Abstract
The antibiotic susceptibility profile and antimicrobial resistance determinants were characterized on Gram-negative bacilli (GNB) isolated from Algerian hospital effluents. Among the 94 isolates, Enterobacteriaceae was the predominant family, with Escherichia coli and Klebsiella pneumoniae being the most isolated species. In non-Enterobacteriaceae, Acinetobacter and Aeromonas were the predominant species followed by Pseudomonas, Comamonas, Pasteurella, and Shewanella spp. The majority of the isolates were multidrug-resistant (MDR) and carried different antimicrobial resistance genes including blaCTX-M, blaTEM, blaSHV, blaOXA-48-like, blaOXA-23, blaOXA-51, qnrB, qnrS, tet(A), tet(B), tet(C), dfrA1, aac(3)-IIc (aacC2), aac(6′)-1b, sul1, and sul2. The qacEΔ1-sul1 and intI2 signatures of class 1 and class 2 integrons, respectively, were also detected. Microarray hybridization on MDR E. coli revealed additional resistance genes (aadA1 and aph3strA, tet30, mphA, dfrA12, blacmy2, blaROB1, and cmlA1) and classified the tested strains as commensals, thus highlighting the potential role of humans in antibiotic resistance dissemination. This study is the first report of blaOXA-48-like in Klebsiella oxytoca in Algeria and blaOXA-23 in A. baumannii in Algerian hospital effluents. The presence of these bacteria and resistance genes in hospital effluents represents a serious public health concern since they can be disseminated in the environment and can colonize other hosts.
Keywords: Multidrug resistance, Hospital effluents, Gram-negative bacilli, Algeria
Introduction
Antimicrobial resistance resulting from intensive and inappropriate use of antibiotics represents an increasing challenge for health care services worldwide. In Gram-negative bacilli (GNB), this resistance is a cause for concern, owing to the emergence and dissemination of different resistance genes identified either in clinical setting or in the environment. Within the environment, aquatic ecosystems have been documented as an important vehicle and reservoir of antibiotic resistance [1]. Different studies have shown that hospital effluents contain high levels of antibiotic-resistant bacteria and contribute to antibiotic resistance dissemination in the environment [2, 3]. Moreover, the non-metabolized fraction of consumed antibiotics excreted directly in wastewater can exert a selective pressure promoting the selection of these resistant bacteria [2]. Generally, hospital effluents are discharged into municipal sewage and collected via wastewater treatment plants (WWTP) or eliminated directly into the environment without any treatments. The latter practice has been criticized and a pre-treatment has been recommended [4].
The resistant bacteria present in hospital wastewater could be a source of genes encoding various resistance mechanisms including extended spectrum β-lactamases (ESBLs), carbapenemases, and plasmid mediated quinolones resistance (PMQRs) [3, 5, 6]. These genes may be transmitted to other bacteria present in sewage and the environment via the aquatic system and the food chain. The spread of these bacteria in the natural environment can have significant consequences on ecological balance and animal and public health [2]. The present study aimed to identify and characterize resistance mechanisms of multidrug-resistant GNB found in hospital effluents and to improve our understanding on the role of hospital effluents as a source of a large diversity of antibiotic resistance genes.
Material and methods
Bacterial sampling
Twenty wastewater samples were collected from untreated effluents from four hospitals in Algeria: two in Bejaia and two in Tizi Ouzou provinces. Sampling was carried out during the period of maximum hospital activity, in 2011 and 2012 in Bejaia and Tizi Ouzou, respectively. In Bejaia, the samples were collected from non-functional WWTP and sewer pipes from Khelil Amrane (H1) and Amizour (H2) hospitals, respectively. In 2011, the H1 hospital had an annual capacity of 160 beds and admitted 5255 patients and the H2 hospital had a capacity of 209 beds and admitted 9360 patients. In Tizi Ouzou, samples were collected from sewer pipes from Nadir Mohammed hospital (H3) and Tigzirt hospital (H4). In 2012, the H3 hospital had 1042 beds and admitted 40,170 patients and the H4 hospital was the only establishment located in a rural region. It had a capacity of 35 beds and admitted 638 patients annually. All the hospitals included in this study were located close to the Mediterranean Sea with approximate distances of 0.4, 8, 33, and 44 km for H4, H1, H2, and H3, respectively. In addition, the H3 hospital was located at about 15 km from Oued Sebaou and the H2 at around 15 km from Oued Soummam, the main rivers of the provinces of Tizi Ouzou and Bejaia, respectively, which flow into the Mediterranean Sea.
Microbiological analysis
A volume of 200 ml of each sample was collected in sterile glass flasks and transported to the laboratory in a cooler, and samples were processed within 2 h upon arrival. Serial dilutions of 10−1 to 10−4 of each sample were performed. One hundred microliters of each sample and their corresponding dilutions were plated on MacConkey agar supplemented with 2 μg/ml of cefotaxime followed by incubation at 37 °C for 18–24 h. A single colony from colonies sharing the same appearance by visual inspection (morphology, color, shape, size) was selected and purified. After purification, the isolates were stored in a nutrient broth containing 10% glycerol (v/v) at − 80 °C. Rapid identification was performed using the API 20E test strip (Biomerieux, France) for Enterobacteriaceae according to the manufacturer’s instructions in conjunction with conventional biochemical testing [7]. Non-Enterobacteriaceae bacteria were identified by sequencing the 16S rRNA and rpoB according to the Laboratoire de Santé Publique du Québec (LSPQ) reference methods or gyrB for Shewanella spp. [6].
The bacterial diversity of different genera of each hospital was evaluated using Shannon diversity index. The BPMSG diversity online calculator website https://bpmsg.com/academic/div-calc.php was used to calculate this index.
Antimicrobial susceptibility testing and extended-spectrum β-lactamase (ESBL) detection
Antimicrobial susceptibility was performed using the disk diffusion method and the results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI, 2014) guidelines [8]. The antibiotics (Bio-Rad, France) tested were as follows: ticarcillin (TIC, 75 μg), ticarcillin-clavulanic acid (TIM, 75/10 μg), amoxicillin-clavulanic acid (AMC, 20/10 μg), ertapenem (ETP, 10 μg), imipenem (IPM, 10 μg), ceftazidime (CAZ, 30 μg), cefepime (FEP, 30 μg), aztreonam (ATM, 30 μg), gentamicin (GEN, 10 μg), amikacin (AMK, 30 μg), ciprofloxacin (CIP, 5 μg), tetracycline (TET, 30 μg), and trimethoprim-sulfamethoxazole (SXT, 1.25/23.75 μg). The ESBL production was detected by the CLSI confirmatory test using both CTX (30 μg) and CAZ (30 μg) disks alone and in combination with clavulanic acid (CA, 10 μg) [8].
Detection of antibiotic resistance genes
DNA extraction was performed by the MagAttract® DNA Mini M48 kit using the biorobot M48 (Qiagen, USA) according to the manufacturer’s instructions. For β-lactam resistance genes, PCR amplification targeting blaCTX-M, blaSHV, and blaTEM genes was carried out in a 50 μl final volume using Phusion DNA Polymerase (New England Biolabs, USA) [9, 10]. For the carbapenemases encoding genes which included blaOXA-23, blaOXA-24, blaOXA-51, and blaOXA-58, multiplex PCR was performed in a 50 μl final volume using HotStar Taq enzyme (Qiagen, USA) [11, 12]. The blaoxa-48-like genes were amplified using simplex PCR with the same amplification conditions.
Genes encoding resistance to non-β-lactam antibiotics were amplified in a 25 μl volume using AmpliTaq DNA polymerase (ThermoFisher scientific, USA) according to the manufacturer’s guidelines. The non-β-lactam resistance genes included qnrA, qnrB, and qnrS encoding resistance to quinolones [13, 14], acc(6′)-lb and aac(3)-IIc (aacC2) encoding resistance to aminoglycosides, dfrA1encoding resistance to trimethoprim, tet(A), tet(B), and tet(C) encoding resistance to tetracycline, and sul1, sul2, and sul3 encoding resistance to sulfonamides [15, 16]. In addition, qacEΔ1-sulI and intI2 genetic markers for class 1 and class 2 integrons, respectively, were also amplified in the same conditions [17, 18]. The primers used in this study and PCR conditions are listed in Table 1.
Table 1.
Primers and PCR conditions used in this study
| Primer | Target | Control strain | Primer sequence (5′–3′) | PCR conditions | Amplicon size (pb) | Reference |
|---|---|---|---|---|---|---|
|
SHVF2 SHVR |
bla SHV | ATCC BAA200 |
GGTTAT GCG TTA TAT TCG CC TTA GCG TTG CCA GTG CTC |
40 cycles of 30 s at 98 °C, 10 s at 98 °C, 30 s at 65 °C, 30 s at 72 °C; 10 min at 72 °C; 4 °C | 867 | [9] |
|
TEMF TEMR |
bla TEM | ATCC BAA197 |
ATG AGT ATT CAA CAT TTC CG CTG ACA GTT ACC AAT GCT TA |
30 cycles of 30 s at 98 °C, 10 s at 98 °C, 30 s at 58 °C, 30 s at 72 °C;10 min at 72 °C; 4 °C | 867 | [9] |
|
CTX-M-U1 CTX-M-U2 |
bla CTx-M | ID098246 |
ATG TGC AGY ACC AGT AAR GTK ATG GC TGG GTR AAR TAR GTS ACC AGA AYC AGC GG |
30 cycles of 30 s at 98 °C, 10 s at 98 °C, 30 s at 65 °C, 30 s at 72 °C;10 min at 72 °C; 4 °C | 593 | [10] |
|
OXA-P1 OXA-P2 |
bla OxA-48 | LSPQ4078 |
ATG CGT GTA TTA GCC TTA TC CTA GGG AAT AAT TTT NTC CT |
10 min at 20 °C; 30 cycles of 15 min at 95 °C, 60 s at 94 °C, 60 s at 57 °C; 10 min at 72 °C; 4 °C | 759 | This study |
|
OXA-23-like-FWD OXA-23-like-REV |
bla OXA-23 | MA092246 |
GAT CGG ATT GGA GAA CCAGA ATT TCT GAC CGC ATT TCC A |
501 | [11] | |
|
OXA-24-like-FWD OXA-24-like-REV |
bla OXA-24 | MA09250 |
GGT TAG TTG GCC CCC TTA AA AGT TGA GCG AAA AGG GGA TT |
246 | [11] | |
|
OXA-51-like-FWD OXA-51-like-REV |
bla OXA-51 | LSPQ4139 |
TAA TGC TTT GAT CGG CCT TG TGG ATT GCA CTT CAT CTT GG |
353 | [12] | |
|
OXA-58-like-FWD OXA-58-like-REV |
bla OXA-58 | LSPQ4139 |
AAG TAT TGG GGC TTG TGC TG CCC CTC TGC GCT CTA CAT AC |
599 | [11] | |
|
QnrAm-F QnrAm-R |
qnrA | J53pMG252 |
AGA GGA TTT CTC ACG CCA GG TGC CAG GCA CAG ATC TTG AC |
10 min at 95 °C and 35 cycles of amplification consisting of 1 min at 95 °C, 1 min at 54 °C and 1 min at 72 °C and 10 min at 72 °C | 580 | [13] |
|
qnrB-forward qnrB-reverse |
qnrB | J53pMG298 |
GAT CGT GAA AGC CAG AAA GG ACG ATG CCT GGT AGT TGT CC |
94 °C for 4 min, 35 cycles of 94 °C of 30 s, 58 °C for 30 s, and 68 °C for 45 s, and a final step of 68 °C for 10 min | 469 | [14] |
|
qnrS-forward qnrS-reverse |
qnrS | J53pMG306 |
ACG ACA TTC GTC AAC TGC AA TAA ATT GGC ACC CTG TAG GC |
417 | [14] | |
|
aac-forward aac-reverse |
acc (6′)-lb | Salmonella SA20042859 |
TTG CGA TGC TCT ATG AGT GGC TA CTC GAA TGC CTG GCG TGT TT |
482 | [15] | |
|
fDFRa rDFRa |
dfrA1 | S17-1 lamda pir |
AAG AAT GGA GTT ATC GGG AAT G GGG TAA AAA CTG GCC TAA AAT TG |
391 | [16] | |
|
fAACa rAACa |
aacC2(aac(3)-IIa) | R176 |
CGG AAG GCA ATA ACG GAG TCG AAC AGG TAG CAC TGA G |
740 | [16] | |
|
fTETa rTETa |
tet(A) | SAS1393 |
GTG AAA CCC AAC ATA CCC C GAA GGC AAG CAG GAT GTA G |
888 | [16] | |
|
fTETb rTETb |
tet(B) | CT4afooB |
CCT TAT CAT GCC AGT CTT GC ACT GCC GTT TTT TCG CC |
774 | [16] | |
|
fTETc rTETc |
tet(C) | pBR322 |
ACT TGG AGC CAC TAT CGA C CTA CAA TCC ATG CCA ACC C |
881 | [16] | |
|
fSULa rSULa |
sulI | SAS1393 |
TTC GGC ATT CTG AAT CTC AC ATG ATC TAA CCC TCG GTC TC |
822 | [16] | |
|
fSULb rSULb |
sulII | RSF1010 |
CGG CAT CGT CAA CAT AAC C GTG TGC GGA TGA AGT CAG |
722 | [16] | |
|
fsul3 rsul3 |
sulIII | sul3 |
GAG CAA GAT TTT TGG AAT CG CTA ACC TAG GGC TTT GGA |
790 | This study | |
|
fQACe rSUL1 |
qacEΔ1-sul1 | SAS1393 |
ATC GCA ATA GTT GGC GAA GT GCA AGG CGG AAA CCC GCG CC |
797 | [17] | |
|
intI2L intI2R |
int2 | PT508-124.61 |
CAC GGA TAT GCG ACA AAA AGG T GTA GCA AAC GAG TGA CGA AAT G |
788 | [18] |
DNA microarray analysis of Escherichia coli isolates
Nine representative multidrug-resistant (MDR) E. coli isolates were analyzed by microarray hybridization. The E. coli Maxivirulence version 3.1 microarray was used as previously described [19]. This microarray can detect 348 virulence genes and 98 antibiotic resistance genes and can determine putative E. coli pathotypes based on virulence gene profiles [20] and phylogenetic groups (A1, B1, B2, and D) based on the pattern of presence or absence of chuA, TspE4.C2, and yjaA [21].
Results
Bacterial diversity
A total of 94 Gram-negative bacilli were cultured from selective plates. The distribution of these isolates was as follows: 25 isolates were from H1, 20 from H2, 22 from H3, and 27 from H4. Among the 26 different species identified, Enterobacteriaceae was the most predominant family with a prevalence of 78% (n = 73). The identified species from this family included E. coli (n = 19), Klebsiella pneumoniae (n = 14), Citrobacter freundii (n = 9), Klebsiella oxytoca (n = 7), Enterobacter cloacae (n = 5), Citrobacter braakii (n = 4), Morganella morganii (n = 3), Citrobacter youngae (n = 2), Proteus vulgaris (n = 2), Providencia rettegeri (n = 2), Cedecea lapagei (n = 1), Enterobacter aerogenes (n = 1), Enterobacter agglomerans (n = 1), Enterobacter asburiae (n = 1), Enterobacter sakazakii (n = 1), and Kluyvera sp. (n = 1). The prevalence of non-Enterobacteriaceae GNB was 22% (n = 21). This group included Acinetobacter baumannii (n = 5), Aeromonas hydrophila (n = 5), Aeromonas sp. (n = 3), Aeromonas veronii (n = 2), Pseudomonas aeruginosa (n = 2), Aeromonas sobria (n = 1), Comamonas aquatica (n = 1), Pasteurella spp. (n = 1), and Shewanella xiamenensis (n = 1). The predominant species from these hospitals were K. pneumoniae (13/25), E. coli (13/20), E. coli (5/22), and K. oxytoca (6/27) for H1, H2, H3, and H4, respectively. In addition, 10/21 non-Enterobacteriaceae isolates (47.6%) were collected from Nadir Mohamed hospital (H3). Shannon diversity index was different between the effluents of different hospitals and revealed greater genera diversity for effluents of H3 (2.07), followed by H4 (1.7), H1 (1.4), and H2 (1.2).
Antibiotic susceptibility of hospital effluents isolates
For β-lactam antibiotics, the highest resistance rate was observed with TIC representing 98.6% and 66.6% for Enterobacteriaceae and non-Enterobacteriaceae, respectively, followed by CAZ with 86.3% and 47.6% for Enterobacteriaceae and non-Enterobacteriaceae, respectively. For non-β-lactam antibiotics, the highest resistance rate observed with ciprofloxacin was 74% and 62% for Enterobacteriaceae and non-Enterobacteriaceae, respectively. All the isolates were resistant to at least two antibiotic classes and the MDR phenotype was observed in 91/94 (96.8%) isolates exhibiting resistance to 3 to 8 antibiotic classes. The MDR phenotype was observed in 72/73 isolates of the Enterobacteriaceae family (98.6%) and in 19/21 isolates in the non-Enterobacteriaceae families (90.5%) which were resistant to three or more antimicrobial classes. In addition, the ESBL phenotype was detected in 45/73 of Enterobacteriaceae (61.5%) and in 9/21 of non-Enterobacteriaceae GNB (42.8%).
Antibiotics resistance mechanisms
Among the genes encoding class A β-lactamases tested in this study, blaCTX-M, blaTEM, and blaSHV were detected in 27/94 (28.7%), 17/94 (18.1%), and 14/94 (14.9%) isolates, respectively. The blaCTX-M gene was detected only in the Enterobacteriaceae family including E. coli (n = 7) and K. pneumoniae (n = 7), K. oxytoca (n = 3), C. youngae (n = 2), C. freundii (n = 2), C. braakii (n = 1), E. aerogenes (n = 1), E. agglomerans (n = 1), E. asburiae (n = 1), M. morganii (n = 1), and P. vulgaris (n = 1). The blaTEM gene was also detected in this family and was detected in E. coli (n = 5) and K. pneumoniae (n = 3) followed by K. oxytoca (n = 1), C. Youngae (n = 1), E. agglomerans (n = 1), and P. rettegeri (n = 1). The blaSHV gene was detected in K. pneumoniae (n = 9), E. cloacae (n = 3), E. agglomerans (n = 1), and E. coli (n = 1). Of the 21 non-Enterobacteriaceae isolates, only blaTEM was detected in five isolates including A. hydrophila (n = 2), Aeromonas spp. (n = 1), A. veronii (n = 1), and A. baumannii (n = 1).
Regarding carbapenemases encoding genes, blaOXA-48-like was detected in K. oxytoca (n = 2). The blaOXA-416 gene was also detected in S. xiamenensis isolate and the genome was fully sequenced and documented in a separate study [6]. The blaOXA-23 gene was detected in two A. baumannii isolates whereas the blaOXA-51 gene was detected in all the five isolates. The blaOXA-24 and blaOXA-58 genes tested negative in all isolates.
For genes conferring resistance to quinolones, the qnrB gene was detected in E. coli (n = 6), C. freundii (n = 5), K. pneumoniae (n = 4), K. oxytoca (n = 3), C. youngae (n = 2), E. cloacae (n = 2), E. asburiae (n = 1), M. morganii (n = 1), P. vulgaris (n = 1), and E. aerogenes (n = 1). However, the qnrS gene was detected only in K. oxytoca (n = 1) and A. baumannii (n = 2) isolates, whereas the qnrA gene tested negative in all isolates. The qnrVC6 variant was also detected in S. xiamenensis isolate as previously documented [6]. Genes conferring resistance to tetracycline tet(A), tet(B), and tet(C) were detected in 12/73 Enterobacteriaceae isolates (16.4%) and 8/21 non-Enterobacteriaceae isolates (38%). The dfrA1gene was found in Enterobacteriaceae isolates and distributed as follows: C. freundii (n = 3), E. coli (n = 4), K. oxytoca (n = 1), P. vulgaris (n = 1), and P. rettgeri (n = 2). In the non-Enterobacteriaceae isolates, this gene was detected in A. hydrophila (n = 2), A. sobria (n = 1), and A. baumannii (n = 2). The sul1gene was found in 14/73 Enterobacteriaceae isolates (19.2%) and in 10/21 non-Enterobacteriaceae isolates (47.6%), and the sul2 gene was detected in 33 /73 Enterobacteriaceae isolates (45.2%) and in 3/21 non-Enterobacteriaceae isolates (14.3%).
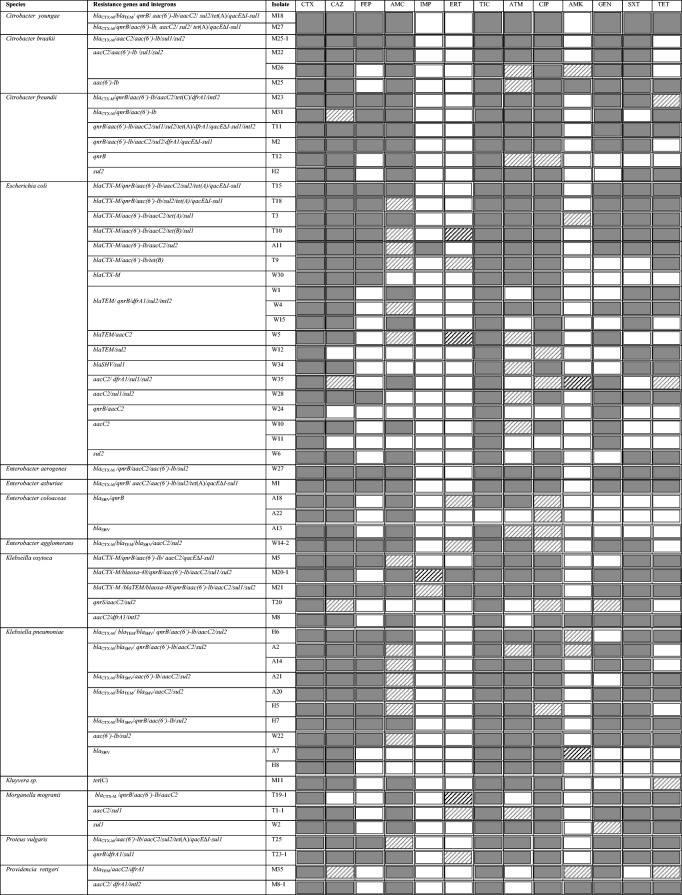
With regard to genes conferring resistance to aminoglycosides, the aac(3)-IIc (aacC2) gene was detected in 36/73 Enterobacteriaceae isolates (49.3%) and 3/21 non-Enterobacteriaceae isolates (14.3%), and the aac(6′)-1b gene was found in 28/73 Enterobacteriaceae isolates (38.3%) and in 8/21 non-Enterobacteriaceae isolates (38%). The qacEΔI-sul1 conserved segment of class 1 integron was detected in 9/73 Enterobacteriaceae isolates (12.3%) and in 1/21 non-Enterobacteriaceae isolates (4.8%), and the intI2 gene was found only in the Enterobacteriaceae group. The different gene combinations of MDR isolates are described in Table 2 and Table 3.
Table 2.
Antimicrobial resistance profiles and resistance genes detected in MDR Enterobacteriaceae isolates
 Resistant
Resistant
 Intermediate
Intermediate
 Susceptible
Susceptible
TIC ticarcillin, AMC amoxicillin-clavulanic acid, ETP ertapenem, IMP imipenem, CAZ ceftazidime, FEP cefepime, ATM aztreonam, GEN gentamicin, AMK amikacin, CIP ciprofloxacin, TET tetracycline, SXT trimethoprim-sulfamethoxazole
Table 3.
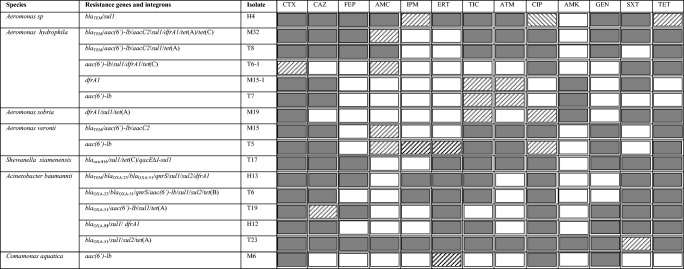
Antimicrobial resistance profile, resistance genes detected in MDR non-Enterobacteriaceae isolates
 Resistant
Resistant
 Intermediate
Intermediate
 Susceptible
Susceptible
TIC ticarcillin, AMC amoxicillin-clavulanic acid, ETP ertapenem, IPM imipenem, CAZ ceftazidime, FEP cefepime, ATM aztreonam, GEN gentamicin, AMK amikacin, CIP ciprofloxacin, TET tetracycline, SXT trimethoprim-sulfamethoxazole
Phylogenetic groups and virulence profile of MDR E. coli
Escherichia coli isolates tested by microarray were classified as commensal and were assigned to the phylogenetic groups A (n = 4), D (n = 3), and B1 (n = 2). However, some virulence genes commonly found in E. coli pathotypes were detected [22]. They included chuA, iha, cvaC, fimH, fyuA, iroN, iss, iutA, kpsM II, traT, ompA, ompT, TSPE4.C2, and yjaA. This analysis also detected other resistance genes not tested by PCR. They included aadA1 and aph3strA conferring resistance to aminoglycosides, tet30 conferring resistance to tetracycline, mphA conferring resistance to macrolides, dfrA12 conferring resistance to trimethoprim, blacmy2 and blaROB1 conferring resistance to β-lactams, and cmlA1 conferring resistance to chloramphenicol. The detail microarray results are presented in Table 4.
Table 4.
Phylogenitic groups, virulence genes, and antimicrobial resistance genes, detected by microarray, of tested Escherichia coli isolates
| Isolate | PG | Virulence genes | Resistance genes |
|---|---|---|---|
| T3 | B1 | fimH/kpsM II/traT/ompA/ompT/TSPE4.C2 | blaCTX-M/aacC2/sul1/tet(A)/tetR/tet30/mphA/dfrA12 |
| T9 | A | iutA/traT/ompA | blaCTX-M/aacC2/tet30/tetR/mphA |
| T10 | D | chuA/fimH/fyuA/iutA/traT/ompA/TSPE4.C2 | blaCTX-M/blacmy2/blaROB1/aacC2/sul1/mphA |
| T18 | A | ompA | blaCTX-M/qnrB/sul2/tet(A)/tet30/tetR/int1 |
| W1 | D | chuA/cvaC/fimH/iroN/iss/iutA/traT/ompA | blaTEM/blacmy2/blaROB1/aadA1/dfrA1/sul2/tet(A)/tet30/mphA/tetR/int2 |
| W4 | D | chuA/iha/cvaC/fimH/iroN/iss/iutA/traT/ompA/ompT/ompA/TSPE4.C2 | blaTEM/blacmy2/qnrB/blaROB1/aacC2/aadA1/dfrA1/int1sul1/sul2/tet(A)/tetR/aph3strA/mphA/cmlA1/int2 |
| W30 | A | cvaC/fimH/traT/ompA/yjaA | bla CTX-M |
| T15 | A | fimH | blaTEM/qnrB/aacC2/aadA1/blaCTX-M/int1/sul2/tet(A)/tet30/tetR |
| W34 | B1 | cvaC/fimH/iroN/iss/traT/TSPE4.C2 | blacmy2/aadA1/int1/sul1/blaROB1/mphA/cmlA1/dfrA12/int2 |
PG phylogenitic group
Discussion
Hospital effluents represent an important source of antibiotics and MDR bacteria carrying several antimicrobial resistance genes and mobile genetic elements (MGEs). This poses a significant threat to public health and the environment, especially when these effluents are discharged in the environment (rivers, lakes, and seas, etc.) without any prior treatments. In this context, our study investigated cephalosporin resistant Enterobacteriaceae and non-Enterobacteriaceae GNB isolated from hospital effluents in Algeria.
In this work, the predominant species isolated were E. coli followed by K. pneumoniae. These bacteria are both pathogenic and commensal organisms and are frequent causes of nosocomial infections [23, 24]. In addition, A. baumannii and A. hydrophila were the predominant species among bacteria other than Enterobacteriaceae isolated in our study. A. baumannii has recently emerged as a leading cause of hospital associated outbreaks infections resulting from contamination and transmission in hospital environments [25]. A. hydrophila was also reported among the most frequent cause of human diseases among Aeromonas spp. [26]. The high levels of resistance to the β-lactam antibiotics (TIC and CAZ), and to the non-β-lactam-antibiotics (CIP and SXT), observed in this study, may be associated to the selective pressure exerted by these antibiotics commonly used in antibiotherapy. The fact that most of the isolates were MDR is worrisome. This is because MDR encoding genes are commonly carried on MGE such as plasmids, integrons, and transposons [27].
In our study, blaCTX-M was the most predominant gene among the β-lactamase encoding genes detected. In fact, in the last decade, blaCTX-M has explosively disseminated and becomes the most common resistance gene around the world [28]. Previous studies in Bejaia (site of our study) have also reported the dissemination of blaCTX-M in patients [29] and in wild fish from the Mediterranean Sea [30]. This result is similar to that of some studies carried out on hospital effluents, particularly in Romania [31] and Poland [3]. However, the prevalence of these genes was lower compared to that reported in Enterobacteriaceae isolated from Zemirli hospital effluents, in Algeria [5].
Interestingly, genes encoding carbapenemases including blaOXA-23 and blaOXA-48-like were detected for the first time in Algerian hospital effluents in A. baumannii, and K. oxytoca and S. xiamenensis, respectively. The emergence of such genes is considered as a threat in the treatment of GNB infections and can lead to MDR and extensive drug resistance; for example, MDR A. baumannii is recognized as the hardest nosocomial pathogen to control and treat [32]. The fact that blaOXA-23 was previously reported in A. baumannii isolated from hospitals located on the Mediterranean coast [33] and from seabream fish from the Mediterranean Sea [34] underlines the probable role of hospital effluents in the transmission of such genes from hospitals into the environment. These genes may also be derived from other sources such as municipal sewage and animal husbandry [2]. Interestingly, S. xiamenensis OXA-416 producing which is a known reservoir of antimicrobial resistance genes carried several other resistance genes clustered on a transposon harbored by a pSx1 plasmid were detected and documented in a separate work [6]. The blaOXA-48 gene was also recently reported in K. pneumoniae outbreaks in Algeria [35] and this also indicates a potential dissemination between patients and the environment.
The genes qnrB, qnrS, tet(A), tet(B), tet(C), dfrA1, aacC2, aac(6′)-1b, sul1, and sul2 conferring resistance to non-β-lactam-antibiotics detected in this study are common in GNB and were also reported in different regions of Algeria [36]. The integrons were previously detected in high proportions of Enterobacteriaceae strains isolated from hospital effluents [37]. In our study, the identification of class 1 and class 2 integrons suggests that the resistance genes could be associated with MGEs and emphasizes their role in the dissemination of antibiotic resistance.
Microarray analysis performed on representative MDR E. coli isolates revealed the presence of additional resistance genes not tested by PCR. This highlights the importance of other methods such as microarray and genomic technology in the detection of resistance genes and MGEs. Previous studies on resistant Enterobacteriaceae isolates conducted on effluents from Algiers’s hospital have reported the same resistance genes as in our study [5, 38] with the exception of blaROB1, blaOXA-48, blaOXA-51, blaOXA-23, mphA, tet30, and cmlA1.
The E. coli phylogenetic groups A, B1, and D identified in this study had a low level of virulence and a high level of antimicrobial resistance, which is in agreement with previous studies [38]. This could be associated to the acquisition of antibiotic resistance [39]. The presence of these commensal MDR E. coli in hospital effluents underlines the role of humans as a source of antibiotic resistance in the environment.
In Algeria as well as many other developing countries, the use of antibiotics for human health and animal husbandry is not properly monitored and hospital effluents are continually discharged into the environment without pre-treatment. Selective pressure exerted by these antibiotics has several repercussions on public health and the environment, especially on the spread of antibiotic resistance. Our study revealed that Algerian hospital effluents carry MDR bacteria with several resistance genes, which could be transferred to different parts of the hydrological system such as surface water (used in agriculture) and sea water where they could reach aquatic animals as well as other neighboring countries. One cannot also exclude a possible transmission of MDR determinants to waterborne pathogens and their dissemination in the environment, which could lead to increased morbidity and mortality.
Owing to the global nature of the antimicrobial resistance problem, it is imperative for all countries to work together within a framework to better manage anthropogenic effluents, including hospital effluents, in order to limit the spread of MDR bacteria and antibiotic resistance genes.
Acknowledgments
The authors would like to thank Nancy Cloutier, Isabelle Robillard, Simon Wang, Karine Desjardins, Dominique Paquette, Annie Vezina, and François Robillard for their Lab assistance and Dr. Valentine Usongo for reviewing the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biyela PT, Lin J, Bezuidenhout CC. The role of aquatic ecosystems as reservoirs of antibiotic resistant bacteria and antibiotic resistance genes. Water Sci Technol. 2004;50(1):45–50. doi: 10.2166/wst.2004.0014. [DOI] [PubMed] [Google Scholar]
- 2.Kümmerer K. Resistance in the environment. J Antimicrob Chemother. 2004;54(2):311–320. doi: 10.1093/jac/dkh325. [DOI] [PubMed] [Google Scholar]
- 3.Korzeniewska E, Harnisz M. Beta-lactamase-producing Enterobacteriaceae in hospital effluents. J Environ Manag. 2013;123:1–7. doi: 10.1016/j.jenvman.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Pauwels B, Verstraete W. The treatment of hospital wastewater: an appraisal. J Water Health. 2006;4(4):405–416. doi: 10.2166/wh.2006.0024. [DOI] [PubMed] [Google Scholar]
- 5.Anssour L, Messai Y, Derkaoui M, Alouache S, Estepa V, Somalo S, Torres C, Bakour R. ESBL, plasmidic AmpC, and associated quinolone resistance determinants in coliforms isolated from hospital effluent: first report of qnrB2, qnrB9, qnrB19, and blaCMY-4 in Algeria. J Chemother. 2014;26(2):74–79. doi: 10.1179/1973947813Y.0000000115. [DOI] [PubMed] [Google Scholar]
- 6.Yousfi K, Touati A, Lefebvre B, Fournier É, Côté JC, Soualhine H, Walker M, Bougdour D, Tremblay C, Bekal S. A novel plasmid, pSx1, harboring a new Tn1696 derivative from extensively drug-resistant Shewanella xiamenensis encoding OXA-416. Microb Drug Resist. 2016;23(4):429–436. doi: 10.1089/mdr.2016.0025. [DOI] [PubMed] [Google Scholar]
- 7.Farmer JJ, Davis BR, Hickman-Brenner FW, et al. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 1985;21(1):46–76. doi: 10.1128/jcm.21.1.46-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. Wayne: CLSI; 2014. [Google Scholar]
- 9.Rasheed JK, Jay C, Metchock B, Berkowitz F, Weigel L, Crellin J, Steward C, Hill B, Medeiros AA, Tenover FC. Evolution of extended-spectrum beta-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother. 1997;41(3):647–653. doi: 10.1128/AAC.41.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monstein HJ, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dornbusch K, Nilsson LE. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS. 2007;115(12):1400–1408. doi: 10.1111/j.1600-0463.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 11.Woodford N, Ellington MJ, Coelho JM, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27(4):351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Brown S, Young HK, Amyes SG. Characterisation of OXA-51, a novel class D carbapenemase found in genetically unrelated clinical strains of Acinetobacter baumannii from Argentina. Clin Microbiol Infect. 2005;11(1):15–23. doi: 10.1111/j.1469-0691.2004.01016.x. [DOI] [PubMed] [Google Scholar]
- 13.Cattoir V, Poirel L, Rotimi V, Soussy CJ, Nordmann P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J Antimicrob Chemother. 2007;60(2):394–397. doi: 10.1093/jac/dkm204. [DOI] [PubMed] [Google Scholar]
- 14.Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob Agents Chemother. 2006;50(8):2872–2874. doi: 10.1128/AAC.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park CH, Robicsek A, Jacoby GA, Sahm D, Hooper DC. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob Agents Chemother. 2006;50(11):3953–3955. doi: 10.1128/AAC.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gow SP, Waldner CL, Harel J, Boerlin P. Associations between antimicrobial resistance genes in fecal generic Escherichia coli isolates from cow-calf herds in western Canada. Appl Environ Microbiol. 2008;74(12):3658–3666. doi: 10.1128/AEM.02505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maynard C, Fairbrother JM, Bekal S, Sanschagrin F, Levesque RC, Brousseau R, Masson L, Lariviere S, Harel J. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149:K91 isolates obtained over a 23-year period from pigs. Antimicrob Agents Chemother. 2003;47(10):3214–3221. doi: 10.1128/AAC.47.10.3214-3221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploy MC, Denis F, Courvalin P, Lambert T. Molecular characterization of integrons in Acinetobacter baumannii: description of a hybrid class 2 integron. Antimicrob Agents Chemother. 2000;44(10):2684–2688. doi: 10.1128/AAC.44.10.2684-2688.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakobsen L, Garneau P, Kurbasic A, et al. Microarray-based detection of extended virulence and antimicrobial resistance gene profiles in phylogroup B2 Escherichia coli of human, meat and animal origin. J Med Microbiol. 2011;60:1502–1511. doi: 10.1099/jmm.0.033993-0. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet C, Diarrassouba F, Brousseau R, Masson L, Topp E, Diarra MS. Pathotype and antibiotic resistance gene distributions of Escherichia coli isolates from broiler chickens raised on antimicrobial-supplemented diets. Appl Environ Microbiol. 2009;75(22):6955–6962. doi: 10.1128/AEM.00375-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66(10):4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapman TA, Wu XY, Barchia I, Bettelheim KA, Driesen S, Trott D, Wilson M, Chin JJC. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl Environ Microbiol. 2006;72(7):4782–4795. doi: 10.1128/AEM.02885-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau HY, Huffnagle GB, Moore TA. Host and microbiota factors that control Klebsiella pneumoniae mucosal colonization in mice. Microbes Infect. 2008;10(12–13):1283–1290. doi: 10.1016/j.micinf.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allocati N, Masulli M, Alexeyev MF, Di Ilio C. Escherichia coli in Europe: an overview. Int J Environ Res Public Health. 2013;10(12):6235–6254. doi: 10.3390/ijerph10126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hrenovic J, Durn G, Goic-Barisic I, Kovacic A. Occurrence of an environmental Acinetobacter baumannii strain similar to a clinical isolate in paleosol from Croatia. Appl Environ Microbiol. 2014;80(9):2860–2866. doi: 10.1128/AEM.00312-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen PL, Wu CJ, Tsai PJ, Tang HJ, Chuang YC, Lee NY, Lee CC, Li CW, Li MC, Chen CC, Tsai HW, Ou CC, Chen CS, Ko WC. Virulence diversity among bacteremic Aeromonas isolates: ex vivo, animal, and clinical evidences. PLoS One. 2014;9(issue 11):e111213. doi: 10.1371/journal.pone.0111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge SR. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol Rev. 2011;35(5):820–855. doi: 10.1111/j.1574-6976.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 28.Canton R, Gonzalez-Alba JM, Galan JC. CTX-M enzymes: origin and diffusion. Front Microbiol. 2012;3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gharout-Sait A, Touati A, Benallaaoua S, et al. CTX-M from community-acquired urinary tract, Algeria. Afr J Microbiol Res. 2012;6(25):5306–5313. [Google Scholar]
- 30.Brahmi S, Dunyach-Remy C, Touati A, Lavigne JP. CTX-M-15-producing Escherichia coli and the pandemic clone O25b-ST131 isolated from wild fish in Mediterranean Sea. Clin Microbiol Infect. 2015;21(3):e18–e20. doi: 10.1016/j.cmi.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Marinescu F, Marutescu L, Savin I, Lazar V. Antibiotic resistance markers among Gram-negative isolates from wastewater and receiving rivers in South Romania. Rom Biotechnol Lett. 2015;20(1):10055–10069. [Google Scholar]
- 32.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 33.Bakour S, Touati A, Bachiri T, Sahli F, Tiouit D, Naim M, Azouaou M, Rolain JM. First report of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and rapid spread of metallo-beta-lactamase NDM-1 in Algerian hospitals. J Infect Chemother. 2014;20(issue 11):696–701. doi: 10.1016/j.jiac.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Brahmi S, Touati A, Cadiere A, et al. First description of two sequence type 2 Acinetobacter baumannii isolates carrying OXA-23 carbapenemase in Pagellus acarne fished from the Mediterranean Sea near Bejaia, Algeria. Antimicrob Agents Chemother. 2016;60(4):2513–2515. doi: 10.1128/AAC.02384-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loucif L, Kassah-Laouar A, Saidi M, Messala A, Chelaghma W, Rolain JM. Outbreak of OXA-48-producing Klebsiella pneumoniae involving a sequence type 101 clone in Batna university hospital, Algeria. Antimicrob Agents Chemother. 2016;60(12):7494–7497. doi: 10.1128/AAC.00525-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baba Ahmed-Kazi Tani Z, Arlet G. News of antibiotic resistance among Gram-negative bacilli in Algeria. Pathol Biol. 2014;62(3):169–178. doi: 10.1016/j.patbio.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Xia R, Han N, Xu H. Genetic diversity analyses of class 1 integrons and their associated antimicrobial resistance genes in Enterobacteriaceae strains recovered from aquatic habitats in China. Lett Appl Microbiol. 2011;52(6):667–675. doi: 10.1111/j.1472-765X.2011.03059.x. [DOI] [PubMed] [Google Scholar]
- 38.Anssour L, Messai Y, Estepa V, Torres C, Bakour R. Characteristics of ciprofloxacin-resistant Enterobacteriaceae isolates recovered from wastewater of an Algerian hospital. J Infect Dev Ctries. 2016;10(7):728–734. doi: 10.3855/jidc.6727. [DOI] [PubMed] [Google Scholar]
- 39.Horcajada JP, Soto S, Gajewski A, Smithson A, Jimenez de Anta MT, Mensa J, Vila J, Johnson JR. Quinolone-resistant uropathogenic Escherichia coli strains from phylogenetic group B2 have fewer virulence factors than their susceptible counterparts. J Clin Microbiol. 2005;43(6):2962–2964. doi: 10.1128/JCM.43.6.2962-2964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]