Abstract
Purpose
Clinical trials have illustrated that Shenmayizhi decoction (SMYZ) could improve the cognitive functions in patients with dementia. However, the mechanism needs to be explored.
Methods
Fifty adult male rats (Wistar strain) were divided into five groups equally and randomly, including control, model, and SMYZ of low dose, medium dose and high dose. Rats in each group received a daily gavage of respective treatment. Rats in control and model group were administrated by the same volume of distilled water. Memory impairment was induced by intraperitoneal administration of scopolamine (0.7 mg/kg) for 5 continuous days. Four weeks later, Morris water maze (MWM) was performed to evaluate the spatial memory in all rats. Then, rats were sacrificed and the hippocampus was removed for further tests. Furthermore, Western blot analysis was employed to assess the levels of acetylcholine M1 receptor (M1), acetylcholine M2 receptor (M2), acetylcholinesterase (AChE) and cholineacetyltransferase (ChAT). AChE and ChAT activities were determined.
Results
The SMYZ decoction significantly improved behavioral performance of rats in high dose. The SMYZ decoction in three doses exhibited anti-acetylcholinesterase activity. In addition, a high dose of SMYZ promoted ChAT activity. Moreover, a high dose of SMYZ increased the level of ChAT and declined the level of AChE assessed by Western blotting. Besides, an increased level of M1 receptor was found after treatment.
Conclusion
Shenmayizhi decoction could mitigate scopolamine-induced cognitive deficits through the preventative effect on cholinergic system dysfunction.
Keywords: dementia, Shenmayizhi decoction, scopolamine, acetylcholine M1 receptor, acetylcholinesterase, cholineacetyltransferase
Introduction
The global prevalence of dementia imposes a considerable burden on society. Nowadays, the number of patients with dementia is estimated to be 24 million and predicted to be quadruple by the year 2050.1 Alzheimer’s disease (AD) is the most common form of dementia and the dominant pathological features of which were amyloid plaques and neurofibrillary tangles. Although AD was observed by Alois Alzheimer in 1901, its pathogenesis was controversial and there is no cure up to now.2 Besides, cognitive decline in AD has no exact relationship with the number of plaques, which indicates that other factors might have effects on AD progression. Among mainstream hypotheses, acetylcholine (ACh) plays a crucial role in cognitive processes, and the cholinergic system is demonstrated as a significant factor in many forms of dementia, including AD.3
At present, cholinesterase inhibitors play an effective role in cognition, daily living activity, behavior, and overall clinical rating. Donepezil, galantamine and rivastigmine are the recognized cholinesterase inhibitors for the treatment of mild, moderate, and severe AD.4 However, herbal medicines have the potential to cure of AD owing to their multi-functional, multi-target characteristics.5 SMYZ which consists of Renshen (Radix Ginseng), Tianma (Rhizoma Gastrodiae), Guijianyu (Ramuli Euonymi) and Chuanxiong (Rhizoma Chuanxiong) based on the mechanism of invigorating Qi, promoting blood circulation and suppressing Yang. Clinical trials and animal experiments have demonstrated the significant effect of SMYZ on the treatment of dementia.6–8 ACh plays a significant role in memory function, particularly in the hippocampus-dependent learning. Physically, the activities of AChE and ChAT regulated the concentration of ACh in the brain.9,10 However, in neurodegenerative disease, especially the AD, the metabolism of ACh undergoes disruption. Evidence showed a loss of ChAT and AChE in the cortex, hippocampus and amygdala of AD brain samples.11 AChE is also able to accelerate amyloid formation.12 It has been shown that ChAT transcription is obviously declined in the cholinergic neurons, which results in diminished ChAT activity and aggravation of dementia.3 Moreover, among the acetylcholine muscarinic receptors, M1 and M2 have the highest expression in the prefrontal cortex (PFC) and hippocampus, brain regions important for cognition.13 M1 are the most abundant subtype in the hippocampus, which plays an important role in regulating the excitability of hippocampal neurons and in spatial learning and memory in elderly primates.9,14 Additionally, research has revealed that loss of M1 exacerbates the cognitive impairment.14–17 In the present research, the effect of SMYZ on spatial learning and memory was examined in a rat model of scopolamine, which can induce cognitive impairments as a muscarinic receptor antagonist. In this study, the expression differences of M1, M2, AChE, and ChAT in the hippocampus were evaluated by Western blot as well as the activity of ChAT and AChE to explore the mechanism of SMYZ in cholinergic system, which is pointed out as a crucial factor in many forms of dementia.
Materials And Methods
Animals
All rat work described in the present study was examined and approved by the Committee on Ethics of Animal Experiments of Xiyuan Hospital of China Academy of Chinese Medical Sciences (No. 2018XLC009-1). Fifty Sprague-Dawley (SD) rats (male) at the age of 10 weeks, weighing 300±20g, were obtained from SPF Biotechnology Co., Ltd. (Beijing), with a certificate of conformity: SCXK (Beijing) 2016-0002. Rats were raised in specific pathogen-free conditions with a temperature of 22–25°C, a 12-hr light cycle and humidity of 50–70%. All animals were given 7 days of adaptive feeding to acclimatize.
Preparation Of SMYZ Decoction
Herb slices of SMYZ (composition: Radix Ginseng, Rhizoma Gastrodiae, Ramuli Euonymi, Rhizoma Chuanxiong in a ratio of 3:3:3:2) were obtained from Xiyuan hospital. Thirty-three grams of crude material of the formulation were soaked in 330 mL of distilled water (1:10, w/v) for 30 mins at room temperature and then extracted twice at 100°C with 330 mL of distilled water under reflux, each time for 2 hrs. Then, the two extracts were combined and freeze-dried. Five grams of SMYZ lyophilized powder were obtained from 33 g of raw herbs. The powder was stored at 4 °C before use.
High-Performance Liquid Chromatography (HPLC) Analysis
Inertsil ODS-3 (4.6 × 150 mm, 5 µm) was used as stationary phase for the chromatographic separation with a column temperature at 30°C. The detection wavelength was 203 nm. The compounds were identified by individual peak retention times compared with reference substances. The flow rate was 1 mL/min, and the total injection volume was 8 µL. The mobile phase consisted of solvent A (0.3% phosphoric acid in water) and solvent B (acetonitrile) with the following gradient elution: 3–20% B at 0–15mins; 20–25% B at 15–20 mins; 25–40% B at 20–25 mins; 40–50% B at 25–30 mins and 50–55% B at 30–37 mins. Commercially available reference compounds were used according to the Chinese Pharmacopeia to identify the major components in the SMYZ decoction.
Drug Administration And Establishment Of Animal Model
Fifty rats were divided into the following five groups using random numbers: a control group, a model group, a low concentration decoction group (SMYZ-L group), a medium concentration decoction group (SMYZ-M group) and a high concentration decoction group (SMYZ-H group). There were 10 rats in each group. Dosages of SMYZ were calculated on the basis of the body surface equivalent dose ratio between rat and adult human. The same volume of distilled water was given to the rats in control group and model group; the drug concentrations in the high, medium and low groups were 16.5, 6.6, and 3.3 g/kg, respectively. All the groups were administrated by daily gavage in the morning, and treatments lasted 4 weeks. After 4 weeks, 30 mins before the behavioral testing, 0.7 mg/kg scopolamine was injected intraperitoneally for 5 consecutive days.18 All efforts were made to minimize the number of animals used and their suffering during this study. Animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals and the Beijing Laboratory Animal Welfare Ethics Review Guidelines issued by the Ministry of Science and Technology of China.
Reagents
Rabbit monoclonal anti-AChE antibody, rabbit monoclonal anti-ChAT antibody, rabbit polyclonal anti-M1 antibody and rabbit monoclonal anti-M2 antibody were acquired from Abcam (Cambridge, GRB). Beta-actin mouse monoclonal antibody was obtained by ImmunoWay (California, USA). The reagent kit for measurement of the AChE activity was purchased from Beijing Solarbio Science & Technology Co., Ltd, and the reagent kit for measurement of ChAT activity was acquired from Nanjing Institute of Jiancheng Bioengineering (Nanjing, China).
The Morris Water Maze Test
Thirty minutes after intraperitoneal injection of scopolamine, MWM was used to detect spatial learning in rats.19 The test consisted of a place navigation and a spatial probe test. The place navigation was evaluated daily for 4 days. The water in the maze was 30 cm deep and approximately 1 cm above the platform surface at 23±1°C. An appropriate amount of ink was mixed in the water to turn the opaque black. This test was conducted in a soundproof room. The procedure for this test involved the following: (1) choosing and marking a point of pool wall from 1/2 radians of the second quadrant, (2) placing the rats against the pool wall into the water on the marked point, and (3) recording the time required to find and climb on the platform. Together this procedure was called escape latency. If the rats could not find the platform in 90 s, they were guided appropriately to the platform and allowed to stay for 10 s. On the fifth day, swimming distance was recorded. Moreover, on the fifth day, the platform was removed to perform a spatial probe test. In this test, the rats were made to face the pool wall and enter the water from a random point of the second quadrant. The camera system automatically recorded the escape latency and swimming distance and time spent in target quadrant of each rat.
Tissue Preparation
After MWM, rats were intraperitoneally anesthetized by 2% pentobarbital sodium (0.2 mL/100g) and then sacrificed by decapitation. The brain tissue was instantly put on ice and incised into left and right hemispheres sagittally. Then, hippocampus was dissected. The right half of the hippocampal tissue was weighed and reserved in liquid nitrogen for protein immunoblotting. The left half of the hippocampal tissue was weighed and reserved in liquid nitrogen for measurement of biochemical analysis.
Western Blot Analysis
Hippocampi were dissected and lysed with RIPA (Radio-Immunoprecipitation Assay) Lysis buffer to extract protein and then centrifuged at 13,000g for 20 mins at 4°C (3–18K, Sigma). The supernatant was collected. Total protein was quantified using a BCA (bicinchoninic acid) Protein Assay kit (Beijing Sinoble Biotechnology Center, Beijing, China). Protein concentration was diluted to 4 mg/mL with 5× loading buffer and boiled for 5mins. According to the molecular weight of the target protein, either 8% or 12% acrylamide resolving gels were prepared. Samples containing 20µg of protein were loaded on a sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) electrophoresis (90-V for the stacking gel-20mins; 160V for the separating gel, timed in accordance with the protein marker) and transferred to 0.45 µm nitrocellulose filter membranes (300mA, 1–2hrs), then blocked with 3% bovine serum albumin for 60 mins and incubated in primary antibody (1:1000, ab183591; 1:5000, ab178850; 1:1000, ab180636; 1:2000, ab109226) overnight at 4°C. After incubation with horseradish peroxidase-conjugated antibody anti-rabbit or anti-mouse IgG antibodies for 40min, protein complexes were visualized using enhanced chemiluminescence Western blotting detection reagents (Millipore). Band intensity was analyzed using Image J.
Determination Of AChE Activity And ChAT Activity
The determination of AChE activity was performed using a commercial kit (Beijing Solarbio Science & Technology Co., Ltd, Beijing city, China). ChAT activity was determined by a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing City, China). All procedures strictly followed the manufacturer’s instructions. The determination of ChAT is based on acetyl coenzyme A and choline as substrates. Under the action of ChAT, the product of the reaction is combined with the chromogenic reagent. The absorbance is determined at 324 nm to calculate the activity of ChAT. AchE catalyzes the hydrolysis of Ach to choline, and choline reacts with P-nitrobenzoic acid disulfide (DTNB) to form TNB. TNB has an absorption peak at 412 nm. AchE activity is calculated by measuring the absorption increase rate at 412 nm.
Statistical Analysis
All data are shown as means ± standard error of mean (SEM). One-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test was performed using SPSS 21.0 software (IBM SPSS, Shanghai, China) for the intergroup comparisons. P < 0.05 was considered statistical difference.
Results
Bioactive Components Of SMYZ
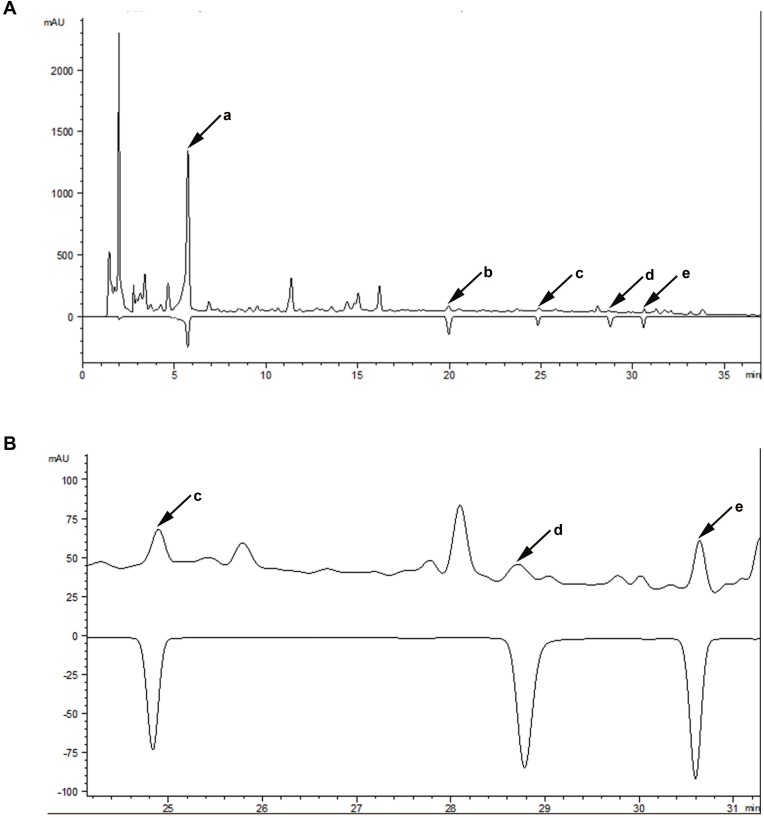
Major components contained in SMYZ water extraction were determined by HPLC. Five bioactive compounds in SMYZ were detected and annotated by comparing the retention time and UV-spectrum with reference chemicals. These compounds were gastrodin, ferulic acid, ginsenosides Rg1, ginsenosides Rb1, and quercetin which are shown in Figure 1. The five standards used in the chromatogram were 300 ug/mL, 500 ug/mL, 1 mg/mL, 1 mg/mL, 1 mg/mL.
Figure 1.
The chromatographic profile of SMYZ decoction.
Notes: (A) Major compounds in SMYZ decoction compared with reference standards; a: gastrodin; b: ferulic acid; c: ginsenosides Rg1; d: ginsenosides Rb1; e: quercetin. (B) Details of retention time from 24 to 31 mins.
Abbreviation: SMYZ, Shenmayizhi decoction.
SMYZ Improves The Spatial Learning And Memory Of The Rats Induced By Scopolamine In The MWM
In order to verify whether SMYZ improves cognitive impairments, the MWM was performed to test spatial learning and memory abilities in the rats. From Figure 2A, the results show that on the first day the escape latency was nearly the same among all groups, which suggested the same start of spatial memory of rats in 5 groups. As the training progressed, the escape latency on the fifth day became shorter than that on the first day for each group (F=669.67, P < 0.01), demonstrating that all rats were capable of learning. On the second day and third day, the escape latency of the model group was longer than that of the control group (F1=15.97, F2=10.76, P < 0.01). Moreover, on the fourth day, the SMYZ-H group and the control group exhibited shorter escape latencies to reach the platform than the model group (F1=15.47, F2=29.35, P < 0.01). On the fifth day, compared to the model group, SMYZ-H group and control group showed shorter latencies to reach the platform (F1=5.62, F2=10.78, P < 0.01). As shown in Figure 2B, on the fifth day, the control group and SMYZ-H group had a larger number of crossing over the platform position compared with the model group (F=8.19, P < 0.05). Besides, from Figure 2C and D, the control group, SMYZ-M group and SMYZ-H group had less present of swimming time and swimming distance in the target quadrant versus the model group (F1=7.66, F2=5.95, F3=8.31, P < 0.05). The data suggest that medium and high dosage of SMYZ could ameliorate the learning and memory abilities of the rats induced by scopolamine.
Figure 2.
SMYZ treatment on learning and memory deficits induced by scopolamine.
Notes: (A) The effects of SMYZ on the escape latency in the MWM test after 4-week treatment in rats, including (A) P < 0.01, control group versus model group; (B) P < 0.01, SMYZ-H group versus model group. (B) The number of crossing over the platform position during the spatial probe test in the MWM test on the fifth day, * P < 0.05, versus the model group. (C) The percent of swimming distance in target quadrant during the spatial probe test in the MWM test on the fifth day, * P < 0.05, versus the model group. (D) The percent of swimming time in target quadrant during the spatial probe test in the MWM test on the fifth day, * P < 0.05, versus model group. Data are expressed as mean ± SEM (n=10).
Abbreviations: SMYZ, Shenmayizhi decoction; SMYZ-L, low-concentration SMYZ group; SMYZ-M, medium-concentration SMYZ group; SMYZ-H, high-concentration SMYZ group.
SMYZ Increases The Expression Of ChAT And Muscarinic M1 Receptor In The Hippocampus While It Decreases The Expression Of AChE In The Hippocampus Of Rats Induced By Scopolamine
The expressions of AChE, ChAT, M1 and M2 were detected by Western blot. The images and quantitative analysis showed the changes in the hippocampal tissue after the treatment of SMYZ for 4 weeks. Figure 3A shows that after treatment there was a significant decline in the AChE of hippocampus in the SMYZ-H group compared to that observed in the model group (F=8.18, P < 0.05). Figure 3B demonstrates that ChAT levels of SMYZ-H were significantly higher than those of the model group after treatment (F=17.78, P < 0.05). Figure 3C reveals a significant increase in the level of M1 in SMYZ groups after treatment comparing to the model group (F=10.14, P < 0.05). Especially the SMYZ-H group had a significant rise in M1 levels compared to the model group (F=53.48, P < 0.01). However, we did not observe a significant between-group difference of M2 expression in hippocampus after treatment.
Figure 3.
AChE, ChAT and M1 levels in the hippocampus of scopolamine-induced amnesia rats.
Notes: (A) AChE expression in the hippocampus, * P < 0.05, versus the model group. (B) ChAT expression in the hippocampus, * P < 0.05, versus the model group. (C) M1 expression in the hippocampus, * P < 0.05 and ** P < 0.01, versus the model group. Data are expressed as mean ± SEM (n=3).
Abbreviations: AChE, acetylcholinesterase; ChAT, cholineacetyltransferase; M1, acetylcholine M1 receptor.
SMYZ Boosts ChAT Activity And Reduces AChE Activity In The Hippocampus Of Rats
In order to clarify the possible mechanisms of SMYZ improving the spatial learning and memory of scopolamine-induced rats, the activities of key enzymes which might affect memory in the hippocampus in the brain were measured. Figure 4 illustrates the AChE and the ChAT activity in the hippocampus of the rats. As shown in Figure 4A, AChE activity in SMYZ-M group, SMYZ-H group and control group is significantly lower than that in the model group (F1=12.63, F2=26.73, F3=31.74, P < 0.01, respectively). Meanwhile, SMYZ-L group had a lower AChE activity than the model group as well (F=5.43, P < 0.05). Moreover, Figure 4B demonstrates that SMYZ-H group and control group also significantly boosted the ChAT activity in the hippocampus (F1=6.98, F2=9.64, P < 0.01) compared to the model group.
Figure 4.
Effect of SMYZ on the activities of AChE and ChAT in the hippocampus of scopolamine-treated rats.
Notes: (A) Activity of choline acetytransferase (AChE). (B) Activity of acetylcholine esterase (ChAT). Data are expressed as mean±SEM (n=5). * P < 0.05, ** P < 0.01 versus model group.
Discussion
It has been proven that the cholinergic system is crucial in the learning process as well as in the memory function and influences acquisition and consolidation of learned task.20–25 Based on experimental and clinical evidence, ACh, as a vital neurotransmitter, plays a vital role in memory function and modulating learning, which both decline in healthy aging and even more in neurodegenerative diseases.9 Decline in the function of the central cholinergic system can influence aspects of dementia such as a deficiency of memory and puzzlement as shown in AD, which is featured by cognitive dysfunction with memory deficiency and behavioral disorders.9,25
Scopolamine, a well-acknowledged anticholinergic drug, is generally used as a standard drug for the experimental purpose to induce cognitive deficits in animals.26 After the cholinergic hypothesis of geriatric memory deficiency was produced, the use of scopolamine as a pharmacological model of “cholinergic amnesia” became very popular.27 Scopolamine, a muscarinic receptor antagonist, damages learning and memory, while, it could stimulate neuronal injury and boost the activity of AChE.27,28 Scopolamine-regulated memory deficiency is one of the most widely used models, which is more convenient than the complex surgical events. Studies have reported that at least a dose of 0.1 mg/kg scopolamine could impair place learning in the Morris task. Besides, some experiments demonstrated that higher doses (i.e., 0.8–1.0 mg/kg, intraperitoneal injection) cannot impair the gain of the Morris task.29 Therefore, this experiment was performed to observe the effects of SMYZ on cholinergic system in rats. Scopolamine (0.7 mg/kg) was used for injection to induce the rats of memory and cognitive impairment.
MWM is a test of spatial learning for rodents. In MWM, animals should rely on distal clues to navigate from start positions to locate a drowned escape platform around the perimeter in an open swimming arena.30 It was first established by neuroscientist Richard G. Morris in 1981 in order to test hippocampal-dependent learning and now it plays a crucial role in the determination of rodent models for neurocognitive impairments like Alzheimer’s disease.31 In the MWM of this study, scopolamine-induced amnesia rats exhibited a significant prolonged escape latency since the second day. Besides, it had a larger number of crossing over the platform position and higher percent of swimming distance and swimming time compared with the control group. All of which was consistent with the results of other researches.32–34 Moreover, after SMYZ-H intervention of 4 weeks, the escape latency significantly decreased and the swimming time and swimming distance in the target quadrant significantly reduced as well, which indicates that SMYZ with high and medium dosage could effectively improve learning and spatial memory capacity in scopolamine-induced amnesia rats.
Research investigated that hippocampus had two core functions, which were spatial navigation and episodic memory.35 Furthermore, the function of hippocampus is prominently affected by cholinergic disorder.36–38 Cholinergic disorder has a crucial role in the pathophysiology of cognitive impairment, while the neuromodulator ACh plays a significant role in memory function, particularly in the hippocampus-dependent learning.17 ACh is generated from choline and acetyl-CoA via the ChAT enzyme and whereafter transfer into vesicles and release into the synapse cleft, where it can bond to ACh receptors. However, ACh can be broken back down into choline and acetic acid by AChE in the synapse. Choline reuptake occurs via a high-affinity choline transporter, and then choline is recycled in the synthesis of new ACh.39 Therefore, the concentration of ACh in the brain is dynamically regulated by the activities of AChE and ChAT.10 As early as in the mid-1970s, autopsy studies provided evidence that cholinergic system was involved in the pathogenesis of AD, which showed loss of ChAT and AChE in the hippocampus, cortex and amygdala of AD brain samples.11 In addition, decreased ChAT activity has been found to be related to growing Aβ plaque load and to cognitive decrease.11 AChE could boost amyloid formation. AChE activity is modulated in the brain and blood, which is co-localized with senile plaques.12 Previous researches have demonstrated that drugs that alter extracellular ACh levels or its receptor activity have a huge influence in memory functions, like donepezil, galantamine and rivastigmine, which are all cholinesterase inhibitors proven to be effective in treating the cognitive and functional symptoms of AD.25 In the present study, we found that SMYZ treatment could reduce AChE activity in the hippocampus of scopolamine-induced amnesia rats. Moreover, after SMYZ-H administration, the levels of AChE in the hippocampus of rats were decreased. Besides, SMYZ-H could increase the ChAT activity and improve the level of ChAT in the hippocampus of scopolamine-induced amnesia rats. It suggests that SMYZ improves the ability of learning and memory in scopolamine-induced amnesia rats by regulating the cholinergic system.
There are two main types of cholinergic receptors which are nicotinic receptors and muscarinic receptors, and they are expressed both in the central and in the peripheral nervous system.9 There are five subtypes of muscarinic receptors, M1 through M5. In particular, among the acetylcholine muscarinic receptors, M1 and M2 are expressed at the highest levels in the prefrontal cortex (PFC) and hippocampus, brain regions important for cognition.13 M1 is the most abundant subtype in the hippocampus, which is mainly expressed in the dendrites or somatic cells and plays an important role in modulating the excitability of hippocampal neurons and in spatial learning and memory in elderly primates.9,14 On the basis of these discoveries and many other research, M1 acetylcholine receptor agonists are becoming a vital method to treat AD. M1 regulates the three major features of AD including cholinergic hypofunction, β-amyloid and tau hyperphosphorylation.14–16 Researches reveal that loss of M1 deteriorates the cognitive deficiency and increases parenchymal and cerebrovascular Aβ as well as increases neuroinflammation in mice with AD. Furthermore, deposition ablating the M1 gene boosts tau pathological characteristics by disrupting PKA-CREB signaling, relative to exacerbation of cognitive impairment.17 Researches have suggested that M1 receptor activation reduces tau hyperphosphorylation by activating PKC and inhibiting GSK-3β.16,40 M1 agonists were considered as potential disease-modifying strategies in AD. It has been shown that the M1 agonist AF267B can decline the cognitive deficiency in a special task and reduce Aβ and tau deposition in the cortex and hippocampus of a mice model of AD.40 In addition, M2 antagonists like SCH-57790 and SC-72788 could induce obstruction of M2-mediated suppression of the presynaptic release of ACh, which can stimulate M1 and nicotinic receptors, improving cognitive deficiency in AD.3 In this study, we measured the Muscarinic M1 receptor and Muscarinic M2 receptor level in the hippocampus by Western blot. Interestingly, we found that SMYZ treatment effectively increased the level of M1 receptor in the hippocampus of rats induced by scopolamine, while there was no significant difference in M2 receptor after treatment. Therefore, the effects of SMYZ in improving cognitive abilities and memory may be achieved through increasing the expression of M1 receptor.
In this research, we found that the major bioactive components of SMYZ were gastrodin, ginsenosides Rg1, ginsenosides Rb1, ferulic acid, as well as quercetin and all of which have treatment of cognitive disorders as described in many reports. Both in vivo and in vitro studies showed that gastrodin can ameliorate spatial learning and memory of scopolamine-treated rats and restrain the production and aggregation of Aβ, as well as protect neurons from Aβ-induced injury.41–43 Besides, gastrodin has a protective effect on inhibiting autophagy and apoptosis of hippocampus neurons.44–46 It has been demonstrated that ginsenoside Rb1 could increase the intake of choline in cerebral cholinergic nerve endings and regulate the release and intake of ACh. Besides, it inhibited AChE activity in the hippocampus and improved cognitive impairment and hippocampus senescence. Rb1 and Rg1 have been reported to elevate the level of ChAT in rodent brains and improved the performance of aged mice in a behavior test. Interestingly, these findings are consistent with our results.47–49 Many studies indicate that in vitro and in vivo experiments of animal models ferulic acid has a beneficial effect on inhibiting the aggregation of neurotoxic Aβ. Moreover, it could obstruct the biological pathways involved in apoptotic programmed cell death which is caused by oxidative stress and inflammatory reaction induced to Aβ aggregation.50–53 Quercetin is known to protect neuronal cells against oxidative stress and inflammatory injuries, which damage the tissues leading to AD. It could ameliorate the learning and spatial memory abilities as well as increase astrogliosis and microgliosis in the hippocampus and the amygdala by its neuroprotective effect.54–57
Conclusion
In conclusion, our findings demonstrate that SMYZ could improve scopolamine-induced learning and memory impairments in rats via its protective effect on cholinergic system dysfunction. This may be explained by the modulation of the cholinergic system, as indicated by increased expression of ChAT, promotion of ChAT activity, decreased expression of AChE and inhibition of AChE activity, and elevated level of M1 receptors in the hippocampus. These may help the future investigations of the clinical use of SMYZ in treating dementia. Moreover, we anticipate that SMYZ might be a promising candidate for the dementia treatment by modulating the cholinergic system.
Acknowledgments
The authors want to thank the Eurasia-Pacific Uninet and the University of Graz, Austria, for financial support. We appreciate the generous help from the Department of Pharmacognosy, the University of Graz. This research is also funded by the Beijing Municipal Science and Technology Plan (No. Z171100001717016), the Self-determined Project of China Academy of Chinese Medical Sciences (ZZ11-025) and the National Natural Science Foundation of China (No. 81603616, No. 81573819).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88(4):640–651. doi: 10.1016/j.bcp.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takashi A, Pavla J, Jiri R, Machova UL, Eva S. Alzheimer’s disease: mechanism and approach to cell therapy. Int J Mol Sci. 2015;16(11):26417–26451. doi: 10.3390/ijms161125961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira-Vieira TH, Guimaraes IM, Silva FR, et al. Ribeiro, Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14(1):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu LW. Alzheimer’s disease: early diagnosis and treatment. Hong Kong Med J. 2012;18(3):228–237. [PubMed] [Google Scholar]
- 5.Kim HG, Oh MS. Nutraceuticals and prevention of neurodegeneration herbal medicines for the prevention and treatment of Alzheimer’s disease. Curr Pharm Des. 2012;18(1):57–75. [PubMed] [Google Scholar]
- 6.Qiong W, Fang L, Jiangang L, et al. Effects of Shenmayizhi extration on cognitive function and hemorheological state in mild and moderate vascular dementia. Chin J Integr Med Cardio-/Cerebrovasc Dis. 2017;15(19):16–20. [Google Scholar]
- 7.Kun L. Shenmayizhi Prescription Alleviates Neurovascular Units Deficits in Vascular Dementia Rats. Beijing: CACMS; 2017. [Google Scholar]
- 8.Kun L, Pei H, Yu C, et al. Effects of Shenmayizhi prescription on morphology and oxidative stress of hippocampus in rats with vascular dementia. J Beijing Univ Tradit Chin Med. 2017;36(5):14–17, 98. [Google Scholar]
- 9.Haam J, Yakel JL. Cholinergic modulation of the hippocampal region and memory function. J Neurochem. 2017;142(Suppl 1):111–121. doi: 10.1111/jnc.14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee B, Shim I, Lee H, Hahm DH. Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats. J Microbiol Biotechnol. 2011;21(8):874–883. doi: 10.4014/jmb.1104.04012 [DOI] [PubMed] [Google Scholar]
- 11.Roy R, Niccolini F, Pagano G, Politis M. Cholinergic imaging in dementia spectrum disorders. Eur J Nucl Med Mol Imaging. 2016;43(7):1376–1386. doi: 10.1007/s00259-016-3349-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvajal FJ, Inestrosa NC. Interactions of AChE with Aβ aggregates in Alzheimer’s brain: therapeutic relevance of IDN 5706. Front Mol Neurosci. 2011;9(4):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley GE, Kroenke C, Schwartz D, Kohama SG, Urbanski HF, Raber J. Hippocampal M1 receptor function associated with spatial learning and memory in aged female rhesus macaques. Age. 2011;33(3):309–320. doi: 10.1007/s11357-010-9184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher A, Brandeis R, Bar-Ner RHN, et al. AF150(S) and AF267B - M1 muscarinic agonists as innovative therapies for Alzheimer’s disease. J Mol Neurosci. 2002;19(1–2):145–153. doi: 10.1007/s12031-002-0025-3 [DOI] [PubMed] [Google Scholar]
- 15.Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer’s disease – the pivotal role of brain M1 receptors. Neurodegener Dis. 2008;5(3–4):237–240. doi: 10.1159/000113712 [DOI] [PubMed] [Google Scholar]
- 16.Caccamo A, Oddo S, Billings LM, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49(5):671–682. doi: 10.1016/j.neuron.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 17.Medeiros R, Kitazawa M, Caccamo A, et al. Loss of muscarinic M1 receptor exacerbates Alzheimer\”s disease–like pathology and cognitive decline. Am J Pathol. 2011;179(2):980–991. doi: 10.1016/j.ajpath.2011.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancianu M, Cioanca O, Mihasan M, Hritcu L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine. 2013;20(5):446–452. doi: 10.1016/j.phymed.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 19.Hernández-Pérez JJ, Gutiérrez-Guzmán BE, López-Vázquez MÁ, Olvera-Cortés ME. Supramammillary serotonin reduction alters place learning and concomitant hippocampal, septal, and supramammillar theta activity in a Morris water maze. Front Pharmacol. 2015;10(6):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine A, Hoyle C, Maclean CJ, Levatte TL, Baker HF, Ridley RM. Learning impairments following injection of a selective cholinergic immunotoxin, ME20.4 IgG-saporin, into the basal nucleus of Meynert in monkeys. Neuroscience. 1997;81(2):331–343. doi: 10.1016/s0306-4522(97)00208-x [DOI] [PubMed] [Google Scholar]
- 21.Miranda MI, Bermudez-Rattoni F. Reversible inactivation of the nucleus basalis magnocellularis induces disruption of cortical acetylcholine release and acquisition, but not retrieval, of aversive memories. Proc Natl Acad Sci. 1999;96(11):6478–6482. doi: 10.1073/pnas.96.11.6478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11(2):180–187. [DOI] [PubMed] [Google Scholar]
- 23.Das A, Dikshit M, Singh HK, Nath C. Scopolamine and activite avoidance evaluation of effect of scopolamine on stages of active avoidance learning in rats. Indian J Pharmacol. 2003;35(1):47–50. [Google Scholar]
- 24.Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem. 2001;75(3):245–252. doi: 10.1006/nlme.2001.4005 [DOI] [PubMed] [Google Scholar]
- 25.Agrawal R, Tyagi E, Saxena G, Nath C. Cholinergic influence on memory stages: a study on scopolamine amnesic mice. Indian J Pharmacol. 2009;41(4):192. doi: 10.4103/0253-7613.56072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandeep M, Hemant K, Duk-Yeon C, Yo-Sep Y, Dong-Kug C. Toxin-induced experimental models of learning and memory impairment. Int J Mol Sci. 2016;17(9):1447. doi: 10.3390/ijms17091447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–417. doi: 10.1126/science.7046051 [DOI] [PubMed] [Google Scholar]
- 28.Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005;27(5):655–665. doi: 10.1097/01.ftd.0000168293.48226.57 [DOI] [PubMed] [Google Scholar]
- 29.Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34(8):1307–1350. doi: 10.1016/j.neubiorev.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 30.Gibson BM, Mair R. A pathway for spatial memory encoding. Learn Behav. 2016;44(2):97–98. doi: 10.3758/s13420-016-0214-5 [DOI] [PubMed] [Google Scholar]
- 31.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp. 2011;(53):e2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deb D, Nayak V, Kurady LB, Rao M. Ameliorative effects of angiotensin receptor blockers against scopolamine-induced memory impairment in rats. Asian J Pharm Clin Res. 2016;9(2):335–341. [Google Scholar]
- 33.Falsafi SK, Deli A, Höger H, Pollak A, Lubec G. Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS One. 2012;7(2):e32082. doi: 10.1371/journal.pone.0032082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wenwen L, Jiansong F, Lvjie X, et al. DL0410 ameliorates memory and cognitive impairments induced by scopolamine via increasing cholinergic neurotransmission in mice. Molecules. 2017;22(3):410. doi: 10.3390/molecules22030410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eichenbaum H. Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci. 2014;15(11):732–744. doi: 10.1038/nrn3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger-Sweeney J, Stearns NA, Murg SL, Floerke-Nashner LR, Lappi D, Baxter MG. Selective immunolesions of cholinergic neurons in mice: effects on neuroanatomy, neurochemistry, and behavior. J Neurosci. 2001;21(20):8164. doi: 10.1523/JNEUROSCI.21-20-08164.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blokland A, Honig W, Raaijmakers WG. Effects of intra-hippocampal scopolamine injections in a repeated spatial acquisition task in the rat. Psychopharmacology. 1992;109(3):373–376. doi: 10.1007/BF02245886 [DOI] [PubMed] [Google Scholar]
- 38.Roloff EVL, Harbaran D, Micheau J, Platt B, Riedel G. Dissociation of cholinergic function in spatial and procedural learning in rats. Neuroscience. 2007;146(3):875–889. doi: 10.1016/j.neuroscience.2007.02.038 [DOI] [PubMed] [Google Scholar]
- 39.Maurer SV, Williams CL. The cholinergic system modulates memory and hippocampal plasticity via Its interactions with non-neuronal cells. Front Immunol. 2017;11(8):1489. doi: 10.3389/fimmu.2017.01489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forlenza OV, Spink JM, Dayanandan R, Anderton BH, Olesen OF, Lovestone S. Muscarinic agonists reduce tau phosphorylation in non-neuronal cells via GSK-3β inhibition and in neurons. J Neural Transm. 2000;107(10):1201–1212. doi: 10.1007/s007020070034 [DOI] [PubMed] [Google Scholar]
- 41.Park YM, Lee BG, Park SH, et al. Prolonged oral administration of Gastrodia elata, extract improves spatial learning and memory of scopolamine-treated rats. Lab Anim Res. 2015;31(2):69–77. doi: 10.5625/lar.2015.31.2.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Li P, Liu J, et al. Gastrodin attenuates cognitive deficits induced by 3,3′-iminodipropionitrile. Neurochem Res. 2016;41(6):1401–1409. doi: 10.1007/s11064-016-1845-9 [DOI] [PubMed] [Google Scholar]
- 43.Hu Y, Li C, Shen W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathology. 2015;34(4):370–377. [DOI] [PubMed] [Google Scholar]
- 44.Liu B, Gao JM, Li F, Gong QH, Shi JS. Gastrodin attenuates bilateral common carotid artery occlusion-induced cognitive deficits via regulating Aβ-related proteins and reducing autophagy and apoptosis in rats. Front Pharmacol. 2018;4(9):405. doi: 10.3389/fphar.2018.00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Wang H, Duan Z, Zhang J, Zhang W. Mechanism of gastrodin in cell apoptosis in rat hippocampus tissue induced by desflurane. Exp Ther Med. 2018;15(3):2767–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan L, Jialiang G, Min P, et al. A review on central nervous system effects of gastrodin. Front Pharmacol. 2018;2(9):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trivena RN, Sean KT, Kai-Chun C, et al. A role of ginseng and its constituents in the treatment of central nervous system disorders. Evid Based Complement Alternat Med. 2016;(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jakaria M, Haque ME, Kim J, et al. Active ginseng components in cognitive impairment: therapeutic potential and prospects for delivery and clinical study. Oncotarget. 2018;9(71):33601. doi: 10.18632/oncotarget.26035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y, Gao H, Turdu G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: a review. Bioorg Chem. 2017;9(75):50–61. doi: 10.1016/j.bioorg.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 50.Sgarbossa A, Giacomazza D, Di Carlo M. Ferulic acid: a hope for Alzheimer’s disease therapy from plants. Nutrients. 2015;7(7):5764–5782. doi: 10.3390/nu7075246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan J, Cho J, Kim H, et al. Protection against β‐amyloid peptide toxicity in vivo with long‐term administration of ferulic acid. Br J Pharmacol. 2010;133(1):89–96. doi: 10.1038/sj.bjp.0704047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagai N, Kotani S, Mano Y, et al. Ferulic acid suppresses amyloid β production in the human lens epithelial cell stimulated with hydrogen peroxide. Biomed Res Int. 2017;2017(3):1–9. doi: 10.1155/2017/5343010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren Z, Zhang RP, Li YY, Yang ZY, Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med. 2017;40(5):1444–1456. doi: 10.3892/ijmm.2017.3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer\”s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer\”s disease model mice. Neuropharmacology. 2015;6(93):134–145. doi: 10.1016/j.neuropharm.2015.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.David AVA, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev. 2016;10(20):84–89. doi: 10.4103/0973-7847.194044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pattanashetti LA, Taranalli AD, Parvatrao V, Malabade RH, Kumar D. Evaluation of neuroprotective effect of quercetin with donepezil in scopolamine-induced amnesia in rats. Indian J Pharmacol. 2017;49(1):60–64. doi: 10.4103/0253-7613.201016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costa LG, Garrick JM, Roquè PJ, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. 2016;2016(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]