The unexpected location of VvK5.1 expression detected in the lateral root primordium, berry phloem and pistil provides new insights into the roles that this outward channel type can play in plants.
Keywords: Flowers, new physiological properties, new tissue location sites, phloem, potassium Shaker channel, root lateral primordium, root to shoot translocation
Abstract
Grapevine (Vitis vinifera L.), one of the most important fruit crops, is a model plant for studying the physiology of fleshy fruits. Here, we report on the characterization of a new grapevine Shaker-type K+ channel, VvK5.1. Phylogenetic analysis revealed that VvK5.1 belongs to the SKOR-like subfamily. Our functional characterization of VvK5.1 in Xenopus oocytes confirms that it is an outwardly rectifying K+ channel that displays strict K+ selectivity. Gene expression level analyses by real-time quantitative PCR showed that VvK5.1 expression was detected in berries, roots, and flowers. In contrast to its Arabidopsis thaliana counterpart that is involved in K+ secretion in the root pericycle, allowing root to shoot K+ translocation, VvK5.1 expression territory is greatly enlarged. Using in situ hybridization we showed that VvK5.1 is expressed in the phloem and perivascular cells of berries and in flower pistil. In the root, in addition to being expressed in the root pericycle like AtSKOR, a strong expression of VvK5.1 is detected in small cells facing the xylem that are involved in lateral root formation. This fine and selective expression pattern of VvK5.1 at the early stage of lateral root primordia supports a role for outward channels to switch on cell division initiation.
Introduction
Potassium (K+) plays an important role in many plant physiological processes. This ion is the most abundant cation in the cytosol, with a concentration ranging between 100 mM and 200 mM (Ache et al., 2001; Wada et al., 2008). K+ ions are involved in basic functions such as the neutralization of non-diffusible negative charges, the control of cell membrane polarization, electrical signalling, and osmoregulation (Sharma et al., 2013; Dreyer et al., 2014; Véry et al., 2014). Indeed, K+ is involved in many integrated functions at the whole-plant level such as cellular expansion/elongation and division (Sano et al., 2007, 2009), the control of guard cell turgor allowing stomatal movements (Hosy et al., 2003; Lebaudy et al., 2008b), maintenance of cytosolic pH homeostasis, and the setting of the membrane potential (Maathuis, 2009; Marschner, 2012). In plants, the first molecular actors involved in K+ translocation and distribution are the Shaker K+ channels. These channels, expressed at the cell plasma membrane, dominate the membrane K+ conductance in most cell types and are implicated in the control of long-distance K+ transport (Sharma et al., 2013; Véry et al., 2014). More precisely, K+ Shaker channels are involved in a number of physiological functions that require sustained large-scale K+ fluxes. Furthermore, they drive the inwardly or outwardly rectifying K+ fluxes of the plasma membrane according to the membrane potential, allowing the inward or outward K+ translocation of the cell (Sharma et al., 2013). For instance, in Arabidopsis thaliana roots, the inward Shaker channel AKT1 and the outward channel SKOR are involved in K+ uptake from the soil (Lagarde et al., 1996; Hirsch et al., 1998; Xu et al., 2006) and the secretion of K+ into the xylem sap, allowing K+ translocation to the shoots (Gaymard et al., 1998).
Shaker K+ channels are multimeric proteins formed by the assembly of four Shaker gene products, known as subunits (Dreyer et al., 1997, 2004). Within this tetrameric structure, the four subunits can either be provided by a single Shaker gene (e.g. a homomeric Shaker channel), or by different Shaker genes (e.g. a heteromeric Shaker channel) (Lebaudy et al., 2008a, 2010). Each subunit has a hydrophobic core comprising six transmembrane domains, including a pore domain between the fifth and sixth transmembrane domains as well as a voltage sensor domain (the S4 transmembrane domain) (Zimmermann and Sentenac, 1999). The N- and C-terminal regions are cytoplasmic. The large C-terminal region begins just after the end of the sixth transmembrane domain (S6) and successively contains a C-linker domain, a cyclic nucleotide-binding domain (CNBD), an ankyrin domain, and a KHA domain (Ehrhardt et al., 1997; Sharma et al., 2013; Nieves-Cordones et al., 2014; Véry et al., 2014).
In grapevine, K+ plays an essential role in the initiation and control of the massive fluxes that are necessary for berry loading during its maturation (Cuéllar et al., 2013; Nieves-Cordones et al., 2019, Zhu et al., 2019). Moreover, in the context of environmental changes, K+ ion accumulation increases during grape ripening, leading to an excessive neutralization of organic acids (Cuéllar et al., 2010; 2013; Rogiers et al., 2017; Nieves-Cordones et al., 2019). This results in grape berries with low acidity at harvest, yielding wines with poor organoleptic properties and low potential ageing. This is a major concern for grape production, although information on the molecular determinants that control berry K+ accumulation during its ripening, as well as acidity in grape berry, is still fragmentary. This lack of information hinders wine producers from making sustainable choices to help face future challenges.
Here, we report on the characterization of the grapevine Shaker K+ channel VvK5.1. Based on our phylogenetic analysis, we show that this Shaker belongs to the SKOR subgroup of the Shaker channel family. Furthermore, electrophysiology analysis reveals that the VvK5.1 channel displays classical slowly activating outward K+ currents upon depolarizing voltage pulses. In grapevine, VvK5.1 is mainly expressed in three organs: the flowers, the grape berry phloem, and the roots. In the flowers, there is a strong expression of this channel in the stigmas and in the transmitting tract. In the grape berry phloem, the expression of VvK5.1 continuously increases after post-veraison and during grape ripening. In the roots, VvK5.1 expression is detected in the pericycle parenchyma cells, which is reminiscent of the role that the A. thaliana SKOR channel plays in secreting K+ into the xylem sap. We also detected an intense expression of VvK5.1 in the lateral root primordium. Finally, the roles played by the VvK5.1 channel in these three organs are discussed.
Materials and methods
Plant material
Four-year-old grapevines (Vitis vinifera cv. Cabernet Sauvignon) were potted in 70 litre plant containers and grown in field conditions. Plant watering was carried out by a controlled drip-irrigation system. The plant water status was checked by measuring leaf water potential (Ψ) at dawn (Cuellar et al., 2010), which remained close to –0.2 MPa throughout grape maturation. All organs were collected at fruit set, with the exception of berry samples, which were harvested at different stages of grape ripening. For VvK5.1 root expression analysis, root samples were harvested from 2-month-old rooted canes grown in ‘perlite’. All collected samples were immediately frozen in liquid nitrogen, crushed, and used for RNA extraction.
Cloning of the VvK5.1 cDNA subunit
First-strand cDNAs were generated from total RNA extracted from ripe berries using SuperScript III RT polymerase (Invitrogen 18080-051). Full-length VvK5.1 Shaker subunit cDNA (2466 bp) was then amplified using VvK5.1-V2-F1 and VvK5.1-V2-R1 specific primers (see Supplementary Table S1 at JXB online) prior to cloning into the pDONR207 entry vector using a Gateway strategy (Invitrogen).
Functional characterization of VvK5.1
VvK5.1 cRNAs produced using the mMessage mMachine T7 ultra transcription Kit (Invitrogen) were injected (15 ng in 14 nl) using a microinjector (Nanoliter2010, WPI, https://www.wpiinc.com) into Xenopus laevis oocytes. As a control, the same volume (14 nl) of water was injected. After injections, oocytes were kept at 20 °C in ND96 solution (pH 7.4; 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, and 50 µg ml–1 gentamicin). Membrane currents were recorded 2–4 d after injections with a two-microelectrode voltage-clamp amplifier (GeneClamp 500B; Axon Instruments, http://www.moleculardevices.com) and a Digidata 1322A interface (Axon Instruments), using pipettes filled with 3 M KCl. Recordings were visualized and saved on a pClampex 10 (Axon Instruments: Molecular Devices Corp., Sunnyvale, CA, USA). During recordings, oocytes were maintained in percolating solutions of 100 (K100), 50 (K50), 10 (K10), 1 (K1), or 0.1 (K0.1) mM potassium gluconate supplemented respectively with 0, 50, 90, 99, or 99.9 mM sodium gluconate. Each of these solutions contained 1 mM CaCl2, 2 mM MgCl2, 31 mM sorbitol, and 5 mM HEPES (pH 6.5 and 7.4) or 5 mM MES (pH 5.0 and 5.5). Furthermore, either 10 mM TEA (tetraethyl ammonium) or 10 mM Ba2+ was added to the K10 solution at pH 7.4 in order to test channel inhibition. Two voltage-clamp protocols were applied with 8 s voltage pulses, one from –110 mV to +40 mV and the other from –100 mV to + 50 mV (in –15 mV steps for each protocol). Each step started with a holding potential of –40 mV, then a 250 ms pulse at –100 mV was conducted before the different voltage steps. After each pulse of 4.5 s, the voltage returned to –100 mV for 2 s before returning to the holding potential.
Analyses were performed with the pClampfit 10 software program (Axon Instruments). Current–voltage (I/V) curves were obtained by plotting the steady-state currents at the end of the activating voltage pulses against the corresponding applied membrane potentials.
Total RNA extraction and real-time quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from grapevine samples using the Spectrum plant total RNA kit (Sigma Aldrich) and quantified with the Quant-iT Ribogreen RNA reagent (Invitrogen). After DNase I treatment (Invitrogen), first-strand cDNAs were synthesized with SuperScript III reverse transcriptase (Invitrogen) and used as a template for the RT-qPCR experiments. All steps were carried out according to the manufacturer’s instructions. RT-qPCR analyses were performed on the Lightcycler480 system using TB Green Premix Ex Taq (TAKARA) with 20 ng of total RNA in 10 µl. Reactions were performed in triplicate using two independent biological samples. All amplification plots were analysed with Lightcycler480 software, using a threshold of 0.25 to obtain CT (cycle threshold) values. The VvK5.1 expression level was then normalized using the corresponding CT values of the control housekeeping gene VvEf1-alpha (Terrier et al., 2005; Nieves-Cordones et al., 2019). Standard curves were obtained by successive dilution with known quantities of VvK5.1 and VvEf1-alpha cDNA amplicons. Specific primers for amplifying VvK5.1 (VvK5.1-V2-qP-3'-F1 and VvK5.1-V2-qP-3'-R1; Supplementary Table S1) and VvEf1-alpha (EF1-F/EF1-R; Cuéllar et al., 2010) were designed using the PRIMER3 website (http://primer3.ut.ee/) and are provided in Supplementary Table S1.
Tissue localization of VvK5.1 mRNA by in situ hybridization
Fresh grapevine organs (flowers, roots, and ripe berries) from plants grown in well-irrigated conditions were harvested, fixed in 4% paraformaldehyde solution (Bio-Rad), dehydrated, embedded in paraffin, and cut into 11 µm thick sections. VvK5.1 RNA sense and antisense probes were synthetized using the specific VvK5.1 primers (T7P-VvK5.1-V2-up/VvK5.1-V2-down and VvK5.1-V2-up/T7P-VvK5.1-V2-down; Supplementary Table S1) and labelled with UTP-digoxigenin during the transcription step (MAXIscript T7 transcription kit; Invitrogen). An 18S rRNA probe was used as positive control. Samples were hybridized overnight with VvK5.1 RNA probes and then incubated for 1 h with an anti-digoxigenin antibody conjugated to alkaline phosphatase (1/500 dilution; Roche, http://www.roche.com). Hybridization signals were revealed using the vector blue kit III (Vector Laboratories, http://www.vectorlabs.com; positive signal is blue). In situ hybridization experiments were independently repeated twice.
The DAPI (Sigma-Aldrich) nuclear counterstain was used to visualize the cell nucleus. DAPI [0.001% in 1× phosphate-buffered saline (PBS)] was applied onto hybridized tissues for 10 min in the dark. Slides were then observed with an Eclipse Ni-E microscope at the ×4, ×10, and ×20 objective magnifications (Nikon, https://www.microscope.healthcare.nikon.com), and images were taken with a Nikon DS-Ri2 camera using the NIS elements software. In situ hybridization pictures were taken in white light or DIC (differential interference contrast) mode. For fluorescent pictures, DAPI was excited at 350 nm in order to recover the emitted signal between 450 nm and 490 nm (blue).
Localization of VvK5.1 activity in planta
A 1.4 kb region upstream of the VvK5.1 gene (XP_010660282.1) ATG codon was amplified through two successive PCR runs using VvK5.1-prom-Fw1/VvK5.1-prom-Rv1 and VvK5.1-prom-Fw2/VvK5.1-prom-Rv2 primers (Supplementary Table S1), and inserted into the pGWB3 destination Gateway vector (Nakagawa et al., 2007) containing the β-glucuronidase (GUS) gene. Arabidopsis thaliana plants (Col ecotype) were transformed using the floral dip method (Clough and Bent, 1998). Transformants were selected on hygromycin, and homozygotes were recovered in the next generation. GUS activity was detected according to Lagarde et al. (1996), and slides were observed using an Eclipse Ni-E microscope (Nikon, https://www.microscope.healthcare.nikon.com).
Results
Molecular cloning of VvK5.1 cDNA
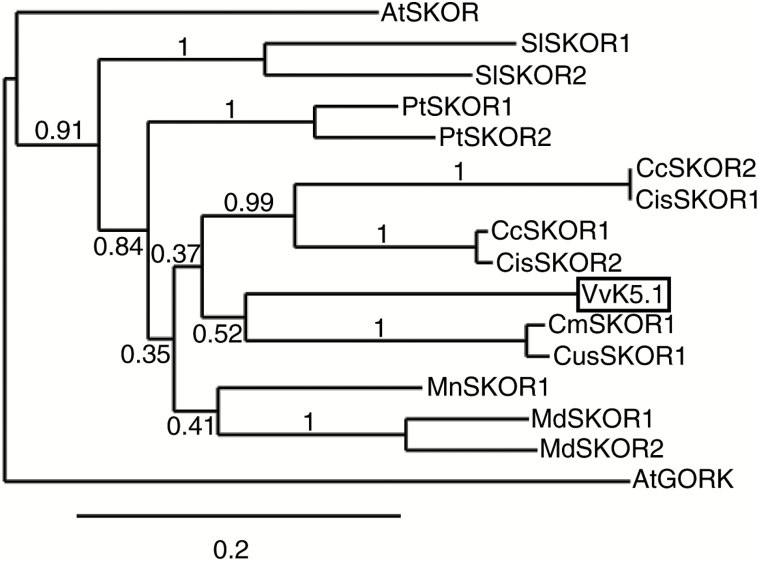
A 2466 bp cDNA was cloned by RT-PCR from the total RNA of post-veraison V. vinifera (cv. Cabernet Sauvignon) using the VvK5.1-V2-F1/VvK5.1-V2-R1 specific primers (Supplementary Table S1). The deduced VvK5.1 polypeptide contains 821 amino acids and shares similarity with other plant Shaker potassium channels. The hydrophobicity profile predicts that VvK5.1 displays the typical structural regions of a Shaker subunit with six membrane-spanning domains (S1–S6) flanked by N- and C-terminal cytosolic sequences of variable lengths. The N-terminal domain is very short, in contrast to the large C-terminal sequence that successively contains a C-linker domain, a CNBD, an ankyrin domain, and a KHA domain (Ehrhardt et al., 1997; Sharma et al., 2013; Nieves-Cordones et al., 2014). The six-transmembrane domain includes a well-defined pore region between the fifth and sixth transmembrane segments, which contains the hallmark TxxTxGYGD of highly K+-selective channels. Identification of the closest relatives of the grapevine VvK5.1 channel by phylogenetic analysis revealed that VvK5.1 is highly similar to the SKOR channels already characterized in several dicotyledonous plants (Fig. 1). These channels belong to the outwardly rectifying K+ channel subgroup of the Shaker family. The VvK5.1 sequence displays 76.5, 75.2, 71.6, and 66.5% amino acid sequence identities (ASI) throughout its entire protein length with CmSKOR1 from melon (Cucumis melo), CusSKOR1 from cucumber (Cucumis sativa), A. thaliana AtSKOR, and AtGORK, respectively.
Fig 1.
The phylogenetic relationships of VvK5.1. An unrooted phylogenetic tree was constructed with 16 polypeptide Shaker K+ channel sequences belonging to the outwardly rectifying potassium channel subfamily. VvK5.1 (XP_010660282.1) is the grapevine Shaker channel. Other sequenced species close to VvK5.1 are AtSKOR (At3g02850) and AtGORK (At5g37500) from Arabidopsis thaliana; SlSKOR1 (XP_004240037.1) and SlSKOR2 (XP_004250206.1) from tomato (Solanum lycopersicum); CcSKOR1 (XP_006421368.1) and CcSKOR2 (XP_006427880.1) from clementine (Citrus clementina); CisSKOR1 (XP_006464550.1) and CisSKOR2 (XP_015389397.1) from orange tree (Citrus sinensis); CmSKOR1 (XP_008460504.1) from melon (Cucumis melo); CusSKOR1 (XP_004140369.2) from cucumber (Cucumis sativa); MnSKOR1 (XP_010108959.1) from blackberry (Morus notabilis); MdSKOR1 (XP_008343075.1) and MdSKOR2 (XP_008381509.1) from apple (Malus domestica); and PtSKOR1 (XP_006372521.1) and PtSKOR2 (XP_002305894.2) from poplar (Populus trichocarpa). Bootstrap values are reported next to the nodes of the tree. Branch length is proportional to the evolutionary distance between the outward rectifying potassium channels of different dicotyledon species.
The grapevine channel VvK5.1 mediates outwardly rectifying K+ currents in Xenopus laevis oocytes
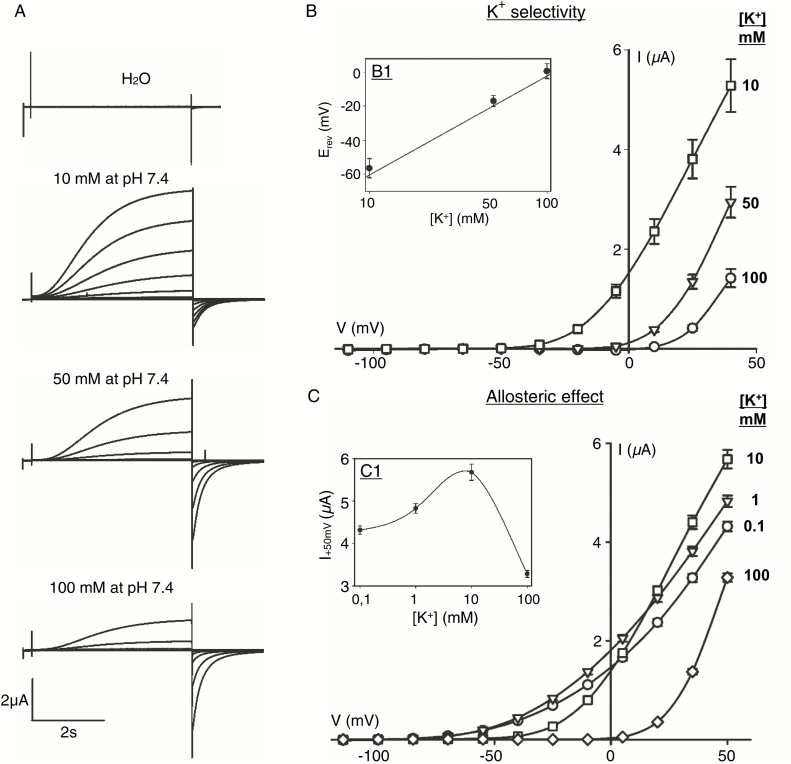
Heterologous expression of the VvK5.1 channel in X. laevis oocytes induced outwardly rectifying currents upon depolarizing voltage pulses (Fig. 2), which were never observed in control oocytes injected with water. These time-dependent slowly activating currents showed sigmoidal activation kinetics (Fig. 2A). Current amplitudes increased with lower external K+ levels according to the driving force for K+ efflux. Current–voltage curves (Fig. 2B) plotted from the steady-state currents at the end of the voltage pulses from –110 mV to +40 mV, as shown in Fig. 2A, revealed strict outward rectification of these K+-dependent currents. The activation potential displays a shift depending on external K+ concentration allowing, at lower K+ concentrations, an efflux of K+ at more hyperpolarized potentials. Further analyses of reversal potentials (Erev) when plotting tail currents from –110 mV to +40 mV after activation of the channel at +30 mV showed a shift towards more hyperpolarized potentials when the external K+ concentration was decreased. This shift of around –56 mV corresponds to the predicted Nernst potential of K+ for a 10-fold change in the K+ gradient (Fig. 2B, insert B1), proving the K+ selectivity of the channel.
Fig 2.
Functional characterization of the grapevine channel VvK5.1 by heterologous expression in Xenopus laevis oocytes. (A) Representative current traces in response to voltage–clamp pulses from –110 mV to +40 mV that depend on varying external K+ concentrations at pH 7.4 as indicated. Time-dependent outwardly rectifying currents are activated upon depolarization. (B) Current–voltage curves for mean currents mediated by VvK5.1 that depend on K+ concentrations; n=10 ±SE. Note the shift in the activation potential at the reduced external K+. (B1) VvK5.1 reversal potentials (Erev) were determined from tail current analysis, indicating a shift in dependence on external K+ concentrations as shown; n=10 ±SE. The line in the insert corresponds to the predicted Nernst potentials of K+. Tail currents were analysed at voltage pulses ranging from –110 mV to +40 mV, following activation of the channel at +30 mV. (C) Current–voltage curves for mean currents mediated by VvK5.1 that depend on K+ concentrations; n=8 ±SE. (C1) The maximal currents at +50 mV decreased between 10 mM and 0.1 mM K+, despite the increase in driving force.
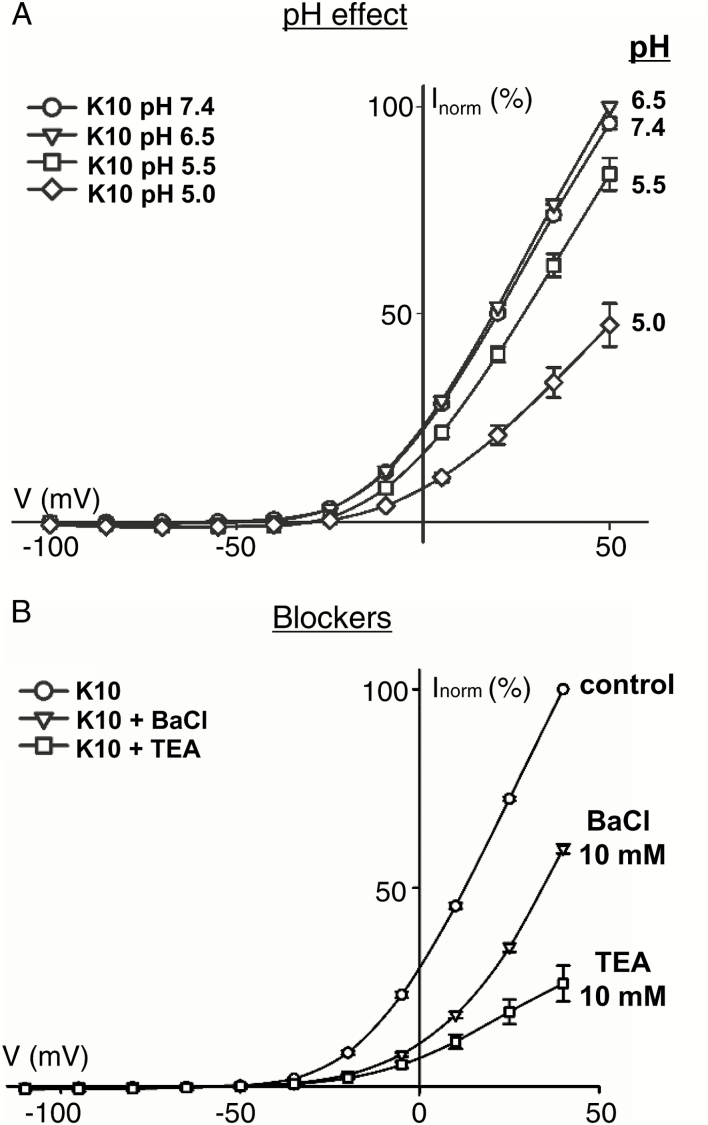
Further analyses of the dependence of the VvK5.1 channel on external K+ revealed a decrease in the maximal currents at depolarized potentials when K+ decreases below 10 mM (Fig. 2C). At concentrations of 1 mM or 0.1 mM K+, current amplitudes at positive potentials decreased even though the channel was activated at more hyperpolarized potentials, suggesting an allosteric regulation (Fig. 2C1). This kind of regulation has already been observed for other voltage-gated channels, such as the A. thaliana SKOR channel (Gaymard et al., 1998). Dependence of the VvK5.1 current amplitudes on external pH was tested at four different pH values at 10 mM K+ (Fig. 3A). No significant differences were observed in solutions adjusted to pH 7.4 or to pH 6.5. However, further acidification of the external solution to pH 5.5 or pH 5.0 reduced the VvK5.1 current, suggesting the regulation of channel permeation by protons. This pH dependence has also been observed for the A. thaliana outward rectifier GORK and SKOR (Ache et al., 2000; Lacombe et al., 2000), and is in contrast to the pH dependence of plant inward rectifiers such as KAT1 (Brüggemann et al., 1999) or VvK1.2 (Cuéllar et al., 2013). Finally, pharmacological properties of the VvK5.1 channel were tested by external perfusion with the known K+ channel blockers, BaCl and TEA (Fig. 3B). In both cases, the current amplitudes were largely inhibited, and maximal currents were respectively reduced by 40% (10 mM BaCl, n=11) and 75% (10 mM TEA, n=5). Altogether, our functional characterization of the grapevine channel VvK5.1 in oocytes demonstrates typical properties of plant outwardly rectifying voltage-gated K+ channels.
Fig 3.
Functional properties of the grapevine channel VvK5.1 when expressed in Xenopus laevis oocytes. (A) Dependence of VvK5.1-normalized mean currents upon external pH in 10 mM K+; n=8 ±SE. Normalization was conducted by setting the currents at +50 mV in the standard solution (pH 6.5) to 100%. The current is reduced upon acidification of the external bath solution. (B) Inhibition of VvK5.1-mediated normalized currents by 10 mM BaCl (n=11 ±SD) or TEA (n=5 ±SD) in an external solution of 10 mM K+ at pH 7.4. Inhibition is shown as a percentage of control currents (n=16).
VvK5.1 expression patterns
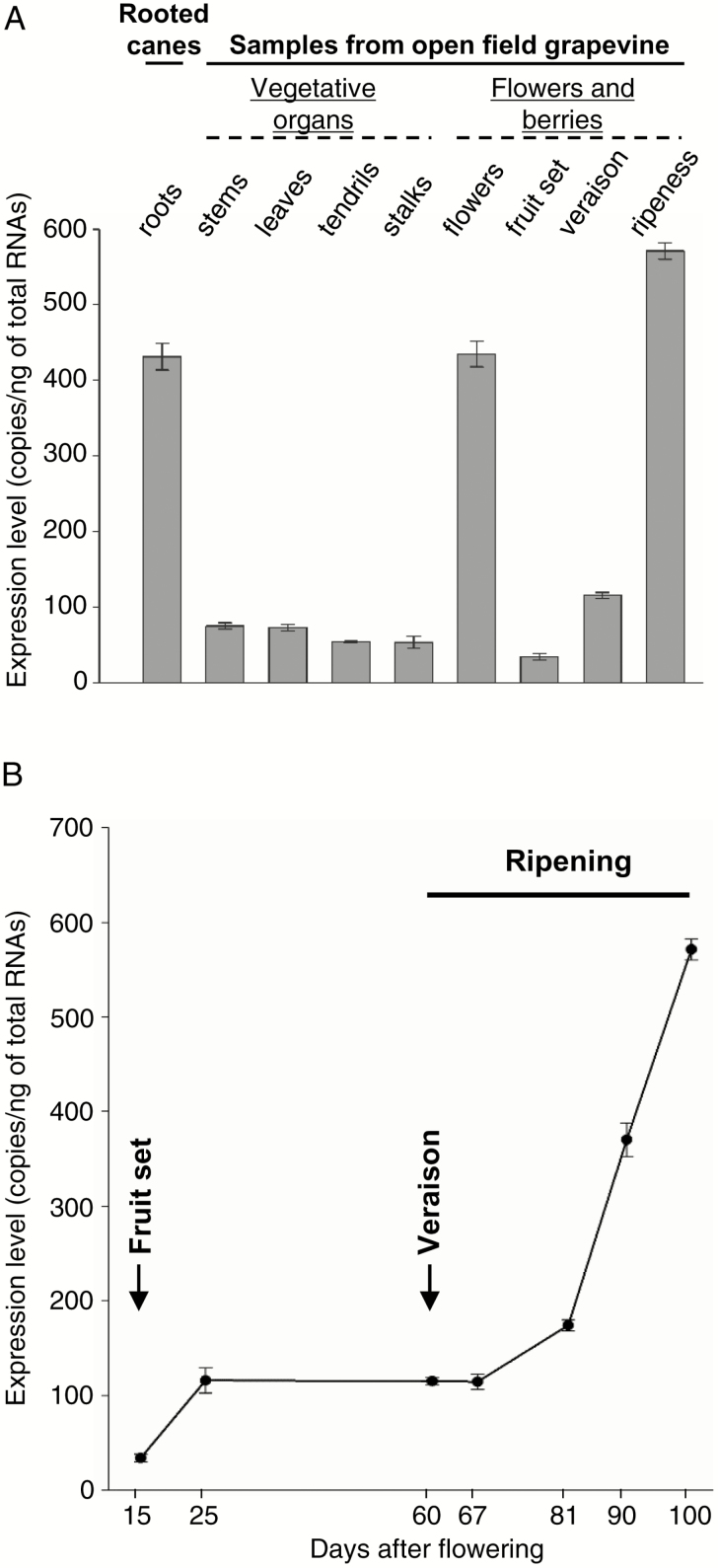
The spatial expression patterns of the VvK5.1 gene were investigated by RT-qPCR on total RNA extracted from roots, stems, leaves, tendrils, and stalks (all at fruit set), and from flowers and berries during their development. Unexpectedly, our results showed that VvK5.1 transcripts are mainly expressed in three organs: the roots, the flowers, and the berries, with expression levels ranging between 450 copies ng–1 and 550 copies ng–1 of total RNA (Fig. 4A). During grape berry development, VvK5.1 expression remains consistently very low until the berry post-veraison period (Fig. 4B). From this stage onward, the accumulation of Vv5.1 transcripts is strongly accelerated, increasing by up to 5-fold at ripeness.
Fig 4.
VvK5.1 transcript levels in grapevine organs and during berry development. RT-qPCR was performed on first-strand cDNAs synthesized from total RNAs of different organs. (A) VvK5.1 transcript levels in roots from rooted canes, or in vegetative organs (stems, leaves, tendrils, and stalks) collected at fruit set (15 d after flowering), flowers, or in berries at three different developmental stages (fruit set, veraison, and ripeness). Vegetative organs, flowers, and berries were collected from grapevines grown in open field conditions under standard irrigation. (B) VvK5.1 transcript levels of berries collected at different developmental stages in field conditions. The fruit set, veraison, and ripening phases are indicated. Note that VvK5.1 expression suddenly and strongly increased in grape berries at veraison. The mean values and SE of two biological replicates are presented.
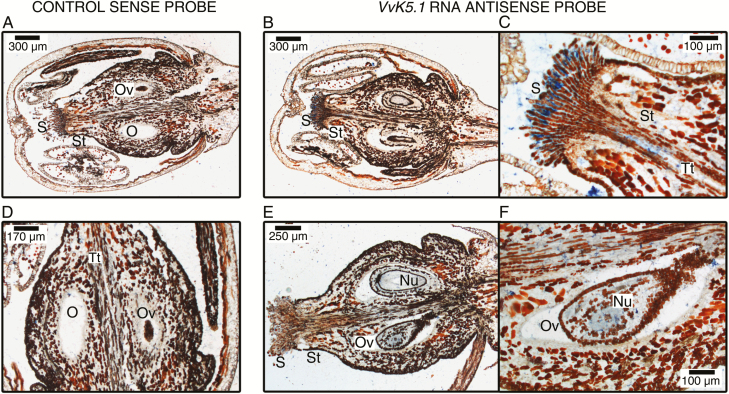
In order to identify the exact localization of VvK5.1 expression at the tissue level, in situ hybridization experiments were performed on flowers, ripe berries, and roots (Figs 5–7). In flowers, VvK5.1 signals were detected in the transmitting tract and in the stigmas (Fig. 5B, C), while in the ovule, the signal was detected in the nucellus (Fig. 5E, F).
Fig 5.
In situ localization of VvK5.1 transcripts in flowers. Longitudinal sections of flowers were hybridized with a VvK5.1 RNA sense probe as negative control (left column: A, D) or antisense probe (two right columns: B, C, E, F). Sections hybridized with the sense probe did not show any signal. Sections hybridized with the VvK5.1 antisense probe showed positive blue signals in the stigmas, the transmitting tract (B and C), and the ovule (E, F). In the ovule, blue signal was observed in the nucellus (F). Nu, nucellus; O, ovary; Ov, ovule; S, stigmas; St, style; Tt, transmitting tract.
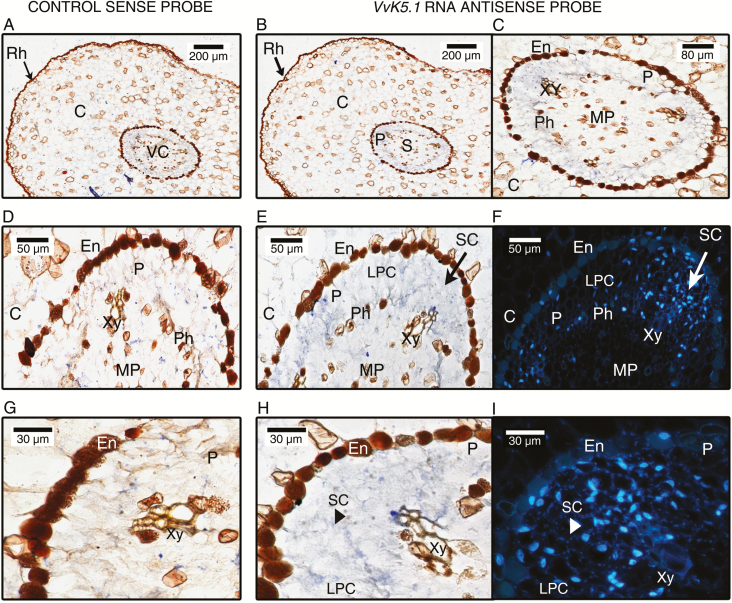
Fig. 7.
In situ localization of VvK5.1 transcripts in roots of rooted canes. Equatorial sections were hybridized with VvK5.1 RNA sense probe as negative control (left column: A, D, G) or with VvK5.1 RNA antisense probe (two right columns: B, C, E, F, H, I). Sections hybridized with VvK5.1 sense probe did not show any blue staining at the different magnifications (A, D, G). In contrast, positive signals were observed in the stele with VvK5.1 RNA antisense probe (B, C, E, H). The blue signals were located in the phloem in the small (SC) and large parenchyma cells (LPC) of the pericycle (C, E, H). A weaker signal was also detectable in the phloem. (E) and (H) were observed by fluorescence microscopy after DAPI staining to visualize nuclei and the different cell density of the pericycle (F, I). Rh, rhizodermis; C, cortex; En, endoderm; P, Pericycle; PM, medullary parenchyma; Xy, xylem; Ph, phloem; SC, small cells; LPC, large parenchyma cells.
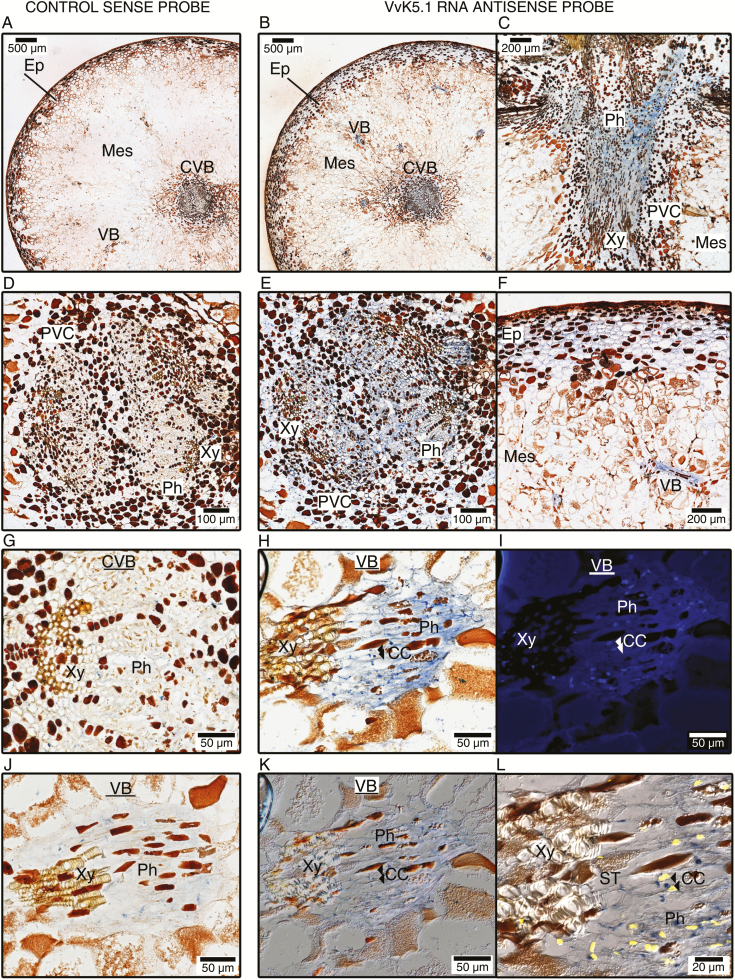
In ripe berries, strong VvK5.1 expression was located in the phloem of the central and peripheral vascular bundles (Fig. 6B, C, E, H). It is worth noting that this VvK5.1 expression was observed throughout the sieve tube system, which is composed of companion cells and enucleated sieve tubes (Fig. 6H, I, K, L). In addition, in situ hybridization results showed that the VvK5.1 gene is expressed in the epicarp cells (Fig. 6F).
Fig. 6.
In situ localization of VvK5.1 transcripts in berries during ripening. Longitudinal and equatorial sections were hybridized with the VvK5.1 RNA sense probe as negative control (left column: A, D, G, J) or VvK5.1 RNA antisense probe (right column: B, C, E, F, H, I, K, L). Sections probed with sense probes did not show any significant signal. Sections hybridized with RNA antisense probe showed positive blue signals in ripening berries. Intense blue signals were specifically found in the phloem (B, C, E), perivascular cells (B, C, E), and, to a lesser extent, in the epicarp cells (F). DAPI staining was performed after in situ hybridization of a longitudinal section revealed a vascular bundle (H), in order to identify companion cells via their nucleus and to distinguish them from enucleated phloem sieve tubes. The section stained with DAPI was observed by fluorescence microscopy to localize cell nuclei (I), and by DIC microscopy to visualize the cell walls (K). A zoomed composite picture (L) merging (H), (I), and (K) was constructed using Image J to help localize phloem companion cells (CCs) and enucleated sieve tubes (STs). As an example, the locations of two CCs are indicated by double arrows in (H), (I), (K), and (L). Note that these two cells display a positive blue signal after in situ hybridization (H and L). CC, companion cells; CVB, central vascular bundle; Ep, epicarp; VB, vascular bundle; Mes, mesocarp; Ph, phloem; PVC, perivascular cells; ST, sieve tubes; Xy, xylem.
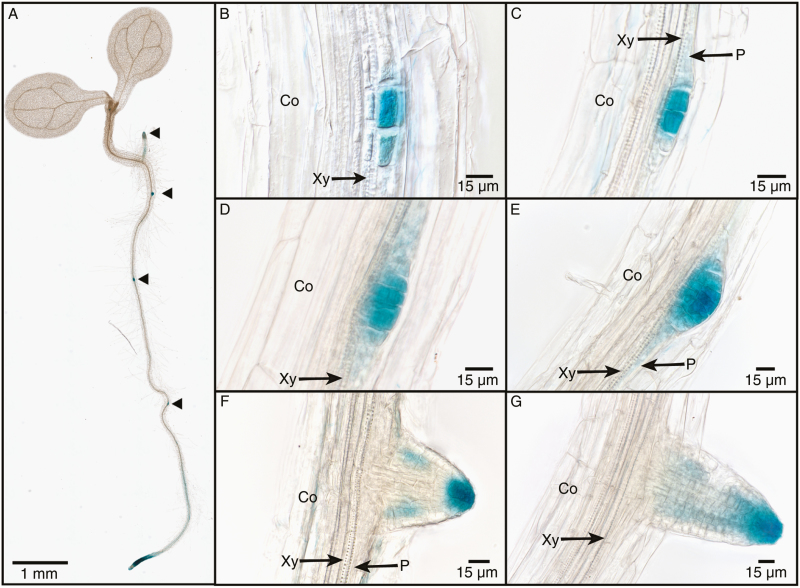
In roots, VvK5.1 expression was detected in the vascular cylinder (Fig. 7B, C). In situ hybridization signals were found in the root pericycle and to a lesser extent in the phloem. In the pericycle, we observed heterogenous VvK5.1 signals distributed between two different cell types: large parenchyma cells (LPCs) and small cells (SCs) of the pericycle. The SCs were always located near the xylem pole (Fig. 7), as revealed by cell nucleus labelling with DAPI (Fig. 7F). In this zone, the high number of observed nuclei indicates the presence of numerous cells. However, previous research has shown that the lateral root primordia arise from localized cell divisions into a pericycle zone located in the proximity of the xylem (Casero et al., 1989; Dubrovsky et al., 2000; Hochholdinger and Zimmermann, 2008), and so the strong expression of VvK5.1 in this zone suggests that this Shaker could be involved in primordia formation (Fig. 7E, F, H, I). To examine this hypothesis, we analysed the tissue-specific activity of the VvK5.1 promoter by histochemical analysis of GUS staining (blue) in transgenic plants of A. thaliana expressing a VvK5.1 promoter::GUS fusion construct. As shown in Fig. 8, GUS expression (blue colour) is observed in A. thaliana root cells beginning at the two-cell stage (Fig. 8B). Subsequently, further oriented divisions followed by cell enlargement give rise to an organized organ, the root primordium, which develops its own root apex and root cap, and grows through the parent root cortex to emerge at the root surface as a lateral root (Fig. 8C–G). This experiment confirms the involvement of the outward Shaker channel VvK5.1 in the lateral root formation. To date, no involvement of any outward K+ channels in lateral root primordia formation has been described.
Fig. 8.
Lateral root primordium-specific activity of the VvK5.1 promoter. Tissue-specific activity of the VvK5.1 promoter was investigated by histochemical analysis of GUS staining (blue colour) in transgenic Arabidopsis seedlings expressing GUS under control of the VvK5.1 promoter region. This activity was observed in 6- (A) and 10-day- (B–G) old seedlings grown in vitro in a growth chamber (16 h light photoperiod, 140 μM photons m−2 s−1, 21 °C ,and 70% humidity during both light and darkness) on half-strength Murashige and Skoog (MS/2) medium, supplemented with hygromycin (25 mg l−1). (A) A full view of the whole plantlet. Lateral primordia locations are indicated by arrows. (B–G) A coordinated cell division programme produces the root primordium, shown from the two-cell stage (B) to the stage where the lateral root primordium emerges at the root surface (G). Note that in each stage of the cell division programme, an intense blue colour is present within the most central cells of the primordium, which are known to undergo further cell divisions. In the stages where the primordium emerges at the root surface (F and G), the most intense blue colour is located within the root apex and the root cap.
Discussion
Two outward Shaker K+ channels have been identified and extensively characterized in the plant model A. thaliana (Gaymard et al., 1998; Hosy et al., 2003). The first one, GORK, encodes the major voltage-gated outwardly rectifying K+ channel of the guard cell membrane, which mediates guard cell K+ release, allowing stomatal closure (Ache et al., 2000; Pilot et al., 2001; Szyroki et al., 2001; Hosy et al., 2003; Lebaudy et al., 2008b). The GORK channel is also expressed in the root hairs, where it could play a role in physiological processes that involve K+ efflux from the roots (Demidchik, 2014). Furthermore, its expression has been reported in the phloem, and its role in the electrical propagation of action potentials has recently been deciphered (Cuin et al., 2018). In contrast, the second A. thaliana K+ channel, SKOR, is mainly expressed in the root pericycle and xylem parenchyma cells, and is involved in K+ secretion into the xylem sap (Gaymard et al., 1998; Nguyen et al., 2017; Long-Tang et al., 2018). The grapevine channel VvK5.1 belongs to the SKOR-like Shaker channel group (Fig. 1). Although the closest VvK5.1 relative is the CmSKOR1 melon channel (C. melo, with 76.5% ASI), which is primarily expressed in the root pericycle and is essentially involved in the translocation of K+ from the root to the shoot like its A. thaliana counterpart SKOR (Long-Tang et al., 2018), the wide-ranging expression profile of the VvK5.1 gene (which is highly linked to the channel’s functional properties) strongly indicates that VvK5.1 could be involved in different processes.
The VvK5.1 channel in flowers
In contrast to the ancestral wild grapevine V. vinifera subsp. sylvestris, which is a dioecious plant, the cultivated grapevine (V. vinifera) used for wine and grape production have hermaphroditic flowers. The main method of pollination in these domesticated vines is self-fertilization. However, in an inflorescence, the percentage of ovaries yielding fruit is strongly variable, and is affected by different factors including environmental changes such as light, temperature, and water stress (Lecourieux et al., 2017; Fábián et al., 2019), but also vineyard management and grape variety features (Dry et al., 2010). Indeed, low fruit set can limit crop yield, resulting in losses to grape and wine production. In grapevine, as for all flowering plants, the fertilization process begins with pollen hydration, once the pollen has landed on the stigma. This hydration stage is a key step, since pollen grains need to absorb water for germination. Germination is then initiated at the surface of the stigmatic papilla cells, and the pollen produces a pollen tube that grows in the intercellular space through the style, where it enters into the transmitting tract and delivers the two sperm cells to the ovule for the typical double fertilization of plants (Dresselhaus and Franklin-Tong, 2013; Doucet et al., 2016). The pistil is the site of pistil–pollen interaction and the cooperative processes that regulate pollen hydration, germination, pollen tube growth, and thereby fertilization. Potassium ions are required at each step (Campanoni and Blatt, 2007; Michard et al., 2009). Here, we demonstrated that the Shaker channel VvK5.1 is only expressed in the stigma and transmitting tract, and not in pollen grains (Fig. 5; Supplementary Fig. S1). Therefore, the channel is likely to be involved in the dialogue between pistil and pollen. VvK5.1 is a voltage-gated outwardly rectifying K+ channel activated under membrane depolarization that drives K+ secretion into the intercellular space. We propose that this K+ efflux could trigger water secretion from stigmatic papilla cells for use in pollen hydration, thus enabling germination. Subsequently, the pollen tube will grow down through the style. Pollen tube development is known to be very fast and highly polarized, with pronounced oscillations in growth rate (Zheng et al., 2018). This extensive growth by the pollen tube requires the influx of water and solutes into the cell before accumulating in the expanding vacuole. To sustain this growth rate, there are efficient ion transport systems at the pollen plasma membrane that have been identified and extensively studied. Among these systems, the inward K+ Shaker channel SPIK (Mouline et al., 2002; Zhao et al., 2013) is specifically expressed in pollen as well as pollen tubes, and its disruption results in impaired pollen tube growth (Mouline et al., 2002). The tandem-pore K+ channel AtTPK4 is also involved in this process (Becker et al., 2004), and the cation/proton exchangers CHX21 and CHX23 have been shown to be essential for pollen tube guidance toward the ovule (Lu et al., 2011). Another study using the loss-of-function triple mutant chx17chx18chx19 in A. thaliana revealed a mutant pollen altered at different levels including pollen tube growth, which is probably affected by the absence of AtCHX19 (Padmanaban et al., 2017). In contrast, information on K+ transport systems expressed in the pistil remains fragmentary. The growth of the pollen tube is dependent on K+ availability, and, when the K+ concentration is too low (preventing K+ influx into the cell), a total absence of tube growth is observed (Mouline et al., 2002). By relying on its functional features, the outward K+ VvK5.1 channel (which is expressed in the transmitting tract cells) drives K+ efflux and is probably involved in the secretion of K+ into the intercellular space of these cells. Thus, driving K+ secretion in the apoplast should make K+ more available, enabling the necessary K+ influx into the pollen tube for its development.
Involvement of the VvK5.1 channel in the berry phloem
Veraison is a key step in berry development, occurring at the onset of ripening. During this stage, long-distance transport from the xylem and phloem vasculatures towards the berries is profoundly restructured. The xylem becomes non-functional (Keller et al., 2006; Chatelet et al., 2008a, b; Choat et al., 2009; Knipfer et al., 2015) and berry loading is fed by a directional flow of water, sugar, and nutrients driven by massive K+ fluxes from the phloem. Since VvK5.1 expression precisely arrives in the berry starting at veraison and continues to increase throughout the grape ripening period (Fig. 4B), it is tempting to assume that this channel takes part in the necessary reorganization of the transport mechanisms for berry loading. The fact that this VvK5.1 expression is strictly located in the berry phloem and the perivascular cells supports this idea (Fig. 6).
In flowering plants, the phloem pipe is comprised of files of sieve elements and companion cells. During phloem development, the nuclei and vacuoles in the sieve elements are degraded, leading to enucleated phloem sieve tubes that have lost their capacity for transcription. The enucleated sieve tubes then become dependent on an association with the neighbouring nucleated companion cells in order to allow the plasma membrane of these sieve elements to remain functional. In the berry phloem, we identified VvK5.1 expression in both the enucleated phloem sieve tubes and the companion cells (Fig. 6H–L). This suggests that the VvK5.1 mRNAs identified within the sieve tube were probably delivered through the sieve plate pores via an associated companion cell, and do not belong to the large population of mRNAs that serve as mobile systemic signalling agents.
At veraison, K+ transport in the berry switches from the symplastic to the apoplastic mode (Zhang et al., 2006), meaning that ions, water, and solutes must cross plasma membranes at least twice before accumulating within the flesh cell vacuoles. Due to this, we and others have invested in the identification and characterization of K+ transport systems involved in grape berry loading during its maturation. Indeed, the weakly rectifying K+ Shaker channel VvK3.1, expressed in the berry phloem, is involved in the massive K+ efflux from the phloem cell cytosol to the berry apoplast (Nieves-Cordones et al., 2019). By switching to its non-rectifying mode, VvK3.1 drives the K+ efflux that allows K+ ions to move down their transmembrane concentration gradient. This is a major challenge for grapevine, as the fruit is energetically limited due to the disappearance of stomata after veraison (Blanke et al., 1999), and the additional energy stored in the transmembrane K+ gradient, known as the K+ battery, also allows sucrose retrieval under energy-limited conditions (Gajdanowicz et al., 2011; Dreyer et al., 2017; Nieves-Cordones et al., 2019). At the same time, the inwardly rectifying K+ channel VvK1.2, which is localized in the plasma membranes of perivascular and flesh cells and is strongly activated by its interactions with specific VvCIPK/VvCBL pairs (Cuéllar et al., 2013), drives the rapid absorption of K+ into these cells to keep the apoplastic K+ concentration at low levels (0.1–1 mM) (Ache et al., 2001; Nieves-Cordones et al., 2019). Thus, the phloem stream flux towards the sink should be triggered but also retained as long as the transmembrane gradient of K+ is maintained at the phloem plasma membrane. The VvK5.1 Shaker channel is an outward rectifying channel that is voltage dependent and only open under membrane depolarization. However, during berry loading, massive K+ fluxes hyperpolarize the plasma membrane potential, which explains why the outward channel VvK5.1 is probably not involved in the phloem unloading. In contrast, this channel is both K+ selective and K+ sensing, similar to the GORK and SKOR channels in A. thaliana (Gaymard et al., 1998; Ache et al., 2000, Johansson et al., 2006). According to the behaviour of outward K+ channels, the VvK5.1 current amplitude decreases when external K+ concentrations increase (Fig. 2B), except in the 0.1–1 mM external K+ concentration range. At these concentrations, which correspond to the expected apoplastic concentrations during phloem unloading, the current amplitudes decrease even though the channel is activated at more hyperpolarized potentials, strongly indicating an allosteric regulation. As shown in Fig. 2C, at external concentrations of 0.1 mM or 1 mM K+, activation of the VvK5.1 channel occurs at a voltage of approximately –65 mV, whereas when the external K+ concentration is of the order of 100 mM, the channel opening takes place at a positive voltage. In this context, in which a low apoplastic K+ concentration is maintained in the berry by storage of K+ in the flesh cells, we propose that the VvK5.1 channel should intervene in the repolarization of the plasma membrane. In plants, as in animals, this repolarization is proposed to be induced by K+ (Cuin et al., 2018). Recently, Cuin et al. (2018) expanded the role of the outward Shaker channels in A. thaliana by studying the involvement of voltage-gated ion channels in the propagation of action potentials. By directly recording action potentials from the midvein of Arabidopsis leaves, the authors demonstrated that the outward rectifying channel GORK is involved in the control of the membrane potential via the repolarization phase. A similar role can be observed for the VvK5.1 channel at the sites of phloem unloading, in which the voltage is driven back to its resting potential. There may be an identical role for VvK5.1 in the perivascular cells that does not exclude its involvement in K+ secretion into flesh cell apoplasts before being stored in the vacuoles of these cells.
A dual role for VvK5.1 in the root
The third organ in which VvK5.1 is significantly expressed is the root. In this organ, VvK5.1 expression is mainly detected in the pericycle, which is clearly composed of two cell types (Fig. 7): the LPCs and many SCs of the pericycle. The expression of VvK5.1 in the LPCs is reminiscent of that of the SKOR channel in the Arabidopsis root pericycle and xylem parenchyma, and clearly indicates that the VvK5.1 channel should be involved in K+ secretion in the xylem sap, thus playing a major role in K+ translocation from the root to the shoot as previously described for SKOR-like channels (Gaymard et al., 1998; Nguyen et al., 2017; Long-Tang et al., 2018). On the other hand, it is worth noting that the intense signal detected in the SCs does not match any known localization of SKOR expression, or that of any other outward Shaker channel. These SCs are precisely located in the protoxylem pole pericycle, and the large number of cells as revealed by the number of nuclei detected in this zone is indicative of an intense cell division process. Previous reports have established that the lateral roots are formed from pericycle cells located near the xylem pole (Casero et al., 1989; Dubrovsky et al., 2000; Hochholdinger and Zimmermann, 2008). These cells begin a coordinated programme of cell division and differentiation in order to produce a root primordium. The SCs, designated as lateral root founder cells, undergo this cell division programme with three successive phases. During the first phase, a single-cell layered primordium containing ~10 cells is produced. In the second phase, the most central cells undergo cell division leading to a two-cell layered primordium. In the third phase of lateral root formation, the central cells undergo several rounds of division that give rise to a primordium with an ellipsoid shape. This primordium grows through the different cell layers of the main root before emerging from the root surface. This process is perfectly illustrated in Fig. 8, where the different steps of lateral root formation are shown in the A. thaliana root expressing the GUS reporter gene under the control of the VvK5.1 promoter. This confirms the involvement of the VvK5.1 channel in establishing the lateral roots that shape the root architecture. K+, the most abundant inorganic cation in plants, is also essential to their existence. Specifically, it is involved in various physiological processes, including osmotically driven functions such as cell movement, regulation of stomatal aperture, cell expansion in growing tissues, and long-distance phloem transport. K+ channels also play a central role in plant growth and development, by driving K+ fluxes into or out of the cells. In particular, their roles in cell division and cell elongation/expansion have been investigated using tobacco BY-2 protoplast cultures (Sano et al., 2007, 2009), demonstrating that the activation of the outward channel could be used as a switch to induce cell division. In parallel, these studies revealed that K+ uptake and the increase in cell K+ content are necessary for cell elongation in relation to cytoplasmic pH regulation. In the context of these studies, we propose here that the K+ efflux driven by the VvK5.1 channel could allow the commencement and maintenance of the cell division programme that enables lateral root primordium formation.
Even though the functional properties of VvK5.1 are reminiscent of those of classical outward K+ Shaker channels, our results demonstrate overall that this channel has acquired a unique expression profile of its own. Some of our observed tissue-specific VvK5.1 expression patterns are to be expected, including in the LPCs of the pericycle, whereas other patterns are completely novel, such as the VvK5.1 expression in the lateral root primordium. It is tempting to assume that these particular tissue-specific types of expression confer VvK5.1 with new properties that are perfectly adapted to the needs of grapevine. Nevertheless, the physiological relevance of these evolutionary differences indicates that this biological diversity must be further investigated, since these differences are likely to have important roles in plant physiological functions.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Primers used in this study.
Fig. S1. In situ localization of VvK5.1 transcripts in flowers, showing pollen.
Supplementary Material
Acknowledgements
We thank Nadine Paris for helpful discussions. This work was supported by SweetKaliGrape ANR (ANR-33 14-CE20-0002-02). JV was the recipient of a PhD fellowship from the Institut National Recherche Agronomique and from Agropolis fondation in the context of APLIM (Advanced Plant Life Imaging) project (contract 1504-005).
References
- Ache P, Becker D, Deeken R, Dreyer I, Weber H, Fromm J, Hedrich R. 2001. VFK1, a Vicia faba K+ channel involved in phloem unloading. The Plant Journal 27, 571–580. [PubMed] [Google Scholar]
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R. 2000. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Letters 486, 93–98. [DOI] [PubMed] [Google Scholar]
- Becker D, Geiger D, Dunkel M, et al. . 2004. AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proceedings of the National Academy of Sciences, USA 101, 15621–15626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke MM, Prung RJ, Baker EA. 1999. Structure and elemental composition of grape berry stomata. Journal of Plant Physiology 154, 477–481. [Google Scholar]
- Brüggemann L, Dietrich P, Becker D, Dreyer I, Palme K, Hedrich R. 1999. Channel-mediated high-affinity K+ uptake into guard cells from Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 96, 3298–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanoni P, Blatt MR. 2007. Membrane trafficking and polar growth in root hairs and pollen tubes. Journal of Experimental Botany 58, 65–74. [DOI] [PubMed] [Google Scholar]
- Casero PJ, García-Sánchez C, Lloret PG, Navascués J. 1989. Morphological features of pericycle cells in relation to their topographical location in onion adventitious roots. New Phytologist 112, 527–532. [DOI] [PubMed] [Google Scholar]
- Chatelet DS, Rost TL, Matthews MA, Shackel KA. 2008a The peripheral xylem of grapevine (Vitis vinifera) berries. 2. Anatomy and development. Journal of Experimental Botany 59, 1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelet DS, Rost TL, Shackel KA, Matthews MA. 2008b The peripheral xylem of grapevine (Vitis vinifera). 1. Structural integrity in post-veraison berries. Journal of Experimental Botany 59, 1987–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Gambetta GA, Shackel KA, Matthews MA. 2009. Vascular function in grape berries across development and its relevance to apparent hydraulic isolation. Plant Physiology 151, 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cuéllar T, Azeem F, Andrianteranagna M, Pascaud F, Verdeil JL, Sentenac H, Zimmermann S, Gaillard I. 2013. Potassium transport in developing fleshy fruits: the grapevine inward K+ channel VvK1.2 is activated by CIPK–CBL complexes and induced in ripening berry flesh cells. The Plant Journal 73, 1006–1018. [DOI] [PubMed] [Google Scholar]
- Cuéllar T, Pascaud F, Verdeil JL, Torregrosa L, Adam-Blondon AF, Thibaud JB, Sentenac H, Gaillard I. 2010. A grapevine Shaker inward K+ channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. The Plant Journal 61, 58–69. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Dreyer I, Michard E. 2018. The role of potassium channels in Arabidopsis thaliana long distance electrical signalling: AKT2 modulates tissue excitability while GORK shapes action potentials. International Journal of Molecular Sciences 19, 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V. 2014. Mechanisms and physiological roles of K+ efflux from root cells. Journal of Plant Physiology 171, 696–707. [DOI] [PubMed] [Google Scholar]
- Doucet J, Lee HK, Goring DR. 2016. Pollen acceptance or rejection: a tale of two pathways. Trends in Plant Science 21, 1058–1067. [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Franklin-Tong N. 2013. Male–female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Molecular Plant 6, 1018–1036. [DOI] [PubMed] [Google Scholar]
- Dreyer I. 2014. Potassium K+ in plants. Journal of Plant Physiology 171, 655. [DOI] [PubMed] [Google Scholar]
- Dreyer I, Antunes S, Hoshi T, Müller-Röber B, Palme K, Pongs O, Reintanz B, Hedrich R. 1997. Plant K+ channel alpha-subunits assemble indiscriminately. Biophysical Journal 72, 2143–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer I, Gomez-Porras JL, Riedelsberger J. 2017. The potassium battery: a mobile energy source for transport processes in plant vascular tissues. New Phytologist 216, 1049–1053. [DOI] [PubMed] [Google Scholar]
- Dreyer I, Porée F, Schneider A, Mittelstädt J, Bertl A, Sentenac H, Thibaud JB, Mueller-Roeber B. 2004. Assembly of plant Shaker-like Kout channels requires two distinct sites of the channel alpha-subunit. Biophysical Journal 87, 858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry PR, Longbottom ML, McLoughlin S, Johnson T, Collins C. 2010. Classification of reproductive performance of ten winegrape varieties. Australian Journal of Grape and Wine Research. 16, 47–55. [Google Scholar]
- Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL. 2000. Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiology 124, 1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt T, Zimmermann S, Müller-Röber B. 1997. Association of plant K+in channels is mediated by conserved C-termini and does not affect subunit assembly. FEBS Letters 409, 166–170. [DOI] [PubMed] [Google Scholar]
- Fábián A, Sáfrán E, Szabó-Eitel G, Barnabás B, Jäger K. 2019. Stigma functionality and fertility are reduced by heat and drought co-stress in wheat. Frontiers in Plant Science 10, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdanowicz P, Michard E, Sandmann M, et al. . 2011. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proceedings of the National Academy of Sciences, USA 108, 864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferrière N, Thibaud JB, Sentenac H. 1998. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655. [DOI] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. 1998. A role for the AKT1 potassium channel in plant nutrition. Science 280, 918–921. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Zimmermann R. 2008. Conserved and diverse mechanisms in root development. Current Opinion in Plant Biology 11, 70–74. [DOI] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, et al. . 2003. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proceedings of the National Academy of Sciences, USA 100, 5549–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Wulfetange K, Porée F, et al. . 2006. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. The Plant Journal 46, 269–281. [DOI] [PubMed] [Google Scholar]
- Keller M, Smith JP, Bondada BR. 2006. Ripening grape berries remain hydraulically connected to the shoot. Journal of Experimental Botany 57, 2577–2587. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fei J, Gambetta GA, McElrone AJ, Shackel KA, Matthews MA. 2015. Water transport properties of the grape pedicel during fruit development: insights into xylem anatomy and function using microtomography. Plant Physiology 168, 1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe B, Pilot G, Gaymard F, Sentenac H, Thibaud JB. 2000. pH control of the plant outwardly-rectifying potassium channel SKOR. FEBS Letters 466, 351–354. [DOI] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C. 1996. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. The Plant Journal 9, 195–203. [DOI] [PubMed] [Google Scholar]
- Lebaudy A, Hosy E, Simonneau T, Sentenac H, Thibaud JB, Dreyer I. 2008a Heteromeric K+ channels in plants. The Plant Journal 54, 1076–1082. [DOI] [PubMed] [Google Scholar]
- Lebaudy A, Pascaud F, Véry AA, Alcon C, Dreyer I, Thibaud JB, Lacombe B. 2010. Preferential KAT1–KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. Journal of Biological Chemistry 285, 6265–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, Thibaud JB, Very AA, Simonneau T, Sentenac H. 2008b Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proceedings of the National Academy of Sciences, USA 105, 5271–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux F, Kappel C, Pieri P, Charon J, Pillet J, Hilbert G, Renaud C, Gomès E, Delrot S, Lecourieux D. 2017. Dissecting the biochemical and transcriptomic effects of a locally applied heat treatment on developing Cabernet Sauvignon grape berries. Frontiers in Plant Science 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-Tang H, Li-Na Z, Li-Wei G, Anne-Aliénor V, Hervé S, Yi-Dong Z. 2018. Constitutive expression of CmSKOR, an outward K+ channel gene from melon, in Arabidopsis thaliana involved in saline tolerance. Plant Science 274, 492–502. [DOI] [PubMed] [Google Scholar]
- Lu Y, Chanroj S, Zulkifli L, Johnson MA, Uozumi N, Cheung A, Sze H. 2011. Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. The Plant Cell 23, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJ. 2009. Physiological functions of mineral macronutrients. Current Opinion in Plant Biology 12, 250–258. [DOI] [PubMed] [Google Scholar]
- Marschner P. 2012. Nutritional physiology. In: Marschner P, ed. Marschner’s mineral nutrition of higher plants, 3rd edn.San Diego: Academic Press, 3–311. [Google Scholar]
- Michard E, Alves F, Feijó JA. 2009. The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. International Journal of Developmental Biology 53, 1609–1622. [DOI] [PubMed] [Google Scholar]
- Mouline K, Véry AA, Gaymard F, Boucherez J, Pilot G, Devic M, Bouchez D, Thibaud JB, Sentenac H. 2002. Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes & Development 16, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. 2007. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. Journal of Bioscience and Bioengineering 104, 34–41. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Huang S, Meynard D, Chaine C, Michel R, Roelfsema MRG, Guiderdoni E, Sentenac H, Véry AA. 2017. A dual role for the OsK5.2 ion channel in stomatal movements and K+ loading into xylem sap. Plant Physiology 174, 2409–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Cordones M, Andrianteranagna M, Cuéllar T, et al. . 2019. Characterization of the grapevine Shaker K+ channel VvK3.1 supports its function in massive potassium fluxes necessary for berry potassium loading and pulvinus-actuated leaf movements. New Phytologist 222, 286–300. [DOI] [PubMed] [Google Scholar]
- Nieves-Cordones M, Chavanieu A, Jeanguenin L, Alcon C, Szponarski W, Estaran S, Chérel I, Zimmermann S, Sentenac H, Gaillard I. 2014. Distinct amino acids in the C-linker domain of the Arabidopsis K+ channel KAT2 determine its subcellular localization and activity at the plasma membrane. Plant Physiology 164, 1415–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanaban S, Czerny DD, Levin KA, et al. . 2017. Transporters involved in pH and K+ homeostasis affect pollen wall formation, male fertility, and embryo development. Journal of Experimental Botany 68, 3165–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot G, Lacombe B, Gaymard F, Cherel I, Boucherez J, Thibaud JB, Sentenac H. 2001. Guard cell inward K+ channel activity in arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. Journal of Biological Chemistry 276, 3215–3221. [DOI] [PubMed] [Google Scholar]
- Rogiers SY, Coetzee ZA, Walker RR, Deloire A, Tyerman SD. 2017. Potassium in the grape (Vitis vinifera L.) berry: transport and function. Frontiers in Plant Science 8, 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Becker D, Ivashikina N, Wegner LH, Zimmermann U, Roelfsema MR, Nagata T, Hedrich R. 2007. Plant cells must pass a K+ threshold to re-enter the cell cycle. The Plant Journal 50, 401–413. [DOI] [PubMed] [Google Scholar]
- Sano T, Kutsuna N, Becker D, Hedrich R, Hasezawa S. 2009. Outward-rectifying K+ channel activities regulate cell elongation and cell division of tobacco BY-2 cells. The Plant Journal 57, 55–64. [DOI] [PubMed] [Google Scholar]
- Sharma T, Dreyer I, Riedelsberger J. 2013. The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Frontiers in Plant Science 4, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyroki A, Ivashikina N, Dietrich P, et al. . 2001. KAT1 is not essential for stomatal opening. Proceedings of the National Academy of Sciences, USA 98, 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrier N, Glissant D, Grimplet J, et al. . 2005. Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222, 832–847. [DOI] [PubMed] [Google Scholar]
- Véry AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H. 2014. Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? Journal of Plant Physiology 171, 748–769. [DOI] [PubMed] [Google Scholar]
- Wada H, Shackel KA, Matthews MA. 2008. Fruit ripening in Vitis vinifera: apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta 227, 1351–1361. [DOI] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. 2006. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125, 1347–1360. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Yu XC, Zhang DP. 2006. A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiology 142, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LN, Shen LK, Zhang WZ, Zhang W, Wang Y, Wu WH. 2013. Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. The Plant Cell 25, 649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YY, Lin XJ, Liang HM, Wang FF, Chen LY. 2018. The long journey of pollen tube in the pistil. International Journal of Molecular Sciences 19, 3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Génard M, Poni S, Gambetta GA, Vivin P, Vercambre G, Trought MCT, Ollat N, Delrot S, Dai Z. 2019. Modelling grape growth in relation to whole-plant carbon and water fluxes. Journal of Experimental Botany 70, 2505–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Sentenac H. 1999. Plant ion channels: from molecular structures to physiological functions. Current Opinion in Plant Biology 2, 477–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.