Abstract
Many anticancer strategies rely on the promotion of apoptosis in cancer cells as a means to shrink tumors. Crucial for apoptotic function are executioner caspases, most notably caspase-3, that proteolyze a variety of proteins, inducing cell death. Paradoxically, overexpression of procaspase-3 (PC-3), the low-activity zymogen precursor to caspase-3, has been reported in a variety of cancer types. Until recently, this counterintuitive overexpression of a pro-apoptotic protein in cancer has been puzzling. Recent studies suggest subapoptotic caspase-3 activity may promote oncogenic transformation, a possible explanation for the enigmatic overexpression of PC-3. Herein, the overexpression of PC-3 in cancer and its mechanistic basis is reviewed; collectively, the data suggest the potential for exploitation of PC-3 overexpression with PC-3 activators as a targeted anticancer strategy.
Keywords: Proteolysis: the enzyme-catalyzed hydrolysis of peptide bonds found in a protein; Zymogen: refers to a precursor protein that upon an activating stimulus (e.g., proteolytic cleavage, liberation of inhibitory entities) forms a more active enzyme; often synonymous with the term proenzyme; Apoptosis: a process of programmed-cell death that culminates in the activation of executioner caspases (caspase-3 and −7) and the cleavage of essential proteins; Procaspase-3 (PC-3): the zymogen form of caspase-3 that has minor proteolytic activity and can autoactivate to form caspase-3; Caspase-3: cysteine protease that cleaves a variety of cellular substrates, leading to phenotypes associated with apoptosis; Labile zinc: the amount of “free” zinc ions within a given system; these ions are in equilibrium with their binding partners and not sequestered in proteins; Genomic instability: a cellular state where the machinery to repair damaged DNA has been compromised (e.g., BRCA mutations) and/or sustains high levels of DNA damage (e.g., double-strand DNA breaks); this landscape is considered a hallmark of cancer as it promotes mutation, a major driver of cancer; Overexpression: an abnormally high level of an entity in a cell, in the context of this review is through the comparison of noncancerous and cancerous tissue; PAC-1: acronym for the first procaspase-activating compound; a small molecule that binds labile zinc, promoting autoactivation of PC-3 to caspase-3 and apoptosis of cancer cells
Graphical Abstract
CANCER, APOPTOSIS, AND PROCASPASE-3
Apoptosis is a central pathway used in organismal development and maintenance of homeostasis, with a crucial role in eliminating genetically unstable or aberrantly growing cells. First postulated in 19721,2 and later evidenced by recognition of the Bcl-2 oncogene,3,4 apoptosis represents a major barrier for the development and progression of cancer. This inhibitory relationship has led to the canonical view that cancers must evade apoptotic induction to root themselves as a developing tumor, with evasion of apoptosis classified as a major hallmark of cancer.5,6 Cancer cells employ a variety of strategies to evade apoptosis, as has been extensively reviewed.5,6 Classically, these strategies follow the dogma that cancers overexpress antiapoptotic proteins or have mutated/downregulated pro-apoptotic proteins, consistent with the notion that apoptosis is tumor suppressive.
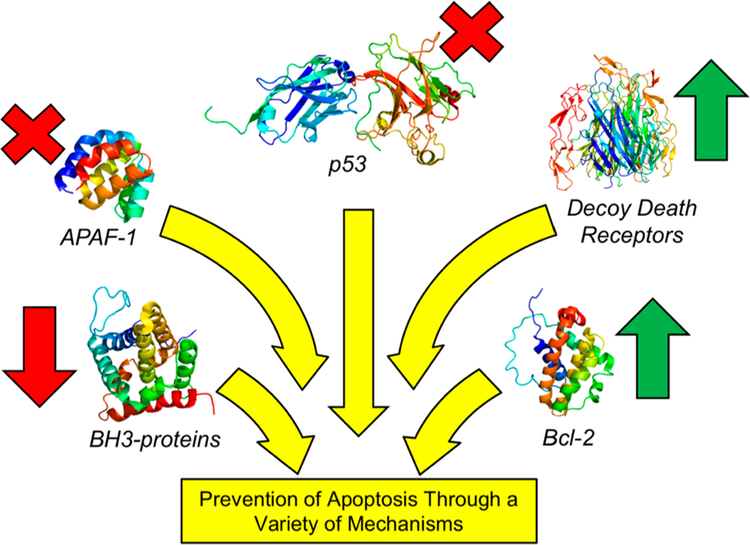
Induction of apoptosis results from a variety of intrinsic or extrinsic signals. Halting intrinsic apoptosis can be the result of multiple mechanisms including (l) shunting pro-apoptotic signals (e.g., p53 loss-of-function mutations),7,8 (2) increased expression of antiapoptotic proteins (e.g., Bcl-2 overexpression),9 or (3) decreased expression of pro-apoptotic proteins (e.g., APAF-1 ).10 Extrinsic apoptosis is often prevented via perturbation of death receptors (e.g., decoy receptors)11,12 or employment of the altered expression patterns described for intrinsic apoptotic evasion. Most importantly for this review, these alterations of apoptosis almost always lie upstream of the proteolytic cleavage of executioner caspases, namely the activation of zymogen procasapase-3 (PC-3) to active caspase-3 (Figure 1).13,14 Of note, loss-of-function mutations of PC-3/caspase-3 are rarely observed in tumors.13,15–17
Figure 1.
Diverse upstream mechanisms employed by cancers to prevent apoptosis. Increased expression of antiapoptotic proteins (green arrows, e.g. Bcl-2, decoy death receptors) or decreased expression or mutation of proapoptotic proteins (red arrows, x’s, e.g. APAF-1, BH3 proteins, p53) drive the net effect of preventing downstream activation of PC-3 to caspase-3 and avoiding apoptotic cell death. Protein structures displayed: p53 (PDB: 4QOl), APAF-1 (PDB: 1CY5), BH3-proteins (Bax, PDB: 1F16), decoy death receptor (Death Receptor 4, PDB: 5CIR, no reported decoy death receptor crystal structures), and Bcl-2 (PDB: 1G5M). For comprehensive reviews on the upstream signaling that results in apoptosis, see refs 14 and 25.
Interestingly, recent studies suggest there are also oncogenic roles for pro-apoptotic machinery,18–24 including PC-3. In this review, we analyze the multidisciplinary work surrounding the study of PC-3 expression and its role in oncogenesis, the biochemistry and cellular biology of PC-3 regulation, and therapeutic development seeking to utilize PC-3 overexpression as a target for selective anticancer therapy. Specifically, the evidence for overexpression of PC-3 in multiple cancers is summarized, and the landscape of PC-3 gene and enzymatic regulation is detailed. These data provide an emerging explanation for PC-3 overexpression in cancer, and this common aberration in cancer suggests a broadly leverageable therapeutic target.
Procaspase-3 Activation to Caspase-3.
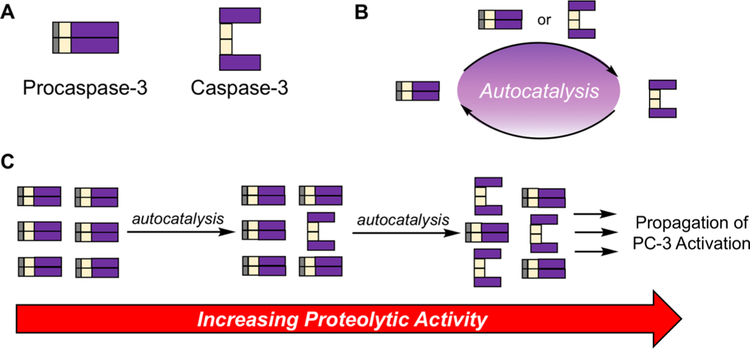
PC-3, the precursor to caspase-3, consists of a prodomain, a large subunit, and a small subunit (Figure 2A). PC-3 activation to caspase-3 results from proteolysis at Asp9, Asp28, and Asp 175.26–28 Caspase-3 is a cysteine protease that cleaves over 200 proteins and ultimately leads to apoptotic cell death.29–31 The conversion of PC-3 to caspase-3 is a crucial node of apoptosis and is often considered as a “point of no return” for a cell. While PC-3 is generally regarded as the inactive zymogen form of caspase-3, multiple groups have demonstrated that PC-3 does have proteolytic activity, albeit at least 200-fold less active than caspase-326,28,32 (although experimental care must be taken to ensure observed activity is not due to small amounts of contaminating caspase-333). This is perhaps best evidenced by experiments in which proteolysis was observed with a noncleavable mutant of PC-3, which is unable to form caspase-3.28 While in canonical apoptosis PC-3 is cleaved to caspase-3 via activity of caspase-8/−9, PC-3 activation can also be the result of an autocatalytic mechanism in which PC-3 or caspase-3 cleaves another equivalent of PC-3 (Figure 2B).28,33 This autocatalysis enables minimal activity of PC-3/caspase-3 to propagate, having profound effects in cells (Figure 2C). As such, analyses that implicate caspase-3 proteolysis are complicated by intrinsic activity of PC-3, and small perturbations in basal PC-3/ caspase-3 levels and activity can lead to significant outcomes as demonstrated by engineered overexpression of PC-3/caspase-3. experiments34–36.
Figure 2.
Autocatalytic PC-3 activation, resulting in large effects from minor initial activity. (A) Pictorial representation of PC-3 and caspase-3 domains. (B) Reaction scheme for PC-3 activation to form caspase-3. While PC-3 is canonically cleaved by caspase-8 or caspase-9, caspase-3 can be formed by PC-3-mediated proteolysis of another PC-3 protein, or caspase-3 mediated cleavage of PC-3. (C) Initial PC-3 activation to form caspase-3 leads to a proteolytic cascade to form increased levels of caspase-3, in turn increasing proteolysis.
Procaspase-3 Overexpression in Cancer.
PC-3 overexpression in cancer has been reported in a variety of contexts, summarized in Table 1. There are a few caveats to this compiled data set. First, in studies on this topic it is not always reported if the antibodies used are specific for caspase-3, PC-3, or both. We have excluded references that are vague in their antibody descriptions, such as reports that solely measure active caspase-3 levels in tumors. Another note is defining the term overexpression. “Overexpression” in Table 1 is noted
Table 1.
Procaspase-3 Expression Levels in Cancer
| cancer typea | PC-3 expression levels | refs | |
|---|---|---|---|
| blood | ALL | overexpressed | 37, 38 |
| AML | overexpressed | 38, 39 | |
| BL/BLL | overexpressed | 40 | |
| CLL | overexpressed | 41, 42 | |
| DLBCL | overexpressed | 43 | |
| NHL | overexpressed | 41, 44 | |
| underexpressed | 45 | ||
| childhood NHL | overexpressed | 46 | |
| multiple myeloma | overexpressed | 47 | |
| brain | astrocytomas | overexpressed | 48, 49 |
| glioblastoma | overexpressed | 48–51 | |
| meningioma | overexpressed | 49, 52, 53 | |
| neuroblastoma | overexpressed | 54 | |
| oligodendrogliomas | overexpressed | 49 | |
| solid tumors | breast | overexpressed | 55–60 |
| underexpressed | 61 | ||
| cervical | overexpressed | 62, 63 | |
| colorectal | overexpressed | 32, 64–67 | |
| underexpressed | 68 | ||
| esophageal | overexpressed | 69, 70 | |
| gallbladder | overexpressed | 71 | |
| gastric | overexpressed | 72 | |
| hepatocellular | overexpressed | 73 | |
| underexpressed | 74, 75 | ||
| NSCLC | overexpressed | 76–81 | |
| melanoma | overexpressed | 82, 83 | |
| pancreatic | similar | 84 | |
| overexpressed | 85, 86 | ||
| prostate | overexpressed | 87 | |
| underexpressed | 88–90 | ||
| SCC | overexpressed | 91–93 | |
| stomach | overexpressed | 94 |
ALL, Acute Lymphocytic Leukemia; AML, Acute Myeloid Leukemia; BL/BLL, Burkitt Lymphoma/Burkitt-Like Lymphoma; CLL, Chronic Lymphocytic Leukemia; DLBCL, Diffuse Large B-Cell Lymphoma; NSCLC, Non-Small Cell Lung Cancer; SCC, Squamous Cell Carcinoma.
when a report describes abnormally high expression of PC-3 as compared to matched normal tissue (the ideal case) or increased expression when comparing clinical stages of cancerous tissues. “Underexpression” is defined as the opposite case. Finally, there are numerous cancer types that do not appear in Table 1. This is simply because there are no published data about those missing cancers, and as such absence from Table 1 does not imply any information on the PC-3 expression.
Table 1 summarizes a growing body of work suggesting that the overexpression of PC-3 is common across a wide range of cancer types. There are still conflicting reports within some cancers, likely due to insufficient data. For example, in colorectal cancers, Yeatman and co-workers highlighted the correlation of PC-3 expression with the mutational status of APC, a critical tumor suppressor that is mutated in 80% of colorectal cancers.68,95 However, other reports demonstrate robust overexpression of PC-3 in colorectal cancer patient samples with no mention of APC mutational status.32,64–67 Such inconsistencies notwithstanding, the totality of the studies in Table 1 demonstrate strong evidence for the near-ubiquitous overexpression of PC-3 in cancers. These data further demonstrate the continued need for robust tumor samples along with matched normal tissue to empower the understanding of cancer s proteomic landscape.
Transcriptional Regulation of Procaspase-3.
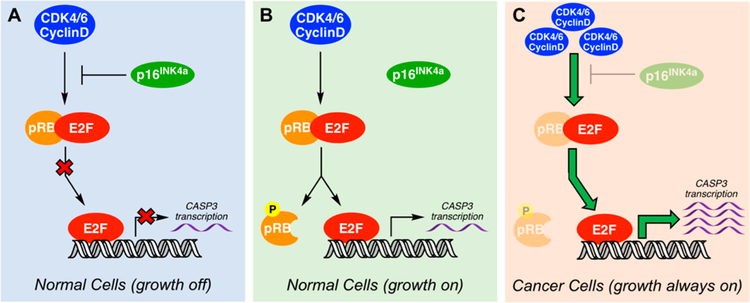
CASP3 (the gene encoding PC-3) is one of the target genes for the E2F family of transcription factors.96 In the absence of growth signals, E2F forms a complex with the retinoblastoma (Rb) family of proteins, specifically pRb,97 which silences its transcriptional activity (Figure 3A). When growth signals are present, CDK4/6 kinases are not inhibited by pl6INK4a (encoded by CDKN2A) and form a complex with cyclin D to phosphorylate pRb. Phosphorylated-pRb dissociates from the pRb–E2F complex, liberating E2F to turn-on transcriptional activity (Figure 3B). Interestingly, the pRb/E2F signaling nexus is often dysregulated in many cancers (for example, through overexpression of CDK4/6 and cyclin D), leading to unfettered transcriptional activity of E2F (Figure 3C).98–103 This common occurrence of pRb/E2F pathway dysregulation in multiple cancers, which funnels to the eventual upregulation of CASP3 transcription, is a possible explanation for the prevalence of PC-3 overexpression across numerous cancers.
Figure 3.
pRB/E2F pathway dysregulation leading to unrestricted CASP3 gene transcription. The pRb/E2F pathway regulation of transcription and cell cycle progression. (A) The transcriptional activity of E2F is inhibited through binding with pRb with further regulation of the pathway via pl6INK4a. (D) In the presence of growth signals, cells turn on E2F transcription via CDK4/6 activation, resulting in phosphorylation of pRB and E2F translocation to the nucleus, turning on transcription of target genes (i.e., CASP3). (C) In cancer cells, the loss of pl6INK4a, pRb, or overexpression of CDK4/6 lead to relief of E2F inhibition, resulting in unregulated transcription. For a comprehensive review of this pathway in cancer, see refs 99 and 101.
Post-Translational Regulation of Procaspase-3 and Caspase-3 with Inhibitory Zinc.
It is important when discussing overexpression of a protein to also consider key post-translational regulation of an enzyme’s activity in cells. Specifically for PC-3 and caspase-3, inhibitory zinc plays a vital regulatory role. Labile zinc pools have been studied in a variety of contexts,104–108 and the labile zinc concentration in cells is estimated to be in the high picomolar to low nanomolar range, suggesting a tightly regulated system of zinc transport.109–112 Preventing aberrant apoptosis is closely tied to maintaining labile zinc,113–115 and there are multiple reports of zinc inhibition of caspase-316 – 20 and PC-328 in biochemical assays.
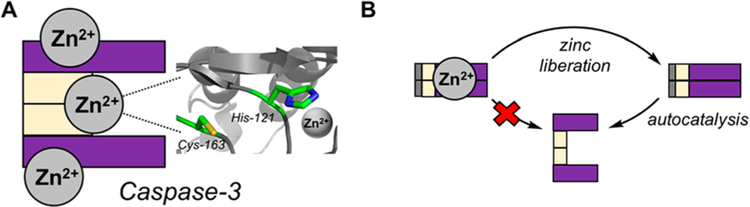
A recent detailed report from Hardy and co-workers121 demonstrates the importance of zinc to modulate the activity of caspases and further establishes the stoichiometry of zinc ion binding, and their experimental values for zinc inhibition of caspase-3 are in agreement with previous experimental methods,118,120 as are their caspase-3/zinc stoichiometries.122 These data suggest zinc inhibits caspase-3 with an IC50 of 12.5 nM, and caspase-3 binds three zinc ions.121 Interestingly, caspase-3 binds these zinc ions even in the presence of a covalent caspase inhibitor (zVAD-FMK). This result suggests that one zinc ion inhibits the active site of caspase-3 while leaving the reactive cysteine unperturbed, consistent with a prior investigation.120 Hardy et al. hypothesize that one zinc ion binds the catalytic histidine (inhibiting proteolysis) while the two other zincs bind in exosites outside the active site of caspase-3 (Figure 4A).121 While studies of inhibitory zinc often focus on caspase-3, PC-3 proteolytic activity and autoactivation to caspase-3 are also inhibited by zinc;28 overall, it appears that zinc plays a significant role in regulating both PC-3 and caspase-3 function (Figure 4B).
Figure 4.
Post-translational regulation of caspase-3 and PC-3 activity via inhibitory zinc. (A) Graphical representation of inhibitory zinc on caspase-3.121 Protein structure displayed is PDB: 2XYG. (B) Zinc binding inhibits PC-3 autocatalysis to form caspase-3. Upon zinc liberation, PC-3 can autoactivate to form caspase-3. Adapted with permission from ref 28. Copyright 2009 Elsevier.
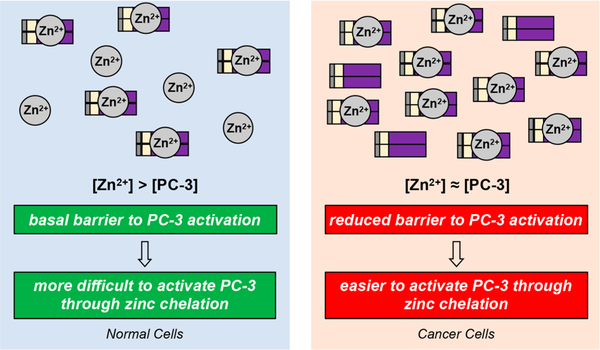
Modulation of zinc levels can be a powerful means to alter caspase activity in a given cell type. Regardless of the absolute zinc levels in cancer cells,123–127 the overexpression of PC-3 across many cancers results in a perturbation of the labile zinc/ PC-3 ratio. This ratio is important for controlling the basal caspase-3 activity in cells, as an increase in PC-3 concentration favors PC-3 activation (Figure 5). Further, this ratio in normal cells (low expressers of PC-3) is a differential that provides a basis for targeting cancer cells specifically with PC-3 activators.
Figure 5.
Perturbation of zinc regulation via increased PC-3 levels in cancer cells. Cancer cells overexpress PC-3, making regulation of PC-3 activity through inhibitory zinc less effective. Given the autocatalytic nature of PC-3 autoactivation and amplification, these changes in zinc/PC-3 ratio may prove sufficient to alter basal PC-3 (and ultimately caspase-3) activity within a cell. Zinc (Zn2+) circles represent the labile zinc pool.
Other Modulations of Caspase-3 Activity.
Post-translational modifications (PTMs) that enhance or inhibit caspase activity can be a mechanism for regulation of caspase activity in cells (comprehensively reviewed by Lavrik and co-workers).128Specifically for caspase-3, there are multiple examples of PTMs that inhibit the enzymatic function of caspase-3, including S-nitrosylation on the catalytic Cysl63,129–33 glutathionylation of cysteine residues,134 and phosphorylation of Seri50 by p38-MAPK.135,136 The overexpression of PC-3 could counteract inhibitory PTMs found on caspase-3 and promote apoptosis in these diseased cells.
The overexpression of the X-linked inhibitor of apoptosis (XIAP) protein is a direct mechanism to inhibit caspase-3 activity and is reported in a variety of cancers. XIAP is an E3 ligase that mediates the ubiquitination of caspase-3,7,9.137,138 However, XIAP does not inhibit PC-3 activity, since the LAP recognition motif is only revealed upon PC-3 cleavage to caspase-3.137,139,140 Therefore, XIAP acts to prevent cytotoxic caspase-3 activity, but this expression does not alter proteolytic events facilitated by PC-3. Perturbing XIAP activity to promote apoptosis has been demonstrated with SMAC mimetics, and there are ongoing explorations of these anticancer agents.138
Proteolysis through Caspase-3 and Its Role in Cancer.
While sufficiently high caspase-3 activity leads to apoptotic death, it now appears that PC-3 and caspase-3 activity may have nonapoptotic roles and broader effects on a cell population (Figure 6A).141 The possibility of PC-3/caspase-3 activity as pro-tumorigenic has significant implications for basic and translational research, and these single cell and tumor-microenvironment mechanisms have been the focus of recent reviews.18–24 Here, we consider implications of minimal caspase-3-like activities (either from PC-3 or caspase-3), providing a possible advantage for cancer cells that overexpress PC-3.
Figure 6.
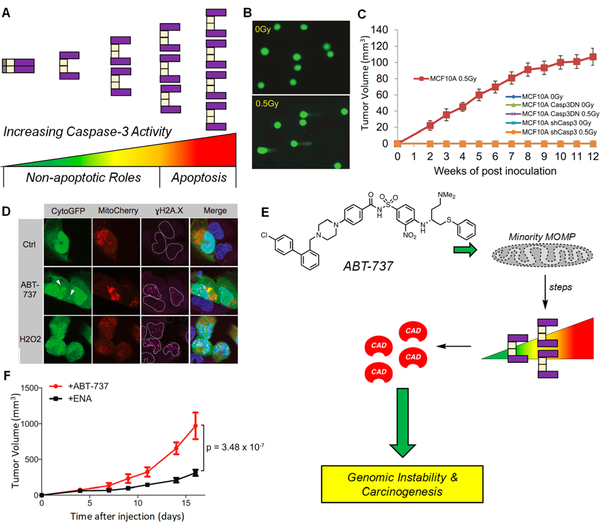
Promotion of DNA damage and genomic instability by subapoptotic caspase-3 activity. (A) Graphical representation of the spectrum of PC-3/caspase-3 activity. Sublethal levels (spanning from green to yellow/orange) represent proteolytic levels not sufficient for apoptotic induction, but sufficient for cleavage of a variety nonlethal protein substrates. Lethal levels (spanning from orange to red) signify irreconcilable caspase-3 activities and result in cell death via apoptosis. (B) Sublethal irradiation of MCF10A induces DNA damage as per the comet tail assay. A larger tail moment indicates increased DNA damage. Reprinted with permission from ref 142. Copyright 2015 Elsevier. (C) Irradiated MCF10A cells form tumors in mice, while shRNA of CASP3 leads to a dramatic reduction in tumor formation (n = 10 per arm, all arms are displayed in the panel). These results indicate an active role of caspase-3 in tumorigenesis. Casp3DN: dominant-negative caspase-3. Reprinted with permission from ref 142. Copyright 2015 Elsevier. (D) Treatment of U20S cells, transiently expressing CytoGFP/Mitocherry, with ABT-737 (5 μΜ) leading to increased /H2AX foci; H202 is the positive control. Increases in yH2AX foci are indicative of heightened DNA damage. Reprinted with permission from ref 145. Copyright 2015 Elsevier. (E) Treatment with ABT-737 causes minority MOMP (displayed as a perforated mitochondria), leading to sublethal levels of caspase-3, resulting in CAD activation and genomic instability and carcinogenesis. (F) Primary pi9Arf null MEF cells were treated with ABT-737 (10 μΜ) or its inactive enantiomer (ENA, 10 μΜ) for 10 passages, then inoculated into mice. Tumor formation is increased when cells were pretreated with ABT-737 (n = 15 per treatment), suggesting that ABT-737 treatment leads to caspase-3 mediated genomic instability that ultimately increases tumorigenicity. Adapted with permission from ref 145. Copyright 2015 Elsevier.
Sub-Lethal Caspase-3 Activity Leads to Genomic Instability.
The elucidation of cellular substrates for caspase-3 reveals that proteins involved in DNA repair are preferentially cleaved by caspase-3 during apoptosis.30 These perturbations in DNA repair protein levels may lead to genomic instability, ultimately enhancing carcinogenesis. For example, Li and coworkers demonstrated that sublethal doses of ionizing radiation led to profound DNA damage (Figure 6B),142 but these genomic instabilities were not observed in cells lacking PC-3 expression or lacking catalytically competent caspase-3. In a follow-up study, the Li group reported sublethal activation of caspase-3 promoted DNA damage as a result of Myc-induced oncogenesis in breast epithelial cells, MCF10A, suggesting caspase-3-dependent oncogenic transformation.143 CRISPR/Cas9-mediated knockout of PC-3 led to reduced DNA damage and abolishment of carcinogenic effects. In both of these studies,142,143 DNA damage was the result of endonuclease G (EndoG), a DNase that is liberated from the mitochondria following caspase-3 activation. EndoG activity has been shown in the presence of pan-caspase inhibitors,144 implying that caspase-3 activation may not be solely responsible for EndoG-mediated DNA damage. Importantly in both studies, tumor formation in mice was compromised when cancer cells had PC-3 knocked down, implying a dependency on PC-3 expression for tumor formation and maintenance (Figure 6C). Thus, the overexpression of PC-3 may not merely be a passive effect but could be a true driver for oncogenic transformation, strengthening the case for PC-3 as a therapeutic target.
In a separate report, Tait and co-workers found that sublethal concentrations of ABT-737, a Bcl-2/Bcl-xL inhibitor, led to minority mitochondrial outer membrane permeablization (MOMP), caspase-3-mediated CAD (caspase-activated DNAase) activation, and extensive DNA damage (Figure 6D,E).145 Treatment with a pan-caspase inhibitor led to no DNA damage upon ABT-737 treatment. Pretreatment of cells with ABT-737 increased tumor formation as compared to pretreatment with the inactive enantiomer of ABT-737 (Figure 6F), consistent with studies by Li and co-workers142,143 and suggesting again that caspase-3 is critical for tumorigenesis. Further validating these reports, other work has revealed that sublethal antimitotic146 and extrinsic-apoptotic agents147 induce DNA damage. Taken together, it appears that caspase-3 promotes DNA damage and oncogenic transformation when sublethal levels of PC-3 activation are induced by a variety of environmental stresses.
These data suggest that there may be an advantage to cancer cells that overexpress PC-3, resulting in higher levels of genetic instability. Given PC-3 autoactivation, a lower barrier to sublethal caspase-3 activity afforded by a decreased zinc/PC-3 ratio (Figure 5) may be sufficient to promote DNA damage and oncogenesis. Whether this lower level of proteolysis is the result of direct PC-3 mediated cleavages resulting from aberrantly high concentrations of PC-3 or low levels of caspase-3 accessed via PC-3 autocatalysis remains unanswered. Regardless, genomic instability resulting from PC-3 overexpression represents a possible explanation for the paradoxical overexpression of PC-3, a canonical pro-apoptotic protein, in cancer. There are other possible pro-oncogenic roles of PC-3/ caspase-3 activity, including promoting a tumorigenic proliferative state, as has been extensively discussed.18–20,22,23,148
Leveraging Procaspase-3 Overexpression for Selective Anticancer Therapy.
The majority of the current anticancer arsenal (both conventional cytotoxins and targeted therapies) relies on robust activation of apoptosis via processes upstream of PC-3 for their antitumor effect.25,149 Due to a variety of mechanisms, these therapies can fail to elicit levels of caspase-3 activity sufficient for apoptotic death, diminishing their efficacy.149 As described above, a low level of caspase-3 activity may actually benefit cancers, which has led to the suggestion of caspase inhibition as a therapeutic strategy.19,20 However, given (l) the downstream location of PC-3 in the apoptotic cascade relative to frequently mutated proteins (Figure 1),150 (2) the low frequency of PC-3 loss-of-function mutations in cancer,15,17 (3) the robust overexpression of PC-3 in a number of cancer types (Table l), and (4) the dependency of tumorigenesis on maintained PC-3 expression (Figure 6), therapeutic interventions that directly activate PC-3 leading to robust caspase-3 activity represent a strategy to overcome apoptotic evasion and synergize with the current suite of anticancer drugs.
Procaspase Activating Compound 1 (PAC-1).
With this backdrop, a first-in-class small molecule activator of PC-3, the first procaspase-activating compound (PAC-1 ), was reported in 2006.64 PAC-1 treatment leads to robust activation of apoptosis in multiple cancer cell lines and patient-derived tumor cells, while having a minimal effect in normal cell lines and matched normal tissues.64,151,152 Through multiple mechanistic studies, it has been shown that PAC-1 leads to PC-3 activation via chelation of the inhibitory zinc of PC-3 (Figure 7A).28 The affinity of PAC-1 for zinc (Kd = 1.28nM)153 allows for chelation of inhibitory zinc from PC-3 but is not strong enough to disrupt proteins containing essential zinc ions.42,15 PAC-1 chelation of intracellular labile zinc has been demonstrated using genetically encoded zinc-selective sensor proteins,155 and this unique mode of action of PAC-1 has been validated via utilization of caspase-specific inhibitors156 caspase-specific substrates151 in work with Bax/Bak double knockout cells157,158 as well as explorations using a PAC-1 derivative in caspase-3/caspase-7 knockout cell lines.42
Figure 7.
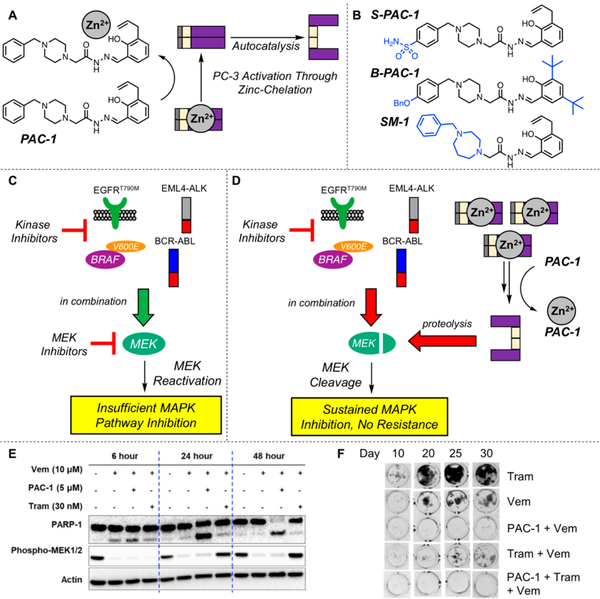
Activation of PC-3 by PAC-1 and its derivatives via chelation of labile inhibitory zinc. (A) PAC-1 binds zinc, alleviating inhibition of PC-3, allowing for autocatalytic formation of caspase-3. (B) Chemical structures of reported derivatives of PAC-1. Deviations from PAC-1 are highlighted in blue. (C) A variety of kinase inhibitors that target mutant proteins found in cancer are effective as single agents and in combination with MEK inhibitors. However, this initial efficacy is short-lived, and resistance invariably occurs through a variety of mechanisms surrounding MEK reactivation.208 (D) PAC-1 treatment synergizes with targeted kinase inhibitors and leads to robust activation of PC-3. These increased levels of caspase-3 activity lead to dramatic reduction of MEK levels via caspase-3 mediated cleavage. This protein degradation strategy sustains inhibition of MEK and the MAPK pathway and delays the onset of resistance.207 (E) Time course of phosphorylated MEK1/2 levels upon treatment of vemurafenib (BRAFv600E inhibitor, Vem), PAC-1, trametinib (MEK1/2 inhibitor, Tram), and combinations as indicated. Experiment was conducted with A375 cells (BRAFv600E cell line). PAC-1 combined with vemurafenib leads to persistent phosphorylated MEK1/2 suppression and increased apoptosis as measured by PARP-1 cleavage. Reprinted with permission from ref 207 (some blots have been removed for simplification). Copyright 2018 Elsevier. (F) Long-term incubation of A375 cells with PAC-1 (l μΜ), vemurafenib (10 μΜ), trametinib (3 nM), and combinations thereof. PAC-1 in combination with kinase inhibitors dramatically decreases the occurrence of resistant cell growth. Reprinted with permission from ref 207 (the orientation of the figure is rotated from the original). Copyright 2018 Elsevier.
The relationship between PAC-1 and other therapeutically relevant metal chelators has been extensively described as has evidence suggesting PAC-1 is not acting through a pan-assay interfering mechanism.25,28,153 Targeting transition metal homeostasis is known to be a viable drug strategy.25,153,159,160 PAC-1 is stable at temperatures and pH values well outside of the physiological range161 and the N-acyl hydrazone functional group is found in other drugs (e.g., rifampicin, eltrombopâ). Over 1000 PAC-1 derivatives have been synthesized,162–169 as recently reviewed.25 In particular, the derivatives S-PAC-1, B-PAC-1, and SM-1 appear to have clinical promise (Figure 7B)>25,47,170–174
PAC-1 has been used as a tool for exploring the effect of direct PC-3 activation in a variety of contexts, highlighted by the recent work of Fuchs and co-workers that elucidated a key role for caspase-3 activity in regulating organ size.175 PAC-1 has also been utilized for the induction of apoptosis176–182 for the direct activation of PC-3 downstream of the mitochondria157,183–189 and has been the subject of detailed preclinical studies.161,190–194 PAC-1 is part of several pharmacological reagent kits (e.g., Sigma LOPAC bioactives library, SCADs inhibitor kit) and has been evaluated in multiple large-scale drug/cell line screens.195–197 The half-life of PAC-1 is ~25 min in mice153 and ~2.1 h in dogs198 but markedly longer in humans (discussed below), suggesting differences between the rodent and human metabolism of PAC-1.
Combination Studies with PAC-1.
Given that PC-3 activation is crucial for apoptotic-inducing agents, investigation of PAC-1 treatment as a means to synergistically enhance the activity of a variety of chemotherapeutics has been explored.51,199 Of interest, PAC-1 + doxorubicin has efficacy in animal models of osteosarcoma, including in canine cancer patients.199 PAC-1 + doxorubicin treatment induced shrinkage of pulmonary macrometastatic lesions in canines with metastatic osteosarcoma (trial size n = 6), and in another small trial PAC-1 + doxorubicin showed impressive results for the treatment of canine lymphoma (trial size n = 4).199
PAC-1 is a blood–brain barrier penetrant155 suggesting the possibility for treating CNS cancers, namely glioblastoma (GBM). PAC-1 induces synergistic apoptosis with the standard-of-care drug temozolomide (TMZ) in many glioma cancer cell lines and in vivo intracranial rodent models.51 Again, a small clinical trial enrolling canine cancer patients with spontaneous glioma allowed for further valuable preclinical assessment of PAC-1, given the outstanding evidence that canine glioma mirrors its human counterpart.49,200–202 Three canine glioma patients were treated with a protocol that mimics human treatment protocols, i.e., PAC-1, radiation, and TMZ cycling. Marked responses were observed with tumor regressions of 43%, 60%, and 100% observed in these three patients51.
As stated by Thornbury and Lazebnik in 1998, proteolysis of a protein represents an irreversible post-translational modification,203 and the ability to degrade a target rather than inhibit its function is an emerging therapeutic strategy.204,205 PAC-1 treatment in combination with a variety of clinically approved kinase inhibitors leads to synergistic caspase-3 activity, resulting in increased apoptotic cell death and delayed onset of resistance.206,207 MEK1/2 are the gatekeeper kinases for ERK½ phosphorylation, and MEK1/2 reactivation is a major driver of resistance to MAPK-pathway targeted agents (Figure 7C).208 Direct PC-3 activation with PAC-1 leads to caspase-3 dependent proteolysis of MEK1/2, irreversibly removing the necessary cellular machinery to reactivate MAPK signaling (Figure 7D). As shown, PAC-1 combinations lead to persistent inhibition of MEK1/2 phosphorylation (Figure 7E), resulting in minimal resistance (Figure 7F).207 This study highlights the ability to leverage caspase-3’s proteolytic substrate scope29,31 to cleave proteins within cells and utilize cancer s overexpression of PC-3 to selectively direct this degradation only to cancer cells.
On the basis of compelling data in mouse models of cancer64,199,206,209 and m canine cancer patients,51,199 a phase 1 clinical trial of PAC-1 for late-stage cancer patients was initiated (). Thus far, it has been reported that PAC-1 has excellent pharmacokinetics in human cancer patients (e.g., a half-life of ~20 h210), and PAC-1 has been dosed as high as 750 mg (oral tablet, once-a-day for 21 days) with signs of efficacy in these late-stage cancer patients.211,212 The FDA granted PAC-1 orphan drug designation for the treatment of GBM, and based on the demonstration that PC-3 expression is increased in glioma samples,48–51 and data showing efficacy of PAC-1 + TMZ in rodent models of glioma and canine glioma patients,51 the combination of PAC-1 and TMZ is being assessed in recurrent human GBM patients. Initial reports of this trial () suggest that the PAC-1/TMZ combination is well-tolerated and that PAC-1 has outstanding pharmacokinetics in this combination study.213
CONCLUSIONS AND OUTLOOK
Apoptosis-inducing drugs are a mainstay of many anticancer regimens. Cancers must evade apoptosis to proliferate, and it appears that certain subapoptotic processes may be advantageous for cancers (e.g., through genetic instability). The zymogen precursor of executioner caspase-3, PC-3, is overexpressed in multiple malignances, and this abnormal expression suggests the possibility for crucial roles of canonical apoptotic machinery in oncogenic transformation. PC-3 presents an opportunity to target cancer through weaponization of PC-3 overexpression to selectively generate cytotoxic caspase-3 in cancer cells. PAC-1 is a first-in-class drug that chelates the inhibitory zinc from PC-3/caspase-3, restoring their enzymatic function. PAC-1 has been useful as a tool to explore procaspase-3 function, and the translational potential of PAC-1 is being evaluated in human cancer patients (, , ). Continued efforts to unravel the overexpression of PC-3 and studies that capitalize on this paradoxical observation will further our understanding of the complex relationship between normal cell growth, apoptosis, and cancer.
ACKNOWLEDGMENTS
We would like to extend our gratitude to B. R. Boudreau for his aid in developing visual aids in this report.
Funding
This work is supported by funding from the National Institutes of Health (R01-CA120439). M.W.B. is a member of the National Institutes of Health Chemistry–Biology Interface Training Program (T32-GM070421).
Footnotes
The authors declare the following competing financial interest(s): The University of Illinois has filed patents on some of the technology described in this manuscript.
REFERENCES
- (1).Kerr JF, Wyllie AH, and Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wyllie AH, Kerr JF, and Currie AR (1980) Cell death: the significance of apoptosis. Int. Rev. Cytol 68, 251–306. [DOI] [PubMed] [Google Scholar]
- (3).Tsujimoto Y, Cossman J, Jaffe E, and Croce CM (1985) Involvement of the bcl-2 gene in human follicular lymphoma. Science 228, 1440–1443. [DOI] [PubMed] [Google Scholar]
- (4).Vaux DL, Cory S, and Adams JM (1988) Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442. [DOI] [PubMed] [Google Scholar]
- (5).Hanahan D, and Weinberg RA (2000) The hallmarks of cancer. Cell 100, 57–70. [DOI] [PubMed] [Google Scholar]
- (6).Hanahan D, and Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- (7).Lowe SW, Cepero E, and Evan G (2004) Intrinsic tumour suppression. Nature 432, 307–315. [DOI] [PubMed] [Google Scholar]
- (8).Evan G, and Littlewood T (1998) A matter of life and cell death. Science 281, 1317–1322. [DOI] [PubMed] [Google Scholar]
- (9).Yip KW, and Reed JC (2008) Bcl-2 family proteins and cancer. Oncogene 27, 6398–6406. [DOI] [PubMed] [Google Scholar]
- (10).Fernald K, and Kurokawa M (2013) Evading apoptosis in cancer. Trends Cell Biol. 23, 620–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kumar R, Herbert PE, and Warrens AN (2005) An introduction to death receptors in apoptosis. Int. J. Surg 3, 268–211. [DOI] [PubMed] [Google Scholar]
- (12).Takeda K, Stagg J, Yagita H, Okumura K, and Smyth MJ (2007) Targeting death-inducing receptors in cancer therapy. Oncogene 26, 3745–3757. [DOI] [PubMed] [Google Scholar]
- (13).Jager R, and Zwacka RM (2010) The enigmatic roles of caspases in tumor development. Cancers 2, 1952–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Olsson M, and Zhivotovsky B (2011) Caspases and cancer. Cell Death Differ. 18, 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Soung YH, Lee JW, Kim SY, Park WS, Nam SW, Lee JY, Yoo NJ, and Lee SH (2004) Somatic mutations of CASP3 gene in human cancers. Hum. Genet 115, 112–115. [DOI] [PubMed] [Google Scholar]
- (16).Chen KX, Zhao H, Hu ZB, Wang LE, Zhang W, Sturgis EM, and Wei QY (2008) CASP3 Polymorphisms and Risk of Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 14, 6343–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, and Los M (2009) Apoptosis and cancer: mutations within caspase genes. J. Med. Genet 46, 497–510. [DOI] [PubMed] [Google Scholar]
- (18).Shalini S, Dorstyn L, Dawar S, and Kumar S (2015) Old, new and emerging functions of caspases. Cell Death Differ. 22, 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ichim G, and Tait SW (2016) A fate worse than death: apoptosis as an oncogenic process. Nat. Rev. Cancer 16, 539–548. [DOI] [PubMed] [Google Scholar]
- (20).Zhao R, Kaakati R, Lee AK, Liu X, Li F, and Li CY (2018) Novel roles of apoptotic caspases in tumor repopulation, epigenetic reprogramming, carcinogenesis, and beyond. Cancer Metastasis Rev. 37, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kalkavan H, and Green DR (2018) MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 25, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Perez-Garijo A (2018) When dying is not the end: Apoptotic caspases as drivers of proliferation. Semin. Cell Dev. Biol 82, 86–95. [DOI] [PubMed] [Google Scholar]
- (23).Cao K, and Tait SWG (2018) Apoptosis and Cancer: Force Awakens, Phantom Menace, or Both? Int. Rev. Cell Mol. Biol 337, 135–152. [DOI] [PubMed] [Google Scholar]
- (24).Kaakati R, Zhao R, Bao X, Lee AK, Liu X, Li F, and Li CY (2019) Non-apoptotic Roles of Caspases in Stem Cell Biology, Carcinogenesis, and Radiotherapy Curr. Stem Cell Rep 5, 31–37. [Google Scholar]
- (25).Roth HS, and Hergenrother PJ (2016) Derivatives of Procaspase-Activating Compound 1 (PAC-l) and their Anticancer Activities. Curr. Med. Chem 23, 201–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bose K, Pop C, Feeney B, and Clark AC (2003) An uncleavable procaspase-3 mutant has a lower catalytic efficiency but an active site similar to that of mature caspase-3. Biochemistry 42, 12298–12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Feeney B, Pop C, Swartz P, Mattos C, and Clark AC (2006) Role of loop bundle hydrogen bonds in the maturation and activity of (Pro)caspase-3. Biochemistry 45, 13249–13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Peterson QP, Goode DR, West DC, Ramsey KN, Lee JJ, and Hergenrother PJ (2009) PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J. Mol. Biol 388, 144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Dix MM, Simon GM, and Cravatt BF (2008) Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell 134, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Mahrus S, Trinidad JC, Barkan DT, Sali A, Burlingame AL, and Wells JA (2008) Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini. Cell 134, 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Dix MM, Simon GM, Wang C, Okerberg E, Patricelli MP, and Cravatt BF (2012) Functional interplay between caspase cleavage and phosphorylation sculpts the apoptotic proteome. Cell ISO, 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Roy S, Bayly CI, Gareau Y, Houtzager VM, Kargman S, Keen SL, Rowland K, Seiden IM, Thornberry NA, and Nicholson DW (2001) Maintenance of caspase-3 proenzyme dormancy by an intrinsic “safety catch” regulatory tripeptide. Proc. Natl. Acad. Sci. U. S. A 98, 6132–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zorn JA, Wolan DW, Agard NJ, and Wells JA (2012) Fibrils colocalize caspase-3 with procaspase-3 to foster maturation. J. Biol. Chem 287, 33781–33795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Gray DC, Mahrus S, and Wells JA (2010) Activation of specific apoptotic caspases with an engineered small-molecule-activated protease. Cell 142, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Smart AD, Pache RA, Thomsen ND, Kortemme T, Davis GW, and Wells JA (2017) Engineering a light-activated caspase-3 for precise ablation of neurons in vivo. Proc. Natl. Acad. Sei. U. S. A 114, E8174–E8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Tenev T, Marani M, McNeish I, and Lemoine NR (2001) Pro-caspase-3 overexpression sensitises ovarian cancer cells to proteasome inhibitors. Cell Death Differ. 8, 256–264. [DOI] [PubMed] [Google Scholar]
- (37).Faderl S, Thall PF, Kantarjian HM, Talpaz M, Harris D, Van Q, Beran M, Kornblau SM, Pierce S, and Estrov Z (1999) Caspase 2 and caspase 3 as predictors of complete remission and survival in adults with acute lymphoblastic leukemia. Clin. Cancer Res. 5, 4041–4047. [PubMed] [Google Scholar]
- (38).Svingen PA, Karp JE, Krajewski S, Mesner PW, Gore SD Jr., Burke PJ, Reed JC, Lazebnik YA, and Kaufmann SH (2000) Evaluation of Apaf-1 and procaspases-2, −3, −7, −8, and −9 as potential prognostic markers in acute leukemia. Blood 96, 3922–3931. [PubMed] [Google Scholar]
- (39).Estrov Z, Thall PF, Talpaz M, Estey EH, Kantarjian HM, Andreeff M, Harris D, Van Q, Walterscheid M, and Kornblau SM (1998) Caspase 2 and caspase 3 protein levels as predictors of survival in acute myelogenous leukemia. Blood 92, 3090–3097. [PubMed] [Google Scholar]
- (40).Nomura Y, Yoshida S, Karube K, Takeshita M, Hirose S, Nakamura S, Yoshino T, Kikuchi M, and Ohshima K (2008) Estimation of the relationship between caspase-3 expression and clinical outcome of Burkitt’s and Burkitt-like lymphoma. Cancer Sei. 99, 1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Krajewski S, Gascoyne RD , Zapata JM, Krajewska M, Kitada S, Chhanabhai M, Horsman D, Berean K, Piro LD, Fugier-Vivier I, Liu YJ, Wang HG, and Reed JC (1997) Immunolocalization of the ICE/Ced-3-family protease, CPP32 (Caspase-3), in non-Hodgkin’s lymphomas, chronic lymphocytic leukemias, and reactive lymph nodes. Blood 89, 3817–3825. [PubMed] [Google Scholar]
- (42).Patel V, Balakrishnan K, Keating MJ, Wierda WG, and Gandhi V (2015) Expression of executioner procaspases and their activation by a procaspase-activating compound in chronic lymphocytic leukemia cells. Blood 125, 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Donoghue S, Baden HS, Lauder I, Sobolewski S, and Pringle JH (1999) Immunohistochemical localization of caspase-3 correlates with clinical outcome in B-cell diffuse large-cell lymphoma. Cancer Res. 59, 5386–5391. [PubMed] [Google Scholar]
- (44).Soini Y, and Paakko P (1999) Apoptosis and expression of caspases 3, 6 and 8 in malignant non-Hodgkin’s lymphomas. APMIS 107, 1043–1050. [DOI] [PubMed] [Google Scholar]
- (45).Chhanabhai M, Krajewski S, Krajewska M, Wang HG, Reed JC, and Gascoyne RD (1997) Immunohistochemical analysis of interleukin-lbeta-converting enzyme/Ced-3 family protease, CPP32/Yama/Caspase-3, in Hodgkin’s disease. Blood 90, 2451–2455. [PubMed] [Google Scholar]
- (46).Wrobel G, Maldyk J, Kazanowska B, Rapala M, Maciejka-Kapuscinska L, and Chaber R (2011) Immunohistochemical expression of procaspase-3 and its clinical significance in childhood non-Hodgkin lymphomas. Pediatr. Dev. Pathol 14, 173–179. [DOI] [PubMed] [Google Scholar]
- (47).Zaman S, Wang R, and Gandhi V (2015) Targeting executioner procaspase-3 with the procaspase-activating compound B-PAC-1 induces apoptosis in multiple myeloma cells. Exp. Hematol 43, 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Bodey B, Bodey V, Siegel SE, Nasir A, Coppola D, Hakam A, and Kaiser HE (2004) Immunocytochemical detection of members of the caspase cascade of apoptosis in high-grade astrocytomas. In Vivo 18, 593–602. [PubMed] [Google Scholar]
- (49).Schiein LJ, Fadl-Alla B, Pondenis HC, Lezmi S, Eberhart CG, LeBlanc AK, Dickinson PJ, Hergenrother PJ, and Fan TM (2019) Immunohistochemical Characterization of Procaspase-3 Overexpression as a Druggable Target With PAC-1, a Procaspase-3 Activator, in Canine and Human Brain Cancers, Front. Oncol 9, DOI: 10.3389/fonc.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Tirapelli LF, Bolini PH, Tirapelli DP, Peria FM, Becker AN, Saggioro FP, and Carlotti CG Jr. (2010) Caspase-3 and Bcl-2 expression in glioblastoma: an immunohistochemical study. Arq. Neuro-Psiquiatr. 68, 603–607. [DOI] [PubMed] [Google Scholar]
- (51).Joshi AD, Botham RC, Schiein LJ, Roth HS, Mangraviti A, Borodovsky A, Tyler B, Joslyn S, Looper JS, Podell M, Fan TM, Hergenrother PJ, and Riggins GJ (2017) Synergistic and targeted therapy with a procaspase-3 activator and Temozolomide extends survival in glioma rodent models and is feasible for the treatment of canine malignant glioma patients. Oncotarget 8, 80124–80138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Konstantinidou AE, Givalos N, Gakiopoulou H, Korkolopoulou P, Kotsiakis X, Boviatsis E, Agrogiannis G, Mahera H, and Patsouris E (2007) Caspase-3 immunohistochemical expression is a marker of apoptosis, increased grade and early recurrence in intracranial meningiomas. Apoptosis 12, 695–705. [DOI] [PubMed] [Google Scholar]
- (53).Vranic A (2013) Caspase-3 and survivin expression in primary atypical and malignant meningiomas. ISRN Neurosci. 2013, 626290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Nakagawara A, Nakamura Y, Ikeda H, Hiwasa T, Kuida K, Su MS, Zhao H, Cnaan A, and Sakiyama S (1997) High levels of expression and nuclear localization of interleukin-1 beta converting enzyme (ICE) and CPP32 in favorable human neuroblastomas. Cancer Res. 57, 4578–4584. [PubMed] [Google Scholar]
- (55).Grigoriev MY, Pozharissky KM, Hanson KP, Imyanitov EN, and Zhivotovsky B (2002) Expression of caspase-3 and −7 does not correlate with the extent of apoptosis in primary breast carcinomas. Cell Cycle 1, 326–331. [PubMed] [Google Scholar]
- (56).Krajewski S, Krajewska M, Turner BC, Pratt C, Howard B, Zapata JM, Frenkel V, Robertson S, Ionov Y, Yamamoto H, Perucho M, Takayama S, and Reed JC (1999) Prognostic significance of apoptosis regulators in breast cancer. Endocr.-Relat. Cancer 6, 29–40. [DOI] [PubMed] [Google Scholar]
- (57).Nakopoulou L, Alexandrou P, Stefanaki K, Panayotopoulou E, Lazaris AC, and Davaris PS (2002) Immunohistochemical expression of caspase-3 as an adverse indicator of the clinical outcome in human breast cancer. Pathobiology 69, 266–273. [DOI] [PubMed] [Google Scholar]
- (58).O’Donovan N, Crown J, Stunell H, Hill ADK, McDermott E, O’Higgins N, and Duffy MJ (2003) Caspase 3 in breast cancer. Clin. Cancer Res. 9, 738–742. [PubMed] [Google Scholar]
- (59).Vakkala M, Paakko P, and Soini Y (1999) Expression of caspases 3, 6 and 8 is increased in parallel with apoptosis and histological aggressiveness of the breast lesion. Br. J. Cancer 81, 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Zapata JM, Krajewska M, Krajewski S, Huang RP, Takayama S, Wang HG, Adamson E, and Reed JC (1998) Expression of multiple apoptosis-regulatory genes in human breast cancer cell lines and primary tumors. Breast Cancer Res. Treat 47, 129–140. [DOI] [PubMed] [Google Scholar]
- (61).Devarajan E, Sahin AA, Chen JS, Krishnamurthy RR, Aggarwal N, Brun AM, Sapino A, Zhang F, Sharma D, Yang XH, Tora AD, and Mehta K (2002) Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene 21, 8843–8851. [DOI] [PubMed] [Google Scholar]
- (62).Shi L, Baohua Z, and Zehua W (2005) Expression of survivin, cyclinDl, p21WAFl, caspase-3 in cervical cancer and its relation with prognosis. J. Huazhong Univ. Sei. Technol., Med. Sei 25, 78–81. [DOI] [PubMed] [Google Scholar]
- (63).Lu H, Gan M, Zhang G, Zhou T, Yan M, and Wang S (2010) Expression of survivin, caspase-3 and p53 in cervical cancer assessed by tissue microarray: correlation with clinicopathology and prognosis. Eur. J. Gynaecol. Oncol 31, 662–666. [PubMed] [Google Scholar]
- (64).Putt KS, Chen GW, Pearson JM, Sandhorst JS, Hoagland MS, Kwon JT, Hwang SK, Jin H, Churchwell MI, Cho MH, Doerge DR, Helferich WG, and Hergenrother PJ (2006) Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat. Chem. Biol 2, 543–550. [DOI] [PubMed] [Google Scholar]
- (65).Flanagan L, Meyer M, Fay J, Curry S, Bacon O, Duessmann H, John IC, Boland KC, McNamara DA, Kay EW, Bantel H, Schulze-Bergkamen H, and Prehn JH (2016) Low levels of Caspase-3 predict favourable response to 5FU-based chemotherapy in advanced colorectal cancer: Caspase-3 inhibition as a therapeutic approach. Cell Death Dis. 7, No. e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Hector S, Conlon S, Schmid J, Dicker P, Cummins RJ, Concannon CG, Johnston PG, Kay EW, and Prehn JH (2012) Apoptosome-dependent caspase activation proteins as prognostic markers in Stage II and III colorectal cancer. Br. J. Cancer 106,1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Sadowska A, Car H, Pryczynicz A, Guzinska-Ustymowicz K, Kowal KW, Cepowicz D, and Kedra B (2014) Expression of apoptotic proteins in human colorectal cancer and metastatic lymph nodes. Pathol, Res. Pract 210, 576–581. [DOI] [PubMed] [Google Scholar]
- (68).Chen TA, Yang I, Irby R, Shain KH, Wang HG, Qpackenbush J, Coppola D, Cheng JQ, and Yeatman TJ (2003) Regulation of caspase expression and apoptosis by adenomatous polyposis coli. Cancer Res. 63, 4368–4374. [PubMed] [Google Scholar]
- (69).Jiang H, Gong M, Cui Y, Ma K, Chang D, and Wang TY (2010) Upregulation of caspase-3 expression in esophageal cancer correlates with favorable prognosis: an immunohistochemical study from a high incidence area in northern China. Dis Esophagus 23,487–492. [DOI] [PubMed] [Google Scholar]
- (70).Wang XS, Luo KJ, Bella AE, Bu SS, Wen J, Zhang SS, and Hu Y (2014) Caspase-3 expression in metastatic lymph nodes of esophageal squamous cell carcinoma is prognostic of survival. World J. Gastroenterol 20, 4414–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Maurya SK, Tewari M, Sharma B, and Shukla HS (2013) Expression of procaspase 3 and activated caspase 3 and its relevance in hormone-responsive gallbladder carcinoma chemotherapy. Korean J. Intern. Med 28, 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Kania J, Konturek SJ, Mariiez K, Hahn EG, and Konturek PC (2003) Expression of survivin and caspase-3 in gastric cancer. Dig. Dis. Sci 48, 266–271. [DOI] [PubMed] [Google Scholar]
- (73).Persad R, Liu C, Wu TT, Houlihan PS, Hamilton SR, Diehl AM, and Rashid A (2004) Overexpression of caspase-3 in hepatocellular carcinomas. Mod. Pathol 17, 861–867. [DOI] [PubMed] [Google Scholar]
- (74).Fujikawa K, Shiraki K, Sugimoto K, Ito T, Yamanaka T, Takase K, and Nakano T (2000) Reduced expression of ICE/ caspase 1 and CPP32/caspase3 in human hepatocellular carcinoma. Anticancer Res. 20, 1927–1932. [PubMed] [Google Scholar]
- (75).Sun BH, Zhang J, Wang BJ, Zhao XP, Wang YK, Yu ZQ, Yang DL, and Hao LJ (2000) Analysis of in vivo patterns of caspase 3 gene expression in primary hepatocellular carcinoma and its relationship to p2l(WAFl) expression and hepatic apoptosis. World J. Gastroenterol 6, 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Krepela E, Prochazka J, Liu X, Fiala P, and Kinkor Z (2004) Increased expression of Apaf-1 and procaspase-3 and the functionality of intrinsic apoptosis apparatus in non-small cell lung carcinoma. Biol. Chem 385, 153–168. [DOI] [PubMed] [Google Scholar]
- (77).Krepela E, Prochazka J, Fiala P, Zatloukal P, and Selinger P (2006) Expression of apoptosome pathway-related transcripts in non-small cell lung cancer. J. Cancer Res. Clin. Oncol 132, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Takata T, Tanaka F, Yamada T, Yanagihara K, Otake Y, Kawano Y, Nakagawa T, Miyahara R, Oyanagi H, Inui K, and Wada H (2001) Clinical significance of caspase-3 expression in pathologic-stage I, nonsmall-cell lung cancer. Int. J. Cancer 96, 54–60. [DOI] [PubMed] [Google Scholar]
- (79).Tormanen-Napankangas U, Soini Y, Kahlos K, Kinnula V, and Paakko P (2001) Expression of caspases-3, –6 and –8 and their relation to apoptosis in non-small cell lung carcinoma. Int. J. Cancer 93, 192–198. [DOI] [PubMed] [Google Scholar]
- (80).Volm M, Mattern J, and Koomagi R (1999) Inverse correlation between apoptotic (Fas ligand, caspase-3) and angiogenic factors (VEGF, microvessel density) in squamous cell lung carcinomas. Anticancer Res. 19, 1669–1671. [PubMed] [Google Scholar]
- (81).Yoo J, Kim S, Shim B, Jo M, Song S, Cho D, Ahn M, Kim C, Cho K, and Kim H (2005) Prognostic significance of caspase-3 and c-myc protein expressions in non-small cell lung cancer. Lung Cancer 49, S299–S299. [Google Scholar]
- (82).Chen N, Gong J, Chen X, Meng W, Huang Y, Zhao F, Wang L, and Zhou Q (2009) Caspases and inhibitor of apoptosis proteins in cutaneous and mucosal melanoma: expression profile and clinicopathologic significance. Hum. Pathol 40, 950–956. [DOI] [PubMed] [Google Scholar]
- (83).Fink D, Schlagbauer-Wadl H, Selzer E, Lucas T, Wolff K, Pehamberger H, Eichler HG, and Jansen B (2001) Elevated procaspase levels in human melanoma. Melanoma Res. 11, 385–393. [DOI] [PubMed] [Google Scholar]
- (84).Jakubowska K, Guzinska-Ustymowicz K, Famulski W, Cepowicz D, Jagodzinska D, and Pryczynicz A (2016) Reduced expression of caspase-8 and cleaved caspase-3 in pancreatic ductal adenocarcinoma cells. Oncol. Lett 11, 1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Virkajarvi N, Paakko P, and Soini Y (1998) Apoptotic index and apoptosis influencing proteins bcl-2, mcl-1, bax and caspases 3, 6 and 8 in pancreatic carcinoma. Histopathology 33, 432–439. [DOI] [PubMed] [Google Scholar]
- (86).Satoh K, Kaneko K, Hirota M, Toyota T, and Shimosegawa T (2000) The pattern of CPP32/caspase-3 expression reflects the biological behavior of the human pancreatic duct cell tumors. Pancreas 21, 352–357. [DOI] [PubMed] [Google Scholar]
- (87).Anwar S, Ambros RA, Jennings TA, Ross JS, Beza A, Mian B, and Nazeer T (2004) Expression of cysteine protease protein 32 in prostatic adenocarcinoma correlates with tumor grade. Arch. Pathol. Lab Med. 128, 649–652. [DOI] [PubMed] [Google Scholar]
- (88).O’Neill AJ, Boran SA, O’Keane C, Coffey RN, Hegarty NJ, Hegarty P, Gaffney EF, Fitzpatrick JM, and Watson RW (2001) Caspase 3 expression in benign prostatic hyperplasia and prostate carcinoma. Prostate 47, 183–188. [DOI] [PubMed] [Google Scholar]
- (89).Rodriguez-Berriguete G, Torrealba N, Ortega MA, Martinez-Onsurbe P, Olmedilla G, Paniagua R, Guil-Cid M, Fraile B, and Royuela M (2015) Prognostic value of inhibitors of apoptosis proteins (iAPs) and caspases in prostate cancer: caspase-3 forms and XIAP predict biochemical progression after radical prostatectomy. BMC Cancer 15, 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Winter RN, Kramer A, Borkowski A, and Kyprianou N (2001) Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 61, 1227–1232. [PubMed] [Google Scholar]
- (91).Poomsawat S, Punyasingh J, and Vejchapipat P (2014) Overexpression of survivin and caspase 3 in oral carcinogenesis. Appl. Immunohistochem Mol. Morphol 22, 65–71. [DOI] [PubMed] [Google Scholar]
- (92).Huang JS, Yang CM, Wang JS, Liou HH, Hsieh IC, Li GC, Huang SJ, Shu CW, Fu TY, Lin YC, Ger LP, and Liu PF (2017) Caspase-3 expression in tumorigenesis and prognosis of buccal mucosa squamous cell carcinoma. Oncotarget 8, 84237–84247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Liu PF, Hu YC, Kang BH, Tseng YK, Wu PC, Liang CC, Hou YY, Fu TY, Liou HH, Hsieh IC, Ger LP, and Shu CW (2017) Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. PLoS One 12, eO 180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).YOO NJ, KIM HS, KIM SY, PARK WS, KIM SH, LEE JY, and LEE SH (2002) Stomach cancer highly expresses both initiator and effector caspases; an immunohistochemical study. Apmis 110, 825–832. [DOI] [PubMed] [Google Scholar]
- (95).Kwong LN, and Dove WF (2009) APC and Its Modifiers in Colon Cancer. Apc Proteins 656, 85–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Müller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, and Helin K (2001) E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15,267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Harbour JW, and Dean DC (2000) The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14, 2393–2409. [DOI] [PubMed] [Google Scholar]
- (98).Ruas M, and Peters G (1998) The pl6INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta, Rev. Cancer 1378, FI 15–177. [DOI] [PubMed] [Google Scholar]
- (99).Nevins JR (2001) The Rb/E2F pathway and cancer. Hum. Mol. Genet 10, 699–703. [DOI] [PubMed] [Google Scholar]
- (100).Deshpande A, Sicinski P, and Hinds PW (2005) Cyclins and cdks in development and cancer: a perspective. Oncogene 24, 2909–2915. [DOI] [PubMed] [Google Scholar]
- (101).Knudsen ES, and Wang JYJ (2010) Targeting the RB-pathway in Cancer Therapy. Clin. Cancer Res. 16, 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Mavrakis KJ, McDonald ER 3rd, Schlabach MR, Billy E, Hoffman GR, deWeck A, Ruddy DA, Venkatesan K, Yu J, McAllister G, Stump M, deBeaumont R, Ho S, Yue Y, Liu Y, Yan-Neale Y, Yang G, Lin F, Yin H, Gao H, Kipp DR, Zhao S, McNamara JT, Sprague ER, Zheng B, Lin Y, Cho YS, Gu J, Crawford K, Ciccone D, Vitari AC, Lai A, Capka V, Hurov K, Porter JA, Tallarico J, Mickanin C, Lees E, Pagliarini R, Keen N, Schmelzle T, Hofmann F, Stegmeier F, and Sellers WR (2016) Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science 351, 1208–1213. [DOI] [PubMed] [Google Scholar]
- (103).Zhao R, Choi BY, Lee MH, Bode AM, and Dong ZG (2016) Implications of Genetic and Epigenetic Alterations of CDKN2A (pl6(lNK4a)) in Cancer. Ebiomedicine 8, 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Fraker PJ, and King LE (2004) Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr 24, 277–298. [DOI] [PubMed] [Google Scholar]
- (105).Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, and Hirano T (2007) Zinc is a novel intracellular second messenger. J. Cell Biol. 177, 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, and Suzuki T (2008) Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv. Immunol 97, 149–176. [DOI] [PubMed] [Google Scholar]
- (107).Prasad AS (2008) Zinc in human health: effect of zinc on immune cells. Mol. Med 14, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Turan B, and Tuncay E (2017) Impact of Labile Zinc on Heart Function: From Physiology to Pathophysiology. Int. J. Mol. Sei 18, DOI: 10.3390/ijmsl8112395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Suhy DA, Simon KD, Linzer DI, and O’Halloran TV (1999) Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J. Biol. Chem 274, 9183–9192. [DOI] [PubMed] [Google Scholar]
- (110).Bozym RA, Thompson RB, Stoddard AK, and Fierke CA (2006) Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem. Biol 1, 103–111. [DOI] [PubMed] [Google Scholar]
- (111).Krezel A, and Maret W (2006) Zinc-buffering capacity of a eukaryotic cell at physiological pZn. JBICJ. Biol. Inorg. Chem 11, 1049–1062. [DOI] [PubMed] [Google Scholar]
- (112).Li Y, and Maret W (2009) Transient fluctuations of intracellular zinc ions in cell proliferation. Exp. Cell Res. 315, 2463–2470. [DOI] [PubMed] [Google Scholar]
- (113).Chai F, Truong-Tran AQ, Ho LH, and Zalewski PD (1999) Regulation of caspase activation and apoptosis by cellular zinc fluxes and zinc deprivation: A review. Immunol. Cell Biol. 77, 272–278. [DOI] [PubMed] [Google Scholar]
- (114).Truong-Tran AQ, Carter J, Ruffin RE, and Zalewski PD (2001) The role of zinc in caspase activation and apoptotic cell death. BioMetals 14, 315–330. [DOI] [PubMed] [Google Scholar]
- (115).Miyai T, Hojyo S, Ikawa T, Kawamura M, Irie T, Ogura H, Hijikata A, Bin BH, Yasuda T, Kitamura H, Nakayama M, Ohara O, Yoshida H, Koseki H, Mishima K, and Fukada T (2014) Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc. Natl. Acad. Sci. U. S. A Ill, 11780–11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, and Hannun YA (1997) Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J. Biol. Chem 272, 18530–18533. [DOI] [PubMed] [Google Scholar]
- (117).Stennicke HR, and Salvesen GS (1997) Biochemical characteristics of caspases-3, –6, –7, and –8. J. Biol. Chem 272, 25719–25723. [DOI] [PubMed] [Google Scholar]
- (118).Maret W, Jacob C, Vallee BL, and Fischer EH (1999) Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc. Natl. Acad. Sci. U. S. A 96, 1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Schrantz N, Auffredou MT, Bourgeade MF, Besnault L, Leca G, and Vazquez A (2001) Zinc-mediated regulation of caspases activity: dose-dependent inhibition or activation of caspase-3 in the human Burkitt lymphoma B cells (Ramos). Cell Death Differ. 8, 152–161. [DOI] [PubMed] [Google Scholar]
- (120).Daniel AG, Peterson EJ, and Farrell NP (2014) The bioinorganic chemistry of apoptosis: potential inhibitory zinc binding sites in caspase-3. Angew. Chem., Int. Ed 53, 4098–4101. [DOI] [PubMed] [Google Scholar]
- (121).Eron SJ, MacPherson DJ, Dagbay KB, and Hardy JA (2018) Multiple Mechanisms of Zinc-Mediated Inhibition for the Apoptotic Caspases-3, −6, −7, and −8. ACS Chem. Biol 13, 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Velazquez-Delgado EM, and Hardy JA (2012) Zinc-mediated allosteric inhibition of caspase-6. J. Biol. Chem 287, 36000–36011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Clegg MS, Hanna LA, Niles BJ, Momma TY, and Keen CL (2005) Zinc deficiency-induced cell death. IUBMB Life 57, 661–669. [DOI] [PubMed] [Google Scholar]
- (124).Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, and Nicholson RI (2007) The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol. Med 13, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Hogstrand C, Kille P, Nicholson RI, and Taylor KM (2009) Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med 15, 101–111. [DOI] [PubMed] [Google Scholar]
- (126).Alam S, and Kelleher SL (2012) Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 4, 875–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Thomas P, Pang Y, Dong J, and Berg AH (2014) Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 155, 4250–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Zamaraev AV, Kopeina GS, Prokhorova EA, Zhivotovsky B, and Lavrik IN (2017) Post-translational Modification of Caspases: The Other Side of Apoptosis Regulation. Trends Cell Biol. 27, 322–339. [DOI] [PubMed] [Google Scholar]
- (129).Kim YM, Talanian RV, and Billiar TR (1997) Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J. Biol. Chem 272, 31138–31148. [DOI] [PubMed] [Google Scholar]
- (130).Li J, Billiar TR, Talanian RV, and Kim YM (1997) Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem. Biophys. Res. Commun 240, 419–424. [DOI] [PubMed] [Google Scholar]
- (131).Mannick JB, Schonhoff C, Papeta N, Ghafourifar P, Szibor M, Fang K, and Gaston B (2001) S-Nitrosylation of mitochondrial caspases. .J. Cell Biol. 154, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Maejima Y, Adachi S, Morikawa K, Ito H, and Isobe M (2005) Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J. Mol. Cell. Cardiol 38, 163–174. [DOI] [PubMed] [Google Scholar]
- (133).Mitchell DA, Morton SU, Fernhoff NB, and Marietta MA (2007) Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc. Natl. Acad. Sei. U. S. A 104, 11609–11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Huang Z, Pinto JT, Deng H, and Richie JP Jr. (2008) Inhibition of caspase-3 activity and activation by protein glutathio-nylation. Biochem. Pharmacol 75, 2234–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Alvarado-Kristensson M, Melander F, Leandersson K, Ronnstrand L, Wernstedt C, and Andersson T (2004) p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J. Exp. Med 199, 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Alvarado-Kristensson M, and Andersson T (2005) Protein phosphatase 2A regulates apoptosis in neutrophils by dephosphorylat-ing both p38 MAPK and its substrate caspase 3. J. Biol. Chem 280, 6238–6244. [DOI] [PubMed] [Google Scholar]
- (137).Gyrd-Hansen M, and Meier P (2010) IAPs: from caspase inhibitors to modulators of NF-kappa B, inflammation and cancer. Nat. Rev. Cancer 10, 561–574. [DOI] [PubMed] [Google Scholar]
- (138).Derakhshan A, Chen Z, and Van Waes C (2017) Therapeutic Small Molecules Target Inhibitor of Apoptosis Proteins in Cancers with Deregulation of Extrinsic and Intrinsic Cell Death Pathways. Clin. Cancer Res. 23, 1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun CH, Fesik SW, Liddington RC, and Salvesen GS (2001) Structural basis for the inhibition of caspase-3 by XIAP. Cell 104, 791–800. [DOI] [PubMed] [Google Scholar]
- (140).Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, and Salvesen GS (2005) XIAP inhibits caspase-3 and −7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 24, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Clark AC (2016) Caspase Allostery and Conformational Selection. Chem. Rev 116, 6666–6706. [DOI] [PubMed] [Google Scholar]
- (142).Liu X, He Y, Li F, Huang Q, Kato TA, Hall RP, and Li CY (2015) Caspase-3 promotes genetic instability and carcinogenesis. Mol. Cell 58, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Cartwright IM, Liu X, Zhou M, Li F, and Li CY (2017) Essential roles of Caspase-3 in facilitating Myc-induced genetic instability and carcinogenesis. eLife 6, DOI: 10.7554/eLife.26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Li LY, Luo X, and Wang X (2001) Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412, 95–99. [DOI] [PubMed] [Google Scholar]
- (145).Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, Haller M, Riley JS, Mason SM, Athineos D, Parsons MJ, van de Kooij B, Bouchier-Hayes L, Chalmers AJ, Rooswinkel RW, Oberst A, Blyth K, Rehm M, Murphy DJ, and Tait SWG (2015) Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell 57, 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Orth JD, Loewer A, Lahav G, and Mitchison TJ (2012) Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol. Biol. Cell 23, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Lovric MM, and Hawkins CJ (2010) TRAIL treatment provokes mutations in surviving cells. Oncogene 29, 5048–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Cheng J, He S, Wang M, Zhou L, Zhang Z, Feng X, Yu Y, Ma J, Dai C, Zhang S, Sun L, Gong Y, Wang Y, Zhao M, Luo Y, Liu X, Tian L, Li C, and Huang Q (2019) The Caspase-3/PKCdelta/Akt/VEGF-A Signaling Pathway Mediates Tumor Repopulation during Radiotherapy. Clin. Cancer Res. 25, 3732–3743. [DOI] [PubMed] [Google Scholar]
- (149).Baig S, Seevasant I, Mohamad J, Mukheem A, Huri HZ, and Kamarul T (2016) Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 7, No. e2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol. Pathol 35, 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Wang F, Wang L, Zhao Y, Li Y, Ping G, Xiao S, Chen K, Zhu W, Gong P, Yang J, and Wu C (2014) A novel small-molecule activator of procaspase-3 induces apoptosis in cancer cells and reduces tumor growth in human breast, liver and gallbladder cancer xenografts. Mol. Oncol 8, 1640–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Wang FY, Liu YJ, Wang LH, Yang JY, Zhao YF, Wang NN, Cao Q, Gong P, and Wu CF (2015) Targeting procaspase-3 with WF-208, a novel PAC-1 derivative, causes selective cancer cell apoptosis. J. Cell Mol. Med 19, 1916–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Roth HS, Botham RC, Schmid SC, Fan TM, Dirikolu L, and Hergenrother PJ (2015) Removal of Metabolic Liabilities Enables Development of Derivatives of Procaspase-Activating Compound 1 (PAC-1) with Improved Pharmacokinetics. J. Med. Chem 58, 4046–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Sjoli S, Solli AI, Akselsen O, Jiang Y, Berg E, Hansen TV, Sylte I, and Winberg JO (2014) PAC-1 and isatin derivatives are weak matrix metalloproteinase inhibitors. Biochim. Biophys. Acta, Gen. Subj 1840, 3162–3169. [DOI] [PubMed] [Google Scholar]
- (155).West DC, Qin Y, Peterson QP, Thomas DL, Palchaudhuri R, Morrison KC, Lucas PW, Palmer AE, Fan TM, and Hergenrother PJ (2012) Differential effects of procaspase-3 activating compounds in the induction of cancer cell death. Mol. Pharmaceutics 9, 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Putinski C, Abdul-Ghani M, Stiles R, Brunette S, Dick SA, Fernando P, and Megeney LA (2013) Intrinsic-mediated caspase activation is essential for cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U. S. A 110, E4079–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Seervi M, Sobhan PK, Mathew KA, Joseph J, Pillai PR, and Santhoshkumar TR (2014) A high-throughput image-based screen for the identification of Bax/Bak-independent caspase activators against drug-resistant cancer cells. Apoptosis 19, 269–284. [DOI] [PubMed] [Google Scholar]
- (158).Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, Parys JB, Leybaert L, and Bultynck G (2012) Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 19, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Weekley CM, and He C (2017) Developing drugs targeting transition metal homeostasis. Curr. Opin. Chem. Biol 37, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Hunsaker EW, and Franz KJ (2019) Emerging Opportunities To Manipulate Metal Trafficking for Therapeutic Benefit. Inorg. Chem DOI: 10.102l/acs.inorgchem.9b01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Song Z, Chen X, Zhang D, Ren L, Fang L, Cheng W, Gong P, and Bi K (2009) Kinetic study of the degradation of PAC-1 and identification of a degradation product in alkaline condition. Chromatographia 70, 1575–1580. [Google Scholar]
- (162).Bao GL, Du BQ, Ma YX, Zhao M, Gong P, and Zhai X (2016) Design, Synthesis and Antiproliferative Activity of Novel Benzothiazole Derivatives Conjugated with Semicarbazone Scaffold. Med. Chem 12, 489–498. [DOI] [PubMed] [Google Scholar]
- (163).Manzoni L, Gornati D, Manzotti M, Cairati S, Bossi A, Arosio D, Lecis D, and Seneci P (2016) Dual action Smac mimetics-zinc chelators as pro-apoptotic antitumoral agents. Bioorg. Med. Chem. Lett 26, 4613–4619. [DOI] [PubMed] [Google Scholar]
- (164).Zhai X, Bao G, Wang L, Cheng M, Zhao M, Zhao S, Zhou H, and Gong P (2016) Design, synthesis and biological evaluation of novel 4-phenoxy-6,7-disubstituted quinolines possessing (thio)semicarbazones as c-Met kinase inhibitors. Bioorg. Med. Chem 24, 1331–1345. [DOI] [PubMed] [Google Scholar]
- (165).Luo HM, Yang CL, Zhang XY, Zhao MM, Jiang D, Xiao JH, Yang XH, and Li S (2013) Design, synthesis and antitumor activity of a novel series of PAC-1 analogues. Chem. Res. Chin. Univ 29, 906–910. [Google Scholar]
- (166).Antonio JPM, Frade R F. M, Santos FMF, Coelho JAS, Afonso CAM, Gois PMP, and Trindade AF (2014) NHC catalysed direct addition of HMF to diazo compounds: synthesis of acyl hydrazones with antitumor activity. RSC Adv. 4, 29352–29356. [Google Scholar]
- (167).Zhai X, Huang Q, Jiang N, Wu D, Zhou H, and Gong P (2013) Discovery of hybrid dual N-acylhydrazone and diaryl urea derivatives as potent antitumor agents: design, synthesis and cytotoxicity evaluation. Molecules 18, 2904–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Astrand OAH, Aziz G, Ali SF, Paulsen RE, Hansen TV, and Rongved P (2013) Synthesis and initial in vitro biological evaluation of two new zinc-chelating compounds: Comparison with TPEN and PAC-1. Bioorg. Med. Chem 21, 5175–5181. [DOI] [PubMed] [Google Scholar]
- (169).Luo H. m., Yang C. l., Zhang X. y., Zhao M. m., Jiang D, Xiao J. h., Yang X. h., and Li S (2013) Design, synthesis and antitumor activity of a novel series of PAC-1 analogues. Chem. Res. Chin. Univ 29, 906–910. [Google Scholar]
- (170).Sarkar A, Balakrishnan K, Chen J, Patel V, Neelapu SS, McMurray JS, and Gandhi V (2016) Molecular evidence of Zn chelation of the procaspase activating compound B-PAC-1 in B cell lymphoma. Oncotarget 7, 3461–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Peterson QP, Hsu DC, Novotny CJ, West DC, Kim D, Schmit JM, Dirikolu L, Hergenrother PJ, and Fan TM (2010) Discovery and canine preclinical assessment of a nontoxic procaspase-3-activating compound. Cancer Res. 70, 7232–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Chen Y, Sun M, Ding J, and Zhu Q (2016) SM-1, a novel PAC-1 derivative, activates procaspase-3 and causes cancer cell apoptosis. Cancer Chemother. Pharmacol 78, 643–654. [DOI] [PubMed] [Google Scholar]
- (173).Wang Y, Yuan S, Li L, Yang D, Xu C, Wang S, and Zhang D (2017) Novel proapoptotic agent SM-1 enhances the inhibitory effect of 5-fluorouracil on colorectal cancer cells in vitro and in vivo. Oncol. Lett 13, 4762–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Ji S, Li MZ, Wen Z, Li J, Li SW, Xie FF, and Cheng ZN (2015) Development and validation of a simple HPLC assay for the quantitation of SM-1, a novel derivative of the PAC-1 anticancer agent, and an initial pharmacokinetics study in rats. Anal. Methods 7, 9562–9567. [Google Scholar]
- (175).Yosefzon Y, Soteriou D, Feldman A, Kostic L, Koren E, Brown S, Ankawa R, Sedov E, Glaser F, and Fuchs Y (2018) Caspase-3 Regulates YAP-Dependent Cell Proliferation and Organ Size. Mol. Cell 70, 573–587. [DOI] [PubMed] [Google Scholar]
- (176).Zhao HJ, Liu T, Mao X, Han SX, Liang RX, Hui LQ, Cao CY, You Y, and Zhang LZ (2015) Fructus phyllanthi tannin fraction induces apoptosis and inhibits migration and invasion of human lung squamous carcinoma cells in vitro via MAPK/MMP pathways. Acta Pharmacol. Sin 36, 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Ma J, Bao G, Wang L, Li W, Xu B, Du B, Lv J, Zhai X, and Gong P (2015) Design, synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur. J. Med. Chem 96, 173–186. [DOI] [PubMed] [Google Scholar]
- (178).Chakkath T, Lavergne SN, Fan TM, Bunick D, and Dirikolu L (2014) Preliminary metabolism of lomustine in dogs and comparative cytotoxicity of lomustine and its major metabolites in canine cells. Vet. Sei 1, 159–173. [Google Scholar]
- (179).Guo WT, Wang XW, Yan YL, Li YP, Yin X, Zhang Q, Melton C, Shenoy A, Reyes NA, Oakes SA, Blelloch R, and Wang Y (2015) Suppression of epithelial-mesenchymal transition and apoptotic pathways by miR-294/302 family synergistically blocks let-7-induced silencing of self-renewal in embryonic stem cells. Cell Death Differ. 22, 1158–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Xu J, Li Z, Wang J, Chen H, and Fang JY (2014) Combined PTEN mutation and protein expression associate with overall and disease-free survival of glioblastoma patients. Transi. Oncol 7, 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Campbell DS, and Okamoto H (2013) Local caspase activation interacts with Slit-Robo signaling to restrict axonal arborization. J. Cell Biol. 203, 657–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Zhu H, Sun G, Dong J, and Fei L (2017) The role of PRRX1 in the apoptosis of A549 cells induced by cisplatin. Am. J. Transi Res. 9, 396–402. [PMC free article] [PubMed] [Google Scholar]
- (183).Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, Parys JB, Leybaert L, and Bultynck G (2012) Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-XL. Cell Death Differ. 19, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Putinski C, Abdul-Ghani M, Stiles R, Brunette S, Dick SA, Fernando P, and Megeney LA (2013) Intrinsic-mediated caspase activation is essential for cardiomyocyte hypertrophy. Proc. Natl. Acad. Set. U. S. A 110, E4079–E4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Dong T, Zhang Q, Hamblin MR, and Wu MX (2015) Low-level light in combination with metabolic modulators for effective therapy of injured brain. J. Cereb. Blood Flow Metab. 35, 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Dick SA, Chang NC, Dumont NA, Bell RA, Putinski C, Kawabe Y, Litchfield DW, Rudnički MA, and Megeney LA (2015) Caspase 3 cleavage of Pax7 inhibits self-renewal of satellite cells. Proc. Natl. Acad. Sci. U. S. A 112, E5246–E5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Xu L, Zeng Z, Zhang W, Ren G, Ling X, Huang F, Xie P, Su Y, Zhang XK, and Zhou H (2017) RXRalpha ligand Z-10 induces PML-RARalpha cleavage and APL cell apoptosis through disrupting PML-RARalpha/RXRalpha complex in a cAMP-independ-ent manner. Oncotarget 8, 12311–12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Namjoshi P, Showalter L, Czerniecki BJ, and Koski GK (2014) T-helper 1-type cytokines induce apoptosis and loss of HER-family oncodriver expression in murine and human breast cancer cells. Oncotarget, DOI: 10.18632/oncotarget. 10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Auner HW, Beham-Schmid C, Dillon N, and Sabbattini P (2010) The life span of short-lived plasma cells is partly determined by a block on activation of apoptotic caspases acting in combination with endoplasmic reticulum stress. Blood 116, 3445–3455. [DOI] [PubMed] [Google Scholar]
- (190).Fang LN, Wang QD, Bi KS, and Zhao X. (2016) Simultaneous Determination of Procaspase Activating Compound 1 and Permeability Markers in Intestinal Perfusion Samples and Application to a Rat Intestinal Absorption Study. Chromatographia 79,1659–1663. [Google Scholar]
- (191).Ren L, Bi K, Gong P, Cheng W, Song Z, Fang L, and Chen X (2008) Characterization of the in vivo and in vitro metabolic profile of PAC-1 using liquid chromatography-mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sei. 876, 47–53. [DOI] [PubMed] [Google Scholar]
- (192).Fang LN, Chen XH, Wang QD, Zhang D, Zhao JJ, Long ZM, Gong P, and Bi KS (2011) A liquid chromatography-tandem mass spectrometry method for the quantification of PAC-1 in rat plasma. J. Pharm. Biomed. Anal 54, 225–229. [DOI] [PubMed] [Google Scholar]
- (193).Song Z, Chen X, Zhang D, Gong P, and Bi K (2010) Isolation and structure elucidation of degradation products in the potential anticancer drug PAC-1. J. Pharm. Biomed. Anal 51, 965–968. [DOI] [PubMed] [Google Scholar]
- (194).Fang LN, Chen XH, Song Z, Wang G, Zhao X, Ren L, Gong P, and Bi KS (2009) Development of a high performance liquid chromatography method for quantification of PAC-1 in rat plasma. J. Pharm. Biomed. Anal 49, 447–450. [DOI] [PubMed] [Google Scholar]
- (195).Iorio F, Knijnenburg TA, Vis DJ, Bignell GR, Menden MP, Schubert M, Aben N, Goncalves E, Barthorpe S, Lightfoot H, Cokelaer T, Greninger P, van Dyk E, Chang H, de Silva H, Heyn H, Deng X, Egan RK, Liu Q, Mironenko T, Mitropoulos X, Richardson L, Wang J, Zhang T, Moran S, Sayols S, Soleimani M, Tamborero D, Lopez-Bigas N, Ross-Macdonald P, Esteller M, Gray NS, Haber DA, Stratton MR, Benes CH, Wessels LFA, Saez-Rodriguez J, McDermott U, and Garnett MJ (2016) A Landscape of Pharmacogenomic Interactions in Cancer. Cell 166, 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Basu A, Bodycombe NE, Cheah JH, Price EV, Liu K, Schaefer GI, Ebright RY, Stewart ML, Ito D, Wang S, Bracha AL, Liefeld T, Wawer M, Gilbert JC, Wilson AJ, Stransky N, Kryukov GV, Dancik V, Barretina J, Garraway LA, Hon CS, Munoz B, Bittker JA, Stockwell BR, Khabele D, Stern AM, Clemons PA, Shamji AF, and Schreiber SL (2013) An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell 154, 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Rees MG, Seashore-Ludlow B, Cheah JH, Adams DJ, Price EV, Gill S, Javaid S, Coletti ME, Jones VL, Bodycombe NE, Soule CK, Alexander B, Li A, Montgomery P, Kotz JD,Hon CS, Munoz B, Liefeld T, Dancik V, Haber DA, Clish CB, Bittker JA, Palmer M, Wagner BK, Clemons PA, Shamji AF, and Schreiber SL (2016) Correlating chemical sensitivity and basal gene expression reveals mechanism of action. Nat. Chem. Biol 12, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Lucas PW, Schmit JM, Peterson QP, West DC, Hsu DC, Novotny CJ, Dirikolu L, Churchwell MI, Doerge DR, Garrett LD, Hergenrother PJ, and Fan TM (2011) Pharmacokinetics and derivation of an anticancer dosing regimen for PAC-1, a preferential small molecule activator of procaspase-3, in healthy dogs. Invest. New Drugs 29, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Botham RC, Roth HS, Book AP, Roady PJ, Fan TM, and Hergenrother PJ (2016) Small-Molecule Procaspase-3 Activation Sensitizes Cancer to Treatment with Diverse Chemo-therapeutics. ACS Cent. Sei 2, 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Lipsitz D, Higgins RJ, Kortz GD, Dickinson PJ, Bollen AW, Naydan DK, and LeCouteur RA (2003) Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet. Pathol 40, 659–669. [DOI] [PubMed] [Google Scholar]
- (201).Stoica G, Kim HT, Hall DG, and Coates JR (2004) Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet. Pathol 41, 10–19. [DOI] [PubMed] [Google Scholar]
- (202).Hicks J, Platt S, Kent M, and Haley A (2017) Canine brain tumours: a model for the human disease? Vet. Comp. Oncol 15, 252–272. [DOI] [PubMed] [Google Scholar]
- (203).Thornberry NA, and Lazebnik Y (1998) Caspases: enemies within. Science 281, 1312–1316. [DOI] [PubMed] [Google Scholar]
- (204).Cromm PM, and Crews CM (2017) Targeted Protein Degradation: from Chemical Biology to Drug Discovery. Cell Chem. Biol 24, 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Lai AC, and Crews CM (2017) Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discovery 16, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Peh J, Fan TM, Wycislo KL, Roth HS, and Hergenrother PJ (2016) The Combination of Vemurafenib and Procaspase-3 Activation Is Synergistic in Mutant BRAF Melanomas. Mol. Cancer Ther. IS, 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Peh J, Boudreau MW, Smith HM, and Hergenrother PJ (2018) Overcoming Resistance to Targeted Anticancer Therapies through Small-Molecule-Mediated MEK Degradation. Cell Chem. Biol 25, 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Caunt CJ, Sale MJ, Smith PD, and Cook SJ (2015) MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat. Rev. Cancer 15, 577–592. [DOI] [PubMed] [Google Scholar]
- (209).Botham RC, Fan TM, Im I, Borst LB, Dirikolu L, and Hergenrother PJ (2014) Dual small-molecule targeting of procaspase-3 dramatically enhances zymogen activation and anticancer activity. J. Am. Chem. Soc 136, 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Danciu OC, Nicholas MK, Holdhoff M, Venepalli NK, Hergenrother PJ, Tarasow TM, and Dudek AZ (2016) Phase I study of procaspase activating compound-1 (PAC-l) in the treatment of advanced malignancies. J. Clin. Oncol 34, TPS2605,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Danciu O, Nicholas M, Holdhoff M, Peterson RA, Venepalli NK, Chowdhery R, Tarasow T, Hergenrother PJ, and Dudek A (2019) Phase I study of procaspase activating compound – 1 (PAC-l) in the treatment of advanced malignancies and in combination with Temozolomide in refractory high-grade astrocytomas, Proceedings: AACR Annual Meeting 2019, March 29–April 3, 2019, Atlanta, GA, p CT027, American Association for Cancer Research,. [Google Scholar]
- (212).Danciu OC, Nicholas MK, Holdhoff M, Peterson RA, Tarasow TM, Hergenrother PJ, and Dudek AZ (2018) Phase I study of procaspase activating compound-1 (PAC-l) for treatment of advanced malignancies. J. Clin. Oncol 36, TPS2621. [Google Scholar]
- (213).Nicholas M, Holdhoff M, Peterson R, Danciu O, Wefel J, Tarasow T, Hergenrother PJ, and Dudek A (2018) Initial Results of a Phase I Study of Procaspase Activating Ccompound-1 (Pac-l) in Combination with Temozolomide for Recurrent Malignant Glioma. Neuro-Oncology 20, 12–12. [Google Scholar]