Abstract
Genetic tools are a prerequisite to engineer cellular factories for synthetic biology and biotechnology. Methylorubrum extorquens AM1 is an important platform organism of a future C1-bioeconomy. However, its application is currently limited by the availability of genetic tools. Here we systematically tested repABC regions to maintain extrachromosomal DNA in M. extorquens. We used three elements to construct mini-chromosomes that are stably inherited at single copy number and can be shuttled between Escherichia coli and M. extorquens. These mini-chromosomes are compatible among each other and with high-copy number plasmids of M. extorquens. We also developed a set of inducible promoters of wide expression range, reaching levels exceeding those currently available, notably the PmxaF-promoter. In summary, we provide a set of tools to control the dynamic expression and copy number of genetic elements in M. extorquens, which opens new ways to unleash the metabolic and biotechnological potential of this organism for future applications.
Keywords: repABC, inducible promoters, synthetic chromosomes, Methylorubrum extorquens, Alphaproteobacteria
Methylorubrum extorquens AM1 (formerly Methylobacterium extorquens) has been used as a model organism to study methylotrophy, i.e., growth on C1 carbon sources, such as methanol and formate, since its isolation in 1961.1 The genome of M. extorquens AM1 has been fully sequenced, and a genome-scale metabolic network was reconstructed for rational engineering.2,3 The unique ability of M. extorquens to grow on C1-units has made it an increasingly relevant organism for biotechnology in a methanol- and formate-based bioeconomy.4 Production of bulk and value-added chemicals such as mevalonate, α-humulene, 3-hydroxypropionate, and 1-butanol has already been realized in M. extorquens.5−8 For the further development of M. extorquens as a platform organism in C1-biotechnology, a broad set of genetic tools are required. Several basic tools are available: one replicative plasmid, cre-loxP and sacB-based suicide vectors for allelic replacement, and genetic parts including constitutive and, to a lesser extent, inducible promoters.9−14 Yet, this basic set of genetic tools is far from being complete, and many applications are challenged by the fact that more elaborate genetic tools are missing for this organism.
One particular challenge is the lack of dynamic control of gene expression in M. extorquens. The Plac promoter and its derivatives, which are widely used in many bacteria, confer only weak or leaky expression in M. extorquens.10 As an alternative to Plac, cumate-inducible promoters have been created by combining the CuO-CymR system from Pseudomonas putida F1 to native PmxaF and PMETA1p2148 promoters.12,14 While these hybrid promoters are strong upon induction, overexpression of the CymR repressor itself is toxic to the cells causing severe growth defects.
Another, equally important challenge is the availability and control over extrachromosomal genetic elements. Only one broad host range plasmid has been reported that replicates in M. extorquens without causing severe growth defects.9,10 This plasmid (referred to as oriV-traJ′ henceforth) has several disadvantages: a multiple and varying copy number, limited compatibility with other extrachromosomal genetic elements, and the need for constant selective pressure, which restricts its application potential. Genomic integration would circumvent using a plasmid. However, the transformation efficiency is several orders of magnitude lower for genomic integration events, and neutral sites for integration have not been characterized in this organism. It also requires the construction of a suicide vector with the desired DNA cargo flanked by homologous regions (≥0.5 kb) and subsequent verification of integration at the desired locus. All of these steps are work intensive and time-consuming.
Many Alphaproteobacteria have multipartite genomes composed of a main chromosome and one or more megaplasmids. The replication of megaplasmids is integrated into the bacterial cell cycle and occurs once to ensure faithful transmission of genetic information.15,16 A small region within these megaplasmids, termed the repABC cassette, drives vertical transmission of these secondary replicons. repA and repB encode for partitioning proteins analogous to the well-known ParA and ParB, while RepC is a replication initiator protein. The origin of replication resides within the repC coding sequence, whose expression is downregulated by a short, nontranslated counter-transcribed RNA (ctRNA) typically located in the repB/repC intergenic region.15−17 On the basis of this distinctive region, a new family of repABC-type shuttle vectors was successfully designed for Sinorhizobium meliloti mimicking the characteristic features of secondary replicons, i.e., single copy number and stable propagation.18 Four of these repABC regions were shown to be able to replicate in M. extorquens but they affected the growth of the organism.18
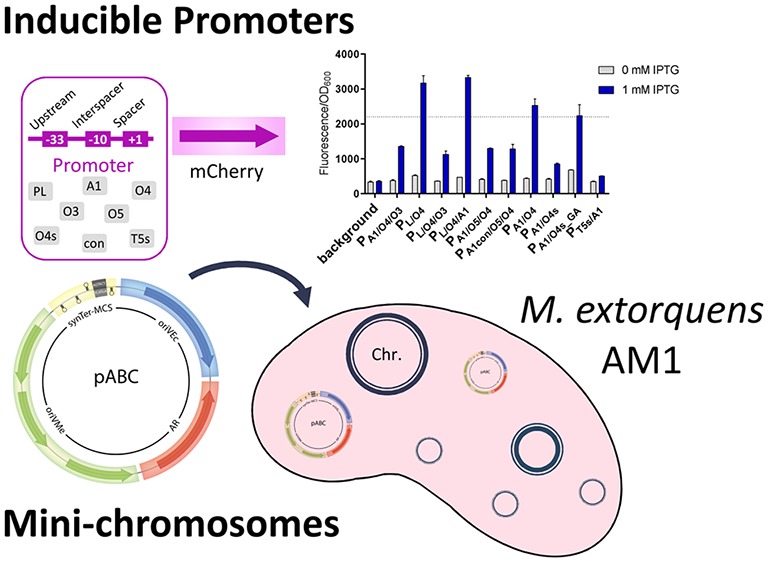
Here, to expand the limited genetic toolbox of M. extorquens AM1, we developed a set of inducible, orthogonal promoters of different strengths, which can be dynamically controlled by IPTG. Furthermore, we systematically tested new repABC regions to establish a set of extrachromosomal elements (“mini-chromosomes”) that are faithfully inherited by daughter cells and compatible with each other. This work provides the tools for the extensive genetic engineering of M. extorquens AM1 in the future.
Results and Discussion
Realization of Tight, IPTG-Inducible Promoters with a Dynamic Range
To overcome the problem of weak and leaky expression from IPTG-inducible promoters in M. extorquens, we sought to develop different lacO-controlled promoters that are tight and show a wide range of expression levels after addition of the inducer.
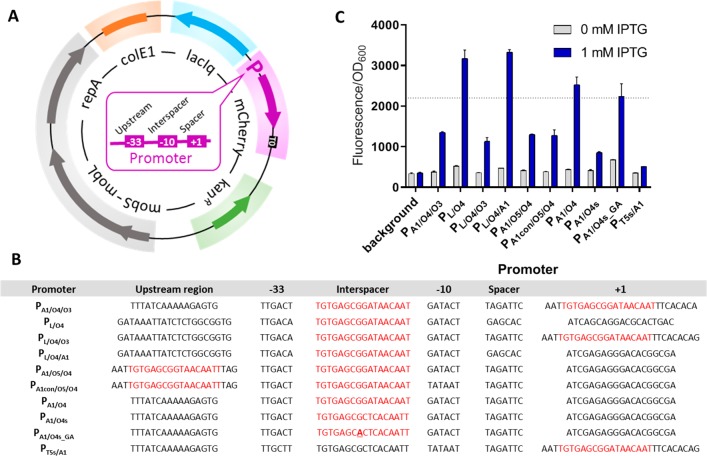
The PA1/O4/O3 promoter,19,20 also known as PA1lacO-1, was functional in M. extorquens with 27-fold induction of the fluorescence reporter gene mCherry (Figure 1C). The PA1/O4 and PA1/O4s promoters21 were also functional and showed 21- and 6-fold induction, respectively. A single point mutation within the O4s region of PA1/O4s, resulting in PA1/O4s_GA, showed increased mCherry expression at the expense of leakiness without inducer.
Figure 1.
IPTG-inducible promoters for M. extorquens. Map of pIND4-derived plasmid for testing new promoters expressing mCherry (A). Sequence of hybrid promoters constructed and tested (B); lacO sequences are shown in red. The point mutation within the lacO4s region is underlined. Fluorescence/OD600 before and after addition of 1 mM IPTG (C). The dashed line marks fluorescence/OD600 signal obtained using the strong PmxaF promoter.
The T5 promoter has been used for many years for protein overexpression in E. coli.22−24 The PT5-lac promoter from pCA24N was weakly functional in M. extorquens, but there was no difference in the fluorescent signal between uninduced and induced states (data not shown). We modified the upstream region and replaced lacO4 for a lacO4s sequence resulting in promoter PT5s/A1 that is tightly repressed but only weakly induced (11-fold). While PT5s/A1 is not suitable for gene overexpression, it might be interesting for applications where a protein is toxic if expressed at high levels or constitutively within the cell.
We also created three PL-lacO hybrid promoters, PL/O4, PL/O4/O3, and PL/O4/A1, which are all strongly repressed in the absence of inducer. Upon induction, PL/O4 and PL/O4/A1 exhibit 15- and 23-fold induction of mCherry, respectively. Notably, these two promoters provide mCherry fluorescence levels that are approximately 1.5-fold higher than those of the PmxaF promoter, which drives 9% of soluble protein expression in M. extorquens(25) (Figure 1C, dashed line shows PmxaF levels). The dynamic range of the PL/O4/A1 promoter is given in Figure S1. See Table S4 and S5 for more details on all inducible promoters tested in this study.
In summary, we created and identified a set of IPTG-inducible promoters for M. extorquens that range between 6- and 36-fold induction (Table S4). Additionally, our PA1- and PL-derived promoters range in maximum strength between 9% and 166% of the strong PmxaF promoter, which opens new possibilities for the controlled overexpression of proteins. Thus far, the highest expression level reported from an inducible promoter in M. extorquens was 33% of the PmxaF promoter.13 To facilitate the use of these new promoters by the scientific community, we created empty vectors featuring the respective promoter and a multiple cloning site with an optional N-terminal Strep-II tag compatible with the Methylobrick system9 and pET vectors (Novagen).
Identification of Suitable repABC Regions for Use in M. extorquens AM1
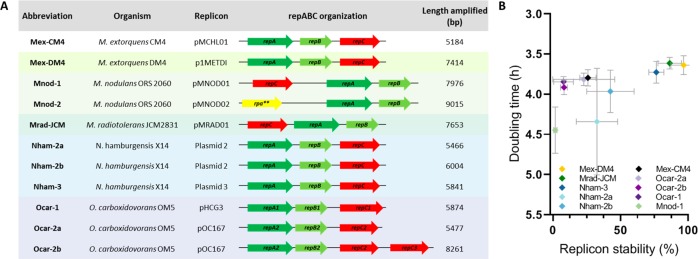
To establish artificial mini-chromosomal elements in M. extorquens, we tested 11 repABC regions from six different organisms. We introduced these regions into pK18mob2, which is a “suicide” vector unable to replicate in M. extorquens (Figure 2A and S2). All repABC regions were maintained as independent replicons in M. extorquens. The repABC-based replicons were recovered for restriction analysis and sequencing of the repABC region to confirm their integrity. The only exception was Mnod-2 from Methylobacterium nodulans, for which no colonies were obtained after electroporation, likely due to incompatibility of the Mnod-2 repABC region with the native genomic system.
Figure 2.
repABC regions tested in M. extorquens. (A) Size and operon organization. (B) Doubling time versus replicon stability (% of kanamycin resistant colonies) of repABC regions in the suicide vector pK18mob2. Mean and SD from three biological replicates.
Next, we determined the growth behavior of M. extorquens carrying each replicon under selective conditions, as well as replicon stability after 96 h under nonselective conditions (Figure 2B). We observed a general trend that replicon stability and growth behavior of M. extorquens were correlated. Mnod-1, also from M. nodulans, was very unstable, showed the lowest doubling time (Figure 2B) and a long lag phase (data not shown), suggesting that repABC cassettes from this organism are generally not well compatible with M. extorquens. Similarly, Nham-2a and Nham-2b from Nitrobacter hamburgensis are very unstable and affected the growth. Replicons originating from Oligotropha carboxidovorans were also unstable in the absence of selective pressure, in particular Ocar-1 and Ocar-2a, discouraging the use of these elements for genetic manipulations of M. extorquens.
The repABC cassette Mex-CM4 originating from M. extorquens CM4 showed an interesting behavior. Mex-CM4 allowed fast doubling of M. extorquens AM1 under selective conditions, while cells quickly lost the replicon in the absence of antibiotic pressure (Figure 1B and S4). The repABC cassettes of Mex-CM4 and Mex-DM4 (discussed below) are 98% identical to each other, except for the fact that Mex-DM4 has a longer region downstream of repC (Figure 1B and Figure S2). It might be the case that Mex-CM4 lacks some parS sites required for a faithful transmission of the respective replicon to the daughter cells. This behavior makes Mex-CM4 an interesting system to establish CRISPR-Cas in M. extorquens where transient expression followed by fast extinction of the genetic element is a desired feature.
The replicons Mex-DM4 (originating from M. extorquens DM4), Mrad-JCM (from M. radiotolerans JCM2831), and Nham-3 (from N. hamburgensis X-14 plasmid 3) showed the highest stability with 97%, 86%, and 77% of cells still harboring the plasmid after 96 h without antibiotic selection, respectively. The replicons were isolated from kanamycin resistant cells after 96 h without selective pressure and their integrity was verified by restriction analysis (data not shown). These three replicons allowed fast doubling times of M. extorquens, reaching almost wildtype-like growth behavior, and were present at a copy number of 1 (Table 1), indicating that they are good candidates for the construction of mini-chromosomes.
Table 1. Copy Number of Stable repABC Cassettes in M. extorquensa.
| replicon | copy number (mean ± SD) |
|---|---|
| oriV-traJ′ (pTE101) | 8 ± 1.21 |
| Mex-DM4 (pAD1) | 1 ± 0.17 |
| Mrad-JCM (pAD3) | 1 ± 0.13 |
| Nham-3 (pAD6) | 1 ± 0.09 |
Mean ± SD from three biological replicates.
Assembly and Testing of Mini-Chromosomes in M. extorquens AM1
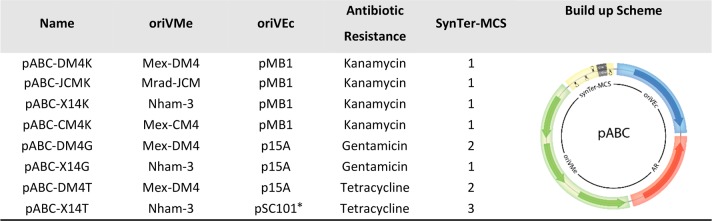
Next, we used the stable repABC regions identified in the pK18mob2 system plus Mex-CM4 to construct mini-chromosomes that can be shuttled between M. extorquens and E. coli. The pABC vector concept and assembly strategy is based on four basic modules each with several standardized parts (see below). Each part is flanked by standardized linker sequences for ligase chain reaction LCR-based assembly. This enables a fast and convenient build-up of custom-made replicons for individual purposes. Additional modules, if necessary, can be implemented with ease. The position and orientation of each module (Table 2) was designed to minimize crosstalk from adjacent regions. The MCS and the promoter of the repABC operon are insulated by flanking transcriptional terminators. The antibiotic resistance cassette is located downstream and in the same orientation as the repABC transcriptional unit to avoid reverse transcriptional read-through.
Table 2. Modular Construction of repABC-Based Mini-Chromosomesa.
Abbreviations: oriVMe, repABC-based origin of replication for M. extorquens AM1; oriVEc, origin of replication for E. coli; SynTer-MCS, multiple cloning site flanked by synthetic terminators.
We decided to assemble several mini-chromosomes from the four basic modules, i.e., (1) a repABC-based origin for M. extorquens AM1 (Mex-DM4, Nham-3, Mrad-JCM, Mex-CM4), (2) an E. coli origin of replication (pMB1, p15A, or pSC101*), (3) a minimal antibiotic resistance cassette (Kanamycin, Gentamicin, Tetracycline), and (4) a multiple cloning site (MCS1, MCS2, MCS3) flanked by synthetic terminators (Table 2). When we assembled and tested the final mini-chromosomes, our constructs showed similar or higher growth rates of transformed M. extorquens compared to replicative plasmids. Replicon stability of the mini-chromosomes was confirmed via flow cytometry, showing similar results as before (Figure S3 and S4). We also assembled pABC mini-chromosomes with a mob site for conjugation and the Cre recombinase under control of constitutive (PcoxB) and inducible (PL/O4/A1) promoters as additional fifth modules (see Table S2).
The modular construction of the mini-chromosomes allowed us to test intercompatibility of replicons while avoiding extended regions of high sequence identity, which could lead to homologous recombination events. Nham-3 was compatible with Mex-DM4 and Mrad-JCM, while the two latter were not compatible with each other (Tables 2 and 3). In addition, we also tested the compatibility of Mex-DM4, Mrad-JCM, and Nham-3 with the pIND4 and pTE101 vectors, respectively. All three mini-chromosomes were fully compatible with those plasmids, further expanding the box of available and compatible extrachromosomal genetic elements in M. extorquens AM1. Replicon integrity was verified by restriction analysis from single and double compatibility test of pABCs and/or replicative plasmids. There were no instances of recombination, proving all compatible replicons functioned autonomously (data not shown). In all cases tested, double transformations were possible, adding to the convenience of these tools for their use in genetic engineering of M. extorquens AM1.
Table 3. Doubling Times (h) of M. extorquens with repABC-Based Mini-Chromosomes in Single and Double Compatibility Testsa.
| origin of replication | Mex-CM4 | Mex-DM4 | Mrad-JCM | Nham-3 | oriV-traJ′ | pMG160 |
|---|---|---|---|---|---|---|
| CM2720 (strain) | 4.11 ± 0.02 | 3.90 ± 0.01 | 3.85 ± 0.03 | 3.94 ± 0.02 | 4.09 ± 0.05 | 4.31 ± 0.17 |
| Mex-DM4 | X | 4.41 ± 0.04 | 4.15 ± 0.03 | 4.46 ± 0.06 | ||
| Mrad-JCM | 5.44 ± 0.31 | 4.68 ± 0.21 | 5.48 ± 0.27 | |||
| Nham-3 | 4.43 ± 0.04 | 4.49 ± 0.03 | ||||
| oriV-traJ′ | 5.54 ± 0.40 |
Mean ± SD from three biological replicates. X = not compatible. pTE100/pTE101 = oriV-traJ′, pIND4/pTE1841 = pMG160. Areas were left blank to avoid redundancy.
Conclusions
Here, we provide a set of novel promoters for M. extorquens AM1 that are tight and show a dynamic range of different expression levels upon induction, even exceeding the promoter elements available thus far. We also identified a set of repABC regions that allowed us to construct different mini-chromosomal elements that can be stably maintained at single copy number and in different combinations with each other. In addition, we also provide repABC regions that allow transient expression in the absence of antibiotic selection, which is a prerequisite to establish CRISPR-Cas-based methods in this organism in the future. Altogether, these tools expand genetic tools available for the engineering of M. extorquens, an important platform organism for a sustainable C1-biotechnology. The genetic tools developed in this study are freely available to the community and hopefully leverage the metabolic and biotechnological potential of M. extorquens AM1.
Materials and Methods
Strains and Cultivation Conditions
M. extorquens AM1 strains (Table S1) were grown at 30 °C in minimal medium with 123 mM methanol.26 Antibiotics were used accordingly: kanamycin 35 μg/mL for M. extorquens or 50 μg/mL for E. coli, gentamicin 7.5 μg/mL for M. extorquens or 8–15 μg/mL for E. coli, tetracycline 4–10 μg/mL for both, chloramphenicol 34 μg/mL for E. coli, ampicillin 100 μg/mL for E. coli. E. coli TOP10 ΔdapA strains were supplemented with 0.3 μM diaminopimelic acid (DAP). E. coli was grown at 37 °C in LB medium. E. coli TOP10 and DH5α (Thermo Scientific) were used for construction and amplification of all plasmids in this study. Solid medium had 1.5% (w/v) select agar.
Strain Construction
Plasmids (Table S2) were transferred into M. extorquens by electroporation or triparental mating.27 The dapA gene of E. coli TOP10 was disrupted by an FRT-flanked chloramphenicol resistance cassette, amplified from pKD3 with MC261 + MC262 primers, using the Quick and Easy E. coli Gene Deletion kit (Gene Bridges K006-GVO-GB) according to manufacturer’s instructions. In brief, 20 μL of recipient M. extorquens from a well-grown culture were spotted on nonselective medium and incubated at 30 °C for 24 h. Donor and helper E. coli TOP10 ΔdapA strains were spotted on top of the recipient cells and incubated at 30 °C for 24 h. pRK2013 was used as helper plasmid. Spotted cells were recovered and plated at appropriate dilutions on selective medium. Donor and helper E. coli TOP10 ΔdapA cannot grow without DAP. Single colonies were screened for chromosomal integration of pTE1899 by colony PCR.
DNA Manipulation and Plasmid Construction
Refer to Table S2 for detailed information on the construction of each plasmid (Supporting Information). Standard molecular techniques were used for amplification, purification, cloning and transformation of DNA.28 Point mutations were generated by QuickChange Site-Directed mutagenesis (Stratagene, La Jolla, USA). Ligase chain reaction (LCR) and primer hybridization reactions were performed as published before.18 T4 Polynucleotide Kinase, FastAP, and FastDigest restriction enzymes were obtained from Thermo Scientific, Q5 DNA polymerase was obtained from NEB, and used according to manufacturer’s instructions. DNA oligos were obtained from Eurofins Genomics (Table S3). Plasmid isolation and PCR product purification was performed with NucleoSpin Plasmid and NucleoSpin Gel and PCR Clean-up kits (Macherey Nagel), E.Z.N.A. Plasmid Mini Kit (Omega Bio-Tek), or illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare Life Sciences) according to manufacturer’s instructions.
Growth in 96-Well Plates
Cultures were inoculated from a single colony or glycerol stocks into Erlenmeyer flasks with 20 mL of the appropriate medium. Cells from late exponential phase were diluted in fresh medium at an OD600 ≈ 0.05 and 180 μL of cell suspension was aliquoted into Nunclon Delta Surface (Thermo Scientific #167008) 96-well plates. The temperature was kept constant at 30 °C and OD600 was recorded every 30 min using a Tecan Infinite M200Pro (Tecan, Männedorf, Switzerland). Data was analyzed using the GraphPad Prism 7 software.
qPCR-Based Copy Number Determination
M. extorquens strains were grown in selective medium until OD600 1.0, adjusted to 1.38 × 106 cells (≙ 10 ng DNA/μL) and boiled at 95 °C for 15 min to serve as templates. qPCR was carried out in a qTOWER Thermal Cycler (Analytik Jena, Germany) using the Takyon No ROX SYBR 2X MasterMix blue dTTP. Reactions were performed according to the manufacturer’s instructions in a 5 μL volume. M. extorquens katA::pTE1179 was used as a reference strain. Replicon copy number was calculated according to Lee et al., 200629 using primer sets JD253 + 254 and MC147 + 148.
Replicon Stability
M. extorquens carrying pK18mob2-repABC derivatives were grown until OD600 = 1.0–1.5 in selective medium. Cell suspensions were diluted every 24 h to an OD600 ≈ 0.02 in nonselective medium. Dilution series were plated on agar with and without kanamycin. Antibiotic resistance was correlated with the presence of the assessed plasmid. The inheritance stability of selected repABC replicons expressing mCherry as mini-chromosomes was assessed via flow cytometry on a BD LSRFortessaTM SORP flow-cytometer (BD Biosciences, NJ, USA). Fluorescence was detected using a 561 nm laser at 100 mW and a 610/20 bandpass filter.30,31 Forward and side scatter values were monitored using a 488 nm laser at 100 mW. The acquired data was analyzed using FACSDiva software v8.0 (BD Biosciences). The percentage of fluorescent and nonfluorescent cells was determined from 30 000 gated events each.
Acknowledgments
We thank S. González Sierra for supporting the FACS analysis and M. Seletskiy for technical assistance. We thank J. Armitage for providing us with pIND4 and S. Vuilleumier for providing us with M. extorquens CM4 and M. nodulans ORS 2060 strains. This work was created in the framework of the CHIRAMET program (German Ministry of Education and Research) and received support from the SYNMIKRO (LOEWE program, State of Hesse, Germany), TRR 174 (German Research Foundation), the European Research Council (Grant No. 637675 SYBORG), the European Coordination and Support Action on biological standardization BIOROBOOST (EU Grant No. 820699), as well as the Max Planck Society.
Glossary
Abbreviations
- SD
standard deviation
- h
hours
- IPTG
isopropyl β-d-1-thiogalactopyranoside.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssynbio.9b00220.
Table S1: Strains used in this study; Table S2: Plasmids used in this study; Table S3: Primers used in this study; Table S4: Fold induction of IPTG-inducible promoters in M. extorquens AM1; Figure S1: PL/O4/A1 promoter in M. extorquens AM1; Figure S2: Overview of the individual repABC regions tested in this study; Figure S3: Verification of flow cytometry sensitivity; Figure S4: Replicon stability measured by flow cytometry of mini-chromosomes; Table S5: DNA sequences of inducible promoters characterized in this study (PDF)
Author Contributions
T.J.E., A.B., M.W., and M.C. designed the research. M.C., M.W., F.P., and A.D. performed the experimental work. M.C. and M.W. analyzed or provided data. M.C. and T.J.E. wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Peel D.; Quayle J. R. (1961) Microbial growth on C1 compounds. 1. Isolation and characterization of Pseudomonas AM1. Biochem. J. 81, 465–469. 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyraud R.; Schneider K.; Kiefer P.; Massou S.; Vorholt J. A.; Portais J. C. (2011) Genome-scale reconstruction and system level investigation of the metabolic network of Methylobacterium extorquens AM1. BMC Syst. Biol. 5, 189. 10.1186/1752-0509-5-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier S.; Chistoserdova L.; Lee M. C.; Bringel F.; Lajus A.; Zhou Y.; Gourion B.; Barbe V.; Chang J.; Cruveiller S.; Dossat C.; Gillett W.; Gruffaz C.; Haugen E.; Hourcade E.; Levy R.; Mangenot S.; Muller E.; Nadalig T.; Pagni M.; Penny C.; Peyraud R.; Robinson D. G.; Roche D.; Rouy Z.; Saenampechek C.; Salvignol G.; Vallenet D.; Wu Z.; Marx C. J.; Vorholt J. A.; Olson M. V.; Kaul R.; Weissenbach J.; Medigue C.; Lidstrom M. E. (2009) Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS One 4, e5584 10.1371/journal.pone.0005584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner A. M.; Sonntag F.; Buchhaupt M.; Schrader J.; Vorholt J. A. (2015) Methylobacterium extorquens: methylotrophy and biotechnological applications. Appl. Microbiol. Biotechnol. 99, 517–534. 10.1007/s00253-014-6240-3. [DOI] [PubMed] [Google Scholar]
- Liang W. F.; Cui L. Y.; Cui J. Y.; Yu K. W.; Yang S.; Wang T. M.; Guan C. G.; Zhang C.; Xing X. H. (2017) Biosensor-assisted transcriptional regulator engineering for Methylobacterium extorquens AM1 to improve mevalonate synthesis by increasing the acetyl-CoA supply. Metab. Eng. 39, 159–168. 10.1016/j.ymben.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Sonntag F.; Kroner C.; Lubuta P.; Peyraud R.; Horst A.; Buchhaupt M.; Schrader J. (2015) Engineering Methylobacterium extorquens for de novo synthesis of the sesquiterpenoid alpha-humulene from methanol. Metab. Eng. 32, 82–94. 10.1016/j.ymben.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Hu B.; Lidstrom M. E. (2014) Metabolic engineering of Methylobacterium extorquens AM1 for 1-butanol production. Biotechnol. Biofuels 7, 156. 10.1186/s13068-014-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. M.; Chen W. J.; Yang J.; Zhou Y. M.; Hu B.; Zhang M.; Zhu L. P.; Wang G. Y.; Yang S. (2017) Production of 3-hydroxypropionic acid in engineered Methylobacterium extorquens AM1 and its reassimilation through a reductive route. Microb. Cell Fact. 16, 179. 10.1186/s12934-017-0798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schada von Borzyskowski L.; Remus-Emsermann M.; Weishaupt R.; Vorholt J. A.; Erb T. J. (2015) A set of versatile brick vectors and promoters for the assembly, expression, and integration of synthetic operons in Methylobacterium extorquens AM1 and other alphaproteobacteria. ACS Synth. Biol. 4, 430–443. 10.1021/sb500221v. [DOI] [PubMed] [Google Scholar]
- Marx C. J.; Lidstrom M. E. (2001) Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147, 2065–2075. 10.1099/00221287-147-8-2065. [DOI] [PubMed] [Google Scholar]
- Marx C. J.; Lidstrom M. E. (2002) Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. BioTechniques 33, 1062–1067. 10.2144/02335rr01. [DOI] [PubMed] [Google Scholar]
- Kaczmarczyk A.; Vorholt J. A.; Francez-Charlot A. (2013) Cumate-inducible gene expression system for sphingomonads and other Alphaproteobacteria. Appl. Environ. Microbiol. 79, 6795–6802. 10.1128/AEM.02296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubiz L. M.; Purswani J.; Carroll S. M.; Marx C. J. (2013) A novel pair of inducible expression vectors for use in Methylobacterium extorquens. BMC Res. Notes 6, 183. 10.1186/1756-0500-6-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. J.; Morel L.; Bourque D.; Mullick A.; Massie B.; Miguez C. B. (2006) Bestowing inducibility on the cloned methanol dehydrogenase promoter (PmxaF) of Methylobacterium extorquens by applying regulatory elements of Pseudomonas putida F1. Appl. Environ. Microbiol. 72, 7723–7729. 10.1128/AEM.02002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto U. M.; Pappas K. M.; Winans S. C. (2012) The ABCs of plasmid replication and segregation. Nat. Rev. Microbiol. 10, 755–765. 10.1038/nrmicro2882. [DOI] [PubMed] [Google Scholar]
- Fournes F.; Val M. E.; Skovgaard O.; Mazel D. (2018) Replicate Once Per Cell Cycle: Replication Control of Secondary Chromosomes. Front. Microbiol. 9, 1833. 10.3389/fmicb.2018.01833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevallos M. A.; Cervantes-Rivera R.; Gutierrez-Rios R. M. (2008) The repABC plasmid family. Plasmid 60, 19–37. 10.1016/j.plasmid.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Döhlemann J.; Wagner M.; Happel C.; Carrillo M.; Sobetzko P.; Erb T. J.; Thanbichler M.; Becker A. (2017) A Family of Single Copy repABC-Type Shuttle Vectors Stably Maintained in the Alpha-Proteobacterium Sinorhizobium meliloti. ACS Synth. Biol. 6, 968–984. 10.1021/acssynbio.6b00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz R.; Bujard H. (1997) Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25, 1203–1210. 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ind A. C.; Porter S. L.; Brown M. T.; Byles E. D.; de Beyer J. A.; Godfrey S. A.; Armitage J. P. (2009) Inducible-expression plasmid for Rhodobacter sphaeroides and Paracoccus denitrificans. Appl. Environ. Microbiol. 75, 6613–6615. 10.1128/AEM.01587-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M.; Bujard H. (1988) Promoters largely determine the efficiency of repressor action. Proc. Natl. Acad. Sci. U. S. A. 85, 8973–8977. 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujard H.; Gentz R.; Lanzer M.; Stueber D.; Mueller M.; Ibrahimi I.; Haeuptle M. T.; Dobberstein B. (1987) A T5 promoter-based transcription-translation system for the analysis of proteins in vitro and in vivo. Methods Enzymol. 155, 416–433. 10.1016/0076-6879(87)55028-5. [DOI] [PubMed] [Google Scholar]
- Kitagawa M.; Ara T.; Arifuzzaman M.; Ioka-Nakamichi T.; Inamoto E.; Toyonaga H.; Mori H. (2006) Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12, 291–299. 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- Gentz R.; Bujard H. (1985) Promoters recognized by Escherichia coli RNA polymerase selected by function: highly efficient promoters from bacteriophage T5. J. Bacteriol. 164, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Kirchhoff J. R.; Faehnle C. R.; Viola R. E.; Hudson R. A. (2006) A rapid method for the purification of methanol dehydrogenase from Methylobacterium extorquens. Protein Expression Purif. 46, 316–320. 10.1016/j.pep.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Peyraud R.; Kiefer P.; Christen P.; Massou S.; Portais J. C.; Vorholt J. A. (2009) Demonstration of the ethylmalonyl-CoA pathway by using 13C metabolomics. Proc. Natl. Acad. Sci. U. S. A. 106, 4846–4851. 10.1073/pnas.0810932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama H.; Anthony C.; Lidstrom M. E. (1998) Construction of insertion and deletion mxa mutants of Methylobacterium extorquens AM1 by electroporation. FEMS Microbiol. Lett. 166, 1–7. 10.1111/j.1574-6968.1998.tb13175.x. [DOI] [PubMed] [Google Scholar]
- Sambook J., and Russel D. W. (2001) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Lee C.; Kim J.; Shin S. G.; Hwang S. (2006) Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 123, 273–280. 10.1016/j.jbiotec.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Piatkevich K. D.; Verkhusha V. V. (2011) Guide to red fluorescent proteins and biosensors for flow cytometry. Methods Cell Biol. 102, 431–461. 10.1016/B978-0-12-374912-3.00017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford W. G.; Hawley T.; Subach F.; Verkhusha V.; Hawley R. G. (2012) Flow cytometry of fluorescent proteins. Methods 57, 318–330. 10.1016/j.ymeth.2012.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.