Abstract
Background/Objective:
To examine loss of community-dwelling status 9 months after hospitalization for high-acuity emergency general surgery (HA-EGS) disease among older Americans.
Design:
Retrospective analysis of claims data.
Setting:
US Communities with Medicare beneficiaries.
Participants:
Medicare Beneficiaries age ≥65 hospitalized urgently/emergently between Jan 1-Mar 31, 2015 with a principle diagnosis representing potential life or organ threat (necrotizing soft tissue infections, hernias with gangrene, ischemic enteritis, perforated viscus, toxic colitis or gastroenteritis, peritonitis, intra-abdominal hemorrhage) and an operation of interest on hospital days #1/2. (N=3319).
Measurements:
Demographic characteristics (age; race; sex), co-morbidities, principle diagnosis, complications, and index hospitalization disposition (died; discharged to skilled nursing [SNF], long-term acute care [LTAC], rehabilitation, hospice, home (with/without services), or acute care hospital; other) were measured. Survivors of index hospitalization were followed until Dec 31, 2015 mortality and community-dwelling status (SNF/LTAC vs not). Descriptive statistics, Kaplan-Meier plots, Chi2 tests were used to describe and compare the cohort based on disposition. A multivariable logistic regression model, adjusted for age, sex, co-morbidities, complications, and discharge disposition, determined independent predictors of loss of community-dwelling status at 9 months.
Results:
2922 (88%) survived index hospitalization. Likelihood of discharge to home decreased with increasing age, baseline co-morbidities, and in-hospital complications. 418 (14.3%) HA-EGS survivors died during the follow-up period. Among those alive at 9 months, 10.3% were no longer community-dwelling. Initial discharge disposition to any location other than home and ≥3 surgical complications during index hospitalization were independent predictors of residing in a SNF/LTAC 9 months after surviving HA-EGS.
Conclusion:
Older Americans, known to prioritize living in the community, will experience substantial loss of independence due to HA-EGS. Long-term expectations after surviving HA-EGS must be framed from the perspective of the outcomes older patients value the most. Further research is needed to examine the quality of life burden of EGS survivorship prospectively.
Keywords: Emergency General Surgery, Quality of Life, Community-Dwelling Status
INTRODUCTION
Americans are living longer and experiencing good quality of life even at advanced ages.1 The number of Americans age 65 and older is projected to increase from 40.2 million in 2010 to 88.5 million by 2050.2 This aging US population is disproportionately contributing to the growing demand and costs for emergency general surgery (EGS) care; of the more than 3 million EGS patients admitted annually in the US, currently 50% are ≥65 years old.3, 4 In anticipation of the aging of the US population, the estimated national costs of EGS care are expected to exceed $41 billion by 2060.5 Aging is associated with problems such as accumulating co-morbidities, polypharmacy, malnutrition, cognitive impairment and frailty which have been implicated in suboptimal outcomes for other emergency conditions as well as elective general surgery.6–13 These factors are less well studied among the EGS population.
Advances in science and technology have enabled heroic interventions—including emergency operations and peri-operative critical care interventions with ventilators, vasopressors, hemofiltration, and nutrition support—which, even with excellent baseline quality of life (QOL), cannot reverse the outcome of a catastrophic intra-abdominal or soft tissue infection. When presenting emergently with potentially life-threatening high-acuity EGS (HA-EGS) diseases such as gastrointestinal perforation, ischemic bowel, or necrotizing fasciitis, community dwelling Americans age 65 and older are at high risk of death. Cognitive decline (which might hinder compliance with treatment recommendations), reduced functional independence before disease onset, co-morbidities, and frailty are presumed etiological factors for the 16–50% 30-day mortality among older EGS patients that continues to rise in the year after discharge.14–19
Given that maintaining independence in their own communities is of paramount importance to aging Americans,20–22 understanding long-term disability among survivors of HA-EGS is perhaps as important as understanding mortality alone. There are a number of factors that might prevent a return to baseline QOL among EGS patients age 65 and older. Across all ages, 1 year after sudden critical illness, caused by intraabdominal and soft-tissue infection for example, 34% of survivors have cognitive function similar to brain injured patients, and 57% require caregiver assistance for one or more activities of daily living.23 Evidence suggests that physiologic strain of sepsis and critical illness is not well handled by the aging body despite high baseline functional status and cognition. Older survivors of sepsis are three times more likely to develop persistent impairments in both physical function and cognition as compared with equivalent aged community dwellers.24 Similarly, older patients with pre-existing frailty showed a downward trajectory in health-related QOL 12 months after ICU discharge.25 The objective of this epidemiologic study using Medicare claims data was to examine loss of community-dwelling status 9 months after hospitalization for HA-EGS disease among Medicare beneficiaries age 65 and older.
METHODS
A 100% sample of the Medicare claims data (MEDPAR) from 2015 was queried for subjects age ≥65yrs admitted emergently or urgently with a primary diagnosis of HA-EGS disease (see Supplementary Appendix S1) between January 1 and March 31 who also underwent a corresponding principal procedure of interest (see Supplementary Appendix S2) within two calendar days of admission. Given that ICD-10 coding was implemented in October of the study year, cohort selection was based on ICD-9 codes. We previously defined the list of EGS diagnoses that would typically be cared for by a general or acute care surgeon, starting with a broader list published by Shafi, et. al. in 2013 and narrowing it based on published reports on EGS care and acute care surgery as well as consensus among a research team conducting research on EGS practice patterns nationally.26 Surgery on the day of admission was used to categorize these as life-threatening EGS diagnoses (i.e., those that in real-world scenarios would require operation within 1–2 hours).26, 27 Those who did not undergo surgery for such a diagnosis may have had non-life threatening or non-critical degrees of illness (ie. a mild form of diverticulitis) or might have chosen to forego operative intervention due to previously determined goals of care. For the present manuscript, the requirement for operation within the first two calendar dates of admission was to confirm high-acuity, but not necessarily an immediately life-threatening, admitting diagnosis. In addition, primary diagnoses of appendicitis, cholecystitis, and simple soft tissue abscess, though noted to contribute to the overall national burden of EGS disease given their incidence,28 were excluded as they are typically well tolerated by most patients, including those in older age groups.29–31 Our goal in defining a group of high-acuity diagnoses was to focus on those older individuals at highest risk of debilitating outcomes. The 3-month inclusion time frame was selected to allow for 9 months of follow-up data for all subjects. For subjects with more than one unique EGS admission in the first 3 months of 2015, only the first admission for HA-EGS was selected. Patients admitted from skilled nursing facilities, long-term acute care facilities, and other or unknown locations that would suggest non-community dwelling status or potentially lead to misclassification of community dwelling status were also excluded.
Descriptive statistics were used to describe the study cohort demographic characteristics (age groups [65–74,75–84, 85–94, ≥95], race, sex); Elixhauser Index,32 admitting diagnosis category (necrotizing soft tissue infections, hernias with gangrene, ischemic enteritis, perforated viscus, toxic colitis or gastroenteritis, peritonitis, intra-abdominal hemorrhage), major surgical complications, major systemic complications (see Supplementary Appendix S3), and discharge disposition (died during index hospitalization, discharged to skilled nursing facility, discharged to long-term acute care facility [includes Medicaid facility and intermediate care facilities], discharged to rehabilitation hospital, discharged to hospice, discharged home with services, discharged to home, discharged to other acute care hospital or other [discharge disposition coded as “other,” “internally transferred,” or “unknown”]).
For the remaining analyses, we excluded those who died during the index hospitalization, were discharged to hospice, and other. We then used Chi2 tests of association first to compare overall characteristics and discharge disposition, and second to compare discharge disposition to status at 9 months (living at home [with our without services] vs. living in skilled nursing or long-term acute care facility). Time to death by initial discharge disposition was plotted using the Kaplan-Meier method. Finally, after excluding patients who died or who lacked continuous part A & B coverage during the follow-up period (to avoid loss of follow-up data), we used multivariable logistic regression analysis to predict likelihood of loss of community-dwelling status at 9 months while adjusting for key patient demographic and clinical characteristics that achieved a 0.2 p-value or smaller in univariate comparisons. Loss of community-dwelling status was determined by Medicare claims for skilled nursing, intermediate care, or long-term acute care facilities at 9 months. We chose this follow-up duration given that functional recovery for surgical patients over age 60 has been reported to be 3–6 months of longer.33
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary NC) and SPSS 25 (Armonk, NY: IBM Corp). This research was approved by the Ohio State University Institutional Review Board.
RESULTS
We identified 3319 Medicare beneficiaries meeting our inclusion criteria with HA-EGS disease (see Supplementary Figure S1: Flow Diagram). Of these, 273 (8.2%) died during the index hospitalization, 81 (2.4%) were discharged to hospice, and 43 (1.3%) had an other/unknown disposition. Table 1 shows the characteristics of this baseline community-dwelling HA-EGS inpatient cohort. The majority were white (88%) women (60%) under the age of 85 (81%) with 3 or more co-morbidities (65%). Colorectal emergencies (53%), perforated viscus (27%), and ischemic enteritis (14%) were the most common HA-EGS conditions. More than half of all patients did not experience a major surgical (59%) or systemic complication (54%).
Table 1.
Description of Overall Cohort of Community-Dwelling Medicare Beneficiaries Age 65 and Older Hospitalized in the First 3 months of 2015 with High-Acuity EGS Disease (N = 3319)
| Characteristic | N | % |
|---|---|---|
| Age | ||
| 65–74 | 1489 | 44.9 |
| 75–84 | 1200 | 36.2 |
| 85–94 | 587 | 17.7 |
| ≥95 | 43 | 1.3 |
| Sex | ||
| Male | 1327 | 40.0 |
| Female | 1992 | 60.0 |
| Race | ||
| White | 2934 | 88.4 |
| Black | 231 | 7.0 |
| Other | 36 | 1.1 |
| Asian | 36 | 1.1 |
| Hispanic | 41 | 1.2 |
| North American Native | 20 | 0.6 |
| Unknown | 21 | 0.6 |
| Elixhauser Index | ||
| 0 | 174 | 5.2 |
| 1 | 419 | 12.6 |
| 2 | 569 | 17.1 |
| ≥3 | 2157 | 65.0 |
| Admitting Diagnosis Category | ||
| Peritonitis | 21 | 0.6 |
| Hernias with gangrene | 104 | 3.1 |
| Ischemic enteritis | 476 | 14.3 |
| Perforated viscus | 895 | 27.0 |
| Toxic colitis or gastroenteritis | - | - |
| Intra-abdominal hemorrhage | - | - |
| Necrotizing soft tissue infections | 65 | 2.0 |
| Colorectal emergencies | 1751 | 52.8 |
| Major surgical complications | ||
| 0 | 1970 | 59.4 |
| 1 | 1087 | 32.8 |
| 2 | 228 | 6.9 |
| ≥3 | 34 | 1.0 |
| Major systemic complications | ||
| 0 | 1808 | 54.5 |
| 1 | 970 | 29.2 |
| 2 | 446 | 13.4 |
| ≥3 | 95 | 2.9 |
| Discharge Disposition | ||
| To home | 967 | 29.1 |
| To home with services | 687 | 20.7 |
| To rehabilitation hospital | 180 | 5.4 |
| To skilled nursing facility | 906 | 27.3 |
| To long-term acute care facility | 122 | 3.7 |
| To other acute care hospital | 60 | 1.8 |
| To hospice | 81 | 2.4 |
| Died during index hospitalization | 273 | 8.2 |
| Other1 | 43 | 1.3 |
Discharge code as “other,” “internally transferred,” or “unknown”
Intentionally blank due to cell sizes <10 per data use agreement
Table 2 compares these characteristics among index hospitalization survivors (N = 2922) by initial discharge disposition. Older age, a greater number of co-morbidities, and more complications (both systemic and post-operative) were associated with a decreased likelihood of discharge to home or home with services. However, even among the youngest age group (65–74), more than 20% of previous community dwellers ended up discharged to SNF or LTAC. Additionally, among those with Elixhauser Index = “0,” 9.2% ended up discharged to SNF or LTAC. Women were less likely than men to be discharged back to home without services.
Table 2.
Comparison of Demographic and Clinical Characteristics of Baseline Community-Dwelling Medicare Beneficiaries Age 65 and older1 Who Survived Hospitalization for High-Acuity Emergency General Surgery Disease2 During the First 3 months of 2015 by Discharge Disposition3 (N = 2922)
| Characteristic | Home N = 967 |
Home w/ Services N = 687 |
Rehab4 N = 180 |
SNF4 N = 906 |
LTAC4 N = 122 |
Acute Care Hospital N = 60 |
p-value5 |
|---|---|---|---|---|---|---|---|
| Age | <0.001 | ||||||
| 65–74 | 636 (45.9) | 344 (24.8) | 75 (5.4) | 248 (17.9) | 60 (4.3) | 24 (1.7) | |
| 75–84 | 274 (26.5) | 252 (24.4) | 64 (6.2) | 376 (36.4) | 39 (3.8) | 28 (2.7) | |
| 85–94 | 54 (11.5) | 85 (18.0) | 39 (8.3) | 265 (53.3) | 20 (4.2) | - | |
| ≥95 | - | - | - | 17 (54.8) | - | - | |
| Sex | <0.001 | ||||||
| Male | 432 (37.0) | 283 (24.2) | 70 (6.0) | 299 (25.6) | 54 (4.6) | 30 (2.6) | |
| Female | 535 (30.5) | 404 (23.0) | 110 (6.3) | 607 (34.6) | 68 (3.9) | 30 (1.7) | |
| Race6 | |||||||
| White | 848 (32.9) | 600 (23.3) | 166 (6.4) | 812 (31.5) | 102 (4.0) | 52 (2.0) | 0.049 |
| Black | 58 (28.4) | 61 (29.9) | - | 62 (30.4) | 13 (6.4) | - | |
| Other/Unknown | 26 (49.1) | 12 (22.6) | - | 11 (20.8) | - | - | |
| Asian | 13 (39.4) | - | - | - | - | - | |
| Hispanic | 13 (36.1) | - | - | - | - | - | |
| Elixhauser Index | <0.001 | ||||||
| 0 | 113 (67.7) | 36 (21.6) | - | 15 (9.0) | - | - | |
| 1 | 197 (49.7) | 103 (26.0) | 10 (2.5) | 76 (19.2) | - | - | |
| 2 | 222 (41.6) | 141 (26.4) | 30 (5.6) | 118 (22.1) | 11 (2.1) | 12 (2.2) | |
| ≥3 | 435 (23.8) | 407 (22.3) | 139 (7.6) | 697 (38.2) | 107 (5.9) | 40 (2.2) | |
| Admitting Diagnosis6 | <0.001 | ||||||
| Hernias with gangrene | 37 (38.5) | 25 (26.0) | - | 30 (31.3) | - | - | |
| Ischemic enteritis | 100 (21.0) | 87 (18.3) | 22 (4.6) | 109 (22.9) | 24 (50) | 15 (3.2) | |
| Perforated viscus | 240 (31.5) | 138 (18.1) | 51 (6.7) | 267 (35.0) | 48 (6.3) | 18 (2.4) | |
| Necrotizing soft tissue infections | 15 (25.4) | - | - | 20 (33.9) | - | - | |
| Colorectal emergencies | 570 (31.5) | 424 (26.1) | 99 (6.1) | 470 (28.9) | 41 (2.5) | 20 (1.2) | |
| Major surgical complications6 | <0.001 | ||||||
| 0 | 630 (36.4) | 388 (22.4) | 96 (5.5) | 519 (30.0) | 64 (3.7) | 34 (2.0) | |
| 1 | 291 (30.1) | 240 (24.8) | 62 (6.4) | 314 (32.4) | 39 (4.0) | 22 (2.3) | |
| 2 | 43 (21.5) | 56 (28.0) | 17 (8.5) | 64 (32.0) | 16 (8.0) | - | |
| Major systemic complications | <0.001 | ||||||
| 0 | 753 (43.9) | 422 (24.6) | 76 (4.4) | 416 (24.2) | 27 (1.6) | 23 (1.3) | |
| 1 | 176 (20.9) | 194 (23.0) | 70 (8.3) | 340 (40.3) | 45 (5.3) | 19 (2.3) | |
| 2 | 33 (11.0) | 65 (21.7) | 25 (8.3) | 123 (41.0) | 42 (14.0) | 12 (4.0) | |
| ≥3 | - | - | - | 27 (44.3) | - | - | |
| 9 month disposition6 | <0.001 | ||||||
| Home7 | 798 (37.6) | 554 (26.1) | 122 (5.7) | 564 (26.5) | 59 (2.8) | 28 (1.3) | |
| SNF/LTAC | 35 (14.3) | 38 (15.5) | 16 (6.5) | 132 (53.9) | 17 (6.9) | 7 (2.9) | |
| Died | 55 (13.2) | 73 (17.5) | 34 (8.1) | 192 (45.9) | 40 (9.6) | 24 (5.7) | |
| Unknown | 79 (59.0) | 22 (16.4) | - | 18 (13.4) | - | - |
Excludes those who died during index hospitalization (N = 273), were discharged to hospice (N = 81), or had an other/unknown discharge disposition (N =43).
Urgent/Emergent admission Code, emergency general surgery diagnosis of interest (see Supplementary Appendix S1), and operation of interest (see Supplementary Appendix S2) on hospital day #1 or #2.
Results presented as row percentages
Rehab = rehabilitation hospital; SNF = skilled nursing facility; LTAC = long-term acute care facility including a Medicaid facility and intermediate care facilities
Chi2 tests of association
Native American, peritonitis, toxic colitis/enteritis and intra-abdominal hemorrhage, and ≥3 major surgical complications not shown due to all cell counts <10
Home with or without services
Intentionally blank due to cell sizes <10 per data use agreement
Also shown in Table 2 are the 9 month dispositions of the 2,922 patients discharged alive but not to hospice or an unknown location. Four hundred eighteen (14.3%) of these index hospitalization survivors died during the 9 month follow-up period. Among those who died, discharge to SNF (45.9% of all patients who died) was most strongly associated with death in the following 9 months. Meanwhile, those discharged home without services had the greatest likelihood of being home (with or without) services at 9 months (37.6% of all patients residing at home), and those discharged to rehab were least likely to die (8.1% of all patients who died). Figure 1 is the Kaplan-Meier survival plot for these beneficiaries by discharge disposition. Discharge to SNF, LTAC, and other acute care hospitals was more strongly associated with 9-month mortality than discharge to home or home with services (Logrank χ2 (5) = 193.2, p<0.001).
Figure 1.
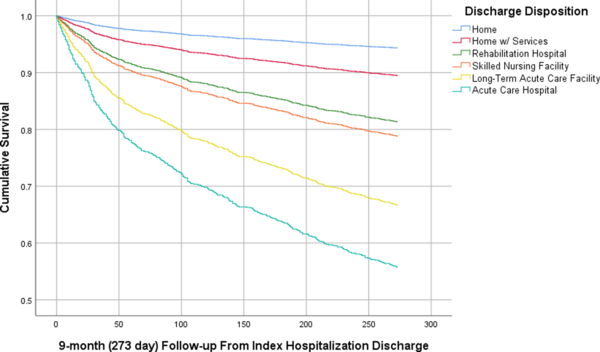
Kaplan-Meier Curve Modeling Time to Death By Discharge Disposition1 Among Medicare Beneficiaries Age 65 and older Hospitalized in the First 3 months of 2015 with High-Acuity Emergency General Surgery Disease who Survived to Discharge (N = 2922)
1. Excludes those who died during index hospitalization (N = 273), were discharged to hospice (N = 81), or had an other/unknown discharge disposition (N =43).
2. Long-term acute care facility includes a Medicaid facilities and intermediate care facilities
After excluding 134 patients lacking continuous Part A/B coverage (therefore, precluding our ability to follow their community dwelling status at 9 months), we were left with 2370 HA-EGS survivors at 9 months, among whom we were able to capture community-dwelling status. 245 (10.3%) were not community-dwelling (i.e., they were residing in an LTAC or SNF) 9 months after their HA-EGS hospitalization. Table 3 compares those living and not living in the community at 9 months. Increasing age, higher number of co-morbidities, and more surgical and systemic complications were all associated with higher likelihood lack of community dwelling status at 9 months. Discharge to another acute care hospital (20%), to an LTAC (22%) or to a SNF (19%) were all associated with lack of community dwelling status at 9 months. In multivariable models, after adjusting for significant factors in univariate comparisons (p-value <0.2), initial discharge disposition to any location other than home with or without services) and 3 or more surgical complications during index hospitalization were independent predictors of being in a SNF/LTAC 9 months after surviving HA-EGS hospitalization. For ease of review, the alternative outcome of being home with or without services (the inverse odds ratios of loss of community dwelling status) is also shown in the table.
Table 3.
Comparison of Characteristics of Medicare Beneficiaries Age 65 and older who Survived1 Hospitalization for High-Acuity Emergency General Surgery Disease2 during the First 3 months of 2015 Community Dwelling Status at 9-month Follow-up (N = 2370)
| Characteristic | Living at Home3 N=2125 (89.7) |
Living in SNF or LTAC4 N=245 (10.3) |
P-value5 |
|---|---|---|---|
| Age | |||
| 65–74 | 1055 (92.1) | 91 (7.9) | <0.001 |
| 75–84 | 769 (89.3) | 92 (10.7) | |
| 85–94 | 287 (83.2) | 58 (16.8) | |
| ≥95 | 14 (77.8) | - | |
| Race | |||
| White | 1887 (89.8) | 215 (10.2) | 0.95 |
| Black | 149 (89.2) | 18 (10.8) | |
| Asian | 21 (84.0) | - | |
| Hispanic | 21 (87.5) | - | |
| North American Native | 11 (91.7) | - | |
| Other/Unknown | 36 (90.0) | - | |
| Sex | |||
| Male | 822 (91.1) | 80 (8.9) | 0.07 |
| Female | 1303 (88.8) | 165 (12.2) | |
| Elixhauser Index | |||
| 0 | 138 (95.8) | - | <0.001 |
| 1 | 334 (94.4) | 20 (5.6) | |
| 2 | 424 (91.6) | 29 (8.4) | |
| ≥3 | 1229 (87.2) | 180 (12.8) | |
| Admitting Diagnosis Category | |||
| Peritonitis | 12 (75.0) | - | 0.26 |
| Hernias with gangrene | 75 (92.6) | - | |
| Ischemic enteritis | 258 (89.9) | 29 (10.1) | |
| Perforated viscus | 520 (90.0) | 58 (10.0) | |
| Colorectal Emergencies | 1221 (89.7) | 140 (10.3) | |
| Intra-abdominal hemorrhage | - | - | |
| Necrotizing soft tissue infections | 37 (84.1) | - | |
| Major surgical complications | |||
| 0 | 1256 (89.5) | 148 (10.5) | 0.05 |
| 1 | 713 (90.4) | 76 (9.6) | |
| 2 | 145 (90.1) | 16 (9.9) | |
| ≥3 | 11 (68.8) | - | |
| Major systemic complications | |||
| 0 | 1335 (91.7) | 121 (8.3) | <0.000 |
| 1 | 558 (85.7) | 93 (14.3) | |
| 2 | 194 (87.8) | 27 (12.2) | |
| ≥3 | 38 (90.5) | - | |
| Discharge Disposition | |||
| To home | 798 (95.8) | 35 (4.2) | <0.001 |
| To home with services | 554 (93.6) | 38 (6.4) | |
| To rehabilitation hospital | 122 (88.4) | 16 (11.6) | |
| To skilled nursing facility | 564 (81.0) | 132 (19.0) | |
| To long-term acute care facility | 59 (77.6) | 17 (22.4) | |
| To other acute care hospital | 28 (80.0) | - |
Excludes 418 survivors of index hospitalization who died during the follow-up period
Urgent/Emergent admission Code, emergency general surgery diagnosis of interest (see Supplementary Appendix S1), and operation of interest (see Supplementary Appendix S2) on hospital day #1 or #2.
With or without services
SNF = skilled nursing facility; LTAC = Long-term acute care facility including a Medicaid facility and intermediate care facilities
Chi2 tests of association
Intentionally blank due to cell sizes <10 per data use agreement
DISCUSSION
In this national study of Medicare beneficiaries age 65 and older who were community-dwelling before being hospitalized for HA-EGS disease, we found that a vast majority of patients, 88%, survived to discharge. However, a quarter of these survivors were dead or had experienced significant loss of independence, requiring SNF or LTAC care, in the nine months following discharge.
Mortality among the older patients with EGS diseases, both high-and low-acuity, is decreasing overall. For example, using a broader definition of EGS diseases, Armenia and colleagues used administrative data to show a mortality decline from 7.7% in 1993 to 6.5% in 2003 to 4.5% in 2013 among 65–79 year olds and from 13% to 11% to 7.3% in the same time periods among those age 80 and older.34 Similarly, Gale and colleagues using broad criteria for EGS disease, where fewer than 30% of EGS patients required surgery, reported overall mortality decline from 2.7% in 2001 to 1.6% in 2010.3 Unlike Gale, to focus on the sickest of older EGS patients, we used surgery within the first two days of admission to confirm the high-acuity nature of our EGS diseases of interest and found an overall mortality rate of 25% at one-year including both in-hospital death and death during our follow-up period. Like us, those who have reported rates of mortality among only those requiring urgent or emergent operations have found higher mortality rates with 12% 30-day mortality among 193 patients age 70 and older,35 and 1-year mortality of 34% both among 400 Medicare beneficiaries age 65 and older18 and 390 patients age 70 and older.16
Still, given that older EGS patients are overall experiencing improved survival, understanding the implications of HA-EGS survivorship is of paramount importance. It is widely acknowledged that the growing number of community-dwelling older Americans prioritize remaining independent in their own homes.20–22 Thus, the impact that HA-EGS disease has on older Americans who are quickly becoming the largest patient base among surgeons who provide EGS care is worthy of attention from a public health perspective and necessary to appropriately counsel older HA-EGS patients and their loved ones in the peri-operative period.4 However, in a recent review article intending to identify both mortality and functional outcomes after emergency major abdominal surgery in older adults, Cooper and colleagues found that none of the 92 studies undergoing full review assessed functional outcomes.19 Given that the reported rate of new, as well as persistent at 6 months follow-up, functional disability among elective abdominal surgery cohorts is substantial (ADL, 9% not at baseline; IADL, 19%; Medical Outcomes Study Short Form-36 [SF-36]; SF-36 Physical Component Scale, 16%; and SF-36 MCS, 17%, timed walk, 39%, Functional Reach, 58%, Grip Strength, 52%),36 presumably EGS patients would fare even worse as they lack opportunity for pre-operative medical optimization of co-morbidities and optimization of functional capacity through prehabilitation.
Therefore, the present findings derived from a national cohort of Medicare beneficiaries age 65 and older with more severe manifestations of EGS disease represent a novel contribution to the literature on long-term EGS outcomes. When assessing decisional regret after major surgical interventions – a phenomenon with an overall average prevalence of 14.4% in a review of 73 studies – post-operative quality of life was the most common reason for regret (38.4%).37 Our results, using loss of community-dwelling status as a proxy for loss of independence and decline in overall quality of life, suggest that at least 10% of those surviving initial hospitalization for HA-EGS may experience similar regret. We found that more than 44% of HA-EGS survivors (excluding those discharged to hospice) were discharged to a location other than home even though they were presumably successfully residing in a community-based setting prior to onset of disease. Furthermore, discharge to a location providing higher level of support was associated with almost dose-response (i.e., odds increased across discharge disposition from rehabilitation to SNF and to LTAC and Acute Care Hospital which shared similar odds) higher odds of having lost community-dwelling status 9 months after discharge which we found in more than 10% of our HA-EGS survivors. This suggests a substantial loss of functional status among our study population affecting even those who were presumed to have the ability to return to home based on discharge to rehabilitation hospital after index hospitalization. Interestingly, age and baseline co-morbidities did not predict odds of loss of community-dwelling status at 9 months, while multiple surgical complications and discharge status did. These findings suggest the physiologic toll of HA-EGS and associated treatments in and of themselves play a major role in later quality of life detriments. Our results are similar to those reported by Merani and colleagues regarding predictors of in-hospital mortality among octa-and nonagenerians undergoing emergency surgery; chronologic age and co-morbidities were not associated with death during index hospitalization.38
While baseline sarcopenia and frailty have been increasingly implicated in adverse EGS outcomes,39–42 these results are derived from small single center cohorts. Furthermore, while reported prevalence of frailty varies based on setting and measurement tool, a review of 21 studies examining frailty among community-dwelling older adults found an average prevalence of frailty of 11%.43 Such robust clinical information is typically not available in claims data thus precluding our ability in the present study to objectively measure either of these baseline risk factors. Future research should consider utilizing newly proposed techniques to measure frailty using claims data to study larger cohorts of elderly EGS patients.44–46 However, the impact of frailty and sarcopenia are ideally prospectively studied on a large scale across many older patients.
The majority of studies focusing on older individuals with EGS disease have focused on intra-abdominal pathologies.19, 35, 38–40 Similarly, the majority of our older cohort underwent urgent or emergent abdominal surgery for a variety of serious conditions. Depending on the indication for surgery and age breakdown, mortality for emergency abdominal surgery among the elderly is reported to range from 10 to 51%.19 Cooper and colleagues examined 400 emergency laparotomy patients and found that index mortality at 16% while it was over 34% at one year.18 Our finding of lower overall mortality may be due to secular trends (e.g., healthier patients overall, improved techniques) in overall EGS outcomes between Cooper’s study period (2000–2010) and ours. However, like Cooper, we found that mortality was associated, again with a dose-response type pattern, with discharge location.
In the present research, we include NSTIs as HA-EGS diseases. Interestingly, unlike with emergency abdominal operations as detailed above, very little is reported on the elderly with diagnosis with NSTI. This is likely due to the fact that overall, NSTIs represent a very small proportion of EGS patients as evident in our own findings of only 2% of HA-EGS patients having an NSTI diagnosis. In a single center study of 64 patients, Krieg and colleagues reported that 42% were over 60 years old.47 Two older studies noted that age was a risk factor for incidence of NSTI.48, 49 However, we reported that the overall incidence of NSTI was decreasing while the proportion of NSTI patients >65 years/Medicare beneficiaries was also decreasing.50 Overall, this suggests that in targeting areas of process improvement and optimal shared decision-making, focusing on intra-abdominal emergencies will be highest yield for aging populations.
Our findings must be interpreted in the context of our study limitations. First, as with all administrative data, diagnosis and procedure codes are subject to systematic coding errors. Second, such data lack clinical granularity for measuring both acute illness severity, frailty, and sarcopenia. In addition, we tried to define a cohort of HA-EGS patients using a combination of diagnosis and procedure codes, where the latter occurring on the day of or the day after admission a priori assumed to be confirmatory of HA-EGS disease. This study design component may lead to misclassification. However, since we focused on diagnosis codes that would be consistent with life-or organ-threatening disease, we hope this risk is minimized and the result is a more homogenous population of HA-EGS patients presented in this study. We also assumed that claims for skilled nursing or long-term care facility at 9 months indicated loss of community-dwelling status. It is possible that some of the patients with such claims on file had a later illness requiring short term institutionalization, another source of potential misclassification. Finally, our results may not be generalizable to populations older than 65 who are not covered by typical Medicare coverage (e.g., those on supplemental plans who might therefore lack continuous part A/B coverage during the follow-up period, or those covered by Veteran’s Administration plans).
Despite these limitations, we provide a perspective on the burden of surviving hospitalization for high-acuity emergency general surgery diagnoses such as perforated viscus and ischemic enteritis among community-dwelling Americans age 65 and older who are known to prioritize their independence. Surgeons and intensivists providing care to older individuals in urgent, possibly life-threatening situations, have little time to address overall goals of care before operation. Modern surgical and intensive care have greatly contributed to our ability provide care that results in survival to discharge. However, survival outcomes may not be concordant with patients’ QOL goals. Thus, it is imperative to appropriately counsel patients and families during the post-operative period on long-term expectations after discharge. Furthermore, in the context of improving overall mortality and QOL, measurement of functional outcomes needs to be prioritized in future research examining EGS outcomes among older populations.
Supplementary Material
Table 4.
Adjusted1 Odds of Loss of Community Dwelling Status2 at 9-months Among Medicare Beneficiaries Age 65 and older who Survived3 Hospitalization for High-Acuity Emergency General Surgery Disease4 during the First 3 months of 2015 (N = 2370)
| Characteristic | aOR Living in SNF or LTAC5 (95% CI) |
aOR Living at Home6 (95% CI) |
|---|---|---|
| Age | ||
| 65–74 | Ref | REF |
| 75–84 | 1.09 (0.79–1.50) | 0.92 (0.67–1.26) |
| 85–94 | 1.42 (0.97–2.07) | 0.71 (0.48–1.03) |
| ≥95 | 2.22 (0.69–7.18) | 0.45 (0.14–1.46) |
| Sex | ||
| Male | Ref | |
| Female | 1.13 (0.85–1.52) | 0.88 (0.66–1.18) |
| Elixhauser Index | ||
| 0 | Ref | |
| 1 | 1.04 (0.40–2.69) | 0.97 (0.37–2.50) |
| 2 | 1.46 (0.59–3.59) | 0.69 (0.28–1.69) |
| ≥3 | 1.82 (0.77–4.30) | 0.55 (0.23–1.31) |
| Major surgical complications | ||
| 0 | Ref | |
| 1 | 0.80 (0.59–1.08) | 1.25 (0.93–1.70) |
| 2 | 0.81 (0.46–1.42) | 1.23 (0.70–2.17) |
| ≥3 | 3.18 (1.03–9.79) | 0.31 (0.10–0.97) |
| Major systemic complications | ||
| 0 | Ref | |
| 1 | 1.26 (0.93–1.72) | 0.79 (0.58–1.08) |
| 2 | 0.83 (0.52–1.34) | 1.20 (0.74–1.94) |
| ≥3 | 0.60 (0.20–1.75) | 1.67 (0.57–4.89) |
| Discharge Disposition | ||
| To home | Ref | |
| To home with services | 1.42 (0.88–2.30) | 0.70 (0.44–1.14) |
| To rehabilitation hospital | 2.32 (1.22–4.41) | 0.43 (0.23–0.82) |
| To skilled nursing facility | 4.15 (2.72–6.31) | 0.24 (0.16–0.37) |
| To long-term acute care facility | 5.51 (2.83–10.8) | 0.18 (0.09–0.35) |
| To other acute care hospital | 5.49 (2.21–13.6) | 0.18 (0.07–0.45) |
Adjusted for all variables in Table 2 with p-value of 0.2 or smaller
SNF/LTAC = Living at home (with or without services)
Excludes 418 survivors of index hospitalization who died during the follow-up period
Urgent/Emergent admission Code, emergency general surgery diagnosis of interest (see Supplementary Appendix S1), and operation of interest (see Supplementary Appendix S2) on hospital day #1 or #2.
SNF = skilled nursing facility; LTAC = Long-term acute care facility including a Medicaid facility and intermediate care facilities
Home with or without services; data represents inverse of results presented in column 2
ACKNOWLEDGMENTS
Conflicts of Interest: Dr. Heena Santry and Dr. Quatman-Yates are paid consultants by the Johnson & Johnson Company for service on a fragility fracture advisory board. The content herein is unrelated to the topic of the advisory board and is in no way supported by the Johnson & Johnson Company. Dr. Quatman-Yates is also a paid consultant for Helius Medical Technologies for participation on medical advisory boards for projects unrelated to the study or information presented in this manuscript. Dr. Clark has received research funding from Regeneron Pharmaceuticals, Astellas Pharma Global Development, Inc., RTI Health Solutions, Ohio Department of Higher Education, and the Osteopathic Heritage Foundations. In the past 5-years Dr. Clark has received consulting fees from Regeneron Pharmaceuticals, Abbott Laboratories, and the Gerson Lehrman Group. Additionally, Brian Clark is co-founder with equity and Chief of Aging Research with AEIOU Scientific, LLC.
Sponsor’s Role: This research is supported by a grant from the Agency for Healthcare Research Quality (R01HS022694) to HPS and from the National Institute on Aging (NIH R01AG044424) to BCC. The content is solely the responsibility of the authors and does not represent the official views of the funding agencies.
Footnotes
SUPPLEMENTARY DATA: Procedure codes (ICD-9 and ICD-10) for emergency general surgery and surgical complications.
REFERENCES
- 1.Rowe JW, Fulmer T, Fried L. Preparing for Better Health and Health Care for an Aging Population. JAMA. 2016;316: 1643–1644. [DOI] [PubMed] [Google Scholar]
- 2.Vincent G, Velkoff V. THE NEXT FOUR DECADES The Older Population in the United States: 2010 to 2050. 2010. [Google Scholar]
- 3.Gale SC, Shafi S, Dombrovskiy VY, Arumugam D, Crystal JS. The public health burden of emergency general surgery in the United States: A 10-year analysis of the Nationwide Inpatient Sample−−2001 to 2010. J Trauma Acute Care Surg 2014;77: 202–208. [DOI] [PubMed] [Google Scholar]
- 4.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg 2003;238: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogola GO, Gale SC, Haider A, Shafi S. The financial burden of emergency general surgery: National estimates 2010 to 2060. J Trauma Acute Care Surg 2015;79: 444–448. [DOI] [PubMed] [Google Scholar]
- 6.Hickey A, Clinch D, Groarke EP. Prevalence of cognitive impairment in the hospitalized elderly. Int J Geriatr Psychiatry. 1997;12: 27–33. [DOI] [PubMed] [Google Scholar]
- 7.Di Iorio A, Longo AL, Mitidieri Costanza A, et al. Characteristics of geriatric patients related to early and late readmissions to hospital. Aging (Milano). 1998;10: 339–346. [DOI] [PubMed] [Google Scholar]
- 8.Sales AE, Tipton EF, Levine DA, et al. Are co-morbidities associated with guideline adherence? The MI-Plus study of Medicare patients. J Gen Intern Med 2009;24: 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med 2007;356: 2496–2504. [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59: 255–263. [DOI] [PubMed] [Google Scholar]
- 11.Amrock LG, Neuman MD, Lin HM, Deiner S. Can routine preoperative data predict adverse outcomes in the elderly? Development and validation of a simple risk model incorporating a chart-derived frailty score. J Am Coll Surg 2014;219: 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JL, Henderson MA, Revenig LM, et al. Frailty and one-year mortality in major intra-abdominal operations. J Surg Res 2016;203: 507–512 e501. [DOI] [PubMed] [Google Scholar]
- 13.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010;210: 901–908. [DOI] [PubMed] [Google Scholar]
- 14.Svenningsen P, Manoharan T, Foss NB, Lauritsen ML, Bay-Nielsen M. Increased mortality in the elderly after emergency abdominal surgery. Danish medical journal. 2014;61: A4876. [PubMed] [Google Scholar]
- 15.Wilson I, Paul Barrett M, Sinha A, Chan S. Predictors of in-hospital mortality amongst octogenarians undergoing emergency general surgery: a retrospective cohort study. Int J Surg 2014;12: 1157–1161. [DOI] [PubMed] [Google Scholar]
- 16.Rangel EL, Cooper Z, Olufajo OA, et al. Mortality after emergency surgery continues to rise after discharge in the elderly: Predictors of 1-year mortality. J Trauma Acute Care Surg 2015;79: 349–358. [DOI] [PubMed] [Google Scholar]
- 17.Joray S, Wietlisbach V, Bula CJ. Cognitive impairment in elderly medical inpatients: detection and associated six-month outcomes. Am J Geriatr Psychiatry. 2004;12: 639–647. [DOI] [PubMed] [Google Scholar]
- 18.Cooper Z, Mitchell SL, Gorges RJ, Rosenthal RA, Lipsitz SR, Kelley AS. Predictors of Mortality Up to 1 Year After Emergency Major Abdominal Surgery in Older Adults. J Am Geriatr Soc 2015;63: 2572–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper Z, Scott JW, Rosenthal RA, Mitchell SL. Emergency Major Abdominal Surgical Procedures in Older Adults: A Systematic Review of Mortality and Functional Outcomes. J Am Geriatr Soc 2015;63: 2563–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox S, Kenny L, Day MR, O’Connell C, Finnerty J, Timmons S. Exploring the Housing Needs of Older People in Standard and Sheltered Social Housing. Gerontol Geriatr Med 2017;3: 2333721417702349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Center BP. Healthy Aging Begins at Home. 2016. [Google Scholar]
- 22.Rivera-Hernandez M, Yamashita T, Kinney JM. Identifying Naturally Occurring Retirement Communities: A Spatial Analysis. The Journals of Gerontology: Series B. 2014;70: 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chelluri L, Im KA, Belle SH, et al. Long-term mortality and quality of life after prolonged mechanical ventilation. Crit Care Med 2004;32: 61–69. [DOI] [PubMed] [Google Scholar]
- 24.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304: 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 2015;43: 973–982. [DOI] [PubMed] [Google Scholar]
- 26.Daniel VT, Rushing AP, Ingraham AM, et al. Association Between Enhanced Overnight Operating Room Access and Mortality for True Life-Threatening Surgical Disease. J Trauma 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rushing AP, Baselice HB, Daniel VT, et al. Peri-operative Critical Care Practices and Mortality Among Patients with General Surgery Emergencies. Society of Critical Care Medicine, 2018. [Google Scholar]
- 28.Scott JW, Olufajo OA, Brat GA, et al. Use of national burden to define operative emergency general surgery. JAMA Surgery. 2016;151: e160480. [DOI] [PubMed] [Google Scholar]
- 29.Shah AA, Zafar SN, Kodadek LM, et al. Never giving up: outcomes and presentation of emergency general surgery in geriatric octogenarian and nonagenarian patients. Am J Surg 2016;212: 211–220.e213. [DOI] [PubMed] [Google Scholar]
- 30.Dowgiallo-Wnukiewicz N, Kozera P, Wojcik W, Lech P, Rymkiewicz P, Michalik M. Surgical treatment of acute appendicitis in older patients. Polski przeglad chirurgiczny. 2019;91: 12–15. [DOI] [PubMed] [Google Scholar]
- 31.Loozen CS, van Ramshorst B, van Santvoort HC, Boerma D. Early Cholecystectomy for Acute Cholecystitis in the Elderly Population: A Systematic Review and Meta-Analysis. Digestive surgery. 2017;34: 371–379. [DOI] [PubMed] [Google Scholar]
- 32.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36: 8–27. [DOI] [PubMed] [Google Scholar]
- 33.Deiner S, Silverstein JH. Long-term outcomes in elderly surgical patients. Mt Sinai J Med 2012;79: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armenia SJ, Pentakota SR, Merchant AM. Socioeconomic factors and mortality in emergency general surgery: trends over a 20-year period. Journal of Surgical Research. 2017;212: 178–186. [DOI] [PubMed] [Google Scholar]
- 35.Sharrock AE, McLachlan J, Chambers R, Bailey IS, Kirkby-Bott J. Emergency Abdominal Surgery in the Elderly: Can We Predict Mortality? World J Surg 2017;41: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. Journal of the American College of Surgeons. 2004;199: 762–772. [DOI] [PubMed] [Google Scholar]
- 37.Wilson A, Ronnekleiv-Kelly SM, Pawlik TM. Regret in Surgical Decision Making: A Systematic Review of Patient and Physician Perspectives. World Journal of Surgery. 2017;41: 1454–1465. [DOI] [PubMed] [Google Scholar]
- 38.Merani S, Payne J, Padwal RS, Hudson D, Widder SL, Khadaroo RG. Predictors of in-hospital mortality and complications in very elderly patients undergoing emergency surgery. World J Emerg Surg 2014;9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dirks RC, Edwards BL, Tong E, et al. Sarcopenia in emergency abdominal surgery. J Surg Res 2017;207: 13–21. [DOI] [PubMed] [Google Scholar]
- 40.Rangel EL, Rios-Diaz AJ, Uyeda JW, et al. Sarcopenia increases risk of long-term mortality in elderly patients undergoing emergency abdominal surgery. J Trauma Acute Care Surg 2017;83: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 41.Joseph B, Zangbar B, Pandit V, et al. Emergency General Surgery in the Elderly: Too Old or Too Frail? J Am Coll Surg 2016;222: 805–813. [DOI] [PubMed] [Google Scholar]
- 42.McIsaac DI, Moloo H, Bryson GL, van Walraven C. The Association of Frailty With Outcomes and Resource Use After Emergency General Surgery: A Population-Based Cohort Study. Anesth Analg 2017;124: 1653–1661. [DOI] [PubMed] [Google Scholar]
- 43.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. Journal of the American Geriatrics Society. 2012;60: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 44.McIsaac DI, Wong CA, Huang A, Moloo H, van Walraven C. Derivation and Validation of a Generalizable Preoperative Frailty Index Using Population-based Health Administrative Data. Ann Surg 2018. [DOI] [PubMed] [Google Scholar]
- 45.Kim DH, Glynn RJ, Avorn J, et al. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. The journals of gerontology Series A, Biological sciences and medical sciences. 2018;73: 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krieg A, Dizdar L, Verde PE, Knoefel WT. Predictors of mortality for necrotizing soft-tissue infections: a retrospective analysis of 64 cases. Langenbeck’s Archives of Surgery. 2014;399: 333–341. [DOI] [PubMed] [Google Scholar]
- 48.Awsakulsutthi S. A retrospective review of necrotizing fasciitis in Thammasat University Hospital. J Med Assoc Thai 93 Suppl 7: S246–253. [PubMed] [Google Scholar]
- 49.Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: Clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med 1997;103: 18–24. [DOI] [PubMed] [Google Scholar]
- 50.Psoinos CM, Flahive JM, Shaw JJ, et al. Contemporary trends in necrotizing soft-tissue infections in the United States. Surgery. 2013;153: 819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


