The clinical presentation of brucellosis in humans is variable and unspecific, and thus, laboratory corroboration of the diagnosis is essential for the patient’s proper treatment. The diagnosis of brucellar infections can be made by culture, serological tests, and nucleic acid amplification assays.
KEYWORDS: human brucellosis, diagnosis, culture, serological tests, nucleic acid amplification methods
SUMMARY
The clinical presentation of brucellosis in humans is variable and unspecific, and thus, laboratory corroboration of the diagnosis is essential for the patient’s proper treatment. The diagnosis of brucellar infections can be made by culture, serological tests, and nucleic acid amplification assays. Modern automated blood culture systems enable detection of acute cases of brucellosis within the routine 5- to 7-day incubation protocol employed in clinical microbiology laboratories, although a longer incubation and performance of blind subcultures may be needed for protracted cases. Serological tests, though they lack specificity and provide results that may be difficult to interpret in individuals repeatedly exposed to Brucella organisms, nevertheless remain a diagnostic cornerstone in resource-poor countries. Nucleic acid amplification assays combine exquisite sensitivity, specificity, and safety and enable rapid diagnosis of the disease. However, long-term persistence of positive molecular test results in patients that have apparently fully recovered is common and has unclear clinical significance and therapeutic implications. Therefore, as long as there are no sufficiently validated commercial tests or studies that demonstrate an adequate interlaboratory reproducibility of the different homemade PCR assays, cultures and serological methods will remain the primary tools for the diagnosis and posttherapeutic follow-up of human brucellosis.
INTRODUCTION
Brucellae are small (0.5 to 0.7 by 0.6 to 1.5 μm), nonmotile, non-spore-forming, and slow-growing Gram-negative coccobacilli belonging to the Brucellaceae family in the alpha-2 subclass of the Proteobacteria, together with the Mycoplana, Pseudochrobactrum, Paenochrobactrum, Daeguia, Crabtreella, and Ochrobactrum genera (1).
Brucellae comprise facultative intracellular bacteria that infect a variety of feral and domestic animals. The discovery of novel brucellae in recent years has considerably expanded the genus, which currently comprises 12 recognized species, of which four—namely, B. melitensis, B. abortus, B. suis, and B. canis—are the main causes of the disease in humans. Brucella melitensis is the most virulent species in humans, whereas no cases of infection caused by B. ovis, B. neotomae, B. microti, or B. papionis have been reported so far. In addition to the well-established species, many isolates derived from animal sources that have not yet been taxonomically allocated have been described (1).
The different Brucella species constitute a closely related monophyletic cluster with DNA-DNA hybridization values approaching 100% (2) and thus can be considered to represent biovars of a single species. However, the traditional nomenclature has been retained for practical reasons, since the different Brucella species are closely associated with specific animal hosts (i.e., B. abortus with cattle, B. melitensis with small ruminants, B. suis with swine, and B. canis with canids). It should be emphasized, however, that Brucella species can cross-infect nonpreferential hosts, a feature that explains the accidental acquisition of the disease by humans from zoonotic sources. In addition, an extended sequence analysis of 21 independent genetic loci has shown that the distribution of genotypes correlates remarkably well with the different species, validating the classic taxonomic division (1).
Members of the genus Ochrobactrum are the closest phylogenetic relatives of brucellae, sharing over 97% identity with the Brucella consensus sequence of the 16S rRNA gene, and species such as O. antrophi and O. intermedium appear more related to brucellae than to other species of their own genus (3–6). This remarkable similarity has important implications for the correct identification of brucellae and the diagnosis of the infection.
The Global Challenge of Human Brucellosis and Its Diagnosis
Brucellosis was probably first acquired by humans shortly after the domestication of cattle, camels, sheep, goats, and swine, and since person-to-person transmission of the infection is exceptional (7), humans represents a dead end in the cycle of the disease. Because brucellosis is not a sustainable infection in humans and the disease is almost always transmitted to humans by direct or indirect exposure to infected animals or consumption of their contaminated products, eradicating the infection in livestock is crucial for preventing human contagion. Whereas strict implementation of control measures, including routine screening of livestock, culling of infected herds, and vaccination of healthy animals, has resulted in the successful control of the disease in most industrialized countries, brucellosis remains endemic in the Mediterranean basin, the Middle East, Latin America, the Indian subcontinent, and many African countries north and south of the Sahara (8). In global terms, 500,000 new human cases of brucellosis are diagnosed each year, representing the world’s most prevalent bacterial zoonosis (8). However, since many cases remain unrecognized due to inaccurate diagnosis, inadequate surveillance, and incomplete reporting, this staggering figure should only be considered a minimal estimate. According to the World Health Organization (WHO), the actual incidence could be at least 1 order of magnitude higher (9). The global disease burden in livestock is even greater, and conservative estimates are that >300 million of the 1.4 billion worldwide cattle population are infected with the pathogen (10).
In recent years, the breakdown of public veterinarian and health systems in resource-poor and politically troubled countries has resulted in the emergence of new foci of disease in central Asia and a worsening of the situation in countries such as Syria (11). Although the incidence of human brucellosis in neighboring Israel has been steadily declining for many years countrywide, because of an inconsistent control policy and underfunding, the attack rate among the seminomadic Bedouin inhabitants of the southern Negev desert has increased, reaching a minimal estimate of 100.4 per 100,000 population in 2008 (12). In the developed world, brucellosis has also managed to elude complete eradication because of persistent infection among wildlife with consecutive spillover to domestic animals (13, 14), international travel and human migration (15), and illegal import of contaminated dairy products (16). In addition, B. abortus vaccines do not fully prevent B. melitensis infection, and the B. melitensis Rev.1 vaccine has not been evaluated for administration in cattle. As a result, bovine B. melitensis disease is becoming a serious public health threat in many regions (17).
Although brucellosis in humans is not usually lethal and is only exceptionally transmitted from person to person, the potential economic damage caused by the loss of productivity in animal husbandry and the debilitating effects of the disease in humans and its complicated treatment can also turn Brucella organisms into candidate agents of biowarfare (18).
Diagnosing Human Brucellosis: Culture, Serology, and NAATs
Because human brucellosis can affect any organ and body system, the presenting symptoms of the infection are not pathognomonic, and therefore, the disease may be easily confused with other medical conditions (8, 16). Conversely, overdiagnosis of brucellosis may result in untoward drug effects and, no less importantly, in overlooking other serious infectious or noninfectious illnesses. The antibiotic therapy of brucellar infections is also challenging and necessitates prolonged administration of antimicrobial drug combinations that are not routinely prescribed for other infectious diseases (16). The correct diagnosis of brucellosis in humans therefore not only is crucial for early and adequate patient management but has also serious public health significance, as it may reveal exposure to sick animals, consumption of contaminated food (especially dairy products), breach of laboratory safety practices, or the intentional release of brucellae as a biological weapon.
The microbiological diagnosis of human brucellosis relies on three different modalities: culture, serology, and nucleic acid amplification tests (NAATs). This review summarizes the recent developments in and present status of these diagnostic approaches and their clinical use and provides an assessment of their relative advantages and drawbacks.
CULTURE DETECTION OF BRUCELLAE
Blood Cultures
Although the culture detection of Brucella organisms is hampered by the slow-growing features of members of the genus, laboratory safety concerns, and reduced sensitivity in prolonged disease and focal infections, isolation of the bacterium is indisputable evidence of the disease. Culture recovery of the bacterium permits its precise identification to species level and genotyping, making it possible to track the source, differentiate between wild and vaccine Brucella strains (19), and perform antibiotic susceptibility testing when indicated.
Detection of brucellae in blood cultures also makes it possible to confirm the presence of the disease in its early stages, when the serological tests results are still negative or show low or borderline antibody titers (20). An additional benefit of the isolation of brucellae is the fact that it establishes a solid diagnosis in patients in whom the infection is not clinically suspected but the organism is recovered from a blood culture obtained as part of the routine workup of a nonspecific febrile syndrome (21, 22). This is an important consideration because the history of antecedent exposure to zoonotic sources cannot always be elicited due to the prolonged incubation of the disease, i.e., weeks or months. The clinical features of brucellosis are often suggestive of other medical conditions, including systemic and localized infections and rheumatic or hematological disorders (8). When the possibility of the disease is not considered, Brucella-specific serodiagnostic assays and nucleic acid amplification tests are not requested, and thus, the diagnosis can be overlooked. Naturally, this situation is not uncommon in countries where brucellosis is rare, awareness of the disease is low, and physicians do not include it in the differential diagnosis, as shown in an outbreak of brucellar infections in Hong Kong (23). However, because human brucellosis is a “great imitator,” the possibility of this infection is not always entertained, even in regions of endemicity. For instance, in a study performed in the southern region of Israel, where the infection is hyperendemic, 27 blood cultures drawn from 21 patients in whom the disease was suspected grew B. melitensis, as did 42 cultures obtained from 27 individuals in whom the infection was not considered (21). A similar experience was reported at a Turkish hospital in which 52 of 88 (59.1%) patients with proven disease had been previously examined by a physician and misdiagnosed (24).
Dynamics of Brucella bacteremia.
Brucella species are highly transmissible organisms that can penetrate the human body through a variety of routes, including the gastrointestinal and respiratory tracts, the conjunctiva, and abraded skin, or may access the bloodstream directly as in transfusion-related cases or transplacental transmission (8, 16). Regardless of the portal of entry of Brucella organisms into the body, the bacterium quickly translocates across the epithelial layer and is endocytosed by mucosal macrophages and dendritic cells (8). Internalized brucellae initially localize in the regional lymph nodes and then spread through the bloodstream, entering macrophages-rich tissues such as the liver, spleen, lymph nodes, or bone marrow. There they adopt a facultative and stealthy intracellular lifestyle, evading the innate and adaptive immune responses and the action of many antibiotics (16). Since the pathogenesis of human brucellosis always involves a bacteremic stage, cultures of peripheral blood represent a suitable tool for confirming the disease, although their sensitivity shows a broad range (10 to 90%) in different reports (16). The factors affecting the recovery of brucellae in blood cultures are summarized in Table 1.
TABLE 1.
Factors that determine the positivity rate of blood cultures for diagnosing brucellosis
| Category | Associated factors |
|---|---|
| Microbial | Brucella species |
| Patient | Age, duration of symptoms, systemic vs focal disease, first infection vs relapse, previous or current antibiotic administration |
| Blood culture method | Vol of blood specimen, no. of cultures obtained, frequency of monitoring, blood culture system sensitivity, incubation period, performance of periodic blind subcultures, performance of terminal blind subcultures |
In the initial stages of brucellosis, patients experience a continuous low-grade bacteremia, which can be easily detected by drawing two or three separate blood culture sets. As the infection progresses, the organism is removed from the bloodstream and sequestrated in macrophages. As a result, the concentration of circulating bacteria gradually diminishes and the pattern of bacteremia becomes less consistent, making the isolation of the organism increasingly difficult (25). The importance of obtaining multiple blood cultures was illustrated in a Turkish study in which brucellae were detected in 26 of 31 (83.9%) patients from whom a pair of blood cultures were drawn and in 17 of 29 (58.6%) patients from whom a single blood culture was obtained (P = 0.03) (26). The natural course of human brucellosis in general, and that of Brucella bacteremia in particular, is unpredictable (27). The organism may reenter the bloodstream intermittently (25), and its reappearance in the bloodstream increases the risk of clinical relapse and seeding to distant sites (28, 29).
Brucellemic patients frequently present with higher fever than those with no demonstrable bacteremia (30). However, because brucellae are pathogens with relatively low virulence in humans, the inflammatory response may be attenuated, and the organism may be recovered from paucisymptomatic and even afebrile patients (31). Therefore, blood cultures should always be obtained whenever the disease is suspected, even in the absence of fever (32).
Assessing the performance of blood culture methods for the detection of brucellae.
Brucella species are characterized by a long generation time (i.e., several hours), low concentration of circulating bacteria, and reduced levels of CO2 emission (CO2 being the metabolic product monitored by current automated blood culture systems). To maximize recovery of the organism, incubation of inoculated medium for 4 weeks and performance of blind subcultures of apparently negative blood culture media have been advised by the American Society for Microbiology (33) and the WHO (34). This strategy, however, has evident shortcomings: it is expensive and labor-intensive, demands extra laboratory space to accommodate additional blood culture instruments, and results in a considerable delay in the diagnosis of the disease. However, limiting the incubation of inoculated bottles to the routine 5- to 7-day period implemented in clinical microbiology laboratories cannot be advocated unless it is convincingly shown that by adopting this policy, vials containing Brucella organisms are not systematically overlooked. Many studies in which a customary short incubation protocol has been followed and no blind subcultures have been performed have reported detection of circulating brucellae within 3 to 7 days (35–38). These communications can be dangerously misleading because the possibility that vials containing living Brucella organisms were prematurely discarded is not addressed. This is particularly significant since a positive blood culture result is often the first and only proof of the infection. The sensitivity of blood culture systems and the time to detection for the method need to be assessed in prospective and well-designed controlled studies in which prolonged incubation and blind subcultures of negative vials are performed. The sensitivity of the blood culture method should be calculated as the fraction of positive blood culture vials identified within the routine 5- to 7-day incubation period out of the total number of positive vials detected by the system under evaluation and/or by blind subcultures in the course of the 4-week period.
Blood culture methods.
(i) Manual monophasic method.
Traditionally, the microbiological procedures used for isolating brucellae from the blood did not differ from the laboratory practices employed for the detection of other bacterial pathogens. Blood culture vials were inoculated with patients’ blood specimens, incubated at 35°C, and periodically examined for the development of visible turbidity of the culture broth, a clear indication that bacteria or fungi have multiplied in the medium and reached a high concentration. Due to the slow growth of members of the genus Brucella and the fact that vials were discarded after 5 to 7 days if not flagged by the automated blood culture instrument, the organism frequently remained undetected. In cases where physicians were aware of the possibility of brucellosis and communicated the information to the laboratory in a timely fashion, vials were kept and incubated for a longer period, and blind subcultures on agar plates were performed.
(ii) Manual biphasic methods.
(a) Castañeda flask.
To obviate the necessity of performing repeat blind subcultures, an ingeniously simple and inexpensive biphasic flask was designed by Ruiz-Castañeda in the late 1940s (39, 40). One side of a culture flask is layered with solid nutrient agar, and an appropriate culture broth, such as serum-dextrose or a high-quality peptone-based medium, is then poured. After inoculating the patient’s blood and, eventually, his or her bone marrow samples, exudates, ground tissues, or other clinically relevant specimens, 10% CO2 is added. The flask is then tightly closed, tilted to bathe the agar slant with the blood-medium mixture, and incubated at 35°C in the standing position. Flasks are examined every 48 h for the appearance of bacterial colonies on the agar surface, turbidity of the broth, or both (40). If no signs of growth are observed, the flasks are tilted again and reincubated, repeating the sequence for at least 35 days (39, 41). Dispensing with the need for performing repeated subcultures not only is labor- and time-saving, but it also decreases the danger of laboratory-acquired brucellosis. Naturally, the Castañeda method is not specific for brucellae, and other microorganisms, including true pathogens and contaminants, may grow in the flask; therefore, full identification of the isolate is required. Although the performance of the Castañeda method has been superseded in recent years by automated blood culture systems, it is still extensively employed in countries of endemicity with limited economic and technical resources due to its low cost and practicality (27, 42–47).
Gotuzzo et al., working in Peru, reported that Brucella colonies were noted after an average of 4.3 days in Castañeda flasks inoculated with bone marrow samples and after a mean of 6.7 days when seeded with blood, and all positive cultures were detected within 15 days (48). In a study conducted in Spain, however, the majority of positive blood cultures required incubation of the flasks for 1 to 3 weeks (49). Differences in the characteristics of the populations enrolled in these studies, the biological features of the Brucella strains, and/or the components of the in-house biphasic media may account for the disparities observed in the performance of the Castañeda technique.
(b) TUMS medium.
Recently, a variant of the Castañeda flask medium has been developed, named Tehran University of Medical Sciences (TUMS) medium, in which a solid urea agar base is used on the slant and brain heart infusion in the liquid phase (50). The principle behind this formulation is that all members of the genus Brucella exhibit positive urease activity that is revealed by the color change of a pH indicator, shortening the time required for identifying the isolate.
(c) Hémoline medium.
A biphasic blood culture system named Hémoline, manufactured commercially by bioMérieux (Marcy l’Etoile, France), has been prospectively evaluated by Ruiz et al. (51). Blood samples obtained from individuals with presumptive brucellosis were inoculated into flasks, incubated for 3 weeks, and subjected to terminal blind subcultures. The median time to detection was only 5 days, but isolation was delayed in 4 out of 17 (23.5%) patients whose cultures became positive after >7 days (51).
(iii) Lysis-based blood cultures.
Because of the low sensitivity and prolonged time to detection of plain blood cultures in liquid media for the recovery of Brucella organisms, an alternative approach consisting of lysing the white blood cells contained in the blood sample prior to seeding the specimen onto solid medium has been developed. The rationale of this strategy is that brucellae do not circulate in the bloodstream as free-living microorganisms. Rather, after an opsonization step, they are readily phagocytosed by polymorphonuclear cells (52). Engulfed brucellae are contained in a special intracellular vacuole where over time the vast majority of organisms do not survive, reducing the sensitivity of the culture and prolonging the time to detection. The lysis of the white blood cells releases already phagocytosed but still cultivable bacteria that, subsequently, can be seeded onto appropriate solid media.
(a) Lysis-filtration.
In the early 1950s, a membrane filtration method for culturing circulating microorganisms was developed by Braun and Kelsh and assessed in an animal model of brucellosis (53). A blood sample was drawn from experimentally infected rabbits, heparinized, and subjected to osmotic lysis. The lysate was then filtered under negative pressure through a sterilized Millipore filter. Filter membranes were deposited on the surfaces of petri dishes, and, after proper incubation, the organisms stuck in the filter grew as individual colonies on the plates. This lysis-filtration method never gained popularity because it was too unwieldy and unsafe, and filter membranes became repeatedly clogged.
(b) In-house lysis-centrifugation method.
The aforementioned lysis-filtration procedure was subsequently improved upon by separating the bacteria from the blood lysate by centrifugation instead of filtration, followed by seeding of the sediment onto agar plates (54, 55). Etemadi et al. compared the performance of this in-house lysis-centrifugation method with that of the classic biphasic Castañeda technique in 14 peripheral blood specimens, two bone marrow aspirates, and 2 cerebrospinal fluid (CSF) samples (54). The results were conclusive: the lysis-centrifugation method detected B. melitensis in all specimens within 2 days, whereas all 18 Castañeda vials were negative despite being incubated for 3 weeks (54).
A similar comparison was performed by Mantur and Mangalgi in a prospective study involving 148 Indian patients with acute and chronic brucellar infections, identified by a positive serodiagnostic test (56). Of the 121 patients with acute disease, the lysis-centrifugation culture recovered brucellae in 110 (90.9%), whereas the Castañeda method identified only 87 (71.8%) patients, and the results were statistically significant (P = 0.001). The time to detection was also shorter in the lysis-centrifugation cultures (2.4 ± 0.9 days, compared to 6.7 ± 2.2 days in the biphasic vial; P < 0.001). Out of a total of 27 patients with chronic brucellosis, the organism was recovered by the lysis-centrifugation method in 20 (74.1%), versus 9 (33.3%) by the Castañeda flask (P = 0.087), and the detection time was 2.7 ± 1.4 versus 7.2 ± 2.6 days, respectively (P = 0.001).
More recently, Mangalgi and Sajjan conducted a comparative study in which the lysis-centrifugation method isolated B. melitensis in 73 (43.2%) of 169 patients with positive serological tests, the Castañeda technique in 42 patients (24.9%), and the blood clot culture in 59 (34.9%). The time to detection was significantly shorter in the lysis-centrifugation cultures than in the two comparators: 4.1 ± 0.9 days versus 9.6 ± 1.7 days versus 5.8 ± 1.4 days, respectively (P < 0.001) (57).
A favorable experience was also reported by Espinosa et al., who compared the Etemadi lysis-centrifugation technique with the Castañeda flask in 88 Peruvian patients exhibiting clinical symptoms compatible with brucellosis and a standard agglutination test (SAT) titer of ≥1:25 (58). The lysis-centrifugation method demonstrated better sensitivity, detecting brucellae in 38 (43.2%) patients, while the Castañeda technique was positive in only 31 (35.2%), but the difference did not reach statistical significance. However, the time to positivity differed significantly, and the use of the lysis-centrifugation method resulted in an average gain of 10 days (time to detection, 3.8 ± 0.8 days for the lysis-centrifugation method versus13.6 ± 6.5 days for the Castañeda flask; P < 0.001). The lysis centrifugation method was also evaluated by Kolman et al. in a prospective comparative study that enrolled symptomatic Israeli patients who exhibited positive serodiagnostic tests for Brucella (55). Blood specimens were drawn, and equal volumes were either processed by the lysis-centrifugation method or inoculated into Bactec 460 (Becton, Dickinson Diagnostic Instrument Systems, Towson, MD, USA) blood culture vials (55). The lysis-centrifugation technique recovered B. melitensis in 15 (27.8%) of 54 patients, whereas the commercial blood culture system was positive in 19 (35.2%) patients. The lysis-centrifugation cultures detected the organism after an average of 3.5 days (range, 2 to 4 days), versus 14 days (range, 7 to 30 days) required by the comparator.
(c) Isolator microbial tube.
The original in-house-prepared lysis-centrifugation methods have since been replaced by the commercial Isolator microbial tube (Wampole Laboratories, Cranbury, NJ, USA). Blood specimens are seeded into special vials that contain a mixture of the sodium polyanethole sulfonate (SPS) anticoagulant and a detergent. Whereas the anticoagulant prevents clotting of the sample, the detergent disrupts the cellular membranes of polymorphonuclear blood cells, releasing phagocytosed organisms. The resulting lysate is then dispersed onto appropriate agar plates and incubated. The Isolator microbial tube has two versions: a 10-ml tube, employed for adult patients, that requires an early centrifugation step to concentrate the lysate before plating, and a smaller 1.5-ml tube, reserved for use for children, that is plated without the centrifugation step.
Navas et al. collected 20 ml of blood from patients with presumptive brucellosis and inoculated 10 ml into an adult Isolator microbial tube and two 5-ml aliquots into one aerobic and one anaerobic Bactec NR660 blood culture vial (59). The two methods exhibited similar sensitivity, but the lysis-concentration cultures had a time to detection of only 2 to 5 days, significantly shorter than the 17 to 29 days (mean, 20.6 days) required by the Bactec system. Although, unexpectedly, Brucella organisms were detected in both the aerobic and the anaerobic media in one patient, because aerobic brucellae do not grow well in anaerobic vials, the actual blood volume processed by the automated blood culture system was, in fact, only one-half of that inoculated into the lysis-centrifugation vial, a factor that could have prolonged the time required to reach the threshold level for automated detection (59).
(d) Bactec Myco/F lytic vial.
The Myco/F lytic blood culture vial is a recent addition to the Bactec 9000 blood culture media, used to enhance the detection of facultative intracellular pathogens such as mycobacteria and fungi. The novel vial appears to combine the advantages of lysing the leukocytes contained in the blood sample with the continuous monitoring of bacterial growth and the laboratory safety of the automated instrument (60). In a prospective, volume-controlled study in which the Myco/F lytic vial was compared with the aerobic pediatric and adult Bactec vials for the recovery of B. melitensis, the sensitivity of the lytic medium vial was similar to that of the comparators, but the time to positivity was significantly longer (101.4 ± 46.7 h versus 65.5 ± 18.9 h; P = 0.004). These results suggest that the composition of the medium contained in the Myco/F lytic vial does not fully support the nutritional requirements of the fastidious bacterium (60).
(iv) Blood clot cultures.
Because the antibodies present in the sera of patients with brucellosis exert an antibacterial effect, culturing blood clots in which leukocytes containing phagocytosed organisms are trapped appears to be a rational and promising approach. The technique involves collection of a blood specimen in a sterile tube and allowing it to clot. The tube is centrifuged to separate the serum phase, which can be used for serodiagnostic tests, and the clot is mechanically disrupted by shaking the tube vigorously and then dispersed onto solid agar medium (56). The published experience with this strategy, however, is limited and its results inconsistent. In a pioneering study, Escamilla et al. employed clot cultures supplemented with either taurocholate-streptokinase or bile and compared their performance with that of cultures of whole blood (61). The clot cultures exhibited lower sensitivity and were more labor-intensive than the conventional blood cultures (61). While the tube with added taurocholate-streptokinase isolated the organism in 21 of 30 (70.0%) Brucella-positive specimens and the bile-clot method succeeded in one (3.3%), the traditional whole-blood culture succeeded in 28 of 30 cultures (93.3%). It can be speculated that the addition of the emulsifying supplements exerted a deleterious effect upon Brucella organisms, thus decreasing the sensitivity of the method.
A better result with the blood clot cultures was reported by Mangalgi and Sajjan in a study that enrolled 169 patients with serologically confirmed brucellosis (57). The investigators reported a sensitivity of 34.9% for the clot culture, 24.8% for the biphasic Castañeda flask, and 43.1% for an in-house lysis-centrifugation method; the mean ± standard deviation (SD) detection times were 5.8 ± 1.4, 9.6 ± 1.7, and 4.1 ± 0.9 days, respectively (57). In another study, Mantur et al. found that the clot cultures were noticeably more sensitive than those of whole blood, improving the sensitivity by >20% and reducing the average time to detection from 8.2 days to 3.1 days (62). In summary, although the clot culture technique appears to combine simplicity and low cost and thus could substantially improve the diagnosis of brucellosis in resource-poor countries, additional data need to be gathered before the method can be routinely adopted.
(v) Automated blood culture systems.
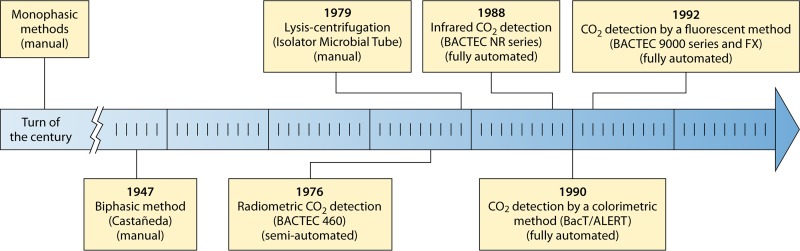
As blood culture technology rapidly evolved over the last 4 decades, successive generations of improved automated instruments have entered the market, gradually replacing the insensitive manual monophasic methods (Fig. 1). The current diagnostic approach relies on determining the metabolic activity of bacterial of fungal microorganisms by detecting an increasing concentration of CO2 or a reduction of the oxygen content in the blood culture vials above the fluid level. Because alterations in the gas composition can be detected before cloudiness of the medium becomes evident, the automated instruments shorten the time needed to diagnose bacteremia. In addition, many automated instruments mechanically agitate the incubating aerobic vials continuously or intermittently to expose bacteria to oxygen and fresh nutrients and to facilitate the release of CO2.
FIG 1.
Timeline of the introduction of blood culture methods employed for the isolation of Brucella organisms.
(a) Factors influencing detection of brucellae by automated blood culture systems.
The method of measuring the metabolic changes occurring in positive blood culture vials differs among automated blood culture systems: in the Bactec 9000 and Bactec FX series of instruments, fluorescence levels increase as the CO2 content increases and the O2 concentration decreases; in the BacT/Alert blood culture system (bioMérieux, Marcy l’Etoile, France), a colorimetric sensor monitors the changing CO2 content; and the Vital instrument (bioMérieux, Marcy l’Etoile, France) measures quenching of the fluorescence caused by acidification of the medium.
The release of CO2 in blood culture bottles depends on multiple factors: the initial quantity of bacteria or fungi inoculated (which is the product of the concentration of circulating microorganisms and the volume of the blood specimen), the species’ replication time and its intrinsic metabolic activity, the adequacy of the broth to meet the nutritional requirements of the organism, the presence of inhibitory factors, the sensitivity and frequency of the CO2 measurements, and the cutoff levels employed to define positivity.
The magnitude of Brucella bacteremia is frequently as low as 1 to 5 CFU per ml (63–65). Obviously, the time to detection of a septic event is negatively correlated with the magnitude of the bacteremia, validating the results of experimental studies with simulated blood cultures (66, 67). Naturally, drawing a large blood sample increases the sensitivity of the culture, and it is therefore recommended to obtain volumes of 20 to 30 ml in adults, 2 to 4 ml in children younger than 3 years, and ≥10 ml in older children (68). Despite these guidelines, in a prospective study investigating the performance of the Myco/F lytic medium for the recovery of brucellae in an adult population, the mean blood volume inoculated into the culture vials was less than 5 ml (60). One should keep in mind that a blood-to-broth ratio of at least 1:5 to 1:10 in the vial is necessary in order to decrease the concentration of detrimental factors contained in the sample, such as complement, antibodies, or, eventually, antibiotics. If a larger blood specimen has been drawn, it should be inoculated into multiple blood culture vials to maintain the critical dilutional effect (68).
Members of the genus Brucella also have a relatively long doubling time (2.5 to 3.5 h) compared to other human pathogens (65), and their CO2 release is also limited due to the fact that they metabolize carbohydrates exclusively by the pentose-phosphate pathway. Employing a simulated blood culture model, the production of CO2 by B. melitensis was found to be slower than that of other bacteria and reached lower peak concentrations (66), explaining the prolonged time to detection of many automated blood culture systems. For instance, in an experimental study in which Bactec NR730 vials were inoculated with brucellae, Gamazo et al. observed cloudiness of the culture broth 1 day, on average, before positivity was detected by the CO2 monitor (65), nullifying the advantages of the automated reading.
In an attempt to improve CO2 release by members of the genus Brucella and to accelerate detection, the effect of supplementing the culture broth with glucose, erythritol, pyruvate, alanine, glutarate, and urea, as well as modifying the pH of the medium, was studied (65). Adding alanine and pyruvate induced a mild increase of the CO2 release, while acidification of the medium from 7.2 to 6.2 combined with an elevated pyruvate concentration resulted in a more marked increase. Although these study results indicate that changes in the composition of the nutrient broth may speed up the detection of brucellae, such modifications may be detrimental to the growth of other bacterial species in the vial.
Because diluting the blood sample into a large volume of liquid medium is essential for reducing the bacteriostatic factors contained in the serum, the volume of nutrient broth has been increased from 30 ml in the Bactec NR660 vials to 40 ml in the Bactec 9000 vials, reducing the blood/broth ratio. This change may have contributed to the better sensitivity for detecting Brucella bacteremia found in recent generations of blood culture systems (69).
To avoid clotting of the blood, SPS is added to commercial blood culture vials. This chemical compound cannot be easily replaced because, in addition to its anticoagulant properties, the SPS supplement has antiphagocytic, anticomplementary, and aminoglycoside-neutralizing effects, an important consideration in patients with brucellosis already being treated with combined antibiotic therapy. However, an inhibitory effect of SPS upon recovery of Brucella organisms has been observed (65). Therefore, the concentration of SPS has been reduced to 0.025% in the blood culture vials of the Bactec 9000 instruments, compared with 0.035% in the old Bactec NR660 and in all the culture media of the BacT/Alert system.
(b) Radiometric detection of CO2.
The semiautomated Bactec 460 instrument, developed in the 1970s, revolutionized the blood culture methodology. A needle, sterilized by heat, penetrated the blood culture vial’s rubber top, and the gas chamber above the fluid level was aspirated and analyzed. This headspace accumulated radioactive CO2 created by the metabolism of 14C-labeled substrata contained in the nutrient broth. Positivity was defined by the radioactivity reaching a critical threshold or showing a significant increase between two consecutive measurements. This pioneering system, however, had many drawbacks. The manual loading of the bottles into the instrument was time-consuming and laborious; thus, CO2 monitoring could be performed only once or twice a day. In addition, breaching the rubber tops entailed the risk of cross-contamination of the vials (70), an unfortunate event that could have serious clinical implications and, in the case of brucellosis, public health ramifications as well.
Although the Bactec 460 system substantially improved the diagnosis of bacteremia caused by traditional human pathogens, its performance for the detection of brucellae was suboptimal (55, 71–73). The sensitivity of the method was lower than that of the traditional Castañeda flask (73), the time to detection of many positive blood culture bottles exceeded the customary 1-week incubation period (71), and the presence of the organism was frequently missed by the CO2 reading and detected only by terminal blind subcultures (72, 73).
(c) Detection of CO2 production by infrared technology.
The subsequent generations of blood culture systems consisted of fully automated instruments in which the incubator was integrated into the instrument, obviating the need for the tedious manual loading of the vials. The novel technologies also offered the clear advantage of continuous monitoring of CO2 release, so that positive vials were detected shortly after the measured metabolite reached the detection threshold, gaining precious time that could be critical in the management of a septic patient. It should be noted, however, that this benefit is lost if the laboratory is not staffed around the clock all week or if the relevant information is not conveyed to the attending physician in a timely manner.
The available information on the performance of infrared detection of generated CO2 by the Bactec NR instruments for detecting Brucella spp. is limited. Evaluations of the system were hampered by the fact that, in most published reports, blood culture vials were incubated for only 7 days and no terminal subcultures of negative vials were performed. Nevertheless, the results were, in general terms, disappointing (21, 55, 59, 67, 74, 75). In comparative studies, only a few Brucella-positive vials were detected by the Bactec NR within the customary 1-week monitoring period (67), demonstrating lower sensitivity and longer detection time than both the biphasic Hémoline flasks (74) and the Isolator microbial tube (55).
In the only methodologically valid evaluation of the performance of the Bactec NR system, blood culture bottles were monitored by the instrument for a 4-week period and blindly subcultured once a week (21). In the course of a 2-year study period, 27 of 373 (7.2%) blood cultures, obtained from 21 Israeli patients, grew B. melitensis. The Bactec NR system detected 21 (78.8%) of these cultures within 1 week, and 6 positive cultures (22.2%) were missed by the automated reading and detected by subculture after 2 or 3 weeks of incubation, demonstrating that the nonradiometric system had a limited and unsatisfactory capacity for detecting Brucella bacteremia.
(d) Continuous monitoring systems.
Experience in isolating Brucella spp. with the current generation of automated blood culture systems has been accumulating at a slow pace. Although brucellosis is still endemic in many countries, the high price of these blood culture systems renders advanced laboratory technology inaccessible in the developing world, whereas in industrialized countries, where automated blood culture instruments have been available for over 3 decades, zoonotic brucellosis has long been controlled and cases of human disease are rare.
The vast majority of evaluations of the continuous monitoring blood culture systems for isolating brucellae have been conducted in countries of endemicity such as Israel (60, 63, 76, 77), Turkey (26), or Saudi Arabia (78), where well-equipped medical facilities and rural populations that maintain a traditional lifestyle exist side by side.
(e) BacT/Alert system.
Published information on the performance of the BacT/Alert system in recovering Brucella spp. is scarce and inconclusive (66, 79, 80). On the one hand, the system successfully detected B. melitensis bacteremia in a case of travel-related infection after only 2.8 days (66), and in a second report, all 9 blood cultures obtained from 5 patients yielded the organism within 3.7 days, including a bottle seeded with pancreatic fluid that was positive after only 13.3 h of incubation (79). On the other hand, Casas et al. reported a poor outcome with the BacT/Alert system (80). Blood cultures obtained from 6 patients with serologically confirmed brucellar infections were monitored by the instrument for 10 consecutive days. At the end of the period, vials that remained negative were transferred to a regular incubator for an additional 10 days, with blind subcultures carried out on days 10 and 20 (80). A single positive bottle was detected by the automated system within 3 days, while 7 positive cultures were detected by subculture performed on day 10 and another on day 20 (80).
(f) Bactec 9000 instruments.
In the 1990s and 2000s, studies conducted in Middle Eastern countries of endemicity reported that the Bactec 9000 series of instruments successfully detected brucellae from blood and other normally sterile body fluids within 10 days. Gedikoglu et al. obtained peripheral blood samples from Turkish patients with suspected brucellosis, processed them with the Bactec 9120 instrument, and monitored the vials for 7 days (75). Thirty cultures, drawn from 15 patients, were found to be positive for B. melitensis within 84 h. In another study, Saudi researchers, working in an area where both B. abortus and B. melitensis are endemic, cultured a mixed population of inpatients and outpatients using the Bactec 9240 system (69). Inoculated vials were monitored for 3 weeks, but blind subcultures of seemingly negative vials were not performed. During a 2-year period, 85 vials were positive for B. melitensis and 12 others grew B. abortus. All 97 positive vials were detected by the blood culture instrument within 9 days of incubation, of which 90 (92.7%) were identified within 5 days (69). In a second Saudi study, blood cultures were processed with the Bactec 9240 and Bactec NR660 instruments, monitored for 6 weeks, and subcultured once a week. Eight cultures were positive for brucellae and were detected, on average, after 1 week (range, 4 to 14 days). Unfortunately, the performances of the two blood culture systems were not reported separately, and the precise time to detection was not stated in the article (78).
In a retrospective Turkish study, Durmaz et al., employing the Bactec 9120 system, incubated blood culture bottles for 1 week, and those that were not flagged as positive at the end of the period were Gram stained and subcultured on solid medium (81). A total of 20 bottles grew B. melitensis after a mean of 30.0 h (range, 31.2 to 117.5 h; median, 69.9 h), and no positive cultures were missed by the automated monitoring. Inferior results, however, were reported in a study by Ayaşlioğlu et al. in which 50 of 58 (84.1%) positive blood cultures were detected by the Bactec 9050 system within a 1-week incubation but 8 additional cultures were detected only by blind subculture performed on day 30 (26).
Working in a rural area of Turkey where brucellosis is hyperendemic, Kurtoglu et al. cultured blood samples from febrile patients and processed them with the small Bactec 9050 and the medium-size Bactec 9120 versions of the system. Vials were routinely monitored for up to 5 days, but when brucellosis was suspected, the incubation period was extended to 2 weeks (82). A total of 60 Brucella-positive vials were identified within a 10-day incubation period, but no precise data on the detection time were reported. It should be noticed that cultures from patients in whom the diagnosis of brucellosis was not considered underwent a very short incubation and no blind subcultures were performed, making it impossible to assess the false-negative rate for the protocol.
Additional retrospective studies performed in countries in the developing world have also reported the recovery of Brucella species within a few days by using the Bactec 9000 series of automated instruments (35–38). All these studies, however, employed short incubation protocols: 5 days (35), 1 week (37, 41, 75), or 5 days that would be extended to 14 days when brucellosis was suspected (38, 82). Terminal blind subcultures of seemingly sterile vials were not performed in any of these cases.
The ability of the Bactec 9240 system to detect brucellae within the customary 7-day protocol was adequately investigated in a prospective study conducted among febrile children attending an emergency department in southern Israel (76). Blood samples were inoculated into aerobic pediatric blood culture bottles and incubated for 4 consecutive weeks; if growth was not detected, the vials were subcultured on a weekly basis (76). Brucella melitensis was recovered in 42 of 2,579 blood cultures (1.6%), of which 41 (97.6%) were identified by the automated reading within 2 to 6 days, and only one culture was detected by the blind subculture performed at the end of the first week. Cumulative detection rates by the automated reading were 0.0%, 23.6%, 78.9%, 86.8%, 92.1%, 97.6%, and 97.6% for days 1 to 7, respectively. A complementary study was conducted to assess the ability of the aerobic vial to detect B. melitensis infection in adult seropositive patients living in the same region of endemicity of the country (77). Inoculated Bactec Plus Aerobic/F medium vials were incubated for 28, days and blind subcultures of negative vials were performed on days 7 and 28. Overall, B. melitensis was isolated from 31 (35.2%) of 88 blood culture vials obtained from 19 (38.0%) of 50 enrolled patients. The automated monitoring identified 30 (96.8%) of 31 positive vials within 1 week; the single positive vial missed by the instrument was identified by the terminal subculture at the end of the 4-week incubation period, indicating a very low initial bacterial inoculum (77).
Ayaşlioğlu et al. reported the results of a Turkish study in which 8 of 136 (5.9%) Brucella-positive blood culture vials, drawn from 60 patients, were undetected by the automated reading and were identified only by blind subcultures performed after a 30-day incubation period, despite the use of the advanced Bactec 9050 system (26). This automated blood culture system differs from the other models of the Bactec 9000 series in that mechanical shaking of the culture bottles is continuous instead of intermittent, a factor that may improve bacterial growth. Similar failure in the automated detection of Brucella bacteremia by the Bactec 9050 blood culture system was also noted in the investigation of a cluster of 16 cases of B. melitensis disease reported in 2001 by Lepe et al. (83). Blood culture vials were monitored for 3 consecutive weeks, and unflagged vials were subcultured blindly at the end of the study period (83). Thirteen patients (81.3%) had confirmable Brucella bacteremia. The automated monitoring detected the bacterium in 9 patients (69.2%) within 7 days, did so in 2 additional patients on days 8 and 11, and failed in the remaining 2 patients, where the organism was recovered only in the final blind subculture.
The explanation for the wide differences in the performance of the current blood culture instruments reported in the aforementioned studies is not obvious. It is speculated that the superior performance of the Bactec system in the two Israeli studies (76, 77) could have resulted from the fact that their patient population presented to the emergency department at the early stages of the infection when high-magnitude bacteremia occurs, whereas studies showing inferior sensitivity of the system mostly enrolled individuals with a long-standing disease characterized by a lower bacterial load, thus decreasing the detection capability and prolonging the time to positivity (76, 77).
(vi) Is the traditional recommendation of prolonged incubation of vials still valid?
The results of multiple studies have shown that bot the evolution of the CO2 measurement strategy and the changes in the composition of liquid culture media have substantially improved the diagnosis of Brucella bacteremia in recent years. Current bacteriological methods enhance the sensitivity of Brucella cultures, reduce the detection time, and considerably reduce the time and labor spent. Use of these automated systems enables the hands-off processing of a large number of blood culture bottles, nearly eliminates contamination of media, and ensures safe handling of dangerous bacteria.
The increased sensitivity and shortened time to detection with modern blood culture systems have led to questioning of the relevance of the traditional recommendation for prolonged incubation and periodic subculturing in order to optimize the detection of elusive Brucella organisms (33, 34). Published experience indicates that the current automated systems detect acute brucellar infections in both children and adults within the customary 1-week incubation period and avoid the need for subculturing seemingly negative vials, provided that the blood samples are obtained in the initial phase of the infection (76, 77). In cases with a longer evolution or a focal infection, some patients may still require prolonged incubation of culture bottles and performance of terminal subcultures to maximize isolation (26, 83).
(vii) Which of the current blood culture systems is superior for recovering brucellae?
Despite the fact that reviews on human brucellosis published in prestigious medical journals in recent years still recommend the use of lysis-centrifugation cultures (8, 16) and consider this technique the method of choice for isolating the bacterium, the results of the only prospective comparison between the Isolator microbial tube and the Bactec system demonstrated a statistically significant superiority of the automated system in terms of overall sensitivity and time to detection of positive cultures (63). In a prospective volume-controlled study, blood aliquots obtained from pediatric patients in whom brucellosis was suspected were inoculated into a Bactec 9240 aerobic bottle or seeded into an Isolator microbial tube (63). A total of 122 cultures were drawn, and 28 (22.8%) grew B. melitensis by one or both techniques. The automated system detected all 28 positive cultures, whereas the lysis-centrifugation method detected only 22 (sensitivity, 78.6%; P < 0.023). The automated system was also superior in terms of time to positivity, detecting 21 of the 22 (95.5%) cultures positive by both methods within 3 days, compared to only 15 (68.2%) detected by the Isolator microbial tube. Eight of the 22 cultures (36.4%) detected by both culture methods were found to be positive at least 24 h earlier by the Bactec instrument, and the remaining 14 were detected by both methods on the same day (P < 0.05).
The performances of the Bactec 9120 and Vital (bioMérieux) automated systems and the Hémoline biphasic flask were prospectively assessed and compared by employing blood cultures obtained from Spanish patients with brucellosis (51). The Hémoline vial detected all 19 positive blood cultures, whereas the Bactec and the Vital systems overlooked one positive culture each (sensitivity, 94.7%). After a 5-day incubation period, 47.4%, 78.9%, and 10.5% of the cultures were detected by the three blood culture systems, respectively. At the end of the first week, the detection rates increased to 73.7%, 94.7%, and 47.4%, respectively, proving that the Bactec 9120 was significantly faster than the two other blood culture systems (P < 0.05). The delayed detection of Brucella by the Vital system was confirmed in two later studies in which the average detection occurred after incubation times of 119.7 and 211.7 h (84, 85).
The capabilities of two of the most popular commercial blood culture systems to detect brucellae were compared in a single head-to-head study in which 10-ml aliquots of adult patients’ blood were inoculated into BacT/Alert and Bactec 9240 bottles (86). The study design, unfortunately, had two important drawbacks: vials were incubated for only 7 days, and no blind subcultures were ever performed. The results were inconclusive: the times to detection were similar (2.5 days by the BacT/Alert system versus 2.8 days by the Bactec 9240 system), and the former detected 9 out of 17 (52.9%) positive cultures whereas the latter detected 14 (82.3%) (P = 0.067).
Clearly, additional studies enrolling a larger number of patients with culture-proven brucellosis are needed to determine which blood culture system is preferable for detecting Brucella bacteremia. However, with very rare exceptions, clinical microbiology laboratories employ only a single automated blood culture system; therefore, a proper comparison will likely never be performed. In any case, the choice of a blood culture system is a costly and strategic decision that has wide and long-lasting implications. The choice should be made on the basis of a variety of professional and economic considerations and not only on the ability of the system to isolate a particular bacterial species.
(viii) Blood versus bone marrow cultures as the diagnostic gold standard.
Up to the advent of novel generations of automated blood culture instruments in the mid-1990s, the recovery of Brucella organisms from peripheral blood samples was frequently suboptimal. In order to improve detection, it was advised to culture alternate sources such as bone marrow aspirates (27, 40, 41, 87–89), liver biopsy specimens (90, 91), or lymph nodes (92). The rationale behind obtaining these specimens was that Brucella organisms multiply and concentrate inside the reticuloendothelial system, and thus, culturing of these macrophage-rich tissues may increase bacterial recovery (16). Despite this theoretical advantage, the question of which specimen is preferable for diagnosing human brucellosis is far from resolved. Ganado and Bannister reported than in one-fifth of patients from whom bone marrow cultures grew brucellae, the bacterium could not be simultaneously recovered from the blood (87). Gotuzzo et al. found that bone marrow aspirates were positive in 46 of 50 (92.0%) patients and that peripheral blood cultures were positive in only 35 (70.0%) (48). Mantur et al. reported that bone marrow cultures isolated Brucella organisms in 85 of 103 (82.5%) patients, versus only 47 (45.6%) detected by blood cultures (P < 0.001), and that the detection was also significantly quicker (2.8 ± 0.7 and 7.2 ± 2.4 days, respectively; P = 0.001) (27). Superior results were also obtained in a prospective study by Özkurt et al., who simultaneously inoculated blood and bone marrow specimens from patients with presumptive brucellosis into BacT/Alert vials as well as an in-house monophasic liquid medium (93). Thirty-five of 50 (70.0%) bone marrow cultures yielded brucellae, versus only 24 of 50 (48.0%) blood cultures (P < 0.05). In a second study, Özturk et al. obtained blood and bone marrow samples from 23 seropositive symptomatic adults and seeded them into Bactec 9240 vials. The sensitivities of the two methods were comparable (19 of 23 [82.6%] isolations in the blood cultures and 13 of 16 [81.2%; in the bone marrow cultures) (94). In the 13 patients from whom paired blood and bone marrow cultures were drawn, the automated instrument detected growth in the blood samples within 3 to 7 days (mean, 4.3 ± 1.1 days; median, 4 days), versus a range of 2 to 4 days (mean, 2.6 ± 0.7 days; median, 3 days) for the vials inoculated with bone marrow (P < 0.05).
It is noticeable that in all these studies, despite the fact that, generally, <1 ml of bone marrow was inoculated into the culture bottles compared to much greater volumes of peripheral blood (between 5 and 10 ml), detection times for bone marrow cultures were shorter, indicating a higher bacterial inoculum (27, 48, 74, 93–95). The improved performance of bone marrow cultures was noted in patients with acute disease as well as in those with a long-standing infection (27).
A different experience was reported by Magill and Killough, who found that cultures of peripheral blood isolated Brucella organisms in 90% of culture-positive cases, versus only 40% by bone marrow cultures (96), and by Shehabi et al., who reported sensitivities of 44.4% and 27.7%, respectively (97). Similar results were reported by Iseri et al., who, employing the Bactec 9050 instrument, found detection rates of 39 out of 102 (48.0%) for blood cultures and 35 out of 102 (34.3%) for bone marrow cultures (95). Similarly, Wang et al. recovered Brucella organisms in 10 out of 16 blood cultures (62.5%), versus only 3 isolations (18.8%) from bone marrow specimens (89).
Although the optimal specimen for isolating Brucella organisms continues to be a matter of debate, it should be noted that blood samples have other substantial advantages: they are easier to draw and repeat, aspiration of the specimen is less invasive and painful, and greater volumes can be obtained. In addition, peripheral blood cultures can detect a brucellar infection in patients for whom the disease was not included in the differential diagnosis, whereas harvesting a bone marrow specimen requires considering the possibility of the infection a priori.
Isolation of Brucellae from Specimens Other than Blood
Traditional culture methods.
Because the initial hematogenous spread of brucellae results in seeding of bacteria to remote organs and the development of focal infections, a variety of biological specimens, such as blood, bone marrow, genital exudates, bone tissue, synovial fluid aspirates, or cerebrospinal fluid, may serve as a host for the organism (32). Samples of these tissues and normally sterile body fluids should be collected following strict aseptic precautions and sent to the laboratory without delay, and inoculation of culture media should be performed within 1 to 2 h of obtaining the specimen. In the case of more prolonged transport times, specimens should be kept moist and cooled to 2 to 8°C (98). Brucella species grow well on solid culture media routinely used in clinical microbiology laboratories for the isolation of traditional human pathogens, such as Trypticase soy agar with added hemoglobin (blood agar) and chocolate agar media. Brucellae do not develop on MacConkey agar, and seeding of selective media is unnecessary. To maximize detection, inoculated plates should be incubated for up to 14 days in a 5%–10% CO2-enriched atmosphere at 35°C and under aerobic conditions. Inoculated plates should be sealed, and all bacteriological procedures should be performed in a class II biological safety cabinet (see “Brucella Cultures and Laboratory Safety” below).
In infected animals, Brucella organisms may also be isolated from vaginal secretions, placental and fetal tissues, milk, semen, and other specimens that usually harbor additional commensal flora as well as environmental bacterial species and fungi (99). Because most of these potential contaminants have short generation times, they tend to overgrow on the agar media, making it difficult to detect slow-growing brucellae. To facilitate the recognition and recovery of Brucella spp., selective media that inhibit competing microorganisms, such as Farrel medium and modified Thayer-Martin medium (MTM), are widely employed in veterinary laboratories (99). Farrel medium is not usually available in clinical microbiology laboratories serving human populations, but MTM agar plates are routinely used for the isolation of Neisseria gonorrhoeae. The unexpected growth of B. melitensis on MTM seeded with female genital specimens resulted in the inadvertent and extensive exposure of personnel in a clinical microbiology laboratory in southern Israel (100).
Use of blood culture methods for culturing other biological specimens.
The use of a variety of bacteriological blood culture techniques, including both manual and automated systems, has been occasionally attempted for the isolation of Brucella species from pus (41), bone marrow (27, 93, 96), liver tissue (27), lymph nodes (27), synovial fluid (101, 102), testicular aspirates (42), pancreatic exudates (79), and CSF (35). Naturally, seeding these specimens into automated blood culture system vials has the advantages of continuous growth monitoring, labor and time savings, and laboratory safety. In general, the results of this unorthodox practice have been comparable to or showed better sensitivity than traditional cultures on solid media and have also shortened the detection time. For instance, when synovial fluid aspirates from patients with joint infections were inoculated into an aerobic Peds Plus blood culture bottle and incubated in the Bactec 9240 instrument for 4 weeks, 15 vials grew B. melitensis, of which 14 were detected by the automated reading within 3 to 7 days (101). A single culture which originally contained only 1.3 CFU of viable organisms per ml (as determined by a lysis-centrifugation culture run in parallel) exhibited nonsignificant CO2 readings during the whole monitoring period (102). Akcam et al. compared the performance of the aerobic pediatric bottle, monitored by the Bactec 9240 instrument for 1 week, to that of conventional solid media for culturing normally sterile body fluids other than blood (41). The study found that the 5 B. melitensis-positive clinical specimens were detected by the automated monitoring but missed by the conventional cultures (41).
From Detection to Identification
Conventional methods.
Prompt and correct identification of Brucella organisms recovered in a blood culture bottle or isolated on a petri dish is essential for establishing a timely diagnosis and avoiding the risk of contagion to laboratory personnel. Whenever isolation of a member of the genus Brucella is suspected on the basis of clinical and/or epidemiological considerations or the phenotypic characteristics of the isolate, strict safety precautions should be taken to prevent transmission (see “Brucella Cultures and Laboratory Safety” below).
Traditionally, a Gram stain of the bacteria developing on the agar surface or in the culture broth is initially performed. Very small, faintly stained Gram-negative coccobacilli that resemble fine sand and may appear as microcolonies suspended in the liquid blood culture medium are usually observed (Fig. 2). Unless the biphasic Castañeda method is employed, the positive culture broth should be streaked for isolation onto solid medium. Inclusion of MacConkey agar is recommended because failure of the still-unidentified isolate to grow on this medium is a distinctive feature of the genus Brucella. After 2 to 4 days of incubation, punctate Brucella colonies may appear on the agar. Fully developed colonies are small (0.5 to 1 mm), convex, nonpigmented, and nonhemolytic and have an entire edge. Presumptive identification of brucellae is based on the typical Gram staining appearance, capnophilia, positive oxidase, catalase, and urease activity, no fermentation of sugars, and lack of motility.
FIG 2.
Gram stain of a positive aerobic Bactec blood culture vial showing Brucella melitensis microcolonies (arrows).
The main drawbacks of the phenotypic identification of brucellae are the long turnaround time and the exposure of laboratory technicians to a highly transmissible bacterium. In addition, commercial systems may misidentify brucellae as the closely related Ochrobactrum anthropi (103, 104) or Ochrobactrum intermedium species (105), as well as the unrelated Haemophilus influenzae (106), Bergeyella zoohelcum (107), Bordetella bronchiseptica (108), or Psychrobacter phenylpyruvicus (formerly Moraxella phenylpyruvica) (109), a serious mistake that has already caused outbreaks of laboratory-acquired disease (110). The presumptive identification of brucellae should be confirmed by a molecular method (see Diagnosis of Brucellosis by Nucleic Acid Amplification Tests below) or by a positive slide agglutination reaction with specific antiserum against the bacterial O-lipopolysaccharide (O-LPS). However, because this polysaccharide component is shared by many other Gram-negative bacteria, this serodiagnostic test should be performed only after the unknown organism has met all the clue phenotypic criteria (Gram stain morphology, typical biochemical profile, etc.), and it should never be used as a shortcut to identify unknown and hitherto-uncharacterized isolates. It is also important to point out that the smooth Brucella spp. frequently dissociate in culture in a mixture of smooth and rough colonies, the latter having lost the ability to synthesize the O-polysaccharide. These rough mutants, as well as the naturally occurring rough species (B. canis and B. ovis), fail to agglutinate with the regular antiserum and require a specific anti-rough LPS reagent for confirmation.
Because of the need for simple and rapid methods for the presumptive identification (or exclusion) of blood isolates as brucellae, Rich et al. proposed to subculture the broth of positive Bactec 9240 blood culture vials on urea slants (111). Overall, the study included 33 vials in which Gram-negative coccobacilli were visualized and 32 vials in which no organisms were disclosed. Thirty-seven of the 44 (84.1%) slants that grew Brucella organisms exhibited urease activity within 4 h, and the remaining 7 turned positive after overnight incubation. Only two blood culture vials that grew Haemophilus influenzae produced a delayed positive urease reaction, demonstrating good specificity of the method. Maleknejad et al. performed acridine orange and Gram stainings of positive blood culture broth and, in parallel, inoculated a urea slant (112). The test was positive within 4 h in all 41 cultures in which Brucella organisms were isolated and was negative in 61 vials in which other bacteria were recovered.
Identification of members of the genus Brucella to the species level is important for epidemiological reasons due to the strong association between the individual species and their naturally occurring hosts (2). The conventional phenotypic method for species identification for Brucella species that are pathogenic for humans is summarized in Table 2, whereas species identification by molecular methods is discussed in Diagnosis of Brucellosis by Nucleic Acid Amplification Tests below.
TABLE 2.
Phenotypic features of Brucella species pathogenic to humans
| Species | Growth on dye at routine test dilution |
H2S production | Urease test (maximum time to positivity)a | Lysis with phage: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Fuchsin | Thionine | Safranin | Tb | Wb | Iz | R/C | |||
| B. melitensis | Yes | Yes | Yes | No | 24 h | No | No | Yes | No |
| B. abortus | Yesb | No | Yes | Yesc | 24 h | Yes | Yes | Yes | Yes |
| B. suis | Nod | Yes | No | No | 15 min | No | Yes | Yes | No |
| B. canis | Variable | Yes | No | No | 15 min | No | No | No | Yes |
| Marine speciese | Yes | Yes | Yes | No | 90 min | Nof | Yes | Yes | No |
Many strains show lack of correlation with species.
Except biotype 2.
Except biotype 5.
Except biotype 3.
B. pinnipedialis and B. ceti.
Lysis occurs in a few strains of B. pinnipedialis.
In 1992, Wong et al. employed the Biolog microtiter plate system (Biolog, Hayward, CA) to identify Brucella organisms to the species level (113). The method is based on the differential oxidation of a panel of carbon source substrata, which, by reducing a tetrazolium dye indicator, results in a color reaction that can be read visually. After a 24-h incubation at 35°C in a 5% CO2-enriched atmosphere, the test unambiguously identified B. melitensis, B. abortus, and B. suis (113). Despite this successful identification to the species level, the test did not gain widespread acceptance, probably because its performance creates dangerous aerosols (113).
In a pioneering study, a novel miniaturized semiautomated system (Taxa Profile) based on 570 metabolic reactions was evaluated for the identification and species determination of members of the genus Brucella (2). The results revealed a high degree of biodiversity among Brucella species and biovars. Overall, 196 metabolic reactions provided stable results between cultures of the same strain, as well as reliable discrimination between the 23 reference Brucella strains. The panel also distinguished brucellae from taxonomically related and difficult-to-differentiate microorganisms such as Ochrobactrum spp. On the basis of the consistent species- and biovar-specific reactions thus identified, a 96-well plate (Micronaut; Merlin Diagnostika GmbH) was designed, and its discriminatory power was challenged with 113 Brucella isolates and other closely related organisms. Although Brucella spp. and biovars generally exhibited distinctive metabolic patterns, the extended biochemical profiling could not separate B. canis from B. suis biovar 3, and B. melitensis isolates showed a remarkable homogeneity and could not be resolved according to their biovars. The system does not require the preparation of specific reagents, has easy-to-handle identification software, and has the potential for detecting novel Brucella species and biovars (114). However, the discriminatory capability of the Micronaut kit exceeds the usual needs of clinical microbiology laboratories, since subtyping of Brucella spp. is not needed to arrive at therapeutic decisions. The use of this identification system appears to be more adequate for referral laboratories, where it may substitute for or complement time-consuming tube tests, especially for the identification of atypical Brucella strains (13).
MALDI-TOF technology.
The introduction of the matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) technology in the clinical microbiology laboratory profoundly changed the way microorganisms are identified. MALDI-TOF instruments make possible the fast, accurate, reproducible, and cost-effective identification of isolates to the species level, replacing tedious biochemical testing. In addition, due to their technical simplicity, they are particularly suitable for use in busy laboratories, where they can be operated by less-skilled technicians (115, 116).
The MALDI-TOF method can be applied directly on bacterial colonies growing on solid media, as well as on positive blood culture broth (117). To avoid the risk of exposure to living Brucella organisms, an initial bacterial inactivation step with absolute ethanol is customarily added prior to the standard protein extraction with formic acid and acetonitrile (118–120).
Early evaluations of the performance of this novel technology in identifying Brucella organisms were inconclusive. In some studies, the method allowed for precise identification of ATCC Brucella type strains growing in simulated blood cultures at the genus level, identification of the isolates to the species level, and even differentiation between B. suis biovars (115, 116). However, other studies reported that the MALDI-TOF technology-based Vitek MS (bioMérieux, France) system, using available databases, misidentified B. melitensis as O. anthropi (121). Its competitor, the Bruker system (Bruker Daltonics, Germany), exhibited unreliable discrimination between Brucella species, indicating that the analyzed protein profile did not accurately reflect the genetic evolution of the genus members (117, 120). In a recent investigation, an improved Vitek MS reference database was constructed on the basis of 590 proteomic spectra from 84 different Brucella strains belonging to all recognized species of the genus, including rare and atypical bacterial isolates (122). The modified database enabled clear-cut differentiation of brucellae from members of the Ochrobactrum genus, as well as precise identification to the species level of the three major zoonotic species: B. melitensis, B. abortus, and B. suis. Obviously, these favorable results still require independent confirmation with multiple wild-type strains derived from human and zoonotic sources of diverse geographic origins. However, it should be realized that data on the performance of this technology are still limited since, although the cost per bacterial identification is low, MALDI-TOF equipment is expensive and, as such, inaccessible in most countries where brucellosis is endemic.
Brucella Cultures and Laboratory Safety
Brucellae are the most common etiology of laboratory-acquired infections, making up 2% of all human cases of brucellosis globally (122). Genus-specific biological features make the organism easily communicable within the close confinement of the clinical microbiology laboratory: the number of viable organisms required to establish an infection in humans is remarkable low (101 to 102 cells); the bacterium may access the host through numerous portals of entry relevant to standard microbiological work, including the respiratory mucosa, conjunctivae, gastrointestinal tract, and abraded skin (123); the microorganism remains viable on inanimate surfaces for periods of weeks and even months (123, 124); and manual laboratory procedures may create dangerous aerosols and spillovers of contaminated culture media and reagents.
Because brucellae may infect any body organ or tissue, a large variety of specimen types submitted to the clinical microbiology laboratory may contain living brucellae, although blood cultures constitute by far the most common clinical specimen. Because the magnitude of Brucella bacteremia is generally low (63), unless a gross violation of safety practices has been committed, blood samples do not present a real risk of contagion. In addition, modern blood culture instruments monitor bacterial growth without piercing the vial’s rubber top and, therefore, do not nebulize bacteria. Nevertheless, the risk of a clinically meaningful exposure increases exponentially during and after incubation of solid and liquid media. Routine bacteriological procedures such as homogenization of tissues, centrifugation and vortexing of bacterial suspensions, performance of subcultures, and biochemical testing may also result in dispersion and spillage of living bacteria, contamination of the laboratory environment, and unintentional transmission to working personnel (125). Particularly dangerous is the catalase test, which is strongly positive in brucellae and causes bubbling and aerosolization of microorganisms.
In regions where the zoonosis is endemic, the number of Brucella-positive cultures processed by clinical microbiology laboratories and, consequently, the risk for transmission to the workforce can be exceedingly high. In a clinical microbiology laboratory situated in Ankara, Turkey, in which a mean of 400 specimens grow Brucella spp. every year, the disease affected 10 of 55 (18%) workers, representing an annual risk of 8% per employee (126). In two studies carried out at the Soroka University Medical Center (SUMC), located in an area of endemicity of B. melitensis in southern Israel, the bacterium was recovered in 127 of 3,974 (3.2%) positive aerobic Bactec blood culture bottles and in 11 of 126 (8.7%) Isolator microbial tube cultures in 1997 (127) and in 514 of 20,620 (2.5%) positive Bactec vials in the period from 2002 to 2009 (128). As expected, the prevalence rate of positive Brucella cultures in the later study was significantly higher between April and September (3.3%) than between October and March (0.9%) (P < 0.001), coinciding with the breeding season of sheep and goats and the resulting increase in human exposure and morbidity (128).
Although the Centers for Disease Control and Prevention (CDC) have advised that all laboratory manipulations of live brucellae should be performed in a class II biological safety cabinet (129), by the time bacterial isolates are suspected or confirmed as Brucella organisms, incautious work with culture media has usually taken place, and inadvertent exposure of laboratory technicians may have already occurred.
Because of the nonspecific manifestations of human brucellosis, clinicians frequently miss the diagnosis and therefore fail to alert the laboratory to anticipate the presence of Brucella spp. in clinical specimens, creating a risk of accidental exposure to technicians. Nevertheless, the responsibility for the early recognition and correct identification of brucellae rests with the clinical microbiology laboratory. Small Gram-negative coccobacilli that grow on blood agar and chocolate agar but fail to develop on MacConkey agar should not be imprudently processed on an open bench but rather should be subjected to a “rule-out-or-refer” testing policy, and a basic biochemical workup should be performed following rigorous safety precautions.
Following an outbreak of 7 cases of laboratory-acquired brucellosis at the SUMC in 1997 involving technicians, administrative personnel, and occasional visitors, a strict infection control policy has been adopted (127). All blood culture vials detected as positive by the automated blood culture instrument are initially processed in safety cabinets until the possibility of brucellae is firmly excluded. Use of the Isolator microbial tube for patients with suspected brucellosis, as well as the routine performance of antibiotic susceptibility testing of Brucella isolates and aerosol-generating procedures, has been discontinued altogether. Since the implementation of these enhanced safety practices, no further cases of infection among the laboratory staff have been detected in over 2 decades, despite an ever-increasing number of isolations (128). It seems prudent, then, to recommend that, in areas of endemicity of brucellosis, all positive blood culture vials should be initially processed in safety cabinets, pending final identification of the organism. In addition, bacterial isolates presumptively identified as Ochrobactrum spp., Psychrobacter phenylpyruvicus, Bordetella bronchiseptica, or Bergeyella zoohelcum should be managed in a similar manner until the possibility of a Brucella organism has been firmly ruled out.
Conclusions
Despite long-term experience with the use of serological tests and the recent design and implementation of exquisitely sensitive molecular assays, the isolation of the causative organism has conserved its clinical and epidemiological relevance. In the past, the recovery of members of the genus Brucella was hampered by the slow bacterial growth, requiring prolonged incubation of blood culture vials and performance of periodic blind subcultures of seemingly negative media or, alternatively, the use of the in-house Castañeda flask or the manual lysis-centrifugation method. Over the last 4 decades, the development of automated blood culture systems, in which bacterial multiplication is detected by monitoring CO2 production, has increased the sensitivity of blood cultures and shortened the time to detection of these fastidious species. Nowadays, over 95% of all blood cultures obtained from patients with acute brucellosis detect the causative organism within the customary 1-week incubation period without the need for subcultures. In patients with a longer evolution of infection and/or focal complications, prolonged incubation and performance of blind subcultures are still required. The introduction of MALDI-TOF technology and nucleic acid amplification assays and hybridization tests enables a rapid, precise, and safe identification and determination of the species of recovered Brucella isolates.
SERODIAGNOSIS OF HUMAN BRUCELLOSIS
Serological Diagnosis of Human Brucellosis: Imperfect but Indispensable
In contrast to culture-based and molecular diagnostic approaches that depend on detection of living bacteria or their specific DNA sequences in the patient’s body fluids or tissues, the serodiagnosis of brucellosis does not provide direct evidence of the presence of the microorganism. Rather, it relies on the indirect strategy of probing the patient’s immune system in search of antibodies that attest to previous contact with the pathogen. The patient’s medical history of past diseases and exposures, the recognition of nonself antigens, the immunological processing, and the resulting patterns and dynamics of antibody production all vary widely among individuals. Therefore, instead of an objective, straightforward, and irrefutable diagnostic proof, results of serological tests for brucellosis require interpretation that is often difficult and frequently inconclusive (13).
The serodiagnosis of brucellosis is mostly based on consensual criteria, such as a given titer in an agglutination assay, a cutoff enzyme-linked immunosorbent assay (ELISA) reading value, or the appearance or intensity of a band in a lateral flow test. The validity of these criteria is frequently questioned, and the threshold values for defining positivity vary according to clinical and epidemiological considerations such as the duration of illness, history of brucellosis, and occupational risk factors (i.e., abattoir workers, farmers, etc.). Asymptomatic and self-limiting episodes of Brucella infection are not uncommon in regions where the zoonosis is endemic (130), and IgG isotype antibodies may persist for many months after the conclusion of successful antibiotic therapy (131, 132). This explains the high seroprevalence of anti-Brucella antibodies found in areas of endemicity (133) and among individuals who are repeatedly exposed to the organism (134). Therefore, the sensitivity and specificity of any criterion for identifying diseased patients depend not only on the intrinsic properties of the investigated laboratory variable but also on the characteristics of the population studied and, in the case of brucellosis, on local epidemiological conditions. For instance, the use of healthy individuals as negative controls could overestimate the test specificity (8). Conversely, enrolling patients who are initially suspected to be infected by brucellae but had an alternative final diagnosis and thus may have cross-reactive antibodies may substantially decrease the measured specificity of the assay (8).
The diagnostic performance of any test should be appraised by comparing its results with those obtained with the gold standard method. The evaluation of the serodiagnostic tests for brucellosis is made difficult by the fact that there is no single unquestionable test for defining disease against which all other laboratory assays should be measured. Although any biological test should be interpreted in light of the clinical context (135), this is not an easy task when dealing with the elusive diagnosis of human brucellosis, and this conundrum cannot always be solved by current microbiological methods (13). Since the manifestations of Brucella infections in humans are protean and nonspecific (8), a clinical diagnosis based on symptoms and signs is clearly inadequate as a yardstick. Although the isolation of brucellae from normally sterile body fluids or tissues is a definitive proof of the infection and has diagnostic specificity of 100%, the sensitivity of cultures decreases with the progression of the infection (8). The yield of cultures is remarkably low in patients with a protracted course or focal complications, which are frequently the cases in which an undisputable diagnosis is both problematic and most necessary. Nucleic acid amplification assays have, in general, an unmatched sensitivity (136). However, the performance of PCR tests for Brucella shows disagreement among laboratories, and no standardization of important technical aspects such as the sample preparation procedure, the choice of genomic targets, and detection techniques has yet been established. Moreover, in a seminal and provocative study by Vrioni et al., 7 of 10 patients continued to exhibit positive PCR results 24 to 36 months after completion of antibiotic therapy, despite the absence of symptoms indicative of persisting disease or relapse (137). Detection of Brucella DNA sequences does not discriminate between viable and dead organisms and, therefore, is not irrefutable evidence of active infection, nor does it effectively support therapeutic decision making (see Diagnosis of Brucellosis by Nucleic Acid Amplification Tests below).
Since there is no gold standard, serodiagnostic tests for brucellosis are frequently evaluated by comparing results with those obtained with other serological assays, used alone or in combination. The performance of different serological tests has been measured in dissimilar populations, employing a wide range of inclusion criteria and cutoff values and a diversity of commercial as well as in-house assays. Not surprisingly, results have been frequently inconsistent. Under these circumstances, a definitive judgment of the performance of individual serodiagnostic assays cannot be reached.
Despite their numerous drawbacks, serological tests remain an indispensable diagnostic tool for human brucellosis in countries of endemicity. In general terms, serology has kept its clinical relevance and popularity for diagnosing the infection because, unlike cultures or nucleic acid amplification methods, it is inexpensive and relatively simple from the technical point of view. These are two important considerations in countries in the developing world and rural regions with a high burden of human and animal morbidity on the one hand and poor availability of laboratory equipment and expertise on the other.
Brucella Antigens for Serodiagnosis: LPS and Cytosol Proteins
Starting with the simple agglutination test developed by Wright and Smith in 1897, a wide variety of serological tests have been proposed for the diagnosis of human brucellosis, many of which were adapted from veterinary medicine. The cornucopia of assays accumulated over a century of ongoing research indicates that the definitive assay has not yet been found and probably will never be. The ideal laboratory test should be sensitive as well as specific, be able to differentiate diseased individuals from merely exposed ones, reliably distinguish between latent cases and eradicated disease, enable the detection of early infections as well as long-lasting cases, diagnose systemic as well as localized disease, and be capable of identifying patients infected with species that do not contain the O-chain component of the LPS, such as B. canis. To date, no single assay or combination of tests meets all these goals, for several interrelated reasons.
Brucella organisms pose a difficult challenge for the serodiagnostic approach because of their complex antigenic structure comprising outer membrane proteins, genus-specific cytosolic proteins, and an immunodominant LPS. The LPS elaborated by the so-called smooth Brucella species (B. melitensis, B. abortus, and B. suis, the two marine species B. ceti and B. pinnipedialis, and B. neotomae) includes the glycolipid A component, the core, and the O chain. The O chain is an unbranched homopolymer of approximately 100 residues of 4,6-dideoxy-4-formamido-d-mannopyranosyl (d-Rha4NFo) that are variably α(1→2) and α(1→3) linked (138). Serodiagnosis of brucellosis based on antibodies to the smooth LPS (S-LPS) component is generally performed by employing antigens extracted from the B. abortus S19 strain, since the Brucella immunodominant S-LPS is common to all the biovars of the three clinically important smooth species that are pathogenic to humans (139). Naturally, serodiagnostic tests based on the recognition of the shared S-LPS antigen do not enable the discrimination of infections caused by these cross-reacting species. The rough Brucella species B. canis and B. ovis naturally lack the O chain, and infections cannot be diagnosed by detecting anti-S-LPS antibodies. Brucella ovis is nonpathogenic for humans, but human infections caused by B. canis have been reported, and their serological diagnosis requires an agglutination test that uses species-specific rough lipopolysaccharide or an ELISA that employs B. canis proteins as an antigen (140).
It should be noted that the LPS molecule carries epitopes that cross-react with those of a variety of Gram-negative organisms, including, among others, Yersinia enterocolitica O:9, Salmonella enterica serovar Urbana O:30, Francisella tularensis, Escherichia coli O116 and O157, Vibrio cholerae, Xanthomonas maltophilia, and Afipia clevelandensis (13, 16). Therefore, results of serodiagnostic assays that target the S-LPS should be interpreted with caution and correlated with the clinical manifestations of the disease and epidemiological data. The anti-S-LPS antibodies responsible for these nonspecific reactions are mostly of the IgM isotype (141).
The cytosolic proteins of brucellae represent an attractive alternative to the LPS because they lack a significant serological cross-reactivity with bacteria other than the genetically related members of the genus Ochrobactrum. Thanks to this specificity, these proteins can be used to distinguish infections caused by Brucella from those caused by organisms that cross-react at the S-LPS level.
Humoral Immune Response in Human Brucellosis
The humoral immune response to infections caused by smooth Brucella species is dominated by elaboration of antibodies to the S-LPS. The sequence of antibody production follows the classic pattern of rising levels of IgM in the first week of infection (which is readily detected by agglutination tests such as the rose bengal test [RBT] and SAT), followed by IgG1 in the second week and later by small quantities of IgG2 and IgA. Production of all three isotypes continues to increase in the early phases of the disease, reaching its peak around the fourth week (13). In prolonged cases, the IgM concentration decreases, resulting in the net predominance of IgG and IgA antibodies, which can be quantified by an enzyme-linked immunosorbent assay (ELISA) that employs S-LPS as the antigen. Over time, the nonagglutinating antibodies (also called incomplete antibodies) become more abundant than the agglutinating ones, which may lead to false-negative results. Detection of these nonagglutinating antibodies then requires the use of additional serological tools such as the Coombs test (13). Early administration of antimicrobial therapy decreases production of antibodies to Brucella cytosolic proteins but has only a marginal effect on the anti-LPS response (142).
It should be emphasized that the individual immune response to brucellae in humans is highly variable. In many patients who have apparently fully recovered from the disease, detectable IgM antibodies may persist for prolonged periods, and between 25% and 50% of patients with brucellosis exhibit IgM antibodies 1 year after treatment (132, 143). A rapid decrease of IgG and IgA antibody titers usually indicates a favorable response to antibiotic therapy, whereas persisting or increasing high titers can be a sign of treatment failure, residual disease, or impending clinical relapse (144). Patients experiencing relapse of the disease usually show a significant increase in IgG levels and a more moderate increment of the IgA, but not the IgM, isotype (145). In general, antibody titers fall more slowly in patients with focal complications (143) and in those who had very high titers at the initial phases of the infection. Thus, measurable antibody levels after therapy do not necessarily indicate treatment failure, evolution toward chronicity, or relapse (13, 132).
Long-term persistence of antibody titers makes it difficult if not impossible to differentiate between active infection, a history of brucellosis, or repeated exposure to the organism; the last is a not-uncommon event in areas of endemicity, where it may lead to overdiagnosis and unnecessary antimicrobial therapy.
In patients with brucellosis without involvement of the central nervous system, no antibrucellar antibodies are secreted in the CSF (146). In contrast, in patients in whom neurobrucellosis develops, low antibody titers against S-LPS and cytosolic proteins can be detected in the fluid by the rose bengal test and ELISA (147).
Outdated and Obsolete Diagnostic Tests
Many of the diagnostic serological assays developed over the years have not stood the test of time and have been utterly abandoned. The intradermal skin test not only failed to distinguish between mere exposure, current infection, and remote disease but also induced elaboration of antibodies to the injected Brucella antigens, making the subsequent interpretation of serodiagnostic tests difficult or impossible (148). The opsonocytophagic index probed the capability of the patient’s serum to facilitate phagocytosis of living Brucella organisms but gave inconsistent results, and its complicated manual execution posed a substantial risk of contagion to laboratory personnel (149). The hemagglutination test was in widespread use in the former Soviet Union and Eastern Bloc but has not gained acceptance elsewhere (150). These and others tests of historical interest not in use today are not discussed further in this review. The current serodiagnostic tests for diagnosing human brucellosis and their clinical use are summarized in Table 3.
TABLE 3.
Serodiagnostic tests for human brucellosis and their recommended use
| Serological test | Use(s) |
|---|---|
| Undiluted rose bengal test | Initial screening |
| Rose bengal test with serial serum dilutions | Confirmation of diagnosis |
| Standard agglutination test | Confirmation of diagnosis |
| 2-Mercaptoethanol test | Follow-up and early detection of treatment failure |
| Complement fixation test | Confirmation of diagnosis |
| Coombs test | Diagnosis of relapses and chronic cases, detection of incomplete antibodies |
| ELISA | Diagnosis confirmation, diagnosis of B. canis infection and neurobrucellosis |
| IgG avidity ELISA | Differentiation between recent disease and past infection |
| Immunocapture agglutination test | Diagnosis confirmation |
| D-TEC CB test | Diagnosis of B. canis infection |
| Dipstick test | Rapid point-of-care diagnosis of acute cases |
| Lateral flow assay | Rapid point-of-care diagnosis |
| Fluorescent polarization immunoassay | Rapid diagnosis |
Serological Tests That Target Brucellar S-LPS
RBT.
The rose bengal test (RBT) is a card agglutination test that uses an 8% suspension of killed B. abortus strain 1119-3 cells stained with rose bengal dye and buffered to pH 3.65 ± 0.05. The RBT detects agglutinating and nonagglutinating antibodies and does not have the drawback of the prozone phenomenon (i.e., the failure to observe agglutination of the antigen at low serum dilutions due to a relative excess of antibodies) that is observed with the SAT. Performance of the test is straightforward and does not require technical expertise or special laboratory equipment (151). Although the assay provides results within 4 min, sera with high titers of nonagglutinating antibodies may require up to 8 min to show characteristic bacterial clumps or a typical rim (151). The RBT is highly sensitive (>99%) irrespectively of the stage of the brucellar infection, but, similarly to all assays that detect antibodies to LPS, it can give spurious positive reactions in patients infected with cross-reacting bacteria. The combination of technical simplicity, high sensitivity, speed of use, and low price makes it an ideal screening tool, but a positive result should be validated by a second, more specific test such as the SAT, particularly in areas where the zoonosis is endemic.
In a recent study, the traditional RBT was modified to provide quantitative information (130). After obtaining a positive qualitative RBT result, serum specimens were subjected to 2-fold dilutions and retested. Positive reactions at a ≥1:8 dilution correlated with acute brucellar infection, whereas lower titers suggested contact with the organism without clinical disease, history of brucellosis, or a long-standing infection and required further investigation (130).
SAT.
Originally developed by Bruce, the standard agglutination test (SAT) is the most common serodiagnostic assay used for diagnosing B. abortus, B. melitensis, and B. suis infections. Because the SAT detects antibodies to brucellar S-LPS, it is not useful for diagnosing disease caused by the rough B. canis species. The SAT is performed by mixing serial 2-fold dilutions of patient’s serum (in the range of 1:20 through 1:2,560) with Brucella antigen derived from heat-phenol-killed B. abortus strain 119-3 in test tubes or in the wells of a microtiter plate. After overnight incubation and without shaking the test tubes, the reaction is read by the unaided eye, under a magnifying glass or by employing a fluorescent light and a dark background. If agglutination has occurred, the clumps of antigen and antibody complexes will settle, leaving a clean supernatant. In case of a negative test, the suspension remains unchanged and cloudy. SAT titers of ≥1:160 are considered diagnostic when coupled with a compatible clinical presentation. To increase the specificity of the SAT, a cutoff of 1:320 has been advocated for the serodiagnosis of human brucellosis in regions of endemicity (8, 13). Naturally, due to the trade-off between specificity and sensitivity, the increase in the threshold may reduce sensitivity and compromise the diagnosis altogether.
In the early stages of the disease, the SAT results may be negative or exhibit titers below the diagnostic cutoff. In a study performed among 264 Israeli patients with acute infection and B. melitensis bacteremia, 8.3% had SAT titers of <1:160 and 17.4% had titers below 1:320 (20). A false-negative result can also be attributed to the predominance of nonagglutinating antibodies or the aforementioned prozone phenomenon. The potential solutions to resolve these issues are summarized in Table 4. It should be stressed that the host’s immune response to Brucella antigens exhibits a broad and unpredictable variability among individuals; while some patients present with high SAT titers, others never reach the diagnostic cutoff values. If a false-negative result is suspected from clinical and/or epidemiological considerations, the SAT should be repeated at least 2 to 3 weeks apart. Testing paired serum samples during the acute stage and then again after several weeks or months may demonstrate seroconversion or a ≥4-fold increase in the antibody titer, enabling the retrospective confirmation of the diagnosis. However, because in many patients the possibility of a Brucella infection is suspected only after considerable delay, frequently no serum samples are available from the early phases of the disease, and thus, no significant changes in the SAT titer are observed.
TABLE 4.
Causes of false-negative results of serodiagnostic tests and possible solutions
| Cause of lack of demonstrable seroconversion | Possible solution |
|---|---|
| Too-early performance of the test | Retest after 2–3 wk |
| Prozone effect | Dilute the serum beyond 1:320 |
| Blocking/incomplete antibodies | Perform a Coombs test |
| Low-affinity antibodies | Acidify diluent to pH 5.0 |
| Brucella canis infection | Perform a species-specific test |
Although an appropriate response to antibiotic therapy correlates well with decreasing SAT titers, significant antibody titers are still present in 3 to 5% of asymptomatic patients 2 years after completing an effective antimicrobial therapy, and therefore, extended serological follow-up might not be necessary in individuals who are clinically well (152).
(i) Microagglutination test.
The traditional SAT has been miniaturized so as to be performed in a microtiter plate format, enabling the use of small amounts of reagents and low serum volumes, which allows for simultaneous testing of multiple samples and results in a shortened turnaround table (153).
2-ME test.
The interpretation of serodiagnostic tests for brucellosis is frequently hampered by the unsatisfactory specificity caused by cross-reacting IgM antibodies to the S-LPS and the long-term persistence of IgM titers observed in many patients, despite a seemingly adequate response to therapy. To eliminate the IgM confounder, it has been proposed to disable the agglutinating capabilities of the IgM pentamer, leaving the IgG isotype intact. The IgM –S–S– bonds can be chemically inactivated by a wide array of technical manipulations: adding 2-mercaptoethanol (2-ME), antiglobulin, or chelating agents; using an acidified antigen; rivanol precipitation; and heating the serum. Nowadays, only the 2-ME modification remains in widespread use for the diagnosis of human brucellosis (154). A major inconvenience of the test is that 2-ME has a strong and unpleasant odor and irritates the eyes and respiratory tract mucosa, requiring working in a fume hood. The 2-ME has been replaced in many laboratories by dithiothreitol (DTT), which also inactivates IgM antibodies but is less toxic and can be worked with on an open bench, although the test has kept its traditional and familiar 2-ME name (155). The results obtained with the DTT additive are comparable to those of the traditional 2-ME test (155).
The 2-ME assay is performed identically to the SAT and is run in parallel, employing as a diluent phosphate buffer containing 2-ME or DTT at a final concentration of 0.05 M in each test tube. The remaining agglutination titer under the effect of 2-ME is interpreted as representing the activity of IgG isotype antibodies. It should be noted that reduction tests are not totally specific for IgM, as they can degrade the IgA dimer as well. Because of the delayed appearance of IgG relative to IgM antibodies, the 2-ME test, by neutralizing the IgM isotype, turns positive later than the SAT and thus is a less sensitive indicator of brucellar infection in the first weeks of illness.
The main use of the 2-ME is for the serological monitoring of the response to antibiotic therapy in already-diagnosed patients. In a classic study by Buchanan and Faber, 84 patients with 2-ME titers of ≤1:80 at 1 year after the onset of illness were asymptomatic and stayed healthy without any further antibiotic therapy. In contrast, four of the eight patients with 2-ME titers of ≥1:160 still had significant clinical symptoms and signs of disease and required supplemental antimicrobial treatment (154). Similar to the case for the SAT, the performance of the 2-ME depends on the characteristics of the patient population, as well as on the quality of the reagents employed. A 2-ME titer of ≤1:20 is considered negative, titers in the range from 1:40 to 1:80 are indicative of active brucellosis in regions of low incidence of disease, and antibody titers of ≥1:80 are interpreted as diagnostic in areas of endemicity or among individuals repeatedly exposed to Brucella organisms (156).
Coombs antiglobulin agglutination test.
Because in patients with long-standing Brucella infection, nonagglutinating antibodies progressively become more abundant than the agglutinating ones, the SAT may give false-negative results. Under these circumstances, the Coombs test is particularly useful for confirmation of the disease because it detects the presence of incomplete antibodies. The test consists of performing serial dilution of the patient’s serum in normal saline solution and incubating the test tubes with B. abortus antigen at 37°C for 24 h, followed by a second incubation at 5°C for an additional hour. The tubes are then centrifuged at 4,000 rpm for 15 min, and the supernatant is discarded and replaced by saline solution. The sediment is resuspended by shaking, followed by a new centrifugation step. The washing operation is repeated three times, and after resuspending the sediment one more time, anti-human globulin rabbit serum is added to each tube and to a negative-control tube with no serum. After mixing, tubes are incubated again for half an hour and examined for agglutination.
The test is generally ordered in patients with chronic brucellosis and in relapses of the infection, in which a titer of 1:80 or greater is usually present (145). Unfortunately, few laboratories in areas of endemicity have the technical tools and expertise to perform this sensitive but intricate and time- and labor-consuming assay.
Brucella Coombs gel test.
The Brucella Coombs gel test (Odak test) is a novel, simple, and rapid agglutination assay that is performed in microcolumns containing a gel matrix and Coombs antibodies. The test uses a centrifugation gel system similar to that employed for blood grouping. The presence of Brucella antibodies in the serum sample is revealed by the formation of a pink antigen/antibody complex, which remains trapped in the gel. In the absence of antibodies, the Brucella antigen precipitates at the bottom of the gel card system. In contrast to the case for the classic cumbersome and time-consuming Coombs test, the Odak results are obtained within 2 h. The current published experience with this test is favorable but is still limited to three small studies (157–159). Clearly, additional experience with the Odak test is mandatory before it can replace the traditional Coombs assay. A comprehensive evaluation of the performance of the Brucella Coombs gel test should be carried out, enrolling a large number of patients at different stages of brucellosis as well as including individuals with focal complications, those with a history of the disease, and those with repeat exposures to the organisms but no clinical disease.
CF test.
The complement fixation (CF) test is performed by inactivating the patient’s complement by heating the serum at 56°C for 30 to 60 min. The serum is then serially diluted, and whole killed Brucella cells and pretitrated rabbit complement are added to the test tubes. An indicator system consisting of sheep erythrocytes sensitized with rabbit antibody is subsequently added. If IgG1 isotype antibodies are present in the patient’s serum, they will attach to the antigen, the complement will be activated, and no residual complement will be available for lysing the erythrocytes. Alternately, if no antibody is present, hemolysis will occur, which can be detected visually or measured with a spectrophotometer. The resulting hemoglobin concentration in solution represents an inverse measure of the antibody activity in the serum (160).
The CF test, which is widely used in control/eradication programs for the serodiagnosis of the zoonosis in livestock, is not commonly used to diagnose human infection due to its technical complexity and problems in its standardization (13, 139). Positive results are obtained later than with the SAT, and titers are usually higher than those of the SAT in the fourth or fifth month of the infection and persist for approximately 12 months after the initial symptoms of the disease (161). A negative SAT coupled with a high CF titer can be observed in individuals with chronic brucellosis but also in recovered patients (32).
Immunocapture agglutination test.
A major downside of the traditional agglutination tests is that they are labor-intensive and time-consuming. In recent years a novel single-step immunocapture assay (Brucellacapt test; Vircell, Santa Fé, Granada, Spain) has been introduced for the serodiagnostic of human brucellosis. The test does not require skilled laboratory personnel or additional components, and results are read after 24 h. Brucellacapt operates in acidic medium to avoid the low specificity of IgM antibodies, employs B. abortus as the antigen, and detects agglutinating IgG and IgM antibodies, as well as nonagglutinating antibodies to the three smooth O-polysaccharide-containing Brucella species (162). The test consists of strips of microtiter wells coated with anti-human immunoglobulins. Diluted patient’s serum and antigen are added to the wells, and the strips are incubated for 24 h at 37°C. Agglutination defines a positive test result, whereas reactions exhibiting a central pellet in the bottom of the well are interpreted as negative.
In general terms, the Brucellacapt assay gives titers that are concordant with or higher than those in the Coombs test in the early stages of the infection, and titers of ≥1:640 are usually observed in patients with brucellosis of long evolution, many of whom may exhibit nondiagnostic SAT titers (<1:160) (162). A pronounced and rapid decrease in the Brucellacapt titers is observed at the end of successful antimicrobial therapy, whereas in patients with relapse or reinfection, a 2- to 5-fold increase in the antibody titers generally occurs (143). It should be noted, however, that the Brucellacapt results show wide differences between individuals, and one of the relapsed patients exhibited a one-dilution decrease in the titer (143). In most cases there is a good correlation between the results of the immunocapture agglutination and those obtained with the Coombs tests; however, in some cases of relapse or prolonged disease, only slight changes in low-affinity antibodies occur, for which the Coombs test is a more sensitive detector. In summary, the Brucellacapt test is a sensitive marker of the progression of the infection and, because of its simplicity compared to the Coombs test, is preferable for the follow-up of treated patients (143). When the performance of the test was assessed in an region of endemicity of Spain, none of the 278 SAT-positive sera were negative by the Brucellacapt assay, demonstrating adequate sensitivity. However, 127 of 606 (21%) SAT-negative sera exhibited a positive Brucellacapt result, and the specificity was only 63% when patients who had diseases other than brucellosis were tested (162). Naturally, at a higher cutoff value, the specificity of the assay improved but at the expenses of its sensitivity (162).
IgG avidity ELISA.
The IgG avidity ELISA, a modification of the traditional ELISA, is based on the concept that, over time, the initial low-affinity IgG antibodies are gradually replaced by high-affinity antibodies. Pairs of wells of a microtiter plate coated with B. abortus S-LPS are incubated with each patient’s serum. For each serum, one well is then washed with phosphate-buffered saline (PBS), and the other well is washed with highly concentrated urea diluted in PBS (163, 164). The low-affinity antibodies are removed from the binding sites by the urea, leaving the high-affinity ones attached to the solid phase. Subsequently, an ELISA for IgG antibodies is performed. An IgG avidity index (AI) is calculated by dividing the absorbance of urea-treated microwells by the absorbance of the untreated ones and multiplying by 100. A high AI suggests immunological memory and a more mature immune response, whereas a low AI is interpreted as consistent with recent infection (165). The test has been advocated to differentiate patients with an active brucellar disease from those with remote or past infections (163). Despite its potential usefulness, the test conditions have not been standardized yet, and the AI cutoff values have varied between studies (163, 164).
Serological Tests That Target Cytosolic Proteins
ELISA.
The enzyme-linked immunosorbent assay (ELISA) has become increasingly accepted for the same-day diagnosis of brucellosis. The test is performed in 96-well microtiter plates that are precoated with Brucella antigen (whole cells or sonicate, protein extracts, or other antigen). Although the ELISA method can be also employed for the detection of S-LPS, plates are usually coated with cytosolic protein antigens. The patient’s serum is serially diluted and poured into the wells, and the plates are incubated. After a washing step, an enzyme (usually alkaline phosphatase or horseradish peroxidase)-conjugated anti-human IgG, IgM or IgA is added. The enzyme-specific substrate is subsequently also added, and following a second incubation, the optical density of the wells is measured at the appropriate wavelength.
The sensitivity of in-house ELISAs is usually high, but the specificity is lower than that of agglutination tests. Commercial ELISA kits usually perform less well and should be evaluated taking in consideration the epidemiological background when employed in regions of endemicity (8). For some authors, ELISA is the preferred choice for complicated, focal, and chronic cases. Use of the ELISA is also indicated when patients present with a clinical picture that suggests a brucellar infection but other serodiagnostic tests are negative. The test is also recommended for the serodiagnosis of neurobrucellosis when performed on CSF specimens (147). Because it enables the simultaneous testing of multiple samples, ELISA is also widely used for epidemiological serosurveys. It is also the preferred technique for detecting specific immunoglobulin isotypes. False-negative results for anti-Brucella IgM antibodies, however, may result from an excess of IgG, and therefore both IgG and IgM isotypes should be tested simultaneously (13). False-positive results due to the presence of rheumatoid factor may also occur, and it should therefore routinely be removed by absorption of the serum before testing for the presence of Brucella IgM antibodies (13).
Novel Serodiagnostic Tests
TR-FRET assay.
Fluorescent resonance energy transfer (FRET) is made possible when donor and acceptor fluorophores with appropriate spectral features, located at close proximity and correctly oriented, transfer energy between them. Labeling a given antigen and its complementary antibody with adequate fluorophores enables accurate measuring of the amount of energy transferred after the excitation of the donor fluorophore (166). In recent years, novel fluorophores with long fluorescent lifetimes have been introduced to improve the specificity of the reaction by removing the background non-FRET fluorescence. The underlying biological principle of the time-resolved FRET (TR-FRET) assay for brucellosis is that serum antibodies against the Brucella S-LPS outcompete a labeled monoclonal antibody that is also specific for this antigen. This competition decreases the attachment between the monoclonal antibody and the S-LPS antigen, and hence, the energy transfer measured by the fluorescence of the conjugate will be reduced. The assay is performed in a 96-well microtiter plate after a single 30-min incubation period and no washing steps, followed by fluorescence reading of the loaded wells.
The performance of the test has been assessed with a panel of sera derived from 73 individuals with proven brucellosis and 480 controls and compared to that of traditional serodiagnostic tests. The results of the TR-FRET assay were similar to those obtained with the comparators, and the method proved also to be effective with serum samples of poor quality collected in the field (166).
FPA.
The principle of the fluorescent polarization immunoassay (FPA) is based on measuring the difference in rotational velocity between a small antigen molecule in solution, labeled with a fluorochrome, and the same antigen molecule conjugated with its antibody. The rather small brucellar S-LPS molecule will rotate at a rapid pace, resulting in rapid depolarization of light, whereas the much larger antigen-antibody complex will rotate at a lower rate, resulting in a reduced rate of light depolarization. The difference can be measured accurately, and results can be obtained in minutes (167). The FPA technology has been successfully tested for the diagnosis of the zoonosis in domestic and feral animals, as well as for brucellosis screening in the dairy industry. In a pioneer study, the potential use of the FPA method was evaluated with 340 sera from blood donors and showed a specificity of 97.9% (168). When sera from patients with acute infections or relapses caused by B. melitensis, B. abortus, or B. suis were tested, an overall sensitivity of 96.1% was found. Because of the ease of the procedure, the researchers concluded that the FPA could be readily adopted for use in clinical laboratories, blood banks, and, given portable equipment, also in the field (168). Although the latter configuration could obviate shipping samples from remote areas and waiting for results, the dependence on highly sophisticated laboratory equipment and reagents might make the assay far too expensive.
Rapid Point-of-Care Tests
Human populations affected by endemic brucellosis frequently consist of shepherds of goats, sheep, or camels living in remote rural areas who migrate seasonally in search of fresh pastures. Shipping cooled or frozen serum specimens from the field to distant centralized testing laboratories, as recommended (98), and getting the test results back soon enough to make an impact on the patient’s management imply insurmountable logistic difficulties. Moreover, performance of traditional confirmatory serological tests such as the SAT, Coombs test, or complement fixation or of the newest Brucellacapt assay, FPA, or TR-FRET assay requires well-equipped laboratory facilities and highly trained personnel, which are generally not available in regions where the zoonosis is endemic. To overcome these impediments, rapid tests that can be performed bedside and provide almost immediate results have been developed in recent years.
Dipstick assay.
The dipstick assay is a rapid test in use for the detection of Brucella-specific IgM antibodies. The assay consists of a strip of nitrocellulose containing S-LPS derived from B. abortus as the antigen applied in a distinct line. A wetted dipstick strip is incubated for 3 h in a mixture of patient’s serum and detection agent (a monoclonal anti-human IgM antibody conjugated to colloidal dye particles of Palanyl red [169]). After completing this step, the strip is rinsed with tap water and dried at room temperature, and the staining intensity is compared to a colored reference. Performance of the test is simple and does not require technical expertise or special equipment. Because only IgM antibodies are detected, the dipstick is particularly useful for the diagnosis of recent-onset brucellosis, but it proved to be inadequate for protracted and chronic cases (169, 170). For instance, in a comparative study performed in an region of endemicity in Albacete, Spain, 1 month after the onset of antibiotic treatment, only 7% of sera obtained from patients with acute brucellosis were still positive by the dipstick test, whereas the detection rate for the SAT and 2-ME test was 46% and that for the Coombs test was 92% (171).
LFA.
The lateral flow assay (LFA) is a simplified version of an ELISA contained in a suitable plastic device. The assay consists of a nitrocellulose detection strip containing B. abortus LPS, flanked at one end by a reagent pad and at the other end by an absorption pad, and a reagent control applied in distinct lines. The reagent pad contains dried colloidal gold anti-human IgM and anti-human IgG conjugates as the means of detection (172).
The LFA was conceived for bedside use: it employs a tiny blood drop drawn by fingerprick; it does not require laboratory expertise, special equipment, or an electric supply; and results are easy to interpret. The different test reagents have been stabilized so they do not need refrigeration for transportation or storage, an important advantage in field studies. In the published experience of Smits et al., the sensitivity of the LFA was similar to that of the SAT at a cutoff value of 1:160 (>95%) and superior to that of the comparator when a SAT titer of 1:320 was employed, with a specificity of 98% for samples obtained from asymptomatic controls (172). Similarly favorable results were also obtained in a Turkish study in which the LFA was found to be slightly more sensitive than the classic SAT and 2-ME test (173).
RBT as a rapid diagnostic test.
In recent years, the rose bengal test (RBT) has become popular in hospital emergency departments for the rapid diagnosis or exclusion of brucellar infections in patients presenting with a febrile syndrome (130). The test is performed with undiluted patient’s serum on a glass slide or on a smooth and clear surface such as a clean white tile, and results are obtained within a few minutes. However, establishing the diagnosis of the disease and prescribing prolonged antibiotic therapy to individuals who are repeatedly exposed to Brucella organisms, such as shepherds, abattoir workers, veterinarians, dairy industry professionals, and personnel in microbiology laboratories, or to those who have a history of brucellosis on the sole basis of a positive RBT is not indicated and may also conceal other clinically significant diseases (130).
Novel Experimental Antigens and Tests and Future Developments
Novel assay platforms and configurations, whether laboratory or point-of-care based, are desirable and welcomed, but the quality of the antigen remains the most crucial component of the diagnostic performance of the test (174). In attempting to increase the specificity of the serological assays for brucellosis, the challenge has been to find a trustworthy replacement for the native Brucella LPS antigen. Many potentially useful proteins have been proposed and tested, but despite the growing number of reports praising the merits of one antigen or another, none have yet gained universal acceptance as an alternative to the traditional LPS.
Novel antigens.
(i) Synthetic oligosaccharides.
Given the dominance of the LPS O chain as a target for polyclonal antibodies, artificial d-Rha4NFo homo-oligosaccharides have been synthesized, each containing the capping M epitope and a single α(1→3) link but with a varied number of α(1→2) links. The diagnostic performance of these synthetic antigens was assessed with panels of bovine serum samples that included specimens that gave false-positive results when tested by traditional serodiagnostic assays, and the results were compared with those obtained with conventional tests (175). The synthetic antigens showed improved specificity, suggesting that these oligosaccharides could be used in the future as surrogates for naturally derived antigens in serodiagnostic tests (175).
(ii) Recombinant Brucella proteins.
In recent years, biotechnological progress in mass spectrometry for protein identification, combined with availability of genomic data, has made production of recombinant proteins more accessible. These advances are coupled with immunoselection procedures aimed at identifying novel and improved antigens to be used for the serodiagnosis of human infections.
Sera obtained from Brucella-infected individuals as well as healthy controls are subjected to immunoscreening against immunoblotted whole proteomes of the relevant organisms mapped by two-dimensional gel electrophoresis methods (174). Unlike the repeating polysaccharide of Brucella LPS, proteins contain several different epitopes. Some of these epitopes are Brucella specific, but others may be present in different protein antigens, even if the entire protein is itself unique. Therefore, research has shifted from studying the antigenicity of complete proteins to focus on the immune response at the peptide level. Several studies have showed that peptides derived from the outer membrane protein BP26 of B. melitensis may be more effective diagnostic antigens than the whole naturally occurring protein (176, 177). Once potentially valuable proteins have been identified, the peptides within are screened in silico for their antigenicity. To facilitate the selection procedure, highly sophisticated software tools have been developed (178). Once in silico research has identified potential candidate peptides, a full in vitro proteomic investigation is performed with the aid of high-throughput microarrays, which currently enable simultaneous analysis of up to 104 different peptides (179). Antigens of interest thus identified are cloned and expressed in an Escherichia coli system, extracted, and purified. As the result of this multistep approach, a wide variety of candidate proteins that are less immunodominant but more specific as brucellar antigens are currently being investigated.
Pioneering studies show that the outer membrane protein Omp31 of B. melitensis reacts with serum of animals with brucellosis when tested by ELISA, and this reactivity is absent from the serum of uninfected animals (180). The performance of Omp31, however, exhibits wide disparities in sensitivity and specificity among the domesticated animal species tested, and no information on its performance with human sera is currently available (176).
The potential use of recombinant whole proteins or peptide derivatives such as B. abortus Omp28 and the periplasmic protein BP26, Brucella lumazine synthase, and the B. melitensis Omp2a protein in bacterial expression systems is currently being investigated for use in veterinary medicine (181, 182). So far, the results are unsatisfactory and inferior to those obtained with traditional serological assays. The suboptimal performance of these novel antigens might be caused by alterations in the tertiary structure of the proteins and their immune-dominant epitopes during the Western blotting process or by their poor binding to polystyrene microtiter plates employed in ELISAs. It should be pointed out that even if these technical problems will be solved in the future, the resulting novel serological assays will continue to suffer from many of the traditional shortcomings described in “Serological Diagnosis of Human Brucellosis: Imperfect but Indispensable” above, which are immanent to the serodiagnostic strategy itself.
Antigen capture antibody technology.
A radically different approach, in which high-affinity antibodies are employed in a capture assay to detect brucellar S-LPS in the blood and possibly in other normally sterile body fluids and exudates, has been proposed by Patra et al. (183). The test was validated in an experimental murine model of high-magnitude B. melitensis bacteremia and was subsequently evaluated in Peruvian patients with culture-proven disease. Seven of 10 bacteremic patients had detectable B. melitensis S-LPS in the blood. One of 10 patients who experienced a relapse of the infection but whose blood cultures remained negative had a positive serum antigen test. No patients who exhibited negative blood cultures showed a positive blood S-LPS test result. As the investigators rightly acknowledge, the test sensitivity would probably be insufficient to detect S-LPS in patients with prolonged infection and focal disease, and its performance with specimens other than serum needs to be further evaluated (183).
Conclusions
The traditional serodiagnosis of human brucellosis has the evident drawbacks of low sensitivity in the early phase of acute disease, suboptimal specificity caused by cross-reacting bacterial species, and difficulties in differentiating active disease from past infections and inconsequential exposure to the organism. However, because of their relative simplicity, low cost, and high negative predictive value, serological tests remain the main diagnostic tools in areas of endemicity with limited economic and technical resources. An initial screening of the patient’s serum by the sensitive RBT should be followed by a more specific confirmatory test such as the SAT or the immunocapture agglutination assay. The recent development of synthetic antigens seem likely to improve the sensitivity of future tests, while novel point-of-care assays hold the promise of providing rapid and reliable results without the need for costly equipment or technical expertise and may dispense with the need of shipping serum specimens under refrigerated conditions to distant central laboratories.
DIAGNOSIS OF BRUCELLOSIS BY NUCLEIC ACID AMPLIFICATION TESTS
Nucleic Acid Amplification Tests: an Alternative to Conventional Microbiological Diagnosis
The last 20 years have witnessed continuous advances in the molecular diagnosis of bacterial infections and especially those caused by uncultivable and difficult-to-culture microorganisms. Because of their fastidious nature, Brucella spp. have been considered natural candidates for diagnosis by nucleic acid amplification tests (NAATs) from the outset. Even though there exists a wide array of serological tests and automated blood culture instruments have reduced the time needed for isolation, conventional methods for the microbiological diagnosis of human brucellosis still have important limitations, as described earlier. It would therefore seem logical to seek new tools for the diagnosis of human brucellosis that are safer, faster, and more efficient. As with other bacterial infections, these alternatives could come from the field of molecular biology.
Genomics and Phylogenomics of the Brucella Genus
In 2002 the genomes of B. melitensis 16M (184) and B. suis 1330 (185) were fully sequenced and analyzed. The genomes showed great similarity and gene synteny, with an identity of 98 to 100% in over 90% of the genes. Three years later the full sequences of B. abortus 2308 and B. abortus 9941 (186) were published, covering the full genome content of the three species responsible for the overwhelming majority of cases of human disease.
Currently, the genus Brucella groups a total of 11 species: the six classic species B. melitensis, B. abortus, B. suis, B. canis, B. ovis, and B. neotomae; two species isolated from marine mammals, B. ceti and B. pinnipedialis; and three new species, B. microti, B. inopinata, and the recently described B. papionis (187).
As of the end of 2018, whole-genome sequencing data are available for five strains of B. melitensis (16M, M28, M5-9, ATCC 23457, and NI), four strains of B. suis (1330, ATCC 23445, VBI22, and 019), four strains of B. abortus (S19, 9-941, A13334, and 2308), one strain of B. ovis (ATCC 25840), two strains of B. canis (ATCC 23365 and HSK A52141), one strain of B. microti (CCM 4915), one strain of B. pinnipedialis (B2/94), and at least another 61 genomes of different members of the genus, some of them at various stages of completion. Genomic comparison between the different species and strains that have already been sequenced has confirmed the similarity of chromosome sizes, nucleotide composition, and gene synteny among Brucella organisms (186). With the exception of Brucella suis biovar 3, which has a single 3.1-Mb chromosome, all other Brucella species possess two circular chromosomes of about 2.1 Mb and 1.2 Mb that share a GC content of 57.2%, a similar proportion of encoding regions, and an equivalent distribution of constitutive genes. The relatively large size of the genome suggests that it comprises a wide repertoire of genes that enable brucellae to thrive in different ecosystems, including the environment and the intracellular milieu, and infect a variety of animal hosts (188). Chromosome I encodes most of the core metabolic machinery, while chromosome II contains gene complexes of plasmid replication and conjugation, as well as genes involved in auxiliary metabolism and cell processes. Although of a different evolutionary origin, there are also essential genes located on both chromosomes, with a similar distribution in the sequenced species (185).
Of note is the presence on chromosome II of a gene complex for flagellar synthesis, with 31 genes grouped in three loci. All Brucella genomes have truncated genes essential for flagellar functionality, except for B. microti, which has no evident defects in the components of the flagellar complex. The flagellar genes present in B. microti are identical to those of Ochrobactrum anthropi, although they display differences in gene organization (189). Although Brucella species do not exhibit motility, the presence of flagellar genes may be necessary for other functions, such as virulence (190).
The insertion sequence element 711 (IS711) is present in all strains of the genus and spread on the two chromosomes with different positions and number of copies. Closely related organisms and, specifically, members of the genus Ochrobactrum do not harbor insertion sequences similar to IS711, suggesting that this genomic component is native to the Brucella genus (191).
Although brucellae show a high degree of genomic homology, various studies have shown a remarkable variety of insertions, deletions, and recombinations among Brucella species and strains. Establishing the precise genetic relation between the different brucellae is important to understand their ecology, evolutionary history, host relationships, and pathogenicity, as well as for the development of genotyping methods.
The relatively small size of the bacterial genomes has facilitated their phylogenetic analysis and led to deeper understanding of their evolution at the genus and even the species level in a variety of organisms (192–194). The phylogeny of the Brucella genus has been analyzed with various molecular typing techniques, including multiplex PCR, single nucleotide polymorphism (SNP) analysis, and multilocus sequence typing or multilocus sequence analysis. Although none of these methods has been able to fully clarify the phenotypic particularities and host tropism exhibited by the different species, the availability of whole-genome sequencing and the global genome-wide SNP analysis is leading to better understanding of the phylogenetic evolution and pathogenicity of the different members of the Brucella genus (195, 196).
Whole-genome analyses possess an extraordinary resolution capacity to differentiate phylogenetically closely related bacterial isolates. Whole-genome comparisons have shown that B. canis and B. suis biovars 3 and 4 have such nucleotide similarity that B. suis can be considered a paraphyletic species and that B. canis seems to have arisen directly from an ancestor of B. suis (197). Likewise, it is possible to assume that the Brucella genus has its origins in soil- or plant-associated bacteria and that both B. suis and B. microti could have a replication cycle outside the mammalian hosts (198). The notable genetic homogeneity of the Brucella genus may possibly be related to the relative youth of the lineage, as well as the poor lateral transfer rate of isolated genes and whole genomic islands from other bacteria (185, 199).
Genomic studies of the newly reported Brucella species could lead to better understanding of the diversity and intricate interconnections within the genus, especially between the marine and terrestrial species. As new sequencing techniques become less expensive and simpler, the whole-genome SNP-based approaches will soon allow for phylogeographic reconstruction of Brucella populations and determine with greater accuracy the origin and the global spread of the different species (200) and the position occupied by the new atypical strains that are currently being isolated from amphibians and other animal hosts (201).
Targets and Primers
As with any other microorganism, PCR-based methods to detect Brucella DNA use a wide array of different targets and primers. Although the medical literature comprises multiple reports on the molecular diagnosis of brucellosis, the overall number of genomic targets remains relatively small. The most commonly employed are summarized in Table 5.
TABLE 5.
Molecular targets and primers most frequently used for the molecular diagnosis of human brucellosis
| Target gene | Primer | Sequence (5′–3′) | PCR product (bp) | Authors (reference) |
|---|---|---|---|---|
| omp2 | JPF | GCGCTCAGGCTGCCGACGCAA | 193 | Leal-Klevezas et al. (202) |
| JPR | ACCAGCCATTGCGGTCGGTA | |||
| omp31 | F | TGGTAAGGTCAAGTCTGCGTT | 281 | Kattar et al. (203) |
| R | CTTCTTCATTCCGTGTTCGTG | |||
| omp28a | 26A | GCCCCTGACATAACCCGCTT | 1,029 | Mitka et al. (205) |
| 26B | GAGCGTGACATTTGCCGATA | |||
| 16S rRNA | F4 | TCGAGCGCCCGCAAG GGG | 905 | Romero et al. (206) |
| R2 | AACCATAGTGTCTCCACTAA | |||
| IS711 | I1 | TCAATCCAACACGTTCC | 52 | Al-Nakkas et al. (210) |
| I2 | TCCTTGTACAGCCTCC | |||
| bcsp31 | B4 | TGGCTCGGTTGCCAATATCAA | 223 | Baily et al. (213) |
| B5 | CGCGCTTGCCTTTCAGGTCTG |
Encoding the Omp28 protein (also named BP26) of B. melitensis.
The genes encoding outer membrane proteins Omp2 and Omp31 were initially used as the amplification targets in diagnostic NAATs (202, 203), though interest declined when deletions of the omp31 gene in some strains of B. abortus were disclosed (204). More recently, the diagnostic performance of the omp28 gene, also named bp26, has been compared with that of the omp2 and bcsp31 genes, without demonstrating any advantage in terms of sensitivity (205).
The use of 16S rRNA as an amplification target is very common in the molecular diagnosis of many bacterial infections. The presence of multiple copies of the gene in the bacterial genome and of genus- and species-specific variable regions makes its use particularly attractive (206–208). However, cross-reactions with other alphaproteobacteria and the greater sensitivity shown by other, more-specific targets limit its application in the diagnosis of brucellosis (209).
Amplification of the insertion sequence IS711, which is present with a variable number of copies in all Brucella species hitherto described (210), is very frequently used in the molecular diagnosis of the disease. A study that evaluated the diagnostic performances of different genomic targets showed IS711 to be more sensitive than its comparators (211). However, the sequence variation in the IS711 element between Brucella species, and even its absence in some strains, has brought the validity of its use as an amplification target into question.
The bcsp31 gene encodes the synthesis of an immunogenic membrane protein of 31 kDa that is specific to the Brucella genus (212). Used initially by Baily et al. (213), it is currently the most frequently used target in all PCR formats, as well as in a variety of clinical settings of Brucella infection.
Specific targets for the vaccine strains (B. melitensis Rev 1, B. abortus B19, and B. abortus RB51) have also been described, but only limited information is currently available on the diagnostic performance of these targets (214, 215).
Use of Nucleic Acid Amplification Tests in Different Clinical Specimens
As with traditional cultures, Brucella spp. can be identified by NAATs in any clinical sample, such as blood or other normally sterile body fluid, as well as tissues and exudates. It should be emphasized that, independently of its tendency to cause focal complications, human brucellosis is a systemic infection. Accordingly, peripheral blood is the clinical sample that usually has the best yield and with which most experience has been gathered over the years.
Whole blood samples.
Because the bacterial concentration in the blood of patients with brucellosis is known to be usually low (65) and members of the genus Brucella are facultative intracellular pathogens, the initial studies used samples of peripheral whole blood, on the assumption that this would enable the maximum amount of circulating DNA to be rescued (216). Unfortunately, the high concentrations of human genomic DNA in whole blood and the strong inhibitory effect on the Taq polymerase exerted by molecules of the heme group severely compromised the performance of NAATs (217). To resolve this issue, some researchers have used other strategies that have shown efficacy. These include using hydrogen peroxide with successive washes of the sample and low concentrations of DNA in the assay mixture (218). However, these additional manipulations not only increase the risk of sample contamination, they also complicate and slow down the work in the clinical laboratory. Although use of the buffy coat could eliminate some inhibitors that might copurify with the targeted bacterial DNA, this specimen type has not become widespread because it usually requires treatment of the sample with Ficoll-Hypaque (219).
Other possible specimens such as coagulated blood also require considerable handling through mechanical or manual homogenization that could cause cross-contamination between samples (220).
Serum samples.
For operational reasons, serum has traditionally been considered the most desirable specimen for NAAT-based diagnosis. However, in the case of human brucellosis, serum was initially looked at with great caution as, at least theoretically, it involved the risk of too small a concentration of circulating DNA. This doubt began to be dispelled after a study demonstrated that the diagnostic yield of PCR assays was better using patient serum than with whole blood samples (221). This may be because DNA is released in the bloodstream as a product of bacterial breakdown during bacteremia, and by the late 1990s many authors had shown the presence of detectable amounts of brucellar DNA in serum in both systemic and focal infections (222, 223).
Constant improvements in the procedures for DNA extraction and the technology used for the amplification process have shown that serum is also the sample of choice for the molecular diagnosis of human brucellosis (224). At the present time, any clinical laboratory, however poorly equipped, can perform suitable serodiagnostic tests when the disease is suspected and can easily store an aliquot of the sample to complete the study by a molecular assay in doubtful cases, either in the same laboratory if the capability is available or by sending the specimen to a reference laboratory.
Specimens other than blood.
Despite its systemic nature, from 25 to 35% of patients with brucellosis (especially those infected by B. melitensis) have at least one focal complication during the course of their disease (225, 226). Focal complications of brucellosis can affect any organ and tissue, including, among others, those of the osteoarticular, genitourinary, cardiovascular, and central nervous systems. Unfortunately, in patients with localized forms of the disease, the frequency of detectable bacteremia is much lower than that found in patients with acute noncomplicated infection. For this reason, it is often necessary to resort to clinical specimens other than blood to achieve a correct diagnosis. The yield of cultures from specimens other than blood tends to be low (usually in the 10 to 20% range), particularly in diluted fluids like urine or CSF (225–227). This explains why NAATs have become an attractive alternative for diagnosing brucellosis in human patients with focal complications. Given the potential heterogeneity of the relevant clinical specimens (bone, synovial fluid, urine, hepatic tissue, CSF, etc.), the DNA extraction protocols have to be adapted and validated for the different samples processed.
FFPE tissue samples.
On occasion, especially in countries where brucellosis has been virtually eradicated or has a low incidence, some patients whose attending physicians had not initially considered the possibility of a brucellar infection have undergone a biopsy of the liver, bone, or other tissue for pathological examination. Brucellar DNA can be extracted and amplified from formalin-fixed paraffin-embedded (FFPE) tissue acquired from surgical biopsy samples (228). This enables the bacteriological diagnosis to be established retrospectively when conventional microbiological methods prove to be inconclusive (229). When resorting to this procedure, it is important to use suitable and well-validated protocols for DNA extraction from FFPE samples (230).
Brucella infections in humans produce granulomatous lesions that can at times be difficult to differentiate from those produced by Mycobacterium tuberculosis. In addition, the anatomic distribution of the focal complications of brucellosis is similar to that observed in some forms of extrapulmonary tuberculosis (231). Accordingly, in areas where both brucellosis and tuberculosis are prevalent, it would be very useful to have a tool able to differentiate the two etiologies in FFPE tissue samples.
Extraction and Amplification Strategies
Although the incidence of human brucellosis is low in most industrialized countries, interest in the development of molecular methods for its diagnosis dates back to the 1990s (232). The incentives were primarily the great economic impact of animal brucellosis, the difficulty of diagnosing a disease that has very few or even no symptoms in most affected mammals, and the fact that the conventional diagnostic methods for this zoonosis are slow and lack the desired sensitivity and specificity. Thus, veterinary medicine led the way to the molecular diagnosis of human brucellosis (202, 232–234).
Extraction methods.
The extraction method is known to substantially affect the amount, purity, and integrity of the DNA obtained and, consequently, the sensitivity of NAATs. Comparative studies have demonstrated differences in the DNA yield between extraction methods as large as 2 orders of magnitude. This is particularly relevant for the diagnosis of the disease when only a small amount of target DNA is present in the clinical specimen (235).
During the early days of the use of NAATs, DNA extraction with phenol was widely used as the preamplification step, although some researchers employed other customary extraction methods to avoid exposure to toxic fumes of the organic solvent. One such strategy was that reported by Miller et al., which consisted of salting out the proteins by dehydration and precipitation with a saturated NaCl solution prior to lysing the bacterial cells with a combination of SDS and proteinase K. This simple procedure succeeded in obtaining DNA amounts comparable to those extracted with the classic phenol-chloroform combination (236).
The increasing use of NAATs has led to the production of commercial extraction kits that guarantee standardization and optimization of reagents for all phases of the process. A wide range of products is now available for the manual and automated extraction of DNA, using different protocols that vary according to the type of sample to be assayed. In clinical laboratories automated extraction has clear advantages over manual methods, including greater processing speed, less interassay variability, and the possibility to work simultaneously on multiple samples.
Briefly, a commercial extraction method can be considered adequate if it rescues the maximum amount of DNA from the microorganism in the sample, eliminates all possible inhibitory factors in the sample, and prevents contamination. In addition, it should be capable of being used with a wide range of different clinical specimens and be subject to automation (237).
Recent years have seen a few comparative studies dealing with the efficiency of various commercial extraction methods for the detection of brucellar DNA. In the first of these reports, the authors compared the efficiency of seven commercial methods (the UltraClean DNA BloodSpin kit, Puregene DNA purification system, Wizard Genomic DNA purification kit, High Pure PCR template preparation kit, GFX GenomicBlood DNA purification kit, NucleoSpin Tissue kit, and QIAamp DNA Blood minikit) for the recovery of DNA from serum samples spiked with known concentrations of Brucella Rev1 cells (238). The authors found that although all the protocols tested were simple and easy to use, there were important differences in the DNA recovery rates, the reproducibility of the method, and the risk of contamination. Although all protocols enabled the recovery of at least 102 fg of Brucella DNA, the extraction methods that used proteolytic enzymes were the most efficient. With the exception of the UltraClean kit, all the other kits exhibited some degree of contamination. The authors concluded that the UltraClean DNA BloodSpin kit was the most efficient commercial method for recovery of Brucella DNA from serum (238).
The authors of another study that assessed the capacities of six commercial automated and manual methods (the MagNA Pure Compact and MagNA Pure LC instruments, IT 1-2-3 DNA sample purification kit, MasterPure Complete DNA and RNA purification kit, QIAamp DNA blood minikit, and UltraClean microbial DNA isolation kit) to extract DNA from B. abortus, B. melitensis, and B. suis suspended in PBS or spiked swab specimens concluded that although all the evaluated methods were highly efficient at inactivating these three highly virulent Brucella species (an important biosafety consideration), there were differences in the recovery rate and purity of the extracted DNA depending on the sample type (239). For instance, the MasterPure kit was the most sensitive when applied to bacterial suspensions, while the MasterPure and MagNA Pure Compact methods were equivalent and superior to the comparators for DNA extraction from spiked swab samples (239).
Finally, another study examined the efficiencies of six commercial extraction kits (the QIAamp DNA minikit, PeqGold Tissue DNA minikit, UltraClean Tissue and Cells DNA isolation kit, DNA Isolation kit for Cells and Tissues, and NucleoSpin Tissue) using various tissues from animals naturally infected by B. melitensis. In this investigation, most of the methods achieved good DNA recovery, though the QIAamp DNA minikit provided the best results for most of the specimens (240).
In conclusion, all the commercial kits currently available inactivate Brucella organisms, even at the high concentration of 106 CFU/ml, and most are able to recover brucellar DNA efficiently from clinical specimens, although depending on the study sample, there may be significant differences. Thus, given the wide variety of clinical settings that can occur in human brucellosis and the potentially wide range of specimen types, further studies are needed to define the most efficient extraction protocols for each of them.
Amplification strategies.
(i) Conventional PCR assays.
The strategies employed to amplify Brucella-specific targets in NAATs have evolved in parallel with the technical advances in the field of molecular diagnosis, from traditional detection of amplification by agarose gel electrophoresis to real-time PCR.
The first in-house PCR assays in which the amplified product was visualized using agarose gel electrophoresis proved to be faster and more sensitive than brucellar detection by conventional cultures (219). However, many of the early assays lacked the desired analytical sensitivity, which resulted in the need for a nested PCR (241) or visualization of the amplified product with dot blot or Southern blot steps (242). Unfortunately, this additional handling favored contamination that, added to the subjectivity of the interpretation of the results, represented a serious drawback that precluded the routine adoption of this diagnostic approach by clinical laboratories.
(ii) PCR-EIA.
In an attempt to overcome the difficulties posed by conventional PCR, shorten the processing time, enable the simultaneous assay of multiple samples, and avoid the use of toxic compounds such as ethidium bromide, researchers developed a PCR-enzyme immunoassay (PCR-EIA) in a microplate format (243, 244). This NAAT strategy consisted basically of hybridization with a biotinylated capture probe that is complementary to the inner part of the amplicon previously labeled with digoxigenin. This is then captured on streptavidin-coated microtiter plates and detected using an anti-digoxigenin Fab-peroxidase (245). These PCR-EIAs had an analytical sensitivity of around 10 fg of DNA, which corresponds to approximately two genomic equivalents, a quantity that is probably present in most specimens derived from patients with active brucellar infection. In addition, interpretation of the PCR-EIA results was far more objective than that of conventional PCR, and the method enabled handling of multiple samples at the same time and automation and did not require the use of UV light or working in a darkroom (246, 247).
(iii) Real-time PCR.
Despite the introduction of conventional PCR and its predictions for the accurate diagnosis of human brucellosis, followed by PCR-EIA, it was real-time PCR that brought about the real advance, enabling molecular methods to be used for the routine diagnosis of brucellosis outside reference laboratories.
First described at the end of the 1990s, real-time technology not only increased the analytical sensitivity of the molecular tests but also improved their reproducibility, simplified the technical aspects of the procedure, shortened the time needed to obtain results, drastically reduced the chances of contamination, and enabled an approximate quantification of the bacterial load originally present in the clinical specimen (248–250). These important advances were possible because real-time PCR uses sealed capillary tubes, the amplification process is monitored continuously, and the measured fluorescence is proportional to the amount of DNA generated during the thermal cycling.
The use of real-time technology for the amplification and detection of nucleic acids has significantly increased test efficiency compared with that of conventional PCR or PCR-EIA, as, after the extraction, the amplification and detection steps can be completed in 2 h, and it enables the simultaneous processing of multiple samples, an important benefit in busy clinical laboratories.
Many methods of quantitative real-time PCR (Q-RT-PCR) have been described over recent years. Some use DNA binding dyes like SYBR green I or EvaGreen, which are simpler and relatively inexpensive but may detect nonspecific amplification products, while others instead employ fluorophores attached to oligonucleotides (primer probes, hydrolysis probes, or hybridization probes), which are more costly but specifically detect the PCR product of interest (251).
With the gradual simplification of real-time PCR technology and the progressive reduction in the price of reagents and thermocycler instruments, this powerful tool is now available in most clinical laboratories in countries in the developed world.
(iv) Multiplex PCR.
The wide range of symptoms and nonspecific presentation of human brucellosis mean that it should be considered in the differential diagnosis of many clinical entities, such as community-acquired febrile syndrome with no apparent focus, lymphocytic meningitis, granulomatous hepatitis, epididymo-orchitis, septic arthritis, and vertebral osteomyelitis. Using NAATs to identify the various etiological agents that could be implicated in a particular syndrome is a slow and costly process. Multiplex real-time PCR (M-RT-PCR) is a type of molecular diagnostic strategy that is being increasingly used in different areas of DNA analysis, including infectious diseases (252–254), because it enables the simultaneous amplification of many relevant species-specific sequences in a single reaction. This capability is of great medical importance, especially in infections such as meningitis or vertebral osteomyelitis in which a diagnostic delay can result in a poor prognosis.
The usefulness of M-RT-PCR has been assessed for the rapid differentiation of brucellosis from extrapulmonary complications of tuberculosis (255) and for the identification of Brucella at the species level. This technique obviates the need not only for time-consuming successive biochemical and serological tests but also for the required handling of human pathogens that are transmissible in the laboratory setting (256).
Diagnostic Yield of PCR-Based Assays in Human Brucellosis
Although brucellosis in humans usually manifests as a febrile syndrome with no apparent focus, either from the onset of the disease or during its course, Brucella infections can affect any organ or body system and result in a variety of focal complications and clinical scenarios. Unfortunately, the diagnostic yield of NAATs has been sufficiently studied in only a few of these possible settings and mainly in infections caused by B. melitensis. Bearing in mind these limitations, we shall concentrate on those clinical conditions where the accumulated evidence is greater.
Acute infection.
The first study designed to assess the diagnostic efficacy of NAATs in human brucellosis was published in 1996 by Matar et al. (219). Using the buffy coat as a clinical sample and the primers B4 and B5 described by Baily et al. (213), Matar el al. assayed a conventional nested PCR assay that amplified the 223-bp target sequence of the gene encoding a 31-kDa protein (BCSP31) which is conserved in all Brucella species. The study included 20 patients with brucellosis, of whom 17 had acute symptoms. The control group comprised 9 patients with typhoid fever and 30 blood donors. The sensitivity and specificity of the PCR assay were 100%. Despite these initial promising results, the study had a few important limitations. Confirmatory blood cultures were performed for only two of the brucellosis patients, the control group mostly included healthy subjects, and the clinical information on the population was rather limited.
One year later and using an identical target and primers, Queipo-Ortuño et al. assessed the performance of a single-step conventional PCR assay employing peripheral whole blood as the clinical specimen (242). This study included 50 blood samples from 47 consecutive patients, 35 of them (70%) with a positive blood culture for B. melitensis and the remaining 15 (30%) diagnosed by clinical and serological criteria. As well as having a larger sample size, the control group in this study included 60 samples taken from 15 patients who had other febrile syndromes, 20 samples from asymptomatic patients who were professionally exposed to Brucella organisms or patients with a history of brucellosis during the previous 12 months and high titers of Brucella antibodies, and 25 blood specimens from healthy individuals with no history of brucellosis or potential exposure to Brucella organisms (242). The sensitivity of the NAAT was 100% and the specificity 98.3%. Although this study, like the earlier one, was also open, its results can be considered of higher validity due to the larger sample size, more rigorous inclusion criteria, and enrollment of a control group more clinically relevant to the diagnosis of brucellosis in daily medical practice.
Based on these findings and in an attempt to eliminate the deficiencies of employing whole blood, Zerva et al. reproduced the aforementioned study with a very similar methodology but using serum as the clinical specimen. This study, comprising 31 consecutive brucellosis patients, showed high sensitivity and specificity of the NAATs (94% and 100%, respectively) (221). Since these pioneering results, many others have considered serum to be the most suitable specimen for the molecular diagnosis of human Brucella infections.
Using a single-step in-house conventional PCR method, Mitka et al. assessed the diagnostic yields of four different genomic targets: bcsp31, omp28, and two different sequences of the omp2 gene, which encodes an outer membrane protein of 26 kDa of B. abortus (205). The authors assayed simultaneously buffy coat, whole blood, and serum samples from 200 patients with brucellosis, 148 of them (74%) with a confirmatory blood culture and the remaining 52 diagnosed by a combination of clinical and serological criteria. The authors reported that the four assays were 100% specific, with sensitivity rates ranging from 95.5% to 100% depending on the amplification target and the clinical sample used (205).
Once NAATs were accepted to be more sensitive than culture and more specific than serological methods for the diagnosis of acute Brucella infection in humans, it was then necessary to simplify the methodology and make interpretation of the results more objective, so as to enable the molecular technology to be used in any clinical laboratory. Accordingly, using the experience of their previous studies with a segment of the gene encoding the protein BCSP31 as a target and the B4 and B5 primers, Morata et al. developed a novel diagnostic PCR-EIA microplate assay. Its efficacy was studied in 59 whole blood samples drawn from 57 consecutive brucellosis patients, of whom two provided two samples, the first obtained at the initial infection and the second during a relapse of the disease, and a control group consisting of 113 blood samples from 30 patients with febrile syndromes of other etiology, 41 asymptomatic individuals with a history of brucellosis treated successfully during the previous 12 months, 14 asymptomatic subjects professionally exposed to Brucella organisms and exhibiting persistent high titers of antibrucellar antibodies, and 28 healthy subjects (245). The results of the study demonstrated that the reproducibility of the PCR-EIA was good and its detection limit was 10 fg, identical to that reported by the same authors using a conventional single-step PCR plus hybridization with dot blot (242). The sensitivity of the PCR-EIA was 94.9% and the specificity 96.5%. Three of the four controls who gave a false-positive result had a history of confirmed brucellar infection occurring between 6 months and 3 years before but had no clinical, serological, or bacteriological evidence of persistent disease or relapse (245).
With a similar method but using a 52-bp fragment of IS711 as the target, Al-Nakkas et al. studied a large group of patients with brucellosis, reporting a sensitivity of 96.9% and a specificity of 100% (210). In a later study that employed the same target and primers as those used by Morata et al. (245) but assaying in parallel one sample of whole blood and another of serum, Vrioni et al. analyzed the diagnostic performance of a PCR-EIA in a population of 243 patients with acute brucellosis (73.7% of them with a positive blood culture) and 50 healthy controls (257). When the results for the whole blood and the serum were considered together, the sensitivity of the NAAT was 99.2%, being 79% for serum and 81.5% for whole blood when considered separately. Although these results appear to be inferior to those reported by Morata et al. analyzing the whole blood and serum samples separately, it should be noted that the volume of the study samples was much smaller in the study by Vrioni et al. and that Morata et al. assayed all the specimens in duplicate.
The ultimate catalyst for the molecular diagnosis of human brucellosis was real-time PCR. The first study with this technology was reported in 2005 by Queipo-Ortuño et al. (258). Using the same target and the same primers as in their previous studies on a LightCycler platform (Roche Diagnostic, Mannheim, Germany) and using SYBR green I as the intercalating fluorophore and 200 μl of serum as the clinical specimen, the authors analyzed 62 serum samples derived from 60 consecutive patients diagnosed with acute brucellosis and 65 serum samples from a rigorously chosen control group. Despite the limitations of the small study population and the rigorous requirements fir the control group, 40% of which involved asymptomatic persons with a history of brucellosis who had been treated during the previous 12 months and individuals repeatedly exposed to Brucella organisms, the NAATs still had a sensitivity of 92% (a much higher value than the detection rate of 65.5% for blood cultures performed in parallel), a specificity of 96.4%, and positive and negative predictive values of 95.0% and 92.5%, respectively. As other studies have now confirmed the high reproducibility and analytical sensitivity of the real-time PCR in serum specimens (259, 260), as well as the superior performance compared to PCR-EIA in whole blood samples (261), real-time PCR is currently the most-used molecular method in the diagnosis of human brucellosis. Table 6 summarizes the yields of different PCR-based NAATs in the diagnosis of acute human brucellosis.
TABLE 6.
Performance of the different blood NAATs used in the diagnosis of human brucellosisa
| Authors (reference) | PCR method | Specimenb | No. of cases/no. of controls | No. (%) of culture-confirmed cases | NAAT performance (%) |
|||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | |||||
| Matar et al. (219) | Conventional nested PCR | Buffy coat | 20/39 | 1 (5.0) | 100 | 100 | 100 | 100 |
| Queipo-Ortuño et al. (242) | Conventional PCR | Whole blood | 50/60 | 30 (60.0) | 100 | 98.3 | 98.0 | 100 |
| Zerva et al. (221) | Conventional PCR | Serum | 31/45c | 13 (41.9) | 94.0 | 100 | 100 | 95.7 |
| Mitka et al. (205) | Conventional PCR | Buffy coat | 200/100 | 148 (74.0) | 100 | 100 | 100 | 100 |
| Morata et al. (245) | PCR-EIA | Whole blood | 59/113 | 40 (67.8) | 94.9 | 96.5 | 93.3 | 94.7 |
| Al-Nakkas et al. (241) | Nested PCR-EIA | Whole blood | 195/250 | 89 (45.6) | 98.9 | 100 | 100 | 99.2 |
| Vrioni et al. (257) | PCR-EIA | Whole blood + serum | 243/50c | 179 (73.7) | 99.2 | 100 | 100 | 96.2 |
| Queipo-Ortuño et al. (258) | Real-time PCR | Serum | 62/65 | 40 (64.5) | 91.9 | 95.4 | 95.0 | 92.5 |
| Debeaumont et al. (259) | Real-time PCR | Serum | 17/60 | 17 (100) | 64.7 | 100 | 100 | 90.9 |
| Surucouglu et al. (260) | Real-time PCRd | Serum | 50/30c | 18 (36.0) | 88.0 | 100 | 100 | 83.3 |
| Hasanjani Roushan et al. (270) | Conventional nested PCR | Whole blood | 50/30 | 5 (10.0) | 100 | 100 | 100 | 100 |
| Navarro et al. (271) | Q-RT-PCR | Whole blood | 18/30c | 16 (88.9) | 100 | 100 | 100 | 100 |
| Vrioni et al. (137) | Q-RT-PCR | Whole blood | 39/50c | 13 (33.3) | 100 | 100 | 100 | 100 |
| Queipo-Ortuño et al. (273) | Q-RT-PCR | Serum | 46/64 | 32 (69.6) | 95.7 | 92.2 | 89.8 | 96.7 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; EIA, enzyme immunoassay; Q-RT-PCR, quantitative real-time PCR.
When multiple targets or specimen types were tested in the study, the data in the table refer to the target and sample that had the best diagnostic efficiency.
All controls were healthy adults.
RoboGene Brucella detection kit (RoboScreen, GmbH, Leipzig, Germany).
Focal complications.
During the course of their disease, some 30% of brucellosis patients have a localized infection. Given that the development of focal complications is associated with a worse prognosis and the yield of blood cultures is much lower than in acute nonfocal infections, there is surprisingly little information on molecular diagnosis techniques in this clinical setting. Although some evaluations of the NAAT methods included a mixed population of patients with focal infections and individuals with acute noncomplicated disease, none of these studies analyzed separately the sensitivity of NAATs in peripheral blood samples in the two patient groups. Additionally, the available information on the diagnostic yield of PCR in specimens other than blood in patients with focal complications is even more scarce, limited in most reports to the mere description of single cases (229, 262).
The most relevant study in this context is one performed by Morata et al. (227). The authors compared the diagnostic capabilities of cultures and a conventional one-step in-house PCR assay in 34 nonblood samples from 32 patients with different focal complications (two had two concomitant foci of infection). The study specimens comprised 8 synovial fluid aspirates from patients with peripheral arthritis, exudates from 5 patients with hepatic or splenic abscesses, 5 urine samples from individuals with epididymo-orchitis, CSF specimens from 5 patients with meningitis or meningoencephalitis, 4 bone tissue samples from individuals with a variety of osteoarticular infections, and the remaining 6 from patients with miscellaneous focal diseases. The samples from the brucellosis patients were paired with an equal number of samples obtained from an identical body site but with a microbiologically proven different etiology. The sensitivity of the NAAT and that of the parallel culture in samples other than blood were 97.1% and 29.4%, respectively (227). Three-quarters of the patients who had negative blood cultures had been administered antimicrobial agents during the previous days. Interestingly, the NAAT detected two false positives in the control group (both were patients with vertebral osteomyelitis due to M. tuberculosis), lowering the NAAT specificity to 94.1%. As the high specificity of the primers B4 and B5, which are known to cross-react only with O. anthropi (263), is accepted, and after discarding the possibility of cross-contamination of the specimens, the authors attributed the seemingly false-positive results to a possible brucellar coinfection, as the patients were a shepherd and a farmer repeatedly exposed to Brucella organisms who also had a positive PCR test for Brucella in blood. Later, using a SYBR green I LightCycler-based real-time PCR assay, the same researchers published very satisfactory results for nonblood specimens from patients with brucellar genitourinary (264) and neurological (265) complications.
Li et al. have recently published excellent results with a real-time PCR assay on 31 formalin-fixed paraffin-embedded samples from patients with brucellar vertebral osteomyelitis who required surgical treatment because of neurological deficits (228). Although the study has some limitations, as no data were given on the specificity of the four primer pairs used, the culture results for the tissue samples studied, or the characteristics of the control group, the results nevertheless suggest that for those patients with a brucellar skeletal infection who had had a previous biopsy but for some reason no etiological diagnosis had been established, this PCR assay could be useful for determining the etiology of the disease a posteriori from paraffinized tissue samples.
Posttherapy follow-up and relapses.
Due to its ability to survive and even multiply within cells of the mononuclear-phagocytic system (266), infection by microorganisms of the genus Brucella has a high tendency to relapse, even with appropriate antimicrobial treatment (267, 268). The relapse rate in human brucellosis ranges between 4% and 30% and depends mainly on the efficacy of the administered treatment, its duration, the patient’s compliance, and the development of focal complications (29). Given that the yield of blood cultures in episodes of relapse is suboptimal and the serodiagnostic tests lack specificity in this clinical setting (28, 145), the molecular diagnosis has raised great expectations for the follow-up of patients with brucellosis after completion of antibiotic treatment, as well as for the early detection of relapses. This is clearly shown by the considerable number of studies aimed at exploring these aspects.
Morata et al. reported the results of the follow-up of a cohort of 30 adult patients diagnosed with brucellosis, of whom 22 (73.3%) had the disease confirmed by culture and the remaining 8 (26.7%) fulfilled clinical and serological criteria. After completion of antimicrobial therapy, the patients were followed by a conventional in-house NAAT, blood cultures, and a wide battery of serological tests, which were repeated at 2, 4, and 6 months, as well as at any time a relapse was suspected (269). On enrollment the NAAT was positive in 29 of the 30 patients (96.5%), whereas the blood cultures were positive in only 21 (66.7%). The NAAT became negative after concluding treatment in 28 (96.5%) of the 29 patients in whom it was initially positive. In the two patients who experienced a relapse of the disease 2 and 5 months after completion of therapy, the NAAT result became positive again, while the blood culture isolated brucellae in only one of them. In 4 other patients a clinical relapse was suspected but the molecular tests remained negative, and on follow-up either an alternative diagnosis was made or the possibility of reactivation of the disease was excluded after a prolonged clinical, microbiological, and serological follow-up (269). Only one patient (3.5%), who underwent surgery for vertebral osteomyelitis, had a positive PCR after adequate antibiotic combination therapy for 3 consecutive months, even though the clinical response was satisfactory.
These preliminary results were later confirmed in a larger study that included 200 patients, 17 of whom (8.5%) relapsed after treatment (205). Using the same 223-bp fragment of the bcsp31 gene as the target and the primers B4 and B5, the NAAT in whole blood was positive in all the patients at the time of the initial diagnosis, became negative in 183 of them (91.5%) at the end of treatment, and remained negative over the 12 months of follow-up. All those patients whose PCR assay remained positive at the end of therapy relapsed during the follow-up period. Of note was the fact that only 7 (41.2%) of the 17 patients who relapsed had positive blood cultures and only 4 (23.5%) showed increasing serological titers (205).
More recently, blood cultures, serodiagnostic tests, and a nested PCR assay targeting the IS711 insertion element were used to examine 50 patients with brucellosis at the time of diagnosis and 6 months after conclusion of treatment, as well as 30 controls (270). At the end of treatment, the PCR test became negative in 43 patients (86.0%); the clinical course was favorable in all of them, and the molecular assay remained repeatedly negative after 6 months. In the 7 patients who had a positive PCR test at the end of treatment, five (71.4%) experienced a clinical relapse and two remained asymptomatic throughout follow-up (270).
These studies seem to confirm that a small percentage of patients have persistent positive NAAT results after completing an apparently successful course of antimicrobial therapy, while others suffer a relapse after their molecular tests have become negative. In an attempt to clarify this conundrum, some researchers have studied the value of quantifying the bacterial load in the posttherapeutic phase of the disease as a possible way to predict effective treatment and therapeutic failure. The outcome of this attempt, however, was not as encouraging as expected, and inconsistent results were noted.
In the first of these studies, Navarro et al. developed a Q-RT-PCR assay with TaqMan probes that amplified a 251-bp region of IS711 specific for B. melitensis. They then studied its diagnostic capability at the time of the initial diagnosis and during posttreatment follow-up in a small sample of 18 patients with brucellosis as well as in 30 blood donors (271). The patients were enrolled at two medical facilities, were treated with a variety of antimicrobial regimens, and a few of them participated in a pilot study on doxycycline monotherapy. At the initial diagnosis, 16 patients (94.4%) had brucellae isolated from the blood, whereas the Q-RT-PCR performed on whole blood samples was positive in all the patients. Seven patients (39%) experienced one or more relapses after completion of treatment that were uniformly detected by the NAAT. Only three patients (42.9%) had a positive blood culture in the course of the relapse, and two (28.6%) exhibited increased SAT titers. No differences were seen in the bacterial load detected at the time of the initial diagnosis between the patients who experienced a relapse and those who remained asymptomatic throughout the follow-up period (1,171 ± 1,662 copies/ml [range, 7 to 4,528 copies/ml] versus 1,706 ± 1,872 copies/ml [range, 7 to 4,982 copies/ml], respectively). Nevertheless, the bacterial load was significantly higher in patients who had positive blood cultures during their relapses than in those with no demonstrable bacteremia: 2,540 ± 2,404 copies/ml (range, 113 to 4,920 copies/ml) versus 26 ± 41 copies/ml (range, 3 to 88 copies/ml), respectively (P = 0.03) (271). The most relevant point of this study was that at the end of follow-up, 7 of the 18 patients (38.9%), including 3 of the asymptomatic patients (27.3%) and 4 of those who relapsed (57.1%), were still positive by Q-RT-PCR, although they exhibited low bacterial loads.
Subsequently, and employing a similar study design, Vrioni et al. studied 39 patients with acute brucellosis and 50 healthy controls using Q-RT-PCR. Of the 39 patients, 13 had been diagnosed by positive blood cultures and the other 26 based on clinical and serological criteria. Blood samples were drawn from all the patients at the time of diagnosis, at the end of treatment, and after 2, 6, 12, and 24 months of follow-up (137). At diagnosis the Q-RT-PCR was positive in all 39 infected patients, with a mean bacterial load of 803 ± 1,236 copies/5 ml (range, 26 to 4,570 copies/5 ml), whereas all the controls had a negative test result. Thus, the sensitivity and specificity of the assay were 100%. After concluding treatment, the bacterial load fell slightly, and 87% of the patients continued having a positive NAAT. During follow-up, 3 patients (7.7%) had a relapse, with the blood culture becoming positive in only 1, and although the Q-RT-PCR remained positive, their bacterial load did not rise compared to the previous result. Notably, although all other patients remained asymptomatic during the posttreatment follow-up, the Q-RT-PCR remained positive in 79.4%, 76.9%, and 61.9% of the cases at 2, 6, 12 months, respectively (137).
Another study addressed the behavior of the bacterial load after finishing antimicrobial therapy. It included 35 patients with brucellosis diagnosed 2 to 33 years previously, of whom 17 were symptomatic (48.6%) and the remaining 18 (51.4%) had no signs of persistent disease (272). Repeated whole blood and serum samples were studied during a variable follow-up period. The authors reported that 28 (26.4%) of the 106 whole blood samples obtained at baseline from the 17 symptomatic patients had a positive Q-RT-PCR result. The NAAT was also positive in 5 of the 36 sera (13.8%) derived from the asymptomatic subjects. All the blood cultures drawn from both the symptomatic and the asymptomatic individuals were negative. Based on the NAAT results, 11 of the 17 (64.7%) symptomatic patients received at least one course of antibiotics, but only 2 of them (18.1%) experienced long-term improvement of the symptoms. In addition, 60% of the treated patients, though showing a low bacterial load, still had a positive Q-RT-PCR at the end of the follow-up period (272). The authors concluded that Q-RT-PCR is of little value for the follow-up of human brucellosis and suggested that in a considerable percentage of patients the persistence of viable Brucella organisms in nonidentifiable bacterial reservoirs would evolve to a chronic infection, while other patients, even if asymptomatic, would experience DNAemia over a prolonged period (137, 271, 272).
Despite being treated with adequate antibiotic regimens, a small percentage of patients are known to continue experiencing nonspecific symptoms that are difficult to interpret and to have no conclusive clinical or microbiological evidence of relapse (131). Attempting to examine in more detail the clinical meaning of the long-term persistence of DNAemia in treated patients with a favorable course and no evidence of relapse, Queipo-Ortuño et al. assessed whether a bacterial load cutoff can differentiate patients with active brucellosis from those with a cured past infection (273). The authors used a Q-RT-PCR assay with serum samples to study 46 consecutive brucellosis patients and a carefully chosen control group of 11 individuals with febrile syndromes initially suggestive of brucellosis but in whom a different etiology was later established, 36 asymptomatic subjects who had recovered from brucellosis during the previous 2 years, 12 members of families in which clusters of brucellosis were detected following the consumption of unpasteurized dairy products, and 5 subjects potentially exposed to Brucella organisms (3 veterinary surgeons and 2 abattoir workers) (273). The Q-RT-PCR was positive in 44 brucellosis patients (95.7%) and five controls (7.8%). At the time of diagnosis, 43 of the 44 (97.7%) infected patients had a bacterial load above 105 copies/ml, whereas none of the 4 controls with a recent history of brucellosis in which Q-RT-PCR was positive had a bacterial load of >4 × 103 copies/ml (273). Analysis of the cutoff points on the receiver operating characteristic (ROC) curve showed that a threshold of 5 × 103 copies/ml differentiated patients with active brucellosis from healthy individuals with a history of the infection; the area under the curve was 0.963. The Q-RT-PCR assay had a sensitivity of 93.5%, a specificity of 98.4%, a positive predictive value of 97.7%, a negative predictive value of 95.5%, and positive and negative likelihood ratios of 59.9 and 0.07, respectively. Thus, the Q-RT-PCR exhibited a much higher discriminatory power than cultures and serological titers in differentiating patients with active disease from those in whom the infection was probably successfully eradicated.
Using a similar methodology, though with different follow-up periods, other research groups have corroborated that the bacterial load in patients with acute brucellosis is generally higher than 5 × 103 copies/ml, falling progressively after administration of adequate antimicrobial therapy, provided that no treatment failures or clinical or bacteriological relapses occur (274, 275). Some individuals, and particularly those with repeated exposure to brucellae, continue to have a positive Q-RT-PCR on follow-up, but the measured bacterial loads are usually very low.
Combined analysis of these studies discloses methodological aspects that could explain the discrepancies in the bacterial loads observed in the posttreatment phase of human brucellosis. In the studies reporting high percentages of asymptomatic patients with a persistent bacterial load, the criteria for positivity of the test were more lax, as a Q-RT-PCR result was considered positive when a single one of three replicates was positive. The samples in the other studies, however, were processed in duplicate, and both replicates had to give a positive result to be considered positive. Moreover, no information was provided in these studies on professional or other potential contacts with Brucella organisms among asymptomatic individuals with a persistent bacterial load (137, 271, 272). In the study that included patients who were subjected to experimental treatments with a single antibiotic, the relapse rate was far higher than that usually found in clinical practice, demonstrating failure to eradicate the organism and confirming the inadequacy of monotherapy for human brucellosis (271). Finally, although the bacterial load of asymptomatic patients was generally found to be very low, no study used an ROC curve to find a threshold for the bacterial load that could indicate an association with active infection with a certain degree of certainty.
Further studies are needed to determine the true meaning of the transitory appearance of low-grade bacterial loads in asymptomatic patients with a history of disease (276). However, strict compliance with the guidelines for minimum information for the publication of quantitative real-time PCR experiments (MIQE) could result in greater homogeneity in the design of research studies and a reduction in interlaboratory variability (277).
Identification of Brucellae, Species Identification, and Typing
From a mere clinical perspective and considering only the patient’s health care, identification of the Brucella species causing the infection is not of interest (apart for exceptional cases), as the treatment of human brucellosis does not vary according to the specific etiological agent. In addition, the susceptibility of brucellae to the first-line antimicrobial agents tetracycline, aminoglycosides, and rifampin has remained stable at a low level for many years, and treatment failures and relapses are not usually caused by acquired antibiotic resistance of the infecting strain (278). In fact, routine performance of antibiotic susceptibility studies of Brucella isolates is strongly discouraged, as it involves a substantial hazard of exposure and contagion without obvious benefit. However, it is recommended that all isolated strains should be sent to a reference laboratory for complete identification to the species level of the recovered isolate and determination of its biovar (279). The reasons for this are many and include (though are not restricted to) the search for the zoonotic source of the infection, epidemiological investigation of outbreaks, monitoring the strains circulating in a particular geographic area and their dissemination over time, differentiation between wild-type isolates and vaccine strains, and the assessment of veterinary control programs. However, using conventional phenotypic methods to differentiate between the different Brucella species is a time-consuming and labor-intensive process demanding substantial manual work with living bacteria, thereby entailing an unacceptable risk of laboratory-acquired infection. In recent years, novel molecular methods that shorten the identification process and enable precise identification of Brucella isolates are increasingly replacing the traditional procedures for identification to the species level.
FISH technology.
A fluorescence in situ hybridization (FISH) test targeting a segment of the 16S rRNA gene and that facilitates rapid and specific detection of all human-pathogenic species of Brucella has been developed (280). This molecular assay was applied directly to positive blood culture broths from two patients with brucellosis and allowed for the rapid and accurate identification of B. melitensis at a low cost, and it was negative in blood cultures that grew a wide array of other bacterial pathogens. However, targeting the “universal” 16S rRNA gene can be misleading because of the low polymorphism of its sequence among members of the Brucellaceae family, and thus, brucellae cannot be accurately distinguished from organisms belonging to the closely related Ochrobactrum genus (281).
Nucleic acid amplification methods.
A novel recA gene-based, multiprimer, single-target PCR assay has recently been developed. It proved to be able to differentiate between members of the genus Brucella and Ochrobactrum anthropi or O. intermedium, although the test has a more prolonged timetable than the FISH method (282). A rapid multiplex PCR assay for differentiation between the principal Brucella species pathogenic for humans has been developed by Kumar et al. (283). In a preliminary study, the novel assay was directly applied to positive blood culture broth or to isolated colonies, and it successfully identified and differentiated B. abortus, B. melitensis, and B. suis in a single test and in less than 2 h.
Bricker and Halling developed the “AMOS PCR” test to identify and differentiate four terrestrial Brucella species, namely, B. abortus, B. melitensis, B. ovis, and B. suis (284). This test was later expanded to also include vaccine strains. Other research groups have also developed PCR-based NAAT assays for the rapid identification of the Brucella species most commonly involved in human infections in a single test (285), as well as marine species and the vaccine strains S19, RB51, and Rev1 (214). A multiplex NAAT developed by López-Goñi et al. was evaluated in seven European laboratories using a varied and wide panel of 625 Brucella strains. These included not only reference organisms but also wild-type isolates from various geographical areas and strains recovered from different animal species and human infections (215). The assay (Bruce ladder multiplex PCR) proved to be species specific and produced reproducible results in all the participating laboratories. Although the Bruce ladder multiplex PCR assay cannot differentiate between the species’ biovars, it proved to be simple, rapid, and safe (215).
In order to reduce cost and further simplify the typing procedure, SNPs characteristic of five terrestrial Brucella species were identified through whole-genome sequencing and used to construct a single-test-tube multiplex real-time PCR assay (286). The test was validated with a collection of 135 Brucella strains, distinguishing the five target organisms from other bacterial species within this panel. Although this multiplex PCR high-resolution melting technology has some limitations, such as the reduced number of targets that can be analyzed in a single reaction and the difficulty to use it directly on clinical specimens, it has the important advantages of being technically simple and rapid and entailing a very low risk of cross-contamination.
Conclusions
NAATs, in any format, are more sensitive than conventional cultures and more specific than the serological tests currently available for diagnosing both acute human Brucella infection and its focal complications. The favorable features of molecular assays, including their unequalled sensitivity, technical simplicity, speed, and safety, make them a true alternative to the conventional culture and serological methods.
Given the exquisite sensitivity of real-time PCR assays, a positive test may not necessarily imply an active infection but may be the mere detection of a minuscule bacterial inoculum in frequently exposed but healthy subjects, DNA from nonviable organisms, or remains of DNA present in circulating mononuclear cells in patients after a successful treatment course. Accordingly, interpretation of results achieved by NAATs should be carefully done, taking into consideration the clinical and epidemiological setting involved. Therefore, no solid evidence exists to justify prolonging medical treatment in asymptomatic patients who have a low bacterial DNA load in blood after concluding therapy. Although no well-defined criteria presently exist to establish with certainty the cure of human brucellosis, the quantification of the bacterial load by means of Q-RT-PCR holds promise to do so in the future. Likewise, multiplex real-time PCR assays are very useful for the identification and differentiation of Brucella species, replacing the traditional, laborious, and risky phenotypic methods.
Unfortunately, the number of commercial NAATs currently available for diagnosing human brucellosis is still limited. In addition, most of these tests have been evaluated in only a small number of patients, usually employing whole blood samples, and no extensive comparative studies of commercial kits and in-house PCR assays have been published (260, 287). As no sufficiently validated commercial tests are available, achieving consistent and reproducible results between laboratories would require standardization of the methodology, including the choice and conservation conditions of the specimen and the optimal sample volume, as well as the DNA extraction process, the molecular target selected, and the conditions of the amplification process.
Biographies

Pablo Yagupsky, M.D., is Professor Emeritus of Pediatrics and Microbiology at the Ben-Gurion University of the Negev and former Director of the Clinical Microbiology Laboratory of the Soroka University Medical Center, Beer-Sheva, Israel. Dr. Yagupsky received his M.D. from the University of Buenos Aires, Argentina, and then had a pediatric residency at the Soroka University Medical Center and a fellowship in medical and public health microbiology at the University of Rochester, Rochester, NY. Between 2007 and 2011, he served as Vice-Dean and Director of the Ben-Gurion University Medical School. Dr. Yagupsky has long-standing research interest in the epidemiology of human brucellosis and the detection of the disease in areas of endemicity, as well as the epidemiology, clinical presentation, and diagnosis of Kingella kingae infections in children.

Pilar Morata, Ph.D., received her Biological Sciences B.Sc and Ph.D degrees from the University of Granada, Spain. She is currently Professor of Biochemistry and Molecular Biology at the School of Medicine of the University of Malaga. She has been a member of the National Agency for the Evaluation of Quality and Accreditation (ANECA) and of the Spanish National Network for the study of Brucella infection. Her main expertise focuses on the development, optimization, and evaluation of molecular methods applicable to the diagnosis of infectious diseases. She has been the principal investigator of many research projects on the molecular diagnosis of brucellosis and tuberculosis.

Juan D. Colmenero, M.D., Ph.D., completed his undergraduate and postgraduate studies in medicine at the School of Medicine of the University of Granada, Spain. He completed his doctorate in Infectious Diseases at the University of Malaga in 1988. He has been Head of the Infectious Diseases Department of the University Regional Hospital of Malaga, Associate Professor of Infectious Diseases and director of the Intercenter Unit of Infectious Diseases, Clinical Microbiology and Preventive Medicine of the University Hospitals of Malaga, and a member of the Spanish National Network for the study of Brucella infection. He has studied the clinical, epidemiological, diagnostic, and therapeutic aspects of brucellosis for over 30 years.
REFERENCES
- 1.Whatmore AM, Koylass MS, Muchowski J, Edwards-Smallbone J, Gopaul KK, Perrett LL. 2016. Extended multilocus sequence analysis to describe the global population structure of the genus Brucella: phylogeography and relationship to biovars. Front Microbiol 7:2049. doi: 10.3389/fmicb.2016.02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Dahouk S, Scholz HC, Tomaso H, Bahn P, Göllner C, Karges W, Appel B, Hensel A, Neubauer H, Nöckler K. 2010. Differential phenotyping of Brucella species using a newly developed semi-automated metabolic system. BMC Microbiol 10:269. doi: 10.1186/1471-2180-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velasco J, Romero C, Lopez-Goni I, Leiva J, Diaz R, Moriyon I. 1998. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol 48:759–768. doi: 10.1099/00207713-48-3-759. [DOI] [PubMed] [Google Scholar]
- 4.Gee JE, De BK, Levett PN, Whitney AM, Novak RT, Popovic T. 2004. Use of 16S rRNA gene sequencing for rapid confirmatory identification of Brucella isolates. J Clin Microbiol 42:3649–3654. doi: 10.1128/JCM.42.8.3649-3654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholz HC, Al Dahouk S, Tomaso H, Neubauer H, Witte A, Schloter M, Kämpfer P, Falsen E, Pfeffer M, Engel M. 2008. Genetic diversity and phylogenetic relationships of bacteria belonging to the Ochrobactrum-Brucella group by recA and 16S rRNA gene-based comparative sequence analysis. Syst Appl Microbiol 31:1–16. doi: 10.1016/j.syapm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Bohlin J, Snipen L, Cloeckaert A, Lagesen K, Ussery D, Kristoffersen AB, Godfroid J. 2010. Genomic comparisons of Brucella spp. and closely related bacteria using base compositional and proteome based methods. BMC Evol Biol 10:249. doi: 10.1186/1471-2148-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesner O, Riesenberg K, Biliar N, Borstein E, Bouhnik L, Peled N, Yagupsky P. 2007. The many faces of human-to-human transmission of brucellosis: congenital infection and outbreak of nosocomial disease related to an unrecognized clinical case. Clin Infect Dis 45:e135–e140. doi: 10.1086/523726. [DOI] [PubMed] [Google Scholar]
- 8.Franco MP, Mulder M, Gilman RH, Smits HL. 2007. Human brucellosis. Lancet Infect Dis 7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. 1997. Fact sheet N173. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 10.de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. 2015. Pathogenesis and immunobiology of brucellosis. Review of brucellae-host interactions. Am J Pathol 185:1505–1517. doi: 10.1016/j.ajpath.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect Dis 6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 12.Shemesh AA, Yagupsky P. 2013. Increasing incidence of human brucellosis in Southern Israel after the cessation of a veterinarian control campaign. J Air Water Borne Dis 2:112. [Google Scholar]
- 13.Al Dahouk S, Nöckler K. 2011. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev Anti-Infect Ther 9:833–845. doi: 10.1586/eri.11.55. [DOI] [PubMed] [Google Scholar]
- 14.Godfroid J. 2018. Brucella spp. at the wildlife-livestock interface: an evolutionary trajectory through a livestock-to-wildlife “host jump”? Vet Sci 5:81. doi: 10.3390/vetsci5030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garofolo G, Fasanella A, Di Giannatale E, Platone I, Sacchini L, Persiani T, Boskani T, Rizzardi K, Wahab T. 2016. Cases of human brucellosis in Sweden linked to Middle East and Africa. BMC Res Notes 9:277. doi: 10.1186/s13104-016-2074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Brucellosis. N Engl J Med 352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 17.Corbel MJ. 1997. Brucellosis: an overview. Emerg Infect Dis 3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappas G, Panagopoulou P, Christou L, Akritidis N. 2006. Brucella as a biological weapon. Cell Mol Life Sci 63:2229–2236. doi: 10.1007/s00018-006-6311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang L, Liu J, Wang Y, Zhang H, Chen C. 2017. Evaluation of a hypervariable octameric oligonucleotide fingerprints assay for identification of and discrimination between wild-type and vaccine strains of Brucella melitensis. Am J Vet Res 78:495–499. doi: 10.2460/ajvr.78.4.495. [DOI] [PubMed] [Google Scholar]
- 20.Shemesh AA, Yagupsky P. 2011. Limitations of the standard agglutination test for detecting patients with Brucella melitensis bacteremia. Vector Borne Zoonotic Dis 11:1599–1601. doi: 10.1089/vbz.2011.0704. [DOI] [PubMed] [Google Scholar]
- 21.Yagupsky P. 1994. Detection of Brucella melitensis by BACTEC NR660 blood culture system. J Clin Microbiol 32:1889–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucero NE, Corazza R, Almuzara MN, Reynes E, Escobar GI, Boeri E, Ayala SM. 2010. Human Brucella canis outbreak linked to infection in dogs. Epidemiol Infect 138:280–285. doi: 10.1017/S0950268809990525. [DOI] [PubMed] [Google Scholar]
- 23.Luk S, To WK. 2010. Diagnostic challenges of human brucellosis in Hong Kong: a case series in two regional hospitals. Hong Kong Med J 16:299–303. [PubMed] [Google Scholar]
- 24.Yilmaz B, Ozdemir G, Aktas E, Komur B, Alfidan S, Memisoglu S, Duymuş TM. 2016. Brucellosis suspicion is the most important criterion for diagnosis particularly in endemic regions. Open Orthop J 29:10. doi: 10.2174/1874325001610010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappas G, Papadimitriou P. 2007. Challenges in Brucella bacteremia. Int J Antimicrob Agents 30(Suppl 1):S29–S31. doi: 10.1016/j.ijantimicag.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Ayaşlioğlu E, Kiliç D, Kaygusuz S, Kücük S, Ceken S, Erol O, Alpay Y. 2004. The detection of Brucella spp. by BACTEC 9050 blood culture system. Mikrobiyol Bul 38:415–419. [PubMed] [Google Scholar]
- 27.Mantur BG, Mulimani MS, Bidari LH, Akki AS, Tikare NV. 2008. Bacteremia is as unpredictable as clinical manifestations in human brucellosis. Int J Infect Dis 12:303–307. doi: 10.1016/j.ijid.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Ariza J, Corredoira J, Pallares R, Viladrich PF, Rufi G, Pujol M, Gudiol F. 1995. Characteristics of and risk factors for relapse of brucellosis in humans. Clin Infect Dis 20:1241–1249. doi: 10.1093/clinids/20.5.1241. [DOI] [PubMed] [Google Scholar]
- 29.Solera J, Martínez-Alfaro E, Espinosa A, Castillejos ML, Geijo P, Rodríguez-Zapata M. 1998. Multivariate model for predicting relapse in human brucellosis. J Infect 36:85–88. doi: 10.1016/s0163-4453(98)93342-4. [DOI] [PubMed] [Google Scholar]
- 30.Apa H, Devrim I, Memur S, Günay I, Gülfidan G, Celegen M, Bayram N, Karaarslan U, Bağ O, Işgüder R, Oztürk A, Inan S, Unal N. 2013. Factors affecting Brucella spp. blood cultures positivity in children. Vector Borne Zoonotic Dis 13:176–180. doi: 10.1089/vbz.2012.0997. [DOI] [PubMed] [Google Scholar]
- 31.Celebi G, Külah C, Kiliç S, Ustündağ G. 2007. Asymptomatic Brucella bacteraemia and isolation of Brucella melitensis biovar 3 from human breast milk. Scand J Infect Dis 39:205–208. doi: 10.1080/00365540600978898. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. 2006. Brucellosis in humans and animals. Diagnosis. WHO/CDS/EPR/2006.7 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 33.Moyer NP, Holcomb LA. 1995. Brucella, p 549–555. In Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (ed), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, DC. [Google Scholar]
- 34.Alton GG, Jones LM. 1967. Laboratory techniques in brucellosis, p 13–17. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 35.Raj A, Gautam V, Gupta PK, Sethi S, Rana S, Ray P. 2014. Rapid detection of Brucella by an automated blood culture system at a tertiary care hospital of north India. Indian J Med Res 139:776–778. [PMC free article] [PubMed] [Google Scholar]
- 36.Pathak AD, Dubal ZB, Doijad S, Raorane A, Rodrigues S, Naik R, Naik-Gaonkar S, Kalorey DR, Kurkure NV, Naik R, Barbuddhe SB. 2014. Human brucellosis among pyrexia of unknown origin cases and occupationally exposed individuals in Goa Region, India. Emerg Health Threats J 7:23846. doi: 10.3402/ehtj.v7.23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sisirak M, Hukić M. 2009. Evaluation and importance of selected microbiological methods in the diagnosis of human brucellosis. Bosn J Basic Med Sci 9:198–203. doi: 10.17305/bjbms.2009.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esel D, Doganay M, Alp E, Sumerkan B. 2003. Prospective evaluation of blood cultures in a Turkish university hospital: epidemiology, microbiology and patient outcome. Clin Microbiol Infect 9:1038–1044. doi: 10.1046/j.1469-0691.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz Castañeda M. 1947. A practical method for routine blood cultures in brucellosis. Proc Soc Exp Biol Med 64:114–115. doi: 10.3181/00379727-64-15717. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz Castañeda M. 1961. Laboratory diagnosis of brucellosis in man. Bull World Health Org 24:73–84. [PMC free article] [PubMed] [Google Scholar]
- 41.Akcam FZ, Yayli G, Uskun E, Kaya O, Demir C. 2006. Evaluation of the Bactec microbial detection system for culturing miscellaneous sterile body fluids. Res Microbiol 157:433–436. doi: 10.1016/j.resmic.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Mantur BG, Mulimani MS, Mangalagi SS, Patil AV. 2001. Brucellar epididymoorchitis—report of five cases. Indian J Med Microbiol 19:208–211. [PubMed] [Google Scholar]
- 43.Barua A, Kumar A, Thavaselvam D, Mangalgi S, Prakash A, Tiwari S, Arora S, Sathyaseelan K. 2016. Isolation & characterization of Brucella melitensis isolated from patients suspected for human brucellosis in India. Indian J Med Res 143:652–658. doi: 10.4103/0971-5916.187115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purwar S, Metgud SC, Mutnal MB, Nagamoti MB, Patil CS. 2016. Utility of serological tests in the era of molecular testing for diagnosis of human brucellosis in endemic area with limited resources. J Clin Diagn Res 10:DC26–DC29. doi: 10.7860/JCDR/2016/15525.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Álvarez-Ojeda MG, Saldaña-Fuentes C, Ballesteros-Elizondo MR, Martínez-Vázquez IO, López-Merino A, Briones Lara E, Morales-Loredo A. 2015. Comparison of the tests polymerase chain reaction, serology, and blood culture with respect to sensitivity and specificity for detection of Brucella spp. in human samples. Gac Med Mex 151:620–627. [PubMed] [Google Scholar]
- 46.Hekmatimoghaddam S, Sadeh M, Khalili MB, Mollaabedin M, Sazmand A. 2013. Comparison of PCR, Wright agglutination test and blood culture for diagnosis of brucellosis in suspected patients. Pak J Biol Sci 16:1589–1592. doi: 10.3923/pjbs.2013.1589.1592. [DOI] [PubMed] [Google Scholar]
- 47.Mantur B, Parande A, Amarnath S, Patil G, Walvekar R, Desai A, Parande M, Shinde R, Chandrashekar M, Patil S. 2010. ELISA versus conventional methods of diagnosing endemic brucellosis. Am J Trop Med Hyg 83:314–318. doi: 10.4269/ajtmh.2010.09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gotuzzo E, Carrillo C, Guerra J, Llosa L. 1986. An evaluation of diagnostic methods for brucellosis—the value of bone marrow culture. J Infect Dis 153:122–125. doi: 10.1093/infdis/153.1.122. [DOI] [PubMed] [Google Scholar]
- 49.Rodríguez-Torres A, Fermoso J, Landinez R. 1983. Brucelosis. Medicine (Spanish ed) 48:3126. [Google Scholar]
- 50.Fatolahzadeh B, Maleknejad P, Hejazi MJ, Pyri H. 2009. Development and evaluation of TUMS medium, a novel biphasic culture medium for isolation of Brucella spp. from patients. Iran J Microbiol 1:21–25. [Google Scholar]
- 51.Ruiz J, Lorente I, Perez J, Simarro E, Martínez-Campos L. 1997. Diagnosis of brucellosis by using blood cultures. J Clin Microbiol 35:2417–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young EJ, Borchert M, Frank L, Kretzer FL, Musher DM. 1985. Phagocytosis and killing of Brucella by human polymorphonuclear leukocytes. J Infect Dis 151:682–690. doi: 10.1093/infdis/151.4.682. [DOI] [PubMed] [Google Scholar]
- 53.Braun W, Kelsh J. 1954. Improved method for cultivation of Brucella from the blood. Proc Soc Exp Biol Med 85:154–155. doi: 10.3181/00379727-85-20815. [DOI] [PubMed] [Google Scholar]
- 54.Etemadi H, Raissadat A, Pickett MJ, Zafari Y, Vahedifar P. 1984. Isolation of Brucella spp. from clinical specimens. J Clin Microbiol 64:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolman S, Maayan MC, Gotesman G, Rozenszajn LA, Wolach B, Lang R. 1991. Comparison of the BACTEC and lysis concentration methods for recovery of Brucella species from clinical specimens. Eur J Clin Microbiol Infect Dis 10:647–648. doi: 10.1007/bf01975817. [DOI] [PubMed] [Google Scholar]
- 56.Mantur BG, Mangalgi SS. 2004. Evaluation of conventional Castañeda and lysis centrifugation blood culture techniques for diagnosis of human brucellosis. J Clin Microbiol 42:4327–4328. doi: 10.1128/JCM.42.9.4327-4328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangalgi S, Sajjan A. 2014. Comparison of three blood culture techniques in the diagnosis of human brucellosis. J Lab Physicians 6:14–17. doi: 10.4103/0974-2727.129084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Espinosa BJ, Chacaltana J, Mulder M, Franco MP, Blazes DL, Gilman RH, Smits HL, Hall ER. 2009. Comparison of culture techniques at different stages of brucellosis. Am J Trop Med Hyg 80:625–627. doi: 10.4269/ajtmh.2009.80.625. [DOI] [PubMed] [Google Scholar]
- 59.Navas E, Guerrero A, Cobo J, Loza E. 1993. Faster isolation of Brucella spp. from blood by Isolator compared with BACTEC NR. Diagn Microbiol Infect Dis 16:79–81. doi: 10.1016/0732-8893(93)90135-t. [DOI] [PubMed] [Google Scholar]
- 60.Yagupsky P. 2004. Use of the MYCO/F LYTIC medium for detection of Brucella melitensis bacteremia. J Clin Microbiol 42:2207–2208. doi: 10.1128/jcm.42.5.2207-2208.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Escamilla J, Florez-Ugarte H, Kilpatrick ME. 1986. Evaluation of blood clot cultures for isolation of Salmonella typhi, Salmonella paratyphi-A, and Brucella melitensis. J Clin Microbiol 24:388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantur BG, Bidari LH, Akki AS, Mulimani MS, Tikare NV. 2007. Diagnostic yield of blood clot culture in the accurate diagnosis of enteric fever and human brucellosis. Clin Lab 53:57–61. [PubMed] [Google Scholar]
- 63.Yagupsky P, Peled N, Press J, Abramson O, Abu-Rashid M. 1997. Comparison of BACTEC 9240 Peds Plus medium and Isolator 1.5 Microbial Tube for detection of Brucella melitensis from blood cultures. J Clin Microbiol 35:1382–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCabe WR, Jackson GG. 1962. Gram negative bacteremia. Arch Intern Med 110:856–864. doi: 10.1001/archinte.1962.03620240038007. [DOI] [Google Scholar]
- 65.Gamazo C, Vitas AI, López-Goñi I, Díaz R, Moriyón I. 1993. Factors affecting detection of Brucella melitensis by BACTEC NR730, a nonradiometric system for hemocultures. J Clin Microbiol 31:3200–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solomon HM, Jackson D. 1992. Rapid diagnosis of Brucella melitensis in blood: some operational characteristics of the BACT/ALERT. J Clin Microbiol 30:222–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zimmerman SJ, Gillikin S, Sofat N, Bartholomew WR, Amsterdam D. 1990. Case report and seeded blood culture study of Brucella bacteremia. J Clin Microbiol 28:2139–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baron EJ, Weinstein MP, Dunne WM Jr, Yagupsky P, Welch DF, Wilson DM. 2005. Cumitech 1C. Blood cultures IV. Coordinating ed, Baron EJ. ASM Press, Washington, DC. [Google Scholar]
- 69.Bannatyne RM, Jackson MC, Memish Z. 1997. Rapid diagnosis of Brucella bacteremia by using the BACTEC 9240 system. J Clin Microbiol 35:2673–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Craven DE, Lichtenberg DA, Browne KF, Coffey DM, Treadwell TL, McCabe WR. 1984. Pseudobacteremia traced to cross-contamination by an automated blood culture analyzer. Infect Control 5:75–78. doi: 10.1017/S0195941700058987. [DOI] [PubMed] [Google Scholar]
- 71.Arnow PM, Smaron M, Ormiste V. 1984. Brucellosis in a group of travelers to Spain. JAMA 251:505–507. doi: 10.1001/jama.1984.03340280055029. [DOI] [PubMed] [Google Scholar]
- 72.Menard RR, Choudhury SP, Naini MG, Wilk JT. 1989. A case of brucellosis: the importance of physician/laboratory communication. Clin Microbiol Newsl 11:157–158. doi: 10.1016/0196-4399(89)90025-1. [DOI] [Google Scholar]
- 73.Serrano ML, Llosa J, Castells C, Mendoza J, Navarro JM, de la Rosa M. 1987. Detección radiométrica de bacteriemias por Brucella. Enferm Infec Microbiol Clin (Spain) 5:139–142. [Google Scholar]
- 74.García Rodríguez JA, García Sánchez JE, Muñoz Bellido JL, Canut-Blasco A. 1988. Comparison of a biphasic system and non-radiometric system for blood culture. Eur J Clin Microbiol Infect Dis 7:666–668. doi: 10.1007/BF01964249. [DOI] [PubMed] [Google Scholar]
- 75.Gedikoğlu S, Helvaci S, Ozakin C, Gökirmak F, Kiliçturgay K. 1996. Detection of Brucella melitensis by BACTEC NR 730 and BACTEC 9120 systems. Eur J Epidemiol 12:649–650. doi: 10.1007/bf00499467. [DOI] [PubMed] [Google Scholar]
- 76.Yagupsky P, Peled N, Press J, Abu-Rashid M, Abramson O. 1997. Rapid detection of Brucella melitensis from blood cultures by a commercial system. Eur J Clin Microbiol Infect Dis 16:605–607. doi: 10.1007/bf02447926. [DOI] [PubMed] [Google Scholar]
- 77.Sagi M, Nesher L, Yagupsky P. 2017. The Bactec FX blood culture system detects Brucella melitensis bacteremia in adult patients within the routine 1-week incubationperiod. J Clin Microbiol 55:942–946. doi: 10.1128/JCM.02320-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Attas RA, Al-Khalifa M, Al-Qurashi AR, Badawy M, Al-Gualy N. 2000. Evaluation of PCR, culture and serology for the diagnosis of acute human brucellosis. Ann Saudi Med 20:224–228. doi: 10.5144/0256-4947.2000.224. [DOI] [PubMed] [Google Scholar]
- 79.Roiz MP, Peralta FG, Valle R, Arjona R. 1998. Microbiological diagnosis of brucellosis. J Clin Microbiol 36:1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casas J, Partal Y, Llosa J, Leiva J, Navarro JM, de la Rosa M. 1994. Detección de Brucella por un sistema automático de hemocultivos: Bact/ALERT. Enferm Infec Microbiol Clin 12:497–500. [PubMed] [Google Scholar]
- 81.Durmaz G, Us T, Aydinli A, Kiremitci A, Kiraz N, Akgun Y. 2003. Optimum detection times for bacteria and yeast species with the BACTEC 9120 aerobic blood culture system: evaluation for a 5-year period in a Turkish university hospital. J Clin Microbiol 41:819–821. doi: 10.1128/JCM.41.2.819-821.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurtoglu MG, Bozkurt H, Tuncer O, Kesli R, Berktas M. 2008. Distribution, optimum detection time and antibiotic susceptibility rates of the microorganisms isolated from blood cultures over a 4-year time period in a Turkish university hospital and a review of the international literature. J Int Med Res 36:1261–1272. doi: 10.1177/147323000803600613. [DOI] [PubMed] [Google Scholar]
- 83.Lepe JA, Guerrero FJ, Garrido A, Perea R. 2001. Deteccion de Brucella melitensis por el sistema BACTEC 9050. Enferm Infecc Microbiol Clin 19:267–269. doi: 10.1016/S0213-005X(01)72633-X. [DOI] [PubMed] [Google Scholar]
- 84.Giménez M, Prat C, Vallés X, Matas L, Arnal J, Ausina V. 2002. Evaluation of the VITAL (bioMérieux) automated blood culture system using blind subculture. Clin Microbiol Infect 8:222–228. doi: 10.1046/j.1469-0691.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 85.Melo-Cristino J, Salgado MJ. 1999. Detection of Brucella bacteremia by using the VITAL automated system and trypticase broth culture. Clin Microbiol Infect 5:706–709. doi: 10.1111/j.1469-0691.1999.tb00519.x. [DOI] [Google Scholar]
- 86.Baysallar M, Aydogan H, Kilic A, Kucukkaraaslan A, Doganci L. 2006. Evaluation of the BacT/ALERT and BACTEC 9240 automated blood culture systems for growth time of Brucella species in a Turkish tertiary hospital. Med Sci Monitor 12:235–238. [PubMed] [Google Scholar]
- 87.Ganado W, Bannister W. 1960. Bacteremia in human brucellosis. Br Med J 1:601–603. doi: 10.1136/bmj.1.5173.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Young EJ. 1995. An overview of human brucellosis. Clin Infect Dis 21:283–290. doi: 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Yan Y, Wu F, Su G, Li S, Yuan X, Lai J, Zhou Z. 2018. Sixteen Chinese pediatric brucellosis patients onset of fever in non-epidemic areas and 8 developed with osteoarticular involvement. Clin Rheumatol 37:145–149. doi: 10.1007/s10067-017-3819-y. [DOI] [PubMed] [Google Scholar]
- 90.Cohen FB, Robins B, Lipstein W. 1957. Isolation of Brucella abortus by percutaneous liver biopsy. N Engl J Med 257:228–230. doi: 10.1056/NEJM195708012570508. [DOI] [PubMed] [Google Scholar]
- 91.Fogel R, Lewis S. 1960. Diagnosis of Brucella melitensis by percutaneous needle biopsy of the liver. Ann Intern Med 53:204–206. doi: 10.7326/0003-4819-53-1-204. [DOI] [PubMed] [Google Scholar]
- 92.Poston MA, Parsons PB. 1940. Isolation of Brucella from lymph nodes. J Infect Dis 66:86–90. doi: 10.1093/infdis/66.1.86. [DOI] [Google Scholar]
- 93.Özkurt Z, Erol S, Tasyaran MA, Kaya A. 2002. Detection of Brucella melitensis by the BacT/ALERT automated system and Brucella broth culture. Clin Microbiol Infect 8:749–752. doi: 10.1046/j.1469-0691.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- 94.Özturk R, Mert A, Kocak F, Ozaras R, Koksal F, Tabak F, Bilir M, Aktuglu Y. 2002. The diagnosis of brucellosis by use of the BACTEC 9240 culture system. Diagn Microbiol Infect Dis 44:133–135. doi: 10.1016/s0732-8893(02)00428-5. [DOI] [PubMed] [Google Scholar]
- 95.Işeri S, Bulut C, Yetkin MA, Kinikli S, Demiröz AP, Tülek N. 2006. Comparison of the diagnostic value of blood and bone marrow cultures in brucellosis. Mikrobiyol Bul 40:201–206. [PubMed] [Google Scholar]
- 96.Magill GB, Killough JH, Said SI. 1954. Cortisone and combined antibiotic therapy of acute brucellosis melitensis. Am J Med 16:810–817. doi: 10.1016/0002-9343(54)90445-3. [DOI] [PubMed] [Google Scholar]
- 97.Shehabi A, Shakir K, el-Khateeb M, Qubain H, Fararjeh N, Shamat AR. 1990. Diagnosis and treatment of 106 cases of human brucellosis. J Infect 20:5–10. doi: 10.1016/s0163-4453(90)92214-6. [DOI] [PubMed] [Google Scholar]
- 98.Young EJ. 1995. Brucellosis: current epidemiology, diagnosis, and management. Curr Clin Topics Infect Dis 15:115–128. [PubMed] [Google Scholar]
- 99.De Miguel MJ, Marín CM, Muñoz PM, Dieste L, Grilló MJ, Blasco JM. 2011. Development of a selective culture medium for primary isolation of the main Brucella species. J Clin Microbiol 49:1458–1463. doi: 10.1128/JCM.02301-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yagupsky P. 2014. Brucellae growing on Thayer-Martin medium: a source of inadvertent exposure for laboratory personnel in endemic areas. J Med Microbiol 63:148–149. doi: 10.1099/jmm.0.064121-0. [DOI] [PubMed] [Google Scholar]
- 101.Yagupsky P, Peled N, Press J. 2001. Use of the BACTEC 9240 blood culture system for detection of Brucella melitensis in synovial fluid. J Clin Microbiol 39:738–739. doi: 10.1128/JCM.39.2.738-739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yagupsky P, Peled N. 2002. Use of the Isolator 1.5 microbial tube for detection of Brucella melitensis in synovial fluid. J Clin Microbiol 40:3878. doi: 10.1128/jcm.40.10.3878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elsaghir AA, James EA. 2003. Misidentification of Brucella melitensis as Ochrobactrum anthropi by API 20NE. J Med Microbiol 52:441–442. doi: 10.1099/jmm.0.05153-0. [DOI] [PubMed] [Google Scholar]
- 104.Vila A, Pagella H, Vera Bello G, Vicente A. 2016. Brucella suis bacteremia misidentified as Ochrobactrum anthropi by the VITEK 2 system. J Infect Dev Ctries 10:432–436. doi: 10.3855/jidc.7532. [DOI] [PubMed] [Google Scholar]
- 105.Hubálek Z, Scholz HC, Sedlácek I, Melzer F, Sanogo YO, Nesvadbová J. 2007. Brucellosis of the common vole (Microtus arvalis). Vector Borne Zoonotic Dis 7:679–687. doi: 10.1089/vbz.2007.0143. [DOI] [PubMed] [Google Scholar]
- 106.Castrodale LJ, Raczniak GA, Rudolph KM, Chikoyak L, Cox RS, Franklin TL, Traxler RM, Guerra M. 2015. A case-study of implementation of improved strategies for prevention of laboratory-acquired brucellosis. Saf Health Work 6:353–356. doi: 10.1016/j.shaw.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dash N, Al-Zarouni M, Rattan A, Panigrahi D. 2012. Misidentification of Brucella melitensis as Bergeylla zoohelcum by Microscan WalkAway: a case report. Med Princ Pract 21:495–497. doi: 10.1159/000338391. [DOI] [PubMed] [Google Scholar]
- 108.Yang J, Ren X-q, Chu M-l, Meng D-y, Xue W-c. 2013. Mistaken identity of Brucella Infection. J Clin Microbiol 51:2011. doi: 10.1128/JCM.03222-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Public Health Laboratory Service. 1991. Microbiological test strip (API20NE) identifies Brucella melitensis as Moraxella phenylpyruvica. Commun Dis Rep 1:165. [PubMed] [Google Scholar]
- 110.Batchelor BI, Brindle RJ, Gilks GF, Selkon JB. 1992. Biochemical mis-identification of Brucella melitensis and subsequent laboratory acquired infections. J Hosp Infect 22:159–162. doi: 10.1016/0195-6701(92)90100-Z. [DOI] [PubMed] [Google Scholar]
- 111.Rich M, Bannatyne RA, Memish ZA. 2000. Direct urease test on BACTEC blood cultures. Early presumptive diagnosis of brucellosis in an area of endemicity. J Clin Microbiol 38:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maleknejad P, Hashemi FB, Fatollahzadeh B, Jafari S, Peeri Dogaheh H. 2005. Direct urease test and acridine orange staining on BACTEC blood culture for rapid presumptive diagnosis of brucellosis. Iranian J Public Health 34:52–55. [Google Scholar]
- 113.Wong JD, Janda JM, Duffey PS. 1992. Preliminary studies on the use of carbon substrate utilization patterns for identification of Brucella species. Diagn Microbiol Infect Dis 15:109–113. doi: 10.1016/0732-8893(92)90032-o. [DOI] [PubMed] [Google Scholar]
- 114.Scholz HC, Nöckler K, Göllner C, Bahn P, Vergnaud G, Tomaso H, Al Dahouk S, Kämpfer P, Cloeckaert A, Maquart M, Zygmunt MS, Whatmore AM, Pfeffer M, Huber B, Busse HJ, De BK. 2010. Brucella inopinata sp. nov., isolated from a breast implant infection. Int J Syst Evol Microbiol 60:801–808. doi: 10.1099/ijs.0.011148-0. [DOI] [PubMed] [Google Scholar]
- 115.Lista F, Reubsaet FAG, De Santis R, Parchen RR, de Jong AL, Kieboom J, van der Laaken AL, Voskamp-Visser IA, Fillo S, Jansen HJ, Van der Plas J, Paauw A. 2011. Reliable identification at the species level of Brucella isolated with MALDI-TOF. BMC Microbiol 11:267. doi: 10.1186/1471-2180-11-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karger A, Melzer F, Timke M, Bettin B, Kostrzewa M, Nockler K, Hohmann A, Tomaso H, Neubauer H, Al Dahouk S. 2013. Interlaboratory comparison of intact-cell matrix-assisted laser desorption ionization-time of flight mass spectrometry results for identification and differentiation of Brucella spp. J Clin Microbiol 51:3123–3126. doi: 10.1128/JCM.01720-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferreira L, Vega Castaño S, Sánchez-Juanes F, González-Cabrero S, Menegotto F, Orduña-Domingo A, González-Buitrago JM, Muñoz-Bellido JL. 2010. Identification of Brucella by MALDI-TOF mass spectrometry. Fast and reliable identification from agar plates and blood cultures. PLoS One 5:e14235. doi: 10.1371/journal.pone.0014235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Drevinek M, Dresler J, Klimentova J, Pisa L, Hubalek M. 2012. Evaluation of sample preparation methods for MALSI-TOF MS identification of highly dangerous bacteria. Lett Appl Microbiol 55:40–46. doi: 10.1111/j.1472-765X.2012.03255.x. [DOI] [PubMed] [Google Scholar]
- 119.Marklein G, Josten M, Klanke U, Müller E, Horré R, Maier T, Wenzel T, Kostrzewa M, Bierbaum G, Hoerauf A, Sahl HG. 2009. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J Clin Microbiol 47:2912–2917. doi: 10.1128/JCM.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sali M, De Maio F, Tarantino M, Garofolo G, Tittarelli M, Sacchini L, Zilli K, Pasquali P, Petrucci P, Marianelli C, Francia M, Sanguinetti M, Adone R. 2018. Rapid and safe one-step extraction method for the identification of Brucella strains at genus and species level by MALDI-TOF mass spectrometry. PLoS One 13:e0197864. doi: 10.1371/journal.pone.0197864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poonawala H, Marrs Conner T, Peaper DR. 2018. The brief case: misidentification of Brucella melitensis as Ochrobactrum anthropi by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol 56:e00914-17. doi: 10.1128/JCM.00914-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mesureur J, Arend S, Cellière B, Courault P, Cotte-Pattat P-J, Totty H, Deol P, Mick V, Girard V, Touchberry J, Burrowes V, Lavigne J-P, O’Callaghan D, Monnin V, Keriel A. 2018. A MALDI-TOF MS database with broad genus coverage for species-level identification of Brucella. PLoS Negl Trop Dis 12:e0006874. doi: 10.1371/journal.pntd.0006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yagupsky P, Baron EJ. 2005. Laboratory-exposures to brucellae and implications for bioterrorism. Emerg Infect Dis 11:1180–1185. doi: 10.3201/eid1108.041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doganay M, Aygen B. 2003. Human brucellosis: an overview. Int J Infect Dis 7:173–182. doi: 10.1016/S1201-9712(03)90049-X. [DOI] [Google Scholar]
- 125.Noviello S, Gallo R, Kelly M, Limberger RJ, DeAngelis K, Cain L, Wallace B, Dumas N. 2004. Laboratory-acquired bucellosis. Emerg Infect Dis 10:1848–1850. doi: 10.3201/eid1010.040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ergönül O, Celikbaş A, Tezeren D, Güvener E, Dokuzoğuz B. 2004. Analysis of risk factors for laboratory-acquired Brucella infections. J Hosp Infect 56:223–227. doi: 10.1016/j.jhin.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 127.Yagupsky P, Peled N, Riesenberg K, Banai M. 2000. Exposure of hospital personnel to Brucella melitensis and occurrence of laboratory-acquired disease in an endemic area. Scand J Infect Dis 32:31–35. [DOI] [PubMed] [Google Scholar]
- 128.Shemesh AA, Yagupsky P. 2012. Isolation rates of Brucella melitensis in an endemic area and implications for laboratory safety. Eur J Clin Microbiol Infect Dis 31:441–443. doi: 10.1007/s10096-011-1325-8. [DOI] [PubMed] [Google Scholar]
- 129.Centers for Disease Control, National Institutes of Health. 1988. Biosafety in microbiological and biomedical laboratories. Publication no. 17-40-508-3. U.S. Government Printing Office, Washington, DC. [Google Scholar]
- 130.Ruiz-Mesa JD, Sánchez-Gonzalez J, Reguera JM, Martín L, Lopez-Palmero S, Colmenero JD. 2005. Rose Bengal test: diagnostic yield and use for the rapid diagnosis of human brucellosis in emergency departments in endemic areas. Clin Microbiol Infect 2005 11:221–225. doi: 10.1111/j.1469-0691.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- 131.Young EJ. 1991. Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination test and review of the literature. Rev Infect Dis 13:359–372. doi: 10.1093/clinids/13.3.359. [DOI] [PubMed] [Google Scholar]
- 132.Ariza J, Pellicer T, Pallares R, Foz A, Gudiol F. 1992. Specific antibody profile in human brucellosis. Clin Infect Dis 14:131–140. doi: 10.1093/clinids/14.1.131. [DOI] [PubMed] [Google Scholar]
- 133.Abo-Shehada MN, Odeh JS, Abu-Essud M, Abuharfeil N. 1996. Seroprevalence of brucellosis among high risk people in northern Jordan. Int J Epidemiol 25:450–454. doi: 10.1093/ije/25.2.450. [DOI] [PubMed] [Google Scholar]
- 134.Kumar P, Singh DK, Barbuddhe SB. 1997. Sero-prevalence of brucellosis among abattoir personnel of Delhi. J Commun Dis 29:131–137. [PubMed] [Google Scholar]
- 135.Eldin C, Parola P, Raoult D. 2018. Limitations of diagnostic tests for bacterial infections. Med Mal Infect doi: 10.1016/j.medmal.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 136.Kaden R, Ferrari S, Alm E, Wahab T. 2017. A novel real-time PCR assay for specific detection of Brucella melitensis. BMC Infect Dis 17:230. doi: 10.1186/s12879-017-2327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vrioni G, Pappas G, Priavali E, Gartzonika C, Levidiotou S. 2008. An eternal microbe: Brucella DNA load persists for years after clinical cure. Clin Infect Dis 46:e131–e136. doi: 10.1086/588482. [DOI] [PubMed] [Google Scholar]
- 138.Bundle DR, Cherwonogrodzky JW, Caroff M, Perry MB. 1987. The lipopolysaccharides of Brucella abortus and B. melitensis. Ann Inst Pasteur Microbiol 138:92–101. doi: 10.1016/0769-2609(87)90083-4. [DOI] [PubMed] [Google Scholar]
- 139.Nielsen K. 2002. Diagnosis of brucellosis by serology. Vet Microbiol 90:447–459. doi: 10.1016/s0378-1135(02)00229-8. [DOI] [PubMed] [Google Scholar]
- 140.Polt SS, Dismukes WE, Flint A, Schaefer J. 1982. Human brucellosis caused by Brucella canis: clinical features and immune response. Ann Intern Med 97:717–719. doi: 10.7326/0003-4819-97-5-717. [DOI] [PubMed] [Google Scholar]
- 141.Lamb VL, Jones LM, Schurig GG, Berman DT. 1979. Enzyme-linked immunosorbent assay for bovine immunoglobulin subclass-specific response to Brucella abortus lipopolysaccharides. Infect Immun 26:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baldi PC, Giambartolomei GH, Wallach JC, Velikovsky CA, Fossati CA. 2001. Limited diagnostic usefulness of antibodies to cytoplasmic proteins of Brucella in early-treated human brucellosis. Scand J Infect Dis 33:200–205. doi: 10.1080/00365540151060842. [DOI] [PubMed] [Google Scholar]
- 143.Casanova A, Ariza J, Rubio M, Masuet C, Díaz R. 2009. Brucellacapt versus classical tests in the serological diagnosis and management of human brucellosis. Clin Vaccine Immunol 16:844–851. doi: 10.1128/CVI.00348-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bosilkovski M, Katerina S, Zaklina S, Ivan V. 2010. The role of Brucellacapt test for follow-up patients with brucellosis. Comp Immunol Microbiol Infect Dis 33:435–442. doi: 10.1016/j.cimid.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 145.Pellicer T, Ariza J, Foz A, Pallares R, Gudiol F. 1988. Specific antibodies detected during relapse of human brucellosis. J Infect Dis 157:918–924. doi: 10.1093/infdis/157.5.918. [DOI] [PubMed] [Google Scholar]
- 146.Araj GF, Lulu AR, Saadah MA, Mousa AM, Strannegard IL, Shakir RA. 1986. Rapid diagnosis of central nervous system brucellosis by ELISA. J Neuroimmunol 12:173–182. doi: 10.1016/s0165-5728(86)80001-7. [DOI] [PubMed] [Google Scholar]
- 147.Araj GF, Lulu AR, Khateeb MI, Saadah MA, Shakir RA. 1988. ELISA versus routine tests in the diagnosis of patients with systemic and neurobrucellosis. APMIS 96:171–176. doi: 10.1111/j.1699-0463.1988.tb05286.x. [DOI] [PubMed] [Google Scholar]
- 148.Harris HJ. 1949. Chronic brucellosis. The unsatisfactory status of current diagnostic methods. Am J Public Health Nations Health 39:870–874. doi: 10.2105/ajph.39.7.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Skurski A. 1958. Mechanism of the opsonocytophagic test in brucellosis. Schweiz Z Pathol Bakteriol 21:17–31. [DOI] [PubMed] [Google Scholar]
- 150.Versilova PA, Cernyseva MI, Aslanjan RG, Knjazeva EN. 1974. Diagnosis of human and animal brucellosis by the indirect haemagglutination test. Bull World Health Organ 51:191–197. [PMC free article] [PubMed] [Google Scholar]
- 151.Díaz R, Casanova A, Ariza J, Moriyón I. 2011. The Rose Bengal Test in human brucellosis: a neglected test for the diagnosis of a neglected disease. PLoS Negl Trop Dis 5:e950. doi: 10.1371/journal.pntd.0000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Roushan MR, Amiri MJ, Laly A, Mostafazadeh A, Bijani A. 2010. Follow-up standard agglutination and 2-mercaptoethanol tests in 175 clinically cured cases of human brucellosis. Int J Infect Dis 14:e250–e253. doi: 10.1016/j.ijid.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 153.Park SH, Lee YH, Chu H, Hwang SD, Hwang KJ, Choi HY, Park MY. 2012. Application of the microagglutination test for serologic diagnosis of human brucellosis. Osong Public Health Res Perspect 3:19–23. doi: 10.1016/j.phrp.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buchanan TM, Faber LV. 1980. 2-Mercaptoethanol Brucella agglutination test: usefulness for predicting recovery from brucellosis. J Clin Microbiol 11:691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Klein GC, Behan KA. 1981. Determination of Brucella immunoglobulin G agglutinating antibody titer by dithiothreitol. J Clin Microbiol 14:24–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moyer NP, Holcomb LA. 2012. Brucellosis, p 143–154. In Ballows A, Hausler WJ, Jr, Ohashi M, Turano A (ed), Laboratory diagnosis of infectious diseases: principles and practice. Springer Verlag, New York, NY. [Google Scholar]
- 157.Borsa BA, Aldag ME, Yilmaz M, Dalar ZG, Ozalp VC. 2016. Comparison of a novel test (ODAK Brucella Coombs Gel Test) with commonly used serological tests in human brucellosis. Clin Lab 62:1671–1674. doi: 10.7754/Clin.Lab.2016.160120. [DOI] [PubMed] [Google Scholar]
- 158.Hanci H, Igan H, Uyanik MH. 2017. Evaluation of a new and rapid serologic test for detecting brucellosis: Brucella Coombs gel test. Pak J Biol Sci 20:108–112. doi: 10.3923/pjbs.2017.108.112. [DOI] [PubMed] [Google Scholar]
- 159.İrvem A, Yücel FM, Aksaray S, Bor E. 2015. Comparison of a new and rapid method, Brucella Coombs gel test with the other methods in the serological diagnosis of brucellosis. Mikrobiyol Bul 49:181–187. doi: 10.5578/mb.8881. [DOI] [PubMed] [Google Scholar]
- 160.Gupte S, Kaur T. 2015. Diagnosis of human brucellosis. J Trop Dis 4:185. doi: 10.4172/2329-891X.1000185. [DOI] [Google Scholar]
- 161.Farrell ID, Hinchliffe PM, Robertson L. 1975. The use of the conglutinating complement fixation test in the diagnosis of human brucellosis. J Hyg 74:29–33. doi: 10.1017/S0022172400046684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Orduña A, Almaraz A, Prado A, Gutierrez MP, Garcia-Pascual A, Dueñas A, Cuervo M, Abad R, Hernández B, Lorenzo B, Bratos MA, Torres AR. 2000. Evaluation of an immunocapture-agglutination test (Brucellacapt) for serodiagnosis of human brucellosis. J Clin Microbiol 38:4000–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pabuccuoglu O, Ecemis T, El S, Coskun A, Akcali S, Sanlidag T. 2011. Evaluation of serological tests for diagnoisis of brucellosis. Jpn J Infect Dis 64:272–276. [PubMed] [Google Scholar]
- 164.Mantecón MA, Gutiérrez MP, Zarzosa MP, Fernández-Lago L, Colmenero JD, Vizcaíno N, Bratos MA, Almaraz A, Cubero A, Muñoz MF, Rodríguez Torres A, Orduña A. 2008. Influence of brucellosis history on serological diagnosis and evolution of patients with acute brucellosis. J Infect 57:397–403. doi: 10.1016/j.jinf.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 165.Kutl SS, Celikbas A, Ergönül O, Kutlu M, Aksaray S, Güvener E, Dokuzoğuz B. 2003. The value of the immunoglobulin G avidity test for the serologic diagnosis of brucellosis. Mikrobiyol Bul 37:261–267. [PubMed] [Google Scholar]
- 166.McGiven JA, Thompson IJ, Commander NJ, Stack JA. 2009. Time-resolved fluorescent resonance energy transfer assay for simple and rapid detection of anti-Brucella antibodies in ruminant serum sample. J Clin Microbiol 47:3098–3107. doi: 10.1128/JCM.00919-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nielsen K, Lin M, Gall D, Jolley M. 2000. Fluorescence polarization immunoassay: detection of antibody to Brucella abortus. Methods 22:71–76. doi: 10.1006/meth.2000.1038. [DOI] [PubMed] [Google Scholar]
- 168.Lucero NE, Escobar GI, Ayala SM, Silva Paulo P, Nielsen K. 2003. Fluorescence polarization assay for diagnosis of human brucellosis. J Med Microbiol 52:883–887. doi: 10.1099/jmm.0.05217-0. [DOI] [PubMed] [Google Scholar]
- 169.Altuglu I, Zeytinoğlu A, Bilgic A, Kamcioglu S, Karakartal G, Smits H. 2002. Evaluation of Brucella dipstick assay for the diagnosis of acute brucellosis. Diagn Microbiol Infect Dis 44:241–243. doi: 10.1016/S0732-8893(02)00456-X. [DOI] [PubMed] [Google Scholar]
- 170.Clavijo E, Díaz R, Anguita A, García A, Pinedo A, Henk L, Smits HL. 2003. Comparison of a dipstick assay for detection of Brucella-specific immunoglobulin M antibodies with other tests for serodiagnosis of human brucellosis. Clin Diagn Lab Immunol 10:612–615. doi: 10.1128/CDLI.10.4.612-615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Casao MA, Smits HL, Navarro E, Solera J. 2003. Clinical utility of a dipstick assay in patients with brucellosis: correlation with the period of evolution of the disease. Clin Microbiol Infect 9:301–305. doi: 10.1046/j.1469-0691.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 172.Smits HL, Abdoel TH, Solera J, Clavijo E, Diaz R. 2003. Immunochromatographic Brucella-specific immunoglobulin M and G lateral flow assays for rapid serodiagnosis of human brucellosis. Clin Diagn Lab Immunol 10:1141–1146. doi: 10.1128/CDLI.10.6.1141-1146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zeytinoğlu A, Turhan A, Altuğlu I, Bilgiç A, Abdoel TH, Smits HL. 2006. Comparison of Brucella immunoglobulin M and G flow assays with serum agglutination and 2-mercaptoethanol tests in the diagnosis of brucellosis. Clin Chem Lab Med 44:180–184. [DOI] [PubMed] [Google Scholar]
- 174.McGiven JA. 2013. New developments in the immunodiagnosis of brucellosis in livestock and wildlife. Rev Sci Tech 32:163–176. doi: 10.20506/rst.32.1.2205. [DOI] [PubMed] [Google Scholar]
- 175.McGiven J, Howells L, Duncombe L, Stack J, Vijaya Ganesh N, Guiard J, Bundle DR. 2015. Improved serodiagnosis of bovine brucellosis by novel synthetic oligosaccharide antigens representing the capping M epitope elements of Brucella O-polysaccharide. J Clin Microbiol 53:1204–1210. doi: 10.1128/JCM.03185-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Seco-Mediavilla P, Verger JM, Grayon M, Cloeckaert A, Marín CM, Zygmunt MS, Fernández-Lago L, Vizcaíno N. 2003. Epitope mapping of the Brucella melitensis BP26 immunogenic protein: usefulness for diagnosis of sheep brucellosis. Clin Diagn Lab Immunol 10:647–651. doi: 10.1128/cdli.10.4.647-651.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tiwari AK, Kumar S, Pal V, Bhardwaj B, Rai GP. 2011. Evaluation of recombinant 10 kDa immunodominant region of BP26 protein of Brucella abortus for specific diagnosis of bovine brucellosis. Clin Vaccine Immunol 18:1760–1764. doi: 10.1128/CVI.05159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang HW, Lin YC, Pai TW, Chang HT. 2011. Prediction of B-cell linear epitopes with a combination of support vector machine classification and amino acid propensity identification. J Biomed Biotechnol 2011:1. doi: 10.1155/2011/432830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morales Betanzos C, González-Moa MJ, Boltz KW, Vander Werf BD, Johnston SA, Svarovsky SA. 2009. Bacterial glycoprofiling by using random sequence peptide microarrays. Chembiochem 10:877–888. doi: 10.1002/cbic.200800716. [DOI] [PubMed] [Google Scholar]
- 180.Gupta VK, Rout PK, Vihan VS. 2007. Induction of immune response in mice with a DNA vaccine encoding outer membrane protein (omp31) of Brucella melitensis 16M. Res Vet Sci 82:305–313. doi: 10.1016/j.rvsc.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 181.Simborio HL, Lee JJ, Bernardo Reyes AW, Hop HT, Arayan LT, Min W, Lee HJ, Yoo HS, Kim S. 2015. Evaluation of the combined use of the recombinant Brucella abortus Omp10, Omp19 and Omp28 proteins for the clinical diagnosis of bovine brucellosis. Microb Pathog 83–84:41–46. doi: 10.1016/j.micpath.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 182.Thepsuriyanont P, Intarapuk A, Chanket P, Tunyong W, Kalambaheti T. 2014. ELISA for brucellosis detection based on three Brucella recombinant proteins. Southeast Asian J Trop Med Public Health 45:130–141. [PubMed] [Google Scholar]
- 183.Patra KP, Saito M, Atluri VL, Rolán HG, Young B, Kerrinnes T, Smits H, Ricaldi JN, Gotuzzo E, Gilman RH, Tsolis RM, Vinetz JM. 2014. A protein-conjugate approach to develop a monoclonal antibody-based antigen detection test for the diagnosis of human brucellosis. PLoS Negl Trop Dis 8:e2926. doi: 10.1371/journal.pntd.0002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.DelVecchio VG, Kapatral V, Redkar RJ, Patra G, Mujer C, Los T, Ivanova N, Anderson I, Bhattacharyya A, Lykidis A, Reznik G, Jablonski L, Larsen N, D'Souza M, Bernal A, Mazur M, Goltsman E, Selkov E, Elzer PH, Hagius S, O'Callaghan D, Letesson J-J, Haselkorn R, Kyrpides N, Overbeek R. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc Natl Acad Sci U S A 99:443–448. doi: 10.1073/pnas.221575398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Paulsen IT, Seshadri R, Nelson KE, Eisen JA, Heidelberg JF, Read TD, Dodson RJ, Umayam L, Brinkac LM, Beanan MJ, Daugherty SC, Deboy RT, Durkin AS, Kolonay JF, Madupu R, Nelson WC, Ayodeji B, Kraul M, Shetty J, Malek J, Van Aken SE, Riedmuller S, Tettelin H, Gill SR, White O, Salzberg SL, Hoover DL, Lindler LE, Halling SM, Boyle SM, Fraser CM. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc Natl Acad Sci U S A 99:13148–13153. doi: 10.1073/pnas.192319099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Halling SM, Peterson-Burch BD, Bricker BJ, Zuerner RL, Qing Z, Li LL, Kapur V, Alt DP, Olsen SC. 2005. Completion of the genome sequence of Brucella abortus and comparison to the highly similar genomes of Brucella melitensis and Brucella suis. J Bacteriol 187:2715–2726. doi: 10.1128/JB.187.8.2715-2726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Whatmore AM, Davison N, Cloeckaert A, Al Dahouk S, Zygmunt MS, Brew SD, Perrett LL, Koylass MS, Vergnaud G, Quance C, Scholz HC, Dick EJ Jr, Hubbard G, Schlabritz-Loutsevitch NE. 2014. Brucella papionis sp. nov., isolated from baboons (Papio spp.). Int J Syst Evol Microbiol 64:4120–4128. doi: 10.1099/ijs.0.065482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ficht T. 2010. Brucella taxonomy and evolution. Future Microbiol 5:859–866. doi: 10.2217/fmb.10.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Audic S, Lescot M, Claverie JM, Scholz HC. 2009. Brucella microti: the genome sequence of an emerging pathogen. BMC Genomics 10:352. doi: 10.1186/1471-2164-10-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fretin D, Fauconnier A, Köhler S, Halling S, Léonard S, Nijskens C, Ferooz J, Lestrate P, Delrue RM, Danese I, Vandenhaute J, Tibor A, DeBolle X, Letesson JJ. 2005. The sheathed flagellum of Brucella melitensis is involved in persistence in a murine model of infection. Cell Microbiol 7:687–698. doi: 10.1111/j.1462-5822.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 191.Ocampo-Sosa AA, García-Lobo JM. 2008. Demonstration of IS711 transposition in Brucella ovis and Brucella pinnipedialis. BMC Microbiol 8:17. doi: 10.1186/1471-2180-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Alland D, Whittam TS, Murray MB, Cave MD, Hazbon MH, Dix K, Kokoris M, Duesterhoeft A, Eisen JA, Fraser CM, Fleischmann RD. 2003. Modelling bacterial evolution with comparative-genome based marker systems: application to Mycobacterium tuberculosis evolution and pathogenesis. J Bacteriol 185:3392–3399. doi: 10.1128/JB.185.11.3392-3399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Holt KE, Parkhill J, Mazzoni CJ, Roumagnac P, Weill FX, Goodhead I, Rance R, Baker S, Maskell DJ, Wain J, Dolecek C, Achtman M, Dougan G. 2008. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat Genet 40:987–993. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pearson T, Busch JD, Ravel J, Read TD, Rhoton SD, U'Ren JM, Simonson TS, Kachur SM, Leadem RR, Cardon ML, Van Ert MN, Huynh LY, Fraser CM, Keim P. 2004. Phylogenetic discovery bias in Bacillus anthracis using single-nucleotide polymorphisms from whole genome sequencing. Proc Natl Acad Sci U S A 101:13536–13541. doi: 10.1073/pnas.0403844101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Foster JT, Price LB, Beckstrom-Sternberg SM, Pearson T, Brown WD, Kiesling DM, Allen CA, Liu CM, Beckstrom-Sternberg J, Roberto FF, Keim P. 2012. Genotyping of Brucella species using clade specific SNPs. BMC Microbiol 12:110. doi: 10.1186/1471-2180-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Scholz HC, Vergnaud G. 2013. Molecular characterisation of Brucella species. Rev Sci Tech 32:149–162. doi: 10.20506/rst.32.1.2189. [DOI] [PubMed] [Google Scholar]
- 197.Foster JT, Beckstrom-Sternberg SM, Pearson T, Beckstrom-Sternberg JS, Chain PS, Roberto FF, Hnath J, Brettin T, Keim P. 2009. Whole-genome-based phylogeny and divergence of the genus Brucella. J Bacteriol 191:2864–2870. doi: 10.1128/JB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Scholz HC, Hubalek Z, Nesvadbova J, Tomaso H, Vergnaud G, Le Flèche P, Whatmore AM, Al Dahouk S, Krüger M, Lodri C, Pfeffer M. 2008. Isolation of Brucella microti from soil. Emerg Infect Dis 14:1316–1317. doi: 10.3201/eid1408.080286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ratushna VG, Sturgill DM, Ramamoorthy S, Reichow SA, He YQ, Lathigra R, Sriranganathan N, Halling SM, Boyle SM, Gibas CJ. 2006. Molecular targets for rapid identification of Brucella spp. BMC Microbiol 6:13. doi: 10.1186/1471-2180-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tan KK, Tan YC, Chang LY, Lee KW, Nore SS, Yee WY, Mat Isa MN, Jafar FL, Hoh CC, AbuBakar S. 2015. Full genome SNP-based phylogenetic analysis reveals the origin and global spread of Brucella melitensis. BMC Genomics 16:93. doi: 10.1186/s12864-015-1294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mühldorfer K, Wibbelt G, Szentiks CA, Fischer D, Scholz HC, Zschöck M, Eisenberg T. 2017. The role of ‘atypical’ Brucella in amphibians: are we facing novel emerging pathogens? J Appl Microbiol 122:40–53. doi: 10.1111/jam.13326. [DOI] [PubMed] [Google Scholar]
- 202.Leal-Klevezas DS, Martínez-Vázquez IO, López-Merino A, Martínez-Soriano JP. 1995. Single-step PCR for detection of Brucella spp. from blood and milk of infected animals. J Clin Microbiol 33:3087–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kattar MM, Zalloua PA, Araj GF, Samaha-Kfoury J, Shbaklo H, Kanj SS, Khalife S, Deeb M. 2007. Development and evaluation of real-time polymerase chain reaction assays on whole blood and paraffin-embedded tissues for rapid diagnosis of human brucellosis. Diagn Microbiol Infect Dis 59:23–32. doi: 10.1016/j.diagmicrobio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 204.Vizcaíno N, Cloeckaert A, Zygmunt MS, Fernández-Lago L. 1999. Molecular characterization of a Brucella species large DNA fragment deleted in Brucella abortus strains: evidence for a locus involved in the synthesis of a polysaccharide. Infect Immun 67:2700–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mitka S, Anetakis C, Souliou E, Diza E, Kansouzidou A. 2007. Evaluation of different PCR assays for early detection of acute and relapsing brucellosis in humans in comparison with conventional methods. J Clin Microbiol 45:1211–1218. doi: 10.1128/JCM.00010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Romero C, Gamazo C, Pardo M, López-Goñi I. 1995. Specific detection of Brucella DNA by PCR. J Clin Microbiol 33:615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Herman L, De Ridder H. 1992. Identification of Brucella spp. by using the polymerase chain reaction. Appl Environ Microbiol 58:2099–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.El Kholy AA, Gomaa HE, El Anany MG, Abd El Rasheed E. 2009. Diagnosis of human brucellosis in Egypt by polymerase chain reaction. East Mediterr Health J 15:1068–1074. doi: 10.26719/2009.15.5.1068. [DOI] [PubMed] [Google Scholar]
- 209.Baddour MM, Alkhalifa DH. 2008. Evaluation of three polymerase chain reaction techniques for detection of Brucella DNA in peripheral human blood. Can J Microbiol 54:352–357. doi: 10.1139/w08-017. [DOI] [PubMed] [Google Scholar]
- 210.Al-Nakkas AF, Wright SG, Mustafa AS, Wilson S. 2002. Single-tube, nested PCR for the diagnosis of human brucellosis in Kuwait. Ann Trop Med Parasitol 96:397–403. doi: 10.1179/000349802125001203. [DOI] [PubMed] [Google Scholar]
- 211.Bounaadja L, Albert D, Chénais B, Hénault S, Zygmunt MS, Poliak S, Garin-Bastuji B. 2009. Real-time PCR for identification of Brucella spp.: a comparative study of IS711, bcsp31, and per target genes. Vet Microbiol 137:156–164. doi: 10.1016/j.vetmic.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 212.Mayfield JE, Bricker BJ, Godfrey H, Crosby RM, Knight DJ, Halling SM, Balinsky D, Tabatabai LB. 1988. The cloning and nucleotide sequence of a gene coding for an immunogenic Brucella abortus protein. Gene 63:1–9. doi: 10.1016/0378-1119(88)90540-9. [DOI] [PubMed] [Google Scholar]
- 213.Baily GG, Krahn JB, Drasar BS, Stoker NG. 1992. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J Trop Med Hyg 95:271–275. [PubMed] [Google Scholar]
- 214.García-Yoldi D, Marín CM, De Miguel MJ, Muñoz PM, Vizmanos JL, López-Goñi I. 2006. Multiplex PCR assay for the identification and differentiation of all Brucella species and the vaccine strains Brucella abortus S19 and RB51 and Brucella melitensis Rev1. Clin Chem 52:779–781. doi: 10.1373/clinchem.2005.062596. [DOI] [PubMed] [Google Scholar]
- 215.López-Goñi I, García-Yoldi D, Marín CM, de Miguel MJ, Muñoz PM, Blasco JM, Jacques I, Grayon M, Cloeckaert A, Ferreira AC, Cardoso R, Corrêa de Sá MI, Walravens K, Albert D, Garin-Bastuji B. 2008. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J Clin Microbiol 46:3484–3487. doi: 10.1128/JCM.00837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Navarro E, Escribano J, Fernández J, Solera J. 2002. Comparison of three different PCR methods for detection of Brucella spp. in human blood samples. FEMS Immunol Med Microbiol 34:147–151. doi: 10.1111/j.1574-695X.2002.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 217.Queipo-Ortuño MI, Garcia-Ordoñez MA, Colmenero JD, Morata P. 1999. Hydrogen peroxide improves the efficiency of a peripheral blood PCR assay for diagnosis of human brucellosis. Biotechniques 27:248–250. doi: 10.2144/99272bm06. [DOI] [PubMed] [Google Scholar]
- 218.Morata P, Queipo-Ortuño MI, de Dios Colmenero J. 1998. Strategy for optimizing DNA amplification in a peripheral blood PCR assay used for diagnosis of human brucellosis. J Clin Microbiol 36:2443–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Matar GM, Khneisser IA, Abdelnoor AM. 1996. Rapid laboratory confirmation of human brucellosis by PCR analysis of a target sequence on the 31-kilodalton Brucella antigen DNA. J Clin Microbiol 34:477–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Salazar LA, Hirata MH, Cavalli SA, Machado MO, Hirata RD. 1998. Optimized procedure for DNA isolation from fresh and cryopreserved clotted human blood useful in clinical molecular testing. Clin Chem 44:1748–1750. [PubMed] [Google Scholar]
- 221.Zerva L, Bourantas K, Mitka S, Kansouzidou A, Legakis NJ. 2001. Serum is the preferred clinical specimen for diagnosis of human brucellosis by PCR. J Clin Microbiol 39:1661–1664. doi: 10.1128/JCM.39.4.1661-1664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brown PD, Gravekamp C, Carrington DG, van de Kemp H, Hartskeerl RA, Edwards CN, Everard CO, Terpstra WJ, Levett PN. 1995. Evaluation of the polymerase chain reaction for early diagnosis of leptospirosis. J Med Microbiol 43:110–114. doi: 10.1099/00222615-43-2-110. [DOI] [PubMed] [Google Scholar]
- 223.Murdoch DR, Walford EJ, Jennings LC, Light GJ, Schousboe MI, Chereshsky AY, Chambers ST, Town GI. 1996. Use of the polymerase chain reaction to detect Legionella DNA in urine and serum samples from patients with pneumonia. Clin Infect Dis 23:475–480. doi: 10.1093/clinids/23.3.475. [DOI] [PubMed] [Google Scholar]
- 224.Sanjuan-Jimenez R, Colmenero JD, Morata P. 2017. Lessons learned with molecular methods targeting the BCSP-31 membrane protein for diagnosis of human brucellosis. Clin Chim Acta 469:1–9. doi: 10.1016/j.cca.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 225.Colmenero JD, Reguera JM, Martos F, Sánchez-De-Mora D, Delgado M, Causse M, Martín-Farfán A, Juárez C. 1996. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore, MD) 75:195–211. doi: 10.1097/00005792-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 226.Buzgan T, Karahocagil MK, Irmak H, Baran AI, Karsen H, Evirgen O, Akdeniz H. 2010. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis 14:e469–e478. doi: 10.1016/j.ijid.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 227.Morata P, Queipo-Ortuño MI, Reguera JM, Miralles F, Lopez-Gonzalez JJ, Colmenero JD. 2001. Diagnostic yield of a PCR assay in focal complications of brucellosis. J Clin Microbiol 39:3743–3746. doi: 10.1128/JCM.39.10.3743-3746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li M, Zhou X, Li J, Sun L, Chen X, Wang P. 2018. Real-time PCR assays for diagnosing brucellar spondylitis using formalin-fixed paraffin-embedded tissues. Medicine (Baltimore, MD) 97:e0062. doi: 10.1097/MD.0000000000010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ögredici Ö, Erb S, Langer I, Pilo P, Kerner A, Haack HG, Cathomas G, Danuser J, Pappas G, Tarr PE. 2010. Brucellosis reactivation after 28 years. Emerg Infect Dis 16:2021–2022. doi: 10.3201/eid1612.100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lehmann U, Kreipe H. 2001. Real-time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods 25:409–418. doi: 10.1006/meth.2001.1263. [DOI] [PubMed] [Google Scholar]
- 231.Sharif HS, Clark DC, Aabed MY, Haddad MC, Al Deeb SM, Yaqub B, Al Moutaery KR. 1990. Granulomatous spinal infections: MR imaging. Radiology 177:101–107. doi: 10.1148/radiology.177.1.2399306. [DOI] [PubMed] [Google Scholar]
- 232.Fekete A, Bantle JA, Halling SM, Sanborn MR. 1990. Preliminary development of a diagnostic test for Brucella using polymerase chain reaction. J Appl Bacteriol 69:216–227. doi: 10.1111/j.1365-2672.1990.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 233.Fekete A, Bantle JA, Halling SM. 1992. Detection of Brucella by polymerase chain reaction in bovine fetal and maternal tissues. J Vet Diagn Invest 4:79–83. doi: 10.1177/104063879200400118. [DOI] [PubMed] [Google Scholar]
- 234.Romero C, Pardo M, Grillo MJ, Diaz R, Blasco JM, Lopez-Goñi I. 1995. Evaluation of PCR and indirect enzyme-linked immunosorbent assay on milk samples for diagnosis of brucellosis in dairy cattle. J Clin Microbiol 33:3198–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rantakokko-Jalava K, Jalava J. 2002. Optimal DNA isolation method for detection of bacteria in clinical specimens by broad-range PCR. J Clin Microbiol 40:4211–4217. doi: 10.1128/jcm.40.11.4211-4217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Miller SA, Dykes DD, Polesky HF. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Thatcher SA. 2015. DNA/RNA preparation for molecular detection. Clin Chem 61:89–99. doi: 10.1373/clinchem.2014.221374. [DOI] [PubMed] [Google Scholar]
- 238.Queipo-Ortuño MI, Tena F, Colmenero JD, Morata P. 2008. Comparison of seven commercial DNA extraction kits for the recovery of Brucella DNA from spiked human serum samples using real-time PCR. Eur J Clin Microbiol Infect Dis 27:109–114. doi: 10.1007/s10096-007-0409-y. [DOI] [PubMed] [Google Scholar]
- 239.Dauphin LA, Hutchins RJ, Bost LA, Bowen MD. 2009. Evaluation of automated and manual commercial DNA extraction methods for recovery of Brucella DNA from suspensions and spiked swabs. J Clin Microbiol 47:3920–3926. doi: 10.1128/JCM.01288-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tomaso H, Kattar M, Eickhoff M, Wernery U, Al Dahouk S, Straube E, Neubauer H, Scholz HC. 2010. Comparison of commercial DNA preparation kits for the detection of brucellae in tissue using quantitative real-time PCR. BMC Infect Dis 10:100. doi: 10.1186/1471-2334-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Al-Nakkas A, Mustafa AS, Wright SG. 2005. Large-scale evaluation of a single-tube nested PCR for the laboratory diagnosis of human brucellosis in Kuwait. J Med Microbiol 54:727–730. doi: 10.1099/jmm.0.45772-0. [DOI] [PubMed] [Google Scholar]
- 242.Queipo-Ortuño MI, Morata P, Ocón P, Manchado P, Colmenero JD. 1997. Rapid diagnosis of human brucellosis by peripheral-blood PCR assay. J Clin Microbiol 35:2927–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Luk JMC, Kongmuang U, Tsang RW, Lindberg AA. 1997. Enzyme-linked immunosorbent assay to detect PCR products of the rfbS gene from serogroup D salmonellae: a rapid screening prototype. J Clin Microbiol 35:714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zambardi G, Druetta A, Roure C, Fouqué B, Girardo P, Chypre C, Marchand J, Freney J, Fleurette J. 1995. Rapid diagnosis of Mycobacterium tuberculosis infections by ELISA-like detection polymerase chain reaction products. Mol Cell Probes 9:91–99. doi: 10.1016/S0890-8508(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 245.Morata P, Queipo-Ortuño MI, Reguera JM, García-Ordoñez MA, Cárdenas A, Colmenero JD. 2003. Development and evaluation of a PCR-enzyme-linked immunosorbent assay for diagnosis of human brucellosis. J Clin Microbiol 41:144–148. doi: 10.1128/jcm.41.1.144-148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cherian T, Lalitha MK, Manoharan A, Thomas K, Yolken RH, Steinhoff M. 1998. PCR-enzyme immunoassay for detection of Streptococcus pneumoniae DNA in cerebrospinal fluid samples from patients with culture negative meningitis. J Clin Microbiol 36:3605–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sails AD, Fox AJ, Bolton FJ, Wareing DR, Greenway DL, Borrow R. 2001. Development of a PCR ELISA assay for the identification of Campylobacter jejuni and Campylobacter coli. Mol Cell Probes 15:291–300. doi: 10.1006/mcpr.2001.0374. [DOI] [PubMed] [Google Scholar]
- 248.Higuchi R, Fockler C, Dollinger G, Watson R. 1993. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Nat Biotechnol 11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- 249.Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP. 1997. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 22:130–138. doi: 10.2144/97221bi01. [DOI] [PubMed] [Google Scholar]
- 250.Lockey C, Otto E, Long Z. 1998. Real-time fluorescence detection of a single DNA molecule. Biotechniques 24:744–746. doi: 10.2144/98245bm09. [DOI] [PubMed] [Google Scholar]
- 251.Navarro E, Serrano-Heras G, Castaño MJ, Solera J. 2015. Real-time PCR detection chemistry. Clin Chim Acta 439:231–250. doi: 10.1016/j.cca.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 252.Liu J, Gratz J, Maro A, Kumburu H, Kibiki G, Taniuchi M, Howlader AM, Sobuz SU, Haque R, Talukder KA, Qureshi S, Zaidi A, Haverstick DM, Houpt ER. 2012. Simultaneous detection of six diarrhea-causing bacterial pathogens with an in-house PCR luminex assay. J Clin Microbiol 50:98–103. doi: 10.1128/JCM.05416-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McDonough EA, Barrozo CP, Russell KL, Metzgar D. 2005. A multiplex PCR for detection of Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, and Bordetella pertussis in clinical specimens. Mol Cell Probes 19:314–322. doi: 10.1016/j.mcp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 254.Markoulatos P, Georgopoulou A, Kotsovassilis C, Karabogia-Karaphillides P, Spyrou N. 2000. Detection and typing of HSV-1, HSV-2, and VZV by a multiplex polymerase chain reaction. J Clin Lab Anal 14:214–219. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sanjuan-Jimenez R, Colmenero JD, Bermúdez P, Alonso A, Morata P. 2013. Amplicon DNA melting analysis for the simultaneous detection of Brucella spp. and Mycobacterium tuberculosis complex. Potential use in rapid differential diagnosis between extrapulmonary tuberculosis and focal complications of brucellosis. PLoS One 8:e58353. doi: 10.1371/journal.pone.0058353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Traxler RM, Lehman MW, Bosserman EA, Guerra MA, Smith TL. 2013. A literature review of laboratory-acquired brucellosis. J Clin Microbiol 51:3055–3062. doi: 10.1128/JCM.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vrioni G, Gartzonika C, Kostoula A, Boboyianni C, Papadopoulou C, Levidiotou S. 2004. Application of a polymerase chain reaction enzyme immunoassay in peripheral whole blood and serum specimens for diagnosis of acute human brucellosis. Eur J Clin Microbiol Infect Dis 23:194–199. doi: 10.1007/s10096-003-1082-4. [DOI] [PubMed] [Google Scholar]
- 258.Queipo-Ortuño MI, Colmenero JD, Reguera JM, García-Ordoñez MA, Pachón ME, Gonzalez M, Morata P. 2005. Rapid diagnosis of human brucellosis by SYBR Green I-based real-time PCR assay and melting curve analysis in serum samples. Clin Microbiol Infect 11:713–718. doi: 10.1111/j.1469-0691.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- 259.Debeaumont C, Falconnet PA, Maurin M. 2005. Real-time PCR for detection of Brucella spp. DNA in human serum samples. Eur J Clin Microbiol Infect Dis 24:842–845. doi: 10.1007/s10096-005-0064-0. [DOI] [PubMed] [Google Scholar]
- 260.Surucuoglu S, El S, Ural S, Gazi H, Kurutepe S, Taskiran P, Yurtsever SG. 2009. Evaluation of real-time PCR method for rapid diagnosis of brucellosis with different clinical manifestations. Pol J Microbiol 58:15–19. [PubMed] [Google Scholar]
- 261.Queipo-Ortuño MI, Colmenero JD, Baeza G, Morata P. 2005. Comparison between LightCycler real-time polymerase chain reaction (PCR) assay with serum and PCR-enzyme-linked immunosorbent assay with whole blood samples for the diagnosis of human brucellosis. Clin Infect Dis 40:260–264. doi: 10.1086/426818. [DOI] [PubMed] [Google Scholar]
- 262.Navarro-Martínez A, Navarro E, Castaño MJ, Solera J. 2008. Rapid diagnosis of human brucellosis by quantitative real-time PCR: a case report of brucellar spondylitis. J Clin Microbiol 46:385–387. doi: 10.1128/JCM.01303-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Casañas MC, Queipo-Ortuño MI, Rodriguez-Torres A, Orduña A, Colmenero JD, Morata P. 2001. Specificity of a polymerase chain reaction assay of a target sequence on the 31-kilodalton Brucella antigen DNA used to diagnose human brucellosis. Eur J Clin Microbiol Infect Dis 20:127–131. doi: 10.1007/pl00011242. [DOI] [PubMed] [Google Scholar]
- 264.Queipo-Ortuño MI, Colmenero JD, Muñoz N, Baeza G, Clavijo E, Morata P. 2006. Rapid diagnosis of Brucella epididymo-orchitis by real-time polymerase chain reaction assay in urine samples. J Urol 176:2290–2293. doi: 10.1016/j.juro.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 265.Colmenero JD, Queipo-Ortuño MI, Reguera JM, Baeza G, Salazar JA, Morata P. 2005. Real time polymerase chain reaction: a new powerful tool for the diagnosis of neurobrucellosis. J Neurol Neurosurg Psychiatry 76:1025–1027. doi: 10.1136/jnnp.2004.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Canning PC, Roth JA, Deyoe BL. 1986. Release of 59-guanosine monophosphate and adenine by Brucella abortus and their role in intracellular survival of the bacteria. J Infect Dis 154:464–470. doi: 10.1093/infdis/154.3.464. [DOI] [PubMed] [Google Scholar]
- 267.Ariza J, Gudiol F, Pallarés R, Fernandez-Viladrich P, Rufi G, Corredoira J, Miratvilles MR. 1992. Treatment of human brucellosis with doxycycline plus rifampin and doxycycline plus streptomycin. A randomized double-blind study. Ann Intern Med 117:25–30. doi: 10.7326/0003-4819-117-1-25. [DOI] [PubMed] [Google Scholar]
- 268.Solera J, Rodriguez-Zapata M, Geijo P, Largo J, Paulino J, Saez L, Martinez- Alfaro E, Sanchez L, Sepulveda MA, Ruiz-Ribo MD. 1995. Doxycycline-rifampin versus doxycycline-streptomycin in treatment of human brucellosis due to Brucella melitensis. The GECMEI Group. Grupo de Estudio de Castilla-la Mancha de Enfermedades Infecciosas. Antimicrob Agents Chemother 39:2061–2067. doi: 10.1128/AAC.39.9.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Morata P, Queipo-Ortuño MI, Reguera JM, García-Ordoñez MA, Pichardo C, Colmenero JD. 1999. Posttreatment follow-up of brucellosis by PCR assay. J Clin Microbiol 37:4163–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hasanjani Roushan MR, Marashi SM, Moulana Z. 2016. Polymerase chain reaction-based assays for the diagnosis of active and relapsed cases of human brucellosis. Am J Trop Med Hyg 95:1272–1276. doi: 10.4269/ajtmh.16-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Navarro E, Segura JC, Castaño MJ, Solera J. 2006. Use of real-time quantitative polymerase chain reaction to monitor the evolution of Brucella melitensis DNA load during therapy and post-therapy follow-up in patients with brucellosis. Clin Infect Dis 42:1266–1273. doi: 10.1086/503035. [DOI] [PubMed] [Google Scholar]
- 272.Castaño MJ, Solera J. 2009. Chronic brucellosis and persistence of Brucella melitensis DNA. J Clin Microbiol 47:2084–2089. doi: 10.1128/JCM.02159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Queipo-Ortuño MI, Colmenero JD, Bravo MJ, García-Ordoñez MA, Morata P. 2008. Usefulness of a quantitative real-time PCR assay using serum samples to discriminate between inactive, serologically positive and active human brucellosis. Clin Microbiol Infect 14:1128–1134. doi: 10.1111/j.1469-0691.2008.02095.x. [DOI] [PubMed] [Google Scholar]
- 274.Sohrabi M, Mohabati Mobarez A, Khoramabadi N, Hosseini Doust R, Behmanesh M. 2014. Efficient diagnosis and treatment follow-up of human brucellosis by a novel quantitative TaqMan real-time PCR assay: a human clinical survey. J Clin Microbiol 52:4239–4243. doi: 10.1128/JCM.01819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Thakur S, Bedi JS, Singh R, Gill JPS, Arora AK, Kashyap N. 2018. Quantitative polymerase chain reaction based quantification of Brucella DNA in serum of pre- and post-therapeutic occupationally exposed infected human population. J Infect Public Health 11:514–520. doi: 10.1016/j.jiph.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 276.Vrioni G, Bourdakis A, Pappas G, Pitiriga V, Mavrouli M, Pournaras S, Tsakris A. 2014. Administration of a triple versus a standard double antimicrobial regimen for human brucellosis more efficiently eliminates bacterial DNA load. Antimicrob Agents Chemother 58:7541–7544. doi: 10.1128/AAC.03841-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Taylor SC, Mrkusich EM. 2014. The state of RT-quantitative PCR: first hand observations of implementation of minimum information for the publication of quantitative real-time PCR experiments (MIQE). J Mol Microbiol Biotechnol 24:46–52. doi: 10.1159/000356189. [DOI] [PubMed] [Google Scholar]
- 278.Memish Z, Mah MW, Al Mahmoud S, Al Shaalan M, Khan MY. 2000. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J Infect 40:59–63. doi: 10.1053/jinf.1999.0586. [DOI] [PubMed] [Google Scholar]
- 279.Al Dahouk S, Neubauer H, Hensel A, Schöneberg I, Nöckler K, Alpers K, Merzenich H, Stark K, Jansen A. 2007. Changing epidemiology of human brucellosis, Germany, 1962–2005. Emerg Infect Dis 13:1895–1900. doi: 10.3201/eid1312.070527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wellinghausen N, Nöckler K, Sigge A, Bartel M, Essig A, Poppert S. 2006. Rapid detection of Brucella spp. in blood cultures by fluorescence in situ hybridization. J Clin Microbiol 44:1828–1830. doi: 10.1128/JCM.44.5.1828-1830.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Horvat RT, El Atrouni W, Hammoud K, Hawkinson D, Cowden S. 2011. Ribosomal RNA sequence analysis of Brucella infection misidentified as Ochrobactrum anthropi. J Clin Microbiol 49:1165–1168. doi: 10.1128/JCM.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Scholz HC, Pfeffer M, Witte A, Neubauer H, Al Dahouk S, Wernery U, Tomaso H. 2008. Specific detection and differentiation of Ochrobactrum anthropi, Ochrobactrum intermedium and Brucella spp. by a multi-primer PCR that targets the recA gene. J Med Microbiol 57:64–71. doi: 10.1099/jmm.0.47507-0. [DOI] [PubMed] [Google Scholar]
- 283.Kumar S, Tuteja U, Sarika K, Singh D, Kumar A, Kumar O. 2011. Rapid multiplex PCR assay for the simultaneous detection of the Brucella genus, B. abortus, B. melitensis, and B. suis. J Microbiol Biotechnol 21:89–92. doi: 10.4014/jmb.1007.07051. [DOI] [PubMed] [Google Scholar]
- 284.Bricker BJ, Halling SM. 1995. Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella abortus vaccine strains S19 and RB51. J Clin Microbiol 33:1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Imaoka K, Kimura M, Suzuki M, Kamiyama T, Yamada A. 2007. Simultaneous detection of the genus Brucella by combinatorial PCR. Jpn J Infect Dis 60:137–139. [PubMed] [Google Scholar]
- 286.Gopaul KK, Sells J, Lee R, Beckstrom-Sternberg SM, Foster JT, Whatmore AM. 2014. Development and assessment of multiplex high resolution melting assay as a tool for rapid single-tube identification of five Brucella species. BMC Res Notes 7:903. doi: 10.1186/1756-0500-7-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Nimri LF. 2003. Diagnosis of recent and relapsed cases of human brucellosis by PCR assay. BMC Infect Dis 3:5. doi: 10.1186/1471-2334-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]