Abstract
BACKGROUND:
Achieving a pathologic complete response (pCR) with neoadjuvant chemotherapy (NAC) in patients with muscle-invasive bladder cancer (MIBC) has been associated with improved overall survival (OS). This study was aimed at evaluating the impact of pathologic downstaging (pDS; ie, a pT stage at least 1 stage lower than the pre-NAC cT stage) on the OS of patients with MIBC treated with NAC.
METHODS:
The Retrospective International Study of Cancers of the Urothelial Tract (RISC) and the National Cancer Database (NCDB) were queried for cT2-4N0M0 patients treated with NAC. A multivariable Cox model including either pDS or pCR was generated. A nested model was built to evaluate the added value of pDS (excluding patients achieving a pCR) to a model including pCR alone. C indices were computed to assess discrimination. NCDB was used for validation. The treatment effect of NAC versus cystectomy alone in achieving pDS was estimated through an inverse probability-weighted regression adjustment.
RESULTS:
Overall, 189 and 2010 patients from the RISC and NCDB cohorts, respectively, were included; pDS and pCR were achieved by 33% and 35% and by 20% and 15% in RISC and NCDB, respectively. In both data sets, pDS and pCR were associated with better OS and C indices. Adding pDS excluding pCR to the model with pCR fit the data better (likelihood ratio, P = .019 for RISC and P < .001 for NCDB), and it yielded better discrimination (incremental C index, 4.2 for RISC and 1.6 for NCDB). The treatment effect of NAC in achieving pDS was 2.07-fold (P < .001) in comparison with cystectomy alone.
CONCLUSIONS:
A decrease of at least 1 stage from the cT stage to the pT stage is associated with improved OS in patients with MIBC treated with NAC.
Keywords: bladder cancer, neoadjuvant chemotherapy, overall survival, radical cystectomy, urothelial cancer
INTRODUCTION
Cisplatin-based neoadjuvant chemotherapy (NAC) before radical cystectomy has been shown to improve the survival of patients with muscle-invasive bladder cancer (MIBC) and has been integrated into standard treatment guidelines.1 In several studies, achieving a pathologic complete response (pCR) after NAC, in comparison with the presence of residual disease, has been associated with a marked improvement in outcomes. This finding has had 2 important ramifications: 1) pCR has been used as an endpoint in phase 2 trials exploring the activity of novel NAC regimens, and 2) the randomized phase 3 NAC trial results have commonly been reframed in the context of the pCR-survival association to conclude that NAC benefits only patients achieving a pCR.
Although pCR is a clinically and potentially biologically important measure of the effects of NAC, a key advantage of pCR as an endpoint is its reliability and reproducibility of measurement. That is, pCR overcomes the complexities associated with the potential discordance between clinical and pathologic stages encountered by response assessments that incorporate both pre- and post-NAC information.2 However, these attributes of pCR may come at the cost of a loss of prognostic information afforded by endpoints that capture a greater spectrum of response categories. Given the potential implications for both practice and clinical trial design, we explored the frequency of pathologic downstaging (pDS), defined as a change in the tumor stage from the pre-NAC clinical stage to the post-NAC pathologic stage, and the impact of downstaging on survival in 2 complementary cohorts of patients with MIBC.
MATERIALS AND METHODS
Study Populations
Discovery cohort
The Retrospective International Study of Cancers of the Urothelial Tract (RISC) is a retrospective cohort study encompassing 3024 patients with clinical stage T2 or higher bladder cancer from 28 international centers treated from January 1, 2006, to January 1, 2011. The RISC study has previously been described in detail.3 The institutional review board of each hospital approved the RISC project.
The database was queried for patients with clinical T2-4N0M0 urothelial cancer of the bladder who received cisplatin-based NAC before radical cystectomy. Variables collected from the RISC cohort included the following: age, sex, Charlson Comorbidity Index (CCI), year of diagnosis, cT stage, surgical margin status, pT stage, pN stage, and survival data. Patients were excluded in the case of missing data concerning the variables of interest.
Validation cohort
The National Cancer Database (NCDB), a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, is a registry encompassing data from more than 1500 US hospitals and includes approximately 70% of all newly diagnosed cases of cancer in the United States; the NCDB has been previously described in detail.4 This database encompasses deidentified data, and the analysis was deemed exempt by the institutional review board of the Mount Sinai Hospital.
The NCDB was queried for patients with clinical T2-4N0M0 urothelial cancer of the bladder who received NAC before radical cystectomy. NAC was defined as initiating multi-agent chemotherapy within the 6 months before the time of cystectomy. Variables collected from the NCDB cohort analysis included the following: age, sex, CCI, year of diagnosis, cT stage, surgical margin status, pT stage, pN stage, and survival data. Patients were excluded in the case of missing data concerning at least 1 of the variables of interest. Imputation was not pursued because of the low overall missing rate (ie, less than 5%).5,6
Response Definitions
A pCR was defined as the absence of pathologic evidence of bladder cancer in both the primary tumor (pT0) and the accompanying nodes (pN0). pDS was defined as a pathologic tumor stage (T stage) that was at least 1 stage lower than the pre-NAC clinical stage in addition to pN0. The degree of pDS was quantified via a comparison of the difference between the cystectomy pT stage and the pre-NAC cT stage in each patient; pDS, pTis, and pTa were grouped in a single category and were considered distinct from pT1. Thus, pDS could be ranked according to downstaging of 1 to 5 T stages, and the maximum degree of pDS achievable in an individual patient was 5 (ie, from cT4 to pT0).
Given that pDS, by definition, includes all patients achieving a pCR, we also explored the impact of the following mutually exclusive response categories on overall survival (OS): no response, any pDS (excluding patients achieving a pCR), and pCR.
Statistical Analyses
Descriptive statistics such as medians and ranges for continuous variables and frequencies for categorical variables were provided to summarize patient characteristics in each patient population. Kruskal-Wallis statistics and chi-square test statistics were used to assess the distributional difference of these baseline variables in each subset of the patient cohorts defined by response categories.
OS was defined as the time from radical cystectomy to death or the last follow-up date. The median follow-up time was estimated with the reverse Kaplan-Meier method for all patients.7 Survival probabilities were estimated with the Kaplan-Meier method, and the log-rank test was used to test the equality of the survival functions according to different response categories.
Assessment of the impact of pDS on OS
Multivariable Cox proportional hazard models were fit to assess the effect of pDS on OS, with adjustments made for age, sex, CCI, year of diagnosis, pN stage, and surgical margin status. Proper functional forms of the variables were explored, and the proportional hazards assumption was examined. When the proportional hazards assumption was not met, the average hazard ratio (HR) was presented instead to summarize the effect over time.8 The discriminatory performance of the model was assessed with Harrell’s C index through leave-one-sample-out validation on the same data set. A similar multivariable model was developed on the same data set to assess the discriminatory ability of pCR on OS with adjustments for the same set of variables.
Assessment of the independent prognostic information conferred by pDS beyond pCR alone
To further gauge the added value of incorporating pDS information beyond pCR alone, an additional model (model 1) was developed with the same data set with responses classified as absent, pDS excluding pCR, or pCR, with adjustments made for the same set of covariates, and it was compared with the model limited to pCR versus no pCR (model 2):
| Model 1: |
| Model 2: |
where λ(t) and λ0(t) are the hazard function and the baseline hazard function, respectively; exp(b1) represents the hazard ratio of pCR versus absence; and exp(b2) represents the hazard ratio of pDS excluding pCR versus absence. We further performed the likelihood ratio test to compare the goodness of fit of the 2 models. Model discriminatory ability on OS was estimated with Harrell’s C index9,10 to measure the probability of survival concordance, that is, the probability for a random subject from the better prognosis group to have longer survival than another random subject from the worse prognosis group. The added discriminatory ability of model 1 in comparison with model 2 was assessed with the incremental C index and its associated 95% CI. These analyses were performed separately for both cohorts.
Landmark analysis to minimize the potential impact of a guarantee-time bias
In addition, a landmark analysis was performed with the 6 months after the diagnosis as the landmark time point. The landmark time point was chosen a priori. This analysis included only patients alive and not censored 6 months after the diagnosis.
Assessment of the effect of NAC on pDS
Given that pT stage alone is prognostic for patients undergoing cystectomy for MIBC, in the context of the potential clinicopathologic stage disconnect, we sought to generate further evidence showing that pDS represents an NAC treatment effect. To estimate the effect of NAC in achieving various degrees of pDS in patients treated with NAC versus cystectomy alone, we relied on the inverse probability–weighted regression adjustment (IPWRA) estimator considering baseline characteristics (age, sex, CCI, year of diagnosis, and cT stage) as independent variables.
Statistical tests were 2-sided; P < .05 was considered significant. All analyses were performed with Stata 14 (StataCorp MP, College Station, Texas).
RESULTS
RISC Cohort Analysis
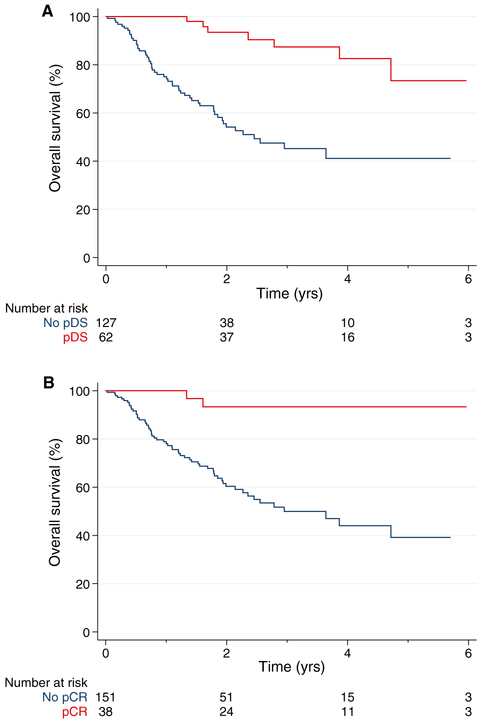
Among the 3024 patients with any stage of urothelial cancer in the RISC cohort, 189 had MIBC treated with cisplatin-based NAC followed by cystectomy meeting the criteria for the analysis (Supporting Fig. 1). The characteristics of the RISC cohort are shown in Supporting Table 1. The 5-year OS probability was 52% (95% CI, 40%-62%). Overall, 62 patients (33%) achieved pDS, and 15, 30, 12, and 5 patients were downstaged 1, 2, 3, and 4 categories, respectively (Supporting Fig. 2). A pCR was achieved by 38 patients (20%). The baseline characteristics were similar among patients achieving a pCR and patients achieving no response and among patients achieving pDS and patients achieving no response (Supporting Table 1). The 5-year OS probability for patients achieving pDS was 73% (Fig. 1A), and it was 93% for patients achieving a pCR (Fig. 1B).
Figure 1.
Survival of patients according to (A) pDS and (B) pCR with neoadjuvant chemotherapy in the Retrospective International Study of Cancers of the Urothelial Tract cohort. pCR indicates pathologic complete response; pDS, pathologic downstaging.
In the Cox multivariable analysis (Table 1), both pCR (HR, 0.19; 95% CI, 0.08-0.44; P < .001) and pDS (HR, 0.11; 95% CI, 0.03-0.47; P = .003) were associated with improved OS. Models including pDS better discriminated OS than models including pCR (C index, 73.2 vs 69.5). To dissect the impact of pDS from the impact of pCR, we examined 3 mutually exclusive response categories: no response, pDS excluding pCR, and pCR. In comparison with patients not achieving a response to NAC, pDS excluding pCR was associated with an HR of 0.35 (95% CI, 0.13-0.93; P < .035) for OS, whereas pCR was associated with an HR of 0.09 (95% CI, 0.02-0.38; P < .001) for OS. The Kaplan-Meier curves for the mutually exclusive response categories are shown in Supporting Figure 3. The C index of the model for OS including these mutually exclusive response categories was 73.7. The incorporation of downstaging into the model with pCR fit the data better with a likelihood ratio test P value of .019, and it yielded better discrimination of OS with an incremental C index of 4.2 (95% CI, 1.2-7.2).
TABLE 1.
Multivariable Cox Regression Predicting Overall Survival Using the Retrospective International Study of Cancers of the Urothelial Tract Cohort
| Covariate | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|
| Age | 1.01 | 0.98-1.04 | .405 | 1.01 | 0.98-1.04 | .589 | 1.01 | 0.98-1.04 | .490 |
| Sex | |||||||||
| Female | 1 | Reference | 1 | Reference | 1 | Reference | |||
| Male | 0.98 | 0.54-1.79 | .948 | 1.03 | 0.56-1.87 | .931 | 1.00 | 0.54-1.83 | .990 |
| CCI | 1.00 | 0.86-1.18 | .968 | ||||||
| 0 | 1 | Reference | 1 | Reference | |||||
| 1 | 1 | Reference | 1.02 | 0.30-3.42 | .977 | 0.88 | 0.26-2.95 | .831 | |
| ≥2 | 0.79 | 0.24-2.64 | .701 | 1.05 | 0.61-1.83 | .853 | 1.03 | 0.59-1.80 | .916 |
| Year of diagnosis | 1.05 | 0.60-1.84 | .859 | 0.98 | 0.84-1.15 | .830 | 1.00 | 0.85-1.17 | .973 |
| cT stage | |||||||||
| 2 | 1 | Reference | 1 | Reference | 1 | Reference | |||
| 3 | 1.66 | 0.98-2.83 | .060 | 1.47 | 0.86-2.51 | .156 | 1.61 | 0.95-2.75 | .079 |
| 4 | 1.45 | 0.42-5.04 | .562 | 0.85 | 0.25-2.87 | .799 | 1.31 | 0.37-4.63 | .673 |
| Surgical margin status | |||||||||
| Negative | 1 | Reference | 1 | Reference | 1 | Reference | |||
| Positive | 1.39 | 0.66-2.93 | .387 | 1.48 | 0.70-3.13 | .311 | 1.38 | 0.65-2.91 | .397 |
| pN stage | |||||||||
| 0 | 1 | Reference | 1 | Reference | 1 | Reference | |||
| ≥1 | 1.25 | 0.67-2.33 | .477 | 1.52 | 0.82-2.80 | .181 | 1.26 | 0.68-2.34 | .465 |
| pDS | |||||||||
| Absent | 1 | Reference | |||||||
| Present | 0.19 | 0.08-0.44 | <.001 | ||||||
| pCR | |||||||||
| Absent | 1 | Reference | |||||||
| Present | 0.11 | 0.03-0.47 | .003 | ||||||
| Response | |||||||||
| Absent | 1 | Reference | |||||||
| Downstaging | 0.35 | 0.13-0.93 | .035 | ||||||
| pCR | 0.09 | 0.02-0.38 | .001 | ||||||
| C index, % | 73.2 | 69.5 | 73.7 | ||||||
| P from Schoenfeld test | .3 | .5 | .1 |
Abbreviations: CCI, Charlson Comorbidity Index; HR, hazard ratio; pCR, pathologic complete response; pDS, pathologic downstaging.
NCDB Cohort Analysis
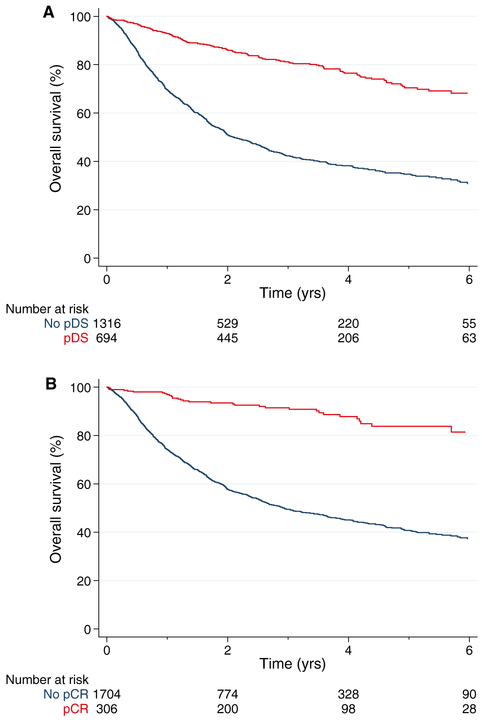
Among 525,332 patients with any stage of bladder cancer in the NCDB cohort, 2010 had MIBC treated with NAC followed by cystectomy meeting the criteria for the analysis (Supporting Fig. 4). The characteristics of the NCDB cohort are shown in Supporting Table 2. The median follow-up for survivors was 36 months with a 5-year OS rate of 47% (95% CI, 44%-50%). Overall, 694 patients (35%) achieved pDS, and 139, 201, 291, 49, and 14 patients were downstaged 1, 2, 3, 4, and 5 categories, respectively (Supporting Fig. 5). The 5-year OS rate for patients who achieved pDS was 70% (Fig. 2A). A pCR was achieved by 306 patients (15%) with a 5-year OS rate of 84% (Fig. 2B). Patients achieving a pCR or pDS were generally younger and were diagnosed with MIBC in more recent years (Supporting Table 2). Patients achieving pDS generally presented with a higher cT stage, whereas patients achieving a pCR generally presented with a lower cT stage.
Figure 2.
Survival of patients according to (A) pDS and (B) pCR with neoadjuvant chemotherapy in the National Cancer Database. pCR indicates pathologic complete response; pDS, pathologic downstaging.
In the Cox multivariable analysis (Table 2), both pDS (HR, 0.37; 95% CI, 0.31-0.45; P < .001) and pCR (HR, 0.25; 95% CI, 0.18-0.35; P < .001) were associated with better survival. Models including pDS better discriminated OS than models including pCR (C index, 71.7 vs 70.4). When we explored the role of mutually exclusive response categories in OS, pDS excluding a complete response was associated with an HR of 0.49 (95% CI, 0.40-0.61; P < .001), whereas pCR was associated with an HR of 0.21 (95% CI, 0.14-0.29; P < .001); Kaplan-Meier curves for the mutually exclusive response categories are shown in Supporting Figure 6. The C index of the model for OS including the mutually exclusive response categories was 72. The likelihood ratio test demonstrated statistical significance (P < .001) with an increment in the C index of 1.6 (95% CI, 1.1-2.1).
TABLE 2.
Multivariable Cox Regression Predicting Overall Survival Using the National Cancer Database
| Covariate | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|
| Age | 1.03 | 1.02-1.03 | <.001 | 1.03 | 1.02-1.04 | <.001 | 1.03 | 1.02-1.03 | <.001 |
| Sex | |||||||||
| Female | 1 | 1 | 1 | ||||||
| Male | 0.96 | 0.83-1.12 | .635 | 0.99 | 0.85-1.15 | .854 | 0.97 | 0.83-1.13 | .652 |
| CCI | |||||||||
| 0 | 1 | 1 | 1 | ||||||
| 1 | 1.19 | 1.02-1.39 | .028 | 1.20 | 1.03-1.40 | .021 | 1.20 | 1.02-1.40 | .025 |
| 2 | 1.34 | 1.01-1.79 | .043 | 1.28 | 0.96-1.71 | .088 | 1.31 | 0.99-1.75 | .063 |
| Year of diagnosis | 0.94 | 0.91-0.97 | <.001 | 0.94 | 0.91-0.97 | <.001 | 0.94 | 0.91-0.97 | <.001 |
| cT stage | |||||||||
| 2 | 1 | 1 | 1 | ||||||
| 3 | 1.42 | 1.19-1.70 | <.001 | 1.22 | 1.02-1.46 | .027 | 1.37 | 1.14-1.64 | .001 |
| 4 | 1.46 | 1.19-1.79 | <.001 | 1.23 | 1.01-1.50 | .043 | 1.39 | 1.13-1.71 | .002 |
| Surgical margin status | |||||||||
| Negative | 1 | 1 | 1 | ||||||
| Positive | 1.88 | 1.54-2.29 | <.001 | 2.00 | 1.64-2.44 | <.001 | 1.87 | 1.53-2.28 | <.001 |
| pN stage | |||||||||
| 0 | 1 | 1 | 1 | ||||||
| 1 | 1.82 | 1.48-2.23 | <.001 | 2.23 | 1.83-2.72 | <.001 | 1.83 | 1.49-2.24 | <.001 |
| 2-3 | 2.66 | 2.24-3.17 | <.001 | 3.22 | 2.72-3.81 | <.001 | 2.68 | 2.25-3.19 | <.001 |
| pDS | |||||||||
| Absent | 1 | ||||||||
| Present | 0.37 | 0.31-0.45 | <.001 | ||||||
| pCR | |||||||||
| Absent | 1 | ||||||||
| Present | 0.25 | 0.18-0.35 | <.001 | ||||||
| Response | |||||||||
| Absent | 1 | ||||||||
| Downstaging | 0.49 | 0.40-0.61 | <.001 | ||||||
| pCR | 0.21 | 0.14-0.29 | <.001 | ||||||
| C index, % | 71.7 | 70.4 | 72 | ||||||
| P from Schoenfeld test | .13 | .24 | .13 |
Abbreviations: CCI, Charlson Comorbidity Index; HR, hazard ratio; pCR, pathologic complete response; pDS, pathologic downstaging.
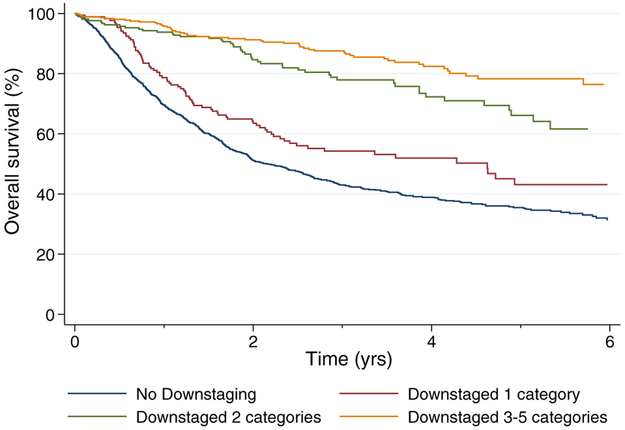
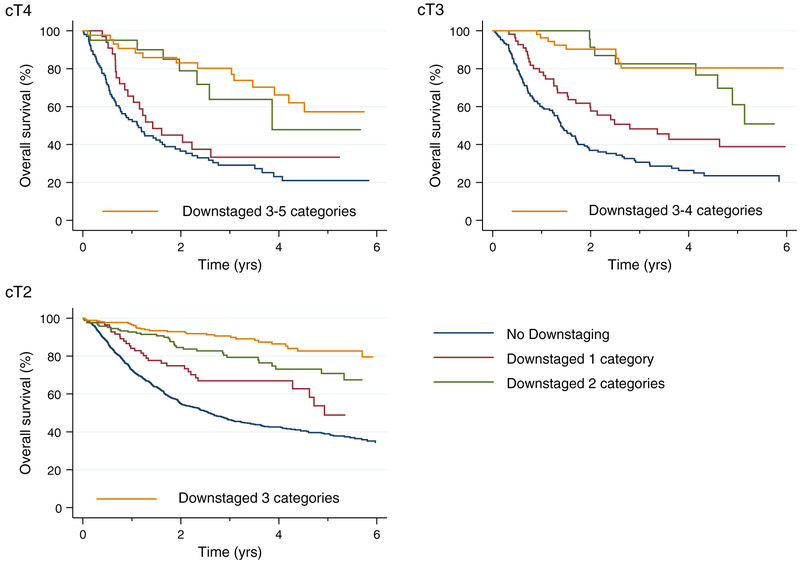
To explore the impact of the extent of downstaging on OS, we considered the post-NAC pT stage with respect to the pre-NAC cT stage and categorized each response by downstaging of 1 to 5 T stages. Patients with downstaging of 3 to 5 T stages were grouped together into a single category because of the number of observations in each group. A greater extent of pDS correlated with improved OS in the Cox multivariable analysis (Supporting Table 3). The C index of this model was 72.6. The Kaplan-Meier curves for OS are shown according to the degree of pDS in Figure 3 and according to the degree of pDS based on the initial cT stage in Figure 4.
Figure 3.
Survival of patients according to the degree of downstaging in the National Cancer Database.
Figure 4.
Survival of patients according to the clinical tumor stage and the degree of downstaging in the National Cancer Database.
Landmark Analysis
Although measuring survival from the time of cystectomy helped to mitigate the potential impact of a guarantee-time bias (ie, all patients in the analysis were required to live long enough from diagnosis to undergo cystectomy), we also conducted a landmark analysis using 6 months from the diagnosis as a landmark time point. Overall, 1972 patients from the NCDB cohort were alive 6 months after diagnosis. Multivariable models predicting OS are shown in Supporting Table 4, which demonstrates results consistent with the analyses measuring survival from the time of cystectomy.
pDS With NAC Versus Cystectomy Alone
From the NCDB cohort, 13,193 patients who received radical cystectomy alone were identified. Among these patients, 1211 (9.2%) achieved pDS, and 422, 386, 358, 31, and 14 patients were downstaged 1, 2, 3, 4, and 5 categories, respectively. Patients’ characteristics are reported in Supporting Table 5. The IPWRA estimator was fed by 3 components: the baseline covariates, the treatment (NAC vs no), and the outcome (pDS vs no pDS). The latter encompassed all 1905 patients who were downstaged (1211 after radical cystectomy only and 694 after NAC). According to the IPWRA analysis, the estimated treatment effect of NAC in determining pDS was 2.07-fold (95% CI, 1.76-2.38; P < .001) with respect to cystectomy alone. The estimated treatment effect of NAC in achieving a downstaging of ≥2 and ≥3 categories was 2.78-fold (95% CI, 2.34-3.23; P < .001) and 3.38-fold (95% CI, 2.69-4.08; P < .001) in comparison with cystectomy alone, respectively.
DISCUSSION
In this analysis, using a discovery and validation cohort, we demonstrate that pDS is associated with improved OS for patients with MIBC treated with NAC. We further show that pDS confers prognostic significance even when it is considered independently of pCR. Moreover, models with pDS included more patients considered to be responders to NAC and are associated with slightly better discrimination in terms of the C index for predicting OS in comparison with models including pCR alone. Our findings reveal a dose-response effect of pDS on OS; that is, greater degrees of pDS are associated with the superior improvements in OS with NAC. Finally, we show that NAC is associated with a significant increase in the likelihood of achieving pDS in comparison with cystectomy alone. Better OS in patients with pDS might indicate eradication of micrometastatic disease even without complete eradication of the more bulky primary tumor.
Other groups have explored downstaging as an intermediate endpoint of the activity of NAC but have defined downstaging solely on the basis of the pT stage.11,12 However, pathologic stage alone is known to be prognostic for patients with MIBC undergoing cystectomy; therefore, we sought to establish an endpoint that more reliably captures an NAC treatment effect by comparing the pre-NAC clinical stage with the post-NAC pathologic stage.
Our findings have several implications for clinical practice, translational research, and clinical trial design. Two large randomized clinical trials have demonstrated a survival benefit with the use of cisplatin-based NAC in patients with MIBC.13,14 However, observational studies have demonstrated that such treatment is substantially underused.15 Although there are several reasons for the suboptimal uptake of NAC, a commonly cited reason is that only a small proportion of patients derive a benefit and that the benefit is limited to those patients achieving a pCR. The latter notion is perpetuated largely by retrospective analyses correlating pCR with improved survival with NAC. The current analysis reinforces that the benefit of NAC is not limited to the subset of patients achieving a pCR. Importantly, the proportion of patients achieving pDS in our cohorts was approximately 2-fold higher than the proportion of patients achieving a pCR (33%-44% vs 15%-20%).2
Clinical staging of bladder cancer is challenging, and the clinicopathologic stage disconnect has previously been highlighted.2 However, despite these challenges, clinical staging of bladder cancer is defined by the American Joint Committee on Cancer staging system and is incorporated into practice guidelines.16-18 We have demonstrated the impact of pDS on OS in 2 highly complementary data sets representing the full spectrum of real-world clinical practice environments and have highlighted the utility of this endpoint despite the aforementioned complexities. Furthermore, notwithstanding the potential difficulties of clinical staging applicable to all patients with MIBC, we have demonstrated a significantly higher likelihood of achieving pDS with NAC in comparison with cystectomy alone. Although pCR can be reliably measured, it is not without limitations, including the potential impact of transurethral resection alone on confounding the interpretation of this endpoint. Yet, we found a rate of downstaging in patients treated with radical cystectomy alone lower than that in the past. In the NCDB, 9.2% of patients were downstaged in case of radical cystectomy alone, whereas Shariat et al19 in a series spanning from 1984 to 2003 reported a rate of 22%. This is likely, at least in large part, related to the full spectrum of practice settings contributing data to the NCDB.
There are potential limitations to our study. Neither the RISC cohort nor the NCDB cohort specified the methods used to determine the cT stage or included central pathology review. Nonetheless, the real-world data included in our analysis yielded very similar results in both the discovery and validation data sets. The NCDB allows identification of patients who received multi-agent NAC but does not indicate the particular treatment regimen. The complementary RISC cohort, on the other hand, includes detailed NAC annotation, and the analysis in the RISC cohort was limited to patients receiving cisplatin-based NAC. We defined pCR as a pT0N0 status, and more permissive definitions of pCR (ie, a lack of pathologic evidence of residual invasive disease) have also correlated with OS. These latter definitions may encompass some, but not all, of the prognostic information derived from pDS.
In an attempt to define predictive biomarkers of benefit with NAC, several groups have performed analyses of NAC-treated patients seeking genetic or genomic differences in baseline tumors from patients who have achieved or not achieved a pCR.20-22 Although such studies have generated critical insights that might ultimately affect clinical practice, the use of pCR alone as an endpoint in such analyses may result in missed opportunities for discovering potentially clinically relevant biomarkers. Indeed, a study of the impact of molecular subtypes of bladder cancer on outcomes with NAC demonstrated a correlation among subtypes with OS but not pCR.23 On the basis of the current analysis, rethinking the endpoints of predictive biomarker discovery in the NAC setting or at least performing analyses including various response classifications may be important to realizing the full impact of such efforts.
Our findings suggest that pDS may play a role as an intermediate endpoint that can be used to assess the activity of novel neoadjuvant regimens in the phase 2 setting. Efforts to improve the documentation and reporting of the clinical staging of bladder cancer and to refine the role of contemporary imaging techniques in assessing the cT stage may further enhance the use of pDS as an intermediate endpoint. Our work does not imply that pDS is a surrogate for survival. Establishing surrogacy requires suitable randomized data sets and is a stepwise process establishing that variability in the intermediate endpoint accounts for the vast majority of variability in the definitive endpoint.24,25 The potential role of pDS and pCR as surrogate endpoints should be explored in the context of ongoing randomized phase 3 trials of novel neoadjuvant systemic regimens in MIBC.
In conclusion, we have demonstrated that achieving pDS, independently of achieving a pCR, is associated with improved OS in patients with MIBC treated with NAC. These findings have implications regarding clinical practice, translational research efforts, and clinical trials endpoints.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Appendix
AUTHOR CONTRIBUTIONS
Alberto Martini: Concept and design, analysis and interpretation of data, drafting of the manuscript, and statistical analysis. Rachel Jia: Concept and design, analysis and interpretation of data, drafting of the manuscript, and statistical analysis. Bart S. Ferket: Concept and design, analysis and interpretation of data, drafting of the manuscript, and statistical analysis. Nikhil Waingankar: Concept and design, analysis and interpretation of data, drafting of the manuscript, and statistical analysis. Elizabeth R. Plimack: Acquisition of data and critical revision of the manuscript. Simon J. Crabb: Acquisition of data and critical revision of the manuscript. Lauren C. Harshman: Acquisition of data and critical revision of the manuscript. Evan Y. Yu: Acquisition of data and critical revision of the manuscript. Thomas Powles: Acquisition of data and critical revision of the manuscript. Jonathan E. Rosenberg: Acquisition of data and critical revision of the manuscript. Sumanta K. Pal: Acquisition of data and critical revision of the manuscript. Ulka N. Vaishampayan: Acquisition of data and critical revision of the manuscript. Andrea Necchi: Acquisition of data and critical revision of the manuscript. N. Peter Wiklund: Critical revision of the manuscript. Reza Mehrazin: Critical revision of the manuscript. Madhu Mazumdar: Critical revision of the manuscript. John P. Sfakianos: Concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript. Matthew D. Galsky: Concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, and supervision.
CONFLICT OF INTEREST DISCLOSURES
Elizabeth R. Plimack has served as a scientific advisor for Bristol-Myers Squibb, Genentech, Incyte, Janssen, Merck, AstraZeneca, and Pfizer; has held continuing medical education presentations for American Urological Association (AUA), Clinical Care Options, the Fox Chase Cancer Center, Medscape, the National Comprehensive Cancer Network, Omniprex, prIME Oncology, and Research to Practice; and reports grants from Astellas, AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Peloton, and Pfizer outside the submitted work. Simon J. Crabb reports funding from AstraZeneca, Astex Pharmaceuticals, and Clovis Oncology; personal fees from Clovis Oncology; and travel funding from Roche, Janssen, and Merck Sharp & Dohme outside the submitted work. Lauren C. Harshman reports personal fees for consulting from Exelixis, Bayer, Genentech, Dendreon, Pfizer, Medivation/Astellas, Kew Group, Corvus, Merck, Novartis, and Jounce; personal fees for speaking from Michael J. Hennessy Associates, Inc (Healthcare Communications Company and several brands such as OncLive and PER); research grants from Bayer, Sotio, Bristol-Myers Squib, Merck, Takeda, Dendreon/Valient, Janssen, Medivation/Astellas, Genentech, and Pfizer; and nonfinancial support from Bayer and Sanofi outside the submitted work. Evan Y. Yu reports personal fees from Amgen, AstraZeneca, Churchill Pharma, EMD Serono, InCyte, Janssen, Pharmacyclics, QED, and Tolmar Pharmaceuticals and grants and personal fees from Bayer, Dendreon, Merck, and Seattle Genetics outside the submitted work. Thomas Powles reports honoraria from Pfizer, Novartis, Roche, Bristol-Myers Squibb, AstraZeneca, and Merck and research funding from AstraZeneca and Roche outside the submitted work. Jonathan E. Rosenberg reports grants and personal fees from Genentech/Roche, Bayer, and Astellas; personal fees from AstraZeneca, Bristol-Myers Squibb, Seattle Genetics, Merck, EMD Serono, Sensei Biotherapeutics, Pharmacyclics, Inovio, Gritstone, Western Oncolytics, BioClin, Mirati Research, QED Therapeutics, Adicet Bio, and Fortress Biotech; and grants from Novartis and GlaxoSmithKline outside the submitted work. Sumanta K. Pal reports honoraria from Genentech, Aveo, Eisai, Myriad Genetics, Roche, Pfizer, Novartis, Exelixis, Ipsen, Medivation, Bristol-Myers Squibb, and Astellas and other from Pfizer, Novartis, Bristol-Myers Squibb, Astellas, and Medivation outside the submitted work. Ulka N. Vaishampayan reports honoraria from Bayer, Exelixis, and Bristol-Myers Squibb and research funding from Merck and Exelixis outside the submitted work. Andrea Necchi reports honoraria from Roche, Merck, AstraZeneca, and Janssen Pharmaceuticals; has served as a consultant or advisor for Bristol-Myers Squibb, Rainier Therapeutics, Merck Sharp & Dohme, Roche, Bayer, AstraZeneca, Clovis Oncology, Janssen Pharmaceuticals, Incyte, BioClin Therapeutics, Seattle Genetics, and Astellas Pharma; has received research funding from Incyte, Merck Sharp & Dohme (institution), and AstraZeneca (institution); and has received travel funding from Roche, Merck Sharp & Dohme, AstraZeneca, and Janssen Pharmaceuticals outside the submitted work. Matthew D. Galsky has served as consultant for BioMotiv, Janssen, Merck, Dendreon, GlaxoSmithKline, Lilly, Astellas, Genentech, Bristol-Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Aileron Therapeutics, Dracen, Inovio Pharmaceuticals, and Numab; has received research funding from Janssen, Merck, Dendreon, Novartis, Bristol-Myers Squibb, AstraZeneca, and Genentech/Roche; and owns stock in Rappta Therapeutics outside the submitted work. Galsky also has a patent entitled Methods and Compositions for Treating Cancer and Related Methods (Mount Sinai School of Medicine; 20120322792). The other authors made no disclosures.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Bajorin DF, Herr HW. Neoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer: ten-year outcome. J Clin Oncol. 1998;16:1298–1301. [DOI] [PubMed] [Google Scholar]
- 2.Gray PJ, Lin CC, Jemal A, et al. Clinical-pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol Biol Phys. 2014;88:1048–1056. [DOI] [PubMed] [Google Scholar]
- 3.Galsky MD, Pal SK, Chowdhury S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121:2586–2593. [DOI] [PubMed] [Google Scholar]
- 4.Steele GD Jr, Winchester DP, Menck HR. The National Cancer Data Base. A mechanism for assessment of patient care. Cancer. 1994;73:499–504. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001;25:464–469. [PubMed] [Google Scholar]
- 6.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 7.Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9:191–192. [DOI] [PubMed] [Google Scholar]
- 8.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, NJ: John Wiley & Sons Inc; 2002. [Google Scholar]
- 9.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 10.Harrell FE. Regression Modeling Strategies. New York, NY: Springer; 2001. [Google Scholar]
- 11.Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61:1229–1238. [DOI] [PubMed] [Google Scholar]
- 12.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. [DOI] [PubMed] [Google Scholar]
- 14.International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party, European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaid HB, Patel SG, Stimson CJ, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology. 2014;83:75–80. [DOI] [PubMed] [Google Scholar]
- 16.Edge BS, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:497–502. [Google Scholar]
- 17.Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71:447–461. [DOI] [PubMed] [Google Scholar]
- 18.Flaig TW, Spiess PE, Agarwal N, et al. NCCN Guidelines Insights: Bladder Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16:1041–1053. [DOI] [PubMed] [Google Scholar]
- 19.Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51:137–151. [DOI] [PubMed] [Google Scholar]
- 20.Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4:1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol. 2015;68:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017;72:544–554. [DOI] [PubMed] [Google Scholar]
- 24.Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1:49–67. [DOI] [PubMed] [Google Scholar]
- 25.Ciani O, Davis S, Tappenden P, et al. Validation of surrogate endpoints in advanced solid tumors: systematic review of statistical methods, results, and implications for policy makers. Int J Technol Assess Health Care. 2014;30:312–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.