Abstract
Background:
The remarkable success of clinical trials in mineralocorticoid receptor (MR) inhibition in heart failure has driven research on the physiological and pathological role(s) of nonepithelial MR expression. MR is widely expressed in the cardiovascular system and is a major determinant of endothelial function, smooth muscle tone, vascular remodeling, fibrosis, and blood pressure. An important new dimension is the appreciation of the role MR plays in immune cells and target organ damage in the heart, kidney and vasculature, and in the development of insulin resistance.
Summary:
The mechanism for MR activation in tissue injury continues to evolve with the evidence to date suggesting that activation of MR results in a complex repertoire of effects involving both macrophages and T cells. MR is an important transcriptional regulator of macrophage phenotype and function. Another important feature of MR activation is that it can occur even with normal or low aldosterone levels in pathological conditions. Tissue-specific conditional models of MR expression in myeloid cells, endothelial cells, smooth muscle cells and cardiomyocytes have been very informative and have firmly demonstrated a critical role of MR as a key pathophysiologic variable in cardiac hypertrophy, transition to heart failure, adipose inflammation, and atherosclerosis. Finally, the central nervous system activation of MR in permeable regions of the blood–brain barrier may play a role in peripheral inflammation.
Key Message:
Ongoing clinical trials will help clarify the role of MR blockade in conditions, such as atherosclerosis and chronic kidney disease.
Keywords: Macrophage inflammation, Kidney fibrosis, Central nervous system inflammation, Atherosclerosis, Vascular remodeling, Cardiac hypertrophy
Introduction
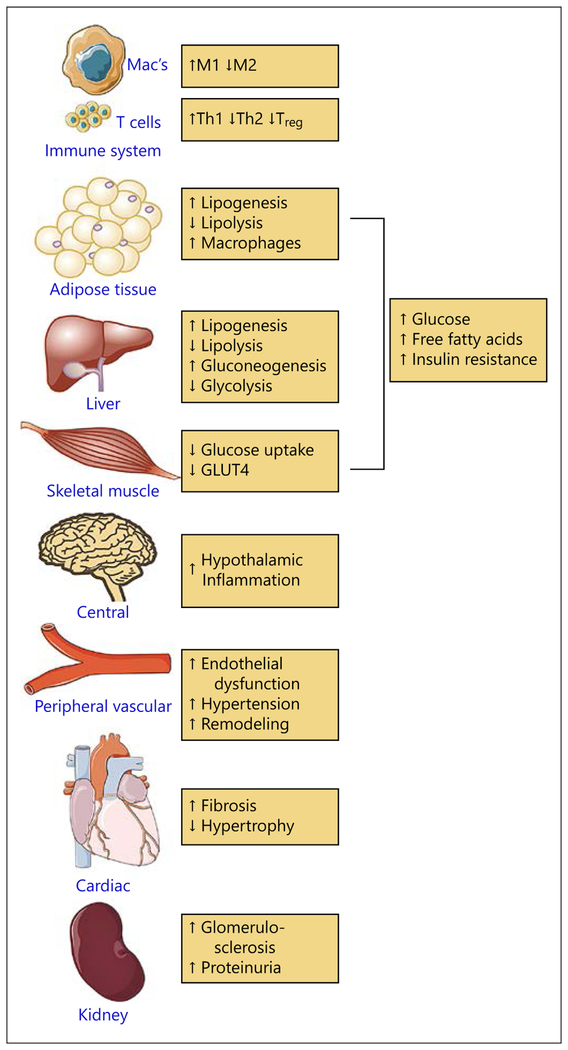
In the 1950s, Selye et al. [1] presciently characterized the effects of aldosterone on nonepithelial tissues and postulated that spironolactone is protective in conditions of aldosterone excess. The cloning of the human miner-alocorticoid receptor (MR) by Arriza et al. [2] exactly 30 years ago, spurred interest in the nonclassical aspects of aldosterone including the role of aldosterone in cardiac remodeling and fibrosis [3]. MRs are members of a superfamily of intracellular ligand-operated steroid receptors that regulate transcription of multiple genes and other transcription factors resulting in a complex repertoire of effects. The remarkable success of clinical trials in MR inhibition through their effects on the cardiovascular system have driven research on the physiological and pathological role(s) of nonepithelial MR expression [4–6]. An important new dimension is the evolving appreciation of the role MR plays in immune cells as it relates to target organ damage (Fig. 1). In this review, we attempt to review the most significant developments related to MR modulation of immune expression and new studies over the last decade that have paved the way for renewed understanding of MR-mediated cardiorenal disease.
Fig. 1.
Mineralocorticoid agonism or elevated aldosterone levels have a deleterious effect on organ systems and cells relevant to cardiometabolic diseases.
MR Structure and Expression
The MR receptor is a 984-amino acid intracytoplasmic receptor divided into 3 domains: the N-terminal domain that regulates transcriptional activity of the receptor; the DNA-binding domain involved in the binding of the specific response element found on the promoter of MR target genes; and finally, a ligand-binding domain responsible for the selectivity of hormone binding. MR also binds to a number of chaperones that play a pivotal role in maintaining MR in an appropriate conformation for ligand binding. Upon hormone binding, the MR dissociates from chaperone proteins, undergoes nuclear translocation, and interacts with numerous molecular partners in a coordinated and sequential manner to ensure appropriate transcriptional regulation. In the nucleus, MR recruits co-regulators (cofactors and/or corepressors) to induce the transactivation and regulation of hundreds of target genes [7]. These genes present a palindromic DNA sequence common for GR and MR called the glucocorticoid response element within their promoter. Although MR is regulated transcriptionally, posttranscriptional mechanisms of regulation are important and include phosphorylation and sumoylation. Very recently, ubiquitination, another posttranslational modification of MR, has been reported [8].
MR Activation/Antagonism and Specificity-Conferring Mechanisms
MR has been shown to bind to aldosterone at low concentrations (high picomolar) and with high affinity. MR also binds with equally high affinity to cortisol, corticosterone, deoxycorticosterone, and progesterone [9]. Since circulating physiologic glucocorticoid levels are ~1,000-fold higher than those of aldosterone on a normal diet, the issue of MR selectivity to aldosterone has been a topic of contention for decades. Studies by Funder and Minireview [9] suggested that 11β-HSD type 2 (11β-HSD2), a high-affinity (nanomolar KM), low-capacity NAD-dependent dehydrogenase is expressed alongside MR, where its activity reduces the availability of glucocorticoids, permitting aldosterone to bind to the MR with relative exclusivity. However, the idea that the presence of 11β-HSD2 is enough to provide unfettered access to aldosterone, through “debulking” cortisol (conversion to cortisone) would require a tremendously efficient system that converts vast excesses of cortisol to cortisone in the area proximal to the MR. This mechanism is not supported by in vivo evidence in the cardiovascular, immune, and central nervous systems. Indeed, the preponderance of evidence suggests little or no 11β-HSD2 activity in the heart, inflammatory cells, and regions of the central nervous system; yet there is extensive evidence to support MR binding in these organs [10]. In addition, in vivo competition studies in adrenal-ectomized animals show high MR selectivity in these organs. For instance, cortisol has ~30% of the apparent affinity of aldosterone in the heart. These studies firmly suggest that specificity-conferring mechanisms, other than pure enzymatic mechanisms are essential for selective aldosterone action. 11β-HSD1, the enzyme that converts inactive cortisone to cortisol in humans, has low affinity for glucocorticoids (micromolar KM) relative to 11β-HSD2, and functions both as an oxidoreductase (transfers electrons from one molecule to another) and as a dehydrogenase. The latter activity is dependent on the supply of NADPH through coupled expression of the enzyme hexose-6-phosphate dehydrogenase. Although there is plentiful evidence that myocardial cells express 11β-HSD1, reactivation of glucocorticoid is apparently limited under physiological conditions both in mice and humans. In an in vivo study in humans, the stable isotope tracer 9, 11, 12, 12-[2H]4-cortisol underwent little metabolism across the human heart [11]. Administration of the MR antagonist canrenoate in the same patients resulted in the elevation of cortisol collected from the coronary sinus, suggesting displacement of endogenous glucocorticoids. These data support the view that the cardiac MR is normally occupied by glucocorticoids rather than by aldosterone. A model that provides an explanation for glucocorticoid-mediated MR signaling in the setting of a protected MR (presence of 11β-HSD2) is the concept that the activity of the enzyme 11β-HSD2 results in a decrease in the NAD/NADH ratio (owing to generation of NADH) which alters the redox state, resulting in blocking activity of MR. There is direct evidence for redox stress/state regulating the activation of other nuclear transactivating factors, and it is likely that similar changes in redox state are operant for cortisol [10]. There are undoubtedly other aspects such as conformation of the ligand-binding interactions and co-regulator recruitment that may contribute to between-ligand (cortisol vs. aldosterone) differentiation in signaling [7, 12].
MR Activation in Kidneys and Cardiovascular Tissues
The mechanism for MR activation in tissue injury continues to evolve and suggests a complex repertoire of effects involving a multitude of mediators that are cell and context dependent. While in both animal models and humans, there is evidence that both plasma and urinary aldosterone concentrations are increased in a variety of cardiometabolic conditions, MR activation may occur in the absence of elevated aldosterone levels [13]. Recently, several studies have suggested that MR activity is also affected by factors other than its ligands including PKA, Rac-1, ubiquitin conjugating enzymes and other factors involved in the regulation of diverse nuclear receptors [14–17]. MR is widely expressed in the cardiovascular system such as in the endothelium, smooth muscle cells, and fibro-blasts, and is a major determinant of endothelial function, smooth muscle tone, vascular remodeling, fibrosis, and blood pressure (BP) [18–25]. Endothelial, vascular smooth muscle, and cardiomyocyte-specific overexpression/deletion studies in animals and studies in humans, support a role for MR activation in promoting vascular oxidative stress, inflammation, proliferation, migration, vasoconstriction, vascular remodeling, and fibrosis [26–34], (online suppl. Table for all online suppl. material, see www.karger.com/doi/10.1159/000480652).
In the kidneys, the classical effects of MR activation are to increase epithelial sodium channel (ENaC) density in the distal convoluted tubule via increased expression and activation of serum and glucocorticoid regulated kinase-1 (SGK1). Phosphorylated SGK1 in turn “inhibits an inhibitor” of ENaC, NEDD4, a protein involved constitutively in the ubiquitination of ENaC [35]. Chronically, SGK1 may also play a role by promoting ENaC transcription through inhibition of H3K79 methyltransferase, which blocks transcription of ENaC. Mineralocorticoid-sensitive inflammation and fibrosis involves the upregulation of the inflammatory transcription factor NFκB, which in turn stimulates the expression of diverse mediators including connective tissue growth factor. SGK1 also inhibits the degradation of the transforming growth factor beta (TGFβ)-dependent transcription factors Smad2/3, further promoting a profibrotic signal [36].
Aldosterone promotes the proliferation of renal fibro-blasts and mesangial cells via transactivation of epidermal growth factor receptor and platelet-derived growth factor receptor, induces myofibroblastic transdifferentiation of mesangial and tubular epithelial cells, and directly stimulates the synthesis of profibrotic cytokines and matrix proteins [37–40]. The profibrotic response of aldosterone at least in animal models, clearly requires sodium. Studies by Shibata et al. [15, 41] have shown that salt can lead to the activation of MR, even in the absence of ligand resulting in a profibrotic response in the kidney and heart. Recent studies have additionally implicated an important role for the immune system in aldosterone-mediated fibrosis and tissue injury. As detailed below, the activation of the immune system appears to be an important mediator of MR-mediated effects and is a requisite for its profibrotic effects.
Role of the MR in Macrophage/Monocytes
MR appears to play a central role in regulating macrophage phenotype and function broadly through transcriptional reprogramming of monocytes/macrophages. Many of the phenotypic effects reported to be regulated by MR may reflect a broad repurposing of cellular function. Thus, while the effects of MR activation/antagonism are reported discretely, they may reflect related and connected effects. In this regard, deletion of MR in myeloid cells (MR knock out or MRKO) has been very useful in ascribing MR-dependent mechanisms in macrophages. Table 1 details the cell-specific and phenotypic effects of conditional tissue-specific deletion of MR in cardiovascular cells, including in myeloid cells.
Table 1.
Lineage-specific MR knockout effects in animal models
| Author, model | Cell-specific effects of MRKO | Phenotype |
|---|---|---|
|
Myeloid MRKO Shen et al. [44], 2016 Uninephrectomized DOCA-salt |
↑ Cardiac M2 macrophage markers PPARy, PDK4, MRC2, CXCL9, ↓ TNFα, MMP12 expression | ↓ Cardiac fibrosis |
| Rickard et al. [93], 2009 Uninephrectomized DOCA-salt |
↓ Cardiac macrophage infiltration, collagen, PAI-1, NADPH oxidase | ↓ Cardiac fibrosis and hypertrophy |
| Usher et al. [94], 2010 Uninephrectomized L-NAME/salt treated |
↓ Peritoneal macrophage TNFα, rantes, IL12, ILIβ, MCP2 expression ↑ Arg1, IL10, Ym1, FIZZ1, F13a1, CCL-17, CCL-7 expression ↓ Cardiac macrophage infiltration ↓ ANP, BNP, collagen III, TGFβ, PAI-1 |
↓ L-NAME/AngII-induced cardiac interstitial fibrosis, aortic fibrosis, and thickening |
| Bienvenu et al. [50], 2012 Uninephrectomized L-NAME/salt treated |
↓ CTGF, collagen III, TNFα expression MRKO made no difference in macrophage infiltration |
↓ Cardiac and large vessel fibrosis |
| Li et al. [49], 2014 AAC mice |
↓ Cardiomyocyte hypertrophy, ANP, βMHC, collagen I/III, CTGF, fibronectin 1, TGFβ1, and TGFβ2 expression, aortic collagen I/III, CTGF staining ↓ Cardiac Nox2, Nox4, p40, p47 ↑ MnSOD expression Rescued AAC-induced cardiac β-oxidation (Acox1, Acadm, and Acadvl) and oxidative phosphorylation (Sdhb, Cox4i1, Atp5j), ↑ Cardiac PPARα and PGCla expression ↓ Cardiac macrophage M1 markers F4/80 and CD68, M1 cytokines TNFα, MIPIβ, COX2, MCP1, and IL6 staining ↓ Cardiac and aortic macrophage infiltration |
⇊ Cardiac fibrosis and hypertrophy ↓ Aortic fibrosis |
| Shen et al. [45], 2017 LDLR null mice |
↓ Plaque necrotic core area, macrophage accumulation, in vitro foam cell formation ↑ Plaque collagen area |
↓ Atherosclerotic lesion area (aorta) |
| Zhang et al. [95], 2017 Lepob/ob |
↑ ERα and ESR1 gene expression ↑ HGF expression ↑ Hepatocyte Met signaling ↑ Insulin sensitivity ↓ Hepatic triglyceride storage and lipogenesis genes - SCD1, Ly6d, and Cidea |
↑ Glucose homeostasis ↓ Hepatic steatosis |
| Sun et al. [47], 2016 Femoral artery wire injury in MRKO mice |
↓ Injury-induced vascular macrophage infiltration and proliferation, smooth muscle proliferation ↓ Injury-induced vascular AP-1, NFκB, SGK1 signaling, IL6, IL1β, ICAM1, MIPlα, MIPIβ, MIP2α, NOS2, MMP9, CXCL1, MCP1, CCR2, CCR4, osteopontin |
↓ Injury-induced intimai hyperplasia and fibrosis |
| Frieler et al. [96], 2011 Focal cerebral ischemia |
↓ Infarct-induced myeloid TNFα, IL1β, MCP1, MIP1α, and IL6 ↑ Myeloid Arg1 and Ym1 |
65% reduction in infarct volume ↓ Infarct-induced microglial activation in the ischemic core |
|
Endothelial MRKO Rickard et al. [97], 2014 Uninephrectomized DOCA-salt or aldosterone Duration: 8 weeks |
↓ Cardiac CCR5 expression at 8 days ↓ Cardiac CTGF expression at 8 weeks ↓ DOCA-salt-induced cardiac macrophage infiltration and fibrosis at 8 weeks Eplerenone inhibited aldosterone-induced ICAM1 and CTGF expression in HUVEC cells |
↓ Aldo-induced endothelial dysfunction in aorta but not mesenteric resistance vessels, as measured by Ach-induced relaxation |
| Schäfer et al. [98], 2013 ND or a HFD and Aldosterone infused mice |
↓ Diet-induced endothelial dysfunction ↓ Aldo-induced COX1 expression Indomethacin reduced aldosterone infusion-induced endothelial dysfunction to same extent as MRKO |
EC MR−/− had no effect on WAT inflammatory state or glucose tolerance of obese or aldo infused mice EC MR−/− prevented HFD and aldo infusion-induced endothelial dysfunction |
| Jia et al. [99], 2015 Control vs. high fat and carbohydrate diet (western; WD) |
↓ WD-induced cardiomyocyte stiffness (by atomic force microscopy), cardiac TGFβ1, and phospho-Smad2/3, collagen I, CTGF, and fibronectin immunostaining ↓ WD-induced cardiac 3-nitrotyrosine staining ↓ WD-induced cardiac pS6K, pIRS1, pERK1/2, M1 markers MCP1, IL17, CD11b immunostaining CD206 and IL1O immunostaining |
↓ WD-induced diastolic dysfunction (evaluated by relaxation time and Doppler), cardiac interstitial fibrosis and hypertrophy |
| Jia et al. [100], 2016 Chow or western diet for 16 weeks |
KO attenuated WD-induced aortic endothelial decreases in p-AKT and p-eNOS ↑ Mesenteric artery flow-induced vasodilation ↓ WD-induced aortic endothelial 3-nitrotyrosine immunostaining ↓ WD-induced aortic pERK immunoblot, and osteopontin, FGF23 immunostaining ↑ M2: M1 marker ratios (↓MI markers CD86, and CD11c; ↑M2 markers CD206 and IL10) ↓ WD-induced aortic ENaC expression |
↓WD-induced aortic stiffness (evaluated by pulse wave velocity and atomic force microscopy), aortic endothelial dysfunction, aortic medial thickening, and fibrosis |
|
VSMC MRKO McCurley et al. [101], 2012 Tamoxifen-induced MRf/f/SMA-Cre-ERT2+ and tamoxifen-induced MRf/f/SMA-Cre-ERT2- littermate controls with no alterations in feeding or growth |
↓ Mesenteric artery L-type Ca-channel Cav1.2 expression ↓ AngII-induced ROS production |
Age-dependent decrease in systolic blood pressure, becoming significant at 7 months of age ↓ Age-related cardiac hypertrophy ↓ Mesenteric artery myogenic tone ↓ AngII-induced pressor response |
| Amador et al. [102], 2016 CsA-induced nephrotoxicity model |
↓ CsA-induced NGAL expression, marker of tubular injury ↓ CsA-induced pMLCKMLCK, pMLC2:MLC2 ratios, markers of SMC contractility ↓ CsA-induced L-type Ca-channel Cav1.2 activation |
SMC MR inactivation but not EC MR inactivation prevents cyclosporine-induced uremia, creatininemia, and tubular vacuolization ↓ Renal vasculature Angll and KCl-induced contractile response |
| Galmiche et al. [103], 2014 NAS hypertension model |
↓ NAS-induced carotid a5-integrin expression | ↓ NAS-induced carotid stiffness No changes in vascular structure |
| Pruthi et al. [104], 2014 Wire-induced carotid injury model and aldosterone enhanced vascular fibrosis model |
↓ Wire injury-induced VEGFR1 expression ↓ Aldo-induced P1GF expression |
Prevented aldosterone-induced 79% increase in SMC proliferation post wire injury Prevents aldosterone-induced vascular fibrosis post wire injury llnjury-induced medial hypertrophy |
|
Cardiomyocyte MRKO Rickard et al. [105], 2012 Uninephrectomized DOCA-salt |
↑ Cardiac MMP9: TIMP-1 mRNA expression ratio ↓ DOCA-salt-induced cardiac PAI-1, CCR5, Nox2, p22Phox, TGFβ1, VEGF, VEGFR2, MCP1, CD14, CD81 expression ↓ DOCA-salt-induced cardiac macrophage infiltration, CD45+ leukocytes, CD8+ T cells |
↓ DOCA-salt-induced cardiac fibrosis, positive inotropic state as assessed by Langendorff apparatus measured pressure and contraction/relaxation times |
| Fraccarollo et al. [106], 2011 Cardiomyocytes LCA ligation model |
↓ MI-induced collagen, ACE, CTGF, fibronectin, periostin, vimentin expression ↓ MI-induced NADPH oxidase subunits Nox2, Nox4, and chronic Mi-induced mitochondrial ROS production ↑ Acute MI-induced NF-κB activation ↓ IκBα and cardiomyocyte apoptosis (TUNEL assay) ↑ Neutrophil and macrophage infiltration 1 day post-acute MI |
↓ Post-MI rightward shift in PV loop, infarct area expansion, Post-MI cardiac hypertrophy, and attenuatation of progressive LV dilation ↑ Scar thickness, post-MI ejection fraction, post-MI capillary density at 1 day post-acute MI |
| Lother et al. [107], 2011 Cardiomyocytes Chronic left ventricular pressure overload by TAC model |
↑ TAC-induced ANP, βMHC gene expression ↑ ERK1/2 phosphorylation and ↓ TAC-induced increase in SGK1 mRNA expression |
Prevented TAC-induced ventricular dilation Prevented TAC-induced LV wall stress Prevented TAC-induced decline in ejection fraction TAC led to cardiac fibrosis and was unaffected by MRKO |
|
CD4+ T cell MRKO Li et al. [108], 2017 T cells AAC mouse model |
↓ Cardiomyocyte hypertrophy ↓ Cardiac CD11b+Ly6C+, CD11b+Ly6C−, Ly6Chi, macrophage, neutrophil, CD4+ & CD8+ T cell, CD4+CD69+, CD8+CD69+, CD4+CD44hiCD62low, CD8+CD44hiCD62low T cell infiltration, ANP, βMHC, collagen I/III, CTGF, TGFβ1 expression ↓ Anti-CD3 stimulated IL2, IFNγ, and IL6 |
↓ AAC-induced cardiac hypertrophy, fibrosis, LV dilation Improved ejection fraction |
| Sun et al. [66], 2017 T cells Angll-induced hypertensive mouse model |
↓ AngII-induced renal CD11b macrophage, CD4+ and CD8+, CD4+IFNγ+, CD8+IFNγ+ T cell infiltration, ↓ NGAL, osteopontin, MCP1, and VCAM1 1 AngII-induced aortic macrophage infiltration, CD4+ and CD8+, CD4+IFNγ+, and CD8+IFNγ+ T cell infiltration ↓ RANTES & MCP1 |
↓ AngII-induced hypertension, glomerular hypertrophy, renal fibrosis, albuminuria aortic fibrosis, endothelial dysfunction |
AAC, abdominal aortic constriction; Ach, acetylcholine; AngII, angiotensin II; ACE, angiotensin I converting enzyme (peptidyl-dipeptidase A) 1; Aldo, aldosterone; ANP, natriuretic peptide precursor type A; Arg1, arginase 1; BNP, natriuretic peptide precursor type B/brain natriuretic peptide; Cav1.2, calcium channel, voltage-dependent, L type, alpha 1C subunit; CCL, chemokine (C-C motif) ligand; Cidea, cell death-inducing DNA fragmentation factor, alpha subunit-like effector A; COX, cytochrome c oxidase; CTGF, connective tissue growth factor; CsA, cyclosporine A; DOCA, deoxycorticosterone acetate; CXCL, chemokine (C-X-C motif) ligand; EC, endothelial cell; ENaC, endothelial sodium channel; ER, estrogen receptor; ERK, extracellular regulated MAP kinase; F13a1, coagulation factor XIII, A1 subunit; ESR1, estrogen receptor 1; FIZZ1, found in inflammatory zone 1; HFD, high fat diet; HGF, hepatocyte growth factor; IFNγ, interferon gamma; IL, interleukin; L-NAME, L-NG-nitroarginine methyl ester; MMP, matrix metalloproteinase; MnSOD, manganese superoxide dismutase; MCP1, chemokine (C-C motif) ligand 2; M2, macrophage phenotype 2 – alternatively activated; βMHC, beta myosin heavy chain; MIP1β, chemokine (C-C motif) ligand 4 (CCL4); NGAL, lipocalin 2; MRC2, mannose receptor, C type 2; NAS, Nephrectomy–aldosterone–salt; ND, normal chow diet; Nox2, NADPH oxidase 2/cytochrome b-245, beta polypeptide; NOX4, NADPH oxidase 4; PAI-1, plasminogen activator inhibitor-1; RANTES, chemokine (C-C motif) ligand 5; SGK1, serum/glucocorticoid regulated kinase 1; TAC, transverse aortic constriction; ROS, reactive oxygen species; SCD1, stearoyl-Coenzyme A desaturase 1; TNF, tumor necrosis factor; TGF, transforming growth factor; TIMP1, tissue inhibitor of metalloproteinase 1; VCAM1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; VEGFR, endothelial growth factor receptor; VSMC, vascular smooth muscle cell; WD, western diet.
Transcriptional Reprogramming of Macrophages
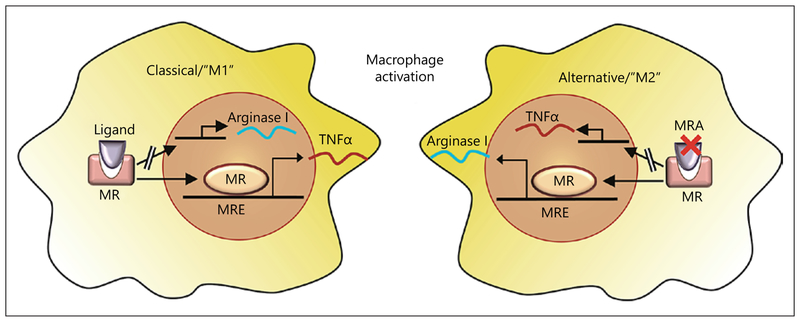
PPARγ, PPARδ, and KLF4 are major regulators of alternate activation and are required for the maintenance of alternatively activated macrophages (Fig. 2) [42]. Macrophages in mice can be rendered pro-inflammatory (M1) in the presence of aldosterone, an effect prevented by MR antagonism which favors an anti-inflammatory, alternatively activated (M2) phenotype [43]. Conversely, MRKO in myeloid cells recapitulated the effects of MR antagonism, by shifting the phenotype to M2 (alternatively activated macrophage) and downregulating proinflammatory and profibrotic genes (TGFβ and PAI-1). In vivo, myeloid MRKO reduced aortic and cardiac macrophage recruitment, cardiac hypertrophy, fibrosis, and fetal gene reprogramming in response to AngII and L-NAME (model of MR activation) suggesting that myeloid MR is crucial to adverse, fibrotic cardiovascular remodeling [43]. Gene expression analysis revealed significant similarity between MRKO and PPARγ activation. These findings were similar to another study investigating macrophages from the heart in myeloid MRKO and wild-type (WT) animals treated with vehicle or DOCA/salt. A pro-inflammatory and profibrotic profile in response to DOCA salt was prevented in the heart of mice with myeloid MR-null macrophages [44]. Further, MRKO in macrophages diminished the activation of both AP1 and NFκB in restenosis models with effects dependent on SGK1, consistent with other studies [45, 46].
Fig. 2.
Mineralocorticoid receptor agonism (ligand binding, left side) increases the classical activation of macrophages, while antagonism MRA promotes an alternative activation (right side).
Regulation of Myeloid Inflammatory Numbers and Chemotaxis
MRKO macrophages demonstrate reduced migration de novo in response to a chemokine gradient with restenotic injury, resulting in lower macrophage content in MRKO animals vs. WT [47]. Corning Transwell® assays with conditioned media derived from LPS-stimulated MRKO macrophages induced markedly less migration of vascular smooth muscle cells and lower expression of pro-inflammatory cytokines, compared to conditional media from control macrophages [47]. Several in vivo studies involving hypertension and stroke models have demonstrated a reduction in macrophage content in mice transplanted with MRKO myeloid cells [43, 48]. Similarly, in a study of cardiac hypertrophy (transaortic constriction), a reduction in macrophages in the heart with myeloid MR deletion was noted [49]. However, two studies using a severe hypertension model (unilateral nephrectomy with 0.9% salt and L-NAME) and a uninephrectomized mouse model of DOCA salt excess, demonstrated no change in the number of tissue macrophages in the heart [44, 50]. In many of these studies, macrophage recruitment seems to play a critical role in BP response, with evidence suggesting a CCL2-dependent movement of myeloid cells to the heart required for myeloid MR-dependent effects [51]. However, all studies demonstrated rather consistent reduction in tissue fibrosis and inflammation suggesting that MR deficiency critically regulates fibrosis [43, 47, 48, 50].
Regulation of ROS and Inflammatory Kinases
Aldosterone has been shown to activate components of NAPDH oxidase in various cell types [27, 28, 52–54], and reductions in NADPH oxidase have been noted in response to MR blockade [54–56]. The degree to which these are direct, nongenomic effects is difficult to understand and is currently uncertain [57]. Rac1, a Rho family small GTPase, is a novel modulator of MR activity and demonstrated the pathological role of Rac1-mediated MR activation in the kidney and in salt-sensitive hyper-tension [58]. In prior studies, overexpression of constitutively active mutant Rac1 in rat cardiomyocytes promoted nuclear accumulation of MR and increased MR-dependent transcriptional activity regardless of the ligand level implicating a potential contribution of Rac1-MR signaling in cardiac diseases [16, 41]. Rac1 associates with Nox isoforms such as Nox4 (present in endothelial cells) rather than Nox2 (present in macrophages and neutrophils), at least in the myocardium and contributes to heart failure in response to pressure overload hypertrophy. Thus, the activation of Rac1 and subsequent activation of MR in addition to ROS generation through Nox4 may contribute to tissue injury and transition to heart failure [41]. cJun N-terminal kinases (JNK) may also be a downstream target of MR, as bone marrow-derived macrophages exposed to LPS (classic type 1 proinflammatory mediator) significantly increased the phosphorylation of JNK, while phosphorylation of JNK is attenuated in MR-null bone marrow-derived macrophages. Analysis of other MAPK pathways such as p38 and ERK1/2 showed equivalent phosphorylation. In this study there were no differences in phosphorylation of the NFκB pathway and IκBα.
Evidence for Inflammasome Activation in Myeloid Cells in Response to Aldosterone and MR Involvement
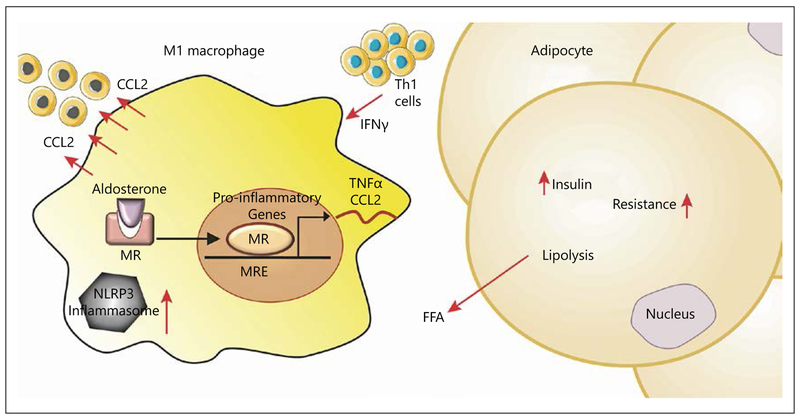
Chronic inflammation caused by inflammasome activation is involved in many diseases including atherosclerosis, diabetes, and obesity (Fig. 3). Recently, a role for inflammasome activation in kidney injury via MR activation has been proposed; aldosterone stimulates various components of the inflammasome complex and products of inflammasome activation (IL18) lead to podocyte injury while blockade of MR reverses these effects [59]. Mice deficient in apoptosis-associated speck-like protein (ASC) had reduced renal fibrosis and inflammation without affecting macrophage numbers. Bone marrow transplantation using ASC-deficient mice marrow reduced inflammasome activation, implicating myeloid cell-derived ASC in tubulointerstitial damage and subsequent fibrotic changes in the kidney [60]. The mechanism of activation was attributed to mitochondrial-driven ROS as mitochondrial-directed antioxidant mito-TEMPO interrupted caspase activation in response to aldosterone in cultured macrophages [60]. A model of anti-glomerular basement membrane glomerulonephritis (anti-GBM GN) was used to interrogate the contribution of podocyte versus myeloid MR. The absence of MR in podocytes did not reduce immune injury in anti-GBM GN. In contrast, injury glomerular crescents, myofibroblast accumulation, and gene expression of profibrotic molecules (COL1A1, FN1, PAI-1) were all decreased in MyMRKO mice versus WT [61].
Fig. 3.
Aldosterone stimulation of adipose tissue macrophages may potentiate local tissue inflammation exacerbating insulin resistance, diabetes, and atherosclerotic processes.
Role of Central Nervous System MR in Regulation of Peripheral Inflammation
MR activation in the brain has been linked to sympathetic hyperactivity and an increase in peripheral tissue aldosterone levels, while central MR blockade attenuates sympathetic hyperactivity [62, 63]. In an interesting study, the time course of macrophage infiltration and apoptosis in the heart in response to central MR blockade (intracerebroventricular infusion of eplerenone, 5 μg/day) was evaluated post-myocardial infarction. Central MR blockade significantly decreased CD80-positive pro-inflammatory M1 macrophages and increased CD163-positive anti-inflammatory M2 macrophages in the infarct. Central MR blockade also reduced apoptosis of myocytes by 40–50% in the peri-infarct zone [64].
Role of the MR in T Cells
T lymphocytes play an important role in target organ damage in hypertension and atherosclerosis. Indeed, MR in T cells is critical in mediating fibrosis in the heart and kidney. T cell deficient in MR reduces ventricular remodeling in hypertension caused by trans-aortic constriction. This was associated with reduced T cell activation markers and inflammation in heart. Activation of MR resulting in increased Th17 T cells could contribute to cardiac fibrosis. Conversely, treatment with IL-17 blocking antibodies prevented DOCA/salt induced cardiac and renal fibrosis in rats [65]. Recently, it has also been shown that MR may interact with a critical transcription factor in T cells, NFAT1, and activator protein-1 to control interferon gamma in T cells and regulate BP and target organ damage in an AngII-infusion murine model [66]. MRA (mineralocorticoid receptor antagonism) by eplerenone and T cell-specific MR ablation (TMRKO) in mice resulted in reduced abdominal aortic constriction (AAC)-induced cardiac hypertrophy, with reduced measures of cardiac fibrosis (% fibrotic area, βMHC, collagen I/III, connective tissue growth factor, and TGFβ1; Table 1) [67]. Measures of cardiac function (e.g., LV end-systolic volume) were partially preserved in TMRKO-AAC versus WT-AAC and LV-dilation/LVH was attenuated. Post-ACC cardiac neutrophil and monocyte/macrophage (CD11b+Ly6G+; CD11b+Ly6Chi respectively) content was dramatically lower in TMRKO mice.
Role of the MR and Effect of MR Antagonism in the Treatment of Cardiovascular and Renal Diseases
Pharmacological and clinical studies over >20 years have defined the importance of the MR in hypertension and heart failure which will not be discussed here. There are studies that demonstrated an impact of MRA in patients with risk factors, with improvement in surrogate outcomes related to LV mass and hypertrophy (Table 2).
Table 2.
Cardiovascular effects of MRA in humans
| Author, study cohorts | Patient population | End point, measurements | Results |
|---|---|---|---|
| Pitt et al. [109], 2003 n = 202 Duration: 9 months |
Patients with left ventricular hypertrophy and hypertension (mean age 58 ± 12 years) Eplerenone 200 mg/day; enalapril 40 mg/day; eplerenone 200 mg/day, and enalapril 10 mg/day | Change in left ventricular mass (MRI), changes in systolic and diastolic BP | Eplerenone significantly reduced LV mass from baseline (p = 0.001); eplerenone-enalapril combination was more effective than eplerenone alone (p = 0.007) Eplerenone significantly reduced systolic and diastolic BP from baseline and more effectively than eplerenone alone (p = 0.048) |
| Kosmala et al. [69], 2013 n = 113 Duration: 6 months |
Patients with BMI ≥30 without comorbidities with impaired early diastolic mitral annular velocity (mean age 58 ± 8 years) Spironolactone 25 mg/day; placebo | LV systolic and diastolic function, myocardial reflectivity and serological fibrosis markers (procollagen type III N-terminal propeptide [PIIINP] and procollagen type I C-terminal propeptide [PICP]) | Significant reduction in PICP (p = 0.04), E/e’ ratio (p = 0.005), LV systolic function (p = 0.02), and myocardial reflectivity (p = 0.02) |
| Montalescot et al. [110], 2014 n = 1,012 Duration: 10.5 months |
Patients with acute STEMI without a history of HF receiving standard therapy Eplerenone 25–50 mg/day in addition to standard therapy; versus standard therapy control (mean age 58 ± 11 years) | Composite of CV mortality, rehospitalization or extended initial hospital stay due to diagnosis ofHF, sustained ventricular tachycardia or fibrillation, LVEF ≤40% or elevated BNP/NT-proBNP levels (primary end point) | The incidence of primary end point was reduced by 42% in the eplerenone group relative to control (p < 0.0001) Primary end point difference was driven by increased BNP/NT-proBNP in control group Adverse event rates were similar in both groups |
| Garg et al. [111], 2014 n = 64 Duration: 6 months |
Patients with diabetes on chronic ACE inhibition (enalapril 20 mg/day, mean age 55 years) Spironolactone 25 mg/day; HCTZ 12.5 mg/day; placebo | Coronary flow reserve (CFR) was assessed by cardiac PET at baseline, and at the end of treatment | Spironolactone significantly improved CFR compared to control (p = 0.04) |
|
Renal Effects of MRA Epstein et al. [112], 2006 n = 268 Duration: 12 weeks |
Patients with diabetes mellitus and UACR ≥ on enalapril (50 mg/day) (mean age of 59 years) Eplerenone 50 mg/day, 100 mg/day or placebo on top of Enalapril therapy | Percentage change from baseline in UACR and incidence of hyperkalemia (primary end points) | By week 12, UACR was reduced 7.4% in placebo group compared to 41.0% in eplerenone (50 mg) group (p = 0.001) and 48.4% in eplerenone 100 mg group (p = 0.001). No significant difference in the incidences of sustained and severe hyperkalemia |
| Bianchi et al. [113], 2006 n = 165 Duration: 1 year |
Patients with CKD receiving ACE inhibitors and/or ARBs (mean age 59 years) Spironolactone 25 mg/day on top background therapy compared with background treatment alone | Proteinuria and eGFR | Spironolactone treatment significantly reduced proteinuria from 2.10 to 0.89 g/g creatinine (p < 0.001), and did not change in patients receiving ACE inhibitors and ARB alone By the end of 1 year, the monthly rate of the decrease in eGFR from baseline was lower in patients treated with spironolactone than in controls |
| Furumatsu et al. [114], 2008 n = 32 Duration: 1 year Trichlorome thiazide 1 mg/day or furosemide 20 mg/day in addition to enalapril 5 mg/day and losartan 50 mg/day |
Patients with nondiabetic nephropathy and proteinuria >0.5 mg/day after receiving an ACE inhibitor and ARB in combination for more than 12 weeks (mean age 52 ± 3 years) Spironolactone 25 mg/day in addition to enalapril 5 mg/day and losartan 50 mg/day (triple blockade) | Proteinuria, urinary type IV collagen level | Urinary protein level decreased by 58% in triple blockade group compared with no change in the control group (p < 0.05) Urinary collagen IV levels decreased by 40% from baseline in triple blockade group compared with no change in the control group (p < 0.05) |
| Mehdi et al. [115], 2009 n = 81 Duration: 48 weeks |
Patients with diabetes mellitus, hypertension and UACR ≥300 mg/g receiving Lisinopril 80 mg/day Spironolactone 25 mg/day; losartan 100 mg/day; placebo | Urine albumin to creatinine ratio, clinic and ambulatory BP, creatinine clearance, and glycemic control | Spironolactone group UACR decreased 34.0% (p = 0.007 vs. placebo) Blood pressure, creatinine clearance, and glycemic control did not change significantly between groups |
| Boesby et al. [116], 2011 n = 40 Duration: 8 weeks |
Patients with nondiabetic CKD and urinary albumin excretion greater than 300 mg/day Eplerenone 25–50 mg/day Mean age 45 years | 24-h urinary albumin excretion, BP, plasma potassium, creatinine clearance | Eplerenone treatment lowered mean urinary albumin excretion by 14% (p = 0.008) |
| Ando et al. [117], 2014 n = 304 Duration: 1 year |
Hypertensive patients with albuminuria (UACR = 30–599 mg/g), eGFR >50 mL/min who had received ACE inhibitor or ARB or both for ≥8 weeks Eplerenone 50 mg/day vs. placebo | Mean percent change in UACR | UACR decreased by 27.6% in eplerenone treatment group (p = 0.022 vs. placebo) |
| Matsumoto et al. [118], 2014 n = 309 Duration: 3 years |
Patients with oligo-anuria undergoing hemodialysis (mean age 67 years ± 12 years) Spironolactone, 25 mg/day or placebo | Composite of death from CV causes and hospitalization for CV causes (primary outcome), and death from all causes (secondary outcome) | Spironolactone decreased the rate of incidence of primary outcome by 62% relative to control (p = 0.016), and of the secondary outcome by 67% (p = 0.003) |
| Bakris et al. [91], 2014 n = 821 Duration: 90 days |
Patients with diabetes and high or very high albuminuria receiving ACE inhibition or ARB (mean age 64 years) Finerenone 1.25–20 mg in graded dose increments (7 different doses) or placebo | Ratio of urinary albumin-creatinine ratio (UACR) at day 90 to baseline | UACR was reduced relative to baseline at each dosage: 7.5 mg/day, 0.79 (p = 0.004) 10 mg/day, 0.76 (p = 0.001) 15 mg/day, 0.67 (p < 0.001) 20 mg/day, 0.62 (p < 0.001) |
UACR, urine albumin creatinine ratio; ACEI, angiotensin converting enzyme inhibitor; LVEF, left ventricular ejection fraction; BNP, brain natriuretic peptide; PET, positron emission tomography.
Effect of MRA in Cardiac Hypertrophy, Fibrosis, and Diastolic Dysfunction
Online supplementary Table details studies involving MRA that have demonstrated efficacy in reducing fibrosis and hypertrophy in a variety of animal models irrespective of aldosterone levels. A consistent effect of MRA is its impact on fibrosis and reduction of left ventricular hypertrophy. In humans, MRA reduced LVH in patients on top of ACE inhibition [68]. While these effects may rely on BP reduction, data from animal models seem to support an effect that may occur independently of BP. A small study in obese patients demonstrated that MRA (spironolactone) for 6 months can improve diastolic dys-function, subclinical markers of systolic dysfunction (global longitudinal strain), a surrogate marker of myocardial fibrosis (integrated backscatter, echo) and circulating markers of fibrosis (PICP) [69]. Although results from the Treatment of Preserved Cardiac Function Heart Failure with an aldosterone antagonist trial were negative, subset analysis suggests benefits in patients with evidence of definitive heart failure who were medication compliant [70–72].
Effect of MRA in Atherosclerosis
Proof-of-concept experiments from our group using eplerenone in a rabbit model of atherosclerosis provided one of the first lines of evidence that MRA may improve vascular function and redox stress independent of aldosterone levels. New Zealand white male rabbits were fed 1% cholesterol chow (HL) or normal chow for 6 weeks to induce endothelial dysfunction and then randomized to receive eplerenone or placebo (100 mg/kg) for 6 additional weeks [56]. Eplerenone normalized peak endothelium-dependent relaxation and reduced O2– in the aorta of high cholesterol fed animals. A number of studies have demonstrated an impact of MRA in reducing atherosclerosis in mouse models, including reduction of inflammatory cell infiltration, increased M2 markers, smooth muscle proliferation, and reduced pro-inflammatory cytokines in plaques (online suppl. Table). Studies in monkeys have also demonstrated important effects of eplerenone in reducing aortic intimal volume (intravascular ultrasound) and improving acetylcholine-induced vasorelaxation [73]. In models of experimental thrombosis, aldosterone enhances thrombosis while spironolactone reverses this effect [74]. In recent studies, LDLR–/– chimeric mice with bone marrow cells from floxed (control) mice or from myeloid MR–/– demonstrated reduced atherogenesis, suggesting that MR in myeloid cells likely promotes atherogenesis. Further chimeric ApoE–/– mice with myeloid MRKO also exhibited reduced atherogenesis in response to AngII, an effect mediated in part by reduced foam cell formation and enhanced cholesterol efflux [45].
While the role of MRA in secondary prevention in patients with post-myocardial infarction and symptoms of heart failure is well known, there are data suggesting that use of MRA early post-MI in patients with STEMI and LV dysfunction may be beneficial [75]. In the REMINDER study (n = 1,012), in patients with STEMI without HF, eplerenone reduced the composite end point of CV mortality, rehospitalization, extended hospital stay, due to HF, sustained ventricular tachycardia or fibrillation, ejection fraction ≤40%, or elevated BNP/NT-proBNP at 1 month. The end point was primarily driven by persistent elevation of BNP/NT-ProBNP in the placebo group. In contrast to earlier studies such as EMPHASIS-HF, this was a low risk population without HF or low EF with a low event rate (0.4% morality rate through the trial). Further the drug was administered early on after presentation, with the first dose of study drug administered within 24 h of the onset of symptoms of acute MI and preferably within 12 h. There is limited data supporting a role for aldosterone in the progression of atherosclerosis. In human studies, polymorphisms of the aldosterone synthase gene (Cyp11β2) have been associated with plaque size on MRI. Plasma aldosterone levels have been associated with nonfatal cardiovascular events and CV death. In a study of 848 patients, plasma aldosterone was the only independent predictor of plaque progression (carotid ultrasound) in the first 2 years of the study [76].
Effect of MRA on Proteinuria and Progression of Chronic Kidney Disease
The beneficial impact of renin-angiotensin-aldosterone system (RAS) blockade with ACE-I and ARB in both diabetic and nondiabetic chronic kidney disease (CKD) has been demonstrated in multiple animal models and human studies (online suppl. Table). Two previously published meta-analysis studies published initially in 2009 and updated in 2014 demonstrated that the addition of MRA to RAS blockade reduced BP and proteinuria in CKD [77, 78]. An updated meta-analysis that included previously unpublished data as well as including data from 3 studies which were not considered in the previous meta-analysis supported previous findings [79]. A total of 19 trials (1,646 patients) were included, of which 8 were done in patients with diabetic nephropathy. Fourteen (889 patients) compared spironolactone plus ACE-I or ARB with ACE-I or ARB alone, and 5 trials (757 patients) compared eplerenone plus ACE-I or ARB to ACE-I or ARB alone. The follow-up period of included trials was <1 year and the mean baseline eGFR was >35 mL/min/1.73 m2, therefore the impact of addition of MRA to RAS blockade on long-term renal outcomes or mortality in the later stages of CKD cannot be evaluated. In random effects meta-analysis, addition of MRA to RAS inhibitors resulted in a reduction in systolic BP from baseline of −5.7 and diastolic BP of −1.7 mm Hg, respectively. The GFR fell by −3.2 mL/min/1.73 m2. MR antagonism reduced the weighted mean protein/albumin excretion by 38.7%. MRA was associated with 3-fold increased risk of hyper-kalemia above the predefined trial limit. Diabetic CKD patients, however, were not at a greater risk of developing hyperkalemia than patients with CKD of alternative etiology (p = 0.38). Number needed to harm for 1 year of treatment, calculated from trials reporting at least one case of hyperkalemia, was 10 (95% CI 5–27). The addition of MRA to RAS blockers led to a moderate increase from baseline potassium compared to ACE-I and/or ARB alone, both at end-of-trial visit (0.19 mmol/L [95% CI 0.07–0.31]; 16 trials; n = 1,356; I2 = 83.8%).
MRA and Insulin Resistance and Type 2 Diabetes
A number of reviews have already detailed in vitro, experimental and human evidence linking aldosterone/MR activation with IR [80–82]. The visceral adipose RAS system synthesizes aldosterone, expresses MR, and predicts IR in both humans and animal models [82]. Adipocyte overexpression of MR results in metabolic syndrome and enhanced vascular contractility, and suggests an independent contribution outside of MR in inflammatory cells in adipose [83]. In patients with nondiabetic stages 2–5 CKD, treatment with spironolactone ameliorated insulin resistance. In the same study, insulin resistance in nephrectomized rats was improved with spironolactone presumably via adipose overexpression of the rate limiting enzyme for aldosterone, CYP11β2 and the downstream effector of MR, SGK-1 [84]. Spironolactone has also been shown to prevent insulin resistance in response to diuretics [85]. However, treatment of individuals with uncomplicated obesity over 6 weeks with spironolactone 50 mg, did not appear to improve insulin sensitivity index assessed by Matsuda method. It is possible that this study was performed in metabolically healthy obesity, although the insulin sensitivity index was <5 indicating potential insulin resistance [86]. MR activation may affect IR through multiple mechanisms that include attenuation of insulin signaling in the heart, vasculature, and skeletal muscle. This may include impairment of expression of insulin receptor and substrate, decreased GLUT4 expression, abnormal phosphorylation of IRS, and activation of multiple stress kinases downstream of insulin receptor/IRS leading to the attenuation of insulin signaling [87]. Recently, caveolin-1 appears to be an additional mediator of MR action. Caveolin-1 knockout mice exhibit features of insulin resistance and oxidative stress with the effects being ameliorated by MR blockade. In humans, individuals with a specific mutation of caveolin-1 also appear to be more insulin-resistant [88].
Conclusion
Based on the central role MR plays in the pathogenesis of target organ damage, there is optimism that the use of MRA could benefit patients with a variety of cardiometabolic diseases to prevent complications. The advantages of MRA use for a broad spectrum of patients are far beyond theoretical and are supported by a vast body of data over the last 5 decades. However, there are challenges in the use of these agents. The identification of patients who may truly benefit from MRA is an important issue, as demonstrated in Treatment of Preserved Cardiac Function Heart Failure [71, 89]. Additionally, hyperkalemia is a concern, particularly in patients with advanced CKD. Whether the use of nonsteroidal MRA’s can minimize hyperkalemia, while allowing continued benefit of MRA is an exciting area of investigation. Quantitative whole-body labeling studies with [14C]-labeled finerenone, a novel nonsteroidal MRA, show equal distribution in heart and kidney tissues in contrast to disproportionate renal deposition for steroidal MRAs such as eplerenone or spironolactone, an aspect believed to be important in the lower incidence of hyperkalemia despite comparable IC50 (24 nM for spironolactone vs. 18 nM for finerenone) [90]. Different dose strengths of finerenone have been investigated in 823 randomized patients with type 2 diabetes mellitus (T2DM) and diabetic kidney disease receiving standard of care (i.e., ACEIs/ARBs) and either once-daily finerenone or placebo [91]. FIGARO-DKD (NCT2545049, n = 6,400) and FIDELIO-DKD (NCT2540993, n = 4,800) are 2 randomized, double-blind, placebo-controlled, parallel-group, multicenter, phase 3 studies that investigate the safety and efficacy of finerenone in the reduction of cardiovascular morbidity and mortality, and progression of CKD in subjects with T2DM and diabetic kidney disease. Apararenone, a novel nonsteroidal MRA (MT-3995), is being tested in nonalcoholic steatohepatitis (NCT2923154) and in diabetic nephropathy (NCT2676401). The Mineralocorticoid Receptor Antagonism in Diabetic Atherosclerosis study will test the utility of MR antagonism in patients with T2DM with CKD, at high risk for cardiovascular complications [92]. The co-primary efficacy end point will be percentage change in total atheroma volume in thoracic aorta and left ventricular mass at 52 weeks in patients treated with spironolactone versus placebo. Secondary outcomes include 24-h mean systolic BP, central aortic BP, and insulin resistance at 6 weeks. A novel measure in the study will be changes in candidate miRNAs that regulate expression of NR3C2 (MR gene) as well as measuring monocyte/macrophage polarization in response to therapy with spironolactone. These studies may extend the utility of MRA and make these agents attractive adjuncts to statins in the prevention of cardiometabolic complications.
Supplementary Material
Footnotes
Disclosure Statement
The authors would like to acknowledge the support of R01HL127422–02 awarded to Dr. Rajagopalan in this grant.
References
- 1.Selye H: Anticortisol action of aldosterone. Science 1955;121:368–369. [DOI] [PubMed] [Google Scholar]
- 2.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, et al. : Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 1987;237:268–275. [DOI] [PubMed] [Google Scholar]
- 3.Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT: Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 1990;67:1355–1364. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. : The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, McMurray JJ V, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. : EMPHASIS-HF (eplerenone CHF). N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 6.Pitt B, Stier CT Jr, Rajagopalan S: Mineralocorticoid receptor blockade: new insights into the mechanism of action in patients with cardiovascular disease. J Renin Angiotensin Aldosterone Syst 2003;4:164–168. [DOI] [PubMed] [Google Scholar]
- 7.Fuller PJ, Yang J, Young MJ: 30 years of the mineralocorticoid receptor: coregulators as mediators of mineralocorticoid receptor signalling diversity. J Endocrinol 2017;234:T23–T34. [DOI] [PubMed] [Google Scholar]
- 8.Faresse N: Post-translational modifications of the mineralocorticoid receptor: How to dress the receptor according to the circumstances? J Steroid Biochem Mol Biol 2014;143:334–342. [DOI] [PubMed] [Google Scholar]
- 9.Funder JW: Minireview: aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology 2010;151:5098–5102. [DOI] [PubMed] [Google Scholar]
- 10.Funder J: 30 years of the mineralocorticoid receptor: mineralocorticoid receptor activation and specificity-conferring mechanisms: a brief history. J Endocrinol 2017;234:T17–T21. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal J, Andrew R, Cruden NL, Kenyon CJ, Hughes KA, Newby DE, et al. : Displacement of cortisol from human heart by acute administration of a mineralocorticoid receptor an tagonist. J Clin Endocrinol Metab 2014;99: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellal-Levy C, Fagart J, Souque A, Rafestin-Oblin ME: Mechanistic aspects of mineralocorticoid receptor activation. Kidney Int 2000;57:1250–1255. [DOI] [PubMed] [Google Scholar]
- 13.Kawarazaki W, Fujita T: The role of aldosterone in obesity-related hypertension. Am J Hypertens 2016;29:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massaad C, Houard N, Lombès M, Barouki R: Modulation of human mineralocorticoid receptor function by protein kinase A. Mol Endocrinol 1999;13:57–65. [DOI] [PubMed] [Google Scholar]
- 15.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. : Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 2008;14:1370–1376. [DOI] [PubMed] [Google Scholar]
- 16.Shibata S, Fujita T: The kidneys and aldosterone/mineralocorticoid receptor system in salt-sensitive hypertension. Curr Hypertens Rep 2011;13:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokota K, Shibata H, Kurihara I, Kobayashi S, Suda N, Murai-Takeda A, et al. : Coactivation of the N-terminal transactivation of miner-alocorticoid receptor by Ubc9. J Biol Chem 2007;282:1998–2010. [DOI] [PubMed] [Google Scholar]
- 18.Lombès M, Oblin ME, Gasc JM, Baulieu EE, Farman N, Bonvalet JP: Immunohistochemical and biochemical evidence for a cardiovascular mineralocorticoid receptor. Circ Res 1992;71:503–510. [DOI] [PubMed] [Google Scholar]
- 19.Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombès M: The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal 2007;5:e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duprez DA, De Buyzere ML, Rietzschel ER, Taes Y, Clement DL, Morgan D, et al. : Inverse relationship between aldosterone and large artery compliance in chronically treated heart failure patients. Eur Heart J 1998;19:1371–1376. [DOI] [PubMed] [Google Scholar]
- 21.Farquharson CA, Struthers AD: Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (Lond) 2002; 103:425–431. [DOI] [PubMed] [Google Scholar]
- 22.MacFadyen RJ, Barr CS, Struthers AD: Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35:30–34. [DOI] [PubMed] [Google Scholar]
- 23.Grandi AM, Imperiale D, Santillo R, Barlocco E, Bertolini A, Guasti L, et al. : Aldosterone antagonist improves diastolic function in essential hypertension. Hypertension 2002;40: 647–652. [DOI] [PubMed] [Google Scholar]
- 24.Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep 2014;163–172. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, et al. : The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J 2010;24:2454–2463. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT: Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 2002;161:1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, et al. : Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation 2004;109:2213–2220. [DOI] [PubMed] [Google Scholar]
- 28.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, et al. : Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 2005;16: 2906–2912. [DOI] [PubMed] [Google Scholar]
- 29.Van Belle E, Bauters C, Wernert N, Hamon M, McFadden EP, Racadot A, et al. : Neointimal thickening after balloon denudation is enhanced by aldosterone and inhibited by spironolactone, and aldosterone antagonist. Cardiovasc Res 1995;29:27–32. [PubMed] [Google Scholar]
- 30.Xiao F, Puddefoot JR, Vinson GP: Aldosterone mediates angiotensin II-stimulated rat vascular smooth muscle cell proliferation. J Endocrinol 2000;165:533–536. [DOI] [PubMed] [Google Scholar]
- 31.Ohmine T, Miwa Y, Takahashi-Yanaga F, Morimoto S, Maehara Y, Sasaguri T: The involvement of aldosterone in cyclic stretch-mediated activation of NADPH oxidase in vascular smooth muscle cells. Hypertens Res 2009;32:690–699. [DOI] [PubMed] [Google Scholar]
- 32.Young M, Head G, Funder J: Determinants of cardiac fibrosis in experimental hypermineralocorticoid states. Am J Physiol 1995;269(4 pt 1):E657–E662. [DOI] [PubMed] [Google Scholar]
- 33.Brown NJ, Nakamura S, Ma L, Nakamura I, Donnert E, Freeman M, et al. : Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int 2000;58:1219–1227. [DOI] [PubMed] [Google Scholar]
- 34.Delcayre C, Silvestre JS, Garnier A, Oubenaissa A, Cailmail S, Tatara E, et al. : Cardiac aldosterone production and ventricular remodeling. Kidney Int 2000;57:1346–13451. [DOI] [PubMed] [Google Scholar]
- 35.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE: Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 2015; 10:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Artunc F, Lang F: Mineralocorticoid and SGK1-sensitive inflammation and tissue fibrosis. Nephron Physiol 2014;128:35–39. [DOI] [PubMed] [Google Scholar]
- 37.Huang LL, Nikolic-Paterson DJ, Ma FY, Tesch GH: Aldosterone induces kidney fibroblast proliferation via activation of growth factor receptors and PI3K/MAPK signalling. Nephron Exp Nephrol 2012;120:e115–e122. [DOI] [PubMed] [Google Scholar]
- 38.Zhang A, Jia Z, Guo X, Yang T: Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am J Physiol Renal Physiol 2007;293:F723–F731. [DOI] [PubMed] [Google Scholar]
- 39.Lai L, Chen J, Hao CM, Lin S, Gu Y: Aldosterone promotes fibronectin production through a Smad2-dependent TGF-beta1 pathway in mesangial cells. Biochem Biophys Res Commun 2006;348:70–75. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Chen Z, Park C, Centrella M, Mc-Carthy T, Chen L, et al. : Aldosterone stimulates fibronectin synthesis in renal fibroblasts through mineralocorticoid receptor-dependent and independent mechanisms. Gene 2013;531:23–30. [DOI] [PubMed] [Google Scholar]
- 41.Ayuzawa N, Nagase M, Ueda K, Nishimoto M, Kawarazaki W, Marumo T, et al. : Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension 2016;67:99–106. [DOI] [PubMed] [Google Scholar]
- 42.Wynn TA, Chawla A, Pollard JW: Macrophage biology in development, homeostasis and disease. Nature 2013;496:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, et al. : Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 2010; 120:3350–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen JZ, Morgan J, Tesch GH, Rickard AJ, Chrissobolis S, Drummond GR, et al. : Cardiac tissue injury and remodeling is dependent upon MR regulation of activation pathways in cardiac tissue macrophages. Endocrinology 2016;157:3213–3223. [DOI] [PubMed] [Google Scholar]
- 45.Shen ZX, Chen XQ, Sun XN, Sun JY, Zhang WC, Zheng XJ, et al. : Mineralocorticoid receptor deficiency in macrophages inhibits atherosclerosis by affecting foam cell formation and efferocytosis. J Biol Chem 2017;292:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terada Y, Ueda S, Hamada K, Shimamura Y, Ogata K, Inoue K, et al. : Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoidinducible protein kinase-1. Clin Exp Nephrol 2012;16:81–88. [DOI] [PubMed] [Google Scholar]
- 47.Sun JY, Li C, Shen ZX, Zhang WC, Ai TJ, Du LJ, et al. : Mineralocorticoid receptor deficiency in macrophages inhibits neointimal hyperplasia and suppresses macrophage inflammation through SGK1-AP1/NF-κB pathways significance. Arterioscler Thromb Vasc Biol 2016;36:874–885. [DOI] [PubMed] [Google Scholar]
- 48.Frieler RA, Meng H, Duan SZ, Berger S, Schütz G, He Y, et al. : Myeloid-specific deletion of the mineralocorticoid receptor reduces infarct volume and alters inflammation during cerebral ischemia. Stroke 2011;42: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Zhang YY, Frieler RA, Zheng XJ, Zhang WC, Sun XN, et al. : Myeloid mineralocorticoid receptor deficiency inhibits aortic constriction-induced cardiac hypertrophy in mice. PLoS One 2014;9:e110950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bienvenu LA, Morgan J, Rickard AJ, Tesch GH, Cranston GA, Fletcher EK, et al. : Macrophage mineralocorticoid receptor signaling plays a key role in aldosterone-independent cardiac fibrosis. Endocrinology 2012;153: 3416–3425. [DOI] [PubMed] [Google Scholar]
- 51.Shen JZ, Morgan J, Tesch GH, Fuller PJ, Young MJ: CCL2-dependent Macrophage recruitment is critical for mineralocorticoid receptor-mediated cardiac fibrosis, inflammation, and blood pressure responses in male mice. Endocrinology 2014;155:1057–1066. [DOI] [PubMed] [Google Scholar]
- 52.Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y: Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 2008; 149:1009–1014. [DOI] [PubMed] [Google Scholar]
- 53.Keidar S, Kaplan M, Aviram M: Angiotensin II-modified LDL is taken up by macrophages via the scavenger receptor, leading to cellular cholesterol accumulation. Arterioscler Thromb Vasc Biol 1996;16:97–105. [DOI] [PubMed] [Google Scholar]
- 54.Bayorh MA, Rollins-Hairston A, Adiyiah J, Lyn D, Eatman D: Eplerenone suppresses aldosterone/salt-induced expression of NOX-4. J Renin Angiotensin Aldosterone Syst 2011; 12:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki J, Iwai M, Mogi M, Oshita A, Yoshii T, Higaki J, et al. : Eplerenone with valsartan effectively reduces atherosclerotic lesion by attenuation of oxidative stress and inflammation. Arterioscler Thromb Vasc Biol 2006;26: 917–921. [DOI] [PubMed] [Google Scholar]
- 56.Rajagopalan S, Duquaine D, King S, Pitt B,6Patel P: Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation 2002;105:2212–2216. [DOI] [PubMed] [Google Scholar]
- 57.Hayashi H, Kobara M, Abe M, Tanaka N, Gouda E, Toba H, et al. : Aldosterone nongenomically produces NADPH oxidase-dependent reactive oxygen species and induces myocyte apoptosis. Hypertens Res 2008;31: 363–375. [DOI] [PubMed] [Google Scholar]
- 58.Nishimoto M, Fujita T: Renal mechanisms of salt-sensitive hypertension: contribution of two steroid receptor-associated pathways. Am J Physiol Renal Physiol 2015;308:F377–F387. [DOI] [PubMed] [Google Scholar]
- 59.Bai M, Chen Y, Zhao M, Zhang Y, He JC, Huang S, et al. : NLRP3 inflammasome activation contributes to aldosterone-induced podocyte injury. Am J Physiol Renal Physiol 2017;312:F556–F564. [DOI] [PubMed] [Google Scholar]
- 60.Kadoya H, Satoh M, Sasaki T, Taniguchi S, Takahashi M, Kashihara N: Excess aldosterone is a critical danger signal for inflamma-some activation in the development of renal fibrosis in mice. FASEB J 2015;29:3899–3910. [DOI] [PubMed] [Google Scholar]
- 61.Huang LL, Nikolic-Paterson DJ, Han Y, Ozols E, Ma FY, Young MJ, et al. : Myeloid mineralocorticoid receptor activation contributes to progressive kidney disease. J Am Soc Nephrol 2014;25:2231–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lal A, Veinot JP, Leenen FH: Critical role of CNS effects of aldosterone in cardiac remodeling post-myocardial infarction in rats. Cardiovasc Res 2004;64:437–447. [DOI] [PubMed] [Google Scholar]
- 63.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, et al. : Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol 2001;281:H2241–H2251. [DOI] [PubMed] [Google Scholar]
- 64.Leenen FH, Huang BS, Yu H, Yuan B: Brain “Ouabain” mediates sympathetic hyperactivity in congestive heart failure. Circ Res 1995; 77:993–1000. [DOI] [PubMed] [Google Scholar]
- 65.Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, et al. : Spironolac-tone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 2014;63:797–803. [DOI] [PubMed] [Google Scholar]
- 66.Sun XN, Li C, Liu Y, Du LJ, Zeng MR, Zheng XJ, et al. : T-cell mineralocorticoid receptor controls blood pressure by regulating inter-feron-gamma. Circ Res 2017;120:1584–1597. [DOI] [PubMed] [Google Scholar]
- 67.Li C, Sun XN, Zeng MR, Zheng XJ, Zhang YY, Wan Q: Mineralocorticoid receptor deficiency in T cells attenuates pressure overload-induced cardiac hypertrophy and dysfunction through modulating T-cell activation. Hyper-tension 2017;70:137–147. [DOI] [PubMed] [Google Scholar]
- 68.Pitt B, Reichek N, Metscher B, Phillips R, Roniker B, Kleiman J, Burns D; on behalf of the Eplerenone 017 Investigators: Efficacy and Safety of Eplerenone, Enalapril, and Eplerenone/Enalapril Combination Therapy in Patients with Left Ventricular Hypertrophy. Presented at the 51st Annual Scientific Session of the American College of Cardiology, Atlanta, GA, 2002. [Google Scholar]
- 69.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH: Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart 2013;99:320–326. [DOI] [PubMed] [Google Scholar]
- 70.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. : Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 71.Anand IS, Solomon SD, Claggett B, Shah SJ, O’Meara E, Boineau R, et al. : Prognostic value of baseline BNP and NT-ProBNP and its interaction with spironolactone in patients with heart failure and preserved ejection fraction in the TOPCAT Trial. Circulation 2015; 132:A14922. [Google Scholar]
- 72.de Denus S, O’Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, et al. : Spironolactone metabolites in TOPCAT – new insights into regional variation. N Engl J Med 2017;376: 1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takai S, Jin D, Muramatsu M, Kirimura K, Sakonjo H, Miyazaki M: Eplerenone inhibits atherosclerosis in nonhuman primates. Hypertension 2005;46:1135–1139. [DOI] [PubMed] [Google Scholar]
- 74.Bodary PF, Sambaziotis C, Wickenheiser KJ, Rajagopalan S, Pitt B, Eitzman DT: Aldosterone promotes thrombosis formation after arterial injury in mice. Arterioscler Thromb Vasc Biol 2006;26:233–233. [DOI] [PubMed] [Google Scholar]
- 75.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. : Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 76.de Rita O, Hackam DG, Spence JD: Effects of aldosterone on human atherosclerosis: plasma aldosterone and progression of carotid plaque. Can J Cardiol 2012;28:706–711. [DOI] [PubMed] [Google Scholar]
- 77.Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF: Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2009;4:542–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF: Aldosterone antagonists for preventing the progression of chronic kidney disease; in Strippoli GF (ed): Cochrane Database of Systematic Reviews. Chichester, UK, John Wiley & Sons, Ltd, 2014, p CD007004. [DOI] [PubMed] [Google Scholar]
- 79.Currie G, Taylor AH, Fujita T, Ohtsu H, Lind-hardt M, Rossing P, et al. : Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol 2016;17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Briet M, Schiffrin EL: The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep 2011;13:163–172. [DOI] [PubMed] [Google Scholar]
- 81.Gilbert KC, Brown NJ: Aldosterone and inflammation. Curr Opin Endocrinol Diabetes Obes 2010;17:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruder-Nascimento T, da Silva MA, Tostes RC: The involvement of aldosterone on vascular insulin resistance: implications in obe sity and type 2 diabetes. Diabetol Metab Syndr 2014;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen Dinh Cat A, Antunes TT, Callera GE, Sanchez A, Tsiropoulou S, Dulak-Lis MG, et al. : Adipocyte-specific mineralocorticoid receptor overexpression in mice is associated with metabolic syndrome and vascular dysfunction: role of redox-sensitive PKG-1 and Rho kinase. Diabetes 2016;65: 2392–2403. [DOI] [PubMed] [Google Scholar]
- 84.Hosoya K, Minakuchi H, Wakino S, Fujimura K, Hasegawa K, Komatsu M, et al. : Insulin resistance in chronic kidney disease is ameliorated by spironolactone in rats and humans. Kidney Int 2015;87:749–760. [DOI] [PubMed] [Google Scholar]
- 85.Raheja P, Price A, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, et al. : Spironolactone prevents chlorthalidone-induced sympathetic activation and insulin resistance in hyper-tensive patients. Hypertension 2012;60:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garg R, Kneen L, Williams GH, Adler GK: Effect of mineralocorticoid receptor antagonist on insulin resistance and endothelial function in obese subjects. Diabetes Obes Metab 2014; 16:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vecchiola A, Lagos CF, Carvajal CA, Baudrand R, Fardella CE: Aldosterone production and signaling dysregulation in obesity. Curr Hypertens Rep 2016;18:20. [DOI] [PubMed] [Google Scholar]
- 88.Baudrand R, Gupta N, Garza AE, Vaidya A, Leopold JA, Hopkins PN, et al. : Caveolin 1 modulates aldosterone-mediated pathways of glucose and lipid homeostasis. J Am Heart Assoc 2016;5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, et al. : Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail 2014;7:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kolkhof P, Bärfacker L: 30 years of the miner-alocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol 2017;234:T125–T140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. : Effect of finerenone on albuminuria in patients with diabetic nephropathy: a Randomized Clinical Trial. JAMA 2015;314:884. [DOI] [PubMed] [Google Scholar]
- 92.Rajagopalan S, Alaiti MA, Broadwater K, Goud A, Gaztanaga J, Connelly K, et al. : Design of the magnetic resonance imaging evaluation of Mineralocorticoid Receptor Antagonism in Diabetic Atherosclerosis (MAGMA) Trial. Clin Cardiol 2017;DOI: 10.1002/clc.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ: Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension 2009;54:537–543. [DOI] [PubMed] [Google Scholar]
- 94.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schütz G, et al. : Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 2010;120:3350–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang YY, Li C, Yao GF, Du LJ, Liu Y, Zheng XJ, et al. : Deletion of macrophage mineralocorticoid receptor protects hepatic steatosis and insulin resistance through ERα/HGF/met pathway. Diabetes 2017;66: 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frieler RA, Meng H, Duan SZ, Berger S, Schutz G, He Y, et al. : Myeloid-specific deletion of the mineralocorticoid receptor reduces infarct volume and alters inflammation during cerebral ischemia. Stroke 2011; 42:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rickard AJ, Morgan J, Chrissobolis S, Miller AA, Sobey CG, Young MJ: Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension 2014;63: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 98.Schäfer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, et al. : Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J 2013;34:3515–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, et al. : Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 2015;66:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, et al. : Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 2016;118:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, et al. : Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 2012;18:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Amador CA, Bertocchio JP, Andre-Gregoire G, Placier S, Duong Van Huyen JP, El Moghrabi S, et al. : Deletion of mineralocorticoid receptors in smooth muscle cells blunts renal vascular resistance following acute cyclosporine administration. Kidney Int 2016;89:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvrard-Pascaud A, Berger S, et al. : Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension 2014;63:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ: Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol 2014;34:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rickard AJ, Morgan J, Bienvenu LA, Fletcher EK, Cranston GA, Shen JZ, et al. : Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension 2012;60:1443–1450. [DOI] [PubMed] [Google Scholar]
- 106.Fraccarollo D, Berger S, Galuppo P, Kneitz S, Hein L, Schutz G, et al. : Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 2011;123:400–408. [DOI] [PubMed] [Google Scholar]
- 107.Lother A, Berger S, Gilsbach R, Rösner S, Ecke A, Barreto F, et al. : Ablation of miner-alocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension 2011;57:746–754. [DOI] [PubMed] [Google Scholar]
- 108.Li C, Sun XN, Zeng MR, Zheng XJ, Zhang YY, Wan Q, et al. : Mineralocorticoid receptor deficiency in T cells attenuates pressure overload-induced cardiac hypertrophy and dysfunction through modulating T-cell activation. Hypertension 2017;70:137–147. [DOI] [PubMed] [Google Scholar]
- 109.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, et al. : Effects of eplerenone, enalapril, and eplerenone/enal-april in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation 2003;108:1831–1838. [DOI] [PubMed] [Google Scholar]
- 110.Montalescot G, Pitt B, Lopez de Sa E, Hamm CW, Flather M, Verheugt F, et al. : Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: the Randomized Double-Blind Reminder Study. Eur Heart J 2014;35:2295–2302. [DOI] [PubMed] [Google Scholar]
- 111.Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, et al. : Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015;64:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, et al. : Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 2006;1: 940–951. [DOI] [PubMed] [Google Scholar]
- 113.Bianchi S, Bigazzi R, Campese VM: Long-term effects of spironolactone on protein-uria and kidney function in patients with chronic kidney disease. Kidney Int 2006;70: 2116–2123. [DOI] [PubMed] [Google Scholar]
- 114.Furumatsu Y, Nagasawa Y, Tomida K, Mikami S, Kaneko T, Okada N, et al. : Effect of renin-angiotensin-aldosterone system triple blockade on non-diabetic renal disease: addition of an aldosterone blocker, spironolac- tone, to combination treatment with an angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker. Hypertens Res 2008;31:59–67. [DOI] [PubMed] [Google Scholar]
- 115.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD: Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 2009;20:2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boesby L, Elung-Jensen T, Klausen TW, Strandgaard S, Kamper AL: Moderate anti-proteinuric effect of add-on aldosterone blockade with eplerenone in non-diabetic chronic kidney disease. A Randomized Cross-Over Study. PLoS One 2011;6:e26904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T, et al. : Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2014;2:944–953. [DOI] [PubMed] [Google Scholar]
- 118.Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, et al. : Spironolac-tone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol 2014;63:528–536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.