Abstract
Steroid biosynthesis and metabolism are reflected by the serum steroid metabolome and, in even more detail, by the 24-hour urine steroid metabolome, which can provide unique insights into alterations of steroid flow and output indicative of underlying conditions. Mass spectrometry–based steroid metabolome profiling has allowed for the identification of unique multisteroid signatures associated with disorders of steroid biosynthesis and metabolism that can be used for personalized approaches to diagnosis, differential diagnosis, and prognostic prediction. Additionally, steroid metabolome analysis has been used successfully as a discovery tool, for the identification of novel steroidogenic disorders and pathways as well as revealing insights into the pathophysiology of adrenal disease. Increased availability and technological advances in mass spectrometry–based methodologies have refocused attention on steroid metabolome profiling and facilitated the development of high-throughput steroid profiling methods soon to reach clinical practice. Furthermore, steroid metabolomics, the combination of mass spectrometry–based steroid analysis with machine learning–based approaches, has facilitated the development of powerful customized diagnostic approaches. In this review, we provide a comprehensive up-to-date overview of the utility of steroid metabolome analysis for the diagnosis and management of inborn disorders of steroidogenesis and autonomous adrenal steroid excess in the context of adrenal tumors.
Essential Points
The steroid metabolome reflects biosynthesis, metabolism, and excretion of steroid hormones and readily reveals underlying conditions altering steroid flow and output
The 24-hour urine steroid metabolome can be used to measure global steroid metabolism and net steroid output
Unique urine steroid metabolome signatures (“steroid fingerprints”) result from inborn disorders of steroid biosynthesis and conditions associated with autonomous adrenal steroid excess and can be used for diagnostic purposes
The combination of mass spectrometry–based steroid profiling with machine learning–based data analysis has created a powerful discovery tool, steroid metabolomics, which offers the prospect of personalized approaches to diagnosis, prognostic prediction, and therapy
The serum and urine steroid metabolomes provide significant insights into the biosynthesis, metabolism, and excretion of steroid hormones and readily reveal underlying enzymatic deficiencies associated with steroidogenesis. Although alterations in the steroid metabolome have been used to diagnose inborn errors in steroidogenesis for several decades, recent advances have refocused attention on the capabilities of steroid metabolome profiling. The combination of mass spectrometry–based steroid profiling with machine learning–based data analysis has created a powerful discovery tool, steroid metabolomics, highly suited as a diagnostic biomarker approach (1–4). Furthermore, novel technological developments have facilitated the development of high-throughput multisteroid profiling methods soon to reach clinical practice (5–7).
Adrenal cortex, gonads, and placenta are the primary sites of de novo steroidogenesis from cholesterol (Fig. 1). Some of the resulting steroids can directly bind and activate steroid receptors in target cells of steroid action, whereas others require downstream activation but may also be inactivated or diverted to other steroid pathways. This intracellular steroid prereceptor and postreceptor metabolism has also been termed “intracrinology” (8), explaining why circulating steroid concentrations are often not representative of observed biological hormone activity. Moreover, adrenal steroidogenesis exhibits a diurnal rhythm and, as a result, single–time point serum steroid measurements only provide snapshots. This problem is circumvented by analyzing 24-hour urine collections, facilitating quantitation of the net 24-hour steroid output.
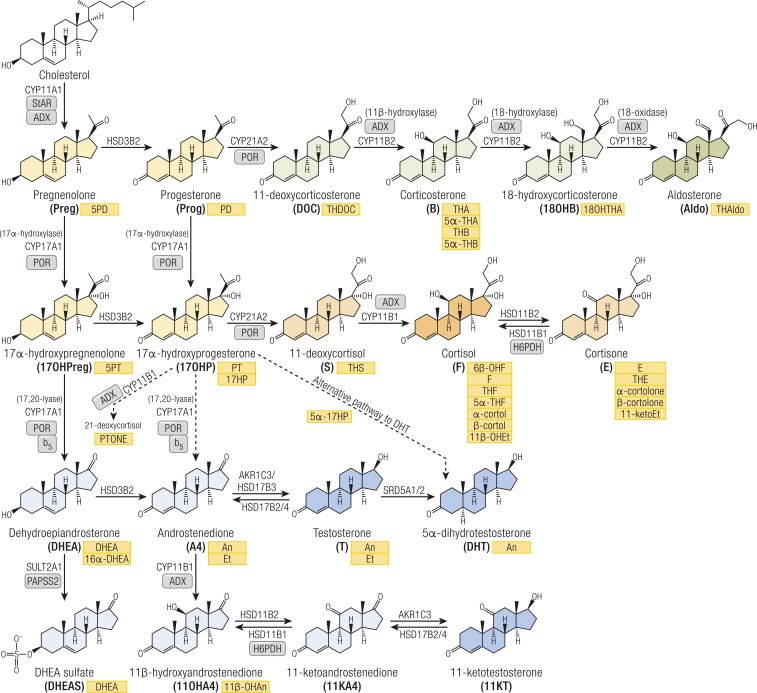
Figure 1.
Schematic overview of steroidogenesis and corresponding urine steroid metabolites. Steroids are color-coded according to their bioactivity or commitment to a specific pathway: general precursors (yellow), mineralocorticoid precursors (light green), active mineralocorticoid (dark green), glucocorticoid precursors and inactive metabolite (light orange), active glucocorticoid (dark orange), androgen precursors (light blue), and active androgens (dark blue). Corresponding urinary metabolites are shown in yellow boxes. Arrows are labeled with the catalyzing enzyme and isoform. Essential cofactor proteins are also indicated: ADX, adrenodoxin; b5, cytochrome b5; PAPSS2, PAPS synthase 2; PRO, cytochrome P450 oxidoreductase; StAR, steroidogenic acute regulatory protein.
Both endogenous and exogenous steroids undergo hepatic metabolism (9), with phase 1 reactions altering the biological activity by adding or revealing functional groups that can function as targets for subsequent conjugation (phase 2) reactions. Ultimately, this results in steroid inactivation and increased water solubility, facilitating urinary excretion, which accounts for ∼80% of steroid excretion. Urine steroid metabolites originate from distinct circulating steroids (Fig. 1; Table 1); the 24-hour urine steroid metabolome serves as a magnifying glass, facilitating the detection of alterations in steroid biosynthesis or metabolism and, thus, of underlying disorders.
Table 1.
Urine Steroid Metabolites as Assessed by Gas Chromatography–Mass Spectrometry (GC-MS)
| No. | Abbreviation | Common Name | Chemical Name | Metabolite of |
|---|---|---|---|---|
| Androgen metabolites | ||||
| 1 | An | Androsterone | 5α-Androstan-3α-ol-17-one | Androstenedione, testosterone, 5α-dihydrotestosterone |
| 2 | Et | Etiocholanolone | 5β-Androstan-3α-ol-17-one | Androstenedione, testosterone |
| Androgen precursor metabolites | ||||
| 3 | 11β-OHAn | 11β-Hydroxyandrosterone | 5α-Androstane-3α,11β-diol-17-one | 11β-Hydroxyandrostenedione |
| 4 | DHEA | Dehydroepiandrosterone | 5-Androsten-3β-ol-17-one | DHEA + DHEA sulfate (DHEAS) |
| 5 | 16α-OHDHEA | 16α-Hydroxy-DHEA | 5-Androstene-3β,16α-diol-17-one | DHEA + DHEAS |
| 6 | 5PT | Pregnenetriol | 5-Pregnene-3β,17α,20α-triol | 17β-Hydroxypregnenolone |
| 7 | 5PD | Pregnenediol | 5-Pregnene-3β,20α-diol | Pregnenolone |
| Mineralocorticoid and mineralocorticoid precursor metabolites | ||||
| 8 | THA | Tetrahydro-11-dehydrocorticosterone | 5β-Pregnane-3α,21-diol-11,20-dione | 11-Dehydrocorticosterone |
| 9 | 5α-THA | 5α-Tetrahydro-11-dehydrocorticosterone | 5α-Pregnane-3α,21-diol-11,20-dione | 11-Dehydrocorticosterone |
| 10 | THB | Tetrahydrocorticosterone | 5β-Pregnane-3α,11β,21-triol-20-one | Corticosterone |
| 11 | 5α-THB | 5α-Tetrahydrocorticosterone | 5α-Pregnane-3α,11β,21-triol-20-one | Corticosterone |
| 12 | THDOC | Tetrahydro-11-deoxycorticosterone | 5β-Pregnane-3α,21-diol-20-one | 11-Deoxycorticosterone |
| 13 | 18OHTHA | 18-Hydroxy-tetrahydro-11-dehydrocorticosterone | 5β-Pregnane-3α,18,21-triol-11, 20-dione | 18-Hydroxycorticosterone |
| 14 | THAldo | 3α,5β-Tetrahydroaldosterone | 5β-Pregnane-3α,11β,21-triol-20-one-18-al | Aldosterone |
| 15 | 18OHF | 18-Hydroxycortisol | 4-Pregnene-11β,17α,18,21-tetrol-3,20-dione | Cortisol (hybrid steroid generated by CYP11B2 18-hydroxylation) |
| 16 | 18oxoF | 18-Oxo-cortisol | 4-Pregnene-11β,17α,21-triol-3,20-dione-18-al | Cortisol (hybrid steroid generated by CYP11B2 18-oxidation) |
| 17 | 18oxoTHF | 18-Oxo-tetrahydrocortisol | 5β-Pregnane-3α,11β,17α,21-tetrol-20-one-18-al | Cortisol (hybrid steroid tetrahydro metabolites) |
| Glucocorticoid precursor metabolites | ||||
| 18 | PD | Pregnanediol | 5β-Pregnane-3α,20α-diol | Progesterone |
| 19 | 5α-17HP | 17α-Hydroxy-3α,5α-pregnanolone | 5α-Pregnane-3α,17α-diol-20-one | 17α-Hydroxyprogesterone |
| 20 | 17HP | 17α-Hydroxypregnanolone | 5β-Pregnane-3α,17α-diol-20-one | 17α-Hydroxyprogesterone |
| 21 | PT | Pregnanetriol | 5β-Pregnane-3α,17α,20α-triol | 17α-Hydroxyprogesterone |
| 22 | PTONE | Pregnanetriolone | 5β-Pregnane-3α,17α,20α-triol-11-one | 21-Deoxycortisol |
| 23 | THS | Tetrahydro-11-deoxycortisol | 5β-Pregnane-3α,17α,21-triol-20-one | 11-Deoxycortisol |
| Glucocorticoid metabolites | ||||
| 24 | F | Cortisol | 4-Pregnene-11β,17α,21-triol-3,20-dione | Cortisol |
| 25 | 6β-OHF | 6β-Hydroxycortisol | 4-Pregnene-6β,11β,17α,21-tetrol-3, 20-dione | Cortisol |
| 26 | THF | Tetrahydrocortisol | 5β-Pregnane-3α,11β,17α,21-tetrol-20-one | Cortisol |
| 27 | 5α-THF | 5α-Tetrahydrocortisol | 5α-Pregnane-3α,11β,17α,21-tetrol-20-one | Cortisol |
| 28 | α-Cortol | α-Cortol | 5β-Pregnane-3α,11β,17α,20α,21-pentol | Cortisol |
| 29 | β-Cortol | β-Cortol | 5β-Pregnane-3α,11β,17α,20β,21-pentol | Cortisol |
| 30 | 11β-OHEt | 11β-Hydroxyetiocholanolone | 5β-androstane-3α,11β-diol-17-one | Cortisol |
| 31 | E | Cortisone | 4-Pregnene-17α,21-diol-3,11,20-trione | Cortisone |
| 32 | THE | Tetrahydrocortisone | 5β-Pregnene-3α,17α,21-triol-11,20-dione | Cortisone |
| 33 | α-Cortolone | α-Cortolone | 5β-Pregnane-3α,17α,20α,21-tetrol-11-one | Cortisone |
| 34 | β-Cortolone | β-Cortolone | 5β-Pregnane-3α,17α,20β,21-tetrol-11-one | Cortisone |
| 35 | 11ketoEt | 11-Ketoetiocholanolone | 5β-Androstan-3α-ol-11,17-dione | Cortisone |
Traditionally, gas chromatography–mass spectrometry (GC-MS) has been employed for comprehensive urine steroid metabolite profiling. Serum steroids are now increasingly analyzed by ultra-HPLC–tandem mass spectrometry (UHPLC-MS/MS), overtaking the use of immunoassays, which are increasingly recognized as compromised by cross-reactivity. In the routine clinical biochemistry context, UHPLC-MS/MS is primarily used for single steroid analysis; however, recent years have seen the emergence of multisteroid mass spectrometry analysis of serum and plasma steroids, and very recently, also of urine steroid metabolites. The translational application of steroid metabolomics has not only facilitated novel diagnostic biomarker approaches, but also facilitated the elucidation of novel steroid pathways and their roles in human disease, the discovery of steroidogenic disorders, as well as the more fine-grained categorization of autonomous steroid excess. In this review, we provide a comprehensive up-to-date overview of the distinct steroid metabolome signatures associated with disorders of steroid biosynthesis and metabolism, summarizing current knowledge about their utility for diagnosis, differential diagnosis, and prognostic prediction.
Steroid Metabolome Signatures of Inborn Errors of Steroid Biosynthesis and Metabolism
The steroid metabolome in congenital adrenal hyperplasia
The variants of congenital adrenal hyperplasia (CAH) comprise five autosomal recessive inborn disorders defined by glucocorticoid deficiency resulting from inactivating mutations in enzymes involved in adrenal steroidogenesis (10). Reduced cortisol feedback within the hypothalamic–pituitary–adrenal (HPA) axis drives continuous stimulation of the adrenal cortex by pituitary ACTH, with subsequent adrenocortical hyperplasia and enhanced activity of the unaffected adrenal steroidogenic pathways. Dependent on the position of the enzymatic block, mineralocorticoid and androgen production can be decreased, increased, or normal, respectively.
CAH due to CYP21A2 deficiency
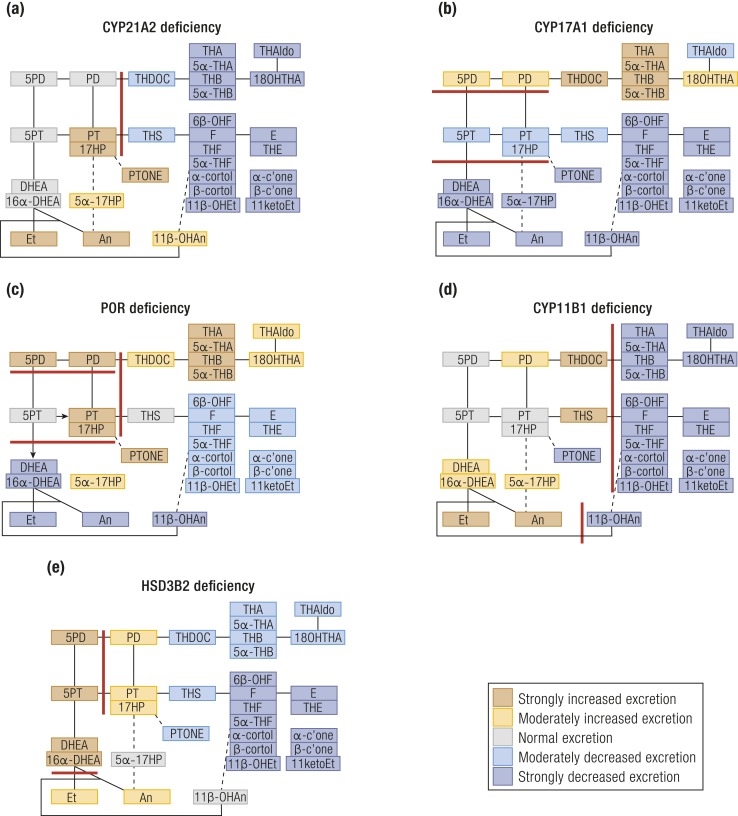
More than 90% of CAH cases are caused by mutant 21-hydroxylase (CYP21A2) (10), a key enzyme in glucocorticoid and mineralocorticoid biosynthesis (Fig. 1); the presence and severity of loss of cortisol and aldosterone production CYP21A2 deficiency is 17OHP, but 17OHPreg, Prog, and Preg are also increased (12). In the absence of CYP21A2 activity, CYP11B1 atypically converts 17OHP to 21-deoxycortisol. Therefore, diagnostic ratios of the urinary metabolites of 21-deoxycortisol (PTONE) or 17OHP (PT and 17HP) over glucocorticoid metabolites are invaluable for the diagnosis of CYP21A2 deficiency (13) [Fig. 2A (13–28); Table 2 (13, 26, 29, 30)].
Figure 2.
(a–e) Schematic visualization of urine steroid metabolome signatures in the five variants of CAH. (a) CYP21A2, (b) CYP17A1, (c) POR, (d) CYP11B1, and (e) HSD3B2 deficiencies. The figure depicts the changes in the major urine steroid metabolites relative to the reference range median of each metabolite and does not represent overall quantitative excretion. Steroid metabolites are mapped onto the steroidogenic pathways leading to mineralocorticoid, glucocorticoid, and androgen biosynthesis as shown in Fig. 1. Data derived from (13–28).
Table 2.
Substrate/Product Ratios of Urine Steroid Metabolites Used for the Biochemical Diagnosis of Inborn Errors of Steroidogenesis
| Enzymatic Activity | Enzymes Involved | Ratio |
|---|---|---|
| 21-Hydroxylase | CYP21A2 and POR | 100*PTONE/(THE+THF+5α-THF) |
| (17HP+PT)/(THE+THF+5α-THF) | ||
| 17α-Hydroxylase | CYP17A1 and POR | (THA+5α-THA+THB+5α-THB)/ |
| (THE+THF+5α-THF) | ||
| (THA+5α-THA+THB+5αTHB)/(An+Et) | ||
| 17,20-Lyase | CYP17A1 and CYB5A and POR | 5PT/DHEA |
| (17HP+PT)/(An+Et) | ||
| P450 oxidoreductase | POR | PD/(THE+THF+5α-THF) |
| 5PD/(THE+THF+5α-THF) | ||
| 11β-Hydroxylase | CYP11B1 | 100*THS/(THE+THF+5α-THF) |
| 3β-Hydroxysteroid dehydrogenase | HSD3B2 | DHEA/(THE+THF+5α-THF) |
| 5PT/(THE+THF+5α-THF) | ||
| 5PT/PTONE | ||
| 11β-Hydroxysteroid dehydrogenase type 2 | HSD11B2 | F/E |
| (THF+5α-THF)/THE | ||
| Cortols/cortolones | ||
| (F+E)/(THF+5α-THF+THE) | ||
| 11β-Hydroxysteroid dehydrogenase type 1 | HSD11B1/H6PDH | THE/(THF+5α-THF) |
| Cortolones/cortols | ||
| 5α-Reductase type 2 | SRD5A2 | Et/An |
| THB/5α-THB | ||
| THF/5α-THF | ||
| 17β-Hydroxysteroid dehydrogenase type 3 | HSD17B3 | (An+Et)/(THE+THF+5α-THF) |
| An/Et |
In the past, diagnosis of CYP21A2 deficiency has been challenging in neonates and preterm infants, as 17OHP radioimmunoassay results are compromised by cross-reactivity of abundant neonatal 3β-OH-Δ5 steroids (31). When using GC-MS, polar 17OHP metabolites such as 5β-pregnane-3β,15β,17α-triol-20-one (13, 32, 33), as well as the ratio of PTONE over 6α-hydroxylated cortisone metabolites, can help discriminate affected from unaffected infants (13, 34), with additional diagnostic value provided by 5α-pregnane-3β,16α,17α-triol-7,20-dione and 5β-pregnane-3α,15β,17α-triol-20-one (35, 36).
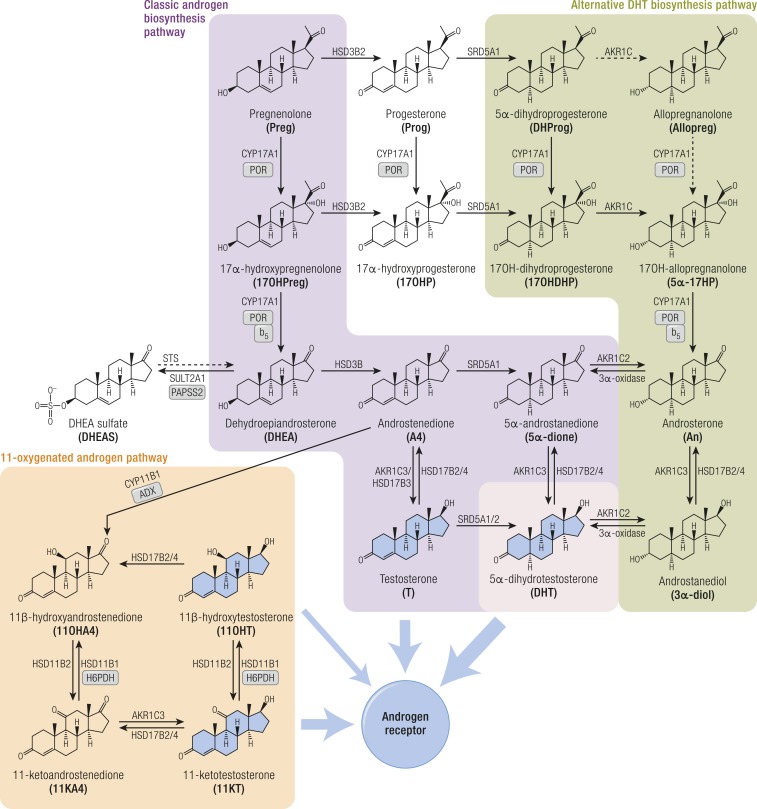
Biologically active androgens are increased in CYP21A2 deficiency, driven by the accumulation of precursor steroids prior to the enzymatic block, feeding into all three major androgen biosynthesis pathways: the classic androgen pathway, the alternative pathway to DHT, and the 11-oxygenated androgen pathway (Fig. 3). The accumulation of 17OHP increases atypical conversion of 17OHP to androstenedione (A4) by CYP17A1 17,20-lyase activity, which physiologically has a much higher preference for the conversion of 17OHPreg to DHEA (29). Accumulating 17OHP also drives increased androgen production by the alternative DHT pathway (26), and increased A4 feeds enhanced 11-oxygenated androgen pathway activity (24) (Fig. 3). An and Et are typically raised in urines of untreated or poorly controlled patients, although note that Et is derived solely from the classic pathway, whereas An can be derived from both classic and alternative androgen pathways (Table 1); urinary DHEA(S) excretion is usually normal or only mildly elevated. Alternative DHT pathway activity is reflected by 5α17HP and An (22, 26), whereas increased 11-oxygenated pathway activity is reflected by the major metabolite of 11OHA4, 11β-OHAn (22, 25, 26) (Figs. 2A and 3; Table 1).
Figure 3.
Schematic overview of the three major pathways of human androgen biosynthesis. The classic, alternative, and 11-oxygenated androgen pathways are each shown in different colors. Androgens that activate the androgen receptor are shown with broad blue arrows leading from them. Other arrows are labeled with the catalyzing enzyme and isoform where appropriate. Essential cofactor proteins are also indicated: b5, cytochrome b5; PAPSS2, PAPS synthase 2; POR, cytochrome P450 oxidoreductase.
CAH due to CYP17A1 deficiency
CYP17A1 is vital for both glucocorticoid and androgen biosynthesis via its 17α-hydroxylase and 17,20-lyase activities, respectively. Therefore, CYP17A1 deficiency mostly presents with combined glucocorticoid and sex steroid deficiency; HPA axis upregulation drives increased mineralocorticoid production via the only remaining functional adrenocortical biosynthesis pathway. Glucocorticoid deficiency is rarely life-threatening in CYP17A1 deficiency, as increased corticosterone exerts some glucocorticoid receptor activation (11, 12).
Affected patients more commonly present with symptoms of sex steroid deficiency with associated hypertension due to very high levels of DOC. Because not only female but also chromosomally male patients appear phenotypically female, it is not uncommon that diagnosis is not made until puberty. In CYP17A1 deficiency, serum DHEA and A4 are low at baseline and after adrenal cosyntropin stimulation (11). Urine steroid profiling (Fig. 2B) shows a substantial reduction of all androgen metabolites, and diagnostic ratios (Table 2) indicate largely abolished 17,20-lyase activity (15, 18).
Although milder forms of CYP17A1 deficiency have been reported, where only sex steroid production seems to be present (so-called “isolated 17,20-lyase deficiency”), stimulated cortisol levels do not rise sufficiently in these patients, indicating glucocorticoid deficiency (15, 37). Similarly, urine steroid profiling in these milder cases suggests attenuation of 17α-hydroxylase activity, assessed by the ratio of mineralocorticoid over glucocorticoid metabolites (15, 19), which is the most important diagnostic ratio for this disorder (Table 2). In classic forms with severe CYP17A1 deficiency, DOC and corticosterone metabolites are markedly increased, with mildly increased Preg and Prog metabolites (38). Circulating 17OHP is significantly decreased, whereas cortisol and aldosterone levels remain normal (17, 39). In neonates with CYP17A1 deficiency, the 11-keto corticosterone metabolite 11-dehydrocorticosterone is dominant in newborns and, therefore, urine steroid metabolites increased in CYP17A1 deficiency include THA, 5α-THA, and 6α-hydroxy-11-dehydro-tetrahydrocorticosterone (6α-OHTHA), with the latter the most important quantitative marker. Thus, useful diagnostic urine steroid ratios in neonates include 6α-OHTHA/cortisol metabolites and 16α-hydroxypregnenolone/16α-OHDHEA (13).
CAH due to P450 oxidoreductase deficiency
P450 oxidoreductase (POR) is the crucial electron donor for microsomal cytochrome P450 (CYP) enzymes, including the steroidogenic enzymes CYP21A2, CYP17A1, and, to a lesser degree, CYP19A1 (11, 12). The discovery of the molecular basis of POR deficiency (PORD) in 2004 (40, 41) solved the puzzle previously posed by patients with a unique urine steroid profile published 20 years earlier, indicating the concurrent presence of CYP21A2 and CYP17A1 deficiencies (42). Most patients have normal baseline cortisol but respond insufficiently to cosyntropin (16), indicative of partial glucocorticoid deficiency, which requires stress dose cover with glucocorticoids in case of intercurrent illness, major stress, or surgery. Mineralocorticoid production is preserved or enhanced, with hypertension typically manifesting in adulthood (16). Preg and Prog are characteristically increased, whereas 17OHP is only mildly elevated. The impairment of other enzymes involved in cholesterol biosynthesis (CYP51A1, SQLE) (30, 43) and retinoic acid metabolism (CYP26A1, CYP26B1, CYP26C1) (44, 45) results in skeletal and multiple other malformations resembling the Antley-Bixler phenotype (16). PORD also results in decreased hepatic drug metabolism, due to reduced capacity of CYP3A4, but also CYP1A2, CYP2D6, CYP2C9, and CYP2C19 (46).
The urine steroid metabolome in PORD shows characteristically increased Preg and Prog metabolites (PD and 5PD; Fig. 2C, Table 1), which together with the increased metabolites attributed to partial CYP21A2 and CYP17A1 deficiencies establishes the diagnosis (14, 16, 23, 27) (Table 2). 5PD, excreted as a bis-sulfate, is particularly prominent in neonates and young infants with PORD (47).
In PORD, DSD has been reported in individuals of both chromosomal sexes, and patients can present as virilized females (46,XX DSD) as well as undermasculinized males (46,XY DSD). This paradox has been explained by the alternative pathway to DHT (Fig. 3), proposed to be mostly active during fetal life, with very low or absent activity in the postnatal situation (40). In individuals with 46,XX, androgen excess generated via the alternative pathway may cause virilization; equally, androgen biosynthesis may be insufficient to masculinize external genitalia in individuals with 46,XY, depending on the effect of the underlying mutations (16, 48, 49). POR mutations allowing for significant residual alternative pathway activity present with 46,XY DSD and normal male genitalia in individuals with 46,XY, whereas major loss-of-function POR mutations result in normal female phenotype and 46,XY DSD. Maternal virilization is also a characteristic feature in many but not all pregnancies with affected babies (41).
Prenatal diagnosis at midpregnancy is straightforward (50, 51). Attenuated activity of POR results in multiple enzyme deficiencies en route to estriol. As a result, unconjugated serum estriol is typically very low. Decreased CYP17A1 activity yields excess excretion of 5α-pregnane-3β,20α-diol (3β5α-PD) bis-sulfate, a fetal pregnenolone metabolite, increasing the urinary 3β5α-PD/estriol ratio (50). Upregulation of the alternative pathway upregulates 5α-17HP and An production, and consequently increased urinary An/Et and 5α-17HP/17HP ratios (50). LC-MS/MS analysis of conjugated urine steroids recently revealed 3β5α-PD bis-sulfate and estriol glucuronides to be useful in the prenatal diagnosis of this disorder (47).
CAH due to CYP11B1 deficiency
CYP11B1 catalyzes key reactions in the mineralocorticoid and glucocorticoid pathways. CYP11B1 deficiency results in cortisol deficiency, mineralocorticoid excess, and androgen excess. The marker steroid is 11-deoxycortisol, accumulating prior to the enzymatic block. DOC also accumulates due to continuous ACTH stimulation, resulting in arterial hypertension (11, 12), with CYP11B2-mediated conversion of DOC to corticosterone not sufficient to compensate for loss of CYP11B1 function.
Serum A4 and testosterone (T) are increased in untreated or poorly controlled individuals, whereas 11-oxygenated androgens are characteristically absent, with low excretion of the 11OHA4 metabolite 11β-OHAn (20, 52). The urine steroid metabolome is dominated by THS; diagnosis is facilitated by the ratio of THS over glucocorticoid metabolites (Table 2; Fig. 2D). THDOC is also increased. In contrast to CYP21A2 deficiency, PTONE is low in CYP11B1 deficiency, as 17OHP cannot be 11β-hydroxylated. In neonates, high 6α-hydroxylase activity increases 6α-hydroxytetrahydrotetrahydro-11-deoxycortisol (6α-OHTHS) excretion, which adds diagnostic value (13, 53).
CAH due to HSD3B2 deficiency
HSD3B2 (Δ5-4 isomerase) is crucial to the production of all three major adrenal steroid classes as well as gonadal androgens (Fig. 1). Deficiency of HSD3B2, therefore, leads to reduced mineralocorticoid, glucocorticoid, and sex steroid production. HPA axis activation drives precursor accumulation, in particular 17OHPreg (54). Classically, patients manifest early in the neonatal period with severe salt-wasting adrenal insufficiency, but broad phenotypic variation is reported (54). The HSD3B1 isoform, expressed in the placenta several peripheral tissues, converts accumulating ∆5 steroids such as 17OHPreg or DHEA to 17OHP and active androgens, respectively. Therefore, serum 17OHP can be elevated and individuals with 46,XX can present virilized. In urine, the 17OHPreg metabolite 5PT and DHEA are raised, and downstream metabolites of all steroidogenic pathways are reduced (21) (Fig. 2E). Surprisingly, excretion rates of PT, 17HP, and PD are elevated, similar to CYP21A2 deficiency; however, in CYP21A2 deficiency, the 5PT/PTONE ratio is typically low (<1.5), whereas it is high (>35) in HSD3B2 deficiency (14, 23) (Table 2). In neonates, elevated 5PT is also an important marker (13), with added diagnostic value of 5-pregnene-3β,15β,17α-triol-20-one and its 20-reduced metabolite (13, 55).
Inborn disorders of androgen biosynthesis and metabolism
SRD5A2 deficiency
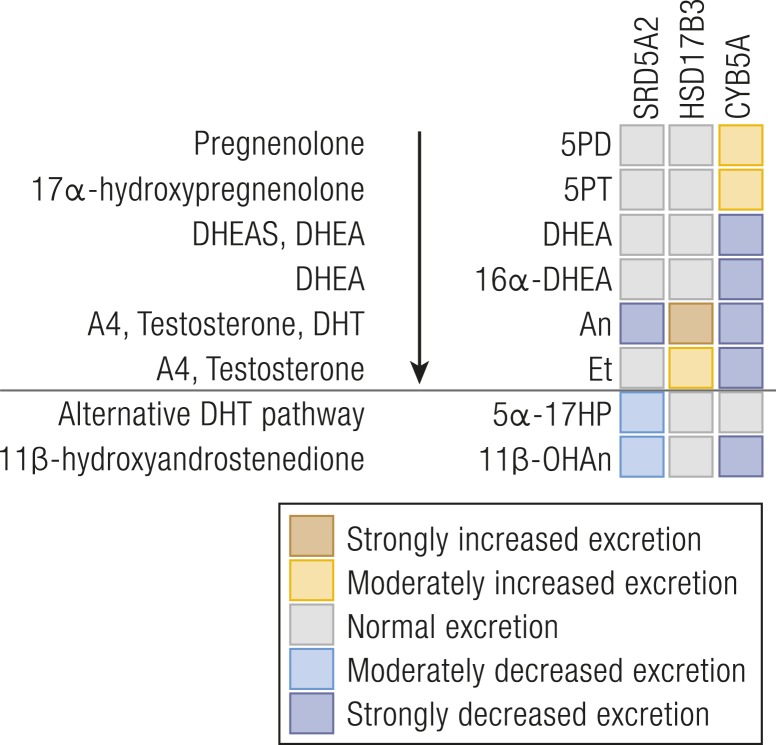
SRD5A2 catalyzes the final activating step of the classic androgen biosynthesis pathway, the 5α-reduction of T to the more potent DHT (Fig. 1). In chromosomally male individuals, SRD5A2 deficiency presents with undermasculinization, that is, ambiguous genitalia at birth (46,XY DSD) (56). SRD5A2 deficiency has elucidated the crucial role of DHT in male genital skin, as in its absence, external genital masculinization does not occur. If not diagnosed and appropriately treated, affected individuals are inadvertently raised as females and, owing to increased expression of SRD5A1 in the genital area at pubertal age, may then present with significant virilization and phallus growth (57, 58).
Serum DHT is low/undetectable at baseline and after human chorionic gonadotropin (hCG) stimulation, with inappropriately high T. However, establishing the diagnosis from the hCG-stimulated serum T/DHT ratio is highly challenging, due to the very low circulating DHT concentrations, in particular in infants and prepubertal children (58–60).
The diagnosis from urine steroid profiling is straightforward [Fig. 4 (15, 61, 62)], with most robust information provided by diagnostic ratios of 5α-reduced over 5β-reduced glucocorticoid and mineralocorticoid metabolites (Table 2) (61), owing to the abundance of those metabolites compared with androgen metabolites, in particular in prepubertal children. Diagnosis can be difficult in neonates and may not be reliably established from urine steroid profiling until the age of 3 months, owing to high activity of the SRD5A1 isoform and lower excretion rates of cortisol metabolites during this stage of development (23, 63, 64).
Figure 4.
Heat map visualization of urine steroid metabolome signatures associated with inborn disorders of androgen biosynthesis. The figure depicts the changes in the major urine steroid metabolites associated with androgen biosynthesis relative to the reference range median of each steroid metabolite and does not represent overall quantitative excretion. For explanation of the link between precursors, active steroids, and their metabolites, please see Fig. 1. Data derived from (15, 61, 62).
HSD17B3 deficiency
Inactivating HSD17B3 mutations results in failure to convert A4 to T in the fetal testis, causing male undermasculinization (46,XY DSD) (65). Owing to increasing expression of other HSD17B isoforms capable of using A4 as a substrate, affected individuals tend to experience significant virilization at the time of puberty.
Serum T is low and A4 increased, both at baseline and after hCG stimulation. An hCG-stimulated T/A4 ratio <0.8 has been suggested as diagnostic in children (66). However, false-positive results have been reported in other disorders of T biosynthesis or structural abnormalities of the testis, that is, Leydig cell hypoplasia (67, 68).
There is a paucity of published data on urine steroid profiling in HSD17B3 deficiency. One might expect that measuring a pair of 17-keto and 17-hydroxy ratios would allow for diagnosis, but in our experience this is not the case, possibly explained by the activities of other HSD17B isoforms. Affected children excrete high levels of the major androgen metabolites, particularly An, increasing the An/Et ratio (Fig. 4). Otherwise, the steroid metabolome is largely normal (14, 23, 62). A notable feature, useful diagnostically, is an increased androgen-over-glucocorticoid metabolite ratio (An+Et/THE+THF+5aTHF) (Table 2). Age-appropriate controls are important when utilizing this ratio.
CYB5A deficiency (true isolated 17,20-lyase deficiency)
Cytochrome b5 (CYB5A) is a modulator crucially required for CYP17A1 17,20-lyase activity (Fig. 1) and hence essential for sex steroid production. Individuals with 46,XY with inactivating CYB5A mutations present with 46,XY DSD at birth, and girls with 46,XX present with lack of adrenarche, pubertal development, and primary gonadal failure in adolescence. Mild but clinically asymptomatic methemoglobinemia is also observed due to the role of CYB5A in Hb metabolism (69).
Serum sex steroids DHEA(S), A4, and T as well as 17β-estradiol are undetectable at baseline and after cosyntropin or hCG stimulation (15, 70). Urine steroid profiling data are available in three siblings (15): excretion rates of the 17OHPreg metabolite 5PT are increased, with reduced androgen excretion but normal glucocorticoids and mineralocorticoids (Fig. 4). In contrast to 17,20-lyase deficiency in the context of CYP17A1 deficiency (see “CAH due to CYP21A2 deficiency” above), which always comes with a degree of impairment of CYP17A1 17α-hydroxylase activity, CYB5A deficiency represents “true” isolated 17,20-lyase deficiency (15, 70); increased steroid ratios for 17,20-lyase activity with normal ratios for 17α-hydroxylase activity are diagnostic (Table 2).
Inborn disorders of sulfation and desulfation
Steroid sulfatase deficiency
Steroid sulfatase (STS) cleaves the sulfate moiety off a variety of sterol and steroid sulfates, including DHEAS (71). Patients with STS deficiency (STSD) are mainly affected by X-linked ichthyosis characterized by dark-brown scaling of the skin due to accumulating cholesterol sulfate in the epidermis (72, 73). STSD could theoretically contribute to a reduced desulfation of DHEAS and, therefore, reduced availability of DHEA for downstream activation to T and DHT. However, several previous studies did not find clinically significant androgen deficiency in affected patients (74–78). A study exploring the serum and urine steroid metabolome in prepubertal and postpubertal children found decreased serum DHEA and T compared with matched controls (77). The urinary excretion of active androgen metabolites An and Et, however, was similar in STSD and controls, possibly due to an upregulation of systemic 5α-reductase activity, as indicated by an increased urinary 5α-THF/THF ratio (77). The serum DHEA/DHEAS ratio reflective of global STS activity was high in prepubertal controls and decreased during the course of puberty; in contrast, STSD patients showed low DHEA/DHEAS ratios both prepubertally and postpubertally (77). This suggests a physiological role of STS prior to puberty, possibly for fine tuning of tissue-specific androgen activation, no longer needed postpubertally in the presence of high gonadal androgen production.
Serum cholesterol sulfate is highly elevated in STS deficiency (79), and diagnosis can readily be achieved by UHPLC-MS/MS analysis of the intact conjugate (80, 81).
There are antenatal indicators of STSD, and prenatal diagnosis of an affected fetus is straightforward. It is the most common cause of low estriol production during pregnancy, with free urine estriol values typically very low. The placenta of an affected fetus is unable to desulfate the fetal estriol precursors (e.g., 16α-OHDHEAS), thus preventing unconjugated 16α-OHDHEA formation and its conversion to 16α-hydroxyandrostenedione. Concomitantly, increased maternal excretion of all Δ5 steroid sulfates, particularly the two precursors of estriol, 16α-OHDHEAS and androstenetriol sulfate (5-androstene-3β,16α,17β-triol sulfate), are characteristic of this disorder during pregnancy (82). The ratio of urinary 16α-OHDHEA/estriol measured by GC-MS is used for diagnosis (83). Moreover, UHPLC-MS/MS of intact steroid conjugates has recently been evaluated for prenatal STSD diagnosis (47). 16α-OHDHEA sulfate, 5-pregnene-3β,20α-diol bis-sulfate, 21-hydroxypregnenolone bis-sulfate, and estriol glucuronides were found to be effective diagnostic markers.
PAPSS2 deficiency (apparent DHEA sulfotransferase deficiency)
PAPSS2 generates the universal sulfate donor PAPS required by DHEA sulfotransferase (SULT2A1), which catalyzes sulfation of DHEA to DHEAS (Fig. 1) (84). PAPSS2 deficiency is a rare monogenic form of androgen excess caused by impaired DHEA sulfation, resulting in an increased downstream activation of unconjugated DHEA to androgens (85).
The seminal case of PAPSS2 deficiency was a girl with early-onset androgen excess who clinically presented with premature adrenarche at the age of 6 years, thereafter progressing to a polycystic ovary syndrome (PCOS)–like phenotype in adolescence (85). The key abnormality was low/undetectable serum DHEAS, but high levels of A4, T, and DHT. Low serum DHEAS is a common finding in patients with PAPSS2 deficiency (85–88).
Detailed investigations of steroid metabolism are available from two brothers with compound heterozygous PAPSS2 mutations and their heterozygous parents (89). After an oral DHEA challenge, the brothers and their mother exhibited a subnormal rate of DHEAS generation, whereas DHEA generation and urinary androgen excretion increased, with evidence of increased 5α-reductase activity (Table 2) in the affected brothers and their mother (89). Of note, this mother and the mother of the first reported case were carriers of major loss-of-function PAPSS2 mutations and presented clinically with PCOS.
Inborn disorders of cortisol activation and inactivation
Cortisone reductase (HSD11B1) deficiency and apparent cortisone reductase (H6PDH) deficiency
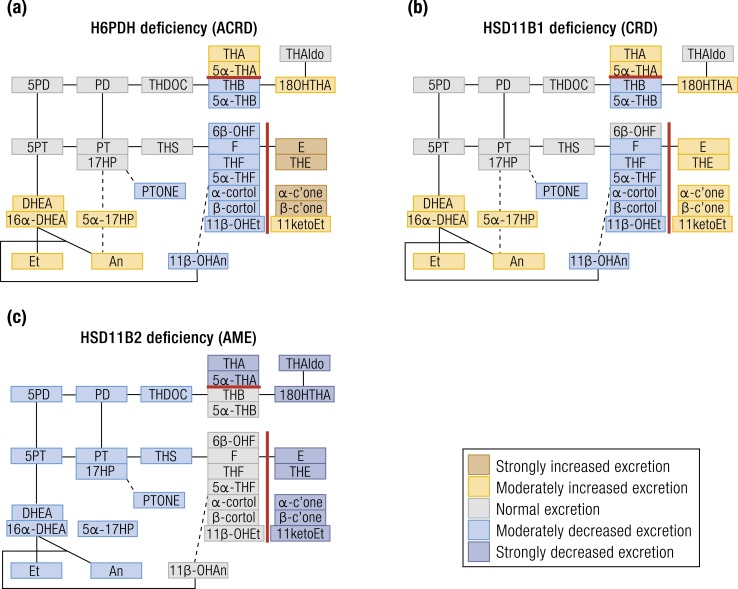
Cortisone reductase deficiency (CRD) and apparent CRD (ACRD) are characterized by the inability to generate active cortisol from cortisone (Fig. 1), with the consequently activated HPA axis driving ACTH-mediated excess adrenal androgen secretion. CRD is caused by HSD11B1 deficiency (90) whereas ACRD results form a deficiency in the activity of H6PDH, which is essential for maintaining the reductive activity of HSD11B1 in vivo by the reduced form of NAD phosphate provision to the enzyme (91, 92).
Clinically, CRD and ACRD present with a similar phenotype, with affected individuals developing androgen excess in childhood, coming to clinical attention with premature adrenarche in children of both sexes or in adolescent and young adult women with a PCOS-like phenotype (90, 92–94). Urine steroid profiling reveals distinct alterations in glucocorticoid metabolism accompanied by an overall increase of androgen excretion rates (90, 92, 95). Notably, the urinary excretion of cortisone metabolites is increased whereas cortisol metabolites are decreased (92) [Fig. 5A and 5B (90, 92, 95–100)], resulting in reduced ratios of cortisol over cortisone metabolites (Table 2).
Figure 5.
Heat map visualization of urine steroid metabolome signatures associated with HSD11B and H6PDH deficiencies. (a) H6PDH deficiency, (b) HSD11B1 deficiency, and (c) HSD11B2 deficiency. The figure depicts the changes in the major urine steroid metabolites relative to the reference range median of each metabolite and does not represent overall quantitative excretion. Steroid metabolites are mapped onto the steroidogenic pathways leading to mineralocorticoid, glucocorticoid, and androgen biosynthesis as shown in Fig. 1. Data derived from (90, 92, 95–100).
Detailed analysis of CRD and ACRD cases suggest that the (5α-THF+THF)/THE ratio and the cortols/cortolones ratio may be used to distinguish the two conditions (≤0.1 for ACRD but 0.1 to 0.5 in CRD) (95). Total androgen metabolite excretion is increased; however, a distinction between CRD and ACRD based on androgen metabolites is not possible, although there is a tendency toward higher androgen output in CRD (95). Global 5α-reductase activity based on 5α-reduced over 5β-reduced THF is increased in most ACRD cases, but normal in CRD (95).
“…machine learning–driven analysis of the urinary steroid excretion data allowed for rapid detection of a ‘malignant steroid fingerprint’….”
HSD11B2 deficiency (apparent mineralocorticoid excess)
The central role of HSD11B2 is to protect mineralocorticoid target tissues from cortisol-mediated activation of the mineralocorticoid receptor (MR) (101). Therefore, HSD11B2 deficiency caused by inborn mutations or excess consumption of HSD11B2 inhibitors (carbenoxolone, licorice) (102, 103) leads to unwanted excess MR activation by cortisol, resulting in apparent mineralocorticoid excess (AME) (104). Clinically, HSD11B2 deficiency manifests with hypertension, hypokalemia, and low renin levels, but no evidence of excess mineralocorticoid secretion, with subnormal levels of aldosterone (99). Most patients are children presenting within the first year of life with failure to thrive, short stature, and hypokalemia leading to thirst, polydipsia, and polyuria (105).
Circulating cortisol remains normal due to increased negative HPA axis feedback. Circulating cortisone is decreased, which increases the cortisol/cortisone ratio (106), the key marker of HSD11B2 function (107). The urine steroid metabolome shows increased excretion of 11β-hydroxysteroids over the respective 11-ketosteroids (Fig. 5C).
The ratio of cortols/cortolones is increased (96, 97, 108), whereas 11ketoEt excretion is undetectable (98). The excretion of metabolites of DOC and aldosterone are subnormal (96) as, in fact, is the excretion of all steroids. Quantitatively, even the diagnostic “hyperproduced” 11β-hydroxy cortisol metabolites are at the lower end of the normal range for age.
Features of impaired cortisol clearance other than decreased cortisol 11-oxidation have also been observed in AME. An increased urinary 5α-THF/THF ratio indicates reduced AKR1D1 activity or a shunt of cortisol into the pathway of 5α-reduction of glucocorticoids (98, 99). There is also an impaired conversion of tetrahydro cortisol metabolites to their corresponding hexahydro metabolites, indicating defective reductive metabolism of the cortisol side chain. There is an increased excretion of urinary free cortisol and cortisol metabolites with an unreduced or incompletely reduced A-ring—for example, 6β-OHF and 5α-dihydrocortisol (96, 109). The urinary free cortisol–to–urinary free cortisone ratio has emerged as a more sensitive marker for AME than the ratio of (THF+5α-THF)/THE (100), as A-ring reduction to tetrahydro metabolites takes place mainly in the liver and their ratio may not accurately reflect renal HSD11B2 activity.
Inborn mineralocorticoid deficiency and excess
CYP11B2 deficiency
CYP11B2 catalyzes the three final steps of aldosterone production, exerting sequential 11β-hydroxylase, 18-hydroxylase, and 18-oxidase activities (Fig. 1). CYP11B2 mutations invariably result in loss of 18-oxidase activity, whereas the 18-hydroxylase activity can be either preserved or lost. In both cases, patients present with identical clinical features of mineralocorticoid deficiency: signs of hyponatremia, hyperkalemia, and hypovolemia, which can lead to shock and death. Plasma renin activity is increased in affected children, but it can be normal in adults (110, 111). As the two-step conversion of corticosterone to aldosterone was initially considered to be catalyzed by two different enzymes—corticosterone methyloxidase I (CMO I, 18-hydroxylase) and corticosterone methyloxidase II (CMO II, 18-oxidase)—a biochemical categorization of CYP11B2 deficiencies as CMO I and CMO II is still widely accepted.
18-Hydroxylase deficiency (CMO I due to CYP11B2 deficiency).
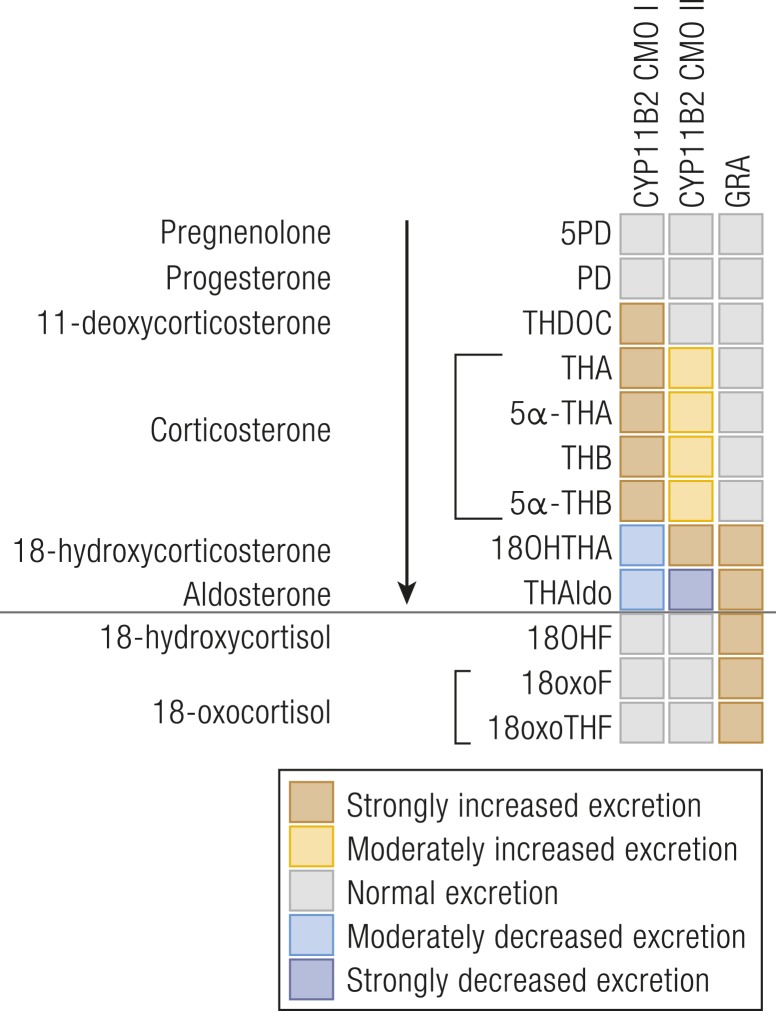
18-hydroxylase deficiency results in low serum 18-hydroxycorticosterone (18OHB) and low to undetectable aldosterone, with concomitant accumulation of corticosterone. As a consequence, urinary excretion of 18OHTHA and THAldo is low whereas corticosterone metabolites are high [Fig. 6 (50, 112–124)].
Figure 6.
Heat map visualization of urine steroid metabolome signatures associated with inborn mineralocorticoid excess. The figure depicts the changes in the major urine steroid metabolites associated with mineralocorticoid biosynthesis relative to the reference range median of each metabolite and does not represent overall quantitative excretion. For explanation of the link between precursors, active steroids, and their metabolites, please see Fig. 1. Data derived from (50, 112–124).
THDOC may also be increased, whereas cortisol metabolite excretion is normal (112, 117). In neonates, 5α-THA is higher than 5α-THB, due to the dominance of the 11-keto derivatives, and 6α-OHTHA is also a quantitatively relevant metabolite, due to high neonatal 6α-hydroxylase activity (117).
18-Oxidase deficiency (CMO II due to CYP11B2 deficiency).
In 18-oxidase deficiency, aldosterone levels are low whereas 18OHB levels are increased, in contrast to low 18OHB in CMO I. Serum corticosterone is normal to high, depending on the severity of 18-oxidase deficiency. Consequently, the urine metabolome shows low to undetectable THAldo, whereas 18OHTHA is significantly increased (Fig. 6). Corticosterone metabolite excretion is normal or increased, whereas cortisol metabolites are normal (118, 121, 122). The urinary ratio of THAldo/18OHTHA or plasma aldosterone/18OHB discriminate CMO I and CMO II conditions (119, 125, 126). When aldosterone is undetectably low and these ratios are incalculable, which can occur in CMO I conditions (127), the circulating 18OHB/B ratio or urinary 18OHTHA/THBs ratio can be used (123).
Pseudohypoaldosteronism
Pseudohypoaldosteronism (PHA) is a rare syndrome of systemic or renal mineralocorticoid resistance characterized by excessive aldosterone secretion, but clinical signs of hypoaldosteronism, which may result from genetic disorders, transient, or secondary salt-losing states (128, 129). PHA is characterized by increased plasma renin and aldosterone as well as increased urinary excretion of aldosterone metabolites (120, 130, 131). Unlike CYP11B2 deficiency CMO II, both 18OHTHA and THAldo are increased in neonates, and thus their ratio is unaffected in PHA, allowing the two conditions to be distinguished (Fig. 6) (119).
Glucocorticoid-remediable aldosteronism
Rare unequal crossover events between the CYP11B1 and CYP11B2 genes yield a hybrid CYP11B gene composed of the ACTH-responsive promoter and first exons of CYP11B1 fused in-frame to the major part of CYP11B2, resulting in ACTH-driven mineralocorticoid production (124, 132). Mineralocorticoid excess is controlled by exogenous glucocorticoid administration, which suppresses endogenous ACTH, reflected in the name of the condition, glucocorticoid-remediable aldosteronism (GRA), sometimes also called familial hyperaldosteronism (FHA) type 1.
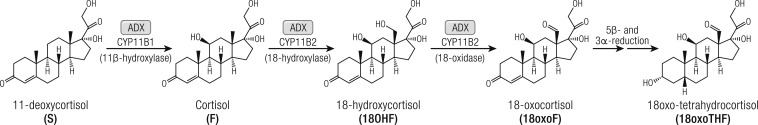
GRA is characterized by aldosterone excess with suppressed renin and increased production of 18-oxygenated cortisol “hybrid” metabolites, 18-hydroxycortisol (18OHF) and 18-oxocortisol (18oxoF) (Fig. 7) (133–135). Urinary excretion of aldosterone metabolites is increased (Fig. 6) (115, 124), with the urinary (18OHF+18oxoF)/THAldo ratio further supporting diagnosis (116); the urinary metabolite 18oxoTHF (Fig. 7) is characteristically increased.
Figure 7.
Schematic overview of the biosynthesis and downstream metabolism of the “hybrid steroids” 18-hydroxycortisol (18OHF) and 18-oxocortisol (18oxoF). Whereas 18OHF is excreted unmodified in urine, 18oxoF is primarily detected as its tetrahydro metabolite (18oxoTHF).
The Steroid Metabolome in Autonomous Adrenal Steroid Excess
Autonomous adrenal cortisol excess
Clinically overt Cushing syndrome presents with characteristic signs and symptoms (facial plethora, broad and purplish stretch marks, easy bruising, proximal muscle weakness) but also less specific ones (centripetal obesity, acne, hirsutism, oligomenorrhea, edema, hypertension, type 2 diabetes, osteoporosis) (136, 137). Most cases of Cushing syndrome are due to excess stimulation of the adrenals by ACTH, either due to a pituitary tumor (= Cushing disease; 70% to 75%) or ectopic ACTH secretion (10% to 15%). However, in the remaining 10% to 15%, primary adrenal cortisol excess is the cause of disease, mostly due to a glucocorticoid-secreting adrenocortical adenoma (ACA) or, less frequently, an adrenocortical carcinoma (ACC) (136). More rarely, autonomous adrenal cortisol hypersecretion is caused by primary bilateral macronodular adrenal hyperplasia, due to AMRC5 mutations (138), or primary pigmented nodular adrenocortical disease due to inactivating PRKAR1A mutations, affecting the regulatory subunit of the cAMP-dependent protein kinase A (136). Mutations in the PRKACA gene, encoding the catalytic subunit of protein kinase A, have been identified as a frequent cause of cortisol excess in unilateral adrenal adenomas due to somatic driver mutations, but also as a rare germline mutation underlying bilateral macronodular adrenal hyperplasia (139).
Adrenal masses are found incidentally in a large number of individuals, and it is estimated that 5% of the general population harbor an adrenal mass. Only 5% of those are cortisol-producing adenomas that manifest as adrenal Cushing syndrome. However, much larger numbers of ACAs are associated with mild autonomous cortisol excess (MACE), also previously termed subclinical Cushing syndrome (140–143). MACE presents with nonspecific signs and symptoms potentially related to cortisol excess, lacking the characteristic clinical Cushing features. The exact prevalence and clinical implications of this condition remain incompletely determined, but an association with metabolic comorbidities (obesity, type 2 diabetes, hypertension, osteoporosis) has been reported by retrospective studies (144–146) and a recent systematic review and meta-analysis (147).
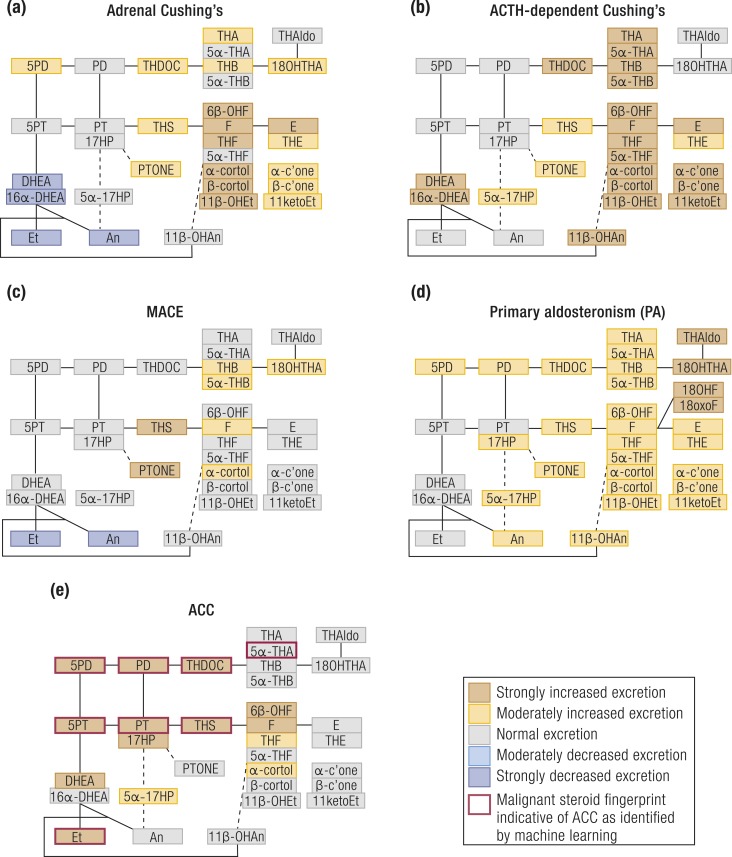
The urine steroid metabolome of patients with overt Cushing syndrome is characterized by excessive excretion of glucocorticoid and mineralocorticoid precursor metabolites, although androgen metabolites tend to be suppressed (148, 149) in adrenal Cushing but increased in ACTH-dependent Cushing syndrome (150, 151) [Fig. 8A and 8B (1, 148–155)]. Similar, albeit less pronounced changes are observed in patients with MACE (148) (Fig. 8C).
Figure 8.
Schematic visualization of urinary steroid metabolome signatures associated with disorders causing autonomous adrenal steroid excess. (a) Adrenal Cushing syndrome, (b) ACTH-dependent Cushing syndrome, (c) MACE, (d) primary aldosteronism, and (e) ACC. The figure depicts the changes in the major urinary metabolites relative to the normal median of each steroid metabolite and does not represent overall quantitative excretion. Steroid metabolites are mapped onto the steroidogenic pathways leading to mineralocorticoid, glucocorticoid, and androgen biosynthesis as shown in Fig. 1. Data derived from (1, 148–155).
In Cushing syndrome, excess cortisol overwhelms the capacity of HSD11B2 (151, 152), the enzyme that inactivates cortisol to cortisone, resulting in a high ratio of urinary (THF+5α-THF)/THE (151). 5α-Reductase activity is mostly decreased in Cushing syndrome, as reflected by an increased THF/5α-THF ratio (151). The possibility that steroid profiling may be used diagnostically for the differential diagnosis of patients with Cushing syndrome has only been tentatively explored (150), and it is not known yet whether different genetic causes of autonomous adrenal cortisol excess manifest with distinct steroid metabolomes.
Autonomous adrenal mineralocorticoid excess
Autonomous aldosterone secretion [primary aldosteronism (PA)] is the most common disorder of adrenal steroidogenesis and represents the predominant cause of secondary hypertension, affecting at least 5% of the hypertensive population (156). The vast majority of PA cases correspond to two types: bilateral hyperaldosteronism (60% to 70%) and unilateral aldosterone-producing adenoma (APA; 30% to 40%) (156). Rare familial forms of PA have been shown to be associated with germline mutations in KCNJ5 (157), CACNA1D (158), CACNA1H (159), and CLCN2 (160). Similarly, somatic driver mutations in KCNJ5, ATP1A1, ATP2B3, CACNA1D, and CTNNB1 have been discovered in APA tissue, altogether accounting for most cases (157, 158, 161–164). Most of these ion channel mutations lead to enhanced calcium influx into zona glomerulosa cells, thereby stimulating aldosterone synthase (CYP11B2) expression (165). Untreated PA exposes patients to a higher cardiovascular risk than for non-PA patients with similar degrees of hypertension (166–170). Differentiation between the different subtypes of PA is clinically important: unilateral disease is an indication for surgery whereas bilateral disease is best managed medically with MR blockers (156).
Biochemically, the hallmark of PA is the combination of high plasma aldosterone and suppressed renin (156). Some PA patients have high circulating levels of the two “hybrid” cortisol metabolites, 18-hydroxycortisol and 18-oxocortisol (Fig. 7) (115, 171, 172). Serum and urinary 18-hydroxycortisol, 18-oxocortisol, and 18-oxo-THF excretion are highest in FHA type 1 (= GRA, see “Glucocorticoid-remediable aldosteronism” above) (124, 173) and FHA type 3 (germline KCNJ5 mutations) (113); they also tend to be higher in APA than in bilateral hyperaldosteronism, albeit with considerable overlap (115). No distinct steroid metabolome signature has been identified for other PA-causing mutations yet, mostly due to the rarity of cases.
Until recently, PA had been regarded as a disorder of mineralocorticoid biosynthesis only; however, it has now been shown that a large proportion of PA patients also demonstrate excessive urinary excretion of glucocorticoid metabolites, as well as of the 11-oxygenated androgen metabolite 11β-OHAn (148) (Fig. 8D). Both the conversions of 11-deoxycortisol to cortisol and A4 to 11OHA4 are catalyzed by the adrenal enzyme CYP11B1, and a recent study showed that the immunohistochemical expression of CYP11B1 in APA tissue correlated with the corresponding excretion of glucocorticoid and 11-oxygenated androgen metabolites (148). This prevalent cosecretion of both cortisol and aldosterone in PA, termed “Connshing syndrome” (148, 174), is likely to explain the reported increased risk of type 2 diabetes, osteoporosis, and depression in PA (153, 175–183), which have no intuitive link to mineralocorticoid activity, but represent commonly noted consequences of cortisol excess.
Autonomous adrenal androgen excess
Adrenal androgen excess is a common feature of steroid excess in patients with ACC, although rarely isolated and more commonly cosecreted with other steroids. Isolated autonomous androgen excess from the adrenal gland can very rarely occur in the context of benign ACAs (1). Isolated macronodular hyperplasia of the zona reticularis is very rare, with only one case reported thus far (184), with the serum steroid profile revealing isolated overproduction of DHEA, DHEAS, and A4, unresponsive to dexamethasone-induced ACTH suppression.
Mixed steroid excess: ACC
ACC is a rare but aggressive malignancy, which accounts for 2% to 11% of adrenal tumors (185). Prompt and accurate differentiation of ACC from benign ACA is the foremost clinical challenge posed by a new adrenal mass. The most useful radiological indicators of malignancy are size and lipid content: the likelihood of malignancy increases with size (186), and lipid-rich lesions are invariably benign (187). A considerable proportion of adrenal tumors, however, are difficult to classify by any imaging modality (187).
Routine serum biochemistry detects steroid hormone excess in 60% to 70% of ACC patients, with glucocorticoid and androgen excess dominating (1, 188); however, urine GC-MS profiling demonstrated steroid excess in >90% (1). Additionally, recent retrospective studies employing GC-MS–based urine steroid profiling revealed that ACCs present a unique steroidogenic “signature” characterized by accumulation of steroid hormone precursors (1, 154, 155) (Fig. 8E). The steroid biomarkers that are most helpful at distinguishing ACCs from ACAs include the glucocorticoid precursor metabolite THS, and the androgen precursor metabolites of Preg and 17OHPreg, 5PD, and 5PT (1). Multiple other Δ5 steroids are also excreted in excess. Recent preliminary retrospective studies also looked at the value of serum multisteroid profiling in detecting ACC (189, 190).
The combination of mass spectrometry–based steroid profiling with machine learning–driven analysis of the urinary steroid excretion data allowed for rapid detection of a “malignant steroid fingerprint” (Fig. 8E) (1) that can differentiate ACC from ACA with high sensitivity and specificity. A large-scale prospective test validation study has recently been completed and results are awaited; if positive, this could represent the first steroid metabolomics approach implemented as a routine diagnostic test.
Urine steroid metabolomics was also used to show that the detection of this malignant steroid fingerprint is not affected by concomitant mitotane treatment in ACC (191), suggesting a potential for noninvasive ACC follow-up monitoring. Additionally, urine steroid metabolome analysis revealed that mitotane strongly inhibits 5α-reductase activity in ACC patients, explaining treatment-related male hypogonadism, and acts as a strong inducer of CYP3A4, resulting in significantly accelerated cortisol inactivation. The latter explains the need for increased doses of glucocorticoid replacement therapy in patients with ACC receiving mitotane treatment (191).
Outlook
The diagnostic potential of steroid metabolome analysis has been recognized since many decades ago and its application has now been extended from inborn steroidogenic disorders to autonomous adrenal steroid excess, yielding fascinating insights. In particular, comprehensive 24-hour urine steroid metabolome analysis has discovered novel steroid pathways and steroidogenic disorders. Recent progress in mass spectrometry technology and methodologies, combined with the development of customized computational approaches to facilitate urine steroid metabolomics analysis, is paving the way for more widespread use of mass spectrometry–based multisteroid profiling and steroid metabolomics approaches in clinical practice. The future is promising for steroid metabolomics, with likely widespread diagnostic and prognostic applications of this fascinating discovery tool.
Acknowledgments
We thank Trish Storbeck for preparation of the figure drafts.
Financial Support: This work was supported by the Wellcome Trust [Investigator Grant WT209492/Z/17/Z (to W.A.); Clinical Research Training Fellowship WT101671AIA (to V.C.)]; the Medical Research Council UK [Clinical Research Training Fellowships G1001964 (to J.I.) and MR/R017913/1 (to E.S.B.)]; Diabetes UK [Sir George Alberti Research Training Fellowship 18/0005782 (to A.P.)]; the Academy of Medical Sciences UK [Newton Advanced Fellowship NAF004/1002 (to K.-H.S.)]; and Starter Grants for Clinical Lecturers SGL020/1018 (to V.C.) and SGL020/1013 (to J.I.). W.A. receives support from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham (Grant BRC-1215-20009). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Glossary
Abbreviations
- 3β5α-PD
5α-pregnane-3β,20α-diol
- 6α-OHTHA
6α-hydroxy-11-dehydro-tetrahydrocorticosterone;
- 18OHB
18-hydroxycorticosterone
- A4
androstenedione
- ACA
adrenocortical adenoma
- ACC
adrenocortical carcinoma
- ACRD
apparent cortisone reductase deficiency
- AME
apparent mineralocorticoid excess
- An
androgen
- APA
aldosterone-producing adenoma
- CAH
congenital adrenal hyperplasia
- CMO I
corticosterone methyloxidase I
- CMO II
corticosterone methyloxidase II
- CRD
cortisone reductase deficiency
- CYB5A
cytochrome b5
- CYP
cytochrome P450
- DOC
deoxycorticosterone
- DSD
disorder of sex development
- FHA
familial hyperaldosteronism
- GC-MS
gas chromatography–mass spectrometry
- GRA
glucocorticoid-remediable aldosteronism
- hCG
human chorionic gonadotropin
- HPA
hypothalamic–pituitary–adrenal
- MACE
mild autonomous cortisol excess
- MR
mineralocorticoid receptor
- PA
primary aldosteronism
- PCOS
polycystic ovary syndrome
- PHA
pseudohypoaldosteronism
- POR
cytochrome P450 oxidoreductase
- PORD
POR deficiency
- STS
steroid sulfatase
- STSD
steroid sulfatase deficiency
- T
testosterone
- UHPLC-MS/MS
ultra-HPLC–tandem mass spectrometry
Additional Information
Disclosure Summary: W.A. is an inventor on a patent for the use of steroid profiling as a biomarker tool in the differential diagnosis of steroid-producing and steroid-dependent tumors (PCT/GB2010/000274). The remaining authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Arlt W, Biehl M, Taylor AE, Hahner S, Libé R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96(12):3775–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biehl M, Schneider P, Smith DJ, Stiekema H, Taylor AE, Hughes BA, Shackleton CHL, Stewart PM, Arlt W. Matrix relevance LVQ in steroid metabolomics based classification of adrenal tumors. Available at: www.i6doc.com/en/livre/?GCOI=28001100967420. Accessed 30 April 2019.
- 3. Bunte K, Smith DJ, Chappell MJ, Hassan-Smith ZK, Tomlinson JW, Arlt W, Tiňo P. Learning pharmacokinetic models for in vivo glucocorticoid activation. J Theor Biol. 2018;455:222–231. [DOI] [PubMed] [Google Scholar]
- 4. Thomson W, Jabbari S, Taylor AE, Arlt W, Smith DJ. Simultaneous parameter estimation and variable selection via the logit-normal continuous analogue of the spike-and-slab prior. J R Soc Interface. 2019;16(150):20180572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Storbeck K-H, Gilligan L, Jenkinson C, Baranowski ES, Quanson JL, Arlt W, Taylor AE. The utility of ultra-high performance supercritical fluid chromatography–tandem mass spectrometry (UHPSFC-MS/MS) for clinically relevant steroid analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1085:36–41. [DOI] [PubMed] [Google Scholar]
- 6. Knuuttila M, Hämäläinen E, Poutanen M. Applying mass spectrometric methods to study androgen biosynthesis and metabolism in prostate cancer. J Mol Endocrinol. 2019;62(4):R255–R267. [DOI] [PubMed] [Google Scholar]
- 7. Shackleton C, Pozo OJ, Marcos J. GC/MS in recent years has defined the normal and clinically disordered steroidome: will it soon be surpassed by LC/tandem MS in this role? J Endocr Soc. 2018;2(8):974–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78(3):C113–C118. [DOI] [PubMed] [Google Scholar]
- 9. Williams RT. Detoxication Mechanisms; the Metabolism and Detoxication of Drugs, Toxic Substances, and Other Organic Compounds. 2nd ed. New York, NY: John Wiley & Sons; 1959. [Google Scholar]
- 10. El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194–2210. [DOI] [PubMed] [Google Scholar]
- 11. Baranowski ES, Arlt W, Idkowiak J. Monogenic disorders of adrenal steroidogenesis. Horm Res Paediatr. 2018;89(5):292–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caulfield MP, Lynn T, Gottschalk ME, Jones KL, Taylor NF, Malunowicz EM, Shackleton CHL, Reitz RE, Fisher DA. The diagnosis of congenital adrenal hyperplasia in the newborn by gas chromatography/mass spectrometry analysis of random urine specimens. J Clin Endocrinol Metab. 2002;87(8):3682–3690. [DOI] [PubMed] [Google Scholar]
- 14. Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CHLL. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol. 2010;121(3-5):496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Idkowiak J, Randell T, Dhir V, Patel P, Shackleton CHL, Taylor NF, Krone N, Arlt W. A missense mutation in the human cytochrome b5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97(3):E465–E475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krone N, Reisch N, Idkowiak J, Dhir V, Ivison HE, Hughes BA, Rose IT, O’Neil DM, Vijzelaar R, Smith MJ, MacDonald F, Cole TR, Adolphs N, Barton JS, Blair EM, Braddock SR, Collins F, Cragun DL, Dattani MT, Day R, Dougan S, Feist M, Gottschalk ME, Gregory JW, Haim M, Harrison R, Olney AH, Hauffa BP, Hindmarsh PC, Hopkin RJ, Jira PE, Kempers M, Kerstens MN, Khalifa MM, Köhler B, Maiter D, Nielsen S, O’Riordan SM, Roth CL, Shane KP, Silink M, Stikkelbroeck NM, Sweeney E, Szarras-Czapnik M, Waterson JR, Williamson L, Hartmann MF, Taylor NF, Wudy SA, Malunowicz EM, Shackleton CH, Arlt W. Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2012;97(2):E257–E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D’Armiento M, Reda G, Kater C, Shackleton CHL, Biglieri EG. 17α-Hydroxylase deficiency: mineralocorticoid hormone profiles in an affected family. J Clin Endocrinol Metab. 1983;56(4):697–701. [DOI] [PubMed] [Google Scholar]
- 18. Neres MS, Auchus RJ, Shackleton CH, Kater CE. Distinctive profile of the 17-hydroxylase and 17,20-lyase activities revealed by urinary steroid metabolomes of patients with CYP17 deficiency. Arq Bras Endocrinol Metabol. 2010;54(9):826–832. [DOI] [PubMed] [Google Scholar]
- 19. Tiosano D, Knopf C, Koren I, Levanon N, Hartmann MF, Hochberg Z, Wudy SA. Metabolic evidence for impaired 17α-hydroxylase activity in a kindred bearing the E305G mutation for isolate 17,20-lyase activity. Eur J Endocrinol. 2008;158(3):385–392. [DOI] [PubMed] [Google Scholar]
- 20. Zhu Y, Cordero JJ, Can S, Cai L, You X, Herrera C, DeFillo-Ricart M, Shackleton C, Imperato-McGinley J. Mutations in CYP11B1 gene: penotype-genotype correlations. Am J Med Genet A. 2003;122A(3):193–200. [DOI] [PubMed] [Google Scholar]
- 21. Rosenfield RL, Rich BH, Wolfsdorf JI, Cassorla F, Parks JS, Bongiovanni AM, Wu CH, Shackleton CH. Pubertal presentation of congenital Δ5–3β-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1980;51(2):345–353. [DOI] [PubMed] [Google Scholar]
- 22. Jones CM, Mallappa A, Reisch N, Nikolaou N, Krone N, Hughes BA, O’Neil DM, Whitaker MJ, Tomlinson JW, Storbeck KH, Merke DP, Ross RJ, Arlt W. Modified-release and conventional glucocorticoids and diurnal androgen excretion in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2017;102(6):1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shackleton C. Genetic disorders of steroid metabolism diagnosed by mass spectrometry. In: Blau N, Duran M, Gibson KM, eds.Laboratory Guide to the Methods in Biochemical Genetics. Berlin, Germany: Springer; 2008:549–605. [Google Scholar]
- 24. Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamrath C, Wettstaedt L, Boettcher C, Hartmann MF, Wudy SA. Androgen excess is due to elevated 11-oxygenated androgens in treated children with congenital adrenal hyperplasia. J Steroid Biochem Mol Biol. 2018;178:221–228. [DOI] [PubMed] [Google Scholar]
- 26. Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012;97(3):E367–E375. [DOI] [PubMed] [Google Scholar]
- 27. Shackleton C, Marcos J, Malunowicz EM, Szarras-Czapnik M, Jira P, Taylor NF, Murphy N, Crushell E, Gottschalk M, Hauffa B, Cragun DL, Hopkin RJ, Adachi M, Arlt W. Biochemical diagnosis of Antley-Bixler syndrome by steroid analysis. Am J Med Genet A. 2004;128A(3):223–231. [DOI] [PubMed] [Google Scholar]
- 28. Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, Tajima T, Takeda R, Fukami M, Ogata T. Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab. 2006;91(7):2643–2649. [DOI] [PubMed] [Google Scholar]
- 29. Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273(6):3158–3165. [DOI] [PubMed] [Google Scholar]
- 30. Keber R, Motaln H, Wagner KD, Debeljak N, Rassoulzadegan M, Ačimovič J, Rozman D, Horvat S. Mouse knockout of the cholesterogenic cytochrome P450 lanosterol 14α-demethylase (Cyp51) resembles Antley-Bixler syndrome. J Biol Chem. 2011;286(33):29086–29097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong T, Shackleton CH, Covey TR, Ellis G. Identification of the steroids in neonatal plasma that interfere with 17 alpha-hydroxyprogesterone radioimmunoassays. Clin Chem. 1992;38(9):1830–1837. [PubMed] [Google Scholar]
- 32. Joannou GE. Identification of 15β-hydroxylated C21 steroids in the neo-natal period: the role of 3α,15β,17α-trihydroxy-5β-pregnan-20-one in the perinatal diagnosis of congenital adrenal hyperplasia (CAH) due to a 21-hydroxylase deficiency. J Steroid Biochem. 1981;14(9):901–912. [DOI] [PubMed] [Google Scholar]
- 33. Małunowicz EM, Mitkowska Z, Bal K, Nizankowska-Błaz T, Moszczyńska E, Iwanicka Z, Romer TE. Definitive diagnosis of enzymatic deficiencies of steroidogenesis in at-risk newborns and infants by urinary marker analysis using GC/MS-SIM. Horm Res. 1997;48(6):243–251. [DOI] [PubMed] [Google Scholar]
- 34. Kamrath C, Hartmann MF, Boettcher C, Zimmer K-P, Wudy SA. Diagnosis of 21-hydroxylase deficiency by urinary metabolite ratios using gas chromatography–mass spectrometry analysis: Reference values for neonates and infants. J Steroid Biochem Mol Biol. 2016;156:10–16. [DOI] [PubMed] [Google Scholar]
- 35. Kraan GPB, Wolthers BG, van der Molen JC, Nagel GT, Drayer NM, Joannou GE. New identified 15β-hydroxylated 21-deoxy-pregnanes in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Steroid Biochem Mol Biol. 1993;45(5):421–434. [DOI] [PubMed] [Google Scholar]
- 36. Christakoudi S, Cowan DA, Christakudis G, Taylor NF. 21-Hydroxylase deficiency in the neonate—trends in steroid anabolism and catabolism during the first weeks of life. J Steroid Biochem Mol Biol. 2013;138:334–347. [DOI] [PubMed] [Google Scholar]
- 37. Geller DH, Auchus RJ, Mendonça BB, Miller WL. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet. 1997;17(2):201–205. [DOI] [PubMed] [Google Scholar]
- 38. Shackleton CHL, Biglieri EG, Roitman E, Honour JW. Metabolism of radiolabeled corticosterone in an adult with the 17α-hydroxylase deficiency syndrome. J Clin Endocrinol Metab. 1979;48(6):976–982. [DOI] [PubMed] [Google Scholar]
- 39. Fennessey PV, Marsh PG, Orr ER, Burnstein P, Betz G. Determination of steroid profiles in healthy and diseased states: identification and quantitation of a block of 17α-hydroxylase. Clin Chim Acta. 1983;129(1):3–11. [DOI] [PubMed] [Google Scholar]
- 40. Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonça BB, Fujieda K, Miller WL. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36(3):228–230. [DOI] [PubMed] [Google Scholar]
- 41. Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CH. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363(9427):2128–2135. [DOI] [PubMed] [Google Scholar]
- 42. Peterson RE, Imperato-McGinley J, Gautier T, Shackleton C. Male pseudohermaphroditism due to multiple defects in steroid-biosynthetic microsomal mixed-function oxidases. A new variant of congenital adrenal hyperplasia. N Engl J Med. 1985;313(19):1182–1191. [DOI] [PubMed] [Google Scholar]
- 43. Kelley RI, Kratz LE, Glaser RL, Netzloff ML, Wolf LM, Jabs EW. Abnormal sterol metabolism in a patient with Antley-Bixler syndrome and ambiguous genitalia. Am J Med Genet. 2002;110(2):95–102. [DOI] [PubMed] [Google Scholar]
- 44. Fukami M, Nagai T, Mochizuki H, Muroya K, Yamada G, Takitani K, Ogata T. Anorectal and urinary anomalies and aberrant retinoic acid metabolism in cytochrome P450 oxidoreductase deficiency. Mol Genet Metab. 2010;100(3):269–273. [DOI] [PubMed] [Google Scholar]
- 45. Ribes V, Otto DME, Dickmann L, Schmidt K, Schuhbaur B, Henderson C, Blomhoff R, Wolf CR, Tickle C, Dollé P. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev Biol. 2007;303(1):66–81. [DOI] [PubMed] [Google Scholar]
- 46. Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol. 2010;163(6):919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pozo OJ, Marcos J, Khymenets O, Pranata A, Fitzgerald CCC, McLeod MD, Shackleton C. Sulfation pathways: alternate steroid sulfation pathways targeted by LC–MS/MS analysis of disulfates: application to prenatal diagnosis of steroid synthesis disorders. J Mol Endocrinol. 2018;61(2):M1–M12. [DOI] [PubMed] [Google Scholar]
- 48. Fukami M, Nishimura G, Homma K, Nagai T, Hanaki K, Uematsu A, Ishii T, Numakura C, Sawada H, Nakacho M, Kowase T, Motomura K, Haruna H, Nakamura M, Ohishi A, Adachi M, Tajima T, Hasegawa Y, Hasegawa T, Horikawa R, Fujieda K, Ogata T. Cytochrome P450 oxidoreductase deficiency: identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. J Clin Endocrinol Metab. 2009;94(5):1723–1731. [DOI] [PubMed] [Google Scholar]
- 49. Idkowiak J, Cragun D, Hopkin RJ, Arlt W.. Cytochrome P450 oxidoreductase deficiency. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993–2019. Available at: www.ncbi.nlm.nih.gov/pubmed/20301592. Accessed 30 August 2019. [Google Scholar]
- 50. Shackleton C, Marcos J, Arlt W, Hauffa BP. Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): a disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley-Bixler syndrome phenotype. Am J Med Genet A. 2004;129A(2):105–112. [DOI] [PubMed] [Google Scholar]
- 51. Reisch N, Idkowiak J, Hughes BA, Ivison HE, Abdul-Rahman OA, Hendon LG, Olney AH, Nielsen S, Harrison R, Blair EM, Dhir V, Krone N, Shackleton CHL, Arlt W. Prenatal diagnosis of congenital adrenal hyperplasia caused by P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2013;98(3):E528–E536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shackleton CH. Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. J Steroid Biochem Mol Biol. 1993;45(1–3):127–140. [DOI] [PubMed] [Google Scholar]
- 53. Honour JW, Anderson JM, Shackleton CH. Difficulties in the diagnosis of congenital adrenal hyperplasia in early infancy: the 11β-hydroxylase defect. Acta Endocrinol (Copenh). 1983;103(1):101–109. [DOI] [PubMed] [Google Scholar]
- 54. Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase gene family. Endocr Rev. 2005;26(4):525–582. [DOI] [PubMed] [Google Scholar]
- 55. Reeder AY, Joannou GE. 15β-Hydroxysteroids (part I). Steroids of the human perinatal period: the synthesis of 3β,15β,17α-trihydroxy-5-pregnen-20-one. Steroids. 1996;61(2):74–81. [DOI] [PubMed] [Google Scholar]
- 56. Thigpen AE, Davis DL, Milatovich A, Mendonca BB, Imperato-McGinley J, Griffin JE, Francke U, Wilson JD, Russell DW. Molecular genetics of steroid 5 alpha-reductase 2 deficiency. J Clin Invest. 1992;90(3):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilson JD, Griffin JE, Russell DW. Steroid 5α-reductase 2 deficiency. Endocr Rev. 1993;14(5):577–593. [DOI] [PubMed] [Google Scholar]
- 58. Mendonca BB, Batista RL, Domenice S, Costa EMF, Arnhold IJP, Russell DW, Wilson JD. Steroid 5α-reductase 2 deficiency. J Steroid Biochem Mol Biol. 2016;163:206–211. [DOI] [PubMed] [Google Scholar]
- 59. Cheon CK. Practical approach to steroid 5alpha-reductase type 2 deficiency. Eur J Pediatr. 2011;170(1):1–8. [DOI] [PubMed] [Google Scholar]
- 60. Maimoun L, Philibert P, Cammas B, Audran F, Bouchard P, Fenichel P, Cartigny M, Pienkowski C, Polak M, Skordis N, Mazen I, Ocal G, Berberoglu M, Reynaud R, Baumann C, Cabrol S, Simon D, Kayemba-Kay’s K, De Kerdanet M, Kurtz F, Leheup B, Heinrichs C, Tenoutasse S, Van Vliet G, Grüters A, Eunice M, Ammini AC, Hafez M, Hochberg Z, Einaudi S, Al Mawlawi H, Nuñez CJ, Servant N, Lumbroso S, Paris F, Sultan C. Phenotypical, biological, and molecular heterogeneity of 5α-reductase deficiency: an extensive international experience of 55 patients. J Clin Endocrinol Metab. 2011;96(2):296–307. [DOI] [PubMed] [Google Scholar]
- 61. Peterson RE, Imperato-McGinley J, Gautier T, Shackleton C. Urinary steroid metabolites in subjects with male pseudohermaphroditism due to 5α-reductase deficiency. Clin Endocrinol (Oxf). 1985;23(1):43–53. [DOI] [PubMed] [Google Scholar]
- 62. Can S, Zhu YS, Cai LQ, Ling Q, Katz MD, Akgun S, Shackleton CH, Imperato-McGinley J. The identification of 5α-reductase-2 and 17β-hydroxysteroid dehydrogenase-3 gene defects in male pseudohermaphrodites from a Turkish kindred. J Clin Endocrinol Metab. 1998;83(2):560–569. [DOI] [PubMed] [Google Scholar]
- 63. Chan AO, But BW, Lee CY, Lam YY, Ng KL, Tung JY, Kwan EY, Chan YK, Tsui TK, Lam AL, Tse WY, Cheung PT, Shek CC. Diagnosis of 5α-reductase 2 deficiency: is measurement of dihydrotestosterone essential? Clin Chem. 2013;59(5):798–806. [DOI] [PubMed] [Google Scholar]
- 64. Perry RJ, Novikova E, Wallace AM, Donaldson MDC. Pitfalls in the diagnosis of 5α-reductase type 2 deficiency during early infancy. Horm Res Paediatr. 2011;75(5):380–382. [DOI] [PubMed] [Google Scholar]
- 65. Geissler WM, Davis DL, Wu L, Bradshaw KD, Patel S, Mendonca BB, Elliston KO, Wilson JD, Russell DW, Andersson S. Male pseudohermaphroditism caused by mutations of testicular 17β-hydroxysteroid dehydrogenase 3. Nat Genet. 1994;7(1):34–39. [DOI] [PubMed] [Google Scholar]
- 66. Boehmer AL, Brinkmann AO, Sandkuijl LA, Halley DJ, Niermeijer MF, Andersson S, de Jong FH, Kayserili H, de Vroede MA, Otten BJ, Rouwé CW, Mendonça BB, Rodrigues C, Bode HH, de Ruiter PE, Delemarre-van de Waal HA, Drop SL. 17β-Hydroxysteroid dehydrogenase-3 deficiency: diagnosis, phenotypic variability, population genetics, and worldwide distribution of ancient and de novo mutations. J Clin Endocrinol Metab. 1999;84(12):4713–4721. [DOI] [PubMed] [Google Scholar]
- 67. Lee YS, Kirk JM, Stanhope RG, Johnston DI, Harland S, Auchus RJ, Andersson S, Hughes IA. Phenotypic variability in 17β-hydroxysteroid dehydrogenase-3 deficiency and diagnostic pitfalls. Clin Endocrinol (Oxf). 2007;67(1):20–28. [DOI] [PubMed] [Google Scholar]
- 68. Khattab A, Yuen T, Yau M, Domenice S, Frade Costa EM, Diya K, Muhuri D, Pina CE, Nishi MY, Yang AC, de Mendonça BB, New MI. Pitfalls in hormonal diagnosis of 17-beta hydroxysteroid dehydrogenase III deficiency. J Pediatr Endocrinol Metab. 2015;28(5–6):623–628. [DOI] [PubMed] [Google Scholar]
- 69. Hegesh E, Hegesh J, Kaftory A. Congenital methemoglobinemia with a deficiency of cytochrome b5. N Engl J Med. 1986;314(12):757–761. [DOI] [PubMed] [Google Scholar]
- 70. Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab. 2010;95(3):994–999. [DOI] [PubMed] [Google Scholar]
- 71. Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA. The regulation of steroid action by sulfation and desulfation. Endocr Rev. 2015;36(5):526–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Williams ML, Elias PM. Stratum corneum lipids in disorders of cornification: increased cholesterol sulfate content of stratum corneum in recessive x-linked ichthyosis. J Clin Invest. 1981;68(6):1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fernandes NF, Janniger CK, Schwartz RA. X-linked ichthyosis: an oculocutaneous genodermatosis. J Am Acad Dermatol. 2010;62(3):480–485. [DOI] [PubMed] [Google Scholar]
- 74. Sánchez-Guijo A, Neunzig J, Gerber A, Oji V, Hartmann MF, Schuppe HC, Traupe H, Bernhardt R, Wudy SA. Role of steroid sulfatase in steroid homeostasis and characterization of the sulfated steroid pathway: evidence from steroid sulfatase deficiency. Mol Cell Endocrinol. 2016;437:142–153. [DOI] [PubMed] [Google Scholar]
- 75. Delfino M, Procaccini EM, Illiano GM, Milone A. X-linked ichthyosis: relation between cholesterol sulphate, dehydroepiandrosterone sulphate and patient’s age. Br J Dermatol. 1998;138(4):655–657. [DOI] [PubMed] [Google Scholar]
- 76. Lykkesfeldt G, Bennett P, Lykkesfeldt AE, Micic S, Møller S, Svenstrup B. Abnormal androgen and oestrogen metabolism in men with steroid sulphatase deficiency and recessive X-linked ichthyosis. Clin Endocrinol (Oxf). 1985;23(4):385–393. [DOI] [PubMed] [Google Scholar]
- 77. Idkowiak J, Taylor AE, Subtil S, O’Neil DM, Vijzelaar R, Dias RP, Amin R, Barrett TG, Shackleton CHL, Kirk JM, Moss C, Arlt W. Steroid sulfatase deficiency and androgen activation before and after puberty. J Clin Endocrinol Metab. 2016;101(6):2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ruokonen A, Oikarinen A, Vihko R. Regulation of serum testosterone in men with steroid sulfatase deficiency: response to human chorionic gonadotropin. J Steroid Biochem. 1986;25(1):113–119. [DOI] [PubMed] [Google Scholar]
- 79. Epstein EH Jr, Krauss RM, Shackleton CH. X-linked ichthyosis: increased blood cholesterol sulfate and electrophoretic mobility of low-density lipoprotein. Science. 1981;214(4521):659–660. [DOI] [PubMed] [Google Scholar]
- 80. Shackleton CHL, Reid S. Diagnosis of recessive X-linked ichthyosis: quantitative HPLC/mass spectrometric analysis of plasma for cholesterol sulfate. Clin Chem. 1989;35(9):1906–1910. [PubMed] [Google Scholar]
- 81. Sánchez-Guijo A, Oji V, Hartmann MF, Traupe H, Wudy SA. Simultaneous quantification of cholesterol sulfate, androgen sulfates, and progestagen sulfates in human serum by LC-MS/MS. J Lipid Res. 2015;56(9):1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Taylor NF, Shackleton CHL. Gas chromatographic steroid analysis for diagnosis of placental sulfatase deficiency: a study of nine patients. J Clin Endocrinol Metab. 1979;49(1):78–86. [DOI] [PubMed] [Google Scholar]
- 83. Marcos J, Craig WY, Palomaki GE, Kloza EM, Haddow JE, Roberson M, Bradley LA, Shackleton CH. Maternal urine and serum steroid measurements to identify steroid sulfatase deficiency (STSD) in second trimester pregnancies. Prenat Diagn. 2009;29(8):771–780. [DOI] [PubMed] [Google Scholar]
- 84. Mueller JW, Idkowiak J, Gesteira TF, Vallet C, Hardman R, van den Boom J, Dhir V, Knauer SK, Rosta E, Arlt W. Human DHEA sulfation requires direct interaction between PAPS synthase 2 and DHEA sulfotransferase SULT2A1. J Biol Chem. 2018;293(25):9724–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Noordam C, Dhir V, McNelis JC, Schlereth F, Hanley NA, Krone N, Smeitink JA, Smeets R, Sweep FC, Claahsen-van der Grinten HL, Arlt W. Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med. 2009;360(22):2310–2318. [DOI] [PubMed] [Google Scholar]
- 86. Tüysüz B, Yılmaz S, Gül E, Kolb L, Bilguvar K, Evliyaoğlu O, Günel M. Spondyloepimetaphyseal dysplasia Pakistani type: expansion of the phenotype. Am J Med Genet A. 2013;161A(6):1300–1308. [DOI] [PubMed] [Google Scholar]
- 87. Iida A, Simsek-Kiper PÖ, Mizumoto S, Hoshino T, Elcioglu N, Horemuzova E, Geiberger S, Yesil G, Kayserili H, Utine GE, Boduroglu K, Watanabe S, Ohashi H, Alanay Y, Sugahara K, Nishimura G, Ikegawa S. Clinical and radiographic features of the autosomal recessive form of brachyolmia caused by PAPSS2 mutations. Hum Mutat. 2013;34(10):1381–1386. [DOI] [PubMed] [Google Scholar]
- 88. Miyake N, Elcioglu NH, Iida A, Isguven P, Dai J, Murakami N, Takamura K, Cho TJ, Kim OH, Hasegawa T, Nagai T, Ohashi H, Nishimura G, Matsumoto N, Ikegawa S. PAPSS2 mutations cause autosomal recessive brachyolmia. J Med Genet. 2012;49(8):533–538. [DOI] [PubMed] [Google Scholar]
- 89. Oostdijk W, Idkowiak J, Mueller JW, House PJ, Taylor AE, O’Reilly MW, Hughes BA, de Vries MC, Kant SG, Santen GW, Verkerk AJ, Uitterlinden AG, Wit JM, Losekoot M, Arlt W. PAPSS2 deficiency causes androgen excess via impaired DHEA sulfation—in vitro and in vivo studies in a family harboring two novel PAPSS2 mutations. J Clin Endocrinol Metab. 2015;100(4):E672–E680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lawson AJ, Walker EA, Lavery GG, Bujalska IJ, Hughes B, Arlt W, Stewart PM, Ride JP. Cortisone-reductase deficiency associated with heterozygous mutations in 11β-hydroxysteroid dehydrogenase type 1. Proc Natl Acad Sci USA. 2011;108(10):4111–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, Lavery GG, Bedendo O, Ray DW, Laing I, Malunowicz E, White PC, Hewison M, Mason PJ, Connell JM, Shackleton CH, Stewart PM. Mutations in the genes encoding 11β-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat Genet. 2003;34(4):434–439. [DOI] [PubMed] [Google Scholar]
- 92. Lavery GG, Walker EA, Tiganescu A, Ride JP, Shackleton CHL, Tomlinson JW, Connell JM, Ray DW, Biason-Lauber A, Malunowicz EM, Arlt W, Stewart PM. Steroid biomarkers and genetic studies reveal inactivating mutations in hexose-6-phosphate dehydrogenase in patients with cortisone reductase deficiency. J Clin Endocrinol Metab. 2008;93(10):3827–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Idkowiak J, Lavery GG, Dhir V, Barrett TG, Stewart PM, Krone N, Arlt W. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165(2):189–207. [DOI] [PubMed] [Google Scholar]
- 94. Phillipov G, Palermo M, Shackleton CH. Apparent cortisone reductase deficiency: a unique form of hypercortisolism. J Clin Endocrinol Metab. 1996;81(11):3855–3860. [DOI] [PubMed] [Google Scholar]
- 95. Lavery GG, Idkowiak J, Sherlock M, Bujalska I, Ride JP, Saqib K, Hartmann MF, Hughes B, Wudy SA, De Schepper J, Arlt W, Krone N, Shackleton CH, Walker EA, Stewart PM. Novel H6PDH mutations in two girls with premature adrenarche: “apparent” and “true” CRD can be differentiated by urinary steroid profiling. Eur J Endocrinol. 2013;168(2):K19–K26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shackleton CHL, Honour JW, Dillon MJ, Chantler C, Jones RWA. Hypertension in a four-year-old child: gas chromatographic and mass spectrometric evidence for deficient hepatic metabolism of steroids. J Clin Endocrinol Metab. 1980;50(4):786–792. [DOI] [PubMed] [Google Scholar]
- 97. Stewart PM, Corrie JE, Shackleton CH, Edwards CR. Syndrome of apparent mineralocorticoid excess. A defect in the cortisol-cortisone shuttle. J Clin Invest. 1988;82(1):340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shackleton CH, Rodriguez J, Arteaga E, Lopez JM, Winter JS. Congenital 11β-hydroxysteroid dehydrogenase deficiency associated with juvenile hypertension: corticosteroid metabolite profiles of four patients and their families. Clin Endocrinol (Oxf). 1985;22(6):701–712. [DOI] [PubMed] [Google Scholar]
- 99. Mantero F, Palermo M, Petrelli MD, Tedde R, Stewart PM, Shackleton CH. Apparent mineralocorticoid excess: type I and type II. Steroids. 1996;61(4):193–196. [DOI] [PubMed] [Google Scholar]
- 100. Palermo M, Shackleton CHL, Mantero F, Stewart PM. Urinary free cortisone and the assessment of 11β-hydroxysteroid dehydrogenase activity in man. Clin Endocrinol (Oxf). 1996;45(5):605–611. [DOI] [PubMed] [Google Scholar]
- 101. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268–275. [DOI] [PubMed] [Google Scholar]
- 102. Epstein MT, Espiner EA, Donald RA, Hughes H. Effect of eating liquorice on the renin-angiotensin aldosterone axis in normal subjects. BMJ. 1977;1(6059):488–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Farese RV Jr, Biglieri EG, Shackleton CHL, Irony I, Gomez-Fontes R. Licorice-induced hypermineralocorticoidism. N Engl J Med. 1991;325(17):1223–1227. [DOI] [PubMed] [Google Scholar]
- 104. Shimojo M, Stewart PM. Apparent mineralocorticoid excess syndromes. J Endocrinol Invest. 1995;18(7):518–532. [DOI] [PubMed] [Google Scholar]
- 105. Stewart PM, Edwards CR. The cortisol-cortisone shuttle and hypertension. J Steroid Biochem Mol Biol. 1991;40(4-6):501–509. [DOI] [PubMed] [Google Scholar]
- 106. Atanasov AG, Ignatova ID, Nashev LG, Dick B, Ferrari P, Frey FJ, Odermatt A. Impaired protein stability of 11β-hydroxysteroid dehydrogenase type 2: a novel mechanism of apparent mineralocorticoid excess. J Am Soc Nephrol. 2007;18(4):1262–1270. [DOI] [PubMed] [Google Scholar]
- 107. Ulick S, Levine LS, Gunczler P, Zanconato G, Ramirez LC, Rauh W, Rösler A, Bradlow HL, New MI. A syndrome of apparent mineralocorticoid excess associated with defects in the peripheral metabolism of cortisol. J Clin Endocrinol Metab. 1979;49(5):757–764. [DOI] [PubMed] [Google Scholar]
- 108. Monder C, Shackleton CH, Bradlow HL, New MI, Stoner E, Iohan F, Lakshmi V. The syndrome of apparent mineralocorticoid excess: its association with 11β-dehydrogenase and 5β-reductase deficiency and some consequences for corticosteroid metabolism. J Clin Endocrinol Metab. 1986;63(3):550–557. [DOI] [PubMed] [Google Scholar]
- 109. Ulick S, Ramirez LC, New MI. An abnormality in steroid reductive metabolism in a hypertensive syndrome. J Clin Endocrinol Metab. 1977;44(4):799–802. [DOI] [PubMed] [Google Scholar]
- 110. White PC. Abnormalities of aldosterone synthesis and action in children. Curr Opin Pediatr. 1997;9(4):424–430. [DOI] [PubMed] [Google Scholar]
- 111. White PC. Aldosterone synthase deficiency and related disorders. Mol Cell Endocrinol. 2004;217(1–2):81–87. [DOI] [PubMed] [Google Scholar]
- 112. Nguyen HH, Hannemann F, Hartmann MF, Wudy SA, Bernhardt R. Aldosterone synthase deficiency caused by a homozygous L451F mutation in the CYP11B2 gene. Mol Genet Metab. 2008;93(4):458–467. [DOI] [PubMed] [Google Scholar]
- 113. Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human Mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93(8):3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hattangady NG, Karashima S, Yuan L, Ponce-Balbuena D, Jalife J, Gomez-Sanchez CE, Auchus RJ, Rainey WE, Else T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J Mol Endocrinol. 2016;57(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lenders JWM, Williams TA, Reincke M, Gomez-Sanchez CE. Diagnosis of endocrine disease: 18-oxocortisol and 18-hydroxycortisol: is there clinical utility of these steroids? Eur J Endocrinol. 2018;178(1):R1–R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rich GM, Ulick S, Cook S, Wang JZ, Lifton RP, Dluhy RG. Glucocorticoid-remediable aldosteronism in a large kindred: clinical spectrum and diagnosis using a characteristic biochemical phenotype. Ann Intern Med. 1992;116(10):813–820. [DOI] [PubMed] [Google Scholar]
- 117. Nguyen HH, Hannemann F, Hartmann MF, Malunowicz EM, Wudy SA, Bernhardt R. Five novel mutations in CYP11B2 gene detected in patients with aldosterone synthase deficiency type I: Functional characterization and structural analyses. Mol Genet Metab. 2010;100(4):357–364. [DOI] [PubMed] [Google Scholar]
- 118. Shackleton CH, Honour JW, Dillon M, Milla P. Multicomponent gas chromatographic analysis of urinary steroids excreted by an infant with a defect in aldosterone biosynthesis. Acta Endocrinol (Copenh). 1976;81(4):762–773. [DOI] [PubMed] [Google Scholar]
- 119. Honour JW, Dillon MJ, Shackleton CH. Analysis of steroids in urine for differentiation of pseudohypoaldosteronism and aldosterone biosynthetic defect. J Clin Endocrinol Metab. 1982;54(2):325–331. [DOI] [PubMed] [Google Scholar]
- 120. Dillon MJ, Leonard JV, Buckler JM, Ogilvie D, Lillystone D, Honour JW, Shackleton CH. Pseudohypoaldosteronism. Arch Dis Child. 1980;55(6):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hauffa BP, Sólyom J, Gláz E, Shackleton CHL, Wambach G, Vecsei P, Stolecke H, Homoki J. Severe hypoaldosteronism due to corticosterone methyl oxidase type II deficiency in two boys: metabolic and gas chromatography-mass spectrometry studies. Eur J Pediatr. 1991;150(3):149–153. [DOI] [PubMed] [Google Scholar]
- 122. Yong AB, Montalto J, Pitt J, Oakes S, Preston T, Buchanan C. Corticosterone methyl oxidase type II (CMO II) deficiency: biochemical approach to diagnosis. Clin Biochem. 1994;27(6):491–494. [DOI] [PubMed] [Google Scholar]
- 123. Peter M, Partsch CJ, Sippell WG. Multisteroid analysis in children with terminal aldosterone biosynthesis defects. J Clin Endocrinol Metab. 1995;80(5):1622–1627. [DOI] [PubMed] [Google Scholar]
- 124. Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. A chimaeric 11β-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355(6357):262–265. [DOI] [PubMed] [Google Scholar]
- 125. Kater CE, Biglieri EG, Rost CR, Schambelan M, Hirai J, Chang BC, Brust N. The constant plasma 18-hydroxycorticosterone to aldosterone ratio: an expression of the efficacy of corticosterone methyloxidase type II activity in disorders with variable aldosterone production. J Clin Endocrinol Metab. 1985;60(2):225–228. [DOI] [PubMed] [Google Scholar]
- 126. Lee PD, Patterson BD, Hintz RL, Rosenfeld RG. Biochemical diagnosis and management of corticosterone methyl oxidase type II deficiency. J Clin Endocrinol Metab. 1986;62(1):225–229. [DOI] [PubMed] [Google Scholar]
- 127. Ulick S, Wang JZ, Morton DH. The biochemical phenotypes of two inborn errors in the biosynthesis of aldosterone. J Clin Endocrinol Metab. 1992;74(6):1415–1420. [DOI] [PubMed] [Google Scholar]
- 128. Furgeson SB, Linas S. Mechanisms of type I and type II pseudohypoaldosteronism. J Am Soc Nephrol. 2010;21(11):1842–1845. [DOI] [PubMed] [Google Scholar]
- 129. Riepe FG. Pseudohypoaldosteronism. Endocr Dev. 2013;24:86–95. [DOI] [PubMed] [Google Scholar]
- 130. Wolthers BG, Kraan GP, van der Molen JC, Nagel GT, Rouwe CW, Lenting F, Boersma ER. Urinary steroid profile of a newborn suffering from pseudohypoaldosteronism. Clin Chim Acta. 1995;236(1):33–43. [DOI] [PubMed] [Google Scholar]
- 131. Akın L, Kurtoglu S, Kendirci M, Akin MA, Hartmann MF, Wudy SA. Hook effect: a pitfall leading to misdiagnosis of hypoaldosteronism in an infant with pseudohypoaldosteronism. Horm Res Paediatr. 2010;74(1):72–75. [DOI] [PubMed] [Google Scholar]
- 132. Pascoe L, Curnow KM, Slutsker L, Connell JM, Speiser PW, New MI, White PC. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci USA. 1992;89(17):8327–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Litchfield WR, New MI, Coolidge C, Lifton RP, Dluhy RG. Evaluation of the dexamethasone suppression test for the diagnosis of glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 1997;82(11):3570–3573. [DOI] [PubMed] [Google Scholar]
- 134. Mosso L, Gómez-Sánchez CE, Foecking MF, Fardella C. Serum 18-hydroxycortisol in primary aldosteronism, hypertension, and normotensives. Hypertension. 2001;38(3 Pt 2):688–691. [DOI] [PubMed] [Google Scholar]
- 135. Litchfield WR. Glucocorticoid-remediable aldosteronism. Curr Opin Endocrinol Diabetes. 1996;3(3):265–270. [Google Scholar]
- 136. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913–927. [DOI] [PubMed] [Google Scholar]
- 137. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Assié G, Libé R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, Barreau O, Lefèvre L, Sibony M, Guignat L, Rodriguez S, Perlemoine K, René-Corail F, Letourneur F, Trabulsi B, Poussier A, Chabbert-Buffet N, Borson-Chazot F, Groussin L, Bertagna X, Stratakis CA, Ragazzon B, Bertherat J. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med. 2013;369(22):2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Beuschlein F, Fassnacht M, Assié G, Calebiro D, Stratakis CA, Osswald A, Ronchi CL, Wieland T, Sbiera S, Faucz FR, Schaak K, Schmittfull A, Schwarzmayr T, Barreau O, Vezzosi D, Rizk-Rabin M, Zabel U, Szarek E, Salpea P, Forlino A, Vetro A, Zuffardi O, Kisker C, Diener S, Meitinger T, Lohse MJ, Reincke M, Bertherat J, Strom TM, Allolio B. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med. 2014;370(11):1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396–405. [DOI] [PubMed] [Google Scholar]
- 141. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Di Dalmazi G, Pasquali R, Beuschlein F, Reincke M. Subclinical hypercortisolism: a state, a syndrome, or a disease? Eur J Endocrinol. 2015;173(4):M61–M71. [DOI] [PubMed] [Google Scholar]
- 143. Hána V, Ježková J, Kosák M, Kršek M, Hána V, Hill M. Novel GC-MS/MS technique reveals a complex steroid fingerprint of subclinical hypercortisolism in adrenal incidentalomas. J Clin Endocrinol Metab. 2019;104(8):3545–3556. [DOI] [PubMed] [Google Scholar]
- 144. Sereg M, Szappanos A, Tóke J, Karlinger K, Feldman K, Kaszper E, Varga I, Gláz E, Rácz K, Tóth M. Atherosclerotic risk factors and complications in patients with non-functioning adrenal adenomas treated with or without adrenalectomy: a long-term follow-up study. Eur J Endocrinol. 2009;160(4):647–655. [DOI] [PubMed] [Google Scholar]
- 145. Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, Salcuni AS, Dolci A, Mendola M, Arosio M, Ambrosi B, Scillitani A, Ghigo E, Beck-Peccoz P, Terzolo M, Chiodini I. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827–834. [DOI] [PubMed] [Google Scholar]
- 146. Morelli V, Eller-Vainicher C, Salcuni AS, Coletti F, Iorio L, Muscogiuri G, Della Casa S, Arosio M, Ambrosi B, Beck-Peccoz P, Chiodini I. Risk of new vertebral fractures in patients with adrenal incidentaloma with and without subclinical hypercortisolism: a multicenter longitudinal study. J Bone Miner Res. 2011;26(8):1816–1821. [DOI] [PubMed] [Google Scholar]
- 147. Elhassan Y, Alahdab F, Prete A, Delivanis D, Khanna A, Prokop L, Murad M, O’Reilly M, Arlt W, Bancos I.. Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess: a systematic review and meta-analysis. Ann Intern Med. 2019;171(2):107–116. [DOI] [PubMed] [Google Scholar]
- 148. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, Feuchtinger A, Chortis V, Gilligan LC, Ludwig P, Riester A, Asbach E, Hughes BA, O’Neil DM, Bidlingmaier M, Tomlinson JW, Hassan-Smith ZK, Rees DA, Adolf C, Hahner S, Quinkler M, Dekkers T, Deinum J, Biehl M, Keevil BG, Shackleton CH, Deeks JJ, Walch AK, Beuschlein F, Reincke M. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2(8):e93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hines JM, Bancos I, Bancos C, Singh RD, Avula AV, Young WF, Grebe SK, Singh RJ. High-resolution, accurate-mass (HRAM) mass spectrometry urine steroid profiling in the diagnosis of adrenal disorders. Clin Chem. 2017;63(12):1824–1835. [DOI] [PubMed] [Google Scholar]
- 150. Eisenhofer G, Masjkur J, Peitzsch M, Di Dalmazi G, Bidlingmaier M, Grüber M, Fazel J, Osswald A, Beuschlein F, Reincke M. Plasma steroid metabolome profiling for diagnosis and subtyping patients with Cushing syndrome. Clin Chem. 2018;64(3):586–596. [DOI] [PubMed] [Google Scholar]
- 151. Stewart PM, Walker BR, Holder G, O’Halloran D, Shackleton CH. 11beta-Hydroxysteroid dehydrogenase activity in Cushing’s syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80(12):3617–3620. [DOI] [PubMed] [Google Scholar]
- 152. Ulick S, Wang JZ, Blumenfeld JD, Pickering TG. Cortisol inactivation overload: a mechanism of mineralocorticoid hypertension in the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1992;74(5):963–967. [DOI] [PubMed] [Google Scholar]
- 153. Wu VC, Chang CH, Wang CY, Lin YH, Kao TW, Lin PC, Chu TS, Chang YS, Chen L, Wu KD, Chueh SJ. Risk of fracture in primary aldosteronism: a population-based cohort study. J Bone Miner Res. 2017;32(4):743–752. [DOI] [PubMed] [Google Scholar]
- 154. Kerkhofs TM, Kerstens MN, Kema IP, Willems TP, Haak HR. Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm Cancer. 2015;6(4):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Velikanova LI, Shafigullina ZR, Lisitsin AA, Vorokhobina NV, Grigoryan K, Kukhianidze EA, Strelnikova EG, Krivokhizhina NS, Krasnov LM, Fedorov EA, Sablin IV, Moskvin AL, Bessonova EA. Different types of urinary steroid profiling obtained by high-performance liquid chromatography and gas chromatography-mass spectrometry in patients with adrenocortical carcinoma. Horm Cancer. 2016;7(5-6):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 157. Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Åkerström G, Björklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Scholl UI, Stölting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife. 2015;4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Scholl UI, Stölting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, Yoo T, Choi J, Xu S, Wu A, Kirchner M, Mertins P, Rump LC, Onder AM, Gamble C, McKenney D, Lash RW, Jones DP, Chune G, Gagliardi P, Choi M, Gordon R, Stowasser M, Fahlke C, Lifton RP. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50(3):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mulatero P, Monticone S, Rainey WE, Veglio F, Williams TA. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol. 2013;9(2):104–112. [DOI] [PubMed] [Google Scholar]
- 162. Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, Lucatello B, Ronconi V, Fallo F, Bernini G, Maccario M, Giacchetti G, Veglio F, Warth R, Vilsen B, Mulatero P. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. 2014;63(1):188–195. [DOI] [PubMed] [Google Scholar]
- 163. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440–444, e1–e2. [DOI] [PubMed] [Google Scholar]
- 164. Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AED, Davenport AP, Dekkers T, Tops B, Küsters B, Ceral J, Yeo GSH, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055–1060. [DOI] [PubMed] [Google Scholar]
- 165. Aristizabal Prada ET, Castellano I, Sušnik E, Yang Y, Meyer LS, Tetti M, Beuschlein F, Reincke M, Williams TA. Comparative genomics and transcriptome profiling in primary aldosteronism. Int J Mol Sci. 2018;19(4):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Milliez P, Girerd X, Plouin P-F, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. [DOI] [PubMed] [Google Scholar]
- 167. Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168(1):80–85. [DOI] [PubMed] [Google Scholar]
- 168. Born-Frontsberg E, Reincke M, Rump LC, Hahner S, Diederich S, Lorenz R, Allolio B, Seufert J, Schirpenbach C, Beuschlein F, Bidlingmaier M, Endres S, Quinkler M; Participants of the German Conn’s Registry. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn’s Registry. J Clin Endocrinol Metab. 2009;94(4):1125–1130. [DOI] [PubMed] [Google Scholar]
- 169. Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62(1):62–69. [DOI] [PubMed] [Google Scholar]
- 170. Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M, Endres S; German Conn’s Registry-Else Kröner-Fresenius-Hyperaldosteronism Registry. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. 2012;60(3):618–624. [DOI] [PubMed] [Google Scholar]
- 171. Nakamura Y, Satoh F, Morimoto R, Kudo M, Takase K, Gomez-Sanchez CE, Honma S, Okuyama M, Yamashita K, Rainey WE, Sasano H, Ito S. 18-Oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J Clin Endocrinol Metab. 2011;96(8):E1272–E1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, Williams TA, Bornstein SR, Haase M, Rump LC, Willenberg HS, Beuschlein F, Deinum J, Lenders JW, Reincke M. Mass spectrometry–based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin Chem. 2016;62(3):514–524. [DOI] [PubMed] [Google Scholar]
- 173. Ulick S, Chu MD. Hypersecretion of a new corticosteroid, 18-hydroxycortisol in two types of adrenocortical hypertension. Clin Exp Hypertens A. 1982;4(9–10):1771–1777. [DOI] [PubMed] [Google Scholar]
- 174. Adolf C, Köhler A, Franke A, Lang K, Riester A, Löw A, Heinrich DA, Bidlingmaier M, Treitl M, Ladurner R, Beuschlein F, Arlt W, Reincke M. Cortisol excess in patients with primary aldosteronism impacts left ventricular hypertrophy. J Clin Endocrinol Metab. 2018;103(12):4543–4552. [DOI] [PubMed] [Google Scholar]
- 175. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454–459. [DOI] [PubMed] [Google Scholar]
- 176. Hanslik G, Wallaschofski H, Dietz A, Riester A, Reincke M, Allolio B, Lang K, Quack I, Rump LC, Willenberg HS, Beuschlein F, Quinkler M, Hannemann A; participants of the German Conn’s Registry. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur J Endocrinol. 2015;173(5):665–675. [DOI] [PubMed] [Google Scholar]
- 177. Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, Wu KD, Yang WS; TAIPAI Study Group. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35(8):1698–1708. [DOI] [PubMed] [Google Scholar]
- 178. Beuschlein F, Reincke M, Arlt W. The impact of Connshing’s syndrome—mild cortisol excess in primary aldosteronism drives diabetes risk. J Hypertens. 2017;35(12):2548. [DOI] [PubMed] [Google Scholar]
- 179. Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, Nirschl N, Bidlingmaier M, Beuschlein F, Thorand B, Peters A, Reincke M, Roden M, Quinkler M. Impaired glucose metabolism in primary aldosteronism is associated with cortisol cosecretion. J Clin Endocrinol Metab. 2019;104(8):3192–3202. [DOI] [PubMed] [Google Scholar]
- 180. Fischer E, Adolf C, Pallauf A, Then C, Bidlingmaier M, Beuschlein F, Seissler J, Reincke M. Aldosterone excess impairs first phase insulin secretion in primary aldosteronism. J Clin Endocrinol Metab. 2013;98(6):2513–2520. [DOI] [PubMed] [Google Scholar]
- 181. Salcuni AS, Palmieri S, Carnevale V, Morelli V, Battista C, Guarnieri V, Guglielmi G, Desina G, Eller-Vainicher C, Beck-Peccoz P, Scillitani A, Chiodini I. Bone involvement in aldosteronism. J Bone Miner Res. 2012;27(10):2217–2222. [DOI] [PubMed] [Google Scholar]
- 182. Sonino N, Tomba E, Genesia ML, Bertello C, Mulatero P, Veglio F, Fava GA, Fallo F. Psychological assessment of primary aldosteronism: a controlled study. J Clin Endocrinol Metab. 2011;96(6):E878–E883. [DOI] [PubMed] [Google Scholar]
- 183. Apostolopoulou K, Künzel HE, Gerum S, Merkle K, Schulz S, Fischer E, Pallauf A, Brand V, Bidlingmaier M, Endres S, Beuschlein F, Reincke M. Gender differences in anxiety and depressive symptoms in patients with primary hyperaldosteronism: a cross-sectional study. World J Biol Psychiatry. 2014;15(1):26–35. [DOI] [PubMed] [Google Scholar]
- 184. Ghayee HK, Rege J, Watumull LM, Nwariaku FE, Carrick KS, Rainey WE, Miller WL, Auchus RJ. Clinical, biochemical, and molecular characterization of macronodular adrenocortical hyperplasia of the zona reticularis: a new syndrome. J Clin Endocrinol Metab. 2011;96(2):E243–E250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology clinical practice guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 186. Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, Giovagnetti M, Opocher G, Angeli A; Study Group on Adrenal Tumors of the Italian Society of Endocrinology. A survey on adrenal incidentaloma in Italy. J Clin Endocrinol Metab. 2000;85(2):637–644. [DOI] [PubMed] [Google Scholar]
- 187. Dinnes J, Bancos I, Ferrante Di Ruffano L, Chortis V, Davenport C, Bayliss S, Sahdev A, Guest P, Fassnacht M, Deeks JJ, Arlt W. Management of endocrine disease: imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis European Journal of Endocrinology. Eur J Endocrinol. 2016;175:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Taylor DR, Ghataore L, Couchman L, Vincent RP, Whitelaw B, Lewis D, Diaz-Cano S, Galata G, Schulte K-M, Aylwin S, Taylor NF. A 13-steroid serum panel based on LC-MS/MS: use in detection of adrenocortical carcinoma. Clin Chem. 2017;63(12):1836–1846. [DOI] [PubMed] [Google Scholar]
- 190. Schweitzer S, Kunz M, Kurlbaum M, Vey J, Kendl S, Deutschbein T, Hahner S, Fassnacht M, Dandekar T, Kroiss M. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur J Endocrinol. 2019;180(2):117–125. [DOI] [PubMed] [Google Scholar]
- 191. Chortis V, Taylor AE, Schneider P, Tomlinson JW, Hughes BA, O’Neil DM, Libé R, Allolio B, Bertagna X, Bertherat J, Beuschlein F, Fassnacht M, Karavitaki N, Mannelli M, Mantero F, Opocher G, Porfiri E, Quinkler M, Sherlock M, Terzolo M, Nightingale P, Shackleton CHL, Stewart PM, Hahner S, Arlt W. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5α-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98(1):161–171. [DOI] [PubMed] [Google Scholar]