Graphical abstract
Abstract
The co-evolution of the microbiota and immune system has forged a mutually beneficial relationship. This relationship allows the host to maintain the balance between active immunity to pathogens and vaccines and tolerance to self-antigens and food antigens. In children living in low-income and middle-income countries, undernourishment and repetitive gastrointestinal infections are associated with the failure of oral vaccines. Intestinal dysbiosis associated with these environmental influences, as well as some host-related factors, compromises immune responses and negatively impacts vaccine efficacy. To understand how immune responses to viral vaccines can be optimally modulated, mechanistic studies of the relationship between the microbiome, host genetics, viral infections and the development and function of the immune system are needed. We discuss the potential role of the microbiome in modulating vaccine responses in the context of a growing understanding of the relationship between the gastrointestinal microbiota, host related factors (including histo-blood group antigens) and resident immune cell populations.
Current Opinion in Virology 2019, 37:16–25
This review comes from a themed issue on Viruses and the microbiome
Edited by Stephanie M Karst and Christiane E Wobus
For a complete overview see the Issue and the Editorial
Available online 1st June 2019
https://doi.org/10.1016/j.coviro.2019.05.001
1879-6257/© 2019 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Numerous factors influence vaccine efficacy including nutrition, sex, age, genetics, and health status and these vary greatly between low-income and middle-income (LMIC) and high-income (HIC) countries as reviewed elsewhere [1••]. Immune responses to oral cholera, poliovirus (PV) and rotavirus (RV) vaccines are significantly lower in children from LMICs than those of children from HICs [2]. For example, only 58% of Nicaraguan and 46% of Bangladeshi children respond to oral RV vaccine while the efficacy of the vaccine in Finland is over 98% [3, 4, 5]. The poor immune responsiveness to vaccination characteristic for LMICs is multifactorial and is associated with host-associated (genetics and commensal microbiota), pathogen-associated (genetic diversity) and environmental factors [6, 7, 8,9••,10,11•] (Figure 1 ).
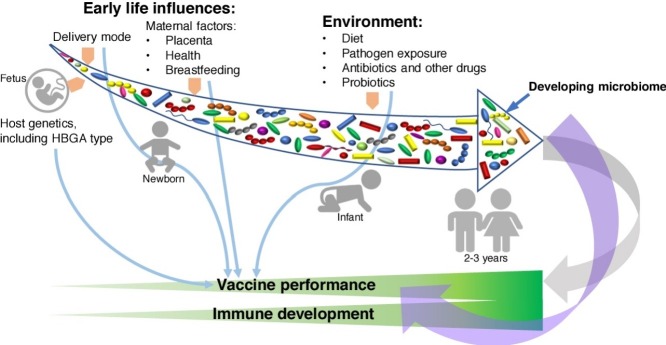
Figure 1.
A schematic illustration of how the microbiota may influence vaccine responses. There are interconnections between the intestinal microbiota and the immune response. This mutualistic relationship is bidirectional with intestinal microbiota influencing immune system development and functions, and the immune system modulating microbial diversity and controlling its anatomical constraint. Effective vaccines should be capable of eliciting protective immune responses against the viral agents administered, whereas microbiota composition and diversity modulate the immune response to vaccines directly and indirectly (via regulating gut barrier function).
The diverse communities of microbes that colonize the gut and other body surfaces (microbiome) have coevolved with their host species in a symbiotic relationship [12•]. The microbiome as a whole or its individual members play important roles in the development and functionality of the immune system [13•]. Colonization occurs mostly during birth and soon after, and the microbiome becomes mature by ∼2 years of age. Until then, the microbial composition is highly variable and can be easily perturbed by various environmental exposures [14••,15•].
The environmental influences of early life that affect the microbiome and can therefore alter vaccine efficacy include diet, delivery method (vaginal versus caesarean section), and hygiene (clean versus unsanitary environment) [16, 17, 18]. Additionally, breastfeeding is known to modulate vaccine responses in infants via altering the microbiome composition and maternal antibody interference with the development of the active (post-vaccine) immune response [19]. High levels of neutralizing antibodies (Ab) in the breast milk of mothers from LMICs were shown to correlate with lower seroconversion rates in their children, suggestive of maternal Ab interference with the ‘take’ of oral RV vaccines. However, restriction of breastfeeding did not enhance the IgA immune response to oral RV vaccines in children [20•,21].
A combination of gut microbiome alterations associated with negative health effects is known as intestinal or enteric dysbiosis. The latter is often accompanied by environmental enteric dysfunction (EED, environmental enteropathy) representing structural and functional disorder of the small intestine that is frequently seen in children from the LMICs or patients with chronic inflammatory diseases. Thus, differences in the efficacy of oral RV and PV as well as other vaccines observed in LMICs can result from enteric dysbiosis and EED [1••,22,23]. In agreement with these observations, celiac disease patients have decreased seroconversion to hepatitis B vaccine [24•]. Also, chronic infections with many viruses including human cytomegalovirus and human immunodeficiency virus induce immune suppression decreasing the effectiveness of many vaccines [25]. However studies on the interactions between the resident virome and host immune function are scarce [26,27]. Collectively, these observations emphasize that ‘healthy’ gut microbiome is the key element that maintains intestinal homeostasis critical for optimal vaccine performance.
Influence of the intestinal microbiome composition on vaccine responses
Since the gut microbiota is intimately linked with immune system development, it is likely that immune responses to all vaccines are modulated by distinct microbial profiles [28]. Systemically immunized GF and antibiotic treated conventional mice showed decreased serum Ab and T-cell responses compared to the age-matched conventional animals [29••]. The introduction of the normal microbiota to the GF mice improved their immune responsiveness following immunization [29••]. In addition, the nasal microbiota also contributes to immune responses to live influenza vaccine, by modifying antiviral IgA Ab production [30]. However, comprehensive studies that directly evaluate how the gut microbiota, as a whole or the individual major microbial taxa, influence immune responses to vaccines are limited.
In a recent study, transplantation of dysbiotic microbiota from stunted Nicaraguan infants decreased numbers of intestinal and systemic effector T cells that coincided with decreased protection against human RV (HRV) challenge in vaccinated human microbiota-associated (HMA) piglets [31]. Numerically decreased IgA and IgG Ab responses were also observed in the dysbiotic HMA piglets further emphasizing the suboptimal HRV vaccine performance associated with intestinal dysbiosis. Importantly this study emphasizes that dysbiotic microbiome is the primary factor associated with the decreased immune responses, because unlike in infant donors, EED was not evident in the HMA piglets. Further, these results and our previous data [32] suggest that specific pathological microbial signatures can be maintained after dysbiosis was induced and can even be transferrable to another host, negatively affecting their immunity. The latter indicates that maternal dysbiosis can be transferred to infants and leading to health and developmental setbacks in early life.
Further, Lactobacillus rhamnosus strain GG (LGG), in a dose-dependent manner, enhanced innate, cytokine, and cellular T cell responses in HMA piglets vaccinated with HRV vaccine [33]. However, the enhanced immune responses did not translate into increased Ab levels [34]. These findings are in contrast to a previous study from the same group that demonstrated that LGG supplementation enhanced HRV Ab responses in GF piglets [35], which emphasizes the importance of evaluating probiotics in the context of the microbiome.
In another study from the Netherlands, higher levels of RV shedding were observed in adults receiving antibiotic treatment before vaccination against RV compared with controls receiving no antibiotic treatment [36••]. This suggests that a dysbiotic microbiome (with decreased abundance of different bacterial taxa or with altered composition) alters the replication of the attenuated RV vaccine. Further, the altered RV replication was not associated with changes in RV-specific IgA Ab levels, suggesting that it could have resulted from direct RV–microbiota interactions [36••].
Impact of nutritional status on the microbiota and vaccine effectiveness
Most frequently, diet is the primary factor that defines the diversity and functions of the gut microbiota and epigenetically re-programs the host metabolism [15,37, 38, 39]. While balanced fiber-rich diets maintain a healthy microbiota [15•,37], disturbances in the nutritional status (especially in children) impair immune responses and compromise gut barrier integrity [40]. Specifically, protein deprivation leads to intestinal dysbiosis, epithelial breaches, altered metabolism, EED, and immune deficiencies in malnourished children [41,42], which in turn promotes opportunistic infections [43]. Clinical studies show that childhood malnutrition is associated with lower seroconversion rates of oral vaccines [45,46] and contributes to almost half of all deaths of children under five years old [42]. Therapeutic food interventions have reduced mortality in children with severe acute malnutrition, but persistent immaturity of the gut microbiota and the associated incomplete restoration of healthy growth and metabolism remains a major problem [14••]. Vaccinated protein-deficient pigs had lower protection rates against human RV diarrhea and significantly increased fecal virus shedding titers compared with their protein-sufficient counterparts, which coincided with suppression of multiple innate and adaptive immune responses [44]. These results confirm the negative effects of protein-calorie malnutrition (PCM) on immune responses to HRV and on vaccine efficacy, which were exacerbated in the HMA versus GF pigs. In this study, PCM decreased the Firmicutes-to-Bacteroides ratios post-challenge, which coincided with increased abundance of Proteus in the gut, while decreased levels of Turicibacter were observed in spleen and ileum [44]. While Proteus species are mostly associated with pro-inflammatory responses, playing roles in the pathogenesis of many gastrointestinal disorders, including Crohn’s disease [45], the relative abundance of Turicibacter species correlates with adequate immune function in mice [46,47].
Additionally, undernourishment is often associated with specific micronutrient deficiencies and deficiencies in key microbiota-derived metabolites. For example, vitamin A deficiency (VAD) affects millions of children in LMICs [48] and leads to significantly impaired mucosal immunity and oral vaccine efficacy [49,50]. Our studies in neonatal GF pigs demonstrated that the protective efficacy of human monovalent and pentavalent RV vaccines and immunoregulatory responses were compromised by VAD [51,52]. Vitamin A derivative, retinoic acid (RA), is required for activation of gut dendritic cells and for imprinting gut homing receptors (CCR9 and α4β7) on vaccine induced B and T cells [53, 54, 55, 56]. Currently, little direct evidence is available on the interactions between the intestinal commensals and vitamin A—both key regulators of intestinal health. In a recent study, VAD induced substantial alterations of the bacterial community structure and meta-transcriptome [57]. Bacteroides vulgatus was identified as a prominent responder to vitamin A status with increased abundance in VAD [57]. VAD was shown to be associated with altered bile acid metabolism in vivo, suggesting that retinol and bile acid metabolites may interact altering microbial communities in the gut [57]. Additionally, growing evidence suggests that the gut microbiota, through its effects on bile acids [58], short chain fatty acids (SCFA) and cholesterol metabolism in the gut lumen, can in turn affect intake and metabolism of all fat-soluble vitamins, including vitamin A [59].
Further, microbiota-associated intestinal inflammation can alter RA levels [60•] affecting the balance between immunoregulatory and inflammatory T cells, emphasizing that the interactions between gut microbiota and vitamin A are bidirectional in nature. Zinc is another micronutrient that is often deficient in low income settings and affects the efficiency of oral vaccines. Mice on a zinc-deficient diet had decreased Th1 and IgG responses to a Hepatitis B vaccine [61]. While zinc deficiency is linked with poor vaccine responses and can modify the composition of the microbiota [62], direct competition between intestinal bacteria and the host for zinc may explain why oral supplementation of zinc does not compensate for its deficiency.
Collectively, these recent studies suggest that while nutrient imbalance and dysbiotic microbiome induce negative effects on host health, immunity and intestinal barrier, their synergistic interactions are implicated in the pathogenesis of severe childhood PCM.
Microbial interactions with resident immune cells
The significance of the microbial–immune interactions is best exemplified by the fact that the immune system of germ-free (GF) mice and pigs is anatomically and functionally immature [63,64••,65,66••], while colonization of the GF animals with commensal microbiota or even individual probiotic species corrects these defects [67•,68,69••,70, 71, 72]. Further, individual commensal microorganisms and their metabolites induce maturation of the intestinal and systemic immune systems [71,73] and modulate cellular signaling as summarized by Valdez et al. [1••]. Besides induction of immune maturation, microbiota regulates the state of hypo-responsiveness against commensals, self-antigens, and food antigens [72,74•].
Immune cells are equipped with various innate immune receptors [including Toll-like receptors (TLR) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs)] to recognize and interact with microbial cells. Our recent study comparing TLR expression profiles in GF and conventional newborn and young pigs demonstrated that exposure to commensal microbiota has a greater influence on TLR mRNA expression than age [75]. TLRs on epithelial and dendritic cells (that can extend their dendrites through the epithelial layer) come into direct contact with luminal microorganisms [76]. These TLR–microbial interactions induce production of anti-microbial peptides and secretory (polymeric) IgA by epithelial and immune cells, respectively [77,78]. Binding of anti-microbial peptides and IgA to the mucus layer that separates commensal bacteria from the apical surface of intestinal epithelial cells (IECs) constrains the topography, composition, and functions of commensal bacteria, thereby preventing inflammatory reactions [79].
Intestinal dysbiosis associated with inadequate diets, antibiotic use, and enteric pathogens cause local inflammation, altering intestinal barrier function. This results in aberrant interactions between immune cells and microbes, which triggers systemic inflammation and autoimmunity, as reported in type 1 diabetes [80]. Further, numerous mouse models deficient in TLRs/NLRs and/or their signaling adaptor proteins (such as MyD88) develop intestinal dysbiosis [81] which leads to the development of various diseases [82]. The latter illustrates the role of genetic factors in dysbiosis development. Often transfer of this ‘dysbiotic microbiota’ into naïve hosts is sufficient to induce disease and reproduce its pathogenesis [32,83,84••]. Therefore, this complex two-way communication plays a critical role in intestinal health and disease.
Finally, although it is recognized that the gut microbiota composition influences systemic immunity, the exact mechanisms of how the gut bacteria exert the immunomodulatory effects at systemic immune sites remain largely unknown [85]. Two alternative theories postulate that these effects can be achieved either via secretion and systemic circulation of microbiota-derived soluble factors [86] or via migration of the activated lamina propria lymphocytes into the periphery [87].
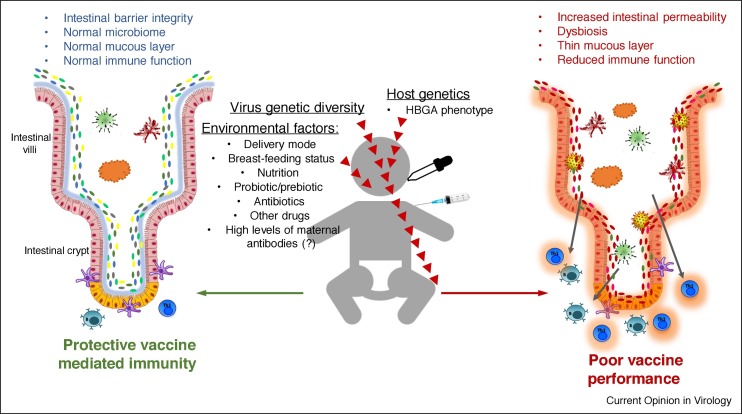
Relationships between gastrointestinal microbiota, RV vaccines and histo-blood group antigens
ABO/H and Lewis family antigens are recognized as receptors by numerous pathogens, including, noroviruses (NoVs), RVs, and coronaviruses [88, 89, 90, 91, 92, 93, 94] and the severity of RV disease is reportedly higher in children with blood group A [95]. Several recent studies demonstrated that host histo-blood group antigen (HBGA) phenotypes impact incidence of all-cause diarrhea [96] and the efficacy of oral RV vaccines by altering susceptibility to RV in unvaccinated children [97]. Lewis A phenotype was shown to be a restriction factor for RotaTeq and Rotarix vaccine-take [98].
The polymorphic HBGAs, including the ABO/H, secretor and Lewis families, are found on red blood cells, mucosal epithelia [99] and in biologic fluids (saliva, intestinal contents, milk, and blood) of secretor individuals [100]. The nonsecretor homozygous genotype in fucosyltransferase 2 (FUT2; occurring due to natural polymorphisms) is associated with resistance to NoV genogroup II and Helicobacter pylori infections that vary with ethnicities [101,102]. Distinct HBGA phenotypes also result in variable binding specificities of innate immune factors such as the galectins [103] or differential profiles of circulating natural Abs [104]. Different HBGAs are also observed in animals, including pigs that express A and H antigens (that share high identity with those of humans), which may facilitate zoonotic and reverse zoonotic infections [105]. Further, certain lactobacilli were shown to bind HBGAs [106,107] while some enteric bacteria [108] produce HBGA-like substances suggesting that such bacteria can directly bind some enteric viruses [108]. Collectively, these data suggest that commensal and pathogenic microorganisms have evolved that exploit human and animal HBGAs as cellular receptors.
Further, recent data showed that healthy subjects with different HBGA possessed distinct profiles of fecal microbiota [109•,110]. The nonsecretor status was also linked to the gut microbiome alterations in patients with inflammatory bowel disease [111]. These provide examples of the influence of host genetics on the intestinal microbiome composition and suggests that HBGA-mediated modulation of the intestinal microbiome may contribute to the observed variations in the vaccine efficacy. Thus, future vaccine trials should account for HBGA status or offer optimized multivalent vaccines that can perform equally well in hosts with different HBGAs.
Probiotic impacts on vaccine responses
As discussed above, live, oral vaccines, including RV and PV vaccines, have historically underperformed in the LMICs. Alterations in the intestinal microbiota are currently being recognized as a leading factor associated with vaccine failures, and their effects are being evaluated in human clinical trials. Supplementation with specific strains of probiotics has been shown to have modulatory effects on intestinal and systemic immune responses in animal models providing the basis for studies with vaccines in humans [72,73,112,113] (Figure 2 ). However, most clinical studies conducted in children and adults generated results that varied by age, antigen, type of Ab response, and probiotic strain [19]. The mechanisms that influence vaccine performance include direct or indirect immunomodulatory actions of probiotics that have yet to be fully evaluated experimentally. Direct effects include alteration of the pathogen-associated molecular patterns presented to the gut-associated lymphoid tissue. Indirect effects can be mediated by changes to the gut microbiota and immunoactive microbial metabolites such as SCFA [114]. Probiotics also affect the functions of IECs through modulation of tight junctions, increasing mucin and defensin production, inducing antimicrobial and heat shock protein production, interfering with pathogenic organisms, and modulating signaling pathways and cell survival [115, 116, 117, 118]. Certain probiotic strains have been shown to enhance Ab responses to oral vaccines against RV [35,119, 120, 121], Salmonella [122], PV [123], and Vibrio cholerae [124] in human volunteers, and this effect was observed after a short period (1–5 weeks) of probiotic treatment. The positive effect of probiotics on immune responses was also seen in parenterally administered vaccines against diphtheria, tetanus, Haemophilus influenzae type B, and hepatitis B [125, 126, 127] in infants after a six-month period. However, no mechanistic explanations for the observed effects or detailed microbiome analysis were available from these studies.
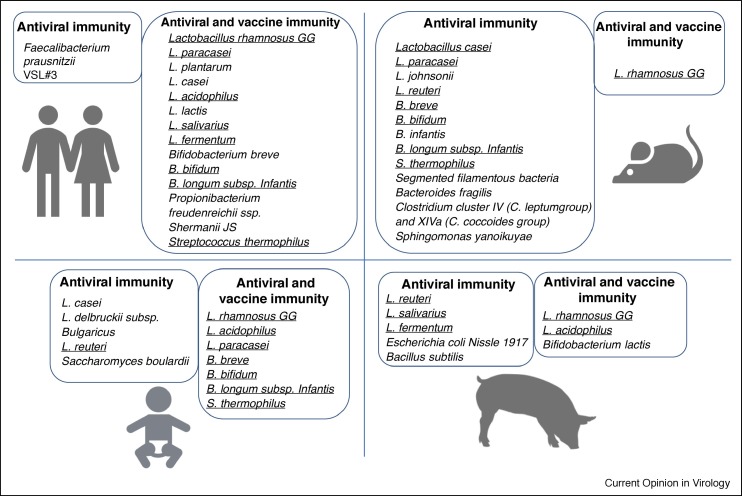
Figure 2.
Probiotic and commensal bacteria that possess immunomodulatory/antiviral [1••,71,73] and vaccine adjuvant [112,113,120,121,128•] properties as demonstrated in human clinical trials and animal experiments in pigs and mice. Bacterial species that were shown to possess immunomodulatory properties in two or more species are underlined.
Concluding remarks and outstanding questions
Despite extensive research and plentiful novel data, there still is a need for improved understanding of the role of microbiota in the immune responses to vaccines. To establish causal relationships between the composition of microbiota and responses to oral and parenteral vaccines, additional clinical and experimental studies are needed. Large-scale studies correlating the effects of diets, probiotics, and antibiotics with pre-vaccine microbial diversity and post-vaccine immune responses will identify environment and microbiota-driven effects. Adoptive transfer of the dysbiotic microbiomes to GF animals would define the function and reversibility of the microbiome alterations. While most studies conducted thus far have been limited to general changes in microbial profiles, a detailed analysis of the role of a particular bacterial species or community and their correlation with vaccine responses is lacking. The lack of clearly defined ‘healthy’ baseline microbiome makes it challenging to identify optimal and suboptimal microbiome composition for viral vaccines. To further improve post-vaccine responses, new adjuvants based on particular microbiota-derived immune-modulating molecules or bioactive micronutrients should be evaluated. The ever-expanding knowledge on how the microbiota is altered by the host and environmental factors and the downstream effects of these alterations need to be considered in the design of future vaccines.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was partially supported by grants from the NIAID at NIH (grant # R01 A1099451) and Bill and Melinda Gates Foundation (OPP1117461), as well as federal funds appropriated to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University. Salaries and research support were provided by state and federal funds provided to the OARDC, The Ohio State University.
References
- 1••.Valdez Y., Brown E.M., Finlay B.B. Influence of the microbiota on vaccine effectiveness. Trends immunol. 2014;35:526–537. doi: 10.1016/j.it.2014.07.003. [DOI] [PubMed] [Google Scholar]; A comprehensive review on the intercations between the gut microbiota and vaccine-associated immune responses and protection: evidence derived from clinical trials and animal experiments.
- 2.Kirkpatrick B.D., Colgate E.R., Mychaleckyj J.C., Haque R., Dickson D.M., Carmolli M.P., Nayak U., Taniuchi M., Naylor C., Qadri F., et al. The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. Am J Trop Med Hyg. 2015;92:744–751. doi: 10.4269/ajtmh.14-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel M., Pedreira C., De Oliveira L.H., Tate J., Orozco M., Mercado J., Gonzalez A., Malespin O., Amador J.J., Umana J., et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 4.Zaman K., Dang D.A., Victor J.C., Shin S., Yunus M., Dallas M.J., Podder G., Vu D.T., Le T.P., Luby S.P., et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 5.Armah G.E., Sow S.O., Breiman R.F., Dallas M.J., Tapia M.D., Feikin D.R., Binka F.N., Steele A.D., Laserson K.F., Ansah N.A., et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 6.Rook G.A., Dheda K., Zumla A. Immune systems in developed and developing countries; implications for the design of vaccines that will work where BCG does not. Tuberculosis. 2006;86:152–162. doi: 10.1016/j.tube.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Parker E.P., Kampmann B., Kang G., Grassly N.C. Influence of enteric infections on response to oral poliovirus vaccine: a systematic review and meta-analysis. J Infect Dis. 2014;210:853–864. doi: 10.1093/infdis/jiu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahishali E., Boztas G., Akyuz F., Ibrisim D., Poturoglu S., Pinarbasi B., Ozdil S., Mungan Z. Response to hepatitis B vaccination in patients with celiac disease. Dig Dis Sci. 2008;53:2156–2159. doi: 10.1007/s10620-007-0128-3. [DOI] [PubMed] [Google Scholar]
- 9••.Bjorksten B. Diverse microbial exposure—consequences for vaccine development. Vaccine. 2012;30:4336–4340. doi: 10.1016/j.vaccine.2011.10.074. [DOI] [PubMed] [Google Scholar]; This review discusses the effects of gut microbiota on immune functionality and vaccine efficacy form the ‘hygiene hypothesis’ perspective.
- 10.McElhaney J.E., Zhou X., Talbot H.K., Soethout E., Bleackley R.C., Granville D.J., Pawelec G. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–2067. doi: 10.1016/j.vaccine.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Pulendran B. Systems vaccinology: probing humanity’s diverse immune systems with vaccines. Proc Natl Acad Sci U S A. 2014;111:12300–12306. doi: 10.1073/pnas.1400476111. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review that highlights how ‘systems biology’ approach can aid in identification of molecular signatures predictive of vaccination outcomes and how such findings can be used to to reprogram suboptimal immune systems.
- 12•.Brown E.M., Sadarangani M., Finlay B.B. The role of the immune system in governing host-microbe interactions in the intestine. Nat Immunol. 2013;14:660–667. doi: 10.1038/ni.2611. [DOI] [PubMed] [Google Scholar]; Complex interactions and co-influences between the gut microbiota and immune system.
- 13•.Jamieson A.M. Influence of the microbiome on response to vaccination. Hum Vaccines Immunother. 2015;11:2329–2331. doi: 10.1080/21645515.2015.1022699. [DOI] [PMC free article] [PubMed] [Google Scholar]; A short review that discusses molecular and immunological mechanisms of vaccine hyporesponsiveness and the role of gut microbiota in it.
- 14••.Subramanian S., Huq S., Yatsunenko T., Haque R., Mahfuz M., Alam M.A., Benezra A., DeStefano J., Meier M.F., Muegge B.D., et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that severe acute childhood malnutrition permanently alters the development of gut microbiome that can only partially be rescued by niutritional interventions.
- 15•.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified and discussed significant differences in the composition of the gut microbiome in children and adults from developing and developed countries.
- 16.Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., van den Brandt P.A., Stobberingh E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 17.Jost T., Lacroix C., Braegger C.P., Chassard C. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guaraldi F., Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:94. doi: 10.3389/fcimb.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Praharaj I., John S.M., Bandyopadhyay R., Kang G. Probiotics, antibiotics and the immune responses to vaccines. Philos Trans R Soc Lond Ser B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Rongsen-Chandola T., Strand T.A., Goyal N., Flem E., Rathore S.S., Arya A., Winje B.A., Lazarus R., Shanmugasundaram E., Babji S., et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine. 2014;32(Suppl. 1):A134–A139. doi: 10.1016/j.vaccine.2014.04.078. [DOI] [PubMed] [Google Scholar]; The authors demonstrated that in contrast to the expectations, witholding of breastffeding did not improve seroconversion rates following rotavirus vaccination.
- 21.Groome M.J., Moon S.S., Velasquez D., Jones S., Koen A., van Niekerk N., Jiang B., Parashar U.D., Madhi S.A. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Organ. 2014;92:238–245. doi: 10.2471/BLT.13.128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass R.I., Parashar U., Patel M., Gentsch J., Jiang B. Rotavirus vaccines: successes and challenges. J Infect. 2014;68(Suppl. 1):S9–S18. doi: 10.1016/j.jinf.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Gilmartin A.A., Petri W.A., Jr. Exploring the role of environmental enteropathy in malnutrition, infant development and oral vaccine response. Philos Trans R Soc Lond Ser B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Vitaliti G., Pratico A.D., Cimino C., Di Dio G., Lionetti E., La Rosa M., Leonardi S. Hepatitis B vaccine in celiac disease: yesterday, today and tomorrow. World J Gastroenterol. 2013;19:838–845. doi: 10.3748/wjg.v19.i6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study demonstrated that celiac disease decreases immune responsiveness to HBV vaccine.
- 25.Falconer O., Newell M.L., Jones C.E. The effect of human immunodeficiency virus and cytomegalovirus infection on infant responses to vaccines: a review. Front Immunol. 2018;9:328. doi: 10.3389/fimmu.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freer G., Maggi F., Pifferi M., Di Cicco M.E., Peroni D.G., Pistello M. The virome and its major component, anellovirus, a convoluted system molding human immune defenses and possibly affecting the development of asthma and respiratory diseases in childhood. Front Microbiol. 2018;9:686. doi: 10.3389/fmicb.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cadwell K. The virome in host health and disease. Immunity. 2015;42:805–813. doi: 10.1016/j.immuni.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira R.B., Antunes L.C., Finlay B.B. Should the human microbiome be considered when developing vaccines? PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Lamouse-Smith E.S., Tzeng A., Starnbach M.N. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027662. [DOI] [PMC free article] [PubMed] [Google Scholar]; A series of experiments in neonatal and germ-fre mice revealed that normal (unaltered) intestinal mirobiota is critical for efficient antibody responses following systemic vaccination.
- 30.Salk H.M., Simon W.L., Lambert N.D., Kennedy R.B., Grill D.E., Kabat B.F., Poland G.A. Taxa of the nasal microbiome are associated with influenza-specific IgA response to live attenuated influenza vaccine. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twitchell E.L., Tin C., Wen K., Zhang H., Becker-Dreps S., Azcarate-Peril M.A., Vilchez S., Li G., Ramesh A., Weiss M., et al. Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs. Gut Pathog. 2016;8:51. doi: 10.1186/s13099-016-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlasova A.N., Paim F.C., Kandasamy S., Alhamo M.A., Fischer D.D., Langel S.N., Deblais L., Kumar A., Chepngeno J., Shao L., et al. Protein malnutrition modifies innate immunity and gene expression by intestinal epithelial cells and human rotavirus infection in neonatal gnotobiotic pigs. mSphere. 2017;2 doi: 10.1128/mSphere.00046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Gao K., Wen K., Allen I.C., Li G., Zhang W., Kocher J., Yang X., Giri-Rachman E., Li G.H., et al. Lactobacillus rhamnosus GG modulates innate signaling pathway and cytokine responses to rotavirus vaccine in intestinal mononuclear cells of gnotobiotic pigs transplanted with human gut microbiota. BMC Microbiol. 2016;16:109. doi: 10.1186/s12866-016-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen K., Tin C., Wang H., Yang X., Li G., Giri-Rachman E., Kocher J., Bui T., Clark-Deener S., Yuan L. Probiotic Lactobacillus rhamnosus GG enhanced Th1 cellular immunity but did not affect antibody responses in a human gut microbiota transplanted neonatal gnotobiotic pig model. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen K., Liu F., Li G., Bai M., Kocher J., Yang X., Wang H., Clark-Deener S., Yuan L. Lactobacillus rhamnosus GG dosage affects the adjuvanticity and protection against rotavirus diarrhea in gnotobiotic pigs. J Pediatr Gastroenterol Nutr. 2015;60:834–843. doi: 10.1097/MPG.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 36••.Harris V.C., Haak B.W., Handley S.A., Jiang B., Velasquez D.E., Hykes B.L., Jr., Droit L., Berbers G.A.M., Kemper E.M., van Leeuwen E.M.M., et al. Effect of antibiotic-mediated microbiome modulation on rotavirus vaccine immunogenicity: a human, randomized-control proof-of-concept trial. Cell Host Microbe. 2018;24:197–207. doi: 10.1016/j.chom.2018.07.005. e194. [DOI] [PMC free article] [PubMed] [Google Scholar]; A clinical trial-derived evidence that microbiome alterations altear rotavirus vaccine immunogenicity.
- 37.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., Almeida M., Arumugam M., Batto J.M., Kennedy S., et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 38.McGowan P.O., Meaney M.J., Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Res. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn O., Gronke S., Stubbs T.M., Ficz G., Hendrich O., Krueger F., Andrews S., Zhang Q., Wakelam M.J., Beyer A., et al. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol. 2017;18:56. doi: 10.1186/s13059-017-1187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Santis S., Cavalcanti E., Mastronardi M., Jirillo E., Chieppa M. Nutritional keys for intestinal barrier modulation. Front Immunol. 2015;6:612. doi: 10.3389/fimmu.2015.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prendergast A.J., Kelly P. Interactions between intestinal pathogens, enteropathy and malnutrition in developing countries. Curr Opin Infect Dis. 2016;29:229–236. doi: 10.1097/QCO.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerrant R.L., Oria R.B., Moore S.R., Oria M.O., Lima A.A. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korpe P.S., Petri W.A., Jr. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyazaki A., Kandasamy S., Michael H., Langel S.N., Paim F.C., Chepngeno J., Alhamo M.A., Fischer D.D., Huang H.C., Srivastava V., et al. Protein deficiency reduces efficacy of oral attenuated human rotavirus vaccine in a human infant fecal microbiota transplanted gnotobiotic pig model. Vaccine. 2018;36:6270–6281. doi: 10.1016/j.vaccine.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton A.L., Kamm M.A., Ng S.C., Morrison M. Proteus spp. as putative gastrointestinal pathogens. Clin Microbiol Rev. 2018;31 doi: 10.1128/CMR.00085-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Presley L.L., Ye J., Li X., Leblanc J., Zhang Z., Ruegger P.M., Allard J., McGovern D., Ippoliti A., Roth B., et al. Host-microbe relationships in inflammatory bowel disease detected by bacterial and metaproteomic analysis of the mucosal-luminal interface. Inflamm Bowel Dis. 2012;18:409–417. doi: 10.1002/ibd.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimitriu P.A., Boyce G., Samarakoon A., Hartmann M., Johnson P., Mohn W.W. Temporal stability of the mouse gut microbiota in relation to innate and adaptive immunity. Environ Microbiol Rep. 2013;5:200–210. doi: 10.1111/j.1758-2229.2012.00393.x. [DOI] [PubMed] [Google Scholar]
- 48.Wirth J.P., Petry N., Tanumihardjo S.A., Rogers L.M., McLean E., Greig A., Garrett G.S., Klemm R.D., Rohner F. Vitamin A supplementation programs and country-level evidence of vitamin A deficiency. Nutrients. 2017;9 doi: 10.3390/nu9030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall J.A., Grainger J.R., Spencer S.P., Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiedermann U., Hanson L.A., Holmgren J., Kahu H., Dahlgren U.I. Impaired mucosal antibody response to cholera toxin in vitamin A-deficient rats immunized with oral cholera vaccine. Infect Immun. 1993;61:3952–3957. doi: 10.1128/iai.61.9.3952-3957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vlasova A.N., Chattha K.S., Kandasamy S., Siegismund C.S., Saif L.J. Prenatally acquired vitamin A deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J Immunol. 2013;190:4742–4753. doi: 10.4049/jimmunol.1203575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kandasamy S., Chattha K.S., Vlasova A.N., Saif L.J. Prenatal vitamin A deficiency impairs adaptive immune responses to pentavalent rotavirus vaccine (RotaTeq®) in a neonatal gnotobiotic pig model. Vaccine. 2014;32:816–824. doi: 10.1016/j.vaccine.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 53.Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., Otipoby K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 54.Hammerschmidt S.I., Friedrichsen M., Boelter J., Lyszkiewicz M., Kremmer E., Pabst O., Forster R. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klebanoff C.A., Spencer S.P., Torabi-Parizi P., Grainger J.R., Roychoudhuri R., Ji Y., Sukumar M., Muranski P., Scott C.D., Hall J.A., et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med. 2013;210:1961–1976. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall J.A., Cannons J.L., Grainger J.R., Dos Santos L.M., Hand T.W., Naik S., Wohlfert E.A., Chou D.B., Oldenhove G., Robinson M., et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hibberd M.C., Wu M., Rodionov D.A., Li X., Cheng J., Griffin N.W., Barratt M.J., Giannone R.J., Hettich R.L., Osterman A.L., et al. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swann J.R., Want E.J., Geier F.M., Spagou K., Wilson I.D., Sidaway J.E., Nicholson J.K., Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A. 2011;108(Suppl. 1):4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 60•.Bhattacharya N., Yuan R., Prestwood T.R., Penny H.L., DiMaio M.A., Reticker-Flynn N.E., Krois C.R., Kenkel J.A., Pham T.D., Carmi Y., et al. Normalizing microbiota-induced retinoic acid deficiency stimulates protective CD8(+) T cell-mediated immunity in colorectal cancer. Immunity. 2016;45:641–655. doi: 10.1016/j.immuni.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent study revealing co-regulatory interactions between vitamin A, gut microbiota and immune cells.
- 61.Zhao N., Wang X., Zhang Y., Gu Q., Huang F., Zheng W., Li Z. Gestational zinc deficiency impairs humoral and cellular immune responses to hepatitis B vaccination in offspring mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gielda L.M., DiRita V.J. Zinc competition among the intestinal microbiota. mBio. 2012;3 doi: 10.1128/mBio.00171-12. e00171-00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith K., McCoy K.D., Macpherson A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 64••.Butler J.E., Weber P., Sinkora M., Baker D., Schoenherr A., Mayer B., Francis D. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. J Immunol. 2002;169:6822–6830. doi: 10.4049/jimmunol.169.12.6822. [DOI] [PubMed] [Google Scholar]; One of several classical studies demonstrating the importance of the intestinal microbiota for the immune system development.
- 65.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Abt M.C., Osborne L.C., Monticelli L.A., Doering T.A., Alenghat T., Sonnenberg G.F., Paley M.A., Antenus M., Williams K.L., Erikson J., et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Another important study describing how the intestinal microbiota controls antiviral immunity.
- 67•.Bouskra D., Brezillon C., Berard M., Werts C., Varona R., Boneca I.G., Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]; The authors showed that bectaerial peptidoglycan is essential and sufficient to induce the genesis of intestinal lymphoid folliles in mice via NOD1 (nucleotide-binding oligomerization domain containing 1) signailing.
- 68.Hansen C.H., Nielsen D.S., Kverka M., Zakostelska Z., Klimesova K., Hudcovic T., Tlaskalova-Hogenova H., Hansen A.K. Patterns of early gut colonization shape future immune responses of the host. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Chung H., Pamp S.J., Hill J.A., Surana N.K., Edelman S.M., Troy E.B., Reading N.C., Villablanca E.J., Wang S., Mora J.R., et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; The maturation of the immune system is achieved more efficiently after colonization with host-specific microbiota.
- 70.Kandasamy S., Vlasova A.N., Fischer D., Kumar A., Chattha K.S., Rauf A., Shao L., Langel S.N., Rajashekara G., Saif L.J. Differential effects of Escherichia coli Nissle and Lactobacillus rhamnosus strain GG on human rotavirus binding, infection, and B cell immunity. J Immunol. 2016;196:1780–1789. doi: 10.4049/jimmunol.1501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vlasova A.N., Shao L., Kandasamy S., Fischer D.D., Rauf A., Langel S.N., Chattha K.S., Kumar A., Huang H.C., Rajashekara G., et al. Escherichia coli Nissle 1917 protects gnotobiotic pigs against human rotavirus by modulating pDC and NK-cell responses. Eur J Immunol. 2016;46:2426–2437. doi: 10.1002/eji.201646498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vlasova A.N., Chattha K.S., Kandasamy S., Liu Z., Esseili M., Shao L., Rajashekara G., Saif L.J. Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vlasova A.N., Kandasamy S., Chattha K.S., Rajashekara G., Saif L.J. Comparison of probiotic Lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet Immunol Immunopathol. 2016;172:72–84. doi: 10.1016/j.vetimm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Gutzeit C., Magri G., Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev. 2014;260:76–85. doi: 10.1111/imr.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]; The review discusses the current knowledge on the role of intestinal IgA in host-microbe interaction.
- 75.Shao L., Fischer D.D., Kandasamy S., Saif L.J., Vlasova A.N. Tissue-specific mRNA expression profiles of porcine Toll-like receptors at different ages in germ-free and conventional pigs. Vet Immunol Immunopathol. 2016;171:7–16. doi: 10.1016/j.vetimm.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allen F., Tong A.A., Huang A.Y. Unique transcompartmental bridge: antigen-presenting cells sampling across endothelial and mucosal barriers. Front Immunol. 2016;7:231. doi: 10.3389/fimmu.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamada N., Seo S.U., Chen G.Y., Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 78.Lavelle E.C., Murphy C., O’Neill L.A., Creagh E.M. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010;3:17–28. doi: 10.1038/mi.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johansson M.E., Sjovall H., Hansson G.C. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26 doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 82.Slack E., Hapfelmeier S., Stecher B., Velykoredko Y., Stoel M., Lawson M.A., Geuking M.B., Beutler B., Tedder T.F., Hardt W.D., et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Couturier-Maillard A., Secher T., Rehman A., Normand S., De Arcangelis A., Haesler R., Huot L., Grandjean T., Bressenot A., Delanoye-Crespin A., et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84••.Blanton L.V., Charbonneau M.R., Salih T., Barratt M.J., Venkatesh S., Ilkaveya O., Subramanian S., Manary M.J., Trehan I., Jorgensen J.M., et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351 doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent sudy that demonstrated that microbiota immaturity caused by childhood undernutrition can be partially rescued by Ruminococcus gnavus and Clostridium symbiosum.
- 85.Wu H.J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarke T.B., Davis K.M., Lysenko E.S., Zhou A.Y., Yu Y., Weiser J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu H.J., Ivanov I.I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D.R., Benoist C., Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang X., Liu Y., Tan M. Histo-blood group antigens as receptors for rotavirus, new understanding on rotavirus epidemiology and vaccine strategy. Emerg Microbes Infect. 2017;6 doi: 10.1038/emi.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang P., Farkas T., Marionneau S., Zhong W., Ruvoen-Clouet N., Morrow A.L., Altaye M., Pickering L.K., Newburg D.S., LePendu J., et al. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 90.Huang P., Xia M., Tan M., Zhong W., Wei C., Wang L., Morrow A., Jiang X. Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner. J Virol. 2012;86:4833–4843. doi: 10.1128/JVI.05507-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y., Huang P., Tan M., Liu Y., Biesiada J., Meller J., Castello A.A., Jiang B., Jiang X. Rotavirus VP8*: phylogeny, host range, and interaction with histo-blood group antigens. J Virol. 2012;86:9899–9910. doi: 10.1128/JVI.00979-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marionneau S., Ruvoen N., Le Moullac-Vaidye B., Clement M., Cailleau-Thomas A., Ruiz-Palacois G., Huang P., Jiang X., Le Pendu J. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology. 2002;122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan M., Jiang X. Association of histo-blood group antigens with susceptibility to norovirus infection may be strain-specific rather than genogroup dependent. J Infect Dis. 2008;198:940–941. doi: 10.1086/589810. author reply 942-943. [DOI] [PubMed] [Google Scholar]
- 94.Guillon P., Clement M., Sebille V., Rivain J.G., Chou C.F., Ruvoen-Clouet N., Le Pendu J. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elnady H.G., Abdel Samie O.M., Saleh M.T., Sherif L.S., Abdalmoneam N., Kholoussi N.M., Kholoussi S.M., El-Taweel A.N. ABO blood grouping in Egyptian children with rotavirus gastroenteritis. Przegl Gastroenterol. 2017;12:175–180. doi: 10.5114/pg.2017.70469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Colston J.M., Francois R., Pisanic N., Yori P.P., McCormick B.J.J., Olortegui M.P., Gazi M.A., Svensen E., Ahmed M.M.M., Mduma E., et al. Effects of child and maternal Histo Blood Group Antigen status on symptomatic and asymptomatic enteric infections in early childhood. J Infect Dis. 2019 doi: 10.1093/infdis/jiz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee B., Dickson D.M., deCamp A.C., Ross Colgate E., Diehl S.A., Uddin M.I., Sharmin S., Islam S., Bhuiyan T.R., Alam M., et al. Histo-blood group antigen phenotype determines susceptibility to genotype-specific rotavirus infections and impacts measures of rotavirus vaccine efficacy. J Infect Dis. 2018;217:1399–1407. doi: 10.1093/infdis/jiy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bucardo F., Nordgren J., Reyes Y., Gonzalez F., Sharma S., Svensson L. The Lewis A phenotype is a restriction factor for Rotateq and Rotarix vaccine-take in Nicaraguan children. Sci Rep. 2018;8 doi: 10.1038/s41598-018-19718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clausen H., Hakomori S. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56:1–20. doi: 10.1111/j.1423-0410.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 100.Marionneau S., Cailleau-Thomas A., Rocher J., Le Moullac-Vaidye B., Ruvoen N., Clement M., Le Pendu J. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie. 2001;83:565–573. doi: 10.1016/s0300-9084(01)01321-9. [DOI] [PubMed] [Google Scholar]
- 101.Ferrer-Admetlla A., Sikora M., Laayouni H., Esteve A., Roubinet F., Blancher A., Calafell F., Bertranpetit J., Casals F. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26:1993–2003. doi: 10.1093/molbev/msp108. [DOI] [PubMed] [Google Scholar]
- 102.Kelly R.J., Rouquier S., Giorgi D., Lennon G.G., Lowe J.B. Sequence and expression of a candidate for the human secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270:4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 103.Stowell S.R., Arthur C.M., Dias-Baruffi M., Rodrigues L.C., Gourdine J.P., Heimburg-Molinaro J., Ju T., Molinaro R.J., Rivera-Marrero C., Xia B., et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med. 2010;16:295–301. doi: 10.1038/nm.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spalter S.H., Kaveri S.V., Bonnin E., Mani J.C., Cartron J.P., Kazatchkine M.D. Normal human serum contains natural antibodies reactive with autologous ABO blood group antigens. Blood. 1999;93:4418–4424. [PubMed] [Google Scholar]
- 105.Forni D., Cleynen I., Ferrante M., Cassinotti A., Cagliani R., Ardizzone S., Vermeire S., Fichera M., Lombardini M., Maconi G., et al. ABO histo-blood group might modulate predisposition to Crohn’s disease and affect disease behavior. J Crohns Colitis. 2014;8:489–494. doi: 10.1016/j.crohns.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 106.Uchida H., Kawai Y., Kinoshita H., Kitazawa H., Miura K., Shiiba K., Horii A., Kimura K., Taketomo N., Oda M., et al. Lactic acid bacteria (LAB) bind to human B- or H-antigens expressed on intestinal mucosa. Biosci Biotechnol Biochem. 2006;70:3073–3076. doi: 10.1271/bbb.60407. [DOI] [PubMed] [Google Scholar]
- 107.Uchida H., Kinoshita H., Kawai Y., Kitazawa H., Miura K., Shiiba K., Horii A., Kimura K., Taketomo N., Oda M., et al. Lactobacilli binding human A-antigen expressed in intestinal mucosa. Res Microbiol. 2006;157:659–665. doi: 10.1016/j.resmic.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 108.Miura T., Sano D., Suenaga A., Yoshimura T., Fuzawa M., Nakagomi T., Nakagomi O., Okabe S. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J Virol. 2013;87:9441–9451. doi: 10.1128/JVI.01060-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109•.Gampa A., Engen P.A., Shobar R., Mutlu E.A. Relationships between gastrointestinal microbiota and blood group antigens. Physiol Genomics. 2017;49:473–483. doi: 10.1152/physiolgenomics.00043.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]; A blood group secretor status is associated with less diversity at higher order taxa; and the presence of blood group A antigens in the secretor subjects leads to expansion families of bacteria within the gut.
- 110.Makivuokko H., Lahtinen S.J., Wacklin P., Tuovinen E., Tenkanen H., Nikkila J., Bjorklund M., Aranko K., Ouwehand A.C., Matto J. Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiol. 2012;12:94. doi: 10.1186/1471-2180-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rausch P., Rehman A., Kunzel S., Hasler R., Ott S.J., Schreiber S., Rosenstiel P., Franke A., Baines J.F. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci U S A. 2011;108:19030–19035. doi: 10.1073/pnas.1106408108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Licciardi P.V., Tang M.L.K. Vaccine adjuvant properties of probiotic bacteria. Discov Med. 2011;12:525–533. [PubMed] [Google Scholar]
- 113.Vitetta L., Saltzman E.T., Thomsen M., Nikov T., Hall S. Adjuvant probiotics and the intestinal microbiome: enhancing vaccines and immunotherapy outcomes (vol 5, 50, 2017) Vaccines–Basel. 2018;6 doi: 10.3390/vaccines5040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pang I.K., Iwasaki A. Control of antiviral immunity by pattern recognition and the microbiome. Immunol Rev. 2012;245:209–226. doi: 10.1111/j.1600-065X.2011.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas C.M., Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes. 2010;1:148–163. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin P.W., Myers L.E., Ray L., Song S.C., Nasr T.R., Berardinelli A.J., Kundu K., Murthy N., Hansen J.M., Neish A.S. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med. 2009;47:1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tao Y., Drabik K.A., Waypa T.S., Musch M.W., Alverdy J.C., Schneewind O., Chang E.B., Petrof E.O. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 118.Kumar A., Vlasova A.N., Liu Z., Chattha K.S., Kandasamy S., Esseili M., Zhang X., Rajashekara G., Saif L.J. In vivo gut transcriptome responses to Lactobacillus rhamnosus GG and Lactobacillus acidophilus in neonatal gnotobiotic piglets. Gut Microbes. 2014;5:152–164. doi: 10.4161/gmic.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Isolauri E., Joensuu J., Suomalainen H., Luomala M., Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–312. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- 120.Chattha K.S., Vlasova A.N., Kandasamy S., Rajashekara G., Saif L.J. Divergent immunomodulating effects of probiotics on T cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. J Immunol. 2013;191:2446–2456. doi: 10.4049/jimmunol.1300678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chattha K.S., Vlasova A.N., Kandasamy S., Esseili M.A., Siegismund C., Rajashekara G., Saif L.J. Probiotics and colostrum/milk differentially affect neonatal humoral immune responses to oral rotavirus vaccine. Vaccine. 2013;31:1916–1923. doi: 10.1016/j.vaccine.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fang H., Elina T., Heikki A., Seppo S. Modulation of humoral immune response through probiotic intake. FEMS Immunol Med Microbiol. 2000;29:47–52. doi: 10.1111/j.1574-695X.2000.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 123.de Vrese M., Rautenberg P., Laue C., Koopmans M., Herremans T., Schrezenmeir J. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2005;44:406–413. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- 124.Paineau D., Carcano D., Leyer G., Darquy S., Alyanakian M.A., Simoneau G., Bergmann J.F., Brassart D., Bornet F., Ouwehand A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunol Med Microbiol. 2008;53:107–113. doi: 10.1111/j.1574-695X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 125.West C.E., Gothefors L., Granstrom M., Kayhty H., Hammarstrom M.L., Hernell O. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatric Allergy Immunol. 2008;19:53–60. doi: 10.1111/j.1399-3038.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 126.Kukkonen K., Nieminen T., Poussa T., Savilahti E., Kuitunen M. Effect of probiotics on vaccine antibody responses in infancy—a randomized placebo-controlled double-blind trial. Pediatric Allergy Immunol. 2006;17:416–421. doi: 10.1111/j.1399-3038.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 127.Soh S.E., Ong D.Q., Gerez I., Zhang X., Chollate P., Shek L.P., Lee B.W., Aw M. Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine. 2010;28:2577–2579. doi: 10.1016/j.vaccine.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 128•.Vitetta L., Saltzman E.T., Thomsen M., Nikov T., Hall S. Adjuvant probiotics and the intestinal microbiome: enhancing vaccines and immunotherapy outcomes. Vaccines–Basel. 2017;5 doi: 10.3390/vaccines5040050. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review summarized the findings from clinicl trials that demonstrated that introduction of probiotics prior to vaccination may provide significant beneficial immune modulatory outcomes in neonates and in the elderly.