A maize introgression line is developed harboring QTLs from Zea nicaraguensis for constitutive aerenchyma, which allows oxygen to diffuse along roots immediately after waterlogging and contributes to oxygen deficiency tolerance.
Keywords: Cell viability, flooding, introgression line, oxygen deficiency, radial oxygen loss, root extension, root internal aeration, teosinte, waterlogging, Zea mays subsp. mays
Abstract
Zea nicaraguensis is a wild relative of Zea mays subsp. mays (maize) that has high waterlogging tolerance. One of its traits is constitutive aerenchyma formation (CAF) in roots and this may be one of the reasons for the tolerance, but it has not yet been proven by comparing plants that differ only in CAF in the same genetic background. We therefore produced an introgression line AE24-50-44-91 (IL-AE91) possessing four quantitative trait loci for CAF from Z. nicaraguensis in the background of maize (inbred line Mi29). The degree of root CAF in IL-AE91 was intermediate between that of Mi29 and Z. nicaraguensis. Seedlings of IL-AE91 grown aerobically were more tolerant to transfer to oxygen-deficient conditions than were Mi29 seedlings. On day 2 of oxygen deficiency, the root extension rate and viability of root-tip cells in IL-AE91 were ~2.7 and ~1.3 times greater, respectively, than they were in Mi29. On day 4, the area of aerenchyma at 80 mm from the root tips was ~1.5 times greater in IL-AE91 and radial oxygen loss from the apical parts of roots was ~3.4 times higher than in Mi29. These results demonstrate that CAF reduces the stress from low external oxygen levels caused by soil waterlogging.
Introduction
Oxygen deficiency stress, which is induced by abiotic factors such as flooding (waterlogging or submergence) and soil compaction, negatively affects the growth of plants and ultimately their yields, especially for upland crops such as maize (Zea mays subsp. mays), wheat (Triticum aestivum), and barley (Hordeum vulgare) (Bailey-Serres et al., 2012; Voesenek and Bailey-Serres, 2015; Herzog et al., 2016; Arora et al., 2017; Mustroph, 2018). Plants have at least three traits that allow them to acclimate to low oxygen levels in the soil. These are the abilities to form aerenchyma for transporting oxygen to the roots, a barrier to radial oxygen loss (ROL) to prevent oxygen leakage to the rhizosphere, and adventitious roots at the soil surface (Armstrong, 1968, 1979; Colmer, 2003; Yamauchi et al., 2018). In this way, the plant can mitigate oxygen deficiency in the root zone by obtaining oxygen from the shoots and/or soil surface. These traits are more pronounced in waterlogging-tolerant plants than in those that are intolerant (Colmer and Voesenek, 2009; Yamauchi et al., 2018).
Generally, cereal crops form lysigenous aerenchyma via cortical cell death and autolysis (Evans, 2003). Some wetland plants such as rice (Oryza sativa) show constitutive aerenchyma formation (CAF, i.e. even in drained soil), and further development of the aerenchyma can be induced by waterlogged or oxygen-deficient conditions, termed inducible aerenchyma formation (IAF) (Colmer et al., 2006; Shiono et al., 2011). In contrast, aerenchyma is not formed in the roots of many upland crops (including maize and wheat) under well-drained soil conditions, but it is formed under stressful conditions, such as those caused by waterlogging (Colmer and Voesenek, 2009; Yamauchi et al., 2018), drought (Zhu et al., 2010), and nutrient deficiency (Drew et al., 1989; Fan et al., 2003; Postma and Lynch, 2011). Aerenchyma formation can be induced in the adventitious roots of upland plants within several hours (Geisler-Lee et al., 2010; Rajhi et al., 2011) or days (Burdick, 1989) after the onset of oxygen deficiency. However, the roots that formed under aerobic conditions may be damaged by the oxygen deficiency during the period prior to the aerenchyma being formed (Yamauchi et al., 2014b; Herzog et al., 2016). In the aerobically grown roots of wetland plants, CAF can supply oxygen to the root tips immediately after the onset of oxygen deficiency (Colmer and Voesenek, 2009). Thus, the trait for CAF is considered to be beneficial for rapid adaption of plants from well-drained soil conditions to waterlogged conditions. However, the advantage of CAF has yet to be clearly demonstrated by comparing plants that differ only in their ability for CAF whilst the rest of their genetic backgrounds are identical. Such a comparison would make it possible to determine the usefulness of CAF for practical breeding to develop waterlogging-tolerant upland crops.
Zea nicaraguensis (Nicaraguan teosinte; Iltis and Benz, 2000) is a wild relative of maize that is tolerant of waterlogging and can be crossed with maize (Mano and Omori, 2007). Z. nicaraguensis differs from maize in that it constitutively forms aerenchyma in adventitious roots (e.g. nodal roots) under aerated conditions (Mano and Omori, 2013b) and it forms a tight ROL barrier in roots under stagnant, deoxygenated conditions (Abiko et al., 2012a; Watanabe et al., 2017). It also has enhanced development of superficial adventitious roots in response to soil flooding (i.e. in the shallow water layer above the waterlogged soil; Mano et al., 2009) and it is also tolerant of toxic constituents in highly reduced soils, which are observed during long-term waterlogging (Mano and Omori, 2013a). It has been proposed that introducing these traits into maize would improve its waterlogging tolerance (Ray et al., 1999; Mano and Omori, 2007; Mano et al., 2016).
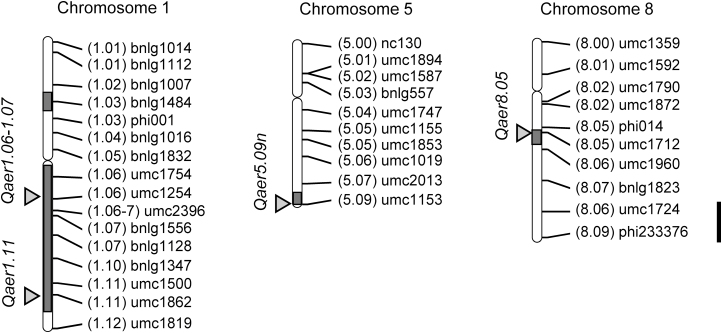
Quantitative trait loci (QTLs) associated with CAF in Z. nicaraguensis have been examined using populations of maize inbred line Mi29 crossed with Z. nicaraguensis (Mano and Omori, 2008, 2009), maize inbred line B64 crossed with Z. nicaraguensis (Mano et al., 2007), and S1 and S2 segregants of Z. nicaraguensis (Mano et al., 2012). So far, seven QTLs have been identified (Mano et al., 2016). Four of these, Qaer1.05-1.06 (Chr. 1), Qaer1.11 (Chr. 1), Qaer5.09n (Chr. 5), and Qaer8.05 (Chr. 8), have been detected in Mi29 × Z. nicaraguensis mapping populations, with Qaer1.05-1.06 showing the largest QTL effect (Mano and Omori, 2008, 2009). We recently found that Qaer1.05-1.06 is located in the interval of bin1.06-1.07 (Y. Mano and F. Omori, unpublished results) and therefore we hereafter term this QTL as Qaer1.06-1.07.
Root aerenchyma has previously been shown to have positive effects on waterlogging tolerance by comparisons of accessions with different genetic backgrounds, for example Z. nicaraguensis accessions with different degrees of CAF (Mano and Omori, 2013b) and barley varieties with different degrees of IAF (Zhang et al., 2015). A QTL related to waterlogging tolerance has also been identified on chromosome 4H of Hordeum spontaneum that overlaps with a locus involved in aerenchyma formation (Zhang et al., 2017). However, these comparisons were made between plants with different degrees of aerenchyma formation and also between different genetic backgrounds.
In the present study, we hypothesized that CAF can improve the tolerance of oxygen deficiency in the root zone, and to test this we compared maize lines with and without QTLs for CAF in the same genetic background under aerated and stagnant, deoxygenated conditions. Four characteristics were compared, namely the growth rate (including root extension), cell viability in the root tips, aerenchyma formation, and ROL in the apical parts of roots. The results of these comparisons clearly showed that CAF improved waterlogging tolerance.
Materials and methods
Plant materials
The maize (Zea mays subsp. mays) inbred line Mi29 was developed at the Kyushu Okinawa Agricultural Research Center, NARO, Japan. Zea nicaraguensis (CIMMYT 13451, a wild relative of maize) was provided by the International Maize and Wheat Improvement Center (CIMMYT), Mexico.
The introgression line (IL) AE24-50-44-91 (hereafter termed as IL-AE91) was developed as shown in Supplementary Table S1 at JXB online and possesses chromosome segments of Z. nicaraguensis of Qaer1.06-1.07, Qaer1.11, Qaer5.09n, and Qaer8.05 for CAF in the genetic background of Mi29 (Fig. 1). Briefly, IL #268-463-135 (possessing Qaer1.06-1.07, Qaer5.09n, and Qaer8.05 regions), which was selected from the segregants during the development of a library of ILs, each containing a chromosome segment from Z. nicaraguensis in the genetic background of maize Mi29 (Mano and Omori, 2013a), was crossed with S72-36 (possessing Qaer1.11) and self-pollinated three times until a homozygous line was produced. As a result, we generated IL-AE91, which contains ~14% (119.6 cM out of 852.7 cM) of Z. nicaraguensis genome (data not shown) according to an estimation based on the BC2F1 linkage map of Mi29×Z. nicaraguensis (Mano and Omori, 2008) and DNA marker information. The number of genes in the QTLs was estimated from the reference genome (v.2) of maize inbred line B73 in MaizeGDB (https://www.maizegdb.org/). Based on the total number of genes in the chromosome that contains these QTLs and the fraction of the distance covered by chromosome segments that contain the QTLs, we estimated that these segments contain ~1300 genes (data not shown).
Fig. 1.
Chromosome locations of the QTLs for constitutive aerenchyma formation (Qaer1.06-1.07, Qaer1.11, Qaer5.09n and Qaer8.05) in IL-AE91 introgressed from Zea nicaraguensis to Z. mays subsp. mays inbred line Mi29. Arrowheads indicate the logarithm of odds (LOD) peak for each QTL. The shaded areas indicate the chromosome segments derived from Z. nicaraguensis. In addition, small segments of Z. nicaraguensis remained on chromosomes 3 and 7 (Supplementary Table S1). Scale bar is 20 cM.
Hydroponic growth conditions
Seeds of IL-AE91 and Z. nicaraguensis were treated with 10% (v/v) hydrogen peroxide for 2 min to synchronize their germination (Naredo et al., 1998), air-dried on a paper towel for 2–3 h, sterilized by coating with Benlate {1-[(butylamino) carbonyl-1H-benzimidazol-2-yl] carbamic acid methyl ester} (Sumitomo Chemical, Tokyo, Japan) for 1 h, and wrapped in wet filter paper (25×25 cm, 3MM CHR; Whatman). They were then transferred to 2-l grey pots (250×120×90 mm height×length×width) containing 600 ml deionized water. The pots were shaded with aluminum foil to exclude light, sealed in plastic bags to maintain high humidity, and incubated in a growth chamber (LH220S; Nippon Medical & Chemical Instruments Co., Ltd, Osaka, Japan) for 4 d at 30 °C. On the fifth day, the aluminum foil was removed and seedlings were exposed to light for 1 d (PAR of 250–300 μmol m−2 s−1; 14/10 h light/dark). The Mi29 seeds were germinated in the same way except that the germination was started 2 d later because their early growth was faster than that of the other seeds. After the shoots had grown to a height of at least 3 cm, seedlings of a uniform size were selected and, using a soft sponge to hold them, were carefully transplanted to 5-l pots (250×190×120 mm; four plants per pot) containing 4.5 l half-strength nutrient solution at 28 °C. The composition of the nutrient solution at full strength was 1.0 mM NH4NO3, 0.5 mM NaH2PO4, 0.3 mM K2SO4, 0.3 mM CaCl2, 0.6 mM MgCl2, 0.045 mM Fe-EDTA, 50 µM H3BO3, 9 µM MnSO4, 0.3 µM CuSO4, 0.7 µM ZnSO4, and 0.1 µM Na2MoO4 (Mae and Ohira, 1981). The solution also contained 5 mM MES buffer and the pH was adjusted to 5.5 using KOH (Abiko et al., 2012a). During plant growth under aerated conditions, an air pump was used to keep the dissolved oxygen concentration in the pots always at more than 7.0 mg l−1 (>87% of the air equilibrium value at 28 °C), as measured using a SevenGo pro-SG6 dissolved oxygen meter (Mettler Toledo, Schwerzenbach, Switzerland). Every 2 d, 1 ml of 25 mM FeSO4 was supplied to each pot to give a final Fe2+ concentration of ~5.0 μM under aerated conditions (Kulichikhin et al., 2014). Nutrient solutions were renewed every 7 d.
When seedlings were 25–28 d old, some of them were kept in aerated solution (referred to as ‘aerated-to-aerated conditions’) and others were transferred to stagnant, deoxygenated nutrient solution (referred to as ‘aerated-to-stagnant conditions’). The stagnant solution consisted of half-strength nutrient solution containing 0.1% (w/v) agar and it was deoxygenated by bubbling with nitrogen gas prior to use (Wiengweera et al., 1997). The bubbling was continued until the dissolved oxygen level was lower than 0.5 mg l−1 (<6% of the air equilibrium value at 28 °C). The stagnant solution was designed to mimic the changes in gas composition (i.e. low oxygen and increased ethylene) that occur in waterlogged soil (Wiengweera et al., 1997). Some of the seedlings were used for anatomical analysis and measurements of ROL and cell viability of the root tips, and the remainder were used for growth measurements. In all experiments, the pots used for the three lines were arranged in a random manner in the growth chamber.
Soil growth conditions
To ensure that all seedlings were at the same three-leaf stage when the flooding treatment began, IL-AE91 and Z. nicaraguensis seeds were first germinated in moistened paper towels at 30 °C for 48 h; Z. nicaraguensis seeds were placed in the paper towels 1 d later than those of IL-AE91. After germination, the seeds were transferred to pots. Mi29 seeds were sown directly into pots at the time when germinated IL-AE91 seeds were transferred. We used pots (159 mm diameter, 300 mm depth) filled with granular soil (Coop Chemical, Tokyo, Japan; pH 6.2–6.8; 0.25 g N, 4.69 g P2O5, 0.19 g K2O per kg soil; soil density 0.8 kg l–1; soil granules 0.5–3.5 mm diameter) with one plant per pot, and four replicate for each line. When the seedlings were at the three-leaf stage (17 d after germination in IL-AE91), they were flooded with 0.1% (w/v) starch (Wako, Osaka, Japan) solution to 1 cm above the soil surface for either 7 d or 11 d, at which point they were sampled for shoot and root growth measurements. We used starch solution according to previous studies of rice (Ishihara et al., 1981), maize (Mano and Omori, 2013a), and barley (Mano and Takeda, 2012) in order to induce oxygen deficiency and to decrease the redox potential (Eh) in the soil, which mimics the conditions of long-duration soil waterlogging. The experiment was conducted from 5 November to 3 December 2018 in a greenhouse at 30/20 °C day/night. Eh was measured at a depth of 10 cm using platinum-tipped electrodes and a PRN-41 millivoltmeter with a 4400–0.65C electrode (Fujiwara Scientific Company, Tokyo, Japan). The measured Eh were corrected for the potential of the standard hydrogen electrode by adding 194 mV (30 °C). Eh values in the flooded soil were 518±18 mV (mean ±SD, n=6) at 1 h, –269±68 mV at 3 d (minimum value), –3±73 mV at 7 d, and –63±108 mV at 11 d after the flooding treatment.
Shoot growth measurements
In the hydroponic experiment, after the plants were exposed to stagnant, deoxygenated conditions for 7 d, the stagnant solution was renewed and treatment was continued for another week. Plant heights were measured every week after the commencement of the treatment. At the end of the experiment, shoots and roots were separated, dried at 70 °C for 5 d, and weighed to obtain their dry weights.
In the soil experiment, plant heights and leaf stages were recorded every day. The shoots and roots of the plants were harvested at 7 d and 11 d of treatment and their dry weights were determined as described above. Chlorophyll contents of the first, second, and third leaves were measured using a SPAD-502Plus meter (Konica Minolta). Measurements were taken at three positions on the leaf, at one-sixth, two-sixths, and three-sixths of the distance from the tip to the base. The mean value of these three positions was used for data analyses.
Root growth measurements
In the hydroponic experiment, we measured the extension of the fourth nodal root. One or two fourth nodal roots with lengths >20 mm were selected on aerobically grown plants at ~22 d old and a thread was loosely tied around them for identification. The lengths of these roots were measured every day. After 4 d, when the lengths were ~100 mm, some of the plants were transferred to stagnant, deoxygenated solution while the others were kept under aerated conditions as a control. We continued to measure the lengths until the root tip died under the stagnant, deoxygenated conditions. ROL, root aerenchyma formation, and root-tip cell viability were measured in the marked roots.
After transferring the plants to stagnant solution, new nodal roots started to form and emerge, which were thicker than the roots that emerged under aerated conditions. These anaerobically grown nodal roots, the lengths of which were ~55 mm after 4–6 d under stagnant conditions, were also used for ROL measurements.
Measurement of radial oxygen loss
Plants at ~26 d old were transferred to transparent plastic boxes with an oxygen-free nutrient solution containing 0.1% (w/v) agar, 5 mM KCl, and 0.5 mM CaSO4 that had previously been deoxygenated by bubbling with nitrogen gas (Colmer et al., 1998). The plants were fixed with a clip and adhesive tape above the root–shoot junction to ensure that the roots were immersed in the deoxygenated solution and that the shoots were in the air. ROL was measured using a cylindrical platinum electrode fitted with root-centralizing guides (Armstrong and Wright, 1975; Watanabe et al., 2017). The electrode (inner diameter 2.25 mm, height 5 mm) was placed around selected nodal roots of ~100 mm in length. ROL was measured at the tips of marked roots on days 0–4 after the plants were transferred to stagnant, deoxygenated conditions by positioning the center of the electrode 5–10 mm from the tip. After measurement, the roots were cut at 10 mm from the tip and this apical segment was used for determination of cell viability. The rest of the root was used for anatomical analyses.
To evaluate ROL barrier formation, ROL was measured by positioning the center of the electrode at different times and at different points along the roots. For roots that had formed under aerated conditions, we measured ROL daily at the root tips as described above, and at distances of 10–80 mm from the tip on days 2 and 4 under stagnant, deoxygenated conditions. To evaluate ROL barrier formation of the newly emerged roots under stagnant, deoxygenated conditions, ROL was measured at 10, 30, and 50 mm from the root tip in deoxygenated solution. All measurements were taken at 28 °C.
Anatomical observations of roots
Aerenchyma formation was examined using the same roots that were used for the ROL measurements described above. Following determination of ROL, the root segments of 10–100 mm from the tips were stored in 50% ethanol for later anatomical observations. Segments of roots that had grown aerobically were taken after transferring to stagnant, deoxygenated conditions on day 0 (i.e. control) and on days 1, 2, 3, and 4. The segments were 4 mm long and were taken at 10-mm intervals with their centers at 20–100 mm from the tip. Transverse sections were made by hand using a razor blade, and were imaged using a BX60 light microscope with a DP70 CCD camera (both Olympus). The area occupied by aerenchyma was measured using the Image J software (version 1.46r; https://imagej.nih.gov/ij/) and is expressed as a percentage of the entire cross-sectional area of the root.
Cell viability assays
The viability of cells at the root tips was measured using 2,3,5-triphenyltetrazolium chloride (TTC; Tokyo Chemical Industry Co., Ltd.) as described previously (Yamauchi et al., 2014b). TTC is a vital stain that is reduced and turns red in living cells. The assays were carried out on the same batch of roots that were used for ROL measurements. The 0–10-mm apical parts of the marked roots were cut, weighed, and immediately transferred to tubes containing 100 μl TTC solution. They were then incubated for 30 min at 40 °C, rinsed with deionized water, and destained in tubes containing 150 μl of 95% (v/v) ethanol overnight at 25 °C in darkness. Cell viability was expressed as the absorbance of the extracted solution at 520 nm as determined using a DU800 spectrophotometer (Beckman Coulter Inc., Brea, CA, USA).
Statistical analyses
Two-sample t-tests were used to compare the effects of the different treatments. Multiple comparisons were performed using one-way ANOVA followed by Tukey’s HSD post hoc test at a confidence level of 95% or Dunnett’s multiple comparison test using SPSS Statistics Version 19 (IBM Software).
Results
Aerenchyma formation under aerated conditions
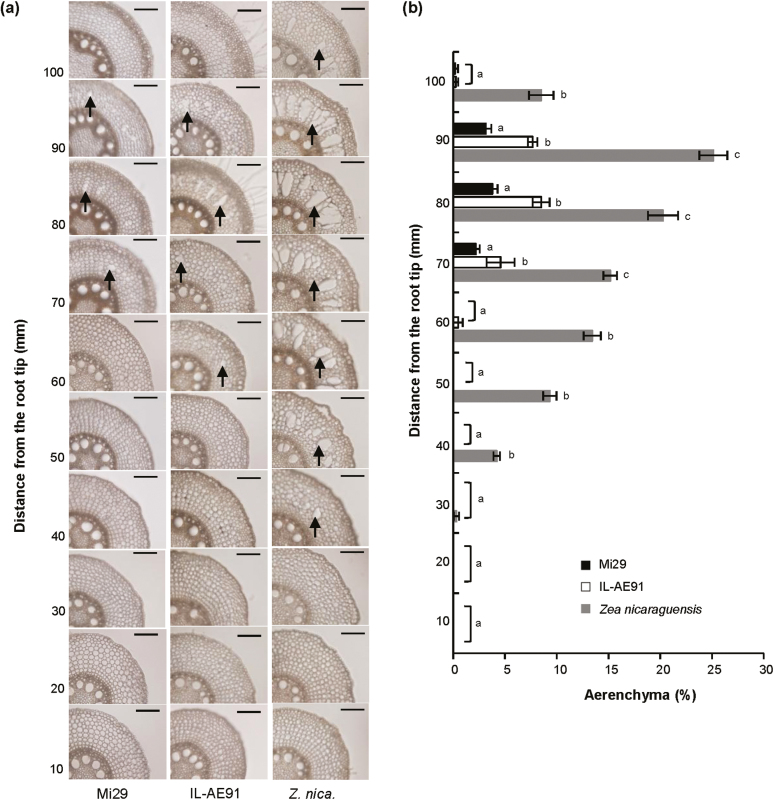
To compare maize lines with and without QTLs for CAF in the same genetic background, we produced the introgression line (IL) AE24-50-44-91 (IL-AE91) that contains four Z. nicaraguensis QTLs (Qaer1.06-1.07, Qaer1.11, Qaer5.09n, and Qaer8.05) for CAF in a Mi29 background (Fig. 1). IL-AE91 differs from Mi29 in the chromosome segments containing the QTLs for CAF. In the roots of maize (Mi29) and IL-AE91, aerenchyma formed at 70, 80, and 90 mm from the root tips but little could be observed elsewhere (Fig. 2a, b). The percentage of the root cross-sectional area occupied by aerenchyma at these distances were significantly higher in IL-AE91 (4.6%, 8.5%, and 7.7%, respectively) than in Mi29 (2.2%, 3.8%, and 3.1%, respectively). In the roots of Z. nicaraguensis, aerenchyma formation started at ~30 mm from the root tip (0.3% of root area), and the amount increased up to 90 mm (25.1%) and then decreased at 100 mm (8.5%). These results indicated that the four loci in the IL-AE91 genome contributed to the development of constitutive aerenchyma in the genetic background of Mi29, although the amounts were lower in IL-AE91 than in Z. nicaraguensis.
Fig. 2.
Constitutive aerenchyma formation in roots of Zea mays subsp. mays inbred line Mi29, introgression line IL-AE91, and Z. nicaraguensis under aerated conditions. (a) Cross-sections at 10–100 mm from the tips of 4th nodal roots ~100 mm long. Examples of aerenchyma are indicated by arrows. Scale bars are 100 μm. (b) Percentage of aerenchyma area in cross-sections of 4th nodal roots at 10–100 mm. Data are means (±SD), n=3. Different letters indicate significant differences between each line within each root position as determined using one-way ANOVA and Tukey’s test for multiple comparisons (P<0.05).
Growth in stagnant, deoxygenized nutrient solution and in flooded soil
In the hydroponic experiments, the plant height and the shoot and root dry weights of the three lines were all reduced by exposure to stagnant, deoxygenated conditions for 7 d and 14 d (Table 1, Fig. 3). The changes in IL-AE91 were intermediate between those of Mi29 and Z. nicaraguensis. For example, after 14 d of treatment, the root dry weights of Mi29, IL-AE91, and Z. nicaraguensis were 50%, 62%, and 86% of the aerated controls, respectively. Similar differences were observed in plants grown in soil after 7 d and 11 d of treatment (Table 2). Leaf chlorophyll contents in the three lines tended to show the same patterns observed in plant height and shoot and root dry weights. At 11 d of soil flooding, the first leaf of Mi29 and IL-AE91 had chlorophyll values of zero (i.e. the leaves were dead; Table 3), whilst in Z. nicaraguensis chlorophyll was still 10% of the control value, i.e. part of the leaf was still alive. Taken together, the results indicated that IL-AE91 was more tolerant of root-zone oxygen deficiency than Mi29 in both the stagnant solution (Table 1) and soil flooding (Tables 2, 3), suggesting that the presence of constitutively formed aerenchyma is beneficial for tolerance of waterlogging.
Table 1.
Growth of Mi29, IL-AE91, and Zea nicaraguensis plants in aerated or stagnant, deoxygenated nutrient solution
| Genotype | Plant height* (cm) | Shoot dry mass (g) | Root dry mass (g) | Plant height* (cm) | Shoot dry mass (g) | Root dry mass (g) | |
|---|---|---|---|---|---|---|---|
| 7 d | 14 d | ||||||
| Mi29 | Aerated | 53.8±1.5 a | 1.25±0.08 a | 0.34±0.01 a | 66.8±1.0 a | 1.92±0.05 a | 1.01±0.05 a |
| Stagnant | 39.8±1.0 d | 0.82±0.05 c | 0.22±0.01 d | 39.8±1.0 e | 0.57±0.10 f | 0.50±0.02 e | |
| % of aerated | 73 | 66 | 66 | 60 | 30 | 50 | |
| IL-AE91 | Aerated | 53.7±1.4 a | 0.91±0.04 b | 0.35±0.03 a | 64.0±0.9 b | 1.74±0.04 b | 0.87±0.06 b |
| Stagnant | 40.0±1.3 d | 0.77±0.24 d | 0.26±0.03 d | 40.0±1.3 e | 1.03±0.09 e | 0.54±0.03 e | |
| % of aerated | 74 | 84 | 74 | 63 | 59 | 62 | |
| Z. nicaraguensis | Aerated | 46.6±0.7 b | 0.84±0.09 c | 0.33±0.04 b | 58.1±0.3 c | 1.31±0.03 c | 0.76±0.07 c |
| Stagnant | 43.1±0.4 c | 0.73±0.07 d | 0.29±0.05 c | 51.4±0.8 d | 1.16±0.03 d | 0.65±0.06 d | |
| % of aerated | 93 | 87 | 88 | 89 | 88 | 86 |
Aerobically grown plants were either maintained in aerated conditions or transferred to stagnant, deoxygenated conditions for 7 d or 14 d. All data are means (±SD), n=4. Different letters indicate significant differences within each parameter at each time-point as determined using one-way ANOVA and Tukey’s test for multiple comparisons (P<0.05).
* Heights were measured for the same plants throughout the experiment.
Fig. 3.
Effects of aerated and stagnant, deoxygenated conditions on growth of Zea mays subsp. mays inbred line Mi29, introgression line IL-AE91, and Z. nicaraguensis. Plants were grown under aerated conditions for 25–28 d and were then transferred to stagnant, deoxygenated conditions for 7 d or 14 d. Seedlings grown continuously under aerated conditions were used as controls. Scale bars are 50 mm. (This figure is available in colour at JXB online.)
Table 2.
Growth of Mi29, IL-AE91, and Zea nicaraguensis plants under drained or flooded soil conditions
| Genotype | Plant height (cm) | Shoot dry mass (g) | Root dry mass (g) | Plant height (cm) | Shoot dry mass (g) | Root dry mass (g) | |
|---|---|---|---|---|---|---|---|
| 7 d | 11 d | ||||||
| Mi29 | Drained | 54.0±2.2 a | 0.67±0.08 a | 0.28±0.04 b | 70.8±2.1 a | 1.98±0.50 a | 0.53±0.04 a |
| Flooded | 40.8±1.7 c | 0.45±0.04 c | 0.16±0.01 d | 47.5±2.9 b | 0.60±0.09 b | 0.26±0.01 b | |
| % of drained | 76 | 68 | 56 | 67 | 30 | 49 | |
| IL-AE91 | Drained | 55.0±2.2 a | 0.72±0.09 a | 0.33±0.04 a | 72.3±2.8 a | 1.86±0.51 a | 0.56±0.07 a |
| Flooded | 46.3±3.1 b | 0.58±0.08 b | 0.21±0.03 c | 50.3±1.7 b | 0.73±0.03 b | 0.31±0.02 b | |
| % of drained | 84 | 81 | 62 | 70 | 39 | 55 | |
| Z. nicaraguensis | Drained | 34.8±3.1 d | 0.24±0.04 d | 0.12±0.02 e | 43.5±3.7 c | 0.50±0.09 b | 0.26±0.05 b |
| Flooded | 29.3±1.5 e | 0.21±0.04 d | 0.08±0.01 f | 36.5±2.4 d | 0.35±0.04 c | 0.12±0.01 c | |
| % of drained | 84 | 87 | 70 | 84 | 71 | 45 |
Seedlings at ~17 d were grown under drained or flooded soil conditions for 7 d or 11 d. All data are means (±SD), n=4. Different letters indicate significant differences within each parameter at each time-point as determined using one-way ANOVA and Tukey’s test for multiple comparisons (P<0.05).
Table 3.
Chlorophyll contents of leaves of Mi29, IL-AE91, and Zea nicaraguensis plants under drained and flooded soil conditions
| Genotype | Leaf | Drained | Flooded | % of drained | Drained | Flooded | % of drained |
|---|---|---|---|---|---|---|---|
| 7 d | 11 d | ||||||
| Mi29 | 1st | 51.7±1.0 | 14.3±11.8** | 28 | 52.7±1.2 | 0.0±0.0** | 0 |
| 2nd | 45.7±1.7 | 42.0±2.6 | 92 | 49.0±2.0 | 6.5±5.6** | 13 | |
| 3rd | - | - | - | 50.1±1.0 | 26.0±5.9** | 52 | |
| IL-AE91 | 1st | 56.6±2.7 | 35.2±8.8** | 62 | 60.5±2.3 | 0.0±0.0** | 0 |
| 2nd | 58.9±1.7 | 49.1±3.8** | 83 | 57.5±2.1 | 17.4±20.1** | 30 | |
| 3rd | - | - | - | 59.1±1.1 | 43.2±3.1** | 73 | |
| Z. nicaraguensis | 1st | 47.9±3.4 | 38.9±2.8** | 81 | 52.0±1.5 | 5.4±8.4** | 10 |
| 2nd | 44.4±0.9 | 37.4±2.3** | 84 | 45.7±1.6 | 36.4±1.5** | 80 | |
| 3rd | - | - | - | 47.3±0.6 | 36.7±1.7** | 78 |
Chlorophyll contents are expressed as SPAD values. All data are means (±SD), n=4. Significant differences between drained and flooded were determined using two-sample t-tests: **P<0.01.
Root extension under aerated and stagnant, deoxygenated conditions
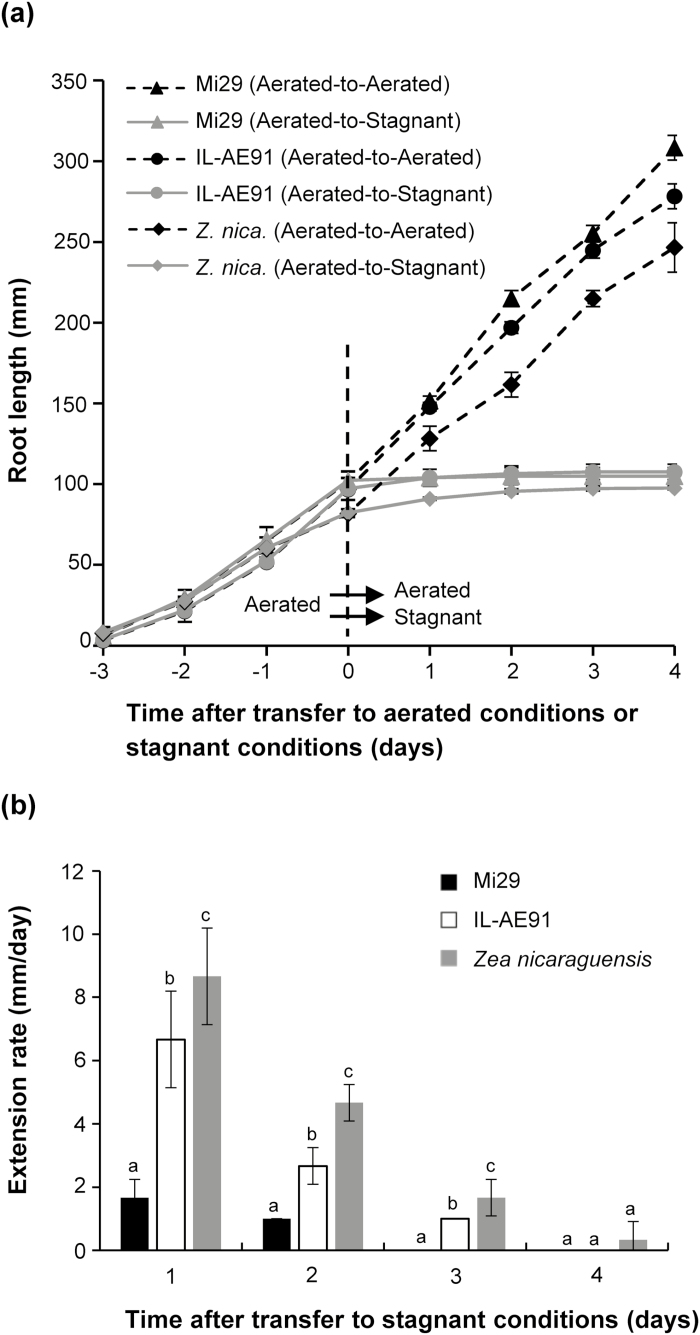
The roots of all three lines extended at a rate of more than 20 mm d–1 when they were maintained in aerated conditions (‘aerated-to-aerated’ treatment), with no significant differences being observed among the lines (Fig. 4a). When the plants were transferred from aerated to stagnant conditions (‘aerated-to-stagnant’ treatment), the extension rates in all three lines were severely reduced (Fig. 4a, b). The extension rate declined to zero on day 3 for Mi29 and on day 4 for IL-AE91, but Z. nicaraguensis roots were still extending slightly on day 4. Roots of IL-AE91 extended at 6.7±1.5 mm d–1 and 2.7±0.6 mm d–1 on day 1 and day 2, respectively, whilst those of Mi29 elongated by less than 2 mm d– for the first two days (Fig. 4b). The extension rates of Z. nicaraguensis roots on day 1 and day 2 were, respectively, 5.2- and 4.7-times higher than those of Mi29, and 1.3- and 1.7-times higher than those of IL-AE91. After 4 d under stagnant, deoxygenated conditions, the root lengths of Mi29, IL-AE91, and Z. nicaraguensis plants had increased by ~3 mm, ~8 mm, and ~13 mm, respectively, and their total lengths were ~100–110 mm.
Fig. 4.
Effects of aerated and stagnant, deoxygenated conditions on root growth of Zea mays subsp. mays inbred line Mi29, introgression line IL-AE91, and Z. nicaraguensis. Plants were grown under aerated conditions for 25–28 d and were then transferred to stagnant, deoxygenated conditions (Aerated-to-Stagnant) or to continued aerated conditions (Aerated-to-Aerated). (a) Root length. (b) Extension rates of roots after transfer to stagnant, deoxygenated conditions. Data are means (±SD), n=4. Different letters indicate significant differences between each line at each time-point as determined using one-way ANOVA and Tukey’s test for multiple comparisons (P<0.05).
Viability of root-tip cells after transfer from aerated to stagnant, deoxygenated conditions
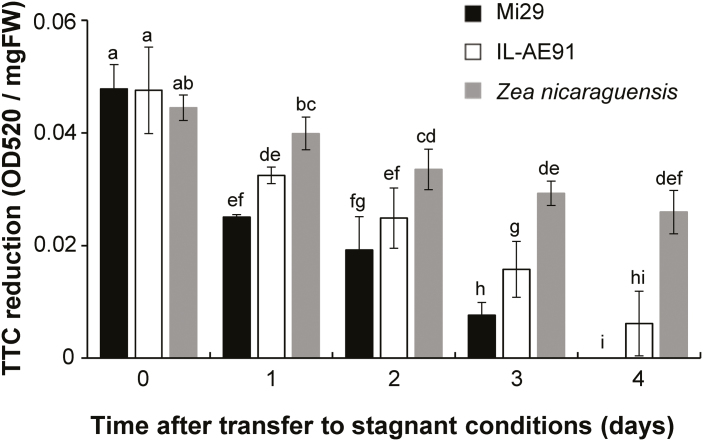
For all three lines, cell viability in the apical part of the roots, as measured by TTC reduction assays, was high and very similar before transfer to stagnant, deoxygenated conditions (Fig. 5; day 0), suggesting that they all consumed similar amounts of oxygen under aerated conditions. The cell viability then gradually decreased over the next 4 d, with the fastest decrease being seen in Mi29 and the slowest in Z. nicaraguensis. The decrease was fastest on day 1, the cell viabilities for Mi29, IL-AE91, and Z. nicaraguensis roots had decreased by 48%, 32%, and 10%, respectively, and a similar trend was seen on subsequent days. On day 4, cell viability in the root tips of Mi29 was negligible, i.e. they were almost dead, whereas the tips of some IL-AE91 and Z. nicaraguensis roots were still alive. These results suggested that the differences in the root extension of the three lines under stagnant, deoxygenated conditions could be attributed to differences in cell viabilities of the root tips.
Fig. 5.
Effects of aerated and stagnant, deoxygenated conditions on cell viability in the root tips of Zea mays subsp. mays inbred line Mi29, introgression line IL-AE91, and Z. nicaraguensis. Plants were grown under aerated conditions for 25–28 d and were then transferred to stagnant, deoxygenated conditions. Cell viability is indicated by the reduction of 2,3,5-triphenyltetrazolium chloride (TTC). Measurements were taken on 4th nodal roots, which were ~100 mm long at the time of transfer. Data are means (±SD), n=3. Different letters indicate significant differences between means across all time-points as determined using one-way ANOVA and Tukey’s test for multiple comparisons (P<0.05).
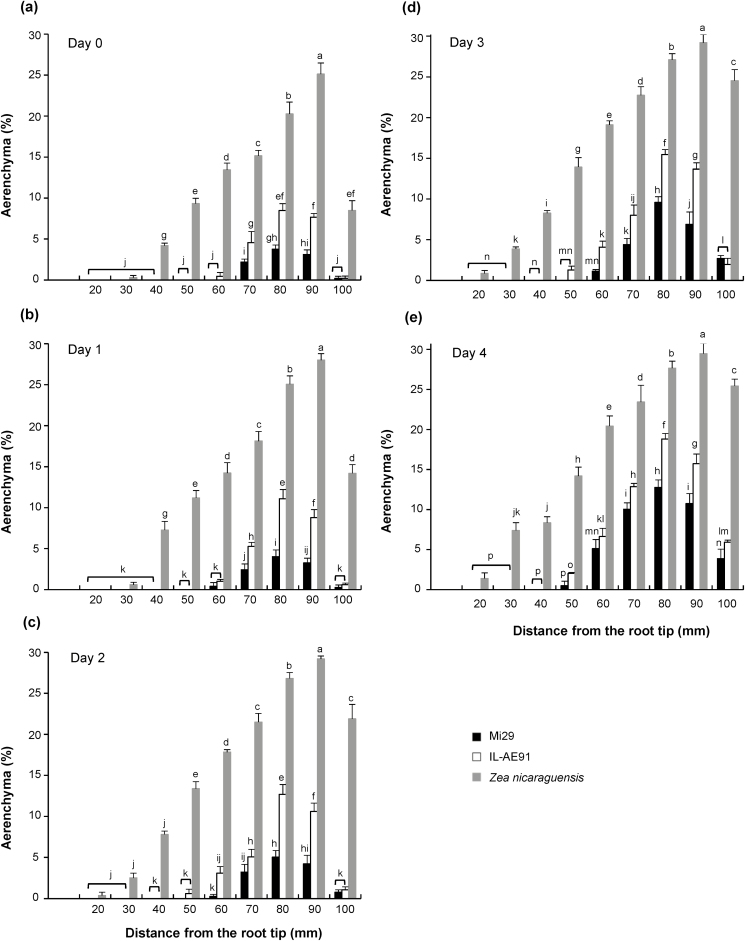
Aerenchyma formation under stagnant, deoxygenated conditions
The area of the aerenchyma was measured at 10-mm intervals from the root tips. Aerenchyma formation (including both CAF and IAF) in the three lines increased daily after transfer to stagnant, deoxygenated conditions (Fig. 6, Supplementary Figs S1, S2), in both the apical and basal parts of the roots. On day 0, none of the lines showed aerenchyma at 20 mm from the root tip (Fig. 6). In Z. nicaraguensis, aerenchyma formed at 20 mm from the root tip on day 2 under stagnant, deoxygenated conditions. Aerenchyma had formed at 50 mm in IL-AE91 roots on day 2, but it did not appear at 50 mm in Mi29 until day 4. At the basal part of the root (i.e. at 100 mm from the tip), by day 4 the total aerenchyma area had increased to 4%, 6%, and 25% in Mi29, IL-AE91, and Z. nicaraguensis, respectively, under stagnant, deoxygenated conditions. By day 1 under stagnant, deoxygenated conditions, the aerenchyma area at 80 mm from the root tip had increased significantly in IL-AE91 and Z. nicaraguensis (Supplementary Fig. S2), and a significant increase was also observed at 60 mm in these lines by day 2. By contrast, increases at 60 mm and 80 mm were not observed in Mi29 until day 3. The induction of aerenchyma formation in response to the root-zone oxygen deficiency therefore started sooner in Z. nicaraguensis and IL-AE91 than in Mi29.
Fig. 6.
Aerenchyma formation in roots of Zea mays subsp. mays inbred line Mi29, introgression line IL-AE91, and Z. nicaraguensis that were grown under aerated conditions and were then transferred to stagnant, deoxygenated conditions. Plants were grown under aerated conditions for 25–28 d before transfer, at which point the roots were ~100 mm long. Percentage of aerenchyma area in cross-sections of roots at 10–100 mm from the tip were made on (a) day 0 (i.e. aerated conditions), (b) day 1, (c) day 2, (d) day 3, and (e) day 4. Data are means (±SD), n=3. Different letters indicate significant differences between means across all root positions as determined using distances within one growth condition (P<0.05, one-way ANOVA and then Tukey’s test for multiple comparisons).
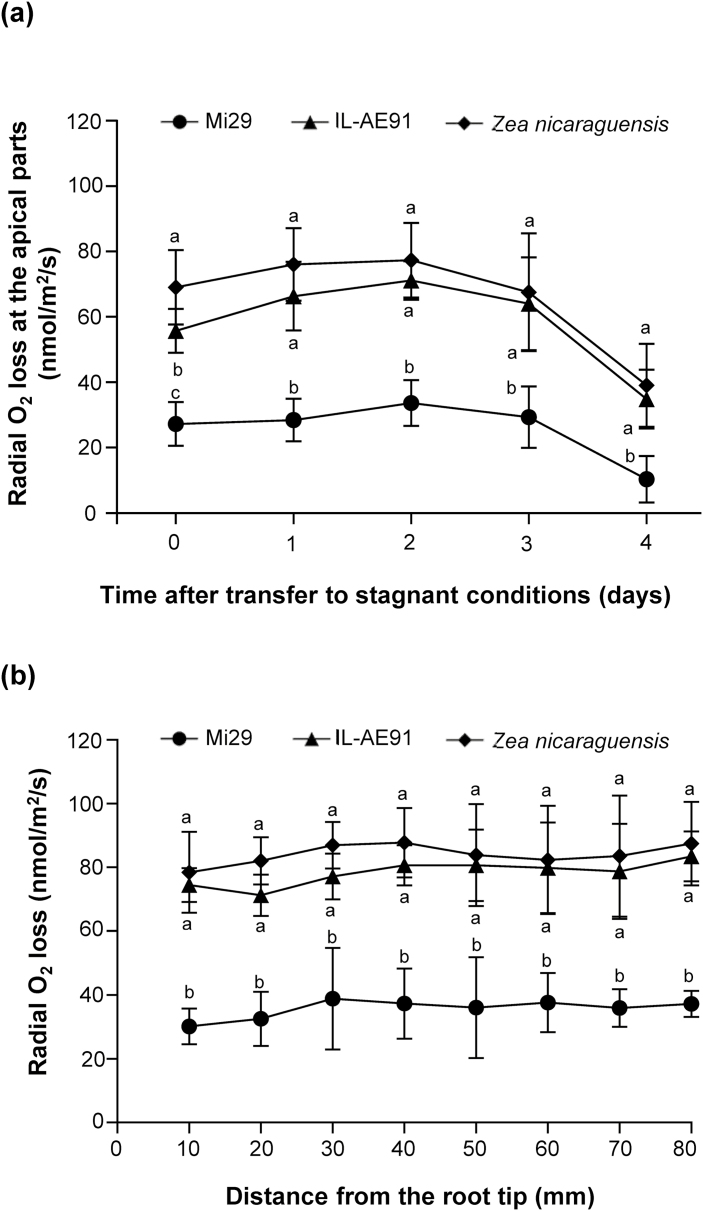
ROL under stagnant, deoxygenated conditions
Under aerated conditions just before transfer to stagnant, deoxygenated conditions, ROL at 5–10 mm from the root tips was significantly different between the three lines, being highest in Z. nicaraguensis and lowest in Mi29 (Fig. 7a, day 0). This suggested that CAF enhanced the supply of oxygen to the root tips, thereby increasing ROL.
Fig. 7.
Effects of aerated and stagnant, deoxygenated conditions on radial oxygen loss (ROL) in the roots of Zea mays subsp. mays inbred line Mi29, introgression line IL-AE91, and Z. nicaraguensis. Plants were grown under aerated conditions for 25–28 d and were then transferred to stagnant, deoxygenated conditions. (a) ROL of root tips at 0–4 d after transfer. (b) ROL at different positions from the root tip on day 2 after transfer. Values are means (±SD), n=3. Different letters indicate significant differences between each line at each time-point (a) or at each root position (b) as determined using one-way ANOVA and Tukey’s test for multiple comparisons (P<0.05).
At 1 d after transfer, ROL in the region near the root tip increased ~1.2-fold in IL-AE91 and ~1.1-fold in Z. nicaraguensis compared to day 0 (Fig. 7a). In contrast, the ROL of Mi29 roots on day 1 was almost the same as it was on day 0. By day 4, the ROLs of all three lines had decreased.
ROL was measured along the length of the roots and day 2 to look for signs of a barrier. Values were consistently much higher in Z. nicaraguensis and IL-AE91 than in Mi29 (Fig. 7b). In all three lines, ROL was slightly higher in the basal parts of the root than in the region near the tip on both day 2 (Fig. 7b) and day 4 (Supplementary Fig. S3), suggesting that none of the three lines formed a significant ROL barrier in aerobically grown roots until at least 4 d after the transfer. Hence, the higher ROL at the apical parts of Z. nicaraguensis and IL-AE91 roots than in Mi29 (Fig. 7a) appeared to be due to higher aerenchyma formation rather than to ROL barrier formation at the basal part of the roots.
In roots that emerged after the transfer to stagnant, deoxygenated conditions in Mi29 and IL-AE91, ROL remained high at all positions examined (i.e. 10–50 mm from the tips; Supplementary Fig. S4a, b). In contrast, in Z. nicaraguensis ROL was high at 10 mm and very low at 30 mm and 50 mm (Supplementary Fig. S4c). Taken together, these results confirmed that the roots of Mi29 and IL-AE91 did not form a significant ROL barrier. IL-AE91 does not possess the locus for ROL barrier formation that is located on the short-arm of chromosome 3 of Z. nicaraguensis (Watanabe et al., 2017). On the other hand, our finding that ROL was high close to the tip but very low in the middle and basal parts of the root indicated that Z. nicaraguensis formed a tight ROL barrier in roots that had newly emerged after transfer to stagnant, deoxygenated conditions, but not in roots that had previously grown aerobically (90–110 mm), even after 4 d under stagnant, deoxygenated conditions.
Discussion
Development of IL-AE91 and its characteristics
We have previously shown that waterlogging tolerance in Z. nicaraguensis accessions increases with increasing degree of constitutive aerenchyma formation (CAF; Mano and Omori, 2013b). However, these accessions did not have the same genetic background, so we could not rule out the possibility that other factors were involved. Therefore, we produced IL-AE91, which possesses four QTLs for CAF of Z. nicaraguensis in the genetic background of maize Mi29 (Fig. 1). The IL-AE91 genome has ~14% of the Z. nicaraguensis genome, including the four CAF QTLs, and ~86% of the maize genome (Y. Mano and F. Omori, unpublished results). To determine the importance of CAF for tolerance of root-zone oxygen deficiency, we compared Mi29, IL-AE91, and Z. nicaraguensis under aerated conditions and then evaluated responses to stagnant, deoxygenated conditions. The area of constitutive aerenchyma at any given position along the root was larger in IL-AE91 than in Mi29 (Fig. 2), suggesting that the four Z. nicaraguensis QTLs contributed to a higher degree and earlier initiation of CAF. However, the area of aerenchyma in IL-AE91 was much smaller than that in Z. nicaraguensis roots (Fig. 2), and a similar result has been found in the introgression line BC4F1 #62, which has one of the four Z. nicaraguensis QTLs (Qaer1.06-1.07) in the genetic background of Mi29 (Abiko et al., 2012b). This may be because other minor Z. nicaraguensis QTLs also control CAF. Indeed, based on the effects observed in our previous studies (Mano and Omori, 2008, 2009), the four QTLs for CAF examined here only account for 50% of the total phenotypic variation, suggesting that other QTLs exist for this trait.
CAF and tolerance of root-zone oxygen deficiency
Plant growth parameters showed that IL-AE91 was more tolerant of root-zone oxygen deficiency than Mi29 (Tables 1–3), suggesting that its greater capacity to form constitutive aerenchyma in roots enhanced its waterlogging tolerance. In addition, ROL measurements suggested that equivalent amounts of oxygen were transported to the root tips in IL-AE91 and Z. nicaraguensis (Fig. 7a); however, IL-AE91 was less tolerant of oxygen deficiency than Z. nicaraguensis (Tables 1, 2). This was probably due to a combination of lower CAF in IL-AE91 (possibly because it did not have all the Z. nicaraguensis QTLs for this trait) and other traits of Z. nicaraguensis that are not shared with IL-AE91, such as formation of a ROL barrier in roots that emerged after the transfer to stagnant, deoxygenated conditions, and emergence of more above-ground adventitious roots under oxygen-deficient conditions.
Effect of CAF on IAF under stagnant, deoxygenated conditions
Following transfer of the plants from aerated conditions to stagnant, deoxygenated conditions, inducible aerenchyma formation (IAF) started sooner in Z. nicaraguensis and IL-AE91 than in Mi29 (Fig. 6, Supplementary Fig. S2), suggesting that the presence of larger constitutively formed aerenchyma speeds up IAF. IAF is triggered by the gaseous phytohormone ethylene (Jackson and Armstrong, 1999; Yamauchi et al., 2018), whose production requires oxygen. The oxygen may be provided by constitutive aerenchyma in the root cortex and the ethylene produced could then easily diffuse through the aerenchyma, stimulating IAF.
The area of aerenchyma was always larger in the order of Z. nicaraguensis > IL-AE91 > Mi29 across days 0–4 after transfer to stagnant, deoxygenated conditions (Fig. 6). In addition, the rates of IAF in the roots under these conditions decreased with increasing distance from the root base across days 0–4. These results suggest that both IAF and CAF are dependent on the genotype. Indeed, IAF in barley has been found to be genetically controlled by the QTL AER (Zhang et al., 2017).
Relationship between cell viability in the root tips and CAF
In each of the lines, the viability of cells in the root tips decreased after transfer to stagnant, deoxygenated conditions (Fig. 5), thereby decreasing root extension rates (Fig. 4). The reductions in viability and extension rates were severe and were in the order of Mi29 > IL-AE91 > Z. nicaraguensis. The reductions were negatively correlated with the area of constitutively formed aerenchyma (Fig. 2), which suggests that CAF contributes to cell viability and root extension.
A similar observation has been made in wheat roots, in which CAF is hardly observed under aerated conditions. Pre-treatment of wheat roots with an ethylene precursor (1-aminocyclopropane-l-carboxylic acid) enhances ethylene-dependent aerenchyma formation, and when seedlings are transferred to stagnant, deoxygenated conditions, the viability of cells in the root tips and the root extension rates are greater than in controls (Yamauchi et al., 2014a). This suggests that, in agreement with our hypothesis, constitutively formed aerenchyma help to maintain cell viability in the root tips and to extend the roots after the onset of oxygen deficiency, thus enhancing tolerance of oxygen deficiency.
Changes in ROL at the root tips are not affected by ROL barriers
ROL at the apical parts can be affected by the presence of a ROL barrier, which prevents radial oxygen leakage at the basal parts of roots and enhances oxygen diffusion to the root tip (Armstrong, 1979; Colmer, 2003; Malik et al., 2011; Shiono et al., 2011). We have previously shown that aerobically grown roots of maize and Z. nicaraguensis do not form a ROL barrier (Abiko et al., 2012a). In the present study, aerobically grown roots in all of the lines had not formed a tight ROL barrier on either day 2 or day 4 after transfer to stagnant, deoxygenated conditions (Fig. 7b, Supplementary Fig. S3). Therefore, it is unlikely that the ROL at the root tips was affected by ROL barrier formation following transfer to stagnant, deoxygenated conditions.
The roots of all three lines still grew slightly after transfer from aerated to stagnant, deoxygenated conditions (Fig. 4), and thus the lengths of the oxygen diffusion paths were slightly increased during the treatment. In general, as root length increases, the amount of oxygen that diffuses from the base to the tip through the aerenchyma decreases as a result of oxygen consumption by cells along the root (Armstrong, 1979). However, for the first 2 d after transfer, the ROL in the apical parts of IL-AE91 and Z. nicaraguensis roots increased slightly, before declining on days 3–4 (Fig. 7a). ROL also decreased in Mi29 on day 4. The changes in ROL might therefore have been affected not only by the changes in length of the oxygen diffusion path but also by other factors, such as the changes in the size of the area of aerenchyma, the oxygen flow from the aerial parts to the root tips, and the viability of the root-tip cells.
ROL barrier formation in Z. nicaraguensis and rice
A tight ROL barrier forms in Z. nicaraguensis in newly emerged roots under stagnant, deoxygenated conditions (Abiko et al., 2012a; Watanabe et al., 2017; Supplementary Fig. S4), but it did not form in roots that had grown aerobically (85–100 mm in length) for at least 4 d after transfer to stagnant, deoxygenated conditions (Fig. 7b, Supplementary Fig. S3). In contrast, a tight ROL barrier forms in rice within 24 h after aerobically grown long roots (105–130 mm in length) are transferred to stagnant, deoxygenated conditions (Shiono et al., 2011); for shorter aerobically grown roots (65–90 mm in length), ROL barrier formation starts between 48 h and 72 h after transfer and is complete by 120 h. These results suggest that the mechanisms regulating ROL barrier formation differ between Z. nicaraguensis and rice.
Conclusions and future directions
Our study demonstrated that the maize introgression line IL-AE91 with QTLs for CAF was more tolerant of root-zone oxygen deficiency than the inbred line Mi29, although the improvement was rather modest. This suggests that prompt supply of oxygen to the root tips via constitutively formed aerenchyma just after exposure to sudden oxygen deficiency contributes to the maintenance of cell viability in the tips and to continued root extension. Therefore, CAF may allow plants to more rapidly adapt to soil waterlogging.
We have previously identified 21 candidate genes in the introgression line BC4F1 #62 that are involved in CAF (Abiko et al., 2012b). In addition, we are planning to conduct transcriptome analyses to identify the genes controlling CAF using IL-AE91 and Mi29. By combining these studies, it should be possible to identify the key genes associated with CAF and to establish the regulatory networks involved. In addition, IL-AE91 can be used as a new germplasm for combining other waterlogging-tolerant traits (e.g. ROL barrier formation) to improve tolerance in maize. To this end, we are currently exploring practical breeding approaches using several QTLs related to waterlogging tolerance in a pyramiding line possessing the loci for both CAF and ROL barrier formation.
Supplementary data
Supplementary data are available at JXB online.
Table S1. The scheme used for the development of IL-AE91.
Fig. S1. Cross-sections of roots of Mi29, IL-AE91, and Z. nicaraguensis under stagnant, deoxygenated conditions.
Fig. S2. Aerenchyma formation at 30, 60 and 80 mm from the root tip of the three lines under stagnant, deoxygenated conditions.
Fig. S3. radial oxygen loss (ROL) from aerobically grown roots of the three lines on day 4 of stagnant, deoxygenated conditions.
Fig. S4. radial oxygen loss (ROL) from roots of the three lines that emerged after the transfer to stagnant, deoxygenated conditions.
Acknowledgements
We thank Dr Timothy D. Colmer for reading the manuscript and for stimulating discussions, and Drs Hui Liu, Jinghua Zhang, Xiaolin Wu, and Takaki Yamauchi also for stimulating discussions. We thank the International Maize and Wheat Improvement Center (CIMMYT) for providing seeds of Z. nicaraguensis and Kyushu Okinawa Agricultural Research Center, NARO, for providing seeds of maize inbred line Mi29. This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (18H02175) to MN and YM. FPG is supported by a fellowship of the Collaborative Innovation Center of Henan Grain Crops, Henan Agricultural University. The tuition fees of FPG to study in Nagoya University were partly supported by a grant from the State Key Laboratory of Wheat and Maize Crop Science, Henan Agricultural University (no. 39990066).
Glossary
Abbreviations:
- CAF
constitutive aerenchyma formation
- IAF
inducible aerenchyma formation
- IL
introgression line
- QTLs
quantitative trait loci
- ROL
radial oxygen loss
- TTC
2,3,5-triphenyltetrazolium chloride
References
- Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M. 2012a Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant, Cell & Environment 35, 1618–1630. [DOI] [PubMed] [Google Scholar]
- Abiko T, Obara M, Abe F, Kawaguchi K, Oyanagi A, Yamauchi T, Nakazono M. 2012b Screening of candidate genes associated with constitutive aerenchyma formation in adventitious roots of the teosinte Zea nicaraguensis. Plant Root 6, 19–27. [Google Scholar]
- Armstrong W. 1968. Oxygen diffusion from the roots of woody species. Physiologia Plantarum 21, 539–543. [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants. Advances in Botanical Research 7, 225–332. [Google Scholar]
- Armstrong W, Wright EJ. 1975. Radial oxygen loss from roots: the theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiologia Plantarum 35, 21–26. [Google Scholar]
- Arora K, Panda KK, Mittal S, Mallikarjuna MG, Rao AR, Dash PK, Thirunavukkarasu N. 2017. RNAseq revealed the important gene pathways controlling adaptive mechanisms under waterlogged stress in maize. Scientific Reports 7, 10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Lee SC, Brinton E. 2012. Waterproofing crops: effective flooding survival strategies. Plant Physiology 160, 1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick DM. 1989. Root aerenchyma development in Spartina patens in response to flooding. American Journal of Botany 76, 777–780. [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26, 17–36. [Google Scholar]
- Colmer TD, Cox MC, Voesenek LA. 2006. Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytologist 170, 767–777. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Gibberd MR, Wiengweera A, Tinh TK. 1998. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany 49, 1431–1436. [Google Scholar]
- Colmer TD, Voesenek LACJ. 2009. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36, 665–681. [DOI] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW. 1989. Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiology 91, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE. 2003. Aerenchyma formation. New Phytologist 161, 35–49. [Google Scholar]
- Fan MS, Zhu J, Richards C, Brown K, Lynch J. 2003. Physiological roles for aerenchyma in phosphorus-stressed roots. Functional Plant Biology 30, 493–506. [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J, Caldwell C, Gallie DR. 2010. Expression of the ethylene biosynthetic machinery in maize roots is regulated in response to hypoxia. Journal of Experimental Botany 61, 857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M, Striker GG, Colmer TD, Pedersen O. 2016. Mechanisms of waterlogging tolerance in wheat – a review of root and shoot physiology. Plant, Cell & Environment 39, 1068–1086. [DOI] [PubMed] [Google Scholar]
- Iltis HH, Benz BF. 2000. Zea nicaraguensis (Poaceae), a new teosinte from pacific coastal Nicaragua. Novon 10, 382–390. [Google Scholar]
- Ishihara K, Hirasawa T, Iida O, Kimura M. 1981. Diurnal course of transpiration rate, stomatal aperture, stomatal conductance, xylem water potential and leaf water potential in the rice plants under the different growth conditions. Japanese Journal of Crop Science 50, 25–37. [Google Scholar]
- Jackson MB, Armstrong W. 1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1, 274-287. [Google Scholar]
- Kulichikhin K, Yamauchi T, Watanabe K, Nakazono M. 2014. Biochemical and molecular characterization of rice (Oryza sativa L.) roots forming a barrier to radial oxygen loss. Plant, Cell & Environment 37, 2406–2420. [DOI] [PubMed] [Google Scholar]
- Mae T, Ohira K. 1981. The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant & Cell Physiology 22, 1067–1074. [Google Scholar]
- Malik AI, Islam AK, Colmer TD. 2011. Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): evaluation of four H. marinum–wheat amphiploids. New Phytologist 190, 499–508. [DOI] [PubMed] [Google Scholar]
- Mano Y, Omori F. 2007. Breeding for flooding tolerant maize using “teosinte” as a germplasm resource. Plant Root 1, 17–21. [Google Scholar]
- Mano Y, Omori F. 2008. Verification of QTL controlling root aerenchyma formation in a maize×teosinte “Zea nicaraguensis” advanced backcross population. Breeding Science 58, 217–223. [Google Scholar]
- Mano Y, Omori F. 2009. High-density linkage map around the root aerenchyma locus Qaer1.06 in the backcross populations of maize Mi29× teosinte “Zea nicaraguensis”. Breeding Science 59, 427–433. [Google Scholar]
- Mano Y, Omori F. 2013a Flooding tolerance in interspecific introgression lines containing chromosome segments from teosinte (Zea nicaraguensis) in maize (Zea mays subsp. mays). Annals of Botany 112, 1125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano Y, Omori F. 2013b Relationship between constitutive root aerenchyma formation and flooding tolerance in Zea nicaraguensis. Plant and Soil 370, 447–460. [Google Scholar]
- Mano Y, Omori F, Loaisiga CH, Bird RM. 2009. QTL mapping of above-ground adventitious roots during flooding in maize×teosinte “Zea nicaraguensis” backcross population. Plant Root 3, 3–9. [Google Scholar]
- Mano Y, Omori F, Takamizo T, Kindiger B, Bird RM, Loaisiga CH, Takahashi H. 2007. QTL mapping of root aerenchyma formation in seedlings of a maize × rare teosinte “Zea nicaraguensis” cross. Plant and Soil 295, 103–113. [Google Scholar]
- Mano Y, Omori F, Takeda K. 2012. Construction of intraspecific linkage maps, detection of a chromosome inversion, and mapping of QTL for constitutive root aerenchyma formation in the teosinte Zea nicaraguensis. Molecular Breeding 29, 137–146. [Google Scholar]
- Mano Y, Omori F, Tamaki H, Mitsuhashi S, Takahashi W. 2016. DNA marker-assisted selection approach for developing flooding-tolerant maize. Japan Agricultural Research Quarterly 50, 175–182. [Google Scholar]
- Mano Y, Takeda K. 2012. Accurate evaluation and verification of varietal ranking for flooding tolerance at the seedling stage in barley (Hordeum vulgare L.). Breeding Science 62, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A. 2018. Improving flooding tolerance of crop plants. Agronomy 8, 160. [Google Scholar]
- Naredo MEB, Juliano AB, Lu BR, De Guzman F, Jackson MT. 1998. Responses to seed dormancy-breaking treatments in rice species (Oryza L.). Seed Science and Technology 26, 675–689. [Google Scholar]
- Postma JA, Lynch JP. 2011. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiology 156, 1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Nakazono M. 2011. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytologist 190, 351–368. [DOI] [PubMed] [Google Scholar]
- Ray JD, Kindiger B, Sinclair TR. 1999. Introgressing root aerenchyma into maize. Maydica 44, 113–117. [Google Scholar]
- Shiono K, Ogawa S, Yamazaki S, Isoda H, Fujimura T, Nakazono M, Colmer TD. 2011. Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Annals of Botany 107, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Bailey-Serres J. 2015. Flood adaptive traits and processes: an overview. New Phytologist 206, 57–73. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Takahashi H, Sato S, Nishiuchi S, Omori F, Malik AI, Colmer TD, Mano Y, Nakazono M. 2017. A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant, Cell & Environment 40, 304–316. [DOI] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H, Thomson CJ. 1997. The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany 80, 115–123. [Google Scholar]
- Yamauchi T, Abe F, Kawaguchi K, Oyanagi A, Nakazono M. 2014a Adventitious roots of wheat seedlings that emerge in oxygen-deficient conditions have increased root diameters with highly developed lysigenous aerenchyma. Plant Signaling & Behavior 9, e28506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Colmer TD, Pedersen O, Nakazono M. 2018. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiology 176, 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Nakazono M. 2014b Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. Journal of Experimental Botany 65, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fan Y, Shabala S, Koutoulis A, Shabala L, Johnson P, Zhou MX. 2017. A new major-effect QTL for waterlogging tolerance in wild barley (H. spontaneum). Theoretical Applied Genetics 130, 1559–1568. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shabala S, Koutoulis A, Shabala L, Johnson P, Hayes D, Zhou M. 2015. Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant and Soil 394, 355–372. [Google Scholar]
- Zhu J, Brown KM, Lynch JP. 2010. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell & Environment 33, 740–749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.