Acute Chest Syndrome (ACS) is a major pulmonary complication and a leading cause of death in sickle cell disease (SCD) (Gladwin and Vichinsky 2008). Extracellular haem has been shown to be independently associated with ACS and vaso-occlusive crisis (VOC). Severe ACS is frequently preceded by painful VOC and acute intravascular haemolysis (Vichinsky, et al 2000). A rapid drop in baseline haemoglobin (mean decrease of 7.8 g/l) and elevated lactate dehydrogenase reported during ACS development indicates a cardinal role of acute intravascular haemolysis in severe ACS (Vichinsky, et al 1997). Accordingly, infusion of purified haem causes respiratory failure and lethal acute lung injury (ALI) reminiscent of severe ACS in transgenic SCD (SS) mice (Ghosh, et al 2013).
P-selectin, a cell adhesion molecule expressed on activated endothelium and platelets, is implicated in obstructing microvascular flow, causing VOC (Embury, et al 2004) by promoting adhesion of sickle erythrocytes and leucocytes to the endothelial wall (Matsui, et al 2001). Extracellular haem activates endothelial P-selectin expression, inducing vascular stasis in murine models of SCD (Belcher, et al 2014). A recent trial showed that an anti-human P-selectin antibody reduced the frequency of painful events in SCD patients (Ataga, et al 2017), although it could not determine the role of P-selectin in ACS. This study addresses this knowledge gap and provides mechanistic evidence demonstrating a pathogenic role for P-selectin in the development of haem-induced ALI in sickle mice.
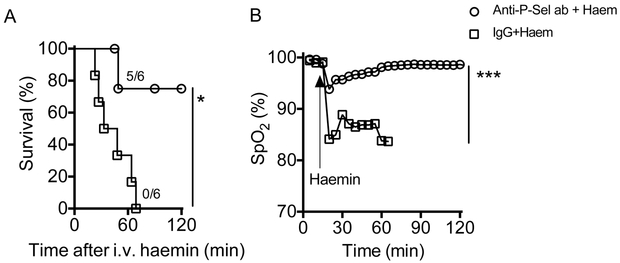
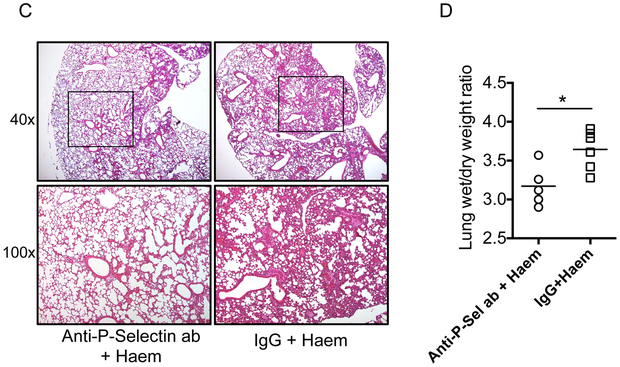
To verify the involvement of P-selectin in ACS, we infused a function-blocking monoclonal murine anti-P selectin antibody (RB 40.34; Millipore, Burlington, MA; 2 mg/kg) or control IgG into SS mice followed by induction of ALI using purified haem (70 μmol/kg) as previously described (Ghosh, et al 2013). All experimental mice were monitored for real-time arterial oxygen saturation (% SpO2) using the MouseOx™ pulse-oximeter (Starr Life Sciences, Oakmont, PA) and the lung wet/dry weight ratio was determined at the end of the experiment. Five of six SS mice pre-treated with the anti-P-selectin antibody did not develop lung injury while all six SS mice pre-treated with the IgG control succumbed (p=0.03), with severe hypoxaemia, post-mortem evidence of alveolar flooding and extensive lung damage (Figure 1A-D). A drop in the platelet count is a predictor of respiratory failure in ACS patients (Chaturvedi, et al 2016). In this study, we found that the platelet count in IgG control group dropped significantly, whereas it remained unaltered in SS mice treated with anti-P-selectin antibody. (Supplementary Figure 1). Infusion of a higher concentration of haem (210 μmol/kg bw) triggered a lethal acute lung injury (ALI) in wild type C57BL/6J mice (P-Sel+/+; B6; JAX stock# 000664), while haem did not cause ALI in congenic P-selectin global knockout mice (P-Sel−/−; B6.129S7-Selptm1Bay/J; JAX stock# 002289) (Supplementary Figure 2).
Figure 1. Functional P-selectin is essential for haem induced ACS in sickle mice.
Wild-type Townes’ sickle mice (SSWT) given intravenous injection of anti-P-selectin antibody or control IgG (n=6), 30 min prior to infusion of purified haemin (70 μmol/kg). (A) Mortality data showing anti-P-selectin antibody (ab) prevents haem-induced death in SS mice (p<0.05; Mantel-Cox test). (B) Real time monitoring of arterial oxygen saturation (% SpO2) indicating hypoxaemia in control IgG treated mice, while antibody-treated mice had no oxygen desaturation. (C) Representative histopathology of lung tissue from mice 2h after haem challenge. The haematoxylin and eosin stained sections under different magnification (40X and 100X) clearly show protection from lung damage following antibody blocking of P-selectin in SS mice. (D) Assessment of lung injury, using gravimetric analysis, in SS mice after indicated treatment and haem challenge.
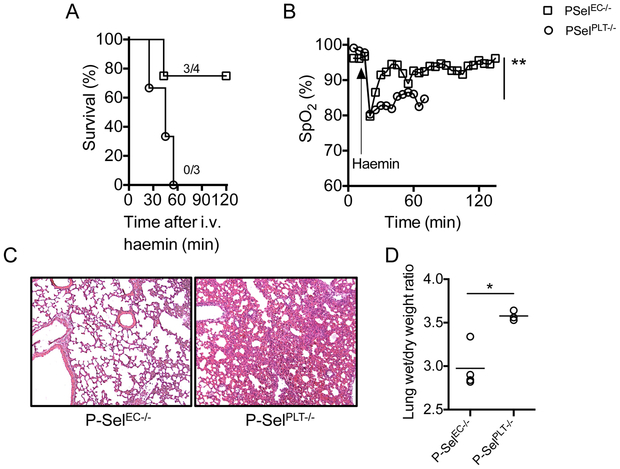
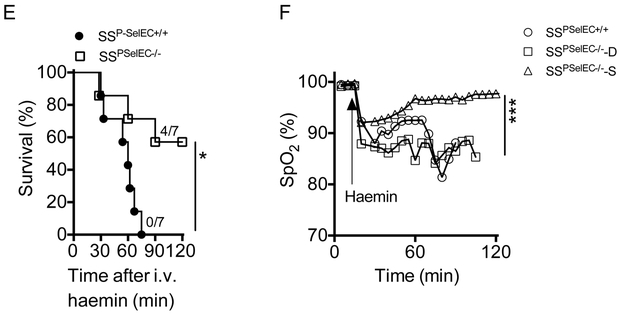
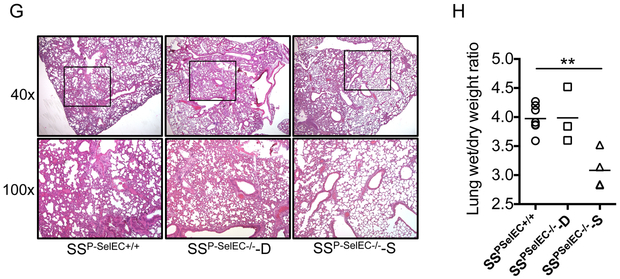
To determine whether platelet P-selectin (in the haematopoietic compartment) or P-selectin expressed by the endothelium (non-haematopoietic compartment) played the dominant role in promoting ALI, we generated bone marrow chimeric C57BL/6 mice lacking P-selectin in haematopoietic (P-SelPLT−/−) and non-haematopoietic (P-SelEC−/−) compartments. For this purpose, we transplanted whole bone marrow cells from P-Sel+/+ or P-Sel−/− donor mice to P-Sel−/− and P-Sel+/+ recipient mice, generating P-SelEC−/− and P-SelPLT−/− mice, respectively. Three of the four P-SelEC−/− mice studied were protected while all three P-SelPLT−/− congenic control mice studied succumbed to the haem infusions. Lung injury in the congenic B6 P-SelPLT−/− mice was characterized by severe hypoxemia (SpO2: 82.75±2.14%) and oedema (Figure 2A-D). Next, we generated bone marrow chimeric SS mice that lack endothelial P-selectin (SSP-SelEC−/−) or express endothelial P-selectin (SSP-SelEC+/+) by transplanting congenic P-Sel−/− and P-Sel+/+ mice with SS mouse bone marrow. Induction of ACS resulted in lethality in 3 of seven SSP-SelEC−/− mice and in all seven SSP-SelEC+/+ mice (Figure 2E). The SSP-SelEC−/− mice that died in this experiment experienced similar degree of lung damage, suggesting a role for other factors in this haemolytic ALI model (Figure 2E-H).
Figure 2. Expression of endothelial P-selectin mediates ACS in sickle mice.
(A) Mortality in B6 mice following haemin (210 μmol/kg) challenge in the presence (P-SelPLT−/−) or absence (P-SelEC−/−) of P-selectin on vascular endothelium. Haem induces (B) severe hypoxaemia, (C) lung damage and (D) pulmonary oedema in presence of endothelial P-selectin (P-SelPLT−/−) in B6 mice. (E) Survival of SSP-SelEC+/+ and SSP-SelEC−/− chimeric mice following infusion of haem (70 μmol/kg) (n=7; Mantel-Cox test; p<0.05). (F) Lung function, determined by SpO2 in SSP-SelEC+/+ mice, and SSP-SelEC−/− mice that died (SSP-SelEC−/− -D) or survived (SSP-SelEC−/− -S) following haem infusion (n=3; p<0.001). (G) Representative haematoxylin and eosin stained lung sections showing histopathology of lung following haem challenge in the indicated mice group. Note both SSP-SelEC+/+ and SSP-SelEC−/− -D mice had severe lung congestion (H) Gravimetric assessment of lung oedema (n = 3–6). Statistical analysis was performed using one-way ANOVA (*P < 0.01).
Haem infusion results in de novo haem release in SS mice during development of acute respiratory failure (Ghosh, et al 2013), suggesting that continuous intravascular haemolysis feeds the inflammation in ACS. In this study, we found that SSP-SelEC−/− cleared ~70% of the haem bolus within 25 min, while the total plasma haem (TPH) increased over 2-fold in the congenic SSP-SelEC+/+ mice. Sequential TPH measurement in SS mice pre-treated with either anti-P-selectin antibody or IgG control revealed rapid clearance of the haem in the anti-P-selectin antibody-treated mice, while TPH was amplified IgG control littermates (Supplementary Figure 3). Together these results indicate that P-selectin potentiates extracellular haem-induced inflammation by releasing more haem into the circulation, most likely via a mechanism involving enhanced adhesions of sickle erythrocytes.
P-selectin has been identified as a key player in acute vascular occlusion in SCD (Chang, et al 2010). A recent clinical trial showed that intermittent intravenous injections of human anti-P-selectin antibody over a period of 52 weeks reduced rates of pain crises in patients with SCD (Ataga, et al 2017). However, there were not enough ACS incidents to evaluate the efficacy of this treatment in ACS. Here we propose a model of ACS pathogenesis in which activation of endothelial P-selectin promotes occlusion and subsequent lysis of sickle erythrocytes yielding supra-physiologically high local concentrations of haem, that disrupts the alveolar capillary barrier to cause alveolar flooding, hypoxaemia and respiratory failure. The anti-P-selectin antibody blocks P-selectin adhesion, which would inhibit the entrapment of sickle cells to promote de novo release of extracellular haem critical to sustaining the inflammation in ACS. This assertion is based on the finding that SSP-SelEC−/− mice cleared ~70% of the haem within the period of the experiment. On the contrary, total plasma haem increased over 2-fold in the SSP-SelEC+/+ mice. Inhibiting P-selectin adhesion may be sufficient to thwart this process and prevent and potentially treat ACS. Finally, this study provides proof-of-principle that anti-P-selectin antibodies can be used to block ACS development.
Supplementary Material
Acknowledgements
This study was conducted with research funding from the National Heart, Lung and Blood Institute (NHLBI) (Award Number: R01HL106192 and U01HL117721).
Footnotes
Disclosure of Conflicts of Interest:
The authors have declared that no conflict of interest exists.
References
- Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, Gualandro S, Colella MP, Smith WR, Rollins SA, Stocker JW & Rother RP (2017) Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med, 376, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP & Vercellotti GM (2014) Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood, 123, 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Patton JT, Sarkar A, Ernst B, Magnani JL & Frenette PS (2010) GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood, 116, 1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S, Ghafuri DL, Glassberg J, Kassim AA, Rodeghier M & DeBaun MR (2016) Rapidly progressive acute chest syndrome in individuals with sickle cell anemia: a distinct acute chest syndrome phenotype. Am J Hematol, 91, 1185–1190. [DOI] [PubMed] [Google Scholar]
- Embury SH, Matsui NM, Ramanujam S, Mayadas TN, Noguchi CT, Diwan BA, Mohandas N & Cheung AT (2004) The contribution of endothelial cell P-selectin to the microvascular flow of mouse sickle erythrocytes in vivo. Blood, 104, 3378–3385. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Adisa OA, Chappa P, Tan F, Jackson KA, Archer DR & Ofori-Acquah SF (2013) Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest, 123, 4809–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT & Vichinsky E (2008) Pulmonary complications of sickle cell disease. N Engl J Med, 359, 2254–2265. [DOI] [PubMed] [Google Scholar]
- Matsui NM, Borsig L, Rosen SD, Yaghmai M, Varki A & Embury SH (2001) P-selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood, 98, 1955–1962. [DOI] [PubMed] [Google Scholar]
- Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O & Nickerson B (1997) Acute chest syndrome in sickle cell disease: clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood, 89, 1787–1792. [PubMed] [Google Scholar]
- Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, Nickerson B, Orringer E, McKie V, Bellevue R, Daeschner C & Manci EA (2000) Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med, 342, 1855–1865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.