Abstract
Background:Statistics from recent years have shown that 40% of infertile couples have a female etiological factor, 40% a male etiology and the remaining 20% a combination of female and male factors. For fertile couples, the chance of getting pregnant is 57% in three months of attempts, 75% in six months, 90% in one year, and 95% in two years. One-third of infertile couples have more than one cause involved in their inability to conceive. In 10-20% of cases, the reason for infertility cannot be determined. Approximately 25% of infertile women have ovulatory dysfunction.
Objectives:This research investigates improvements on the fertility of couples following treatment with a daily tablet of the patented blend consisting in a combination of Vitex agnus-castus (Vitex) extract, Lepidium meyenii (Maca) extract and active folate alone or with a gel capsule of vitamin, minerals, oligo-elements plus DHA and EPA Omega 3 fatty acids.
Materials and methods:Multicenter prospective interventional study with a duration of 18 months, conducted between June 2016 and December 2017. A total of 189 women were enrolled in the study. Participants were assigned to two treatment groups: group A, consisting of 103 patients who received a daily tablet of the patented blend consisting in a combination of Vitex extract, Maca extract and active folate, and group B, consisting of 86 patients who were given a daily tablet of the patented blend also administered to Group A and a gel capsule of vitamins, minerals, oligo-elements plus DHA and EPA omega 3 fatty acids. Paraclinical tests were conducted upon inclusion in the study and six months after treatment initiation in case of non-pregnancy. Every patient received ovulation kits and ovulation tests were performed on day 14 of the menstrual cycle.
Outcomes:Average age of the women was 31.18 years (SD 5.18 years). There was a successful pregnancy rate of 37%, with no difference between the two arms of the study. The number of new pregnancies was relatively constant through the study duration. The number of women with ovulation increased from 10% to 42.9% by the end of the study. During the six-month-period of the study, there were no side effects reported between patients of the two groups.
Conclusions:The supplement may be used by women trying to conceive. The patented blend consisting of a combination of Vitex, Maca and active folate regulates the menstrual cycle, stimulates ovulation and increases the likelihood of getting pregnant.
Keywords:infertility, Vitex Agnus-Castus, Lepidium meyenii, active folate, patented blend, ovulation.
INTRODUCTION
Statistics from recent years (1-3) have shown that 40% of infertile couples have a female etiological factor, 40% have a male etiology and the remaining 20% have a combination of female and male factors. For fertile couples, the chance of getting pregnant is 57% in three months of attempts, 75% in six months, 90% in one year, and 95% in two years. One in six couples attempting to have a baby have trouble conceiving (4). Fertility declines with age, 25% of women having difficulty getting pregnant between 35 and 39 years old, and 34% of women around the age of 40. One-third of infertile couples have more than one cause involved in their inability to conceive. In 10-20% of cases, the reason for infertility cannot be determined.
The active components Lepidium meyenii (Maca) and Vitex agnus-castus (Vitex) of patented blend studied proved to have the potential of enhancing female fertility (5-8). This research investigates improvements on the fertility of couples following treatment with a daily tablet of the patented blend consisting in a combination of Vitex extract, Maca and active folate alone or with a gel capsule of vitamin, minerals, oligo-elements plus DHA and EPA Omega 3 fatty acids.
MATERIAL AND METHODS
This prospective multicentre study with a duration of 18 months was conducted between June 2016 and December 2017, and it was carried out by 11 investigating physicians, specialists in Obstetrics and Gynaecology and Endocrinology, in nine national centers. For each patient, medical history was taken, and anthropometric measurements, with information regarding weight, laboratory tests, and ultrasonography were performed. During the study, monitoring visits were conducted each month. Hormonal profile changes and ovulatory cycles were monitored throughout the study. Laboratory tests were conducted at the beginning of the study, before any treatment, on days 3-5 of the menstrual cycle, and at the end of the study, six months after treatment initiation in case of the non-pregnancy outcome. Upon inclusion in the study, an ultrasound examination was performed in order to identify and measure the existing ovarian follicles. Every participant in the study received an ovulation kit each month. Women were instructed to perform ovulation tests on day 14 of the menstrual cycle (it was taken into account that for women with polycystic ovaries, LH values are high throughout the menstrual cycle, which could cause false-positive results at the ovulation test).
The study was conducted in compliance with rules of medical ethics, with patients expressing their informed consent regarding the use of both individual data from their follow-up records and results of clinical, laboratory and imaging investigations. Inclusion criteria were: age between 20 and 45 years; female, in a relationship; menstrual disorders (hypothalamus-pituitary-ovarian axis disorders); normal AMH and MTHFR values (investigations conducted before inclusion in the study); the desire to have children and unprotected sexual intercourse 2-3 times a week; normal values for the partner’s semen analysis; also, women with hypothyroidism, hyperprolactinemia and clinical and ultrasound diagnosed polycystic ovary syndrome (PCOS) were accepted in the study.
Exclusion criteria were: AMH values outside normal parameters; ligated Fallopian tubes or tube obstructions; uterine synechiae; MTHFR mutation (methylfolate deficiency); patients undergoing anxiolytic treatment; pituitary dwarfism; salpingitis; diabetes mellitus, uterine fibromatosis, endometriosis; participation in another clinical study; antecedents or presence of severe pathologies such as cancer, tuberculosis, multiple sclerosis, schizophrenia, etc.; presence of a single ovary. A sample of 193 women was monitored, including the totality of women of fertile age with or without present ovulation, who had been registered at the Ob-Gyn and Endocrinology clinical sections in nine national centers. The dropout rate was four patients (2.07%), resulting in 189 women completing the study.
Women were assigned to two treatment groups: group A, consisting of 103 patients who received a daily tablet of the patented blend consisting in a combination between Vitex extract, Maca extract and active folate; and group B, consisting of 86 patients who received a daily tablet of the patented blend also administered in group A and a gel capsule of vitamins, minerals, oligo-elements plus DHA and EPA omega 3 fatty acids. The data obtained were entered into an Excel sheet, analyzed and statistically processed using the statistical program R and Microsoft Excel. The values were expressed as mean ± SD for normally distributed data. P values lower than 0.05 were considered as statistically significant.
All (male) partners of patients included in the study from both groups were administered the combination of grape seed extract, coenzyme Q10, Maca, zinc and selenium, a product designed to support spermatogenesis and develop semen within normal values in terms of sperm count, morphology and motility (9-12).
OUTCOMES
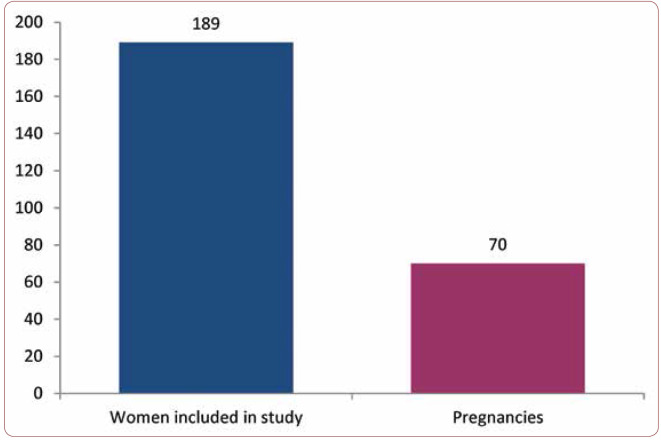
The average age of the women in the study, by study group, was 31,74 years (SD 5,03 years) for group A and 30,71 years (SD 5,28 years) for group B. The distribution was normal for both groups, with a range between 20 and 45 years. The groups did not differ in a statistically significant way (p=0,17), with a mean difference of 1.03 years. Out of the total number of 189 patients who finalized the study, 70 got pregnant during the study. This means a success rate of 37% (Figure 1).
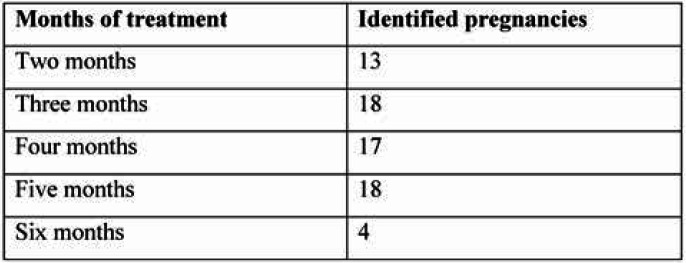
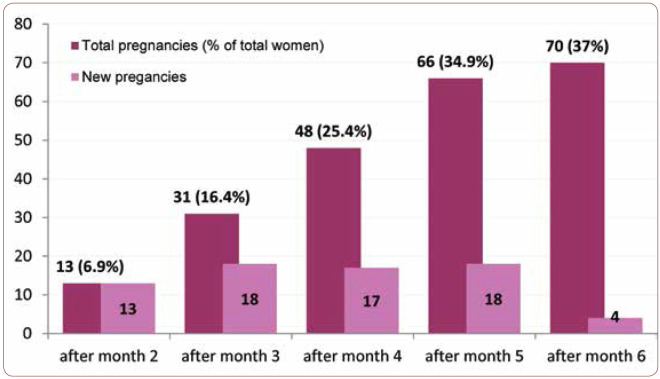
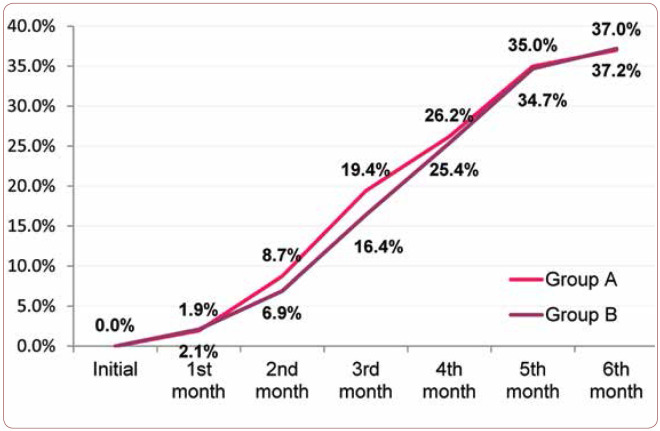
Distribution of the identified pregnancies by treatment duration reveals a relatively uniform distribution, except for the last month of the study, when the number of pregnancies decreased significantly (Table 1). Therefore, the cumulative incidence of pregnancies reveals an approximately constant increase through the course of the study (Figure 2) between the second and the fifth month, with 37% of women who completed the study reaching the outcome of becoming pregnant within six months. When comparing the two arms (groups) of the study, A and B, the cumulative incidence shows similar trends, with minimum differences (RR=1.00, C.I. 95% 0,76 – 1,32) which were not statistically significant (p=0,922). The incidence rate of pregnancies was 7.23/100 patients-month for patients within group A and 7.17/100 patients-month for group B. The mean age of women who got pregnant was 28.91 years (SD 4.75 years), while for those who did not get pregnant it was 32.4 years (SD 5.01 years). Thus, a mean difference of 4.3 years was observed between the two groups, the result being statistically significant (p<0.001). It is worth mentioning that the oldest enrolled patient who became pregnant was 43 years old.
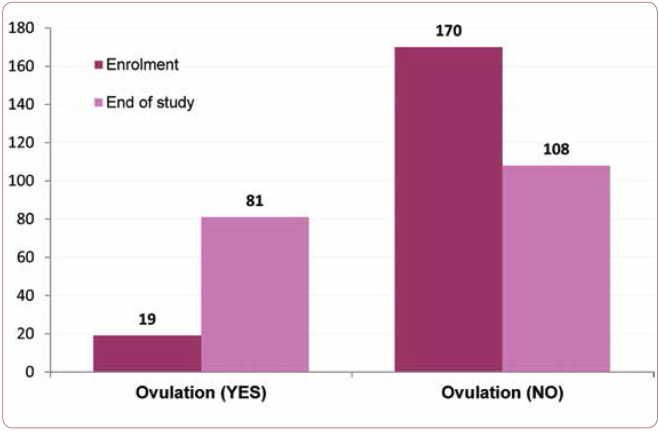
Another aspect investigated in this study was the possibility of getting pregnant even if a history of previous fertility treatments was present. Fifteen (8%) patients had a medical history of fertility treatments. Within this subgroup, we observed one case of pregnancy in a patient who had followed a fertility treatment some time ago. No pregnancies were observed in patients with two or three previous fertility treatments. Key elements of couples with fertility problems are the fact that they did not have sexual contact during the ovulation period, or the female had no ovulation. At the start of the study, 19 patients (10,05%) had ovulation. Through the study period, women received ovulation kits and were instructed how to determine their fertile period. At the end of the study, 81 (42,9%) patients had documented normal ovulation. The difference is statistically significant (p<0.001) (Figure 4). Polycystic ovary syndrome (PCOS) was evaluated at the beginning and at the end of the study (either because of reaching the outcome or the end of the study period).
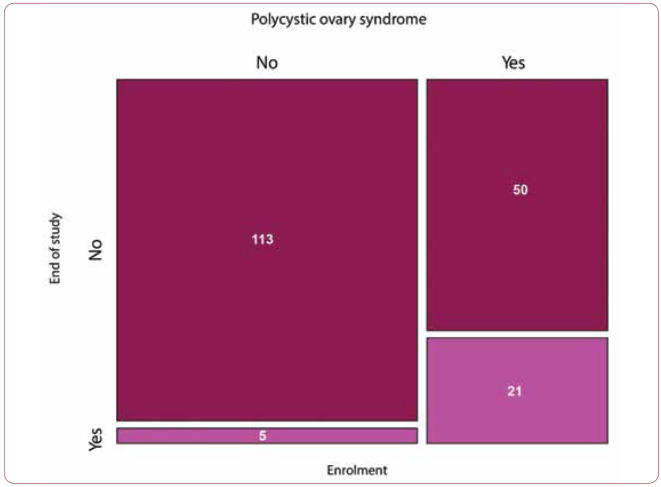
At enrolment, 71 (37.6%) patients out of 189 were diagnosed with PCOS. At the end of the study, the number decreased to 26 (14%). When data was analyzed case by case, we noticed that 21 out of the 71 patients with PCOS at enrolment had the same status at the end of the study, meaning that 71% of women with PCOS did not have the same condition at the end of the study. Among the 118 women who were not diagnosed with PCOS at enrolment, five were diagnosed with PCOS at the end of the study (Figure 5).
DISCUSSION
In this study, we focused on the effect that a patented blend consisting of a combination of Vitex agnus-castus (Vitex) extract, Lepidium meyenii (Maca) extract and active folate has on human reproduction. The sample consisted of 189 women with menstrual disorders who completed the study (six months or until the main outcome was observed, namely diagnosis of pregnancy). Referring to the main outcome, the study results show an overall success rate of 37%, which might be explained by summing up the treatment taken by couples, ovulation monitoring and constant (2-3 times per week) sexual intercourse. One key element that we evaluated during the study was to determine the women’s ovulational status. At enrolment, we determined that only one in 10 women was ovulating. By the end of the study, approximately 43% of women had ovulation. This result is in correlation with the reduction of cases with polycystic ovary syndrome (from 37.6% to 14%).
Effects of Vitex and Maca on mammal fertility is generally investigated in mice, both males and females (6, 13), and less on humans. Reviews of the existing literature (13-16) suggest that these plants have a beneficial effect on fertility. This study brings new data about the usage of medicinal plants with the purpose of increasing fertility, being, to the best of our knowledge, the only study that investigates this topic using a fixed-dose combination of Vitex and Maca. The results of the current study are promising, and further randomized controlled studies are needed for a better understanding of the effects exerted by this combination on human fertility.
CONCLUSION
1. Seventy women got pregnant at the end of the study, meaning a successful rate of 37%.
2. The incidence rate of pregnancies was 7.23 pregnancies/100 female patients per month for group A and 7.17 for group B. No statistically significant differences were found between the two groups (p = 0.922) regarding the chances of pregnancy occurrence. The results indicate a similar probability of pregnancy, regardless of the treatment regimen (group A or group B).
3. Following treatment with the proprietary formula, the number of women with polycystic ovaries decreased from 163 to 118 (86%), with a concomitant decrease in those with PCOS symptomatology to 26 (14%). After treatment, 50 patients with PCOS at baseline, from both groups, had no ovarian pathology at the exit from the study. The result had a high statistical significance (p <0.001).
4. The patented blend consisting of a combination of Vitex, Maca and active folate may be used by women trying to conceive, in either fertility treatments or pregnancy.
5. The patented blend consisting of a combination of Vitex, Maca and active folate regulates the menstrual cycle, stimulates ovulation and increases the likelihood of pregnancy.
6. Women should cooperate with the fertility specialist physician (gynecologist), investigate their own person instead of relying only on assumptions that all prenatal vitamins are the same, so as to fully understand the benefits of prenatal vitamin intake and the recommended doses for each supplement.
Conflict of interests: none declared
Financial support: This work was supported by Hyllan Pharma [to E.A. and C.T]. Hyllan Pharma provided the products for all patients, ovulation kits and laboratory tests that were conducted during the study.
Acknowledgments: The authors thank Emil Aurelian Ranetti, Ancuta Pati Cucu, Marie Jeanne Gardescu, Serban Nastasia, Melihan Bechir, George Vasilescu, Daniela Uzdris, Mircea Muncean, and Marius Dobrogeanu for their contribution.
FIGURE 1.
Number of pregnancies installed from the total number of women
TABLE 1.
New pregnancies by month
FIGURE 2.
Cumulative incidence of pregnancies
FIGURE 3.
Cumulative incidence of pregnancies by study group
FIGURE 4.
Distribution of women by the presence or absence of ovulation
FIGURE 5.
Distribution of patients with PCOS
Contributor Information
Edu ANTOINE, Obstetrics and Gynaecology Department, “Nicolae Malaxa” Clinical Hospital, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Sergiu CHIRILA, Faculty of Medicine, “Ovidius” University of Constanta, Romania.
Cristina TEODORESCU, Obstetrics and Gynaecology Department, “Nicolae Malaxa” Clinical Hospital, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
References
- 1.Patanwala I, King MJ, Barrett DA, Rose J, Jackson R, Hudson M, et al. Folic acid handling by the human gut: implications for food fortification and supplementation. Am J Clin Nutr. 2014;100:593–599. doi: 10.3945/ajcn.113.080507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44:480–488. doi: 10.3109/00498254.2013.845705. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006;15:189–193. doi: 10.1158/1055-9965.EPI-152CO. [DOI] [PubMed] [Google Scholar]
- 4.Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertility and Sterility. 2013;99:1324–1331. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cicero AFG, Piacente S, Plaza A, et al. Hexanic Maca extract improves rat sexual performance more effectively than methanolic and chloroformic Maca extracts. Andrologia. 2002;34:177–179. doi: 10.1046/j.1439-0272.2002.00490.x. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Luna AC, Salazar S, Aspajo NJ, et al. Lepidium meyenii (Maca) increases litter size in normal adult female mice. Reprod Biol Endocrinol. 2005;3:16. doi: 10.1186/1477-7827-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng BL, He K, Kim CH, et al. Effect of a lipidic extract from Lepidium meyenii on sexual behavior in mice and rats. Urology. 2000;55:598–602. doi: 10.1016/s0090-4295(99)00549-x. [DOI] [PubMed] [Google Scholar]
- 8.Rafieian-Kopaei M, Movahedi M. Systematic Review of Premenstrual, Postmenstrual and Infertility Disorders of Vitex Agnus Castus. Electron Physician. 2017;9:3685–3689. doi: 10.19082/3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boitani C, Puglisi R. Selenium, a key element in spermatogenesis and male fertility. Adv Exp Med Biol. 2008;636:65–73. doi: 10.1007/978-0-387-09597-4_4. [DOI] [PubMed] [Google Scholar]
- 10.Colagar AH, Marzony ET, Chaichi MJ. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. 2009;2:82–88. doi: 10.1016/j.nutres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Majzoub A, Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Indian J Urol. 2017;33:207–214. doi: 10.4103/iju.IJU_15_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Authority EFS. Selenium-enriched yeast as source for selenium added for nutritional purposes in foods for particular nutritional uses and foods (including food supplements) for the general population - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA Journal. 2008;6:766. doi: 10.2903/j.efsa.2008.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasprzak D, Jodlowska-Jedrych B, Borowska K, Wojtowicz A. Lepidium meyenii (Maca) - multidirectional health effects - review. Curr Iss Pharm Med Sci. 2018;31:107–112. [Google Scholar]
- 14.Farahbod F, Soureshjani SH. Medicinal Herbs Affecting Gonadotropin Hormones In Women: An Updated Systematic Review. Int J Life Sci Pharma Res. 2018;8:P20–P80. [Google Scholar]
- 15.Mazaro-Costa R, Andersen ML, Hachul H, Tufik S. Medicinal Plants as Alternative Treatments for Female Sexual Dysfunction: Utopian Vision or Possible Treatment in Climacteric Women? J Sex Med. 2010;7:3695–3714. doi: 10.1111/j.1743-6109.2010.01987.x. [DOI] [PubMed] [Google Scholar]
- 16.West E, Krychman M. Natural Aphrodisiacs-A Review of Selected Sexual Enhancers. Sexual Medicine Reviews. 2015;3:279–288. doi: 10.1002/smrj.62. [DOI] [PubMed] [Google Scholar]