Abstract
The norepinephrine transporter (NET) has been suggested to play a critical role in attention-deficit/hyperactivity disorder (ADHD). In this prospective controlled study we tested the a-priori-hypothesis that central NET availability is altered in adult ADHD patients compared to healthy controls. Study participants underwent single positron emission tomography-magnetic resonance imaging (PET-MRI). MRI sequences included high resolution T1-MPRAGE data for regions of interest (ROI) delineation and voxel-based morphometry (VBM) and T2-weighted fluid-attenuated inversion-recovery for detection and exclusion of pathological abnormalities. NET availability was assessed by NET-selective (S,S)-O-[11C]methylreboxetine; regional distribution volume ratios (DVR) were calculated based on individual PET-MRI data co-registration and a multi-linear reference tissue model with two constraints (MRTM2; reference region: occipital cortex). VBM analysis revealed no difference in local distribution of gray matter between the 20 ADHD patients (9 females, age 31.8 ± 7.9 years, 488 ± 8 MBq injected activity) and the 20 age-matched and sex-matched control participants (9 females, age 32.3 ± 7.9 years, 472 ± 72 MBq). In mixed-model repeated-measures analysis with NET availability as dependent and ROI as repeated measure we found a significant main effect group in fronto-parietal-thalamic-cerebellar regions (regions on the right: F1,25 = 12.30, p = .002; regions on the left: F1,41 = 6.80, p = .013) indicating a reduced NET availability in ADHD patients. None of the other investigated brain regions yielded significant differences in NET availability between groups after applying a Benjamini-Hochberg correction at a significance level of 0.05. Overall our findings demonstrate the pathophysiological involvement of NET availability in adult ADHD.
Subject terms: ADHD, Physiology
Introduction
Adult attention-deficit/hyperactivity disorder (ADHD) is an underrecognized chronic disorder with childhood-onset characterized by attention deficit, impulsivity and hyperactivity. The worldwide prevalence of ADHD is estimated to be 2.8%1, in Germany as high as 4.7%2. Moreover, a mortality rate of 5.8% has been found in ADHD, highest in individuals diagnosed in adulthood and mainly driven by deaths from unnatural causes, e.g., accidents3. ADHD is often accompanied by other comorbidities, such as depression, anxiety and conduct disorder4. ADHD symptoms have a detrimental impact on social, financial and professional functioning5—the risk of losing one’s job for example is three times higher in ADHD. Not surprisingly, the economic impact of adult ADHD places a significant burden on society. The gold standard treatment is pharmacotherapy, but 30% of adult patients with ADHD do not respond to medication6,7.
Patients with ADHD show executive functioning deficits, impaired attention and behavioral control, relating to brain areas modulated by noradrenergic transmission8,9 and norepinephrine transporter (NET) expression. Animal models of ADHD demonstrate the involvement of the central locus-coeruleus norepinephrine (LC-NE) system in behavioral control and the attentive process10,11. Drugs modulating NE transmission and targeting NET, such as atomoxetine or methylphenidate, are effective in ADHD patients6 and have been shown to significantly occupy NET in vivo at clinically relevant doses12,13. There is ample and clear evidence that the LC-NE system is involved in regulating wakefulness and arousal14,15 shown to be reduced in patients with adult ADHD16–18.
While NE is released throughout the entire mammalian brain, evidence from structural and functional neuroimaging studies suggests that there are right hemispheric deficits in ADHD, specifically reduced overall cerebral volume including fronto-parietal areas, basal nuclei, globus pallidus and cerebellum19. Moreover, studies show functional abnormalities in two distinct domain-dissociated networks: (1) the fronto-parietal-thalamic-cerebellar network for attention and (2) the fronto-basal-ganglia network for inhibition20–22. The normalizing effect of methylphenidate and atomoxetine on the right fronto-parietal-thalamic activation during sustained attention23, known to be challenging for patients with ADHD24, corroborates the validity of the first model, further supported by the benefits of stimulation of the right dorsolateral frontal cortex on symptoms of inattention in patients with adult ADHD25. Concerning the second model, evidence from lesion and imaging studies suggest a direct pathway from the right inferior frontal cortex to the basal ganglia26,27 in stopping tasks. Importantly, dysfunctions of the prefrontal-striatal systems are implied in ADHD28–31 and basal ganglia are areas of NET expression, albeit less dense than in the LC32,33.
So far, changes in NET availability have not been described in adults with ADHD. One study exploring NET binding potential using radioligand (S,S)-[18F]FMeNER-D233 in selected regions of interest (ROI) did not yield any significant differences between adults ADHD patients and healthy controls. However, using NET-selective (S,S)-O-[11C]methylreboxetine, a previous study found associations between central NET availability and trait impulsivity in twenty healthy individuals34.
In the present study we tested the hypothesis that central NET availability is altered in adult patients with ADHD compared to healthy control subjects. We further explored whether NET availability is associated with ADHD symptom severity, neuropsychological and neurophysiological measures. To evaluate differences in NET availability, PET and NET-selective (S,S)-O-[11C]methylreboxetine (MRB) was applied in unmedicated adult patients with ADHD and age-matched and sex-matched healthy controls. We applied a ROI approach, focusing on areas known to be functionally relevant for ADHD that are linked to the LC-NE system.
Materials/subjects and methods
Study participants
The study was conducted in accordance with the ICH Guideline for Good Clinical Practice (GCP) and the declaration of Helsinki, and was approved by the local ethics committee (EC number 155/15-ff) and the German Bundesamt für Strahlenschutz/Federal Office for Radiation Protection (number Z 5–22461 2-2016-008). Between February and December 2018, outpatients (age ≥ 18) meeting DSM-V criteria for ADHD based on mental status examination, semi-structured Diagnostic Interview for ADHD in adults (DIVA 2.035, adult ADHD Self-Report Scale Symptom Checklist (ASRS 1.136), German version of the Wender-Utah-Rating-Scale (WURS-k37 ≥ 30; or known ADHD diagnosis in childhood) were consecutively recruited from the Adult ADHD outpatient center of the Department of Psychiatry and Psychotherapy of Leipzig University. Age- and sex-matched healthy control participants were recruited from the local community through advertisements. Patients had to be free of any psychotropic medication (ADHD-specific medication, antidepressants, sedatives, z-hypnotics, neuroleptics) and drug use for at least one month prior to the first screening visit; controls had to be naïve to psychotropic medication and had to be free of any drug use for at least one month prior to the first screening visit. Healthy controls received financial reimbursement.
Written informed consent was obtained from all participants at the beginning of the first screening visit after ensuring that all participants were able to understand the study protocol. To evaluate the general health of all participants, physical and neurologic status and routine laboratory tests were performed. Patients with serious psychiatric (acute severe depressive episode, suicidality, psychotic symptoms, bipolar disorder, schizophrenia, psychosis), neurological (history of brain surgery, significant brain malformation or neoplasm, head injury, stroke, epilepsy, neurodegenerative disorder), and/or severe somatic comorbidities were excluded. Any contraindication to MRI (e.g., heart or brain pacemaker, metal implants in the head/neck) and pregnancy also lead to patients’ exclusion. A multi-drug urine test was performed at the first screening. Women underwent a urine pregnancy test before the PET-MRI measurement.
Image acquisition, image data processing, and quantification
PET-MRI data were acquired on a hybrid PET-MRI device (Biograph mMR, Siemens, Erlangen) and processed as previously described38. MRI sequences included high resolution T1-MPRAGE data for ROI delineation, and voxel-based morphometry (VBM) and T2-weighted fluid-attenuated inversion-recovery (FLAIR) for detection and exclusion of pathological abnormalities. Dynamic PET imaging was performed after intravenous bolus injection (90 s) of 480 ± 51 MBq [11C]MRB for 90 min (23 frames: 4 × 15 s, 4 × 60 s, 5 × 120 s, 5 × 300 s, 5 × 600 s). For attenuation correction we applied the MR-based μ-map estimation by Catana et al.39. Using PMOD (version 3.5, PMOD Technologies, Zurich, Switzerland), PET data motion correction was performed, as well as the generation of the regional DVR maps. Accordingly, we applied the multi-linear reference tissue model with 2 constraints (MRTM240) using a fixed clearance rate of k2’ = 0.0238 min−1 and the occipital cortex serving as the reference region. We used PMOD’s Neuro module for automatic individual ROI delineation of the merged Automated Anatomical Labeling (AAL41) atlas. For the Harvard Ascending Arousal Network (AAN42) probability atlas we calculated the inverse transformation matrix of the spatial normalization to the MNI-space based on the T1-MPRAGE in SPM12 (Wellcome Centre for Human Neuroimaging University College London https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and applied the inverse matrix to the AAN atlas to transfer the atlas to the participant’s individual space.
For VBM analysis we used the CAT12 (http://www.neuro.uni-jena.de/cat/) running in SPM12. In short, individual T1 data were spatially normalized, segmented in gray matter, white matter and cerebrospinal fluid and smoothed using a Gaussian filter with 8 mm full-width half-maximum filter size.
Questionnaires
The DSM-IV subscales of the Conners’ Adult ADHD Rating Scale Self-Report (CAARS-SR–long version43,44) were applied at the screening visit to assess ADHD symptom severity. The Structured Clinical Interview for DSM-IV Axis I and Axis II disorders45,46 was performed to exclude comorbid psychiatric disorders. IQ was determined with vocabulary-based IQ screening (Wortschatztest, WST47). Substance use was evaluated with Alcohol Use Disorders Identification Test48 and Drug Use Disorders Identification Test49. The Beck Depression Inventory-II50 (BDI) was applied to assess depressive symptom severity.
Neuropsychological measures
The sustained attention subtest of the computerized test of attentional performance (TAP51) was used to assess attention and behavioral control at the screening visit. In this 15-min test (3 × 5-min blocks), a sequence of stimuli ranging in color, shape, size and filling, is presented on the monitor. A target stimulus occurs whenever it corresponds in shape with the preceding stimulus (e.g., the same shape but with different color, size and filling). Participants were instructed to press a button as quickly as possible when the target stimulus appeared. The probability for the appearance of the target-stimuli was 12 percent (450 trials, 54 targets, stimulus onset asynchrony: 2 s). Errors of omission were defined as number of targets that were presented but not responded to and were used as measure of sustained attention. Errors of commissions were defined as number of non-targets that were responded to and used as measure of behavioral control (inhibition/impulsivity).
Neurophysiological EEG measures
We utilized standardized low-resolution brain electromagnetic tomography (sLORETA) to estimate the prefrontal current density of the theta band (6.5–8.0 Hz). To calculate the current source density of prefrontal EEG theta we recorded EEG signals at the screening visit with Ag/AgCl electrodes using a QuickAmp amplifier (Brain Product GmbH, Gilching, Germany) from 31 electrode positions according to 10–20 system under a 15-min resting condition. Impedance of each electrode was kept below 10kΩ. The raw EEG was firstly down sampled to 256 Hz and then a band pass filtering between 0.1 and 100 Hz was performed. For all participants, the first 5 minutes of entire EEG was segmented and imported into sLORETA, to analyze the cortical three-dimensional distribution of scalp current density52. Each EEG was processed via a sLORETA cross-spectrum analysis; the software then calculated, based on the cross-spectrum data for the theta band (6.5–8.0 Hz), the current source values for each voxel. The current density values in the right and left dorsolateral prefrontal cortices (indexed by Brodmann Area 9; BA9) were averaged over all voxels within this ROI.
Statistical analysis
To investigate whether there are significant differences between ADHD patients and healthy controls regarding demographic variables and clinical variables Chi-square tests, t-tests for independent sample comparison, or Mann–Whitney U tests (dependent on scale of measurement and normality of data) were performed to identify relevant covariates. To determine whether the NET availability is different between ADHD patients and healthy controls mixed-model repeated-measures (MMRM) analyses were applied with NET availability as outcome and ROI as repeated factor, and an unstructured covariance matrix. Models were implemented with restricted maximum likelihood algorithm. Fixed class effects of group (patients versus controls), ROIs, the interaction of group and ROI, BDI score as covariate, as well as random effects for subjects, matched participants and intercept were included. Separate models for attention—(including superior frontal gyrus, precuneus, angular and supramarginal gyri, cerebellum with crus and thalamus) and behavioral control-related (including inferior frontal gyrus, anterior cingulate, supplementary motor area, nucleus caudate, putamen, pallidum) ROIs based on the AAL probability atlas were calculated for the right and left side, as well as a model for LC and Raphe Nuclei based on the AAN probability atlas. The statistical significance of the main effect group or the interaction group x ROI was utilized to determine whether the NET availabilities in selected ROIs are different across the groups. In cases of significant main effects or interactions, Spearman correlation analyses between the mean DVR of respective ROIs and clinical (T-scores of CAARS DSM-IV subscales inattention and hyperactivity/impulsivity), neuropsychological (T-scores of numbers of omission and commission errors during the 15-min sustained attention test) and neurophysiological measures (EEG theta current source density of BA9) were conducted; in case of significant findings, group was included as covariate to estimate the effect of group. All statistical analyses were computed using SPSS, version 24.0 for Windows (IBM Corp., Armonk, NY, USA). The two-tailed significance level was set at p = .05. The corrected significance level for multiple tests according to Benjamini and Hochberg53 was p = .014.
We used VBM to investigate differences in local distribution of gray matter between ADHD patients and age-matched and sex-matched healthy control participants. The modulated and spatially normalized gray matter images were fed into SPM12, where we applied a two-sample t-test with total intracranial volume as a confound to correct for different brain sizes. All clusters with a combined threshold of p < .001 uncorrected at peak level and p < .05 family-wise error corrected at cluster level were considered as significantly different.
Results
Description of sample
The final study sample consisted of 20 adult patients with ADHD (11 males, age 31.8 ± 7.9 years, 488 ± 8 MBq injected activity) and 20 age- and sex-matched healthy controls (11 males, age 32.3 ± 7.9 years, 472 ± 72 MBq). Sample characteristics are presented in Table 1. Groups did not differ in basic demographic and biological characteristics (all: p > .10), however, a significant between-group difference in BDI sum scores was found (p < .0001). Thus, we decided to integrate BDI sum score in multivariate analysis.
Table 1.
Demographic and clinical characteristics
| ADHD (n = 20) | Control (n = 20) | All (n = 40) | p-value | |
|---|---|---|---|---|
| Characteristic | ||||
| Number of males | 11 (55%) | 11 (55%) | 22 (55%) | 1.000 |
| Age (years) | 31.8 (7.9) 22–49 | 32.3 (7.9) 20–52 | 32.1 (7.8) 20–52 | .843 |
| Weight (kg) | 80.4 (14.0) 49–107.5 | 79.4 (11.16) 58–98 | 79.9 (12.5) | .373 |
| Height (cm) | 174.8 (8.1) 156–187 | 175.3 (10.3) 156–196 | 175.1 (0.9) | .213 |
| Smoking status, yes/no | 7/13 (35/65%) | 6/14 (30/70%) | 13/27 (32.5/67.5%) | .736 |
| BMI (kg/m2) | 26.2 (3.7) 19.5–32.7 | 25.7 (2.0) 21.9–28.9 | 26.0 (3.0) | .610 |
| Handedness | .198 | |||
| Right handed | 17 (85%) | 20 (100%) | 37 (92.5%) | |
| Left handed | 2 (10%) | 0 (0%) | 2 (5%) | |
| Ambidextrous | 1 (5%) | 0 (0%) | 1 (2.5%) | |
| Beck depression inventory, sum score | 9.9 (11.3) 0–40 | 1.4 (2.5) 0–9 | 5.7 (9.2) | <.0001 |
| WST_IQ | 107.3 (9.4) 95–133 | 106.9 (8.1) 92–122 | 107.1 (8.7) | .889 |
| WURS-K, sum score | 42.3 (9.5) 25–60 | 9.6 (8.5) 0–24 | 25.9 (18.8) | <.0001 |
| CAARS DSM-IV Inattention, T-score | 84.4 (6.7) 70–90 | 44.5 (8.6) 36–66 | 64.4 (21.6) | <.0001 |
| CAARS DSM-IV hyperactivity/ impulsivity, T-score | 74.8 (12.1) 54–90 | 43.1 (5.4) 35–54 | 58.9 (18.5) | <.0001 |
Annotations: Entries are means (±standard deviations) or numbers (%) and range
BMI body mass index, WST Wortschatztest; WURS-k Wender Utah rating scale, CAARS Conners’ Adult ADHD rating scales
Description of distribution volume ratios DVRs
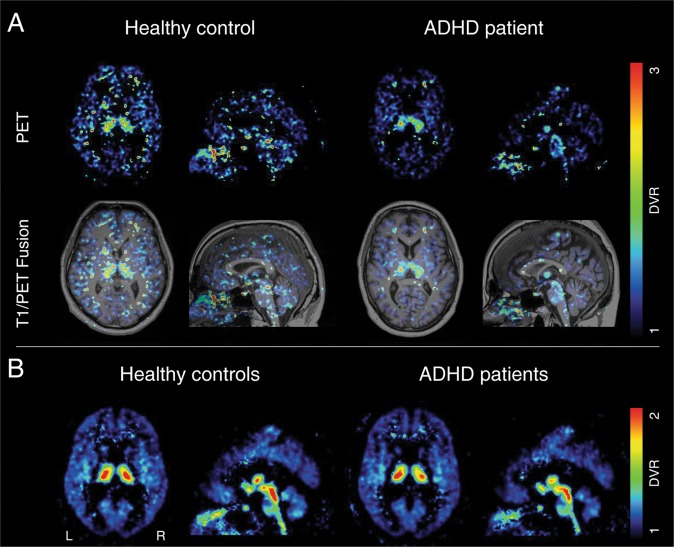
Examples of parametric images of the DVRs in axial and sagittal view, together with their corresponding T1-MPRAGE images, are presented in Fig. 1. In general, most of the investigated brain regions showed a tendency towards lower values in adult ADHD patients compared with healthy controls. NET-selective MRB density in ADHD and healthy controls was highest in the ascending arousal system (i.e., dorsal raphe > median raphe > LC) and the thalamus (please cf. Figs. 1 and 2).
Fig. 1. Parametric images of the distribution volume ratio (DVR) in axial and sagittal view after spatial normalization to the MNI-space.
a An exemplary healthy control and ADHD together with their corresponding T1-MPRAGE image. b Group mean of all healthy controls and ADHD patients. ADHD attention-deficit/hyperactivity disorder, MNI Montreal neurological institute, R right, L left.
Fig. 2. Distribution, mean and error bars of DVR values in selected regions of interest for both, healthy controls (cyan) and ADHD patients (red).
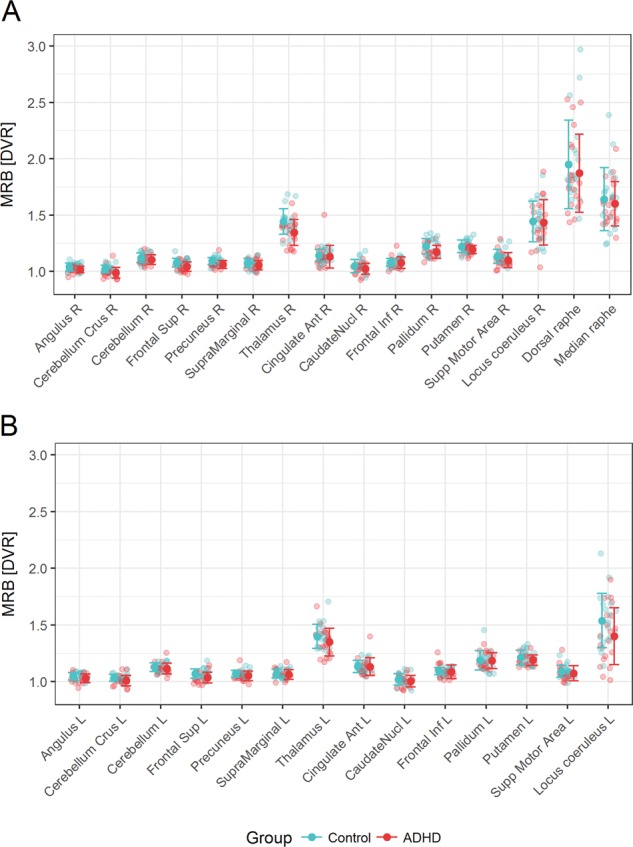
a Right hemisphere. b Left hemisphere. ADHD attention-deficit/hyperactivity disorder, DVR distribution volume ratio, R right, L left.
VBM analysis
We found no difference in local distribution of gray matter between ADHD patients and control participants.
NET availability ADHD patients vs. healthy controls
Ascending arousal system
MMRM analysis using AAN atlas-based ROIs of the ascending reticular activating system (left, right LC; dorsal, median raphe nuclei) indicated a significant main effect of ROI (F3,38 = 10.72; p < .001) and a significant interaction of group x ROI (F3,38 = 3.38; p = .028) which did not survive adjustment for multiple tests. No main effects were obtained for group (F1,39 = 0.91; p = .347) or BDI (F1,37 = 0.41; p = .525).
Attention-related ROIs
In the right fronto-parietal-thalamic-cerebellar ROIs, MMRM analysis revealed a main effect group (F1,25 = 12.30, p = .002), a main effect of ROI (F6,30 = 143.48; p < .001), but no main effect of BDI (F1,21 = 1.41; p = .245) or group × ROI interaction (F6,30 = 1.74; p = .154). When analyzing an analogous model for the left side, a main effect of ROI (F6,38 = 118.77; p < .001) and a main effect group emerged (F1,41 = 6.80, p = .013), but no main effect of BDI (F1,37 = 1.29; p = .264) or group x ROI interaction (F6,38 = 1.46; p = .217).
Behavioral control-related ROIs
In the right fronto-basal-ganglia ROIs, MMRM analysis revealed a statistical trend for main effect group (F1,41 = 3.09, p = .086) and group × ROI interaction (F5,38 = 2.14; p = .081), a main effect of ROI (F5,38 = 138.31; p < .001), but no main effect of BDI (F1,37 = 0.03; p = .868). The analogous model for the left side yielded a main effect of ROI (F5,30 = 104.18; p < .001) but no significant main effect group, main effect BDI or group × ROI interaction (.559 ≤ p ≤ .706).
Exploratory correlation analysis
Mean DVR in attention-related ROIs and ADHD symptom severity
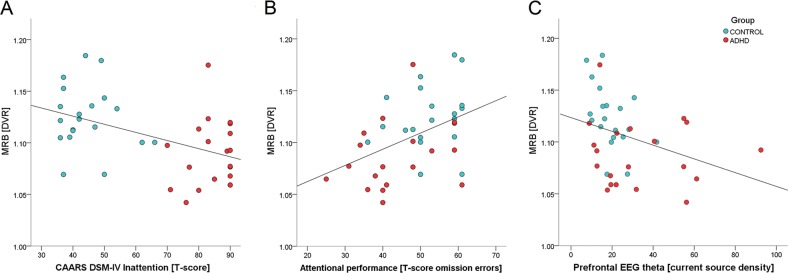
T-scores of CAARS DSM-IV subscales inattention and hyperactivity/impulsivity are presented in Table 1. Correlation analysis revealed a significant negative association between mean DVR in the right fronto-parietal-thalamic-cerebellar ROIs and ADHD symptom severity (inattention: rho = −0.46; p = .003; impulsivity/hyperactivity: rho = −0.40; p = .011); participants with lower NET availability scored higher on the CAARS DSM-IV scores, indicating higher symptom severity. The correlation coefficients decreased to −0.01, and 0.10, respectively, when group was included as covariate, which indicated a medium to large size effect of group. Analogous analysis for the left side did not yield any significant findings (inattention: rho = −0.29; p = .068; impulsivity/hyperactivity: rho = −0.34; p = .034) after adjustment for multiple tests.
Mean DVR in attention-related ROIs and neuropsychological measures
T-scores of omission and commission errors during the 15-min sustained attention test were calculated (please cf. Fig. 3). Correlation analysis revealed a significant association between mean DVR in right fronto-parietal-thalamic-cerebellar ROIs and omission errors (rho = 0.43; p = .007); participants with lower NET availability had worse performance (=more omission errors) in the sustained attention test.
Fig. 3. The sustained attention subtest of the computerized test of attentional performance (TAP, Zimmermann and Fimm, 2012).
a Experimental design. Stimulus onset asynchrony = 2 s. b Mean omission errors during the 15-min test in adult ADHD patients (n = 20), and age- and sex-matched healthy controls (n = 20). c Mean commission errors during the 15-min test in adult ADHD patients (n = 20) and healthy controls (n = 20).
The correlation coefficient decreased to 0.28 when group was included as covariate, indicating that the effect of group was small. No significant correlations were obtained for mean DVR and commission errors (rho = 0.06; p = .732). Analogous analysis for the left side did not survive the correction for multiple tests (omission: rho = 0.35; p = .027) and was not significant regarding commission errors (rho = 0.02; p = .885).
Mean DVR in attention-related ROIs and neurophysiological measures
The current source density values of resting EEG theta in the right and left dorsolateral prefrontal cortices (indexed by current source density of right/left BA9) were calculated. Correlation analysis revealed a significant negative association between mean DVR in right fronto-parietal-thalamic-cerebellar ROIs and theta current density (rho = −0.41; p = .009) indicating that participants with lower NET availability had greater theta current density in the right BA9 (please cf. Fig. 4c). The correlation coefficient decreased to −0.32 when group was included as covariate, indicating that the effect of group was small. Analogous analysis for the left side did not survive the correction for multiple tests (rho = −0.31; p = .051).
Fig. 4. Scatterplots of mean distributed volume ratios (DVR) in right fronto-parietal-thalamic-cerebellar regions of interest versus measures of inattention and EEG extracted theta current density.
a Inattention assessed with the DSM-IV subscale inattention (total T-score) of the Conners’ Adult ADHD Rating Scale Self-Report (CAARS-SR–long version). b Inattention assessed with the 15-min subtest of the computerized test of attentional performance (TAP; Zimmermann and Fimm, 2012). T-scores of omission errors (number of targets that were presented but not responded to) were used as a measure of sustained attention. c Mean absolute right BA9 theta (6.5–8.0 Hz) current density. Red circles: adult ADHD patients (n = 20); cyan circles: sex-matched and age-matched healthy controls (n = 20). BA Brodmann area.
Discussion
Our main goal was to test the hypothesis that central NET availability is altered in unmedicated adult patients with ADHD compared to age- and sex-matched healthy control subjects. We further explored whether NET availability is associated with ADHD symptom severity or neuropsychological and neurophysiological measures. We obtained several noteworthy findings. In line with our hypothesis, we demonstrated that patients with adult ADHD have decreased NET availability in relevant ROIs for attention, more pronounced in the right hemisphere, in comparison to healthy controls. In addition, in exploratory analyses we showed that lower NET availability in right fronto-parietal-thalamic-cerebellar ROIs is associated with more omission (but not commission) errors in a sustained attention test and greater EEG theta current density in the right dorsolateral prefrontal cortex.
Importantly, we found no differences in local gray matter distribution, which is in line with the systematic review by de Melo et al.19. The authors analyzed 17 reviews of whom none reported structural changes in adults with ADHD, while changes were reported in ADHD children. Thus, the obtained differences in NET availability between groups cannot be attributed to structural volume changes in our sample of adult ADHD patients, but rather suggest the involvement of the central LC-NE system corroborating the hypothesis of ADHD as a noradrenergic disorder. This is further supported by the fact that the LC–NE system mediates arousal11,15, which is impaired in adult ADHD, as shown in many EEG-studies of brain arousal16,17,54. Moreover, drugs, such as methylphenidate and atomoxetine, that modulate the LC-NE system12, are effective in ADHD, as demonstrated in randomized controlled clinical trials55,56. It is of note that a recent placebo-controlled double-blind fMRI study, testing the comparative neurofunctional effects of methylphenidate and atomoxetine during sustained attention, showed normalization in right fronto-parietal-thalamic areas under methylphenidate, but not under placebo23. In line with these findings, in the current study the associations between NET availability in right fronto-parietal-thalamic-cerebellar regions and neuropsychological and neurophysiological parameters, suggest a pathophysiological role of NET availability in adult ADHD and support the model of right hemisphere deficits19,57 in ADHD. While brain arousal has been understood as a generalized state of the brain58, it is a consistent finding that the ability to maintain an alert state relies heavily on the right cerebral hemisphere14,59,60. Our findings of greater right-frontal theta current density in individuals with lower NET availability in attention-related right-hemispheric regions point to a NET-related origin of this deficit. Interestingly, early lesion studies in rats revealed a right hemisphere bias in the LC-NE system61,62 indicating a top-down regulation of the NE activation by the right frontal cortex since lesions in this area resulted in lower NE levels in both hemispheres and the LC.
However, functional changes in fronto-basal-ganglia regions on the right side have equally been suggested in ADHD, more often in children31, which we could not confirm in the current study of adult ADHD patients. It is conceivable that the fronto-striatal deficits in ADHD children are due to the overall maturation delay31 and no longer present in adult ADHD patients. Our findings also do not replicate the findings of Vanicek et al. who found no significant differences in NET binding potential between ADHD and HC using (S,S)-[18F]FMeNER-D2 between 22 ADHD patients und 22 HC33. Similar to this study, we did not find any differences in NET availability in key regions of the ascending arousal system (i.e., right and left LC). One reason may be that the spatial resolution of the applied method reaches its limitation when quantifying small brain regions such as the LC. As seen in Fig. 2b, the parametric images of the DVRs are able to clearly delineate the hypothalami, while the LC is conflated with the raphe nuclei. In contrast to our approach, the study by Vanicek et al., besides using a different tracer, did not examine any cortical regions, known to be impaired in adult ADHD20 and their analyses did not consider the effect of hemisphere. Therefore, the question of consistency cannot be conclusively clarified given the differences in study design.
Limitations
Some limitations have to be mentioned. The relatively low spatial resolution of PET may have led to a DVR underestimation in the LC. The choice of specific templates (AAL, AAN) may have influenced the results, other parcellation schemes may generate different results, reducing the generalizability of our findings. However, the regional distribution of [11C]MRB in the current study is consistent with the known distribution of NET in the brain and in line with previous studies reporting highest distribution in midbrain raphe, the thalamus and the LC63–65. Despite these limitations, our results warrant further studies to explore how NE/NET modulates resting-state activity (simultaneously measured by means of echo planar MR sequences) and appropriate behavior as assessed by thorough neuropsychological testing.
Conclusion
For the first time, the current study demonstrates decreased NET availability in adult ADHD patients in specific brain areas. Our findings indicate the pathophysiological involvement of NET availability in adult ADHD and warrant further clinical intervention studies targeting these specific areas.
Acknowledgements
This study was funded by the Roland Ernst Foundation, Project 04/17.
Author’s contributions
Drs. C.U. and M.R. contributed equally to this work and served as co–first authors. Dr. C.U. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. M.R. had access to all the PET-MRI data and takes responsibility for the integrity and the accuracy of the modeling of the PET-MRI data. Study concept and design: M.S., S.H., O.S., M.R., C.U. Acquisition, analysis, or interpretation of data: All authors. Drafting of the paper: C.U., J.H. and M.R. Critical revision of the paper for important intellectual content: All authors. Statistical analysis: C.U. and M.R. Obtained funding: M.S. Administrative, technical, or material support: J.H., J.L., A.B., M.P., M.M., S.T. Supervision: M.S., S.H., O.S.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Christine Ulke, Michael Rullmann
References
- 1.Fayyad J, et al. The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. ADHD Atten. Deficit Hyperactivity Disord. 2017;9:47–65. doi: 10.1007/s12402-016-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Zwaan M, et al. The estimated prevalence and correlates of adult ADHD in a German community sample. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262:79–86. doi: 10.1007/s00406-011-0211-9. [DOI] [PubMed] [Google Scholar]
- 3.Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385:2190–2196. doi: 10.1016/S0140-6736(14)61684-6. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am. J. Psychiatry. 2006;163:716–723. doi: 10.1176/ajp.2006.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harpin V, Mazzone L, Raynaud J-P, Kahle J, Hodgkins P. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J. Atten. Disord. 2016;20:295–305. doi: 10.1177/1087054713486516. [DOI] [PubMed] [Google Scholar]
- 6.Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. Med. Gen. Med. 2006;8:4. [PMC free article] [PubMed] [Google Scholar]
- 7.Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad. Med. 2014;126:64–81. doi: 10.3810/pgm.2014.09.2801. [DOI] [PubMed] [Google Scholar]
- 8.Benarroch EE. The locus ceruleus norepinephrine system Functional organization and potential clinical significance. Neurology. 2009;73:1699–1704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- 9.Mueller A, Hong DS, Shepard S, Moore T. Linking ADHD to the neural circuitry of attention. Trends Cogn. Sci. 2017;21:474–488. doi: 10.1016/j.tics.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viggiano D, Ruocco L, Arcieri S, Sadile A. Involvement of norepinephrine in the control of activity and attentive processes in animal models of attention deficit hyperactivity disorder. Neural Plasticity. 2004;11:133–149. doi: 10.1155/NP.2004.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oken B, Salinsky M, Elsas S. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin. Neurophysiol. 2006;117:1885–1901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannestad J, et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol. Psychiatry. 2010;68:854–860. doi: 10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding Y-S, et al. Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: implications on treatment of depression and ADHD. NeuroImage. 2014;86:164–171. doi: 10.1016/j.neuroimage.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Posner MI, Petersen SE. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 15.Berridge CW. Noradrenergic modulation of arousal. Brain Res. Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauß M, et al. Brain arousal regulation in adults with attention-deficit/hyperactivity disorder (ADHD) Psychiatry Res. 2018;261:102–108. doi: 10.1016/j.psychres.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Geissler J, Romanos M, Hegerl U, Hensch T. Hyperactivity and sensation seeking as autoregulatory attempts to stabilize brain arousal in ADHD and mania? Atten. Deficit Hyperactivity Disord. 2014;6:159–173. doi: 10.1007/s12402-014-0144-z. [DOI] [PubMed] [Google Scholar]
- 18.Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin. Neurophysiol. 2003;114:171–183. doi: 10.1016/S1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- 19.Vieira de Melo BB, Trigueiro MJ, Rodrigues PP. Systematic overview of neuroanatomical differences in ADHD: definitive evidence. Dev. Neuropsychol. 2018;43:52–68. doi: 10.1080/87565641.2017.1414821. [DOI] [PubMed] [Google Scholar]
- 20.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 21.Kucyi A, Hove MJ, Biederman J, Van Dijk KR, Valera EM. Disrupted functional connectivity of cerebellar default network areas in attention‐deficit/hyperactivity disorder. Hum. Brain Mapp. 2015;36:3373–3386. doi: 10.1002/hbm.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scolari M, Seidl-Rathkopf KN, Kastner S. Functions of the human frontoparietal attention network: Evidence from neuroimaging. Curr. Opin. Behav. Sci. 2015;1:32–39. doi: 10.1016/j.cobeha.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowalczyk, O. S. et al. Methylphenidate and atomoxetine normalise fronto-parietal activation in ADHD during sustained attention. 7th World Congress on ADHD (Lisbon, 2019). [DOI] [PubMed]
- 24.Shaw TH, Curby TW, Satterfield K, Monfort SS, Ramirez R. Transcranial Doppler sonography reveals sustained attention deficits in young adults diagnosed with ADHD. Exp. Brain Res. 2019;237:511–520. doi: 10.1007/s00221-018-5432-y. [DOI] [PubMed] [Google Scholar]
- 25.Cachoeira CT, et al. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder–A pilot randomized controlled study. Psychiatry Res. 2017;247:28–32. doi: 10.1016/j.psychres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Aron AR, et al. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J. Neurosci. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene DJ, et al. Developmental changes in the organization of functional connections between the basal ganglia and cerebral cortex. J. Neurosci. 2014;34:5842–5854. doi: 10.1523/JNEUROSCI.3069-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellanos FX, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch. Gen. Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 30.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Rubia K, Alegría AA, Brinson H. Brain abnormalities in attention-deficit hyperactivity disorder: a review. Rev. Neurol. 2014;58(Suppl 1):S3–S16. [PubMed] [Google Scholar]
- 32.Schou M, et al. Post-mortem human brain autoradiography of the norepinephrine transporter using (S, S)-[18F] FMeNER-D2. Eur. Neuropsychopharmacol. 2005;15:517–520. doi: 10.1016/j.euroneuro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Vanicek T, et al. The norepinephrine transporter in attention-deficit/hyperactivity disorder investigated with positron emission tomography. JAMA Psychiatry. 2014;71:1340–1349. doi: 10.1001/jamapsychiatry.2014.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hesse S, et al. The association between in vivo central noradrenaline transporter availability and trait impulsivity. Psychiatry Res. 2017;267:9–14. doi: 10.1016/j.pscychresns.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Kooij J. S. Adult ADHD: Diagnostic Assessment and Treatment. (Springer Science and Business Media, 2012).
- 36.Kessler RC, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychological Med. 2005;35:245–256. doi: 10.1017/S0033291704002892. [DOI] [PubMed] [Google Scholar]
- 37.Retz-Junginger P, et al. Wender Utah rating scale. The short-version for the assessment of the attention-deficit hyperactivity disorder in adults. Der Nervenarzt. 2002;73:830–838. doi: 10.1007/s00115-001-1215-x. [DOI] [PubMed] [Google Scholar]
- 38.Hesse S, et al. Central noradrenaline transporter availability in highly obese, non-depressed individuals. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:1056–1064. doi: 10.1007/s00259-016-3590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catana C, et al. Toward implementing an MRI-based PET attenuation-correction method for neurologic studies on the MR-PET brain prototype. J. Nucl. Med. 2010;51:1431–1438. doi: 10.2967/jnumed.109.069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichise M, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J. Cereb. Blood Flow. Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- 41.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 42.Edlow BL, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J. Neuropathol. Exp. Neurol. 2012;71:531–546. doi: 10.1097/NEN.0b013e3182588293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christiansen, H. Conners Skalen zu Aufmerksamkeit und Verhalten für Erwachsene: deutschsprachige Adaption der Conners’ Adult ADHD Rating Scales (CAARS TM) von C. Keith Conners. Drew Erhardt und Elisabeth Sparrow; CAARS. (Huber, 2014).
- 44.Christiansen H, et al. German validation of the Conners Adult ADHD Rating Scales (CAARS) II: reliability, validity, diagnostic sensitivity and specificity. Eur. Psychiatry. 2012;27:321–328. doi: 10.1016/j.eurpsy.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Wittchen, H.-U., Wunderlich, U., Gruschwitz, S. & Zaudig, M. SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I. (1997).
- 46.First, M. B., Gibbon, M., Spitzer, R. L., Williams, J. B. & Benjamin, L. S. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II). Am. Psych. Pub. (1997) (American Psychiatric Publishing, Inc., Arlington, VA, 1992). https://openscholarship.wustl.edu/bsltests/2661/.
- 47.Schmidt, K. & Metzler, P. Wortschatztest (WST). (Beltz, Weinheim, 1992).
- 48.Babor T. F., Higgins-Biddle J. C., Saunders J. B. & Monteiro M. G. The alcohol use disorders identification test. In Guidelines for use in primary health care Geneva (World Health Organization, 1992).
- 49.Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur. Addiction Res. 2005;11:22–31. doi: 10.1159/000081413. [DOI] [PubMed] [Google Scholar]
- 50.Beck AT, Ward CH, Mendelson MM, Mock JJ, Erbaugh JJ. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann, P. & Fimm, B. Testbatterie zur Aufmerksamkeitsprüfung Version 2.3. Psytest: Herzogenrath (2013).
- 52.Pascual-Marqui RD, Esslen M, Kochi K, Lehmann D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find. Exp. Clin. Pharmacol. 2002;24(Suppl C):91–95. [PubMed] [Google Scholar]
- 53.Benjamini Yoav, Hochberg Yosef. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 54.Hegerl U, Hensch T. The vigilance regulation model of affective disorders and ADHD. Neurosci. Biobehav. Rev. 2014;44:45–57. doi: 10.1016/j.neubiorev.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Volkow ND, Swanson JM. Adult attention deficit–hyperactivity disorder. New Engl. J. Med. 2013;369:1935–1944. doi: 10.1056/NEJMcp1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faraone SV, Spencer T, Aleardi M, Pagano C, Biederman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2004;24:24–29. doi: 10.1097/01.jcp.0000108984.11879.95. [DOI] [PubMed] [Google Scholar]
- 57.Sandson TA, Bachna KJ, Morin MD. Right hemisphere dysfunction in ADHD: Visual hemispatial inattention and clinical subtype. J. Learn. Disabilities. 2000;33:83–90. doi: 10.1177/002221940003300111. [DOI] [PubMed] [Google Scholar]
- 58.Pfaff D, Ribeiro A, Matthews J, Kow LM. Concepts and mechanisms of generalized central nervous system arousal. Ann. N.Y. Acad. Sci. 2008;1129:11–25. doi: 10.1196/annals.1417.019. [DOI] [PubMed] [Google Scholar]
- 59.Sturm W, et al. Functional anatomy of intrinsic alertness: evidencefor a fronto-parietal-thalamic-brainstem network in theright hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/S0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 60.Heilman KM, Valenstein E, Watson RT. Neglect and related disorders. Proc. Semin. Neurol. 1984;4:209–219. doi: 10.1055/s-2008-1041551. [DOI] [PubMed] [Google Scholar]
- 61.Robinson, R. G. Lateralized behavioral and neurochemical consequences of unilateral brain injury in rats. In Cerebral Lateralization in Nonhuman Species. 135–156 (Elsevier, 1985).
- 62.Robinson RG, Coyle JT. The differential effect of right versus left hemispheric cerebral infarction on catecholamines and behavior in the rat. Brain Res. 1980;188:63–78. doi: 10.1016/0006-8993(80)90557-0. [DOI] [PubMed] [Google Scholar]
- 63.Ding YS, et al. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S, S)‐[11C] O‐methylreboxetine and HRRT. Synapse. 2010;64:30–38. doi: 10.1002/syn.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiang-shan RL, et al. Decreased norepinephrine transporter availability in obesity: positron Emission Tomography imaging with (S, S)-[11C] O-methylreboxetine. NeuroImage. 2014;86:306–310. doi: 10.1016/j.neuroimage.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logan J, et al. Imaging the norepinephrine transporter in humans with (S, S)-[11C] O-methyl reboxetine and PET: problems and progress. Nucl. Med. Biol. 2007;34:667–679. doi: 10.1016/j.nucmedbio.2007.03.013. [DOI] [PubMed] [Google Scholar]