Abstract
Background:
C-reactive protein (CRP) is an inflammatory biomarker used in vascular risk prediction, though with less data in non-white populations. Blacks have higher stroke incidence and also higher CRP than whites. We studied the association of CRP on ischemic stroke risk in blacks and whites.
Methods:
REGARDS, an observational cohort study, recruited and followed 30,239 black and white Americans aged 45 and older for ischemic stroke. We calculated hazard ratios (HR) and 95% confidence intervals of ischemic stroke by CRP category (<1, 1 to 3, 3-10, and ≥10 mg/L), adjusted for age, sex and stroke risk factors.
Results:
There were 292 incident ischemic strokes among blacks and 439 in whites over 6.9 years follow-up. In whites, the risk was elevated for CRP in the range from 3 to 10 mg/L and even higher for CRP >10 mg/L, while in blacks an association was only seen for CRP >10 mg/L. Considered as a continuous variable, the the risk factor-adjusted HRs per SD higher lnCRP were 1.18 (95% CI 1.09 - 1.28) overall, 1.14 (95% CI 1.00 - 1.29) in blacks and 1.22 (95% CI 1.10 - 1.35) in whites. Spline regression analysis visually confirmed the race difference in the association.
Conclusions:
CRP may not be equally useful in stroke risk assessment in blacks and whites. Confirmation, similar study for coronary heart disease, and identification of reasons for these racial differences require further study.
Keywords: Stroke, Inflammation, Racial Disparity, Epidemiology, Risk Factors, C-reactive protein
Introduction
Stroke is the fifth leading cause of death in the United States,1 with a disproportionate burden in blacks compared to whites; at ages 45 to 65 years death from stroke is three times higher for blacks, a difference that diminishes at older ages.2 This racial disparity in stroke has existed for decades and is increasing, with temporal declines in stroke mortality marginally higher in whites than blacks.3 Only recently, studies elucidated that about one-half of this disparity is attributable to traditional stroke risk factors 4, suggesting that novel risk factors including biomarkers could play a role.5-8
C-reactive protein (CRP) is an extensively studied inflammation biomarker associated with risk of cardiovascular events and stroke. CRP measurement is advocated by a multisociety guideline to risk classify patients for primary prevention interventions such as statin use.9 Even though CRP measurement is recommended, blacks have substantially higher CRP levels than whites independent of other factors11, and there is minimal literature on the association of CRP with cardiovascular disease and stroke in blacks.12
We addressed gaps in the literature by studying whether the association of CRP with stroke risk differed in blacks compared to whites. Specifically, given that CRP is higher in blacks than whites, a single threshold value for risk prediction (currently ≥2 or >3 mg/L) may not accurately reflect risk in both racial groups, and we hypothesized that a higher value might be more appropriate among blacks.
Methods
Study participants and data collection
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a longitudinal observational study addressing why blacks have higher stroke mortality than whites in the United States. A population sample of 30,239 participants were recruited by mail and telephone between 2003-7 as previously described.13 We included 42% black and 58% white participants ≥45 years of age with oversampling of participants residing in the Southeastern stroke belt (56%; a region with higher stroke mortality than the rest of the country); 44% of the cohort resided in the remaining 40 contiguous states.
Participant characteristics were obtained via a computer-assisted telephone interview followed by in-home examination. The interview obtained demographic information, medical information, and verbal informed consent. The in-home examiner measured blood pressure, performed an electrocardiogram (ECG), collected blood and urine samples, and obtained written informed consent. Blood was processed and shipped to a central laboratory14 and CRP measured using a high-sensitivity immunonephelometric method. Characterization of CRP correlates in REGARDS by race has been published.11 Study methods were approved by institutional review boards at all participating institutions.
The following participants were excluded from analysis: those with data anomalies (n=56), pre-baseline stroke or TIA (n=l,930), missing CRP (n=l,773), and no follow-up (n=407). After exclusions, the analysis dataset included 26,069 participants.
All study funding was from the National Institutes of Health. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Definitions of Baseline Variables
Race was determined by self-report as black or white. Hypertension was defined as blood pressure >140/90 mmHg or use of antihypertensive medications. Heart disease was defined by ECG evidence of MI, self-report of a physician diagnosis of MI, or self-reported coronary artery bypass, angioplasty or stent. Prebaseline stroke was determined by self-report of a physician diagnosis. Diabetes was defined as fasting glucose >126 mg/dL or self-reported use of diabetic medications. Atrial fibrillation was ascertained by ECG or self-report of a physician diagnosis. Left ventricular hypertrophy was defined by ECG.
CRP was categorized as <1 mg/L, 1 to 3 mg/L, ≥3-10 mg/L, and ≥10 mg/L.15 The cutoff of ≥10 mg/L was used to determine if marked elevation in CRP previously reported in relation to coronary disease risk (but also suggested by guidelines to indicate inflammation due to other causes than vascular risk) reflected a degree of inflammation important in stroke risk.16
Stroke ascertainment
Participants and/or proxies were contacted every 6 months by telephone to identify potential strokes and medical records were obtained in the case of positive responses. Two expert physicians independently reviewed medical records to validate stroke. Stroke verification and etiologic subtyping was based on methods developed by previous stroke studies.2,13 The endpoint in this analysis was fatal and non-fatal ischemic stroke through October 31, 2014.
Statistical Analysis
Participant characteristics were tabulated. Associations of baseline CRP with stroke risk in the entire population, and in blacks and whites was analyzed. With our focus on etiologic relationships between CRP and ischemic stroke, cumulative incidence functions were used to display the time to incident ischemic stroke, accounting for the competing risks of hemorrhagic/unknown type stroke and death.17 Cause-specific Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) of ischemic stroke by CRP, accounting for the competing risks above, and censoring at hemorrhagic stroke, death or loss to follow-up.17, 18 Models were adjusted for baseline age, race, sex and then further adjusted for baseline Framingham stroke risk factors: hypertension status, systolic blood pressure, history of heart disease, diabetes, current smoking, left ventricular hypertrophy and atrial fibrillation. A third model additionally adjusted for household income and education. Interaction terms for race*CRP as a continuous variable (log-transformed CRP) were evaluated to determine the statistical difference in the association of CRP with stroke by race. Proportional hazard assumptions for the cause-specific Cox models were assessed with formal statistical tests.19 In addition to standard clinical cut-points, the relationship between continuous CRP and ischemic stroke risk was modeled using penalized splines separately by race.Statistical analyses were performed with SAS 9.4 and R 3.5.1.20, 21
Results
Participant Characteristics and Stroke Incidence
Baseline characteristics by race are shown in Supplemental Table 1, and by CRP categories stratified by race in Table 1. While blacks had a more unfavorable risk profile than whites, correlations of risk factors with CRP were similar by race. With average follow-up of 6.9 years, 730 (2.8%) participants had an incident ischemic stroke, for an incidence rate of 4.0 per 1000 person years (95% CI 3.8 – 4.4). Three participants with cerebral hemorrhage prior to their ischemic stroke were censored and not included as having ischemic stroke. There were 292 ischemic strokes among blacks and 438 in whites; respective incidence rates 3.9 per 1000 person-years (95% CI 3.5 – 4.3) and 4.2 per 1000 (95% CI 3.8 – 4.7). The mean ± SD follow-up was 6.7 ± 2.8 years among blacks and 7.1 ± 2.5 years among whites. Death rates were 19.4 per 1000 person-years among blacks (95% CI 18.4 – 20.5) and 16.9 per 1000 among whites (95% Cl 16.1 −17.6). The hemorrhagic stroke rate was 0.5 per 1000 for whites (95% CI 0.4 – 0.6) and 0.5 per 1000 for blacks (95% CI 0.4 – 0.7). Supplemental Table 2 shows that participants with incident ischemic stroke were more likely to be older males with traditional stroke risk factors and were more likely to have CRP ≥3 mg/L.
Table 1.
Baseline characteristics by C-reactive protein category and race.
| Characteristic mean or frequency (%) |
C-Reactive Protein Categories | ||||
|---|---|---|---|---|---|
| <1 mg/L | 1-3 mg/L | 3-10 mg/L | ≥10 mg/L | ||
| Age (mean) | Black | 64.2 ± 9.8 | 64.2 ± 9.3 | 63.5 ± 8.9 | 63.1 ± 8.8 |
| White | 64.8 ± 9.7 | 65.6 ± 9.3 | 65.1 ± 9.3 | 65.2 ± 9.4 | |
| Sex (male) | Black | 50 | 43 | 31 | 24 |
| White | 58 | 51 | 41 | 42 | |
| Hypertension | Black | 61 | 69 | 73 | 78 |
| White | 40 | 50 | 56 | 58 | |
| Diabetes | Black | 24 | 27 | 30 | 36 |
| White | 12 | 13 | 17 | 23 | |
| Current smoker | Black | 15 | 15 | 19 | 20 |
| White | 9 | 11 | 16 | 21 | |
| Left ventricular hypertrophy | Black | 11 | 14 | 16 | 15 |
| White | 5 | 6 | 8 | 7 | |
| Atrial Fibrillation | Black | 6 | 7 | 8 | 9 |
| White | 8 | 9 | 10 | 13 | |
| History of cardiovascular disease | Black | 12 | 14 | 14 | 18 |
| White | 17 | 19 | 19 | 23 | |
| High school graduate education or less | Black | 42 | 45 | 48 | 51 |
| White | 25 | 31 | 35 | 41 | |
| Income <$20,00 | Black | 21 | 23 | 28 | 34 |
| White | 8 | 10 | 13 | 18 | |
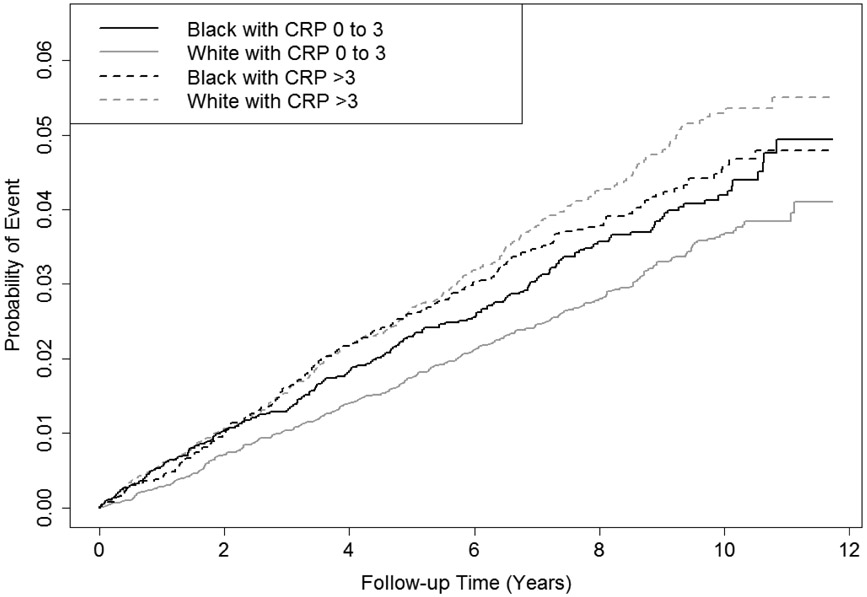
Figure 1 shows the cumulative ischemic stroke incidence stratified by race and CRP, accounting for the competing risks of hemorrhagic stroke and death. Among whites with elevated CRP, the rate was higher throughout follow-up compared to whites with normal CRP (p < 0.0001). In blacks, the difference in ischemic stroke incidence by CRP was also apparent, but the magnitude of difference was smaller than in whites and was not statistically significant (p = 0.39). There was no evidence to contradict the proportionality assumptions of the models used.
Figure 1.
Cumulative Incidence of Ischemic Stroke Stratified by Race and CRP.
Association of CRP with Stroke Incidence
Associations of CRP categories with ischemic stroke risk overall and by race is shown in Table 2. Compared to CRP <1 mg/L, in all models the ischemic stroke risk increased monotonically by increasing CRP category among whites. The risk was elevated for CRP 3-10 mg/L and even higher for CRP >10 mg/L (fully-adjusted HR 2.07 (95% CI 1.46 - 2.95)). In contrast, for all models in blacks ischemic stroke risk was only increased for CRP >10 mg/L. Although the HRs of stroke by CRP categories in blacks compared to whites were different, the interaction term for lnCRP*race suggested these differences were not statistically significant (p race*lnCRP >0.10 in all models). In the sequential models in blacks and whites separately, adjustment for stroke risk factors modestly attenuated the association of CRP with ischemic stroke, and there was minimal confounding by socioeconomic factors. Considered as a continuous variable, the risk factor-adjusted HRs per SD higher lnCRP were 1.18 (95% CI 1.09 - 1.28) overall, 1.14 (95% CI 1.00 - 1.29) in blacks and 1.22 (95% CI 1.10 - 1.35) in whites. In all models, added adjustment for baseline use of statins, regular aspirin or warfarin did not materially alter the hazard ratios (data not shown).
Table 2.
Cause-Specific Hazard Ratio of Ischemic Stroke by CRP Categories for Races Combined and Separately
| Hazard Ratios of Ischemic Stroke by C-reactive Protein Categories | |||||||
|---|---|---|---|---|---|---|---|
| Group | <1 mg/L | 1-3 mg/L | 3-10 mg/L | ≥10 mg/L | P for trend |
Interaction p Values for Race * lnCRP / Race * Categorical CRP |
|
| n Cases / N Non-Cases (%) | |||||||
| Adjustment Variables | All | 156 / 6,893 (2.3%) | 240 / 8,710 (2.8%) | 233 / 7,991 (2.9%) | 101 / 2,475 (4.1%) | ||
| Black | 51 / 2,131 (2.4%) | 94 / 3,207 (2.9%) | 99 / 3,651 (2.7%) | 52 / 1,396 (3.7%) | |||
| White | 105 / 4,762 (2.2%) | 146 / 5,503 (2.7%) | 134 / 4,340 (3.1%) | 49 / 1,079 (4.5%) | |||
| Demographic* | All | 1.0 (ref) | 1.23 (1.01–1.51) | 1.44 (1.17–1.78) | 2.32 (1.79–3.00) | <0.0001 | |
| Black | 1.0 | 1.23 (0.87–1.74) | 1.21 (0.85–1.71) | 1.99 (1.34–2.95) | 0.007 | 0.48 / 0.45 | |
| White | 1.0 | 1.22 (0.95–1.58) | 1.61 (1.24–2.08) | 2.57 (1.82–3.63) | <0.0001 | ||
| + Risk Factors† | All | 1.0 | 1.19 (0.96–1.46) | 1.26 (1.02–1.57) | 1.91 (1.46–2.50) | <0.0001 | |
| Black | 1.0 | 1.25 (0.87–1.79) | 1.10 (0.77–1.59) | 1.65 (1.08–2.51) | 0.07 | 0.40 / 0.32 | |
| White | 1.0 | 1.15 (0.88–1.49) | 1.38 (1.06–1.79) | 2.15 (1. 51–3.06) | <0.0001 | ||
| + Socioeconomic status‡ | All | 1.0 | 1.16 (0.94–1.43) | 1.23 (0.99–1.52) | 1.84 (1.40–2.41) | 0.0001 | |
| Black | 1.0 | 1.22 (0.85–1.75) | 1.08 (0.75–1.56) | 1.59 (1.04–2.42) | 0.09 | 0.48 / 0.39 | |
| White | 1.0 | 1.12 (0.87–1.46) | 1.33 (1.02–1.73) | 2.07 (1.46–2.95) | 0.0003 | ||
Adjusted for age, sex, race.
Additionally adjusted for hypertension status, systolic blood pressure, history of heart disease, diabetes, current smoking status, left ventricular hypertrophy and atrial fibrillation
Additionally adjusted for income and education
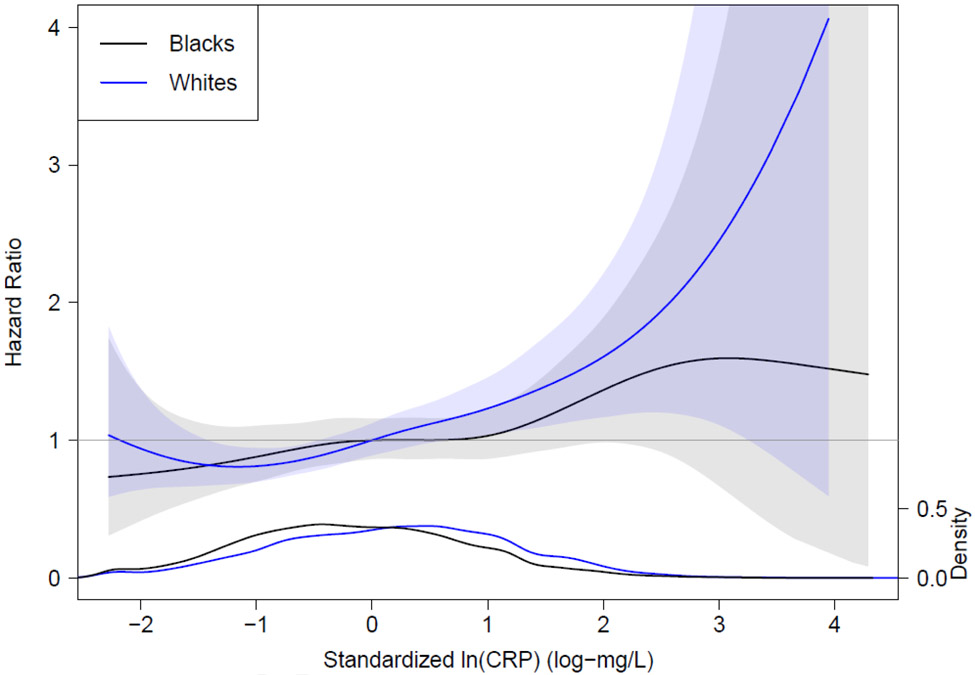
Results of race-stratified spline regression analysis examining the continuous relation of CRP to ischemic stroke are shown in Figure 2. Interpretation was similar to the analyses above, namely that the association of CRP with ischemic stroke risk was more apparent among whites than blacks.
Figure 2.
Spline Regression Analysis Illustrating the Hazard Ratio of Ischemic Stroke Based on Baseline Standardized ln C-reactive protein Relative to Standardized ln C-reactive protein 0 mg/L (the mean ln C-reactive protein). Shaded area represents the 95% confidence interval. The plot at the bottom of the figure is a density plot of the distribution of standardized ln C-reactive protein by race. The black line and shading denotes blacks and the purple line and shading denotes whites.
The Supplementary Figure shows race stratified risk factor-adjusted HRs of ischemic stroke by CRP as a binary variable with increasing threshold values. Using the commonly used clinical threshold of CRP ≥3 mg/L, whites were 40% more likely to have an ischemic stroke than whites with lower CRP, while blacks had no increased risk at this level. Among blacks a CRP threshold of ≥9 mg/L would have to be employed to show a similar increase in ischemic stroke risk as whites with CRP ≥3 mg/L.
Discussion
In this large contemporary cohort of black and white Americans, elevated CRP was associated with increased risk of fatal or non-fatal ischemic stroke, but the association was weaker in blacks than whites. Although the race difference in associations of CRP with ischemic stroke risk was not statistically significant based on multiplicative interaction terms, CRP ≥3 mg/L was not associated with ischemic stroke risk in blacks (even before adjustment for risk factors), while it was associated with a 40% increased risk of ischemic stroke in whites after multivariable adjustment. Consideration of CRP as a continuous variable yielded similar conclusions.
To the best of our knowledge, the association of CRP and ischemic stroke risk has not yet been established in a black population this large. Current CRP threshold values used in clinical practice to predict cardiovascular diseases were derived almost exclusively from white populations.22 A large meta-analysis by the Emerging Risk Factors Collaboration showed a positive relationship between increasing CRP and ischemic stroke risk,23 however race-specific analysis was not presented and the 15 included studies enrolled mostly white participants, such that the findings do not necessarily apply to non-whites, who have a different CRP distribution. Given our findings, and ongoing trials of inflammation lowering for vascular risk reduction, it will be important in that work to evaluate effects separately in blacks and whites.24, 25
We anticipated studying whether CRP mediated the excess ischemic stroke risk in blacks compared to whites in REGARDS, but since the association of CRP differed in blacks versus whites, this analysis was not conducted given the likely violation of traditional mediation method assumptions.19
Mechanisms for the differential association of CRP with ischemic stroke risk should be considered. Although we adjusted for socioeconomic status using education and income, early life adversity is predictive of elevated CRP in blacks.26 Single-nucleotide polymorphisms associated with CRP may also be responsible for variation in CRP and its different association with ischemic stroke in blacks compared to whites.27-29
The current findings have clinical relevance for two key reasons. First, findings extend prior research on racial differences in CRP to a clinical context. Blacks with CRP ≥3 mg/L versus <1 mg/L did not have a similarly increased ischemic stroke as whites with these CRP levels. Second, CRP ≥10 mg/L was associated with incident ischemic stroke in blacks and whites; the 6.9-year stroke rate was substantial at 4.1% in those with CRP ≥10 mg/L, compared to 2.3% with CRP <1. Current guidelines suggest that clinicians should consider causes of overt inflammation or illness when CRP is ≥10 mg/L, however, our findings along with others for coronary heart disease,16- 30 suggest that values in this range have important significance and should not be ignored or trigger other clinical workup. We caution clinicians that our findings are only relevant to ischemic stroke. It is well-accepted that statin initiation may be considered to reduce cardiovascular risk in those with CRP >2 mg/L.31 We advise against utilizing a different cutpoint of CRP for blacks until further research is available including evaluation of CRP and risk of coronary heart disease in blacks.
Study Strengths and Limitations
Strengths of this study include the prospective design and large geographically dispersed cohort of blacks and whites with representation of men and women. Strokes were carefully adjudicated and cohort retention was high. We studied a uniform phenotype by treating hemorrhagic and unknown type strokes as competing risks.
Study limitations require consideration. Although there were a large number of incident ischemic strokes, interaction testing on the multiplicative scale for race*CRP was underpowered, however we observed a racial difference that was clear. We assigned race based on participant self-identification and did not address genetic determinants of CRP, genetic admixture or the full social construct connected to racial identification. Several types of bias could have influenced results. Stroke ascertainment relied on participant contact by phone and incident cases may have been missed. Misclassification of stroke type as ischemic would have introduced bias, but this is unlikely as cases were carefully adjudicated using well established methods and expert adjudicators who were not aware of CRP levels. Ascertainment bias could have been present as follow-up time was marginally shorter in blacks, however this is most likely due to higher occurrence of events or increased withdrawal from the study, factors that would not likely impact the association of CRP with stroke. A single measurement of CRP was used which may have resulted in misclassification and an inability to capture variation that may occur with time. Other inflammation markers were not considered. Lack of inclusion of other racial groups means that our findings are not applicable to these groups. For example, the general population of most Asian races have CRP <1 mg/L;32 in the Women’s Health Study the average CRP by race/ethnicity in women was 1.1 mg/L in Asians, 2.0 mg/L in whites, 2.1 mg/L in Hispanics and 3.0 mg/L in blacks.33 Among Chinese adults CRP >3 mg/L (HR 1.33) was associated with increased risk of ischemic stroke,34 supporting this threshold for stroke risk prediction in the Chinese population. Finally, we could not adjust for use of inflammation-lowering medications after baseline as we did not have this information, however adjustment for baseline aspirin and statin use had no impact on the findings so this is likely not a drawback.
Conclusions and Implications
We present a detailed examination of the association of CRP with ischemic stroke in a large biracial population sample of Americans. Use of a cutoff value of 3.0 mg/L for CRP may not be appropriate for stroke risk prediction in blacks. We are not aware of similar data as presented here for a differential relationship of CRP to coronary heart disease risk in blacks compared to whites. Further research is needed to determine the optimal threshold for CRP and ischemic stroke and cardiovascular risk prediction in blacks and to determine more broadly the role of inflammation in explaining racial disparities in health. They also point out a need to assess race-specific findings in clinical trials of inflammation lowering to reduce cardiovascular risk.
Supplementary Material
Highlights, Cahill et al.
The association of C-reactive protein (CRP) with stroke risk in African-Americans is uncertain
In the REGARDS cohort we observed weaker relationships of CRP with strokes in blacks than whites
More research is needed on the clinical utility of CRP testing to predict stroke in blacks
Acknowledgements
The authors thank the investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Funding
This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data. Other support included a Hematology Opportunities for the Next-Generation of Research Scientists (HONORS) Award from American Society of Hematology, and a Fellowship from the Cardiovascular Research Institute of Vermont at the University of Vermont awarded to Dr. Cahill.
Footnotes
Disclosure
The Authors declare that there is no conflict of interest. All authors contributed in more than one of: study design, analysis, interpretation of the data and preparation and final approval of the final manuscript.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015; 131: e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011; 69: 619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan AS, Quick H, Pathak EB, Kramer MR and Casper M. Disparities in Temporal and Geographic Patterns of Declining Heart Disease Mortality by Race and Sex in the United States, 1973-2010. J Am Heart Assoc. 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard G, Cushman M, Kissela BM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011; 42: 3369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora P, Kalra R, Callas PW, et al. Lp(a) [Lipoprotein(a)] and risk of ischemic stroke in the REGARDS study. In Press, Arterioscler, Thromb, Vasc Biol. 2019: Atvbaha118311857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinkuolie AO, Buring JE, Ridker PM and Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc. 2014; 3: e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan G and Debette S. Genetic risk factors for ischemic and hemorrhagic stroke. Current Cardiol Reports. 2016; 18: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cacciapuoti F Hyper-homocysteinemia: a novel risk factor or a powerful marker for cardiovascular diseases? Pathogenetic and therapeutical uncertainties. J Thromb Thrombolysis. 2011; 32: 82–8. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Stone NJ, Bailey AL, et al. A Guideline on the Management of Blood Cholesterol. Circulation. 2018: Cir0000000000000625. [Google Scholar]
- 10.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63: 2889–934. [DOI] [PubMed] [Google Scholar]
- 11.Cushman M, Mcclure LA, Howard VJ, Jenny NS, Lakoski SG and Howard G. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. clin Chem. 2009; 55: 1627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005; 46: 464–9. [DOI] [PubMed] [Google Scholar]
- 13.Howard VJ, Cushman M, Pulley L, et al. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005; 25: 135–43. [DOI] [PubMed] [Google Scholar]
- 14.Gillett SR, Boyle RH, Zakai NA, Mcclure LA, Jenny NS and Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. clin Biochem. 2014; 47: 243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker PM, Buring JE, Cook NR and Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003; 107: 391–7. [DOI] [PubMed] [Google Scholar]
- 16.Vanderschueren S, Deeren D, Knockaert DC, Bobbaers H, Bossuyt X and Peetermans W. Extremely elevated C-reactive protein. Eur J Intern Med. 2006; 17: 430–3. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Lee DS and Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016; 133: 601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC and Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017; 36: 4391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin DY, Wei L, Ying Z Model checking techniques based on cumulative residuals. Biometrics 2002;58:1–12. [DOI] [PubMed] [Google Scholar]
- 20.Therneau T A Package for Survival Analysis in S., Version 2.38. 2015. [Google Scholar]
- 21.Therneau. Modeling Survivial Data: Extending the Cox Model. Springfield, New York: 2000. [Google Scholar]
- 22.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107: 499–511. [DOI] [PubMed] [Google Scholar]
- 23.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010; 375: 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018; 39: 3499–507. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Everett BM, Pradhan A, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. New Engl J Med. 2019; 380: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slopen N, Lewis TT, Gruenewald TL, et al. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomatic Med. 2010; 72: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kocarnik JM, Pendergrass SA, Carty CI, et al. Multiancestral analysis of inflammation-related genetic variants and C-reactive protein in the population architecture using genomics and epidemiology study. Circulation Cardiovasc Genet. 2014; 7: 178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li HY, Wang J, Meng F, et al. An intrinsically disordered motif mediates diverse actions of monomeric C-reactive protein. J Biol Chem. 2016; 291: 8795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiner AP, Beleza S, Franceschini N, et al. Genome-wide association and population genetic analysis of C-reactive protein in African American and Hispanic American women. Am J Human Genet. 2012; 91: 502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the Cardiovascular Health Study. Circulation. 2005; 112: 25–31. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New Engl J Med. 2008; 359: 2195–207. [DOI] [PubMed] [Google Scholar]
- 32.Saito I, Maruyama K and Eguchi E. C-reactive protein and cardiovascular disease in East asians: a systematic review. clin Med Insights Cardiol. 2014; 8: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albert MA, Glynn RJ, Buring J and Ridker PM. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women's Health Study). Am J Cardiol. 2004; 93: 1238–42. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Wang J, Zhang L, et al. Relationship between C-reactive protein and stroke: a large prospective community based study. PloS one. 2014; 9: el07017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.