaneous deletion of all three FOXO isoforms in macrophages promotes atherosclerosis.60 Clearly further studies are warranted to clarify this issue before FOXO3a can be considered as a potential target for drug development to treat immune deficiency and/or inflammation‐related human diseases.
Disclosure
None declared.
Acknowledgements
This work was supported, in part, by grants from the National Natural Science Foundation of China (81670618, 81700554), from the Nanjing Municipal Administration of Health and Human Services (YKK17061), and from the Fundamental Research Funds for Central Universities (021414380323).
References
- 1. Litman GW, Rast JP, Fugmann SD. The origins of vertebrate adaptive immunity. Nat Rev Immunol 2010; 10:543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol 2001; 19:331–73. [DOI] [PubMed] [Google Scholar]
- 3. Masternak K, Muhlethaler‐Mottet A, Villard J, Peretti M, Reith W. Molecular genetics of the bare lymphocyte syndrome. Rev Immunogenet 2000; 2:267–82. [PubMed] [Google Scholar]
- 4. Friese MA, Jones EY, Fugger L. MHC II molecules in inflammatory diseases: interplay of qualities and quantities. Trends Immunol 2005; 26:559–61. [DOI] [PubMed] [Google Scholar]
- 5. Reith W, Muhlethaler‐Mottet A, Masternak K, Villard J, Mach B. The molecular basis of MHC class II deficiency and transcriptional control of MHC class II gene expression. Microbes Infect 1999; 1:839–46. [DOI] [PubMed] [Google Scholar]
- 6. Masternak K, Muhlethaler‐Mottet A, Villard J, Zufferey M, Steimle V, Reith W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev 2000; 14:1156–66. [PMC free article] [PubMed] [Google Scholar]
- 7. Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell 1993; 75:135–46. [PubMed] [Google Scholar]
- 8. LeibundGut‐Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A et al Mini‐review: specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol 2004; 34:1513–25. [DOI] [PubMed] [Google Scholar]
- 9. Piskurich JF, Linhoff MW, Wang Y, Ting JP. Two distinct γ interferon‐inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by STAT1, interferon regulatory factor 1, and transforming growth factor β . Mol Cell Biol 1999; 19:431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter ME, Brunet A. FOXO transcription factors. Curr Biol 2007; 17:R113–4. [DOI] [PubMed] [Google Scholar]
- 11. Dejean AS, Hedrick SM, Kerdiles YM. Highly specialized role of Forkhead box O transcription factors in the immune system. Antioxid Redox Signal 2011; 14:663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pun NT, Subedi A, Kim MJ, Park PH. Globular adiponectin causes tolerance to LPS‐induced TNF‐α expression via autophagy induction in RAW 264.7 macrophages: involvement of SIRT1/FoxO3A axis. PLoS ONE 2015; 10:e0124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li C, Wang Z, Wang C, Ma Q, Zhao Y. Perivascular adipose tissue‐derived adiponectin inhibits collar‐induced carotid atherosclerosis by promoting macrophage autophagy. PLoS ONE 2015; 10:e0124031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim EJ, Park DW, Lee JG, Lee CH, Bae YS, Hwang YC et al Toll‐like receptor 9‐mediated inhibition of apoptosis occurs through suppression of FoxO3a activity and induction of FLIP expression. Exp Mol Med 2010; 42:712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui M, Huang Y, Zhao Y, Zheng J. Transcription factor FOXO3a mediates apoptosis in HIV‐1‐infected macrophages. J Immunol 2008; 180:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang W, Konopleva M, Burks JK, Dywer KC, Schober WD, Yang JY et al Blockade of mitogen‐activated protein kinase/extracellular signal‐regulated kinase kinase and murine double minute synergistically induces Apoptosis in acute myeloid leukemia via BH3‐only proteins Puma and Bim. Cancer Res 2010; 70:2424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joseph J, Ametepe ES, Haribabu N, Agbayani G, Krishnan L, Blais A et al Inhibition of ROS and upregulation of inflammatory cytokines by FoxO3a promotes survival against Salmonella typhimurium . Nat Commun 2016; 7:12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nie Y, Sun L, Wu Y, Yang Y, Wang J, He H et al AKT2 regulates pulmonary inflammation and fibrosis via modulating macrophage activation. J Immunol 2017; 198:4470–80. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Zhang W, Wu X, Gong J. Foxo3a‐dependent Bim transcription protects mice from a high fat diet via inhibition of activation of the NLRP3 inflammasome by facilitating autophagy flux in Kupffer cells. Oncotarget 2017; 8:34258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joshi PK, Pirastu N, Kentistou KA, Fischer K, Hofer E, Schraut KE et al Genome‐wide meta‐analysis associates HLA‐DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat Commun 2017; 8:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu L, Weng X, Liang P, Dai X, Wu X, Xu H et al MRTF‐A mediates LPS‐induced pro‐inflammatory transcription by interacting with the COMPASS complex. J Cell Sci 2014; 127:4645–57. [DOI] [PubMed] [Google Scholar]
- 22. Fan Z, Kong X, Xia J, Wu X, Li H, Xu H et al The arginine methyltransferase PRMT5 regulates CIITA‐dependent MHC II transcription. Biochim Biophys Acta 2016; 1859:687–96. [DOI] [PubMed] [Google Scholar]
- 23. Kong X, Fang M, Li P, Fang F, Xu Y. HDAC2 deacetylates class II transactivator and suppresses its activity in macrophages and smooth muscle cells. J Mol Cell Cardiol 2009; 46:292–9. [DOI] [PubMed] [Google Scholar]
- 24. Li P, Zhao Y, Wu X, Xia M, Fang M, Iwasaki Y et al Interferon γ (IFN‐γ) disrupts energy expenditure and metabolic homeostasis by suppressing SIRT1 transcription. Nucleic Acids Res 2012; 40:1609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan Z, Li J, Li P, Ye Q, Xu H, Wu X et al Protein arginine methyltransferase 1 (PRMT1) represses MHC II transcription in macrophages by methylating CIITA. Sci Rep 2017; 7:40531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng S, Yang Y, Cheng X, Zhou B, Li P, Zhao Y et al HIC1 epigenetically represses CIITA transcription in B lymphocytes. Biochim Biophys Acta 2016; 1859:1481–9. [DOI] [PubMed] [Google Scholar]
- 27. Fang M, Kong X, Li P, Fang F, Wu X, Bai H et al RFXB and its splice variant RFXBSV mediate the antagonism between IFNγ and TGFβ on COL1A2 transcription in vascular smooth muscle cells. Nucleic Acids Res 2009; 37:4393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y, Li X, Peng L, An L, Sun N, Hu X et al Tanshindiol C inhibits oxidized low‐density lipoprotein induced macrophage foam cell formation via a peroxiredoxin 1 dependent pathway. Biochim Biophys Acta Mol Basis Dis 2018; 1864:882–90. [DOI] [PubMed] [Google Scholar]
- 29. Chen S, Gai J, Wang Y, Li H. FoxO regulates expression of decidual protein induced by progesterone (DEPP) in human endothelial cells. FEBS Lett 2011; 585:1796–800. [DOI] [PubMed] [Google Scholar]
- 30. Li N, Kong M, Zeng S, Xu Z, Li M, Hong W et al The chromatin remodeling protein BRG1 regulates APAP‐induced liver injury by modulating CYP3A11 transcription in hepatocyte. Biochim Biophys Acta Mol Basis Dis 2018; 1864:3487–95. [DOI] [PubMed] [Google Scholar]
- 31. Li N, Li M, Hong W, Shao J, Xu H, Shimano H et al Brg1 regulates pro‐lipogenic transcription by modulating SREBP activity in hepatocytes. Biochim Biophys Acta Mol Basis Dis 2018; 1864:2881–9. [DOI] [PubMed] [Google Scholar]
- 32. Li Z, Zhang X, Liu S, Zeng S, Yu L, Yang G et al BRG1 regulates NOX gene transcription in endothelial cells and contributes to cardiac ischemia‐reperfusion injury. Biochim Biophys Acta Mol Basis Dis 2018; 1864:3477–86. [DOI] [PubMed] [Google Scholar]
- 33. Liu L, Wu X, Xu H, Yu L, Zhang X, Li L et al Myocardin‐related transcription factor A (MRTF‐A) contributes to acute kidney injury by regulating macrophage ROS production. Biochim Biophys Acta Mol Basis Dis 2018; 1864:3109–21. [DOI] [PubMed] [Google Scholar]
- 34. Yu L, Li Z, Fang M, Xu Y. Acetylation of MKL1 by PCAF regulates pro‐inflammatory transcription. Biochim Biophys Acta Gene Regul Mech 2017; 1860:839–47. [DOI] [PubMed] [Google Scholar]
- 35. Yu L, Yang G, Zhang X, Wang P, Weng X, Yang Y et al Megakaryocytic Leukemia 1 (MKL1) bridges epigenetic activation of NADPH oxidase in macrophages to cardiac ischemia‐reperfusion injury. Circulation 2018; 138:2820–36. [DOI] [PubMed] [Google Scholar]
- 36. Li M, Hong W, Hao C, Li L, Xu H, Li P et al Hepatic stellate cell‐specific deletion of SIRT1 exacerbates liver fibrosis in mice. Biochim Biophys Acta 2017; 1863:3202–11. [DOI] [PubMed] [Google Scholar]
- 37. Li Z, Chen B, Weng X, Yu L, Song M, Fang M et al The histone methyltransferase SETD1A regulates thrombomodulin transcription in vascular endothelial cells. Biochim Biophys Acta Gene Regul Mech 2018; 1861:752–61. [DOI] [PubMed] [Google Scholar]
- 38. Li Z, Chen B, Dong W, Xu W, Song M, Fang M et al Epigenetic activation of PERP transcription by MKL1 contributes to ROS‐induced apoptosis in skeletal muscle cells. Biochim Biophys Acta Gene Regul Mech 2018; 1861:905–15. [DOI] [PubMed] [Google Scholar]
- 39. Weng X, Zhang Y, Li Z, Yu L, Xu F, Fang M et al Class II transactivator (CIITA) mediates IFN‐γ induced eNOS repression by enlisting SUV39H1. Biochim Biophys Acta Gene Regul Mech 2019; 1862:163–72. [DOI] [PubMed] [Google Scholar]
- 40. Shao J, Weng X, Zhuo L, Yu L, Li Z, Shen K et al Angiotensin II induced CSF1 transcription is mediated by a crosstalk between different epigenetic factors in vascular endothelial cells. Biochim Biophys Acta Gene Regul Mech 2019; 1862:1–11. [DOI] [PubMed] [Google Scholar]
- 41. Zeng S, Wu X, Chen X, Xu H, Zhang T, Xu Y. Hypermethylated in cancer 1 (HIC1) mediates high glucose induced ROS accumulation in renal tubular epithelial cells by epigenetically repressing SIRT1 transcription. Biochim Biophys Acta Gene Regul Mech 2018; 1861:917–27. [DOI] [PubMed] [Google Scholar]
- 42. Fan Z, Li Z, Yang Y, Liu S, Guo J, Xu Y. HIF‐1α coordinates epigenetic activation of SIAH1 in hepatocytes in response to nutritional stress. Biochim Biophys Acta Gene Regul Mech 2017; 1860:1037–46. [DOI] [PubMed] [Google Scholar]
- 43. Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue‐specific impairment of MHC class II expression. Immunity 1996; 4:167–78. [DOI] [PubMed] [Google Scholar]
- 44. Clavel S, Siffroi‐Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Derijard B. Regulation of the intracellular localization of Foxo3a by stress‐activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol 2010; 30:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Daitoku H, Sakamaki J, Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein–protein interactions. Biochim Biophys Acta 2011; 1813:1954–60. [DOI] [PubMed] [Google Scholar]
- 46. Muhlethaler‐Mottet A, Otten LA, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J 1997; 16:2851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fallarino F, Bianchi R, Orabona C, Vacca C, Belladonna ML, Fioretti MC et al CTLA‐4‐Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med 2004; 200:1051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang JB, Zhao ZB, Liu QZ, Hu TD, Long J, Yan K et al FoxO1 is a regulator of MHC‐II expression and anti‐tumor effect of tumor‐associated macrophages. Oncogene 2018; 37:1192–204. [DOI] [PubMed] [Google Scholar]
- 49. Wright KL, Ting JP. Epigenetic regulation of MHC‐II and CIITA genes. Trends Immunol 2006; 27:405–12. [DOI] [PubMed] [Google Scholar]
- 50. Dong G, Wang Y, Xiao W, Pacios Pujado S, Xu F, Tian C et al FOXO1 regulates dendritic cell activity through ICAM‐1 and CCR50. J Immunol 2015; 194:3745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dejean AS, Beisner DR, Ch'en IL, Kerdiles YM, Babour A, Arden KC et al Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol 2009; 10:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C et al Activation of the AMPK‐FOXO3 pathway reduces fatty acid‐induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes 2009; 58:2246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peng S, Zhao S, Yan F, Cheng J, Huang L, Chen H et al HDAC2 selectively regulates FOXO3a‐mediated gene transcription during oxidative stress‐induced neuronal cell death. J Neurosci 2015; 35:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ambrosio R, Damiano V, Sibilio A, De Stefano MA, Avvedimento VE, Salvatore D et al Epigenetic control of type 2 and 3 deiodinases in myogenesis: role of lysine‐specific demethylase enzyme and FoxO3. Nucleic Acids Res 2013; 41:3551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riedel CG, Dowen RH, Lourenco GF, Kirienko NV, Heimbucher T, West JA et al DAF‐16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat Cell Biol 2013; 15:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hatta M, Cirillo LA. Chromatin opening and stable perturbation of core histone: DNA contacts by FoxO1. J Biol Chem 2007; 282:35583–93. [DOI] [PubMed] [Google Scholar]
- 57. Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K et al Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell 2008; 32:221–31. [DOI] [PubMed] [Google Scholar]
- 58. van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300‐mediated acetylation. Trends Biochem Sci 2005; 30:81–6. [DOI] [PubMed] [Google Scholar]
- 59. Kang H, Corr M, Mansson R, Welinder E, Hedrick SM, Stone EL. Loss of murine FOXO3 in cells of the myeloid lineage enhances myelopoiesis but protects from K/BxN‐serum transfer‐induced arthritis. PLoS ONE 2015; 10:e0126728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tsuchiya K, Westerterp M, Murphy AJ, Subramanian V, Ferrante AW Jr, Tall AR et al Expanded granulocyte/monocyte compartment in myeloid‐specific triple FoxO knockout increases oxidative stress and accelerates atherosclerosis in mice. Circ Res 2013; 112:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]


