Abstract
Zinc is an essential cofactor required for life, and as such, mechanisms exist for its homeostatic maintenance in biological systems. Despite the evolutionary distance between vertebrates and microbial life, parallel mechanisms exist to balance the essentiality of zinc with its inherent toxicity. Vertebrates regulate zinc homeostasis through a complex network of metal transporters and buffering systems that respond to changes in nutritional zinc availability or inflammation. The fine-tuning of this network becomes critical during infections, where host nutritional immunity attempts to limit zinc availability from pathogens. However, accumulating evidence demonstrates that pathogens evolved mechanisms to subvert host-mediated zinc withholding, and these metal homeostasis systems are important for survival within the host. Here we discuss mechanisms of vertebrate and bacterial zinc homeostasis and mobilization, as well as recent developments in our understanding of microbial zinc acquisition.
Zinc is Required for Life
Transition metals (see Glossary) are essential micronutrients required to carry out biological processes in all domains of life [1]. Their requirement stems from unique biochemical properties attributed to late d-block elements that selected for their incorporation into catalytic and structural components of proteins during evolution. The essential metal zinc (Zn) is unique among the first row d-block elements in that it possesses a filled d-orbital and does not undergo redox cycling. Zn is ubiquitous in life and is required for the structure or function of thousands of metalloproteins [2]. Zn is an essential micronutrient for the survival and proliferation of bacteria, including pathogens that are major causes of morbidity and mortality worldwide [3]. Given this essentiality, both vertebrate hosts and pathogens evolved processes to maintain Zn homeostasis. While mechanisms of maintenance and storage of Zn within vertebrates have been well described in recent decades [4], the diverse array of systems that pathogens possess to compete for this essential nutrient during infections has only recently been appreciated [3]. In this review, we discuss vertebrate Zn homeostasis and mobilization during infections, followed by a summary of our current understanding of bacterial Zn uptake and utilization systems.
Vertebrate Zinc Homeostasis is a Highly Regulated and Dynamic Process
As the second most abundant trace metal in humans, around 2 to 4 grams of Zn are distributed throughout the body in various tissue sites [5]. In vertebrates, the gastrointestinal tract serves as a major regulator of Zn homeostasis by tuning absorption and excretion via metal transporter proteins [4] (Figure 1). While the range of total Zn within the body is relatively stable for most humans, changes in dietary Zn availability are associated with poor health [4]. Human genetic deficiencies in Zrt/lrt-like Protein (ZIP) family members, which are the primary Zn importers in eukaryotes, cause severe Zn deficiency [6]. Additionally, dietary Zn deficiency is a major public health burden, with at least 25% of the global population being at risk of inadequate dietary Zn [7, 8]. Consequences of Zn deficiency include impaired immune function, delayed wound healing, diarrhea, and increased susceptibility to infection [9]. Conversely, Zn toxicity occurs in acute and chronic forms and is linked to several sources, including Zn supplements and parenteral nutrition, among others [10, 11]. Acute symptoms of Zn intoxication include symptoms like nausea and vomiting [12], while chronic intoxication may result in reduced immune function and altered copper (Cu) and iron (Fe) levels, demonstrating the interconnectedness between nutrient metals within the body [13].
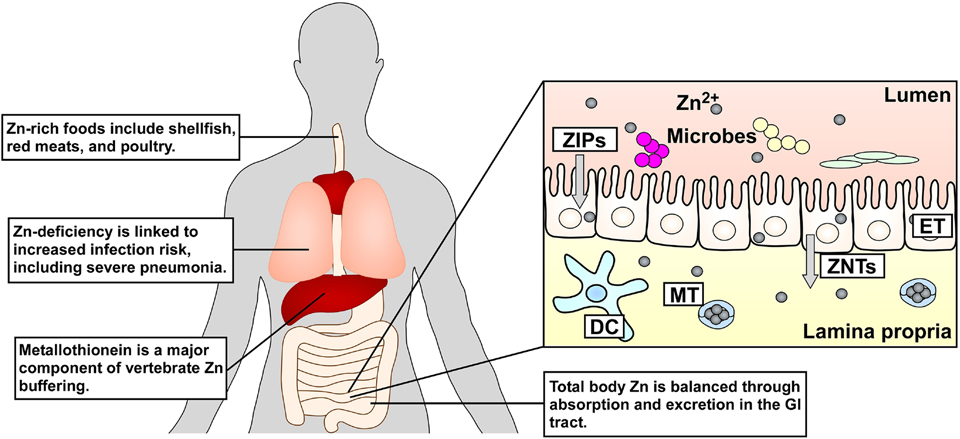
Figure 1.
Vertebrate Zn homeostasis systems. Changes in dietary Zn (grey) can be associated with adverse clinical outcomes and diarrheal disease. Zn buffering is regulated by changes in epithelial cell (ET) Zn uptake/efflux associated with the gastrointestinal (GI) tract primarily by the action of ZIP Zn importers and ZNT Zn exporters. ZIP activity results in Zn uptake from the intestinal lumen into ETs, and ZNT activity results in efflux of Zn from the ETs via the lamina propria into circulation and the extracellular space. Small molecule Zn chelators such as metallothioneins (MTs) that are produced in high abundance by the liver and kidneys also contribute to Zn buffering [4], Immune cells such as dendritic cells (DCs) may respond differentially depending on nutrient Zn availability.
Host tissues and circulating cells evolved mechanisms to control Zn levels to mitigate against the adverse effects of Zn deficiency and toxicity on human health. In circulation, serum Zn is mostly bound by albumin, transferrin, and α2-macroglobulin but remains accessible to transporters to balance Zn levels within cells [14]. The primary regulators of mammalian Zn transport are the ZIP Zn importers and the Zn-transport (ZNT) exporters (Figure 1). There are 14 isoforms of mammalian ZIP transporters that share many structural and functional properties, where they facilitate the import of Zn, and sometimes other cations, into the cytoplasm from the extracellular space or from intracellular vesicles and organelles [15]. However, aside from ZIP1 which is found on all cells, expression of the other ZIPs varies depending on the cell type [16], Likewise, the ZNT family includes 10 isoforms, some of which are expressed ubiquitously, while others are cell-type specific [16], ZNTs generally function in reverse of ZIPs, where they export Zn out of the cytoplasm into circulation or cellular vesicles and organelles. Expression of a subset of ZIPs and ZNTs is controlled by the Metal response element-binding Transcription Factor-1 (MTF-1) [17]. MTF-1 is a cytoplasmic transcription factor that undergoes nuclear translocation following Zn binding, where it regulates certain ZIPs and ZNTs to maintain Zn homeostasis [15]. However, not all Zn transporters are MTF-1-regulated, and the diversity of expression and localization of ZIPs and ZNTs highlights the importance of fine-tuning Zn levels depending on the tissue and cell type.
Aside from direct import and export of Zn, intracellular Zn sequestration also must occur to prevent toxicity. A major component of Zn chelation within eukaryotic cells occurs through the action of metallothionein and glutathione. Metallothionein and glutathione are cysteine-containing molecules that link reversible Zn binding with the cellular redox state [18, 19]. This reversible Zn binding allows these molecules to serve as a component of the intracellular Zn buffering system that includes the potential delivery of Zn to apoforms of metalloenzymes [20]. Metallothionien is synthesized in several organs, including the liver and kidneys, and can be found in circulation [21]. In vitro estimates demonstrate that sulfur-containing molecules like metallothionein control approximately 30% of a cell’s Zn buffering capacity [22]. However, the exact dynamics of Zn buffering are likely dependent on cell type and environmental conditions.
Alterations in nutrient Zn availability affect many biological processes, and mechanistic studies have identified how immune system function changes in response to Zn fluctuations [14]. The impact of Zn deficiency on immune system function has been reviewed in-depth previously [14, 16]. Importantly, altered Zn concentrations affect both innate and adaptive branches of the immune system. Within innate immunity, excess Zn can induce chemotaxis of neutrophils, which are one of the primary cell types responsible for innate immune-mediated pathogen clearance [23]. Zn deficiency impedes the antimicrobial activity of neutrophils and macrophages by altering the oxidative burst and inhibiting phagocytosis [24, 25]. The maturation of dendritic cells (DCs), which connect the innate and adaptive immune system through antigen presentation, is also modulated by Zn availability. DCs experiencing Zn deficiency increase expression of major histocompatibility complexes and costimulatory molecules, while Zn excess inhibits this upregulation [26]. In the adaptive immune system, T cells and various T cells subsets are susceptible to alterations in Zn availability; insufficient Zn decreases T cell maturation while increasing apoptosis [27]. Changes in Zn homeostasis alter the balance of TH1, TH2, and TH17 subsets, and Zn supplementation promotes regulatory T cell induction and TH9 differentiation [28–31]. B cells appear to be impacted less by Zn deprivation, but they too experience a reduction in total cell numbers as well as alterations in development and antibody production [32, 33]. Collectively, these studies establish Zn homeostasis as a critical determinant of vertebrate survival and immune cell function.
Zinc is Mobilized During Microbial Infections
In addition to altered immune function in response to changes in dietary Zn, Zn availability can also modulate the response of the vertebrate host to infection and inflammation. During the acute phase of inflammation, serum Zn drops substantially due to proinflammatory cytokines altering Zn transporter expression, which results in the accumulation of Zn-bound metallothioneins by hepatocytes that capture incoming Zn [34] (Figure 2). This decrease in bioavailable Zn is part of an immune response known as nutritional immunity, which was first described for sequestration of Fe from invading microbes [35]. However, our understanding of nutritional immunity has expanded in recent years to include other nutrients, such as manganese (Mn) and Zn [36]. Nutritional immunity is implicated as a critical host defense mechanism for many types of pathogens, and the role of nutrient metal withholding during fungal infections has been reviewed previously [37].
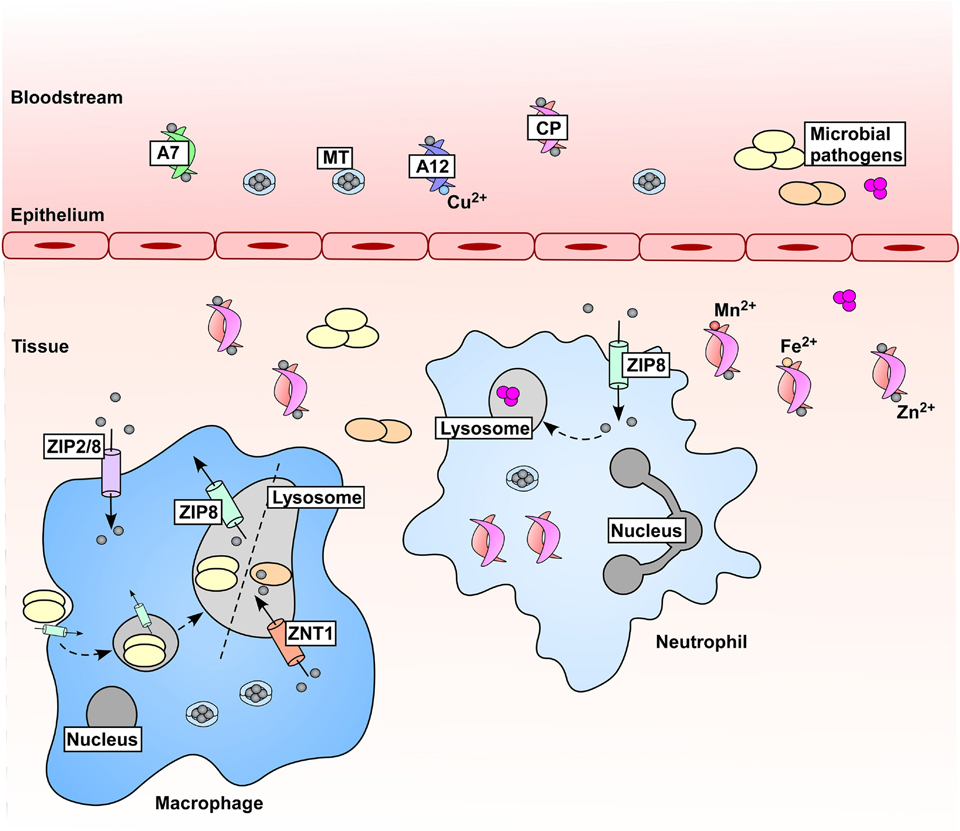
Figure 2.
Zn is mobilized in hosts during microbial infections and inflammation. In response to microbial challenge, serum Zn drops through increased expression of zinc transporters (not depicted) and mobilization and uptake of Zn-binding metallothioneins (MTs). Immune cells contribute to changes in Zn availability, where expression of ZIP Zn importers drives accumulation of Zn within these cells. Zn is also mobilized within immune cells; Mycobacterium tuberculosis (orange ovals) experiences Zn intoxication within macrophages in a ZNT1-dependent manner, and Streptococcus pyogenes (pink spheres) is poisoned by Zn within the neutrophil lysosome [69, 70]. Conversely, the fungal pathogen Histoplasma capsulatum (yellow ovals) has been shown to be Zn-starved within the macrophage lysosome [71]. These two mechanisms (Zn intoxication and Zn starvation) within the macrophage lysosome are denoted by a dashed line. Additionally, metal-chelating proteins are produced as part of the inflammatory response to further reduce Zn availability for pathogens, including those within the S100 protein family. S100A7 (A7) binds Zn and is produced in high abundance by keratinocytes, and S100A12 (A12) binds both Zn and Cu. The heterodimer of S100A8/S100A9, known as calprotectin (CP) [159], binds Zn with high affinity, as well as other divalent cations. CP is a major component of the neutrophil cytoplasmic protein content and is abundant at sites of infection and inflammation within vertebrates.
Host strategies to limit Zn availability from bacterial pathogens include both cell-mediated Zn restriction and extracellular Zn sequestration. Cell-mediated Zn restriction occurs mainly through the activity of Zn transporters. For example, the ZIP8 Zn transporter is expressed by immune cells, where it associates with the lysosomal transmembrane glycoprotein Lamp1 and decreases lysosomal Zn levels [38] (Figure 2). These findings suggest that Zn is actively removed from the lysosome as a strategy to limit nutrient Zn from pathogens trapped within this cellular compartment.
Extracellular Zn sequestration mechanisms implicate members of the S100 protein family as being critical for Zn limitation during infections (Figure 2). S100 proteins are EF-hand calcium-binding proteins found in vertebrates that serve important functions in basic physiology and in the host inflammatory response [39]. A unifying feature among S100 proteins is that they form dimers and may form transition metal binding sites at the dimer interface [39]. S100A8 and S100A9 are unique among S100 proteins in that they preferentially form heterodimers [40]. The heterodimeric S100A8/S100A9 protein complex is called calprotectin (also known as calgranulin A/B or myeloid-related protein 8/14). Calprotectin (CP) is involved in many biological processes, including serving as a damage-associated molecular pattern (DAMP) and as ligands for Toll-like receptor 4 (TLR4), the receptor for advanced glycation end products (RAGE), and CD33 [41–43]. CP is readily detected at infectious foci during infections [44] and accounts for more than 40% of the cytoplasmic protein content of neutrophils [45], underscoring CP’s importance during an immune response.
Part of CP’s immunological importance is due to the protein’s ability to chelate nutrient metals. At the dimer interface between S100A8 and S100A9, two metal binding sites are formed that are termed Site I and Site II. Site I possess broad metal-binding capabilities, including ability to bind Zn, Mn, Fe, Cu, and nickel (Ni) [44, 46–50]. Site II only coordinates Zn with high affinity [47, 51, 52]. The two metal binding sites are important for CP’s antimicrobial activity, as demonstrated by numerous microbial pathogens displaying growth inhibition in vitro in the presence of CP [44, 50, 53–57]. Importantly, addition of exogenous Zn and Mn is generally sufficient to reverse CP-mediated growth inhibition, which demonstrates that metal-binding by CP is adequate to limit microbial growth [39]. However, other nutrient metals have been implicated in binding by CP, including Ni and Fe, among others. While the relative contribution of CP to Ni withholding is not yet known, Fe is increasingly recognized as a metal restricted by CP [57, 58]. Further, mice deficient in producing the CP heterodimer (S100A9−/−) have altered infection susceptibility, demonstrating that CP is critical to infection outcome [44, 46, 53, 54, 59–62].
Other S100 proteins are also implicated in Zn withholding at the host-pathogen interface, including S100A7 and S100A12 (Figure 2). S100A7, also called psoriasin, functions as a homodimer and is constitutively expressed in the skin and at mucosal surfaces. S100A7 binds two Zn ions across the dimer interface [63]. Similar to CP, Zn withholding by S100A7 may contribute to limiting metal availability from bacteria [64]. S100A12, also known as calgranulin C, also functions as a homodimer and binds Zn and Cu at its dimer interface [65]. Recombinant S100A12 can inhibit microbial growth through Zn chelation [66, 67], but its broader contribution to immunity has been difficult to define due to its absence in mice [39]. Additionally, the contribution of S100A12 to Cu withholding during bacterial infections is largely unexplored.
Zn mobilization occurs during infections not only to sequester the metal from invading pathogens, but perhaps to be trafficked within immune cells to impart toxicity (Figure 2). While Zn is redox-inactive, the Irving-Williams series predicts that the high affinity of Zn for metal binding sites promotes aberrant loading of Zn to non-Zn proteins, which leads to toxic effects through mismetallation or other indirect mechanisms [68]. Macrophages infected with Mycobacterium tuberculosis accumulate Zn within the cell that is sufficient to induce bacterial Zn intoxication [69]. Additionally, internalization of Streptococcus pyogenes by human neutrophils results in Zn mobilization that may induce bacterial Zn poisoning [70]. Conversely, macrophages infected with the fungal pathogen Histoplasma capsulatum also accumulate Zn, but this Zn is shuttled from the phagosome to the Golgi apparatus in a granulocyte macrophage-colony stimulating factor (GM-CSF)-dependent manner [71]. Downstream consequences of this Zn shuttling include generation of reactive oxygen species (ROS) to inhibit H. capsulatum growth, presumably by preventing appropriate metalation of Zn/Cu superoxide dismutase enzymes [72]. These results imply that Zn accumulation within immune cells, mediated by shuttling of Zn out of the phagosome, indirectly diminishes pathogen viability [72]. These findings suggest that both Zn starvation and toxicity are employed by the host to limit microbial survival, but precise situations in which starvation or toxicity may be utilized is not well-defined.
Metalloregulators Control Bacterial Zinc Homeostasis
In response to metal restriction by the host, microbes have evolved mechanisms to subvert nutritional immunity during infections. The bacterial response to changes in metal availability is primarily mediated by metalloregulatory proteins, although metabolite-sensing and riboswitch-mediated sensing systems are also described [73]. Generally, these metalloregulators sense changes in cellular metal concentrations and alter gene expression of metal homeostatic systems. These metalloregulators are widely distributed in bacteria, and they respond to metal limitation, metal intoxication, or both conditions. The diversity in bacterial metalloregulators has been reviewed previously [74], therefore we will focus here specifically on mechanisms of bacterial Zn homeostasis.
As a bacterial cell experiences Zn starvation, transcriptional changes must occur to counterbalance these conditions. Many diverse Zn-responsive transcriptional regulators have been identified and reviewed previously [75]. In many bacterial pathogens, the primary regulator for Zn homeostasis is the Zn uptake regulator (Zur) [76, 77]. Zur is a member of the ferric uptake regulator (Fur) family of metallosensing DNA-binding proteins. Metal-sensing by Fur family members is directly mediated by metal binding to the metalloregulator, which induces conformational changes and alters the regulator’s affinity for DNA [78]. Zur is exquisitely sensitive to Zn fluctuations and senses changes to Zn concentrations in the femtomolar (10−15) range to regulate transcription [79]. In Zn-replete conditions, the metal binding sites of Zur are predicted to be occupied. The increased affinity of Zn-bound Zur for DNA permits the metalloregulator to recognize and bind to conserved palindromic inverted repeat regions, termed Zur boxes, in the DNA promoter region of its regulon [78]. The Zur box location generally overlaps with motifs required for effective RNA polymerase recruitment, thereby inhibiting gene transcription. Upon Zn starvation, an increasing proportion of Zur is no longer bound by Zn, which diminishes the affinity of Zur binding to DNA and results in derepression of the Zur regulon [80]. Derepression of the Zur regulon induces several physiological changes, and general themes will be explored for Gram-positive and Gram-negative bacteria.
Zinc Sensing in Gram-Positive Bacteria
Mechanistic studies into the response of Gram-positive bacteria to Zn starvation have been conducted in several organisms [73]. In Bacillus subtilis, Zn starvation is sensed by the metalloregulator Zur [77]. However, non-Fur family Zn metalloregulators have been identified as well; for example, Streptomyces pneumoniae controls Zn uptake through the MarR family member AdcR [81]. Precise investigations into Zn sensing by B. subtilis Zur revealed that the regulator possesses differential activity, corresponding to varying DNA affinity, depending on the number of Zn-binding sites occupied [82] (Figure 3). This differential activity permits a fine-tuned response to Zn starvation that occurs in a step-wise fashion with three distinct stages [83]. First, non-Zn utilizing ribosomal proteins L31* and L33* are expressed, which replace Zn-requiring isoforms to effectively decrease the total cellular requirement of Zn and promote Zn mobilization. Second, the high affinity Zn uptake ABC transport system genes, znuABC, are derepressed to promote Zn acquisition. The ZnuABC system is widely conserved across many species and therefore represents a major metal acquisition system, although other transporters also promote Zn uptake (Table 1). Along with znuABC, the predicted Zn metallochaperone gene zagA (formerly yciC) is also derepressed [84, 85]. Finally, further Zn starvation leads to induction of an additional alternative ribosomal protein S14* to sustain protein synthesis and the Zn-independent GTP cyclohydrolase I FolE2, which permits the continuation of de novo folate biosynthesis, which is a critical metabolite for life [83]. While the extent to which this graded response occurs in other bacteria is not well-defined, the Gram-negative pathogen Salmonella enterica serovar Typhimurium has some features of a graded transcriptional response [86]. Additionally, these same general transcriptional changes occur in other Gram-positive organisms during Zn limitation. These responses include expression of the Zur-regulated Zn uptake systems in Listeria monocytogenes, Bacillus anthracis, Staphylococcus aureus, and Streptococcus pyogenes [87–90] and Zn mobilization via induction of non-Zn requiring ribosomal proteins in Streptomyces coelicolor [91].
Figure 3.
Zn sensing in Gram-positive bacteria. In response to Zn starvation, the Bacillus subtilis Zur regulon experiences derepression in three distinct waves. 1) The non-Zn binding ribosomal proteins L31* and L33* are synthesized to displace the Zn-binding L31/L33 ribosomal proteins, followed by 2) upregulation of the ZnuABC high affinity Zn transport system and the putative metallochaperone ZagA [85]. Lastly, 3) the Zn-independent GTP cyclohydrolase I enzyme FolE2 and the non-zinc requiring S14* ribosomal protein are expressed. Some Gram-positive organisms also produce metal-binding small molecules to capture Zn from the extracellular space. For example, Staphylococcus aureus produces the metal-binding small molecule staphylopine that aids in Zn acquisition [88, 114]. Staphylopine is secreted via CntE, captured by CntA, and imported by the CntBCDF system. Gram-positive bacteria also experience transcriptional changes in response to Zn excess; in B. subtilis, CzrA-regulated genes are expressed during Zn intoxication and includes the P-type ATPase CadA and the cation diffusion family (CDF) transporter CzcD [93]. Expression of these proteins results in Zn efflux from the bacterial cell.
Table 1.
Bacterial pathogen zinc uptake systems.
| Transporter | Pathogen | Role in Pathogenesis | Reference |
|---|---|---|---|
| ZnuABC | Acinetobacter baumannii | Lung colonization | [53] |
| Brucella abortus | Macrophage survival and systemic infection | [137] | |
| Campylobacter jejuni | Cecal colonization | [138] | |
| Escherichia coli | Epithelial cell interactions and urinary tract infections | [76, 139, 140] | |
| Francisella tularensis | Macrophage survival | [141] | |
| Moraxella catarrhalis | Intracellular invasion and lung colonization | [142] | |
| Neisseria gonorrhoeae | Not defined | [143] | |
| Pasturella multocida | Systemic infection | [144] | |
| Proteus mirabilis | Urinary tract infections | [145] | |
| Pseudomonas aeruginosa | Not defined | [146] | |
| Salmonella enterica serovar Typhimurium | Systemic infection and cecal inflammation | [95, 147, 148] | |
| Treponema pallidum | Not defined | [149] | |
| Vibrio cholerae | Gut colonization | [150] | |
| Vibrio parahaemolyticus | Systemic infection | [151] | |
| Yersinia pestis | Systemic infection when yersiniabactin production is inactivated | [112] | |
| ZnuD | Neisseria meningitis | Complement resistance | [124, 152] |
| AdcABC | Streptococcus agalactiae | Survival in human biological fluids | [153] |
| Streptococcus pneumoniae | Survival in biological fluids | [154] | |
| Streptococcus pyogenes | Systemic infection | [90] | |
| ZupT | Escherichia coli | Urinary tract infection | [140] |
| Francisella tularensis | Macrophage survival | [141] | |
| Salmonella enterica serovar Typhimurium | Systemic infection | [155] | |
| ZinT | Escherichia coli | Epithelial cell adherence | [139] |
| Salmonella enterica serovar Typhimurium | Systemic infection when znuA is inactivated | [156] | |
| ZinABC/ZurA | Listeria monocytogenes | Lethality following oral infection | [157] |
| ZevAB | Haemophilus influenzae | Lung colonization | [158] |
| TroABCD | Treponema pallidum | Not defined | [149] |
Bacteria also respond to Zn toxicity through metal-sensing transcriptional regulators. While some metalloregulators can function as both repressors and activators [92], others are functionally divided. In B. subtilis, excess Zn is sensed by the ArsR family of metalloregulators, CzrA [93], which effectively functions in reverse of Zur (Figure 3). When CzrA is not metallated, the protein represses its regulon. Upon metalation, CzrA undergoes a conformational change that lowers its DNA binding affinity and leads to derepression of metal efflux genes that encode a P-type ATPase named CadA and a cation diffusion facilitator type transporter named CzcD [93]. Analogous proteins are involved in detoxification of other divalent cations [94], demonstrating that metal efflux is a broadly conserved bacterial strategy to overcome metal intoxication.
Zinc Sensing in Gram-Negative Bacteria
Consistent with findings in Gram-positive bacteria, Gram-negative organisms primarily sense Zn starvation through the metalloregulator Zur (Figure 4). Structural insights into Escherichia coli Zur-DNA interactions have provided important details about protein conformational changes that occur during Zn binding by Zur. High sequence similarity among Zur homologs from different species suggests these mechanisms are conserved [80]. As is the case with Gram-positive Zur homologs, Zur-regulated derepression in Gram-negative organisms includes induction of genes encoding the high affinity ZnuABC Zn transporters, and these transporters are important for virulence of important human pathogens [53, 95] (Table 1). Additionally, the Zur regulon has been defined for several Gram-negative bacteria, including Yersinia pestis [96], Neisseria meningitidis [97], and Acinetobacter baumannii [98]; in addition to the highly conserved ZnuABC transporters, non Zn-binding ribosomal proteins are also typically increased in expression in response to Zn limitation [74]. Further, Zn starvation induces expression of an outer membrane TonB-dependent Zn transporter named ZnuD in N. meningitidis [99] (Figure 4). Structural studies into N. meningitides ZnuD demonstrate that large extracellular loops directly interact with Zn ions and suggest active uptake of free Zn from the extracellular space. Interestingly, ZnuD possesses structural homology to bacterial heme transporters but does not bind heme [100]; these findings demonstrate that ZnuD is capable of binding free Zn but does not exclude the possibility that ZnuD may bind Zn in some chelated form. A. baumannii also encodes ZnuD homologues that are directly regulated by Zur [98]. In addition to the ZnuD with high homology to the N. meningitidis ZnuD, certain A. baumannii strains encode an additional candidate znuD, denoted znuD2 [98]. However, the relative contribution of each of these ZnuD transporters to A. baumannii Zn homeostasis is unknown.
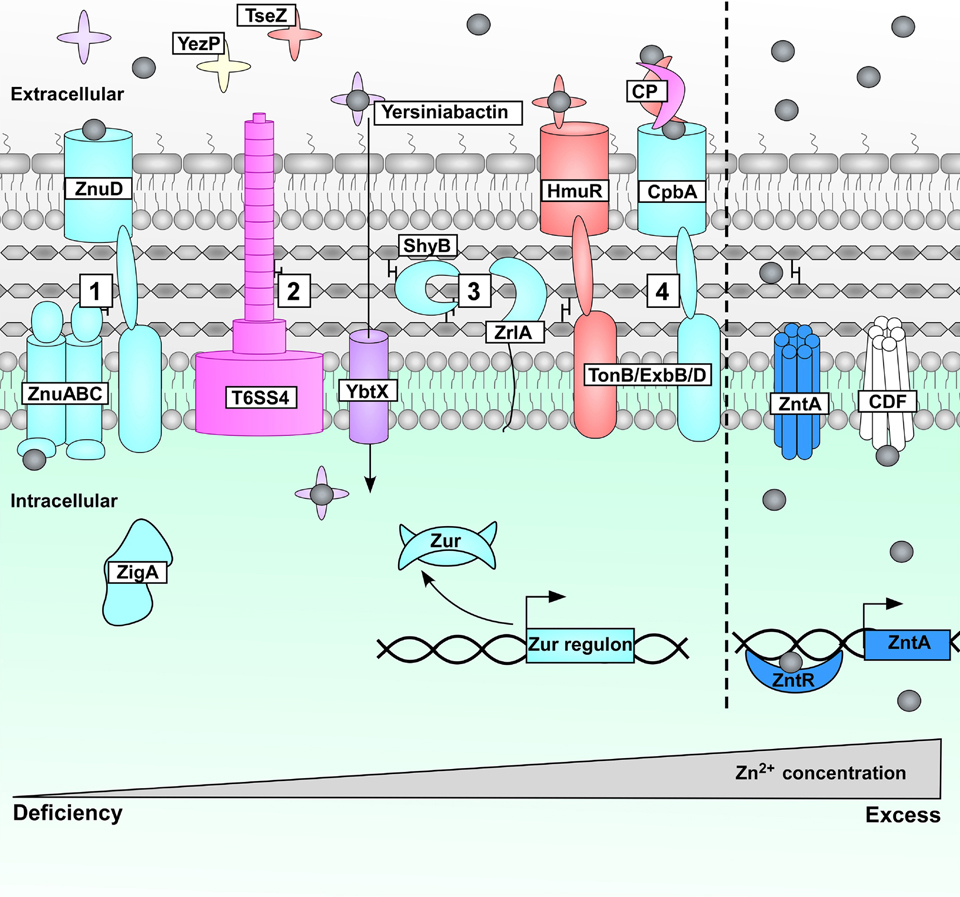
Figure 4.
Zn sensing in Gram-negative bacteria. Zn starvation in Gram-negative bacteria leads to a number of physiological changes. 1) Zur-derepression leads to expression of genes encoding the Zn uptake ZnuABCD system, as well as the predicted Zn metallochaperone ZigA in Acinetobacter baumannii [128]. 2) Zn-binding molecules are produced such as YezP and TseZ, which are secreted by T6SS4, and yersiniabactin, which is captured by YbtX [112, 115, 116]. 3) Enzymes implicated in cell wall homeostasis are induced, including Vibrio cholerae ShyB [121] and A. baumannii ZrlA [120]. 4) TonB-dependent transporters aid in the uptake of Zn-bound molecules; Burkholderia thailandensis HmuR captures the TSS64-secreted TseZ [115], and Neisseria meningititis and Neisseria gonorrhoeae use CpbA/TdfH to bind calprotectin (CP) for Zn acquisition as a form of Zn piracy [123, 124]. During Zn intoxication, Escherichia coli Zn-ZntR causes DNA conformational changes leading to expression of the P-type ATPase ZntA to alleviate the metal toxicity [101]. Many Gram-negative organisms also encode cation diffusion family (CDF) transporters that contribute to overcoming Zn intoxication.
Similar to B. subtilis CzrA, E. coli possesses a separate metalloregulator named ZntR to respond to Zn excess [101] (Figure 4). ZntR is a member of the MerR family of transcriptional regulators. ZntR recognizes conserved inverted repeat sequences in the promoter region of the gene encoding a P-type ATPase named ZntA [101]. Apo-ZntR binds this inverted repeat and causes DNA distortions that prevent gene transcription [102]. Following Zn binding, Zn-ZntR induces DNA untwisting and unkinking that promotes efficient RNA polymerase recruitment and zntA expression [102]. Induction of P-type ATPases and cation diffusion family (CDF) transporters during Zn intoxication have been shown in other Gram-negative organisms as well. In A. baumannii, Zn intoxication leads to significant induction of a wide array of P-type ATPases and CDF transporters that also deplete cellular copper levels [103]. While the transcriptional regulator responsible for these changes is undefined, an A. baumannii strain lacking Zur has increased expression of predicted cation efflux systems and other transporters, which suggesting Zur plays a regulatory role in Zn efflux [98].
Bacterial Zinc Homeostasis Beyond Transporters
More recently, our understanding of bacterial Zn uptake has been expanded to include additional systems other than the ZnuABCD transporters. One strategy that has become increasingly appreciated for Zn acquisition is the production and secretion of Zn-binding small molecules (Figures 3 & 4). This strategy is well-defined for Fe, where Fe-binding molecules termed siderophores are produced that facilitate Fe acquisition in diverse environments [104]. However, some siderophores are capable of binding other nutrient metals, including Zn. The Gram-negative bacterium Pseudomonas putida produces the siderophore pyridine-2,6-bis(thiocarboxylic acid) (PDTC) that is capable of binding both ferric Fe and Zn [105, 106]. Other siderophores including pyochelin, micacocidin, and yersiniabactin have been shown to bind Fe, Zn, and potentially other metals [107–111]. Mechanistic studies into the role of yersiniabactin in Zn uptake revealed that the Gram-negative pathogen Yersinia pestis uses a dedicated Zn-yersiniabactin importer named YbtX to acquire Zn from the molecule; further, genetic inactivation of the znu system and yersiniabactin biosynthetic genes reduces Y. pestis virulence in a septicemic plague model [112]. Zn-binding metallophores have also been implicated in Gram-positive Zn acquisition. For example, Streptomyces coelicolor produces a small molecule termed coelibactin which may bind Zn [113], and S. aureus produces the metallophore staphylopine with broad metal-chelating abilities that affect Zn homeostasis [88, 114].
Type VI secretion systems (T6SSs) are multiprotein machines used by many Gram-negative bacterial species to translocate effectors into neighboring cells and have been implicated in Zn acquisition (Figure 4). Burkholderia thailandensis produces a Zn-scavenging molecule named TseZ that is secreted through a specific T6SS, termed T6SS4. Zn-bound TseZ is then imported into the bacterial cell using the heme transporter HmuR specifically during conditions of oxidative stress, where Zn may be used to populate Cu/Zn superoxide dismutase enzymes and ameliorate potential damage from reactive oxygen species [115]. A similar model also occurs in Y. pseudotuberculosis, where the oxidative stress regulator OxyR induces expression of T6SS4 [116]. Additionally, ZntR was recently identified as a transcriptional activator of the Y. pseudotuberculosis T6SS4 [117], which is consistent with the observation that Zn deficiency promotes increased oxidative damage [118]. This T6SS4 can translocate a Zn-binding molecule named YezP that aids in Zn uptake. While a dedicated importer for YezP is not known, there likely exists an energy-dependent transporter that facilitates YezP uptake, as is the case for other metal-binding small molecules [104, 116].
Cell wall modifications are necessary to construct complex secretion systems and other macromolecular structures. Given the induction of T6SSs and Zn uptake machinery during Zn limitation, there may be changes to the bacterial cell envelope that occur specifically during nutrient starvation. Indeed, members of the genus Acinetobacter are morphologically-constricted to shortened, rounded cells during nutrient limitation and significantly alter the abundance of major peptidoglycan muropeptides [119, 120]. Further, the M15 family Zn-binding peptidase ZrlA contributes to these muropeptide changes and promotes efficient Zn uptake and cell envelope barrier function [120]. In Vibrio cholerae the M23 family Zn-binding endopeptidase ShyB is implicated in cell wall maintenance during Zn limitation [121]. Importantly, both ZrlA and ShyB are directly regulated by Zur and collectively demonstrate that bacterial pathogens encode peptidoglycan-modifying enzymes that are important for surviving Zn limitation (Figure 4).
In addition to the production of metal-chelating molecules by bacterial pathogens, some bacteria can utilize host-derived molecules as a metal source. For example, S. aureus can use human hemoglobin as its sole Fe source through deployment of the iron-regulated surface determinant (Isd) system [122]. As the second most abundant trace metal in humans, Zn scavenging within the vertebrate host may be an effective strategy to subvert nutritional immunity. Consistent with this prediction, the N. meningitidis TonB-dependent outer membrane receptor protein CpbA is expressed during Zn starvation [99] (Figure 4). CpbA is capable of binding human CP, and the presence of CpbA permits N. meningitidis to use CP as its sole Zn source. Additionally, the CpbA homolog in Neisseria gonorrhoeae named TdfH also binds CP and permits Zn acquisition from the protein [123]. These “Zn piracy” mechanisms [124] represent an exciting new area of future investigation towards understanding bacterial Zn acquisition during vertebrate colonization.
Bacterial Zinc Buffering and Allocation
Metal availability varies widely across environments and niches. Therefore, bacterial survival is largely dependent on systems to maintain cellular metabolism despite fluctuations in available nutrients. Considering there is essentially no free Zn within a bacterial cell despite the relatively high total Zn level, Zn must exist in chelated forms that is accessible during Zn starvation [79]. In B. subtilis, Zn limitation induces expression of non-Zn binding ribosomal proteins and the Zn-independent folate biosynthesis enzyme FolE2 [83, 125](Figure 5). In a system analogous to eukaryotic metallothionein, B. subtilis uses the low-molecular weight molecule bacillithiol to maintain an intracellular labile Zn pool [126]. Similarly, E. coli uses glutathione to buffer Zn and other divalent cations [127], A. baumannii utilizes the amino acid L-histidine as a component of its labile pool [128]; during conditions of Zn starvation, A. baumannii upregulates the histidine utilization (Hut) system, thereby catabolizing cellular Zn-histidine complexes and increasing levels of bioavailable Zn [128] (Figure 5). However, the complete inventory of molecules capable of aiding in Zn buffering is not well-defined and warrants further investigation.
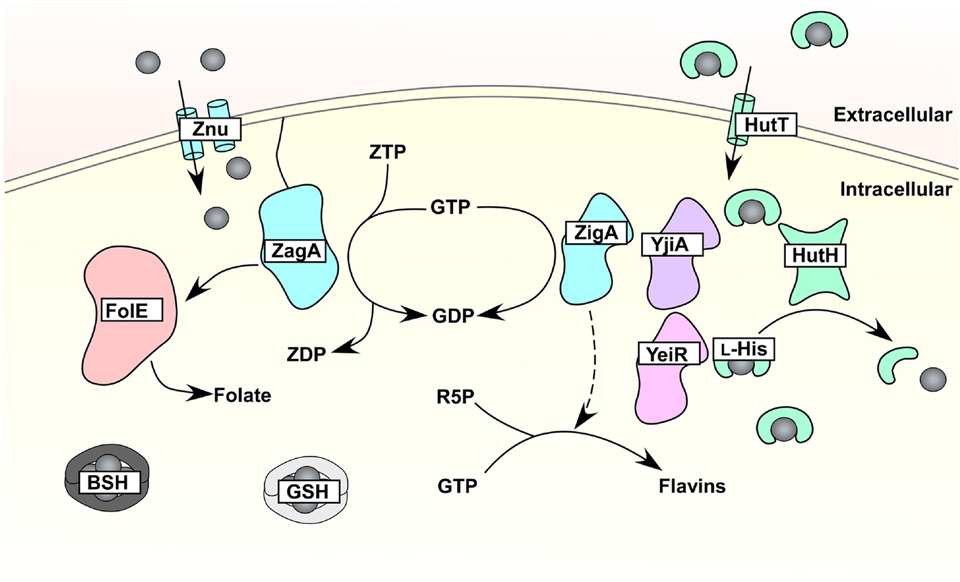
Figure 5.
Bacterial systems for Zn buffering. In order to buffer cellular changes to Zn availability, bacteria employ small molecules to bind excess Zn that can be accessed during zinc limitation. Bacillithiol (BSH) in Bacillus subtilis, glutathione (GSH) in Escherichia coli, and L-histidine in Acinetobacter baumannii serve as components of the labile Zn pool [126–128]. In A. baumannii, the histidine transporter HutT captures Zn-L-His, and its subsequent HutH-mediated degradation is hypothesized to liberate Zn [128]. The delivery of Zn to metalloenzymes remains an area of active investigation, but the mobilization of enzymes involved in essential cofactor biosynthesis implicates these pathways targets for Zn metallochaperone activity. Consistent with this prediction, B. subtilis folate biosynthesis and A. baumannii riboflavin biosynthesis are stressed during Zn limitation. In B. subtilis, the metallochaperone ZagA responds to Zn limitation and the purine alarmone ZTP to interact with, and possibly metallate, the Zn-dependent folate biosynthetic enzyme FolE [85]. In A. baumannii, the predicted metallochaperone ZigA contributes to maintenance of cellular flavin levels during Zn limitation through an undefined mechanism [58]. Possible metallochaperone interactions are denoted with arrows in the figure.
The requirement of Zn for many cellular processes suggests that a mechanism exists to ensure appropriate metalation of cognate metalloproteins, particularly during times of Zn starvation. For metalloregulators, differences in standard free energies for metal complex formation compared to relative metal-binding affinities dictates regulator-metal specificity [86]. However, the process of appropriate metallation is likely more complex for diverse metalloenzymes. To aid in proper metal allocation, members of the G3E GTPase superfamily have been identified as metallochaperones and/or metal insertases [129]. Four subfamilies exist within the G3E superfamily. Two of the subfamilies, represented by the metallochaperones UreG and HypB, are involved in Ni incorporation into the Ni metalloenzymes urease and hydrogenase, respectively [130, 131]. A third family is represented by MeaB, which is involved in methylmalonyl-CoA mutase activation [132]. The fourth subfamily, denoted the COG0523 subfamily, is less defined but is conserved in all domains of life [129]. Genomic analyses suggest that a subset of COG0523 members are Zur-regulated and may therefore serve as Zn metallochaperones [129]. Representative members include E. coli YjiA and YeiR, B. subtilis ZagA, and A. baumannii ZigA. Each of these proteins bind Zn and possess GTPase activity [128, 133–135] (Figure 5). Since Zn is required for many essential cellular processes, COG0523 members may aid in the prioritization of Zn to core metabolic processes when the metal is limited [58]. Consistent with this prediction, analyses of the response of B. subtilis and A. baumannii to Zn starvation revealed that folate and riboflavin biosynthesis are hindered, respectively [58, 85]. In B. subtilis, ZagA interacts with the Zn-dependent FolE enzyme and aids in folate biosynthesis during Zn starvation, and this interaction is dependent on the Z nucleotide ZTP [85]. In A. baumannii, severe Zn restriction hinders de novo flavin biosynthesis [58]. This flavin deficiency is exacerbated in a strain lacking zigA [58], which suggests that ZigA impacts flavin biosynthesis. These studies position Zn metallochaperones at important metabolic hubs, and uncovering other processes altered by COG0523 members represents an exciting area of future research.
Concluding Remarks
Zn is required for life, which necessitates that both bacterial pathogens and vertebrate hosts have evolved strategies to acquire and maintain appropriate Zn levels. Members of the S100 protein family such as CP are capable of withholding Zn from invading pathogens; however, the extent to which Zn starvation occurs in diverse sites within the host is unexplored, but it is likely niche- and pathogen-specific. For example, S. aureus microcolonies experience heterogeneous Fe starvation even within a single tissue [136], which suggests a complex interplay in metallostasis between host and pathogen that that has yet to be defined.
Within a bacterial cell, Zn starvation upregulates Zn uptake machinery, but it also has major consequences for Zn-dependent metabolic processes. Zn starvation has been shown to change ribosome composition, alter bacterial cell wall dynamics, and impact labile Zn pools within the cell. Additionally, representative COG0523 members ZigA and ZagA have been implicated in Zn allocation to metalloenzymes but further exploration is required to determine their precise mechanisms of action as well as the identity of their client proteins. Interestingly, COG0523 members are also present in humans [129], which suggests that understanding their functionality within bacteria may inform metal homeostasis more broadly. Interrogating systems used by both vertebrates and microbes to balance nutrient metals has the potential to improve human health while simultaneously broadening our understanding of metal biology (see Outstanding Questions).
Outstanding Questions.
How do local changes in local metal availability within vertebrates impact infection progression?
What is the precise chemical speciation of zinc and other metal ions within biological systems?
Do zinc-specific siderophores (“zincophores”) exist broadly in bacteria and aid in zinc acquisition?
How do zinc-binding metalloenzymes ensure correct cofactor incorporation, and what is the relative contribution of COG0523 family members to this process?
How is host-mediated zinc intoxication and starvation balanced to limit virulence of diverse pathogens?
Highlights.
Zinc is a redox-inactive nutrient metal required for catalytic activity and/or structural stability for thousands of proteins throughout life.
Vertebrate hosts and bacterial pathogens have evolved parallel mechanisms for balancing the essentiality of zinc with its inherent toxicity.
Zinc homeostasis relies on a complex network of metal transporters linked to zinc buffering systems.
Members of a GTPase subfamily are implicated as zinc-specific metallochaperones, which aid in metal delivery to cognate metalloenzymes.
Acknowledgments
We thank members of the Skaar laboratory for critical evaluation of this manuscript. Z.R.L. was supported by F31 A1136255 and T32 ES007028 from the National Institutes of Health (NIH). E.P.S. was supported by NIH grants R01 AI101171, R01 AI069233, R01 AI073843, and R01 A1138581.
Glossary
- Damage-associated molecular patterns (DAMPs)
molecules produced by damaged, stressed, or dying cells that can escalate an inflammatory response and can occur in the absence of a microbial infection
- Glutathione
tripeptide containing glycine, glutamate, and cysteine that possesses metal-binding properties
- Irving-Williams series
the relative stability by which transition metals form stable complexes. The series is as follows (from least stable to most stable): Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > zn2+
- Metalloenzyme
Enzymes that contain metal ions that are either covalently-bound or bound to prosthetic groups. The metal serves as a cofactor for enzymatic activity, as opposed to a metalloprotein, which may only contain metals for structural stability
- Metallophore
Small molecules with the ability to bind diverse metals; derived from ‘siderohpore,’ which refers to iron-binding small molecules
- Metalloregulatory proteins
Transcriptional regulators that respond to changes in metal availability, usually by direct physical interactions with the metal the regulator is sensing
- Metallothionein
small, cysteine-rich metal binding protein
- Mismetallation
The process whereby the incorrect metal is bound to a metal-binding protein
- Nutritional immunity
the process whereby a host limits the availability of nutrients to defend against infection
- Regulon
The collective genes that are regulated by a specific transcriptional regulator
- Trace metal
metals that are required for biological functions but exist in low abundance within a given system. The precise definition of a trace metal is field-specific, but within vertebrates trace metals are also known as micronutrients and include elements such as iron, copper, magnesium, and selenium
- Transition metals
elements within the central block of the periodic table that have variable outer shell electrons. Zn is not a true transition metal, owing to its filled outer shell. Transition metals are also known as d-block elements
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreini C, et al. (2006) Zinc through the three domains of life. Journal of proteome research 5, 3173–3178 [DOI] [PubMed] [Google Scholar]
- 2.Andreini C, et al. (2006) Counting the zinc-proteins encoded in the human genome. Journal of proteome research 5, 196–201 [DOI] [PubMed] [Google Scholar]
- 3.Hood M.l. and Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10, 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King JC, et al. (2000) Zinc homeostasis in humans. The Journal of nutrition 130, 1360S–1366S [DOI] [PubMed] [Google Scholar]
- 5.Eggleton WG (1940) The zinc and copper contents of the organs and tissues of Chinese subjects. Biochem J 34, 991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kury S, et al. (2002) Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 31, 239–240 [DOI] [PubMed] [Google Scholar]
- 7.Wessells KR and Brown KH (2012) Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PloS one 7, e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maret W and Sandstead HH (2006) Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 20, 3–18 [DOI] [PubMed] [Google Scholar]
- 9.Hambidge M (2000) Human zinc deficiency. The Journal of nutrition 130, 1344S–1349S [DOI] [PubMed] [Google Scholar]
- 10.Broun ER, et al. (1990) Excessive zinc ingestion. A reversible cause of sideroblastic anemia and bone marrow depression. JAMA 264, 1441–1443 [DOI] [PubMed] [Google Scholar]
- 11.Potter JL (1981) Acute zinc chloride ingestion in a young child. Ann Emerg Med 10, 267–269 [DOI] [PubMed] [Google Scholar]
- 12.McKinney PE, et al. (1994) Acute zinc chloride ingestion in a child: local and systemic effects. Ann Emerg Med 23, 1383–1387 [DOI] [PubMed] [Google Scholar]
- 13.Trumbo P, et al. (2001) Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101, 294–301 [DOI] [PubMed] [Google Scholar]
- 14.Rink L and Haase H (2007) Zinc homeostasis and immunity. Trends Immunol 28, 1–4 [DOI] [PubMed] [Google Scholar]
- 15.Kimura T and Kambe T (2016) The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. IntJ Mol Sei 17, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaventura P, et al. (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14, 277–285 [DOI] [PubMed] [Google Scholar]
- 17.Giedroc DP, et al. (2001) Metal response element (MRE)-binding transcription factor-1 (MTF-1): structure, function, and regulation. Antioxidants & redox signaling 3, 577–596 [DOI] [PubMed] [Google Scholar]
- 18.Maret W (2000) The function of zinc metallothionein: a link between cellular zinc and redox state. The Journal of nutrition 130, 1455S–1458S [DOI] [PubMed] [Google Scholar]
- 19.Jiang LJ, et al. (1998) The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proceedings of the National Academy of Sciences of the United States of America 95, 3483–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob C, et al. (1998) Control of zinc transfer between thionein, metallothionein, and zinc proteins. Proceedings of the National Academy of Sciences of the United States of America 95, 3489–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman MT, et al. (2017) Origin, Function, and Fate of Metallothionein in Human Blood. Rev Physiol Biochem Pharmacol 173, 41–62 [DOI] [PubMed] [Google Scholar]
- 22.Krezel A, et al. (2007) The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch Biochem Biophys 463, 188–200 [DOI] [PubMed] [Google Scholar]
- 23.Hujanen ES, et al. (1995) Polymorphonuclear leukocyte chemotaxis induced by zinc, copper and nickel in vitro. Biochim Biophys Acta 1245, 145–152 [DOI] [PubMed] [Google Scholar]
- 24.Sheikh A, et al. (2010) Zinc influences innate immune responses in children with enterotoxigenic Escherichia coli-induced diarrhea. The Journal of nutrition 140, 1049–1056 [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa H, et al. (2000) Effects of zinc on the reactive oxygen species generating capacity of human neutrophils and on the serum opsonic activity in vitro. Luminescence 15, 321–327 [DOI] [PubMed] [Google Scholar]
- 26.Kitamura H, et al. (2006) Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol 7, 971–977 [DOI] [PubMed] [Google Scholar]
- 27.King LE, et al. (2005) Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J Am Coll Nutr 24, 494–502 [DOI] [PubMed] [Google Scholar]
- 28.Prasad AS (2000) Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis 182 Suppl 1, S62–68 [DOI] [PubMed] [Google Scholar]
- 29.Kitabayashi C, et al. (2010) Zinc suppresses Th17 development via inhibition of STAT3 activation. Int Immunol 22, 375–386 [DOI] [PubMed] [Google Scholar]
- 30.Rosenkranz E, et al. (2016) Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol Nutr Food Res 60, 661–671 [DOI] [PubMed] [Google Scholar]
- 31.Maywald M, et al. (2018) Zinc supplementation plays a crucial role in T helper 9 differentiation in allogeneic immune reactions and non-activated T cells. J Trace Bern Med Biol 50, 482–488 [DOI] [PubMed] [Google Scholar]
- 32.Stefanidou M, et al. (2006) Zinc: a multipurpose trace element. Arch Toxicol 80, 1–9 [DOI] [PubMed] [Google Scholar]
- 33.DePasquale-Jardieu P and Fraker PJ (1984) Interference in the development of a secondary immune response in mice by zinc deprivation: persistence of effects. The Journal of nutrition 114, 1762–1769 [DOI] [PubMed] [Google Scholar]
- 34.Aydemir TB, et al. (2012) Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia). PloS one 7, e48679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg ED (1974) Iron and susceptibility to infectious disease. Science 184, 952–956 [DOI] [PubMed] [Google Scholar]
- 36.Palmer LD and Skaar EP (2016) Transition Metals and Virulence in Bacteria. Annu Rev Genet 50, 67–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford A and Wilson D (2015) Essential metals at the host-pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens. FEMS Yeast Res 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyle CJ, et al. (2017) Elemental Ingredients in the Macrophage Cocktail: Role of ZIP8 in Host Response to Mycobacterium tuberculosis. Int J Mol Sei 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zackular JP, et al. (2015) Nutritional Immunity: S100 Proteins at the Host-Pathogen Interface. The Journal of biological chemistry 290, 18991–18998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter MJ and Chazin WJ (1998) High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. The Journal of biological chemistry 273, 12427–12435 [DOI] [PubMed] [Google Scholar]
- 41.Vogl T, et al. (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13, 1042–1049 [DOI] [PubMed] [Google Scholar]
- 42.Leclerc E, et al. (2009) Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta 1793, 993–1007 [DOI] [PubMed] [Google Scholar]
- 43.Eksioglu EA, et al. (2017) Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fc-engineered CD33 antibody Bl 836858. Leukemia 31, 2172–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corbin BD, et al. (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 [DOI] [PubMed] [Google Scholar]
- 45.Yui S, et al. (2003) Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull 26, 753–760 [DOI] [PubMed] [Google Scholar]
- 46.Kehl-Fie TE, et al. (2011) Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 10, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damo SM, et al. (2013) Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proceedings of the National Academy of Sciences of the United States of America 110, 3841–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakashige TG, et al. (2015) Human calprotectin is an iron-sequestering host-defense protein. Nature chemical biology 11, 765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakashige TG, et al. (2017) Nickel Sequestration by the Host-Defense Protein Human Calprotectin. Journal of the American Chemical Society 139, 8828–8836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Besold AN, et al. (2017) The role of calprotectin in withholding zinc and copper from Candida albicans. Infection and immunity [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korndorfer IP, et al. (2007) The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol 370, 887–898 [DOI] [PubMed] [Google Scholar]
- 52.Brophy MB, et al. (2012) Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. Journal of the American Chemical Society 134, 18089–18100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hood MI, et al. (2012) Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS pathogens 8, e1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaddy JA, et al. (2014) The host protein calprotectin modulates the Helicobacter pylori cag type IV secretion system via zinc sequestration. PLoS pathogens 10, e1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clohessy PA and Golden BE (1995) Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol 42, 551–556 [DOI] [PubMed] [Google Scholar]
- 56.Clark HL, et al. (2016) Zinc and Manganese Chelation by Neutrophil S100A8/A9 (Calprotectin) Limits Extracellular Aspergillus fumigatus Hyphal Growth and Corneal Infection. Journal of immunology 196, 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zygiel EM, et al. (2019) The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa. The Journal of biological chemistry [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, et al. (2019) Multi-metal Restriction by Calprotectin Impacts De Novo Flavin Biosynthesis in Acinetobacter baumannii. Cell Chem Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zackular JP, et al. (2016) Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urban CF, et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS pathogens 5, e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Achouiti A, et al. (2012) Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS pathogens 8, e1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wakeman CA, et al. (2016) The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nature communications 7, 11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brodersen DE, et al. (1999) Zinc-binding site of an S100 protein revealed. Two crystal structures of Ca2+-bound human psoriasin (S100A7) in the Zn2+-loaded and Zn2+-free states. Biochemistry 38, 1695–1704 [DOI] [PubMed] [Google Scholar]
- 64.Glaser R, et al. (2005) Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol 6, 57–64 [DOI] [PubMed] [Google Scholar]
- 65.Moroz OV, et al. (2003) Structure of the human S100A12-copper complex: implications for host-parasite defence. Acta Crystallogr D Biol Crystallogr 59, 859–867 [DOI] [PubMed] [Google Scholar]
- 66.Haley KP, et al. (2015) The Human Antimicrobial Protein Calgranulin C Participates in Control of Helicobacter pylori Growth and Regulation of Virulence. Infection and immunity 83, 2944–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shank JM, et al. (2018) The Host Antimicrobial Protein Calgranulin C Participates in the Control of Campylobacter jejuni Growth via Zinc Sequestration. Infection and immunity 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braymer JJ and Giedroc DP (2014) Recent developments in copper and zinc homeostasis in bacterial pathogens. CurrOpin Chem Biol 19, 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Botella H, et al. (2011) Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10, 248–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ong CL, et al. (2014) An antimicrobial role for zinc in innate immune defense against group A streptococcus. J Infect Dis 209, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 71.Subramanian Vignesh K, et al. (2013) Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39, 697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subramanian Vignesh K, et al. (2013) Zinc sequestration: arming phagocyte defense against fungal attack. PLoS pathogens 9, e1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chandrangsu P, et al. (2017) Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 15, 338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capdevila DA, et al. (2017) Metallochaperones and metalloregulation in bacteria. Essays Biochem 61, 177–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi S and Bird AJ (2014) Zinc’ing sensibly: controlling zinc homeostasis at the transcriptional level. Metallomics : integrated biometal science 6, 1198–1215 [DOI] [PubMed] [Google Scholar]
- 76.Patzer SI and Hantke K (1998) The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Molecular microbiology 28, 1199–1210 [DOI] [PubMed] [Google Scholar]
- 77.Gaballa A and Helmann JD (1998) Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport opérons in Bacillus subtilis. Journal of bacteriology 180, 5815–5821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JW and Helmann JD (2007) Functional specialization within the Fur family of metalloregulators. Biometals 20, 485–499 [DOI] [PubMed] [Google Scholar]
- 79.Outten CE and O’Halloran TV (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488–2492 [DOI] [PubMed] [Google Scholar]
- 80.Gilston BA, et al. (2014) Structural and mechanistic basis of zinc regulation across the E. coli Zur regulon. PLoS biology 12, e1001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reyes-Caballero H, et al. (2010) The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol 403, 197–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma Z, et al. (2011) Sequential binding and sensing of Zn(ll) by Bacillus subtilis Zur. Nucleic acids research 39, 9130–9138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shin JH and Helmann JD (2016) Molecular logic of the Zur-regulated zinc deprivation response in Bacillus subtilis. Nature communications 7, 12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gabriel SE, et al. (2008) Regulation of the Bacillus subtilis yciC gene and insights into the DNA-binding specificity of the zinc-sensing metalloregulator Zur. In Journal of bacteriology, pp. 3482–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chandrangsu P, et al. (2019) Bacillus subtilis FolE is sustained by the ZagA zinc metallochaperone and the alarmone ZTP under conditions of zinc deficiency. Molecular microbiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Osman D, et al. (2019) Bacterial sensors define intracellular free energies for correct enzyme metalation. Nature chemical biology 15, 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalet K, et al. (1999) Characterisation of a new operon encoding a Zur-like protein and an associated ABC zinc permease in Listeria monocytogenes. FEMS Microbiol Lett 174, 111–116 [DOI] [PubMed] [Google Scholar]
- 88.Grim KP, et al. (2017) The Metallophore Staphylopine Enables Staphylococcus aureus To Compete with the Host for Zinc and Overcome Nutritional Immunity. mBio 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kandari D, et al. (2018) Identification, Functional Characterization, and Regulon Prediction of the Zinc Uptake Regulator (zur) of Bacillus anthracis - An Insight Into the Zinc Homeostasis of the Pathogen. Front Microbiol 9, 3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Makthal N, et al. (2017) A Critical Role of Zinc Importer AdcABC in Group A Streptococcus-Host Interactions During Infection and Its Implications for Vaccine Development. EBioMedicine 21,131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin JH, et al. (2007) The zinc-responsive regulator Zur controls a zinc uptake system and some ribosomal proteins in Streptomyces coelicolor A3(2). Journal of bacteriology 189, 4070–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang X, et al. (2017) Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Molecular microbiology 103, 253–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moore CM, et al. (2005) Genetic and physiological responses of Bacillus subtilis to metal ion stress. Molecular microbiology 57, 27–40 [DOI] [PubMed] [Google Scholar]
- 94.Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27, 313–339 [DOI] [PubMed] [Google Scholar]
- 95.Liu JZ, et al. (2012) Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11, 227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y, et al. (2009) Characterization of Zur-dependent genes and direct Zur targets in Yersinia pestis. BMC microbiology 9, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pawlik MC, et al. (2012) The zinc-responsive regulon of Neisseria meningitidis comprises 17 genes under control of a Zur element. Journal of bacteriology 194, 6594–6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mortensen BL, et al. (2014) Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. Journal of bacteriology 196, 2616–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stork M, et al. (2010) An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS pathogens 6, e1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calmettes C, et al. (2015) The molecular mechanism of Zinc acquisition by the neisserial outer-membrane transporter ZnuD. Nature communications 6, 7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brocklehurst KR, et al. (1999) ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Molecular microbiology 31, 893–902 [DOI] [PubMed] [Google Scholar]
- 102.Outten CE, et al. (1999) DNA distortion mechanism for transcriptional activation by ZntR, a Zn(ll)-responsive MerR homologue in Escherichia coli. The Journal of biological chemistry 274, 37517–37524 [DOI] [PubMed] [Google Scholar]
- 103.Hassan KA, et al. (2017) Zinc stress induces copper depletion in Acinetobacter baumannii. BMC microbiology 17, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sheldon JR., et al. (2016) Iron Acquisition Strategies of Bacterial Pathogens. Microbiol Spectr 4 [DOI] [PubMed] [Google Scholar]
- 105.Cortese MS, et al. (2002) Metal chelating properties of pyridine-2,6-bis(thiocarboxylic acid) produced by Pseudomonas spp. and the biological activities of the formed complexes. Biometals 15, 103–120 [DOI] [PubMed] [Google Scholar]
- 106.Leach LH, et al. (2007) The role of the siderophore pyridine-2,6-bis (thiocarboxylic acid) (PDTC) in zinc utilization by Pseudomonas putida DSM 3601. Biometals 20, 717–726 [DOI] [PubMed] [Google Scholar]
- 107.Braud A, et al. (2010) Presence of the siderophores pyoverdine and pyochelin in the extracellular medium reduces toxic metal accumulation in Pseudomonas aeruginosa and increases bacterial metal tolerance. Environ Microbiol Rep 2, 419–425 [DOI] [PubMed] [Google Scholar]
- 108.Braud A, et al. (2009) New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine-iron uptake pathway. Environmental microbiology 11, 1079–1091 [DOI] [PubMed] [Google Scholar]
- 109.Chaturvedi KS, et al. (2012) The siderophore yersiniabactin binds copper to protect pathogens during infection. Nature chemical biology 8, 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kreutzer MF, et al. (2011) Biosynthesis of a complex yersiniabactin-like natural product via the mic locus in phytopathogen Ralstonia solanacearum. Applied and environmental microbiology 77, 6117–6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kobayashi S, et al. (1998) Micacocidin A, B and C, novel antimycoplasma agents from Pseudomonas sp. II. Structure elucidation. J Antibiot (Tokyo) 51, 328–332 [DOI] [PubMed] [Google Scholar]
- 112.Bobrov AG, et al. (2014) The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice. Molecular microbiology 93, 759–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao B, et al. (2012) Structural analysis of cytochrome P450 105N1 involved in the biosynthesis of the zincophore, coelibactin. Int J Mol Sei 13, 8500–8513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghssein G, et al. (2016) Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science 352, 1105–1109 [DOI] [PubMed] [Google Scholar]
- 115.Si M, et al. (2017) The Type VI Secretion System Engages a Redox-Regulated Dual-Functional Heme Transporter for Zinc Acquisition. Cell Rep 20, 949–959 [DOI] [PubMed] [Google Scholar]
- 116.Wang T, et al. (2015) Type VI Secretion System Transports Zn2+ to Combat Multiple Stresses and Host Immunity. PLoS pathogens 11, e1005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang T, et al. (2017) ZntR positively regulates T6SS4 expression in Yersinia pseudotuberculosis. J Microbiol 55, 448–456 [DOI] [PubMed] [Google Scholar]
- 118.Eide DJ (2011) The oxidative stress of zinc deficiency. Metallomics : integrated biometal science 3,1124–1129 [DOI] [PubMed] [Google Scholar]
- 119.James GA, et al. (1995) Digital image analysis of growth and starvation responses of a surface-colonizing Acinetobacter sp. Journal of bacteriology 177, 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lonergan ZR, et al. (2019) An Acinetobacter baumannii, Zinc-Regulated Peptidase Maintains Cell Wall Integrity during Immune-Mediated Nutrient Sequestration. Cell Rep 26, 2009–2018 e2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Murphy SG, et al. (2019) Endopeptidase Regulation as a Novel Function of the Zur-Dependent Zinc Starvation Response. mBio 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mazmanian SK, et al. (2003) Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299, 906–909 [DOI] [PubMed] [Google Scholar]
- 123.Jean S, et al. (2016) Neisseria gonorrhoeae Evades Calprotectin-Mediated Nutritional Immunity and Survives Neutrophil Extracellular Traps by Production of TdfH. Infection and immunity 84, 2982–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stork M, et al. (2013) Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS pathogens 9, e1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nanamiya H, et al. (2004) Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Molecular microbiology 52, 273–283 [DOI] [PubMed] [Google Scholar]
- 126.Ma Z, et al. (2014) Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Molecular microbiology 94, 756–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Helbig K, et al. (2008) Glutathione and transition-metal homeostasis in Escherichia coli. Journal of bacteriology 190, 5431–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nairn BL, et al. (2016) The Response of Acinetobacter baumannii to Zinc Starvation. Cell Host Microbe 19, 826–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haas CE, et al. (2009) A subset of the diverse COG0523 family of putative metal chaperones is linked to zinc homeostasis in all kingdoms of life. BMC genomics 10, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maier T, et al. (1995) GTP hydrolysis by HypB is essential for nickel insertion into hydrogenases of Escherichia coli. Eur J Biochem 230, 133–138 [PubMed] [Google Scholar]
- 131.Mehta N, et al. (2003) Roles of conserved nucleotide-binding domains in accessory proteins, HypB and UreG, in the maturation of nickel-enzymes required for efficient Helicobacter pylori colonization. Microb Pathog 35, 229–234 [DOI] [PubMed] [Google Scholar]
- 132.Hubbard PA, et al. (2007) Crystal structure and mutagenesis of the metallochaperone MeaB: insight into the causes of methylmalonic aciduria. The Journal of biological chemistry 282,31308–31316 [DOI] [PubMed] [Google Scholar]
- 133.Khil PP, et al. (2004) Crystal structure of the Escherichia coli YjiA protein suggests a GTP-dependent regulatory function. Proteins 54, 371–374 [DOI] [PubMed] [Google Scholar]
- 134.Sydor AM, et al. (2013) Metal binding properties of Escherichia coli YjiA, a member of the metal homeostasis-associated COGQ523 family of GTPases. Biochemistry 52, 1788–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blaby-Haas CE, et al. (2012) YeiR: a metal-binding GTPasefrom Escherichia coli involved in metal homeostasis. Metallomics : integrated biometal science 4, 488–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cassat JE, et al. (2018) Integrated molecular imaging reveals tissue heterogeneity driving host-pathogen interactions. Sci Transi Med 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang X, et al. (2006) Deletion of znuA virulence factor attenuates Brucella abortus and confers protection against wild-type challenge. Infection and immunity 74, 3874–3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davis LM, et al. (2009) A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. Journal of bacteriology 191, 1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gabbianelli R, et al. (2011) Role of ZnuABC and ZinT in Escherichia coli 0157:H7 zinc acquisition and interaction with epithelial cells. BMC microbiology 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sabri M, et al. (2009) Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infection and immunity 77, 1155–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moreau GB, et al. (2018) Zinc Acquisition Mechanisms Differ between Environmental and Virulent Francisella Species. Journal of bacteriology 200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murphy TF, et al. (2013) Role of the zinc uptake ABC transporter of Moraxella catarrhalis in persistence in the respiratory tract. Infection and immunity 81, 3406–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen CY and Morse SA (2001) Identification and characterization of a high-affinity zinc uptake system in Neisseria gonorrhoeae. FEMS Microbiol Lett 202, 67–71 [DOI] [PubMed] [Google Scholar]
- 144.Garrido ME, et al. (2003) The high-affinity zinc-uptake system znuACB is under control of the iron-uptake regulator (fur) gene in the animal pathogen Pasteurella multocida. FEMS Microbiol Lett 221, 31–37 [DOI] [PubMed] [Google Scholar]
- 145.Nielubowicz GR, et al. (2010) Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infection and immunity 78, 2823–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pederick VG, et al. (2015) ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Scientific reports 5, 13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Campoy S, et al. (2002) Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar typhimurium virulence. Infection and immunity 70, 4721–4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ammendola S, et al. (2007) High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infection and immunity 75, 5867–5876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Desrosiers DC., et al. (2007) The general transition metal (Tro) and Zn2+ (Znu) transporters in Treponema pallidum: analysis of metal specificities and expression profiles. Molecular microbiology 65, 137–152 [DOI] [PubMed] [Google Scholar]
- 150.Sheng Y, et al. (2015) Dual Zinc Transporter Systems in Vibrio cholerae Promote Competitive Advantages over Gut Microbiome. Infection and immunity 83, 3902–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu M, et al. (2013) Characterization of a novel zinc transporter ZnuA acquired by Vibrio parahaemolyticus through horizontal gene transfer. Front Cell Infect Microbiol 3, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hubert K, et al. (2013) ZnuD, a potential candidate for a simple and universal Neisseria meningitidis vaccine. Infection and immunity 81, 1915–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moulin P, et al. (2016) The Adc/Lmb System Mediates Zinc Acquisition in Streptococcus agalactiae and Contributes to Bacterial Growth and Survival. Journal of bacteriology 198, 3265–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bayle L, et al. (2011) Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Molecular microbiology 82, 904–916 [DOI] [PubMed] [Google Scholar]
- 155.Cerasi M, et al. (2014) The ZupT transporter plays an important role in zinc homeostasis and contributes to Salmonella enterica virulence. Metallomics : integrated biometal science 6, 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Petrarca P, et al. (2010) The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. Journal of bacteriology 192, 1553–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Corbett D, et al. (2012) Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo. Infection and immunity 80, 14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rosadini CV, et al. (2011) A novel zinc binding system, ZevAB, is critical for survival of nontypeable Haemophilus influenzae in a murine lung infection model. Infection and immunity 79, 3366–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vokaty S, et al. (1993) Ovine trypanosomosis: a seroepidemiological survey in coastal Guyana. Rev Elev Med Vet Pays Trop 46, 57–59 [PubMed] [Google Scholar]