Abstract
Background and Objectives
Family caregiving stress has been widely reported to have negative effects on circulating biomarkers of immune system function and inflammation. Our goals were to systematically review this literature and conduct a meta-analysis on the extracted effects.
Research Design and Methods
A systematic search of published studies comparing caregivers and noncaregivers on biomarkers measured from blood samples was conducted in the PubMed, Embase, and Cochrane databases. This search identified 2,582 articles and abstracts. After removing duplicative papers and studies not meeting inclusion criteria, 30 articles were identified that reported analyses on 86 relevant biomarkers from 1,848 caregivers and 3,640 noncaregivers.
Results
Random-effects models revealed an overall effect size across all biomarkers of 0.164 SD units (d). A slightly larger overall effect (d = 0.188) was found for dementia caregivers only. Immune system comparisons yielded somewhat larger differences than inflammation comparisons. Most studies used small convenience samples, and effect sizes were larger for studies with moderate or high bias ratings than for studies with low bias ratings. No significant associations were found in studies that used population-based samples.
Discussion and Implications
Caregivers had small but significantly reduced immune system functioning and greater inflammation than noncaregivers, but associations were generally weak and of questionable clinical significance. The absence of clear associations from low bias studies and population-based studies underscores concerns with possible selection biases in many of the convenience samples. Population-based studies that assess biomarkers before and after the onset of caregiving might add much clarity to this literature.
Keywords: Biomarkers, Immune system, Systematic review, Stress
Beginning with the ground-breaking publication by Kiecolt-Glaser and colleagues (1987) reporting that caregivers of persons with Alzheimer’s disease had decreased levels of several immune system biomarkers compared with noncaregiving controls, many subsequent studies have examined whether family caregivers experience altered biomarker indicators of compromised immune system functioning, increased inflammation, or other negative impacts on biological and physiological functioning. Most of these studies are inspired by a conceptual narrative of caregiving as being a long-term, stressful situation that can have negative impacts on the caregivers’ health. Many investigators interested in the biological consequences of stress have viewed caregiving as a “naturalistic stressor” that allows scientific inquiry into how the human body responds to chronic stress over many years in ways that would not be possible using controlled experimentation (Allen et al., 2017). Caregiving is often conceptualized as being particularly stressful and burdensome for persons caring for a family member with dementia or serious cognitive impairment, which is consistent with evidence that dementia caregivers report higher strain and more depressive symptoms than other groups of caregivers (Ory et al., 1999; Pinquart & Sörensen, 2003).
The chronic stress narrative of caregiving is further supported by many studies suggesting that caregiving is linked to adverse mental and physical health consequences. Family caregiving has been repeatedly linked to elevations in depressive symptoms and other indicators of psychological distress (Pinquart & Sörensen, 2003; Roth, Brown, Rhodes, & Haley, 2018; Roth, Perkins, Wadley, Temple, & Haley, 2009). The reported health effects of the chronic stress of caregiving have been extended to a variety of other potentially significant clinical outcomes including slower wound healing (Kiecolt-Glaser, Marucha, Malarkey, Mercado, & Glaser, 1995), decreased telomere length and possibly shortened life expectancy (Epel et al., 2004), and an increased risk for mortality among strained caregivers (Schulz & Beach, 1999). The idea that caregivers, particularly dementia caregivers, have clinically significant physiological changes that could increase risk for disease and even mortality has been widely cited and accepted both in scholarly reviews (Fonareva & Oken, 2014; Lovell & Wetherell, 2011) and in other influential sources including the annual report of the Alzheimer’s Association (2018) and the web pages for organizations such as the Caregiver Action Network (2018) and the U.S. Office of Women’s Health (2018). Ongoing research is often focused on filling in gaps of this narrative, such as which biomarker/physiological pathways are most impacted by caregiving stress, and what interventions or treatments might be designed or prescribed to prevent these adverse health consequences. This work is critical due to the large and increasing number of older adults with chronic disabilities who are likely to survive for many years and will depend on family members and other informal caregivers for assistance to meet their daily functional needs.
Although the caregiving-is-a-health-risk-factor narrative is important and compelling, it does not constitute the complete view of caregiving or all of the mechanisms by which the caregiving experience might influence health. Alternative, counteracting perspectives have emerged that increasingly recognize resilience in the face of psychological stress (Bonanno, 2004) and the potential health benefits of prosocial helping relationships (Brown & Brown, 2015; Poulin, Brown, Dillard, & Smith, 2013). Caregiving, like other family relationships, may involve stress in many ways, but these kinds of social engagements also undoubtedly have other effects on health and well-being, including possible positive effects. For example, a growing series of population-based studies has indicated rather conclusively that family caregiving is not linked to an increased risk for mortality. Conversely, several population-based studies in both the United States and Europe have found that family caregivers tend to live longer than comparable samples of persons who do not report family caregiving responsibilities (Brown et al., 2009; Fredman, Lyons, Cauley, Hochberg, & Applebaum, 2015; O’Reilly, Connolly, Rosato, & Patterson, 2008; O’Reilly, Rosato, Ferry, Moriarty, & Leavy, 2017; O’Reilly, Rosato, & Maguire, 2015; Ramsay, Grundy, & O’Reilly, 2013; Roth, Fredman, & Haley, 2015; Roth et al., 2013). Recent work inspired by models of the health benefits of volunteering and other prosocial helping behaviors (Brown & Brown, 2015; Poulin et al., 2013) has shown that caregiving is associated with a stress-buffering effect such that psychological distress is not associated with mortality in caregivers in the same way that it is for noncaregiving comparison participants (Roth et al., 2018). If one fundamental purpose of the research on caregiving, chronic stress, and biomarkers is to identify possible mechanisms that could explain the link between caregiving and increased mortality, then that literature requires reexamination in light of these replicated findings that caregivers live longer (and do not die faster) than more carefully matched noncaregiving comparison participants.
The literature on the association between family caregiving and circulating biomarkers of inflammation or immunity has been reviewed and summarized in at least four previous articles. An early meta-analysis by Vitaliano, Zhang, and Scanlan (2003) examined 12 studies that compared dementia caregivers and controls on “physiological indicators” including some measures of immunity. They found small but statistically significant associations, further clarified these effects as “weak,” and cautioned that “questions remain regarding their magnitude and their clinical significance” (p. 961). Since the review by Vitaliano and colleagues (2003), many subsequent studies have been conducted, including numerous studies that have examined circulating biomarkers of inflammation (e.g., C-reactive protein [CRP], interleukin-6 [IL-6], D-dimer) as a function of caregiving. Although we are not aware of any more recent meta-analyses on the effect sizes emerging from this expanding literature, at least three more recent narrative reviews have been published.
Lovell and Wetherell (2011) reviewed 42 papers of “endocrine and immune” measures in relation to caregiving and concluded that “findings … consistently revealed dysregulation of cellular and humoral immune markers in elderly, spousal caregivers” (p. 1350). They interpreted these findings as supporting a “cumulative wear and tear” process that fosters “increased risk for deleterious health outcomes” including a “significantly greater risk for mortality” (p. 1350). Interestingly, only one of the 42 papers reviewed by Lovell and Wetherell (2011) used a population-based sample (as opposed to a convenience sample) of participants. Furthermore, only five of the 42 studies had a sample size of over 100 caregiving participants, and 10 of those 42 studies had sample sizes of 20 or fewer caregivers. Also of note, many findings summarized in the Lovell and Wetherell (2011) review are of complex effects, for example, the relationship between caregiving stressors and biomarkers, or the effects of interventions on biomarkers, but do not focus on the simpler question of whether there are biomarker differences as a main effect of caregiving itself.
As part of larger reviews on the physiological effects of dementia caregiving, both Fonareva and Oken (2014) and Allen and colleagues (2017) reviewed effects on measures of immune system function and inflammation. Both reviews included many of the same articles reviewed by Lovell and Wetherell (2011), but their conclusions were somewhat different. Fonareva and Oken (2014) concluded that there is “agreement about CRP levels being increased in dementia caregivers with moderate effect sizes” (p. 741) and that “there is accumulating evidence that chronic dementia caregiver stress increases their vulnerability to disease” (p. 741). Allen and colleagues reviewed 54 studies of immune system activation and inflammation but offered conclusions that were considerably more tentative. They stated that “baseline measures of cytokines… such as IL-6, TNF-alpha, and CRP… have infrequently been assessed in caregivers and the results have been mixed” (p. 130) and that “although baseline low grade immune system activation is apparent in some caregiver studies, the evidence for this is mixed” (p. 130). Only 10 of the 54 papers reviewed by Allen and colleagues (2017) had sample sizes of 100 or more caregivers, but some of these were from the same research group and appear to represent duplicative data.
Recently, Potier, Degryse, and de Saint-Hubert (2018) reviewed 24 studies of inflammatory biomarkers associated with caregiving. They concluded that increases in inflammatory biomarkers were associated with some of the problems associated with caregiving (e.g., disturbed sleep, caregiving burden), but overall, they “found little evidence concerning the association between caregiving status and biomarkers of stress and inflammation” (p. 119).
None of the reviews since the Vitaliano and colleagues’ (2003) article provided a meta-analytic summary of observed effect sizes, and some reviews included studies that did not include noncaregiving comparison participants. Although study quality ratings were made in some previous reviews, little attention was given to a potentially important factor—the use of convenience versus population-based sampling methods to assemble caregiving and noncaregiver comparison groups. In caregiving research that uses convenience samples, participants are enrolled from local clinics, support groups, and notices for research volunteers, with different recruitment sources and enrollment methods usually being used for caregivers and noncaregivers. In population-based studies, samples are assembled with the goal of being representative of a larger population, and both caregivers and noncaregiving controls are recruited and enrolled using the same methods. In addition, none of the four more recent review papers explicitly addressed another important issue—the potential for duplicative reporting of the same data from the same samples across multiple papers published by some research groups.
In the present study, we focused on the association between family caregiving status and baseline measures of circulating biomarkers in two widely studied domains in this literature—immune system functioning and inflammation. In light of the fundamental importance of this primary research question—is the chronic stress of family caregiving associated with compromised immune system functioning and/or inflammation as assessed by circulating biomarkers—we conducted an updated systematic review of this literature and used meta-analysis methods to estimate overall effect sizes from the distinct effects that could be extracted from the relevant studies in this area of investigation. Consistent with the guidelines of the PROSPERO registry, we systematically searched the published literature in multiple databases, reviewed article titles and abstracts for relevance, identified overlap or duplicative findings from multiple papers from the same investigative group, rated retained studies for quality or potential bias in six methodological areas, and extracted caregiver versus noncaregiver comparison effect sizes on biomarkers of either inflammation or immunity. In addition to examining overall effects across all caregivers, we also evaluated effects specifically for dementia caregivers and from studies that differed on potential bias ratings.
Methods
Literature Search
This systematic review was preregistered with PROSPERO in 2017 (registration number CRD42017071123; http://www.crd.york.ac.uk/PROSPERO). Systematic searches of the literature were performed in April of 2017. The final PubMed search terms are provided in Figure 1. PubMed search terms were further refined to facilitate searches of EMBASE and the Cochrane library. Inclusion criteria for studies to be included were as follows: (i) full research articles published in English, such that abstracts without accompanying full articles and papers in non-English languages were excluded; (ii) biomarkers of immunity or inflammation were measured in blood samples from family or informal caregivers; and (iii) the same biomarkers of immunity or inflammation were measured from noncaregiving comparison participants. In addition, only original data-based research reports were included. Review papers and editorial commentaries were excluded. Caregivers were defined as persons caring for an adult with a chronic disease or disability, and studies that reported data from parents caring for children who were predominantly under 18 years of age, including children with developmental disorders such as autism or other disabilities, were also excluded from this review.
Figure 1.
Final PubMed search terms. Search limited to articles in the English language. No time limits were applied.
Article Selection Process
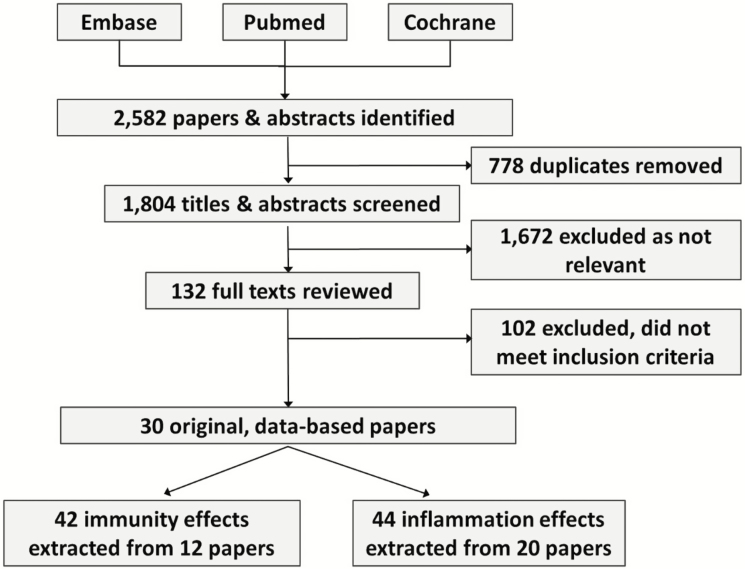
Figure 2 illustrates our article review and selection process. The systematic searches of these databases identified 2,582 papers and abstracts. All articles were entered into a reference manager, Endnote v7, and 778 duplicates were removed. Each title and abstract of the 1,804 remaining articles was then reviewed independently by at least two coauthors, and 1,672 titles or abstracts were identified as not being eligible for inclusion in the present systematic review. This left 132 published papers that were then selected for full-text review by at least two coauthors. Specific biomarker measures used were listed for these papers, and two of the coauthors who are experts in biomarker research (N.S.J. and J.D.W.) determined whether each measure from this list qualified as a biomarker of immune system functioning, inflammation, or neither. In addition, biomarkers that were reported but did not represent baseline indicators of inflammation or immune system function (e.g., responses to vaccination over time) were not included in our meta-analysis.
Figure 2.
Flow diagram of study selection process.
In some cases, we discovered multiple articles from the same research group where data on the same biomarkers from the same participants were reported in more than one paper. Many papers quite reasonably added participants to their samples of caregivers and noncaregivers over time, and later papers sometimes focused on more complex research questions such as subgroup analyses, interaction effects, longitudinal changes, or added additional biomarkers. In cases where there were multiple publications from the same research group, we extracted an effect for each biomarker from the article with the largest sample size that contained data for both caregivers and controls on that biomarker or from the first article reporting that biomarker. When necessary, the authors were contacted to clarify whether they had reported on distinct versus overlapping samples across different papers.
In total, of the 132 papers that underwent full-text review, 102 were excluded as not meeting our inclusion criteria or containing any independent data from other papers. This yielded 30 original, data-based papers that met all of our inclusion criteria. These papers are listed in Table 1, including the biomarkers extracted from each paper for the meta-analysis and the immune system or inflammation category assigned to that biomarker.
Table 1.
Serum Biomarkers of Immune System and Inflammation Extracted From Blood Samples in 30 Studies
| Study | Caregivers | Controls | Biomarkers | Category | Potential bias |
|---|---|---|---|---|---|
| 1. Kiecolt-Glaser et al. (1987) | 34 dementia | 34 demographically matched | % helper T lymphocytes | Immune | Moderate |
| % NK cells | Immune | ||||
| % suppressor T | Immune | ||||
| % total T lymphocytes | Immune | ||||
| EBV VAC | Immune | ||||
| Helper/suppressor ratio | Immune | ||||
| 2. Reese et al. (1994) | 50; 25 dementia, 25 stroke | 25 noncaregivers | % CD3 | Immune | Moderate |
| % CD4 | Immune | ||||
| % CD8 | Immune | ||||
| % leukocytes | Immune | ||||
| % NK cells | Immune | ||||
| 3. Irwin et al. (1997) | 100 dementia, spouses only | 33 volunteers | NK activity | Immune | Low |
| 4. Mills et al. (1997) | 27 dementia, spouses only | 10 matched volunteers | CD16 count | Immune | Moderate |
| CD4 count | Immune | ||||
| CD8 count | Immune | ||||
| 5. Cacioppo et al. (1998) | 27 dementia, spouses only, women only | 37 controls | Cell Prolif to Con A | Immune | Moderate |
| Cell Prolif to PHA | Immune | ||||
| NK cell cytotoxicity | Immune | ||||
| 6. Scanlan, Vitaliano, Ochs, Savage, and Borson (1998) | 81 dementia, spouses only | 82 age- and gender-matched spouses of persons without dementia | %CD4 | Immune | Low |
| %CD8 | Immune | ||||
| 7. Vitaliano et al. (1998) | 80 dementia, spouses only | 85 age- and gender-matched spouses of persons without dementia | NK activity | Immune | Low |
| 8. Lutgendorf et al. (1999) | 18 dementia, women only | 15 noncaregiving (and nonmoving) women | IL-6 | Inflammation | Moderate |
| 9. Bauer et al. (2000) | 49 dementia, spouses only | 67 “closely matched” controls | Cell Prolif to PHA | Immune | Low |
| IL-2 | Inflammation | ||||
| 10. Stowell, Kiecolt-Glaser, and Glaser (2001) | 61 dementia | 78 “comparison” participants | % CD3 | Immune | Moderate |
| % helper T cells | Immune | ||||
| % T (suppressor/cytotoxic) | Immune | ||||
| Log Con A | Immune | ||||
| Log PHA | Immune | ||||
| Helper/suppressor ratio | Immune | ||||
| 11. Vedhara et al. (2002) | 41 multiple sclerosis, spouses only | 62 noncaregiving controls | IFN-γ | Inflammation | Low |
| IL-4 | Inflammation | ||||
| 12. Provinciali et al. (2004) | 38 caregivers of home health care patients, nonspouses only | 37 age- and sex-matched controls | % CD16 | Immune | Minimal |
| % CD56 | Immune | ||||
| NK activity | Immune | ||||
| 13. Vitaliano, Persson, Kiyak, Saini, and Echeverria (2005) | 123 dementia, spouses only | 117 noncaregiving spouses | CRP | Inflammation | Low |
| WBC | Inflammation | ||||
| 14. Graham et al. (2006) | 113 dementia, spouses only | 101 controls | CRP | Inflammation | Moderate |
| IL-6 | Inflammation | ||||
| 15. von Kanel et al. (2006) | 116 dementia, spouses only | 54 noncaregivers | CRP | Inflammation | Low |
| D-dimer | Inflammation | ||||
| IL-6 | Inflammation | ||||
| 16. Aschbacher et al. (2007) | 51 dementia, spouses only, women only | 27 noncaregiving women | % Aggregates | Inflammation | Low |
| 17. Damjanovic et al. (2007) | 38 dementia | 38 age- and gender-matched controls | B cell (CD19) count | Immune | Low |
| CD4 count | Immune | ||||
| CD8 count | Immune | ||||
| GM-CSF | Inflammation | ||||
| IFN-γ | Inflammation | ||||
| IL-2 | Inflammation | ||||
| IL-4 | Inflammation | ||||
| IL-6 | Inflammation | ||||
| IL-8 | Inflammation | ||||
| Monocyte (CD14) | Immune | ||||
| NK cell | Immune | ||||
| 18. Miller et al. (2008) | 11 glioblastoma (brain tumor) caregivers | 10 matched controls | IL-1 | Inflammation | Moderate |
| 19. Segerstrom, Schipper, and Greenberg (2008) | 14 dementia | 30 controls | IL-6 | Inflammation | Low |
| 20. Rohleder, Marin, Ma, and Miller (2009) | 18 glioblastoma (brain tumor) caregivers | 19 matched controls | CRP | Inflammation | Moderate |
| IL-6 | Inflammation | ||||
| 21. Fonareva, Amen, Zajdel, Ellingson, and Oken (2011) | 20 dementia | 20 noncaregivers free of “any major stress” | CRP | Inflammation | Low |
| IL-6 | Inflammation | ||||
| TNF-α | Inflammation | ||||
| 22. Kiecolt-Glaser et al. (2011) | 58 dementia | 74 noncaregivers | IL-6 | Inflammation | Moderate |
| TNF-α | Inflammation | ||||
| 23. Gouin, Glaser, Malarkey, Beversdorf, and Kiecolt-Glaser (2012) | 53 dementia | 77 noncaregivers | CRP | Inflammation | Minimal |
| 24. von Kanel, Mausbach, et al. (2012) | 93 dementia, spouses only | 43 married controls | Endothelin-1 | Inflammation | Low |
| IC adhesion | Inflammation | ||||
| IL-8 | Inflammation | ||||
| IFN-γ | Inflammation | ||||
| Plasminogen activator inhibitor | Inflammation | ||||
| Serum amyloid A | Inflammation | ||||
| TNF-α | Inflammation | ||||
| VC adhesion | Inflammation | ||||
| 25. von Kanel, Mills, et al. (2012) | 118 dementia, spouses only | 51 controls | IL-10 | Inflammation | Low |
| IL-12 | Inflammation | ||||
| 26. Wong et al. (2013) | 55 caregivers of multiple conditions, spouses only | 61 age- and sex-matched noncaregivers | % B lymphocyte | Immune | Minimal |
| % cyto T | Immune | ||||
| % NK | Immune | ||||
| % T helper | Immune | ||||
| % T suppressor | Immune | ||||
| % total T cell | Immune | ||||
| 27. Kang and Marks (2014) | 49 adult child caregivers | 914 noncaregivers | Inflammatory composite | Inflammation | Minimal |
| 28. Kim and Ferraro (2014) | 313 caregivers of “day-to-day activities” | 1477 noncaregivers | CRP | Inflammation | Minimal |
| 29. Miller et al. (2014) | 33 glioblastoma (brain tumor) caregivers | 47 controls “without major stressors … during the previous year” | CRP | Inflammation | Moderate |
| NF-κB | Inflammation | ||||
| 30. Bevans et al. (2016) | 20 stem cell transplant caregivers | 20 noncaregivers | IL-6 | Inflammation | Low |
| TNF-α | Inflammation |
Notes: CRP = C-reactive protein; IFN = interferon; IL = interleukin; NF-κB = nuclear factor kappa B; NK = natural killer; TNF = tumor necrosis factor; WBC = white blood cell; EBV VAC = Epstein-Barr virus capsid antigen; GM-CSF = Granulocyte-macrophage colony-stimulating factor; PHA = phytohemaglutinin; VC = Vascular Cellular.
Quality Reviews
Using a procedure adapted from Allen and colleagues (2017), at least two coauthors independently rated the quality of the methods reported in each of these 30 articles on six separate dimensions: (i) caregiving definition and selection methods, (ii) control definition and selection methods, (iii) descriptors or adequate definitions of caregiving exposure (e.g., hours per week or care, number of years of caregiving), (iv) adequacy of the descriptions of the biomarker assessment methods, (v) potential for confounding and the adequacy of methods to control for confounding such as matching or statistical covariate adjustment, and (vi) sufficient explanation of the statistical methods for testing group differences, either before or after adjustment for potential confounders. Each rating was made on a 4-point scale of potential bias (1 = minimal, 2 = low, 3 = moderate, and 4 = high potential bias). Discrepancies between two raters that exceeded just one category difference (i.e., minimal-moderate, minimal-high, or low-high) were reviewed jointly by a panel of four coauthors and resolved by consensus. An overall quality rating was achieved by averaging the scores across the six dimensions. All quality ratings were obtained and consensus meetings occurred before any effects were extracted for meta-analysis purposes. This minimized any possibilities that the bias ratings themselves might be affected by any emerging trends from the analyses.
Meta-Analysis Procedures
Version 3.3 of the Comprehensive Meta-Analysis (2014) system was used to conduct the meta-analyses. Biomarker data were extracted by the first author for each biomarker from the 30 articles using published means and SD (or SE) when available, and p values and sample sizes from analytic models in cases where descriptive means and SD were not available. These methods allow for the calculation of a standardized effect size expressed as a difference in SD units (d) between the caregiver and noncaregiving control groups. Ninety-five percent confidence intervals (95% CI) were calculated for each standardized effect size, and the multiple standardized effect sizes were then pooled across the multiple measures within and across the 30 studies. We assumed that the effects from the different studies represented randomly sampled estimates of an underlying caregiver versus noncaregiving control effect size distribution and used random-effects methods to estimate the overall average effect size and 95% confidence interval. The Q-statistic was examined as a test of the heterogeneity of individual study effect sizes.
We conducted one overall analysis for all 86 effects that were extracted from the 30 studies. We also conducted separate meta-analyses for the 42 effects that were characterized as tests of immune system functioning and the 44 effects that were characterized as tests of chronic inflammation. In all analyses, the standardized effects from each study and measure were coded such that higher scores on the biomarker indicated the direction expected from greater stress (e.g., more inflammation, lower measures of immune system functioning). Higher overall average effects, therefore, represented potential stress-induced effects on that biomarker in the caregivers compared with the noncaregiving controls. Additional analyses also examined the effect sizes found in studies that focused only on dementia caregivers and on subgroups of studies defined by different levels of potential bias.
Results
Descriptive Information of the Included Studies
Table 1 lists the 30 studies that were included in the meta-analysis in chronological order. These studies reported biomarker data collected from 1,848 caregivers and 3,640 noncaregiving controls. Biomarker indices of immune system function dominated the literature in the late 1980s and throughout the 1990s, whereas studies assessing inflammation became more prevalent in the decade of 2000–2010. Only 6 of the 30 studies (20%) enrolled 100 or more caregivers, and only 3 of the 30 studies (10%) were characterized by us as using population-based samples (Kang & Marks, 2014; Kim & Ferraro, 2014; Provinciali et al., 2004),
The studies varied substantially in their recruitment and inclusion methods and in the level of detail provided for the quality ratings (e.g., caregiving exposure). The overall average quality rating for each study in terms of potential bias is summarized in Table 1 (1.00–1.99 = minimal bias; 2.00–2.50 = low bias; 2.51–3.25 = moderate bias). The average quality ratings tended to improve over time, with more recent studies generally receiving lower potential bias ratings than earlier studies. Quality ratings within individual categories indicated higher possibilities for bias, on average, for caregiver and noncaregiver sample selection issues (e.g., poorly described inclusion criteria, insufficient matching, or possible confounding) and for caregiving exposure descriptions (e.g., hours of care per week, whether activities of daily living [ADL] or instrumental ADL care was provided) than for other aspects that were rated such as quality of the biomarker assays or statistical analysis methods. It should be noted that several studies with overall “low” bias ratings nonetheless had “moderate” or even “high” bias ratings on sample selection or caregiving exposure features.
All 30 studies referred to the persons providing care as “caregivers” even if robust definitions of a caregiving were not always provided. Kim and Ferraro (2014) reported demographic information across four potentially stressed groups combined, so demographics specific to caregivers alone could not be determined in that study. Of the remaining 29 studies, 25 reported on the relationship between the caregiver and the care recipient, with 15 studies including only spouse caregivers, 2 (Kang & Marks, 2014; Provinciali et al., 2004) including only nonspouse caregivers, and 8 studies including a mix of spouses, adult children, and others. Across the 29 studies, 70% of the caregivers were female and the mean age was 64.8 years. Only one study specified a requirement that the caregiver had to provide assistance with ADL (Wong et al., 2013). The number of caregiving hours per day was reported by eight studies with a mean number of 8.7 hr/day. Twenty studies referred to the living arrangements of the care recipient and caregiver. The spouse-only studies all described living with the care recipient, whereas 30%–72% of the caregivers in the five other studies reporting living arrangements indicated that the caregivers lived with the care recipients.
Meta-analytic Findings
The results of the random-effects models for the meta-analyses are summarized in Table 2. The analysis of all 86 effects extracted from the 30 studies in Table 1 yielded a point estimate of d = 0.164 (SE = 0.042, 95% CI = 0.081, 0.247). This overall effect is somewhat below the typical “small” effect size of d = 0.20 as defined by Cohen (1988). An effect of d = 0.20 corresponds to a group difference that explains 1% of the variance in the outcome measure, so the caregiving versus control difference of d = 0.164 accounted for less than 1% of the total variance of the biomarker measures. An examination of the 95% CIs from the individual studies indicated a high degree of study-to-study variability, but the Q-statistic of heterogeneity only approached conventional levels of statistical significance (Q = 41.3, df = 29, p = .064).
Table 2.
Meta-analysis Random-Effects Estimates
| Type of analysis | Number of studies | Number of effects | d | SE | p | 95% CI | Q-statistic of heterogeneity | p |
|---|---|---|---|---|---|---|---|---|
| Total | ||||||||
| Overall | 30 | 86 | 0.164 | 0.042 | <.001* | 0.081–0.247 | 41.3 | .064 |
| Immune | 12 | 42 | 0.217 | 0.066 | .001* | 0.088–0.347 | 13.4 | .267 |
| Inflammation | 20 | 44 | 0.142 | 0.053 | .008* | 0.057–0.246 | 30.0 | .052 |
| Dementia caregivers | ||||||||
| Overall | 21 | 66 | 0.188 | 0.042 | <.001* | 0.105–0.271 | 19.0 | .519 |
| Immune | 10 | 33 | 0.241 | 0.065 | <.001* | 0.004–0.113 | 12.6 | .184 |
| Inflammation | 13 | 33 | 0.155 | 0.052 | .003* | 0.052–0.257 | 11.1 | .521 |
| Minimal or low bias studies | ||||||||
| Overall | 19 | 53 | 0.086 | 0.038 | .028* | 0.009–0.162 | 19.1 | .386 |
| Immune | 7 | 19 | 0.197 | 0.081 | .015* | 0.038–0.355 | 7.7 | .262 |
| Inflammation | 14 | 34 | 0.059 | 0.045 | .194 | -0.030–0.147 | 14.1 | .370 |
| Moderate bias studies | ||||||||
| Overall | 11 | 33 | 0.302 | 0.095 | .002* | 0.116–0.489 | 16.9 | .076 |
| Immune | 5 | 23 | 0.272 | 0.129 | .035* | 0.019–0.525 | 5.5 | .236 |
| Inflammation | 6 | 10 | 0.356 | 0.153 | .020* | 0.056–0.655 | 11.4 | .045 |
Notes: CI = confidence interval.
*p < .05.
The separate meta-analyses for grouped immunity and inflammation biomarkers across all studies indicated that the random-effects model estimate from the 42 immunity effects (d = 0.217) was somewhat larger than the corresponding estimate from the 44 inflammation effects (d = 0.142). An examination of the individual biomarkers listed in Table 1 indicates that only two biomarkers were assessed in more than five studies. For IL-6, an effect of d = 0.134 (p = .129) was observed across nine studies, and for CRP, an effect of d = 0.152 (p = .117) was observed across eight studies.
Twenty of the studies included in this meta-analysis included samples of dementia caregivers only, and one study (Reese, Gross, Smalley, & Messer, 1994) reported data separately for dementia and stroke caregivers. Table 2 summarizes the effect sizes from the meta-analyses for these 21 studies with samples of dementia caregivers. Those effect sizes are only slightly larger than those obtained across all caregiving samples and are still in the “small” effect size range.
Separate meta-analyses were conducted on the effects from the 19 studies that had overall quality ratings of 2.50 or less (i.e., the minimal and low potential bias designations in Table 1) and for the 11 studies with moderate overall potential bias. These analyses are also summarized in Table 2. Interestingly, the overall analysis for minimal or low bias studies revealed a very small effect for caregiving (d = 0.086), and the analysis focusing on the 34 effects of inflammation from these minimal or low bias studies yielded an effect (d = 0.059) that was not significantly different statistically from a null effect of no difference between caregivers and noncaregivers. Conversely, the moderate bias studies showed much stronger effects, with the overall effect (d = 0.302, p = .002) being more than 3.5 times larger than the corresponding effect from the low bias studies.
Of the five effects extracted from the three population-based studies (Kang & Marks, 2014; Kim & Ferraro, 2014; Provinciali et al., 2004), none showed a statistically significant difference between caregivers and noncaregiving controls. Only one population-based study (Kim & Ferraro, 2014) involved a comparison of more than 100 caregivers and 100 noncaregivers. That study was limited to CRP and found no difference between caregivers and controls on this measure of inflammation.
Discussion
The results of this systematic review and meta-analysis suggest that family caregiving is associated with statistically significant effects on measures of immunity and inflammation collected from baseline blood samples. The overall effect size of 0.164 was highly significant statistically (p < .001), and analyses grouped by biomarker type tended to indicate somewhat stronger effects for immune system biomarker than for measures of inflammation. In general, though, the magnitude of the meta-analytic effects was close to or less than the 0.20 “small” effect size (Cohen, 1988). Contrary to widely accepted beliefs that dementia caregiving can have particularly strong effects on health-related biomarkers, associations remained in the small range (d = 0.188) when analyses were restricted to dementia caregivers only.
There are recurring features in the studies reviewed that warrant caution and indicate that more research is needed. The sample sizes used in this area of investigation are often quite small, with 15 of the 30 studies included in the meta-analysis having 50 or fewer caregivers. This led to wide study-specific confidence intervals and may have contributed to a pattern of findings being inconsistent from one study to the next (Allen et al., 2017). Although the findings were highly variable across studies, formal tests of heterogeneity were generally not statistically significant. Power for the heterogeneity tests may have been limited by the relatively small sample sizes used in many studies.
The generally small effect sizes suggest questionable clinical significance for individual family caregivers. Most effect sizes from the meta-analyses were less than 0.20, indicating that the caregiver versus control difference generally accounted for less than 1% of the variance in the outcome measures. By way of comparison, a recent meta-analysis indicated that obesity accounts for 13% of the variance in CRP (Choi, Joseph, & Pilote, 2013), whereas our analysis across eight studies found that caregiving status accounted for less than 1% of the variance in CRP. In a meta-analysis of possible race differences on caregiving variables, Pinquart and Sörensen (2006) characterized effects that explained “less than 1% of the variance” as being “too small to be meaningful” (p. 96). Vitaliano and colleagues (2003) also characterized similar effect sizes as “weak” and that “questions remain regarding their magnitudes and their clinical relevance” (p. 961). Vitaliano and colleagues proceeded to explain how even very small relationships that have little or no relevance at the individual person level might, nonetheless, still account for a nontrivial number of health events, such as myocardial infarctions, at the population level, due to the fact that millions of persons are serving in family caregiving roles.
The findings that the overall effect size was much smaller from studies with lower potential bias ratings and that none of the population-based studies found any significant effects for caregiving on these biomarkers are noteworthy and underscore possible concerns with sample selection biases from many of the convenience samples. There is considerable evidence that caregivers recruited in convenience samples have more psychological distress than those identified in population-based studies (Pinquart & Sörensen, 2003; Pruchno et al. 2008). In addition, many of the studies reviewed here that used convenience samples provided very little information on the recruitment or characteristics of the noncaregiving control participants. In almost all cases, recruitment and enrollment methods appeared to differ for caregivers and noncaregiving controls. When recruitment information was provided for noncaregivers, these participants were often characterized as consisting of socially active persons who were recruited from community organizations, senior centers, alumni newsletters, church groups, or other social organizations. “Volunteers” for research projects from such sources can be expected to be relatively healthy and active (Anderson et al., 2014), and findings of biomarker vulnerability in caregivers using this type of research design may simply reflect greater biomarker resilience among the more socially active, healthy volunteer control groups.
When examining our findings alongside the conclusions from the previous narrative reviews of this literature, it is difficult to concur with the more definitive conclusions offered by Lovell and Wetherell (2011) or Fonareva and Oken (2014) that caregiving is consistently associated with biomarker changes of inflammation or dysregulation of the immune system. The more indeterminate “mixed” interpretations offered by Allen and colleagues (2017) or the lack of an association as described by Potier and colleagues (2018) seem to be more in line with the bulk of the research evidence to date. Future research should continue to introduce greater methodological rigor when examining possible biomarker vulnerabilities associated with long-term caregiving. Studies of large and representative, population-based samples with similar recruitment methods for caregivers and noncaregiving controls are needed. Such studies should specify clear inclusion criteria to ensure that the caregivers are actually providing care, and more careful noncaregiving matching criteria to control possible confounding with social activity or other variables. Future studies should also look more carefully at key caregiving subgroups such as those defined by relationship type (e.g., spouse vs. nonspouse caregiving) and the clinical condition of the care recipients (e.g., caring for persons with or without dementia). In certain longitudinal epidemiological investigations, it would also be advantageous to look at changes in biomarkers within individuals who have transitioned from a noncaregiving status into a caregiving role during the course of their participation in those studies. To our knowledge, such intraperson changes on biomarkers in response to the onset of caregiving have never been reported in the research literature.
Limitations to our systematic review and meta-analysis include unknown impacts of some study eligibility decisions. Some otherwise relevant studies were excluded from the meta-analyses because insufficient data were reported in the publication or for other eligibility reasons. For example, two additional population-based studies were identified by our systematic review but not included in the meta-analysis. Dich, Lange, Head, and Rod (2015) collected data on CRP and IL-6 as part of an assessment of allostatic load (AL) but did not report these individual biomarker data. High caregiving burden was found to be associated with higher AL levels, but low caregiving burden was associated with lower AL levels such that the overall caregiving effect appeared to be minimal. Shivpuri, Gallo, Crouse, and Allison (2012) examined CRP as a function of gender and stress associated with a “serious ongoing health problem in someone close to you.” This undoubtedly included many caregivers but also included “individuals who are not direct caregivers.” They termed this “sympathetic-caregiving stress” and found a very small but statistically significant overall association with CRP that was significantly stronger for women than for men.
Other potential limitations may have been introduced by our decisions to merge effect sizes across multiple different biomarkers into overall, immune, and inflammation outcome categories. Immune and inflammation biomarkers interact, and these dynamic relationships among the biomarkers were not accounted for in our meta-analysis or in most of the studies we reviewed. Although most individual biomarkers were not assessed frequently enough to conduct separate meta-analyses for each measure, we did conduct separate analyses for IL-6 and CRP and found similar effect sizes as those obtained from more inclusive and heterogenous analyses. Modifying the biomarker categorization decisions or the eligibility criteria for including additional studies would lead to some adjustments in the effect sizes estimated, although we believe such adjustments would be minimal and that the estimated meta-analytic effects would remain near or less than the d = 0.20 threshold for a small effect size.
Another limitation of our review and meta-analysis is the preponderance of studies of caregivers of persons with dementia. These studies, therefore, are over-representative of dementia caregivers, who represent only a minority of all caregivers (National Alliance for Caregiving and the AARP, 2015). Because the effect size was slightly larger for dementia caregivers than for all caregivers, the caregiver versus control effects appear to even smaller for the caregivers of persons who are not afflicted with dementia, but additional research is needed for this group to confirm such a trend.
In our view, it is all too common for ongoing research efforts and public health statements on caregiving to perpetuate the narrative that caregiving is an established risk factor for compromised physical health, with clear immune system or proinflammatory pathways often highlighted as possible mechanisms. A recent article in the New York Times (2016), for example, stated that “researchers have found that the human immune system can be weakened by stress and strain for up to three years after caregiving ends. As a result, caregivers can be more prone to having serious illnesses. Yet they rarely complain.” Such media reports are often based on statements from researchers that selectively draw on findings from studies reviewed here. Caregiving can be stressful, and caregivers sometimes do need additional supports and services to manage the care of their family members, but caregiving can also lead to personal growth, purpose in life, and health benefits (Roth et al., 2015). Developing a balanced and empirically supported narrative about caregiving that recognizes it as a stressful but also an expected and potentially healthy part of the adult life cycle (Qualls, 2016) is an important challenge for scholars who continue to conduct research on health effects of caregiving.
In summary, there seems to be little replicable research evidence that supports the hypothesis that the stress of family caregiving leads to clinically significant alterations of circulating biomarkers of immunity or inflammation. Overall, average effect sizes are small, and associations reported from multiple convenience samples are often inconsistent. Higher quality studies and population-based studies rarely find statistically significant elevations in circulating inflammatory biomarkers in caregivers compared with appropriately matched noncaregiving controls. Public statements by researchers and health policy experts that continue to perpetuate the physical health risks of caregiving and point to biomarker studies as evidence often pay insufficient attention to the complexities of the studies in this area, the small average effect sizes, the potential for significant selection biases in studies with small convenience samples, and the mixed or inconsistent findings that are usually uncovered in this area of investigation.
Funding
This work was supported by grant RF1 AG050609 from the National Institute on Aging (NIA). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIA.
Acknowledgment
We thank our clinical informationist, Ms. Carrie Price, for her invaluable advice and assistance with the literature search.
Conflict of Interest
None reported.
References
- Allen A. P., Curran E. A., Duggan Á., Cryan J. F., Chorcoráin A. N., Dinan T. G., … Clarke G (2017). A systematic review of the psychobiological burden of informal caregiving for patients with dementia: Focus on cognitive and biological markers of chronic stress. Neuroscience and Biobehavioral Reviews, 73, 123–164. doi:10.1016/j.neubiorev.2016.12.006 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association (2018). 2018 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 14, 367–429. doi:10.1016/j.jalz.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Anderson N. D., Damianakis T., Kröger E., Wagner L. M., Dawson D. R., Binns M. A., … Cook S. L.; BRAVO Team (2014). The benefits associated with volunteering among seniors: A critical review and recommendations for future research. Psychological Bulletin, 140, 1505–1533. doi: 10.1037/a0037610 [DOI] [PubMed] [Google Scholar]
- Aschbacher K., von Kanel R., Mills P. J., Hong S., Roepke S. K., Mausbach B. T., … Grant I (2007). Combination of caregiving stress and hormone replacement therapy is associated with prolonged platelet activation to acute stress among postmenopausal women. Psychosomatic Medicine, 69, 910–917. doi: 10.1097/PSY.0b013e31815a8ba8 [DOI] [PubMed] [Google Scholar]
- Bauer, M. E., Vedhara, K., Perks, P., Wilcock, G. K., Lightman, S. L., & Shanks, N. (2000). Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. Journal of Neuroimmunology, 103, 84–92. doi:10.1016/S0165-5728(99)00228-3 [DOI] [PubMed] [Google Scholar]
- Bevans M. F., Ross A., Wehrlen L., Klagholz S. D., Yang L., Childs R., … Pacak K (2016). Documenting stress in caregivers of transplantation patients: Initial evidence of HPA dysregulation. Stress, 19, 175–184. doi: 10.3109/10253890.2016.1146670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno, G. A. (2004). Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist, 59, 20–28. Retrieved from https://psycnet.apa.org/doi/10.1037/0003-066X.59.1.20. doi:10.1037/0003-066X.59.1.20 [DOI] [PubMed] [Google Scholar]
- Brown S. L., & Brown R. M (2015). Connecting prosocial behavior to improved physical health: Contributions from the neurobiology of parenting. Neuroscience and Biobehavioral Reviews, 55, 1–17. doi: 10.1016/j.neubiorev.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Brown S. L., Smith D. M., Schulz R., Kabeto M. U., Ubel P. A., Poulin M., … Langa K. M (2009). Caregiving behavior is associated with decreased mortality risk. Psychological Science, 20, 488–494. doi: 10.1111/j.1467-9280.2009.02323.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J. T., Poehlmann K. M., Kiecolt-Glaser J. K., Malarkey W. B., Burleson M. H., Berntson G. G., & Glaser R (1998). Cellular immune responses to acute stress in female caregivers of dementia patients and matched controls. Health Psychology, 17, 182–189. doi: 10.1037/0278-6133.17.2.182 [DOI] [PubMed] [Google Scholar]
- Caregiver Action Network (2018). Caregiver Statistics. Retrieved from http://caregiveraction.org/resources/caregiver-statistics#Impact%20on%20Family%20Caregiver’s%20Health
- Choi J., Joseph L., & Pilote L (2013). Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obesity Reviews, 14, 232–244. doi:10.1111/ obr.12003 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Comprehensive Meta-Analysis (2014). Version 3.3.070. Englewood, NJ: Biostat, Inc. [Google Scholar]
- Damjanovic A. K., Yang Y., Glaser R., Kiecolt-Glaser J. K., Nguyen H., Laskowski B., … Weng N. P (2007). Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. Journal of Immunology, 179, 4249–4254. doi: 10.4049/jimmunol.179.6.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dich N., Lange T., Head J., & Rod N. H (2015). Work stress, caregiving, and allostatic load: Prospective results from the Whitehall II cohort study. Psychosomatic Medicine, 77, 539–547. doi: 10.1097/PSY.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E. S., Blackburn E. H., Lin J., Dhabhar F. S., Adler N. E., Morrow J. D., & Cawthon R. M (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America, 101, 17312–17315. doi: 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonareva I., Amen A. M., Zajdel D. P., Ellingson R. M., & Oken B. S (2011). Assessing sleep architecture in dementia caregivers at home using an ambulatory polysomnographic system. Journal of Geriatric Psychiatry and Neurology, 24, 50–59. doi: 10.1177/0891988710397548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonareva I., & Oken B. S (2014). Physiological and functional consequences of caregiving for relatives with dementia. International Psychogeriatrics, 26, 725–747. doi: 10.1017/S1041610214000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman L., Lyons J. G., Cauley J. A., Hochberg M., & Applebaum K. M (2015). The relationship between caregiving and mortality after accounting for time-varying caregiver status and addressing the healthy caregiver hypothesis. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 70, 1163–1168. doi: 10.1093/gerona/glv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J. P., Glaser R., Malarkey W. B., Beversdorf D., & Kiecolt-Glaser J (2012). Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology, 31, 264–268. doi: 10.1037/a0025536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. E., Robles T. F., Kiecolt-Glaser J. K., Malarkey W. B., Bissell M. G., & Glaser R (2006). Hostility and pain are related to inflammation in older adults. Brain, Behavior, and Immunity, 20, 389–400. doi: 10.1016/j.bbi.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Irwin M., Hauger R., Patterson T. L., Semple S., Ziegler M., & Grant I (1997). Alzheimer caregiver stress: Basal natural killer cell activity, pituitary-adrenal cortical function, and sympathetic tone. Annals of Behavioral Medicine, 19, 83–90. doi: 10.1007/BF02883324 [DOI] [PubMed] [Google Scholar]
- Kang S., & Marks N. F (2014). Filial caregiving is associated with greater neuroendocrine dysfunction: Evidence from the 2005 National Survey of Midlife in the U.S. SAGE Open Medicine, 2, 1–10. doi: 10.1177/2050312113520152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Glaser R., Shuttleworth E. C., Dyer C. S., Ogrocki P., & Speicher C. E (1987). Chronic stress and immunity in family caregivers of Alzheimer’s disease victims. Psychosomatic Medicine, 49, 523–535. doi: 10.1097/00006842-198709000-00008 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Gouin J. P., Weng N. P., Malarkey W. B., Beversdorf D. Q., & Glaser R (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine, 73, 16–22. doi: 10.1097/PSY.0b013e31820573b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Marucha P. T., Malarkey W. B., Mercado A. M., & Glaser R (1995). Slowing of wound healing by psychological stress. The Lancet, 346, 1194–1196. doi: 10.1016/s0140-6736(95)92899-5 [DOI] [PubMed] [Google Scholar]
- Kim S., & Ferraro K. F (2014). Do productive activities reduce inflammation in later life? Multiple roles, frequency of activities, and C-reactive protein. The Gerontologist, 54, 830–839. doi: 10.1093/geront/gnt090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell B., & Wetherell M. A (2011). The cost of caregiving: Endocrine and immune implications in elderly and non-elderly caregivers. Neuroscience and Biobehavioral Reviews, 35, 1342–1352. doi: 10.1016/j.neubiorev.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Lutgendorf S. K., Garand L., Buckwalter K. C., Reimer T. T., Hong S. Y., & Lubaroff D. M (1999). Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 54, M434–M439. doi: 10.1093/gerona/54.9.m434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Chen E., Sze J., Marin T., Arevalo J. M., Doll R., … Cole S. W (2008). A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biological Psychiatry, 64, 266–272. doi: 10.1016/j.biopsych.2008.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Murphy M. L., Cashman R., Ma R., Ma J., Arevalo J. M., … Cole S. W (2014). Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain, Behavior, and Immunity, 41, 191–199. doi: 10.1016/j.bbi.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills P. J., Ziegler M. G., Patterson T., Dimsdale J. E., Hauger R., Irwin M., & Grant I (1997). Plasma catecholamine and lymphocyte beta 2-adrenergic receptor alterations in elderly Alzheimer caregivers under stress. Psychosomatic Medicine, 59, 251–256. doi: 10.1097/00006842-1997/05000-00008 [DOI] [PubMed] [Google Scholar]
- National Alliance for Caregiving and the AARP (2015). 2015 report: Caregiving in the U.S. NAC and AARP Public Policy Institute; Retrieved from http://www.caregiving.org/wp-content/uploads/2015/05/2015_CaregivingintheUS_Final-Report-June-4_WEB.pdf (accessed January 31, 2019). [Google Scholar]
- New York Times (2016). Love and burnout: Caregivers, too, need care Retrieved from https://www.nytimes.com/2016/09/03/your-money/caregivers-alzheimers-burnout.html
- Office of Women’s Health (2018). Caregiver Health. Retrieved from https://www.womenshealth.gov/a-z-topics/caregiver-stress
- Ory M. G., Hoffman R. R. III, Yee J. L., Tennstedt S., & Schulz R (1999). Prevalence and impact of caregiving: A detailed comparison between dementia and nondementia caregivers. The Gerontologist, 39, 177–185. doi: 10.1093/geront/39.2.177 [DOI] [PubMed] [Google Scholar]
- O’Reilly D., Connolly S., Rosato M., & Patterson C (2008). Is caring associated with an increased risk of mortality? A longitudinal study. Social Science & Medicine, 67, 1282–1290. doi: 10.1016/j.socscimed.2008.06.025 [DOI] [PubMed] [Google Scholar]
- O’Reilly D., Rosato M., Ferry F., Moriarty J., & Leavy G (2017). Caregiving, volunteering or both? Comparing effects on health and mortality using census-based records from almost 250,000 people aged 65 and over. Age and Ageing, 46, 821–826. doi: 10.1093/ageing/afx017 [DOI] [PubMed] [Google Scholar]
- O’Reilly D., Rosato M., & Maguire A (2015). Caregiving reduces mortality risk for most caregivers: A census-based record linkage study. International Journal of Epidemiology, 44, 1959–1969. doi: 10.1093/ije/dyv172 [DOI] [PubMed] [Google Scholar]
- Pinquart M., & Sörensen S (2003). Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging, 18, 250–267. doi: 10.1037/0882-7974.18.2.250 [DOI] [PubMed] [Google Scholar]
- Pinquart M., & Sörensen S (2006). Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: A meta-analysis. The Gerontologist, 45, 90–106. doi: 10.1093/geront/45.1.90 [DOI] [PubMed] [Google Scholar]
- Potier F., Degryse J. M., & de Saint-Hubert M (2018). Impact of caregiving for older people and pro-inflammatory biomarkers among caregivers: A systematic review. Aging Clinical and Experimental Research, 30, 119–132. doi: 10.1007/s40520-017-0765-0 [DOI] [PubMed] [Google Scholar]
- Poulin M. J., Brown S. L., Dillard A. J., & Smith D. M (2013). Giving to others and the association between stress and mortality. American Journal of Public Health, 103, 1649–1655. doi: 10.2105/AJPH.2012.300876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provinciali M., Moresi R., Muzzioli M., Tarabelli D., Sirolla C., Melchiorre M. G., … Lamura G (2004). Psychological, neuroendocrine and immune measures in nonspousal carers of disabled elderly in Italy. Neuro Endocrinology Letters, 25, 391–396. [PubMed] [Google Scholar]
- Pruchno R. A., Brill J. E., Shands Y., Gordon J. R., Genderson M. W., Rose M., & Cartwright F (2008). Convenience samples and caregiving research: How generalizable are the findings? The Gerontologist, 48, 820–827. doi: 10.1093/geront/48.6.820 [DOI] [PubMed] [Google Scholar]
- Qualls S. H. (2016). Caregiving families within the long-term services and support system for older adults. The American Psychologist, 71, 283–293. doi: 10.1037/a0040252 [DOI] [PubMed] [Google Scholar]
- Ramsay S., Grundy E., & O’Reilly D (2013). The relationship between informal caregiving and mortality: An analysis using the ONS Longitudinal Study of England and Wales. Journal of Epidemiology and Community Health, 67, 655–660. doi: 10.1136/jech-2012-202237 [DOI] [PubMed] [Google Scholar]
- Reese D. R., Gross A. M., Smalley D. L., & Messer S. C (1994). Caregivers of Alzheimer’s disease and stroke patients: Immunological and psychological considerations. The Gerontologist, 34, 534–540. doi: 10.1093/geront/34.4.534 [DOI] [PubMed] [Google Scholar]
- Rohleder N., Marin T. J., Ma R., & Miller G. E (2009). Biologic cost of caring for a cancer patient: Dysregulation of pro- and anti-inflammatory signaling pathways. Journal of Clinical Oncology, 27, 2909–2915. doi: 10.1200/JCO.2008.18.7435 [DOI] [PubMed] [Google Scholar]
- Roth D. L., Brown S. L., Rhodes J. D., & Haley W. E (2018). Reduced mortality rates among caregivers: Does family caregiving provide a stress-buffering effect? Psychology and Aging, 33, 619–629. doi: 10.1037/pag0000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. L., Fredman L., & Haley W. E (2015). Informal caregiving and its impact on health: A reappraisal from population-based studies. The Gerontologist, 55, 309–319. doi: 10.1093/geront/gnu177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. L., Haley W. E., Hovater M., Perkins M., Wadley V. G., & Judd S (2013). Family caregiving and all-cause mortality: Findings from a population-based propensity-matched analysis. American Journal of Epidemiology, 178, 1571–1578. doi: 10.1093/aje/kwt225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. L., Perkins M., Wadley V. G., Temple E. M., & Haley W. E (2009). Family caregiving and emotional strain: Associations with quality of life in a large national sample of middle-aged and older adults. Quality of Life Research, 18, 679–688. doi: 10.1007/s11136-009-9482-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan J. M., Vitaliano P. P., Ochs H., Savage M. V., & Borson S (1998). CD4 and CD8 counts are associated with interactions of gender and psychosocial stress. Psychosomatic Medicine, 60, 644–653. doi: 10.1097/00006842-199809000-00023 [DOI] [PubMed] [Google Scholar]
- Schulz R., & Beach S. R (1999). Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA, 282, 2215–2219. doi: 10.1001/jama.282.23.2215 [DOI] [PubMed] [Google Scholar]
- Segerstrom S. C., Schipper L. J., & Greenberg R. N (2008). Caregiving, repetitive thought, and immune response to vaccination in older adults. Brain, Behavior, and Immunity, 22, 744–752. doi: 10.1016/j.bbi.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivpuri S., Gallo L. C., Crouse J. R., & Allison M. A (2012). The association between chronic stress type and C-reactive protein in the multi-ethnic study of atherosclerosis: Does gender make a difference? Journal of Behavioral Medicine, 35, 74–85. doi: 10.1007/s10865-011-9345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell J. R., Kiecolt-Glaser J. K., & Glaser R (2001). Perceived stress and cellular immunity: When coping counts. Journal of Behavioral Medicine, 24, 323–339. doi:10.1023/A:1010630801589 [DOI] [PubMed] [Google Scholar]
- Vedhara K., McDermott M. P., Evans T. G., Treanor J. J., Plummer S., Tallon D., … Schifitto G (2002). Chronic stress in nonelderly caregivers: Psychological, endocrine and immune implications. Journal of Psychosomatic Research, 53, 1153–1161. doi: 10.1016/S0022-3999(02)00343-4 [DOI] [PubMed] [Google Scholar]
- Vitaliano P. P., Persson R., Kiyak A., Saini H., & Echeverria D (2005). Caregiving and gingival symptom reports: Psychophysiologic mediators. Psychosomatic Medicine, 67, 930–938. doi: 10.1097/01.psy.0000188485.65153.7b [DOI] [PubMed] [Google Scholar]
- Vitaliano P. P., Scanlan J. M., Ochs H. D., Syrjala K., Siegler I. C., & Snyder E. A (1998). Psychosocial stress moderates the relationship of cancer history with natural killer cell activity. Annals of Behavioral Medicine, 20, 199–208. doi: 10.1007/BF02884961 [DOI] [PubMed] [Google Scholar]
- Vitaliano P. P., Zhang J., & Scanlan J. M (2003). Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological Bulletin, 129, 946–972. doi: 10.1037/0033-2909.129.6.946 [DOI] [PubMed] [Google Scholar]
- von Kanel R., Dimsdale J. E., Mills P. J., Ancoli-Israel S., Patterson T. L., Mausbach B. T., & Grant I (2006). Effect of Alzheimer caregiving stress and age on frailty markers interleukin-6, C-reactive protein, and D-dimer. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 61, 963–969. doi: 10.1093/gerona/61.9.963 [DOI] [PubMed] [Google Scholar]
- von Kanel R., Mausbach B. T., Dimsdale J. E., Mills P. J., Patterson T. L., Ancoli-Israel S., … Grant I (2012). Ways of coping and biomarkers of an increased atherothrombotic cardiovascular disease risk in elderly individuals. Cardiovascular Psychiatry and Neurology, 2012, 875876. doi: 10.1155/2012/875876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R., Mills P. J., Mausbach B. T., Dimsdale J. E., Patterson T. L., Ziegler M. G., … Grant I (2012). Effect of Alzheimer caregiving on circulating levels of C-reactive protein and other biomarkers relevant to cardiovascular disease risk: A longitudinal study. Gerontology, 58, 354–365. doi: 10.1159/000334219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. Y., Wong C. K., Chan F. W., Chan P. K., Ngai K., Mercer S., & Woo J (2013). Chronic psychosocial stress: Does it modulate immunity to the influenza vaccine in Hong Kong Chinese elderly caregivers? Age, 35, 1479–1493. doi: 10.1007/s11357-012-9449-z [DOI] [PMC free article] [PubMed] [Google Scholar]