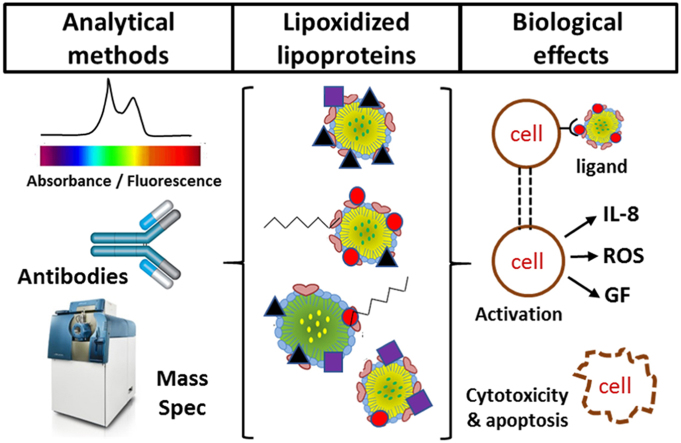
Abstract
Lipoproteins are essential systemic lipid transport particles, composed of apolipoproteins embedded in a phospholipid and cholesterol monolayer surrounding a cargo of diverse lipid species. Many of the lipids present are susceptible to oxidative damage by lipid peroxidation, giving rise to the formation of reactive lipid peroxidation products (rLPPs). In view of the close proximity of the protein and lipid moieties within lipoproteins, the probability of adduct formation between rLPPs and amino acid residues of the proteins, a process called lipoxidation, is high. There has been interest for many years in the biological effects of such modifications, but the field has been limited to some extent by the availability of methods to determine the sites and exact nature of such modification. More recently, the availability of a wide range of antibodies to lipoxidation products, as well as advances in analytical techniques such as liquid chromatography tandem mass spectrometry (LC-MSMS), have increased our knowledge substantially. While most work has focused on LDL, oxidation of which has long been associated with pro-inflammatory responses and atherosclerosis, some studies on HDL, VLDL and Lipoprotein(a) have also been reported. As the broader topic of LDL oxidation has been reviewed previously, this review focuses on lipoxidative modifications of lipoproteins, from the historical background through to recent advances in the field. We consider the main methods of analysis for detecting rLPP adducts on apolipoproteins, including their advantages and disadvantages, as well as the biological effects of lipoxidized lipoproteins and their potential roles in diseases.
Abbreviations: AGEs, advanced glycation end products; ALEs, advanced lipoxidation end products; Apo, Apolipoprotein; COL, Nε-(8-carboxyoctanyl)-Lysine; DNPH, 2,4-dinitrophenylhydrazine; ELISA, enzyme-linked immunosorbent assay; GC-MS, gas chromatography mass spectrometry; HDDE, 4-hydroxy-2E,6Z-dodecadienal; HDL, high density lipoprotein; HHE, 4-hydroxy-trans-2-hexenal; HNE, 4-hydroxy-trans-2-nonenal; HOCl, hypochlorous acid; HPNE, 4-hydroperoxy-trans-2-nonenal; IDL, intermediate density lipoprotein; 3-HOSCA, 3β-hydroxy-5-oxo-5,6-secocholestan-6-al; KLH, keyhole limpet hemocyanin; HPLC-ESI-MSMS, (high pressure) liquid chromatography (electrospray) (tandem) mass spectrometry; LDL, low density lipoprotein; Lp(a), lipoprotein(a); MALDI-TOF, matrix-assisted laser desorption ionization time of flight; MDA, malondialdehyde; MS, mass spectrometry; OSE, oxidation-specific epitope; oxLDL/HDL, oxidized low/high density lipoprotein; (ox)PC, (oxidized) phosphatidylcholine; (ox)PL, (oxidized) phospholipid; PAH, polycyclic aromatic hydrocarbons; PL, phospholipid; PONPC, 1-palmitoyl-2-(9-oxo-nonanoyl)-sn-glycero-3-phosphocholine; POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine; rLPPs, reactive lipid peroxidation products; TBArS, thiobarbituric acid reactive substances; TNBS, 2,4,6-trinitrobenzene sulfonic acid; VLDL, very low density lipoprotein
Keywords: LDL, HDL, ApoB-100, Lipid peroxidation, Liquid chromatography mass spectrometry, Immunoassays, Atherosclerosis
Graphical abstract
Highlights
-
•
Lipoproteins can be modified by reactive Lipid Peroxidation Products (rLPPs).
-
•
Lipoprotein lipoxidation is known to occur in several inflammatory diseases.
-
•
Biochemical, immunochemical and mass spectrometry methods can detect rLPP adducts.
-
•
Due to higher information output, MS can facilitate localization of modifications.
-
•
Antibodies against some rLPPs have been used to identify lipoxidation in vivo.
1. Introduction to lipoproteins and lipid oxidation
Lipoproteins are a family of complex particles consisting of amphipathic apolipoproteins that carry a wide range of lipids, and therefore have important physiological functions in systemic lipid transport. The basic structure is a shell formed of a monolayer of phospholipids and free cholesterol surrounding a core of cholesteryl esters and triglycerides [1], with one or more proteins associated with the surface phospholipids and partially embedded in the hydrophobic lipid core. The role of the proteins is thus to solubilize and stabilize the lipoproteins in an aqueous environment such as plasma, which is a major location of lipoproteins. Lipoproteins are classified according to their size and density, which is determined by the ratio of protein to lipid, and therefore depends on the lipid and apolipoprotein composition. The plasma lipoproteins are typically divided into 7 classes, which are chylomicrons, chylomicron remnants, VLDL, IDL, LDL, HDL, and Lp(a). The chylomicrons are responsible for transport of dietary lipids from the intestine to lipid-metabolizing tissues muscle and adipose, and the resulting chylomicron remnants deliver remaining lipid to the liver. VLDL, IDL, LDL are involved in the second phase of triglyceride and cholesterol delivery from the liver to peripheral tissues, whereas HDL particles are responsible for reverse cholesterol transport from cells in the peripheral tissues to the liver to lipoproteins of the endogenous pathway. The functions and activities of the lipoprotein particles are largely determined by the combination of apolipoproteins they contain, which direct their interaction with receptors as well as containing a variety of enzymatic activities. The lipid cargo is very diverse, and in addition of several classes of phospholipid, cholesterol and cholesteryl esters, it can include free fatty acids, sphingolipids, ceramides, and sulfolipids [2].
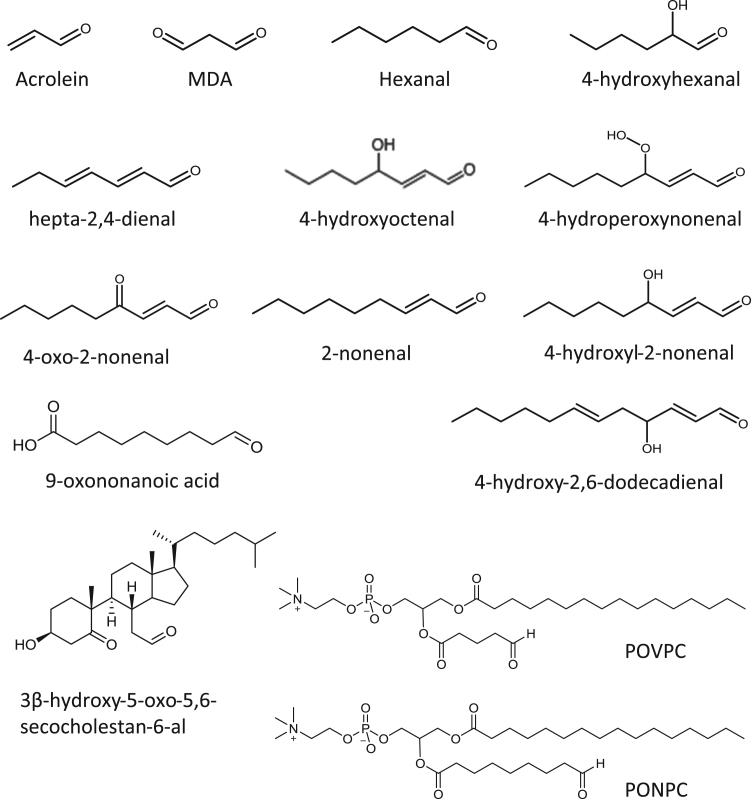
Many of the components of lipoproteins are susceptible to oxidative damage by free radicals of non-radical oxidants, and this has been an energetic field of study since pioneering studies in the 1980s demonstrated that oxidation of LDL contributed to changes in its biological properties [3], [4], [5] as described in Section 4. Lipids containing unsaturated fatty acyl chains (especially poly-unsaturated ones) can be peroxidized following hydrogen abstraction by radical attack and subsequent addition of di-oxygen; the downstream reactions are complex and lead to a plethora of short- and long-chain oxidation products; this topic has been extensively reviewed previously [6], [7], [8], [9]. Peroxidation at bis-allylic sites in the fatty acyl chains tends to result in cleavage of the hydrocarbon backbone by Hock rearrangement or other mechanisms, resulting in formation of an aldehyde on one or both chains [10]. Intrachain cyclization reactions can also occur, for example leading to formation of isoprostanes or isolevuglandins. Secondary radical attack and addition of oxygen can occur at other sites, resulting in complex structures with multiple reactive groups including aldehydes, ketones, epoxides and cyclopentenone rings. A common motif that rises following cleavage is the γ-substituted α,β-alkenal, as in 4-hydroxynonenal (HNE) or 4-oxo-nonenal, although a variety of analogous structures are possible [6], [8], [11]. The headgroups of amine-containing phospholipids, e.g phosphatidylserine, can be modified by oxidative deamination, which also generates an aldehyde [12]. Alternatively, unsaturated lipids can be attacked by electrophilic agents such as hypochlorous acid (HOCl), which causes the formation of chlorohydrins (hydroxyl and chlorine groups on adjacent carbons) on fatty acyl chains, or cleavage of the vinyl ether bonds of plasmalogens to yield chloro-aldehydes. Radical nitrogen species such as nitrogen dioxide can cause peroxidation via hydrogen abstraction, but may also undergo addition reactions leading to nitrated or nitrosylated fatty acyl chains, some of which also contain motifs similar to the substituted alkenals mentioned above. The key point about lipid oxidation products containing aldehydes, ketones, epoxides and substituted alkenals is that they are electrophilic and moderately to highly reactive with nucleophilic groups, which endows them with unusual properties through the ability to react with biological molecules. While several different collective names for such compounds exist, in this review we have used the term “reactive lipid peroxidation products”, or rLPPs. Fig. 1 shows the structure of the various rLPPs discussed in this review.
Fig. 1.
Structures of the reactive lipid peroxidation products (rLPPs) reported to form adducts with lipoproteins. Note that Ne-(3-methylpyridinium) is shown as the lysine adduct form.
Lipid oxidation in lipoproteins has been extensively investigated and the reader is directed to reviews on this topic for more detail [13], [14]. Consequently, lipid oxidation is not the main focus of this review, which instead addresses the more specific issue of how the protein moieties of lipoproteins are modified by the resulting rLPPs, a process known as lipoxidation and explained in more detail in Section 2. In view of the close proximity of protein and lipids within lipoproteins, the probability of adduct formation between rLPPs and residues of the proteins is relatively high, but to date no lipoprotein reviews have focused specifically on lipoxidation, despite the fact that there is growing awareness of the signaling importance of this modification [15], [16], [17], [18]. This review addresses mainly HDL and LDL lipoxidation, as the majority of research has investigated these lipoproteins, and aims to provide a concise overview of lipoprotein modification by oxidized lipid adducts, from early pioneering studies to recent advances in molecular knowledge.
2. Protein oxidation and lipoxidation
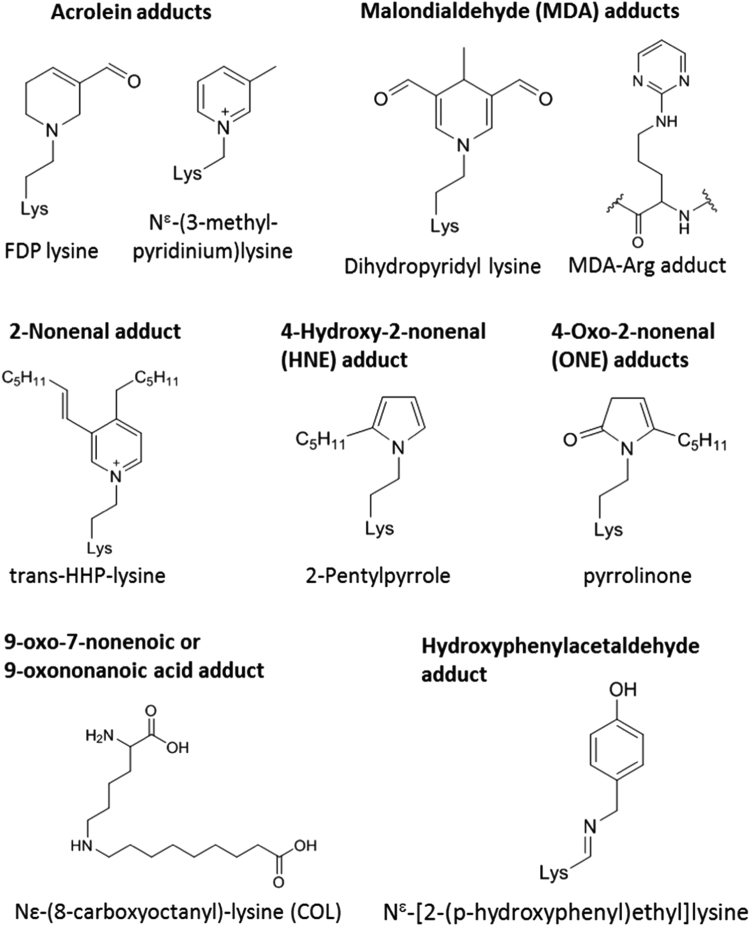
In parallel to lipid oxidation, the apoprotein moieties of lipoproteins can also be oxidized directly by a range of oxidants; usually the basic side chains (lysine, arginine and histidine), and sidechains containing sulfur (cysteine, methionine) or aromatic groups (tyrosine, tryptophan, phenylalanine) are most readily modified. Several studies have investigated the relative susceptibility of lipoprotein residues to oxidants, both in vitro and ex-vivo, in an attempt to identify markers of damage as well as to understand changes in lipoprotein function [19], [20], [21], [22]. Nucleophilic sidechains, i.e. those containing amine or thiol groups, can also be modified by reaction with rLPPs. Protein lipoxidation can occur by one of two chemical reactions: Michael addition occurs when the electrophilic β-carbon of a reactive lipid oxidation product reacts with the amino group of histidine and lysine or the thiol group of a cysteine; Schiff's base formation involves reaction of the free amino group of a lysine, arginine or N-terminal amine with the carbonyl group of an aldehyde or ketone. Schiff's base adducts are less stable than Michael adducts, and both are potentially reversible depending on the chemical environment. Moreover, depending on the electrophilic species involved, secondary reactions such as cyclization, dehydration, oxidation, or condensation with an additional electrophile may take place, leading to formation of more stable adducts that are referred to as advanced lipoxidation end products, or ALEs, by analogy with the better-known advanced glycation end products (AGEs). For example, the HNE Michael adducts tend to cyclize to hemiacetals, while the HNE Schiff's base with lysine can generate a stable 2-pentylpyrrole derivative [23]; the Michael adduct of acrolein can rearrange to form formyl-dehydropiperidino lysine (FDP-lysine) [24]. The process of lipoxidation has been very well explained previously [23], [25], [26], and consequently will not be covered in depth here, although the structures most commonly discussed in the review are shown in Fig. 2.
Fig. 2.
Structures of common advanced lipoxidation end products (ALEs) or adducts detected on lipoproteins. The type of rLPP causing adduct formation is written in bold above the structure(s) and the resulting adduct names are give in plain text below. Trans-HHP-lysine is trans-Ne3-[(hept-1-enyl)-4-hexylpyridinium]lysine.
Many approaches have been used to study lipoprotein oxidation and lipoxidation [10], [16], [27]. The ultimate goal is to be able to detect and identify lipoxidation under physiological conditions and in biological or clinical samples, but many studies of modification in vitro have been carried out as a stepping stone to this stage, to develop methodology or establish the most likely products. Oxidation of lipoproteins in vitro is often achieved by treatment with transition metal ions, mostly commonly Cu2+, but occasionally Fe2+ in combination with hydrogen peroxide as a Fenton reagent to generate hydroxyl radicals is used. The use of iron also represents a model for ferretin-induced oxidation of LDL. Other methods for oxidation of lipoproteins include radical initiators, such as 2,2′-Azobis(2-methylpropionamidine) dihydrochloride (AAPH), or treatment with UV light. These are all able to initiate lipid peroxidation, and therefore lead to lipoxidation of the apolipoprotein. More physiological approaches include enzymes such as lipoxygenases and cytochrome P450 enzymes, which catalyse radical oxidations and hydroxylations of fatty acids, or myeloperoxidase, which is well-known for its ability to generate hypohalous acids such as HOCl, but is also able to catalyse direct oxidations of some substrates [28]. There have also been many studies in vitro where lipoproteins, especially LDL, have been modified by direct treatment with reactive lipid peroxidation products; most work has been carried out with small unsaturated aldehydes, such as acrolein, malondialdehyde (MDA) and HNE, but a small number of studies with less well-known rLPPs and even esterified oxidized phospholipids have been reported, as described in the following sections.
3. Approaches to the analysis of LDL and HDL lipoxidation
The analysis of LDL and HDL is dependent on the separation of these lipoproteins from whole plasma. A common method for separation of these particles is by gradient ultracentrifugation. Using different salt concentrations and centrifugation at high g-forces, the lipoproteins of different density migrate in the gradient, allowing their separation and forming bands that can be collected and analysed further. This methodology, which dates back to 1955, was developed by Havel et al. [29] and has been used extensively for the study of lipoproteins [30], [31]. There are other methods that take advantage of different characteristics of lipoprotein particles for their separation, such as size exclusion chromatography, which depends on the size difference between the lipoproteins, and gradient gel electrophoresis, which uses both size and charge for its separation [30], [32], [33].
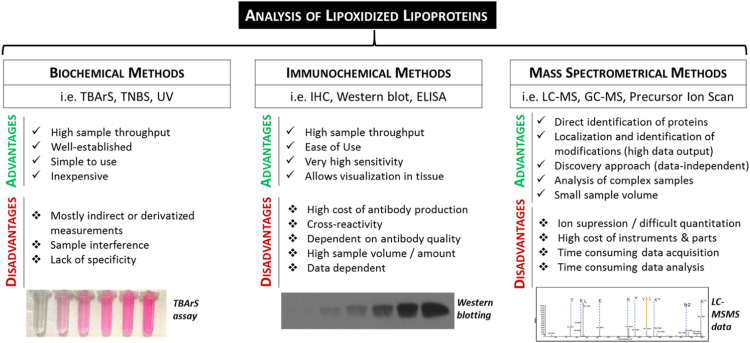
All the studies included in this review used a form of the gradient ultracentrifugation method to separate lipoproteins, followed by the analysis of oxidized lipoproteins by one or more of the methods reviewed in this section. The analytical methods can be broadly divided into (1) biochemical methods, involving colorimetric or fluorescence reagents and labeling with or without additional separation by liquid chromatography; (2) antibody-based methods, including enzyme-linked immunosorbent assay (ELISA) and immunostaining; and (3) mass spectrometric approaches, which usually require the combination of liquid chromatography with tandem mass spectrometry. Each of these approaches have advantages and disadvantages in terms of the complexity of the methodology and the quality or depth of information generated, two aspects that are usually directly related (Fig. 3). Much early work used simple biochemical assays until the generation of antibodies specific for lipoxidation products allowed the application of immunoassays. Mass spectrometry techniques require complex and expensive instruments as well as extensive data handling, and consequently are less widely accessible, although very valuable as they yield site-specific information that cannot be obtained by the other approaches. Ultimately, all the techniques provide complementary information, and are most powerful if used in combination.
Fig. 3.
The most used techniques for the analysis of lipozidized lipoproteins, and their advantages and disadvantages. TBArs, thiobarbituric acid reactive substance assay; TNBS, 2,4,6-trinitrobenzene sulfonic acid; UV, Ultraviolet; IHC, Immunohistochemistry; ELISA, enzyme-linked immunosorbent assay; LC-MS, liquid chromatography-mass spectrometry; GC-MS, Gas chromatography-mass spectrometry.
3.1. Biochemical methods with chromophore or fluorophore labeling
In this section, techniques involving the use of reagents that generate a chromophore or fluorescent signal on reaction with the lipoxidized protein or lipoxidation agent are considered. However, it is important to bear in mind that not all methods are specific for lipoxidation: in fact, the most used reagents are ones that react with aldehydes, which could also be formed by direct oxidation of amino acid residues. Moreover, some of the methods used were indirect, for example, deducing the amount of modification by changes in amount of free rLPP or unmodified residues.
For example, in one of the earliest studies of lipoprotein lipoxidation [27], involving the direct reaction of HNE with LDL, the formation of HNE-LDL adducts was determined indirectly by comparison of the amount of unreacted HNE in the supernatant of treated and untreated lipoproteins, which was quantified by high-performance liquid chromatography on an octadecyl silica (ODS) column with detection at 220 nm; the HNE incorporation was found to increase with increased aldehyde concentration, with 78% bound to the protein moiety, 21% to the lipids, and 1% as free aldehyde within the lipid fraction. To investigate more specifically the residues modified, the apolipoprotein B-100 (ApoB-100) was delipidated, reacted with dinitrofluorobenzene to dinitrophenylate and stabilize free hydroxyl and amine groups before total hydrolysis and quantitation using a standard amino acid analyser. This approach was adopted as it was noted that the adducts were not stable during the acid hydrolysis. Comparison of the levels of dinitrophenylated amino acids (i.e. those not modified by HNE) suggested that HNE attacked mostly lysine and tyrosine, and to a lesser extent, cysteine, serine and histidine [27]. The observation that tyrosine and serine could be modified is interesting as these residues are not widely considered as targets of hydroxyalkenals, but could simply have been an artefact of the experimental design with dinitrofluorobenzene. An alternative reagent that is more specific for detection of free amine groups, i.e. lysines, arginines and the N-terminal of proteins, is 2,4,6-trinitrobenzene sulfonic acid (TNBS). This has been used in several studies to monitor indirectly protein modification by lipid peroxidation products [34], [35], [36], [37], [38], [39], [40].
Amino acid analysis was also used by Uchida et al. to investigate the sites of HNE modification in Cu2+-treated LDL, but in this case the adducts were stabilized by treatment with sodium borohydride (NaBH4) to prevent the issues noted by Jürgens et al. [27], and the amino acids were labeled with o-phthaldehyde for fluorescence detection during HPLC [41]. This enabled the quantification of approximately 40 lysines and 50 histidines (mol/mol LDL) modified in HNE-treated LDL, of which 49% and 80% respectively were identified as Michael adducts, with the remainder as Schiff's base adducts. However, this study also relied on complementary findings from the use of specific antibodies, as described in the next section.
A commonly used reagent for detecting the presence of protein carbonyls is 2,4-dinitrophenylhydrazine (DNPH) [42], [43], [44]. It reacts readily with protein-bound aldehydes or ketones to form a hydrazone derivative with a characteristic absorbance at 365 nm, and despite its mutagenic properties and explosive nature (mostly now only available as a 0.2 M solution), continues to be widely used in studies of protein oxidation. However, its application to oxidation of lipoproteins has been limited. The formation of protein carbonyls in HOCl-treated LDL was investigated by Yang et al. using DNPH labeling followed by delipidation and tryptic cleavage of the ApoB-100; modified peptides were identified on HPLC by the absorbance at 365 nm, isolated and sequenced using a gas phase automated sequencer [45]. The modified peptides contained cysteine, lysine, tryptophan and methionine residues; it was not clear from this study whether the modifications were due to direct oxidation of the residues by HOCl, or formation of rLPPs that reacted with the cysteine to give Michael adducts. In the use of DNPH as a labeling reagent for lipoxidation, it is also important to bear in mind that Schiff's base adducts of hydroxyalkenals do not contain a free aldehyde to react with DNPH; instead, DNPH may react via Michael addition with them to generate 2,4-dinitrophenyl pyrazolines, which have a slightly shifted absorbance maximum compared to hydrazones. Moreover, when using DNPH to study lipoxidation in Cu-oxidized LDL it has been observed from the judicious use of controls in the absence of DNPH that the tryptophan oxidation products N-formyl kynurenine and kynurenine have significant absorbance at 365 nm, which can confound the detection of protein carbonyls, so careful profiling of the labeled peptides is necessary [46].
Alternatively, the thiobarbituric acid reactive substances (TBArS) assay can be used to measure malondialdehyde (MDA) equivalents present in the sample, including MDA adducts on the protein, which are reversible under the conditions used for the assay. The reaction between thiobarbituric acid and MDA yields a pink chromophore with an absorbance maximum at 535 nm. In its simplest form, the TBArS assay has been widely criticized as a measure of lipid peroxidation in complex samples (reviewed by Halliwell and Chirico [47] and Spickett et al. [48]), but under stringent conditions, especially when combined with HPLC separation, it can be used to monitor LDL oxidation [38], [40], [49], [50], [51], [52]. Hoff et al. used the TBArS assay combined with TBNS analysis of free amines to show that, similarly to the HNE-LDL modification reported previously [27], MDA modification of ApoB-100 increased with increased MDA treatment concentration, and appeared to be correlated with the decrease of available amines [53]. This is in accordance with previous reports of decreases in lysine availability upon treatment of LDL with MDA [36].
While these colorimetric assays may now seem somewhat simplistic, they were nevertheless crucial in building evidence on the occurrence of covalent modifications of LDL by short chain non-esterified aldehydes. In contrast, a rather different approach was adopted by Karakatsani et al. to investigate lipoxidation by phospholipid-esterified rLPPs [54]. They used both a phosphorus assay and 31P nuclear magnetic resonance (NMR) to measure the phosphorus in the delipidated ApoB-100 fraction of Cu-oxidized LDL. They identified an NMR peak that appeared to correspond to the phospholipids bound to the protein by hydrophobic bonds, which disappeared upon treatment with phospholipase A2 (PLA2), showing that even in the delipidated protein there is still a fraction of bound phospholipid. Furthermore, they identified a second peak, not hydrolyzed by PLA2, which appeared to correspond to the oxidized phosphatidylcholine fraction that formed covalent adducts with the proteins. This finding was not corroborated until several years later by complementary techniques involving antibodies and LC-MSMS analysis, described in later sections.
3.2. Antibody-based detection of apolipoprotein lipoxidation
Antibodies are an extremely useful tool for biomolecular and biochemical techniques used throughout the biosciences and biomedical sector. From detection methods to therapeutics, they are utilized in a variety of fields owing to their ease of use and, at least for polyclonal antibodies, relatively quick and inexpensive production. These characteristics also make them very attractive for commercial development. The generation of antibodies against specific aldehydes, usually in the form of protein adducts, has allowed the identification of lipoxidized lipoproteins by an array of immunotechniques, and without doubt has underpinned many key advances in this field [10], [16]. Several groups have worked on the development of such antibodies, characterized them, and used them to investigate possible biological effects of oxidized lipoproteins, and their role in certain pathological conditions, mainly atherosclerosis.
3.2.1. Development and application of anti-MDA Antibodies
The first application of antibodies against rLPP-modified lipoproteins were using either previously existing antibodies against MDA-lysine adducts [55], or polyclonal antibodies raised against MDA-treated LDL [56]. The MDAlys monoclonal IgG2A was selected for binding ability to MDA-modified LDL but not to native human LDL, and was able to detect MDA-modified proteins by immunocytochemistry and their colocalization with ApoB-100 in atheromas from rat [55]. In contrast, an ELISA was developed using the polyclonal anti-MDA-LDL that appeared to bind to MDA-ApoB adducts in MDA-treated LDL, while not recognizing native LDL, acetyl-LDL or MDA-HDL, suggesting that the antibody was both modification- and protein-specific [56]. Both studies were important for the establishment of this approach, which has subsequently been used extensively for lipoproteins. Anti-MDA monoclonal and polyclonal (e.g. MAL-2) antibodies produced using either rabbits or guinea pigs were later used to identify the presence of adducts of this aldehyde bound to ApoB100 in copper-oxidized LDL, using techniques such as western blotting using the monoclonal antibodies MDA2 and OLF4-3C10 [36], [57] and a solid-phase fluorescence assay [58].
3.2.2. Development and application of anti-HNE Antibodies
In view of the identification of HNE as a major cytotoxic lipid peroxidation product, it is not surprising that much effort was invested in producing anti-HNE antibodies, with both polyclonal sera and monoclonal antibodies being produced against HNE-treated LDL, or HNE bound to other proteins or peptides. In parallel with their work on MDA-LDL antibodies, Palinski et al. produced an antiserum (HNE-6) and a monoclonal antibody (NA59) against HNE-LDL, which they reported were specific for HNE-lysine adducts [57]. The monoclonal and polyclonal antibodies were used to identify the presence of HNE-LDL adducts in copper-oxidized LDL and atherosclerotic lesions, and did not recognize unmodified LDL [36]. Polyvalent antisera to HNE-modified LDL were also raised in rabbits by Esterbauer's group [45], [59]. It was found that the antiserum recognized LDL treated with either HNE or CuSO4, but not MDA, hexanal, 2,4-heptadienal or 4-hydroxyhexenal, while there was a small cross-reactivity with 4-hydroxyoctenal. This demonstrated the specificity of the polyvalent serum to the nine carbon hydroxyalkenal, but interestingly it was also shown that copper-oxidized Lp(a) and VLDL both cross-reacted with the antiserum, indicating that the specificity was due to the adduct and not the lipoprotein. The work also provided confirmation that oxidation of these lipoproteins resulted in HNE formation and lipoxidation [51]. Later the group prepared a further polyclonal antiserum to HNE-LDL and reported weaker binding to oxidized and HNE-treated HDL3 or bovine serum albumin, but noted some cross-reactivity to hexanal- or 2,4-heptadienal-modified LDL [59], illustrating the variability inherent in polyclonal preparations. They hypothesized that MDA might react differently to HNE and other aldehydes due to its chemical structure, being a bivalent aldehyde. Based on competition studies using HNE-treated poly(L-amino acids) lysine, tyrosine, arginine and histidine, it was concluded that these residues were possible sites of modification; no binding to the corresponding untreated poly(L-amino acids) was observed [59]. Other researchers also prepared and tested antisera against HNE-LDL and MDA-LDL from rabbit by fluorescence sandwich immunoassays or solid-phase radioassays, and used them to investigate the binding of lipoxidized LDL to extracellular matrix components [58].
Other studies also investigated the sites of HNE-lipoxidation in other modified proteins and the epitopes responsible for antibody recognition. The group of Uchida raised monoclonal antibodies against HNE-keyhole limpet hemocyanin (KLH) and demonstrated using ELISAs that they were able to recognize HNE adducts in HNE-LDL, copper-LDL and endothelial cell-treated LDL in a concentration dependent manner [41]. Further experiments showed that the antibodies only recognized the HNE moiety of the adduct, as their binding to the modified LDL was blocked when incubated in the presence of HNE-modified amino-acids (HNE-N-acetyl-lysine, HNE-N-acetyl-histidine) or HNE-glutathione. This agreed with the results of Chen et al. [59] and suggested that for these antibodies the binding was not protein-specific, or indeed particularly residue-specific [41]. However, shortly afterwards the development of monoclonal antibodies with specificity against HNE-histidine adducts and very limited recognition of HNE-lysine and -cysteine adducts was reported [60]. In this study, the antibodies were raised against HNE-modified bovine serum albumin, but were able to recognize HNE-protein adducts in peroxidized liver microsomes as well as in oxidized LDL. There was no detectable cross reaction with adducts of malondialdehyde, nonanal or 4-hydroxyhexenal, although eight and ten carbon hydroxyalkenals showed some reaction. While not specific for lipoxidized lipoproteins, it can also be considered an advantage of these antibodies that they can be used for the identification of any HNE-lipoxidized proteins.
3.2.3. Development and application of antibodies against other alkenals and hydroxyalkenals
While the early focus was very much on developing antibodies against MDA and HNE, subsequently the interest expanded to include other aldehydes known to be formed upon phospholipid peroxidation. The carcinogenic, three carbon alkenal acrolein can be formed from lipid peroxidation but also occurs in tobacco smoke and as an environmental pollutant, and is therefore of considerable interest. A monoclonal antibody (mAb5F6) that reacts selectively with acrolein-lysine adducts was raised in mice using KLH treated with acrolein as an immunogen [61], and then utilized to demonstrate lipoxidation of the N-terminal region of Apolipoprotein E-III (ApoE-III) (normally present in HDL, VLDL and IDL) by this aldehyde with corresponding impairment of ApoEIII-heparin binding [62]. 4-hydroxyhexanal (HHE) is the 6-carbon analogue of HNE and is also a significant product of lipid peroxidation; antibodies raised against a protein modified with this aldehyde were used to identify the presence of these adducts in copper oxidized LDL [50]. Specifically, the antibody clone used (HHE53) had been shown to be inhibited in the presence of HHE-N-acetyl-histidine, but not other amino acids, suggesting that HHE bound to the histidine residues of ApoB-100 in copper oxLDL [50]. The same approach was used to raise an antibody against 4-hydroperoxynonenal (HPNE) adducts (clone PM9), and it was found that 4-hydroperoxy group leads to formation of structurally unusual lysine adducts that were specifically recognized by the antibody aldehydes [63]. It was used to demonstrate the occurrence of HPNE lipoxidation in copper-oxidized LDL, which was verified by western blot analysis, as well as in lesions in samples of the aortic wall of patients with generalized arteriosclerosis, demonstrated by immunohistochemistry [63]. More recently, monoclonal antibodies were developed against protein adducts of the twelve carbon compound 4-hydroxy-2E,6Z-dodecadienal (HDDE); these were used to identify adducts in human aortic section, but as yet have not been demonstrated to bind to lipoproteins or used in specific studies of their oxidation [64].
3.2.4. Development and application of antibodies against oxidized lipoproteins
Besides the development of antibodies against specific aldehydes described above, several groups also developed antibodies against copper-oxidized LDL [36], [57], [58], [65]. Further characterization of two antibodies developed (OB/04 and OB/09) uncovered their reaction not only with copper-oxidized LDL, but also with azo-initiator oxidized LDL and copper-oxidized VLDL, while not reacting with the oxidized HDL3 or any of the native forms of the lipoproteins [65]. This suggested that the epitopes of these antibodies were generated against modified portions of apolipoproteins present in LDL and VLDL, but not in HDL3.
An alternative approach that has proved very valuable is the isolation of autoantibodies produced during a diseased state; for example, autoantibodies present in mice with aortic atherosclerosis caused by a high fat diet, designated E0 antibodies, were found to bind a variety of modified forms of LDL, including LDL modified by copper, acrolein, MDA and HNE [66], [67], [68]. One of the autoantibodies, E06, was subsequently extensively studied, and was found to bind to the phosphocholine head group of oxidized phosphatidylcholines [35], thus binding to both oxidized phospholipids and oxidized phospholipid-protein adducts. As it is not ApoB-specific, it is also able to recognize copper-oxidized HDL, demonstrating the existence of phospholipid-esterified aldehyde adducts in lipoproteins [66]. Antibody E06 [68] is now commercially available and has been used for detection of oxLDL in clinical samples, as discussed in Section 5. It is worth noting that in early publications this antibody was referred to as EO6 [68], but now the name E06 is more commonly used, including by the commercial supplier Avanti Polar Lipids.
3.2.5. Considerations in the utilization of antibodies against lipoxidized proteins
It is important to bear in mind that there has been considerable variability in the methods of production of the antibodies discussed above, although much of the testing was carried out against human lipoproteins or tissue samples. As a consequence, there are substantial difference in specificity and sensitivity between the antibodies that must be borne in mind when interpreting the results, and which explain the different cross-reactivities reported.
Nevertheless, antibodies show great potential as a sensitive technique for the identification of lipoxidation in lipoproteins and have facilitated the development of methods for detecting and identifying lipoxidation, both in plasma and tissue samples. This has supported our understanding of the biological effects of these modifications [62], which is explored further in Section 4, as well as helping to evaluate the potential of therapeutic techniques [69], discussed in Section 5. A key advantage of immunoassays is their simplicity and accessibility, with most biological laboratories well set-up for ELISAs, western blotting and immunocyto/histochemistry. The potential for quantitative analysis is also important, although dependent on well-characterized standards. On the other hand, these methods provide limited information on the site of modifications within the lipoproteins. Where the antibody is specific for a type of adduct, the residues modified can be inferred, and has for example been used to calculate that oxidized LDL contains 3 nmol HNE-histidine per mg protein [60]. However, identification of the precise residues that are modified requires the application of advanced protein analysis by LC-MSMS, as explained in the next section.
3.3. Advances in mass spectrometry approaches for analysis of lipoxidation
3.3.1. LC-ESI-MS(MS) analysis of amino acid lipoxidation (non-site-specific)
With the development of LC-ESI-MS, later followed by tandem mass spectrometry and peptide sequencing, their application for the identification of modified lipoproteins was inevitable. One of the first reported uses of mass spectrometry for the direct measurement of aldehyde adducts identified MDA and HNE adducts to the lysine residues of copper oxidized LDL, by protein hydrolysis and GC-MS [38]. The study reports not only the identification of adducts of MDA (3-(Nε-lysino)propan-1-ol) and HNE (3-(Nε-lysino)-4-hydroxynonan-1-ol), but also a lysine-MDA-lysine iminopropene crosslink (1,3-di(Nε-lysino)propane). While lysine-MDA adducts were found even in LDL from healthy subjects, their increase and the formation of lysine-HNE adducts during copper oxidation of LDL was found to account for the modification of less than 1% of the lysine amino acids present in LDL [38]. This emphasizes the challenges of studying such modifications [70].
GC-MS has been used in the identification of several fatty acids adducted to copper oxidized LDL [49]. Acid hydrolysis was performed on delipidated apolipoproteins, and the fatty acids released were analysed by selected ion monitoring-GS-MS (SIM-GC-MC), together with a phosphorus assay. The carboxylic acids found to be increased in oxidized LDL included pentanedioic (glutaric) acid (PDA), nonanedioic (azelaic) acid (NDA), hexanoate, palmitate, stearate and oleate; the latter three showed a 10–20-fold increase. These results were interpreted as the formation of adducts between proteins and phospholipids, which had also been suggested previously by antibody studies [49], although it is known that total delipidation of lipoproteins is very difficult and non-covalently bound lipids often remain [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71]. LC-MS of hydrolyzed samples was also used to demonstrate that pyridoxamine could the inhibit the formation of several advanced lipoxidation end products, specifically Nε-(carboxymethyl)lysine (CML), Nε-(carboxyethyl)lysine (CEL), malondialdehyde-lysine, and 4-hydroxynonenal-lysine, in the copper-catalysed oxidation of LDL[72].
Uchida's group in Japan have worked intensively in this area using the approach of total protein digestion by 6 N HCl followed by LC-MSMS analysis of modified amino acids. Lipoxidation adducts of trans-2-nonenal [73], 4-oxo-2(E)-nonenal [74], and acrolein [75] were detected in LDL oxidized in vitro. More recently, they have developed a methodology to investigate the lipid peroxidation “adductome” of LDL [76], based on the fact that different lipoxidation adducts of, for example, lysine have different retention times in the LC run and different mass-to-charge ratios. In LC-MSMS, a precursor ion scan for diagnostic fragments of the amino acid moiety was used to identify the precursors, i.e. the modified amino acids. The effectiveness of the method was demonstrated with Cu(II)-oxidized LDL and allowed the quantification of known adducts such as those of 4-hydroxynonenal and acrolein, as well as identifying new adducts such as 9-oxononanoic acid-lysine, which was detected in the reduced form as Nε-(8-carboxyoctanyl)-lysine (COL), and thought to originate from either 9-oxo-7-nonenoic or 9-oxononanoic acid. The methodology was further applied to identify increases in COL in LDL from hyperlipidemic rats, as well as from hyperlipidemic subjects [76].
While clearly providing very useful information on the types and amounts of modifications present in the lipoproteins, analysis of hydrolyzed residues cannot locate the individual residues modified within the apoprotein, information that is important for understanding the various biological effects of lipoxidation. Localization of modified residues within a protein requires the sequencing of peptides in a proteomics approach using tandem MS analysis, as described below.
3.3.2. LC-MSMS proteomic approach for localization of lipoxidized residues within peptides (site-specific)
More recently, tandem mass spectrometric methods have become more common, such as MALDI-MS/MS and LC-ESI-MS/MS. The basic principles of the methods applied to the analysis of oxidized and lipoxidized proteins have been explained in previous reviews [77] and will not be covered here. Most work has been carried out by the “bottom-up” approach, which involves enzymatic digestion of proteins to peptides, which can be separated by LC, and then fragmented within the mass spectrometer to enable sequencing. This approach is not trivial for the localization of any oxidative modification [77], and analysis of lipoxidation is even more challenging [70]. For lipoxidation adducts, which are not particularly stable (Schiff's base adducts are readily reversible and even Michael adducts can degrade), it is important to “fix” the adducts by a reduction step before commencing the proteolysis steps. A smaller number of studies have used a “top-down” approach, which analyses the intact protein complete with modifications, and then partially fragments it to provide further information. Some of these techniques have also been discussed by Colzani et al. in a review on mass spectrometric approaches for the analysis of protein adducts with reactive carbonyl species, which provides a detailed consideration of the approaches for the analysis of more complex biological samples, including lipoproteins, and the use of derivatization with DNPH for the MS identification of acrolein, HNE and MDA adducts with proteins [24]. The derivatization approaches can also be carried out with alternative labeling agents such as the hydroxylamine-functionalized biotin-containing probe, aldehyde reactive probe (ARP), and have been tested by several groups in general redox proteomic studies [16], [70], [78], but have not been applied to studies of lipoproteins.
3.3.3. Application of proteomic approaches to identify sites of LDL lipoxidation by small rLPPs
One of the earliest studies to attempt to identify specific sites of lipoxidation in ApoB-100 used a targeted bottom up approach to look for HNE adducts of histidine in copper-oxidized LDL [79]. Following treatment, the LDL was extracted and the protein digested with trypsin in solution, before being studied using both ESI-MS to identify the mass of the peptides and precursor ion scanning (PIS) of a fragment ion at m/z 268, which corresponds to the reduced HNE-modified histidine immonium ion and allows peptides containing this modification to be found [80]. The group attributed most of the parent ion peaks resulting from the PIS to theoretical HNE-modified ApoB-100 peptides, tentatively localizing the modifications to six histidine containing peptides [79]. Later they used this approach to study the effect of HDL on LDL oxidation. By carrying out a quantitative analysis of specific histidines, they were able to demonstrate that the presence of human HDL, which contains the anti-oxidative enzyme paraoxonase, abrogated the lipoxidation of LDL histidines, whereas avian HDL, which lacks paraoxonase, did not [80]. Modifications in ApoB-100 induced by copper oxidation of LDL were also investigated using an untargeted LC-MSMS approach by Obama et al., and a variety of amino acid oxidations were observed (mono-oxidations of histidine and tryptophan and kynurenine), as well as HNE-histidine and Nε-(3-methylpyridinium)-lysine lipoxidations resulting from acrolein modification [81]. A key novelty in this work was the testing of both in-gel and on-membrane (polyvinylidene difluoride; PVDF) trypsin digestion, which enabled much smaller amounts of lipoprotein to be used [81], compared to the previous work by Bolgar et al. where milligram amounts were used [79].
3.3.4. Application of proteomic approaches to identification of sites of HDL modification by small rLPPs
LDL is a particularly challenging lipoprotein to work with for MS-based proteomic analysis, as ApoB-100 is a very large protein consisting of 4536 amino acid residues with a molecular weight of over 500 kDa. In contrast, the proteins from HDL present a more amenable target: approximate molecular weight for ApoA-I is 28 kDa, ApoA-II is 18 kDa, and ApoC forms are 8–9 kDa. Consequently, in parallel other groups have studied the oxidative modifications of HDL, with reasonable success. Heinecke's group carried out several investigations of apolipoprotein A-I (ApoA-I) using untargeted LC-MSMS methods, and succeeded in obtaining 80% sequence coverage, enabling a fairly thorough assessment of the modifications. While their focus was mainly on the chlorination and nitration of tyrosine residues by myeloperoxidase [82], they also investigated the reaction of acrolein with ApoA-I [83]. Using the endoproteinase GluC to digest the protein, they obtained 90% sequence coverage and were able to identify eight specific lysine residues modified to Nε-(3-methylpyridinium)lysine (MP-lysine); Nε-(3-formyl-3,4-dehydropiperidino)lysine (FDP-lysine) did not appear to be formed and no cysteine modifications were detected because ApoA-I lacks this amino acid. Interestingly, using the monoclonal antibody mAb5F6 mentioned in Section 3.2.3, they were able to show that ApoA-I colocalized with acrolein-lysine adducts in human atherosclerotic lesions. The modification of both ApoA-I and ApoA-II by acrolein has been investigated by Chadwick et al. to determine its role in HDL cross-linking and impairment of the reverse cholesterol transport pathway in peripheral tissues [84]. This demonstrated the occurrence of mass shifts corresponding to the addition of acrolein molecules by both Michael addition and Schiff base on intact ApoA-I and ApoA-II proteins, and related it to the formation of crosslinks seen in western blots, but as only intact protein analysis was carried out, the sites of adduct formation were not identified.
Lipoxidation of the HDL protein component ApoC-II has also been studied, using a more unusual rLLP derived from the ozonolysis of cholesterol, 3β-hydroxy-5-oxo-5,6-secocholestan-6-al (3-HOSCA) [85]. There is evidence that co-localization of ApoC-II and serum amyloid P in atherosclerotic plaques can lead to the formation of fibrils, and previous data from studies in vitro demonstrated that treatment of ApoC-II with 3-HOSCA accelerates fibril formation. To investigate the formation of covalent adducts, ApoC-II was incubated with 3-HOSCA, digested with GluC, separated by HPLC and then analysed by MALDI-TOF-MS to identify modified peptides; however, as the peptides were large (~40 residues), the likely site of modification within them could only be inferred. Nevertheless, the study demonstrated that ApoC-II could be randomly modified at six different lysine residues, typically resulting in one 3-HOSCA attached per ApoC-II molecule, and it was concluded that the presence of this adduct in HDL leads to the formation of fibrils by both covalent Schiff base formation, and other non-covalent mechanisms [85].
Acrolein adducts have also been identified and located in rat ApoE, which is a 294 amino acid protein with a molecular weight of 34 kDa, and about 74% sequence homology with human ApoEIII. The protein was digested with endopeptidases AspN and GluC to improve sequence coverage, before analysis by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry (MALDI TOF/TOF MS). Using this untargeted approach, the researchers were able to find acrolein modifications yielding an aldimine adduct at K149 and K155; a propanal adduct at K135 and K138; MP-lysine at K64, K67, and K254, and an FDP-lysine derivative at position K68. These lipoxidations were concluded to contribute to impairment of binding to the LDL receptor and heparin, and may also be responsible for protein unfolding [86].
3.3.5. Application of proteomic approaches to localization of adducts formed by phospholipid-esterified rLPPs
The research described above has focused on adducts formed by small, non-esterified alkenals, but it is well-established that phospholipid-esterified alkanals or alkenals are also major products of lipid oxidation, and antibody-based studies described in Section 3.2 suggested that they can also form adducts with proteins. To confirm their formation and identify the sites of modification, studies of LDL oxidation were carried out by the group of Spickett [71]. LDL was treated with the esterified nine-carbon alkanal 1-palmitoyl-2-(9-oxo-nonanoyl)-sn-glycero-3-phosphocholine (PONPC), delipidated and in gel-trypsin digestion carried out, followed by LC-MS/MS; approximately 70% sequence coverage of ApoB-100 was obtained, which was good considering the size of the protein. In this work a novel targeted mass spectrometry approach was developed, involving the use of narrow-window extracted ion chromatograms (XICs) to pinpoint the presence of characteristic fragments of certain modifications. These included the m/z 184 ion, an intense fragment commonly formed during fragmentation of phosphatidylcholines, corresponding to the head group. They were able to identify two different peptides modified with PONPC and one modified with the analogous 5-carbon alkanal, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (POVPC). This was the first published report to identify directly and localize lipoxidation adducts of chain-shortened phospholipids.
There is also substantial evidence that adducts of oxidized PC are present on Lp(a), a lipoprotein formed by covalent linking of ApoA to ApoB-100 [87]. The adducts are formed as Schiff bases to two lysine residues within Kringle IV or Kringle V domains of the ApoA. Recombinant Apo(a)s were used to investigate the lysine binding site (LBS) responsible for adduct formation, using both E06 antibody binding and analysis of tryptic peptides by LC-MSMS. Precursor ion scanning for m/z 184 was again employed to show the presence of a number of prominent peptide peaks containing PC, and analysis of the lipid phase of the lipoprotein demonstrated the presence of the short chain aldehyde POVPC, which is E06-detectable [88].
Shortly afterwards, an alternative method for identifying oxidized phospholipid adducts was developed and applied to using the modification of ApoA in HDL [89]. A key advantage in this study was the use of an enrichment process consisting of two separations steps. The first one separated the phospholipid-modified peptide, which is highly hydrophobic, from less hydrophobic molecules using a C18 column. Subsequently aminolysis was carried out; this is a specific reaction that breaks the bond between the phosphoglycerol backbone and the fatty acyl chain at the sn-2 position, thus separating the PC head group and intact long hydrophobic chain from the modified peptide, making the latter more hydrophilic and allowing it to be eluted from the column. Using this method, the group was able to identify a plethora of peptides of ApoA-I, II and III from copper-oxidized HDL [89] and myeloperoxidase-oxidized HDL [90], demonstrating the modification with several different oxidized phospholipids.
It can be seen that mass spectrometry analysis of lipoxidation is a challenging but evolving field: the rate at which papers are published in this area is increasing, the data obtained on the modifications is getting more detailed, and the development of the technology is allowing the analysis of more complex samples. The different approaches: amino acid analysis versus peptide sequencing are quite complementary in the information produced; the former is very good for quantitative studies, whereas the latter is able to identify specific residues that are modified, and therefore enables the mechanisms of function or dysfunction to be elucidated. From the peptide sequencing methods, a substantial body of evidence on the sites of modification of several apolipoproteins has now been obtained and is illustrated in Fig. 4. A key challenge is now to understand the functional effects of these modifications.
Fig. 4.
Scheme of the amino acid location of several types of modifications found in different apolipoproteins. The abbreviated modifications are shown in bold and given in full as follows: CHa,Cyclic hemiacetal adduct; FDP-Lys, Ne-(3-formyl-3, 4-dehydropiperidino)lysine (formed by MA-MA); HF, 2,3-dihydrofuran adduct; HNE, 4-Hydroxy-2-nonenal; HODA-PL, 9-hydroxy-12-oxo-10-dodecenoic acidb; HOHA-PLb, 4-hydroxy-7-oxo-5-heptenoic acid; HOOA-PLb, 5-hydroxy-8-oxo-6-octenoic acid; 3-HOSCA, 3β-hydroxy-5-oxo-5,6-secocholestan-6-al; KAa, Ketoamide adduct; KODA-PLb, 9-keto-12-oxo-10-dodecenoic acid; KOOA-PLb, 5-keto-8-oxo- 6-octenoic acid; KOHA-PLb, 4-keto-7-oxo-5-heptenoic acid; MA, Michael Addition; MP-Lys, Ne-(3-methylpyr-idinium)lysine (formed by MA-SB); OV-PLb, 5-oxovaleric acid; ON-PLb, 9-oxononanoic acid; POVPC, 1-palmitoyl-2-(5'-oxo-valeroyl)-sn-glycero-3-phosphocholine; PONPC, 1-palmitoyl-2-(9-oxo-nonanoyl)-sn-glycero-3-phosphocholine; SB, Schiff's base adduct. a CH and KA forms are indistinguishable by LC-MS. b These adducts were detected by aminolysis and assumed to be derived from esters of lysophospholipids. This figure was prepared using data obtained from references [71], [80], [81], [85], [89], [90].
4. Biological effects of lipoxidized lipoproteins
Since the discovery of the role of oxidized LDL in pathologies like atherosclerosis, some effort has been put into understanding the biological effects this lipoprotein can have, especially in pathology. Many reviews have been published on the subject, from its role in atherosclerosis [13], [91], [92], [93], apoptosis [94] and inflammation [95], its growth and proliferation promoting effects [96], its involvement on endothelial dysfunction and ageing [97], among others. Although for oxidized HDL not as much research has been done, some of its effects have also been uncovered, and it is clear that the beneficial effects of this lipoprotein can be lost following covalent modification. Oxidation of this lipoprotein induces the loss of its protective effect against oxidized LDL [98], and that in turn induces further oxidative stress and cytotoxicity (although to a lower extent than oxLDL) [99]. The effect of oxHDL has also been shown on the dysfunction of endothelial progenitor cells (EPCs) and human renal proximal tube epithelial cells (HK-2), through activation of CD-36 receptors and mitogen-activated protein kinase (MAPK) pathways [100], [101], as well as on the induction of adipogenesis in females with high body mass index (BMI) [102].
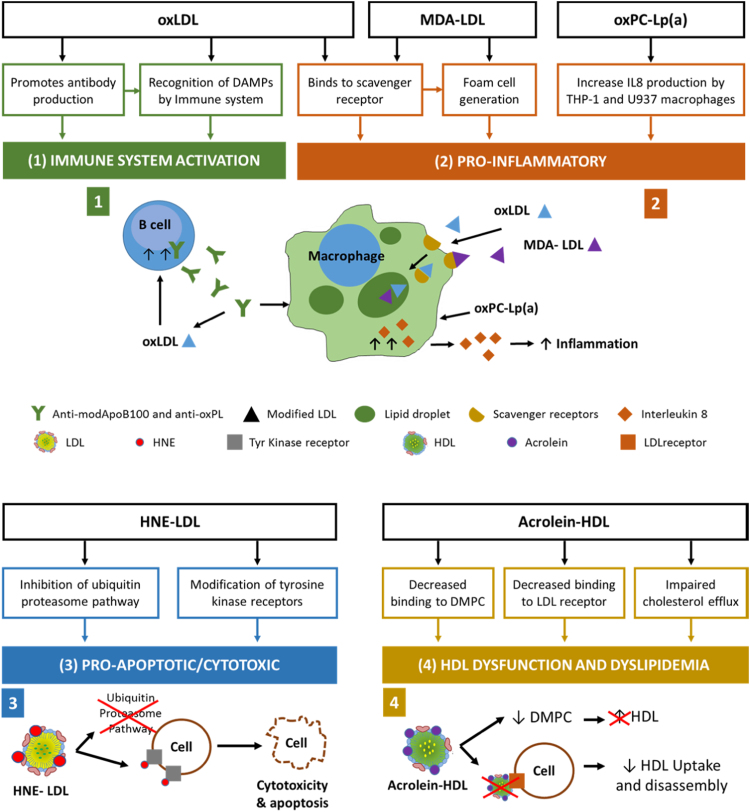
While a lot has been done to uncover the biological effects of oxidized lipoproteins, relatively little of this work is on the effect of lipoxidized lipoproteins specifically. Fig. 5 summarizes the main biological effects that have been reported to date. Much of the work has focused on MDA- and HNE-modified LDL. As early as 1980, MDA-treated LDL was used as a model system and was shown to be taken up by macrophages, but the mechanisms were poorly understood; it was hypothesized that the malondialdehyde modified LDL particles shifted their binding from the native-LDL receptor to a scavenger receptor, thus promoting their uptake [103]. While characterizing the E06 antibody, known to bind to oxidized phospholipids and oxLDL, it was shown that this antibody blocked the uptake of oxLDL by the macrophages, and that the decrease of the phospholipid moiety bound to ApoB-100, decreased the affinity of oxLDL to macrophages, and the reactivity with the antibody [40]. Further studies found that several scavenger receptors bind oxLDL, namely SRA-1, SRA-2, SRA-3, MARCO, CD36, SR-B1, CD68, and LOX-1 [104], [105]. Two in particular, CD36 and SR-B1, were shown to interact with both the protein and the lipid present in oxLDL separately, and their binding was also inhibited by the E06 antibody and the oxidized phospholipid POVPC, suggesting it was the oxidized moiety present in the lipid fraction or bound to the protein fraction that mediated the high affinity binding of oxLDL to the receptor CD36 [106], [107].
Fig. 5.
Physiological effects of lipoxidized lipoproteins, with consequences for the immune system, inflammation, apoptosis and HDL function. (1) Oxidized LDL stimulates the production of antibodies against itself and oxidized phospholipids, that can be recognized by the immune system; (2) oxLDL and MDA-LDL bind to scavenger receptors, leading to their internalization and formation of foam cells; oxPC-Lp(a) promotes the expression of IL-8 by macrophages, increasing inflammation; (3) HNE-LDL leads to apoptosis by both inhibition the ubiquitin proteasome pathway and by modification of tyrosine kinase receptors, leading to their inhibition; (4) Acrolein-HDL inhibits the increase of HDL caused by 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), and HDL uptake and disassembly due to the decreased binding to the LDL receptor.
Other studies showed that in oxidized LDL and MDA-treated LDL with similar degrees of modification, oxLDL showed higher binding to macrophages and degradation rate when compared to MDA-LDL [108]. It has been reported that MDA-treated LDL induced THP-1 cell growth, which could be suppressed by polycyclic aromatic hydrocarbons (PAHs) in an arylhydrocarbon receptor (AhR)-dependent manner; it was suggested that this resulted from the link between AhR and p21 leading to cell cycle arrest [109]. An association between the levels of circulating MDA-LDL and vascular inflammation has been reported, using 18F-FDG PET/CT imaging [110], and it was suggested that part of this inflammation might be due to activation of the complement system, by the binding of MDA and malondialdehyde acetaldehyde (MAA) adducts in treated LDL to the complement anaphylatoxin C3a [111]. HNE-modified LDL is also thought to have altered functionality; it has been reported that HNE-containing oxLDL can induce cell proliferation at low levels in smooth muscle cells, while at higher levels it caused apoptosis in various cell types [112], [113]. This oxLDL-induced apoptosis might be linked to the derivatization of proteins such as tyrosine kinase receptors by HNE [112], [113] and possibly other oxidized lipids, and to the inhibition of the ubiquitin-proteasome pathway [114].
Oxidation of LDL has also been shown to stimulate the production of antibodies against oxidized PC species. Structural and functional similarities between the E06 antibody and T15, an anti-oxPC secreted by B-1 cells and involved in the immune response provoked by bacterial infection with S. pneumoniae, have been uncovered [115]. They suggested that oxidation of PC species, and/or possibly their addition to protein residues, alters their conformation, exposing the PC headgroup and making it accessible to the antigen binding site of E06/T15 antibodies. The production of such antibodies has an important biological effect, since they have the ability to inhibit the uptake of oxLDL by macrophages, as mentioned previously [68]. At this time, it was still unclear whether they were autoantibodies, i.e. antibodies produced by the body against part of “self”, or if the modified proteins and lipids were being recognized as external factors [115]. Similarly, antibodies found in human plasma that bound to oxidized and MDA modified LDL have been tested, and results show that one in particular, IK17, binds to MDA-LDL, MDA-HDL and Cu-oxidized LDL, whilst not binding to LDL treated with HNE or other proteins modified with MDA, suggesting a specific affinity to protein-bound MDA [116]. This antibody was also able to block the uptake of oxLDL by macrophages, which is important as it could potentially be used in therapeutics. In contrast, another study reported that when human LDL was injected into immune-competent and immune-deficient mice (lacking B and T cells), extensively oxidized LDL was cleared faster than native LDL but with similar clearance rates in the two mouse types, suggesting that the clearance of oxLDL is not simply mediated by antibody production [117]. However, there is now extensive evidence that covalent adducts such as ALEs or AGEs constitute “damage associated molecular patterns” (DAMPs) and are recognized by the immune system. They are often referred to as oxidation-specific epitopes, or OSEs; they occur on the surfaces of lipoproteins, apoptotic cells [118] or microvesicles [119], and can be recognized by a wide variety of cell surface pattern recognition receptors central in innate immunity, such as scavenger receptors and some Toll-like receptors (reviewed by [120], [121]). The lipoprotein modifications most typically studied in this context are MDA, HNE and oxPCs, probably because these are most readily analysed, but it is highly likely that modifications by other rLPPs also contribute but have not yet been identified. The autoantibodies (also called natural antibodies or Nabs) produced downstream of the immune activation, and reported in patients with a variety of inflammatory diseases, are often IgMs although IgGs also occur [121].
Another lipoprotein that has been quite well-studied owing to its proatherogenic effects is Lp(a), which is an ApoA covalently linked to ApoB-100 by a disulfide bond [122]. It appears to be quite well-established that Lp(a) can bind covalently to oxidized phosphatidylcholines via lysine residues in the Kringle V domain [123], and it has been found that Lp(a) is the preferred carrier of oxidized phospholipids (oxPLs) in plasma [124]. Lp(a) has been reported to have various pro-inflammatory effects that appear to depend on the presence of the oxPL adduct. For example, Edelstein et al. reported that human apo(a) induced the production of interleukin 8 by cultured THP-1 macrophage-like cells [123]; subsequently this was confirmed in THP-1 and U937 cell lines and the use of siRNA indicated that both CD36 and TLR2 contributed to the effect. Inhibitors of MAPKs, Jun N-terminal kinase and ERK1/2 were found to abolish IL-8 gene expression, suggesting that these signaling pathways are involved in the response downstream of the receptors [87]. Overall, there is mounting evidence that a Schiff base adduct on lysine in the Kringle IV type 10 is responsible for the pro-inflammatory and pro-atherogenic properties of this lipoprotein. There has also been interest in anti-neoplastic effects of Lp(a), which may be caused by degradation products of the lipoprotein, although convincing evidence is only just emerging [122] and the importance oxPC adducts in this effect is not established.
Some studies of HDL have reported altered biological activity following lipoxidation by acrolein. In particular, acrolein modification of ApoE was found to destabilize the protein, and caused a significant decrease in its ability to bind 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and support cholesterol efflux from cholesterol-loaded J774 mouse macrophages. The modified HDL also showed decreased binding to the LDL receptor in a co-immunoprecipitation assay and decreased binding affinity on a HiTrap heparin-sepharose column [86]. Similar findings were reported by Chadwick et al. [84], who showed that HDL cross-linked by acrolein was less able to act as an acceptor of free cholesterol from COS-7 cells, compared to native HDL. In fact, acrolein-HDL increased the neutral lipid uptake into macrophages. Thus it can be seen that acrolein modification caused substantial disruption of HDL structure, leading to a dysfunctional particle with impaired ability to support the reverse cholesterol transport pathway, with consequent atherogenic effects.
5. Lipoxidized lipoproteins as markers of disease and their use in therapeutics discovery
The altered biological effects described in the previous section are in the main pro-inflammatory, and therefore can be expected to contribute to a variety of diseases with underlying inflammatory etiology. It has long been accepted that LDL oxidation and ApoB-100 modification lead to impaired and altered biological functions that contribute to the progression and pathology of atherosclerosis [125], but lipoproteins can also be involved in the pathophysiology of other diseases. For example, dyslipidemia is associated with chronic kidney disease, diabetes mellitus and metabolic syndrome, and is characterized by lipoprotein abnormalities including an increase of triacylglyceride-rich lipoproteins such as VLDL and chylomicrons, LDL particles that are smaller and more dense, and an overall decrease in HDL cholesterol [126], [127]. HDL deficiency or dysfunction have also been implicated in neurodegenerative disorders: high levels of this lipoprotein appear to correlate with increased cognitive function and memory in the senior demographic [128], but in Alzheimer's disease a certain genotype of ApoE, allele APOE-ԑ4, has been shown to predict an accelerated decline of cognitive function in carriers [128], although there is no evidence that it is related to increased susceptibility to lipoxidation.
The development of techniques to localize and identify lipoxidation, reviewed in Section 3, has allowed its analysis in clinical samples. A strong focus, going back more than twenty years, has been on lipid-protein modification in samples from atherosclerotic plaques. The presence of MDA and HNE adducts and the presence of oxLDL was reported in atheroma tissue by immunocytochemistry with antibodies against HNE, MDA and copper-oxidized LDL [57]. The binding was found to be specific for the lipid-rich region of the atherosclerotic lesion and this pattern was maintained across the different antibodies [57], consistent with the expected localization of the oxidized LDL. Several other groups also reported similar results using anti-HNE-LDL antibodies [129], OB/04 and OB/09 antibodies against oxLDL [65], an autoantibody against MDA-LDL [116], or using E06 and an antibody against MDA-LDL [130]. A review from 2000 summarized additional identifications of different advanced lipoxidation end-products found in atherosclerotic lesions, including MDA-lysine [38], HNE-lysine [37], [38], and levuglandin E2 [131], which were analysed by both immunohistochemical and chemical techniques [132]. Antibodies raised against less studied aldehydes have also been tested in tissue from atherosclerotic lesions: the antibodies developed against HHE-histidine adducts [50] and HPNE-lysine adducts [63], which were shown to recognize copper-oxidized LDL, were also used to identify the presence of these adducts in human atherosclerotic aorta. An antibody to 4-HDDE-protein adducts showed increased staining in abdominal aorta of a cardiovascular patient with atherosclerosis and versus aorta from a healthy normotensive 41-year-old male, with no apparent atherosclerosis [64]. Thus despite the strong focus on smaller aldehyde adducts [133], evidence is emerging that other adducts also occur in disease, and may become useful markers in the future.
Anti-HNE antibodies have also been used to demonstrate that HNE-treated LDL could promote the HNE modification of other proteins, such as HSP60, a protein that is a target of autoimmune adaptive responses, and in its modified form a ligand to scavenger receptors alongside oxidized LDL, suggesting a synergetic effect in the progression of atherosclerosis [134]. The mechanisms by which this happens are not well understood, but it is possible that HNE treatment results in solubilization of HNE molecules in the LDL particles, and can then modify HSP60 when cells are treated with HNE-LDL. Another option is that as Michael additions are reversible, HNE bound to the ApoB100 in HNE-LDL could be released and modify other proteins.
The development of a well-validated ELISA assay to detect oxidized phospholipids on ApoB-100 containing LDL, using the E06 antibody, has enabled a substantial number of studies on the presence of oxidized phospholipids on LDL particles with several different cardiovascular diseases (CVD), mostly carried out by the groups of Tsimikas and Witztum [135]. For example, a reclassification of cardiovascular event in subjects from the Bruneck study followed over 15 years, which reported that the highest tertile of OxPL/ApoB was associated with higher risk of cardiovascular disease and stroke [136]. A study of stable subjects with coronary artery disease showed that oxidized phospholipids on ApoB-100 (oxPL-apoB) and plasminogen (oxPL-PLG) in plasma correlated positively with D-dimer, an end product of fibrin degradation that indicates a pro-thrombotic state [137]. The relationship of oxPLs in Lp(a) to calcific aortic valve disease (CAVD) has also been investigated in the Copenhagen General population study, and showed that oxPL-apoB, oxPL-apo(a) and lipoprotein(a) levels all associated with risk of CAVD, suggesting that they may be causal risk factors for the condition [138]. These are recent studies that expand the earlier work reviewed in [135]. The simplicity, high-throughput and easy analysis of results of this assay makes it suitable for clinical studies, and although the technique does not give residue-specific information on the sites of lipoxidation, knowing the association with these pathologies should promote more detailed research on these modifications.
Mass spectrometry techniques to detect lipoxidation markers have already been used to study cardiovascular disease and atherosclerosis. The Uchida group used targeted LC-MSMS to identify an rLPP adduct, Nε-(8-carboxyoctanyl)lysine (COL), in oxidized LDL. They applied this method to investigate its occurrence in sera from atherosclerosis-prone mice as well as from patients with hyperlipidemia, and found that the modification was specifically associated with the lipoprotein fraction of the sera. A significantly higher amount of COL was detected in both disease conditions compared with the controls [76]. It was interesting that in hyperlipidemic humans, the COL levels were tightly clustered while the healthy controls showed more variability, whereas in mice it was the other way round. Despite the relatively small patient and animal numbers, this study shows the potential of MS analysis for novel biomarker discovery.
The detection of autoantibodies against known lipoxidation adducts, namely MDA, MAA, MDA-LDL, and MAA-LDL, has been used to evaluate their potential as biomarkers of atherosclerosis [136], [139], [140]. The MAA-protein adducts specifically were previously shown to be present in aortic tissue of rabbits on a high fat diet [141] and atherosclerotic rats [142], and led to an innate and acquired immune response, provoking the production of antibodies against it. One study developed an ELISA assay capable of binding the anti-MAA and anti-MAA-LDL antibodies from the plasma of human patients with non-obstructive coronary artery disease (CAD), acute myocardial infarction and obstructive multi-vessel CAD [139]. While the data for the MDA-LDL and MAA-LDL adducts did not show a significant difference between the different conditions, which is consistent with published results from other groups [136], [140], the results on the circulating anti-MAA IgG, IgM and IgA antibodies showed a significant increase in the diseased states when compared with the controls. The IgG/IgM/IgA profile between the different conditions also showed variability, information that could be used as a potential biomarker.
Other conditions besides atherosclerosis have also been subject of lipoprotein lipoxidation studies. One study investigated the association between systemic lupus erythematosus (SLE) and both arterial and renal disease [143]. SLE is an autoimmune condition more prevalent in women, known to be linked with an early onset of atherosclerosis. The group looked at the presence of oxidized LDL using the E06 antibody, and determined the levels of autoantibodies against oxLDL, MDA-LDL and cardiolipin in patients with SLE. The results showed an increase of the E06 binding levels in the patients with the condition compared with the controls, and the levels of autoantibodies against oxLDL, MDA-LDL and cardiolipin were also significantly increased in the patients with SLE. It was suggested that the results obtained and the prevalence of premature atherosclerosis can relate to excess lipid peroxidation as playing an important role in SLE [143]. Antibodies against MDA-LDL and HNE-LDL were also used to stain multiple sclerosis plaques at different stages of disease, and these showed a localization of staining on the foam cells present [112], suggesting that the plasma LDL that enters the parenchyma in multiple sclerosis plaques could be oxidized in situ, leading to the development of foam cells. There has also been much interest in the occurrence of lipoxidation in neurodegenerative diseases such as Alzheimer's disease; while extensive work has been done on detection of a variety of rLPP-protein adducts [144], [145], [146], there is less evidence for specific modifications of lipoproteins in these conditions, although it has been reported that ApoA-I is highly oxidatively modified and particularly susceptible to modification by HNE in several neurodegenerative diseases. This may cause increased levels of tumor necrosis factor-α (TNF- α) that can cross the blood-brain barrier and can contribute to neuronal death [147].
The identification and study of lipoxidation adducts ex vivo has opened doors to a new field, the study of possible inhibitors, which could be used in the therapeutics field. One study that exemplifies this is by Onorato et al. They reported the use of pyridoxamine, a known inhibitor of AGEs, already used in clinical studies with diabetic subjects, as an inhibitor of protein modification by CML and CEL, MDA and HNE, both in arachidonate-treated RNase, and in copper oxidized LDL [72]. Similarly, glucosamine has also been studied as an inhibitor of lipid peroxidation and lipoxidation on osteoarthritis, using both an in vitro model of chondrocyte degradation by lipid peroxidation and human LDL samples [52]. Immunoblot assays were used to assess protein oxidation and adduct formation, alongside a TBARS assay, to measure MDA formation. Their findings showed a concentration-dependent decrease of MDA production, and inhibition of protein modification, suggesting that glucosamine might have a protective role, in line with other published studies that show its role as a new antioxidant [148]. There has also been ongoing interest in carnosine and its derivatives over several years, as it is well established that it can react with several aldehydes including MDA, methylglyoxal, HNE, and acetaldehyde, which is thought to contribute to its protective functions [149], [150], [151]. While the use of carnosine itself presents some limitations, recent work has led to the development of (2S)-2-(3-amino propanoylamino)-3-(1H-imidazol-5-yl)propanol (carnosinol), which is a derivative of carnosine with improved oral bioavailability and resistance to carnosinases [24], [152], [153]. The compound was tested in both rat and mouse models of diet-induced obesity and metabolic syndrome, and was found to reduce HNE adduct formation in liver and skeletal muscle, while also improving typical symptoms of metabolic syndrome, namely dyslipidemia, insulin resistance, steatohepatitis and inflammation. Although as yet these scavenging compounds have not been tested specifically with lipoproteins, the likelihood is that formation of adducts will also be attenuated, although the non-polar environment may reduce the effectiveness. Another compound group, the kavalactones (including kawain, methysticin and dihydromethysticin) has been identified as both advanced glycation and lipid peroxidation inhibitors. The latter was shown by the inhibition of TBArS formation in LDL and linoleic acid, which, in conjunction with its metal chelating properties, indicate a possible indirect inhibition of ALE formation [154].
A somewhat different strategy has been developed by Nankar and Pande [155]. They synthesized 11 peptides from conserved regions of human ApoE, which is known to have oxPL binding activity. The peptides showed differential oxPL binding and native PL binding, and those that bound exclusively to the oxPLs also were able to inhibit IL-8 secretion in human blood, thus demonstrating anti-inflammatory activity.
6. Summary and perspectives
In conclusion, interest in the formation of rLPP adducts with lipoproteins is growing steadily, with many publications reporting their formation in a wide variety of conditions. Lipoproteins are already considered as biomarkers in some conditions, but understanding of lipoxidative modifications will add an additional layer of specificity to improve their value. Development of a range of antibodies has been invaluable for immunohistochemistry, and has provided substantial evidence for formation of certain adducts in vivo, frequently with increased levels in inflammatory diseases. The current limitation is that antibodies are only available for a relatively small number of rLPP adducts, compared to the number that exist and have been found by other methods, but work is ongoing to improve this. Despite the challenging nature of the analysis, improvements in mass spectrometry technologies are yielding very detailed qualitative and quantitative information on the sites of modification, which is important both in understanding the biological effects of the modifications, and designing therapeutic interventions. It is essential to appreciate the complementary nature of the different techniques, in terms of the types of information that they offer. It is also worth noting that currently the interest in rLLPs and lipoxidation is largely skewed towards pathology, as such modifications have widely been considered as deleterious, but the concept of rLPPs as signaling molecules with a role in hormesis is gaining favor. Ultimately, the understanding in this area will depend on the sensitivity of methods for detecting the modifications, and specific inhibitors to unravel their outcomes.
Acknowledgements
The authors acknowledge funding from the European Commission's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie project MSCA-ITN-ETN MASSTRPLAN, Grant Agreement Number 675132.
Acknowledgments
Declarations of interest
None.
References
- 1.K.R. Feingold, C. Grunfeld, Introduction to Lipids and Lipoproteins, in: A.V. Leslie, J. De Groot, Editor-in-chief, George Chrousos, Kathleen Dungan, Kenneth R. Feingold, Ashley Grossman, Jerome M. Hershman, Christian Koch, Márta Korbonits, Robert McLachlan, Maria New, Jonathan Purnell, Robert Rebar, Frederick Singer (Ed.), Endotext, South Dartmouth (MA): MDText.com, Inc., 2000.
- 2.Reis A., Rudnitskaya A., Blackburn G.J., Fauzi N.M., Pitt A.R., Spickett C.M. A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. J. Lipid Res. 2013;54:1812–1824. doi: 10.1194/jlr.M034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown M.S., Goldstein J.L., Krieger M., Ho Y.K., Anderson R.G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J. Cell Biol. 1979 doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinbrecher U.P., Witztum J.L., Parthasarathy S., Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis. 1987 doi: 10.1161/01.atv.7.2.135. [DOI] [PubMed] [Google Scholar]
- 5.Parthasarathy S., Steinberg D., Witztum J.L. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu. Rev. Med. 1992 doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- 6.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Reis A., Spickett C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta - Biomembr. 2012;1818:2374–2387. doi: 10.1016/j.bbamem.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Sousa B.C., Pitt A.R., Spickett C.M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 2017 doi: 10.1016/j.freeradbiomed.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Guéraud F., Atalay M., Bresgen N., Cipak A., Eckl P.M., Huc L., Jouanin I., Siems W., Uchida K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010 doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- 10.Spickett C.M. The lipid peroxidation product 4-hydroxy-2-nonenal: advances in chemistry and analysis. Redox Biol. 2013;1:145–152. doi: 10.1016/j.redox.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fruhwirth G.O., Loidl A., Hermetter A. Oxidized phospholipids: from molecular properties to disease. Biochim. Biophys. Acta - Mol. Basis Dis. 2007;1772:718–736. doi: 10.1016/j.bbadis.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Maciel E., Da Silva R.N., Simões C., Domingues P., Domingues M.R.M. Structural characterization of oxidized glycerophosphatidylserine: evidence of polar head Oxidation. J. Am. Soc. Mass Spectrom. 2011 doi: 10.1007/s13361-011-0194-9. [DOI] [PubMed] [Google Scholar]
- 13.Nègre-Salvayre A., Augé N., Camaré C., Bacchetti T., Ferretti G., Salvayre R. Dual signaling evoked by oxidized LDLs in vascular cells. Free Radic. Biol. Med. 2017;106:118–133. doi: 10.1016/j.freeradbiomed.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Zmysłowski A., Szterk A. Current knowledge on the mechanism of atherosclerosis and pro-atherosclerotic properties of oxysterols. Lipids Health Dis. 2017 doi: 10.1186/s12944-017-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domingues R.M., Domingues P., Melo T., Pérez-Sala D., Reis A., Spickett C.M. Lipoxidation adducts with peptides and proteins: deleterious modifications or signaling mechanisms? J. Proteom. 2013;92:110–131. doi: 10.1016/j.jprot.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Aldini G., Domingues M.R., Spickett C.M., Domingues P., Altomare A., Sánchez-Gómez F.J., Oeste C.L., Pérez-Sala D. Protein lipoxidation: detection strategies and challenges. Redox Biol. 2015;5:253–266. doi: 10.1016/j.redox.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 2013;47(Suppl. 1):3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 18.Sasson S. Nutrient overload, lipid peroxidation and pancreatic beta cell function. Free Radic. Biol. Med. 2017 doi: 10.1016/j.freeradbiomed.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Hazell L.J., van den Berg J.J., Stocker R. Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation. Biochem. J. 1994 doi: 10.1042/bj3020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knott H.M., Baoutina A., Davies M.J., Dean R.T. Comparative time-courses of copper-ion-mediated protein and lipid oxidation in low-density lipoprotein. Arch. Biochem. Biophys. 2002 doi: 10.1016/S0003-9861(02)00018-8. [DOI] [PubMed] [Google Scholar]
- 21.Malle E., Marsche G., Arnhold J., Davies M.J. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2006 doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Ismael F.O., Proudfoot J.M., Brown B.E., Van Reyk D.M., Croft K.D., Davies M.J., Hawkins C.L. Comparative reactivity of the myeloperoxidase-derived oxidants HOCl and HOSCN with low-density lipoprotein (LDL): implications for foam cell formation in atherosclerosis. Arch. Biochem. Biophys. 2015 doi: 10.1016/j.abb.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs A.T., Marnett L.J. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc. Chem. Res. 2010;43:673–683. doi: 10.1021/ar900286y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colzani M., Aldini G., Carini M. Mass spectrometric approaches for the identification and quantification of reactive carbonyl species protein adducts. J. Proteomics. 2013;92:28–50. doi: 10.1016/j.jprot.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Sayre L.M., Lin D., Yuan Q., Zhu X., Tang X. Protein Adducts Generated from Products of Lipid Oxidation: Focus on HNE and ONE. Drug Metab. Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- 26.Parvez S., Long M.J.C., Poganik J.R., Aye Y. Redox signaling by reactive electrophiles and oxidants. Chem. Rev. 2018;118:8798–8888. doi: 10.1021/acs.chemrev.7b00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jürgens G., Lang J., Esterbauer H. Modification of human low-density lipoprotein by the lipid peroxidation product 4-hydroxynonenal. Biochim. Biophys. Acta - Lipids Lipid Metab. 1986;875:103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- 28.Heinecke J.W. Tyrosyl radical production by myeloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross-linking of proteins. Toxicology. 2002 doi: 10.1016/s0300-483x(02)00192-0. [DOI] [PubMed] [Google Scholar]
- 29.Havel R.J., Eder H.A., Bragdon J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ordovas J.M. Humana Press; New Jersey: 1998. Lipoprotein Protocols. [Google Scholar]
- 31.Chung B.B.H., Segrest J.P., Ray M.J., Brunzell J.D., Hokanson J.E., Krauss R.M., Beaudrie K.E.N., Cone J.T. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986;128 doi: 10.1016/0076-6879(86)28068-4. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki M., Yamashita S. Recent advances in analytical methods on lipoprotein subclasses: calculation of particle numbers from lipid levels by gel permeation HPLC using “spherical particle model,”. J. Oleo Sci. 2016;65:265–282. doi: 10.5650/jos.ess16020. [DOI] [PubMed] [Google Scholar]
- 33.Superko H.R. Advanced LIpoprotein Testing and subfractionation are clinically useful. Circulation. 2009;119:2383–2395. doi: 10.1161/CIRCULATIONAHA.108.809582. [DOI] [PubMed] [Google Scholar]
- 34.Annangudi S.P., Deng Y., Gu X., Zhang W., Crabb J.W., Salomon R.G. Low-density lipoprotein has an enormous capacity to bind (E)-4-hydroxynon-2-enal (HNE): detection and characterization of lysyl and histidyl adducts containing multiple molecules of HNE. Chem. Res. Toxicol. 2008;21:1384–1395. doi: 10.1021/tx8000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman P., Hörkkö S., Steinberg D., Witztum J.L., Dennis E.A. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J. Biol. Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 36.Palinski W., Yla-Herttuala S., Rosenfeld M.E., Butler S.W., Socher S.A., Parthasarathy S., Curtiss L.K., Witztum J.L. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 1990;10:325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 37.Sayre L.M., Sha W., Xu G., Kaur K., Nadkarni D., Subbanagounder G., Salomon R.G. Immunochemical evidence supporting 2-pentylpyrrole formation on proteins exposed to 4-Hydroxy-2-nonenal. Chem. Res. Toxicol. 1996;9:1194–1201. doi: 10.1021/tx960094j. [DOI] [PubMed] [Google Scholar]
- 38.Requena J.R., Fu M.X., Ahmed M.U., Jenkins A.J., Lyons T.J., Baynes J.W., Thorpe S.R. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem. J. 1997;322:317–325. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoff H.F., Zyromski N., Armstrong D., O’Neil J. Aggregation as well as chemical modification of LDL during oxidation is responsible for poor processing in macrophages. J. Lipid Res. 1993;34:1919–1929. [PubMed] [Google Scholar]
- 40.Gillotte K.L., Hörkkö S., Witztum J.L., Steinberg D. Oxidized phospholipids, linked to apolipoprotein B of oxidized LDL, are ligands for macrophage scavenger receptors. J. Lipid Res. 2000;41:824–833. 〈http://www.ncbi.nlm.nih.gov/pubmed/10787443〉 [PubMed] [Google Scholar]
- 41.Uchida K., Toyokuni S., Nishikawa K., Kawakishi S., Oda H., Hiai H., Stadtman E.R. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994;33:12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- 42.Shacter E. Quantification and significance of protein oxiation in biological samples. Drug Metab. Rev. 2000;32:307–326. doi: 10.1081/dmr-100102336. [DOI] [PubMed] [Google Scholar]
- 43.Yan L.J., Forster M.J. Chemical probes for analysis of carbonylated proteins: a review. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011;879:1308–1315. doi: 10.1016/j.jchromb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wall S.B., Smith M.R., Ricart K., Zhou F., Vayalil P.K., Oh J.Y., Landar A. Detection of electrophile-sensitive proteins. Biochim. Biophys. Acta - Gen. Subj. 2014;1840:913–922. doi: 10.1016/j.bbagen.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang C.-Y., Gu Z.-W., Yang H.-X., Yang M., Gotto A.M., Smith C.V. Oxidative modifications of APOB-100 by exposure of low density lipoproteins to HOCl in vitro. Free Radic. Biol. Med. 1997;23:82–89. doi: 10.1016/s0891-5849(96)00624-7. [DOI] [PubMed] [Google Scholar]
- 46.Yang C.Y., Gu Z.-W.W., Yang M., Lin S.-N.N., Siuzdak G., Smith C.V. Identification of modified tryptophan residues in apolipoprotein B-100 derived from copper ion-oxidized low-density lipoprotein †. Biochemistry. 1999;38:15903–15908. doi: 10.1021/bi991464g. [DOI] [PubMed] [Google Scholar]
- 47.Halliwell B., Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993;57:715S–725S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 48.Spickett C.M., Wiswedel I., Siems W., Zarkovic K., Zarkovic N. Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic. Res. 2010;44:1172–1202. doi: 10.3109/10715762.2010.498476. [DOI] [PubMed] [Google Scholar]
- 49.Januszewski A.S., Alderson N.L., Jenkins A.J., Thorpe S.R., Baynes J.W. Chemical modification of proteins during peroxidation of phospholipids. J. Lipid Res. 2005;46:1440–1449. doi: 10.1194/jlr.M400442-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Yamada S., Funada T., Shibata N., Kobayashi M., Kawai Y., Tatsuda E., Furuhata A., Uchida K. Protein-bound 4-hydroxy-2-hexenal as a marker of oxidized n-3 polyunsaturated fatty acids. J. Lipid Res. 2004;45:626–634. doi: 10.1194/jlr.M300376-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Jürgens G., Ashy A., Esterbauer H. Detection of new epitopes formed upon oxidation of low-density lipoprotein, lipoprotein (a) and very-low-density lipoprotein. Use of an antiserum against 4-hydroxynonenal-modified low-density lipoprotein. Biochem. J. 1990;265:605–608. doi: 10.1042/bj2650605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiku M.L., Narla H., Jain M., Yalamanchili P. Glucosamine prevents in vitro collagen degradation in chondrocytes by inhibiting advanced lipoxidation reactions and protein oxidation. Arthritis Res. Ther. 2007;9:R76. doi: 10.1186/ar2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoff H.F., O’Neil J. Structural and functional changes in LDL after modification with both 4-hydroxynonenal and malondialdehyde. J. Lipid Res. 1993;34:1209–1217. [PubMed] [Google Scholar]
- 54.Karakatsani A.I., Liapikos T.A., Troganis A.N., Tsoukatos D.C. Involvement of phospholipids in apolipoprotein B modification during low density lipoprotein oxidation. Lipids. 1998;33:1159–1162. doi: 10.1007/s11745-998-0318-3. [DOI] [PubMed] [Google Scholar]
- 55.Haberland M., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science (80-.) 1988;241:215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- 56.Salmon S., Maziere C., Theron L., Beucler I., Ayrault-Jarrier M., Goldstein S., Polonovski J. Immunological detection of low-density lipoproteins modified by malondialdehyde in vitro or in vivo. Biochim. Biophys. Acta - Lipids Lipid Metab. 1987;920:215–220. doi: 10.1016/0005-2760(87)90097-x. [DOI] [PubMed] [Google Scholar]
- 57.Palinski W., Rosenfeld M.E., Ylä-Herttuala S., Gurtner G.C., Socher S.S., Butler S.W., Parthasarathy S., Carew T.E., Steinberg D., Witztum J.L. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greilberger J., Schmut O., Jürgens G. In vitro interactions of oxidatively modified LDL with type I, II, III, IV, and V collagen, laminin, fibronectin, and Poly-d-Lysine. Arterioscler. Thromb. Vasc. Biol. 1997;17:2721–2728. doi: 10.1161/01.atv.17.11.2721. [DOI] [PubMed] [Google Scholar]
- 59.Chen Q., Esterbauer H., Jürgens G. Studies on epitopes on low-density lipoprotein modified by 4-hydroxynonenal. Biochem. Charact. Determ. Biochem. J. 1992;288(Pt 1):249–254. doi: 10.1042/bj2880249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waeg G., Dimsity G., Esterbauer H. Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Radic. Res. 1996;25:149–159. doi: 10.3109/10715769609149920. [DOI] [PubMed] [Google Scholar]
- 61.Uchida K., Kanematsu M., Morimitsu Y., Osawa T., Noguchi N., Niki E. Acrolein is a product of lipid peroxidation reaction. J. Biol. Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 62.Tamamizu-Kato S., Wong J.Y., Jairam V., Uchida K., Raussens V., Kato H., Ruysschaert J.-M.M., Narayanaswami V. Modification by acrolein, a component of tobacco smoke and age-related oxidative stress, mediates functional impairment of human apolipoprotein E †. Biochemistry. 2007;46:8392–8400. doi: 10.1021/bi700289k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimozu Y., Hirano K., Shibata T., Shibata N., Uchida K. 4-Hydroperoxy-2-nonenal is not just an intermediate but a reactive molecule that covalently modifies proteins to generate unique intramolecular oxidation products. J. Biol. Chem. 2011;286:29313–29324. doi: 10.1074/jbc.M111.255737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchida K., Shibata T., Toyokuni S., Daniel B., Zarkovic K., Zarkovic N., Sasson S. Development of a novel monoclonal antibody against 4-hydroxy-2E,6Z-dodecadienal (4-HDDE)-protein adducts: immunochemical application in quantitative and qualitative analyses of lipid peroxidation in vitro and ex vivo. Free Radic. Biol. Med. 2018;124:12–20. doi: 10.1016/j.freeradbiomed.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 65.Hammer A., Kager G., Dohr G., Rabl H., Ghassempur I., Jürgens G. Generation, characterization, and histochemical application of monoclonal antibodies selectively recognizing oxidatively modified apoB-containing serum lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1995;15:704–713. doi: 10.1161/01.atv.15.5.704. [DOI] [PubMed] [Google Scholar]
- 66.Palinski W., Hörkkö S., Miller E., Steinbrecher U.P., Powell H.C., Curtiss L.K., Witztum J.L. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Investig. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hörkkö S., Miller E., Dudl E., Reaven P., Curtiss L.K., Zvaifler N.J., Terkeltaub R., Pierangeli S.S., Branch D.W., Palinski W., Witztum J.L. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids recognition of cardiolipin by monoclonal antibodies to epitopes of oxidized low density lipoprotein. J. Clin. Investig. 1996;98:815–825. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hörkkö S., Bird D.A., Miller E., Itabe H., Leitinger N., Subbanagounder G., Berliner J.A., Friedman P., Dennis E.A., Curtiss L.K., Palinski W., Witztum J.L. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid–protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Investig. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonen A., Hansen L.F., Turner W.W., Montano E.N., Que X., Rafia A., Chou M.-Y., Wiesner P., Tsiantoulas D., Corr M., VanNieuwenhze M.S., Tsimikas S., Binder C.J., Witztum J.L., Hartvigsen K. Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine. J. Lipid Res. 2014;55:2137–2155. doi: 10.1194/jlr.M053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vasil’ev Y.V., Tzeng S.-C., Huang L., Maier C.S. Protein modifications by electrophilic lipoxidation products: adduct formation, chemical strategies and tandem mass spectrometry for their detection and identification. Mass Spectrom. Rev. 2014;33:157–182. doi: 10.1002/mas.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spickett C.M., Reis A., Pitt A.R. Use of narrow mass-window, high-resolution extracted product ion chromatograms for the sensitive and selective identification of protein modifications. Anal. Chem. 2013;85:4621–4627. doi: 10.1021/ac400131f. [DOI] [PubMed] [Google Scholar]
- 72.Onorato J.M., Jenkins A.J., Thorpe S.R., Baynes J.W. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. J. Biol. Chem. 2000;275:21177–21184. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- 73.Ishino K., Wakita C., Shibata T., Toyokuni S., Machida S., Matsuda S., Matsuda T., Uchida K. Lipid peroxidation generates body odor component trans -2-nonenal covalently bound to protein in vivo. J. Biol. Chem. 2010;285:15302–15313. doi: 10.1074/jbc.M109.068023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shibata T., Shimozu Y., Wakita C., Shibata N., Kobayashi M., Machida S., Kato R., Itabe H., Zhu X., Sayre L.M., Uchida K. Lipid peroxidation modification of protein generates Nε-(4- oxononanoyl)lysine as a pro-inflammatory ligand. J. Biol. Chem. 2011;286:19943–19957. doi: 10.1074/jbc.M110.187047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maeshima T., Honda K., Chikazawa M., Shibata T., Kawai Y., Akagawa M., Uchida K. Quantitative analysis of acrolein-specific adducts generated during lipid peroxidation-modification of proteins in vitro: identification of Nτ-(3-Propanal)histidine as the major adduct. Chem. Res. Toxicol. 2012;25:1384–1392. doi: 10.1021/tx3000818. [DOI] [PubMed] [Google Scholar]
- 76.Shibata T., Shimizu K., Hirano K., Nakashima F., Kikuchi R., Matsushita T., Uchida K. Adductome-based identification of biomarkers for lipid peroxidation. J. Biol. Chem. 2017;292:8223–8235. doi: 10.1074/jbc.M116.762609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verrastro I., Pasha S., Jensen K., Pitt A., Spickett C. Mass spectrometry-based methods for identifying oxidized proteins in disease: advances and challenges. Biomolecules. 2015;5:378–411. doi: 10.3390/biom5020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chand Bollineni R., Fedorova M., Hoffmann R. Qualitative and quantitative evaluation of derivatization reagents for different types of protein-bound carbonyl groups. Analyst. 2013 doi: 10.1039/c3an00724c. [DOI] [PubMed] [Google Scholar]
- 79.Bolgar M.S., Yang C.-Y.Y., Gaskell S.J. First direct evidence for lipid/protein conjugation in oxidized human low density lipoprotein. J. Biol. Chem. 1996;271:27999–28001. doi: 10.1074/jbc.271.45.27999. [DOI] [PubMed] [Google Scholar]
- 80.Sangvanich P., Mackness B., Gaskell S.J., Durrington P., Mackness M. The effect of high-density lipoproteins on the formation of lipid/protein conjugates during in vitro oxidation of low-density lipoprotein. Biochem. Biophys. Res. Commun. 2003;300:501–506. doi: 10.1016/s0006-291x(02)02849-8. [DOI] [PubMed] [Google Scholar]
- 81.Obama T., Kato R., Masuda Y., Takahashi K., Aiuchi T., Itabe H. Analysis of modified apolipoprotein B-100 structures formed in oxidized low-density lipoprotein using LC-MS/MS. Proteomics. 2007;7:2132–2141. doi: 10.1002/pmic.200700111. [DOI] [PubMed] [Google Scholar]
- 82.Shao B., Bergt C., Fu X., Green P., Voss J.C., Oda M.N., Oram J.F., Heinecke J.W. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J. Biol. Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 83.Shao B., O’Brien K.D., McDonald T.O., Fu X., Oram J.F., Uchida K., Heinecke J.W. Acrolein modifies apolipoprotein A-I in the human artery wall. Ann. N. Y. Acad. Sci. 2005;1043:396–403. doi: 10.1196/annals.1333.046. [DOI] [PubMed] [Google Scholar]
- 84.Chadwick A.C., Holme R.L., Chen Y., Thomas M.J., Sorci-Thomas M.G., Silverstein R.L., Pritchard K.A., Sahoo D. Acrolein impairs the cholesterol transport functions of high density lipoproteins. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0123138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stewart C.R., Wilson L.M., Zhang Q., Pham C.L.L., Waddington L.J., Staples M.K., Stapleton D., Kelly J.W., Howlett G.J. Oxidized cholesterol metabolites found in human atherosclerotic lesions promote apolipoprotein C-II amyloid fibril formation. Biochemistry. 2007;46:5552–5561. doi: 10.1021/bi602554z. [DOI] [PubMed] [Google Scholar]
- 86.Tran T.N., Kosaraju M.G., Tamamizu-Kato S., Akintunde O., Zheng Y., Bielicki J.K., Pinkerton K., Uchida K., Lee Y.Y., Narayanaswami V. Acrolein modification impairs key functional features of rat apolipoprotein E: identification of modified sites by mass spectrometry. Biochemistry. 2014;53:361–375. doi: 10.1021/bi401404u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scipione C.A., Sayegh S.E., Romagnuolo R., Tsimikas S., Marcovina S.M., Boffa M.B., Koschinsky M.L. Mechanistic insights into Lp(a)-induced IL-8 expression: a role for oxidized phospholipid modification of apo(a) J. Lipid Res. 2015;56:2273–2285. doi: 10.1194/jlr.M060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leibundgut G., Scipione C., Yin H., Schneider M., Boffa M.B., Green S., Yang X., Dennis E., Witztum J.L., Koschinsky M.L., Tsimikas S. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J. Lipid Res. 2013;54:2815–2830. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao D., Willard B., Podrez E.A. Analysis of covalent modifications of proteins by oxidized phospholipids using a novel method of peptide enrichment. Anal. Chem. 2014;86:1254–1262. doi: 10.1021/ac4035949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao D., Podrez E.A. Characterization of covalent modifications of HDL apoproteins by endogenous oxidized phospholipids. Free Radic. Biol. Med. 2018;115:57–67. doi: 10.1016/j.freeradbiomed.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holvoet P., Collen D. Oxidation of low density lipoproteins in the pathogenesis of atherosclerosis. Atherosclerosis. 1998;137:S33–S38. doi: 10.1016/s0021-9150(97)00305-5. [DOI] [PubMed] [Google Scholar]
- 92.Mitra S., Goyal T., Mehta J.L. Oxidized LDL, LOX-1 and Atherosclerosis. Cardiovasc. Drugs Ther. 2011;25:419–429. doi: 10.1007/s10557-011-6341-5. [DOI] [PubMed] [Google Scholar]
- 93.Meyer J.M., Ji A., Cai L., van der Westhuyzen D.R. Minimally oxidized LDL inhibits macrophage selective cholesteryl ester uptake and native LDL-induced foam cell formation. J. Lipid Res. 2014;55:1648–1656. doi: 10.1194/jlr.M044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salvayre R., Auge N., Benoist H., Negre-Salvayre A. Oxidized low-density lipoprotein-induced apoptosis. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2002;1585:213–221. doi: 10.1016/s1388-1981(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 95.Estruch M., Sánchez-Quesada J.L., Ordóñez Llanos J., Benítez S. Electronegative LDL: a circulating modified LDL with a role in inflammation. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/181324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chisolm G.M., Chai Y. Regulation of cell growth by oxidized LDL. Free Radic. Biol. Med. 2000;28:1697–1707. doi: 10.1016/s0891-5849(00)00227-6. 〈http://www.ncbi.nlm.nih.gov/pubmed/10946211〉 [DOI] [PubMed] [Google Scholar]
- 97.Gradinaru D., Borsa C., Ionescu C., Prada G.I. Oxidized LDL and NO synthesis-Biomarkers of endothelial dysfunction and ageing. Mech. Ageing Dev. 2015;151:101–113. doi: 10.1016/j.mad.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Perségol L., Brindisi M.C., Rageot D., Pais de Barros J.P., Monier S., Vergès B., Duvillard L. Oxidation-induced loss of the ability of HDL to counteract the inhibitory effect of oxidized LDL on vasorelaxation. Heart Vessels. 2015;30:845–849. doi: 10.1007/s00380-014-0543-2. [DOI] [PubMed] [Google Scholar]
- 99.Soumyarani V.S., Jayakumari N. Oxidized HDL Induces Cytotoxic Effects: implications for Atherogenic Mechanism. J. Biochem. Mol. Toxicol. 2014;28:481–489. doi: 10.1002/jbt.21588. [DOI] [PubMed] [Google Scholar]
- 100.Wu J., He Z., Gao X., Wu F., Ding R., Ren Y., Jiang Q., Fan M., Liang C., Wu Z. Oxidized high-density lipoprotein impairs endothelial progenitor cells' function by activation of CD36-MAPK-TSP-1 pathways. Antioxid. Redox Signal. 2015;22:308–324. doi: 10.1089/ars.2013.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao X., Wu J., Qian Y., Fu L., Wu G., Xu C., Mei C. Oxidized high-density lipoprotein impairs the function of human renal proximal tubule epithelial cells through CD36. Int. J. Mol. Med. 2014;34:564–572. doi: 10.3892/ijmm.2014.1799. [DOI] [PubMed] [Google Scholar]
- 102.Peterson S.J., Vanella L., Gotlinger K., Jiang H., Singh S.P., Sodhi K., Maher E., O’Hanlon K., Shapiro J.I., Abraham N.G. Oxidized HDL is a potent inducer of adipogenesis and causes activation of the Ang-II and 20-HETE systems in human obese females. Prostaglandins Other Lipid Mediat. 2016;123:68–77. doi: 10.1016/j.prostaglandins.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 103.Fogelman A.M., Shechter I., Seager J., Hokom M., Child J.S., Edwards P.A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc. Natl. Acad. Sci. USA. 1980;77:2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamada Y., Doi T., Hamakubo T., Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell. Mol. Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 106.Boullier A., Gillotte K.L., Hörkkö S., Green S.R., Friedman P., Dennis E.A., Witztum J.L., Steinberg D., Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J. Biol. Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 107.Gillotte-Taylor K., Boullier A., Witztum J.L., Steinberg D., Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 108.Zhou M., Chen Y., Liu S., Ding Z., Pang Z., Wan J. Oxidative and malondialdehyde modification of low-density lipoprotein: a comparative study of binding and degradation by macrophages and endothelial cells. Br. J. Biomed. Sci. 1998;55:192–198. [PubMed] [Google Scholar]
- 109.Suzuki H., Sasaki T., Kumagai T., Sakaguchi S., Nagata K. Malondialdehyde-modified low density lipoprotein (MDA-LDL)-induced cell growth was suppressed by polycyclic aromatic hydrocarbons (PAHs) J. Toxicol. Sci. 2010;35:137–147. doi: 10.2131/jts.35.137. [DOI] [PubMed] [Google Scholar]
- 110.Kaida H., Tahara N., Tahara A., Honda A., Nitta Y., Igata S., Ishibashi M., ichi Yamagishi S., Fukumoto Y. Positive correlation between malondialdehyde-modified low-density lipoprotein cholesterol and vascular inflammation evaluated by18F-FDG PET/CT. Atherosclerosis. 2014;237:404–409. doi: 10.1016/j.atherosclerosis.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Veneskoski M., Turunen S.P., Kummu O., Nissinen A., Rannikko S., Levonen A.L., Hörkkö S. Specific recognition of malondialdehyde and malondialdehyde acetaldehyde adducts on oxidized LDL and apoptotic cells by complement anaphylatoxin C3a. Free Radic. Biol. Med. 2011;51:834–843. doi: 10.1016/j.freeradbiomed.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 112.Newcombe J., Li H., Cuzner M.L. Low density lipoprotein uptake by macrophages in multiple sclerosis plaques: implications for pathogenesis. Neuropathol. Appl. Neurobiol. 1994;20:152–162. doi: 10.1111/j.1365-2990.1994.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 113.Liu W., Akhand A.A., Kato M., Yokoyama I., Miyata T., Kurokawa K., Uchida K., Nakashima I. 4-Hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J. Cell Sci. 1999;112(Pt 1):2409–2417. doi: 10.1242/jcs.112.14.2409. 〈http://www.ncbi.nlm.nih.gov/pubmed/10381396〉 [DOI] [PubMed] [Google Scholar]
- 114.Vieira O., Escargueil-Blanc I., Jürgens G., Borner C., Almeida L., Salvayre R., Nègre-Salvayre A. Oxidized LDLs alter the activity of the ubiquitin-proteasome pathway: potential role in oxidized LDL-induced apoptosis. FASEB J. 2000;14:532–542. doi: 10.1096/fasebj.14.3.532. [DOI] [PubMed] [Google Scholar]
- 115.Shaw P.X., Hörkkö S., Chang M.-K., Curtiss L.K., Palinski W., Silverman G.J., Witztum J.L. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Investig. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shaw P.X., Hörkkö S., Tsimikas S., Chang M.K., Palinski W., Silverman G.J., Chen P.P., Witztum J.L. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler. Thromb. Vasc. Biol. 2001;21:1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 117.Reardon C.A., Miller E.R., Blachowicz L., Lukens J., Binder C.J., Witztum J.L., Getz G.S. Autoantibodies to OxLDL fail to alter the clearance of injected OxLDL in apolipoprotein E-deficient mice. J. Lipid Res. 2004;45:1347–1354. doi: 10.1194/jlr.M400075-JLR200. [DOI] [PubMed] [Google Scholar]
- 118.Chang M.-K., Binder C.J., Miller Y.I., Subbanagounder G., Silverman G.J., Berliner J.A., Witztum J.L. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsiantoulas D., Perkmann T., Afonyushkin T., Mangold A., Prohaska T.A., Papac-Milicevic N., Millischer V., Bartel C., Hörkkö S., Boulanger C.M., Tsimikas S., Fischer M.B., Witztum J.L., Lang I.M., Binder C.J. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 2015;56:440–448. doi: 10.1194/jlr.P054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chou M.Y., Hartvigsen K., Hansen L.F., Fogelstrand L., Shaw P.X., Boullier A., Binder C.J., Witztum J.L. Oxidation-specific epitopes are important targets of innate immunity. J. Intern. Med. 2008;263:479–488. doi: 10.1111/j.1365-2796.2008.01968.x. [DOI] [PubMed] [Google Scholar]
- 121.Binder C.J., Papac-Milicevic N., Witztum J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016;16:485–497. doi: 10.1038/nri.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Orsó E., Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin. Res. Cardiol. 2017;Suppl. 12:31–37. doi: 10.1007/s11789-017-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Edelstein C., Pfaffinger D., Hinman J., Miller E., Lipkind G., Tsimikas S., Bergmark C., Getz G.S., Witztum J.L., Scanu A.M. Lysine-phosphatidylcholine adducts in Kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a) J. Biol. Chem. 2003;278:52841–52847. doi: 10.1074/jbc.M310425200. [DOI] [PubMed] [Google Scholar]
- 124.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E.R., Shin M.-J., Binder C.J., Hörkkö S., Krauss R.M., Chapman M.J., Witztum J.L., Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 2008 doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 125.Goldstein J.L., Brown M.S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu. Rev. Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 126.Barter P. Lipoprotein metabolism and CKD: overview. Clin. Exp. Nephrol. 2014;18:243–246. doi: 10.1007/s10157-013-0866-9. [DOI] [PubMed] [Google Scholar]
- 127.Arca M. Alterations of intestinal lipoprotein metabolism in diabetes mellitus and metabolic syndrome. Atheroscler. Suppl. 2015;17:12–16. doi: 10.1016/S1567-5688(15)50004-4. [DOI] [PubMed] [Google Scholar]
- 128.Hottman D.A., Chernick D., Cheng S., Wang Z., Li L. HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 2014;72:22–36. doi: 10.1016/j.nbd.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jürgens G., Chen Q., Esterbauer H., Mair S., Ledinski G., Dinges H.P. Immunostaining of human autopsy aortas with antibodies to modified apolipoprotein B and apoprotein(a) Arterioscler. Thromb. A J. Vasc. Biol. 1993;13:1689–1699. doi: 10.1161/01.atv.13.11.1689. [DOI] [PubMed] [Google Scholar]
- 130.van Dijk R.A., Kolodgie F., Ravandi A., Leibundgut G., Hu P.P., Prasad A., Mahmud E., Dennis E., Curtiss L.K., Witztum J.L., Wasserman B.A., Otsuka F., Virmani R., Tsimikas S. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J. Lipid Res. 2012;53:2773–2790. doi: 10.1194/jlr.P030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salomon R.G., Subbanagounder G., O’Neil J., Kaur K., Smith M.A., Hoff H.F., Perry G., Monnier V.M. Levuglandin E 2 -protein adducts in human plasma and vasculature. Chem. Res. Toxicol. 1997;10:536–545. doi: 10.1021/tx960157y. [DOI] [PubMed] [Google Scholar]
- 132.Baynes J.W., Thorpe S.R. Glycoxidation and lipoxidation in atherogenesis. Free Radic. Biol. Med. 2000;28:1708–1716. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 133.Nègre-Salvayre A., Garoby-Salom S., Swiader A., Rouahi M., Pucelle M., Salvayre R. Proatherogenic effects of 4-hydroxynonenal. Free Radic. Biol. Med. 2017;111:127–139. doi: 10.1016/j.freeradbiomed.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 134.Arcaro A., Daga M., Cetrangolo G.P., Ciamporcero E.S., Lepore A., Pizzimenti S., Petrella C., Graf M., Uchida K., Mamone G., Ferranti P., Ames P.R.J., Palumbo G., Barrera G., Gentile F. Generation of adducts of 4-hydroxy-2-nonenal with heat shock 60 kDa protein 1 in human promyelocytic HL-60 and monocytic THP-1 cell lines. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/296146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taleb A., Witztum J.L., Tsimikas S. Oxidized phospholipids on apoB-100-containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomark. Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsimikas S., Willeit P., Willeit J., Santer P., Mayr M., Xu Q., Mayr A., Witztum J.L., Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J. Am. Coll. Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 137.Kothari H., Nguyen A.T., Yang X., Hisada Y., Tsimikas S., Mackman N., Taylor A., McNamara C.A. Association of D-dimer with plaque characteristics and plasma biomarkers of oxidation-specific epitopes in stable subjects with coronary artery disease. J. Cardiovasc. Transl. Res. 2018;11:221–229. doi: 10.1007/s12265-018-9790-4. [DOI] [PubMed] [Google Scholar]
- 138.Kamstrup P.R., Hung M.-Y., Witztum J.L., Tsimikas S., Nordestgaard B.G. Oxidized phospholipids and risk of calcific aortic valve disease. Arterioscler. Thromb. Vasc. Biol. 2017;37:1570–1578. doi: 10.1161/ATVBAHA.116.308761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anderson D.R., Duryee M.J., Shurmur S.W., Um J.Y., Bussey W.D., Hunter C.D., Garvin R.P., Sayles H.R., Mikuls T.R., Klassen L.W., Thiele G.M. Unique antibody responses to malondialdehyde-acetaldehyde (MAA)-protein adducts predict coronary artery disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsimikas S., Brilakis E.S., Lennon R.J., Miller E.R., Witztum J.L., McConnell J.P., Kornman K.S., Berger P.B. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 2007 doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 141.Hill G.E., Miller J.A., Baxter B.T., Klassen L.W., Duryee M.J., Tuma D.J., Thiele G.M. Association of malondialdehyde-acetaldehyde (MAA) adducted proteins with atherosclerotic-induced vascular inflammatory injury. Atherosclerosis. 1998 doi: 10.1016/s0021-9150(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 142.Duryee M.J., Klassen L.W., Schaffert C.S., Tuma D.J., Hunter C.D., Garvin R.P., Anderson D.R., Thiele G.M. Malondialdehyde–acetaldehyde adduct is the dominant epitope after MDA modification of proteins in atherosclerosis. Free Radic. Biol. Med. 2010;49:1480–1486. doi: 10.1016/j.freeradbiomed.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frostegård J., Svenungsson E., Wu R., Gunnarsson I., Lundberg I.E., Klareskog L., Hörkkö S., Witztum J.L. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005;52:192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 144.Moreira P.I., Sayre L.M., Zhu X., Nunomura A., Smith M.A., Perry G. Detection and localization of markers of oxidative stress by in situ methods: application in the study of Alzheimer disease. Free Radic. Antioxid. Protoc. 2010 doi: 10.1007/978-1-60327-029-8_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sultana R., Perluigi M., Butterfield D.A. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 2013 doi: 10.1016/j.freeradbiomed.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Di Domenico F., Tramutola A., Butterfield D.A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 2017;111:253–261. doi: 10.1016/j.freeradbiomed.2016.10.490. [DOI] [PubMed] [Google Scholar]
- 147.Keeney J.T.R., Swomley A.M., Förster S., Harris J.L., Sultana R., Butterfield D.A. Apolipoprotein A-I: insights from redox proteomics for its role in neurodegeneration. Proteom. - Clin. Appl. 2013;7:109–122. doi: 10.1002/prca.201200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xing R., Liu S., Guo Z., Yu H., Li C., Ji X., Feng J., Li P. The antioxidant activity of glucosamine hydrochloride in vitro, Bioorganic. Med. Chem. 2006;14:1706–1709. doi: 10.1016/j.bmc.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 149.Hipkiss A.R. Chapter 3 carnosine and its possible roles in nutrition and health. Adv. Food Nutr. Res. 2009 doi: 10.1016/S1043-4526(09)57003-9. [DOI] [PubMed] [Google Scholar]
- 150.Marchette L.D., Wang H., Li F., Babizhayev M.A., Kasus-Jacobi A. Carcinine has 4-Hydroxynonenal scavenging property and neuroprotective effect in mouse retina. Investig. Ophthalmol. Vis. Sci. 2012 doi: 10.1167/iovs.11-9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie Z., Baba S.P., Sweeney B.R., Barski O.A. Detoxification of aldehydes by histidine-containing dipeptides: from chemistry to clinical implications. Chem. Biol. Interact. 2013 doi: 10.1016/j.cbi.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anderson E.J., Vistoli G., Katunga L.A., Funai K., Regazzoni L., Monroe T.B., Gilardoni E., Cannizzaro L., Colzani M., De Maddis D., Rossoni G., Canevotti R., Gagliardi S., Carini M., Aldini G. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018;128 doi: 10.1172/JCI94307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aldini G., Vistoli G., Regazzoni L., Benfatto M.C., Bettinelli I., Carini M. Edaravone inhibits protein carbonylation by a direct carbonyl-scavenging mechanism: focus on reactivity, selectivity, and reaction mechanisms. Antioxid. Redox Signal. 2010 doi: 10.1089/ars.2009.2814. [DOI] [PubMed] [Google Scholar]
- 154.Upadhyay A., Tuenter E., Ahmad R., Amin A., Exarchou V., Apers S., Hermans N., Pieters L. Kavalactones, a novel class of protein glycation and lipid peroxidation inhibitors. Planta Med. 2014;80:1001–1008. doi: 10.1055/s-0034-1382949. [DOI] [PubMed] [Google Scholar]
- 155.Nankar S.A., Pande A.H. Properties of apolipoprotein e derived peptide modulate their lipid-binding capacity and influence their anti-inflammatory function. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2014;1841:620–629. doi: 10.1016/j.bbalip.2014.01.006. [DOI] [PubMed] [Google Scholar]