ABSTRACT
Background
Three recent meta-analyses found significant prospective inverse associations between chocolate intake and cardiovascular disease risk. Evidence from these meta-analyses suggests that such inverse associations may only apply to elderly individuals or those with pre-existing major chronic disease.
Objective
We assessed the association between habitual chocolate intake and subsequent incident coronary heart disease (CHD) and stroke, and the potential effect of modification by age.
Design
We conducted multivariable Cox regression analyses using data from 83,310 postmenopausal women free of baseline pre-existing major chronic disease in the prospective Women's Health Initiative cohort. Chocolate intake was assessed using a food-frequency questionnaire. Physician-adjudicated events or deaths were ascertained up to 30 September 2013.
Results
After exclusions, there were 3246 CHD and 2624 stroke events or deaths, representing incidence rates of 3.9% and 3.2% during 1,098,091 and 1,101,022 person-years (13.4 y), respectively. We found no association between consumption of chocolate and risk of CHD (P for linear trend = 0.94) or stroke (P = 0.24). The results for CHD and stroke combined were similar (P = 0.30), but were significantly modified by age (P for interaction = 0.02). For women age <65 y at baseline, those who ate 1 oz (28.35 g) of chocolate <1/mo, 1 to <1.5/mo, 1.5 to <3.5/mo, 3.5/mo to <3/wk, and ≥3/wk had HRs (95% CIs) of 1.00 (referent), 1.17 (1.00, 1.36), 1.05 (0.90, 1.22), 1.09 (0.94, 1.25), and 1.27 (1.09, 1.49), respectively (P for linear trend = 0.005). No association was apparent for older women.
Conclusion
We observed no association between chocolate intake and risk of CHD, stroke, or both combined in participants free of pre-existing major chronic disease. The relation for both combined was modified by age, with a significant positive linear trend and an increased risk in the highest quintile of chocolate consumption among women age <65 y. This trial was registered at clinicaltrials.gov as NCT03453073.
Keywords: coronary heart disease, stroke, postmenopausal women, nutrition, dietary intake and cardiovascular disease, epidemiology
INTRODUCTION
Cardiovascular disease (CVD) is the main cause of mortality worldwide (1) and also in the United States, even though cancer has surpassed CVD in some states and population subgroups (2). A growing body of evidence suggests that chocolate may decrease the risk of CVD via an ability to lower blood pressure, improve endothelial function, and improve insulin sensitivity (3). Three recent meta-analyses (4–6) involving 10 epidemiologic long-term prospective studies found evidence of a negative association between chocolate intake and the risk of CVD, but cautioned that residual confounding or unobserved factors might explain their findings. In fact, none of the studies evaluated in these meta-analyses had excluded participants with pre-existing non-CVD major chronic disease, such as cancer and diabetes. Also, both of the analyzed studies which showed strong inverse associations had a high proportion of participants who were elderly or had pre-existing major chronic disease (7, 8). Such inverse associations may be artefactual if participants with prevalent disease and/or a greater burden of aging-related comorbidities decreased their chocolate consumption following disease diagnosis prior to follow-up baseline. Based on this empirical evidence, we decided to test the following a priori hypotheses: 1) the associations between chocolate intake and coronary heart disease (CHD) and stroke risk are inverse for persons free of major chronic disease, including CVD, diabetes, and cancer; 2) these associations are less strongly inverse at younger age; and 3) persons decrease their chocolate, energy, and fat intake and their body weight after being first diagnosed with one of these diseases.
We used data from postmenopausal women in the prospective Women's Health Initiative (WHI) cohort to evaluate the association between baseline chocolate intake and subsequent risk of CHD and stroke. We focused on these 2 CVD conditions because they are the 2 major components of CVD, and recent research suggests that the risk of both of these conditions should decrease as chocolate intake increases (3).
METHODS
Participants
Detailed descriptions of the design of the WHI have been published previously (9). In summary, between 1993 and 1998, 161,808 postmenopausal women 50–79 y of age were enrolled into the WHI Observational Study (OS) or one or more overlapping clinical trials (CTs). The OS is a longitudinal cohort study with ongoing collection of data on participant characteristics, lifestyle habits and morbidity and mortality from major chronic disease. The CTs tested: 1) estrogen plus progestin compared with placebo for women with a uterus; 2) estrogen-alone compared with placebo for women with hysterectomy; 3) calcium plus vitamin D supplementation compared with placebo; and 4) low-fat-dietary intervention compared with usual diet.
Of the original 161,808 women, 93,676 OS and 68,132 CT participants, we included all of the OS and CT control participants except those: 1) with implausible energy intakes, defined as <600 or >5000 kcal/d; 2) with implausible BMI (<15 or >50 kg/m2) or height <122 cm (4 ft); 3) who at baseline reported pre-existing major chronic disease, including diabetes, angina, myocardial infarction (MI), stroke, heart failure, coronary artery bypass graft, percutaneous coronary intervention, or cancer; and 4) those with missing values on pre-existing major chronic disease or any exposure, outcome, or confounder variables. The remaining 83,310 women provided data for our analysis (Figure 1). The missing data percentage was <1.4% for all variables, except for 2 of our confounder variables, recreational physical activity and income level, for which the missing percentages were 3.3% and 4.3%, respectively.
FIGURE 1.
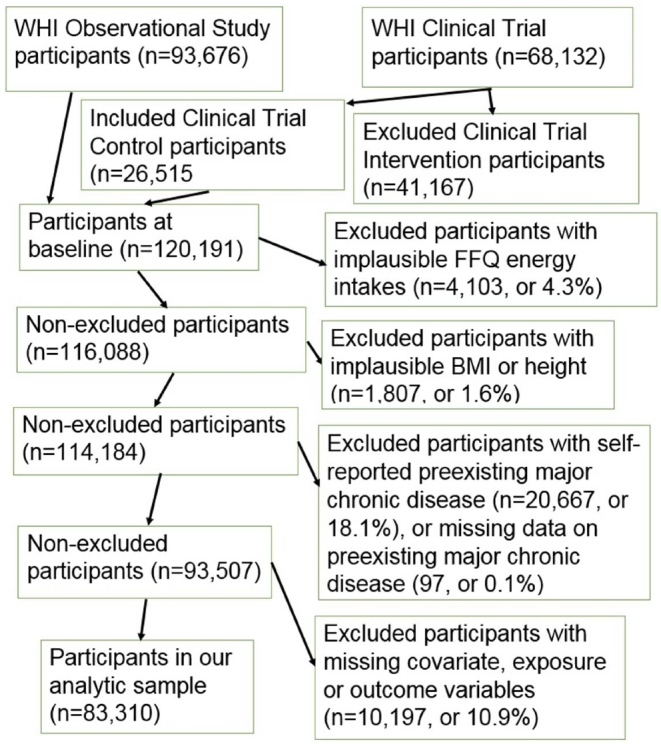
Participant flow chart. FFQ, food-frequency questionnaire; WHI, Women's Health Initiative.
Chocolate intake
Our exposure variable, chocolate consumption, was assessed using data from the WHI food frequency questionnaire (FFQ), administered at baseline (year 0) and year 3. On a single line item, the FFQ asked how often over the past 3 mo the participant ate a 1 oz. (28.35 g) portion, referred to as a medium portion, of chocolate candy or candy bars. From the participants’ responses, we derived 5 categories of intake frequency of a 1 oz. serving of chocolate: <1/mo, 1 to <1.5/mo, 1.5 to <3.5/mo, 3.5 times/mo to <3 times/wk, and ≥3/wk. We selected cut-off points that resulted in categories corresponding as closely as possible to quintiles, given that the lowest level (<1 serving/mo) accounted for more than 20% of cases. We also calculated a continuous version of chocolate intake, the number of 1-oz servings consumed/d.
Outcome variables
Our 3 primary outcome events were the first occurrence of, or death due to CHD, stroke, or both combined. For CHD, it was the first occurrence of clinical MI, definite silent MI, or death due to definite or possible CHD. For stroke, it was the first occurrence of ischemic, hemorrhagic. or other stroke, or death due to a cerebrovascular event. Outcomes were ascertained between the baseline survey (1993–1998) and 30 September 2013, and all events were adjudicated by a trained physician adjudicator using available medical records and death certificates. The WHI criteria for cardiovascular endpoints were adapted from standardized criteria, and have been reported elsewhere (10).
Our secondary objective involved assessing whether women free of major chronic disease at baseline decreased their chocolate, energy, and fat intake and their body weight after being diagnosed with a major chronic disease. Our outcome measures were the differences between the year 0 and year 3 assessments of these variables. The first 3 variables were derived from FFQ data, and body weight was measured at both visits.
Covariates
We built 2 regression models. In our basic model, the covariates were age (y), race/ethnicity (white, black or other), and WHI study arm (OS or CT control). Our full model included additional continuous and binary variables. The continuous variables were: the revised Alternative Healthy Eating Index (11); nonchocolate daily energy intake; recreational physical activity; smoking status; alcohol intake; educational level; and income status. There was one additional binary variable: family history of CVD.
We identified potential covariates using our knowledge of the empirical literature on CVD risk factors (12). We used polynomial or categorical versions of nonbinary variables if they met our inclusion criterion, a >10% change in the regression coefficient for chocolate intake.
Statistical methods
Cox proportional hazards regression was used to estimate the multivariable adjusted HRs for CHD, stroke, and both combined for different levels of chocolate intake frequency and for a 1 oz/d (28.35 g/d) increment in chocolate intake.
Tests of interaction were conducted by inserting a cross product of the continuous version of chocolate intake (number of 1-oz servings consumed/d) and the variable being tested (age in years or BMI in kg/m2) into our full model. Our interaction variables were selected a priori.
Tests for linear trend across quintiles of chocolate intake used the median value in each quintile as a continuous variable.
We used the t test for 2 independent samples with the Satterthwaite method to test the hypothesis that the prospective changes in chocolate, energy, and fat intake and body weight between year 0 and year 3 were different for women who experienced a first incidence of major chronic disease before year 3 and those who did not.
A 2-sided P value of <0.05 was considered significant. SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) was used for all analyses. The WHI study is registered at CLINICTRIALS.gov as NCT00000611. Our manuscript was prepared using the STROBE guidelines for observational studies (13).
Ethics
The WHI study protocol (available at www.whi.org) was approved by institutional review boards at each participating institution, and all participants provided written informed consent. The study was conducted in accordance with the Helsinki Declaration of 1975 as revised in 1989.
RESULTS
Participant baseline characteristics
More frequent chocolate intake was associated with: 1) higher BMI, higher energy intake, and greater likelihood of being white and smoking >15 cigarettes/d; and 2) lower levels of recreational physical activity, healthy eating, and prevalence of major chronic disease, especially diabetes and revascularization (Table 1).
TABLE 1.
Baseline characteristics of postmenopausal women with different levels of chocolate consumption in the WHI1
| Frequency of consumption of a 1-oz (28.35 g) serving of chocolate | |||||
|---|---|---|---|---|---|
| <1/mo | 1 to <1.5/mo | 1.5 to <3.5/mo | 3.5/mo to <3/wk | ≥3/wk | |
| Characteristics | (n = 31,332) | (n = 15,093) | (n = 16,774) | (n = 22,925) | (n = 14,468) |
| Chocolate, 1-oz servings/d | 0.00 ± 0.01 | 0.03 ± 0.00 | 0.07 ± 0.01 | 0.20 ± 0.07 | 0.79 ± 0.53 |
| Age, y | 64.0 ± 7.2 | 63.6 ± 7.2 | 63.4 ± 7.2 | 63.5 ± 7.3 | 63.4 ± 7.3 |
| BMI, kg/m2 | 26.9 ± 5.5 | 27.2 ± 5.3 | 27.5 ± 5.4 | 27.7 ± 5.5 | 28.4 ± 5.8 |
| Physical activity,2 MET-h/wk | 14.6 ± 15.1 | 13.7 ± 14.1 | 13.0 ± 13.5 | 12.1 ± 13.2 | 11.1 ± 12.9 |
| Total dietary calories, kcal/d | 1424.2 ± 539.6 | 1483.6 ± 533.0 | 1576.3 ± 558.5 | 1704.8 ± 610.9 | 2016.3 ± 724.0 |
| Nonchocolate calories, kcal/d | 1423.8 ± 539.6 | 1479.1 ± 533.0 | 1566.8 ± 558.6 | 1679.3 ± 610.7 | 1913.4 ± 711.8 |
| Healthy Eating Index3 | 51.4 ± 10.0 | 50.0 ± 9.6 | 49.2 ± 9.6 | 48.2 ± 9.6 | 46.3 ± 9.6 |
| Family income2 | 4.2 ± 1.9 | 4.3 ± 1.9 | 4.3 ± 1.8 | 4.3 ± 1.8 | 4.3 ± 1.8 |
| Race/ethnicity, % | |||||
| White | 80.2 | 84.9 | 86.4 | 89.2 | 89.3 |
| Black | 10.6 | 8.1 | 7.7 | 5.6 | 6.0 |
| Other | 9.2 | 6.9 | 6.0 | 5.2 | 4.7 |
| Education,2 % | |||||
| Less than high school | 21.8 | 20.6 | 20.5 | 20.4 | 21.3 |
| High school to less than college | 36.9 | 36.4 | 36.9 | 37.2 | 38.0 |
| College to less than postgraduate study | 22.8 | 24.0 | 24.0 | 23.4 | 23.2 |
| Postgraduate study/degree or higher | 18.6 | 19.0 | 18.7 | 19.1 | 17.5 |
| Family history of CVD, % | 66.1 | 67.4 | 66.9 | 66.6 | 66.5 |
| Pre-existing major chronic disease,4 % | 21.6 | 18.3 | 16.3 | 16.5 | 16.3 |
| Pre-existing diabetes, % | 7.0 | 3.9 | 2.9 | 2.6 | 2.3 |
| Pre-existing heart attack, % | 2.8 | 2.3 | 1.9 | 2.0 | 1.9 |
| Pre-existing stroke, % | 1.6 | 1.4 | 1.1 | 1.2 | 1.2 |
| Pre-existing heart failure, % | 1.3 | 0.9 | 0.9 | 0.9 | 0.9 |
| Pre-existing revascularization, % | 2.4 | 2.0 | 1.5 | 1.3 | 1.3 |
| Pre-existing cancer, % | 11.5 | 11.4 | 10.8 | 11.2 | 11.1 |
| Alcohol, % | |||||
| Nondrinker | 12.5 | 10.7 | 9.6 | 9.5 | 9.6 |
| Former drinker | 21.0 | 16.9 | 16.6 | 16.8 | 19.0 |
| <1 drink/mo | 11.4 | 11.1 | 11.8 | 12.2 | 14.1 |
| 1 drink/mo to <1 drink/wk | 18.0 | 21.0 | 21.3 | 20.9 | 21.7 |
| 1 drink/wk to <1 drink/d | 23.2 | 27.4 | 28.0 | 28.8 | 25.8 |
| ≥1 drink/d | 13.9 | 13.0 | 12.8 | 11.8 | 9.8 |
| Smoking, % | |||||
| Never | 50.6 | 51.3 | 50.0 | 51.1 | 50.0 |
| Former | 43.5 | 42.6 | 43.5 | 42.0 | 42.2 |
| Current, <15/d | 3.2 | 3.4 | 3.6 | 3.5 | 3.5 |
| Current, ≥15/d | 2.7 | 2.7 | 2.8 | 3.4 | 4.3 |
1All characteristics showed significant differences between levels of chocolate intake (P < 0.05) except for family history of CVD, based on analysis of variance, the Kruskal-Wallis test, or the chi-square test. Data are given as means ± SDs for continuous variables and as percentages for categorical variables. Data are for observational study and clinical trial control participants without: 1) implausible FFQ energy intakes (defined as mean intakes <600 or >5000 kcal/d), BMI (kg/m2; <15 or >50), or height <122 cm (4 ft); and 2) no missing values for any of the characteristics in this table. CVD, cardiovascular disease; FFQ, food-frequency questionnaire; MET, metabolic equivalent; WHI, Women's Health Initiative.
2Educational level, physical activity and family income were quantified by WHI researchers. Physical activity was total energy expended in recreational physical activity. Family income ranged from 1 to 9.
3The Modified Alternative Healthy Eating Index (10).
4Pre-existing serious chronic disease [yes/no—diabetes, heart attacks, stroke, heart failure, coronary artery bypass graft, revascularization (percutaneous transluminal coronary angioplasty), or cancer].
Prospective association between chocolate consumption and CVD risk
After exclusions, there were 83,310 participants. There were 3246 CHD, 2624 stroke, and 5563 combined CHD and stroke events or deaths, representing incidence rates of 3.9%, 3.2%, and 6.7%, respectively. The person-years of follow-up were 1098,091, 1101,022 and 1087,545, respectively. The mean ± SD follow-up period was 13.4 ± 4.3 y for uncensored participants in our full-model survival analyses.
As shown in Table 2, there were no significant associations between chocolate intake and risk of CHD, stroke, or both combined in our full-model analyses for participants without major chronic disease at baseline.
TABLE 2.
Chocolate intake, HRs, and 95% CIs for incident CHD, stroke, or both in the WHI1
| Frequency2 of chocolate consumption [1-oz (28.35 g) servings] | ||||||
|---|---|---|---|---|---|---|
| Covariates in the model | <1/mo3 | 1 to <1.5/mo | 1.5 to <3.5/mo | 3.5/mo to <3/wk | ≥3/wk | P for linear trend4 |
| Incident coronary heart disease events or death | ||||||
| Age, race, and WHI study arm (n = 91,815) | ||||||
| HR (95% CI)5 | 1.00 | 1.06 (0.95, 1.17) | 1.08 (0.98, 1.19) | 1.01 (0.92, 1.11) | 1.08 (0.98, 1.20) | 0.27 |
| Women, n6 | 27,506 | 13,734 | 15,609 | 21,332 | 13,634 | 91,815 |
| Events, n6 | 1052 | 555 | 635 | 835 | 562 | 3639 |
| Full model7 (n = 83,310) | ||||||
| HR (95% CI) | 1.00 | 1.07 (0.96, 1.19) | 1.06 (0.96, 1.18) | 0.98 (0.89, 1.08) | 1.03 (0.92, 1.15) | 0.94 |
| Women, n | 24,958 | 12,501 | 14,212 | 19,377 | 12,262 | 83,310 |
| Events, n | 923 | 502 | 566 | 745 | 510 | 3246 |
| Incident stroke events or death | ||||||
| Age, race, and WHI study arm (n = 91,815) | ||||||
| HR (95% CI) | 1.00 | 1.07 (0.95, 1.20) | 1.05 (0.94, 1.17) | 1.02 (0.92, 1.13) | 1.20 (1.07, 1.35) | 0.002 |
| Women, n | 27,506 | 13,734 | 15,609 | 21,332 | 13,634 | 91,815 |
| Events, n | 845 | 446 | 488 | 664 | 490 | 2933 |
| Full model (n = 83,310) | ||||||
| HR (95% CI) | 1.00 | 1.07 (0.95, 1.21) | 1.04 (0.92, 1.17) | 0.97 (0.87, 1.09) | 1.09 (0.96, 1.23) | 0.24 |
| Women, n | 24,958 | 12,501 | 14,212 | 19,377 | 12,262 | 83,310 |
| Events, n | 752 | 406 | 446 | 593 | 427 | 2624 |
| Incident coronary heart disease or stroke events or death | ||||||
| Age, race, and WHI study arm (n = 91,815) | ||||||
| HR (95% CI) | 1.00 | 1.07 (0.99, 1.16) | 1.06 (0.98, 1.15) | 1.03 (0.96, 1.11) | 1.14 (1.06, 1.24) | 0.003 |
| Women, n | 27,506 | 13,734 | 15,609 | 21,332 | 13,634 | 91,815 |
| Events, n | 1789 | 950 | 1054 | 1435 | 998 | 6226 |
| Full model (n = 83,310) | ||||||
| HR (95% CI) | 1.00 | 1.08 (0.99, 1.17) | 1.05 (0.96, 1.13) | 0.99 (0.92, 1.07) | 1.07 (0.98, 1.16) | 0.30 |
| Women, n | 24,958 | 12,501 | 14,212 | 19,377 | 12,262 | 83,310 |
| Events, n | 1584 | 860 | 949 | 1279 | 891 | 5563 |
1Incident CHD was the first occurrence of, or death due to, CHD between the baseline survey (1993–1998) and 30 September 2013. Incident stroke was the first occurrence of, or death due to, stroke in the same time period. Our sample included observational study and clinical trial control participants without: 1) implausible FFQ energy intakes (defined as mean intakes <600 or >5000 kcal/d) or extreme energy intake (<600 or >5000 kcal/d), BMI (kg/m2; <15 or >50), or height <122 cm (4 ft); or 2) any of the following pre-existing major chronic conditions at follow-up baseline: diabetes, angina, myocardial infarction, stroke, heart failure, coronary artery bypass graft, percutaneous coronary intervention, or cancer. CHD, coronary heart disease; FFQ, food-frequency questionnaire; MET, metabolic equivalent; WHI, Women's Health Initiative.
2Frequency of chocolate intake was assessed by means of a semi-quantitative FFQ.
3Referent chocolate consumption category.
4Tests for linear trend were performed by using the median for each chocolate intake level as the sole predictor in the model.
5HRs and 95% CIs were determined by means of Cox regression.
6Women, n is the number of participants at year 0 (baseline) without pre-existing serious chronic disease at year 0 who provided data on all variables at year 0. Events, n is the number of incident events or deaths of the condition during the 13.4-y follow-up period. n for the full model is lower than for the model with age, race, and WHI study arm as covariates because of missing values for some of the extra covariates in the full model.
7Basic model covariates were age (years), race/ethnicity (white, black, other), and WHI study arm. The full model included additional continuous and categorical variables. The continuous variables were: the revised Alternative Healthy Eating Index (10); nonchocolate daily energy intake (derived from FFQ data); physical activity (total energy expended from recreational physical activity in MET-h/wk); smoking status (0 = never, 1 = past, 2 = current); alcohol intake (1 = nondrinker, 2 = past drinker, 3 = <1 drink/mo, 4 = <1 drink/wk, 5 = 1 to <7 drinks/wk, 6 = ≥7 drinks/wk), educational level (11 levels), and income status (9 levels). The one additional categorical variable was family history of CVD (yes/no).
There was a significant interaction for age (P = 0.02) but not for BMI (P = 0.93) in the analysis with CHD and stroke combined as the outcome variable. We therefore performed survival analyses in 2 subgroups, defined by age <65 y and ≥65 y. Table 3 shows that there was no significant association for age ≥65 y, but there was a significant positive linear association (β = +0.27) with an increased risk of CVD in the highest quintile of chocolate intake for age <65 y.
TABLE 3.
Chocolate intake, HRs, and 95% CIs for incident CHD or stroke showing the effects of age (P for interaction = 0.02) in the WHI1
| Frequency2 of chocolate consumption [1-oz (28.35 g) servings] | ||||||
|---|---|---|---|---|---|---|
| Covariates in the model | <1/mo3 | 1 to <1.5/mo | 1.5 to <3.5/mo | 3.5/mo to <3/wk | ≥3/wk | P for linear trend4 |
| Women aged <65 y | ||||||
| Age, race, and WHI study arm (n = 52,173) | ||||||
| HR (95% CI)5 | 1.00 | 1.14 (0.98, 1.32) | 1.06 (0.92, 1.22) | 1.10 (0.96, 1.25) | 1.34 (1.17, 1.54) | <0.0001 |
| Women, n6 | 15,027 | 7871 | 9082 | 12,253 | 7940 | 52,173 |
| Events, n6 | 482 | 292 | 317 | 439 | 347 | 1877 |
| Full model7 (n = 47,506) | ||||||
| HR (95% CI) | 1.00 | 1.17 (1.00, 1.36) | 1.05 (0.90, 1.22) | 1.09 (0.94, 1.25) | 1.27 (1.09, 1.49) | 0.005 |
| Women, n | 13,733 | 7189 | 8287 | 11,148 | 7149 | 47,506 |
| Events, n | 423 | 264 | 281 | 401 | 319 | 1688 |
| Women aged ≥65 y | ||||||
| Age, race, and WHI study arm (n = 39,642) | ||||||
| HR (95% CI) | 1.00 | 1.04 (0.95, 1.15) | 1.07 (0.97, 1.17) | 1.01 (0.93, 1.09) | 1.06 (0.97, 1.17) | 0.35 |
| Women, n | 12,479 | 5863 | 6527 | 9079 | 5694 | 39,642 |
| Events, n | 1307 | 658 | 737 | 996 | 651 | 4349 |
| Full model (n = 72,988) | ||||||
| HR (95% CI) | 1.00 | 1.04 (0.95, 1.15) | 1.05 (0.96, 1.16) | 0.95 (0.87, 1.04 | 0.99 (0.89, 1.10) | 0.49 |
| Women, n | 11,225 | 5312 | 5925 | 8229 | 5113 | 35,804 |
| Events, n | 1161 | 596 | 668 | 878 | 572 | 3875 |
1Incident CHD was the first occurrence of, or death due to, CHD between the baseline survey (1993–8) and 30 September 2013. Incident stroke was the first occurrence of, or death due to, stroke in the same time period. Our sample included observational study and clinical trial control participants without: 1) implausible FFQ energy intakes (defined as mean intakes <600 or >5000 kcal/d), or extreme energy intake (<600 or >5000 kcal/d), BMI ( kg/m2; <15 or >50), or height <122 cm (4 ft); or 2) any of the following pre-existing major chronic conditions at follow-up baseline: diabetes, angina, myocardial infarction, stroke, heart failure, coronary artery bypass graft, percutaneous coronary intervention, or cancer. CHD, coronary heart disease; FFQ, food-frequency questionnaire; MET, metabolic equivalent; WHI, Women's Health Initiative.
2Frequency of chocolate intake was assessed by means of a semi-quantitative FFQ.
3Referent chocolate consumption category.
4Tests for linear trend were performed by using the median for each chocolate intake level as the sole predictor in the model.
5HRs and 95% CIs were determined by means of Cox regression.
6Women, n is the number of participants at year 0 (baseline) without pre-existing serious chronic disease at year 0 who provided data on all variables at year 0. Events, n is the number of incident events or deaths of the condition during the 13.4-y follow-up period. n for the full model is lower than for the model with age, race, and WHI study arm as covariates because of missing values for some of the extra covariates in the full model.
7Basic model covariates were age (years), race/ethnicity (white, black, other), and WHI study arm. The full model included additional continuous and categorical variables. The continuous variables were: the revised Alternative Healthy Eating Index (10); nonchocolate daily energy intake (derived from FFQ data); physical activity (total energy expended from recreational physical activity in MET-h/wk); smoking status (0 = never, 1 = past, 2 = current); alcohol intake (1 = nondrinker, 2 = past drinker, 3 = <1 drink/mo, 4 = <1 drink/wk, 5 = 1 to <7 drinks/wk, 6 = ≥7 drinks/wk), educational level (11 levels), and income status (9 levels).The one additional categorical variable was family history of CVD (yes/no).
Incidence of major chronic disease and prospective changes in chocolate, energy, and fat intake and body weight
Table 4 shows that women free of major chronic disease at year 0 decreased their chocolate, energy, and fat intake and body weight between year 0 and year 3 to a greater extent if they were diagnosed with a major chronic disease during the 3-y period compared with those without incident disease. The decrease was noticeably larger for chocolate intake than for energy and fat intake and body weight. Specifically, women who developed a major chronic disease had a percentage reduction in chocolate intake, energy intake, fat intake, and body weight that was larger by about 15%, 2.8%, 5%, and 1.9%, respectively, than those women who remained free of these conditions during follow-up. The percentage reduction for each characteristic was estimated as the “Yes-no year 3–year 0 difference” divided by the mean of the 2 “Year 0” values.
TABLE 4.
Changes in characteristics of participants with and without incidence of serious chronic disease between year 0 and year 3 in the WHI cohort1
| Diagnosis of serious chronic disease between year 0 and year 3 | |||||||
|---|---|---|---|---|---|---|---|
| No (n = 56,374) | Yes (n = 3536) | ||||||
| Characteristic | Year 0 | Year 3 | Year 3–year 0 difference | Year 0 | Year 3 | Year 3–year 0 difference | Yes-no year 3–year 0 difference |
| Chocolate intake, oz/mo | 5.19 ± 9.65 | 5.56 ± 9.89 | 0.37 ± 10.04 | 5.40 ± 9.79 | 4.95 ± 9.62 | –0.44 ± 10.33 | –0.81 ± 10.06**** |
| Energy intake, kcal/d | 1590.8 ± 578.7 | 1525.4 ± 558.2 | –65.5 ± 519.2 | 1647.9 ± 622.4 | 1537.5 ± 575.4 | –110.4 ± 556.0 | –44.98 ± 521.4**** |
| Fat intake, kcal/d | 54.32 ± 28.84 | 54.71 ± 28.00 | 0.39 ± 24.98 | 58.47 ± 31.70 | 56.00 ± 29.57 | –2.47 ± 26.81 | –2.86 ± 25.09**** |
| Body weight, kg | 69.82 ± 14.04 | 70.83 ± 15.56 | 1.01 ± 7.70 | 73.95 ± 16.02 | 73.63 ± 17.21 | –0.32 ± 8.42 | –1.33 ± 7.74**** |
1Serious chronic disease was defined as incidence of heart attack, stroke, heart failure, revascularization, diabetes, or cancer. P values (****<0.0001) are based on the 2-independent sample t test, with the Satterthwaite method, of the difference between participants with and without incident serious chronic disease in the difference in the characteristic between year 0 and year 3. Values are means ± SDs. Data are for participants who at baseline had no self-reported pre-existing serious chronic disease, nonextreme self-reported energy intake (<600 or >5000 kcal/d) derived from an FFQ, nonextreme values of measured BMI (kg/m2; <15 or >50) and height (<122 cm), and with no missing values for any of the characteristics in this table at year 0 or year 3. FFQ, food-frequency questionnaire; WHI, Women's Health Initiative.
The decrease in chocolate intake after diagnosis of a major chronic disease prior to baseline could bias the association between chocolate and CHD or stroke to become more inverse. We therefore investigated whether a possible difference in this effect at different ages could explain the age modification we found in the association between chocolate intake and CHD and stroke combined. We repeated our full-model survival analyses for women aged <65 y and those aged ≥65 y who had major chronic disease at baseline. For women aged <65 y, the HRs (95% CI) for those who ate 1 oz. (28.35 g) of chocolate <1/mo, 1 to <1.5/mo, 1.5 to <3.5/mo, 3.5/mo to <3/wk and ≥3/wk were 1.00 [referent; n (number of events) = 293], 0.89 (0.72, 1.11; n = 113), 0.75 (0.59, 0.95; n = 97), 0.82 (0.67, 1.01; n = 143), and 0.75 (0.58, 0.96; n = 90), respectively (P for linear trend = 0.07). For women aged ≥65 y, the equivalent HRs were 1.00 (referent; n (number of events) = 747), 0.96 (0.84, 1.10; n = 283), 0.92 (0.80, 1.06; n = 277), 0.89 (0.78, 1.01; n = 369), and 0.76 (0.65, 0.89; n = 197), respectively (P for linear trend = 0.0008). Comparison of these results with those in Table 3 shows that for women aged <65 y, those without pre-existing major chronic disease exhibited a significant positive association whereas those with such a disease showed no significant association. For women aged ≥65 y, those without a pre-existing disease exhibited no significant association whereas those with one had a significant inverse association.
Sensitivity analyses
In order to assess the effects of using different definitions for stroke and CHD, we repeated our main full-model analyses with: 1) stroke defined as the first occurrence of ischemic or hemorrhagic stroke, or death due to a cerebrovascular event; and 2) CHD defined as the first occurrence of coronary revascularization, clinical MI, definite silent MI, or death due to coronary revascularization or definite or possible CHD. The results were essentially the same as those in Table 2. For instance, the HRs (95% CI) for ischemic or hemorrhagic stroke or death due to a cerebrovascular event for women who ate 1 oz. (28.35 g) of chocolate <1/mo, 1 to <1.5/mo, 1.5 to <3.5/mo, 3.5/mo to <3/wk, and >3 times/wk were 1.00 (referent; n (number of events) = 664), 1.11 (0.98, 1.26; n = 373), 1.05 (0.93, 1.19; n = 400), 1.00 (0.89, 1.13; n = 539), and 1.08 (0.94, 1.23; n = 371), respectively (P for linear trend = 0.50). Using the expanded version of CHD, the equivalent HRs were 1.00 (referent; n (number of events) = 1533), 1.01 (0.93, 1.10; n = 796), 1.03 (0.94, 1.11; n = 918), 0.97 (0.90, 1.05; n = 1235), and 1.01 (0.93, 1.11; n = 843), respectively (P for linear trend = 0.85).
We included the second FFQ assessment of chocolate intake, which was made at the third visit, into our predictor by averaging the 2 estimates. We then repeated our full-model analyses in Tables 2 and 3. The HRs were similar to those in Tables 2 and 3. For instance, for CHD and stroke combined, the HRs for healthy women aged <65 y who ate 1 oz. (28.35 g) of chocolate <1/mo, 1 to <1.5/mo, 1.5 to <3.5/mo, 3.5/mo to <3/wk, and >3 times/wk were 1.00 (referent; n (number of events) = 434), 1.02 (0.86, 1.21; n = 199), 1.01 (0.87, 1.16; n = 334), 0.91 (0.79, 1.04, n = 396), and 1.19 (1.01, 1.40; n = 265), respectively (P for linear trend = 0.047).
We tested the effects of including 2 variables (one at a time) that could be in the causal pathway between our predictor and outcome variable as covariates in our main full-model analyses in Tables 2 and 3. The variables were pre-existing hypertension (yes/no) and BMI. The results were essentially the same as those presented in Tables 2 and 3. For instance, after including pre-existing hypertension as a covariate in the model for women <65 y of age, the HRs (95% CI) for CHD and stroke combined for those who ate 1 oz. (28.35 g) of chocolate <1/mo, 1 to <1.5/mo, 1.5 to <3.5/mo, 3.5/mo to <3/wk, and >3 times/wk were 1.00 (referent; n (number of events) = 422), 1.16 (1.00, 1.36; n = 262), 1.05 (0.90, 1.22; n = 281), 1.10 (0.96, 1.27; n = 400), and 1.29 (1.11, 1.51; n = 317), respectively (P for linear trend = 0.003).
In order to test the ability of the revised Alternative Healthy Eating Index to fully capture the effects of dietary factors on the association between chocolate and CHD and stroke risk combined, we added sodium intake, saturated fat intake, and total sugar intake, one at a time, as extra covariates in our full-model analysis in Table 2. Sodium intake was expressed in g/d. We used the residual method for saturated fat and sugar intake. None of these factors had a significant coefficient, or changed any of the chocolate HRs by more than one unit in the second decimal place.
DISCUSSION
Among postmenopausal US women in the WHI aged 50–79 y without CVD, diabetes, or cancer at baseline, chocolate intake was not associated with risk of incident CHD or stroke, both combined, or death. However, the relation for both conditions combined, the 2 major components of CVD, was modified by baseline age, with a significant positive linear trend and an increased risk in the highest quintile of chocolate consumption among women below age 65 y, but not among older women. Women below age 65 y constituted 52.4% of our sample. These findings were not changed by any of 8 sensitivity analyses. The fact that an elevated risk only occurred among the younger women is unlikely to be explained by higher levels of any of the factors for which risk was adjusted, such as smoking, BMI, physical activity, or alcohol intake. All of our risk factor estimates were adjusted for the same factors. It seems more likely that a decrease in chocolate intake after the first diagnosis of a serious chronic illness among women in both age groups could explain why we only found an elevated risk among the younger women, as explained below. However, all of our findings require confirmation owing to the limitations inherent in our study, including the fact that we had no data on the types of chocolate consumed by our participants, as outlined below.
Previous findings of significant inverse associations between chocolate consumption and CVD may be at least partially attributable to decreases in chocolate intake that can occur after disease diagnosis/onset. We found some evidence to support this hypothesis. WHI participants initially free of major chronic disease showed a prospective 3-y 25.1% decrease in chocolate intake if they were diagnosed with a major chronic disease during the 3-y period. These women also showed significant but smaller decreases in their energy and fat intake and body weight during the 3-y period. Therefore, it seems likely that some of the significant inverse associations between chocolate intake and CVD risk observed in prior studies among those with pre-existing major chronic disease and/or a greater burden of aging-related comorbidities may be because of post-illness decreases in chocolate intake prior to the baseline assessment. Chocolate is a discretionary nutritional item with high energy and fat density. Whether intake is modified in an attempt to lose weight and decrease risk of disease progression or because serious illness directly affects food intake, the inclusion of participants with prevalent major chronic disease at baseline may introduce bias.
To our knowledge, our finding of a positive association between a high level of chocolate consumption and the risk of CHD or stroke has not been reported previously. However, a positive association is consonant with the available empirical evidence. Two prior epidemiologic analyses (14, 15) found that higher levels of chocolate consumption were followed by prospective significant increases in body weight, with a significant linear trend; and elevated body weight is a major CVD risk factor (12). The possibility that long-term chocolate consumption can cause an increase in adiposity is not surprising given the high energy and fat density of chocolate.
We tested the possibility that high levels of chocolate intake were positively associated with the risk of CHD or stroke for women aged <65 y, but not for those aged ≥65 y, could be explained by a difference in a decrease in chocolate consumption after disease diagnosis/onset in these 2 age subgroups. We found that among women aged ≥65 y, those without pre-existing major chronic disease exhibited no association, whereas those with these pre-existing conditions had a significant inverse association. For age <65 y, those without pre-existing major chronic disease exhibited a significant positive association at high levels of intake, whereas those with such a pre-existing condition demonstrated no significant association. This result suggests that, for women in both age groups, the effect of disease diagnosis/onset was probably similar: a decrease in chocolate intake that changed the chocolate-CVD association to appear more inverse or less positive. We did not find any evidence suggesting that a difference in a decrease in chocolate consumption after disease diagnosis/onset in the 2 age subgroups could explain the difference between the chocolate-CVD association for women aged <65 y and those aged ≥65 y. Further research is needed to confirm and/or explain these observations.
One of the limitations of our study is that heavier WHI participants tend to underreport the intake of energy on the FFQ to a greater extent than lighter persons (16), and this could have affected our results. Another limitation is that the FFQ only asked about chocolate candy or candy bars, so we had no data on the types of chocolate consumed by our participants. Dark chocolate may have yielded different results in our analysis, as many of the phytochemicals in dark chocolate are at higher concentrations than in milk chocolate (17). There is evidence suggesting that dark chocolate or cocoa containing higher levels of cocoa compounds (such as flavanols) than regular milk chocolate might decrease CVD risk, by decreasing blood pressure (18), body weight (19), or diabetes risk (3, 20). Nonetheless, high levels of consumption of dark chocolate may also be associated with elevated energy intake.
In summary, we found no overall association between chocolate intake and risk of CHD, stroke, or both combined among postmenopausal women in the WHI who were free of major chronic disease at baseline. The results for both conditions combined were modified by age, with a significant positive linear trend and increased risk in the highest quintile of chocolate consumption among women below age 65 y, but not among women aged 65 y or above. Inverse associations found in previous studies that included participants with pre-existing major chronic diseases or comorbidities may be at least partially attributable to disease-induced decreases in chocolate intake. Randomized clinical trials are needed to establish the effects of chocolate or cocoa flavanol intake on incident CVD. Our results do not support any changes in chocolate intake when giving dietary advice.
Acknowledgements
We thank the WHI Investigators (see Online Supplement) for their efforts in the collection of the WHI data.
The authors’ responsibilities were as follows—JAG, JEM, LT, and MLN: designed the research and interpreted the data; JAG: analyzed the data, wrote the paper, and had primary responsibility for final content; and all authors: read, edited, and approved the final manuscript before submission. JEM and colleagues at Brigham and Women's Hospital, Harvard Medical School are recipients of funding from Mars Symbioscience for an investigator-initiated randomized trial of cocoa flavanols and cardiovascular disease. JAG is a recipient of funding from the City University of New York Research Award Program to conduct a pilot randomized trial of cocoa compounds and appetite. JEM received partial support from HHSN268201100001C from NIH/NHLBI, the Women's Health Initiative program. LT and MLN received support from NIH/NHLBI, US Department of Health and Human Services contract HHSN268201100046C.
Notes
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
The funding agencies had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
Abbreviations used: CHD, coronary heart disease; CT, Women's Health Initiative clinical trials; CVD, cardiovascular disease; FFQ, food frequency questionnaire; MI, myocardial infarction; OS, Women's Health Initiative observational study; WHI, Women's Health Initiative.
REFERENCES
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S et al.. for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics—2013 Update. A Report from the American Heart Association. Circulation 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heron M, Anderson RN. Changes in the leading cause of death: recent patterns in heart disease and cancer mortality. NCHS Data Brief 2016;(254):1–8. [PubMed] [Google Scholar]
- 3. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal 2011;15(10):2779–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, Franco OH. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ 2011;343:d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Z1, Xu G, Liu X. Chocolate intake reduces risk of cardiovascular disease: evidence from 10 observational studies. Int J Cardiol 2013;168(6):5448–50. [DOI] [PubMed] [Google Scholar]
- 6. Kwok CS, Boekholdt SM, Lentjes MA, Loke YK, Luben RN, Yeong JK, Wareham NJ, Myint PK, Khaw KT. Habitual chocolate consumption and risk of cardiovascular disease among healthy men and women. Heart 2015;101(16):1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med 2006;166(4):411–7. [DOI] [PubMed] [Google Scholar]
- 8. Janszky I, Mukamal KJ, Ljung R, Ahnve S, Ahlbom A, Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: the Stockholm Heart Epidemiology Program. J Intern Med 2009;266(3):248–57. [DOI] [PubMed] [Google Scholar]
- 9. The Women's Health Initiative Study Group. Design of the Women's Health Initiative Clinical Trial and Observational Study. Control Clin Trials 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 10. Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M et al.. for the WHI Morbidity and Mortality Committee Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol 2003 13(suppl): S122–8. [DOI] [PubMed] [Google Scholar]
- 11. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 14. Greenberg J, Buijsse B. Habitual chocolate consumption may increase body weight in a dose-response manner. PLoS One 2013;8(8):e70271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenberg JA, Manson JE, Buijsse B, Wang L, Allison MA, Neuhouser ML, Tinker L, Waring ME, Isasi CR, Martin LW et al.. Chocolate-candy consumption and 3-year weight gain among postmenopausal U.S. women. Obesity (Silver Spring) 2015;23(3):677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9(3):178–87. [DOI] [PubMed] [Google Scholar]
- 17. Steinberg FM, Bearden MM, Keen CL. Cocoa and chocolate flavonoids: implications for cardiovascular health. J Am Diet Assoc 2003;103(2):215–23. [DOI] [PubMed] [Google Scholar]
- 18. Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond) 2008;32(8):1289–96. [DOI] [PubMed] [Google Scholar]
- 19. Greenberg JA, O'Donnell R, Shurpin M, Kordunova D. Epicatechin, procyanidins, cocoa and appetite—a randomized controlled trial. Am J Clin Nutr 2016;104(3):613–9. [DOI] [PubMed] [Google Scholar]
- 20. Lin X, Zhang I, Li A, Manson JE, Sesso HD, Wang L, Liu S. Cocoa flavanol intake and biomarkers for cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Nutr 2016;146(11):2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


