Abstract
Objective:
Elevated anxiety and breast cancer worry can impede mammographic screening and early breast cancer detection. Genetic advances and risk models make personalized breast cancer risk assessment and communication feasible, but it is unknown whether such communication of risk affects anxiety and disease-specific worry. We studied the effect of a personalized breast cancer screening intervention on risk perception, anxiety and breast cancer worry.
Methods:
Women with a normal mammogram but elevated risk for breast cancfer (N=122) enrolled in the Athena Breast Health risk communication program were surveyed before and after receiving a letter conveying their breast cancer risk and a Breast Health Genetic Counselor consultation. We compared breast cancer risk estimation, anxiety and breast cancer worry before and after risk communication, and evaluated the relationship of anxiety and breast cancer worry to risk estimation accuracy.
Results:
Women substantially overestimated their lifetime breast cancer risk, and risk communication somewhat mitigated this overestimation (49% pre-intervention, 42% post-intervention, 13% Gail model risk estimate, p<0.001). Both general anxiety and breast cancer worry declined significantly after risk communication in women with high baseline anxiety. Baseline anxiety and breast cancer worry were essentially unrelated to risk estimation accuracy, but risk communication increased alignment of worry with accuracy of risk assessment.
Conclusions:
Personalized communication about breast cancer risk was associated with modestly improved risk estimation accuracy in women with relatively low anxiety, and less anxiety and breast cancer worry in women with higher anxiety. We detected no negative consequences of informing women about elevated breast cancer risk.
Keywords: Anxiety, Breast Cancer Worry, Oncology, Perceived Risk, Risk Communication
1. Background
One in eight women in the U.S. will be diagnosed with breast cancer over their lifetime1. Personalized risk assessment can identify women at increased risk of breast cancer and communicating that risk to patients is important to guide screening and breast cancer prevention2. It has been suggested that this individual approach could optimize disease detection and reduce over-screening3, 4.
However, communicating breast cancer risk and promulgating risk mitigation strategies to at-risk women could, in and of themselves, provoke anxiety and breast cancer worry5–7. Combined with overestimation of breast cancer risk, such an intervention might intensify psychological distress8, 9. Given the increasing opportunity to personalize breast cancer screening afforded by advances in genetics, it is critical to examine the effects of risk communication on women’s perceptions of breast cancer risk, anxiety and disease-specific worry.
Breast cancer worry and general anxiety are higher among women who have a personal and family history of breast cancer10. Further, many women, including those without a family history of breast cancer, experience anxiety and worry between the mammogram procedure and receipt of a normal result8, 11. If the mammogram result is inconclusive and follow-up procedures are needed, anxiety increases12, 13. In order to implement risk feedback, we need to understand the effect of learning that one’s mammogram result is normal but one’s overall risk for breast cancer is elevated.
The Athena Breast Health Network14 is a statewide quality improvement initiative across the University of California medical and cancer centers. It aims to assess breast cancer risk among women receiving mammograms and to provide risk-based personalized breast cancer screening. As part of Athena, this pilot study examined the relationship of personalized risk communication in the setting of a normal mammogram result with women’s breast cancer risk estimation, anxiety, and breast cancer worry.
2. Methods
This is a multicenter prospective pilot study conducted in University of California breast-imaging centers in Los Angeles (UCLA), San Francisco (UCSF) and San Diego (UCSD). Women were eligible if they received a mammogram and completed an Athena Breast Health screening form. Eligible women were introduced to the study at the time of their mammogram. They were told that if their mammogram result was normal, they would receive a telephone call requesting participation in the study, which would measure their anxiety related to breast cancer. Institutional review board approval was obtained at UCLA, UCSF and UCSD (#12–001516).
2.1. Risk Assessment and Enrollment
The Athena Breast Health screening form assessed participants’ personal and family history of breast cancer. Women were classified into the breast cancer elevated risk group if either of the following criteria was met: (1) United States Preventive Services Task Force (USPSTF) guideline threshold for genetics referral for consideration of BRCA1/2 testing15, or (2) 5-year breast cancer risk score above 1.67 on the Gail model Breast Cancer Risk Assessment Tool16 and in the top 5% of scores from the Gail model within their age group.
Women were contacted to participate in this study if they had a normal mammogram result (BIRAD 1–3) and were at elevated risk for breast cancer according to the above criteria. Women were excluded if they had a previous history of breast cancer, ductal carcinoma in situ, genetic testing, or previously received breast cancer risk prevention services (i.e., prescribed tamoxifen, received breast high-risk clinic consultation or had a screening breast MRI). Women were contacted via telephone call by research staff and provided oral consent to participate in the study17.
2.2. Breast Cancer Risk Communication
The breast cancer risk communication intervention consisted of a mailed letter followed by a telephone call from a Breast Heath Genetic Counselor (BHGC). A lay language breast cancer risk letter (Supplementary material) was created by the Athena Breast Health Program and mailed to participants within two weeks of their receipt of normal mammogram results. The letter informed women of their elevated risk of breast cancer based on the information provided on the questionnaire at the time of screening. It also emphasized the need to verify the self-reported information from which the risk estimate was generated. A similar letter was sent to participants’ primary care physicians to inform them of the patient’s risk. During the BHGC telephone consultation, information from the risk assessment survey was validated and participants’ breast cancer risk was reassessed. Concerns about breast cancer were discussed and the BHGC provided medical and psychological referrals to participants, as needed.
2.3. Measures
We evaluated participants’ breast cancer risk estimation, anxiety, and breast cancer worry using validated questionnaires. Women were called by research staff to complete the same assessment at two time points: before receiving the risk letter (approximately 2 weeks after receiving mammogram results) and after the risk letter and BHGC consultation (6–8 weeks after receiving their mammogram result).
The baseline and follow up surveys contained the following components:
2.3.1. Risk Estimation
Risk estimation was assessed by two methods. First, a numerical risk estimation was generated by asking: “What do you think your chance is of developing breast cancer in your lifetime? Please choose a number between 0% (no chance of breast cancer) and 100% (definitely will get breast cancer)”18. Second, a comparative risk estimation was generated by asking “Compared to other women of the same age, do you think your chance of getting breast cancer is?” with three response options: “lower,” “the same,” and “higher.”19
2.3.2. Anxiety
Anxiety was assessed using the Patient-Reported Outcomes Measurement Information System (PROMIS) anxiety scale (short form 8a)20. The raw score was rescaled into a standardized T score, with higher scores indicating more anxiety. A score of 50 (standard deviation of 10) on the PROMIS 8a represents the mean score for the general population, and a score of 60 is defined as a cut-point for anxiety21, 22.
2.3.3. Breast Cancer Worry
Breast cancer worry was assessed using the Lerman breast cancer worry scale23, 24. The five items assessed: frequency of breast cancer worry, frequency of thinking about breast cancer, impact of breast cancer worry on mood, impact of breast cancer worry on daily activity, and self-description as “generally happy and free from worry, occasionally distressed, often distressed or extremely distressed.” For questions 1–4, responses were scored on a scale of 1 (not at all) to 4 (almost all the time). For question 5, responses were scored from 1 (generally happy and free from worry) to 4 (extremely distressed). The total breast cancer worry score ranged from 5 to 20 with a higher score indicating more worry.
2.4. Statistics and Data Analysis
We computed “risk accuracy” as the difference between participants’ perceived risk and their lifetime breast cancer risk calculated by the Gail model16. Because women tend to overestimate their risk of breast cancer25, we categorized participants into two subgroups: accurate estimators (difference between perceived and Gail-estimated risk ±20%), and over-estimators (difference >20%). Concerning anxiety, for analytic purposes, participants were classified into high (T>60) and not-high (T=<60) anxiety subgroups based on their baseline anxiety level22.
Numerical risk estimation and anxiety were summarized as mean with standard deviation. Breast cancer worry was summarized as median with interquartile range. These measures were compared between baseline and follow up using paired t-tests or Wilcoxon signed rank tests, as appropriate. Pearson correlations were used to examine the correlation between numerical risk estimation, Gail model lifetime risk, anxiety, and breast cancer worry. These correlations were performed separately for each time point. Next, we used a mixed effects linear regression model to assess the relationship between anxiety and risk estimation accuracy over time. In order to account for within-participant repeated measurements, a random intercept for participant was fit along with a nested random slope for time (i.e., post vs. pre-intervention). The differential association of anxiety on risk estimation over time was examined using a time-by-risk estimate fixed effect interaction term. The association between breast cancer worry and risk estimation accuracy was examined with the same method. Two-sided p-values were reported and variables were considered statistically significant if the p-value<0.05. Analyses were performed using STATA 15.
3. Results
Of 284 women with a normal mammogram result who were identified by population-based screening to be at elevated risk for breast cancer, 175 were reached by telephone to request participation, and 122 consented to participate (43% response rate). Study participants’ mean age was 54.8 years, 76% were non-Hispanic white, and 95% were college graduates. (Table 1)
Table 1.
Characteristics of Study Subjects and Gail Model Breast Cancer Risk (N=122)
| Age, mean years (standard deviation) | 54.8 (9.8) |
| Site, N (%) | |
| UCLA | 81 (66%) |
| UCSF | 25 (21%) |
| UCSD | 16 (13%) |
| Education, N (%) | |
| Less than or graduate from high school | 6 (4.8%) |
| Graduate from college | 31 (25%) |
| Post-graduate degree | 85 (70%) |
| Race/Ethnicity, N (%) | |
| Non-Latino White | 93 (76%) |
| Latino | 12 (10%) |
| African American | 11 (9%) |
| Asian American | 6 (5%) |
| Breast cancer risk estimate, median (Interquartile range)† | |
| 5 year breast cancer risk | 1.9% (1.3%, 3.0%) |
| 10 year breast cancer risk | 4.0% (2.7%, 5.8%) |
| Lifetime breast cancer risk | 12.8% (8.5%, 17.7%) |
Risk estimation is based on Gail model
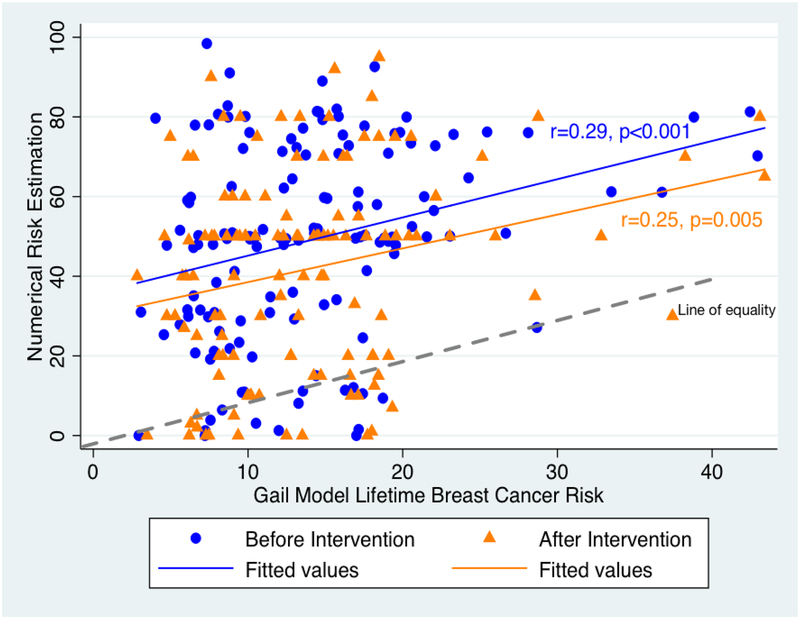
The median calculated Gail model lifetime breast cancer risk was 12.8%. At baseline, 16% of respondents felt that their breast cancer risk was lower, 40% about the same, and 43% higher than other women their age. After risk communication, 16% felt that their breast cancer risk was lower, 32% about the same, and 51% higher than other women their age. Women’s mean numerical lifetime breast cancer risk estimate before risk communication was 49.1%, significantly higher than the mean post-risk communication risk estimate of 41.9% (p<0.001), and both were much higher than the Gail model risk estimate of 12.8% (p<0.001). The plot of numerical lifetime breast cancer risk estimate versus Gail model estimate showed that women’s subjective numerical risk estimation was markedly exaggerated, widely distributed, and only moderately linked to the Gail model risk estimate at baseline (r=0.29, p=0.001) and after risk communication (r=0.25, p=0.005) (Figure 1).
Figure 1.
Plot of Women’s Numerical Risk Estimate v Gail Model Lifetime Breast Cancer Risk, Before and After Risk Communication
Values on the line of equality represent accurate breast cancer risk estimations. Points above the line are overestimates and points below the line are underestimates.
Mean level of anxiety at baseline was 52.4, which was close to the population norm, and it did not change after risk communication. Breast cancer worry at baseline had a median of 7, which decreased significantly to 6 (p=0.01) after risk communication (Table 2a). Anxiety was more highly correlated with breast cancer worry before (r=0.45, p<0.001) than after risk communication (r=0.24, p=0.01). Breast cancer worry was not significantly correlated with risk perception at baseline (r=0.06, p=0.53), but the correlation was statistically significant after risk communication (r=0.22, p=0.02). Anxiety was not correlated significantly with risk perception at baseline (r=0.02, p=0.83) or follow-up (r=0.10, p=0.28) (Appendix Figs. 1–3).
Table 2.
Comparison of Numerical Risk Estimation, Anxiety, and Breast Cancer Worry Before and After Risk Communication
| Before Risk Communication | After Risk communication | P value | |
|---|---|---|---|
| Numerical Risk Estimation, mean (SD) | |||
| Overall | 49.1% (24.6%) | 41.9% (25.2%) | <0.001 |
| Lower anxiety subgroup | 47.6% (24.7%) | 39.1% (25.0%) | <0.001 |
| High anxiety subgroup | 53.0% (24.4%) | 52.5% (21.8%) | 0.9 |
| Anxiety, mean (SD) | |||
| Overall | 52.4 (8.2) | 51.4 (8.8) | 0.12 |
| Lower anxiety subgroup | 50.0 (6.9) | 49.6 (8.1) | 0.6 |
| High anxiety subgroup | 63.2 (3.4) | 59.1 (7.3) | 0.01 |
| Breast cancer worry, median (IQR) | |||
| Overall | 7.0 (5–8) | 6.0 (5–8) | 0.01 |
| Lower anxiety subgroup | 6.0 (5,7) | 6.0 (5,7) | 0.3 |
| High anxiety subgroup | 9.0 (7,10) | 7.0 (6,8) | <0.001 |
| Before Risk Communication | After Risk communication | P value | |
| Numerical Risk Estimation, mean (SD) | |||
| Accurate estimate subgroup | 14.0% (11.2%) | 16.2% (13.8%) | 0.46 |
| Over-estimated subgroup | 61.0% (16.1%) | 50.0% (23.6%) | <0.001 |
| Anxiety, mean (SD) | |||
| Accurate estimate subgroup | 48.3 (7.4) | 49.0 (8.2) | 0.7 |
| Over-estimated subgroup | 54.4 (7.5) | 53.0 (8.9) | 0.1 |
| Breast cancer worry, median (IQR) | |||
| Accurate estimate subgroup | 6 (5,7) | 6 (6,8) | 1.0 |
| Over-estimated subgroup | 7 (6,8) | 7 (5,8) | 0.09 |
SD = standard deviation, IQR=interquartile range
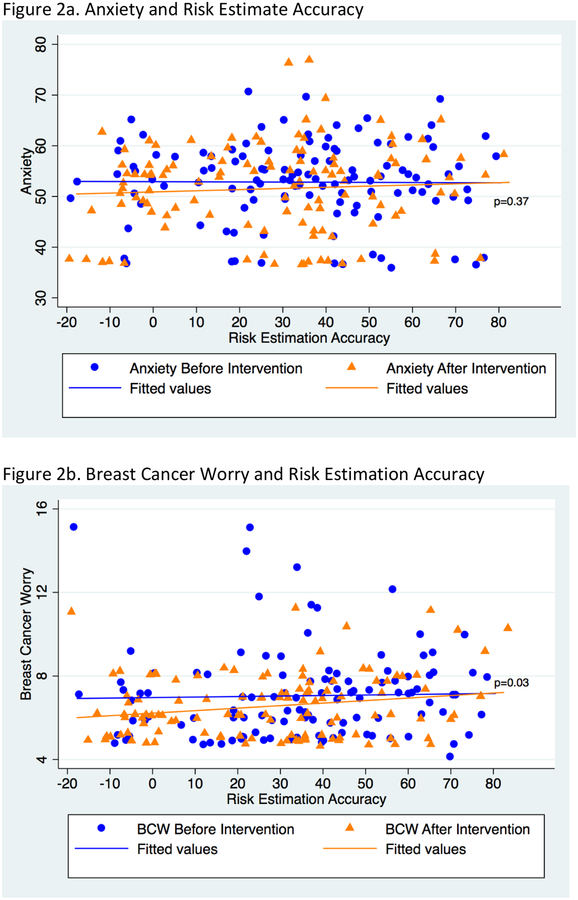
Figures 2a and 2b demonstrate the relationship of anxiety and breast cancer worry to risk estimation accuracy, before and after risk communication. The fitted line for before and after data is derived from the mixed effects linear regression model. At baseline, the relationship between anxiety and breast cancer worry with risk estimation accuracy is nearly flat. After risk communication, anxiety is slightly lower among those with more accurate risk estimation, although there is no statistically significant difference between the slopes of the before and after lines (p=0.37). In contrast, the before and after risk communication lines are significantly different for breast cancer worry, with women who reported more realistic estimates of breast cancer risk having lower breast cancer worry after risk communication (p=0.03).
Figure 2.
Correlations between Anxiety and Breast Cancer Worry with Accuracy of Breast Cancer Risk Estimation before and after risk communication
Risk-estimation accuracy is Personal lifetime risk estimate minus Gail model breast cancer lifetime risk estimate.
In the stratified analyses of participants with high anxiety at baseline (N=22) versus those with lower anxiety (N=100), breast cancer risk perception decreased significantly after risk communication in the subgroup with lower anxiety (47.6% v 39.1%, p<0.001), but not high anxiety. In the high anxiety subgroup, both anxiety (mean 63.2 vs. 59.1, p=0.01) and breast cancer worry (median 9 v 7, p<0.001) decreased significantly after risk communication, but neither changed in the lower anxiety subgroup (Table 2a).
In the stratified analyses based on risk estimation accuracy, mean breast cancer risk perception at baseline was 14% in the accurate estimator subgroup (N=34) and 61% in the over-estimator subgroup (N=88). After risk communication, nearly all women continued to overestimate their personal breast cancer risk (Figure 1 and Table 2b), although the risk perception in over-estimators decreased significantly (61.0% v 50.0%, p<0.001). The over-estimator subgroup was more anxious than accurate-estimator subgroup at baseline (54.4 v 48.3, p=0.003). There was no change in anxiety or breast cancer worry after risk communication in either subgroup (Table 2b).
4. Discussion
Physicians, geneticists and behavioral scientists are on the brink of being able to provide personalized risk information to patients in order to guide preventive behavior26–28. It is critical to know the effect of such an intervention on patients’ risk estimation accuracy, anxiety, and worry29. In the context of a single-arm personalized breast cancer risk communication trial, the present findings suggest that sending a risk letter and providing genetic counseling by telephone to inform women that they are at elevated risk of breast cancer did not provoke negative psychological responses. Building on the work of Livaudais et al., which found that risk communication did not increase breast cancer concern30, we found that risk communication combined with counseling was associated with a significant reduction in breast cancer worry among women with higher general anxiety and improved the accuracy of breast cancer risk estimation among women with lower anxiety (although substantial over-estimation persisted).
In line with previous literature8, about 20% of women in our study reported high anxiety, even after receiving a negative mammogram result, and higher anxiety was significantly associated with breast cancer worry. The finding that the correlation between anxiety and breast cancer worry was lower after risk communication compared to baseline suggests that such communication might help to de-couple general anxiety from cancer-specific worry as women’s perceptions of their risk become more accurate. Such a de-coupling could help explain the seemingly paradoxical finding that communication of elevated breast cancer risk was associated with subsequently lower breast cancer worry in the subgroup of women with higher baseline anxiety (while general anxiety was unchanged).
This study found that breast cancer risk communication reduced disease-specific worry but did not significantly decrease anxiety. This is consistent with Lerman et al’s finding that risk communication could decrease breast cancer worry but not general anxiety unless the anxiety was addressed31, 32. In the context of cancer prevention, reducing high anxiety can be important because high anxiety is associated with less compliance with cancer screening and may negatively affect other health behaviors33.
Numerous studies evaluating breast cancer risk estimation reveal that women typically over-estimate their risk34, 35. The present study not only demonstrates the over-estimation, but also shows that full correction of overestimation does not occur with a one-time risk communication. Others have shown that over-estimation of cancer risk is relatively resistant to change36, 37. Another study suggested that inaccurate cancer risk estimation could itself be a cause of increased anxiety and a significant predictor of breast cancer worry34; however, we found that risk estimation accuracy was only weakly correlated with anxiety and breast cancer worry. Furthermore, prior studies show inconsistency in whether genetic counseling improves risk estimation38–40. Rather than trying to correct misperceptions of risk, interventions to prevent breast cancer might focus more productively on improving screening or reducing risky behaviors.
4.1. Study Limitations
The primary limitation of this study is the absence of a control group, which precludes causal inference. In addition, we used both the USPSTF guideline and Gail model for risk assessment to identify elevated-risk women, which might have led to underestimation of some women’s model-based risk estimates. Furthermore, using the National Comprehensive Cancer Network guideline for risk assessment would likely identify more elevated-risk patients. In that the present sample was largely non-Hispanic white and well-educated, caution is needed when generalizing to other populations. Finally, the study was conducted over two months; later effects of risk communication are unknown.
4.2. Clinical Implications
This study suggests that, at least among well-educated women, developers of personalized breast cancer risk reduction programs need not fear unintended consequences of informing women about elevated breast cancer risk.
4.3. Conclusion
Personalized risk communication about breast cancer risk is associated with modest improvement in risk estimation (in women with lower anxiety) and a reduction in breast cancer worry (in anxious women). Despite communicating a message of risk, the intervention did not appear to create greater anxiety.
Supplementary Material
Acknowledgments
The study was supported by University of California, Los Angeles, Jonsson Comprehensive Cancer Center, National Cancer Institute P30 CA16042; Clinical Translational Science Institute, National Institutes of Health/National Center for Advancing Translational Sciences Grant #UL1TR001881.
We acknowledge the assistance of the following medical students in collecting data for the study: Shannon Ordon, Cary Kraft, Jessica Lucier, Shilpa Agrawal, and Daria Gaut. The authors are grateful to all the patients who participated in the study and the staff that facilitated this research.
Footnotes
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest
The authors declare no potential conflicts of interest related to the data reported in this manuscript.
References
- 1.Surveillance E, and End Results Program. Cancer Stat Facts: Female Breast Cancer: Surveillance, Epidemiology, and End Results Program; 2018. [Available from: https://seer.cancer.gov/statfacts/html/breast.html.
- 2.Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database of Systematic Reviews. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilaprinyo E, Forné C, Carles M, Sala M, Pla R, Castells X, et al. Cost-Effectiveness and Harm-Benefit Analyses of Risk-Based Screening Strategies for Breast Cancer. PLoS ONE. 2014;9:e86858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Annals of internal medicine. 2009;151:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thirlaway K, Fallowfield L. The psychological consequences of being at risk of developing breast cancer. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP). 1993;2:467–71. [DOI] [PubMed] [Google Scholar]
- 6.Lerman C, Rimer BK, Engstrom PF. Cancer risk notification: psychosocial and ethical implications. 1991;9. [DOI] [PubMed] [Google Scholar]
- 7.Zakowski SG, Valdimarsdottir HB, Bovbjerg DH, Borgen P, Holland J, Kash K, et al. Predictors of intrusive thoughts and avoidance in women with family histories of breast cancer. Ann Behav Med. 1997;19(4):362–9. [DOI] [PubMed] [Google Scholar]
- 8.B J, B C, H B, W E. The psychological impact of mammographic screening. A systematic review. Psycho-Oncology. 2005;14:917–38. [DOI] [PubMed] [Google Scholar]
- 9.Absetz P, Aro AR, Sutton SR. Experience with breast cancer, pre-screening perceived susceptibility and the psychological impact of screening. Psychooncology. 2003;12(4):305–18. [DOI] [PubMed] [Google Scholar]
- 10.Kash KM, Holland JC, Halper MS, Miller DG. Psychological distress and surveillance behaviors of women with a family history of breast cancer. J Natl Cancer Inst. 1992;84(1):24–30. [DOI] [PubMed] [Google Scholar]
- 11.Olivotto TGHRHJETJRJCAVJWA. Satisfaction and Anxiety for Women During Investigation of an Abnormal Screening Mammogram. Breast Cancer Research and Treatment. 2002;76(3):245–54. [DOI] [PubMed] [Google Scholar]
- 12.Austoker J, Ong G. Written Information Needs of Women Who are Recalled for Further Investigation of Breast Screening: Results of a Multicentre Study. Journal of Medical Screening. 1994;1:238–44. [DOI] [PubMed] [Google Scholar]
- 13.Scaf-Klomp W, Sanderman R, Van De Wiel M, Otter R, Van Den Heuvel WJA. Distressed or relieved? Psychological side effects of breast cancer screening in the Netherlands. 3rournal of Epidemiology and Community Health. 1997;51:705–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elson SL, Hiatt RA, Anton-Culver H, Howell LP, Naeim A, Parker BA, et al. The Athena Breast Health Network: developing a rapid learning system in breast cancer prevention, screening, treatment, and care. Breast Cancer Research and Treatment. 2013;140:417–25. [DOI] [PubMed] [Google Scholar]
- 15.Moyer VA, Services USP, Force T. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013. [DOI] [PubMed] [Google Scholar]
- 16.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting Individualized Probabilities of Developing Breast Cancer for White Females Who Are Being Examined Annually. Journal of the National Cancer Institute. 1989;81:1879–86. [DOI] [PubMed] [Google Scholar]
- 17.Silver E, Wenger N, Xie Z, Elashoff D, Lee K, Madlensky L, et al. Implementing a Population-based Breast Cancer Risk Assessment Program. Clinical Breast Cancer. 2019;S1526–8209(18):30761–4. [DOI] [PubMed] [Google Scholar]
- 18.Gurmankin Levy A, Shea J, Williams SV, Quistberg A, Armstrong K. Measuring Perceptions of Breast Cancer Risk. Cancer Epidemiology Biomarkers & Prevention. 2006;15:1893–8. [DOI] [PubMed] [Google Scholar]
- 19.Lipkus IM ID, Terrenoire J, Feaganes JR. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(6):533–9. [PubMed] [Google Scholar]
- 20.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, et al. Item Banks for Measuring Emotional Distress From the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, Anxiety, and Anger. Assessment. 2011;18:263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PROMIS SCORING GUIDE VERSION 1.0 SHORT FORMS PROFILE SHORT FORMS COMPUTERIZED ADAPTIVE TESTING 2011. [Available from: https://www.assessmentcenter.net/documents/PROMIS%20Profile%20Scoring%20Manual.pdf.
- 22.Marrie RA, Zhang L, Lix LM, Graff LA, Walker JR, Fisk JD, et al. The validity and reliability of screening measures for depression and anxiety disorders in multiple sclerosis. Multiple Sclerosis and Related Disorders. 2018;20:9–15. [DOI] [PubMed] [Google Scholar]
- 23.Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 1991;10:259–67. [DOI] [PubMed] [Google Scholar]
- 24.Andersen MR, Smith R, Meischke H, Bowen D, Urban N. Breast cancer worry and mammography use by women with and without a family history in a population-based sample. Cancer Epidemiology Biomarkers & Prevention. 2003;12:314–20. [PubMed] [Google Scholar]
- 25.Evans DGR, Burnell LD, Hopwood P, Howell A. Perception of risk in women with a family history of breast cancer. British Journal of Cancer. 1993;67:612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan CP, Haas JS, Pérez-Stable EHBJ, Des Jarlais G, Gregorich SE. Factors affecting breast cancer risk reduction practices among California physicians. Preventive medicine. 2004;41:7–15. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan CP, Haas JS, Perez-Stable EJ, Gregorich SE, Somkin C, Des Jarlais G, et al. Breast cancer risk reduction options: awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol Biomarkers Prev. 2006;15(1):162–6. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong M Katrina, MSCE; Quistberg D. Alex, BA; Micco Ellyn, BA; et al. Prescription of Tamoxifen for Breast Cancer Prevention by Primary Care Physicians. Arch Intern Med. 2006;166(20):2260–5. [DOI] [PubMed] [Google Scholar]
- 29.Frank L, Basch E, Selby JV. The PCORI Perspective on Patient-Centered Outcomes Research. JAMA. 2014;312:1513. [DOI] [PubMed] [Google Scholar]
- 30.Livaudais-Toman J, Karliner LS, Tice JA, Kerlikowske K, Gregorich S, Perez-Stable EJ, et al. Impact of a primary care based intervention on breast cancer knowledge, risk perception and concern: A randomized, controlled trial. Breast. 2015;24(6):758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerman C, Lustbader E, Rimer B, Daly M, Miller S, Sands C, et al. Effects of individualized breast cancer risk counseling: a randomized trial. J Natl Cancer Inst. 1995;87(4):286–92. [DOI] [PubMed] [Google Scholar]
- 32.Watson M, Duvivier V, Wade Walsh M, Ashley S, Davidson J, Papaikonomou M, et al. Family history of breast cancer: what do women understand and recall about their genetic risk? JrMed Genet. 1998;35:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay JL, Buckley TR, Ostroff JS. The role of cancer worry in cancer screening: A theoretical and empirical review of the literature. Psycho-Oncology. 2005;14:517–34. [DOI] [PubMed] [Google Scholar]
- 34.By McGregor BA, Bowen Deborah J.,Ankerst Donna P.,Andersen M. Robyn,Yasui Yutaka,McTiernan Anne. Optimism, Perceived Risk of Breast Cancer, and Cancer Worry Among a Community-Based Sample of Women. [DOI] [PubMed]
- 35.Black WC, Nease RF, Tosteson ANA. Perceptions of Breast Cancer Risk and Screening Effectiveness in Women Younger Than 50 Years of Age. JNCI Journal of the National Cancer Institute. 1995;87:720–31. [DOI] [PubMed] [Google Scholar]
- 36.Weinstein ND, Sandman PM, Hallman WK. Testing a Visual-Display To Explain Small Probabilities. Risk Analysis. 1994;14:895–6. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein ND, Sandman PM, Roberts NE. Perceived susceptibility and self-protective behavior: a field experiment to encourage home radon testing. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 1991;10:25–33. [DOI] [PubMed] [Google Scholar]
- 38.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological Impact of Genetic Counseling for Familial Cancer: A Systematic Review and Meta-analysis. [DOI] [PubMed]
- 39.Butow PN L E, Meiser B, Barratt A, Tucker KM. Psychological outcomes and risk perception after genetic testing and counselling in breast cancer: a systematic review. Med J Aust. 2003;178(2):77–81. [DOI] [PubMed] [Google Scholar]
- 40.Smerecnik CMR, Mesters I, Verweij E, de Vries NK, de Vries H. A Systematic Review of the Impact of Genetic Counseling on Risk Perception Accuracy. Journal of Genetic Counseling. 2009;18:217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.