Abstract
Infections from multidrug resistant (MDR) pathogens and emerging viruses present challenges for effective clinical treatments. Drug repurposing and combination screens may provide therapies at a fraction of the time and cost of traditional methods of drug development. Synergistic combinations of two or three known compounds can increase therapeutic efficacy and reduce concentrations required for individual drugs, in turn, reducing the risk of drug toxicity. Using libraries of approved drugs, traditionally-non-antibiotic compounds identified in repurposing screens can quickly move into clinical trials, since safety profiles have been previously established. Herein we summarize recent advances in identifying synergistic drug combinations and the use of drug screens for personalized medicine treatments of infections caused by MDR pathogens and emerging viruses.
Keywords: drug repurposing, drug combination therapy, drug synergy, multidrug resistant bacteria, non-antibiotic agents for MDR pathogen
Introduction
Despite marked improvements in treatment and disease management, infectious diseases remain a critical, global health problem [1]. HIV/AIDS, tuberculosis, malaria, and influenza, the most common infectious diseases, are ongoing challenges [2]. Infections with MDR pathogens and emerging diseases, such as illnesses caused by Ebola and Zika viruses, continue to threaten public health. The growing incidence of MDR bacterial infections underscores the need for new antibiotics. However, the commercial launching of new antibacterial agents in the last few decades has decreased due to difficulties in drug discovery and development [3–5]. From 1995 to 2001, no candidates were developed from approximately 200 screens against infectious targets conducted by Pfizer, GlaxoSmithKline, and AstraZeneca, illustrating the challenge of finding effective new anti-infectious agents [6–8].
Recently, drug repurposing and synergistic drug screens have provided alternative approaches to combat infections caused by MDR pathogens and emerging viral outbreaks [9–11]. Despite the promise of finding useable approved drugs through repurposing screens alone, identified drugs individually often show weak activity, preventing widespread application as anti-infectives [9]. However, screens designed to test two- or three-drug combinations may yield novel synergistic regimens, permitting lower drug concentrations than required for solo compounds. Herein, we provide a brief overview of drug combination screens employing high-throughput screening technology to identify potential drug combinations for clinically relevant treatment of infectious pathogens.
Multidrug-resistant gram-negative bacteria
Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae (including Klebsiella, E. coli, Serratia, and Proteus) are representative gram-negative bacteria (GNB) whose emerging resistance to third generation cephalosporins and carbapenems has resulted in an urgent need for new treatments [5,12]. Previously abandoned antibiotics, such as the polymyxins, have been utilized to treat carbapenem-resistant GNB as a last resort [13,14]. Polymyxins are a group of cationic polypeptides with five members (polymyxin A through E), discovered in 1947 [15]; but subsequently, were replaced by newer antibiotics due to their significant nephrotoxicity. However, development of resistance to the newer agents has led to a return of polymyxin B and more frequently polymyxin E (colistin) to clinical use, despite their toxicity [16]. In addition to toxicity concerns, the recommended dosages of the older agents may be suboptimal [17–19], making the drug unsuitable for monotherapy due to emerging hetero-resistance recently observed in clinical isolates. These issues underscore the urgent need for development of new treatment options [20,21].
Two- or three-drug combinations of antibiotics have been advocated for severe clinical infections such as bacteremias. Multiple in vitro studies have demonstrated synergistic effects of polymyxin in combination with other antibiotics for MDR GNB [22]. Polymyxin-carbapenem and polymyxin-rifampin combinations are the most promising pairs for MDR GNB treatment. Two randomized clinical trials ([23] and [24]) are currently in progress to elucidate possible clinical benefits. In a recent study, Brennan-Krohn et al. explored the combination of colistin with several other antibiotics against colistin-resistant Enterobacteriaceae [25]. They reported that linezolid, rifampin, or azithromycin re-sensitized acquired colistin-resistant bacteria to colistin, indicating that the suboptimal concentrations of colistin used clinically may be sufficient when combined synergistically with these other antibiotics.
Although drug combination therapy with two or three antibiotics is a useful approach for clinical translation, the number of FDA approved antibiotics are limited and it is difficult in any given clinical situation to know what combination would be most effective. In addition to antibiotic combinations, synergistic effects from combinations with non-antibiotic compounds have been reported, offering a new direction for combatting emergent drug-resistant pathogens [26,27]. Ejim et al. explored a minocycline-based combinational screen using an approved drug library [28]. Six non-antibiotic compounds were identified that potentiated the activity of minocycline against a reference P. aeruginosa strain (PA01); four of them reached a synergy threshold with a fractional inhibitory concentration (FIC) index < 0.5. Benserazide (a DOPA decarboxylase inhibitor for Parkinson’s disease) and loperamide (an opioid receptor agonist used to treat diarrhea) restored minocycline susceptibility in a panel of five MDR P. aeruginosa strains. True synergy was also observed between loperamide and minocycline against a number of common GNB.
In the treatment of GNB infections, several other non-antibiotic compounds were reported to have synergistic effects with polymyxin, including ivacaftor (a cystic fibrosis drug) [29], selective estrogen receptor modulators (breast cancer drugs) [30], zidovudine (an antiretroviral drug) [31], curcumin (a bioactive substance from turmeric), and suloctidil (a vasodilator) [32]. Otto et al. investigated the synergistic antimicrobial effects of polymyxin B with 30 non-antibiotic drugs representing different classes against K. pneumoniae, E. coli, A. baumannii, and P. aeruginosa isolates [33]. Fourteen non-antibiotic drugs exhibited synergy with polymyxin B. Among them, citalopram and sertraline (two selective serotonin reuptake inhibitors) and levomepromazine (an antipsychotic agent) showed synergy with polymyxin B against three types of bacteria including K. pneumoniae, E. coli, and A. baumannii. While P. aeruginosa was relatively more resistant to most of the combinations, levomepromazine and chlorpromazine (both antipsychotic agents) exhibited synergy with polymyxin B. After filtering for drugs with attainable plasma concentrations that correlated with effective concentrations against the bacteria, only spironolactone (a diuretic agent) exhibited synergistic bacterial suppression against E. coli. Additionally, additive or synergistic activities were found against all four species (K. pneumoniae, E. coli, A. baumannii, and P. aeruginosa) among three phenothiazine antipsychotic derivatives (chlorpromazine, levomepromazine, and promethazine), providing potential chemical scaffolds for further drug development. This work demonstrated the virtue of screening of drug combinations with non-antibiotic compounds from screens against MDR pathogens for identifying novel chemical scaffold and new drug targets.
Furthermore, Sun et al. conducted a drug repurposing and combinational screen that identified 17 synergistic three-drug combinations against an MDR K. pneumoniae at clinically achievable concentrations [34]. Two sets of three-drug combinations exhibited broad-spectrum activities against a panel of ten common MDR clinical isolates, including K. pneumoniae, A. baumannii, P. aeruginosa, Citrobacter freundii, Enterobacter cloacae, and E. coli. These three-drug combinations with broad-spectrum activity could thus provide an initial therapy for severe infections before patient bacterial identification is complete.
A number of reports have described the effectiveness of pairwise combinations between antibiotics or with unconventional antimicrobials against MDR strains [35–37]. However, clinical translation has been limited due to the potential side effects of unidentified drug-drug interactions and a lack of clinical experience. Indeed, the effects on mortality of these novel drug combinations have been controversial in some clinical studies [22,38]. Problems could be caused by unpredictable drug-drug interactions, drug metabolism, and unknown pharmacokinetic profiles associated with the new combinations. Each drug has a distinct pharmacokinetic profile which is determined by its absorption, distribution, metabolism, and excretion in the human body. Therefore, selecting a dose regimen in which both drugs achieve optimal concentrations while simultaneously replicating the desired synergistic effects found in vitro remains challenging. New drug delivery systems, formulations, or antibiotic hybrid compounds (two antibiotic moieties combined within a single compound) [39–41] might provide new approaches to overcome the lack of pharmacokinetic complementarity or potential increases in drug toxicity caused by combination therapy.
Strain-dependent growth inhibition by combination drug pairs is another difficulty when selecting a specific combination for a given patient. Indeed, when confronted with clinical deterioration of an individual patient, published studies are often of limited usefulness to the practicing physician because it is difficult to predict the most effective combination for a given strain in the setting of the unique requirements to reduce toxicities within the individua’s drug regimen. Because the effectiveness of any given drug combination can vary for different isolates within the same species, personalized drug combinatorial screens may identify isolate-specific combinations for treatment. This new approach was implemented, for the first time, by screening hundreds of drug combinations within an actionable three-day period against a set of clinical isolates from a patient having a severe infection with multiple MDR GNB (see Figure 1). A useful drug combination was identified as an alternative therapy, circumventing a drug (imipenem) suspected of causing cholestatic liver injury in the patient [42]. This drug combinatorial screen allowed for expanded drug susceptibility synergy testing beyond what is typically available in a clinical microbiology laboratory, using a quantitative high throughput screen in a 1536-well plate format. A set of nine drugs in fourteen combinations of two- and three-drug pairs were identified from a total of 2,304 combinations. A three-drug combination of piperacillin-tazobactam-gentamicin replaced a multi-drug combination containing imipenem suspected of causing cholestatic liver toxicity. After beginning this new regimen, the patient’s liver function improved promptly, allowing reinstitution of therapy for the patient’s aplastic anemia [42]. This case thus demonstrated the potential for “real-time” combinatorial drug screens against patient isolates to identify effective, less toxic drug combinations to control severe infections within a time frame useful for clinicians. Since many academic facilities now have high-throughput capability, combinational screens offer the promise of discovering life-saving, therapeutic options to treat MDR infections. Indeed, two to three antibiotics combination are standard for the treatment of severe infections, especially for those with multidrug resistant bacteria. For drug combination therapy using approved antibiotics, physicians can now optimize drug combinations based on the results from high throughput antimicrobial susceptibility testing without the need to go through clinical trials. However, for drug combinations with non-antibiotics, new clinical trials may be needed to allow approval for new indications beyond an individual patient.
Figure 1.
Hypothetical workflow for applying repurposing and combinational screens to multi-drug resistant (MDR) gram negative bacteria (GNB) and emerging viruses.
Ebola virus disease
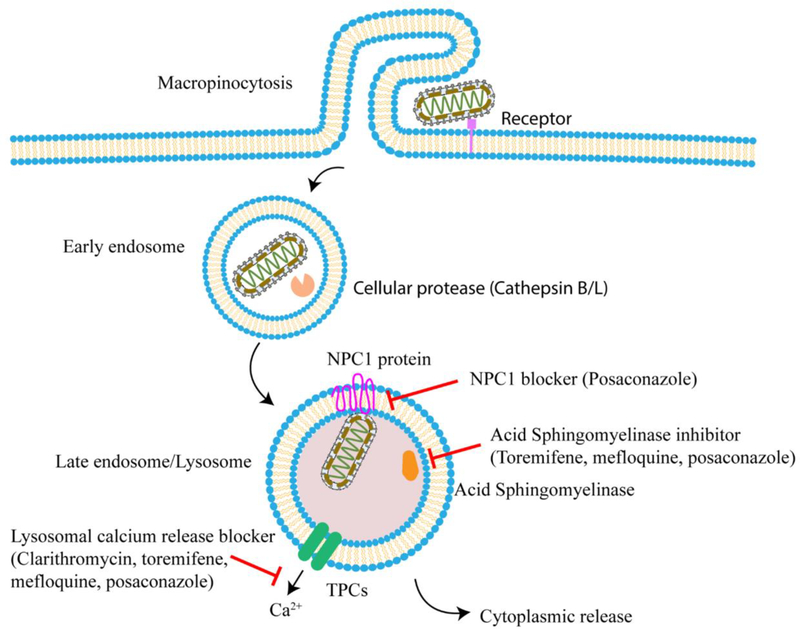
Ebola viral disease is a severe, deadly illness. The 2014–2016 outbreak in West Africa caused 11,310 confirmed deaths [43]. Currently, there is an ongoing Ebola epidemic in the Democratic Republic of the Congo [44], but no effective treatment is available. In response to the Ebola virus outbreak in 2014, our group performed a drug repurposing screen which identified a set of 53 Ebola-entry inhibitors [45]. However, the human plasma drug concentrations of most of these compounds could not safely reach levels showing activity against Ebola virus as suggested by the in vitro experiments, limiting the application of these compounds in clinical practice. Subsequently, a drug combination screen with selected candidates was performed to identify synergistic regimens with reduced toxicity. Three sets of three-drug combination almost completely blocked Ebola viral entry into host cells employing potentially non-toxic drug concentrations. Two-drug combinations were not able to achieve the same inhibition with similar individual drug concentrations. Two sets of three-drug combinations, toremifene-clarithromycin-posaconazole and toremifene-mefloquine-posaconazole both demonstrated significant activity in the live Ebola virus infection assay. Although the actual mechanisms and molecular targets are unclear, these drugs are associated with inhibition of several key steps in the host cell entry process during Ebola virus infection, including Ebola entry receptor Niemann Pick C1 (NPC1) protein, acid sphingomyelinase (ASM), and lysosomal calcium release (Figure 2) [46]. In a second study, nine drug pairs stood out from a pairwise combination test of twelve compounds using a viral inhibition assay [47]. Intriguingly, eight sets of two-drug combinations were composed of two viral entry inhibitors. Among the synergistic pairs, only the sertraline-toremifene combination was effective at clinically attainable concentrations. Therefore, the three-drug combinations of approved drugs may represent better candidates to control or prevent Ebola infections.
Figure 2.
Schematic representation of multiple mechanism of action (MOA) from the three-drug combination against Ebola Virus (EBV). Posaconazole blocks NAADP-stimulated lysosomal calcium release, the function of NPC protein and ASM activity. Toremifene and mefloquine inhibit NAADP-stimulated lysosomal calcium release and ASM activity. Clarithromycin inhibits NAADP-stimulated lysosomal calcium release.
Zika virus infection
Zika virus (ZIKV) infection was a neglected disease until the recent outbreak in 2015–2016 in South America, which caused severe complications in developing fetuses including microcephaly and other neuronal complications [48]. To address an unmet need for anti-Zika therapy, assay methods were developed to enable drug repurposing screens to identify compounds either inhibiting ZIKV replication or protecting cells from Zika-induced cytopathic apoptosis [49–51]. Emricasan, a pan-caspase inhibitor, tested in clinical trials for liver diseases [52], exhibited a protective activity against ZIKV-caused neuronal cell death. Niclosamide (an anthelmintic drug) and PHA-690509 (a cyclin-dependent kinase inhibitor tested in phase II clinical trials) were found to inhibit ZIKV replication in host cells [49]. The combination of emricasan with PHA-690509 demonstrated additive activity protective against cell death caused by ZIKV due to their non-overlapping activities. Another drug repurposing screen utilized the ZIKV NS-1 protein as an indicator of viral replication and identified emetine, an anti-protozoal agent, as a potent inhibitor of ZIKV and EBOV infections [53]. Interestingly, emetine acted at multiple steps related to viral damage including the inhibition of viral NS5 polymerase and the disruption of lysosomal function in host cells. For Ebola virus, emetine inhibited EBOV entry via the NPC1 receptor protein [53]. Others had reported that emetine inhibits protein synthesis by binding to the 40S ribosomal subunit in host cells [54]. Therefore, emetine as a single compound appears to possess multiple mechanisms of action, potentially enhancing its antiviral activity. Therefore, a multi-mechanism-based drug combination approach is often more effective against infectious with certain viruses. Screening drug combinations grouped by differing drug mechanisms of action may save time and increase screening efficiency. However, an unbiased compound collection was selected for screening against the most drug resistant bacteria due to the potential to identify novel noncanonical mechanisms of action. For example, in double carbapenem combination therapy, one carbapenem acts as a suicide substrate [55].
Conclusions and perspectives
Clinical therapeutic options for infections with highly resistant pathogens as well as new emerging infectious diseases remain limited due to a lack of suitable medications. Conventional rates of development for new therapeutics cannot, in many cases, accommodate the rapid evolution of these disease pathogens. Hence, drug repurposing screens present a novel approach to more rapidly identify new drugs and/or drug combinations applicable to these difficult infections. In many cases, these compounds are already used in clinical practice, facilitating pre-clinical and clinical study. Drug repurposing has been widely applied not only in infectious disease research but also in that of cancer and rare diseases [56–58]. However, patent protections are major concerns for companies to invest in such drug repurposing approaches [57,59]. Although “use” patents can be filed for repurposing drugs, it is known that the enforcement of this type of patent is difficult. Instead, repurposing of approved drugs with synergy combinations have advantages with regards to patent issues since a combination of two approved drugs for a new indication may be patentable, especially with a new formulation.
In this review, we also present a recent example where repurposing screens of drug synergistic combination sets have provided real-time, personalized results using pathogens isolated from an individual patient. Recent availability of high- throughput screening capability in most academic clinical centers thus may allow successful and rapid identification of two- or three-drug synergistic combinations tailored for a specific patient. In summary, drug repurposing combinatorial studies represent the next generation of repurposing approaches that can provide class-specific agents for pre-clinical and clinical development as well as personalized therapeutic design options for individual patients within a realistic time-frame for patient-centered clinical treatment.
Highlights.
Drug combinations improve efficacy and permit dose reductions of each drug
Lowering the dose of combined drugs prevents concentration-associated toxicity
Drug combinations can overcome the growing resistance to antibiotics
Real-time screens, using patient isolates, identify treatments for severe infections
Drug repurposing and combinations can identify treatments for emerging pathogens
Acknowledgments
This work was partly supported by the Intramural Research Programs of the National Center for Advancing Translational Sciences (NCATS), National Institute of Allergy and Infectious Diseases (NIAID), and the National Institutes of Health (NIH). The authors thank Dr. DeeAnn Visk, a medical writer and editor, for editing the manuscript.
Footnotes
Conflict of Interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Dye C: After 2015: infectious diseases in a new era of health and development. Philos Trans R Soc Lond B Biol Sci 2014, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The top 10 causes of death. Edited by: World Health Organization; 2018. [Google Scholar]
- 3.Ventola CL: The Antibiotic Resistance Crisis: Part 1: Causes and Threats. P T 2015, 40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 4.Butler MS, Blaskovich MA, Cooper MA: Antibiotics in the clinical pipeline in 2013. J Antibiot (Tokyo) 2013, 66:571–591. [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J: Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2008, 48:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL: Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 2006, 6:29–40.• This review article pinpoints the difficulty and provides directions for antibacterial drug discovery based on the experience from HTS run by GlaxoSmithKine between 1995 to 2001.
- 7.Tommasi R, Brown DG, Walkup GK, Manchester JI, Miller AA: ESKAPEing the labyrinth of antibacterial discovery. Nat Rev Drug Discov 2015, 14:529–542.•• This review article discusses the challenges and provides an overview of innovative approaches to development new antibacterial drugs.
- 8.Baker SJ, Payne DJ, Rappuoli R, De Gregorio E: Technologies to address antimicrobial resistance. Proc Natl Acad Sci U S A 2018, 115:12887–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun W, Sanderson P, Zheng W: Drug combination therapy increases successful drug repositioning. Drug Discov Today 2016, 21:1189–1195.• Herein, drug repurposing and drug combination benefits of the drug repurposing approach is considered.
- 10.Law GL, Tisoncik-Go J, Korth MJ, Katze MG: Drug repurposing: a better approach for infectious disease drug discovery? Curr Opin Immunol 2013, 25:588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekerman E, Einav S: Combating emerging viral threats. Science 2015, 348:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govindaraj Vaithinathan A, Vanitha A: WHO global priority pathogens list on antibiotic resistance: an urgent need for action to integrate One Health data. Perspect Public Health 2018, 138:87–88. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL: Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006, 6:589–601. [DOI] [PubMed] [Google Scholar]
- 14.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM: Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther 2012, 10:917–934. [DOI] [PubMed] [Google Scholar]
- 15.Storm DR, Rosenthal KS, Swanson PE: Polymyxin and related peptide antibiotics. Annu Rev Biochem 1977, 46:723–763. [DOI] [PubMed] [Google Scholar]
- 16.Wertheim H, Van Nguyen K, Hara GL, Gelband H, Laxminarayan R, Mouton J, Cars O: Global survey of polymyxin use: A call for international guidelines. J Glob Antimicrob Resist 2014, 1:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L: Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2006, 50:2946–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivas P, Hunt LN, Pouch SM, Thomas K, Goff DA, Pancholi P, Balada-Llasat JM, Bauer KA: Detection of colistin heteroresistance in Acinetobacter baumannii from blood and respiratory isolates. Diagn Microbiol Infect Dis 2018, 91:194–198. [DOI] [PubMed] [Google Scholar]
- 19.Meletis G, Tzampaz E, Sianou E, Tzavaras I, Sofianou D: Colistin heteroresistance in carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother 2011, 66:946–947. [DOI] [PubMed] [Google Scholar]
- 20.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, et al. : Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2015, 16:161–168. [DOI] [PubMed] [Google Scholar]
- 21.Souli M, Galani I, Giamarellou H: Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill 2008, 13. [PubMed] [Google Scholar]
- 22.Lenhard JR, Nation RL, Tsuji BT: Synergistic combinations of polymyxins. Int J Antimicrob Agents 2016, 48:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Multicenter Open-label Randomized Controlled Trial (RCT) to Compare Colistin Alone Versus Colistin Plus Meropenem. Edited by: https://ClinicalTrials.gov/show/NCT01732250.
- 24.Trial for the Treatment of Extensively Drug-Resistant Gram-negative Bacilli. Edited by: https://ClinicalTrials.gov/show/NCT01597973.
- 25.Brennan-Krohn T, Pironti A, Kirby JE: Synergistic Activity of Colistin-Containing Combinations against Colistin-Resistant Enterobacteriaceae. Antimicrob Agents Chemother 2018, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown D: Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void? Nat Rev Drug Discov 2015, 14:821–832. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Bello C: Antibiotic adjuvants - A strategy to unlock bacterial resistance to antibiotics. Bioorg Med Chem Lett 2017, 27:4221–4228. [DOI] [PubMed] [Google Scholar]
- 28.Ejim L, Farha MA, Falconer SB, Wildenhain J, Coombes BK, Tyers M, Brown ED, Wright GD: Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat Chem Biol 2011, 7:348–350. [DOI] [PubMed] [Google Scholar]
- 29.Schneider EK, Azad MA, Han ML, Tony Zhou Q, Wang J, Huang JX, Cooper MA, Doi Y, Baker MA, Bergen PJ, et al. : An “Unlikely” Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS Infect Dis 2016, 2:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussein MH, Schneider EK, Elliott AG, Han M, Reyes-Ortega F, Morris F, Blastovich MAT, Jasim R, Currie B, Mayo M, et al. : From Breast Cancer to Antimicrobial: Combating Extremely Resistant Gram-Negative “Superbugs” Using Novel Combinations of Polymyxin B with Selective Estrogen Receptor Modulators. Microb Drug Resist 2016, 23:640–650. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Coates A: Combination comprising zidovudine and polymyxin. US Patent 2014, US9757427B2.
- 32.Schneider EK, Reyes-Ortega F, Velkov T, Li J: Antibiotic-non-antibiotic combinations for combating extremely drug-resistant Gram-negative ‘superbugs’. Essays Biochem 2017, 61:115–125. [DOI] [PubMed] [Google Scholar]
- 33.Otto RG, van Gorp E, Kloezen W, Meletiadis J, van den Berg S, Mouton JW: An alternative strategy for combination therapy: Interactions between polymyxin B and non-antibiotics. Int J Antimicrob Agents 2018, 53:34–39. [DOI] [PubMed] [Google Scholar]
- 34.Sun W, Weingarten RA, Xu M, Southall N, Dai S, Shinn P, Sanderson PE, Williamson PR, Frank KM, Zheng W: Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerg Microbes Infect 2016, 5:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doern CD: When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 2014, 52:4124–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worthington RJ, Melander C: Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol 2013, 31:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bollenbach T: Antimicrobial interactions: mechanisms and implications for drug discovery and resistance evolution. Curr Opin Microbiol 2015, 27:1–9. [DOI] [PubMed] [Google Scholar]
- 38.Tamma PD, Cosgrove SE, Maragakis LL: Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 2012, 25:450–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domalaon R, Idowu T, Zhanel GG, Schweizer F: Antibiotic Hybrids: the Next Generation of Agents and Adjuvants against Gram-Negative Pathogens? Clin Microbiol Rev 2018, 31.• This review summarizes the concepts, advances, and challenges of the hybrid antibacterial agents.
- 40.Butler MM, Shinabarger DL, Citron DM, Kelly CP, Dvoskin S, Wright GE, Feng H, Tzipori S, Bowlin TL: MBX-500, a hybrid antibiotic with in vitro and in vivo efficacy against toxigenic Clostridium difficile. Antimicrob Agents Chemother 2012, 56:4786–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorityala BK, Guchhait G, Goswami S, Fernando DM, Kumar A, Zhanel GG, Schweizer F: Hybrid Antibiotic Overcomes Resistance in P. aeruginosa by Enhancing Outer Membrane Penetration and Reducing Efflux. J Med Chem 2016, 59:8441–8455. [DOI] [PubMed] [Google Scholar]
- 42.Sun W, Hesse S, Xu M, Childs RW, Zheng W, Williamson PR: “Real-Time” High-Throughput Drug and Synergy Testing for Multidrug-Resistant Bacterial Infection: A Case Report. Front Med (Lausanne) 2018, 5:267.•• Using a modified HTS, a less toxic drug combination treatment was identified for a patient with a severe infection caused by MDR GNB. This study demonstrates a personalized medicine approach to finding drug combinations for the treatment of severe infections using a real time screen.
- 43.Ebola Outbreak in West Africa- Case Counts. Edited by: Center for Disease Control and Prevention. [Google Scholar]
- 44.Ebola virus disease - Democratic Republic of the Congo. Edited by: World Health Organization. [Google Scholar]
- 45.Kouznetsova J, Sun W, Martinez-Romero C, Tawa G, Shinn P, Chen CZ, Schimmer A, Sanderson P, McKew JC, Zheng W, et al. : Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect 2015, 3:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun W, He S, Martmez-Romero C, Kouznetsova J, Tawa G, Xu M, Shinn P, Fisher E, Long Y, Motabar O, et al. : Synergistic drug combination effectively blocks Ebola virus infection. Antiviral Res 2017, 137:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dyall J, Nelson EA, DeWald LE, Guha R, Hart BJ, Zhou H, Postnikova E, Logue J, Vargas WM, Gross R, et al. : Identification of Combinations of Approved Drugs With Synergistic Activity Against Ebola Virus in Cell Cultures. J Infect Dis 2018, 218:S672–s678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, Baruah K, Mahmud G, Kandun N, Vasconcelos PF, et al. : Zika virus and microcephaly: why is this situation a PHEIC? Lancet 2016, 387:719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, et al. : Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 2016, 22:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, Galarza-Munoz G, McGrath EL, Urrabaz-Garza R, Gao J, et al. : A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe 2016, 20:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adcock RS, Chu YK, Golden JE, Chung DH: Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antiviral Res 2016, 138:47–56. [DOI] [PubMed] [Google Scholar]
- 52.Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, Carreras MC, Poderoso JJ, Gores GJ: The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of nonalcoholic steatohepatitis. Liver Int 2014, 35:953–966. [DOI] [PubMed] [Google Scholar]
- 53.Yang S, Xu M, Lee EM, Gorshkov K, Shiryaev SA, He S, Sun W, Cheng YS, Hu X, Tharappel AM, et al. : Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov 2018, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukhopadhyay R, Roy S, Venkatadri R, Su YP, Ye W, Barnaeva E, Mathews Griner L, Southall N, Hu X, Wang AQ, et al. : Efficacy and Mechanism of Action of Low Dose Emetine against Human Cytomegalovirus. PLoS Pathog 2016, 12:e1005717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bulik CC, Nicolau DP: Double-Carbapenem Therapy for Carbapenemase-Producing Klebsiella pneumoniae▿. Antimicrob Agents Chemother 2011, 55:3002–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sleire L, Forde HE, Netland IA, Leiss L, Skeie BS, Enger PO: Drug repurposing in cancer. Pharmacol Res 2017, 124:74–91. [DOI] [PubMed] [Google Scholar]
- 57.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, et al. : Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019, 18:41–58. [DOI] [PubMed] [Google Scholar]
- 58.Pessetto ZY, Ma Y, Hirst JJ, von Mehren M, Weir SJ, Godwin AK: Drug repurposing identifies a synergistic combination therapy with imatinib mesylate for gastrointestinal stromal tumor. Mol Cancer Ther 2014, 13:2276–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novac N: Challenges and opportunities of drug repositioning. Trends Pharmacol Sci 2013, 34:267–272. [DOI] [PubMed] [Google Scholar]