Abstract
In contrast to many protein translocases that use ATP or GTP hydrolysis as the driving force to transport proteins across biological membranes, the peroxisomal matrix protein import machinery relies on a regulated self-assembly mechanism for this purpose and uses ATP hydrolysis only to reset its components. The ATP-dependent protein complex in charge of resetting this machinery—the Receptor Export Module (REM)—comprises two members of the “ATPases Associated with diverse cellular Activities” (AAA+) family, PEX1 and PEX6, and a membrane protein that anchors the ATPases to the organelle membrane. In recent years, a large amount of data on the structure/function of the REM complex has become available. Here, we discuss the main findings and their mechanistic implications.
Keywords: AAA+ ATPase, peroxisome, PEX1, PEX6, PEX5, protein translocation
1. Introduction
Many newly synthesized proteins have to be translocated across at least one biological membrane in order to reach their final destination. This is the case for thousands of eukaryotic proteins that are synthesized on cytosolic ribosomes but belong to organelles such as mitochondria, the endoplasmic reticulum, and peroxisomes, and for bacterial proteins that are destined to the periplasm or outer membrane [1,2,3,4,5,6]. Translocation of these proteins across (a) membrane(s) is a highly specific process relying on (1) the presence of targeting sequences in their primary structures, (2) receptors that specifically recognize these targeting signals, and (3) protein modules that form the membrane channels/pores through which translocation occurs [7]. In addition, some type of energy is used to provide directionality to the protein translocation step. Frequently, energy from ATP or GTP hydrolysis is used to trigger conformational alterations in components of the translocation machinery which in turn either push the protein substrate through the membrane (power stroke mechanisms) or bind and retain the substrate at the trans side of that membrane (molecular ratchet mechanisms) [8,9,10]. In at least one case—protein import into mitochondria—the electric component of the membrane potential is explored to induce electrophoretic movement of the translocation substrate [11]. In other systems, translocation directionality is attained through strong molecular interactions established between components of the translocation machinery which end up pushing the substrate across the membrane (regulated self-assembly mechanisms; e.g., peroxisomal matrix protein import, type V and VI bacterial secretion systems, and AB-type toxins; [3,5,12]). In some of the latter cases energy from ATP hydrolysis is still needed but only to reset the protein translocation machinery [13]. This is the case of the peroxisomal matrix protein import machinery, which undergoes structural remodeling after each protein translocation event in order to remain active. The proteins involved in this resetting process are long known but only recently did we start to understand how they work. Here we describe these proteins discussing the major findings gathered in recent years.
2. Going through the Peroxisomal Membrane—the Docking/Translocation Module and PEX5
Peroxisomal matrix proteins are synthesized on cytosolic ribosomes and rapidly transported to the organelle by a shuttling receptor [14,15]. The mechanism of this pathway can be described by drawing an analogy with a syringe (see Figure 1; [3]): the shuttling receptor (the “plunger”) binds a cargo protein in the cytosol, an event that triggers its insertion into a peroxisomal transmembrane complex (the “syringe barrel”) with the concomitant translocation of the cargo across the organelle membrane. No nucleoside triphosphate hydrolysis or membrane potential are needed for the translocation step—the driving force derives from the strong and multivalent interactions established between the “plunger” and the “barrel” [16,17,18]. After delivery of the cargo, the “syringe” is disassembled, i.e., the “plunger” is extracted from the “barrel” so that a new cycle of protein transportation can be started. This is the only part of the pathway where energy from ATP hydrolysis is required (reviewed in ref. [3]).
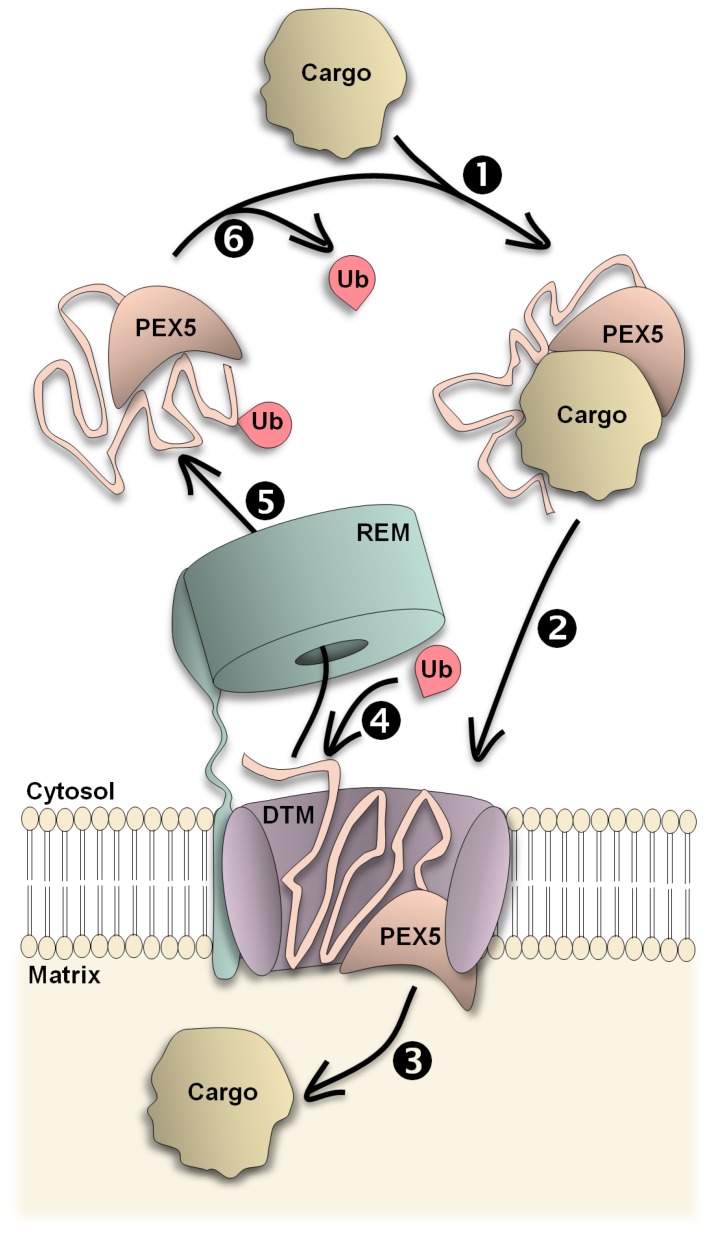
Figure 1.
Import of peroxisomal matrix proteins. A newly synthesized matrix protein (“Cargo”) is recognized by PEX5 in the cytosol (step 1). The PEX5-cargo complex interacts with a multisubunit protein complex at the peroxisome membrane, the Docking/Translocation Module (“DTM”; step 2), comprising PEX13, PEX14, and the RING peroxins PEX2, PEX10, and PEX12. Then, PEX5 becomes inserted into the DTM thus pushing the cargo protein across the membrane and into the organelle lumen (step 3). Afterwards, PEX5 is monoubiquitinated (Ub) at an N-terminally conserved cysteine residue (step 4) and is then extracted back to the cytosol by the action of the Receptor Export Module (REM; step 5), which comprises the AAA+ proteins PEX1 and PEX6 plus a membrane anchor (PEX26/PEX15/APEM9; see main text for details). In the cytosol, PEX5 is rapidly deubiquitinated (step 6) and a new round of import can be initiated. The irregular zigzag line represents the intrinsically disordered N-terminal half of PEX5, whereas the crescent-shaped form represents the globular PTS1-binding domain of PEX5.
In mammals, plants and many other organisms the “plunger” is PEX5, a monomeric and largely intrinsically disordered protein that recognizes in an autonomous manner the vast majority of proteins destined to the peroxisomal lumen [19,20,21,22,23,24,25,26,27,28]. These proteins possess the Peroxisomal Targeting Signal (PTS) Type 1, a small peptide at their C-termini frequently ending with the sequence SKL [29]. A small number of proteins possess instead a PTS Type 2, a degenerated nona-peptide present at their N-termini [30]. PTS2 proteins are also delivered to the peroxisome matrix by PEX5 but in this case an auxiliary protein PEX7 is required to strengthen the PEX5–PTS2 cargo protein interaction [31,32,33,34,35,36,37,38,39,40]. The situation in yeasts and fungi is slightly different. These organisms also rely on PEX5 to transport PTS1 proteins to the peroxisome [19,22,28,41]. However, they use a different “plunger” to import PTS2 proteins, also in a PEX7-dependent manner, and may possess still another shuttling receptor to transport some PTS1 proteins to the organelle under certain growth conditions [42,43,44,45,46,47]. Importantly, all these additional yeast/fungi “plungers” display structural similarities to PEX5 and thus, they are believed to function in an identical manner [42,43,45,48,49].
The polypeptide composition of the “syringe barrel”, referred to as the Docking/Translocation Module (DTM), has been known for many years. It comprises five core components, all transmembrane proteins. These are PEX13 and PEX14, each probably present in multiple copies per DTM, and three proteins containing a really-interesting-new-gene finger domain (RING), PEX2, PEX10, and PEX12 [50,51]. The overall structure of the DTM remains unknown. However, considering that peroxisomal matrix proteins acquire at least part of their tertiary structure already in the cytosol, prior to the translocation step, it is clear that the DTM must contain a rather large pore to accommodate PEX5 plus its cargo ([52,53,54,55]; reviewed in ref. [56]). Interestingly, both PEX14 and PEX13 contain large intrinsically disordered regions, a property that may explain the flexibility that the DTM must possess in order to accommodate folded cargo proteins of so different sizes and shapes (reviewed in ref. [57]).
The interaction between cytosolic PEX5 and the DTM is a regulated event—only cargo loaded PEX5 molecules have access to the DTM ([58] and reviewed in ref. [57])—and occurs in two sequential steps: (1) docking, a reversible interaction and (2) insertion, an essentially irreversible event in the absence of ATP [18,59]. Importantly, PEX5 at the insertion stage displays a transmembrane topology, exposing a small N-terminal domain into the cytosol whereas most of its polypeptide chain faces the organelle matrix [60]. Similarly, a portion of the polypeptide chain of PEX7 also becomes transiently exposed to the organelle matrix during the PTS2 protein transport cycle [61].
Several interactions between PEX5 and DTM components have been characterized in vitro. By far the strongest one involves a small globular domain in the N-terminus of PEX14, which faces the organelle matrix, and a set of eight “Short Linear Motifs” (SLiMs; [62]), the so-called pentapeptide motifs, which are present in the N-terminal half of PEX5 ([63] and reviewed in ref. [57]). Each of these PEX5 SLiMs interacts with PEX14 quite strongly (Kd values from 7 to 157 nM) resulting in a high affinity/high avidity interaction between PEX5 and PEX14 at the DTM [63,64].
Insertion of cargo loaded PEX5 into the DTM culminates in the release of the cargo protein into the organelle matrix [17,18,61]. The available evidence suggests that no ATP hydrolysis is required also at this step and that release of the cargo is probably triggered by allosteric regulation of PEX5 by a DTM component (e.g., PEX14, PEX13, or in yeasts also PEX8) [16,18,65,66,67,68,69,70,71].
At the end of the cargo translocation step, the strong interactions between PEX5 and the DTM have to be disrupted. This is a complex process involving many proteins. Some of these are required to ubiquitinate DTM-embedded PEX5 at a cysteine residue near its N-terminus (Cys11 in the mammalian protein), a mandatory modification for the extraction event [72,73]. These proteins include defined ubiquitin-conjugating enzymes, which may be different in different organisms and one or several RING proteins of the DTM that function as ubiquitin-ligases in this reaction (reviewed in ref. [74]). Another set of proteins is required to extract monoubiquitinated PEX5 (Ub-PEX5) from the DTM. This set comprises PEX1, PEX6 and a poorly conserved tail-anchored membrane protein called PEX26 in mammals and many other organisms, PEX15 in yeasts/fungi and APEM9 in plants [75,76,77,78,79,80]. PEX1, PEX6 and this membrane protein form a protein complex at the peroxisomal membrane, the Receptor Export Module (REM) [81]. The available structural/functional data on the REM are discussed below.
3. The Receptor Export Module—the Initial Findings
The genes encoding REM components were identified in yeasts, mammalian cell lines, and plants using genetic approaches many years ago [41,75,79,82,83,84,85,86,87,88]. Subsequent homology searches revealed their conservation in many other organisms (reviewed in refs. [89,90]). Of relevance, mutations in two REM components, PEX1 and PEX6, have been shown to be the most frequent cause of peroxisomal biogenesis disorders accounting for 76% of all cases [91,92,93].
Primary structure analyses of PEX15/PEX26/APEM9 did not provide much insight into their function [75,79,94]. In contrast, PEX1 and PEX6 turned out to be members of the large family of ATPases Associated with diverse cellular Activities (AAA+), and the only proteins involved in peroxisomal matrix protein import possessing ATP binding/hydrolysis domains [95,96]. This finding, together with previous experiments showing that the overall process of peroxisomal protein import requires ATP hydrolysis [97,98,99,100,101] led to initial models proposing that PEX1 and PEX6 might participate at a pre-translocation step (e.g., in the disassembly of PEX5-cargo protein complex), or in the vectorial transport of proteins across the organelle membrane, or simply in the assembly of the DTM (reviewed in ref. [102]). However, subsequent experiments using in vitro import assays revealed that ATP hydrolysis in the peroxisomal protein import pathway is required only at a post-translocation stage, to extract PEX5 from the peroxisomal DTM [16]. In agreement with this, it was later reported that adding PEX1/PEX6-containing cytosolic fractions to organelles isolated from cells lacking PEX1/PEX6 restored the extraction process of PEX5 from those organelles [77,78]. It became then accepted that the function of PEX1/PEX6 is to extract PEX5 from the DTM, i.e., to reset the peroxisomal protein import machinery. We note that for many years PEX1/PEX6 were also believed to play a role in membrane vesicle fusion events, which presumably occur at early stages of peroxisome biogenesis [103,104]. However, a recent study found that cells lacking PEX1 or PEX6 actually contain peroxisomal “ghosts” which already possess membrane proteins; these “ghosts” are replenished with matrix proteins upon reintroduction of PEX1 or PEX6 [105]. In another work, it was shown that depleting PEX1 from yeast cells blocks import of matrix proteins without affecting biogenesis of the peroxisomal membrane [106]. Thus, a role of PEX1 and PEX6 in early steps of peroxisomal membrane biogenesis seems rather unlikely [105,106].
4. The Mechanism of the Receptor Export Module
As stated above PEX1 and PEX6 are members of the AAA+ family, a large group of ATPases involved in a myriad of biological pathways such as DNA replication (e.g., DnaA), membrane fusion events (e.g., N-ethylmaleimide sensitive factor (NSF)), protein unfolding/disaggregation (e.g., p97/CDC48) and proteolysis (e.g., the 26S proteasome) [107,108,109,110]. AAA+-type ATPases use energy from ATP binding and hydrolysis to perform mechanical work which is used to unfold or remodel substrates [111]. The defining structural feature of these proteins is the AAA+ domain, a 200–250 amino acid residues ATP-binding domain comprising Walker A and Walker B motifs, and the second region of homology (SRH) domain that contains the sensor 1 and arginine finger motifs, key elements for the hydrolysis of ATP (reviewed in ref. [112]). The number of AAA+ domains in these proteins is variable but most have one or two such domains; these are referred to as type I or type II AAA+ proteins, respectively. Frequently, the AAA+ domain(s) is(are) flanked by additional domains which may be involved in interactions with adaptors and substrates or in regulating the activity of the AAA+ proteins (see refs. [113,114] for some recent examples; reviewed in refs. [115,116,117,118]) (see Figure 2).
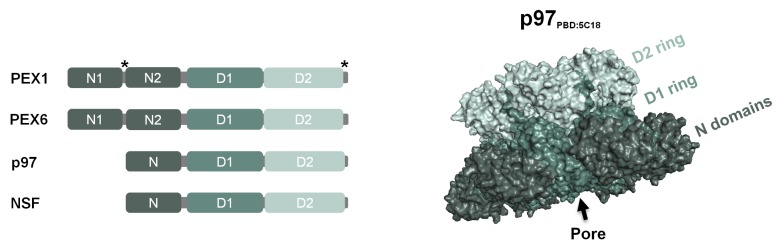
Figure 2.
Domain architecture of the type II AAA+ proteins PEX1, PEX6, p97 and NSF. The two N-domains of PEX1 and PEX6 (N1 and N2) homologous to the N-domain of p97 and NSF (N-domain), and the two AAA+ domains (D1 and D2) are shown (left). Note that mammalian PEX1 is longer than the yeast protein, possessing a different spacing between its N-domains, it also has an extended C-terminal tail after the D2 domain (marked with asterisks). Atomic model of a p97 hexamer (right; PDB code 5C18, [138]) showing the common type II AAA+ structure consisting of one ring of D1 domains on top of another ring of D2 domains, and the six N-domains located on the side of the D1 ring. Note that this p97 structure was obtained in the presence of a non-hydrolysable ATP analogue and in the absence of substrate [138].
Proteins with one or two AAA+ domains assemble into homo- or hetero-oligomers often yielding ring-shaped hexamers [108,112]. Such organization places the ATP-binding/hydrolysis site at the interface of two subunits, one of which provides the Walker A/B and sensor 1 residues and the other the arginine finger and creates a central pore that is frequently of major functional importance [112,119]. Indeed, many AAA+-type ATPases use specific residues in their pores, the so-called pore loops, to grasp substrates while others use the pore loops as paddles to translocate substrates through the pore, thus unfolding them ([120,121,122]; reviewed in refs. [96,112]). Paradigmatic examples of these two mechanisms are provided by NSF and p97/CDC48, two type II AAA+ proteins. NSF is believed to interact with its substrate, a SNARE complex, through its pore loops and to use ATP binding/hydrolysis to induce large movements in its N-domains which in turn are propagated to the substrate to disassemble it [123,124,125,126,127]. P97/CDC48 captures an extended/disordered domain of a substrate in its pore and then uses ATP binding/hydrolysis to thread the substrate through the pore [121,128,129,130]. Like NSF and p97/CDC48, PEX1 and PEX6 are also AAA+-type II ATPases. Besides the two AAA+ domains, both PEX1 and PEX6 possess also two N-terminal domains (N1 and N2), each displaying sequence similarities with the single N-domain present in p97/CDC48 and NSF (see Figure 2; [131] and reviewed in ref. [109]). Intriguingly, while the second AAA+ domain of both PEX1 and PEX6 (the D2 domain) displays all the canonical residues involved in ATP binding/hydrolysis, their first AAA+ domain (the D1 domain) is somewhat degenerated lacking many of the residues required for ATP hydrolysis [41,82,83,84,87,88,132,133,134,135,136,137].
Despite all the information provided by the primary structures of PEX1/PEX6, the actual mechanism used by the REM to extract monoubiquitinated PEX5 from the DTM remained a mystery for many years. A major breakthrough on this issue came in 2015 with the structural characterization of the yeast PEX1/PEX6 complex by negative-stain and cryo-electron microscopy [132,133,137]. That work revealed that the two proteins form a hetero-hexameric ring with alternating PEX1 and PEX6 subunits and a rather large pore at the center. Besides showing the typical arrangement of the AAA+ domains in type II ATPases—a ring of D1 domains on top of another ring made of D2 domains—the structures also revealed the position of the two PEX6 N-domains and the N2 domain of PEX1—they stand on the top/side of the D1 ring giving the complex a triangular shape [132,133,137]. More recently, the structure of the cytosolic domain of the tail-anchored PEX15 (amino acid residues 1-331) was also delineated [139]. The protein comprises a N-terminal disordered region of 42 amino acid residues, which mediates the interaction with PEX6 [76,139,140,141,142], a compact curved domain comprising 12 alpha-helices (residues 43–253) followed by a long intrinsically disordered region (residues 254–331) that connects the compact domain to the single transmembrane domain at the C-terminus of PEX15 [139]. It was also shown that three molecules of the soluble PEX15 cytosolic domain bind to the hexameric PEX1/PEX6 complex through the N1 and N2 domains of PEX6, and that the C-terminal intrinsically disorder region of the PEX15 fragment sits just above the central pore of the PEX1/PEX6 complex [139,143].
Another finding of crucial importance to understand the mechanism of the REM was made by Gardner et al. when studying the ATPase activity of the isolated yeast PEX1/PEX6 complex in vitro [133,139]. These authors noted that the complex displays a rather large basal ATPase activity which, however, is largely decreased in the presence of a recombinant protein comprising the cytosolic domain of PEX15 (amino acid residues 1–327). Initially, it was reasoned that besides being the membrane anchor, PEX15 might also be a regulator/inhibitor of PEX1/PEX6 thus avoiding futile ATP-consuming cycles by the ATPase [133]. However, subsequent work by the same group demonstrated that the in vitro “inhibitory” effect of the PEX15 fragment required intact pore loops at the D2 domains of PEX1/PEX6 [139]. This observation strongly suggested that the PEX15 fragment was actually acting as a substrate for PEX1/PEX6 in those assays. Indeed, those authors were able to show that the complete polypeptide chain of that PEX15 fragment is unfolded by the PEX1/PEX6 complex, a phenomenon that was not observed with shorter PEX15 fragments lacking most of the C-terminal intrinsically disordered region [139]. Apparently, placing the long free disordered C-terminus of the PEX15 fragment just above the PEX1/PEX6 pore results in its engagement with the pore loops of the AAA+ complex and in the complete threading of the PEX15 fragment. These important results established PEX1/PEX6 as a processive protein translocase, thus placing the REM in the mechanistic group of well-characterized AAA+ proteins such as p97/CDC48 and HSP104 [139,144].
Considering that PEX15 as well as its mammalian orthologue, PEX26, interact with PEX14 [139,141,142,145,146], which in turn interacts with PEX5 at the DTM [50,51,63,64,142,145,146,147,148], the results above might suggest that PEX15 is a physiologically relevant substrate for the PEX1/PEX6 complex and that remodeling of PEX15 by the REM somehow disrupts the interaction between PEX5 and the DTM. However, it must be noted that the C-terminus of the PEX15 fragment engaged by PEX1/PEX6 in those in vitro assays is actually linked to a transmembrane domain in the native/peroxisomal PEX15 protein. Thus, one would have to hypothesize that the PEX1/PEX6 complex engages a PEX15 loop in vivo, which remains to be demonstrated. Perhaps more importantly, it would be difficult to understand how unfolding the cytosolic domain of PEX15, or part of it, by PEX1/PEX6 might lead to the disruption of the PEX5-PEX14 interaction, most of which occurs on the other side of the peroxisome membrane [149]. Thus, as discussed by Gardner et al., the physiologically relevant substrate(s) of PEX1/PEX6 might well be other protein(s) [139].
A prime candidate substrate for the REM is of course DTM-embedded monoubiquitinated PEX5 itself, the only protein that is actually extracted from the DTM by the REM [16,59,77,78]. Indeed, using a mammalian cell-free in vitro system that recapitulates all the steps of the PEX5-mediated protein import pathway, we recently reported several observations that support this possibility [80]. First, it was shown that the ubiquitin moiety of DTM-embedded monoubiquitinated PEX5 interacts directly with both PEX1 and PEX6. Second, fusion proteins comprising PEX5 or its N-terminal half and GFP or mouse DHFR, respectively, were shown to enter the DTM and acquire a monoubiquitin; however, although export of these species can be initiated it does not terminate efficiently leading to the accumulation of partially extracted PEX5 proteins at the organelle surface [80,150]. In the case of the PEX5-DHFR fusion protein this effect was particularly noted when the assay contained methotrexate, a folate analog that stabilizes the structure of DHFR [151]. Thus, difficult to unfold moieties attached to the C-termini of PEX5 proteins interfere with the REM. Finally, cysteine residues present in the globular C-terminal half of PEX5 and located dozens/hundreds of amino acid residues away from the PEX5 domains that interact with DTM components, become particularly exposed to an alkylating reagent during (but not before nor after) the ATP-dependent export of Ub-PEX5 by the REM [80]. Altogether, these data suggest that Ub-PEX5 interacts with the REM and is completely unfolded during the export step.
5. Unsolved Mechanistic Aspects: Substrate Engagement and Regulation
Many AAA+-type ATPases, such as those of the proteasome 19S regulatory particle or the microtubule severing katanin, engage substrates by capturing their extended N- or C-termini into their pores [152,153,154,155]. This, however, probably does not apply to the REM because (1) a truncated human PEX5 protein lacking its first nine amino acids (a deletion that does not interfere with monoubiquitination of PEX5 at residue 11) is still efficiently extracted by the REM and (2) several C-terminally truncated PEX5 proteins (one of which comprising solely amino acid residues 1–125 of PEX5) or PEX5 fusion proteins containing folded domains at the C-termini are, likewise, monoubiquitinated at the DTM and engaged by the REM [55,59,80,150]. These data nevertheless suggest that amino acid residues 10–125 of PEX5 plus the covalently attached ubiquitin moiety are sufficient for engagement with the REM. Exactly how this occurs awaits further experimentation but two obvious scenarios are the following (see also Figure 3): (1) the REM engages Ub-PEX5 at a protein loop in the 10–125 region of PEX5 (see [156] for a similar mechanism) or (2) PEX1/PEX6 oligomerize around a PEX5 domain located after the ubiquitination site. A third, less evident mechanism may also be envisaged when we take into consideration very recent data showing that CDC48 engages a polyubiquitinated substrate by unfolding one of its ubiquitin moieties [129]. In such a scenario, the REM would first unfold the ubiquitin moiety of Ub-PEX5 at the entrance of the PEX1/PEX6 pore, and then would engage the ubiquitin N-terminus (see Figure 3). Although this possibility might seem unlikely due to the fact that the D1 domains of PEX1/PEX6 lack many of the residues required for ATP hydrolysis [82,83,84,86,88,132,133,137], we note that inactivating mutations in the walker B domain or arginine finger of the D1 domain in all six subunits of the bacterial ClpB protein do not completely abolish its disaggregase activity [113]. Clearly a type II ATPase containing only fully functional D2 domains can still do the job (see also refs. [157,158]).
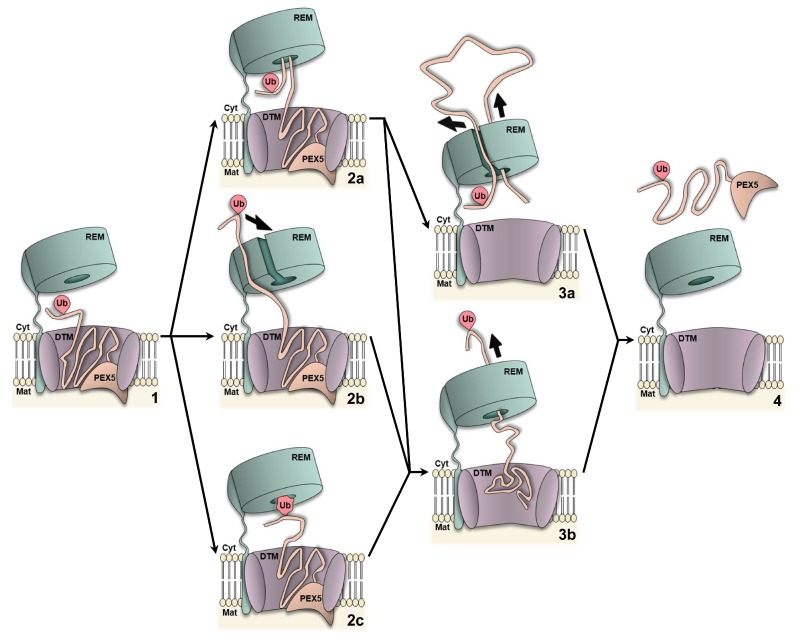
Figure 3.
Possible mechanisms of PEX5 engagement and extraction by the REM. DTM-embedded Ub-PEX5 (1) may engage the REM in different ways. Given the intrinsically disordered nature of its N-terminal half, an extended loop of PEX5 could serve as the initial grabbing site for the AAA+ pore loops (1→ 2a; see [156] for a mechanism of this type). Alternatively, the AAA+ complex could assemble around PEX5 or undergo conformational changes to accommodate the substrate in its pore, as suggested for microtubule-severing enzymes [159]; this would place the Ub moiety already at the trans side of the AAA+ ring (1→ 2b). In a third scenario, local unfolding and threading of the Ub moiety itself by the AAA+ proteins could also be used to extract Ub-PEX5 (1→ 2c), akin to a mechanism recently proposed for the CDC48 ATPase complex [129]. Threading of Ub-PEX5 by the AAA+ proteins could either be partial (paths 2a→ 3a and 2b→ 3b; the ubiquitin moiety is not threaded) or complete (paths 2a→ 3b and 2c→ 3b; the ubiquitin is threaded). 1—Recognition; 2—Engagement; 3—Translocation; 4—Release.
As stated above, the isolated yeast PEX1/PEX6 complex displays a large basal ATPase activity in vitro in the absence of any substrate [133,139,160]. Naturally, futile hydrolysis of ATP should not occur in vivo thus raising the question of how the ATPase activity of the REM is regulated. One possibility is that a still unidentified protein (or protein domain) interacts with the PEX1/PEX6 complex repressing its ATPases activity and disengaging from the ATPase in the presence of the substrate. Such a regulatory protein might be any component of the DTM or even PEX15/PEX26 itself—the properties of this PEX1/PEX6-interacting protein in its native environment, i.e., embedded in the peroxisomal membrane, may be different from those presented by its soluble cytosolic domain in the in vitro assays reported above [139]. Another possibility comes from models proposed for other members of the AAA+ family such as the microtubule severing enzymes, katanin and spastin [159,161] or the GspE protein from bacterial type II secretion systems [162]. It has been proposed that these AAA+ proteins undergo oligomerization, yielding an active ATPase, only under specific conditions. In the case of the microtubule severing enzymes, oligomerization seems to occur only after the corresponding subunits bind microtubules [161,163]. In the case of GspE, oligomerization might also be substrate-induced but in an indirect manner, probably via a substrate-sensing subunit of the secretion system [162]. Clearly, additional work is needed to understand these important mechanistic aspects of the REM.
6. The Energetic Cost of Protein Translocation across the Peroxisomal Membrane
As discussed in the previous sections, the peroxisomal protein import machinery relies on the strong protein–protein interactions that are established between PEX5 and the DTM as the driving force for protein translocation, and uses ATP hydrolysis only to recycle PEX5 back into the cytosol [16,17,18]. One implication of such a mechanism is that the energetic cost of translocating small or large proteins across the peroxisomal membrane is the same. What is this cost exactly? The number of ATPs consumed during dislocation of Ub-PEX5 from the DTM depends on the number of amino acid residues of Ub-PEX5 that have to be threaded through the PEX1/PEX6 pore, and on the step size of the PEX1/PEX6 complex during threading. As discussed in detail above, the available data suggest that engagement of Ub-PEX5 by the REM involves the ubiquitin moiety itself plus amino acid residues 10–125 of PEX5 and that threading continues to the very C-terminus of the PEX5 protein [55,80,150]. Thus, practically all PEX5 residues (approximately 600) are threaded through the PEX1/PEX6 pore. The translocation step size of PEX1/PEX6 is still unknown. There are indirect estimates suggesting an energetic cost of 1 ATP per seven amino acid residues threaded [139], similarly to other AAA+ proteins (e.g., ClpA [164]). However, other type II translocases, such as p97/CDC48 and bacterial ClpB work with step sizes of just two amino acid residues, with ATP hydrolysis occurring in both D1 and D2 domains [113,129,130]. Thus, a conservative estimate for the PEX1/PEX6 complex, taking also into consideration that its D1 ring probably does not hydrolyze ATP, would be one ATP per 2–7 amino acid residues of substrate translocated through the pore. This means that the complete dislocation of Ub-PEX5 from the DTM requires the hydrolysis of some 85–300 ATPs. These are relatively small numbers when compared, for instance, to the energy required for translocating a protein (prOmpA) across the bacterial plasma membrane through the Sec pathway (700–5000 ATPs) [165] or to transport a protein (OE17) using the chloroplast Tat pathway (690,000 kJ/mol, equivalent to 11500 ATPs) [166]. Apparently, the peroxisomal protein import machinery is rather economical.
7. Conclusions
Over the last 5 years, we have learned more on the REM structure and function than in the two previous decades. We now know that the PEX1/PEX6 complex is a p97/CDC48-like ATPase that threads monoubiquitinated PEX5 through its central pore. Naturally, there are still many questions waiting for an answer. As discussed above we still have no idea of how Ub-PEX5 is engaged by the PEX1/PEX6 complex, and how this ATPase is kept in an inactive state in the absence of substrates. Also, it is still unknown whether the PEX1/PEX6 complex has other substrates besides PEX5 and all the yeast/fungi PEX5-like proteins. Hopefully, we will not need two more decades to answer these questions.
Abbreviations
| AAA+ | ATPases Associated with diverse cellular Activities |
| Cys11 | Cysteine 11 |
| Cyt | Cytosol |
| D1 | AAA+ domain 1 |
| D2 | AAA+ domain 2 |
| DHFR | Dihydrofolate reductase |
| DTM | Docking/translocation module |
| GFP | Green fluorescent protein |
| Kd | Dissociation constant |
| Mat | Matrix |
| N1 | N-terminal domain 1 |
| N2 | N-terminal domain 2 |
| NSF | N-ethylmaleimide sensitive factor |
| PTS | Peroxisomal targeting signal |
| REM | Receptor export module |
| RING | Really interesting new gene |
| SLiMs | Short linear motifs |
| SRH | Second region of homology |
| Tat | Twin-arginine translocation |
| Ub | Ubiquitin |
| Ub-PEX5 | Monoubiquitinated PEX5 |
Author Contributions
Conceptualization, A.G.P., T.F., T.A.R., J.E.A.; writing—original draft preparation, A.G.P., J.E.A.; writing—review and editing, A.G.P., A.B.-B., M.J.F., T.F., T.A.R., J.E.A.; graphical illustration, A.G.P., A.B.-B., M.J.F.; funding acquisition, J.E.A.
Funding
This work was financed by FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the project PTDC/BEX-BCM/2311/2014 (POCI-01-0145-FEDER-016613) and the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). This work is a result of the project NORTE-01-0145-FEDER-000008—Porto Neurosciences and Neurologic Disease Research Initiative at I3S, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). A.B.-B., A.G.P., M.J.F., T.F. and T.A.R. are supported by Fundação para a Ciência e Tecnologia, Programa Operacional Potencial Humano do QREN, and Fundo Social Europeu.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Becker T., Song J., Pfanner N. Versatility of Preprotein Transfer from the Cytosol to Mitochondria. Trends Cell Biol. 2019;29:534–548. doi: 10.1016/j.tcb.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Haßdenteufel S., Nguyen D., Helms V., Lang S., Zimmermann R. ER import of small human presecretory proteins: components and mechanisms. FEBS Lett. 2019:1–19. doi: 10.1002/1873-3468.13542. [DOI] [PubMed] [Google Scholar]
- 3.Francisco T., Rodrigues T.A., Dias A.F., Barros-Barbosa A., Bicho D., Azevedo J.E. Protein transport into peroxisomes: Knowns and unknowns. BioEssays. 2017;39:1700047. doi: 10.1002/bies.201700047. [DOI] [PubMed] [Google Scholar]
- 4.Hansen K.G., Herrmann J.M. Import of proteins into mitochondria. Protein J. 2019;38:330–342. doi: 10.1007/s10930-019-09819-6. [DOI] [PubMed] [Google Scholar]
- 5.Christie P.J. The Rich Tapestry of Bacterial Protein Translocation Systems. Protein J. 2019;38:389–408. doi: 10.1007/s10930-019-09862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smets D., Loos M.S., Karamanou S., Economou A. Protein Transport Across the Bacterial Plasma Membrane by the Sec Pathway. Protein J. 2019;38:262–273. doi: 10.1007/s10930-019-09841-8. [DOI] [PubMed] [Google Scholar]
- 7.Wickner W., Schekman R. Protein Translocation Across Biological Membranes. Science. 2005;310:1452–1456. doi: 10.1126/science.1113752. [DOI] [PubMed] [Google Scholar]
- 8.Corey R.A., Allen W.J., Collinson I. Protein translocation: What’s the problem? Biochem. Soc. Trans. 2016;44:753–759. doi: 10.1042/BST20160047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hepp C., Maier B. Bacterial Translocation Ratchets: Shared Physical Principles with Different Molecular Implementations. BioEssays. 2017;39:1700099. doi: 10.1002/bies.201700099. [DOI] [PubMed] [Google Scholar]
- 10.Crow A., Greene N.P., Kaplan E., Koronakis V. Structure and mechanotransmission mechanism of the MacB ABC transporter superfamily. Proc. Natl. Acad. Sci. USA. 2017;114:12572–12577. doi: 10.1073/pnas.1712153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfanner N., Warscheid B., Wiedemann N. Mitochondrial proteins: from biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019;20:267–284. doi: 10.1038/s41580-018-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galán J.E., Waksman G. Protein-Injection Machines in Bacteria. Cell. 2018;172:1306–1318. doi: 10.1016/j.cell.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen V.S., Douzi B., Durand E., Roussel A., Cascales E., Cambillau C. Towards a complete structural deciphering of Type VI secretion system. Curr. Opin. Struct. Biol. 2018;49:77–84. doi: 10.1016/j.sbi.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Dodt G., Gould S.J. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 receptor: evidence that PTS1 protein import is mediated by a cycling receptor. J. Cell Biol. 1996;135:1763–1774. doi: 10.1083/jcb.135.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzioch M., Erdmann R., Veenhuis M., Kunau W.H. PAS7 encodes a novel yeast member of the WD-40 protein family essential for import of 3-oxoacyl-CoA thiolase, a PTS2-containing protein, into peroxisomes. EMBO J. 1994;13:4908–4918. doi: 10.1002/j.1460-2075.1994.tb06818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira M.E., Gouveia A.M., Pinto R.A., Sá-Miranda C., Azevedo J.E. The energetics of Pex5p-mediated peroxisomal protein import. J. Biol. Chem. 2003;278:39483–39488. doi: 10.1074/jbc.M305089200. [DOI] [PubMed] [Google Scholar]
- 17.Alencastre I.S., Rodrigues T.A., Grou C.P., Fransen M., Sá-Miranda C., Azevedo J.E. Mapping the cargo protein membrane translocation step into the PEX5 cycling pathway. J. Biol. Chem. 2009;284:27243–27251. doi: 10.1074/jbc.M109.032565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francisco T., Rodrigues T.A., Freitas M.O., Grou C.P., Carvalho A.F., Sá-Miranda C., Pinto M.P., Azevedo J.E. A cargo-centered perspective on the PEX5 receptor-mediated peroxisomal protein import pathway. J. Biol. Chem. 2013;288:29151–29159. doi: 10.1074/jbc.M113.487140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCollum D., Monosov E., Subramani S. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein import deficiencies of Zellweger syndrome cells--the PAS8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal, and is a member of the TPR protein family. J. Cell Biol. 1993;121:761–774. doi: 10.1083/jcb.121.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fransen M., Breesm C., Baumgart E., Vanhooren J.C.T., Baes M., Mannaerts G.P., Van Veldhoven P.P. Identification and characterization of the putative human peroxisomal C-terminal targeting signal import receptor. J. Biol. Chem. 1995;270:7731–7736. doi: 10.1074/jbc.270.13.7731. [DOI] [PubMed] [Google Scholar]
- 21.Wiemer E.A.C., Nuttley W.M., Bertolaet B.L., Li X., Francke U., Wheelock M.J., Anné U.K., Johnson K.R., Subramani S. Human peroxisomal targeting signal-1 receptor restores peroxisomal protein import in cells from patients with fatal peroxisomal disorders. J. Cell Biol. 1995;130:51–65. doi: 10.1083/jcb.130.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Der Klei I.J., Hilbrands R.E., Swaving G.J., Waterham H.R., Vrieling E.G., Titorenko V.I., Cregg J.M., Harder W., Veenhuis M. The Hansenula polymorpha PER3 gene is essential for the import of PTS1 proteins into the peroxisomal matrix. J. Biol. Chem. 1995;270:17229–17236. doi: 10.1074/jbc.270.29.17229. [DOI] [PubMed] [Google Scholar]
- 23.Wimmer C., Schmid M., Veenhuis M., Gietl C. The plant PTS1 receptor: similarities and differences to its human and yeast counterparts. Plant J. 1998;16:453–464. doi: 10.1046/j.1365-313x.1998.00320.x. [DOI] [PubMed] [Google Scholar]
- 24.Kragler F., Lametschwandtner G., Christmann J., Hartig A., Harada J.J. Identification and analysis of the plant peroxisomal targeting signal 1 receptor NtPEX5. Proc. Natl. Acad. Sci. USA. 1998;95:13336–13341. doi: 10.1073/pnas.95.22.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jardim A., Liu W., Zheleznova E., Ullman B. Peroxisomal targeting signal-1 receptor protein PEX5 from Leishmania donovani. Molecular, biochemical, and immunocytochemical characterization. J. Biol. Chem. 2000;275:13637–13644. doi: 10.1074/jbc.275.18.13637. [DOI] [PubMed] [Google Scholar]
- 26.Costa-Rodrigues J., Carvalho A.F., Fransen M., Hambruch E., Schliebs W., Sá-Miranda C., Azevedo J.E. Pex5p, the peroxisomal cycling receptor, is a monomeric non-globular protein. J. Biol. Chem. 2005;280:24404–24411. doi: 10.1074/jbc.M501985200. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho A.F., Costa-Rodrigues J., Correia I., Faria T.Q., Martins C.L., Fransen M., Sá-Miranda C., Azevedo J.E. The N-terminal half of the peroxisomal cycling receptor Pex5p is a natively unfolded domain. J. Mol. Biol. 2006;356:864–875. doi: 10.1016/j.jmb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Terlecky S.R., Nuttley W.M., McCollum D., Sock E., Subramani S. The Pichia pastoris peroxisomal protein PAS8p is the receptor for the C-terminal tripeptide peroxisomal targeting signal. EMBO J. 1995;14:3627–3634. doi: 10.1002/j.1460-2075.1995.tb00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brocard C., Hartig A. Peroxisome targeting signal 1: is it really a simple tripeptide? Biochim. Biophys. Acta. 2006;1763:1565–1573. doi: 10.1016/j.bbamcr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Lazarow P.B. The import receptor Pex7p and the PTS2 targeting sequence. Biochim. Biophys. Acta. 2006;1763:1599–1604. doi: 10.1016/j.bbamcr.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Braverman N.E., Dodt G., Gould S.J., Valle D. An isoform of pex5p, the human PTS1 receptor, is required for the import of PTS2 proteins into peroxisomes. Hum. Mol. Genet. 1998;7:1195–1205. doi: 10.1093/hmg/7.8.1195. [DOI] [PubMed] [Google Scholar]
- 32.Otera H., Okumoto K., Tateishi K., Ikoma Y., Matsuda E., Nishimura M., Tsukamoto T., Osumi T., Ohashi K., Higuchi O., et al. Peroxisome targeting signal type 1 (PTS1) receptor is involved in import of both PTS1 and PTS2: studies with PEX5-defective CHO cell mutants. Mol. Cell. Biol. 1998;18:388–399. doi: 10.1128/MCB.18.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumura T., Otera H., Fiyiki Y. Disruption of the interaction of the longer isoform of Pex5p, Pex5pl, with Pex7p abolishes peroxisome targeting signal type 2 protein import in mammals. Study with a novel Pex5-impaired Chinese hamster ovary cell mutant. J. Biol. Chem. 2000;275:21715–21721. doi: 10.1074/jbc.M000721200. [DOI] [PubMed] [Google Scholar]
- 34.Woodward A.W., Bartel B. The Arabidopsis peroxisomal targeting signal type 2 receptor PEX7 is necessary for peroxisome function and dependent on PEX5. Mol. Biol. Cell. 2005;16:573–583. doi: 10.1091/mbc.e04-05-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galland N., Demeure F., Hannaert V., Verplaetse E., Vertommen D., Van Der Smissen P., Courtoy P.J., Michels P.A.M. Characterization of the role of the receptors PEX5 and PEX7 in the import of proteins into glycosomes of Trypanosoma brucei. Biochim. Biophys. Acta. 2007;1773:521–535. doi: 10.1016/j.bbamcr.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Khan B.R., Zolman B.K. pex5 Mutants that differentially disrupt PTS1 and PTS2 peroxisomal matrix protein import in Arabidopsis. Plant Physiol. 2010;154:1602–1615. doi: 10.1104/pp.110.162479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramón N.M., Bartel B. Interdependence of the peroxisome-targeting receptors in Arabidopsis thaliana: PEX7 facilitates PEX5 accumulation and import of PTS1 cargo into peroxisomes. Mol. Biol. Cell. 2010;21:1263–1271. doi: 10.1091/mbc.e09-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan D., Nakatsu T., Kato H. Crystal structure of peroxisomal targeting signal-2 bound to its receptor complex Pex7p-Pex21p. Nat. Struct. Mol. Biol. 2013;20:987–993. doi: 10.1038/nsmb.2618. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues T.A., Grou C.P., Azevedo J.E. Revisiting the intraperoxisomal pathway of mammalian PEX7. Sci. Rep. 2015;5:11806. doi: 10.1038/srep11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunze M., Malkani N., Maurer-Stroh S., Wiesinger C., Schmid J.A., Berger J. Mechanistic insights into PTS2-mediated peroxisomal protein import: the co-receptor PEX5L drastically increases the interaction strength between the cargo protein and the receptor PEX7. J. Biol. Chem. 2015;290:4928–4940. doi: 10.1074/jbc.M114.601575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voorn-Brouwer T., van der Leij I., Hemrika W., Distel B., Tabak H.F. Sequence of the PAS8 gene, the product of which is essential for biogenesis of peroxisomes in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1993;1216:325–328. doi: 10.1016/0167-4781(93)90166-B. [DOI] [PubMed] [Google Scholar]
- 42.Purdue P.E., Yang X., Lazarow P.B. Pex18p and Pex21p, a novel pair of related peroxins essential for peroxisomal targeting by the PTS2 pathway. J. Cell Biol. 1998;143:1859–1869. doi: 10.1083/jcb.143.7.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dodt G., Warren D., Becker E., Rehling P., Gould S.J. Domain mapping of human PEX5 reveals functional and structural similarities to Saccharomyces cerevisiae Pex18p and Pex21p. J. Biol. Chem. 2001;276:41769–41781. doi: 10.1074/jbc.M106932200. [DOI] [PubMed] [Google Scholar]
- 44.Otzen M., Wang D., Lunenborg M.G.J., Van Der Klei I.J. Hansenula polymorpha Pex20p is an oligomer that binds the peroxisomal targeting signal 2 (PTS2) J. Cell Sci. 2005;118:3409–3418. doi: 10.1242/jcs.02463. [DOI] [PubMed] [Google Scholar]
- 45.Einwächter H., Sowinski S., Kunau W.H., Schliebs W. Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex21p and mammalian Pex5pL fulfil a common function in the early steps of the peroxisomal PTS2 import pathway. EMBO Rep. 2001;2:1035–1039. doi: 10.1093/embo-reports/kve228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Effelsberg D., Cruz-Zaragoza L.D., Schliebs W., Erdmann R. Pex9p is a new yeast peroxisomal import receptor for PTS1-containing proteins. J. Cell Sci. 2016;129:4057–4066. doi: 10.1242/jcs.195271. [DOI] [PubMed] [Google Scholar]
- 47.Yifrach E., Chuartzman S.G., Dahan N., Maskit S., Zada L., Weill U., Yofe I., Olender T., Schuldiner M., Zalckvar E. Characterization of proteome dynamics during growth in oleate reveals a new peroxisome-targeting receptor. J. Cell Sci. 2016;129:4067–4075. doi: 10.1242/jcs.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schäfer A., Kerssen D., Veenhuis M., Kunau W.H., Schliebs W. Functional similarity between the peroxisomal PTS2 receptor binding protein Pex18p and the N-terminal half of the PTS1 receptor Pex5p. Mol. Cell. Biol. 2004;24:8895–8906. doi: 10.1128/MCB.24.20.8895-8906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schliebs W., Kunau W.H. PTS2 Co-receptors: Diverse proteins with common features. Biochim. Biophys. Acta-Mol. Cell Res. 2006;1763:1605–1612. doi: 10.1016/j.bbamcr.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 50.Agne B., Meindl N.M., Niederhoff K., Einwächter H., Rehling P., Sickmann A., Meyer H.E., Girzalsky W., Kunau W.H. Pex8p: an intraperoxisomal organizer of the peroxisomal import machinery. Mol. Cell. 2003;11:635–646. doi: 10.1016/S1097-2765(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 51.Reguenga C., Oliveira M.E., Gouveia A.M., Sá-Miranda C., Azevedo J.E. Characterization of the mammalian peroxisomal import machinery: Pex2p, Pex5p, Pex12p, and Pex14p are subunits of the same protein assembly. J. Biol. Chem. 2001;276:29935–29942. doi: 10.1074/jbc.M104114200. [DOI] [PubMed] [Google Scholar]
- 52.McNew J.A., Goodman J.M. An oligomeric protein is imported into peroxisomes in vivo. J. Cell Biol. 1994;127:1245–1257. doi: 10.1083/jcb.127.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glover J.R., Andrews D.W., Rachubinski R.A. Saccharomyces cerevisiae peroxisomal thiolase is imported as a dimer. Proc. Natl. Acad. Sci. USA. 1994;91:10541–10545. doi: 10.1073/pnas.91.22.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart M.Q., Esposito R.D., Gowani J., Goodman J.M. Alcohol oxidase and dihydroxyacetone synthase, the abundant peroxisomal proteins of methylotrophic yeasts, assemble in different cellular compartments. J. Cell Sci. 2001;114:2863–2868. doi: 10.1242/jcs.114.15.2863. [DOI] [PubMed] [Google Scholar]
- 55.Dias A.F., Rodrigues T.A., Pedrosa A.G., Barros-Barbosa A., Francisco T., Azevedo J.E. The peroxisomal matrix protein translocon is a large cavity-forming protein assembly into which PEX5 protein enters to release its cargo. J. Biol. Chem. 2017;292:15287–15300. doi: 10.1074/jbc.M117.805044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dias A.F., Francisco T., Rodrigues T.A., Grou C.P., Azevedo J.E. The first minutes in the life of a peroxisomal matrix protein. Biochim. Biophys. Acta. 2016;1863:814–820. doi: 10.1016/j.bbamcr.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 57.Barros-Barbosa A., Rodrigues T.A., Ferreira M.J., Pedrosa A.G., Teixeira N.R., Francisco T., Azevedo J.E. The intrinsically disordered nature of the peroxisomal protein translocation machinery. FEBS J. 2019;286:24–38. doi: 10.1111/febs.14704. [DOI] [PubMed] [Google Scholar]
- 58.Gouveia A.M., Guimarães C.P., Oliveira M.E., Sá-Miranda C., Azevedo J.E. Insertion of Pex5p into the peroxisomal membrane is cargo protein-dependent. J. Biol. Chem. 2003;278:4389–4392. doi: 10.1074/jbc.C200650200. [DOI] [PubMed] [Google Scholar]
- 59.Costa-Rodrigues J., Carvalho A.F., Gouveia A.M., Fransen M., Sá-Miranda C., Azevedo J.E. The N terminus of the peroxisomal cycling receptor, Pex5p, is required for redirecting the peroxisome-associated peroxin back to the cytosol. J. Biol. Chem. 2004;279:46573–46579. doi: 10.1074/jbc.M406399200. [DOI] [PubMed] [Google Scholar]
- 60.Gouveia A.M., Guimarães C.P., Oliveira M.E., Reguenga C., Sá-Miranda C., Azevedo J.E. Characterization of the peroxisomal cycling receptor, Pex5p, using a cell-free in vitro import system. J. Biol. Chem. 2003;278:226–232. doi: 10.1074/jbc.M209498200. [DOI] [PubMed] [Google Scholar]
- 61.Rodrigues T.A., Alencastre I.S., Francisco T., Brites P., Fransen M., Grou C.P., Azevedo J.E. A PEX7-centered perspective on the peroxisomal targeting signal type 2-mediated protein import pathway. Mol. Cell. Biol. 2014;34:2917–2928. doi: 10.1128/MCB.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Der Lee R., Buljan M., Lang B., Weatheritt R.J., Daughdrill G.W., Dunker A.K., Fuxreiter M., Gough J., Gsponer J., Jones D.T., et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014;114:6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neuhaus A., Kooshapur H., Wolf J., Meyer N.H., Madl T., Saidowsky J., Hambruch E., Lazam A., Jung M., Sattler M., et al. A novel Pex14 protein-interacting site of human Pex5 is critical for matrix protein import into peroxisomes. J. Biol. Chem. 2014;289:437–448. doi: 10.1074/jbc.M113.499707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saidowsky J., Dodt G., Kirchberg K., Wegner A., Nastainezyk W., Kunau W.H., Schliebs W. The di-aromatic pentapeptide repeats of the human peroxisome import receptor PEX5 are separate high affinity binding sites for the peroxisomal membrane protein PEX14. J. Biol. Chem. 2001;276:34524–34529. doi: 10.1074/jbc.M104647200. [DOI] [PubMed] [Google Scholar]
- 65.Waterham H.R., Titorenko V.I., Haima P., Cregg J.M., Harder W., Veenhuis M. The Hansenula polymorpha PER1 gene is essential for peroxisome biogenesis and encodes a peroxisomal matrix protein with both carboxy- and amino-terminal targeting signals. J. Cell Biol. 1994;127:737–749. doi: 10.1083/jcb.127.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elgersma Y., Kwast L., Klein A., Voorn-Brouwer T., van den Berg M., Metzig B., America T., Tabak H.F., Distel B. The SH3 domain of the Saccharomyces cerevisiae peroxisomal membrane protein Pex13p functions as a docking site for Pex5p, a mobile receptor for the import PTS1-containing proteins. J. Cell Biol. 1996;135:97–109. doi: 10.1083/jcb.135.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang C.C., Warren D.S., Sacksteder K.A., Gould S.J. PEX12 interacts with PEX5 and PEX10 and acts downstream of receptor docking in peroxisomal matrix protein import. J. Cell Biol. 1999;147:761–774. doi: 10.1083/jcb.147.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rehling P., Skaletz-Rorowski A., Girzalsky W., Voorn-Brouwer T., Franse M.M., Distel B., Veenhuis M., Kunau W.H., Erdmann R. Pex8p, an intraperoxisomal peroxin of Saccharomyces cerevisiae required for protein transport into peroxisomes binds the PTS1 receptor pex5p. J. Biol. Chem. 2000;275:3593–3602. doi: 10.1074/jbc.275.5.3593. [DOI] [PubMed] [Google Scholar]
- 69.Mano S., Nakamori C., Nito K., Kondo M., Nishimura M. The Arabidopsis pex12 and pex13 mutants are defective in both PTS1- and PTS2-dependent protein transport to peroxisomes. Plant J. 2006;47:604–618. doi: 10.1111/j.1365-313X.2006.02809.x. [DOI] [PubMed] [Google Scholar]
- 70.Freitas M.O., Francisco T., Rodrigues T.A., Alencastre I.S., Pinto M.P., Grou C.P., Carvalho A.F., Fransen M., Sá-Miranda C., Azevedo J.E. PEX5 protein binds monomeric catalase blocking its tetramerization and releases it upon binding the N-terminal domain of PEX14. J. Biol. Chem. 2011;286:40509–40519. doi: 10.1074/jbc.M111.287201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lanyon-Hogg T., Hooper J., Gunn S., Warriner S.L., Baker A. PEX14 binding to Arabidopsis PEX5 has differential effects on PTS1 and PTS2 cargo occupancy of the receptor. FEBS Lett. 2014;588:2223–2229. doi: 10.1016/j.febslet.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carvalho A.F., Pinto M.P., Grou C.P., Alencastre I.S., Fransen M., Sá-Miranda C., Azevedo J.E. Ubiquitination of mammalian Pex5p, the peroxisomal import receptor. J. Biol. Chem. 2007;282:31267–31272. doi: 10.1074/jbc.M706325200. [DOI] [PubMed] [Google Scholar]
- 73.Williams C.P., Van Den Berg M., Sprenger R.R., Distel B. A conserved cysteine is essential for Pex4p-dependent ubiquitination of the peroxisomal import receptor Pex5p. J. Biol. Chem. 2007;282:22534–22543. doi: 10.1074/jbc.M702038200. [DOI] [PubMed] [Google Scholar]
- 74.Francisco T., Rodrigues T.A., Pinto M.P., Carvalho A.F., Azevedo J.E., Grou C.P. Ubiquitin in the peroxisomal protein import pathway. Biochimie. 2014;98:29–35. doi: 10.1016/j.biochi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto N., Tamura S., Fujiki Y. The pathogenic peroxin Pex26p recruits the Pex1p-Pex6p AAA ATPase complexes to peroxisomes. Nat. Cell Biol. 2003;5:454–460. doi: 10.1038/ncb982. [DOI] [PubMed] [Google Scholar]
- 76.Birschmann I., Stroobants A.K., van den Berg M., Schäfer A., Rosenkranz K., Kunau W.H., Tabak H.F. Pex15p of Saccharomyces cerevisiae provides a molecular basis for recruitment of the AAA peroxin Pex6p to peroxisomal membranes. Mol. Biol. Cell. 2003;14:2226–2236. doi: 10.1091/mbc.e02-11-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miyata N., Fujiki Y. Shuttling mechanism of peroxisome targeting signal type 1 receptor Pex5: ATP-independent import and ATP-dependent export. Mol. Cell. Biol. 2005;25:10822–10832. doi: 10.1128/MCB.25.24.10822-10832.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Platta H.W., Grunau S., Rosenkranz K., Girzalsky W., Erdmann R. Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol. Nat. Cell Biol. 2005;7:817–822. doi: 10.1038/ncb1281. [DOI] [PubMed] [Google Scholar]
- 79.Goto S., Mano S., Nakamori C., Nishimura M. Arabidopsis ABERRANT PEROXISOME MORPHOLOGY9 is a peroxin that recruits the PEX1-PEX6 complex to peroxisomes. Plant Cell. 2011;23:1573–1587. doi: 10.1105/tpc.110.080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pedrosa A.G., Francisco T., Bicho D., Dias A.F., Barros-Barbosa A., Hagmann V., Dodt G., Rodrigues T.A., Azevedo J.E. Peroxisomal monoubiquitinated PEX5 interacts with the AAA ATPases PEX1 and PEX6 and is unfolded during its dislocation into the cytosol. J. Biol. Chem. 2018;293:11553–11563. doi: 10.1074/jbc.RA118.003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grou C.P., Carvalho A.F., Pinto M.P., Alencastre I.S., Rodrigues T.A., Freitas M.O., Francisco T., Sá-Miranda C., Azevedo J.E. The peroxisomal protein import machinery--a case report of transient ubiquitination with a new flavor. Cell. Mol. Life Sci. 2009;66:254–262. doi: 10.1007/s00018-008-8415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erdmann R., Wiebel F.F., Flessau A., Rytka J., Beyer A., Fröhlich K.U., Kunau W.H. PAS1, a yeast gene required for peroxisome biogenesis, encodes a member of a novel family of putative ATPases. Cell. 1991;64:499–510. doi: 10.1016/0092-8674(91)90234-P. [DOI] [PubMed] [Google Scholar]
- 83.Portsteffen H., Beyer A., Becker E., Epplen C., Pawlak A., Kunau W.H., Dodt G. Human PEX1 is mutated in complementation group 1 of the peroxisome biogenesis disorders. Nat. Genet. 1997;17:449–452. doi: 10.1038/ng1297-449. [DOI] [PubMed] [Google Scholar]
- 84.Reuber B.E., Germain-Lee E., Collins C.S., Morrell J.C., Ameritunga R., Moser H.W., Valle D., Gould S.J. Mutations in PEX1 are the most common cause of peroxisome biogenesis disorders. Nat. Genet. 1997;17:445–448. doi: 10.1038/ng1297-445. [DOI] [PubMed] [Google Scholar]
- 85.Tamura S., Okumoto K., Toyama R., Shimozawa N., Tsukamoto T., Suzuki Y., Osumi T., Kondo N., Fujiki Y. Human PEX1 cloned by functional complementation on a CHO cell mutant is responsible for peroxisome-deficient Zellweger syndrome of complementation group I. Proc. Natl. Acad. Sci. USA. 1998;95:4350–4355. doi: 10.1073/pnas.95.8.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez-Huertas E., Charlton W.L., Johnson B., Graham I.A., Baker A. Stress induces peroxisome biogenesis genes. EMBO J. 2000;19:6770–6777. doi: 10.1093/emboj/19.24.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zolman B.K., Yoder A., Bartel B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics. 2000;156:1323–1337. doi: 10.1093/genetics/156.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zolman B.K., Bartel B. An Arabidopsis indole-3-butyric acid-response mutant defective in PEROXIN6, an apparent ATPase implicated in peroxisomal function. Proc. Natl. Acad. Sci. USA. 2004;101:1786–1791. doi: 10.1073/pnas.0304368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schlüter A., Fourcade S., Ripp R., Mandel J.L., Poch O., Pujol A. The evolutionary origin of peroxisomes: An ER-peroxisome connection. Mol. Biol. Evol. 2006;23:838–845. doi: 10.1093/molbev/msj103. [DOI] [PubMed] [Google Scholar]
- 90.Gabaldón T., Snel B., van Zimmeren F., Hemrika W., Tabak H.F., Huynen M.A. Origin and evolution of the peroxisomal proteome. Biol. Direct. 2006;1:8. doi: 10.1186/1745-6150-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waterham H.R., Ebberink M.S. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim. Biophys. Acta. 2012;1822:1430–1441. doi: 10.1016/j.bbadis.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 92.Argyriou C., D’Agostino M.D., Braverman N.E. Peroxisome biogenesis disorders. Transl. Sci. Rare Dis. 2016;1:111–144. doi: 10.3233/TRD-160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schieferdecker A., Wendler P. Structural Mapping of Missense Mutations in the Pex1/Pex6 Complex. Int. J. Mol. Sci. 2019;20:3756. doi: 10.3390/ijms20153756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elgersma Y., Snyder W.B., Subramani S., Kwast L., Van Den Berg M., Distel B., Tabak H.F. Overexpression of Pex15p, a phosphorylated peroxisomal integral membrane protein required for peroxisome assembly in S.cerevisiae, causes proliferation of the endoplasmic reticulum membrane. EMBO J. 1997;16:7326–7341. doi: 10.1093/emboj/16.24.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Collins C.S., Kalish J.E., Morrell J.C., McCaffery J.M., Gould S.J. The peroxisome biogenesis factors pex4p, pex22p, pex1p, and pex6p act in the terminal steps of peroxisomal matrix protein import. Mol. Cell. Biol. 2000;20:7516–7526. doi: 10.1128/mcb.20.20.7516-7526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanson P.I., Whiteheart S.W. AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 97.Imanaka T., Small G.M., Lazarow P.B. Translocation of acyl-CoA oxidase into peroxisomes required ATP hydrolysis but not a membrane potential. J. Cell Biol. 1987;105:2915–2922. doi: 10.1083/jcb.105.6.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Behari R., Baker A. The carboxyl terminus of isocitrate lyase is not essential for import into glyoxysomes in an in vitro system. J. Biol. Chem. 1993;268:7315–7322. [PubMed] [Google Scholar]
- 99.Rapp S., Soto U., Just W.W. Import of firefly luciferase into peroxisomes of permeabilized Chinese hamster ovary cells: A model system to study peroxisomal protein import in vitro. Exp. Cell Res. 1993;205:59–65. doi: 10.1006/excr.1993.1058. [DOI] [PubMed] [Google Scholar]
- 100.Wendland M., Subramani S. Cytosol-dependent peroxisomal protein import in a permeabilized cell system. J. Cell Biol. 1993;120:675–685. doi: 10.1083/jcb.120.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brickner D.G., Harada J.J., Olsen L.J. Protein transport into higher plant peroxisomes: In vitro import assay provides evidence for receptor involvement. Plant Physiol. 1997;113:1213–1221. doi: 10.1104/pp.113.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gould S.J., Collins C.S. Opinion: peroxisomal-protein import: is it really that complex? Nat. Rev. Mol. Cell Biol. 2002;3:382–389. doi: 10.1038/nrm807. [DOI] [PubMed] [Google Scholar]
- 103.Titorenko V.I., Rachubinski R.A. Peroxisomal membrane fusion requires two AAA family ATPases, Pex1p and Pex6p. J. Cell Biol. 2000;150:881–886. doi: 10.1083/jcb.150.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van Der Zand A., Gent J., Braakman I., Tabak H.F. Biochemically distinct vesicles from the endoplasmic reticulum fuse to form peroxisomes. Cell. 2012;149:397–409. doi: 10.1016/j.cell.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 105.Knoops K., De Boer R., Kram A., Van Der Klei I.J. Yeast pex1 cells contain peroxisomal ghosts that import matrix proteins upon reintroduction of Pex1. J. Cell Biol. 2015;211:955–962. doi: 10.1083/jcb.201506059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Motley A.M., Galvin P.C., Ekal L., Nuttall J.M., Hettema E.H. Reevaluation of the role of Pex1 and dynamin-related proteins in peroxisome membrane biogenesis. J. Cell Biol. 2015;211:1041–1056. doi: 10.1083/jcb.201412066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogura T., Wilkinson A.J. AAA+ superfamily ATPases: common structure—Diverse function. Genes to Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 108.Sysoeva T.A. Assessing heterogeneity in oligomeric AAA+ machines. Cell. Mol. Life Sci. 2017;74:1001–1018. doi: 10.1007/s00018-016-2374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saffert P., Enenkel C., Wendler P. Structure and Function of p97 and Pex1/6 Type II AAA+ Complexes. Front. Mol. Biosci. 2017;4:1–13. doi: 10.3389/fmolb.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bard J.A., Goodall E.A., Greene E.R., Jonsson E., Dong K.C., Martin A. Structure and Function of the 26S Proteasome. Annu. Rev. Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olivares A.O., Baker T.A., Sauer R.T. Mechanistic insights into bacterial AAA+ proteases and protein-remodelling machines. Nat. Rev. Microbiol. 2016;14:33–44. doi: 10.1038/nrmicro.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miller J.M., Enemark E.J. Fundamental Characteristics of AAA+ Protein Family Structure and Function. Archaea. 2016:9294307. doi: 10.1155/2016/9294307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deville C., Franke K., Mogk A., Bukau B., Saibil H.R. Two-Step Activation Mechanism of the ClpB Disaggregase for Sequential Substrate Threading by the Main ATPase Motor. Cell Rep. 2019;27:3433–3446.e4. doi: 10.1016/j.celrep.2019.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown B.L., Vieux E.F., Kalastavadi T., Kim S., Chen J.Z., Baker T.A. N domain of the Lon AAA+ protease controls assembly and substrate choice. Protein Sci. 2019;28:1239–1251. doi: 10.1002/pro.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dougan D.A., Mogk A., Zeth K., Turgay K., Bukau B. AAA+ proteins and substrate recognition, it all depends on their partner in crime. FEBS Lett. 2002;529:6–10. doi: 10.1016/S0014-5793(02)03179-4. [DOI] [PubMed] [Google Scholar]
- 116.Stach L., Freemont P.S. The AAA+ ATPase p97, a cellular multitool. Biochem. J. 2017;474:2953–2976. doi: 10.1042/BCJ20160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao C., Smith E.C., Whiteheart S.W. Requirements for the catalytic cycle of the N-ethylmaleimide-Sensitive Factor (NSF) Biochim. Biophys. Acta. 2012;1823:159–171. doi: 10.1016/j.bbamcr.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sweeny E.A., Shorter J. Mechanistic and Structural Insights into the Prion-Disaggregase Activity of Hsp104. J. Mol. Biol. 2016;428:1870–1885. doi: 10.1016/j.jmb.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wendler P., Ciniawsky S., Kock M., Kube S. Structure and function of the AAA+ nucleotide binding pocket. Biochim. Biophys. Acta. 2012;1823:2–14. doi: 10.1016/j.bbamcr.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 120.Hinnerwisch J., Fenton W.A., Furtak K.J., Farr G.W., Horwich A.L. Loops in the central channel of ClpA chaperone mediate protein binding, unfolding, and translocation. Cell. 2005;121:1029–1041. doi: 10.1016/j.cell.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 121.DeLaBarre B., Christianson J.C., Kopito R.R., Brunger A.T. Central pore residues mediate the p97/VCP activity required for ERAD. Mol. Cell. 2006;22:451–462. doi: 10.1016/j.molcel.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 122.Roll-Mecak A., Vale R.D. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature. 2008;451:363–367. doi: 10.1038/nature06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao C., Matveeva E.A., Ren Q., Whiteheart S.W. Dissecting the N-ethylmaleimide-sensitive factor: required elements of the N and D1 domains. J. Biol. Chem. 2010;285:761–772. doi: 10.1074/jbc.M109.056739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cipriano D.J., Jung J., Vivona S., Fenn T.D., Brunger A.T., Bryant Z. Processive ATP-driven substrate disassembly by the N-ethylmaleimide- sensitive factor (NSF) molecular machine. J. Biol. Chem. 2013;288:23436–23445. doi: 10.1074/jbc.M113.476705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao M., Wu S., Zhou Q., Vivona S., Cipriano D.J., Cheng Y., Brunger A.T. Mechanistic insights into the recycling machine of the SNARE complex. Nature. 2015;518:61–67. doi: 10.1038/nature14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao M., Brunger A.T. Recent Advances in Deciphering the Structure and Molecular Mechanism of the AAA+ ATPase N-Ethylmaleimide-Sensitive Factor (NSF) J. Mol. Biol. 2016;428:1912–1926. doi: 10.1016/j.jmb.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.White K.I., Zhao M., Choi U.B., Pfuetzner R.A., Brunger A.T. Structural principles of SNARE complex recognition by the AAA+ protein NSF. Elife. 2018;7:e38888. doi: 10.7554/eLife.38888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van den Boom J., Meyer H. VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol. Cell. 2018;69:182–194. doi: 10.1016/j.molcel.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 129.Twomey E.C., Ji Z., Wales T.E., Bodnar N.O., Ficarro S.B., Marto J.A., Engen J.R., Rapoport T.A. Substrate processing by the Cdc48 ATPase complex is initiated by ubiquitin unfolding. Science. 2019;365:eaax1033. doi: 10.1126/science.aax1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cooney I., Han H., Stewart M.G., Carson R.H., Hansen D.T., Iwasa J.H., Price J.C., Hill C.P., Shen P.S. Structure of the Cdc48 segregase in the act of unfolding an authentic substrate. Science. 2019;365:502–505. doi: 10.1126/science.aax0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shiozawa K., Maita N., Tomii K., Seto A., Goda N., Akiyama Y., Shimizu T., Shirakawa M., Hiroaki H. Structure of the N-terminal domain of PEX1 AAA-ATPase. Characterization of a putative adaptor-binding domain. J. Biol. Chem. 2004;279:50060–50068. doi: 10.1074/jbc.M407837200. [DOI] [PubMed] [Google Scholar]
- 132.Ciniawsky S., Grimm I., Saffian D., Girzalsky W., Erdmann R., Wendler P. Molecular snapshots of the Pex1/6 AAA+ complex in action. Nat. Commun. 2015;6:7331. doi: 10.1038/ncomms8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gardner B.M., Chowdhury S., Lander G.C., Martin A. The Pex1/Pex6 complex is a heterohexameric AAA+ motor with alternating and highly coordinated subunits. J. Mol. Biol. 2015;427:1375–1388. doi: 10.1016/j.jmb.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsukamoto T., Miura S., Nakai T., Yokota S., Shimozawa N., Suzuki Y., Orii T., Fujiki Y., Sakai F., Bogaki A., et al. Peroxisome assembly factor-2, a putative ATPase cloned by functional complementation on a peroxisome-deficient mammalian cell mutant. Nat. Genet. 1995;11:395–401. doi: 10.1038/ng1295-395. [DOI] [PubMed] [Google Scholar]
- 135.Tamura S., Shimozawa N., Suzuki Y., Tsukamoto T., Osumi T., Fujiki Y. A cytoplasmic AAA family peroxin, Pex1p, interacts with Pex6p. Biochem. Biophys. Res. Commun. 1998;245:883–886. doi: 10.1006/bbrc.1998.8522. [DOI] [PubMed] [Google Scholar]
- 136.Yahraus T., Braverman N.E., Dodt G., Kalish J.E., Morrell J.C., Moser H.W., Valle D., Gould S.J. The peroxisome biogenesis disorder group 4 gene, PXAAA1, encodes a cytoplasmic ATPase required for stability of the PTS1 receptor. EMBO J. 1996;15:2914–2923. doi: 10.1002/j.1460-2075.1996.tb00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blok N.B., Tan D., Wang R.Y.-R., Penczek P.A., Baker D., DiMaio F., Rapoport T.A., Walz T. Unique double-ring structure of the peroxisomal Pex1/Pex6 ATPase complex revealed by cryo-electron microscopy. Proc. Natl. Acad. Sci. USA. 2015;112:E4017–E4025. doi: 10.1073/pnas.1500257112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hänzelmann P., Schindelin H. Structural Basis of ATP Hydrolysis and Intersubunit Signaling in the AAA+ ATPase p97. Structure. 2016;24:127–139. doi: 10.1016/j.str.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 139.Gardner B.M., Castanzo D.T., Chowdhury S., Stjepanovic G., Stefely M.S., Hurley J.H., Lander G.C., Martin A. The peroxisomal AAA-ATPase Pex1/Pex6 unfolds substrates by processive threading. Nat. Commun. 2018;9:135. doi: 10.1038/s41467-017-02474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Birschmann I., Rosenkranz K., Erdmann R., Kunau W.H. Structural and functional analysis of the interaction of the AAA-peroxins Pex1p and Pex6p. FEBS J. 2005;272:47–58. doi: 10.1111/j.1432-1033.2004.04393.x. [DOI] [PubMed] [Google Scholar]
- 141.Tamura S., Yasutake S., Matsumoto N., Fujiki Y. Dynamic and functional assembly of the AAA peroxins, Pex1p and Pex6p, and their membrane receptor Pex26p. J. Biol. Chem. 2006;281:27693–27704. doi: 10.1074/jbc.M605159200. [DOI] [PubMed] [Google Scholar]
- 142.Tamura S., Matsumoto N., Takebas R., Fujiki Y. AAA peroxins and their recruiter Pex26p modulate the interactions of peroxins involved in peroxisomal protein import. J. Biol. Chem. 2014;289:24336–24346. doi: 10.1074/jbc.M114.588038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grimm I., Saffian D., Girzalsky W., Erdmann R. Nucleotide-dependent assembly of the peroxisomal receptor export complex. Sci. Rep. 2016;6:19838. doi: 10.1038/srep19838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Erzberger J.P., Berger J.M. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- 145.Hagmann V., Sommer S., Fabian P., Bierlmeier J., van Treel N., Mootz H.D., Schwarzer D., Azevedo J.E., Dodt G. Chemically monoubiquitinated PEX5 binds to the components of the peroxisomal docking and export machinery. Sci. Rep. 2018;8:16014. doi: 10.1038/s41598-018-34200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schwerter D.P., Grimm I., Girzalsky W., Erdmann R. Receptor recognition by the peroxisomal AAA complex depends on the presence of the ubiquitin moiety and is mediated by Pex1p. J. Biol. Chem. 2018;293:15458–15470. doi: 10.1074/jbc.RA118.003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Urquhart A.J., Kennedy D., Gould S.J., Crane D.I. Interaction of Pex5p, the type 1 peroxisome targeting signal receptor, with the peroxisomal membrane proteins Pex14p and Pex13p. J. Biol. Chem. 2000;275:4127–4136. doi: 10.1074/jbc.275.6.4127. [DOI] [PubMed] [Google Scholar]
- 148.Fransen M., Brees C., Ghys K., Amery L., Mannaerts G.P., Ladant D., Van Veldhoven P.P. Analysis of mammalian peroxin interactions using a non-transcription-based bacterial two-hybrid assay. Mol. Cell. Proteomics. 2002;1:243–252. doi: 10.1074/mcp.M100025-MCP200. [DOI] [PubMed] [Google Scholar]
- 149.Barros-Barbosa A., Ferreira M.J.M.J., Rodrigues T.A., Pedrosa A.G., Grou C.P., Pinto M.P.M.P., Fransen M., Francisco T., Azevedo J.E. Membrane topologies of PEX13 and PEX14 provide new insights on the mechanism of protein import into peroxisomes. FEBS J. 2019;286:205–222. doi: 10.1111/febs.14697. [DOI] [PubMed] [Google Scholar]
- 150.Nordgren M., Francisco T., Lismont C., Hennebel L., Brees C., Wang B., Van Veldhoven P.P., Azevedo J.E., Fransen M. Export-deficient monoubiquitinated PEX5 triggers peroxisome removal in SV40 large T antigen-transformed mouse embryonic fibroblasts. Autophagy. 2015;11:1326–1340. doi: 10.1080/15548627.2015.1061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johnston J.A., Johnson E.S., Waller P.R., Varshavsky A. Methotrexate inhibits proteolysis of dihydrofolate reductase by the N-end rule pathway. J. Biol. Chem. 1995;270:8172–8178. doi: 10.1074/jbc.270.14.8172. [DOI] [PubMed] [Google Scholar]
- 152.Yedidi R.S., Wendler P., Enenkel C. AAA-ATPases in Protein Degradation. Front. Mol. Biosci. 2017;4:1–14. doi: 10.3389/fmolb.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johjima A., Noi K., Nishikori S., Ogi H., Esaki M., Ogura T. Microtubule severing by katanin p60 AAA+ATPase requires the C-terminal acidic tails of both α-and β-tubulins and basic amino acid residues in the AAA+ring pore. J. Biol. Chem. 2015;290:11762–11770. doi: 10.1074/jbc.M114.614768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zehr E.A., Szyk A., Piszczek G., Szczesna E., Zuo X., Roll-Mecak A. Katanin spiral and ring structures shed light on power stroke for microtubule severing. Nat. Struct. Mol. Biol. 2017;24:717–725. doi: 10.1038/nsmb.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhattacharyya S., Yu H., Mim C., Matouschek A. Regulated protein turnover: snapshots of the proteasome in action. Nat. Rev. Mol. Cell Biol. 2014;15:122–133. doi: 10.1038/nrm3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Han H., Fulcher J.M., Dandey V.P., Iwasa J.H., Sundquist W.I., Kay M.S., Shen P.S., Hill C.P. Structure of Vps4 with circular peptides and implications for translocation of two polypeptide chains by AAA+ ATPases. Elife. 2019;8:e44071. doi: 10.7554/eLife.44071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blythe E.E., Olson K.C., Chau V., Deshaies R.J. Ubiquitin- and ATP-dependent unfoldase activity of P97/VCP•NPLOC4•UFD1L is enhanced by a mutation that causes multisystem proteinopathy. Proc. Natl. Acad. Sci. USA. 2017;114:E4380–E4388. doi: 10.1073/pnas.1706205114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bodnar N.O., Rapoport T.A. Molecular Mechanism of Substrate Processing by the Cdc48 ATPase Complex. Cell. 2017;169:722–735. doi: 10.1016/j.cell.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Monroe N., Hill C.P. Meiotic Clade AAA ATPases: Protein Polymer Disassembly Machines. J. Mol. Biol. 2016;428:1897–1911. doi: 10.1016/j.jmb.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saffian D., Grimm I., Girzalsky W., Erdmann R. ATP-dependent assembly of the heteromeric Pex1p-Pex6p-complex of the peroxisomal matrix protein import machinery. J. Struct. Biol. 2012;179:126–132. doi: 10.1016/j.jsb.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 161.Bailey M.E., Jiang N., Dima R.I., Ross J.L. Invited review: Microtubule severing enzymes couple atpase activity with tubulin GTPase spring loading. Biopolymers. 2016;105:547–556. doi: 10.1002/bip.22842. [DOI] [PubMed] [Google Scholar]
- 162.Lu C., Turley S., Marionni S.T., Park Y.J., Lee K.K., Patrick M., Shah R., Sandkvist M., Bush M.F., Hol W.G.J. Hexamers of the type II secretion ATPase GspE from Vibrio cholerae with increased ATPase activity. Structure. 2013;21:1707–1717. doi: 10.1016/j.str.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hartman J.J., Vale R.D. Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science. 1999;286:782–785. doi: 10.1126/science.286.5440.782. [DOI] [PubMed] [Google Scholar]
- 164.Olivares A.O., Nager A.R., Iosefson O., Sauer R.T., Baker T.A. Mechanochemical basis of protein degradation by a double-ring AAA+ machine. Nat. Struct. Mol. Biol. 2014;21:871–875. doi: 10.1038/nsmb.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Driessen A.J. Precursor protein translocation by the Escherichia coli translocase is directed by the protonmotive force. EMBO J. 1992;11:847–853. doi: 10.1002/j.1460-2075.1992.tb05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alder N.N., Theg S.M. Energetics of protein transport across biological membranes. a study of the thylakoid DeltapH-dependent/cpTat pathway. Cell. 2003;112:231–242. doi: 10.1016/S0092-8674(03)00032-1. [DOI] [PubMed] [Google Scholar]