Abstract
Alcohol use disorder, characterized by modest levels of alcohol use, commonly occurs in patients with schizophrenia and dramatically worsens their course. Recent data indicate that the atypical antipsychotic clozapine, but not the typical antipsychotic haloperidol, decreases alcohol drinking both in patients with schizophrenia and also in the Syrian golden hamster, an animal of moderate alcohol drinking. The present study was designed to assess the comparative effects of clozapine and haloperidol in the alcohol-preferring (P) rat, an animal model of alcoholism. First, the study investigated the comparative effects of clozapine and haloperidol on initiation of alcohol consumption in P rats, which models the early stage of alcoholism. Second, the study assessed the comparative effects of clozapine and haloperidol on maintenance of chronic alcohol consumption in P rats to provide a clue as to whether either drug may also limit alcohol consumption in alcohol-dependent patients. Clozapine attenuated the initiation of alcohol drinking and development of alcohol preference while haloperidol did not. However, neither clozapine nor haloperidol attenuated maintenance of chronic alcohol drinking. Taken together, the current data suggest that clozapine, but not haloperidol, may be effective at reducing alcohol abuse or non-dependent drinking and the P rat, used within an alcohol initiation paradigm, and may differentiate the effects of clozapine and haloperidol on alcohol drinking.
Keywords: alcohol, antipsychotic, substance abuse, alcoholism, schizophrenia
Introduction
The rates of alcohol use disorder co-occurring with schizophrenia (SCZ) are 3-fold higher than that of primary alcoholism occurring in the general population (Drake et al., 1989; Regier et al., 1990). Unlike primary alcoholism, alcohol use disorder in schizophrenia is characterized by alcohol abuse occurring more frequently than alcohol dependence — patients with schizophrenia tend to consume moderate quantities of alcohol on a regular basis without developing physical withdrawal symptoms upon cessation of drinking (Drake et al., 1989; Lehman et al., 1996; Test et al., 1989). Nevertheless, even this moderate alcohol use can dramatically worsen their clinical course (Drake et al., 1989; Drake and Wallach, 1993).
Patients with schizophrenia and co-occurring alcohol use disorder are extremely difficult to treat, in part because most of the antipsychotic medications they take for their psychotic symptoms do not decrease their alcohol use. In fact, there is some suggestion that the typical antipsychotics, such as haloperidol (HAL), may even increase substance use (Brady et al., 1990; Buckley et al., 1994; Dixon et al., 1991; McEvoy et al., 1995). Moreover, the one antipsychotic medication that has been shown in preliminary studies to decrease alcohol use (and other substance use) in patients with schizophrenia, the atypical agent clozapine (CLOZ), is used infrequently in patients because of its side-effect profile (Albanese et al., 1994; Drake et al., 2000; Marcus and Snyder, 1995; Yovell and Opler, 1994; Zimmet et al., 2000). Thus, new approaches to the treatment of patients with SCZ and co-occurring alcohol use disorder are needed.
Our group (Chau et al., 2004; Green et al., 2008; Green et al., 1999) along with others (Blum et al., 2000; Der-Avakian and Markou, 2012) has proposed that patients with SCZ have a dysregulated brain reward circuitry that underpins their substance use, that alcohol and other substances tend to improve the functioning of this circuit (although they make SCZ worse), and that CLOZ, because of its broad-spectrum effects including its weak dopamine D2 receptor antagonism, potent norepinephrine α−2 antagonism, and ability to increase levels of norepinephrine in plasma and brain, may improve the functioning of this circuitry and thereby limit alcohol/other substance use (Chau et al., 2011; Chau et al., 2004; Green et al., 2008; Green et al., 1999). We have further proposed that the typical antipsychotics, such as HAL, are not able to decrease alcohol drinking, in part because of their potent dopamine D2 receptor antagonism, which may itself produce a reward deficit (Grace, 1991). A recent study showed that HAL may, in fact, augment the pursuit of reward cues, and as a result increase reward seeking and intake, probably owing to its effect on dopaminergic signaling and compensatory neuroadaptation in response to the potent D2 blockade (Bedard et al., 2013).
In order to begin to develop new treatments to limit alcohol drinking in patients with SCZ, we have studied the effects of CLOZ and HAL on alcohol drinking in the Syrian golden hamster, an outbred animal that, like patients with SCZ, drinks alcohol moderately and does not develop alcohol dependence (McMillan et al., 1977). In this animal model, we have shown that CLOZ, but not HAL, will decrease alcohol drinking (Green et al., 2004).
As a further extension of this work, we have studied the effects of CLOZ and HAL on alcohol drinking in the alcohol-preferring P rat, a heavy alcohol-drinking genetic strain that has been proposed as a model of alcoholism (McBride and Li, 1998). This report delineates our experience with this animal model, in which we have assessed the ability of CLOZ and HAL to limit both the initiation of alcohol drinking as well as chronic alcohol drinking.
Given the effects of CLOZ in patients with SCZ, as well as in the Syrian golden hamster, an animal that does not become dependent on alcohol despite chronic drinking, we hypothesized that CLOZ, but not HAL, would limit the initiation of alcohol drinking in the P rat. We secondarily evaluated the effects of these two drugs on chronic alcohol drinking in the P rat, which does become alcohol dependent. The knowledge gained from such investigations may potentially advance treatment for alcohol-abusing and/or alcohol-dependent patients with SCZ (and potentially those without SCZ).
Materials and methods
Animals
Male alcohol-preferring P rats from the 58th generation (360–590 g, Indiana University) were used for these studies. Rats were housed individually in a normal 12-h light/12-h dark cycle (lights on at 6:00 AM), with ad libitum access to food and water. Alcohol (10% v/v) was presented as a second choice of fluid in a separate bottle at specific times indicated in the experimental protocols in an unforced initiation design. The positions of the water and alcohol bottles were rotated on a daily basis to prevent positional preference. A technician, blind to the experimental conditions, measured fluid intake (every 24 h), food intake (every 48 h), and body weight (every 3 days to minimize disruption to the animals) at the same time (4 h before dark) on the days of the measurements. The design is similar to that employed by other groups that also did not employ daily body weight measurements (Dhaher et al., 2012; Lynch et al., 2011; Rodd et al., 2009; Rodd-Henricks et al., 2001). Nonetheless, we acknowledge the potential for differential body weight changes across groups to confound the comparisons of alcohol intake, measured in terms of g/kg, and address that through data analysis.
All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996 and approved by the Institutional Animal Care and Use Committee of Dartmouth College.
Experimental Protocols
Experiment 1: Comparative effects of CLOZ and HAL on the initiation of alcohol drinking
Thirty-nine P rats were used for Experiment 1 to compare the effects of CLOZ and HAL on initiation of alcohol drinking. Rats were assigned to 1 of 5 groups (n = 7–8 per group) with similar body weight and water intake: 4 mg/kg/day CLOZ (4 mg/kg CLOZ); 12 mg/kg/day CLOZ (12 mg/kg CLOZ); 0.2 mg/kg/day HAL (0.2 mg/kg HAL); 1 mg/kg/day HAL (1 mg/kg HAL); and vehicle (VEH). The doses of CLOZ and HAL used in these P rats were similar to doses previously used in the Syrian golden hamsters (Chau et al., 2010; Chau et al., 2004). The dose ranges of HAL and CLOZ used here also cover the clinically relevant ranges of these drugs as determined by in vivo DA D2 receptor occupancy studies (Kapur et al., 2003). The rats’ baseline water and food intakes were established prior to drug administration (baseline was the averaged value over 4 consecutive days). Afterward, with only water and food present in the cage, groups were pretreated subcutaneously (s.c.) for 10 days (Pretreatment Period), at 2 hours before dark each day, with the respective doses of CLOZ or HAL or vehicle. We administered drugs before the beginning of the dark cycle to allow CLOZ and HAL to have maximal brain exposure around the beginning of the dark cycle, when the rats are most actively drinking and eating. A graded dosing schedule was used to adapt the animals to the drugs. According to this schedule, the doses of CLOZ began at 4 mg/kg/day and were increased by 2 or 4 mg/kg every 2 days up to the targeted values (e.g., the 8 mg/kg/day CLOZ group received 4 and 6 mg/kg/day CLOZ during treatment days 1–2 and 3-4, respectively, with 8 mg/kg/day beginning on day 5 and continuing at the same dosage through day 20). Similarly, the doses of HAL began at 0.2 mg/kg/day and were increased by 0.2 mg/kg every 2 days up to the targeted values (e.g., the 1 mg/kg/day HAL group received 0.2, 0.4, 0.6, and 0.8 mg/kg/day HAL during treatment days 1–2, 3–4, 5–6, and 7–8, respectively, and 1.0 mg/kg/day HAL beginning on day 9 and continuing throughout the study). The animals were treated with the targeted drug doses for another 7 days prior to the introduction of alcohol. Then, over the next 20 days (i.e., during the Alcohol-Access Period), rats received continuous access (24 hours per day) to a 10% (v/v) ethanol solution as the second choice of drinking fluid in addition to continued access to water, while they continued to receive daily treatment with the respective drug or vehicle.
Experiment 2: Comparative effects of CLOZ and HAL on the maintenance of alcohol drinking
Another 41 P rats were used for Experiment 2 to assess the comparative effects of CLOZ and HAL on maintenance of chronic alcohol drinking. After adapting to their home cage environment, all rats received continuous access to 10% (v/v) alcohol as the second choice of fluid in a bottle separated from that containing water. Following 40 days of access to alcohol, these rats were divided into 6 treatment groups (n = 6–7 per group) with similar alcohol intake baselines. Baseline for each group in Experiment 2 was the averaged value calculated using the last 4 days prior to treatment. The 40-day pre-treatment period in which alcohol was made available to P rats was based on studies indicating that P rats drinking alcohol for 5 to 6 weeks (35 to 42 days) exhibit signs of physical dependence on alcohol. Voluntary alcohol drinking for 6 weeks, but not for 2 or 4 weeks, produces a significant reduction in bicuculline-induced seizure thresholds during alcohol withdrawal. Moreover, after 6 weeks of alcohol drinking, alterations in the microstructure of alcohol intake (i.e., 90% increase in the size of alcohol drinking bouts compared to the baseline with no change in bout frequency) are associated with the development of alcohol dependence (Kampov-Polevoy et al., 2000). Following randomization, groups were injected s.c. daily over the next 20 days, 2 hours before dark, with the following: 4 mg/kg/day CLOZ (4 mg/kg CLOZ); 8 mg/kg/day CLOZ (8 mg/kg CLOZ); 12 mg/kg/day CLOZ (12 mg/kg CLOZ); 0.2 mg/kg/day HAL (0.2 mg/kg HAL); 1 mg/kg/day HAL (1 mg/kg HAL); or vehicle (VEH). The additional dose of CLOZ (8 mg/kg CLOZ) was added to the second experiment to be consistent with a prior study, in which we assessed the effects of 4, 8, and 12 mg/kg/day of CLOZ on maintenance of chronic alcohol drinking in Syrian golden hamsters (Chau et al., 2010). To adapt rats to CLOZ, the doses of CLOZ began at 4 mg/kg/day and were increased by 4 mg/kg every other day up to the targeted values; the doses of HAL began at 0.2 mg/kg and increased by 0.2 mg/kg/day (for the animals treated with 1 mg/kg/day of HAL).
Drug Preparation
Drug solutions were prepared for injection by first dissolving CLOZ or HAL, both from Sigma Chemical Inc., in 0.5 N acetic acid, then adjusting the volumes to the desired concentrations using a vehicle solution (VEH, 0.5 M sodium acetate, pH 5.0). The pH of the drug solutions was adjusted to 5.0 to prevent the drugs from precipitating out of solution. The injections were given in volumes of 1 mL/kg of body weight.
Data analysis
Separate 2-way (day × group) repeated-measures ANOVAs (RMANOVA) were performed for alcohol intake (g/kg), water intake (mL), alcohol preference (the ratio of the volume of the alcohol solution consumed to the volume of the total fluid consumed), food intake (g), and body weight (g) data. For alcohol intake, body weight was assigned according to the most proximal measurement (e.g., day 0 was used for day 1, day 3 was used for days 2–4, etc.). When the ANOVA analysis indicated that significant differences existed among treatment groups, post hoc pair-wise comparisons between groups were made using the Tukey adjustment. The pair-wise comparisons were tested each day to help interpret group × time interactions from the RMANOVAs (Maxwell, 1980); adjustment top values was carried out separately each day. Additionally, when ANOVA analysis indicated significant time effects, post hoc pair-wise comparisons between all possible pairs of days for each group were made using the Tukey adjustment. Dunnett’s test was also used to compare alcohol intake and preference between treatment groups and controls (VEH) in order to evaluate whether any detected effects were transient. Significance was determined atp < 0.05. For multiple pair-wise comparisons, the largest p value is presented whenever more than one post hoc comparison is significant. Data points and error bars depicted in the figures represent the means ± standard errors of the means, respectively.
Results
Experiment 1: Comparative effects of CLOZ and HAL on the initiation of alcohol drinking
There were no significant between-group differences at baseline for either body weight or water intake, indicating that the randomization scheme used to assign individual rats to treatment groups was effective.
Alcohol intake in g/kg (Fig. 1A)
Fig. 1.
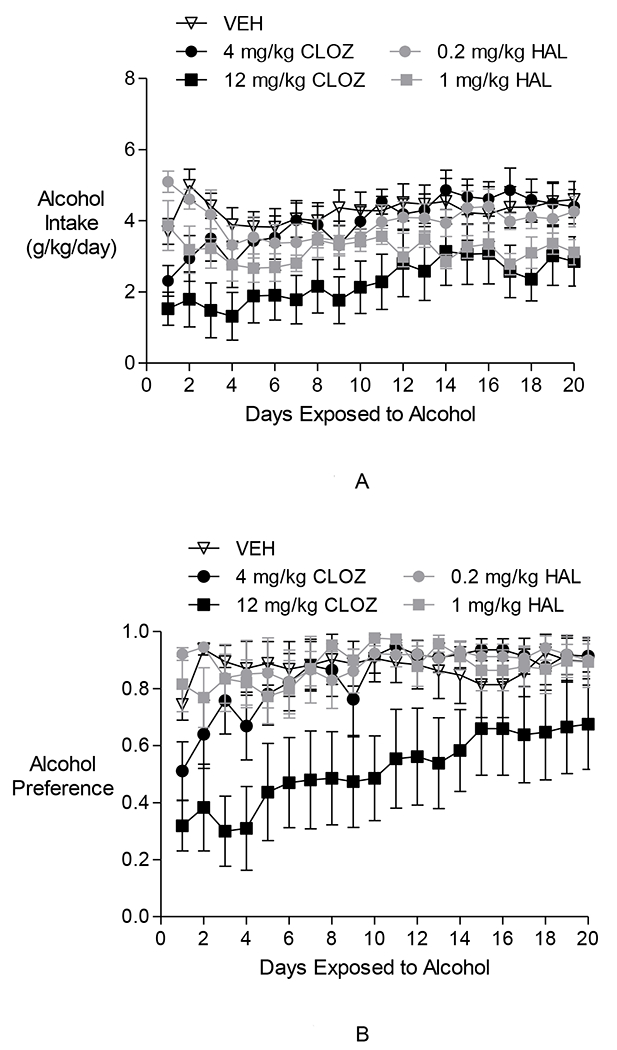
The effects of clozapine and haloperidol on initiation of alcohol drinking in P rats in Experiment 1. (A) Alcohol Intake in g/kg: The 12 mg/kg CLOZ group, but not the HAL groups, drank less alcohol than the VEH group. There was an increase in alcohol intake in the 12 mg/kg CLOZ group over the 20-day Alcohol Access Period. However, alcohol intake appears to remain lower than levels seen in the VEH-treated animals. (B) Alcohol preference: Only the 12 mg/kg CLOZ group had significantly lower alcohol preference than the VEH group over the 20-day Alcohol Access Period. Alcohol preference in the 12 mg/kg CLOZ group was also lower than that in all HAL groups. There was a gradual increase over time in alcohol preference in the 12 mg/kg CLOZ group. However, alcohol preference in this group appears to be lower than the VEH group during the latter part of the Alcohol Access Period. Alcohol preference in the VEH group and in the haloperidol-treated groups remained steadily high.
Two-way repeated-measures ANOVAs performed on daily alcohol intake during the 20-day Alcohol Access Period revealed a significant effect of time [F(19,627) = 4.43, p < 0.0001], a significant effect of group [F(4,33) = 3.58, p < 0.05], and a significant group × day interaction [F(76,627) = 1.56, p < 0.01]. Post hoc pair-wise comparisons between groups revealed that during this period, intake of alcohol in the 4 mg/kg/day CLOZ group, as well as the 0.2 and 1.0 mg/kg/day HAL groups was not significantly different from the VEH group at any time point. However, the 12 mg/kg/day CLOZ group drank less alcohol than the VEH group (alcohol access days 1–4, 7, 9–10 and 18: p < 0.05). Post hoc pair-wise analysis between groups showed that the 12 mg/kg CLOZ group also drank significantly less alcohol than the 0.2 mg/kg HAL group (days 1–3: p < 0.05), the 1 mg/kg HAL group (day 1; p < 0.05) and the 4 mg/kg CLOZ group (days 7, 10–11, 17–18; p < 0.05). Dunnett’spost hoc test used to assess transience showed that the 12 mg/kg CLOZ group differed from VEH on days 1–4, 7, 9–11, and 18 (p < 0.05), and trended toward being significantly different on days 5–6, 12, 17, and 20 (p < 0.07). Additionally, post hoc comparisons between all possible pairs of days revealed an increase in alcohol intake in the 4 mg/kg/day CLOZ group over the course of the study period (e.g., day 1 vs. days 7–8, 10–20: p < 0.05). The 12 mg/kg/day CLOZ group also exhibited such an increase (e.g., day 1 vs. days 14–16, 19–20: p < 0.05). Post hoc comparisons between days indicated no such increase in alcohol intake in the HAL groups over the 20-day Alcohol Access Period. The results for the 2 CLOZ groups are consistent with the trends observed in Fig. 1A.
To assess for the potential confound due to differential changes in weight across groups, the analyses were repeated with the g/kg metric for alcohol intake using the average weight for the days that fell between measurements. For example, the weight for days 1 and 2 was calculated as the mean of the 2 weights for day 0 and day 3. There was no significant difference in results.
Alcohol preference (Fig. 1B)
Two-way repeated-measures ANOVAs performed on alcohol preference during the 20-day Alcohol Access Period revealed a significant effect of day [F(19,627) = 5.50, p < 0.0005], a significant effect of group [F(4,33) = 3.77, p < 0.05], and a significant group × time interaction [F(76,627) = 1.54, p < 0.005]. Post hoc pair-wise comparisons between groups revealed only the 12 mg/kg/day CLOZ group having significantly lower alcohol preference than the VEH group over the 20-day Alcohol Access Period (days 1–4, 8, and 10–11; p < 0.05). Between-group comparisons also revealed that alcohol preference in the 12 mg/kg CLOZ group was lower than that in the 0.2 mg/kg/day HAL group (days 1–4, 10–11, and 13–14; p < 0.05), the 1 mg/kg/day HAL group (days 1, 3–4, 8, 10–11, and 13; p < 0.05), and the 4 mg/kg CLOZ group (days 3, 10–11, and 13–14; p < 0.05). Evaluation of transience using Dunnett’s test showed that the 12 mg/kg CLOZ group was different from VEH on days 1–5, 7–11, and 13 (p < 0.05). Post hoc pair-wise comparisons between days revealed a gradual increase in alcohol preference in the 4 mg/kg/day CLOZ group (day 1 vs. days 3, 5–20: p < 0.05) and in the 12 mg/kg/day CLOZ group (day 1 vs. days 11–20: p < 0.05). Alcohol preference in the VEH group and in the HAL-treated groups remained steadily high (day 1 vs. days 2–20; N.S.) The results for the two CLOZ groups are consistent with the trends observed in Fig. 1B.
Water intake
Two-way repeated-measures ANOVAs performed on daily water intake (mL/day) during the 20-day Alcohol Access Period revealed a significant effect of day [F(19,627) = 5.71, p < 0.0001], a significant effect of group [F(4,33) = 3.28, p < 0.05], and a significant group × time interaction [F(76,627) = 1.61, p < 0.005]. During the Alcohol Access Period, the 12 mg/kg CLOZ group drank more water than the VEH group (days 1–4, 8, and 10; p < 0.05), the 0.2 mg/kg/day HAL group (days 1–2, 4, 10, and 11: p < 0.05), the 1.0 mg/kg/day HAL group (days 1–2, 4, 8, 10, and 13: p < 0.05), and the 4 mg/kg CLOZ group (days 9–11; p < 0.05). This occurred while the 12 mg/kg CLOZ group decreased its alcohol drinking. Thus, the 12 mg/kg CLOZ group maintained steady fluid intake.
Food intake (Fig. 2A)
Fig. 2.
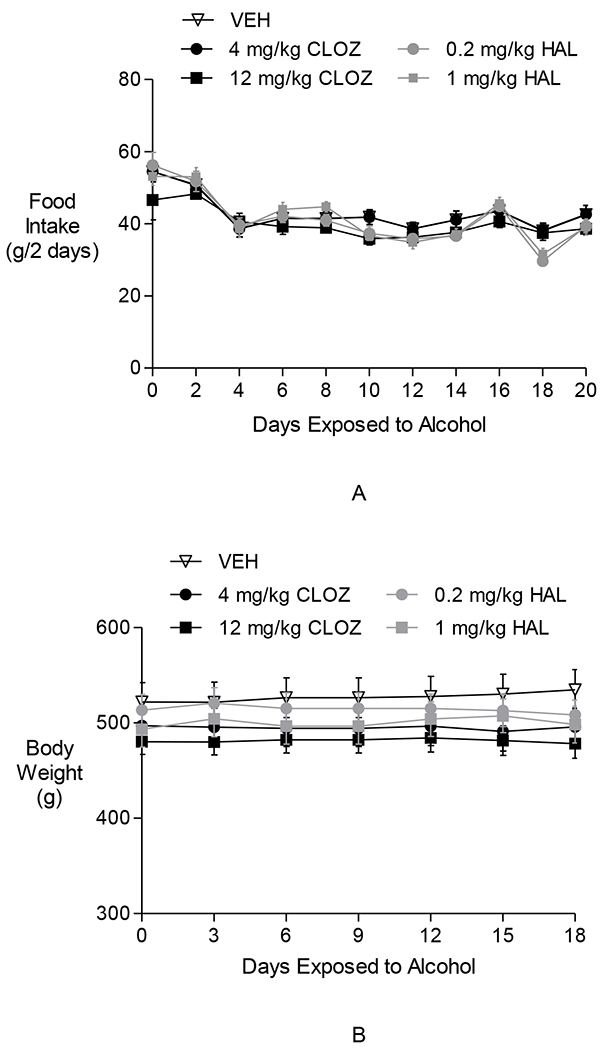
The effects of clozapine and haloperidol on food intake and body weight in Experiment 1. Neither clozapine nor haloperidol affected food intake (A) or body weight (B).
Two-way repeated-measures ANOVAs performed on 2-day food intake during the Alcohol Access Period revealed a significant effect of time [F(9,297) = 39.35, p < 0.0001], no effect of group, and a significant group × time interaction [F(36,27) = 1.89, p < 0.005]. Post hoc pair-wise comparisons between days indicated that during the Alcohol Access Period, as alcohol was introduced into the cage, food intake in all groups decreased (day 1 vs. day 20; p < 0.05). The absence of a group effect indicates that decreased alcohol drinking in the 12 mg/kg/day CLOZ group was not due to the animals eating (and drinking) less due to becoming ill when treated with the drug.
Body Weight (Fig. 2B)
Two-way repeated-measures ANOVAs performed on body weight measurements taken every 3 days during the Alcohol Access Period revealed a significant effect of time [F(7,231) = 6.93, p < 0.0001], no effect of group, and no significant group × time interaction. Body weight within individual groups did not decrease over time, indicating that the treatment did not adversely affect the animals’ health.
Experiment 2: Comparative effects of CLOZ and HAL on the maintenance of alcohol drinking
There were no significant between-group differences at baseline for alcohol intake, indicating that the randomization scheme used to assign individual rats to treatment groups was effective.
Alcohol intake in g/kg (Fig. 3A)
Fig. 3.
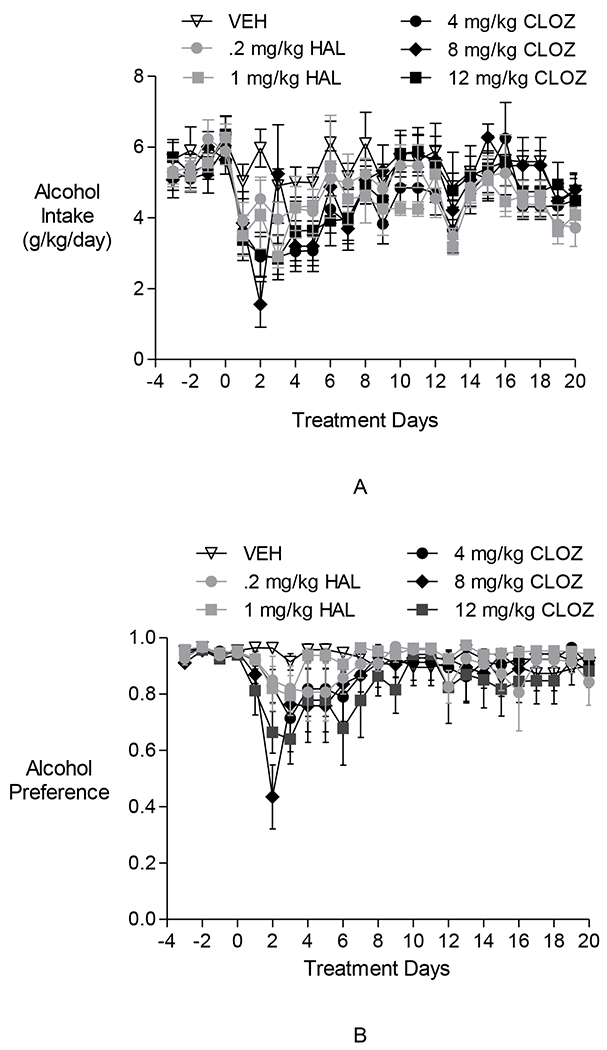
The effects of clozapine and haloperidol on maintenance of alcohol drinking in P rats in Experiment 2. (A) Alcohol intake in g/kg: Neither clozapine nor haloperidol decreased alcohol intake. (B) Alcohol preference: Neither clozapine nor haloperidol decreased alcohol preference.
Two-way repeated-measures ANOVAs performed on daily alcohol intake (g/kg/day) during the 20-day Treatment Period revealed a significant effect of day [F(19,646) = 13.564, p <0.0001], no effect of group, and a significant group × day interaction [F(95,646) = 1.440, p < 0.001]. Dunnett’spost hoc comparisons showed the VEH group drank more alcohol than the 4 mg/kg CLOZ group, the 8 mg/kg CLOZ group, and the 12 mg/kg CLOZ group only on day 2 of the treatment period (p < 0.05). Additionally, post hoc comparisons between all possible pairs of days revealed an increase across time in alcohol intake in the 8 mg/kg/day CLOZ group (e.g., day 1 vs. days 10–12, and 15–16: p < 0.05) and 12 mg/kg/day CLOZ group (e.g., day 1 vs. days 10–12, 14–16: p < 0.05). Post hoc comparisons between days indicated no such increase across time in alcohol intake in the HAL groups over the 20-day Treatment Period. The results with the CLOZ group are consistent with the trends observed in Fig. 3A.
Alcohol preference (Fig. 3B)
Two-way repeated-measures ANOVAs performed on alcohol preference during the 20-day Treatment Period revealed a significant effect of day [F(19,646) = 6.73, p < 0.0001], no effect of group, and a significant group × day interaction [F(95,646) = 1.64, p < 0.001]. Post hoc comparisons between all possible pairs of days revealed an increase over time in alcohol preference in the 8 mg/kg/day CLOZ group (e.g., day 2 vs. days 3–20: p < 0.05) and 12 mg/kg/day CLOZ group (e.g., day 2 vs. days 8, 10–11, 13–14, 16–20: p < 0.05). Post hoc pair-wise comparisons between days indicated no such increase in alcohol preference in the HAL groups over the 20-day Treatment Period. These results are consistent with the trends observed in Fig. 3B.
Water intake
Two-way repeated-measures ANOVAs performed on daily water intake (mL/day) during the 20-day Treatment Period revealed a significant effect of day [F(19,646) = 5.67, p < 0.0001], no effect of group, and a significant group × time interaction [F(95,646) = 1.28, p < 0.05]. Post hoc pair-wise comparisons between all possible pairs of days revealed a transient increase in water intake in the 4 mg/kg/day CLOZ group (e.g., day 0 vs. days 3 and 6: p < 0.05), 8 mg/kg/day CLOZ group (e.g., day 0 vs. days 2 and 3: p < 0.05) and 12 mg/kg/day CLOZ group (e.g., day 0 vs. days 2, 3, 6–8: p < 0.05). Post hoc pair-wise comparisons between days indicated no such increase in water intake in the HAL groups over the 20-day Treatment Period.
Food intake (Fig. 4A)
Fig. 4.
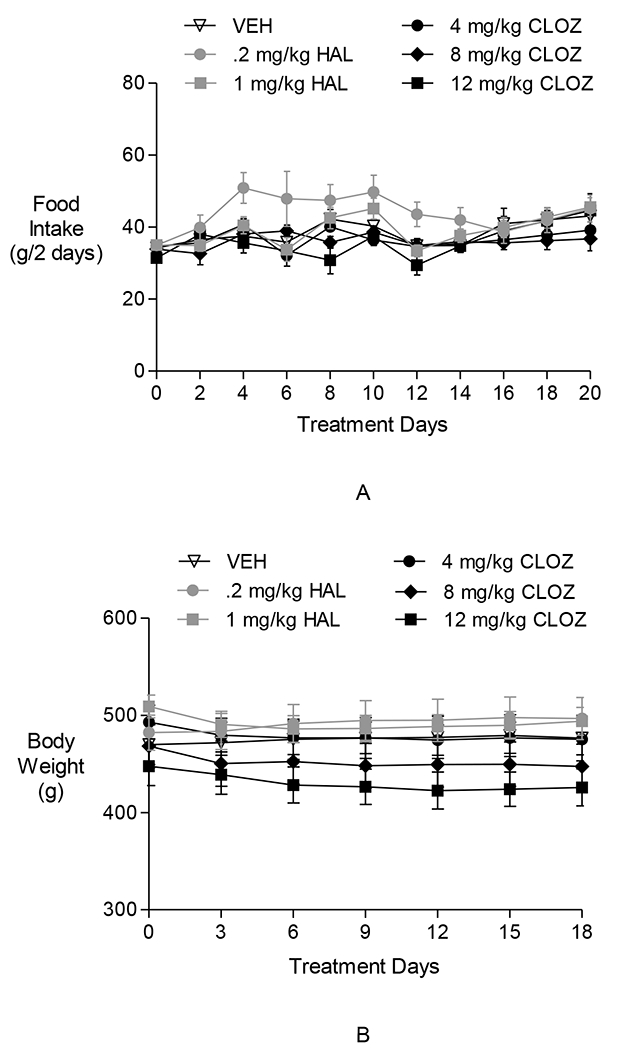
The effects of clozapine and haloperidol on food intake and body weight in Experiment 2. Neither clozapine nor haloperidol affected food intake (A) or body weight (B).
Two-way repeated-measures ANOVAs performed on 2-day food intake during the 20-day Treatment Period revealed a significant effect of time [F(10,340) = 6.39, p < 0.0001], a significant effect of group [F(50,340) = 56.21, p = 0.04], and a significant group × time interaction [F(45,306) = 1.77, p < 0.01]. Post hoc analysis indicated that only the 0.2 mg/kg/day HAL group ate more food than the VEH group (days 4–6, p < 0.05). Additionally, post hoc pair-wise comparisons revealed an increase over time in food intake in the 0.2 mg/kg/day HAL group (e.g., day 0 vs. days 4–12, and 20: p < 0.05), but no such increase in the 1 mg/kg/day HAL group or in the CLOZ groups over the 20-day Treatment Period.
Body Weight (Fig. 4B)
Two-way repeated-measures ANOVAs performed on body weight during the 20-day Treatment Period revealed a significant effect of time [F(7,238) = 2.41, p < 0.021], no effect of group, and a significant group × time interaction [F(35,238) = 2.69, p < 0.0001], possibly due to group means crossing each other over time. Post hoc pair-wise comparisons between all possible pairs of days indicated an increase in body weight in the VEH-treated group (day 0 vs. days 9–18; p < 0.05), and decreases in both the 8 mg/kg CLOZ (day 0 vs. days 6–18; p < 0.05) and the 12 mg/kg CLOZ groups (day 0 vs. days 6–18; p < 0.05).
Discussion
To the best of our knowledge, the current study is the first to assess the comparative effects of CLOZ and HAL on initiation and maintenance of alcohol drinking in the alcohol-preferring P rat.
Experiment 1: Initiation of Alcohol Drinking
In the current study, 12 mg/kg CLOZ significantly attenuated initiation of alcohol drinking and preference more than vehicle, while HAL did not. Over time, following the early attenuation, there was an increase in alcohol intake and alcohol preference in the 12 mg/kg/day CLOZ group, suggesting the possibility that some tolerance to the CLOZ-induced attenuation of alcohol drinking developed. Nevertheless, alcohol intake and preference appeared to stabilize at levels lower than the VEH-treated animals over the course of the study. In particular, post hoc tests showed that alcohol intake in the 12 mg/kg/day CLOZ group either differed significantly from the VEH group or trended toward such differences throughout the treatment period. Thus, CLOZ limited the initiation of alcohol drinking, while HAL did not.
The alcohol-drinking and alcohol-preference suppressing effects of CLOZ were not likely due to the drugs decreasing general drinking and eating, because prior to the introduction of alcohol the drugs did not alter either water or food intake. Moreover, once alcohol was available, the total fluid intake increased significantly in the control VEH group and in those drug-treated groups that did not show attenuated alcohol intake.
Taken together, data from Experiment 1 suggest that CLOZ attenuates the initiation of alcohol drinking and the development of alcohol preference in the P rat, while HAL does not. These data in the P rat are consistent with our prior data indicating that CLOZ, but not HAL, attenuates initiation of alcohol intake and acquisition of alcohol preference in the Syrian golden hamster (Chau et al., 2011), an animal model of alcohol preference that does not exhibit alcohol dependence (McMillan et al., 1977). Thus, the paradigm involving initiation of alcohol drinking in the P rat appears to mimic the effects of CLOZ and HAL on alcohol drinking seen in the hamster.
As noted in the introduction, we have suggested that CLOZ’s effect on alcohol drinking (as observed here) may be related to its broad-spectrum pharmacological effects on both the dopaminergic, and particularly noradrenergic system (Green et al., 1999). Moreover, we have suggested that HAL’s potent DA D2 receptor antagonism (in contrast to CLOZ’s weak antagonism at the receptor) may be, in part, related to the lack of effect of HAL on alcohol drinking (Chau et al., 2011; Chau et al., 2010; Chau et al., 2004).
Experiment 2: Maintenance of Alcohol Drinking
Experiment 2 studied P rats that had consumed alcohol for 40 days prior to treatment - the length of time required for P rats to develop clear signs of physical dependence (Kampov-Polevoy et al., 2000). In these alcohol-dependent P rats, neither CLOZ nor HAL was able to significantly reduce alcohol drinking.
The inability of HAL to disrupt maintenance of alcohol drinking is consistent with our previous study with HAL in Syrian golden hamsters and with studies by others in rats and mice that also employed similar continuous free-access paradigms (Naeger and Martinez, 1990; Ng and George, 1994; Panocka et al., 1993a; Panocka and Massi, 1992; Panocka et al., 1993b; Potthoff et al., 1983; Salimov et al., 2000).
The current data regarding the inability of CLOZ to disrupt maintenance drinking in the P rat are consistent with a study by Ingman and Korpi (2006) in which a single dose of CLOZ did not alter alcohol drinking (in a limited access paradigm) in the alcohol-preferring AA (Alko, Alcohol) rat, and repeated CLOZ treatment was only moderately effective in decreasing it. Interestingly, however, CLOZ is able to suppress chronic alcohol drinking in Syrian golden hamsters (Chau et al., 2010), as well as in common stock, Sprague-Dawley rats drinking a low concentration of alcohol while ingesting a high dose of CLOZ in drinking water (Ufer et al., 1999). Taken together, these data suggest that experimental paradigms involving maintenance of alcohol drinking in animal models of alcohol dependence (e.g., the P rat) do not reliably predict the alcohol-intake reducing effects of CLOZ and HAL on alcohol drinking in patients with SCZ. However, paradigms involving free-choice alcohol drinking (both acquisition and chronic intake) in the golden hamster and acquisition of alcohol drinking and alcohol preference in the P rat both appear to predict the differential effects of these drugs seen in patients with SCZ.
Limitations
In both experiments, body weight was measured less frequently than the volume of alcohol intake. While this study design has been used by others (Dhaher et al., 2012; Lynch et al., 2011; Rodd et al., 2009; Rodd-Henricks et al., 2001), we recognize that differences in timing of the measurement of weight and alcohol measures could potentially complicate analysis of absolute alcohol intake expressed in g/kg/day. The less frequent assessment of body weight was done to minimize disturbing the animals. We found that two separate methods of assessing body weights — using the most proximal measurement (e.g., using day 3 for days 2–4) and using averaged body weight (e.g., using the average of days 3 and 6 for days 4 and 5) — yielded similar statistical results. In addition, body weight for all groups was relatively stable throughout the first experiment. Post hoc tests showed that there was a significant decrease in body weight in the 8 and 12 mg/kg CLOZ treated groups over the course of Experiment 2. This decrease, however, did not seem to affect our finding that there was a lack of effect of CLOZ on alcohol intake in this experiment. Moreover, the pattern of effect was consistent between alcohol intake and alcohol preference (a measure not affected by changes in body weight), suggesting that this potential confound did not have a significant impact on the results.
Concerning baseline alcohol intake, alcohol drinking in the vehicle group in Experiment 2 was slightly higher than that seen in Experiment 1, even though both groups came from similar generations of the P rat. This difference in alcohol intake between the 2 groups was likely due to the fact that rats in Experiment 2 drank alcohol for a significantly longer time (40 days) than rats in Experiment 1 (0 days) prior to the initiation of the experiment. Thus, the lengths of time rats were exposed to alcohol and differences in baseline alcohol intake could potentially contribute to the varying results between the two experiments.
Conclusion
In summary, the data indicate that CLOZ attenuates initiation, but not maintenance, of chronic alcohol consumption in the P rat, a heavy alcohol-drinking animal that develops physiological dependence on alcohol. The study further suggests that HAL alters neither initiation nor maintenance of alcohol drinking in this animal. These data are consistent with our studies in the Syrian golden hamster, an alcohol-drinking rodent that does not develop physiological dependence on alcohol, as well as in patients with SCZ, who are more likely to develop alcohol abuse than dependence. In the hamster, CLOZ disrupts both the initiation and the maintenance of chronic drinking of alcohol (Chau et al., 2011; Chau et al., 2010). In patients with SCZ, CLOZ but not HAL limits alcohol drinking (Brady et al., 1990; Dixon et al., 1991; McEvoy et al., 1995). We have proposed that a dysregulated mesocorticolimbic brain reward circuit underpins alcohol drinking in patients with schizophrenia, and that CLOZ is able to decrease alcohol drinking in these patients because it is able to alleviate this brain reward circuit dysregulation (Green et al., 1999). Interestingly, the P rat is known to have a dysregulated mesocorticolimbic system. It is not known whether the ability of CLOZ to decrease initiation of alcohol drinking, but not chronic drinking, in the P rat relates to CLOZ’s ability to modulate the brain reward circuit in P rats. This is consistent with CLOZ’s ability to reduce alcohol drinking in the Syrian golden hamster, an animal that does not develop physiological dependence on alcohol, and in patients with SCZ, in whom alcohol abuse is more likely to occur than alcohol dependence.
Acknowledgments
This work was supported by the National Institute of Alcohol Abuse and Alcoholism (R03AA014644 and 1R01AA018151-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albanese MJ, Khantzian EJ, Murphy SL, and Green AI (1994). Decreased substance use in chronically psychotic patients treated with clozapine. Am J Psychiatry 151, 780–781. [DOI] [PubMed] [Google Scholar]
- Bedard AM, Maheux J, Levesque D, and Samaha AN (2013). Prior haloperidol, but not olanzapine, exposure augments the pursuit of reward cues: implications for substance abuse in schizophrenia. Schizophr Bull 39, 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, and Comings DE (2000). Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 32 Suppl, i–iv, 1–112. [DOI] [PubMed] [Google Scholar]
- Brady K, Anton R, Ballenger JC, Lydiard RB, Adinoff B, and Selander J (1990). Cocaine abuse among schizophrenic patients. Am J Psychiatry 147, 1164–1167. [DOI] [PubMed] [Google Scholar]
- Buckley P, Thompson P, Way L, and Meltzer HY (1994). Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry 151, 385–389. [DOI] [PubMed] [Google Scholar]
- Chau DT, Ahmed J, Wang TT, Xie H, Dawson R, and Green AI (2011). Raclopride lessens the ability of clozapine to suppress alcohol drinking in Syrian golden hamsters. Neuropharmacology 61, 646–652. [DOI] [PubMed] [Google Scholar]
- Chau DT, Gulick D, Xie H, Dawson R, and Green AI (2010). Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology 58, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau DT, Roth RM, and Green AI (2004). The neural circuitry of reward and its relevance to psychiatric disorders. Curr Psychiatry Rep 6, 391–399. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, and Markou A (2012). The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, McConnell KK, Rodd ZA, McBride WJ, and Bell RL (2012). Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav 102, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L, Haas G, Weiden PJ, Sweeney J, and Frances AJ (1991). Drug abuse in schizophrenic patients: clinical correlates and reasons for use. Am J Psychiatry 148, 224–230. [DOI] [PubMed] [Google Scholar]
- Drake RE, Osher FC, and Wallach MA (1989). Alcohol use and abuse in schizophrenia. A prospective community study. J Nerv Ment Dis 177, 408–414. [DOI] [PubMed] [Google Scholar]
- Drake RE, and Wallach MA (1993). Moderate drinking among people with severe mental illness. Hosp Community Psychiatry 44, 780–782. [DOI] [PubMed] [Google Scholar]
- Drake RE, Xie H, McHugo GJ, and Green AI (2000). The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull 26, 441–449. [DOI] [PubMed] [Google Scholar]
- Grace AA (1991). Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41, 1–24. [DOI] [PubMed] [Google Scholar]
- Green AI, Chau DT, Keung WM, Dawson R, Mesholam RI, and Schildkraut JJ (2004). Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res 128, 9–20. [DOI] [PubMed] [Google Scholar]
- Green AI, Noordsy DL, Brunette MF, and O’Keefe C (2008). Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat 34, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Zimmet SV, Strous RD, and Schildkraut JJ (1999). Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine? Harv Rev Psychiatry 6, 287–296. [DOI] [PubMed] [Google Scholar]
- Ingman K, and Korpi ER (2006). Alcohol drinking of alcohol-preferring AA rats is differentially affected by clozapine and olanzapine. Eur J Pharmacol 534, 133–140. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, and Overstreet DH (2000). P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res 24, 278–284. [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, and Nobrega JN (2003). Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther 305, 625–631. [DOI] [PubMed] [Google Scholar]
- Lehman AF, Myers CP, Dixon LB, and Johnson JL (1996). Detection of substance use disorders among psychiatric inpatients. J Nerv Ment Dis 184, 228–233. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Bond C, Breslin FJ, and Johnson BA (2011). Severity of drinking as a predictor of efficacy of the combination of ondansetron and topiramate in rat models of ethanol consumption and relapse. Psychopharmacology (Berl) 217, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P, and Snyder R (1995). Reduction of comorbid substance abuse with clozapine. Am J Psychiatry 152, 959. [DOI] [PubMed] [Google Scholar]
- Maxwell S (1980). Pairwise multiple comparisons in repeated measures designs. Journal of Educational Statistics 5, 269–287. [Google Scholar]
- McBride WJ, and Li TK (1998). Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 12, 339–369. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Freudenreich O, Levin ED, and Rose JE (1995). Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology (Berl) 119, 124–126. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Ellis FW, Frye GD, and Pick JR (1977). Failure of signs of physical dependence to develop in hamsters after prolonged consumption of large doses of ethanol. Pharmacol Biochem Behav 7, 55–57. [DOI] [PubMed] [Google Scholar]
- Naeger S, and Martinez TT (1990). The effect of tetrahydropapaveroline, bromocriptine, haloperidol, and lithium on voluntary ethanol ingestion. Proc West Pharmacol Soc 33, 205–208. [PubMed] [Google Scholar]
- Ng GY, and George SR (1994). Dopamine receptor agonist reduces ethanol self-administration in the ethanol-preferring C57BL/6J inbred mouse. Eur J Pharmacol 269, 365–374. [DOI] [PubMed] [Google Scholar]
- Panocka I, Ciccocioppo R, Pompei P, and Massi M (1993a). 5-HT2 receptor antagonists do not reduce ethanol preference in Sardinian alcohol-preferring (sP) rats. Pharmacol Biochem Behav 46, 853–856. [DOI] [PubMed] [Google Scholar]
- Panocka I, and Massi M (1992). Long-lasting suppression of alcohol preference in rats following serotonin receptor blockade by ritanserin. Brain Res Bull 28, 493–496. [DOI] [PubMed] [Google Scholar]
- Panocka I, Pompei P, and Massi M (1993b). Suppression of alcohol preference in rats induced by risperidone, a serotonin 5-HT2 and dopamine D2 receptor antagonist. Brain Res Bull 31, 595–599. [DOI] [PubMed] [Google Scholar]
- Potthoff AD, Ellison G, and Nelson L (1983). Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav 18, 489–493. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, and Goodwin FK (1990). Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 264, 2511–2518. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, and McBride WJ (2009). Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of high-alcohol-drinking (HAD) rats. Addict Biol 14, 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, and Li TK (2001). Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res 25, 1140–1150. [PubMed] [Google Scholar]
- Salimov RM, Salimova NB, Shvets LN, and Maisky AI (2000). Haloperidol administered subchronically reduces the alcohol-deprivation effect in mice. Alcohol 20, 61–68. [DOI] [PubMed] [Google Scholar]
- Test MA, Wallisch LS, Allness DJ, and Ripp K (1989). Substance use in young adults with schizophrenic disorders. Schizophr Bull 15, 465–476. [DOI] [PubMed] [Google Scholar]
- Ufer M, Dadmarz M, and Vogel WH (1999). Voluntary consumption of amphetamine, cocaine, ethanol and morphine by rats as influenced by a preceding period of forced drug intake and clozapine. Pharmacology 58, 285–291. [DOI] [PubMed] [Google Scholar]
- Yovell Y, and Opler LA (1994). Clozapine reverses cocaine craving in a treatment-resistant mentally ill chemical abuser: a case report and a hypothesis. J Nerv Ment Dis 182, 591–592. [DOI] [PubMed] [Google Scholar]
- Zimmet SV, Strous RD, Burgess ES, Kohnstamm S, and Green AI (2000). Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol 20, 94–98. [DOI] [PubMed] [Google Scholar]


