Abstract
Dengue has emerged as a major public health challenge in terms of both changing clinical pattern and epidemiological features. The state of Odisha reported first dengue epidemic in the year 2010 and this continued each year in epidemic form during post monsoon period gradually becoming an endemic phenomenon. Present study depicts the changing epidemiological and clinical pattern of dengue with reference to its serotypes and genotypes. The study included 5320 suspected dengue cases from different health facilities of the state during 2010–2017. Dengue NS1 antigen and IgM antibody was done through ELISA. Serotyping was done through RTPCR by amplifying a part of core-pre-membrane gene (CprM) followed by sequencing and phylogenetic analysis. Dengue IgM antibody in 17.7% cases and NS1 antigen in 53.20% cases was detected. Dengue serotype 2 (DEN-2) was the only serotype detected in 2010 and 2011 where as all four serotypes 1, 2, 3, 4 were detected in 2012–2017, DEN-2 being dominant but in 2017 DEN-3 was found to be dominant. Phylogenetic analysis revealed genotype IV of DEN-2 and genotype III of DEN-1 and DEN-3 circulating in this region. In 6 cases involvement of DEN-2 in clinically evident encephalitis cases is an important observation in this region and needs public health attention. High prevalence of dengue was observed without any previous reported outbreaks in the state with increased number of cases from 2010 to 2012 affecting both urban and rural areas. High incidence in 2012 was due to co-circulation of more than one serotype which continued in the following years. Severity in some cases was associated with mixed infection but in most cases it was mild indicating the endemic nature of the virus in most parts of Odisha.
Keywords: Dengue, Epidemiology, Molecular, Serotype, Odisha
Introduction
The fast changing epidemiological features have put dengue as a global threat affecting around 50 million people worldwide [8]. Currently it is the most important vector borne, human viral disease in the world. Especially in tropical and subtropical countries it has emerged as a major public health concern in terms of both the number of cases and number of deaths. A wide range of clinical presentation may result from dengue virus infection which can be differentiated from asymptomatic or a mild, non specific fever to classic Dengue Fever (DF) and severe presentations such as Dengue Hemorrhagic Fever (DHF) or Dengue Shock Syndrome (DSS). In South East Asia, DHF and DSS are leading causes of hospitalization and death among children [13].
Dengue virus (Family Flaviviridae, genus Flavivirus) constitutes four antigenically distinct serotypes. Infection is thought to confer lifelong immunity against the same serotype but only partial or transient (few months) cross protection against infection by other serotypes indicating chances of infection by other serotypes subsequently. Secondary infection with different serotype may also carry an increased risk of developing severe forms of the disease [12].
In India the first dengue epidemic was reported in 1963 in Calcutta, West Bengal with significantly high number of reported DHF cases (30%) and with 200 deaths followed by a number of DF outbreaks affecting different states like Haryana, Punjab, Rajasthan, Uttar Pradesh and Tamil Nadu [18]. Delhi was severely affected with largest epidemic of DF/DHF cases during 1996 with 10,252 cases and 423 deaths [2].
The state of Odisha did not have any report of dengue outbreak until 2010 when first report of sporadic dengue was reported from Malkangiri and Gajapati districts that was followed by several epidemics in subsequent years. The present study describes epidemiological changes of dengue virus infection that emerged in Odisha from 2010 to 2017 with special reference to serotypes and genotypes of the virus.
Materials and methods
Geographical distribution
The state of Odisha is situated on the eastern coast of India in between 17°48′ and 22°34′ North latitude and 81°24′ and 87°29′ East longitude. Standing on the coastal belt, the weather is greatly influenced by the sea (Bay of Bengal). The climate of the region is tropical resulting in very high temperature in the months of April and May. During summer maximum temperature ranges between 35 and 40 °C and during winter the low temperatures are usually between 12 and 14 °C. The average rainfall is 150 cm, experienced as the result of south west monsoon during July–September.
Odisha has been divided into five major geographical regions: coastal plain in the east, the middle mountainous and highland region, the central plateaus, the western rolling uplands and the major flood plains. The study areas like Baleswar, Ganjam and Gajapati are situated on the coastal plain whereas Malkangiri mainly consists of mountains and highlands.
Sample collection and laboratory test
Blood samples were collected from two sources (a) samples referred from secondary and tertiary health care centers of the state (n = 4463) and (b) from outbreak affected areas by direct outbreak investigation (n = 857). The centre’s virology laboratory has been networked with different secondary and tertiary level hospitals and medical colleges of the state. The centre is also designated as the regional referral laboratory for laboratory diagnosis of vector borne diseases in the state. Samples were collected along with clinical information in a predesigned format from 33 hospitals and 6 medical colleges covering all 30 districts of Odisha.
Besides the above hospital based surveillance the centre also investigated different reported outbreaks in the state by direct field investigation in collaboration with the state health department and integrated diseases surveillance program (IDSP). During outbreak investigation the medical doctor of the team investigated cases and obtained clinical information in predesigned questionnaire. A case of dengue was defined as an acute febrile illness having duration of 2–7 days with two or more manifestations among headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, leucopenia [7]. Other related infectious causes like malaria and urinary tract infections were ruled out by laboratory investigation. A total of 5320 blood samples were collected from 2010 to 2017. Samples were transported to the laboratory in cold chain. The samples were tested for dengue specific IgM antibodies (NIV, Pune) or NS1antigen (PanBio, Australia) through ELISA following the manufacturer instruction. Viral RNA was extracted by QIA amp viral RNA mini kit (Qiagen, Germany) as per the protocol provided by the manufacturer. RTPCR assay was performed targeting core pre-membrane (CprM) gene and 4 dengue serotypes were distinguished by the size of the products as described by Lanciotti et al. [14]. PCR contamination was avoided by spatially separating the pre and post amplification steps and to detect possible contamination a no template negative control was incorporated in all the PCR reactions. The products were electrophoresed through 2% agarose gel, stained with ethidium bromide and examined under ultraviolet light using gel documentation system (Alpha Imager, USA).
The gel purified products were directly sequenced using the specific primers and Big dye terminator kit (Applied Biosystems, USA) as per the manufacturers instruction in automated DNA sequencer (ABI, Genetic Analyzer 3730). The sequences were analyzed by using Clustal W function of the Bio-Edit 6.0.7software. Phylogenetic analysis was carried out by using Neighbor joining method in MEGA 3.1 program with Kimura 2 parameter distance correction and 1000 boots strap replications.
Result and discussion
Serological investigation of enrolled cases
Suspected cases of dengue infection were first reported from Malkangiri district of Odisha during 2010. A total of 5320 cases were collected/received during 2010–2017. Highest numbers of cases i.e. 2255 were reported in 2012 from all 30 districts whereas lowest numbers of cases i.e. 81 were reported from 8 Districts during 2010. Cases included both rural and urban population (Source: IDSP, Odisha). Among the cases 3498 were male (65.7%) where as females represented 34.2% (n = 1822). Throughout all the years 2644 cases (49.7%) of dengue positives were detected either through IgM or NS1. Percent positivity of NS1 ranged from 15.38 to 72.52% in comparison to IgM in 6.52–29.58% of cases (Table 1). Distribution of positive cases in all the 8 years have shown majority number of cases were received during post monsoon period (July–Nov) irrespective of the year. The month wise distribution of positive cases is given in Fig. 1. Age-wise distribution of dengue positive cases in all the years indicates that age group > 15 years were more commonly affected (53–80%) than the age group < 15 years. Except during 2013, children below 15 years were also seen to be affected (48.8%) in a greater proportion. During this year all age groups more than 15 years age group were equally affected (74.4–79.8%). Median age of presentation during 2011 was the lowest (11 years) and highest (28 years) during 2010 and 2013. Age wise distribution of positive cases is presented in Table 2.
Table 1.
Laboratory identification of dengue over years
| Year of investigation | Total sample | IgM tested | IgM Positive | % positive | NS1 tested | NS1 positive | % positive | Dengue positive | % positive |
|---|---|---|---|---|---|---|---|---|---|
| 2010 | 81 | 81 | 15 | 18.52 | 0 | 0 | 15 | 18.52 | |
| 2011 | 226 | 216 | 25 | 11.57 | 57 | 28 | 49.12 | 46 | 20.35 |
| 2012 | 1283 | 905 | 59 | 6.52 | 1052 | 392 | 37.26 | 440 | 34.29 |
| 2013 | 1728 | 628 | 115 | 18.31 | 1601 | 1161 | 72.52 | 1267 | 73.32 |
| 2014 | 290 | 266 | 63 | 23.68 | 100 | 20 | 20.00 | 79 | 27.24 |
| 2015 | 289 | 234 | 41 | 17.52 | 143 | 22 | 15.38 | 55 | 19.03 |
| 2016 | 735 | 426 | 126 | 29.58 | 584 | 290 | 49.66 | 372 | 50.61 |
| 2017 | 688 | 386 | 112 | 29.02 | 585 | 280 | 47.86 | 370 | 53.78 |
| Total | 5320 | 3142 | 556 | 17.70 | 4122 | 2193 | 53.20 | 2644 | 49.70 |
Fig. 1.
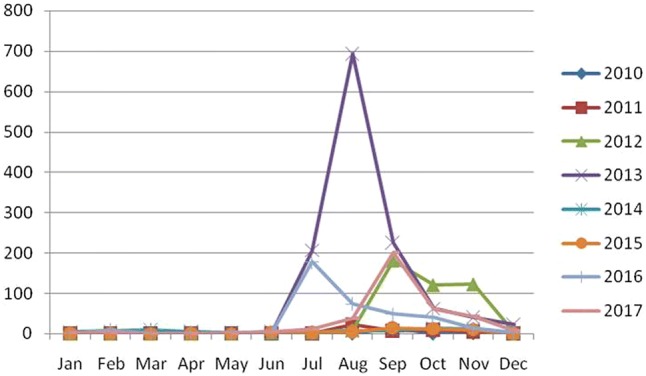
Monthly distribution of suspected dengue cases from 2010 to 2017
Table 2.
Age wise distribution of dengue positive cases
| Age group Number positive (%) | |||||
|---|---|---|---|---|---|
| Age (years) | 0–15 | 16–30 | 31–45 | 46–60 | >60 |
| 2010 | 4 (23.53) | 6 (20.69) | 3 (17.65) | 1 (8.33) | 1 (16.67) |
| 2011 | 14 (11.67) | 14 (31.11) | 12 (36.36) | 5 (26.32) | 1 (11.11) |
| 2012 | 82 (23.3) | 167 (38.48) | 109 (40.82) | 56 (36.36) | 26 (34.21) |
| 2013 | 145 (48.82) | 553 (79.11) | 353 (79.86) | 166 (74.44) | 50 (74.63) |
| 2014 | 36 (28.35) | 22 (29.73) | 13 (27.66) | 6 (20.69) | 2 (15.38) |
| 2015 | 11 (12.22) | 18 (16.67) | 18 (36) | 6 (18.18) | 2 (25) |
| 2016 | 83 (33.2) | 145 (61.7) | 96 (70.07) | 36 (41.38) | 12 (46.15) |
| 2017 | 77 (27.5) | 154 (75.12) | 81 (77.14) | 43 (61.43) | 15 (53.57) |
Sequencing and phylogenetic analysis
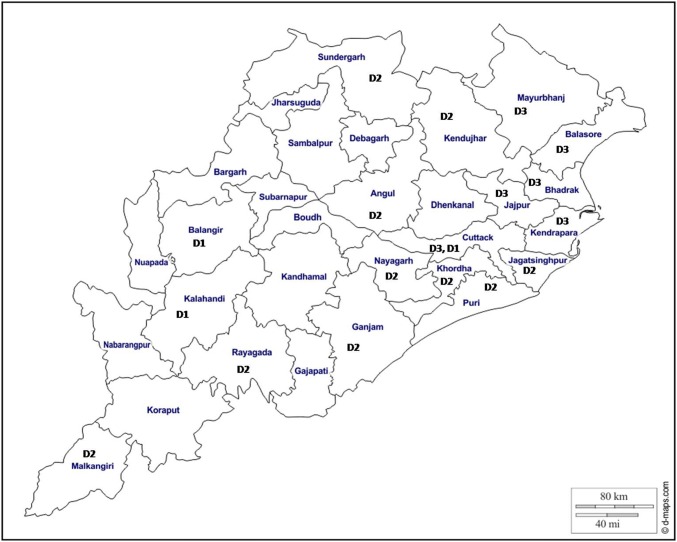
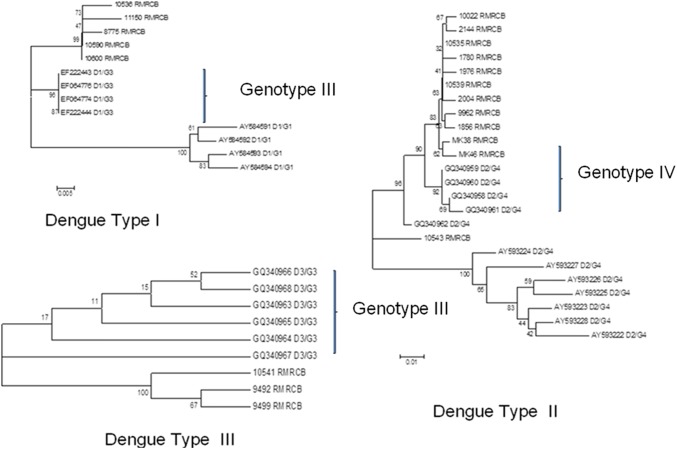
A representative subset of samples positive for NS1 antigen (n = 940) were subjected to RTPCR for serotyping. Serotype determined in 70.1% of samples shown circulation of all four serotypes in the state. During 2010 and 2011, DEN-2 was the only serotype detected where as from 2012 to 2017 all four serotypes were detected but DEN-2 remained the dominating serotype (17.58-100%) till 2016. In 2017, DEN-3 was more detected in comparison to other three serotypes. Distribution in terms of number of serotypes detected over the years is presented in the Table 3. Though more than one serotype was detected in different districts of the state but each district was affected by a dominant serotype either DEN-1, DEN-2 or DEN-3. Also it has been observed that one serotype was present in 2–3 adjoining districts. The distribution of dominant serotype in different districts is presented in Fig. 2. The districts showing no serotype indicates though there were dengue positive cases but it could not be detected through PCR. Sequencing was done in a subset of randomly selected samples from each serotype (5 from DEN-1, 12 from DEN-2, 3 from DEN-3). Three neighbor joining phylogenetic trees were constructed for three serotypes with available reference sequences isolated from different regions of India. A 362 bp product from CprM region was identical in 5 isolates of DEN-1 and the sequences had 99% similarity with North Indian strains. All isolates of DEN-1 fell into genotype III cluster and grouped with North Indian strains isolated during 2005 and 2010. Sequences of samples positive for DEN-2 had clustered with sequences of genotype IV with 99% identity with strains isolated from Kerala and 98% identity with North Indian strains. Sequences for DEN-3 were of genotype III and maximum identity (99%) was observed with strains isolated from Delhi during 2003 (Fig. 3).
Table 3.
Dengue serotypes detected over the years
| Year of investigation | Serotypes (no. of cases) | |||
|---|---|---|---|---|
| DEN-1 | DEN-2 | DEN-3 | DEN-4 | |
| 2010 | 0 | 5 | 0 | 0 |
| 2011 | 0 | 22 | 0 | 0 |
| 2012 | 36 | 58 | 34 | 19 |
| 2013 | 66 | 88 | 62 | 19 |
| 2014 | 2 | 3 | 1 | 1 |
| 2015 | 1 | 6 | 1 | 0 |
| 2016 | 13 | 46 | 7 | 4 |
| 2017 | 26 | 29 | 98 | 12 |
| Total | 144 | 257 | 203 | 55 |
Fig. 2.
District wise distribution of serotypes (D1-DEN-1, D2-DEN-2, D3-DEN-3)
Fig. 3.
Phylogenetic tree of CprM nucleotide sequences of dengue strains isolated from Odisha and other strains with their Genebank accession number
Correlation of laboratory investigation with clinical presentation
Clinical presentations of most of the cases with confirmed dengue infection in our study population were mild to moderate. Fever, headache, arthralgia, myalgia, nausea and vomiting were the common symptoms. The range of platelet count was observed from 46 to 113 (109/L) with a mean platelet count of 146.5 (109/L, SD: 119.6). Hematocrit was observed to be in the range of 32–42% with a mean value of 38.2% (SD: 5.25). In one patient two episodes of bleeding (hematemesis) was observed and found to be infected with both DEN-1 and DEN-2. Sixteen patients showed signs of increased capillary permeability (ascites) during 2012. Out of these 16 cases of ascites, in 12 cases intraperitoneal fluid collection was observed clinically where as 4 cases were detected through ultrasound. Three hundred one dengue positive cases had features of encephalitis having mental disorientation and irritability including two patients with DEN-2 infection. The mean duration of persistence of symptoms was 6.4 days (SD: 3.9).
Most of vector borne diseases exhibit a distinctive seasonal pattern and climatic factors such as rainfall, temperature, relative humidity and other weather variables affecting both the vector and the pathogen they transmit in many ways favoring outbreaks of vector borne diseases [3]. A similar seasonal pattern was observed from September to November during which 51% and 96% of the total positive cases were detected during 2011 and 2012 respectively indicating the post monsoon period as the most favorable condition for spread of the disease. These findings indicate that during epidemic and non-epidemic years, dengue infections are mostly seen in post monsoon season hence preventive measures should be in full swing at the very onset of the monsoon.
Dengue is known to be an urban disease and most of the outbreaks and endemic infections have been reported in India from Vellore, Calcutta, Delhi [12, 18] and other parts of the world. But the present report has shown dengue infection affecting the major parts of rural population of the state of Odisha. This indicated a suitable ecological environment for vector breeding shifting from urban to rural.
In many previous reported outbreaks in India males were more commonly affected than females in contrast to some studies where females outnumbered males [20] but coming to confirmed cases this gender differentiation is not significant [17]. In our study subjects, throughout the study period, males outnumbered females. This raises two possibilities either males have a greater exposure to mosquito bites because of outdoor activity or sampling bias involved in hospital cases where socio cultural practices can influence gender discrimination in health care seeking behavior among the general population ruling out dengue as a gender specific disease [10].
In India dengue was reported as predominantly a disease of young adults. Many reported outbreaks in Delhi had confirmed 21–30 years age group as the most affected category [11] which was also observed in our study. Infection in older age group (> 40) during 2012 was an important finding of this investigation. This indicated all ages were susceptible due to lack of previous immunity in the population which is supported by no record of dengue infection in the community before 2010. However age is not the only factor to determine the susceptibility to infection, pre existing dengue immunity at individual and community level resulting from past outbreaks and the changing pathogenic potential of dengue viruses with reference to the change in environment plays an important role [7].
The present study reports emergence of DEN-2 as the most common type along with presence of other three serotypes. During 2010 and 2011, DEN-2 was the only dengue strain found to be circulating in the region. This was added by other three serotypes in 2012. Since its first isolation during 1956 [19] DEN-2 remains a predominant serotype from early 1970 to 2000 during which it was responsible for large epidemic of DF in south India (1993) [16] and of DF/DHF in north India (1996) [5]. DEN-3 was also associated with many outbreaks causing high morbidity and mortality not only in India but also in Sri Lanka, East Africa and Latin America [11]. DEN-1 and DEN-4 had not been associated with any major outbreaks in the country [4]. Introduction of novel serotypes may be due to population movement in boarder areas of the state [15] which may be one of the causes for circulation of all four serotypes. Odisha comprising of 30 districts and all districts reported dengue cases over the time period of 8 years where as in 18 districts serotypes have been identified. In 3, 10 and 6 districts; DEN-1, DEN-2 and DEN-3 were circulating as the predominant serotype and in majority of the districts DEN-2 was the dominant serotype circulating in the state. High incidence of dengue infection during 2012 may be due to co-circulation of various serotypes and high vector prevalence as Maximum Likely Estimate of Aedes ageptyi (1.55; 95% CI 0.09, 7.54) and Aedes albopictus (4.72; 95% CI 1.94, 9.80) mosquitoes in the state had been documented [6]. Some previous studies documented that these vectors can get simultaneously infected with more than one serotype [22] as the feeding behavior may involve multiple blood meals in a single gonotropic cycle enabling them to acquire multiple serotypes in a disease hyperendemic area. However further investigations in the vector need to be done to establish the co-infection of mosquito vector as a contributing factor for the larger outbreaks in Odisha. Many studies have reported co-circulation of multiple serotypes coupled with high vector index leading to the infection in a person with multiple serotypes of the virus [9].
Strains of DEN-2 circulated during 8 years were similar except one strain that had formed a separate clade indicating circulation of Genotype IV till now in this region. Genotype I of DEN-1 and 3 had maximum identity with isolates found in Kerala, the southern part of India [1]. This indicates entry of these dengue strains from southern part of India. DEN-4 was identified only from the northern districts of the state bordering to West Bengal and Jharkhand, where this serotype has been reported previously [17] and it might have got entry into this region through population migration.
The signs and symptoms manifested by the patients in this study were non-specific like myalgia, anorexia, abdominal pain, cough and headache which were similar to other studies [21].
The patients presented with encephalopathy were also confirmed by laboratory tests and in 2 cases the virus was detected as DEN-2 and the patients recovered completely with conventional AES management. Similar observation was noted in Vietnam where DEN-2 was an important cause of neurologic manifestation presenting with encephalopathy [21]. Co-infection with more than one serotype can enhance the severity of the disease [23] however, in our study in spite of multiple serotypes in one individual; the disease severity was mild which suggested for further study on complete genetic characterization of the serotypes. Studies on multiple infections in mosquito vectors in this region are also a need of hour.
The result of the study revealed that multiple serotypes are circulating in the state with DEN-2 as the major strain involved in co-infection. In 2012, though the number of cases increased due to co-circulation of different serotypes affecting older age groups beyond 40 years of age but the disease severity was not observed. The reason for this can be better explained with the complete genetic characterization, mutations if any and host immunological factors.
Acknowledgements
Authors wish to thank IDSP, Govt. of Odisha for providing assistance during outbreak investigation. Authors also thank DHR, ICMR, New Delhi for financial support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anoop M, Issac A, Mathew T, Philip Kareem NA, Unnikrishnan R, Sreekumar E. Genetic characterisation of dengue virus serotypes causing concurrent infection in an outbreak in Ernakulam, Kerala, South India. Indian J Exp Biol. 2010;48:849–857. [PubMed] [Google Scholar]
- 2.Broor S, Dar L, Sengupta S, Chakaraborty M, Wali JP, Biswas A, Kabra SK, Jain Y, Seth P. Recent dengue epidemic in Delhi, India. In: Saluzzo JE, Dodet B, editors. Factors in the emergence of arbovirus diseases. Paris: Elsevier; 1997. pp. 123–127. [Google Scholar]
- 3.Chakravarti A, Kumaria R. Eco-epidemiological analysis of dengue infection during an outbreak of dengue fever, India. Virol J. 2005;2:32. doi: 10.1186/1743-422X-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakravarti A, Arora R, Luxemburger C. Fifty years of dengue in India. Trans R Soc Trop Med Hyg. 2012;106:273–282. doi: 10.1016/j.trstmh.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Dar L, Broor S, Sengupta S, Xess I, Seth P. The first major outbreak of dengue hemorrhagic fever in Delhi, India. Emerg Infect Dis. 1999;5:589–590. doi: 10.3201/eid0504.990427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das B, Das M, Dwibedi B, Kar SK, Hazra RK. Molecular investigations of dengue virus during outbreaks in Orissa state, Eastern India from 2010 to 2011. Infect Genet Evol. 2013;16C:401–410. doi: 10.1016/j.meegid.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/CMR.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330. doi: 10.1016/S0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 9.Gubler DJ, Kuno G, Sather GE, Waterman SH. A case of natural concurrent human infection with two dengue viruses. Am J Trop Med Hyg. 1985;34:170. doi: 10.4269/ajtmh.1985.34.170. [DOI] [PubMed] [Google Scholar]
- 10.Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta E, Dar L, Kapoor G, Broor S. The changing epidemiology of dengue in Delhi, India. Virol J. 2006;3:92–96. doi: 10.1186/1743-422X-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 13.Henchal EA, Putnak JR. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/CMR.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30(3):545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi EE, Gubler DJ. Dengue in Southeast Asia: epidemiological characteristics and strategic challenges in disease prevention. Cad Saude Publica. 2009;25(Suppl 1):S115–S124. doi: 10.1590/S0102-311X2009001300011. [DOI] [PubMed] [Google Scholar]
- 16.Padbidri VS, Adhikari P, Thakare JP. The 1993 epidemic of dengue fever in Mangalore, Karnataka state, India. SE Asian J Trop Med. 1995;26:699–704. [PubMed] [Google Scholar]
- 17.Paramasivan R, Thenmozhi V, Hiriyan J, Dhananjeyan K, Tyagi B, Dash AP. Serological and entomological investigations of an outbreak of dengue fever in certain rural areas of Kanyakumari district, Tamil Nadu. Indian J Med Res. 2006;123:697–701. [PubMed] [Google Scholar]
- 18.Raheel U, Faheem M, Riaz MN, Kanwa N, Javed F, Zaidi NS, Qadri I. Dengue fever in the Indian subcontinent: an overview. J Infect Dev Countr. 2011;5:239–247. doi: 10.3855/jidc.1017. [DOI] [PubMed] [Google Scholar]
- 19.Rao CV. Dengue fever in India. Indian J Pediatr. 1987;54:11–14. doi: 10.1007/BF02751227. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Sharma SK, Mohan A. Clinical profile of dengue hemorrhagic fever in adults during 1996—outbreak in Delhi, India. Dengue Bull. 1998;22:20–27. [Google Scholar]
- 21.Solomon T, Dung N, Vaughn D, Kneen R, Thao L, Raengsakulrach B, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–1058. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 22.Thavaru U, Siriyasatien P, Tawatsin A, Asavadachanukorn P, Anantapreecha S, Wongwanich R, Mulla MS. Double infection of heteroserotypes of dengue viruses in field populations of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and serological features of dengue viruses found in patients in southern Thailand. SE Asian J Trop Med. 2006;37:468. [PubMed] [Google Scholar]
- 23.Thisyakorn U, Nimmannitya S, Ningsanond V, Soogarun S. Atypical lymphocyte in dengue hemorrhagic fever: its value in diagnosis. SE Asian J Trop Med. 1984;15:32–36. [PubMed] [Google Scholar]