Abstract
Background
Cardiac dysfunction during endotoxemia is a major cause of cardiovascular disease with high morbidity and mortality. Alisol B 23-acetate (AB23A) is a triterpenoid extracted from the Rhizoma Alismatis, a kind of traditional Chinese medicine, exhibits anti-inflammatory activity on endotoxemia. This investigation aimed to uncover the protective effects of AB23A against sepsis-induced cardiac dysfunction.
Material/Methods
Adult male C57BL/6 mice received lipopolysaccharide (LPS) (20 mg/kg intravenous) stimulation, with or without pre-treatment of AB23A (10 mg/kg, 20 mg/kg, or 40 mg/kg). Histopathological staining and cardiac function were performed 4 hours after LPS stimulation. Then the levels of interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α were monitored with enzyme-linked immunosorbent assay (ELISA). In addition, H9C2 cells were treated with LPS (5 μg/mL) with or without pre-treated with AB23A (0.1 μM, 1 μM, or 10 μM), and the production of reactive oxygen species (ROS) was detected by DCFH-DA combined with flow cytometry. The expression of Toll-like receptor 4 (TLR4), NADPH oxidase 2 (NOX2), NOX4, P38, p-P38, extracellular-signal-regulated kinase (ERK), and p-ERK were assessed by western blotting.
Results
AB23A improved the survival rate and ameliorated myocardial injury, decreased inflammatory infiltration and the level of IL-6, IL-1β, and TNF-α in the LPS-stimulated mouse model. Moreover, AB23A inhibited the ROS production in LPS-treated H9C2 cells. In addition, AB23A suppressed the levels of TLR4 and NOX2 as well as the activation levels of P38 and ERK both in vivo and in vitro.
Conclusions
AB23A reduced LPS-induced myocardial dysfunction by inhibiting inflammation and ROS production through the TLR4/NOX2 pathway.
MeSH Keywords: Alisma; Myocytes, Cardiac; Sepsis; Toll-Like Receptor 4; NADPH Oxidase
Background
Cardiovascular disease is one of the most common diseases and has high morbidity and is life-threatening [1,2]. Several mechanisms have been proposed to clarify the pathophysiology ultimately leading to cardiac dysfunction, including endotoxins and inflammatory cytokines, hypoxia and acidosis, and increased production of reactive oxygen (ROS) [3–5]. Among these, accumulating evidence has indicated that endotoxemia and sepsis are the major causes of cardiac dysfunctions [6]. Significantly, sepsis-induced cardiac dysfunction is relevant to the survival rate of patients and can play a significant part in further complicating therapy [7]. The therapeutic schedule of this type of disease is still insufficiency. There is an urgent need for effective therapies to treat such diseases.
Lipopolysaccharide (LPS) is one of the most important causes of the pathogenesis of septic shock and endotoxemia. Toll-like receptors (TLRs) play a central role in inflammation stimulated by the various infectious pathogens as the primary receptors of innate immunity, leading to the activation of the inflammation pathway [8,9]. TLRs are expressed in many cells, but mainly in immune cells, including cardiomyocytes, which express TLR2, TLR3, TLR4, and TLR6 [10]. TLR4 was the first TLR detected in humans where activation has been reported to be associated with LPS [11]. Interfering endotoxin-mediated activation of TLR, blocking either TLR4 or its adapter protein MyD88, improved cardiovascular outcomes and improved survival rate in the LPS-induced sepsis model [12]. There are other reports that have indicated that LPS could stimulate the inflammatory process, accompanied by abundant reaction oxygen species (ROS) production [13]. NADPH oxidases (NOXs) are the superoxide-generating enzymes that include NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2. Among all these homologs, NOX2 and NOX4 are highly expressed in cardiomyocytes [5,14]. Thus, blocking the TLR4/NOX4 signaling might be a potential therapy for endotoxin-induced cardiac dysfunction.
In recent years, molecules from natural sources with biological potential have been reported to be useful in the management of several diseases as alternative medicine. Alisol B 23-acetate (AB23A) (Figure 1A) is a natural triterpenoid extracted from the dried rhizomes of Alisma orientalis (Sam.) which is a plant with medicinal value in different traditional Chinese herbal [15]. The literature reveals that AB23A exhibits several pharmacological activities, including anti-hepatitis virus [16], antibacterial [17], diuretic [18], hyperlipidemia [19], antitumoral [20], and hepatoprotective effects [21]. Furthermore, AB23A has been shown to have anti-inflammatory actions [22]. In the present study, we demonstrated that AB23A can be used against LPS-induced cardiac dysfunction by inhibiting ROS production and inflammatory response by blocking the TLR4/NOX2 pathway. Additionally, the study revealed potential pharmacological effect of AB23A on cardiac dysfunction.
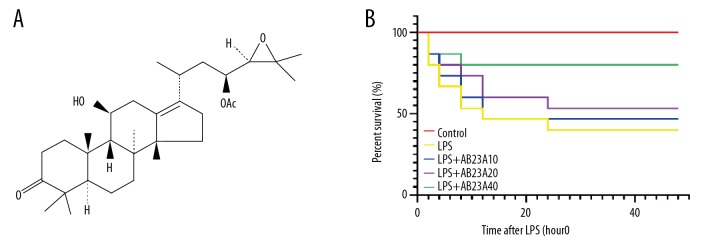
Figure 1.
AB23A improves the survival rate in the lipopolysaccharide (LPS)-induced sepsis mouse model. (A) Chemical structure of Alisol B 23-acetate: C32H50O5, molecular weight =51.74. (B) In the 48 hours after LPS-injection, the survival rate in the control group was 100%, in the LPS-induced group it was 40% (6 out of 15), in the low-dose AB23A (10 mg/kg) pre-treatment group it was 46.667% (7 out of 15), in the medium-dose AB23A (20 mg/kg) pre-treatment group it was 53.333% (8 out of 15), and in the high-dose AB23A (20 mg/kg) pre-treatment group it was 80% (12 out of 15).
Material and Methods
Animal model
Adult male C57BL/6 mice (6–8 weeks old, weighing 20–25 g, n=68) of specific pathogen-free (SPF) grade, were purchased from Shanghai Slake Animal Laboratory Co., Ltd. (SCXK) and raised in the Animal Laboratory Research Center, Zhejiang University of Traditional Chinese Medicine (SYXK). All animals were housed under standard conditions (humidity: 60±5%; temperature: 24±3°C) with a 12-hour light/dark cycle and free access to standard diet and tap water and acclimatized for 7 days before the experiment. The mice were given humanistic concern according to the animal laboratory guidelines. Mice were randomly divided into 5 groups. 1) The control group (n=8) had normal saline intraperitoneally injected. 2) The LPS group (n=15) were injected intraperitoneally with LPS (Sigma-Aldrich Co., USA, 20 mg/kg). 3) the low-dose AB23A+LPS group (n=15) were pretreated with 1 mg/kg of AB23A (Hushi Medical Technology Co., Ltd., Shanghai, China) by gavage for 30 days. 4) The medial-dose AB23A+LPS group (n=15) were pretreated with 20 mg/kg of AB23A by gavage for 30 days. 5) The high-dose AB23A+LPS group (n=15) were pretreated with 40 mg/kg of AB23A by gavage for 30 days. LPS was intraperitoneal injected 1 hour after the last gavage; Mice were sacrificed at 48 hours after LPS injection for LPS-subjected animals. Dosages of AB23A were decided according to our pre-experiment and previous descriptions [23]. Cardiac apexes were harvested and kept at −80°C.
Welfare assessment
Animals were monitored for responses to LPS every 2 hours. All assessments were performed by the evaluators in a double-blind manner. Systematic evaluation of common symptoms of C57BL/6 mice diseases, including piloerection, orbital constriction, and somnolence, has been carried out according to previous reports. A systematic evaluation of all mice was completed within 48 hours. Animals that showed no signs of disease were excluded from the stimulation of LPS, while those showing signs of disease were excluded from the severe limits of the project license.
Histopathology and cardiac function
C57BL/6J mice were anesthetized with sevoflurane 4 hours after LPS injected. Left ventricular ejection fraction (LVEF), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEED), and left ventricular fraction shortening (LVFS) were measured by echocardiography. Then all the animals were sacrificed, and their hearts were isolated. Isolated hearts were fixed in formalin (10%) for 3 days. Slices of cardiac apexes were seeded in the paraffin and a microtome was used to make 3-μm sections. Hematoxylin and eosin (H&E) was used to stain the heart tissue sections. The inflammatory cell infiltration was evaluated by the BX51 light microscope (OLYMPUS, Japan).
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were taken from the main abdominal vein of mice 4 hours after LPS injection and kept at 4°C overnight. Among the different groups, the concentration changes of serum TNF-α, IL-6, and IL-1β were evaluated by ELISA according to the ELISA kit instructions (Cat. No. ab208348, ab100712, and ab197742; Abcam). After coating samples, we used PBST buffer to wash the plates 3 times and then blocked samples with 1% BSA incubated for 1 hour at 37°C. Then, anti-TNF-alpha (Cat. No. ab1793, Abcam), anti-IL-6 (Cat. No. ab7737, Abcam), anti-IL-1-beta (Cat. No. ab9722, Abcam) and HRP-conjugated antibody were sequentially added and incubated for 1 hour at 37°C. The detection was achieved by adding chromogenic substrate, 3,3′,5,5′-tetramethylbenzidine (TMB). Signals were then detected at 450 nm with an EnSpire multimode plate reader (Perkin Elmer, Waltham, MA, USA).
Western blotting
Protein expression of TLR4, NOX2, P38, p-P38, extracellular-signal-regulated kinase (ERK), and p-ERK in cardiac apexes tissue and H9C2 cells were detected by western blotting. For protein extraction, cultured H9C2 cells and cardiac apexes tissue homogenates were first lysed in lysis buffer, and then the lysate was centrifuged at 10 000g for 15 minutes at 4°C; then the clear supernatant was collected. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), then blotted onto polyvinylidene fluoride (PVDF) membranes. After blocking with TBST containing 5% non-fat milk powder for 1 hour at room temperature, the membrane was incubated with primary antibodies for TLR4 (1: 1000, Cat. No. #14358S, Cell Signaling Technology), NOX2 (1: 2000, Cat. No. MA5-18052, Invitrogen), P38 (1: 2000, Cat. No. #8690, Cell Signaling Technology), p-P38 (1: 500, Cat. No. #4092, Cell Signaling Technology), ERK (1: 1,000, Cat. No. #4695, Cell Signaling Technology), p-ERK (1: 500, Cat. No. #4370, Cell Signaling Technology), and GAPDH (1: 2000, Cat. No. #8884, Cell Signaling Technology) at 4°C overnight. Then membranes were incubated with a secondary antibody labeled with HRP at room temperature for 1 hour. Membranes were incubated with electrochemiluminescence (ECL, Bio-Rad) solution, and films were exposed in a dark room.
Cell culture
H9C2 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), and were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) with 10% fetal bovine serum (FBS, Gibco) medium at 37°C in a humidified atmosphere of 5% CO2. H9C2 cells were pre-treated with AB23A in concentrations of 0 μM, 0.1 μM, 1 μM, and 10 μM (LPS group, LPS+AB23A-L group, LPS+AB23A-M group, LPS+AB23A-H group respectively) at 6 hours before treatment with LPS (5 μg/mL). Dosages of AB23A were decided according to our pre-experiment and previous descriptions [24]. Cells were harvested for further analysis 4 hours later.
Cell Counting Kit-8 assay
H9C2 was inoculated into a 96-well culture plate and treated with different drug concentrations. 10 μL of Cell Counting Kit-8 solution was added to each well at 1/10 dilution. After incubation for 2 hours at 37°C, the absorbance was measured at 450 nm using a microtitration reader (Spectra May 190, Molecular Device, USA). The cell viability was calculated by dividing the optical density of the sample by the optical density of the control group.
ROS production or measurement in H9C2 cells
The ROS production or measurement in H9C2 cells was evaluated by dichloro-dihydro-fluorescein diacetate (DCFH-DA, Cat. No. S0033-1, Beyotime, Shanghai, China) method according to the ROS assay kit instructions (Cat. No. S0033, Beyotime, Shanghai, China). DCFH-DA is a non-polar fluorescence probe that can penetrate cell membranes, where it is converted into DCFH then detected by flow cytometry. Cells were incubated with 10 μM DCFH-DA at 37°C for 30 minutes in the dark. Analysis was performed using a quantitative method via flow cytometry (FCM, Becton Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
Each in vivo and in vitro experiment was repeated at least 3 times. Statistical analyses were performed with SPSS software (version 16.0) or GraphPad Prism 8.1 (GraphPad Software Inc., CA, USA). Differences between variables were assessed by 2-tailed Student’s t-test and one-way ANOVA, where appropriate. Data were expressed as mean±standard error of the mean (SEM). P values less than 0.05 were considered statistically significant (* P<0.05, ** P<0.01, *** P<0.001).
Results
AB23A improved the survival rate in LPS-induced sepsis mouse model
To investigate the role of ABA23 in the LPS-induced mouse model, the survival curves were plotted at 48 hours after LPS injection. The survival rate significantly decreased in LPS-induced septic mice compared with the control group. However, the AB23A treatment dramatically increased the survival rate of mice that received the LPS procedure. The 48-hour survival rate of LPS-induced mice was decreased to 40%; the low-dose AB23A group and medium-dose AB23A group did not show a significant difference from the model group, but the high-dose AB23A group dramatically enhanced the survival rate to 80% (Figure 1B), implying that AB23A might attenuate injury in these LPS-induced sepsis mice.
AB23A ameliorated cardiac dysfunctions in vitro and in vivo
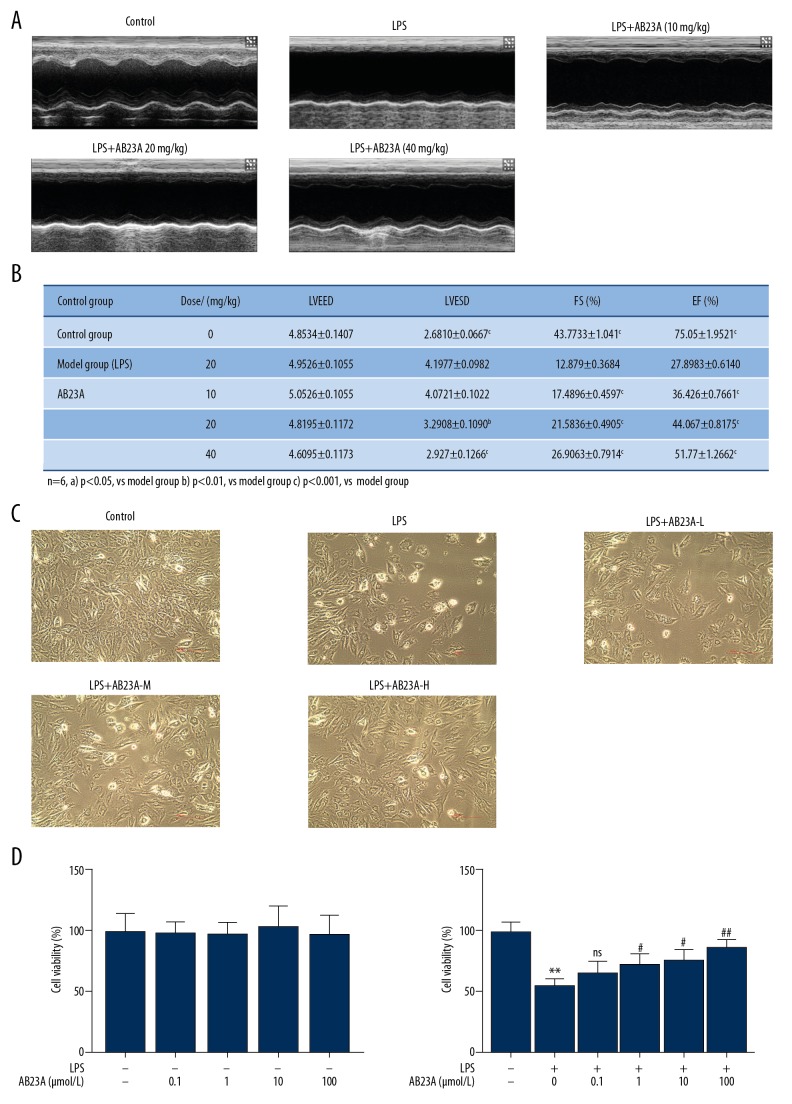
We next performed experiments in the LPS-induced sepsis mouse model and the LPS-stimulated H9C2 cell model to analyze the function of AB23A in cardiac dysfunctions. Echocardiographic results showed that AB23A could effectively prevent the myocardial dysfunction caused by LPS. The results of LVEF, LVFS, and LVESDs in the LPS group changed significantly compared with those of the control group. And the myocardial function of mice induced by LPS decreased significantly (P<0.05). Compared with the results of the LPS group, LVEF, LVFS, and LVESD in the AB23A group (10 mg/kg, 20 mg/kg, 40 mg/kg) recovered significantly (Figure 2A, 2B). In order to investigate whether AB23A had a protective effect against cardiac injury in vitro, the present study determined the effect of AB23A on the survival of LPS-treated H9C2 cells. Cell viability was determined by CCK-8. The result shows no significant cell viability changes in the control group (Figure 2C, P>0.05); LPS-treatment significantly decreased the cell viability while AB23A-treatment significantly increased the cell viability in a dose-dependent manner (Figure 2D, P<0.05, compared with the control group). As a consequence, AB23A reduced the cardiac dysfunctions in vivo and in vitro.
Figure 2.
(A–D) AB23A ameliorates cardiac dysfunctions in lipopolysaccharide (LPS)-induced mouse sepsis model (A) Echocardiography of the heart of LPS-induced sepsis animal model and (B) hemodynamic parameter containing LVESD, LVEED, LVEF, and LVFS (n=8). (A) P<0.05 versus model group. (B) P<0.01 versus model group. (C) P<0.001 versus model group. The effect of AB23A treatment on cell viability in the H9C2 cell was detected by Cell Counting Kit-8. (C) AB23A increased the level of cell viability in LPS-treated H9C2 cells. ** P<0.01 versus control group. # P<0.05 versus model group. ## P<0.01 versus model group. LVESD – left ventricular end-systolic diameter; LVEED – left ventricular end-diastolic diameter; LVEF – left ventricular ejection fraction; LVFS – left ventricular fraction shortening.
AB23A decreased inflammatory cells infiltration and attenuated inflammatory mediator expression of septic mice
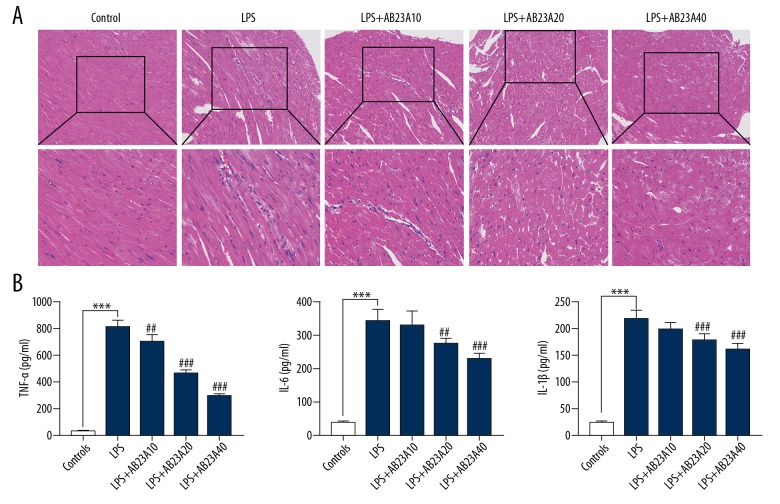
To validate the notion that AB23A reduces cardiac dysfunction through its anti-inflammation effect. H&E staining was used to evaluate the histological changes in cardiac apex tissues. Inflammatory cell infiltration was present in the LPS-induced septic mice and AB23A reduced myocardial injury in LPS-induced sepsis mice (Figure 3A). Blood samples were collected for each group from the main abdominal vein 4 hours after LPS injected. The concentrations of TNF-α, IL-6, and IL-1β in the serum were detected by ELISA. LPS injection-induced production of TNF-α, IL-6, and IL-1β compared with the control group, whereas AB23A treatment attenuated the increase in a dose-dependent manner (Figure 3B).
Figure 3.
AB23A decreases inflammatory cells infiltration in myocardial tissue and attenuates inflammatory mediator expression in serum of lipopolysaccharide (LPS)-induced mouse sepsis model (A) Inflammatory cells infiltration in myocardial tissue by hematoxylin and eosin (H&E) staining. (B) Concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β were increased by LPS stimulation and reduced by AB23A treatment (10 mg/kg, 20 mg/kg, 40 mg/kg). *** P<0.001 versus control group. # P<0.05 versus model group. ## P<0.01 versus model group. ### P<0.001 versus model group.
AB23A regulated TLR4, NOX2, p-P38 and p-ERK protein expression in myocardial tissue of LPS-induced mouse sepsis model
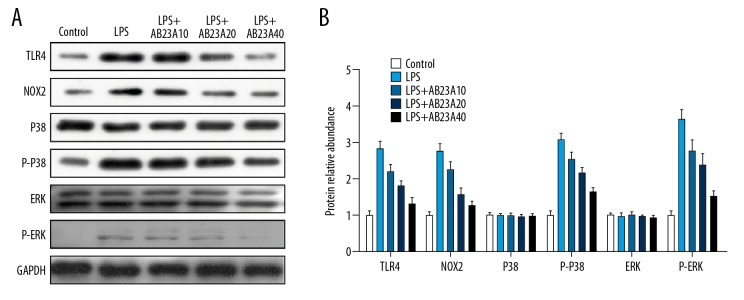
As shown in Figure 3, AB23A suppressed the inflammation process in the sepsis model. In addition, after the activation of TLR4, signal transduction of downstream pathway was closely related to an inflammatory response. Thus, we measured the protein levels of p-P38 and p-ERK, to evaluate whether AB23A could regulate the TLR signaling to attenuate the cardiac dysfunction. As shown in Figure 4, AB23A regulated TLR4, NOX2, p-P38, and p-ERK protein expression in myocardial tissue of the LPS-induced mouse sepsis model. Expression levels of TLR4 and NOX2, as well as the phosphorylation level of ERK and P38, were elevated significantly in myocardial tissue from mice that received LPS. However, the AB23A treatment could obviously downregulate the expression levels of TLR4, NOX2, p-P38, and p-ERK expression in a dose-dependent manner (Figure 4).
Figure 4.
(A, B) AB23A attenuates the lipopolysaccharide (LPS)-induced alteration in TLR4/NOX2 signaling. The western blotting assay showed the pre-treatment of AB23A to reduce the increasing expressions of TLR4, NOX2, p-P38, and p-ERK protein in LPS-induced septic mice (n=3).
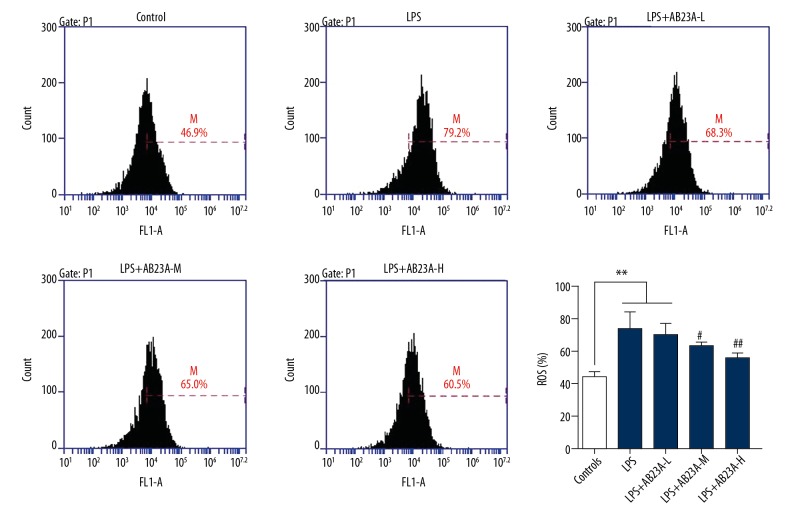
AB23A decreased the level of reactive oxygen species (ROS) in H9C2 cell with LPS stimulation
ROS production is an important marker of intracellular reactive oxygen species (ROS). The changes of ROS can be determined by dichloro-dihydro-fluorescein diacetate (DCFH-DA) staining combined with flow cytometry analysis. DCFH-DA is free to enter the cell and is oxidized by ROS in the cell to become DCF that produces green fluorescence signals. To further explore whether AB23A has an effect on ROS levels in the LPS-stimulated model, ROS production was measured in the H9C2 cell. The results show that ROS production significantly increased in the LPS-subjected group and AB23A attenuated the changes in a dose-dependent manner (Figure 5).
Figure 5.
AB23A decreases the level of reactive oxygen species (ROS) in the H9C2 cell line with lipopolysaccharide (LPS) stimulation. The chart shows the ROS production of normal control, LPS injection group, pre-treatment with AB23A at the concentration of 0.1 μM (LPS+AB23A-L), 1 μM (LPS+AB23A-M), and 10 μM (LPS+AB23A-H). With pre-treatment with AB23A of LPS stimulated H9C2 cells, the ROS began to decline within a certain range. ** P<0.01, versus control group. # P<0.05 versus model group. ## P<0.01 versus model group.
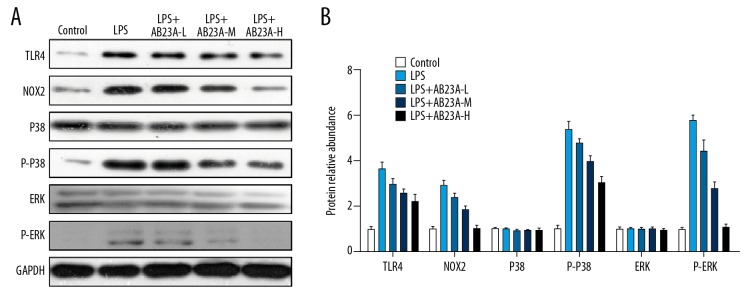
AB23A regulated TLR4, NOX2, p-P38, and p-ERK protein expression in H9C2 cell with LPS stimulation
AB23A showed significant inhibition to both TLR4 and NOX2 during LPS stress in vivo. To investigate the inhibition of AB23A with the TLR4/NOX2 pathway in vitro, the effects of AB23A on the expression of TLR4, NOX2, p-P38, and p-ERK protein in H9C2 cell were determined by western blot assay (Figure 6). There was a significant (P<0.01) increase in the expression of TLR4, NOX2, p-P38, and p-ERK in H9C2 cell with LPS stimulation compared to control group, whereas treatment with AB23A significantly attenuated the altered expression of TLR4, NOX2, p-P38, and p-ERK. Consequently, AB23A ameliorates LPS-induced cardiac dysfunction by blocking the TLR4/NOX2 signaling pathway.
Figure 6.
(A, B) AB23A regulates TLR4, NOX2, p-P38, and p-ERK protein expression in the H9C2 cell line with LPS stimulation. The western blotting assay showed the pre-treatment of AB23A to reduce the increasing expressions of TLR4, NOX2, p-P38, and p-ERK protein in H9C2 cells (n=3).
Discussion
Bacterial endotoxin-induced systemic inflammatory response syndrome sepsis is the main cause of heart failure and myocardial cell damage. Over the past 30 years, the number of sepsis patients has been increasing year by year, with high morbidity and mortality [25]. The mechanism of myocardial injury in sepsis is presently believed to be closely related to inflammatory factors [26]. LPS is the most important component of endotoxin and LPS has been shown to aggravate cardiac dysfunction by increasing the expression of TNF-α, IL-6, and IL-1β in myocardial tissue [27]. This study performed investigations in the LPS-stimulated H9C2 cell model and in the LPS-induced mouse sepsis model to reveal the role of AB23A in cardiac dysfunction of endotoxin sepsis. AB23A adjunctive treatment decreased LPS-induced TNF-α, IL-6, and IL-1β levels in the mouse model, attenuated LPS-simulated cardiac injure.
Studies have shown that endotoxin can aggravate myocardial injury through TNF-α, IL-6, and IL-1β progression, which might be related to TLRs, but the mechanism is unclear [28]. TLRs are important proteins involved in non-specific immunity (innate immunity) and connect with specific immunity. TLR4 is a member of the TLR family which is expressed in a variety of cells, such as macrophages, fibroblasts, endothelial cells, and cardiomyocytes. It is also a pattern recognition receptor closely related to immune or inflammatory diseases. The ligands identified by TLR4 are mainly LPS from gram-negative bacteria [11]. Previous studies have shown that TLR can activate NOX2 to produce ROS and regulate inflammation by influencing the expression of NOX2 [29]. Studies have shown that NOX2 can be activated in pulmonary vascular endothelial cells and cardiac fibroblasts stimulated by LPS, leading to myocardial fibrosis [30,31]. The depletion of NOX2 in macrophages can reduce the expression of cytokines and chemokines, and thus significantly inhibit their antimicrobial activity [32]. P38MAPK is a significant member of the MAPK family, which is activated by external stimulation. P38MAPK can promote the aggregation and activation of leukocytes, regulate the activity of transcription factors and the synthesis of cytokines, and play a key role in the regulation of inflammatory response [33]. ERK is a member of the mammalian mitogen-activated protein kinase (MAPK) family. In the intracellular phosphorylation cascade, as a sensor of extracellular stimuli, it plays a key role, leading to cell differentiation, proliferation, survival, and death [34,35]. In order to investigate the mechanism of AB23A in suppressing cardiac dysfunction, we detected the inflammatory progression in the mouse model. On the other hand, we detect ROS production in the cell model. The results showed that AB23A could significantly reduce the infiltration of inflammatory cells in myocardial tissue and the release of important inflammatory factors in serum. At the same time, AB23A could reduce the production of ROS in the cell model. These results indicate that AB23A alleviates myocardial damage caused by sepsis by reducing the inflammatory response and oxidative damage in the LPS-induced model. For further molecular mechanisms, we explored the downstream molecular pathway of AB23A. We found that AB23A can reduce the expression of TLR4, NOX2, p-ERK, and p-P38 induced by LPS. This suggests that AB23A alleviates the inflammatory response and oxidative damage in sepsis-induced myocardial injury through the TLR4/NOX2 pathway, thereby reducing myocardial injury.
Rhizoma Alismatis was wildly used for the treatment of cancer, pyelonephritis, inflammatory, enteritis diarrhea, dysuria, hyperlipidemia, and diabetes. Alisol B 23-acetate were identified as novel inducers of autophagy, with Alisol B being the most potent natural component in Rhizoma Alismatis [36]. It has been reported that Alisol B acetate can inhibit the production of endothelin-1 in nephritic rats, elicited an antinephritic action resulting from the inhibition of endothelin-1 production in nephritic rats [37]. Two other in vivo studies showed that the hemolysis and allergic reactions induced by complement in rats were significantly alleviated after oral administration of AB23A [38,39]. However, even heart failure is a serious health hazard, the important functions affecting myocardial injury induced by sepsis of AB23A has not been characterized [40].
In this study, we found that the better cardiac function can be explained in AB23A pre-treatment LPS-induced model by the decrease of inflammatory cells infiltration in heart tissue. AB23A could suppress the expression of IL-6, IL-1β, and TNF-α in serum and reduced oxidative injury in a cell model, which may promote enhanced survival rate and protect against sepsis-induced cardiac dysfunction. Furthermore, AB23A elevated the expression of TLR4, NOX2, as well as the phosphorylation levels of ERK and P38 in the LPS injection model, however, AB23A administration dramatically inhibited the activation of the TLR4/NOX2 signaling pathway in vivo and in vitro. Our current study revealed for the first time that AB23A was protective against septic cardiac dysfunction by suppressing inflammatory cytokine production and oxidative injury through inhibiting TLR4/NOX2 signal transduction.
Conclusions
The investigations of AB23A in the LPS-induced mouse sepsis model and LPS-simulated H9C2 cells revealed that AB23A protected against cardiac dysfunction during bacterial endotoxemia by attenuating the TLR4/NOX2 pathway. AB23A suppressed the release of inflammatory medium, ameliorated the heart damage, enhanced the survival rate of the LPS-induced sepsis model, and inhibited ROS-oxidative stress, and reduced the levels of inflammatory mediators in this in vitro study. These results suggest a potential clinical management for heart injury in sepsis and suggest future research needed to explore the critical role of AB23A in the pathogenesis of sepsis.
Footnotes
Source of support: Departmental sources
References
- 1.van Vught LA, Klein Klouwenberg PM, Spitoni C, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315:1469–79. doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 2.Jina Q, Lia R, Hud N, et al. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biology. 2018;14:576–87. doi: 10.1016/j.redox.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35(6):1599–608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 4.Kakihana Y, Ito T, Nakahara M, et al. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 2016;4:22. doi: 10.1186/s40560-016-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H, Wang J, Zhu P, et al. NR4A1 aggravates the cardiac microvascular ischemia-reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mf-required mitochondrial fission by CK2α. Basic Res Cardiol. 2018;113:23. doi: 10.1007/s00395-018-0682-1. [DOI] [PubMed] [Google Scholar]
- 6.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116(7):793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Su J, Huang P, Chen G, et al. Buddleoside prevents TNF-α-induced human aortic endothelial cells inflammatory injury through inhibiting TLR4/IκBα/NF-κB signaling pathway. Chin J Mod Appl Pharm. 2017;34(5):637–43. [Google Scholar]
- 9.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Frantz S, Ertl G, Bauresachs J. Mechanism of disease: Toll-like receptors in cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2007;4:444–54. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 11.Miyake K. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol. 2004;12:186–92. doi: 10.1016/j.tim.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Zou L, Chen C, et al. Role of cardiac- and myeloid-MyD88 signaling in endotoxin shock-a. Anesthesiology. 2014;121(6):1258–69. doi: 10.1097/ALN.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu SX, Du CT, Chen W, et al. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci Rep. 2015;5:17935. doi: 10.1038/srep17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anilkumar N, Weber R, Zhang M, et al. Nox4 and Nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–54. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Feng L, Ma L, et al. Alisol a 24-acetate and Alisol b 23-acetate induced autophagy mediates apoptosis and nephrotoxicity in human renal proximal tubular cells. Front Pharmacol. 2017;8:172. doi: 10.3389/fphar.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang ZY, Zhang XM, Zhang FX, et al. A new triterpene and anti-hepatitis B virus active compounds from Alisma Orientalis. Planta Med. 2006;72(10):951–54. doi: 10.1055/s-2006-947178. [DOI] [PubMed] [Google Scholar]
- 17.Jin HG, Jin Q, Ryun Kim A, et al. A new triterpenoid from Alisma Orientale and their antibacterial effect. Arch Pharm Res. 2012;35(11):1919–26. doi: 10.1007/s12272-012-1108-5. [DOI] [PubMed] [Google Scholar]
- 18.Feng YL, Chen H, Tian T, et al. Diuretic and anti-diuretic activities of the ethanol and aqueous extracts of Alismatis rhizoma. Ethnopharmacol. 2014;154:386–90. doi: 10.1016/j.jep.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, Gao H, Wand J, Zhao J. Separated prescription research on black currant seed and Zexie decoction, Crataegi fructus combination about depressurization and adjusting blood lipid. Chin J Mod Appl Pharm. 2016;33(4):414–19. [Google Scholar]
- 20.Zhao Y, Li E, Wang M. Alisol B 23-acetate induces autophagic-dependent apoptosis in human colon cancer cells via ROS generation and JNK activation. Oncotarget. 2017;8(41):70239–49. doi: 10.18632/oncotarget.19605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng Q, Chen X, Wang C, et al. Protective effects of Alisol B 23-acetate via Farnesoid X receptor-mediated regulation of transporters and enzymes in estrogen-induced cholestatic liver injury in mice. Pharm Res. 2015;32:3688–98. doi: 10.1007/s11095-015-1727-x. [DOI] [PubMed] [Google Scholar]
- 22.Li HM, Fan M, Xue Y, et al. Guaiane-type sesquiterpenoids from Alismatis rhizoma and their anti-inflammatory activity. Chem Pharm Bull. 2017;65:403–7. doi: 10.1248/cpb.c16-00798. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Li XY, Lin N, et al. Diuretic activity of compatible triterpene components of Alismatis rhizome. Molecules. 2017;22:1459. doi: 10.3390/molecules22091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng Q, Chen X, Wang C, et al. Alisol B 23-acetate promotes liver regeneration in mice after partial hepatectomy via activating farnesoid X receptor. Biochemical Pharmacology. 2014;92:289–98. doi: 10.1016/j.bcp.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Tsalik EL, Woods CW. Sepsis redefined: the search for surrogate markers. Int J Antimicrob Agents. 2009;34(4):16–20. doi: 10.1016/S0924-8579(09)70560-6. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Zhang H, Chen P. Sulfur dioxide attenuates sepsis-induced cardiac dysfunction via inhibition of NLRP3 inflammasome activation in rats. Nitric Oxide. 2018;81:11–20. doi: 10.1016/j.niox.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Adhikari A, Martel C, Marette A, et al. Hepatocyte SHP-1 is a critical modulator of inflammation during endotoxemia. Sci Rep. 2017;7(1):2218. doi: 10.1038/s41598-017-02512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaez H, Najafi M, Rameshrad M, et al. AMPK activation by metformin inhibits local innate immune responses in the isolated rat heart by suppression of TLR4 related pathway. Int Immunopharmacol. 2016;40(11):501–7. doi: 10.1016/j.intimp.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh LT, Frey H, Nastase MV, et al. Bimodal role of NADPH oxidases in the regulation of biglycan-triggered IL-1beta synthesis. Matrix Biol. 2016;49:61–81. doi: 10.1016/j.matbio.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lew WY, Bayna E, Dalle Molle E, et al. Myocardial fibrosis induced by exposure to subclinical lipopolysaccharide is associated with decreased miR-29c and enhanced NOX2 expression in mice. PLoS One. 2014;9:e107556. doi: 10.1371/journal.pone.0107556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhirajan RK, Meng S, Chandramoorthy HC, et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. Clin Invest. 2013;123:887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CS, Shin DM, Kim KH, et al. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. Immunol. 2009;182:3696–705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 33.Corre I, Paris F, Huot J, et al. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget. 2017;22:55684–714. doi: 10.18632/oncotarget.18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 35.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22(4):954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 36.Law BYK, Wang MF, Ko BCB. Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2C ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol Cancer Ther. 2010;9:718–30. doi: 10.1158/1535-7163.MCT-09-0700. [DOI] [PubMed] [Google Scholar]
- 37.Hattori T, Nishimura H, Makino B, et al. [Sairei-to inhibits the production of endothelin-1 by nephritic glomeruli (2): Alisols, possible candidates as active compounds]. Nippon Jinzo Gakkai Shi. 1998;40:33–41. [in Japanese] [PubMed] [Google Scholar]
- 38.Matsuda H, Tomohiro N, Yoshikawa M, Kubo M. Anti-complementary activities of methanol extract and terpene components from Alismatis rhizoma (dried rhizome of Alisma Orientale) Biol Pharm Bull. 1998;21:1317–21. doi: 10.1248/bpb.21.1317. [DOI] [PubMed] [Google Scholar]
- 39.Kubo M, Matsuda H, Tomohiro N, Yoshikawa M. Anti-allergic effects of methanol extract and six terpene components from Alismatis rhizome (dried rhizome of Alisma orientale) Biol Pharm Bull. 1007;20:511–16. doi: 10.1248/bpb.20.511. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Zhu P, Wang J, et al. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2α-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25:1080–93. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]