Summary
This review takes the reader through 45 years of islet autoantibody research, from the discovery of islet‐cell antibodies in 1974 to today’s population‐based screening for presymptomatic early‐stage type 1 diabetes. The review emphasizes the current practical value of, and factors to be considered in, the measurement of islet autoantibodies.
Keywords: Autoantibodies, ICA, Islet‐cell antibodies, Type 1 diabetes
This review takes the reader through 45 years of islet autoantibody research, from the discovery of islet‐cell antibodies in 1974 to today’s population‐based screening for presymptomatic early‐stage type 1 diabetes. The review emphasizes the current practical value of, and factors to be considered in, the measurement of islet autoantibodies.

Historical perspectives
Discovery of islet‐cell antibodies
The concept of autoimmunity as a pathogenetic mechanism in a subgroup of patients with diabetes was first raised in the 1960s and early 1970s with the observation of insulitis 1, and the association of juvenile‐onset diabetes with certain human leukocyte antigen (HLA) alleles and T cell abnormalities 2, 3, 4, 5, 6. The definitive autoimmune pathogenetic discovery was made in 1974, when two research groups in the United Kingdom reported the identification of islet‐cell antibodies (ICA) in patients with so‐called ‘multiple organ‐specific autoimmunity’ 7, 8.
The first of these publications was by Gian Franco Bottazzo and Deborah Doniach. This research group had discovered thyroid autoimmunity almost 20 years earlier 9, and several other autoantibodies 10, 11, 12, and had a treasure chest of samples from patients with various and multiple endocrine autoimmune diseases. Using indirect immunofluorescence, Bottazzo et al. detected ICA in these human pancreas samples (Fig. 1a). The manuscript was published in The Lancet in November 1974 7. The abstract stated: ‘Antibodies to pancreatic islet cells were found by immunofluorescence in the sera of 13 patients with multiendocrine deficiencies associated with organ‐specific autoimmunity. 10 of these patients were diabetic… The presence of organ‐specific pancreatic antibodies supports the hypothesis of an autoimmune form of diabetes mellitus put forward to explain the histological ‘insulitis’ found in selected cases of this disease.’
Figure 1.
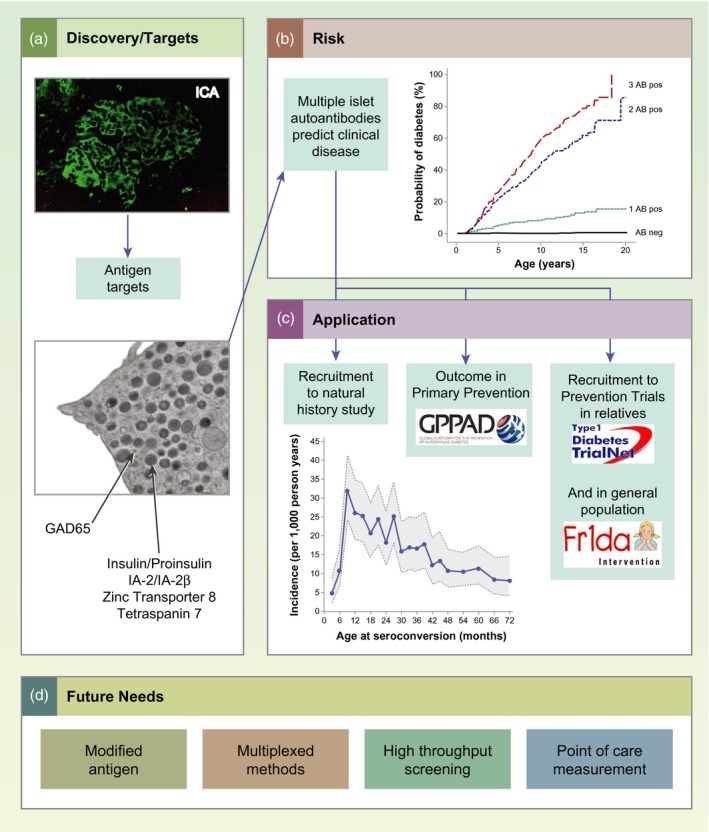
(a) Discovery of islet‐cell antibodies (ICA) and subsequent identification of major β cell autoantigen targets has provided diagnostic markers with which to distinguish autoimmune type 1 diabetes from other non‐autoimmune types. (b) Probability of developing clinical type 1 diabetes increases with increasing numbers of different islet autoantibodies, and children with multiple islet autoantibodies will develop clinical diabetes. (c) Currently, islet autoantibody measurements are used to recruit study participants for natural history studies; as outcome markers in primary prevention trials; and for the recruitment of individuals to prevention trials. (d) Future requirements for islet autoantibody diagnostics are stated.
The second publication was by William J. Irvine’s research group, which had previously reported T cell responses against pancreatic antigens in patients with diabetes 6. His group also examined a collection of samples from polyendocrine patients, and published their work in The Lancet 1 month after the study by Bottazzo et al. 8. Their abstract stated: ‘Using an indirect immunofluorescence technique, circulating antibodies to pancreatic islet cells were found in the sera of 5 patients with insulin‐dependent diabetes mellitus and coexistent autoimmunity… These findings provide further direct evidence to support the hypothesis of an autoimmune form of diabetes mellitus.’
Thus, the discovery and validation of ICA were reported by two independent research groups within the space of a month. These were discovered in polyendocrine patients rather than in typical patients with type 1 diabetes. Moreover, the classification of diabetes into types 1 and 2, which had been introduced decades earlier, had not yet taken root, and terms such as ‘juvenile’, ‘adult‐onset’, ‘insulin‐dependent’ and ‘non‐insulin‐dependent’ were used to distinguish age‐ and therapy‐related forms of the disease. In 1975, Lendrum et al. reported ICA in the sera of 51 of 105 children with recent‐onset diabetes 13, revealing an autoimmune pathogenetic component in a large proportion of childhood cases of diabetes.
Prediabetes
Perhaps the most important discoveries were those that led to the notion of a ‘prediabetic’ stage of the disease. Lendrum et al. examined ICA in the diabetic twin cohort of David Pyke and in 1976, reported that the antibodies could be present years before the onset of diabetes 14. Also in 1976, Irvine et al. reported that the antibodies could precede diabetes onset by several years 15. In 1981, Gorsuch et al. measured ICA in the first‐degree relatives of patients with insulin‐dependent diabetes and discovered that patients who developed insulin‐dependent diabetes had ICA up to 30 months before the onset of diabetes 16. These early findings eventually led to the notion that type 1 diabetes is a chronic autoimmune disease, as described by George Eisenbarth in 1986 17.
Standardization
The number of methods for detecting ICA and reports of ICA has increased rapidly since the pivotal studies described above. These reports discussed complement‐fixing antibodies 18, bovine‐pancreas‐positive ICA 19, the two‐color fluorescence detection method 20, the protein‐A detection method 21 and islet‐cell‐surface antibodies 22, among many others. What started as a clear concept soon became complex and confused. A workshop to standardize ICA measurements was convened, and after the sobering realization of how variable these measurements could be 23, an exemplary standardization program that introduced common standards 24 and international units 25, 26 was established, and was subsequently used for antigen‐specific islet autoantibody measurements 27, 28, 29. Importantly, the program gave credibility to the antibodies as markers of prediabetes 30, 31, 32 and many poorly performing detection methods became obsolete.
Islet‐cell antibodies are heterogeneous and target multiple antigens
The ICA immunofluorescence test had become standard, but the identification of their target antigens became an urgent undertaking (Fig. 1a). MacCuish et al. had demonstrated T cell responses to insulin fragments in patients with and without insulin treatment in 1975 33. In 1983, Palmer et al. showed that children who developed type 1 diabetes had insulin autoantibodies (IAA) before they were treated with insulin 34. This was an important breakthrough in the field. It also signaled the presence of multiple autoantibodies, because insulin is only expressed in pancreatic islet β cells, whereas all islet cells stained for ICA 7, 8.
In 1982, Baekkeskov et al. reported autoantibodies against a 64‐kDa islet protein 35, 36, and in 1990 Christie et al. described autoantibodies against 40‐kDa and 37‐kDa fragments of islet proteins 37. The 64‐kDa target of autoantibodies was later identified as glutamate decarboxylase (GAD65) 38, a known antigenic target of autoantibodies in the neurological disorder stiff‐person syndrome 39. The 40‐kDa and 37‐kDa fragments were identified as ICA512 (now also known as IA‐2) 40 and the related protein phogrin (also known as IA‐2β) 41, respectively, both of which were identified separately as the targets of autoantibodies in type 1 diabetes 42, 43. GAD65‐directed autoantibodies (GADA) were shown to be part of the ICA reaction, with a β cell‐specific staining pattern 44. The IA‐2‐directed autoantibodies (IA‐2A) were shown to be part of the pan‐islet‐cell staining of ICA 45. Other proteins, such as ICA69, were claimed to be targets of ICA 46, but were not confirmed by other groups or in standardization workshops 47. The lipid antigens GM2‐1 and sulfatides were also reported to be targeted by ICA 48, 49, but no methods have been developed for robust assessment of their validity. In contrast, the β cell zinc transporter 8 (ZnT8) protein has been confirmed to be a target of autoantibodies (ZnT8A) in more than 50% of patients with type 1 diabetes 50, 51, and tetraspanin 7 was identified as the 38‐kDa target of autoantibodies against glima 52, 53. These autoantibodies were present in more than 30% of patients with type 1 diabetes 54.
Prediction of clinical disease
The notion that ICA and other islet autoantibodies precede the onset of type 1 diabetes allows the prediction of future disease. As early as 1977, Irvine’s group showed that the presence of ICA identified adult patients treated with oral hypoglycemic agents who would later require insulin treatment 55. Numerous studies, including a prominent study in triplets 56, had identified occasional cases of ICA‐positive individuals who later developed diabetes, but it was not until 1988 that an analysis of the Barts–Windsor Family Study showed that it was indeed possible to estimate the risk in ICA‐positive relatives of patients with type 1 diabetes 57. This was followed by the establishment of risk estimates using the standardized international units for ICA 30. The higher the titre of ICA, the higher the risk that an ICA‐positive relative would develop type 1 diabetes. The risk reached 100% within 10 years in relatives who had ICA titres of > 80 Juvenile Diabetes Foundation (JDF) units/ml. These studies provided the foundation for later prevention trials in ICA‐positive first‐degree relatives 58.
The inclusion of IAA, GADA and IA‐2A further improved our ability to stratify the risk of type 1 diabetes. The first reported use of autoantibody combinations to improve diabetes prediction was in twins in 1992, when a combination of ICA, IAA, GADA and antibodies against the 37‐kDa and 40‐kDa fragments was used 59. This was followed in 1994 by a study in relatives of patients with type 1 diabetes, which found that 8% of relatives with ICA only and 88% of those with ICA plus IAA, GADA or antibodies to the 37‐kDa or 40‐kDa fragments developed diabetes 60. It is noteworthy that not all the antibodies are useful in every situation. For example, the prediction of insulin requirement in adult‐onset diabetes is made by testing for ICA 61, GADA 62 and IA‐2A 63, but IAA are rare in patients in this age group 64.
Antibody combinations were subsequently used to select at‐risk relatives for clinical trials 58, 65, and it is now well established that the diabetes risk associated with the presence of multiple islet autoantibodies (two or more of IAA, GADA, IA‐2A and ZnT8A) is markedly greater than the risk in people with a single autoantibody 66, 67, 68, 69. A landmark study, that involved combined analysis of more than 13 000 individuals from three birth cohorts, demonstrated that almost all children with genetic susceptibility to type 1 diabetes who developed multiple islet autoantibodies progressed to diabetes (Fig. 1b) 70. This has paved the way for population‐based screening 71.
Natural history of islet autoantibodies
Prospective birth‐cohort studies have made invaluable contributions to our knowledge of the appearance and progression of islet autoantibodies in childhood, including the German BABYDIAB Study 72, the Finnish DIPP Project 73, the DAISY from Colorado 74 and the TEDDY Study 75, which have now been running for up to three decades. These studies have shown that in genetically predisposed children, autoantibody seroconversion occurs relatively frequently between the ages of 6 months and 3 years, with the incidence of autoantibodies peaking at an age of 1 year (Fig. 1c) 76, 77, 78. The typical natural history of type 1 diabetes in children is the appearance of the first high‐affinity autoantibody 79, which is usually IAA in the youngest children, followed by the appearance of other islet autoantibodies 80, usually within 3 years 81, and eventually the development of diabetes. Two islet‐autoimmunity endotypes are distinguished 82. One is characterized by the first appearance of IAA in children carrying HLA‐DR4, and occurs in the first years of life. The second is characterized by the first appearance of GADA in children carrying HLA‐DR3, and is the endotype most frequently observed in children who seroconvert after age 2 years. Based on the different associations of the two endotypes and environmental factors, it has been suggested that the endotypes have different etiologies 82. However, age is an important confounder and it is possible that these differences are merely age‐related. In contrast to IAA and GADA, IA‐2A usually occurs together with autoantibodies against other β cell antigens and is therefore a very specific and highly predictive immune marker for progression to clinical type 1 diabetes 83, 84, particularly if its reactivity spreads to epitopes on the homologous protein IA‐2β 84, 85, 86. ZnT8A also usually appears later in the development of the disease 87.
Practical perspectives
Antibody titre, affinity and specificity
There are differences in the target autoantigens and epitopes, the titres, affinities and subclasses of islet autoantibodies. These characteristics are associated with the subject’s age and HLA genotype, and in some cases can help to distinguish diabetes‐associated islet autoantibodies from non‐disease‐associated autoantibody signals 88.
The intensity and maturity of the antibody response are reflected in the antibody titre, affinity, immunoglobulin (Ig)G subclass, and target epitopes on single or multiple islet autoantigens. Islet autoantibodies with high titres usually involve multiple IgG subclasses and are directed against multiple epitopes on the target antigen. Similar to ICA 30, high titres of IAA 64, 84 or IA‐2A 84 are associated with faster progression to clinical type 1 diabetes. Moreover, IAA or IA‐2A responses that include IgG2, IgG3 and/or IgG4 as well as IgG1 are associated with an increased risk, even if the antibody titres are not high. By combining these antibody characteristics, the 5‐year diabetes risk in islet‐autoantibody‐positive relatives can be stratified from less than 10% to more than 90% 84.
The affinity (binding strength) of the autoantibody to the target antigen is closely related to the intensity of the antibody response. Accordingly, high‐affinity islet autoantibodies are associated with progression to clinical type 1 diabetes, even if the antibody titre is relatively low, whereas low‐affinity antibodies are unrelated to the development of diabetes, even if the capacity and titre of the antibody are high 79, 89, 90, 91, 92. Consistent with their high disease specificity, IA‐2A are characterized by high affinity 92. In contrast, both IAA and GADA can range in affinity by more than 1000‐fold 79, 89, 90, 91. The highest affinities are > 1011 l/mol. For IAA and GADA the low‐ and high‐affinity autoantibodies appear to bind to different epitopes 79, 90, 91. For example, high‐affinity IAA require the preservation of amino acids 8–13 in the insulin A chain to bind to human insulin, and also bind proinsulin. In contrast, the majority of low‐affinity IAA are dependent on the COOH‐terminal residues of the insulin B chain and usually do not bind proinsulin 79. Low‐affinity antibodies are seen more frequently in individuals who do not have a strong genetic susceptibility to type 1 diabetes and in children who remain positive for only IAA or GADA 79, 80, 93. The affinities and epitope specificities of IAA and GADA can be used to stratify the progression to type 1 diabetes 79, 90, 94, 95, and those for GADA can predict insulin therapy in individuals with adult‐onset diabetes 96, 97. The spread of IA‐2A reactivity against epitopes on the homologous IA‐2β protein is associated with the rapid development of diabetes 84, 85, 86.
Therefore, it is useful to identify and/or exclude low‐affinity signals in risk screening for clinical trials, particularly in individuals with only IAA or GADA, who may be at an early stage of the disease process and may progress to producing multiple islet autoantibodies 79, 80, 90. The identification of markers associated with the risk of progression from single to multiple islet autoantibodies has been investigated in studies within the TrialNet Consortium 68, 98, 99, 100, 101, 102, 103. Genetic risk may also be used to select single islet autoantibody‐positive children who are most likely to progress to producing multiple islet autoantibodies 68, 102, 103. A low GAD autoantibody titre is associated with a low risk of progression to multiple islet autoantibodies 98.
Why do multiple antibodies or multiple tests work?
Multiple antibodies and multiple tests have the mathematical advantage of increasing the a priori probability of a true result in the samples selected for the second measurement, as may be expected from Bayes’ theorem 104. This can be illustrated theoretically in the example shown in Fig. 2. The example assumes that 0·3% of preschool children are true positives and will develop type 1 diabetes. In a population of 100 000, this corresponds to 300 children. A single islet autoantibody measurement (e.g. IAA) with a sensitivity of 70% will identify 1000 children when its threshold is set to the 99th percentile of the population. These children will have a 21% risk (positive predictive value) of developing type 1 diabetes. A second test with similar characteristics will identify a similar number of children with a similar risk. However, there will be a marked enrichment of future cases of type 1 diabetes in the children who have both autoantibodies: there would be 155 children with both IAA and GADA, 147 of whom would develop diabetes (95% risk; 49% sensitivity); and of 1690 with only IAA or GADA, 126 would develop diabetes (7·5% risk; 42% sensitivity). Adding more antibodies, such as IA‐2A, would identify multiple islet autoantibodies in another 70% (88) of children with a single IAA or GADA who will develop diabetes, thereby increasing the sensitivity (78%) with only a slight reduction in the risk. Adding another antibody (e.g. ZnT8A) will provide a limited improvement in test performance. In reality, IAA and GADA, etc. are not completely independent and there are age relationships, so it is not quite this simple. Nevertheless, for many children who develop the disease, the high risk associated with multiple antibodies has more to do with Bayes’ theorem and perhaps less to do with a multiple‐hit disease process that progresses from single to multiple antibodies. A similar principle applies when a second test is performed (e.g. an electrochemiluminescence assay 105, 106, luciferase immunoprecipitation system assay 107, 108 or IAA affinity assay 109, 110) using samples that were previously identified as positive on a radiobinding assay. A very similar outcome would also be expected if the order of the tests were reversed. The thresholds for the different antibodies can also be adjusted to obtain the best possible combination of sensitivity and risk 111.
Figure 2.
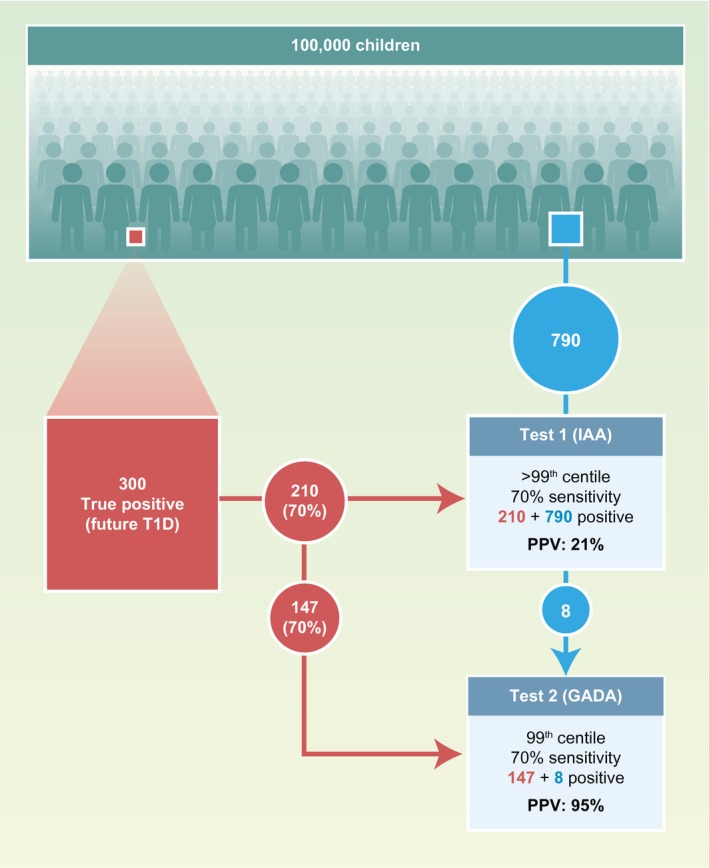
Illustration of how the application of Bayes’ theorem to islet autoantibodies can confer high positive predictive values in the diagnosis of future type 1 diabetes using multiple islet autoantibodies. A population of 100 000 unselected children, including 300 (0·3%) who will develop type 1 diabetes, is tested for insulin autoantibodies (IAA) with a test that has a threshold selected at the upper 99th percentile (1% positive) and 70% sensitivity. Under these assumptions, 210 of the true future cases of type 1 diabetes will be identified (filled red box) and 790 of the 99 700 who will not develop type 1 diabetes will be positive (filled blue box). This provides a positive predictive value [(PPV) or risk] of 21%. A second test with a similar threshold and sensitivity [e.g. for glutamic acid decarboxylase antibodies (GADA)], applied to the same 100 000 children, will yield a similar number of positives and predictive value. However, among the IAA‐positive children, there will be a marked enrichment of true positives who are also GADA‐positive, yielding a PPV of 95%.
Modelling islet autoantibody profiles
Prospective studies have shown that the natural progression to type 1 diabetes is not uniform in children and adolescents. Based on the individuals’ different genetic backgrounds and environments, islet autoimmunity may develop at different ages, show different longitudinal autoantibody profiles and progress to clinical diabetes at various rates. Today, we are unable to predict the individual’s progression exactly or to link etiological factors to the dynamics of islet autoantibody patterns over time. However, recent studies have started to develop mathematical algorithms to model complex longitudinal autoantibody profiles and stratify progression rates 112, 113. Children who develop multiple islet autoantibodies can be clustered according to their longitudinal profiles, and it has been shown that the likelihood of progressing from seroconversion to clinical diabetes within 5 years ranges in these clusters from below 10% to above 80%. Those children who seroconverted in the first years of life and expressed stable IAA and IA‐2A responses had the highest risk of diabetes. Interestingly, this risk was unaffected by the child’s GADA status 113. A cluster analysis also revealed that losing IAA reactivity was associated with delayed progression to type 1 diabetes in children who were positive for multiple islet autoantibodies 112. Mathematical approaches applied to data from prospective cohorts have strong potential utility as novel tools for the stratification of islet‐autoantibody‐positive individuals and offer new opportunities to clarify the disease mechanisms.
Islet autoantibodies used to select for trials and as study outcomes
Clinical trials to investigate the treatment of type 1 diabetes commonly use the participant’s autoantibody positivity as an inclusion criterion (Fig. 1c) 58, 65, 114. In trials that recruit individuals with clinical diabetes, islet autoantibodies are used to distinguish type 1 diabetes from other types. In addition to the recruitment of trial participants, islet autoantibodies have been used as outcome markers in several studies (Fig. 1c). These include natural history studies such as TEDDY 115 and primary prevention studies such as BABYDIET 116, TRIGR 117 and POInT 118. The availability of high‐quality, high‐throughput and harmonized autoantibody tests 119 mean that stable longitudinal measurements of the outcomes are possible. As discussed in above, the definition of outcome can be improved by including confirmation with a second laboratory test or the use of multiple assays.
The age at screening is also important. For first‐degree relatives of patients with type 1 diabetes, the risk of developing islet autoantibodies decreases exponentially with age, with a half‐life of 3–4 years 120. This has practical implications. First, if we consider that the peak incidence of islet autoantibody seroconversion occurs in the first 3 years of life, screening is likely to be most effective in preschool years. Secondly, relatives who remain negative through to their teenage years will have an eightfold lower risk of developing islet autoantibodies than they had when they were born.
Extension to the population at large
The development of multiple islet autoantibodies has long been recognized as a critical step in the pathogenesis and diagnosis of type 1 diabetes 59, 60, culminating in the finding that almost all children who develop multiple islet autoantibodies will develop clinical symptomatic diabetes, regardless of whether they have an a priori family history of the disease 70. This has led to a new staging strategy for type 1 diabetes, in which the presence of multiple islet autoantibodies is now used as a criterion for the diagnosis of presymptomatic early‐stage type 1 diabetes 121. An early diagnosis of type 1 diabetes can prevent the severe metabolic decompensation that is frequently observed at the onset of clinical diabetes 122, 123, 124. Screening for islet autoantibodies can be performed with capillary blood samples or dried blood spots 125, 126, 127, 128. The Fr1da Study started in 2015 as a model project investigating public health screening for early‐stage type 1 diabetes (confirmed with positivity for multiple islet autoantibodies) in Bavaria, Germany 71. It assesses: (1) whether the early diagnosis of type 1 diabetes in the context of regular medical check‐ups in childhood is feasible and efficient; (2) whether ketoacidosis and the hospitalization of children can be prevented by screening; and (3) whether psychological distress can be reduced with the early diagnosis of diabetes, education and care. Similar studies have already commenced in Lower Saxony, Germany, with additional screening for low‐density lipoprotein–hypercholesterolemia (Fr1dolin Study) 129 and in Colorado, with additional screening for celiac disease (ASK Study) 130.
The future
We envisage two areas of activity in the next few years (Fig. 1d). From a practical perspective, a technology is required that facilitates the widespread use of islet autoantibody testing for the diagnosis of presymptomatic type 1 diabetes in the public health context 131. From the research perspective, activities to identify modified protein targets, both to generate better assays and to identify pathogenetic disease mechanisms, are highly likely.
Technological advances should drive down costs and allow simple high‐throughput screening, which will favor its widespread application. Cost is a clear factor, because population‐based screening requires that tens or hundreds of thousands of children be tested, more than 99% of whom will be negative. This will require a sensitive first‐line test that covers the majority of the major islet autoantibodies (IAA, GADA, IA‐2A, ZnT8A), with follow‐up tests for those who are positive to confirm and stratify their risk 71, 127, 132, 133. Point‐of‐care testing may be one approach to achieving this. This technology should be coupled to careful application of Bayes’ modeling, including additional risk factors such as genetics 134, family history and age. This sort of modeling will be possible once much larger numbers of children have been tested and followed, emphasizing the need to introduce broad testing programs in many regions and countries.
Modified islet antigens have been reported in the literature 135, 136, but we conclude that a number of these are unlikely to be validated because of weaknesses in the assays. Increased antibody binding to a modified form of the tetraspanin 7 protein was observed in some patients 137, but it is difficult to determine whether this is a favored in‐vivo target or an artificial in‐vitro modification. Smart systems that reliably identify antibodies that bind to proteins from unperturbed and perturbed islets should be possible, and will probably reveal a range of variations in the autoantibody–autoantigen targets that we know today.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Historical and new insights into pathogenesis of type 1 diabetes. Clinical and Experimental Immunology 2019, 198: 292–293.
HIPs and HIP‐reactive T cells. Clinical and Experimental Immunology 2019, 198: 306–313.
Immune cell trafficking to the islets during type 1 diabetes. Clinical and Experimental Immunology 2019, 198: 314–325.
Islet‐immune interactions in type 1 diabetes: the nexus of beta cell destruction. Clinical and Experimental Immunology 2019, 198: 326–340.
Contributor Information
E. Bonifacio, Email: ezio.bonifacio@tu-dresden.de.
P. Achenbach, Email: peter.achenbach@helmholtz-muenchen.de.
References
- 1. Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965; 14:619–33. [DOI] [PubMed] [Google Scholar]
- 2. Cudworth AG, Woodrow JC. HL‐A antigens and diabetes mellitus [Letter]. Lancet 1974; 2:1153. [DOI] [PubMed] [Google Scholar]
- 3. Strosberg JM, Harris ED Jr. Letter: HL‐A genotypes and diabetes. Lancet 1974; 2:1212. [DOI] [PubMed] [Google Scholar]
- 4. Menser MA, Forrest JM, Honeyman MC, Burgess JA. Diabetes, HL‐A antigens, and congenital rubella [Letter]. Lancet 1974; 2:1508–9. [DOI] [PubMed] [Google Scholar]
- 5. Cudworth AG, Woodrow JC. Evidence for HL‐A‐linked genes in ‘juvenile’ diabetes mellitus. BMJ 1975; 3:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacCuish AC, Jordan J, Campbell CJ, Duncan LJ, Irvine WJ. Cell‐mediated immunity to human pancreas in diabetes mellitus. Diabetes 1974; 23:693–7. [DOI] [PubMed] [Google Scholar]
- 7. Bottazzo GF, Florin‐Christensen A, Doniach D. Islet‐cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974; 2:1279–83. [DOI] [PubMed] [Google Scholar]
- 8. MacCuish AC, Irvine WJ, Barnes EW, Duncan LJ. Antibodies to pancreatic islet cells in insulin‐dependent diabetics with coexistent autoimmune disease. Lancet 1974; 2:1529–31. [DOI] [PubMed] [Google Scholar]
- 9. Pulvertaft RJ, Doniach D, Roitt IM, Hudson RV. Cytotoxic effects of Hashimoto serum on human thyroid cells in tissue culture. Lancet 1959; 2:214–6. [DOI] [PubMed] [Google Scholar]
- 10. Forbes IJ, Roitt IM, Doniach D, Solomon IL. The thyroid cytotoxic autoantibody. J Clin Invest 1962; 41:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor KB, Roitt IM, Doniach D, Couchman KG, Shapland C. Autoimmune phenomena in pernicious anaemia: gastric antibodies. BMJ 1962; 2:1347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doniach D, Roitt IM, Walker JG, Sherlock S. Tissue antibodies in primary biliary cirrhosis, active chronic (lupoid) hepatitis, cryptogenic cirrhosis and other liver diseases and their clinical implications. Clin Exp Immunol 1966; 1:237–62. [PMC free article] [PubMed] [Google Scholar]
- 13. Lendrum R, Walker G, Gamble DR. Islet‐cell antibodies in juvenile diabetes mellitus of recent onset. Lancet 1975; 1:880–2. [DOI] [PubMed] [Google Scholar]
- 14. Lendrum R, Nelson PG, Pyke DA, Walker G, Gamble DR. Islet‐cell, thyroid, and gastric autoantibodies in diabetic identical twins. BMJ 1976; 1:553–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irvine WJ, Gray RS, McCallum CJ. Pancreatic islet‐cell antibody as a marker for asymptomatic and latent diabetes and prediabetes. Lancet 1976; 2:1097–102. [DOI] [PubMed] [Google Scholar]
- 16. Gorsuch AN, Spencer KM, Lister J et al Evidence for a long prediabetic period in type I (insulin‐dependent) diabetes mellitus. Lancet 1981; 2:1363–5. [DOI] [PubMed] [Google Scholar]
- 17. Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986; 314:1360–8. [DOI] [PubMed] [Google Scholar]
- 18. Bottazzo GF, Dean BM, Gorsuch AN, Cudworth AG, Doniach D. Complement‐fixing islet‐cell antibodies in type‐I diabetes: possible monitors of active beta‐cell damage. Lancet 1980; 1:668–72. [PubMed] [Google Scholar]
- 19. Kawahara DJ, Buckingham B, Kershnar A. Heterogeneity in the specificity of the islet cell cytoplasmic antibody response in insulin‐dependent diabetes mellitus. Pancreas 1990; 5:647–51. [DOI] [PubMed] [Google Scholar]
- 20. Madsen OD, Olsson ML, Bille G et al A two‐colour immunofluorescence test with a monoclonal human proinsulin antibody improves the assay for islet cell antibodies. Diabetologia 1986; 29:115–8. [DOI] [PubMed] [Google Scholar]
- 21. Srikanta S, Rabizadeh A, Omar MA. Eisenbarth GS. Assay for islet cell antibodies. Protein A–monoclonal antibody method. Diabetes 1985; 34:300–5. [DOI] [PubMed] [Google Scholar]
- 22. Lernmark A, Freedman ZR, Hofmann C et al Islet‐cell‐surface antibodies in juvenile diabetes mellitus. N Engl J Med 1978; 299:375–80. [DOI] [PubMed] [Google Scholar]
- 23. Gleichmann H, Bottazzo GF. Progress toward standardization of cytoplasmic islet cell‐antibody assay. Diabetes 1987; 36:578–84. [DOI] [PubMed] [Google Scholar]
- 24. Bonifacio E, Lernmark A, Dawkins RL. Serum exchange and use of dilutions have improved precision of measurement of islet cell antibodies. J Immunol Methods 1988; 106:83–8. [DOI] [PubMed] [Google Scholar]
- 25. Bonifacio E, Boitard C, Gleichmann H, Shattock MA, Molenaar JL, Bottazzo GF. Assessment of precision, concordance, specificity, and sensitivity of islet cell antibody measurement in 41 assays. Diabetologia 1990; 33:731–6. [DOI] [PubMed] [Google Scholar]
- 26. Greenbaum CJ, Palmer JP, Nagataki S et al Improved specificity of ICA assays in the Fourth International Immunology of Diabetes Serum Exchange Workshop. Diabetes 1992; 41:1570–4. [DOI] [PubMed] [Google Scholar]
- 27. Wilkin TJ, Schoenfeld SL, Diaz JL, Kruse V, Bonifacio E, Palmer JP. Systematic variation and differences in insulin‐autoantibody measurements. Diabetes 1989; 38:172–81. [DOI] [PubMed] [Google Scholar]
- 28. Schmidli RS, Colman PG, Bonifacio E, Bottazzo GF, Harrison LC. High level of concordance between assays for glutamic acid decarboxylase antibodies. The First International Glutamic Acid Decarboxylase Antibody Workshop. Diabetes 1994; 43:1005–9. [DOI] [PubMed] [Google Scholar]
- 29. Verge CF, Stenger D, Bonifacio E et al Combined use of autoantibodies (IA‐2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes 1998; 47:1857–66. [DOI] [PubMed] [Google Scholar]
- 30. Bonifacio E, Bingley PJ, Shattock M et al Quantification of islet‐cell antibodies and prediction of insulin‐dependent diabetes. Lancet 1990; 335:147–9. [DOI] [PubMed] [Google Scholar]
- 31. McCulloch DK, Klaff LJ, Kahn SE et al Nonprogression of subclinical beta‐cell dysfunction among first‐degree relatives of IDDM patients. 5‐yr follow‐up of the Seattle Family Study. Diabetes 1990; 39:549–56. [DOI] [PubMed] [Google Scholar]
- 32. Bosi E, Becker F, Bonifacio E et al Progression to type I diabetes in autoimmune endocrine patients with islet cell antibodies. Diabetes 1991; 40:977–84. [DOI] [PubMed] [Google Scholar]
- 33. MacCuish AC, Jordan J, Campbell CJ, Duncan LJ, Irvine WJ. Cell‐mediated immunity in diabetes mellitus; lymphocyte transformation by insulin and insulin fragments in insulin‐treated and newly‐diagnosed diabetes. Diabetes 1975; 24:36–43. [DOI] [PubMed] [Google Scholar]
- 34. Palmer JP, Asplin CM, Clemons P et al Insulin antibodies in insulin‐dependent diabetics before insulin treatment. Science 1983; 222:1337–9. [DOI] [PubMed] [Google Scholar]
- 35. Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature 1982; 298:167–9. [DOI] [PubMed] [Google Scholar]
- 36. Baekkeskov S, Lernmark A. Rodent islet cell antigens recognized by antibodies in sera from diabetic patients. Acta Biol Med Germ 1982; 41:1111–5. [PubMed] [Google Scholar]
- 37. Christie MR, Vohra G, Champagne P, Daneman D, Delovitch TL. Distinct antibody specificities to a 64‐kD islet cell antigen in type 1 diabetes as revealed by trypsin treatment. J Exp Med 1990; 172:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baekkeskov S, Aanstoot HJ, Christgau S et al Identification of the 64K autoantigen in insulin‐dependent diabetes as the GABA‐synthesizing enzyme glutamic acid decarboxylase. Nature 1990; 347:151–6. [DOI] [PubMed] [Google Scholar]
- 39. Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA‐ergic neurons and pancreatic beta cells in stiff‐man syndrome. N Engl J Med 1990; 322:1555–60. [DOI] [PubMed] [Google Scholar]
- 40. Payton MA, Hawkes CJ, Christie MR. Relationship of the 37,000‐ and 40,000‐M(r) tryptic fragments of islet antigens in insulin‐dependent diabetes to the protein tyrosine phosphatase‐like molecule IA‐2 (ICA512). J Clin Invest 1995; 96:1506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hawkes CJ, Wasmeier C, Christie MR, Hutton JC. Identification of the 37‐kDa antigen in IDDM as a tyrosine phosphatase‐like protein (phogrin) related to IA‐2. Diabetes 1996; 45:1187–92. [DOI] [PubMed] [Google Scholar]
- 42. Rabin DU, Pleasic SM, Shapiro JA et al Islet cell antigen 512 is a diabetes‐specific islet autoantigen related to protein tyrosine phosphatases. J Immunol 1994; 152:3183–8. [PubMed] [Google Scholar]
- 43. Lu J, Li Q, Xie H et al Identification of a second transmembrane protein tyrosine phosphatase, IA‐2beta, as an autoantigen in insulin‐dependent diabetes mellitus: precursor of the 37‐kDa tryptic fragment. Proc Natl Acad Sci USA 1996; 93:2307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Genovese S, Bonifacio E, McNally JM et al Distinct cytoplasmic islet cell antibodies with different risks for type 1 (insulin‐dependent) diabetes mellitus. Diabetologia 1992; 35:385–8. [DOI] [PubMed] [Google Scholar]
- 45. Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E. Identification of protein tyrosine phosphatase‐like IA2 (islet cell antigen 512) as the insulin‐dependent diabetes‐related 37/40K autoantigen and a target of islet‐cell antibodies. J Immunol 1995; 155:5419–26. [PubMed] [Google Scholar]
- 46. Pietropaolo M, Castano L, Babu S et al Islet cell autoantigen 69 kD (ICA69). Molecular cloning and characterization of a novel diabetes‐associated autoantigen. J Clin Invest 1993; 92:359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lampasona V, Ferrari M, Bosi E, Pastore MR, Bingley PJ, Bonifacio E. Sera from patients with IDDM and healthy individuals have antibodies to ICA69 on western blots but do not immunoprecipitate liquid phase antigen. J Autoimmun 1994; 7:665–74. [DOI] [PubMed] [Google Scholar]
- 48. Dotta F, Gianani R, Previti M et al Autoimmunity to the GM2‐1 islet ganglioside before and at the onset of type I diabetes. Diabetes 1996; 45:1193–6. [DOI] [PubMed] [Google Scholar]
- 49. Buschard K, Josefsen K, Horn T, Fredman P. Sulphatide and sulphatide antibodies in insulin‐dependent diabetes mellitus. Lancet 1993; 342:840. [DOI] [PubMed] [Google Scholar]
- 50. Wenzlau JM, Juhl K, Yu L et al The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007; 104:17040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wenzlau JM, Liu Y, Yu L et al A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 2008; 57:2693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McLaughlin KA, Richardson CC, Ravishankar A et al Identification of tetraspanin‐7 as a target of autoantibodies in type 1 diabetes. Diabetes 2016; 65:1690–8. [DOI] [PubMed] [Google Scholar]
- 53. Aanstoot HJ, Kang SM, Kim J et al Identification and characterization of glima 38, a glycosylated islet cell membrane antigen, which together with GAD65 and IA2 marks the early phases of autoimmune response in type 1 diabetes. J Clin Invest 1996; 97:2772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walther D, Eugster A, Jergens S et al Tetraspanin 7 autoantibodies in type 1 diabetes. Diabetologia 2016; 59:1973–6. [DOI] [PubMed] [Google Scholar]
- 55. Irvine WJ, McCallum CJ, Gray RS, Duncan LJ. Clinical and pathogenic significance of pancreatic‐islet‐cell antibodies in diabetics treated with oral hypoglycaemic agents. Lancet 1977; 1:1025–7. [DOI] [PubMed] [Google Scholar]
- 56. Srikanta S, Ganda OP, Eisenbarth GS, Soeldner JS. Islet‐cell antibodies and beta‐cell function in monozygotic triplets and twins initially discordant for Type I diabetes mellitus. N Engl J Med 1983; 308:322–5. [DOI] [PubMed] [Google Scholar]
- 57. Tarn AC, Thomas JM, Dean BM et al Predicting insulin‐dependent diabetes. Lancet 1988; 1:845–50. [DOI] [PubMed] [Google Scholar]
- 58. Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial G. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004; 363:925–31. [DOI] [PubMed] [Google Scholar]
- 59. Christie MR, Tun RY, Lo SS et al Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes 1992; 41:782–7. [DOI] [PubMed] [Google Scholar]
- 60. Bingley PJ, Christie MR, Bonifacio E et al Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody‐positive relatives. Diabetes 1994; 43:1304–10. [DOI] [PubMed] [Google Scholar]
- 61. Landin‐Olsson M, Nilsson KO, Lernmark A, Sundkvist G. Islet cell antibodies and fasting C‐peptide predict insulin requirement at diagnosis of diabetes mellitus. Diabetologia 1990; 33:561–8. [DOI] [PubMed] [Google Scholar]
- 62. Turner R, Stratton I, Horton V et al UKPDS 25: autoantibodies to islet‐cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 1997; 350:1288–93. [DOI] [PubMed] [Google Scholar]
- 63. Bottazzo GF, Bosi E, Cull CA et al IA‐2 antibody prevalence and risk assessment of early insulin requirement in subjects presenting with type 2 diabetes (UKPDS 71). Diabetologia 2005; 48:703–8. [DOI] [PubMed] [Google Scholar]
- 64. Vardi P, Ziegler AG, Mathews JH et al Concentration of insulin autoantibodies at onset of type I diabetes. Inverse log‐linear correlation with age. Diabetes Care 1988; 11:736–9. [DOI] [PubMed] [Google Scholar]
- 65. Diabetes Prevention Trial‐Type 1 Diabetes Study G . Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002; 346:1685–91. [DOI] [PubMed] [Google Scholar]
- 66. Orban T, Sosenko JM, Cuthbertson D et al Diabetes Prevention Trial‐Type 1 Study G. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial – Type 1. Diabetes care 2009; 32:2269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu L, Boulware DC, Beam CA et al Diabetes TrialNet Study G. Zinc transporter‐8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care 2012; 35:1213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bingley PJ, Boulware DC, Krischer JP, Type 1 Diabetes TrialNet Study G. The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: development of other autoantibodies and progression to type 1 diabetes. Diabetologia 2016; 59:542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gorus FK, Balti EV, Messaaoui A et al Twenty‐year progression rate to clinical onset according to autoantibody profile, age, and HLA‐DQ genotype in a registry‐based group of children and adults with a first‐degree relative with type 1 Diabetes. Diabetes Care 2017; 40:1065–72. [DOI] [PubMed] [Google Scholar]
- 70. Ziegler AG, Rewers M, Simell O et al Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013; 309:2473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Raab J, Haupt F, Scholz M et al Fr1da Study G. Capillary blood islet autoantibody screening for identifying pre‐type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ open 2016; 6:e011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ziegler AG, Hillebrand B, Rabl W et al On the appearance of islet associated autoimmunity in offspring of diabetic mothers: a prospective study from birth. Diabetologia 1993; 36:402–8. [DOI] [PubMed] [Google Scholar]
- 73. Kupila A, Muona P, Simell T et al, Juvenile Diabetes Research Foundation Centre for the Prevention of Type IDiF . Feasibility of genetic and immunological prediction of type I diabetes in a population‐based birth cohort. Diabetologia 2001; 44:290–7. [DOI] [PubMed] [Google Scholar]
- 74. Rewers M, Bugawan TL, Norris JM et al Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 1996; 39:807–12. [DOI] [PubMed] [Google Scholar]
- 75. Hagopian WA, Erlich H, Lernmark A et al, Group TS . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011; 12:733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ziegler AG, Bonifacio E, Group B‐BS . Age‐related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia 2012; 55:1937–43. [DOI] [PubMed] [Google Scholar]
- 77. Parikka V, Nanto‐Salonen K, Saarinen M et al Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 2012; 55:1926–36. [DOI] [PubMed] [Google Scholar]
- 78. Krischer JP, Lynch KF, Schatz DA et al, Group TS . The 6 year incidence of diabetes‐associated autoantibodies in genetically at‐risk children: the TEDDY study. Diabetologia 2015; 58:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high‐affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004; 114:589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Giannopoulou EZ, Winkler C, Chmiel R et al Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia 2015; 58:2317–23. [DOI] [PubMed] [Google Scholar]
- 81. Chmiel R, Giannopoulou EZ, Winkler C, Achenbach P, Ziegler AG, Bonifacio E. Progression from single to multiple islet autoantibodies often occurs soon after seroconversion: implications for early screening. Diabetologia 2015; 58:411–3. [DOI] [PubMed] [Google Scholar]
- 82. Krischer JP, Lynch KF, Lernmark A et al, Group TS . Genetic and environmental interactions modify the risk of diabetes‐related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 2017; 40:1194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Decochez K, De Leeuw IH, Keymeulen B et al IA‐2 autoantibodies predict impending type I diabetes in siblings of patients. Diabetologia 2002; 45:1658–66. [DOI] [PubMed] [Google Scholar]
- 84. Achenbach P, Warncke K, Reiter J et al Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 2004; 53:384–92. [DOI] [PubMed] [Google Scholar]
- 85. Achenbach P, Bonifacio E, Williams AJ, Ziegler AG, Gale EA, Bingley PJ, Group E . Autoantibodies to IA‐2beta improve diabetes risk assessment in high‐risk relatives. Diabetologia 2008; 51:488–92. [DOI] [PubMed] [Google Scholar]
- 86. De Grijse J, Asanghanwa M, Nouthe B et al Predictive power of screening for antibodies against insulinoma‐associated protein 2 beta (IA‐2beta) and zinc transporter‐8 to select first‐degree relatives of type 1 diabetic patients with risk of rapid progression to clinical onset of the disease: implications for prevention trials. Diabetologia 2010; 53:517–24. [DOI] [PubMed] [Google Scholar]
- 87. Achenbach P, Lampasona V, Landherr U et al Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009; 52:1881–8. [DOI] [PubMed] [Google Scholar]
- 88. Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity 2010; 32:468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schlosser M, Koczwara K, Kenk H et al In insulin‐autoantibody‐positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia 2005; 48:1830–2. [DOI] [PubMed] [Google Scholar]
- 90. Mayr A, Schlosser M, Grober N et al GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 2007; 56:1527–33. [DOI] [PubMed] [Google Scholar]
- 91. Bender C, Schlosser M, Christen U, Ziegler AG, Achenbach P. GAD autoantibody affinity in schoolchildren from the general population. Diabetologia 2014; 57:1911–8. [DOI] [PubMed] [Google Scholar]
- 92. Krause S, Chmiel R, Bonifacio E et al IA‐2 autoantibody affinity in children at risk for type 1 diabetes. Clin Immunol 2012; 145:224–9. [DOI] [PubMed] [Google Scholar]
- 93. Adler K, Mueller DB, Achenbach P et al Insulin autoantibodies with high affinity to the bovine milk protein alpha casein. Clin Exp Immunol 2011; 164:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Williams AJ, Lampasona V, Wyatt R et al Reactivity to N‐terminally truncated GAD65(96–585) identifies GAD autoantibodies that are more closely associated with diabetes progression in relatives of patients with type 1 diabetes. Diabetes 2015; 64:3247–52. [DOI] [PubMed] [Google Scholar]
- 95. Wyatt RC, Brigatti C, Liberati D et al The first 142 amino acids of glutamate decarboxylase do not contribute to epitopes recognized by autoantibodies associated with Type 1 diabetes. Diabetes Med 2018; 35:954–63. [DOI] [PubMed] [Google Scholar]
- 96. Krause S, Landherr U, Agardh CD et al GAD autoantibody affinity in adult patients with latent autoimmune diabetes, the study participants of a GAD65 vaccination trial. Diabetes Care 2014; 37:1675–80. [DOI] [PubMed] [Google Scholar]
- 97. Achenbach P, Hawa MI, Krause S et al Action Lc. Autoantibodies to N‐terminally truncated GAD improve clinical phenotyping of individuals with adult‐onset diabetes: Action LADA 12. Diabetologia 2018; 61:1644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xu P, Krischer JP, Type 1 Diabetes TrialNet Study G. Prognostic classification factors associated with development of multiple autoantibodies, dysglycemia, and type 1 diabetes – a recursive partitioning analysis. Diabetes Care 2016; 39:1036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Meah FA, DiMeglio LA, Greenbaum CJ et al, Type 1 Diabetes TrialNet Study G . The relationship between BMI and insulin resistance and progression from single to multiple autoantibody positivity and type 1 diabetes among TrialNet Pathway to Prevention participants. Diabetologia 2016; 59:1186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Steck AK, Fouts A, Miao D et al, TrialNet Study G . ECL‐IAA and ECL‐GADA can identify high‐risk single autoantibody‐positive relatives in the TrialNet Pathway to Prevention Study. Diabetes Technol Ther 2016; 18:410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bosi E, Boulware DC, Becker DJ et al, Diabetes TrialNet Study G . Impact of age and antibody type on progression from single to multiple autoantibodies in type 1 diabetes relatives. J Clin Endocrinol Metab 2017; 102:2881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Redondo MJ, Geyer S, Steck AK et al, Diabetes TrialNet Study G . A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care 2018; 41:1887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Redondo MJ, Steck AK, Sosenko J et al, Diabetes TrialNet Study G . Transcription factor 7‐like 2 (TCF7L2) gene polymorphism and progression from single to multiple autoantibody positivity in individuals at risk for type 1 diabetes. Diabetes Care 2018; 41:2480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bayes T. An essay towards solving a problem in the doctrine of chances 1763. MD Computing 1991; 8:157–71. [PubMed] [Google Scholar]
- 105. Miao D, Steck AK, Zhang L et al, Diabetes TrialNet Study G . Electrochemiluminescence assays for insulin and glutamic acid decarboxylase autoantibodies improve prediction of type 1 diabetes risk. Diabetes Technol Ther 2015; 17:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fouts A, Pyle L, Yu L et al, Diabetes TrialNet Study G . Do electrochemiluminescence assays improve prediction of time to type 1 diabetes in autoantibody‐positive TrialNet subjects? Diabetes Care 2016; 39:1738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Burbelo PD, Hirai H, Issa AT et al Comparison of radioimmunoprecipitation with luciferase immunoprecipitation for autoantibodies to GAD65 and IA‐2beta. Diabetes Care 2010; 33:754–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Liberati D, Wyatt RC, Brigatti C et al A novel LIPS assay for insulin autoantibodies. Acta Diabetol 2018; 55:263–70. [DOI] [PubMed] [Google Scholar]
- 109. Achenbach P, Guo LH, Gick C et al A simplified method to assess affinity of insulin autoantibodies. Clin Immunol 2010; 137:415–21. [DOI] [PubMed] [Google Scholar]
- 110. Curnock RM, Reed CR, Rokni S, Broadhurst JW, Bingley PJ, Williams AJ. Insulin autoantibody affinity measurement using a single concentration of unlabelled insulin competitor discriminates risk in relatives of patients with type 1 diabetes. Clin Exp Immunol 2012; 167:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care 2015; 38:989–96. [DOI] [PubMed] [Google Scholar]
- 112. Endesfelder D, Hagen M, Winkler C et al A novel approach for the analysis of longitudinal profiles reveals delayed progression to type 1 diabetes in a subgroup of multiple‐islet‐autoantibody‐positive children. Diabetologia 2016; 59:2172–80. [DOI] [PubMed] [Google Scholar]
- 113. Endesfelder D, Zu Castell W, Bonifacio E et al, Group TS . Time‐resolved autoantibody profiling facilitates stratification of preclinical type 1 diabetes in children. Diabetes 2019; 68:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Greenbaum C, VanBuecken D, Lord S. Disease‐modifying therapies in type 1 diabetes: a look into the future of diabetes practice. Drugs 2019; 79:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Krischer JP, Liu X, Vehik K et al., Group TS . Predicting islet cell autoimmunity and type 1 diabetes: an 8‐year TEDDY study progress report. Diabetes Care 2019; 42:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care 2011; 34:1301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Writing Group for the TSG , Knip M, Akerblom HK, Al Taji E et al Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes: the TRIGR randomized clinical trial . JAMA 2018; 319:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ziegler AG, Achenbach P, Berner R et al, the GSg . Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD‐POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol. BMJ open 2019; 9:e028578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bonifacio E, Yu L, Williams AK et al Harmonization of glutamic acid decarboxylase and islet antigen‐2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010; 95:3360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hoffmann VS, Weiss A, Winkler C et al Landmark models to define the age‐adjusted risk of developing stage 1 type 1 diabetes across childhood and adolescence. BMC Med 2019; 17:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Insel RA, Dunne JL, Atkinson MA et al Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015; 38:1964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Elding Larsson H, Vehik K, Bell R et al, Group TS, Group SS, Swediabkids Study G, Group DPVS, Finnish Diabetes Registry Study G . Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow‐up. Diabetes Care 2011; 34:2347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes 2012; 13:308–13. [DOI] [PubMed] [Google Scholar]
- 124. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long‐term glycemic control. Diabetes Care 2017; 40:1249–55. [DOI] [PubMed] [Google Scholar]
- 125. Bazzigaluppi E, Bonfanti R, Bingley PJ, Bosi E, Bonifacio E. Capillary whole blood measurement of islet autoantibodies. Diabetes Care 1999; 22:275–9. [DOI] [PubMed] [Google Scholar]
- 126. Bingley PJ, Rafkin LE, Matheson D et al, TrialNet Study G . Use of dried capillary blood sampling for islet autoantibody screening in relatives: a feasibility study. Diabetes Technol Ther 2015; 17:867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ziegler AG, Haupt F, Scholz M et al 3 Screen ELISA for high‐throughput detection of beta cell autoantibodies in capillary blood. Diabetes Technol Ther 2016; 18:687–93. [DOI] [PubMed] [Google Scholar]
- 128. Liu Y, Rafkin LE, Matheson D et al, Diabetes TrialNet Study G . Use of self‐collected capillary blood samples for islet autoantibody screening in relatives: a feasibility and acceptability study. Diabetic Med 2017; 34:934–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kordonouri O, Lange K, Boettcher I et al New approach for detection of LDL‐hypercholesterolemia in the pediatric population: the Fr1dolin‐Trial in Lower Saxony, Germany. Atherosclerosis 2019; 280:85–91. [DOI] [PubMed] [Google Scholar]
- 130. Rasmussen CRG, Rewers M, Baxter J et al Population screening for T1D and celiac disease – Autoimmunity Screening for Kids (ASK). Diabetes 2018; 67 (Suppl. 1):A48–9. [Google Scholar]
- 131. Ziegler AG, Hoffmann GF, Hasford J et al Screening for asymptomatic beta‐cell autoimmunity in young children. Lancet Child Adolesc Health 2019; 3:288–90. [DOI] [PubMed] [Google Scholar]
- 132. Amoroso M, Achenbach P, Powell M et al 3 Screen islet cell autoantibody ELISA: a sensitive and specific ELISA for the combined measurement of autoantibodies to GAD65, to IA‐2 and to ZnT8. Clin Chim Acta 2016; 462:60–4. [DOI] [PubMed] [Google Scholar]
- 133. Zhao Z, Miao D, Michels A et al A multiplex assay combining insulin, GAD, IA‐2 and transglutaminase autoantibodies to facilitate screening for pre‐type 1 diabetes and celiac disease. J Immunol Methods 2016; 430:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bonifacio E, Beyerlein A, Hippich M et al, Group TS . Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLOS Med 2018; 15:e1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Strollo R, Vinci C, Arshad MH et al Antibodies to post‐translationally modified insulin in type 1 diabetes. Diabetologia 2015; 58:2851–60. [DOI] [PubMed] [Google Scholar]
- 136. Buitinga M, Callebaut A,Marques Camara Sodre F et al. Inflammation‐induced citrullinated glucose‐regulated protein 78 elicits immune responses in human type 1 diabetes. Diabetes 2018; 67:2337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Eugster A, Kraus G, Lidzba V et al Cytoplasmic ends of tetraspanin 7 harbour epitopes recognised by autoantibodies in type 1 diabetes. Diabetologia 2019; 62:805–10. [DOI] [PubMed] [Google Scholar]


