Abstract
Objectives
The characteristics and prognostic factors of small-cell lung cancer (SCLC) patients with bone metastases at first diagnosis have scarcely been reported. This study aimed to analyze the prognostic factors of these patients and to develop a scoring system for survival to provide evidence for clinical treatment decisions.
Materials and Methods
The records of 102 SCLC patients with bone metastasis at the time of diagnosis who were seen in our hospital between May 2010 and May 2015 were retrospectively reviewed. The log-rank test and multivariate Cox regression analysis were used to evaluate potential clinical predictors of survival. A scoring system was developed based on the hazard ratios of significant independent prognostic factors.
Result
The most common site of bone metastases was the spine (64.7%), and 26 patients (25.6%) had a single bone metastasis. The median survival was 10.4 months, and the 2-year survival rate was 10.3%. Age, number of bone metastases, and occurrence of extraosseous distant metastases were significant independent prognostic factors for overall survival. Based on their scores, patients were divided into three groups. The median survival times of the three groups were 6.4 months, 8.5 months and 12.4 months, and the 2-year survival rates were 0%, 2.9%, and 19.3% (p=0.000). Twenty-six patients (25.5%) developed skeletal-related events (SREs), and the most common SREs were radiation to the bone (22.5%) and spinal cord compression (11.8%).
Conclusion
This study includes preliminary clinical data of SCLC patients with bone metastases at the time of diagnosis, and more studies are needed.
Keywords: small-cell lung cancer, bone metastases, prognosis, scoring system
1. Introduction
Small-cell lung cancer (SCLC) accounts for approximately 15-20% of lung cancer, which is highly malignant, prone to distant metastasis, and has a poor survival rate [1]. During the past 30 years, the survival of SCLC patients has not improved significantly. Until recently, it had been reported that the addition of immunotherapy may lead to a significant survival benefit in the treatment of extensive SCLC [2]. However, survival is still poor, especially for extensive SCLC. For extensive SCLC, the skeletal system is one of the most common sites for metastases, and approximately two-thirds of patients with SCLC have bone metastases at diagnosis. Once bone metastases occur, they may produce considerable morbidity, such as substantial bone pain, pathologic fracture, and spinal cord compression, which have a negative impact on the quality of life. The choice of treatment method for bone metastases should be based on patient life expectancy [3,4]. Therefore, predicting the survival time of SCLC patients with bone metastases is of great clinical significance. Many studies have reported the natural history of NSCLC patients with bone metastases [5], [6], [7], [8], [9], [10]. However, the prognostic factors and characteristics of SCLC have scarcely been reported, and there is no scoring system for life expectancy in SCLC. This study aimed to examine the characteristics and prognostic factors of SCLC patients with bone metastases at initial diagnosis and to develop a scoring system to guide physicians in estimating the survival time of these patients.
2. Materials and Methods
2.1. Study population
We retrospectively reviewed 103 patients with SCLC and bone metastases at the time of diagnosis at our hospital between May 2010 and May 2015. We excluded one patient because of incomplete information. Thus, in our study, 102 patients were analyzed. All patients were diagnosed with typical clinical indicators and an assessment of histopathological results. Chest and abdominal computed tomography (CT), radionuclide bone scan, and brain magnetic resonance imaging (MRI) or integrated positron emission tomography (PET)-CT were undertaken to assess tumor stage. Thus, radionuclide bone scan or PET-CT was performed routinely to stage bone metastases. If the radionuclide bone scan or PET-CT manifested suspicious bone lesions, especially a single suspicious bone lesion, magnetic resonance imaging, CT or radiography was mandatory to determine whether bone metastases were present. At least one radiologist and one physician confirmed the diagnosis. The imaging was reviewed for this report. In regard to the number of bone metastases, the patients with two adjacent vertebral metastases were classified into the multiple metastases group. There was one patient with two adjacent vertebral metastases, and that patient was classified into the multiple metastases group. The patient characteristics are summarized in Table 1. Twenty (19.6%) patients were female, and eighty-two (80.4%) patients were male. The median age was 60 years (range 42-85 years).
Table 1.
Characteristics of SCLC patients with bone metastases at initial diagnosis.
| Subgroups | Number of patients (%) |
|---|---|
| Gender | |
| Female | 20 (19.6) |
| Male | 82 (80.4) |
| Age | |
| <65 | 71 (69.6) |
| ≥65 | 31 (30.4) |
| Smoke | |
| Yes | 83 (81.4) |
| No | 19 (18.6) |
| KPS | |
| <80 | 12 (11.8) |
| ≥80 | 90 (88.2) |
| Coexisting with extraosseous metastases | |
| No | 47 (46.1) |
| Yes | 55 (53.9) |
| Number of bone metastases | |
| Single | 26 (25.5) |
| Multiple | 76 (74.5) |
| Appendicular bone metastases | |
| Yes | 45 (44.1) |
| No | 57 (55.9) |
| Number of vertebra metastases | |
| <3 | 68 (66.7) |
| ≥3 | 34 (33.3) |
| T stage | |
| T1 | 5 (4.9) |
| T2 | 78 (76.5) |
| T3 | 15 (14.7) |
| T4 | 4 (3.9) |
| N stage | |
| N0 | 2 (2.0) |
| N1 | 8 (7.8) |
| N2 | 56 (54.9) |
| N3 | 36 (35.3) |
| Supraclavicular lymph nodes metastases | |
| Yes | 34 (33.3) |
| No | 68 (66.7) |
| Location of extraosseous metastases | |
| Liver | 25 (24.5) |
| Brain | 11 (10.8) |
| Adrenals | 7 (6.9) |
| Abdominal lymph node | 4 (3.9) |
| Skin | 1(1.0) |
| Pleural/chest wall | 8 (7.8) |
| Pancreas | 2 (2.0) |
| Contralateral lung | 8 (7.8) |
| LDH level | |
| Normal | 47 (46.1) |
| Elevated | 55 (53.9) |
| ALP level | |
| Normal | 87 (85.3) |
| Elevated | 15 (14.7) |
Abbreviations: LDH= lactate dehydrogenase; ALP= alkaline phosphatase
The following potential prognostic factors were evaluated: sex (male vs female), age (<65 vs ≥65), smoking status (yes vs no), coexisting extraosseous metastases (yes vs no), number of bone metastases (single vs multiple), appendicular bone metastases (yes vs no), number of vertebral metastases (<3 vs ≥3), T stage (T1/T2 vs T3/T4), N stage (N0/N1/N2 vs N3), LDH level (normal (≤250 U/L) vs elevated (>250 U/L)), and ALP level (normal female (≤120 U/L) and male (≤132 U/L) vs elevated female (>120 U/L) and male (>132 U/L)). Because there were only 12 patients with a KPS <80, the KPS was not analyzed in this study. Tumor response was assessed as described in the Response Evaluation and Criteria in Solid Tumors (RECIST).
2.2. Treatment
All patients underwent chemotherapy, and all patients received EP (30 mg/m2 cisplatin from days 1 to 3; 100 mg etoposide from days 1 to 5), CE (500 mg carboplatin for day 1; 100 mg etoposide from days 1 to 5) or platinum-based chemotherapy as the first-line treatment. Patients received a median of six cycles of chemotherapy. Forty-nine patients received TRT, and TRT started after at least 2 cycles of chemotherapy. The total dose administered was 40 to 60 Gy delivered at 1.8 to 2 Gy per fraction or 30 to 45 Gy delivered at 3 Gy per fraction. Only three patients (2.9%) received prophylactic cranial irradiation. Eighty-seventeen patients received treatment with bisphosphonate, of whom 12 patients received zoledronic acid and 75 patients received pamidronate disodium.
2.3. Statistical Analysis
The primary outcome was overall survival (OS), which was defined as the time from the first day of treatment to the date of death or last follow-up. Patients were followed up until death, and surviving patients were censored at the time of their last follow-up. The Kaplan–Meier method was used to estimate the distribution of time to death. Multivariate Cox regression analysis was performed to determine the significant factors associated with longer survival. An adjusted hazard ratio with a 95% confidence interval was reported for each factor. All statistical tests were 2-sided, and a result was considered statistically significant at p< 0.05. The statistical software SPSS version 18.0 was used for statistical analysis.
The scoring system was made according to the hazard ratios of significant prognostic factors in the multivariate analysis. The score of a factor was 2 or 0 when the hazard ratio was <0.5. The score of a factor was -2 or 0 when the hazard ratio was >2. The score of a factor was 1 or 0 when the hazard ratio was >0.5 and <1. The score of a factor was -1 or 0 when the hazard ratio was >1 and <2. The prognostic score was calculated by adding the scores for individual factors.
3. Results
3.1. Distribution of skeletal metastases
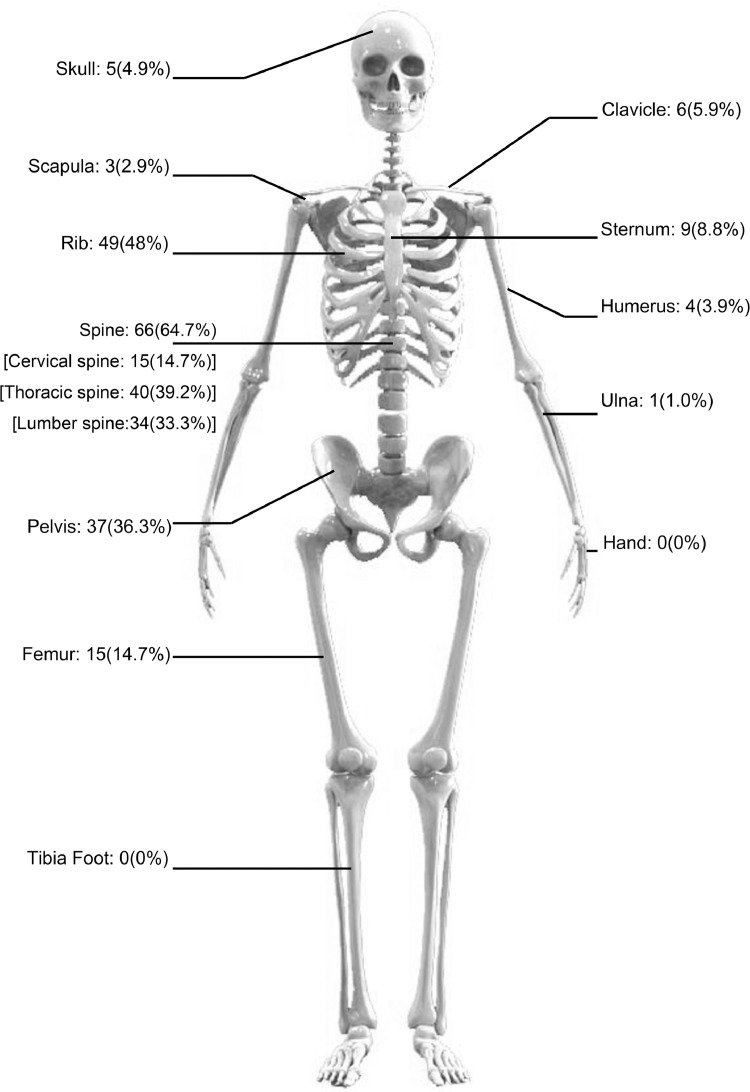
There were 26 patients (25.6%) with a single bone metastasis and 76 patients (74.5%) with multiple bone metastases. There were 68 patients with less than 3 vertebral metastases and 34 patients with more than three vertebral metastases. Sixty-six (64.7%) patients were found to have spine metastases, which was the most common site of bone metastases. Other common bone metastasis sites included the ribs (48%), pelvis (36.3%) and femur (14.7%) (Fig 1).
Fig 1.
Distribution of bone metastases in patients with small cell lung cancer at initial diagnosis. The most common sites of bone metastases were spine (64.7%).
3.2. Survival and prognostic factors of survival
The median survival time was 10.4 months, and the 2-year survival rate was 10.3%. Seventeen patients (16.6%) with only a single bone metastasis and no extraosseous distant metastases had a median survival of 17.8 months and a 2-year survival rate of 38%. Age, sex, smoking, coexistence of extraosseous distant metastases, number of bone metastases, appendicular bone metastases, number of vertebral metastases, T stage and N stage, LDH level and ALP level were included in the univariate analysis. The results showed that age, coexistence of extraosseous distant metastases, number of vertebral bone metastases, number of bone metastases, N stage, and LDH level were significant prognostic factors in the univariate analysis (see Table 2). Multivariate analysis showed that age, coexisting with extraosseous distant metastases, and number of bone metastases were significant prognostic factors for overall survival (see Table 3). The median survival time was 11.5 months and the 2-year survival rate was 28.3 months in the single bone metastasis group, while the median survival time was 9.3 months and the 2-year survival rate was 5.3% in the multiple bone metastases group (p=0.026). Patients with coexisting extraosseous distant metastases had a median survival time of 8 months and a 2-year survival rate of 2.1%, while for patients without coexisting distant metastases, the median survival time was 12.2 months, and the 2-year survival rate was 17.7% (p=0.000).
Table 2.
Univariate analysis of survival in SCLC patients with bone metastases at initial diagnosis.
| Subgroup | Median Survival (months) | 2-year survival rate (%) | p Value |
|---|---|---|---|
| Gender | |||
| Male | 10.4 | 7.2 | 0.226 |
| Female | 10.3 | 23.7 | |
| Age | |||
| <65 | 10.9 | 13.1 | 0.002 |
| ≥65 | 9.1 | 3.4 | |
| Smoke | |||
| Yes | 13.0 | 8.5 | 0.103 |
| No | 10.3 | 18.9 | |
| Coexisting extraosseous metastases | |||
| Yes | 8.0 | 2.1 | 0.000 |
| No | 12.2 | 17.7 | |
| Number of bone metastases | |||
| Single | 11.5 | 28.3 | 0.026 |
| Multiple | 9.3 | 5.3 | |
| Appendicular bone metastases | |||
| Yes | 9.3 | 10.6 | 0.992 |
| No | 10.5 | 9.7 | |
| Number of vertebra metastases | |||
| <3 | 10.9 | 14.5 | 0.01 |
| ≥3 | 8.67 | 2.9 | |
| T stage | |||
| T1/T2 | 10.0 | 8.9 | 0.231 |
| T3/T4 | 10.6 | 15.4 | |
| N stage | |||
| N0/N1/N2 | 66 | 15.8 | 0.009 |
| N3 | 36 | 0 | |
| LDH level | |||
| Normal | 11.5 | 16.0 | 0.048 |
| Elevated | 8.8 | 5.2 | |
| ALP | |||
| Normal | 10.4 | 10.1 | 0.832 |
| Elevated | 10.3 | 13.3 |
Abbreviations: LDH= lactate dehydrogenase; ALP= alkaline phosphatase
Table 3.
Multivariate analysis of survival in SCLC patients with bone metastases at initial diagnosis.
| Potential prognostic factor | Hazard Ratio | 95%CI | P |
|---|---|---|---|
| Age | 1.847 | 1.159-2.942 | 0.010 |
| Coexisting with extraosseous metastases | 2.324 | 1.475-3.661 | 0.000 |
| Number of bone metastases | 1.776 | 1.040-3.034 | 0.036 |
| N stage | 1.046 | 0.628-1.744 | 0.832 |
| Number of vertebrate bone metastases | 1.165 | 0.718-1.891 | 0.449 |
| LDH level | 0.731 | 0.469-1.137 | 0.136 |
Abbreviations: LDH= lactate dehydrogenase
3.3. Scoring system for survival
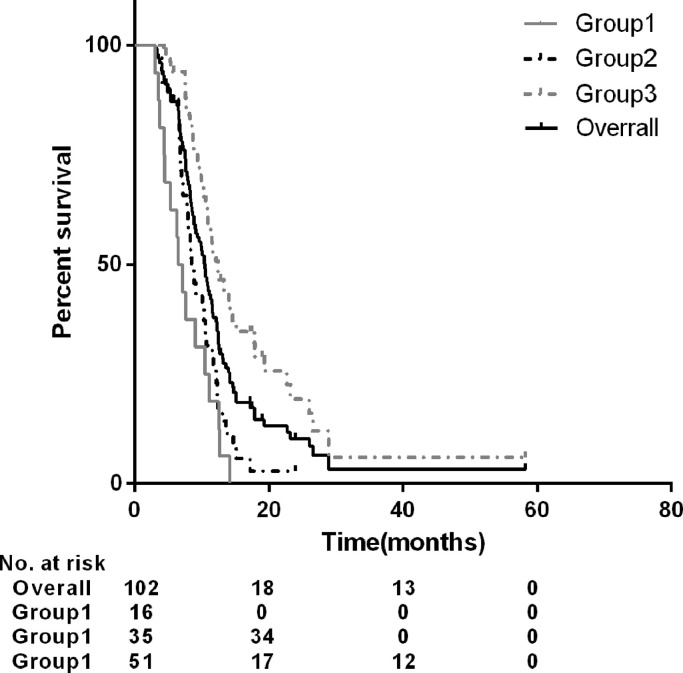
We developed a scoring system according to three of the significant factors identified from the multivariate analysis according to their hazard ratios. Patients with age ≥ 65 years received -1 point, and other patients received 0 points. Patients with multiple bone metastases received -1 point, and those with a single bone metastasis received 0 points. Patients with coexisting extraosseous distant metastases received -2 points, and other patients received 0 points (see Table 4). To verify the validity of the survival score, we divided patients into 3 groups according to the score (see Table 5). The median survival times of the three groups were 6.4 months, 8.5 months and 12.4 months, respectively, and the 2-year survival rates were 0%, 2.9%, and 19.3%, respectively (p=0.000) (Fig 2).
Table 4.
Score of significant survival factors in patients with bone metastases of small cell lung cancer
| Potential prognostic factor | Subgroup | Score | Hazard Ratio |
|---|---|---|---|
| Age | <65 ≥65 |
0 -1 |
1.847 |
| Coexisting with extraosseous distant metastases | No Yes |
0 -2 |
2.324 |
| Number of bone metastases | Single multiple |
0 -1 |
1.776 |
Table 5.
Score and survival of different groups
| Subgroup | No. patients | Score | Survival | |
|---|---|---|---|---|
| Median survival(months) | Two-year survival rate (%) | |||
| Group 1 | 16 | -4 | 6.4 | 0 |
| Group 2 | 35 | -2/-3 | 8.5 | 2.9 |
| Group 3 | 51 | -1/0 | 12.4 | 19.3 |
Fig 2.
Survival curves of different groups. The median survival time of the whole group and three subgroups were 10.4 months, 6.4 months, 8.5 months and 12.4 months, respectively (p=0.000).
3.4. Characteristics of SREs
At initial diagnosis, pathological fractures occurred in 2 patients, and spinal cord compression syndrome occurred in 5 patients. Hypercalcemia was observed in 1 patient. Seven patients underwent bone radiotherapy, including 5 patients with spinal cord compression syndrome and 2 patients with pathological fracture. In the course of treatment, there were 7 patients with spinal cord compression syndrome and 2 patients with pathological fracture. Sixteen patients underwent bone metastasis radiotherapy, including 6 patients with spinal cord compression and 2 patients with pathological fracture. One patient underwent surgical treatment for spinal cord compression. Therefore, 26 patients (25.5%) experienced skeletal-related events (see Table 6). A total of 41 SREs were observed in the whole group, and the skeletal morbidity rate was 0.88.
Table 6.
Characteristics of SREs in SCLC patients with bone metastases at diagnosis and during treatment
| SREs | Number of patients (n) | Percentage (%) |
|---|---|---|
| Radiation to bone | 23 | 22.5 |
| Spinal cord compression | 12 | 11.8 |
| Pathologic fracture | 4 | 3.9 |
| Hypercalcemia | 1 | 1.0 |
| Surgical stabilization | 1 | 1.0 |
For patients who were treated with bisphosphonate, 23 patients (26.4%) had bone-related events. For the fifteen patients who did not use bisphosphonate, 3 of them (20.0%) had bone-related events. Patients with SREs had a two-year survival rate of 5.2% and a median survival time of 10.5 months, while patients without SREs had a two-year survival rate of 9.6% and a median survival time of 10.4 months (p=0.490).
4. Discussion
The skeletal system is the most common metastasis site for several types of cancer, such as breast cancer, prostate cancer, and lung cancer. Small-cell lung cancer is more prone to metastasis and has a poorer survival than breast cancer and prostate cancer. The median survival time of extensive SCLC ranges from 5 to 10 months [5,10,11]. In addition, the effects of systemic therapy on each primary site are different, so it is important to analyze the prognosis and characteristics of SCLC alone. This study retrospectively analyzed the clinical characteristics and prognostic factors of 102 SCLC patients with bone metastasis at initial diagnosis and developed a scoring system to predict survival.
Little information on ES-SCLC with bone metastases has been reported in previous studies, and we summarized what has been reported in Table 7. To our knowledge, only one study has reported prognostic factors in SCLC patients with bone metastases at initial diagnosis [12]. Kang EJ et al. analyzed 61 SCLC patients with bone metastases at initial diagnosis. The median survival time was 4.13 months, and poor PS and high ALP (two times above the upper normal limit) were poor prognostic factors. In our study, the median survival time of SCLC patients with bone metastases was 10.4 months, and there were three significant beneficial prognostic factors: age<65; single bone metastasis; and no extraosseous metastases. We think that there are several reasons for the difference in median survival time between the two studies. First, in the former study, 26 patients (42.6%) had a PS ≥2, and 23 patients (22.5%) did not receive chemotherapy; in our study, only 11.8% of patients had a KPS less than 80, and all patients received chemotherapy. Second, in the former study, the disease stage was more advanced, and there were only two patients (3.3%) without extraosseous metastasis, while in this study, there were 47 patients (46.1%) without extraosseous metastasis. In our study, elevated ALP (above the upper normal limit) was not a prognostic factor, which is consistent with the findings of Kang EJ's study. However, there were only 2 patients with high ALP (two times higher than the upper normal limit), which may be another reason for the better survival in our study than in the other study. Primary site, PS, presence or absence of metastases to organs, and number of bone metastases have been reported as important prognostic indicators in patients with bone metastases from various cancers [13], [14], [15]. Several studies have demonstrated that there is a significant difference in survival between ED-SCLC patients with different numbers of organs with metastases [16], [17], [18]. The results of this study agree with a previous study showing that the state of organ metastases is a significant prognostic factor and plays the most important role in ED-SCLC with bone metastases.
Table 7.
Results of SCLC with bone metastases in previous studies
| Year/Country/Author | Study population | No. SCLC with BM | Findings of SCLC with BM | |
|---|---|---|---|---|
| Survival | Incidence of SREs | |||
| 2016 Korea Kang EJ et al [12]. |
ES-SCLC | 61 (BM at initial diagnosis) |
Median:4.13m; Poor prognostic factors: PS≥2; higher ALP |
34.4% |
| 2012, Japan K. Nakazawa et al [18] |
LS-SCLC+ ES-SCLC |
46 (BM at initial diagnosis); |
1 yr: 25% (without extraosseous metastases) | NR |
| 2018,Thailand Pruksakorn D et al.[8] | SCLC+NSCLC | 30 (BM at initial diagnosis) |
1 yr: 10.7% | NR |
| 2019, Brazil Silva GT et al [31] |
SCLC+NSCLC | 22 (BM at initial diagnosis /follow up) |
Median: 2.13m (with SREs), 8.57m (without SREs) p = 0.146 |
63.6% |
| 2014, Danish Cetin K et al.[32] |
SCLC+NSCLC | 340 (BM at initial diagnosis /follow up) |
NR | 50%. |
| 2014, Japan N. Katakami et al.[24] |
SCLC+NSCLC | 47 (BM at initial diagnosis /follow up) |
NR | 8.5% |
| 2016, Swiss (Conen K et al.) [33] |
SCLC | 92 (BM at initial diagnosis /follow up) |
NR | 18.4% (total) 8.7%(initial diagnosis) |
Abbreviations: BM=bone metastases; NR=not reported; m=months.
Our study confirmed that multiple bone metastases were much more common than a single bone metastasis (74.5% vs 25.5%). It has been reported that lung cancer patients with a single bone metastasis have a better survival than lung cancer patients with multiple metastases [6,19], while some studies have found no significant difference in survival expectations between patients with a single bone metastasis or multiple bone metastases [8]. In this study, patients with a single bone metastasis had a longer survival than patients with multiple bone metastases. The number of bone metastases was a significant prognostic factor in univariate and multivariate analyses. However, when radiotherapy and chemotherapy response status were analyzed in the multivariate analysis at the same time, the number of bone metastases was not a significant prognostic factor. This suggests that patients with multiple bone metastases may benefit from radiotherapy and chemotherapy. Furthermore, in this study, seventeen patients with a single bone metastasis and no coexisting distant metastases had good survival, with a median survival time of 17.8 months and a 2-year survival rate of 38%, suggesting that a more aggressive treatment approach may be needed.
Several scoring systems have been developed to predict the survival of patients with bone metastases after palliative surgery or radiotherapy, and they are based on the study of various primary tumors [15,[20], [21], [22], [23], [24], [25], [26], [27]]. To our knowledge, this study is the first to develop a scoring system for SCLC with bone metastases. The scoring system was derived from the three significant factors identified using the hazard ratio method, and according to the score, patients were classified into three groups. Different groups had significantly different survival rates. Patients with low scores had a short life expectancy. For those patients, surgical intervention and long-course radiotherapy should be avoided; however, noninvasive ablation for pain control and short-course radiotherapy are recommended. Patients with a high score had a good prognosis; therefore, surgical intervention and long-course radiotherapy are recommended.
The incidence of SREs in non-small-cell lung cancer ranges from 40% to 65% [28], [29], [30], and it ranges from 8.5% to 63.5% in SCLC according to reported studies [12,[30], [31], [32], [33]] (see Table 7). In a study by Katakami N et al., the incidence of SREs in SCLC was lower than that in NSCLC, and the incidence of SREs in extensive small-cell lung cancer was only 8.5% [30]. Kang EJ et al. [33] reported an SRE incidence of 34.4% in 61 SCLC patients with bone metastases at diagnosis. In this study, SREs occurred in 26 patients (25.5%), which is consistent with the findings of a previous study. We speculate that the low incidence of SREs is due to the short survival time of small-cell lung cancer patients and the lack of timely bone-related examinations after disease progression in many patients. In the present study, the most common SREs were radiation to the bone (22.5%) and spinal cord compression (11.8%), which is consistent with the findings of other studies [32]. Many studies have reported that SREs are prognostic factors for survival, while some studies have not found that the occurrence of SREs affects survival [7,31,32,34]. In our study, there was no significant difference in the 2-year survival rate and median survival time between those patients with and without SREs. The role of SREs in survival requires further investigation.
In conclusion, this study reported the clinical features and prognostic factors of survival in SCLC patients with bone metastases at the time of diagnosis and developed a simple scoring system for predicting survival, which can provide important evidence for clinical treatment decisions in these patients. However, there are some limitations of this study. First, this study was based on the experience of a single institution and had a small number of patients. Second, most patients in this study had good performance. The scoring system still requires further validation.
Acknowledgements
This research was not supported by any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Govindan R., Page N., Morgensztern D., Read W., Tierney R., Vlahiotis A., Spitznagel E.L., Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006 doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Pacheco J., Bunn P.A. Advancements in Small-cell Lung Cancer: The Changing Landscape Following IMpower-133. Clin Lung Cancer. 2019;20:148-160 e2. doi: 10.1016/j.cllc.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya I.S., Hoskin P.J. Stereotactic Body Radiotherapy for Spinal and Bone Metastases. Clin. Oncol. 2015;27:298–306. doi: 10.1016/j.clon.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Rose C.M., Kagan A.R. The final report of the expert panel for the radiation oncology bone metastasis work group of the American College of Radiology. Int J Radiat Oncol Biol Phys. 1998;40:1117–1124. doi: 10.1016/s0360-3016(97)00952-8. https://www.ncbi.nlm.nih.gov/pubmed/9539567 [DOI] [PubMed] [Google Scholar]
- 5.Santini D., Barni S., Intagliata S., Falcone A., Ferrau F., Galetta D., Moscetti L., La Verde N., Ibrahim T., Petrelli F., Vasile E., Ginocchi L., Ottaviani D., Longo F., Ortega C., Russo A., Badalamenti G., Collova E., Lanzetta G., Mansueto G., Adamo V., De Marinis F., Satolli M.A., Cantile F., Mancuso A., Tanca F.M., Addeo R., Russano M., Sterpi M., Pantano F., Vincenzi B., Tonini G. Natural History of Non-Small-Cell Lung Cancer with Bone Metastases. Sci Rep. 2015;5:18670. doi: 10.1038/srep18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komatsu T., Kunieda E., Oizumi Y., Tamai Y., Akiba T. An analysis of the survival rate after radiotherapy in lung cancer patients with bone metastasis: is there an optimal subgroup to be treated with high-dose radiation therapy? Neoplasma. 2012;59:650–657. doi: 10.4149/neo_2012_082. [DOI] [PubMed] [Google Scholar]
- 7.Kuchuk M., Kuchuk I., Sabri E., Hutton B., Clemons M., Wheatley-Price P. The incidence and clinical impact of bone metastases in non-small cell lung cancer. Lung Cancer. 2015;89:197–202. doi: 10.1016/j.lungcan.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Pruksakorn D., Phanphaisarn A., Settakorn J., Arpornchayanon U., Tantraworasin A., Chaiyawat P., Klangjorhor J., Teeyakasem P. Prognostic score for life expectancy evaluation of lung cancer patients after bone metastasis. J Bone Oncol. 2018;10:1–5. doi: 10.1016/j.jbo.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rief H., Muley T., Bruckner T., Welzel T., Rieken S., Bischof M., Lindel K., Combs S.E., Debus J. Survival and prognostic factors in non-small cell lung cancer patients with spinal bone metastases: A retrospective analysis of 303 patients. Strahlentherapie Und Onkol. 2014;190:59–63. doi: 10.1007/s00066-013-0431-1. [DOI] [PubMed] [Google Scholar]
- 10.Tsuya A., Kurata T., Tamura K., Fukuoka M. Skeletal metastases in non-small cell lung cancer: A retrospective study. Lung Cancer. 2007;57:229–232. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Stanley K.E. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. https://www.ncbi.nlm.nih.gov/pubmed/6930515 [PubMed] [Google Scholar]
- 12.Kang E.J., Lee S.Y., Kim H.J., Min K.H., Hur G.Y., Shim J.J., Kang K.H., Oh S.C., Seo J.H., Lee S.Y., Kim J.S. Prognostic Factors and Skeletal-Related Events in Patients with Small Cell Lung Cancer with Bone Metastases at the Time of Diagnosis. Oncology. 2016;90:103–111. doi: 10.1159/000442949. [DOI] [PubMed] [Google Scholar]
- 13.Hansen B.H., Keller J., Laitinen M., Berg P., Skjeldal S., Trovik C., Nilsson J., Walloe A., Kalén A., Wedin R. The scandinavian sarcoma group skeletal metastasis registry functional outcome and pain after surgery for bone metastases in the pelvis and extremities. Acta Orthop. 2009;80:85–90. doi: 10.1080/00016470410001708270. [DOI] [PubMed] [Google Scholar]
- 14.Katagiri H., Takahashi M., Wakai K., Sugiura H., Kataoka T., Nakanishi K. Prognostic factors and a scoring system for patients with skeletal metastasis. J Bone Jt. Surg Br. 2005;87:698–703. doi: 10.1302/0301-620X.87B5.15185. [DOI] [PubMed] [Google Scholar]
- 15.Van Der Linden Y.M., Dijkstra S.P.D.S., Vonk E.J.A., Marijnen C.A.M., Leer J.W.H. Prediction of survival in patients with metastases in the spinal column: Results based on a randomized trial of radiotherapy. Cancer. 2005;103:320–328. doi: 10.1002/cncr.20756. [DOI] [PubMed] [Google Scholar]
- 16.Tas F., Aydiner A., Topuz E., Camlica H., Saip P., Eralp Y. Factors influencing the distribution of metastases and survival in extensive disease small cell lung cancer. Acta Oncol. (Madr). 1999;38:1011–1015. doi: 10.1080/028418699432275. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Dai C.H., Chen P., Wu J.N., Ban Q.L., Qiu H., Li X.Q. Survival and prognostic factors in small cell lung cancer. Med. Oncol. 2010;27:73–81. doi: 10.1007/s12032-009-9174-3. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa K., Kurishima K., Tamura T., Kagohashi K., Ishikawa H., Satoh H., Hizawa N. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4:617–620. doi: 10.3892/ol.2012.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiura H., Yamada K., Sugiura T., Hida T., Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 2008;466:729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokuhashi Y., Matsuzaki H., Oda H., Oshima M., Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. https://www.ncbi.nlm.nih.gov/pubmed/16205345 [DOI] [PubMed] [Google Scholar]
- 21.Tokuhashi Y., Matsuzaki H., Toriyama S., Kawano H., Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. https://www.ncbi.nlm.nih.gov/pubmed/1702559 [DOI] [PubMed] [Google Scholar]
- 22.Tomita K., Kawahara N., Kobayashi T., Yoshida A., Murakami H., Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. https://www.ncbi.nlm.nih.gov/pubmed/11224867 [DOI] [PubMed] [Google Scholar]
- 23.Rades D., Fehlauer F., Schulte R., Veninga T., Stalpers L.J., Basic H., Bajrovic A., Hoskin P.J., Tribius S., Wildfang I., Rudat V., Engenhart-Cabilic R., Karstens J.H., Alberti W., Dunst J., Schild S.E. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24:3388–3393. doi: 10.1200/JCO.2005.05.0542. [DOI] [PubMed] [Google Scholar]
- 24.Katagiri H., Okada R., Takagi T., Takahashi M., Murata H., Harada H., Nishimura T., Asakura H., Ogawa H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3:1359–1367. doi: 10.1002/cam4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W.Y., Li H.F., Su M., Lin R.F., Chen X.X., Zhang P., Zou C.L. A simple scoring system predicting the survival time of patients with bone metastases after RT. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westhoff P.G., De Graeff A., Monninkhof E.M., Bollen L., Dijkstra S.P., Van Der Steen-Banasik E.M., Van Vulpen M., Leer J.W.H., Marijnen C.A., Van Der Linden Y.M. An easy tool to predict survival in patients receiving radiation therapy for painful bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 2014;90:739–747. doi: 10.1016/j.ijrobp.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan M.S., Epstein-Peterson Z., Chen Y.H., Tseng Y.D., Wright A.A., Temel J.S., Catalano P., Balboni T.A. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: The TEACHH model. Cancer. 2014;120:134–141. doi: 10.1002/cncr.28408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tominaga H., Setoguchi T., Shimada H., Nagano S., Sasaki H., Ishidou Y., Sato M., Mizuno K., Inoue H., Komiya S. Prognostic factors in patients with skeletal-related events at non-small-cell lung cancer diagnosis. Mol Clin Oncol. 2017;7:897–902. doi: 10.3892/mco.2017.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae H.M., Lee S.H., Kim T.M., Kim D.W., Yang S.C., Wu H.G., Kim Y.W., Heo D.S. Prognostic factors for non-small cell lung cancer with bone metastasis at the time of diagnosis. Lung Cancer. 2012;77:572–577. doi: 10.1016/j.lungcan.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 30.Katakami N., Kunikane H., Takeda K., Takayama K., Sawa T., Saito H., Harada M., Yokota S., Ando K., Saito Y., Yokota I., Ohashi Y., Eguchi K. Prospective study on the incidence of bone metastasis (BM) and skeletal-related events (SREs) in Patients (pts) with Stage IIIB and IV Lung Cancer - CSP-HOR 13. J. Thorac. Oncol. 2014;9:231–238. doi: 10.1097/JTO.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva G.T., Silva L.M., Bergmann A., Thuler L.C. Bone metastases and skeletal-related events: incidence and prognosis according to histological subtype of lung cancer. Futur. Oncol. 2019;15:485–494. doi: 10.2217/fon-2018-0613. [DOI] [PubMed] [Google Scholar]
- 32.Cetin K., Christiansen C.F., Jacobsen J.B., Norgaard M., Sorensen H.T. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer. 2014;86:247–254. doi: 10.1016/j.lungcan.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Conen K., Hagmann R., Hess V., Zippelius A., Rothschild S.I. Incidence and predictors of Bone Metastases (BM) and Skeletal-Related Events (SREs) in Small Cell Lung Cancer (SCLC): A Swiss patient cohort. J Cancer. 2016;7:2110–2116. doi: 10.7150/jca.16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J.M., Ahn J.S., Lee S., Kim J.A., Lee J., Park Y.H., Park H.C., Ahn M.J., Ahn Y.C., Park K. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer. 2011;71:89–93. doi: 10.1016/j.lungcan.2010.04.003. [DOI] [PubMed] [Google Scholar]