Abstract
Background
Persistent left superior vena cava (PLSVC) is a vascular anomaly that is usually asymptomatic and detected incidentally. The incidence of PLSVC has seldom been evaluated in normal populations. In this study, we determined the incidence of PLSVC in a normal neonatal population using transthoracic echocardiography. We also evaluated the associations between PLSVC and asymptomatic congenital heart diseases.
Materials and methods
In this retrospective study, we identified healthy neonates based on echocardiography results from 2008 to 2017. Based on the echocardiography findings, we categorized the patients into a PLSVC group and a control group (patients without PLSVC). Chi-square and logistic regression tests were used for data analysis.
Results
Of the 19,488 neonates assessed in this study, 56 were found to have PLSVC, and the remaining 19,432 neonates comprised the control group. The incidence of PLSVC was 0.29% in our population. In the PLSVC group, 3.6% of the patients exhibited bicuspid aortic valves, and 10.7% of the patients exhibited secundum-type atrial septal defects. Both the incidence and association of these conditions were higher in the PLSVC group than in the control group.
Conclusions
Based on the echocardiography examination results, we discovered that the incidence of PLSVC in Taiwanese neonates was 0.29%. Although the neonates with PLSVC were asymptomatic and exhibited no health concerns, they were associated with higher incidence rates of bicuspid aortic valves and secundum-type atrial defects. Additional follow-up and evaluation regarding these findings may be warranted.
Keywords: Echocardiography, Neonate, Persistent left superior vena cava
INTRODUCTION
Persistent left superior vena cava (PLSVC) is a vascular variant, and the most common congenital anomaly of the thoracic venous system.1 It results from the failure of regression of the left superior cardinal vein during early cardiac development. It is usually asymptomatic and detected incidentally. The presence of PLSVC complicates catheter placement within the right side of the heart when a left subclavian approach is used for vascular access, during the placement of percutaneous central venous catheters in newborns from the right upper limb, or during the placement of pacemakers or defibrillators for treating arrhythmias. PLSVC is sometimes discovered incidentally during surgery. The incidence of PLSVC has rarely been evaluated in the normal population. In this retrospective study, we investigated the incidence of PLSVC in a normal neonatal population through transthoracic echocardiography. We also examined the associations between PLSVC and asymptomatic congenital heart diseases and compared the echocardiographic parameters of the patients with PLSVC and those of controls without PLSVC.
METHODS
Patient population
From January 2008 to December 2017, 20,936 patients aged 1-3 months received newborn screening echocardiography in our hospital. Overall, 1,448 neonates with congenital heart diseases were excluded from this study due to chromosome anomalies, desaturation, heart murmur, cyanosis, or any other symptoms/signs. Among 19,488 patients enrolled in this study, none of the infants required oxygen supplementation or respiratory or circulatory support, except in the early neonatal period. Satisfactory sucking, urination, and weight gain were observed when the infants were assessed at 1 month (corrected age), and no abnormal heart sounds such as cardiac murmur or gallop were noted. The median (range) age, weight, and height of the enrolled neonates were 39 (14-91) days, 4.3 (1.93-8.2) kg, and 54.5 (40-87) cm, respectively.
Among the participants, we identified 56 patients with PLSVC through echocardiography. The results of echocardiography revealed dilated coronary sinuses in the parasternal long axis (Figure 1A), the A4C view (Figure 1B), and subcostal view (Figure 1C). In the suprasternal short axis view, we also observed PLSVC on the left side of the left pulmonary artery (Figure 1D). We defined a secundum atrial septal defect (ASD) as a central defect of the atrial septum with left to right shunting in the short-axis view, with a rim of tissue separating the superior vena cava entrance to the right atrium and right upper pulmonary venous entrance to the left atrium from the ASD. A patent foramen ovale (PFO) was defined as a small atrial defect (< 0.3 cm) with a redundant septal flap.
Figure 1.
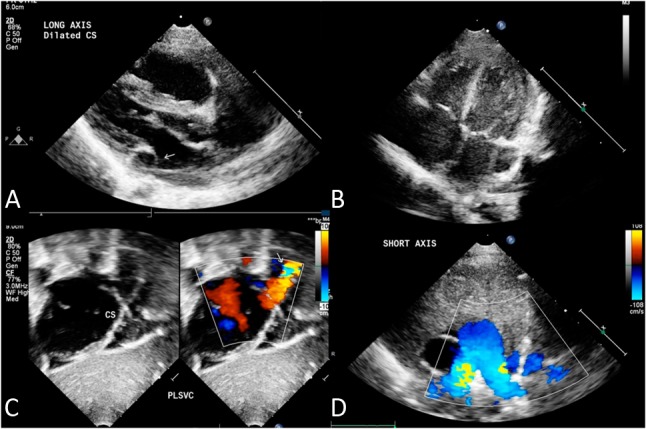
Transthoracic echocardiogram in parasternal long-axis view showing the dilated coronary sinus in (A) parasternal long-axis view and (B) apical four chamber view. (C) Dilated coronary sinus draining into the right atrium. (D) Suprasternal view showing persistent left superior vena cava (PLSVC) on the left side of the left pulmonary artery.
Data acquisition
We used a Philips Sonos 5500, iE33, or CX50 system (Andover, MA, USA) equipped with an 8 MHz electronic ultrasound transducer for conventional 2-dimensional echocardiographic examinations. All infants underwent the examinations while they were asleep or in a calm condition by echocardiographic technicians and rechecked by a pediatric cardiologist.
Statistical analysis
We recorded sex, age, height, weight, and body surface area at the time of the study. To reduce selection bias, propensity score matching was applied to create a 1:4 matched sample. We compared the PLSVC and non-PLSVC groups to determine the incidence of associated congenital heart diseases and echocardiographic parameters.
Continuous data were expressed as the median (interquartile range) and analyzed using the non-parametric Mann-Whitney U test. Categorical data were expressed as percentages and were analyzed using the chi-square or Fisher’s exact test. Two-tailed p values < 0.05 were considered significant. SPSS software version 24.0 was used for all statistical analyses.
Ethics statement
This study was approved by the MacKay Memorial Hospital Institutional Review Board (IRB number 19 MMHIS003e).
RESULTS
Of the 19,488 neonates assessed in this study, 56 were found to have PLSVC, and the remaining 19,432 neonates comprised the control group. The incidence of PLSVC in the assessed neonates was approximately 0.29%. Significant sex-based differences in the incidence of PLSVC were not observed (male:female, 30:26), and the male to female ratio in the non-PLSVC group was 10,082: 9,350 (p = 0.593). In the PLSVC group, 48 (85.7%) patients exhibited normal echocardiographic findings or a PFO in the echocardiographic examination. PLSVC was observed in two (3.6%) infants with bicuspid aortic valves (BAVs) and seven (10.7%) infants with ASD (Table 1); thus, the incidence rates of BAV and ASD were significantly higher in the PLSVC group than in the non-PLSVC group. In the PLSVC group, four patients had silent patent ductus arteriosus (PDA), all of whom had spontaneous closure of the PDA after follow-up. One patient exhibited a small muscular ventricular septal defect, which also caused spontaneous closure at 3 months of age. In addition, one infant exhibited transient mild pulmonary valve stenosis. None of the patients had mild aortic coarctation.
Table 1. Echocardiographic findings in PLSVC and non-PLSVC patients.
| Before propensity score matching | After propensity score matching | |||||
| PLSVC patients numbers | Control group (non-PLSVC patients) | p value | PLSVC patients numbers | Control group (non-PLSVC patients) | p value | |
| Total | 56 | 19432 | 56 | 224 | ||
| Normal heart structures or with PFO | 48 (85.7%) | |||||
| ASD | 7 (10.7%) | 759 (3.9%) | 0.006 | 7 | 8 (3.5%) | 0.015 |
| BAV | 2 (3.6%) | 51 (0.26%) | 0.002 | 2 | 0 (0%) | 0.039 |
ASD, atrial septal defect secundum type; BAV, bicuspid aortic valve; PFO, patent foramen ovale; PLSVC, persistent left superior vena cava.
We compared the echocardiographic parameters in the PLSVC and non-PLSVC groups and found no significant differences except for aortic root dimension and left atrium to aortic root ratio. The aortic dimension at the sinus level was smaller in the PLSVC group than in the non-PLSVC group (Table 2).
Table 2. Echocardiographic parameters in the PLSVC and non-PLSVC groups.
| Echocardiographic parameters | Before propensity score matching | After propensity score matching | ||||
| Non-PLSVC group (n = 19,432) | PLSVC group (n = 56) | p value | Non-PLSVC group (n = 224) | PLSVC group (n = 56) | p value | |
| n (%) | n (%) | n (%) | n (%) | |||
| Rvdd | 0.77 | 0.78 | 0.854 | 0.75 | 0.78 | 0.871 |
| Ivsd | 0.39 | 0.41 | 0.119 | 0.41 | 0.41 | 0.557 |
| Lvidd | 2.05 | 2.08 | 0.933 | 2.08 | 2.08 | 0.34 |
| Lvpwd | 0.31 | 0.31 | 0.626 | 0.31 | 0.31 | 0.367 |
| Lvids | 1.34 | 1.34 | 0.815 | 1.36 | 1.34 | 0.412 |
| Lvpws | 0.48 | 0.48 | 0.325 | 0.48 | 0.48 | 0.519 |
| Ao | 1.07 | 1.04 | 0.089 | 1.10 | 1.04 | 0.002 |
| LA | 1.25 | 1.30 | 0.124 | 1.25 | 1.30 | 0.337 |
| LA/Ao | 1.17 | 1.27 | 0.001 | 1.15 | 1.27 | < 0.001 |
| Esv | 4.50 | 4.50 | 0.708 | 4.69 | 4.50 | 0.322 |
| Ef | 0.66 | 0.67 | 0.857 | 0.66 | 0.67 | 0.991 |
| Sv | 8.92 | 8.97 | 0.867 | 9.28 | 8.97 | 0.408 |
| Edv | 13.5 | 14.0 | 0.93 | 14.00 | 14.00 | 0.393 |
Ao, aortic root diameter; Edv, end diastolic volume; Ef, left ventricular ejection fraction; Esv, end systolic volume; Ivsd, interventricular septal end diastole and end systole; LA, left atrium diameter; LA/Ao, left ventricular dimension and aortic dimension ratio; LVIdd, left ventricular internal diameter end diastole; Lvids, left ventricular internal diameter in systolic phase; Lvpwd, left ventricular posterior wall end diastole; Lvpws, left ventricular posterior wall systolic phase; PLSVC, persistent left superior vena cava; Rvdd, right ventricular end diastole; Sv, stroke volume.
DISCUSSION
PLSVC is an uncommon vascular variant. During early embryonic growth, at approximately 3-4 weeks of gestation, venous return to the right atrium from the arms and head is drained from the right and left anterior cardinal veins. The left anterior cardinal vein should regress at 8 weeks of gestation; failure of regression leads to PLSVC.
In patients with congenital heart diseases, such as atrial and ventricular septal defects, tetralogy of Fallot, aortic coarctation, and cor triatriatum, the incidence of PLSVC is up to 10%.2,5 The incidence of PLSVC has rarely been discussed in normal populations. In most cases, isolated PLSVC is asymptomatic. In a previous report, the incidence of PLSVC in cadavers was 0.29%.3 In 1958, Keith et al. reported an incidence of 0.29% (1/350) based on autopsies performed.4 In another report, patients with arrhythmia who underwent placement of a pacemaker or implantable cardiac defibrillators had an incidence of approximately 4%.5 On the basis of results of echocardiographic examinations, Nagasawa et al. reported an incidence of 0.21% (6/2864).6 These results are similar to those of our study (0.29%; 56/19,488).
Patients with PLSVC and a BAV were first reported in 2009.7 However, the statistical association of PLSVC and BAV has not been previously reported. In the present study, the incidence of BAV was higher in the PLSVC group than in the non-PLSVC group. In adults, BAV is the most common congenital cardiovascular anomaly, and patients with BAV require follow-up from cardiologists.8 If a patient’s valve becomes dysfunctional, they may require aortic valve replacement.9,10 However, PLSVC may complicate this procedure by increasing the difficulty of inserting the guidewire from the right internal jugular vein into the right superior vena cava if the patient does not have a right SVC.11 Two patients had PLSVC with BAV in the current study, and they both had bilateral SVC in echocardiography. Our results emphasize that patients with PLSVC and BAV should be followed up due to the potential to develop aortic valve stenosis, and that careful evaluation is needed if the right SVC is present.
If a transcatheter device is required to close an ASD in a patient with PLSVC, the device must not obstruct coronary sinus drainage. Carlson et al. reported that the coronary sinus was significantly dilated in all of the patients with PLSVC and ASD in their study, indicating possible interference from the device. Before the release of an AmplatzerTM septal occluder device, non-obstruction of coronary sinus flow should be carefully confirmed.12
Differences in hemodynamic variables and echocardiographic parameters in the PLSVC and non-PLSVC groups in the current study were nonsignificant, however the aortic root size in the PLSVC group was significantly smaller. None of our patients had aortic valve stenosis in follow-up echocardiography. No large study has discussed the relationship between a small aortic root and PLSVC, and there have only been a few case studies.11 The reason for the small aortic root in the PLSVC group is uncertain, and further studies are needed to elucidate this issue.
Coronary sinus dilatation has been postulated to cause cardiac arrhythmia by stretching the atrioventricular node and His bundle.13 Central line placement in PLSVC may result in arrhythmia.14,15 In the current study, none of the patients with PLSVC had symptomatic arrhythmia.
Study limitations
Despite the strengths of our methodology and consistency of our findings, this study involved some limitations. Analyses were performed using a single-institution retrospective database, and this database may have missing data, including follow-up data for some patients. In addition, cases of PLSVC without remarkable coronary sinus orifice would have been missed, and other imaging studies such as computed tomography (CT) would have been needed for conformation. Furthermore, PLSVC was found in the study population by newborn screening echocardiography. We had no reason to use CT or other imaging examinations to confirm the diagnosis in such asymptomatic neonates. This is a limitation of our study design.
CONCLUSIONS
This study was conducted to determine the prevalence of PLSVC in normal populations. We found higher incidence rates of BAV and ASD in the PLSVC group, which required further follow-up. Although PLSVC is usually hemodynamically nonsignificant, undiagnosed PLSVC can cause catastrophic consequences during invasive procedures. If PLSVC is suspected, the anatomy of the thoracic venous system must be identified before conducting invasive cardiac procedures. Furthermore, newborn screening through echocardiography can allow for the early diagnosis of PLSVC to prevent complications during invasive procedures in the future.
Acknowledgments
The authors thank Ms. Fang-Ju Sun for her professional assistance in biostatistics.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Goyal SK, Punnam SR, Verma G, Ruberg FL. Persistent left superior vena cava: a case report and review of literature. Cardiovasc Ultrasound. 2008;6:50. doi: 10.1186/1476-7120-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards J, Du Shane J. Thoracic venous anomalies. Arch Pathol. 1950;49:514–537. [Google Scholar]
- 3.Sanders JM. Bilateral superior vena cavae. Anat Rec. 1946;94:657–652. doi: 10.1002/ar.1090940407. [DOI] [PubMed] [Google Scholar]
- 4.Keith JB, Rows RD, Vlad P. Heart disease in infancy and childhood. J Med Educ. 1958;33:608. [Google Scholar]
- 5.Hsu LF, Jaïs P, Keane D, et al. Atrial fibrillation originating from persistent left superior vena cava. Circulation. 2004;109:828–832. doi: 10.1161/01.CIR.0000116753.56467.BC. [DOI] [PubMed] [Google Scholar]
- 6.Nagasawa H, Kuwabara N, Goto H, et al. Incidence of persistent left superior vena cava in the normal population and in patients with congenital heart diseases detected using echocardiography. Pediatr Cardiol. 2018;39:484–490. doi: 10.1007/s00246-017-1778-3. [DOI] [PubMed] [Google Scholar]
- 7.Mandila C, Papanikolaou J, Saranteas T, et al. Bicuspid aortic valve associated with persistent left and absent right superior vena cava. J Cardiothoracic and Vasc Anesth. 2009;23:579–580. doi: 10.1053/j.jvca.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Yin WH. Transcatheter aortic valve implantation in Taiwan: still evolving! Acta Cardiol Sin. 2017;33:350–352. doi: 10.6515/ACS20170707A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YH, Chang HH, Chen PL, et al. Procedural characteristics and outcomes of transcatheter aortic valve implantation: a single-center experience of the first 100 inoperable or high surgical risk patients with severe aortic stenosis. Acta Cardiol Sin. 2017;33:339–349. doi: 10.6515/ACS20170620A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paresj KK, Jasvinder S, Ashok KS. Persistent left superior vena cava with absent right superior vena cava and bicuspid aortic valve: a case report and review of literature. Oman Med J. 2013;28:3. doi: 10.5001/omj.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushant G, Vijayakanth B, Varun B, et al. Absent right superior vena cava and persistent left superior vena cava in a patient with bicuspid aortic valve with aortic stenosis. Ann Card Anaesth. 2018;21:212–214. doi: 10.4103/aca.ACA_154_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson KM, Johnston TA, Jones TK, Grifka RG. Amplatzer septal occluder closure of secundum atrial septal defects in the presence of persistent left superior vena cava to coronary sinus. Pediatr Cardiol. 2004;25:686–689. doi: 10.1007/s00246-003-0658-1. [DOI] [PubMed] [Google Scholar]
- 13.Morgan LG, Gardner J, Calkins J. The incidental finding of a persistent left superior vena cava: implications for primary care providers—case and review. Case Rep Med. 2015;Epub doi: 10.1155/2015/198754 doi: 10.1155/2015/198754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moorthy N, Kapoor A, Kumar S. Isolated persistent left-sided superior vena cava, giant coronary sinus, atrial tachycardia and heart failure in a child. Indian Heart J. 2013;65:603–606. doi: 10.1016/j.ihj.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postema PG, Rammeloo LA, van Litsenburg R, et al. Left superior vena cava in pediatric cardiology associated with extra-cardiac anomalies. Int J Cardiol. 2008;123:302–306. doi: 10.1016/j.ijcard.2006.12.020. [DOI] [PubMed] [Google Scholar]


