Abstract
Background
Fibroblast growth factor 21 (FGF21) is a novel metabolic regulator that has beneficial effects on glucose and lipid metabolism. However, plasma FGF21 levels are paradoxically increased in type 2 diabetes mellitus (T2DM) and obesity, suggesting resistance to this ligand. FGF21 acts mainly on adipose tissue and ectopic fat accumulation is a typical feature in metabolic deterioration such as diabetes, metabolic syndrome, and cardiovascular disease.
Objective
To investigate the relationship between FGF21 resistance and ectopic fat accumulation.
Research design and methods
Subjects who underwent 64-slice multidetector CT (MDCT) were enrolled (n=190). Plasma FGF21 levels and MDCT data of ectopic fats at various sites were analyzed. Human visceral and subcutaneous fat tissues from abdominal and coronary artery bypass surgery were obtained. FGF21 receptor expression and postreceptor signaling in different fat deposits of both control and T2DM subjects were analyzed.
Results
Plasma FGF21 levels were significantly associated with body mass index, triglyceride, homeostatic model assessment of insulin resistance, and Matsuda index. Plasma FGF21 levels were significantly higher in patients with T2DM than in the pre-diabetes and normal glucose tolerance groups. The ectopic fat phenotypes (visceral, epicardial, intrahepatic, and intramuscular fat) of T2DM were significantly higher than controls. Plasma FGF21 levels were elevated and exhibited a strong positive correlation with ectopic fat accumulation in T2DM. The expression of genes comprising the FGF21 signaling pathway was also lower in visceral fat than in subcutaneous fat in this disease.
Conclusions
Human FGF21 resistance in T2DM could result from increases in FGF21-resistant ectopic fat accumulation. Our study provides novel clinical evidence linking FGF21 resistance and T2DM pathogenesis.
Keywords: insulin resistance, type 2 diabetes, adipocytokine, body fat distribution
Significance of this study.
What is already known about this subject?
Fibroblast growth factor 21 (FGF21) is a metabolic regulator that has beneficial effects on glucose and lipid metabolism. However, plasma FGF21 levels are paradoxically increased in type 2 diabetes mellitus (T2DM) and obesity, suggesting resistance to this ligand.
What are the new findings?
Various types of ectopic fat, especially epicardial fat, were positively associated with plasma FGF21 levels in patients with T2DM.
Protein expression of the FGF21 receptor dimer (FGFR1, β-klotho) and postreceptor signaling pathway-related proteins (p-P38, p-ERK) was not significantly different between the visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) in normal glucose tolerance subjects.
The expression of FGFR1, β-klotho, and p-P38 was lower in VAT than in SAT in subjects with T2DM, suggesting that in T2DM, visceral fat is more resistant to FGF21 signaling than subcutaneous fat.
How might these results change the focus of research or clinical practice?
An increase in the amount of ‘FGF21-resistant’ visceral fat in patients with T2DM could be one of the mechanisms explaining the observed paradoxical elevations in plasma FGF21.
Our study provides novel clinical evidence linking FGF21 resistance and T2DM pathogenesis.
These findings could provide novel insights for FGF21-related therapeutics.
Introduction
Fibroblast growth factor 21 (FGF21) is a metabolic regulator produced primarily by the liver that acts mainly through white adipose tissue.1 2 FGF21 treatment reduces plasma glucose, insulin, and lipid levels, as well as hepatic steatosis, in murine models of diabetes.3–5 Accordingly, FGF21 transgenic mice are resistant to diet-induced obesity (DIO) and fat accumulation.3 These beneficial effects of FGF21 suggest that FGF21 or an agonist could be a potential therapeutic agent for diabetes. In fact, drug discovery efforts have yielded an FGF21 analog,6 but its effect on glycemic control was not as robust as anticipated based on prior experiments using diabetic rodents. Another FGF21 analog was shown to decrease body weight and improve lipid profiles in subjects with type 2 diabetes mellitus (T2DM) without significant effects on glycemic control.7
Several human studies have revealed that plasma FGF21 levels are paradoxically increased in T2DM8 9 and insulin-resistant states such as non-alcoholic fatty liver disease.10 Furthermore, FGF21 concentrations are positively correlated with body mass index (BMI) or triglyceride (TG) levels in obese non-diabetic individuals.11 Serum FGF21 levels were also found to be significantly higher in patients with end-stage renal disease and coronary heart disease.12 13
It has been suggested that this paradoxical increase in plasma FGF21 levels could be a result of ‘FGF21 resistance’.14 In a previous study, the effect of FGF21 on signaling responses and target gene expression was attenuated in mice with DIO.15 The results of this animal study and the paradoxical increase in plasma FGF21 in patients with T2DM support the hypothesis that FGF21 resistance might play a role in obesity and T2DM. Further, fibroblast growth factor receptor 1 (FGFR1) and β-klotho dimers function as a receptor for FGF21.16 Accordingly, the suppression of FGFR1 and β-klotho expression has been suggested as a plausible mechanism underlying this FGF21 resistance.17 Among intracellular signaling pathways, extracellular signal-regulated kinase (ERK)15 and mitogen-activated protein kinases have also been suggested to be downstream of FGF21.18
In contrast to orthotopic subcutaneous fat, ectopic fat is defined as the deposition of TGs in ectopic sites or within cells of non-adipose tissue. Fat tissues in ectopic sites include visceral and epicardial fat and fat depositions in non-adipose tissue cells include intrahepatic and intramuscular fat.19 It is evident that ectopic fat is strongly associated with diabetes, metabolic syndrome, and cardiovascular disease.20–23 These observations suggest that ectopic fat plays a major pathogenic role in T2DM. However, little is known regarding the relationship between FGF21 and ectopic fat or the role of ectopic fat in FGF21 metabolism.
The aim of the present study was to investigate the relationship between FGF21 concentrations and various types of ectopic fat accumulation. Furthermore, to identify the mechanism underlying paradoxical FGF21 elevation in T2DM subjects, we tested FGF21 receptor expression and postreceptor signaling in different fat deposits of both control and T2DM subjects.
Research design and methods
Subjects
Three independent cohorts were investigated. Cohort 1 was enrolled to investigate the association between plasma FGF21 levels and ectopic fat phenotypes. Cohorts 2 and 3 were enrolled to investigate fat tissue FGF21 signaling-related gene expression profiles. Cohorts are described as follows.
Cohort 1: Subjects referred to Seoul National University Bundang Hospital from a routine physical check-up center and patients who received regular follow-up for pre-diabetes and drug-naive patients with T2DM were screened. Among them, subjects over 30 years of age who underwent 64-slice multidetector CT (MDCT) for the assessment of coronary artery disease (CAD) were enrolled. We excluded patients with malignant disease, chronic wasting diseases such as tuberculosis or malabsorption syndrome, advanced liver disease, and advanced renal disease. We excluded patients with cocaine consumption, chronic steroid use, or anabolic drug use. Finally, 190 unrelated subjects were enrolled in the study. We identified risk factors based on medical history, demographics, baseline clinical profiles, and concomitant medications. We analyzed plasma FGF21 levels and MDCT data for this cohort.
Cohort 2: Subjects who underwent coronary artery bypass graft surgery at Seoul National University Bundang Hospital were enrolled. Plasma FGF21 levels were analyzed in 40 subjects. Visceral adipose tissue (VAT) (intrathoracic preperitoneal) and subcutaneous adipose tissue (SAT) samples were obtained from eight non-diabetic controls and eight patients with diabetes.
Cohort 3: Subjects who underwent general abdominal surgery at Seoul National University Bundang Hospital were enrolled. For an additional non-diabetic control group, we obtained VAT and SAT from the eight non-diabetic subjects.
Measurement of anthropometric and biochemical parameters
Body weight, height, waist circumference, and blood pressure were measured when subjects were enrolled. BMI was calculated as the weight in kilograms divided by the square of the height in meters (kg/m2). Total cholesterol, TG, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate transaminase, alanine aminotransferase, fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), plasma insulin and C peptide levels were measured after at least a 12-hour fasting period. Postprandial 30 min and 2-hour glucose (PP2) levels were sampled during a 75 g oral glucose tolerance test (OGTT) in cohort 1. We defined T2DM as FPG≥126 mg/dL or PP2≥200 mg/dL, normal glucose tolerance (NGT) as FPG<100 mg/dL or PP2<140 mg/dL, and the remaining subjects as prediabetic. We calculated insulin resistance with the homeostasis model using the following validated formula: homeostatic model assessment of insulin resistance (HOMA-IR)=(fasting glucose (mg/dL)×fasting insulin (µIU/mL))/(22.5×18).24 In subjects with OGTT, Matsuda index (10 000/square root of [fasting glucose×fasting insulin]×[mean glucose×mean insulin during OGTT]) was used as the insulin sensitivity index.25 Plasma FGF21 levels were measured using an ELISA kit (BioVendor, Germany) with samples frozen at −80°C until the assay was performed.
CT protocol
A 64-slice MDCT was used to detect CAD during a routine health examination. Subjects with a heart rate >70 beats per minute received 10–30 mg of intravenous esmolol (Jeil Pharma, Seoul, Korea) before MDCT imaging. CT angiography was performed with a 64-slice MDCT scanner (Brilliance 64, Philips Medical Systems, Best, Netherlands). A standard scanning protocol described previously was employed.26 A 10 mm CT slice image of the abdomen (L4–L5) and a mid-thigh level were added to this protocol to assess other ectopic fat accumulation.
Fat assessment
Abdominal subcutaneous and visceral fat areas were assessed at the L4–L5 intervertebral disc space within a threshold of −250 to −50 Hounsfield units (HU) with a dedicated computer 3D workstation (Rapidia V.2.8, Infinitt, Seoul, Korea).27
Epicardial fat volume was measured by a single observer with the same software. Epicardial fat volume was segmented by isolating the epicardial fat and heart from the thorax using specific anatomical landmarks. The superior extent of the volume was determined by the point initiating the division of the main pulmonary artery. Inferiorly, the analysis volume was segmented from the liver and abdominal cavity by manually tracing the pericardium in the axial view every 5 mm from the top to bottom with software that automatically interpolated between the user-defined traces.28 After segmentation of the heart and epicardial adipose tissue from the remainder of the thorax, a threshold of −190 to −30 HU was applied to isolate adipose tissue-containing voxels. The adipose tissue voxels were then summed to provide an epicardial fat volume value in milliliters. The examinations were placed in random order with the observer blinded to other participant information.
Non-contrast CT was used to measure intrahepatic fat. Images were reviewed by a single observer blinded to other data. CT attenuation of three distinct circular areas was measured in addition to the spleen to generate mean values. Care was taken to avoid the inclusion of visually distinct vasculature and biliary structures in the regions of interest. Liver–spleen HU differences were calculated as the mean hepatic HU–mean splenic HU.29 Lower liver–spleen HU differences were considered to indicate higher intrahepatic fat accumulation.
Skeletal muscle attenuation was determined by measuring the mean value of all pixels within the range of 0 to +100 HU. The mid-thigh skeletal muscle area was compartmentalized into a normal density muscle area (+31 to +100 HU) and a low-density muscle area (0 to +30 HU).27 The low-density muscle area indicated an intramuscular fat area.
Fat tissue samples
During surgery, biopsies of adipose tissues were obtained after an overnight fast, washed in 9 g/L NaCl solution, sectioned into pieces, immediately frozen in a deep freezer, and stored −40°C. In cohort 2, visceral fat sample was obtained from the intrathoracic preperitoneal fat which is located in the fat compartment under xiphoid process from the anterior surface of the left lobe of the liver. Subcutaneous fat sample was obtained from the incision field. In cohort 3, visceral fat sample was obtained during laparoscopic surgery. Subcutaneous fat sample was obtained from the incision field. The surgeon aimed to obtain the samples from similar anatomical locations in all the subjects.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from frozen tissue samples using TRIzol (Ambion, CA, USA). For quantitative real-time PCR analysis, 3 µg of total RNA was reverse-transcribed using a High Capacity cDNA Reverse Transcription Kit (Thermo Scientific, CA, USA). SYBR Green reactions using the SYBR Green PCR Master mix (Enzynomics, Korea) were assembled along with 10 pM primers according to the manufacturer’s instructions and reactions were performed using the Applied Biosystems ViiA7 system (Thermo Scientific). Relative mRNA levels were calculated using the comparative CT method and normalized to cyclophilin mRNA. mRNA expression levels are displayed relative to those in visceral fat as a control. The sequences of all primers used are listed in online supplementary table 1.
bmjdrc-2019-000776supp002.pdf (59.2KB, pdf)
Western blotting
In brief, tissues were homogenized in tissue lysis buffer (Cell Signaling, MA, USA) supplemented with 1 mM phenylmethylsulfonyl fluoride. Protein concentrations were determined with the Bradford protein assay (Amresco, OH, USA). Then, 20 µg of protein was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis on a 10% Tris/HCl gel and transferred onto nitrocellulose membranes (Whatman, USA). Blotting with anti-FGFR1 (Abcam, UK), anti-β-klotho (R&D, MN, USA), anti-p-ERK (Cell Signaling), anti-p-P38 (Santa Cruz, Germany), and anti-β-actin (Sigma-Aldrich, MO, USA) antibodies was performed and the blots were developed with ECL prime (Amersham, USA). Results are expressed as fold change compared with data from visceral fat.
Statistical analysis
All values are expressed as means±SD or medians (IQR). For the analysis of variance (ANOVA) tests, clinical parameters and regional fat distribution that were not normally distributed were log-transformed to reduce skewedness; we presented the results by taking the antilogarithm for simple interpretation. Differences in parameters among groups were analyzed by one-way ANOVA or t-tests. When significantly different values were observed, a Bonferroni post hoc analysis was applied to determine the significance of the relationship between the means. Associations between parameters were identified using Pearson correlations. A p value less than 0.05 was considered significant. All analyses were performed using SPSS V.18.0 for Windows.
Results
Study population and clinical characteristics
According to glucose tolerance, the subjects in cohort 1 were divided three groups as follows: NGT, pre-diabetes, and T2DM. The clinical characteristics for the groups are shown in table 1. Eighty-nine (47%) subjects were classified as having T2DM, 53 (28%) subjects were classified as having pre-diabetes, and 48 (25%) subjects were in the NGT group. Age and blood pressure were similar but sex was significantly different among the three groups. BMI was significantly higher in the T2DM and pre-diabetes groups than in the NGT group. Based on lipid profiles, TG levels were significantly higher and HDL-C levels were significantly lower in the T2DM groups than in the NGT group. All glucose metabolic parameters including FPG, PP2, HbA1c, fasting insulin, fasting C peptide, and HOMA-IR levels were significantly higher for patients with T2DM and pre-diabetes compared with those in the NGT groups. The Matsuda index was significantly lower in the T2DM and pre-diabetes groups than in the NGT group.
Table 1.
Basal characteristics of subjects
| NGT (n=48) |
Pre-diabetes (n=53) |
T2DM (n=89) |
P value | |
| Age (years) | 57.8±7.5 | 57.6±10.4 | 56.4±9.3 | 0.606 |
| Male:female | 19:29 | 38:15 | 62:27 | 0.001 |
| WC (cm) | 88.5±8.8 | 89.7±6.0 | 89.7±8.0 | 0.813 |
| BMI (kg/m2) | 24.1±2.8 | 25.4±2.9* | 25.5±2.6† | 0.016 |
| SBP (mm Hg) | 124.5±11.6 | 128.1±15.6 | 127.7±13.0 | 0.353 |
| DBP (mm Hg) | 76.8±8.2 | 77.3±11.0 | 79.0±10.4 | 0.418 |
| Total cholesterol (mg/dL) | 214.6±30.5 | 212.8±41.1 | 211.5±47.7 | 0.718 |
| TG (mg/dL) | 137.3±78.3 | 165.7±104.1 | 233.6±280.9† | 0.028 |
| HDL-C (mg/dL) | 55.4±13.2 | 50.1±11.4 | 48.9±9.2† | 0.023 |
| LDL-C (mg/dL) | 118.8±26.5 | 122.2±35.6 | 113.4±32.0 | 0.312 |
| AST (IU/L) | 23.8±8.2 | 25.5±11.8 | 24.9±11.0 | 0.712 |
| ALT (IU/L) | 27.0±22.1 | 31.0±19.2 | 32.7±20.9 | 0.079 |
| FPG (mg/dL) | 92.1±5.4 | 110.5±8.5* | 146.9±44.2†‡ | <0.001 |
| PP2 (mg/dL) | 134.5±31.6 | 145.5±29.6 | 277.0±87.8†‡ | <0.001 |
| HbA1c (%) | 5.7±0.3 | 6.0±0.4* | 7.3±1.4†‡ | <0.001 |
| Fasting insulin (μIU/mL) | 8.5±3.1 | 11.9±5.1* | 11.2±4.7† | 0.003 |
| Fasting C peptide (ng/mL) | 1.4±0.5 | 2.2±0.9* | 2.2±0.9† | <0.001 |
| HOMA-IR | 1.9±0.7 | 3.3±1.4* | 4.1±2.1† | <0.001 |
| Matsuda index | 5.8±1.9 | 4.3±1.9* | 4.1±1.9† | 0.002 |
| FGF21 (pg/mL) | 102.2±90.6 | 114.7±107.5 | 161.2±152.2† | 0.041 |
Data are presented as mean±SD.
*NGT versus pre-diabetes.
†NGT versus type 2 diabetes.
‡Pre-diabetes versus type 2 diabetes.
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FGF21, fibroblast growth factor 21; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; NGT, normal glucose tolerance; PP2, postprandial 2-hour glucose; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TG, triglyceride; WC, waist circumference.
Relationship between FGF21 and the clinical parameters
Cohort 1: Plasma FGF21 levels were significantly associated with BMI, TG, HOMA-IR, and Matsuda index (r=0.212, p=0.008; r=0.292, p=0.001; r=0.246, p=0.004; r=−0.240, p=0.015, respectively; online supplementary table 2). The relationships between plasma FGF21 levels and serum glycemic variables such as FPG, PP2, and HbA1c were not significant. However, plasma FGF21 levels were significantly higher in patients with T2DM than in the pre-diabetes and NGT groups (161.2±152.2 pg/mL vs 114.7±107.5 and 102.2±90.6 pg/mL, respectively; p=0.041; table 1 and online supplementary figure 1A). Plasma FGF21 levels were not significantly different between males and females (129.8±123.1 pg/mL vs 137.4±137.6 pg/mL; p=0.862).
bmjdrc-2019-000776supp001.pdf (221.4KB, pdf)
Cohort 2: The clinical characteristics of this cohort are shown in online supplementary table 3. Similar to the results of cohort 1, plasma FGF21 levels were significantly higher in the T2DM group than in the NGT group (431.6±371.1 pg/mL vs 178.4±169.4 pg/mL; p=0.009; online supplementary figure 1B).
Ectopic fat accumulation
The ectopic fat phenotypes (visceral, epicardial, intrahepatic, and intramuscular fats) of cohort 1 are presented in the online supplementary table 4. Abdominal visceral fat area was significantly greater in T2DM and pre-diabetes groups than in subjects with NGT. Epicardial fat volume was significantly greater in subjects with T2DM than in subjects with NGT. Liver–spleen HU differences (intrahepatic fat) were marginally lower in the T2DM group than in NGT subjects, representing higher fat accumulation. Mid-thigh low-density muscle area (intramuscular fat) was also significantly greater in patients with T2DM than in subjects with NGT. However, abdominal subcutaneous fat was not significantly different between the groups. Further, each ectopic fat phenotype was strongly associated with each other (online supplementary figure 2).
Correlation between plasma FGF21 and ectopic fat accumulation
Among regional fat amounts, only epicardial fat was significantly associated with plasma FGF21 levels (r=0.189, p=0.019; online supplementary figure 2A). Based on subgroup analysis of patients with T2DM, all ectopic fat amounts were highly correlated with plasma FGF21 levels including visceral (r=0.240, p=0.046), epicardial (r=0.336, p=0.004), intrahepatic (r=0.308, p=0.028), and intramuscular (r=0.282, p=0.017) fats (online supplementary figure 2D and 3). However, subcutaneous fat and plasma FGF21 levels were not significantly associated (r=0.197, p=0.102). There were no significant correlations between plasma FGF21 levels and the indices of ectopic fat based on subgroup analysis with NGT and prediabetic subjects (online supplementary figure 2B,C).
Alterations in FGF receptor and signaling components in adipose tissue
We next investigated protein and RNA levels of FGF21 receptors and postreceptor signaling components in human VAT and SAT. In cohort 3 (control), protein expression of the FGF21 receptor dimer (FGFR1, β-klotho) and postreceptor signaling pathway-related proteins (p-P38, p-ERK) was not significantly different between the VAT and SAT (online supplementary figure 4). Similar results were found for the adipose tissues of the non-diabetic control group in cohort 2 (online supplementary figure 5). However, the protein expression of FGFR1, β -klotho, and p-P38 was significantly lower in the VAT than in the SAT of patients with T2DM in cohort 2 (figure 1). The expression of p-ERK was variable and not statistically different in each T2DM subject. The gene expression of FGF21 receptor dimer and PPARγ signaling components was not reached to statistical significance between VAT and SAT (online supplementary figure 6). However, it showed similar pattern of changes with respect to the protein expression of FGF21 receptors and postreceptor signaling in patients with T2DM.
Figure 1.
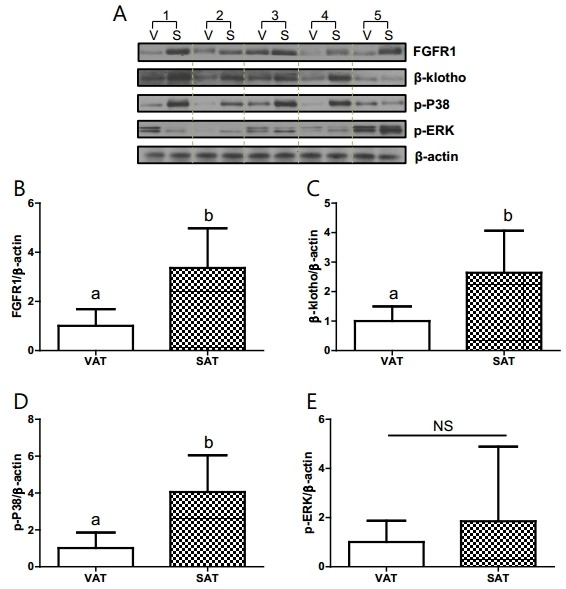
Protein expression of the FGFR dimer and signaling components in visceral and subcutaneous fat tissue of the type 2 diabetes mellitus (T2DM) group of subjects who underwent coronary artery bypass graft surgery (cohort 2). (A) Western blot analysis. (B) FGFR1. (C) β-klotho. (D) p-P38. (E) p-ERK. n=5/group. a,bSignificant differences (p<0.05). FGFR, fibroblast growth factor receptor; NS, non-significant; SAT, subcutaneous adipose tissue (S); VAT, visceral adipose tissue (V).
Discussion
The main findings of the present study are as follows: (1) plasma FGF21 levels are elevated in patients with T2DM; specifically plasma FGF21 was strongly associated with TG, BMI, and insulin resistance; (2) various types of ectopic fat, especially epicardial fat, were positively associated with plasma FGF21 levels in patients with T2DM; (3) the expression of FGFR1, β-klotho, and p-P38 was lower in VAT than in SAT in subjects with T2DM, suggesting that in T2DM, visceral fat is more resistant to FGF21 signaling than subcutaneous fat. Together, an increase in the amount of ‘FGF21-resistant’ visceral fat in patients with T2DM could be one mechanism explaining the observed paradoxical elevations in plasma FGF21.
In a previous study based on 76 healthy individuals, serum FGF21 levels were highly variable and did not strongly correlate with age, BMI, or sex.30 Further, FGF21 levels in 17 anorexic women (mean BMI, 16 kg/m2) were significantly lower than those in age-matched controls.31 Another study based on 232 Chinese subjects and our group also showed that FGF21 concentrations were significantly higher in obese subjects and correlated positively with adiposity, serum insulin, and TGs.11 32 In concordance with these previous studies, our clinical data also showed that plasma FGF21 levels are positively correlated with BMI, insulin resistance, and hypertriglyceridemia. These results suggest that adipose tissue might contribute to increased plasma FGF21 levels.
Ectopic fat has been suggested to play a key role in the development of insulin resistance and metabolic syndrome.33–35 Therefore, the quantification of ectopic fat accumulation could have a substantial role in risk stratification for metabolic syndrome. Nevertheless, it is difficult to obtain an accurate measurement of ectopic fat. Visceral fat amounts are relatively easy to measure using CT. Epicardial fat can also be measured by CT; however, more effort is required because of its three-dimensional shape. The gold standard of estimating intrahepatic fat and intramuscular fat is MR spectrometry. However, because this technique is not commonly applicable, these types of fat could be measured by CT.36 37 Few studies have comprehensively measured ectopic fat accumulation in various sites in a single subject. In this study, we evaluated ectopic fat accumulation comprehensively at four different sites in each individual subject.
Similar to a previous study,33 our data showed that all types of ectopic fat, and especially epicardial fat, are higher in subjects with T2DM. Further, plasma FGF21 levels were positively correlated with all four ectopic fat accumulation indices in patients with T2DM, but not in non-diabetic subjects. This result suggests that the cause of paradoxical elevated plasma FGF21 levels in T2DM could be a combination of two factors, specifically decreased ectopic fat FGF21 sensitivity (‘FGF21 resistance’) and increased amounts of FGF21-resistant ectopic fat tissue.
In animal studies, the systemic administration of FGF21 was found to result in a sustained decrease in blood glucose/TGs, improved insulin sensitivity, the amelioration of obesity/hepatosteatosis,1 4 5 decreased LDL-C, and elevated HDL-C,38 suggesting that FGF21 might have beneficial effects on metabolic diseases. However, ob/ob and DIO mice exhibit elevated FGF21 blood levels and are less sensitive to acute FGF21 administration.15 39 Such results suggest that these animals exhibit ‘FGF21 resistance’ resulting in a paradoxical FGF21 increase. Mechanistically, a reduced response to FGF21 treatment in a metabolically compromised state can be explained at the FGF21 receptor level, as FGFR1 and β-klotho transcripts are reduced in FGF21-responsive tissues in ob/ob and DIO mice, as compared with those in normal animals.15
Here, we analyzed human fat tissue grouped based on NGT and T2DM subjects. Components of the FGF21 receptor (FGFR1 and β-klotho) and proteins involved in postreceptor signaling (p-P38) were lower in visceral fat than in subcutaneous fat, suggesting that in T2DM, visceral fat is more resistant to FGF21 signaling. These results suggest that excessive ectopic fat accumulation, which is easily detected in patients with obesity, insulin resistance, and diabetes, could contribute to the exaggeration of FGF21 resistance. A previous study found that β-klotho levels were reduced in the visceral fat of high-fat diet (HFD)-induced obese mice.40 Further, a study on monkeys chronically fed an HFD demonstrated that HFD-resistant monkeys exhibited increased β-klotho levels in subcutaneous fat compared with those in HFD-sensitive monkeys.41 Moreover, elevated FGF21 in circulation and its action on adipose tissue lead to the accumulation of subcutaneous fat mass during DIO.42 The increase in β-klotho in subcutaneous fat and its decrease in visceral fat suggest that FGF21 preserves insulin sensitivity via its action on subcutaneous fat.42 FGF21 also acts in an autocrine fashion in white adipose tissue to stimulate PPARγ activity.43
In conclusion, plasma FGF21 levels are higher in patients with T2DM. Plasma FGF21 is also positively correlated with ectopic fat accumulation in this disease and FGF21 signaling in visceral fat is attenuated. Thus, ‘FGF21 resistance’ in T2DM could be a result of a combination of two factors, specifically decreased ectopic fat tissue FGF21 sensitivity (‘increased fat tissue FGF21 resistance’) and increased amounts of FGF21-resistant ectopic fat tissue. However, our study was performed in relatively small numbers of Asian control and patients with T2DM. Large human studies in various ethnic backgrounds are required to generalize this concept in human metabolic diseases. Our study supports to understand the mechanisms regulating FGF21 resistance and provides clinical insights to discovery of FGF21 therapeutics.
Footnotes
Contributors: ESH designed the study, carried out the research, analyzed and interpreted the results, and wrote the manuscript. CL contributed to obtaining tissue samples and for study design. HYC and YKL carried out sample preparation and the laboratory work. EJK, JHM, KSP, and HCJ helped plan the study and data analysis. SHC designed the study, contributed to the clinical research (patient enrollment, sample collection, and data analysis), and reviewed the manuscript.
Funding: This study is funded by the National Research Foundation of Korea (NRF-2018R1A5A2024425) to SHC.
Disclaimer: The funder had no involvement in study design, analysis of data, writing the report, and publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the local ethics committee (SNUBH IRB number B-1203/147-006, number A111218-CP02) and all subjects provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Badman MK, Pissios P, Kennedy AR, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–37. 10.1016/j.cmet.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 2.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007;5:415–25. 10.1016/j.cmet.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 3.Kharitonenkov A, Shiyanova TL, Koester A. Shanafelt AB: FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–27. 10.1210/en.2008-0816 [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009;58:250–9. 10.2337/db08-0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Dunbar JD. Kharitonenkov A: FGF21 as a therapeutic reagent. Adv Exp Med Biol 2012;728:214–28. [DOI] [PubMed] [Google Scholar]
- 7.Talukdar S, Zhou Y, Li D, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab 2016;23:427–40. 10.1016/j.cmet.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Chen W-W, Li L, Yang G-Y, et al. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2008;116:65–8. 10.1055/s-2007-985148 [DOI] [PubMed] [Google Scholar]
- 9.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, et al. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009;32:1542–6. 10.2337/dc09-0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010;139:456–63. 10.1053/j.gastro.2010.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Yeung DCY, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008;57:1246–53. 10.2337/db07-1476 [DOI] [PubMed] [Google Scholar]
- 12.Han SH, Choi SH, Cho BJ, et al. Serum fibroblast growth factor-21 concentration is associated with residual renal function and insulin resistance in end-stage renal disease patients receiving long-term peritoneal dialysis. Metabolism 2010;59:1656–62. 10.1016/j.metabol.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 13.Lin Z, Wu Z, Yin X, et al. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One 2010;5:e15534 10.1371/journal.pone.0015534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharitonenkov A, Larsen P. Fgf21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab 2011;22:81–6. 10.1016/j.tem.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 15.Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010;59:2781–9. 10.2337/db10-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fon Tacer K, Bookout AL, Ding X, et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 2010;24:2050–64. 10.1210/me.2010-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallego-Escuredo JM, Gómez-Ambrosi J, Catalan V, et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int J Obes 2015;39:121–9. 10.1038/ijo.2014.76 [DOI] [PubMed] [Google Scholar]
- 18.Chau MDL, Gao J, Yang Q, et al. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci U S A 2010;107:12553–8. 10.1073/pnas.1006962107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutrition, Metabolism and Cardiovascular Diseases 2010;20:481–90. 10.1016/j.numecd.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 20.Wajchenberg BL, Wajchenberg Bernardo Léo. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697–738. 10.1210/edrv.21.6.0415 [DOI] [PubMed] [Google Scholar]
- 21.Lautamäki R, Borra R, Iozzo P, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 2006;291:E282–90. 10.1152/ajpendo.00604.2005 [DOI] [PubMed] [Google Scholar]
- 22.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 1999;48:1600–6. 10.2337/diabetes.48.8.1600 [DOI] [PubMed] [Google Scholar]
- 23.Kim TH, Yu SH, Choi SH, et al. Pericardial fat amount is an independent risk factor of coronary artery stenosis assessed by multidetector-row computed tomography: the Korean atherosclerosis study 2. Obesity 2011;19:1028–34. 10.1038/oby.2010.246 [DOI] [PubMed] [Google Scholar]
- 24.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;23:57–63. 10.2337/diacare.23.1.57 [DOI] [PubMed] [Google Scholar]
- 25.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–70. 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 26.Choi E-K, Choi SI, Rivera JJ, et al. Coronary computed tomography angiography as a screening tool for the detection of occult coronary artery disease in asymptomatic individuals. J Am Coll Cardiol 2008;52:357–65. 10.1016/j.jacc.2008.02.086 [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Nam S, Ahn C, et al. Correlation between midthigh low-density muscle and insulin resistance in obese nondiabetic patients in Korea. Diabetes Care 2003;26:1825–30. 10.2337/diacare.26.6.1825 [DOI] [PubMed] [Google Scholar]
- 28.Wheeler GL, Shi R, Beck SR, et al. Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Invest Radiol 2005;40:97–101. 10.1097/00004424-200502000-00007 [DOI] [PubMed] [Google Scholar]
- 29.Shores NJ, Link K, Fernandez A, et al. Non-contrasted computed tomography for the accurate measurement of liver steatosis in obese patients. Dig Dis Sci 2011;56:2145–51. 10.1007/s10620-011-1602-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gälman C, Lundåsen T, Kharitonenkov A, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab 2008;8:169–74. 10.1016/j.cmet.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 31.Dostálová I, Kaválková P, Haluzíková D, et al. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab 2008;93:3627–32. 10.1210/jc.2008-0746 [DOI] [PubMed] [Google Scholar]
- 32.Lee Y, Lim S, Hong E-S, et al. Serum FGF21 concentration is associated with hypertriglyceridaemia, hyperinsulinaemia and pericardial fat accumulation, independently of obesity, but not with current coronary artery status. Clin Endocrinol 2014;80:57–64. 10.1111/cen.12134 [DOI] [PubMed] [Google Scholar]
- 33.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372–9. 10.2337/diacare.26.2.372 [DOI] [PubMed] [Google Scholar]
- 34.Wang C-P, Hsu H-L, Hung W-C, et al. Increased epicardial adipose tissue (eat) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol 2009;70:876–82. 10.1111/j.1365-2265.2008.03411.x [DOI] [PubMed] [Google Scholar]
- 35.Kelley DE, McKolanis TM, Hegazi RAF, et al. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 2003;285:E906–16. 10.1152/ajpendo.00117.2003 [DOI] [PubMed] [Google Scholar]
- 36.Ricci C, Longo R, Gioulis E, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol 1997;27:108–13. 10.1016/S0168-8278(97)80288-7 [DOI] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Kelley DE, Thaete FL, et al. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 2000;89:104–10. 10.1152/jappl.2000.89.1.104 [DOI] [PubMed] [Google Scholar]
- 38.Kharitonenkov A, Wroblewski VJ, Koester A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007;148:774–81. 10.1210/en.2006-1168 [DOI] [PubMed] [Google Scholar]
- 39.Berglund ED, Li CY, Bina HA, et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 2009;150:4084–93. 10.1210/en.2009-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Díaz-Delfín J, Hondares E, Iglesias R, et al. TNF-α represses β-Klotho expression and impairs FGF21 action in adipose cells: involvement of JNK1 in the FGF21 pathway. Endocrinology 2012;153:4238–45. 10.1210/en.2012-1193 [DOI] [PubMed] [Google Scholar]
- 41.Nygaard EB, Møller CL, Kievit P, et al. Increased fibroblast growth factor 21 expression in high-fat diet-sensitive non-human primates (Macaca mulatta). Int J Obes 2014;38:183–91. 10.1038/ijo.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Wu G, Fang Q, et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat Commun 2018;9:272 10.1038/s41467-017-02677-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutchak PA, Katafuchi T, Bookout AL, et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 2012;148:556–67. 10.1016/j.cell.2011.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000776supp002.pdf (59.2KB, pdf)
bmjdrc-2019-000776supp001.pdf (221.4KB, pdf)


